Abstract
The association between exposure to ambient air pollution and respiratory or cardiovascular endpoints is well-established. An increasing number of studies have shown that this exposure is also associated with adverse pregnancy outcomes. However, the majority of research has been undertaken in high-income western countries, with relatively lower levels of exposure. There is now a sufficient number of studies to warrant an assessment of effects in China, a relatively higher exposure setting. We conducted a systematic review of 25 studies examining the association between ambient air pollution exposure and adverse pregnancy outcomes (lower birth weight, preterm birth, mortality, and congenital anomaly) in China, published between 1980 and 2015. The results indicated that sulphur dioxide (SO2) was more consistently associated with lower birth weight and preterm birth, and that coarse particulate matter (PM10) was associated with congenital anomaly, notably cardiovascular defects.
Keywords: Pollution, Low birth weight, Preterm birth, Mortality, Congenital anomaly, Birth defects
Graphical abstract
1. Introduction
Exposure to air pollutants has been linked to a range of health problems including respiratory and cardiovascular morbidity (Cesaroni et al., 2013; Gurjar et al., 2010). There is also increasing evidence that exposure to air pollutants is associated with adverse pregnancy outcomes such as low birth weight (Bell et al., 2007; Pedersen et al., 2013), preterm birth (Bobak, 2000; Pereira et al., 2013), mortality (Pope et al., 2010; Woodruff et al., 1997) and congenital anomaly (Padula et al., 2013; Rankin et al., 2009). In addition, molecular epidemiological studies have shown that PAH-DNA adduct levels (biomarkers of exposure) are associated with adverse effects including intrauterine growth retardation (Šrám et al., 1999) and HPRT locus mutation frequency in infants (Perera et al., 2002). This suggests plausible biological mechanisms for the effects of air pollution on fetal growth and health (Šrám et al., 2005).
A recent systematic review (Stieb et al., 2012) examined the association between ambient air pollution and low birth weight, change in birth weight and preterm birth for pollutants including carbon monoxide (CO), nitrogen dioxide (NO2), sulphur dioxide (SO2) and particulate matter <10 and 2.5 μm in aerodynamic diameter (PM10 and PM2.5). Of the 62 reviewed studies, nearly half (27) were conducted in North America, followed by Europe (18), Asia (10), Australia (4) and South America (3). Only one study was conducted in China.
A systematic review of 17 studies (Chen et al., 2014) examined the association between congenital anomaly and maternal exposure to ambient air pollutants during pregnancy. The most frequently-studied anomalies were cardiovascular, followed by nervous system defects. Seven of the studies in this review were conducted in the United States, four in the United Kingdom and the remainder in six different countries. None were conducted in China.
There have been relatively few systematic reviews on the association between ambient air pollution and mortality of infants and/or fetuses. The most recent one (Šrám et al., 2005) examined sudden infant death as well as intrauterine, perinatal, postneonatal and infant mortality. The authors observed a notable consistency in the results – the three largest studies produced very similar estimates of relative risk – and the evidence was sufficient to infer a causal relationship between particulate air pollution and respiratory deaths in the postneonatal period. For this review, studies were conducted in the Czech Republic, Britain, and the United States, Mexico and Brazil. None were conducted in China.
Compared with western countries, China has relatively high air pollution levels. According to the World Health Organization air pollution database (WHO, 2014), China had annual population weighted PM10 concentrations of 90 μg/m3 compared to 21 μg/m3 for the United States and the United Kingdom, 33 μg/m3 for Italy and 23 μg/m3 for Canada. Particulate matter is generally regarded as an important measure of air quality as studies have consistently demonstrated its toxicity (Hester and Harrison, 1998).
In addition to differences in the levels and physico-chemical composition of air pollutants between China and other countries studied, there exist relevant genetic and physiological differences in between cohort. For example, it has been shown that North-East Asians have significantly different lung dynamics than Caucasians (Quanjer et al., 2012). While this difference is less pronounced than for other groups (e.g. South-East Asians), it would potentially result in different dosage/deposition rates for the same exposure.
Previous reviews indicate accumulating evidence for an association between air pollution and adverse pregnancy outcomes but most evidence is derived from research conducted in western countries with relatively lower levels of exposure. A significant gap in the literature is the lack of review of effects for exposure in a country such as China, with among the highest levels of air pollution globally. To our knowledge, there has been no systematic review of the effects of ambient air pollution on pregnancy outcomes in China. Exposure-response associations from studies conducted in Western countries may not apply to populations in China due to the worse air quality, but also due to potential differences in the underlying population such as different baseline health status and health care systems (O’Neill et al., 2003).
We investigated the association between ambient air pollutant exposure (NO2, SO2, CO, PM10, PM2.5 and ozone (O3)) and the following adverse pregnancy outcomes in China: decrease in birth weight, low birth weight, preterm birth, mortality and congenital anomaly. This review included both Chinese and English language articles.
2. Method
This systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (Moher et al., 2009).
2.1. Study selection
A search of the following databases was undertaken: PubMed, Medline (Web of Science), Embase, CNKI (China National Knowledge Infrastructure) and Wanfang Data. The search was limited to English and Chinese papers published between 1980 and 2015 using the search terms in Fig. 1. The inclusion criteria were: 1) peer-reviewed articles in journals published from 1st January 1980 to 31st December 2015, 2) conducted in China, 3) ambient exposure to at least one of the following air pollutants: NO2, SO2, CO, PM10, PM2.5, O3; and 4) health outcomes: low birth weight, preterm birth, stillbirth or death (in utero to age one year), or birth defects (congenital anomaly). Studies that measured occupational or accidental exposure, tobacco, exposure to other pollutants or a proxy for exposure were excluded. No case reports were included.
Fig. 1.

Search terms used to identify eligible studies.
2.2. Quality assessment
We evaluated the quality of the selected studies using the following criteria (Berman and Parker, 2002): 1) source of the information (reputable, reliable, clearly identified); 2) study design clearly described and appropriate to the study questions; 3) exposures and outcomes well-defined, including methods of measurement; 4) adjustment for confounding variables; and 5) statistical methods appropriate.
2.3. Data extraction
The following items were extracted from each study: location, sample size (number of births), year/s of study, study design, adjustment variables, population, outcome ascertainment, exposure assessment, and quantitative findings.
3. Results
3.1. Included and excluded studies
Using the search terms in Fig. 1, a total of 1029 articles were identified, consisting of 193 papers written in English and 836 papers written in Chinese (Fig. 2). We included articles written in Chinese. The first screening, by title, eliminated duplicates and non-relevant titles, leaving a total of 83 possibly relevant articles. The second screening, an abstract review, resulted in 46 articles eligible for further review. A full-text review of these articles at the third screening identified 21 articles for inclusion. The reference lists of these articles were then examined and four additional papers were identified for inclusion. This resulted in the inclusion of 25 articles consisting of 14 papers written in Chinese and 11 papers written in English. Each study was assigned a number between one and 25 (Table 1), which was used to refer to the study in this review.
Fig. 2.
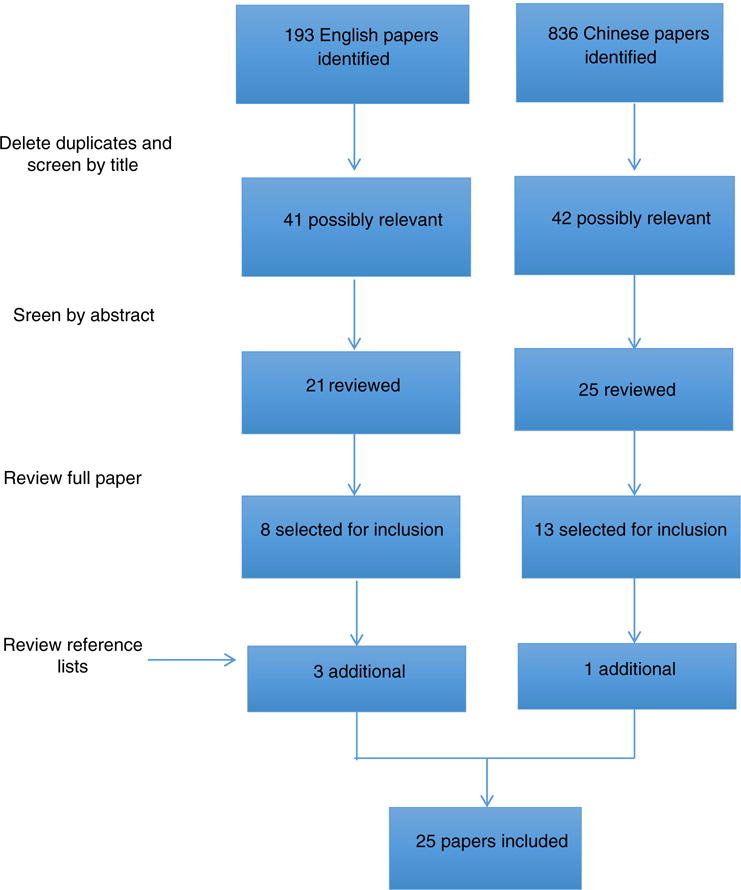
Flow diagram for the inclusion and exclusion of studies.
Table 1.
Summary of studies included in this review, by outcome.
| Outcome | Study | Reference | Study period | Area | Study type | Sample size | SO2 | NO2 | PM10 | PM2.5 | CO | O3 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BW | 3 | (Huang et al., 2015) | 2006–2010 | Beijing | Cross-sectional | 50,874 | + | − | ||||
| BW | 4 | (Rich et al., 2015) | 2007–2010 | Beijing | Cross-sectional | 83,672 | + | − | + | + | ||
| BW | 6 | (Wang et al., 1997) | 1988–1991 | Beijing | Prospective cohort | 74,671 | + | |||||
| BW | 13a | (Zhao and Li, 2008) | 2003–2005 | Guangzhou | Retrospective cohort | 58,869 | + | |||||
| BW | 19a | (Zhao et al., 2008) | 2003–2005 | Guangzhou | Cross-sectional | 58,837 | + | + | + | |||
| CA | 9a | (Chen et al., 2012) | 2002–2007 | Tianjin | Cross-sectional | 235,998 | ||||||
| CA | 10a | (Zheng et al., 2013) | 2001–2006 | Tianjin | Case-control | 1539 (459 cases, 1080 controls) | − | − | ||||
| CA | 11a | (Zhang et al., 2007) | 1997–2004 | Taiyuan | Retrospective cohort | 48,491 | + | + | ||||
| CA | 12a | (Shi et al., 2013) | 2007–2012 | Fuzhou | Case-control | 534 (178 cases, 356 controls) | + | |||||
| CA | 14a | (Aimaier et al., 2013) | 2007–2009 | Beijing | Retrospective cohort | 23,187 | − | + | − | |||
| CA | 23 | (Jin et al., 2015) | 2010–2012 | Lanzhou | Retrospective cohort | 8969 | + | + | + | |||
| CA | 24 | (Liang et al., 2014) | 2009–2011 | Haikou | Case-control | 63,900 (509 cases, 63,391 controls) | − | − | + | |||
| LBW | 6 | (Wang et al., 1997) | 1988–1991 | Beijing | Prospective cohort | 74,671 | + | |||||
| LBW | 11a | (Zhang et al., 2007) | 1997–2004 | Taiyuan | Retrospective cohort | 49,881 | − | + | ||||
| LBW | 14a | (Aimaier et al., 2013) | 2007–2009 | Beijing | Retrospective cohort | 23,283 | + | − | − | |||
| LBW | 18a | (Zhao et al., 2010b) | 2007 | Guangdong | Cross-sectional | 7004 | − | − | − | |||
| LBW | 22a | (Ruan et al., 2008) | 2003–2005 | Guangzhou | Case-control | 2964 (1482 cases, 1482 controls) | + | + | + | |||
| mortality | 7 | (Hou et al., 2014) | 2001–2006 | Tianjin | Case-control | 1918(959 cases, 959 controls) | + | + | − | |||
| mortality | 9a | (Chen et al., 2012) | 2002–2007 | Tianjin | Cross-sectional | 235,998 | ||||||
| mortality | 11a | (Zhang et al., 2007) | 1997–2004 | Taiyuan | Retrospective cohort | 49,910 | − | + | ||||
| mortality | 16a | (Hou et al., 2012) | 2001–2006 | Tianjin | Case-control | 1918 (959 cases, 959 controls) | − | |||||
| mortality | 25 | (Hwang et al., 2011) | 2001–2007 | Taiwan | Case-control | 102,575 (9325 cases, 93,250 controls) | + | − | + | − | + | |
| PTB | 1 | (Xu et al., 1995) | 1988 | Beijing | Prospective cohort | 25,370 | + | |||||
| PTB | 2 | (Zhao et al., 2011) | 2007 | Guangzhou | Cross-sectional | 7836 | + | − | − | |||
| PTB | 3 | (Huang et al., 2015) | 2006–2010 | Beijing | Cross-sectional | 50,874 | − | − | ||||
| PTB | 5 | (Zhao et al., 2015) | 2010–2012 | Lanzhou | Case-control | 8969 (677 cases, 8292 controls) | + | |||||
| PTB | 8 | (Jiang et al., 2007) | 2004 | Shanghai | Cross-sectional | 3346 | + | + | + | + | ||
| PTB | 11a | (Zhang et al., 2007) | 1997–2004 | Taiyuan | Retrospective cohort | 48,029 | + | + | ||||
| PTB | 14a | (Aimaier et al., 2013) | 2007–2009 | Beijing | Retrospective cohort | 23,896 | + | − | + | |||
| PTB | 15a | (Ruan et al., 2010) | 2007–2012 | Guangzhou | Case-control | 9848 (4924 cases, 4924 controls) | − | + | − | |||
| PTB | 17a | (Zhao et al., 2010a) | 2007 | Guangdong | Cross-sectional | 7836 | + | − | − | |||
| PTB | 20a | (Xu et al., 2008) | 2005–2007 | Taiyuan | Retrospective cohort | 31,145 | − | − | − | |||
| PTB | 21a | (Zhang et al., 2008) | 2005–2007 | Taiyuan | Case-crossover | 716 | + | + | + |
+Statistically significant association (p < 0.05) – not statistically significant [blank] = not investigated, BW = birth weight, LBW = low birth weight, PTB = preterm birth, CA = congenital anomaly.
Article written in Chinese.
Of the 25 reviewed studies, the majority were conducted in large urban areas. Seven studies were based in the Guangdong province (including Guangzhou) and five were in Beijing. The most common study design was case-control (nine studies), followed by cross-sectional (eight studies). There were only two prospective cohort studies and both were conducted in Beijing.
3.2. Exposure ascertainment
The majority of the reviewed studies examined PM10, NO2 and SO2 because these were the only pollutants routinely measured by the Chinese government monitoring network from 2000 to 2012 (PM2.5, O3 and CO were less well-studied). The most common method of exposure ascertainment was calculating the average concentration for each pollutant across all monitoring sites in the study area (17 out of 25 studies). Other methods involved using the nearest monitor (four studies) or inverse distance weighting (IDW, five studies), which is the weighted average of concentrations at monitoring sites whereby each site’s concentration is assigned the reciprocal of the distance as the weight. One study used both the nearest monitor approach and inverse distance weighting (Study 5).
The majority of reviewed studies reported average pollutant concentrations over the study periods, which ranged from one to eight years (Tables 2–5). Mean concentrations of PM10, NO2 and SO2 across all studies were 113 μg/m3, 50 μg/m3 and 61 μg/m3, respectively, with maximum concentrations of 600, 468 and 630 μg/m3, respectively. On average, studies in Taiyuan reported the highest concentrations of PM10 and SO2 (165 μg/m3 and 114 μg/m3, respectively), while studies in Shanghai reported the highest concentrations of NO2 (71 μg/m3). The highest average pollutant levels overall were for studies in Beijing (population 19 million) and the lowest were for studies in Haikou (population 2 million).
Table 2.
Nitrogen dioxide (NO2) concentrations (μg/m3) reported over the study periods for the reviewed studies.
| Study | Location | Mean | SD | Min | 25th | Median | 75th | Max |
|---|---|---|---|---|---|---|---|---|
| 4 | Beijing | 49 | 17 | 26 | 34 | 49 | 61 | 84 |
| 14 | Beijing | 54 | 23 | 10 | 38 | 50 | 66 | 152 |
| 3 | Beijing | 58 | 13 | 23 | 83 | |||
| 17 | Guangdong | 61 | 64 | 15 | 27 | 38 | 60 | 468 |
| 18 | Guangdong | 61 | 64 | 15 | 27 | 38 | 60 | 468 |
| 2 | Guangzhou | 61 | 64 | 15 | 27 | 38 | 60 | 468 |
| 15 | Guangzhou | 60 | ||||||
| 19 | Guangzhou | 57 | 34 | 60 | 86 | |||
| 22 | Guangzhou | 57 | 34 | 60 | 86 | |||
| 24 | Haikou | 32 | 11 | |||||
| 23 | Lanzhou | 42 | ||||||
| 8 | Shanghai | 71 | 1 | 17 | 52 | 67 | 84 | 169 |
| 25 | Taiwan | 44 | 16 | 7 | 81 | |||
| 20 | Taiyuan | 24 | 2 | |||||
| 21 | Taiyuan | 25 | 7 | 7 | 20 | 24 | 29 | 53 |
| 7 | Tianjin | 50 | ||||||
| 10 | Tianjin | 51 | 18 |
Table 5.
Concentrations (μg/m3) of carbon monoxide (CO), ozone (O3) and particulate matter < 2.5 μm (PM2.5) reported over the study periods for the reviewed studies.
| Study | Location | Pollutant | Mean | SD | Min | 25th | Median | 75th | Max |
|---|---|---|---|---|---|---|---|---|---|
| 25 | Taiwan | CO | 814 | 222 | 333 | 1490 | |||
| 3 | Beijing | CO | 1730 | 680 | 800 | 3900 | |||
| 4 | Beijing | CO | 987 | 247 | 740 | 740 | 863 | 1110 | 1600 |
| 8 | Shanghai | O3 | 65 | 2 | 5 | 38 | 56 | 87 | 251 |
| 25 | Taiwan | O3 | 76 | 20 | 30 | 130 | |||
| 4 | Beijing | PM2.5 | 61 | 11 | 44 | 52 | 60 | 71 | 85 |
3.3. Outcome assessment
3.3.1. Birth weight
Effects on birth weight were investigated as either the occurrence of low birth weight (<2500 g) or the change in birth weight (measured in grams). Study 11 further classified low birth weight as either low birth weight (<2500 g) or very low birth weight (<1500 g).
Of the nine studies that examined either birth weight or low birth weight, six studies restricted analyses to term births (≥37 weeks). The other three studies (Study 13, 18, 19) used the term ‘Weichaner’, which is defined in Chinese as ≥28 weeks gestational age. Of the five studies that investigated change in birth weight (continuous variable), only two (Study 6, 19) adjusted for gestational age.
3.3.2. Preterm birth
Preterm birth is defined as birth occurring before 37 weeks gestational age. However, in Study 5, preterm birth was classified as either moderate preterm (32–36 weeks) or very preterm (<32 weeks) and then further classified as either medically-indicated or spontaneous (with or without prelabour rupture of membranes). Medically-indicated preterm birth was defined as preterm birth without spontaneous onset of labour, and is often due to maternal or fetal complications including placental abruption, placenta previa, placental accreta, pregnancy hypertension and preeclampsia, intrauterine growth restriction, oligohydramnion, uterine rupture, and pre-gestational diabetes (Zhao et al., 2015).
Of the 11 studies that examined preterm birth, eight studies calculated gestational age as the number of completed weeks between the first day of the last menstrual period (LMP) and the date of birth. Study 1 did not specify how gestational age was calculated. In Study 3, gestational age was confirmed by sonographic examination prior to 20 weeks gestation. Study 8 used LMP and a clinical estimate of gestational age if LMP was missing. Compared to ultrasound scanning, LMP estimates overstate the duration of gestation by 2.8 days on average (Savitz et al., 2002).
3.3.3. Congenital anomaly
There were seven studies that examined congenital anomaly. Four studies examined a range of diagnosed birth defects (Study 10, 12, 14, 24). One study examined only congenital heart defects, divided into the following groups: pooled cases, congenital malformations of the great arteries, congenital malformations of cardiac septa, and isolated cases of patent ductus arteriosus (Study 23). Two studies did not specify the types of anomaly examined (Study 9, 11).
A clinical diagnosis of birth defects was generally made within 7 days after delivery. Within this period, all diagnosed birth defects were required to be reported. All studies undercounted birth defects considerably as not all birth defects are diagnosed shortly after birth. For example, a one year follow-up identified approximately 50% fewer births with birth defects compared to a six-year follow-up (King Edward Memorial Hospital, 2010).
3.3.4. Mortality
Five of the reviewed studies examined mortality. Types of mortality studied were (i) fetal loss <14 weeks gestation (Study 7); (ii) missed abortion ≤14 weeks where the embryo died but miscarriage had not yet occurred (Study 16); (iii) mortality between 28 weeks gestation to 7 days after birth, referred to as ‘perineonate death’ (Study 9, 11); and (iv) stillbirth, defined as death after 20 weeks (Study 25).
3.4. Adjustment variables
Of the 25 reviewed studies, 17 studies adjusted for maternal age (Table 6). Other variables commonly adjusted for were fetal/infant sex (11 studies), temperature (10 studies), gestational age, humidity and other pollutants (8 studies). Eight studies accounted for socioeconomic status by adjusting for education, income and/or occupation variables. Time variables such as day of week/year, season and/or year were adjusted for in eight studies. Study 9 did not report any adjustment variables.
Table 6.
Variables adjusted for in the reviewed studies (by study number).
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Maternal age | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||||||
| Maternal race | X | X | |||||||||||||||||||||||
| Gravidity | X | X | X | X | X | X | X | ||||||||||||||||||
| Parity | X | X | X | X | X | X | X | ||||||||||||||||||
| BMI | X | ||||||||||||||||||||||||
| Maternal health | X | ||||||||||||||||||||||||
| Drinking | X | ||||||||||||||||||||||||
| Folic acid intake | X | X | |||||||||||||||||||||||
| Therapeutic drug use | X | ||||||||||||||||||||||||
| Mother’s education | X | X | X | X | X | X | X | ||||||||||||||||||
| Father’s education | X | ||||||||||||||||||||||||
| Mother’s occupation | X | X | X | ||||||||||||||||||||||
| Father’s occupation | X | ||||||||||||||||||||||||
| Household income | X | X | X | X | |||||||||||||||||||||
| Smoking | X | X | X | ||||||||||||||||||||||
| Residential area | X | X | X | X | |||||||||||||||||||||
| Mother’s birth place | X | X | |||||||||||||||||||||||
| Prenatal health care | X | ||||||||||||||||||||||||
| Gestational age | X | X | X | X | X | X | X | X | |||||||||||||||||
| Height of neonate | X | ||||||||||||||||||||||||
| Fetal/infant sex | X | X | X | X | X | X | X | X | X | X | X | ||||||||||||||
| Day of week | X | X | X | ||||||||||||||||||||||
| Day of year | X | X | |||||||||||||||||||||||
| Year | X | X | |||||||||||||||||||||||
| Season | X | X | X | X | X | X | |||||||||||||||||||
| Temperature | X | X | X | X | X | X | X | X | X | X | |||||||||||||||
| Humidity | X | X | X | X | X | X | X | X | |||||||||||||||||
| Wind speed | X | ||||||||||||||||||||||||
| Other pollutants | X | X | X | X | X | X | X | X | |||||||||||||||||
| Type of cooking fuel | X | X | |||||||||||||||||||||||
| Type of heating | X |
3.5. Quantitative results on association between pollution and pregnancy outcomes
Results were converted to effect estimates per 10 μg/m3 increase in exposure over the relevant exposure period. All reported associations are with adverse health outcomes and are statistically significant (p < 0.05), unless stated otherwise.
3.5.1. Birth weight
Five of the reviewed studies examined change in birth weight (BW). All three studies that examined SO2 reported significant associations with BW (e.g., 1.9 g decrease in BW for exposure over the entire pregnancy in Study 19) (Table 7). A prospective cohort study (Study 6) reported an association (0.7 g decrease in BW) for exposure in the third trimester.
Table 7.
Results for change in birth weight for a 10 μg/m3 increase in exposure.
| Study | Pollutant | Result (95% CI) | Exposure period | Details | |
|---|---|---|---|---|---|
| 19 | * | NO2 | 3.3 g (1.1, 5.5) | Entire pregnancy | ≥28 weeks |
| 19 | * | NO2 | −3.3 g (−4.9, −1.7) | Trimester 1 | ≥28 weeks |
| 19 | NO2 | 1.6 g (0.0, 3.2) | Trimester 2 | ≥28 weeks | |
| 19 | * | NO2 | 6.3 g (4.7, 7.9) | Trimester 3 | ≥28 weeks |
| 13 | PM10 | −0.1 g (−1.9, 1.7) | Entire pregnancy | ≥28 weeks | |
| 19 | PM10 | −0.1 g (−1.9, 1.7) | Entire pregnancy | ≥28 weeks | |
| 13 | * | PM10 | −2.7 g (−3.7, −1.7) | Trimester 1 | ≥28 weeks |
| 19 | * | PM10 | −2.7 g (−3.7, −1.7) | Trimester 1 | ≥28 weeks |
| 13 | * | PM10 | −2.2 g (−3.4, −1.0) | Trimester 2 | ≥28 weeks |
| 19 | * | PM10 | −2.2 g (−3.4, −1.0) | Trimester 2 | ≥28 weeks |
| 13 | * | PM10 | 3.8 g (2.8, 4.8) | Trimester 3 | ≥28 weeks |
| 19 | * | PM10 | 3.8 g (2.8, 4.8) | Trimester 3 | ≥28 weeks |
| 19 | * | SO2 | −1.9 g (−3.5, −0.3) | Entire pregnancy | ≥28 weeks |
| 19 | * | SO2 | −3.3 g (−4.5, −2.1) | Trimester 1 | ≥28 weeks |
| 19 | SO2 | −1.4 g (−2.8, 0.0) | Trimester 2 | ≥28 weeks | |
| 19 | SO2 | 1.4 g (0.0, 2.8) | Trimester 3 | ≥28 weeks | |
| 4 | CO | −0.1 g (−0.5, 0.2) | 1st month | Term births | |
| 4 | CO | 0.0 g (−0.4, 0.3) | 2nd month | Term births | |
| 4 | CO | 0.0 g (−0.3, 0.4) | 3rd month | Term births | |
| 4 | CO | −0.2 g (−0.6, 0.1) | 4th month | Term births | |
| 4 | CO | −0.1 g (−0.4, 0.3) | 5th month | Term births | |
| 4 | CO | 0.2 g (−0.2, 0.5) | 6th month | Term births | |
| 4 | CO | −0.2 g (−0.5, 0.2) | 7th month | Term births | |
| 4 | * | CO | −0.5 g (−0.8, −0.2) | 8th month | Term births |
| 4 | NO2 | −6.9 g (−21.7, 8) | 1st month | Term births | |
| 4 | NO2 | 0.0 g (−10.5, 10.1) | 2nd month | Term births | |
| 4 | NO2 | −4.3 g (−19.6, 10.5) | 3rd month | Term births | |
| 4 | NO2 | −6.5 g (−21, 8.3) | 4th month | Term births | |
| 4 | NO2 | −2.5 g (−16.7, 11.2) | 5th month | Term births | |
| 4 | NO2 | 8.0 g (−6.2, 21.7) | 6th month | Term births | |
| 4 | NO2 | −8.3 g (−21.7, 5.4) | 7th month | Term births | |
| 4 | NO2 | −12.3 g (−26.1, 1.1) | 8th month | Term births | |
| 3 | NO2 | −1.4 g (−17.2, 14.3) | Trimester 1 | Term births | |
| 3 | NO2 | −9.7 g (−28.9, 9.4) | Trimester 2 | Term births | |
| 3 | * | NO2 | −14.8 g (−29.1, −0.4) | Trimester 3 | Term births |
| 3 | PM10 | −1.6 g (−5.7, 2.4) | Trimester 1 | Term births | |
| 3 | PM10 | 2.5 g (−3.6, 8.7) | Trimester 2 | Term births | |
| 3 | PM10 | 4.7 g (−2.8, 12.1) | Trimester 3 | Term births | |
| 4 | PM2.5 | −7.1 g (−15.2, 0.5) | 1st month | Term births | |
| 4 | PM2.5 | 0.0 g (−8.1, 7.6) | 2nd month | Term births | |
| 4 | PM2.5 | −1.0 g (−9.1, 7.6) | 3rd month | Term births | |
| 4 | PM2.5 | −0.5 g (−8.6, 7.1) | 4th month | Term births | |
| 4 | PM2.5 | −0.5 g (−8.1, 7.1) | 5th month | Term births | |
| 4 | PM2.5 | −0.5 g (−7.6, 7.1) | 6th month | Term births | |
| 4 | PM2.5 | −4.0 g (−11.1, 3.5) | 7th month | Term births | |
| 4 | * | PM2.5 | −9.1 g (−16.2, −1.5) | 8th month | Term births |
| 4 | SO2 | 2.0 g (−27.6, 29.6) | 1st month | Term births | |
| 4 | SO2 | 0.0 g (−27.6, 27.6) | 2nd month | Term births | |
| 4 | SO2 | −3.9 g (−33.5, 25.6) | 3rd month | Term births | |
| 4 | SO2 | −7.9 g (−35.5, 19.7) | 4th month | Term births | |
| 4 | SO2 | 3.9 g (−21.7, 31.5) | 5th month | Term births | |
| 4 | SO2 | 13.8 g (−11.8, 41.4) | 6th month | Term births | |
| 4 | SO2 | −13.8 g (−37.4, 13.8) | 7th month | Term births | |
| 4 | * | SO2 | −45.3 g (−70.9, −19.7) | 8th month | Term births |
| 6 | * | SO2 | −0.7 g (−1.0, −0.4) | Trimester 3 | Term births |
Statistically significant (p < 0.05).
The results were inconsistent for NO2. Two out of three studies reported associations with BW (3.3 g decrease in BW for Study 19 for the first trimester and 14.8 g decrease for Study 3 for the third trimester). However, a protective association between NO2 and BW was reported in the third trimester as well as the entire pregnancy (6.3 g increase in BW for the third trimester in Study 19).
Two studies (Studies 13 and 19) reported identical results for PM10, namely an association with BW in the first two trimesters of pregnancy (2.7 g and 2.2 g decrease in BW in the first and second trimester, respectively) and a protective association in the third trimester (3.8 g increase in BW). However, another study did not report any significant associations between BW and PM10 (Study 3). Only one study examined the effects of CO and PM2.5 on BW, and reported associations for both pollutants but only for the eighth month of pregnancy (0.5 g and 9.1 g decrease in BW for CO and PM2.5, respectively, for Study 4).
The results indicate that there is more consistent evidence of a statistically significant association between exposure to SO2 and lower birth weight.
3.5.2. Low birth weight
Five of the reviewed studies investigated low birth weight (LBW) (Table 8). Of the five studies that examined SO2, three reported associations with LBW (adjusted odds ratio (AOR) = 1.04 in the first month for Study 14 and AOR = 1.08 in the first trimester for Study 22). A prospective cohort study (Study 6) reported an association (AOR = 1.01) for exposure in the third trimester.
Table 8.
Quantitative results for low birth weight for a 10 μg/m3 increase in exposure.
| Study | Pollutant | Result (95% CI) | Exposure period | Details | |
|---|---|---|---|---|---|
| 18 | NO2 | RR = 1.00 (1.00,1.01) | Entire pregnancy | ≥28 weeks | |
| 18 | PM10 | RR = 1.01 (1.00,1.01) | Entire pregnancy | ≥28 weeks | |
| 18 | SO2 | RR = 1.01 (1.00,1.02) | Entire pregnancy | ≥28 weeks | |
| 14 | NO2 | AOR = 1.01 (0.93,1.10) | 1 month BD | Term births | |
| 14 | NO2 | AOR = 0.95 (0.88,1.04) | 1st month | Term births | |
| 14 | NO2 | AOR = 0.97 (0.89,1.06) | 2 months BD | Term births | |
| 14 | NO2 | AOR = 0.93 (0.85,1.02) | 2nd month | Term births | |
| 14 | NO2 | AOR = 0.94 (0.86,1.03) | 3rd month | Term births | |
| 14 | NO2 | AOR = 0.98 (0.87,1.10) | Trimester 1 | Term births | |
| 14 | NO2 | AOR = 0.99 (0.89,1.09) | Trimester 3 | Term births | |
| 22 | * | NO2 | AOR = 0.92 (0.85,0.99) | Trimester 3 | Term births |
| 14 | PM10 | AOR = 0.98 (0.95,1.01) | 1 month BD | Term births | |
| 14 | PM10 | AOR = 0.98 (0.95,1.01) | 1st month | Term births | |
| 14 | PM10 | AOR = 1.03 (0.99,1.06) | 2 months BD | Term births | |
| 14 | PM10 | AOR = 0.99 (0.95,1.03) | 2nd month | Term births | |
| 14 | PM10 | AOR = 1.00 (0.97,1.04) | 3rd month | Term births | |
| 14 | PM10 | AOR = 0.94 (0.89,1.00) | Trimester 1 | Term births | |
| 14 | PM10 | AOR = 1.00 (0.95,1.05) | Trimester 3 | Term births | |
| 22 | * | PM10 | AOR = 0.95 (0.90,0.99) | Trimester 3 | Term births |
| 14 | SO2 | AOR = 1.02 (0.97,1.06) | 1 month BD | Term births | |
| 14 | * | SO2 | AOR = 1.04 (1.01,1.07) | 1st month | Term births |
| 14 | SO2 | AOR = 0.98 (0.95,1.01) | 2 months BD | Term births | |
| 14 | SO2 | AOR = 1.02 (0.98,1.02) | 2nd month | Term births | |
| 14 | SO2 | AOR = 0.97 (0.93,1.01) | 3rd month | Term births | |
| 22 | * | SO2 | AOR = 1.08 (1.01,1.15) | Trimester 1 | Term births |
| 14 | SO2 | AOR = 1.05 (1.00,1.10) | Trimester 1 | Term births | |
| 14 | SO2 | AOR = 0.99 (0.95,1.04) | Trimester 3 | Term births | |
| 6 | * | SO2 | AOR = 1.01 (1.01,1.01) | Trimester 3 | Term births |
| 11 | PM10 | AOR = 0.99 (0.94,1.04) | 1st month | Very low birth weight | |
| 11 | PM10 | AOR = 0.98 (0.93,1.04) | 2nd month | Very low birth weight | |
| 11 | PM10 | AOR = 1.02 (0.96,1.07) | 3rd month | Very low birth weight | |
| 11 | PM10 | AOR = 1.00 (0.92,1.04) | 4–6th months | Very low birth weight | |
| 11 | * | PM10 | AOR = 0.94 (0.89,0.99) | 7–9th months | Very low birth weight |
| 11 | PM10 | AOR = 1.02 (0.97,1.06) | C-3 | Very low birth weight | |
| 11 | SO2 | AOR = 1.00 (0.95,1.06) | 1st month | Very low birth weight | |
| 11 | SO2 | AOR = 0.99 (0.95,1.02) | 2nd month | Very low birth weight | |
| 11 | SO2 | AOR = 0.96 (0.92,1.00) | 3rd month | Very low birth weight | |
| 11 | SO2 | AOR = 0.95 (0.91,1.00) | 4–6th months | Very low birth weight | |
| 11 | SO2 | AOR = 1.02 (0.98,1.06) | 7–9th months | Very low birth weight | |
| 11 | SO2 | AOR = 1.01 (0.98,1.05) | C-3 | Very low birth weight | |
| 11 | PM10 | AOR = 0.99 (0.98,1.00) | 1st month | ||
| 11 | PM10 | AOR = 0.99 (0.98,1.00) | 2nd month | ||
| 11 | PM10 | AOR = 1.00 (0.99,1.02) | 3rd month | ||
| 11 | PM10 | AOR = 1.01 (0.99,1.03) | 4–6th months | ||
| 11 | PM10 | AOR = 0.99 (0.98,1.00) | 7–9th months | ||
| 11 | PM10 | AOR = 1.00 (0.99,1.01) | C-3 | ||
| 11 | SO2 | AOR = 1.00 (1.00,1.01) | 1st month | ||
| 11 | SO2 | AOR = 1.00 (0.99,1.01) | 2nd month | ||
| 11 | SO2 | AOR = 0.99 (0.98,1.00) | 3rd month | ||
| 11 | SO2 | AOR = 0.99 (0.98,1.00) | 4–6th months | ||
| 11 | SO2 | AOR = 1.00 (0.99,1.01) | 7–9th months | ||
| 11 | SO2 | AOR = 1.00 (0.99,1.01) | C-3 |
Statistically significant (p < 0.05), C-3 = 3 months before conception, BD = before delivery, AOR = adjusted odds ratio
Of the three studies that examined NO2, one reported a weak protective association with LBW in the third trimester (AOR = 0.92 for Study 22). Two out of four studies that examined PM10 reported significant associations with LBW in late pregnancy. One study reported a weak protective association in the third trimester (AOR = 0.95 for Study 22), while another reported a weak protective association for the sub-outcome very low birth weight (<1500 g) in the 7th–9th months of pregnancy (AOR = 0.94 for Study 11).
The results indicate that there is more consistent evidence of a statistically significant association between exposure to SO2 and low birth weight.
3.5.3. Preterm birth
Eleven of the reviewed studies examined preterm birth (PTB). Only one study examined O3 and this study reported an association for exposure within the last two months before delivery (3.1% and 4.6% increase in the number of preterm births for the last four and eight weeks before delivery, respectively, for Study 8) (Table 9).
Table 9.
Quantitative results for preterm birth for a 10 μg/m3 increase in exposure.
| Study | Pollutant | Result (95% CI) | Exposure period | Details | |
|---|---|---|---|---|---|
| 5 | * | PM10 | AOR = 1.14 (1.02,1.28) | entire pregnancy | medically indicated |
| 5 | PM10 | AOR = 1.01 (0.94,1.08) | trimester 2 | medically-indicated | |
| 5 | PM10 | AOR = 1.04 (1.00,1.09) | last 4 weeks BD | medically-indicated | |
| 5 | PM10 | AOR = 1.04 (1.00,1.09) | last 6 weeks BD | medically-indicated | |
| 5 | PM10 | AOR = 1.03 (0.98,1.09) | last 8 weeks BD | medically-indicated | |
| 5 | * | PM10 | AOR = 1.07 (1.01,1.14) | trimester 1 | medically-indicated |
| 5 | PM10 | AOR = 1.05 (0.99,1.12) | trimester 3 | medically-indicated | |
| 5 | PM10 | AOR = 1.03 (0.96,1.10) | entire pregnancy | moderate preterm | |
| 5 | PM10 | AOR = 1.01 (0.99,1.04) | last 4 weeks BD | moderate preterm | |
| 5 | PM10 | AOR = 1.01 (0.98,1.03) | last 6 weeks BD | moderate preterm | |
| 5 | PM10 | AOR = 1.00 (0.97,1.03) | last 8 weeks BD | moderate preterm | |
| 5 | PM10 | AOR = 1.01 (0.97,1.05) | trimester 1 | moderate preterm | |
| 5 | PM10 | AOR = 1.01 (0.97,1.06) | trimester 2 | moderate preterm | |
| 5 | PM10 | AOR = 0.98 (0.95,1.02) | trimester 3 | moderate preterm | |
| 5 | PM10 | AOR = 1.02 (0.94,1.10) | entire pregnancy | spontaneous | |
| 5 | PM10 | AOR = 1.01 (0.99,1.04) | last 4 weeks BD | spontaneous | |
| 5 | PM10 | AOR = 1.01 (0.98,1.04) | last 6 weeks BD | spontaneous | |
| 5 | PM10 | AOR = 1.00 (0.97,1.04) | last 8 weeks BD | spontaneous | |
| 5 | PM10 | AOR = 0.97 (0.93,1.02) | trimester 1 | spontaneous | |
| 5 | PM10 | AOR = 1.02 (0.97,1.07) | trimester 2 | spontaneous | |
| 5 | PM10 | AOR = 0.97 (0.93,1.01) | trimester 3 | spontaneous | |
| 5 | PM10 | AOR = 0.96 (0.82,1.12) | entire pregnancy | very preterm | |
| 5 | * | PM10 | AOR = 1.07 (1.02,1.13) | last 4 weeks BD | very preterm |
| 5 | * | PM10 | AOR = 1.09 (1.02,1.15) | last 6 weeks BD | very preterm |
| 5 | * | PM10 | AOR = 1.10 (1.03,1.17) | last 8 weeks BD | very preterm |
| 5 | PM10 | AOR = 0.97 (0.87,1.07) | trimester 1 | very preterm | |
| 5 | PM10 | AOR = 1.02 (0.92,1.13) | trimester 2 | very preterm | |
| 5 | PM10 | AOR = 1.06 (0.97,1.16) | trimester 3 | very preterm | |
| 14 | NO2 | AOR = 0.97 (0.91,1.03) | 1 month BD | ||
| 14 | NO2 | AOR = 0.95 (0.89,1.01) | 1st month | ||
| 14 | NO2 | AOR = 0.91 (0.86,1.00) | 2 months BD | ||
| 14 | NO2 | AOR = 0.96 (0.90,1.03) | 2nd month | ||
| 14 | NO2 | AOR = 0.96 (0.90,1.03) | 3rd month | ||
| 8 | NO2 | −0.6% change in number of events (−3.6,2.4) | 4 weeks BD | ||
| 8 | NO2 | −2.0% change in number of events (−5.4,1.4) | 6 weeks BD | ||
| 8 | * | NO2 | 5.4% change in number of events (1.8,9.1) | 8 weeks BD | |
| 21 | * | NO2 | AOR = 1.24 (1.03,1.48) | entire pregnancy | |
| 2 | NO2 | RR = 1.00 (1.00,1.01) | entire pregnancy | ||
| 17 | NO2 | RR = 1.00 (1.00,1.01) | entire pregnancy | ||
| 3 | NO2 | AOR = 0.93 (0.70,1.25) | trimester 1 | ||
| 14 | NO2 | AOR = 0.99 (0.91,1.08) | trimester 1 | ||
| 3 | NO2 | AOR = 0.22 (0.68,1.08) | trimester 2 | ||
| 15 | * | NO2 | AOR = 0.93 (0.90,0.97) | trimester 2 | |
| 3 | NO2 | AOR = 0.96 (0.72,1.29) | trimester 3 | ||
| 14 | NO2 | AOR = 0.94 (0.87,1.02) | trimester 3 | ||
| 15 | * | NO2 | AOR = 1.06 (1.03,1.09) | trimester 3 | |
| 8 | * | O3 | 3.1% change in number of events (0.2,6.0) | 4 weeks BD | |
| 8 | O3 | 3.0% change in number of events (−0.6,6.5) | 6 weeks BD | ||
| 8 | * | O3 | 4.6% change in number of events (0.4,8.9) | 8 weeks BD | |
| 14 | PM10 | AOR = 0.98 (0.95,1.00) | 1 month BD | ||
| 11 | PM10 | AOR = 1.00 (0.99,1.01) | 1st month | ||
| 14 | PM10 | AOR = 1.00 (0.98,1.03) | 1st month | ||
| 14 | * | PM10 | AOR = 1.04 (1.01,1.06) | 2 months BD | |
| 11 | * | PM10 | AOR = 1.02 (1.01,1.02) | 2nd month | |
| 14 | PM10 | AOR = 0.98 (0.95,1.00) | 2nd month | ||
| 11 | * | PM10 | AOR = 1.05 (1.04,1.06) | 3rd month | |
| 14 | PM10 | AOR = 0.98 (0.96,1.00) | 3rd month | ||
| 8 | PM10 | −0.2% change in number of events (−2.2,1.8) | 4 weeks BD | ||
| 11 | * | PM10 | AOR = 0.97 (0.96,0.99) | 4–6th months | |
| 8 | PM10 | −0.9% change in number of events (−3.4,1.5) | 6 weeks BD | ||
| 11 | * | PM10 | AOR = 1.04 (1.03,1.05) | 7–9th months | |
| 8 | * | PM10 | 4.4% change in number of events (1.6,7.3) | 8 weeks BD | |
| 11 | * | PM10 | AOR = 1.06 (1.05,1.07) | C-3 | |
| 5 | PM10 | AOR = 1.02 (0.96,1.08) | entire pregnancy | ||
| 21 | * | PM10 | AOR = 1.03 (1.01,1.05) | entire pregnancy | |
| 2 | PM10 | RR = 1.01 (1.00,1.01) | entire pregnancy | ||
| 17 | PM10 | RR = 1.01 (1.00,1.01) | entire pregnancy | ||
| 5 | PM10 | AOR = 1.02 (1.00,1.04) | last 4 weeks BD | ||
| 5 | PM10 | AOR = 1.02 (0.99,1.04) | last 6 weeks BD | ||
| 5 | PM10 | AOR = 1.01 (0.98,1.04) | last 8 weeks BD | ||
| 3 | PM10 | AOR = 1.00 (0.92,1.08) | trimester 1 | ||
| 5 | PM10 | AOR = 1.00 (0.97,1.04) | trimester 1 | ||
| 14 | PM10 | AOR = 0.94 (0.90,1.00) | trimester 1 | ||
| 3 | PM10 | AOR = 0.97 (0.88,1.08) | trimester 2 | ||
| 5 | PM10 | AOR = 1.01 (0.97,1.05) | trimester 2 | ||
| 3 | PM10 | AOR = 1.01 (0.90,1.12) | trimester 3 | ||
| 5 | PM10 | AOR = 0.99 (0.96,1.03) | trimester 3 | ||
| 14 | PM10 | AOR = 1.01 (0.98,1.05) | trimester 3 | ||
| 14 | * | SO2 | AOR = 1.06 (1.03,1.09) | 1 month BD | |
| 14 | SO2 | AOR = 1.01 (0.99,1.04) | 1st month | ||
| 11 | * | SO2 | AOR = 1.02 (1.02,1.03) | 1st month | |
| 14 | SO2 | AOR = 1.00 (0.98,1.03) | 2 months BD | ||
| 11 | SO2 | AOR = 1.01 (1.00,1.01) | 2nd month | ||
| 14 | SO2 | AOR = 1.02 (1.00,1.05) | 2nd month | ||
| 14 | SO2 | AOR = 1.00 (0.97,1.03) | 3rd month | ||
| 11 | * | SO2 | AOR = 0.98 (0.97,0.99) | 3rd month | |
| 8 | SO2 | 2.4% change in number of events (−1.0,5.9) | 4 weeks BD | ||
| 11 | SO2 | AOR = 1.00 (0.99,1.01) | 4th–6th months | ||
| 8 | SO2 | 0.9% change in number of events (−3.3, 5.1) | 6 weeks BD | ||
| 11 | SO2 | AOR = 1.00 (1.00,1.01) | 7th–9th months | ||
| 8 | * | SO2 | 11.9% change in number of events (6.7,17.1) | 8 weeks BD | |
| 11 | SO2 | AOR = 1.00 (1.00,1.01) | C-3 | ||
| 2 | * | SO2 | RR = 1.01 (1.01,1.02) | entire pregnancy | |
| 17 | * | SO2 | RR = 1.01 (1.01,1.02) | entire pregnancy | |
| 21 | * | SO2 | AOR = 1.06 (1.02,1.10) | entire pregnancy | |
| 14 | SO2 | AOR = 1.04 (1.00,1.08) | trimester 1 | ||
| 14 | SO2 | AOR = 1.02 (0.99,1.06) | trimester 3 | ||
| 15 | SO2 | AOR = 1.03 (1.00,1.05) | trimester 3 |
Statistically significant (p < 0.05), C-3 = 3 months before conception, BD = before delivery, AOR = adjusted odds ratio
Three out of eight studies that examined NO2 found a significant association with PTB. One study reported a 5.4% increase in the number of preterm births for exposure to NO2 in the eight weeks before delivery (Study 8). Associations were also reported for the third trimester (AOR = 1.06 for Study 15) and for the entire pregnancy (AOR = 1.24 for Study 21). There was a weak protective association for the second trimester (AOR = 0.93 for Study 15).
Five out of 10 studies that examined PM10 reported significant associations. For PTB overall, associations were reported for different exposure periods, from three months before conception (AOR = 1.06 for Study 11) to two months before delivery (AOR = 1.04 for Study 14), as well as for the entire pregnancy (AOR = 1.03 for Study 21). One study reported a 4.4% increase in the number of preterm births for exposure to PM10 in the eight weeks before delivery (Study 8). For the sub-outcome medically-indicated preterm birth (defined in Section 3.3.1), respective adjusted odds ratios were 1.07 and 1.14 for the first trimester and the entire pregnancy (Study 5). For very preterm birth (<32 weeks), associations were observed in the last four, six and eight weeks before delivery (AOR = 1.07, AOR = 1.09 and AOR = 1.10, respectively for Study 5).
For SO2, the majority (seven of nine) of studies reported significant associations for different exposure periods, ranging from the first month of pregnancy (AOR = 1.02 for Study 11) to one month before delivery (AOR = 1.06 for Study 14), as well as for the entire pregnancy (AOR = 1.06 for Study 21). Two studies reported identical results (RR = 1.01 for the entire pregnancy for Study 2 and 17). Moreover, a prospective cohort study (Study 1) also reported an association (AOR = 1.21 for each unit increase in ln(SO2)) over the entire pregnancy.
The results indicate that there is more consistent evidence of a statistically significant association between exposure to SO2 and preterm birth.
3.5.4. Congenital anomaly
Seven studies examined congenital anomaly for NO2, PM10 and/or SO2. Of the four studies that examined NO2, two reported associations with congenital anomaly (Table 10). Notably, strong associations were reported with congenital malformations of the great arteries (AOR = 2.03), isolated cases of patent ductus arteriosus (PDA) (AOR = 2.16) and pooled congenital heart defects (AOR = 2.11) for exposure over the entire pregnancy (Study 23).
Table 10.
Quantitative results for congenital anomaly for a 10 μg/m3 increase in exposure.
| Study | Pollutant | Result (95% CI) | Exposure period | Details | |
|---|---|---|---|---|---|
| 23 | SO2 | AOR = 0.90 (0.71,1.13) | Trimester 1 | Pooled heart defects | |
| 23 | * | NO2 | AOR = 2.11 (1.24,3.63) | Entire pregnancy | Pooled heart defects |
| 23 | NO2 | AOR = 1.36 (0.93,1.99) | Trimester 1 | Pooled heart defects | |
| 23 | * | NO2 | AOR = 1.59 (1.13,2.23) | Trimester 2 | Pooled heart defects |
| 23 | NO2 | AOR = 1.14 (0.84,1.56) | Weeks 3–8 | Pooled heart defects | |
| 23 | * | PM10 | AOR = 1.35 (1.15,1.60) | Entire pregnancy | Pooled heart defects |
| 23 | * | PM10 | AOR = 1.17 (1.04,1.33) | Trimester 1 | Pooled heart defects |
| 23 | * | PM10 | AOR = 1.26 (1.13,1.42) | Trimester 2 | Pooled heart defects |
| 23 | PM10 | AOR = 1.04 (0.96,1.14) | Weeks 3–8 | Pooled heart defects | |
| 23 | SO2 | AOR = 1.10 (0.70,1.73) | Entire pregnancy | Pooled heart defects | |
| 23 | SO2 | AOR = 0.98 (0.75,1.28) | Trimester 2 | Pooled heart defects | |
| 23 | SO2 | AOR = 0.96 (0.80,1.14) | Weeks 3–8 | Pooled heart defects | |
| 23 | * | NO2 | AOR = 2.16 (1.13,4.14) | Entire pregnancy | Patent ductus arteriosus |
| 23 | NO2 | AOR = 1.50 (0.95,2.35) | Trimester 1 | Patent ductus arteriosus | |
| 23 | * | NO2 | AOR = 1.63 (1.08,2.46) | Trimester 2 | Patent ductus arteriosus |
| 23 | NO2 | AOR = 1.18 (0.83,1.69) | Weeks 3–8 | Patent ductus arteriosus | |
| 23 | * | PM10 | AOR = 1.31 (1.07,1.60) | Entire pregnancy | Patent ductus arteriosus |
| 23 | * | PM10 | AOR = 1.21 (1.04,1.41) | Trimester 1 | Patent ductus arteriosus |
| 23 | * | PM10 | AOR = 1.23 (1.08,1.41) | Trimester 2 | Patent ductus arteriosus |
| 23 | PM10 | AOR = 1.07 (0.96,1.19) | Weeks 3–8 | Patent ductus arteriosus | |
| 23 | SO2 | AOR = 0.92 (0.52,1.62) | Entire pregnancy | Patent ductus arteriosus | |
| 23 | SO2 | AOR = 0.88 (0.66,1.17) | Trimester 1 | Patent ductus arteriosus | |
| 23 | SO2 | AOR = 0.92 (0.67,1.28) | Trimester 2 | Patent ductus arteriosus | |
| 23 | SO2 | AOR = 0.94 (0.76,1.16) | Weeks 3–8 | Patent ductus arteriosus | |
| 23 | * | NO2 | AOR = 2.03 (1.11,3.71) | Entire pregnancy | Defect of great arteries |
| 23 | NO2 | AOR = 1.33 (0.87,2.05) | Trimester 1 | Defect of great arteries | |
| 23 | * | NO2 | AOR = 1.67 (1.13,2.47) | Trimester 2 | Defect of great arteries |
| 23 | NO2 | AOR = 1.12 (0.80,1.58) | Weeks 3–8 | Defect of great arteries | |
| 23 | * | PM10 | AOR = 1.36 (1.12,1.65) | Entire pregnancy | Defect of great arteries |
| 23 | * | PM10 | AOR = 1.22 (1.06,1.41) | Trimester 1 | Defect of great arteries |
| 23 | * | PM10 | AOR = 1.25 (1.10,1.43) | Trimester 2 | Defect of great arteries |
| 23 | PM10 | AOR = 1.08 (0.98,1.19) | Weeks 3–8 | Defect of great arteries | |
| 23 | SO2 | AOR = 1.13 (0.67,1.91) | Entire pregnancy | Defect of great arteries | |
| 23 | SO2 | AOR = 0.94 (0.79,1.11) | Trimester 1 | Defect of great arteries | |
| 23 | SO2 | AOR = 0.98 (0.71,1.34) | Trimester 2 | Defect of great arteries | |
| 23 | SO2 | AOR = 0.98 (0.80,1.20) | Weeks 3–8 | Defect of great arteries | |
| 23 | NO2 | AOR = 1.48 (0.55,3.97) | Entire pregnancy | Defect of cardiac septa | |
| 23 | NO2 | AOR = 0.96 (0.44,2.10) | Trimester 1 | Defect of cardiac septa | |
| 23 | NO2 | AOR = 1.41 (0.73,2.71) | Trimester 2 | Defect of cardiac septa | |
| 23 | NO2 | AOR = 0.96 (0.49,1.85) | Weeks 3–8 | Defect of cardiac septa | |
| 23 | * | PM10 | AOR = 1.44 (1.04,1.99) | Entire pregnancy | Defect of cardiac septa |
| 23 | PM10 | AOR = 1.13 (0.89,1.45) | Trimester 1 | Defect of cardiac septa | |
| 23 | * | PM10 | AOR = 1.32 (1.05,1.66) | Trimester 2 | Defect of cardiac septa |
| 23 | PM10 | AOR = 1.04 (0.88,1.24) | Weeks 3–8 | Defect of cardiac septa | |
| 23 | * | SO2 | AOR = 2.35 (1.01,5.49) | Entire pregnancy | Defect of cardiac septa |
| 23 | SO2 | AOR = 0.97 (0.70,1.33) | Trimester 1 | Defect of cardiac septa | |
| 23 | SO2 | AOR = 1.16 (0.69,1.95) | Trimester 2 | Defect of cardiac septa | |
| 23 | SO2 | AOR = 1.07 (0.77,1.49) | Weeks 3–8 | Defect of cardiac septa | |
| 10 | NO2 | AOR = 1.00 (1.00,1.00) | 1st month | Congenital heart disease, polydactyly, limb reduction defects and hypospadias | |
| 24 | NO2 | AOR = 1.00 (0.86,1.16) | 1st month | All diagnosed birth defects | |
| 24 | NO2 | AOR = 0.99 (0.85,1.15) | 2nd month | All diagnosed birth defects | |
| 24 | NO2 | AOR = 0.98 (0.85,1.13) | 3rd month | All diagnosed birth defects | |
| 24 | PM10 | AOR = 1.08 (0.99,1.18) | 1st month | All diagnosed birth defects | |
| 24 | PM10 | AOR = 1.06 (0.97,1.16) | 2nd month | All diagnosed birth defects | |
| 24 | * | PM10 | AOR = 1.13 (1.03,1.23) | 3rd month | All diagnosed birth defects |
| 24 | SO2 | AOR = 1.10 (0.93,1.30) | 1st month | All diagnosed birth defects | |
| 24 | SO2 | AOR = 1.09 (0.92,1.29) | 2nd month | All diagnosed birth defects | |
| 24 | SO2 | AOR = 1.11 (0.94,1.32) | 3rd month | All diagnosed birth defects | |
| 14 | * | NO2 | AOR = 1.11 (1.01,1.22) | Weeks 3–8 | 24 types of birth defects |
| 11 | PM10 | AOR = 1.01 (0.98,1.03) | 1st month | Birth deformities | |
| 11 | PM10 | AOR = 1.03 (1.00,1.06) | 2nd month | Birth deformities | |
| 11 | * | PM10 | AOR = 1.05 (1.02,1.08) | 3rd month | Birth deformities |
| 11 | PM10 | AOR = 1.03 (0.99,1.07) | 4–6th months | Birth deformities | |
| 11 | * | PM10 | AOR = 1.03 (1.01,1.05) | 7–9th months | Birth deformities |
| 11 | PM10 | AOR = 1.02 (0.99,1.04) | C-3 | Birth deformities | |
| 14 | PM10 | AOR = 0.99 (0.95,1.03) | Weeks 3–8 | 24 types of birth defects | |
| 11 | SO2 | AOR = 0.99 (0.97,1.01) | 1st month | Birth deformities | |
| 11 | SO2 | AOR = 0.98 (0.96,1.00) | 2nd month | Birth deformities | |
| 11 | * | SO2 | AOR = 0.97 (0.95,0.99) | 3rd month | Birth deformities |
| 11 | SO2 | AOR = 0.99 (0.97,1.02) | 4–6th months | Birth deformities | |
| 11 | SO2 | AOR = 0.99 (0.97,1.01) | 7–9th months | Birth deformities | |
| 11 | SO2 | AOR = 1.00 (0.98,1.02) | C-3 | Birth deformities | |
| 14 | SO2 | AOR = 0.99 (0.94,1.03) | Weeks 3–8 | 24 types of birth defects |
Statistically significant (p < 0.05), C-3 = 3 months before conception, AOR = adjusted odds ratio
Four out of five studies that examined PM10 reported associations for different exposure periods during pregnancy. Associations for all diagnosed birth defects were relatively weak (highest AOR = 1.13 in the third month of pregnancy for Study 24). However, higher effect estimates were reported for malformations of the cardiac septa (AOR = 1.44), great arteries (AOR = 1.36), and PDA (AOR = 1.31) as well as pooled heart defects (AOR = 1.35) over the entire pregnancy (Study 23). There was a strong association with fetal cardiovascular deformity for exposure in the first three months of pregnancy (AOR = 2.22 for a one quartile increase in PM10 for Study 12).
Two out of five studies examining SO2 reported significant associations. One study reported a weak protective association with all diagnosed birth defects for exposure in the third month of pregnancy (AOR = 0.97 for Study 11). However, another reported an association with malformations of the cardiac septa over the entire pregnancy (AOR = 2.35 for Study 23).
The results indicate that there is more consistent evidence of a statistically significant association between exposure to PM10 and congenital cardiovascular defects.
3.5.5. Mortality
Five of the reviewed studies examined mortality. Results for individual pollutants were not available for Study 9; however, the study reported that ‘perineonate death’ (defined in Section 3.3.4) was significantly higher in rural compared to city areas (p < 0.001). Of the two studies that examined NO2, only one observed associations with mortality (Table 11). There was an association between fetal loss in early pregnancy (<14 weeks) and exposure to NO2 one and two months before conception as well as one month after conception (all ORs = 1.05 for Study 7). Only one study examined O3 and reported a weak protective association with stillbirth for full term births with exposure in the second trimester (AOR = 0.96 for Study 25).
Table 11.
Quantitative results for mortality for a 10 μg/m3 increase in exposure.
| Study | Pollutant | Result (95% CI) | Exposure period | Details | |
|---|---|---|---|---|---|
| 25 | CO | AOR = 1.00 (1.00,1.00) | Entire pregnancy | Term births | |
| 25 | CO | AOR = 1.00 (1.00,1.00) | Trimester 1 | Term births | |
| 25 | CO | AOR = 1.00 (1.00,1.00) | Trimester 2 | Term births | |
| 25 | CO | AOR = 1.00 (1.00,1.00) | Trimester 3 | Term births | |
| 25 | NO2 | AOR = 0.98 (0.94,1.02) | Entire pregnancy | Term births | |
| 25 | NO2 | AOR = 0.99 (0.95,1.03) | Trimester 1 | Term births | |
| 25 | NO2 | AOR = 0.98 (0.94,1.01) | Trimester 2 | Term births | |
| 25 | NO2 | AOR = 0.99 (0.95,1.03) | Trimester 3 | Term births | |
| 25 | O3 | AOR = 0.97 (0.93,1.01) | Entire pregnancy | Term births | |
| 25 | O3 | AOR = 1.00 (0.96,1.03) | Trimester 1 | Term births | |
| 25 | * | O3 | AOR = 0.96 (0.93,0.99) | Trimester 2 | Term births |
| 25 | O3 | AOR = 1.00 (0.96,1.03) | Trimester 3 | Term births | |
| 25 | PM10 | AOR = 1.01 (0.97.,1.04) | 1st month | Term births | |
| 25 | PM10 | AOR = 1.01 (0.97,1.04) | 2nd month | Term births | |
| 25 | PM10 | AOR = 0.98 (0.95,1.02) | 3rd month | Term births | |
| 25 | PM10 | AOR = 0.96 (0.91,1.00) | Entire pregnancy | Term births | |
| 25 | PM10 | AOR = 1.00 (0.96,1.04) | Trimester 1 | Term births | |
| 25 | * | PM10 | AOR = 0.95 (0.91,0.99) | Trimester 2 | Term births |
| 25 | PM10 | AOR = 0.98 (0.94,1.01) | Trimester 3 | Term births | |
| 25 | SO2 | AOR = 1.00 (0.93,1.11) | 1st month | Term births | |
| 25 | SO2 | AOR = 1.00 (0.93,1.11) | 2nd month | Term births | |
| 25 | SO2 | AOR = 0.96 (0.90,1.07) | 3rd month | Term births | |
| 25 | SO2 | AOR = 0.96 (0.87,1.07) | Entire pregnancy | Term births | |
| 25 | SO2 | AOR = 1.00 (0.90,1.07) | Trimester 1 | Term births | |
| 25 | SO2 | AOR = 0.96 (0.87,1.07) | Trimester 2 | Term births | |
| 25 | SO2 | AOR = 0.96 (0.90,1.07) | Trimester 3 | Term births | |
| 25 | CO | AOR = 1.00 (1.00,1.00) | Entire pregnancy | Preterm births | |
| 25 | CO | AOR = 1.00 (1.00,1.00) | Trimester 1 | Preterm births | |
| 25 | CO | AOR = 1.00 (1.00,1.00) | Trimester 2 | Preterm births | |
| 25 | CO | AOR = 1.00 (1.00,1.00) | Trimester 3 | Preterm births | |
| 25 | NO2 | AOR = 1.01 (0.97,1.05) | Entire pregnancy | Preterm births | |
| 25 | NO2 | AOR = 1.02 (0.99,1.06) | Trimester 1 | Preterm births | |
| 25 | NO2 | AOR = 1.00 (0.96,1.03) | Trimester 2 | Preterm births | |
| 25 | NO2 | AOR = 0.99 (0.94,1.04) | Trimester 3 | Preterm births | |
| 25 | O3 | AOR = 1.00 (0.96,1.04) | Entire pregnancy | Preterm births | |
| 25 | O3 | AOR = 1.01 (0.98,1.04) | Trimester 1 | Preterm births | |
| 25 | O3 | AOR = 1.00 (0.97,1.03) | Trimester 2 | Preterm births | |
| 25 | O3 | AOR = 0.99 (0.97,1.04) | Trimester 3 | Preterm births | |
| 25 | PM10 | AOR = 1.03 (1.00,1.06) | 1st month | Preterm births | |
| 25 | PM10 | AOR = 1.03 (1.00,1.06) | 2nd month | Preterm births | |
| 25 | PM10 | AOR = 1.02 (0.99,1.05) | 3rd month | Preterm births | |
| 25 | PM10 | AOR = 1.00 (0.96,1.05) | Entire pregnancy | Preterm births | |
| 25 | PM10 | AOR = 1.03 (1.00,1.07) | Trimester 1 | Preterm births | |
| 25 | PM10 | AOR = 0.99 (0.95,1.02) | Trimester 2 | Preterm births | |
| 25 | PM10 | AOR = 0.97 (0.92,1.02) | Trimester 3 | Preterm births | |
| 25 | * | SO2 | AOR = 1.15 (1.07,1.23) | 1st month | Preterm births |
| 25 | * | SO2 | AOR = 1.15 (1.04,1.23) | 2nd month | Preterm births |
| 25 | * | SO2 | AOR = 1.11 (1.04,1.23) | 3rd month | Preterm births |
| 25 | SO2 | AOR = 1.11 (1.00,1.23) | Entire pregnancy | Preterm births | |
| 25 | * | SO2 | AOR = 1.15 (1.04,1.27) | Trimester 1 | Preterm births |
| 25 | SO2 | AOR = 1.00 (0.80,1.27) | Trimester 2 | Preterm births | |
| 25 | SO2 | AOR = 1.04 (0.90,1.15) | Trimester 3 | Preterm births | |
| 11 | PM10 | AOR = 1.00 (0.96,1.04) | 1st month | Perineonate death | |
| 11 | PM10 | AOR = 0.99 (0.95,1.04) | 2nd month | Perineonate death | |
| 11 | PM10 | AOR = 1.02 (0.98,1.07) | 3rd month | Perineonate death | |
| 11 | PM10 | AOR = 1.03 (0.97,1.09) | 4th–6th months | Perineonate death | |
| 11 | * | PM10 | AOR = 1.05 (1.02,1.08) | 7th–9th months | Perineonate death |
| 11 | PM10 | AOR = 1.01 (0.97,1.04) | C-3 | Perineonate death | |
| 11 | SO2 | AOR = 1.00 (0.97,1.02) | 1st month | Perineonate death | |
| 11 | SO2 | AOR = 1.00 (0.98,1.03) | 2nd month | Perineonate death | |
| 11 | SO2 | AOR = 0.98 (0.95,1.01) | 3rd month | Perineonate death | |
| 11 | SO2 | AOR = 0.99 (0.96,1.02) | 4th–6th months | Perineonate death | |
| 11 | SO2 | AOR = 0.97 (0.95,1.00) | 7–9th months | Perineonate death | |
| 11 | SO2 | AOR = 0.99 (0.96,1.02) | C-3 | Perineonate death | |
| 25 | CO | AOR = 1.00 (1.00,1.00) | Entire pregnancy | All births | |
| 25 | CO | AOR = 1.00 (1.00,1.00) | Trimester 1 | All births | |
| 25 | CO | AOR = 1.00 (1.00,1.00) | Trimester 2 | All births | |
| 25 | CO | AOR = 1.00 (1.00,1.00) | Trimester 3 | All births | |
| 25 | NO2 | AOR = 0.99 (0.96,1.02) | Entire pregnancy | All births | |
| 25 | NO2 | AOR = 1.00 (0.98,1.03) | Trimester 1 | All births | |
| 25 | NO2 | AOR = 0.98 (0.96,1.01) | Trimester 2 | All births | |
| 25 | NO2 | AOR = 0.99 (0.96,1.02) | Trimester 3 | All births | |
| 25 | O3 | AOR = 0.99 (0.96,1.02) | Entire pregnancy | All births | |
| 25 | O3 | AOR = 1.00 (0.98,1.03) | Trimester 1 | All births | |
| 25 | O3 | AOR = 0.98 (0.96,1.00) | Trimester 2 | All births | |
| 25 | O3 | AOR = 0.99 (0.97,1.02) | Trimester 3 | All births | |
| 25 | PM10 | AOR = 1.02 (1.00,1.05) | 1st month | All births | |
| 25 | PM10 | AOR = 1.02 (1.00,1.04) | 2nd month | All births | |
| 25 | PM10 | AOR = 1.00 (0.98,1.03) | 3rd month | All births | |
| 25 | PM10 | AOR = 0.98 (0.94,1.01) | Entire pregnancy | All births | |
| 25 | PM10 | AOR = 1.02 (0.99,1.04) | Trimester 1 | All births | |
| 25 | * | PM10 | AOR = 0.96 (0.94,0.99) | Trimester 2 | All births |
| 25 | PM10 | AOR = 0.97 (0.94,1.00) | Trimester 3 | All births | |
| 25 | SO2 | AOR = 1.07 (1.00,1.15) | 1st month | All births | |
| 25 | SO2 | AOR = 1.07 (1.00,1.15) | 2nd month | All births | |
| 25 | SO2 | AOR = 1.04 (1.00,1.11) | 3rd month | All births | |
| 25 | SO2 | AOR = 1.04 (0.96,1.11) | Entire pregnancy | All births | |
| 25 | SO2 | AOR = 1.07 (1.00,1.15) | Trimester 1 | All births | |
| 25 | SO2 | AOR = 1.00 (0.93,1.07) | Trimester 2 | All births | |
| 25 | SO2 | AOR = 1.00 (0.93,1.07) | Trimester 3 | All births | |
| 7 | NO2 | OR = 1.01 (0.98,1.05) | C + 0 | ≤14 weeks pregnancy | |
| 7 | * | NO2 | OR = 1.05 (1.01,1.09) | C + 1 | ≤14 weeks pregnancy |
| 7 | * | NO2 | OR = 1.05 (1.01,1.08) | C−1 | ≤14 weeks pregnancy |
| 7 | * | NO2 | OR = 1.05 (1.01,1.10) | C−2 | ≤14 weeks pregnancy |
| 7 | NO2 | OR = 1.00 (0.97,1.05) | C−3 | ≤14 weeks pregnancy | |
| 7 | PM10 | OR = 1.03 (1.00,1.06) | C + 0 | ≤14 weeks pregnancy | |
| 7 | PM10 | OR = 1.01 (0.99,1.03) | C + 1 | ≤14 weeks pregnancy | |
| 7 | PM10 | OR = 0.99 (0.961.01) | C−1 | ≤14 weeks pregnancy | |
| 7 | PM10 | OR = 0.99 (0.971.01) | C−2 | ≤14 weeks pregnancy | |
| 7 | PM10 | OR = 1.00 (0.991.02) | C−3 | ≤14 weeks pregnancy | |
| 7 | * | SO2 | OR = 1.03 (1.011.04) | C + 0 | ≤14 weeks pregnancy |
| 7 | * | SO2 | OR = 1.03 (1.01,1.05) | C + 1 | ≤14 weeks pregnancy |
| 7 | SO2 | OR = 1.01 (1.00,1.03) | C−1 | ≤14 weeks pregnancy | |
| 7 | SO2 | OR = 1.01 (0.99,1.02) | C−2 | ≤14 weeks pregnancy | |
| 7 | SO2 | OR = 1.01 (0.99,1.02) | C−3 | ≤14 weeks pregnancy |
Statistically significant (p < 0.05) C ± X indicates X ± months post/prior to conception, AOR = adjusted odds ratio.
For PM10, two out of four studies reported significant associations with mortality. One study reported an association with perineonate death for exposure between the 7th and 9th months of pregnancy (AOR = 1.05 for Study 11). However, another reported weak protective associations with stillbirth for exposure in the second trimester (AOR = 0.96 for all births and AOR = 0.95 for full term births only for Study 25).
For SO2, two out of three studies reported associations with mortality. There was an association between fetal loss in early pregnancy (<14 weeks) and exposure to SO2 around the time of conception as well as one month after conception (both ORs = 1.03 for Study 7). There were also associations with stillbirth for preterm births for exposure in the first, second and third months of pregnancy as well as for the first trimester overall (including non-preterm births) (AOR = 1.15 for the first trimester for Study 25).
The results indicate that there is currently insufficient evidence for a statistically significant association between ambient air pollution exposure during pregnancy and mortality of the child.
4. Discussion
This is the first systematic review of the association between ambient air pollution and adverse pregnancy outcomes in China. We investigated the association between six different pollutants (SO2, NO2, PM10, PM2.5, CO and O3) and five outcomes (decrease in birth weight, low birth weight, preterm birth, congenital anomaly and mortality) based on 25 reviewed studies. The results indicated that SO2 was more consistently associated with lower birth weight and preterm birth, and that PM10 was associated with congenital anomaly, notably cardiovascular defects. Significant associations were reported for different periods during pregnancy as well as for the entire pregnancy.
The results for other pollutants and outcomes were inconsistent. In some cases, weak protective associations were reported, particularly in the third trimester. Further studies are needed to clarify associations for other outcomes and pollutants, particularly CO, PM2.5 and O3, for which there were relatively few studies.
We note that effect sizes in the reviewed studies were higher than those reported in previous systematic reviews, conducted mainly in western countries. A meta-analysis (Chen et al., 2014) of 21 combinations of air pollutants and congenital anomalies reported only one statistically significant result: the pooled odds ratio for coarctation of the aorta was 1.09 (95% CI: 1.01, 1.18) per 10 μg/m3 of NO2. In contrast, the reviewed studies indicated a consistent, statistically significant association between exposure to PM10 and a range of congenital cardiovascular defects. The adjusted odds ratio for pooled heart defects was 1.35 (1.15, 1.60) and ranged from 1.31 (1.07, 1.60) for patent ductus arteriosus to 1.44 (1.04, 1.99) for defects of the cardiac septa per 10 μg/m3 of PM10 over the entire pregnancy.
It is important to note that exposures for different time periods and concentrations of different air pollutants are correlated, making it difficult to determine the effects of individual pollutants (Šrám et al., 2005) or the most relevant window of exposure. Also, since many different types of pollutants are often emitted from the same sources, it is not possible to determine if the observed effects are due to the pollutants studied or if they are due to the effects of other unmeasured pollutants, or interactive effects of multiple mixed pollutants. Human chamber studies (Hackney et al., 1975) have been used to assess the effects of specific pollutants; however, this is not ethical when the subjects are pregnant women and infants/fetuses. In addition, pollution sources can vary by city, leading to potentially different chemical components and toxicity of PM10 or PM2.5.
Although the mechanisms by which air pollution cause adverse pregnancy outcomes are not well-understood, inflammation is a biologically plausible mechanism. Air pollution might lead to preterm birth through early activation of cytokines favouring inflammation, which are otherwise part of the body’s normal preparation for parturition (Engel et al., 2005; Keelan et al., 2003). Placental inflammation in particular can affect transplacental nutrient exchange (Bobak, 2000), which can lead to fetal growth restriction, early delivery and their associated morbidities (Mestan et al., 2010). The role of inflammation in promoting adverse pregnancy outcomes is supported by observational evidence from the Boston Birth Cohort, for which intrauterine inflammation at birth was associated with fine particulate matter exposure (Nachman et al., 2016). Inflammatory effects are not restricted to particulate matter exposure. Animal studies have demonstrated lung inflammation after exposure to sulphur dioxide (Li et al., 2014) and controlled human exposure studies to sulphur dioxide have consistently observed increase in bronchoconstriction (Johns and Linn, 2011).
An important issue in epidemiological studies is adjustment for risk factors. The majority of studies reviewed adjusted for various factors such as meteorological variables, maternal age, gestational age, gravidity, parity and fetal sex. Some studies also adjusted for socioeconomic variables such as education, occupation and family income. Only a few studies adjusted for smoking as a risk factor, but smoking rates among women living in Chinese cities are generally very low (Rich et al., 2015).
All of the reviewed studies used ambient air pollutant concentrations measured at routine monitoring sites with exposures estimated using average concentrations across monitoring sites or, if the subject’s location was known, using the nearest monitor or an inverse-distance weighting approach. None of the reviewed studies undertook personal monitoring or satellite remote sensing. However, molecular epidemiological studies have shown that ambient air pollution levels translate to higher individual exposures (Šrám et al., 2005). The approaches used by these studies are consistent with those of similar studies conducted in other parts of the world due to the feasibility constraints of personal monitoring over long timeframes. Measurement error may differ by study due to differences in the monitoring network or spatial heterogeneity of pollutant by study area.
We attempted to mitigate publication bias by including articles written in Chinese. For low birth weight and preterm birth, there were a large number of articles in both English and Chinese. However, for congenital anomaly and mortality, the majority of articles were written in Chinese. An advantage of this study was that by including peer reviewed articles written in Chinese, we were able to include 14 additional studies on the topic that would not have been included had the review been limited to English language articles.
The study population in China differs in some aspects from the study populations in western countries. Notably, the rate of preterm birth differs for Chinese-born women living China versus those living in different countries, which may be due to factors such as smoking and sexual practices (Newnham et al., 2011). Although the preterm birth rate is lower in China (3%) than the United States (12%) (Martin et al., 2010), the number of preterm births in China per year is double that of the USA (about 500,000 compared to one million preterm births) (Blencowe et al., 2012). However, pregnancy outcomes at the population level are not comparable because China’s Family Planning Policy from 1979 to 2015 restricted parity, and it is well-established that birth weights increase and preterm birth rates decrease with increasing parity, with the greatest decrease in risk observed from first to second birth. In contrast to many western countries such as Australia, the UK and the United States, the race/ethnicity of the population in China is relatively homogeneous, with the majority being Han Chinese. These population differences may result in different health effects for air pollution and pregnancy outcomes in China than in Western countries, due both to physiological differences highlighted earlier and different cultural or social practices.
5. Conclusion
China has a relatively higher level of air pollution, resulting in greater exposure to pollutant concentrations during pregnancy. Our results indicated that effect sizes for preterm birth, change in birth weight and congenital anomaly were demonstrably greater than those reported in western countries. Given the large number of births in China, at a population scale this has a considerable impact on fetal and infant health and consequent morbidities. We conclude that, for pregnancies in China, there were consistent associations between (i) SO2 exposure during pregnancy and preterm birth and lower birth weight; and (ii) PM10 exposure during pregnancy and congenital anomaly.
Table 3.
Sulphur dioxide (SO2) concentrations (μg/m3) reported over the study periods for the reviewed studies.
| Study | Location | Mean | SD | Min | 20th | 25th | 40th | Median | 60th | 75th | 80th | Max |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Beijing | 102 | 630 | |||||||||
| 3 | Beijing | 38 | 32 | 8 | 142 | |||||||
| 4 | Beijing | 17 | 3 | 10 | 14 | 16 | 20 | 23 | ||||
| 6 | Beijing | 9 | 18 | 55 | 146 | 239 | 308 | |||||
| 14 | Beijing | 37 | 38 | 6 | 12 | 21 | 46 | 222 | ||||
| 17 | Guangdong | 52 | 35 | 9 | 29 | 44 | 63 | 195 | ||||
| 18 | Guangdong | 52 | 35 | 9 | 29 | 44 | 63 | 195 | ||||
| 2 | Guangzhou | 52 | 35 | 9 | 29 | 44 | 63 | 195 | ||||
| 15 | Guangzhou | 66 | ||||||||||
| 19 | Guangzhou | 64 | 99 | 41 | 53 | |||||||
| 22 | Guangzhou | 64 | 99 | 41 | 53 | |||||||
| 24 | Haikou | 20 | 11 | |||||||||
| 23 | Lanzhou | 55 | ||||||||||
| 8 | Shanghai | 56 | 1 | 11 | 36 | 52 | 71 | 163 | ||||
| 25 | Taiwan | 16 | 8 | 6 | 45 | |||||||
| 11 | Taiyuan | 182 | 169 | |||||||||
| 20 | Taiyuan | 74 | 37 | |||||||||
| 21 | Taiyuan | 87 | 55 | 15 | 45 | 67 | 119 | 317 | ||||
| 7 | Tianjin | 53 | ||||||||||
| 10 | Tianjin | 70 | 59 |
Table 4.
Particulate matter <10 μm (PM10) concentrations (μg/m3) reported over the study periods for the reviewed studies.
| Study | Location | Mean | SD | Min | 25th | Median | 75th | Max |
|---|---|---|---|---|---|---|---|---|
| 3 | Beijing | 135 | 35 | 69 | 264 | |||
| 14 | Beijing | 126 | 81 | 12 | 70 | 111 | 150 | 600 |
| 12 | Fuzhou | 46 | 63 | 73 | 80 | 95 | ||
| 17 | Guangdong | 83 | 53 | 20 | 49 | 70 | 94 | 405 |
| 18 | Guangdong | 83 | 53 | 20 | 49 | 70 | 94 | 405 |
| 2 | Guangzhou | 83 | 53 | 20 | 49 | 70 | 94 | 405 |
| 13 | Guangzhou | 105 | 77 | 39 | 51 | 66 | ||
| 15 | Guangzhou | 112 | ||||||
| 19 | Guangzhou | 105 | 77 | 39 | 51 | |||
| 22 | Guangzhou | 105 | 77 | 39 | 51 | |||
| 24 | Haikou | 40 | 18 | |||||
| 5 | Lanzhou | 142 | 18 | |||||
| 23 | Lanzhou | 144 | ||||||
| 8 | Shanghai | 101 | 3 | 22 | 59 | 83 | 130 | 333 |
| 25 | Taiwan | 73 | 23 | 34 | 126 | |||
| 11 | Taiyuan | 212 | 74 | |||||
| 20 | Taiyuan | 140 | 13 | |||||
| 21 | Taiyuan | 142 | 67 | 38 | 109 | 129 | 160 | 508 |
| 7 | Tianjin | 108 | ||||||
| 16 | Tianjin | 114 | 45 |
HIGHLIGHTS.
Ambient air pollution was significantly associated with adverse pregnancy outcomes in China.
Sulphur dioxide (SO2) was consistently associated with lower birth weight and preterm birth.
Particulate matter (PM10) was consistently associated with congenital anomaly, especially cardiovascular defects.
Results for nitrogen dioxide (NO2) were inconsistent.
Further studies are needed on the effects of fine particulate matter (PM2.5), ozone (O3) and carbon monoxide (CO).
Acknowledgments
The authors are grateful to the Australia-China Centre for Air Quality Science and Management (ACC-AQSM) for helpful discussions in preparing the manuscript. Dr Pereira is supported by NHMRC Early Career Fellowship (Sidney Sax) #1052236 and project grant #1099655. Professor Bell would like to acknowledge the funding source National Institutes of Health (NIEHS R01ES019587). A special thanks is also extended to Symon Aked for his assistance with the graphical abstract.
References
- Aimaier Y-k-F, Wang J-j, Peng Z-y, Li G-x, Pan X-c. Case-control study of ambient air pollution and adverse pregnancy outcomes of women in Beijing. J Environ Health. 2013;30:389–393. [Google Scholar]
- Bell ML, Ebisu K, Belanger K. Ambient air pollution and low birth weight in Connecticut and Massachusetts. Environ Health Perspect. 2007:1118–1124. doi: 10.1289/ehp.9759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman NG, Parker RA. Meta-analysis: neither quick nor easy. BMC Med Res Methodol. 2002;2 doi: 10.1186/1471-2288-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blencowe H, Cousens S, Oestergaard MZ, Chou D, Moller AB, Narwal R, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012;379:2162–2172. doi: 10.1016/S0140-6736(12)60820-4. [DOI] [PubMed] [Google Scholar]
- Bobak M. Outdoor air pollution, low birth weight, and prematurity. Environ Health Perspect. 2000;108:173–176. doi: 10.1289/ehp.00108173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesaroni G, Badaloni C, Gariazzo C, Stafoggia M, Sozzi R, Davoli M, et al. Long-term exposure to urban air pollution and mortality in a cohort of more than a million adults in Rome. Environ Health Perspect. 2013;121:324. doi: 10.1289/ehp.1205862. (Online) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Chen Y-q, Hou H-y, Wang D. Relationship between air pollution and perineonate death and birth defects in Tianjin. J Int Obstet Gynecol. 2012;39:308–310. [Google Scholar]
- Chen EKC, Zmirou-Navier D, Padilla C, Deguen S. Effects of air pollution on the risk of congenital anomalies: a systematic review and meta-analysis. Int J Environ Res Public Health. 2014;11:7642–7668. doi: 10.3390/ijerph110807642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel SAM, Erichsen HC, Savitz DA, Thorp J, Chanock SJ, Olshan AF. Risk of spontaneous preterm birth is associated with common proinflammatory cytokine polymorphisms. Epidemiology. 2005;16:469–477. doi: 10.1097/01.ede.0000164539.09250.31. [DOI] [PubMed] [Google Scholar]
- Gurjar B, Jain A, Sharma A, Agarwal A, Gupta P, Nagpure A, et al. Human health risks in megacities due to air pollution. Atmos Environ. 2010;44:4606–4613. [Google Scholar]
- Hackney JD, Linn WS, Buckley RD, Pedersen EE, Karuza SK, Law DC, et al. Experimental studies on human health effects of air pollutants: I. Design considerations. Arch Environ Health. 1975;30:373–378. doi: 10.1080/00039896.1975.10666728. [DOI] [PubMed] [Google Scholar]
- Hester RE, Harrison RM. Services RSoCI Air Pollution and Health. Royal Society of Chemistry; 1998. [Google Scholar]
- Hou HY, Chen YQ, Wang D, Zou XP. Association between ambient air particulate matter and missed abortion in early pregnancy in Tianjin. J Int Obstet Gynecol. 2012;39:609–612. [Google Scholar]
- Hou HY, Wang D, Zou XP, Yang ZH, Li TC, Chen YQ. Does ambient air pollutants increase the risk of fetal loss? A case–control study. Arch Gynecol Obstet. 2014;289:285–291. doi: 10.1007/s00404-013-2962-1. [DOI] [PubMed] [Google Scholar]
- Huang C, Nichols C, Liu Y, Zhang Y, Liu X, Gao S, et al. Ambient air pollution and adverse birth outcomes: a natural experiment study. Popul Health Metrics. 2015;13 doi: 10.1186/s12963-015-0050-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang BF, Lee YL, Jaakkola JJ. Air pollution and stillbirth: a population-based case-control study in Taiwan. Environ Health Perspect. 2011;119:1345–1349. doi: 10.1289/ehp.1003056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Zhang Y, Song G, Chen G, Chen B, Zhao N, et al. A time series analysis of outdoor air pollution and preterm birth in Shanghai, China. Biomed Environ Sci. 2007;20:426–431. [PubMed] [Google Scholar]
- Jin L, Qiu J, Zhang Y, Qiu W, He X, Wang Y, et al. Ambient air pollution and congenital heart defects in Lanzhou, China. Environ Res Lett. 2015;10:074005. doi: 10.1088/1748-9326/10/7/074005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns DO, Linn WS. A review of controlled human SO2 exposure studies contributing to the US EPA integrated science assessment for sulfur oxides. Inhal Toxicol. 2011;23:33–43. doi: 10.3109/08958378.2010.539290. [DOI] [PubMed] [Google Scholar]
- Keelan J, Blumenstein M, Helliwell R, Sato T, Marvin K, Mitchell M. Cytokines, prostaglandins and parturition—a review. Placenta. 2003;24:S33–S46. doi: 10.1053/plac.2002.0948. [DOI] [PubMed] [Google Scholar]
- King Edward Memorial Hospital. Report of the Birth Defects Registry of Western Australia 1980–2009 2010 [Google Scholar]
- Li R, Kou X, Tian J, Meng Z, Cai Z, Cheng F, et al. Effect of sulfur dioxide on inflammatory and immune regulation in asthmatic rats. Chemosphere. 2014;112:296–304. doi: 10.1016/j.chemosphere.2014.04.065. [DOI] [PubMed] [Google Scholar]
- Liang Z, Wu L, Fan L, Zhao Q. Ambient air pollution and birth defects in Haikou city, Hainan province. BMC Pediatr. 2014;14 doi: 10.1186/s12887-014-0283-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JA, Osterman M, Sutton PD. Are preterm births on the decline in the United States? Recent data from the National Vital Statistics System. NCHS Data Brief. 2010:1–8. [PubMed] [Google Scholar]
- Mestan K, Yu Y, Matoba N, Cerda S, Demmin B, Pearson C, et al. Placental inflammatory response is associated with poor neonatal growth: preterm birth cohort study. Pediatrics. 2010;125:e891–e898. doi: 10.1542/peds.2009-0313. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- Nachman R, Mao G, Zhang X, Hong X, Chen Z, Soria C, et al. Intrauterine Inflammation and Maternal Exposure to ambient PM2.5 during preconception and specific periods of pregnancy: the Boston birth cohort. Environ Health Perspect. 2016;124:1608–1615. doi: 10.1289/EHP243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newnham JP, Sahota DS, Zhang CY, Xu B, Zheng M, Doherty DA, et al. Preterm birth rates in Chinese women in China, Hong Kong and Australia–the price of Westernisation. Aust N Z J Obstet Gynaecol. 2011;51:426–431. doi: 10.1111/j.1479-828X.2011.01327.x. [DOI] [PubMed] [Google Scholar]
- O’Neill MS, Jerrett M, Kawachi I, Levy JI, Cohen AJ, Gouveia N, et al. Health, wealth, and air pollution: advancing theory and methods. Environ Health Perspect. 2003;111:1861–1870. doi: 10.1289/ehp.6334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padula AM, Tager IB, Carmichael SL, Hammond SK, Lurmann F, Shaw GM. The association of ambient air pollution and traffic exposures with selected congenital anomalies in the San Joaquin Valley of California. Am J Epidemiol. 2013;177:1074–1085. doi: 10.1093/aje/kws367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen M, Giorgis-Allemand L, Bernard C, Aguilera I, Andersen AMN, Ballester F, et al. Ambient air pollution and low birthweight: a European cohort study (ESCAPE) Lancet Respir Med. 2013;1:695–704. doi: 10.1016/S2213-2600(13)70192-9. [DOI] [PubMed] [Google Scholar]
- Pereira G, Belanger K, Ebisu K, Bell ML. Fine particulate matter and risk of preterm birth in Connecticut in 2000–2006: a longitudinal study. Am J Epidemiol. 2013:kwt216. doi: 10.1093/aje/kwt216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera F, Hemminki K, Jedrychowski W, Whyatt R, Campbell U, Hsu Y, et al. In utero DNA damage from environmental pollution is associated with somatic gene mutation in newborns. Cancer Epidemiol Biomark Prev. 2002;11:1134–1137. [PubMed] [Google Scholar]
- Pope DP, Mishra V, Thompson L, Siddiqui AR, Rehfuess EA, Weber M, et al. Risk of low birth weight and stillbirth associated with indoor air pollution from solid fuel use in developing countries. Epidemiol Rev. 2010:mxq005. doi: 10.1093/epirev/mxq005. [DOI] [PubMed] [Google Scholar]
- Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40:1324–1343. doi: 10.1183/09031936.00080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin J, Chadwick T, Natarajan M, Howel D, Pearce MS, Pless-Mulloli T. Maternal exposure to ambient air pollutants and risk of congenital anomalies. Environ Res. 2009;109:181–187. doi: 10.1016/j.envres.2008.11.007. [DOI] [PubMed] [Google Scholar]
- Rich DQ, Liu K, Zhang J, Thurston SW, Stevens TP, Pan Y, et al. Differences in birth weight associated with the 2008 Beijing Olympics air pollution reduction: results from a natural experiment. Environ Health Perspect. 2015;123:880–887. doi: 10.1289/ehp.1408795. (Online) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan HY, Zhao QG, Chen LN, Zheng YX. A case control study on association between air pollution and birth weight. Chin J Woman Child Health Res. 2008;19:306–308. [Google Scholar]
- Ruan HY, Zhao QG, Chen LN. A case-control study on the relationship between air pollutants and preterm birth in Guangzhou Fanyu district. China Mod Med. 2010;17:140–141. [Google Scholar]
- Savitz DA, Terry JW, Dole N, Thorp JM, Siega-Riz AM, Herring AH. Comparison of pregnancy dating by last menstrual period, ultrasound scanning, and their combination. Am J Obstet Gynecol. 2002;187:1660–1666. doi: 10.1067/mob.2002.127601. [DOI] [PubMed] [Google Scholar]
- Shi M, Liu C-b, Chen X-q, Song Y-f, Hong X-r, Sun Q-h. Fetal congenital deformity resulting from ambient inhalable particulate matter: a case-control study. Chin J Perinat Med. 2013;16:200–205. [Google Scholar]
- Šrám RJ, Binková B, Rössner P, Rubeš J, Topinka J, Dejmek J. Adverse reproductive outcomes from exposure to environmental mutagens. Mutat Res Fundam Mol Mech Mutagen. 1999;428:203–215. doi: 10.1016/s1383-5742(99)00048-4. [DOI] [PubMed] [Google Scholar]
- Šrám RJ, Binková B, Dejmek J, Bobak M. Ambient air pollution and pregnancy outcomes: a review of the literature. Environ Health Perspect. 2005:375–382. doi: 10.1289/ehp.6362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stieb DM, Chen L, Eshoul M, Judek S. Ambient air pollution, birth weight and preterm birth: a systematic review and meta-analysis. Environ Res. 2012;117:100–111. doi: 10.1016/j.envres.2012.05.007. [DOI] [PubMed] [Google Scholar]
- Wang X, Ding H, Ryan L, Xu X. Association between air pollution and low birth weight: a community-based study. Environ Health Perspect. 1997;105:514–520. doi: 10.1289/ehp.97105514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. Ambient Air Pollution Database 2014 [Google Scholar]
- Woodruff TJ, Grillo J, Schoendorf KC. The relationship between selected causes of postneonatal infant mortality and particulate air pollution in the United States. Environ Health Perspect. 1997;105:608–612. doi: 10.1289/ehp.97105608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Ding H, Wang X. Acute effects of total suspended particles and sulfur dioxides on preterm delivery: a community-based cohort study. Arch Environ Health. 1995;50:407–415. doi: 10.1080/00039896.1995.9935976. [DOI] [PubMed] [Google Scholar]
- Xu LZ, Xue XP, Zhang YP, Liu L, Shen J. Association between air pollutants exposure during pregnancy and preterm delivery in Taiyuan. J Environ Health. 2008;25:298–301. [Google Scholar]
- Zhang Y-p, Lui X-h, Ren Z-h. Effects of air pollution on pregnancy outcome in Taiyuan, China. J Environ Health. 2007;24:128–131. [Google Scholar]
- Zhang Y-p, Zhang Z-q, Wu Y-c. Association of air pollution with preterm birth in Taiyuan. J Environ Health. 2008;25:194–198. [Google Scholar]
- Zhao QG, Li B. The influence of inhalable PM10 in outdoor air on birth weight of the newborn. Chin J Woman Child Health Res. 2008;19:4–6. [Google Scholar]
- Zhao QG, Li B, Du YK. Study on the association between air pollutants and birth weight. Chin J Prev Med. 2008;9:607–613. [Google Scholar]
- Zhao QG, Liang ZJ, Li B, Du YK. Acute effect of air pollution on preterm birth in a city. J Environ Health. 2010a;27:488–492. [Google Scholar]
- Zhao QG, Liang ZJ, Li B, Du YK. An analysis of influence of air pollution on incidence of low birth weight of the neonate. Chin J Woman Child Health Res. 2010b;21:138–142. [Google Scholar]
- Zhao Q, Liang Z, Tao S, Zhu J, Du Y. Effects of air pollution on neonatal prematurity in Guangzhou of China: a time-series study. Environ Health. 2011;10 doi: 10.1186/1476-069X-10-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao N, Qiu J, Zhang Y, He X, Zhou M, Li M, et al. Ambient air pollutant PM10 and risk of preterm birth in Lanzhou, China. Environ Int. 2015;76:71–77. doi: 10.1016/j.envint.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J-y, Hui W-l, Lan X-x, Hou H-y, Wang D, Chen Y-q. Periconceptional exposure to air pollutants SO2, NO2 associated with birth defects. J Int Obstet Gynecol. 2013;40:71–74. [Google Scholar]


