Abstract
Antigenic sites on the nucleocapsid (N) protein of severe acute respiratory syndrome (SARS) coronavirus (SARS-CoV) were mapped by Pepscan analysis with overlapping peptides that span the N protein sequence. Two major immunodominant epitopes located in the C-terminal region (amino acids [aa] 362 to 412) and middle region (aa 153 to 178) reacted with more than 75% of sera from SARS patients. Several minor immunodominant epitopes were reactive with about 50% of the SARS sera. Antisera from mice immunized with inactivated SARS-CoV recognized the two major immunodominant epitopes and one antigenic site located adjacent to the N-terminal region (aa 76 to 101), which did not react with the sera from SARS patients. Several monoclonal antibodies against SARS-CoV bound to the N- or C-terminal antigenic sites. These results suggest that the above antigenic sites on the N protein are important in eliciting humoral immune response against SARS-CoV in humans and animals and can be used as antigens for developing diagnostic tests.
Severe acute respiratory syndrome (SARS), a new emerging infectious disease, caused a global outbreak in 2003 (9, 16, 23). A novel SARS coronavirus (SARS-CoV) was proven to be the causative agent (3, 7, 19). Its genomic organization is similar to those of other known CoVs (12, 17, 19); however, phylogenetic analyses and sequence comparisons indicate that SARS-CoV does not closely resemble any of the previously characterized CoVs.
CoVs are enveloped positive-stranded RNA viruses featuring the largest viral RNA genomes known to date (27 to 31 kb) (8, 21). Two-thirds of the viral genome starting from the 5′ end encodes replicase proteins for amplication of CoV RNA. The structural proteins of CoVs include spike, membrane, envelope, nucleocapsid (N), and several uncharacterized proteins. The N protein of CoVs is a structural component of the helical N and plays important roles in viral pathogenesis, replication, and RNA packaging (8, 11, 21). Antigenic studies have showed that the N protein is one of the immunodominant antigens in the CoV family (4, 18, 22, 27, 28). Furthermore, the N proteins are capable of inducing protective immune responses against CoV infection (1, 25, 26). These features make it a suitable candidate for developing diagnostic agents and possibly subunit vaccines.
Similar to other CoVs, the N protein of SARS-CoV is the most abundantly expressed of the structural proteins during infection. It is composed of 422 amino acids (aa) and aligns well with N proteins from other representative CoVs. Recently, several reports have shown that the antibodies (Abs) to the SARS-CoV N protein were highly detectable in SARS patients (10, 20, 24), suggesting that this protein may serve as one of the immunodominant antigens in the early diagnosis of infection. However, the antigenic structure of N protein remains to be elucidated. In the present study, we identified several immunodominant epitopes on the N protein by Pepscan analysis of convalescent-phase sera from SARS patients, Abs from mice immunized with inactivated SARS-CoV, and a series of synthetic overlapping peptides spanning the entire sequence of the N protein.
MATERIALS AND METHODS
Recombinant N protein and peptides.
Recombinant N protein was expressed and purified from bacteria. Briefly, the N gene was obtained by reverse transcription-PCR amplification from blood samples taken from a SARS patient in Beijing, China, by using the following primer pair: 5′-ACGGATCCATGTCTGATAATGGACCCCA-3′ and 5′-CTGAATCCTTATGCCTGAGTTGAATCAG-3′ (the underlined sequences are BamHI and EcoRI restriction sites, respectively). The DNA fragment was then digested with restriction enzymes BamHI and EcoRI and ligated with linearized pET32a to generate a vector expressing N protein fused with His tag at the N terminus, which was verified by DNA sequencing. The resulting recombinant plasmid was transformed into Escherichia coli strain BL21, and protein expression was induced with 1 mM isopropyl-l-thio-d-galactopyranoside (IPDG) at 37°C for 5 h. The recombinant N protein was purified from bacterial lysates with Ni-nitrilotriacetic acid agarose (QIAGEN, Valencia, Calif.) according to the procedure provided by the manufacturer and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blotting.
A set of 57 overlapping peptides spanning the entire sequence of the N protein was obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Serum specimens from SARS patients.
Serum samples were collected from 42 convalescent SARS patients 30 to 60 days after the onset of symptoms, based on the clinical diagnosis. The diagnostic criteria for SARS-CoV infection followed the clinical description of SARS released by the World Health Organization (http://www.who.int/csr/sars/guidelines/en/). All of the sera were verified positive for SARS-CoV by immunofluorescence assay and enzyme-linked immunosorbent assay (ELISA) with a commercially available diagnostic kit (Beijing Genomics Institute, Beijing, China). Sera collected from 30 healthy blood donors were used as controls.
Preparation of inactivated SARS-CoV.
SARS CoV strain BJ01 (GenBank accession number AY278488) was used as a virus source and propagated in Vero-E6 cells as described previously (17). The infected cells were harvested and completely lysed by three freeze-thaw cycles. β-Propiolactone was then added to the lysates at a 1:2,000 ratio and incubated at 37°C for 2 h. The inactivated virus was centrifuged at 10,000 rpm for 20 min to remove cell debris, further purified by desalting with Sephadex G-50, concentrated with polyethylene glycol 8000, and filtrated sequentially with Sepharose-CL 2B. The purified virus particles were at >95% purity by high-performance liquid chromatography analysis.
Immunizations.
BALB/c mice were immunized intradermally with 10 μg of purified inactivated viruses as an immunogen in the presence of complete Freund's adjuvant and boosted with a freshly prepared emulsion of the immunogen and Freund's incomplete adjuvant at 2-week intervals. Mouse antisera were collected after three immunizations.
Production of hybridomas.
Hybridomas were generated by standard techniques. Briefly, the splenocytes from the immunized mice were harvested and fused with SP2/0 myeloma cells. Cell culture supernatants from wells containing hybridoma colonies were screened by ELISA in which viral lysates were used as coating antigens, and cells from positive wells were expanded and retested. Cultures that remained positive were subcloned to generate stable hybridoma cell lines expressing monoclonal Abs (MAbs). Mouse ascites was generated by injecting BALB/c mice with hybridomas, and the MAbs were purified by protein A-Sepharose 4 Fast Flow (Amersham Biosciences, Piscataway, N.J.).
Antibody (Ab) detection by ELISA.
The reactivity of immune sera with various antigens was determined by ELISA. Briefly, the recombinant N protein (2 μg/ml) and peptides (10 μg/ml) were used, respectively, to coat 96-well microtiter plates (Corning Costar, Acton, Mass.) in 0.1 M carbonate buffer (pH 9.6) at 4°C overnight. After being blocked with 2% nonfat milk, the plates were incubated with serially diluted antisera at 37°C for 1 h and then washed three times with phosphate-buffered saline containing 0.1% Tween 20. Bound antibodies were detected with alkaline phosphatase-conjugated goat anti-human immunoglobulin G or goat anti-mouse immunoglobulin G (Zymed, South San Francisco, Calif.) at 37°C for 1 h, followed by washing. The reaction was visualized by the use of the p-nitrophenyl phosphate substrate (Zymed), and absorbance at 405 nm was measured by an ELISA plate reader.
Computational analysis of antigenic sites.
Antigenic sequences of the N protein were predicted by the method of Kolaskar and Tongaonkar, based on a table that reflects the occurrence of amino acid residues in experimentally known segmental epitopes (6).
RESULTS
Mapping of immunodominant epitopes on the N protein in SARS patients.
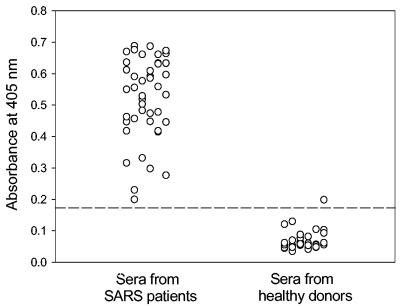
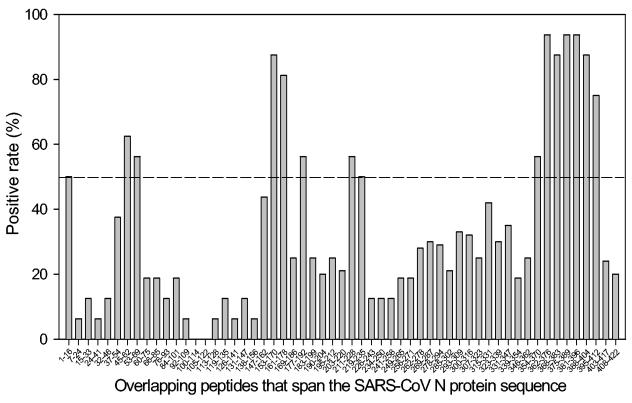
A recombinant N protein fused with His tag at the N terminus was expressed in the bacteria and purified by a Ni-nitrilotriacetic acid column. The protein was verified to be reactive with sera from SARS patients by Western blotting (data not shown). The Abs specific for the N protein in the sera from convalescent-phase patients were detected by ELISA using the recombinant N protein as an antigen. While only one of control serum samples from healthy blood donors reacted weakly with the N protein, all of the 42 serum samples from SARS patients contained considerable anti-N Abs (Fig. 1). To determine the immunodominant epitopes of the N protein, SARS sera were screened against a set of overlapping peptides that cover the entire N protein sequence. As shown in Fig. 2, several antigenic sites induced Ab responses dominant in the SARS patients. A cluster of peptides corresponding to aa 362 to 412 in the C-terminal region and two peptides spanning aa 153 to 178 in the middle region of the N protein reacted with more than 75% of the serum samples from SARS patients. These suggest that these two regions are the major immunodominant epitopes on the N protein. In addition, several peptides corresponding to aa 1 to 16, 45 to 69, 177 to 192, and 211 to 235, respectively, were reactive with about 50% of the SARS sera, suggesting that these regions represent the minor immunodominant epitopes.
FIG. 1.
Detection of anti-N Abs in convalescent-phase sera from SARS patients by ELISA. Recombinant SARS-CoV N protein was used for coating plates, and the sera from 42 SARS patients and 30 healthy blood donors were tested at a 1:50 dilution. Sera were considered positive when the optical density values were above the cutoff value (mean absorbance at 405 nm of sera from healthy blood donors plus 3 standard deviations).
FIG. 2.
Mapping of the immunodominant epitopes on the SARS-CoV N protein by Pepscan analysis against antisera from SARS patients. A set of overlapping peptides that covers the entire N protein sequence was used for the coating plate, and the sera (1:50 dilution) from 42 SARS patients and 30 healthy blood donors were tested by ELISA. Sera were considered positive when the optical density values were above the cutoff value (mean optical density of absorbance at 405 nm of sera from healthy blood donors plus 3 standard deviations). The positive rate of SARS sera for each peptide was calculated.
Mapping of antigenic sites on the N protein in mice.
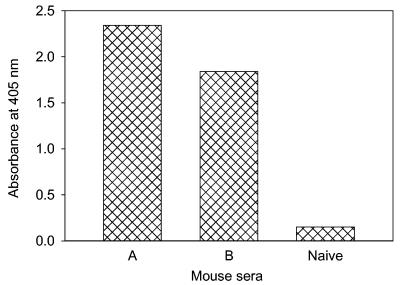
To map the antigenic sites on the SARS-CoV N protein that elicit humoral immune response in animals, two mice were immunized with purified β-propiolactone-inactivated SARS-CoV. As shown in Fig. 3, the mice developed appreciable Ab responses to the N protein after three immunizations. Pepscan analyses against mouse antisera revealed three major antigenic sites on the N protein (Fig. 4). Interestingly, the antigenic sites located in the C-terminal region (aa 362 to 412) and the middle region (aa 153 to 178) overlapped exactly with the immunodominant epitopes that react with most SARS sera. But the one site adjacent to the N-terminal region (aa 76 to 101) was reactive with mouse antiserum only, rather than human antiserum, suggesting that this region may be a specific antigenic site eliciting Ab response in mice.
FIG. 3.
Anti-N Ab responses in the sera of mice immunized with inactivated SARS-CoV. Two immune serum samples and one normal mouse serum sample at a 1:100 dilution were tested against the recombinant N protein by ELISA.
FIG. 4.
Mapping of the antigenic sites on the SARS-CoV N protein by Pepscan analysis against mouse antisera. The reactivity of the mouse antisera with the overlapping peptides that cover the entire sequences of the N protein was determined by ELISA. Mouse sera were tested at a 1:100 dilution.
Epitope mapping of anti-N MAbs.
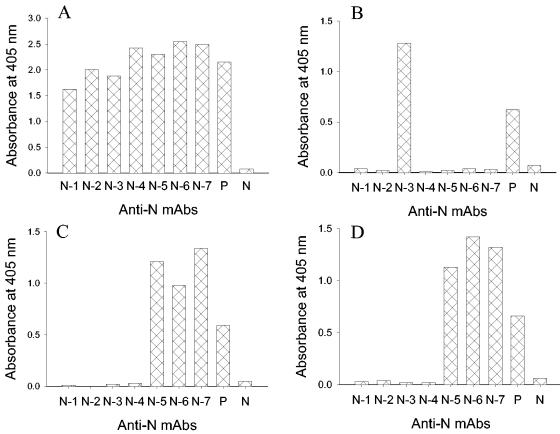
To further gain an insight into the antigenicity of SARS-CoV N protein, we generated seven hybridomas that produce MAbs reactive with the recombinant N protein (Fig. 5A). Pepscan analysis with the whole set of overlapping peptides that span the N protein sequence was used to map epitopes for these MAbs. Three MAbs (N-5, N-6, and N-7) only reacted with two peptides corresponding to aa 76 to 93 and aa 84 to 101 (Fig. 5C and D), respectively, corresponding to the N-terminal antigenic site described above, indicating that the overlapping sequence aa 84 to 93 is their epitope. One MAb (N-3) reacted with the peptide corresponding to aa 388 to 404 only, suggesting that its epitope is located in the C-terminal antigenic site (Fig. 5B). Given that this peptide was also reactive with human SARS sera and mouse antisera against SARS-CoV, this site is highly immunogenic in humans and animals. The remaining three MAbs (N-1, N-2, and N-4) did not react with any of the peptides, implying that their epitopes may contain some unique structures or conformations. Further characterization of these MAbs is needed to elucidate their target sites on the N protein.
FIG. 5.
Epitope mapping of anti-N MAbs. Reactivity of anti-N MAbs with the recombinant N protein and peptides derived from the N protein was determined by ELISA. (A) The recombinant N protein; (B) the peptide corresponding to aa 388 to 404; (C) the peptide corresponding to aa 76 to 93; (D) the peptide corresponding to aa 84 to 101. Pooled sera from two mice immunized with inactivated SARS-CoV were used as the positive control (P), while a normal mouse serum was used as the negative control (N). The MAbs were tested at a concentration of 10 μg/ml, and the control sera were tested at a 1:100 dilution.
Computational analysis of antigenic sites.
Using the method of Kolaskar and Tongaonkar (6), we predicted 16 antigenic sequences in the N protein (Table 1). Interestingly, most of the immunodominant sites that were identified by Pepscan analysis of the sera from SARS patients and mice immunized by inactivated SARS-CoV contain the predicted antigenic sequences. For instance, the C-terminal and middle immunodominant domains contain the predicted antigenic sequences 14 to 16 and 7, respectively. The N-terminal antigenic site (aa 76 to 101) contains predicted antigenic sequence 3. This suggests that this semiempirical method is applicable for designing peptides as probes to study the antigenicity and immunogenicity of the SARS-CoV N protein.
TABLE 1.
Prediction of antigenic sequences on the N protein of SARS-CoV strain BJ01
| Sequence no. | Region | Sequence |
|---|---|---|
| 1 | 52-59 | SWFTALTQa |
| 2 | 69-75 | RGQGVPI |
| 3 | 83-89 | DQIGYYRa |
| 4 | 106-115 | SPRWYFYYLG |
| 5 | 118-124 | PEASLPY |
| 6 | 130-136 | GIVWVAT |
| 7 | 156-166 | AATVLQLPQGTa |
| 8 | 218-227 | TALALLLLDRa |
| 9 | 229-237 | NQLESKVSG |
| 10 | 243-249 | QGQTVTK |
| 11 | 267-273 | KQYNVTQ |
| 12 | 299-315 | YKHWPQIAQFAPSASAF |
| 13 | 323-339 | MEVTPSGTWLTYHGAIK |
| 14 | 347-363 | FKDNVILLNKHIDAYKTa |
| 15 | 379-385 | EAQPLPQa |
| 16 | 389-398 | KQPTVTLLPAa |
Predicted antigenic sequences corresponding to the immunodominant sites that were identified by Pepscan analysis.
DISCUSSION
Identification of antigenic sites on the SARS-CoV N protein that elicit Ab responses in humans and animals is critical for developing diagnostic tests and vaccines. Here, we show that SARS patients have developed significant anti-N Ab responses during infection, consistent with the recent reports from other groups (10, 20, 24) suggesting that the N protein of SARS-CoV, similar to other CoVs, is a major antigenic determinant. To study the immunological properties of N protein in humans and animals infected or immunized by SARS-CoV, we have mapped the immunodominant epitopes on the N protein by Pepscan analysis. Serum samples from 42 SARS patients and 30 healthy blood donors were screened against a set of synthetic peptides covering the entire sequence of N protein. Two major immunodominant epitopes that reacted with more than 75% of sera from SARS patients were localized at the C-terminal and middle regions, corresponding to aa 362 to 412 and 153 to 178, respectively. There are several minor immunodominant epitopes located at the N-terminal and middle regions of the N protein that were reactive with about 50% of the SARS sera.
The inactivated SARS-CoV was shown to generate protective immunity against SARS-CoV in immunized animals and was recently approved by the State Food and Drug Administration of China as a vaccine for clinical trials (13). However, the major immunological determinants of the antigens have not been clearly determined. In this study, we observed that the mice immunized with the inactivated SARS-CoV induced substantial Ab responses to the N protein, which contains three major antigenic sites. Interestingly, two of the major antigenic sites located in the C-terminal and middle regions overlap exactly the sequences of the major immunodominant epitopes that are reactive with the Abs in sera from SARS patients. The antigenic site located adjacent to the N-terminal region in the N protein did not react with human Abs, but rather only with the murine Abs, including both polyclonal Abs and MAbs produced from the mice immunized with inactivated SARS-CoV. This suggests that some antigenic sites of the N protein may not be immunodominant in the SARS patients but are capable of inducing substantial Ab responses in immunized small animals.
The antigenicity and immunogenicity of the N proteins in the CoV family have been extensively studied (2, 14, 15, 18, 27, 28). All of these CoV N proteins contain multiple immunodominant epitopes and antigenic sites; however, they contain a common epitope located in the C-terminal region that induces strong Ab responses. Since the N protein of SARS-CoV shares similar antigenic properties and high sequence similarity with other CoVs, the full-length N protein may cross-react with Abs against other CoV N proteins, thus limiting the use of SARS-CoV N protein for developing diagnostic tests. The peptides that are derived from antigenic sites may serve as ideal antigens to develop site-specific immunoassays for serological diagnosis. By aligning the N protein sequences of SARS-CoV with those of other CoVs, a short lysine-rich region, corresponding to aa 362 to 381 (KTFPPTEPKKDKKKKTDEAQ), appears to be unique to SARS-CoV. The function of this region is unknown. The highly basic nature of the peptide suggests its putative role in RNA binding. This unique sequence resides in the highly immunogenic C-terminal region, suggesting that it may serve as a SARS-CoV-specific antigen for diagnosis of SARS-CoV infection.
Since the SARS-CoV N protein is highly immunogenic in humans, it may be developed as a subunit vaccine if the anti-N Abs have virus-neutralizing activity. Most recently, Kim et al. (5) demonstrated that DNA vaccines using SARS-CoV N protein as a target antigen were capable of generating strong N-specific humoral and cellular immunity against infection by vaccinia virus expressing the N protein of SARS-CoV. However, it is unclear whether the Ab response induced by these vaccines is effective in neutralizing infectivity of SARS-CoV. It is essential to dissect the Ab responses elicited by the immunodominant epitopes on the N protein. Those antigenic sites that induce harmful humoral immune responses in humans should be excluded from the antigens to design effective and safe vaccines for the prevention of SARS.
REFERENCES
- 1.Boots, A. M., B. J. Benaissa-Trouw, W. Hesselink, E. Rijke, C. Schrier, and E. J. Hensen. 1992. Induction of anti-viral immune responses by immunization with recombinant-DNA encoded avian coronavirus nucleocapsid protein. Vaccine 10:119-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Casal, J. I., M. J. Rodriguez, J. Sarraseca, J. Garcia, J. Plana-Duran, and A. Sanz. 1998. Identification of a common antigenic site in the nucleocapsid protein of European and North American isolates of porcine reproductive and respiratory syndrome virus. Adv. Exp. Med. Biol. 440:469-477. [DOI] [PubMed] [Google Scholar]
- 3.Drosten, C., S. Gunther, W. Preiser, S. van derWerf H. R. Brodt, S. Becker, H. Rabenau, M. Panning, L. Kolesnikova, R. A. Fouchier, A. Berger, A. M. Burguiere, J. Cinatl, M. Eickmann, N. Escriou, K. Grywna, S. Kramme, J. C. Manuguerra, S. Muller, V. Rickerts, M. Sturmer, S. Vieth, H. D. Klenk, A. D. Osterhaus, H. Schmitz, and H. W. Doerr. 2003. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N. Engl. J. Med. 348:1967-1976. [DOI] [PubMed] [Google Scholar]
- 4.Ignjatovic, J., and L. Galli. 1993. Structural proteins of avian infectious bronchitis virus: role in immunity and protection. Adv. Exp. Med. Biol. 342:449-453. [DOI] [PubMed] [Google Scholar]
- 5.Kim, T. W., J. H. Lee, C. F. Hung, S. Peng, R. Roden, M. C. Wang, R. Viscidi, Y. C. Tsai, L. He, P. J. Chen, D. A. Boyd, and T. C. Wu. 2004. Generation and characterization of DNA vaccines targeting the nucleocapsid protein of severe acute respiratory syndrome coronavirus. J. Virol. 78:4638-4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kolaskar, A. S., and P. C. Tongaonkar. 1990. A semi-empirical method for prediction of antigenic determinants on protein antigens. FEBS Lett. 276:172-174. [DOI] [PubMed] [Google Scholar]
- 7.Ksiazek, T. G., D. Erdman, C. S. Goldsmith, S. R. Zaki, T. Peret, S. Emery, S. Tong, C. Urbani, J. A. Comer, W. Lim, P. E. Rollin, S. F. Dowell, A. E. Ling, C. D. Humphrey, W. J. Shieh, J. Guarner, C. D. Paddock, P. Rota, B. Fields, J. DeRisi, J. Y. Yang, N. Cox, J. M. Hughes, J. W. LeDuc, W. J. Bellini, and L. J. Anderson. 2003. A novel coronavirus associated with severe acute respiratory syndrome. N. Engl. J. Med. 348:1953-1966. [DOI] [PubMed] [Google Scholar]
- 8.Lai, M. M., and D. Cavanagh. 1997. The molecular biology of coronaviruses. Adv. Virus Res. 48:1-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee, N., D. Hui, A. Wu, P. Chan, P. Cameron, G. M. Joynt, A. Ahuja, M. Y. Yung, C. B. Leung, K. F. To, S. F. Lui, C. C. Szeto, S. Chung, and J. J. Sung. 2003. A major outbreak of severe acute respiratory syndrome in Hong Kong. N. Engl. J. Med. 348:1986-1994. [DOI] [PubMed] [Google Scholar]
- 10.Liu, X., Y. Shi, P. Li, L. Li, Y. Yi, Q. Ma, and C. Cao. 2004. Profile of antibodies to the nucleocapsid protein of the severe acute respiratory syndrome (SARS)-associated coronavirus in probable SARS patients. Clin. Diagn. Lab. Immunol. 11:227-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Macneughton, M. R., and H. A. Davies. 1978. Ribonucleoprotein-like structures from coronavirus particles. J. Gen. Virol. 39:545-549. [DOI] [PubMed] [Google Scholar]
- 12.Marra, M. A., S. J. Jones, C. R. Astell, R. A. Holt, A. Brooks-Wilson, Y. S. Butterfield, J. Khattra, J. K. Asano, S. A. Barber, S. Y. Chan, A. Cloutier, S. M. Coughlin, D. Freeman, N. Girn, O. L. Griffith, S. R. Leach, M. Mayo, H. McDonald, S. B. Montgomery, P. K. Pandoh, A. S. Petrescu, A. G. Robertson, J. E. Schein, A. Siddiqui, D. E. Smailus, J. M. Stott, G. S. Yang, F. Plummer, A. Andonov, H. Artsob, N. Bastien, K. Bernard, T. F. Booth, D. Bowness, M. Czub, M. Drebot, L. Fernando, R. Flick, M. Garbutt, M. Gray, A. Grolla, S. Jones, H. Feldmann, A. Meyers, A. Kabani, Y. Li, S. Normand, U. Stroher, G. A. Tipples, S. Tyler, R. Vogrig, D. Ward, B. Watson, R. C. Brunham, M. Krajden, M. Petric, D. M. Skowronski, C. Upton, and R. L. Roper. 2003. The genome sequence of the SARS-associated coronavirus. Science 300:1399-1404. [DOI] [PubMed] [Google Scholar]
- 13.Marshall, E., and M. Enserink. 2004. Medicine. Caution urged on SARS vaccines. Science 303:944-946. [DOI] [PubMed] [Google Scholar]
- 14.Martin Alonso, J. M., M. Balbin, D. J. Garwes, L. Enjuanes, S. Gascon, and F. Parra. 1992. Antigenic structure of transmissible gastroenteritis virus nucleoprotein. Virology 188:168-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meulenberg, J. J., A. P. van Nieuwstadt, A. Essen-Zandbergen, J. N. Bos-de Ruijter, J. P. Langeveld, and R. H. Meloen. 1998. Localization and fine mapping of antigenic sites on the nucleocapsid protein N of porcine reproductive and respiratory syndrome virus with monoclonal antibodies. Virology 252:106-114. [DOI] [PubMed] [Google Scholar]
- 16.Poutanen, S. M., D. E. Low, B. Henry, S. Finkelstein, D. Rose, K. Green, R. Tellier, R. Draker, D. Adachi, M. Ayers, A. K. Chan, D. M. Skowronski, I. Salit, A. E. Simor, A. S. Slutsky, P. W. Doyle, M. Krajden, M. Petric, R. C. Brunham, and A. J. McGeer. 2003. Identification of severe acute respiratory syndrome in Canada. N. Engl. J. Med. 348:1995-2005. [DOI] [PubMed] [Google Scholar]
- 17.Qin, E., Q. Zhu, M. Yu, B. Fan, G. Chang, B. Si, B. Yang, W. Peng, T. Jiang, B. Liu, Y. Deng, H. Liu, Y. Zhang, C. Wang, Y. Li, Y. Gan, X. Li, F. Lu, G. Tan, W. Cao, and R. Yang. 2003. A complete sequence and comparative analysis of a SARS-associated virus (isolate BJ01). Chin. Sci. Bull. 48:941-948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodriguez, M. J., J. Sarraseca, J. Garcia, A. Sanz, J. Plana-Duran, and C. J. Ignacio. 1997. Epitope mapping of the nucleocapsid protein of European and North American isolates of porcine reproductive and respiratory syndrome virus. J. Gen. Virol. 78:2269-2278. [DOI] [PubMed] [Google Scholar]
- 19.Rota, P. A., M. S. Oberste, S. S. Monroe, W. A. Nix, R. Campagnoli, J. P. Icenogle, S. Penaranda, B. Bankamp, K. Maher, M. H. Chen, S. Tong, A. Tamin, L. Lowe, M. Frace, J. L. DeRisi, Q. Chen, D. Wang, D. D. Erdman, T. C. Peret, C. Burns, T. G. Ksiazek, P. E. Rollin, A. Sanchez, S. Liffick, B. Holloway, J. Limor, K. McCaustland, M. Olsen-Rasmussen, R. Fouchier, S. Gunther, A. D. Osterhaus, C. Drosten, M. A. Pallansch, L. J. Anderson, and W. J. Bellini. 2003. Characterization of a novel coronavirus associated with severe acute respiratory syndrome. Science 300:1394-1399. [DOI] [PubMed] [Google Scholar]
- 20.Shi, Y., Y. Yi, P. Li, T. Kuang, L. Li, M. Dong, Q. Ma, and C. Cao. 2003. Diagnosis of severe acute respiratory syndrome (SARS) by detection of SARS coronavirus nucleocapsid antibodies in an antigen-capturing enzyme-linked immunosorbent assay. J. Clin. Microbiol. 41:5781-5782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siddell, S., H. Wege, and V. Ter Meulen. 1983. The biology of coronaviruses. J. Gen. Virol. 64:761-776. [DOI] [PubMed] [Google Scholar]
- 22.Stohlman, S. A., C. Bergmann, D. Cua, H. Wege, and R. van der Veen. 1994. Location of antibody epitopes within the mouse hepatitis virus nucleocapsid protein. Virology 202:146-153. [DOI] [PubMed] [Google Scholar]
- 23.Tsang, K. W., P. L. Ho, G. C. Ooi, W. K. Yee, T. Wang, M. Chan-Yeung, W. K. Lam, W. H. Seto, L. Y. Yam, T. M. Cheung, P. C. Wong, B. Lam, M. S. Ip, J. Chan, K. Y. Yuen, and K. N. Lai. 2003. A cluster of cases of severe acute respiratory syndrome in Hong Kong. N. Engl. J. Med. 348:1977-1985. [DOI] [PubMed] [Google Scholar]
- 24.Wang, J., J. Wen, J. Li, J. Yin, Q. Zhu, H. Wang, Y. Yang, E. Qin, B. You, W. Li, X. Li, S. Huang, R. Yang, X. Zhang, L. Yang, T. Zhang, Y. Yin, X. Cui, X. Tang, L. Wang, B. He, L. Ma, T. Lei, C. Zeng, J. Fang, J. Yu, J. Wang, H. Yang, M. B. West, A. Bhatnagar, Y. Lu, N. Xu, and S. Liu. 2003. Assessment of immunoreactive synthetic peptides from the structural proteins of severe acute respiratory syndrome coronavirus. Clin. Chem. 49:1989-1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wasmoen, T. L., N. P. Kadakia, R. C. Unfer, B. L. Fickbohm, C. P. Cook, H. J. Chu, and W. M. Acree. 1995. Protection of cats from infectious peritonitis by vaccination with a recombinant raccoon poxvirus expressing the nucleocapsid gene of feline infectious peritonitis virus. Adv. Exp. Med. Biol. 380:221-228. [DOI] [PubMed] [Google Scholar]
- 26.Wesseling, J. G., G. J. Godeke, V. E. Schijns, L. Prevec, F. L. Graham, M. C. Horzinek, and P. J. Rottier. 1993. Mouse hepatitis virus spike and nucleocapsid proteins expressed by adenovirus vectors protect mice against a lethal infection. J. Gen. Virol. 74:2061-2069. [DOI] [PubMed] [Google Scholar]
- 27.Wootton, S., G. Koljesar, L. Yang, K. J. Yoon, and D. Yoo. 2001. Antigenic importance of the carboxy-terminal beta-strand of the porcine reproductive and respiratory syndrome virus nucleocapsid protein. Clin. Diagn. Lab. Immunol. 8:598-603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wootton, S. K., E. A. Nelson, and D. Yoo. 1998. Antigenic structure of the nucleocapsid protein of porcine reproductive and respiratory syndrome virus. Clin. Diagn. Lab. Immunol. 5:773-779. [DOI] [PMC free article] [PubMed] [Google Scholar]