Abstract
Positive Platelia Aspergillus test results were observed in consecutive serum samples from an immunocompromised host during amoxicillin-clavulanic acid treatment, and a correlation between plasmatic amoxicillin concentration and galactomannan optical density index was observed. Amoxicillin-clavulanic acid vials tested positive for galactomannan but were negative for Aspergillus DNA.
In recent years, the value of screening for circulating Aspergillus galactomannan (GM) antigen in prolonged neutropenic patients with the Platelia Aspergillus test (Bio-Rad, Marnes-La-Coquette, France) has been published, and, although contrasting results have been reported, this approach proved to have a sensitivity of 89.7% and a specificity of 98.1% (5).
Platelia Aspergillus is a double-sandwich enzyme-linked immunosorbent assay based on the rat anti-GM monoclonal antibody EB-A2, which recognizes the 1→5-β-d galactofuranoside side chains of the Aspergillus GM molecule but is reported to also cross-react with several fungal exoantigens from other genera (4, 7, 8, 10).
In December 2003, a 55-year-old woman treated with immunosuppressive drugs for hypoplastic myelodysplastic syndrome was admitted to our ward because of pneumonia.
Blood, stool, and urine cultures, cytomegalovirus (CMV) pp65 antigenemia, and twice-weekly Platelia Aspergillus screening for circulating GM were negative. A negative tuberculin skin test was observed, and a fine-needle lung biopsy under computed tomography (CT) guidance contained no organisms. Empirical therapy was begun with ceftazidime and amikacin and was later switched to imipenem. Throughout the course, the patient received antifungal prophylaxis with itraconazole oral solution.
Treatment with intravenous amoxicillin-clavulanic acid (AMC) at 2 g of amoxicillin and 200 mg of clavulanic acid three times daily was begun on February 2 because of a painful skin nodule of the left leg, and it was given until 13 February 2004. A diagnostic procedure on the skin lesion was performed and revealed necrotic tissue.
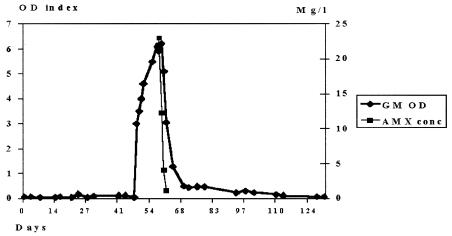
The GM optical density (OD) index increased suddenly from 0.06 on February 3 up to 4.01 2 days later, with an increase of up to 6.1 on 12 February 2004. Platelia Aspergillus assay reactivity was observed in eight consecutive serum samples; the GM antigenemia increase was sustained, and following AMC discontinuation the time to OD index reduction to <0.7 was 10 days.
Samples obtained from 13 to 16 February were examined by high-performance liquid chromatography assay; the concentrations of plasmatic amoxicillin (AMX) were 23, 12.3, 4.1, and 1.1 mg per liter, respectively. On the same days the GM OD indices were 5.9, 6.2, 5.1, and 3.2, respectively (Fig. 1). Clavulanic acid was detected only on 13 February 2004, at a concentration of 5 mg per liter; thereafter it became undetectable.
FIG. 1.
Correlation between plasmatic amoxicillin concentration (AMX conc) and GM OD index. AMC treatment was begun on day 47 and was discontinued on day 58.
During this period, the patient was well and apyretic. No breakthrough bacteremia was documented, CMV pp65 antigenemia was negative, and chest CT scan did not reveal any new pulmonary lesion, which would be consistent with fungal infection.
In order to prove whether or not the false-positive Platelia Aspergillus assay reactions were caused by AMC treatment, we tested one vial of each of three different batches used during this period. The vials were taken from the hospital pharmacy and were diluted according to manufacturer's instructions (2 g of amoxicillin and 200 mg of clavulanic acid in 100 ml of 0.9% NaCl).
All Platelia Aspergillus tests performed on the AMC vials were positive, with OD indices of 6, 1.9, and 0.74, respectively, whereas all the assays on the 0.9% NaCl diluent had negative results. To evaluate the possibility of Aspergillus contamination of AMC vials, blood samples drawn from healthy nonneutropenic adults were mixed with AMC solution from the same batches and were examined with a PCR assay using a DNA probe specifically hybridizing with Aspergillus fumigatus, Aspergillus flavus, and Aspergillus versicolor (TGGGGAACCTCATGGCCTTCACTGGCTGTG; MWG Biotech, Florence, Italy) (3).
PCR results were negative for all three of the samples tested. The finding that AMX concentration and GM OD index correlate appears to be suggestive of an association between AMC therapy and transient GM antigenemia. Nevertheless, we cannot definitively rule out the possibility that our patient had invasive aspergillosis, as she had appropriate high risks and clinical findings (lung infiltrate and skin lesion), and the diagnostic tests performed, including biopsy, are not highly sensitive.
This report appears to be the first case correlating AMC contamination with clinical false-positive tests. However, a high rate of false-positive test results during a surveillance for circulating GM with the Platelia Aspergillus assay in hematology patients receiving piperacillin-tazobactam (TZP) has been recently reported, with cross-reactivity of many of the TZP batches tested (1, 9, 11).
AMX and piperacillin (PIP) are semisynthetic penicillins obtained from the genus Penicillium that contain galactofuran-bearing molecules in the cell wall that cross-react with EB-A2 in vitro (4, 10). Reactions to GM in AMC and PIP vials tested with the EB-A2-coated latex agglutination test, Pastorex Aspergillus (Sanofi Diagnostics Pasteur, Marnes-La-Coquette, France), had been previously reported, and it had been suggested that GM or other similar moieties bearing the epitope reacting with EB-A2 could be carried through the production process of antibiotics into vials designed for therapeutic use (2). GM contamination of AMC vials could also be a possible cause of false-positive Platelia Aspergillus test results in vivo for our patient. PCR findings do not appear to support A. fumigatus, A. flavus, or A. versicolor contamination of the AMC vials during the production process, but they do not exclude other non-Aspergillus causes of contamination, including Penicillium species.
In our patient, plasmatic concentrations of AMX as low as 1.1 mg per liter yielded a positive Platelia Aspergillus test result; this value appears to be far lower than the minimum serum concentration of TZP, 75 mg per liter, that tested positive with the Platelia Aspergillus assay and could be a consequence of variable GM concentrations in contaminated products or different rates of GM bloodstream clearance (6).
In conclusion, we suggest that intravenous AMC treatment could be a possible cause of false-positive Platelia Aspergillus test results in a diagnostic setting in vivo and should be considered when interpreting an increase of GM antigenemia in patients receiving antibiotics and at risk of invasive aspergillosis.
Acknowledgments
We thank Antonia Schlueter for checking the English.
REFERENCES
- 1.Adam, O., A. Auperin, F. Wilquin, J. H. Bourhis, B. Gachot, and E. Chachaty. 2004. Treatment with piperacillin-tazobactam and false-positive Aspergillus galactomannan antigen test results for patients with hematological malignancies. Clin. Infect. Dis. 38:917-920. [DOI] [PubMed] [Google Scholar]
- 2.Ansorg, R., R. Van Den Boom, and P. M. Rath. 1997. Detection of Aspergillus galactomannan antigen in foods and antibiotics. Mycoses. 40:353-357. [DOI] [PubMed] [Google Scholar]
- 3.Heinsele, H., H. Hebart, G. Roller, J. Loffler, I. Rothenhofer, C. A. Muller, R. A. Bowden, J. van Burik, D. Engelhard, L. Kanz, and U. Schumacher. 1997. PCR for detection and differentiation of various fungal pathogens in blood samples. J. Clin. Microbiol. 35:1353-1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kappe, R., and A. Schulze-Berge. 1993. New cause for false-positive results with the Pastorex Aspergillus antigen latex agglutination test. J. Clin. Microbiol. 31:2489-2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maertens, J., J. Verhaegen, K. Lagrou, J. Van Eldere, and M. Boogaerts. 2001. Screening for circulating galactomannan as a noninvasive diagnostic tool for invasive aspergillosis in prolonged neutropenic patients and stem cell transplantation recipients: a prospective validation. Blood. 97:1604-1610. [DOI] [PubMed] [Google Scholar]
- 6.Singh, N., A. Obman, S. Husain, S. Aspinall, S. Mietzner, and J. E. Stout. 2004. Reactivity of platelia Aspergillus galactomannan antigen with piperacillin-tazobactam: clinical implication based on achievable concentrations in serum. Antimicrob Agents Chemother. 48:1989-1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stynen, D., A. Sarfati, A. Goris, M. C. Prevost, M. Lesourd, H. Kamphuis, V. Darras, and J. P. Latgé. 1992. Rat monoclonal antibodies against Aspergillus galactomannan. Infect Immunol. 60:2237-2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stynen, D., A. Goris, J. Sarfati, and J. P. Latgé. 1995. A new sensitive sandwich enzyme-linked immunosorbent assay to detect galactofuran in patients with invasive aspergillosis. J. Clin. Microbiol. 33:497-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sulahian, A., S. Touratier, and P. Ribaud. 2003. False positive test for Aspergillus antigenemia related to concomitant administration of piperacillin and tazobactam. N. Engl. J. Med. 349:2366-2367. [DOI] [PubMed] [Google Scholar]
- 10.Swanink, C. M., J. F. Meis, A. J. Riis, J. P. Donnelly, and P. E. Verweij. 1997. Specificity of a sandwich enzyme-linked immunosorbent assay for detecting Aspergillus galactomannan. J. Clin. Microbiol. 35:257-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Viscoli, C., M. Machetti, P. Cappellano, M. Bucci, P. Bruzzi, M. T. Van Lint, and A. Bacigalupo. 2004. False positive galactomannan platelia Aspergillus test results for patients receiving piperacillin-tazobactam. Clin. Infect. Dis. 38:913-916. [DOI] [PubMed] [Google Scholar]