Abstract
This study represents a long-term follow-up of human patients receiving pig islet xenotransplantation. Eighteen patients had been monitored for up to 9 years for potentially xenotic pig viruses: pig endogenous retrovirus, pig cytomegalovirus, pig lymphotropic herpesvirus, and pig circovirus type 2. No evidence of viral infection was found.
Human xenotransplant recipients are theoretically at risk of infection with animal viruses. Pig endogenous retroviruses (PERVs) have been a major source of anxiety, since they are present in the genome of all pigs and infect human cells (25, 29). Whether PERV truly poses an infection risk will ultimately be determined by in vivo monitoring of patients and experimental animals receiving xenografts. To date, more than 200 patients exposed to pig cells have been tested for evidence of PERV infection (3, 5, 14, 15, 16, 17, 21, 24, 26, 27, 33, 34). Additionally, some 50 nonhuman primates have also been tested for PERV infection (20, 32). No evidence of infection has been found. Three different species of nonhuman primates, which were heavily immunosuppressed and injected with a human cell-adapted virus, also failed to show any evidence of PERV transmission (4, 30). Similar results were obtained when PERVs were injected into small animals, regardless of whether they were immunosuppressed (29-31).
Other pig viruses are able to establish persistent infections or, alternatively, are potentially oncogenic. These viruses include pig lymphotropic herpesvirus (PLHV), pig cytomegalovirus (PCMV), and pig circovirus (PCV) (10, 23, 35), all of which are highly prevalent in pig populations (1, 6, 7, 36). Since PLHV is very similar to lymphoproliferative herpesviruses of other species, this virus should be included in the follow-up program for pig cell transplant recipients. In vitro experiments have shown no transmission of PCMV to human cells (37). However, data on activation of cytomegalovirus in pig-to-primate organ transplantation demonstrate that PCMV may be an important pathogen in immunosuppressed xenograft recipients (22). It has been shown that PCV type 2 can be transmitted to human cells in vitro (14). It seems prudent, therefore, to include testing for a range of pig viruses in the follow-up of transplantation patients.
The aim of this study was to look for evidence of viral infection (PERV, PCMV, PLHV, and PCV type 2) in patients that had received pig islet cell transplants.
Eighteen type 1 diabetic patients received neonatal pig islet cell transplants via three different modes of delivery: encapsulated islets (8), Sertoli islet structures in a specially designed device (38), and free islet cells (Table 1). Pig cells were obtained from pigs of Large White and Cambrough breeds that had been screened extensively for conventional microorganisms and for viruses of particular relevance to xenotransplantation, namely PLHV, PCMV, and PCV. In none of the tested donor pigs were these viruses found (12).
TABLE 1.
Antibodies against PERV proteins and pig cellular proteins detected in xenotransplantation patients
| Patient no. | Transplantation date | Mode of delivery | Wk before (−) or after transplantation | Presence of antibodies against:a
|
|||
|---|---|---|---|---|---|---|---|
| PERV
| |||||||
| gp70 | p27Gag | p15E | Pig proteins | ||||
| 1 | 1993 | Nonencapsulated islets | −214.6 | − | − | − | − |
| 15.9 | − | − | − | − | |||
| 460.3 | ++ | − | − | + | |||
| 2 | 1994 | Nonencapsulated islets | −6.9 | − | − | − | − |
| 2.0 | − | − | − | − | |||
| 14.4 | − | − | − | − | |||
| 410.0 | +++ | − | − | + | |||
| 3 | 1994 | Nonencapsulated islets | −0.4 | − | − | − | − |
| 2.7 | − | − | − | − | |||
| 15.4 | − | − | − | − | |||
| 427.6 | − | − | − | − | |||
| 4 | 1994 | Nonencapsulated islets | 2.4 | + | − | − | − |
| 163.6 | + | − | − | − | |||
| 394.4 | − | − | − | − | |||
| 5 | 1996 | Encapsulated islets | 2.7 | − | + | − | ++ |
| 44.3 | − | +++ | − | ++ | |||
| 312.0 | − | ++ | − | + | |||
| 6 | 1996 | Encapsulated islets | −2.3 | − | − | − | + |
| 2.6 | − | − | − | + | |||
| 42.9 | − | − | − | + | |||
| 311.9 | − | − | − | − | |||
| 7 | 2000 | Islets + Sertoli cells | 0.0 | − | − | − | − |
| 1.1 | − | − | − | + | |||
| 8 | 2000b | Islets + Sertoli cells | −13.0 | − | + | − | − |
| 1.9 | − | ++ | − | − | |||
| 6.0 | − | ++ | − | − | |||
| 19.1 | − | ++ | − | − | |||
| 9 | 2000 | Islets + Sertoli cells | −13.0 | − | − | − | − |
| 1.9 | − | − | − | + | |||
| 5.9 | − | − | − | + | |||
| 10 | 2000 | Islets + Sertoli cells | 1.7 | − | − | − | + |
| 6.6 | − | − | − | + | |||
| 15.3 | − | − | − | + | |||
| 11 | 2000 | Islets + Sertoli cells | −8.9 | − | ++ | − | − |
| 2.7 | − | ++ | − | +/− | |||
| 4.6 | − | ++ | − | +/− | |||
| 12 | 2001b | Islets + Sertoli cells | −1.1 | − | − | − | − |
| 1.7 | − | − | − | − | |||
| 6.6 | − | − | − | − | |||
| 15.3 | − | − | − | − | |||
| 13 | 2001 | Islets + Sertoli cells | −8.9 | − | − | − | − |
| 2.7 | − | − | − | + | |||
| 4.6 | − | − | − | + | |||
| 7.6 | − | − | − | + | |||
| 14 | 2001 | Islets + Sertoli cells | −6.0 | − | − | − | − |
| 2.7 | − | − | − | ++ | |||
The presence of antibodies is characterized as follows: −, none; +/−, none or weakly positive; +, weakly positive; ++, moderately positive; +++, strongly positive.
Microchimerism (presence of pig cells) was detected on one occasion; repeated bleeding after 1 month did not show any further evidence of PERV or pig cellular marker.
Only one recipient had been immunosuppressed. This patient had previously received an allogeneic kidney transplant. All patients were given approximately 10,000 islet equivalents per kg of body weight. The Sertoli islet structures consisted of 20 to 100 Sertoli cells per islet equivalent.
To date, recipients have been monitored up to a maximum of 9 years. Recipient monitoring has included testing peripheral blood mononuclear cells (PBMC) and blood plasma for PCV 2 (19), PLHV (7), PCMV (2, 11, 13), and PERV by PCR or reverse transcription-PCR (25). This testing has been carried out on each recipient before transplantation, at nine intervals during the first year posttransplantation, and once each subsequent year. From 14 patients, serum was collected before transplantation and at several intervals after transplantation for PERV serology (Table 1).
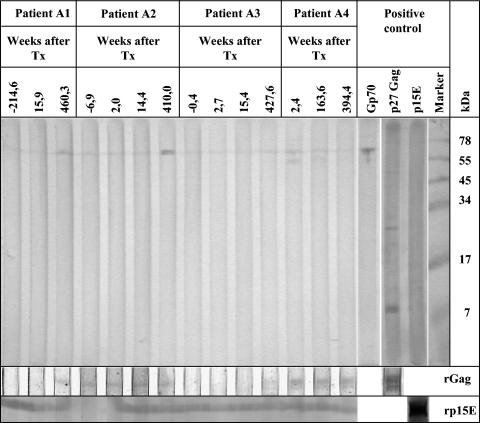
Screening for PERV infection was performed by using Western blot analysis and purified human cell-adapted PERV as an antigen (Fig. 1). In addition, two recombinant proteins were used as antigens, the transmembrane envelope protein p15E (9) and the major core protein p27Gag (17). The sequence amplified from pig PK-15 cells corresponded to the ectodomain of the transmembrane envelope protein p15E (amino acids 488 to 597) of PERV-A. For the generation of the PERV core protein p27Gag, the entire sequence encoding this protein was amplified and cloned. Both were expressed in Escherichia coli. Several PERV-specific antisera were used as positive controls, e.g., goat antisera against the recombinant p15E, the viral p15E, the surface envelope protein gp70, and the recombinant p27Gag.
FIG. 1.
Western blot analysis of sera from pig islet cell transplant recipients (only four selected patients are shown). In the upper panel, purified virus was used as the antigen; in the lower panels, recombinant p15E (rp15E) or p27Gag (rGag) was used as the antigen. Animal sera against viral proteins were used to evaluate the quality of the blots used. Negative values indicate the number of weeks before transplantation (Tx).
In the follow-up period of up to 9 years, pig islet cell transplantation was well tolerated by all 18 type 1 diabetics. No patient was admitted to the hospital for febrile disease. No patient had signs of lymphoproliferative or neurological disease. Studies of all patients receiving porcine islet cell xenotransplantation have not shown transmission of porcine viruses. PCR and reverse transcription-PCR analysis of PBMCs and plasma showed no evidence of PERV infection in transplanted patients. PBMC DNA from two patients was found to be positive for both PERV and pig cytochrome oxidase subunit II on one occasion each. Repeated analyses of samples taken at a later time point did not show any evidence of this porcine virus or porcine cells. Analysis for PERV-specific antibodies did not show evidence for PERV transmission since none of the patient's sera reacted simultaneously against Gag and Env (Fig. 1; Table 1). Three of the 14 patients tested had antibodies against p27Gag of PERV which were already detected before treatment and which have been described also for normal blood donors (34). Antibodies against p24Gag of HIV-1 were also described for healthy uninfected persons (28). The reaction against the gp70 is certainly directed against the carbohydrate part of the molecule (18). Some of the sera reacted with pig antigens (Table 1).
All 18 patients (four patients not shown in Table 1 had also received islet and Sertoli cells) were repeatedly checked for the presence of PCV, PCMV, and PLHV DNA, and all PCR results were negative.
This study represents a long-term follow-up of human patients receiving pig islet cell xenotransplantation and further confirms the absence of transmission of PERV and of other potentially xenotic pig viruses.
Acknowledgments
We thank R. Valdes for his collaboration.
REFERENCES
- 1.Allan, G. M., and J. A. Ellis. 2000. Porcine circoviruses: a review. J. Vet. Diagn. Investig. 12:3-14. [DOI] [PubMed] [Google Scholar]
- 2.Clark, D. A., J. F. Fryer, A. W. Tucker, P. D. McArdle, A. E. Hughes, V. C. Emery, and P. D. Griffiths. 2003. Porcine cytomegalovirus in pigs being bred for xenotransplantation organs: progress towards control. Xenotransplantation 10:142-148. [DOI] [PubMed] [Google Scholar]
- 3.Denner, J. 2003. Porcine endogenous retroviruses (PERVs) and xenotransplantation: screening for transmission in several clinical trials and in experimental models using non-human primates. Ann. Transplant. 8(3):39-48. [PubMed] [Google Scholar]
- 4.Denner, J., V. Specke, J. Schwendemann, and S. J. Tacke. 2001. Porcine endogenous retroviruses (PERVs): adaptation to human cells and attempts to infect small animals and non-human primates. Ann. Transplant. 6(3):25-33. [PubMed] [Google Scholar]
- 5.Dinsmore, J. H., C. Manhart, R. Raimeri, D. B. Jacoby, and A. Moore. 2000. No evidence for infection for human cells with porcine endogenous retrovirus (PERV) after exposure to porcine fetal neuronal cells. Transplantation 70:1382-1389. [DOI] [PubMed] [Google Scholar]
- 6.Edington, N. 1999. Cytomegalovirus, p. 125-131. In B. E. Straw, S. D'Allaire, W. L. Mengeling, and D. J. Taylor (ed.), Diseases of swine, 8th ed. Iowa State University Press, Ames, Iowa.
- 7.Ehlers, B., S. Ulrich, and M. Goltz. 1999. Detection of two novel porcine herpesviruses with high similarity to gammaherpesviruses. J. Gen. Virol. 80:971-974. [DOI] [PubMed] [Google Scholar]
- 8.Elliot, R. B., L. Escobar, O. Garkavenko, M. C. Croxson, B. A. Schroeder, M. McGregor, G. Ferguson, N. Beckman, and S. Ferguson. 2000. No evidence of infection with porcine retrovirus in recipients of encapsulated porcine islet-cell xenograft. Cell Transplant. 9:895-901. [DOI] [PubMed] [Google Scholar]
- 9.Fiebig, U., O. Stephan, R. Kurth, and J. Denner. 2003. Neutralizing antibodies against conserved domains of p15E of porcine endogenous retroviruses (PERVs): basis for a vaccine for xenotransplantation? Virology 307:406-413. [DOI] [PubMed] [Google Scholar]
- 10.Fishman, J. A. 2001. Prevention of infection in Xenotransplantation, p. 261-290. In J. L. Platt (ed.), Xenotransplantation. ASM Press, Washington, D.C.
- 11.Fryer, J. F. L., P. D. Griffiths, J. A. Fishman, V. C. Emery, and D. A. Clark. 2001. Quantitation of porcine cytomegalovirus in pig tissue by PCR. J. Clin. Microbiol. 39:1155-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garkavenko, O., M. Muzina, Z. Muzina, K. Powels, R. B. Elliott, and M. C. Croxson. 2004. Monitoring for potentially xenozoonotic viruses in New Zealand pigs. J. Med. Virol. 72:338-344. [DOI] [PubMed] [Google Scholar]
- 13.Hamel, A. L., L. Lin, C. Sachvie, E. Grudeski, and G. P. Nayer. 1999. PCR assay for detecting porcine cytomegalovirus. J. Clin. Microbiol. 37:3767-3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hattermann, K., C. Roedner, C. Schmitt, T. Finsterbusch, T. Steinfeldt, and A. Mankertz. 2004. Infection studies on human cell lines with porcine circovirus type 1 and porcine circovirus type 2. Xenotransplantation 11:284-294. [DOI] [PubMed] [Google Scholar]
- 15.Heneine, W., A. Tibell, W. M. Switzer, P. Sandstrom, G. V. Rosales, A. Mathews, O. Korsgren, L. E. Chapman, T. M. Folks, and C. G. Groth. 1998. No evidence of infection with porcine endogenous retrovirus in recipients of porcine islet-cell xenografts. Lancet 352:695-699. [DOI] [PubMed] [Google Scholar]
- 16.Heneine, W., W. M. Switzer, J. M. Sourcie, B. L. Evatt, V. Shanmugam, G. V. Rosales, A. Matthews, P. Sandstrom, and T. M. Folks. 2001. Evidence of porcine endogenous retroviruses in porcine factor VIII and evaluation of transmission to recipients with hemophilia. J. Infect. Dis. 183:648-652. [DOI] [PubMed] [Google Scholar]
- 17.Irgang, M., I. M. Sauer, A. Karlas, K. Zeilinger, J. C. Gerlach, R. Kurth, P. Neuhaus, and J. Denner. 2003. Porcine endogenous retroviruses (PERVs): no infection in patients treated with a bioreactor based on porcine liver cells. J. Clin. Virol. 28:141-154. [DOI] [PubMed] [Google Scholar]
- 18.Kurth, R., N. M. Teich, R. Weiss, and R. T. Oliver. 1977. Natural human antibodies reactive with primate type-C viral antigens. Proc. Natl. Acad. Sci. USA 74:1237-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Larochelle, R., M. Antaya, M. Morin, and R. Magar. 1999. Typing of porcine circovirus in clinical specimens by multiplex PCR. J. Virol. Methods 80:69-75. [DOI] [PubMed] [Google Scholar]
- 20.Martin, U., S. J. Tacke, A. R. Simon, C. Schroder, K. Wiebe, B. Lapin, A. Haverich, J. Denner, and G. Steinhoff. 2002. Absence of PERV specific humoral immune response in baboons after transplantation of porcine cells or organs. Transplant. Int. 15:361-368. [DOI] [PubMed] [Google Scholar]
- 21.Moza, A. K., H. Mertsching, T. Herden, A. Bader, and A. Haverich. 2001. Heart valves from pigs and porcine endogenous retrovirus: experimental and clinical data to assess the probability of porcine endogenous retrovirus infection in human subjects. J. Thorac. Cardiovasc. Surg. 121:697-701. [DOI] [PubMed] [Google Scholar]
- 22.Mueller, N. J., R. N. Barth, S. Yamamato, H. Kitimura, C. Patience, K. Yamada, D. K. Cooper, D. H. Sachs, A. Kaur, and J. A. Fishman. 2002. Activation of cytomegalovirus in pig-to-primate organ transplantation. J. Virol. 76:4734-4740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Onions, D., D. K. Cooper, T. J. Alexander, C. Brown, E. Classen, J. E. Foweraker, D. L. Harris, B. W. Mahy, P. D. Minor, A. D. Osterhaus, P. P. Pastoret, and K. Yamanouchi. 2000. An approach to the control of disease transmission in pig-to-human xenotransplantation. Xenotransplantation 7:143-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paradis, K., G. Langford, Z. Long, W. Heneine, P. Sandstrom, W. M. Switzer, L. E. Chapman, C. Lockey, D. Onions, E. Otto, et al. 1999. Search for cross-species transmission of porcine endogenous retrovirus in patients treated with living pig tissue. Science 285:1236-1241. [DOI] [PubMed] [Google Scholar]
- 25.Patience, C., Y. Takeuchi, and R. A. Weiss. 1997. Infection of human cells by an endogenous retrovirus of pigs. Nat. Med. 3:282-286. [DOI] [PubMed] [Google Scholar]
- 26.Patience, C., G. S. Patton, Y. Takeuchi, R. A. Weiss, M. O. McClure, L. Rydberg, and M. E. Breimer. 1998. No evidence of pig DNA or retroviral infection in patients with short-term extracorporeal connection to pig kidneys. Lancet 352:699-701. [DOI] [PubMed] [Google Scholar]
- 27.Sauer, M., D. Kardassis, K. Zeillinger, A. Gruenwald, G. Pless, A. Pascher, M. Irgang, M. Kraemer, G. Puhl, J. Frank, A. R. Müller, T. Steinmüller, J. Denner, P. Neuhaus, and J. Gerlach. 2003. Extracorporeal hybrid liver support therapy—phase I study with primary porcine liver cells. Xenotransplantation 10:460-469. [DOI] [PubMed] [Google Scholar]
- 28.Sayre, K. R., R. Y. Dodd, G. Tegtmeier, L. Layug, S. S. Alexander, and M. P. Busch. 1996. False-positive human immunodeficiency virus type 1 western blot tests in noninfected blood donors. Transfusion 36:45-52. [DOI] [PubMed] [Google Scholar]
- 29.Specke, V., S. J. Tacke, K. Boller, J. Schwendemann, and J. Denner. 2001. Porcine endogenous retroviruses: in vitro host range and attempts to establish small animal models. J. Gen. Virol. 82:837-844. [DOI] [PubMed] [Google Scholar]
- 30.Specke, V., H. J. Schuurman, R. Plesker, C. Coulibaly, M. Ozel, G. Langford, R. Kurth, and J. Denner. 2002. Virus safety in xenotransplantation: first exploratory in vivo studies in small laboratory animals and non-human primates. Transplant. Immunol. 9:281-288. [DOI] [PubMed] [Google Scholar]
- 31.Specke, V., R. Plesker, C. Coulibaly, K. Boller, and J. Denner. 2002. Productive infection of a mink cell line with porcine endogenous retroviruses (PERVs) but lack of transmission to mink in vivo. Arch. Virol. 147:305-319. [DOI] [PubMed] [Google Scholar]
- 32.Switzer, W. M., R. E. Michler, V. Shanmugam, A. Matthews, A. I. Hussain, A. Wright, P. Sandstrom, L. E. Chapman, C. Weber, S. Safley, R. R. Denny, A. Navarro, V. Evans, A. J. Norin, P. Kwiatkowski, and W. Heneine. 2001. Lack of cross-species transmission of porcine endogenous retrovirus infection to nonhuman primate recipients of porcine cells, tissues, or organs. Transplantation 71:959-965. [DOI] [PubMed] [Google Scholar]
- 33.Tacke, S., R. Kurth, and J. Denner. 2000. Porcine endogenous retrovirus inhibits human immune cells: risk for xenotransplantation. Virology 268:87-93. [DOI] [PubMed] [Google Scholar]
- 34.Tacke, S., K. Bodusch, A. Berg, and J. Denner. 2001. Sensitive and specific detection methods for porcine endogenous retroviruses applicable to experimental and clinical xenotransplantation. Xenotransplantation 8:125-135. [PubMed] [Google Scholar]
- 35.Tischer, I., L. Bode, J. Apodaca, H. Timm, D. Peters, D. Rasch, S. Pociuli, and E. Gerike. 1995. Presence of antibodies reacting with porcine circovirus in sera of humans, mice, and cattle. Arch. Virol. 140:1427-1439. [DOI] [PubMed] [Google Scholar]
- 36.Trujano, M., G. Iglesias, J. Segales, and J. M. Palacios. 2001. PCV2 from emaciated pigs in Mexico. Vet. Record 148:792. [PubMed] [Google Scholar]
- 37.Tucker A. W., F. McNeilly, B. Meehan, D. Galbraith, P. D. McArdle, G. Allan, and C. Patience. 2003. Methods for the exclusion of circoviruses and gammaherpesviruses. Xenotransplantation 10:343-348. [DOI] [PubMed] [Google Scholar]
- 38.Valdes, R. 2002. Xenotransplantation trials. Lancet 359:2281. [DOI] [PubMed] [Google Scholar]