Abstract
We describe a case of disseminated Beauveria bassiana infection in a patient with acute lymphoblastic leukemia. Her infection was successfully treated with amphotericin B and itraconazole. B. bassiana is rarely reported as a human pathogen. It is commonly found in soil and because of its pathogenicity to many insect species is incorporated into several pesticides.
CASE REPORT
A 44-year-old Caucasian woman was diagnosed with acute lymphoblastic leukemia. She had a history of recurrent sinusitis but was otherwise well. She lived in a rural area of the South Island of New Zealand and owned a garden center.
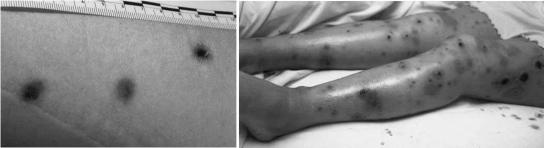
At presentation she had a neutrophil count of 0.36 × 109/liter but was clinically well with no signs of infection and commenced treatment with prophylactic ciprofloxacin and fluconazole. She was nursed in a positive-pressure single room and given a low-bacteria diet. At day 1 of induction chemotherapy (United Kingdom Acute Lymphoblastic Leukemia 12 protocol), ciprofloxacin and fluconazole were stopped, and co-trimoxazole and nystatin were started. At day 15 of treatment the patient became febrile, and Streptococcus viridans was isolated from blood cultures. She was treated with piperacillin and gentamicin, and her temperature stabilized. She remained neutropenic, and at day 20, small (<1-cm) purple macular “cigarette burn ” lesions were noted on her left upper arm (Fig. 1A). An aspirate sent for bacteriology and skin scrapings sent for fungal culture yielded no microorganisms. Four days after the skin lesions developed, she complained of symptoms of sinusitis, headache, and facial pain and had percussion tenderness over her maxillary sinuses. Paranasal sinus disease was not identified by computed tomography scanning, and in view of her persisting neutropenia further invasive investigations were not performed. Serum transaminases were elevated on day 21, but an abdominal ultrasound scan was normal.
FIG. 1.
(A) Initial skin lesions resembling cigarette burns. (B) Disseminated skin lesions.
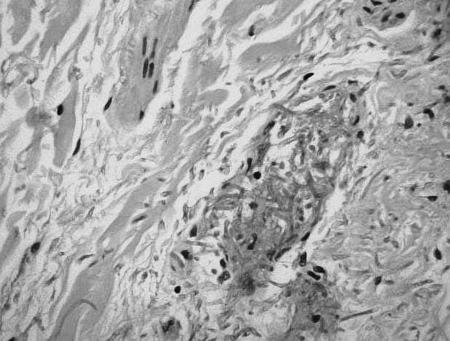
Fluconazole was restarted on day 28, and an excision biopsy of one of the skin lesions was performed the next day. Histopathological examination of the biopsy specimen revealed sharply demarcated areas of necrosis with lack of cellular reaction at the interface. The necrotic tissue was heavily permeated by fungal hyphae, which also invaded the local blood vessels (Fig. 2). Cultures of the tissue biopsy sample on blood agar and Sabouraud glucose agar plates at 30°C produced a pure growth of a white mould identified preliminarily as a Beauveria sp. No recovery of the mould was obtained on plates incubated at 35°C. The susceptibility of the isolate was assessed using Sensititre Yeast One susceptibility plates (Trek Diagnostic Systems, West Sussex, England). Testing was performed at 30°C, and the MIC was read after 72 h of incubation. The susceptibility results were as follows: the amphotericin B MIC was 2.0 mg/liter, the itraconazole MIC was 0.06 mg/liter, the fluconazole MIC was 8.0 mg/liter, the ketoconazole MIC was 0.125 mg/liter, and the 5-flucytosine MIC was >64 mg/liter.
FIG. 2.
Skin biopsy sample stained with hematoxylin and eosin showing blood vessel infiltration by fungal hyphae.
Prednisone was stopped on day 37 of phase 1 induction, and conventional treatment with intravenous amphotericin B was commenced at 15 mg daily, escalating to a dose of 55 mg daily for 10 days. The skin lesions progressed, involving the patient's arms, legs, buttocks, and face, and became necrotic and exudative (Fig. 1B). The patient developed a persistent hemorrhagic left-sided pleural effusion which was consistently sterile in microbial culture. A computed tomography scan of her chest was performed and was suggestive of lung necrosis. Therapy was then changed to liposomal amphotericin (AmBisome) at a dose escalating to 200 mg daily for 10 days before returning to conventional intravenous amphotericin in combination with itraconazole for a further 25 days. Antifungal therapy was continued throughout phase 2 induction and for the duration of her neutropenia. Her skin lesions continued to heal over several months with some scarring. She is now receiving maintenance chemotherapy and has had no recurrence of fungal infection.
The isolate (MR2097) was identified as Beauveria bassiana at the Mycology Reference Laboratory, Auckland Hospital, and referred for confirmation to the University of Alberta Microfungus Collection and Herbarium, Edmonton, Canada, where it was retained as UAMH 10179. Colonial and microscopic appearances were typical of B. bassiana. Colonies were fast growing, reaching diameters of 4.5 cm in 13 days at 30°C on potato dextrose agar (Difco Laboratories, Detroit, Mich.). They were densely cottony to flocculent, with droplets of exudate on the surface, yellowish white, raised, and dome shaped. Conidiogenous cells occurred in sporodochial clusters and were slightly swollen at the base and narrowed at the tip to form a zigzag rachis. Conidia were oval to subglobose and apiculate and measured 2 to 3 μm long and 1.5 to 2 μm wide. Neither the patient isolate nor an environmental isolate (UAMH 7866) grew when stab inoculated onto PDA and incubated at 35°C (tested twice). Both isolates were confirmed as B. bassiana on the basis of their physiological profiles using the automated microtiter plate system BIOLOG (Biolog Inc., Hayward, Calif.); however, a positive result was obtained after 96 h for the patient isolate compared with 72 h for the environmental isolate. In addition to morphological and physiological similarities, a sequence of the nuclear ribosomal internal transcribed spacer (ITS) region from the case isolate demonstrated the highest homology (98% identity) to the GenBank sequences of B. bassiana and its teleomorph Cordyceps bassiana.
Beauveria species are rarely verified as agents of human infection. B. bassiana has been reported as the cause of mycotic keratitis in three cases (4, 5, 6), but other reports concerning this species have not been substantiated (2, 3, 9, 10). Our case of disseminated Beauveria infection is the second involving an immunosuppressed leukemic patient. In the first report, a patient undergoing therapy for acute myeloid leukemia presented with a dry cough and fever and was found by lung biopsy to have allergic alveolitis (3). Ultrasonography revealed multiple liver and splenic lesions, and the fungus was isolated from a liver biopsy specimen. No skin lesions were reported. Our patient's first indication of infection was the appearance of skin lesions and respiratory symptoms developed much later. The skin lesions were widespread, appearing and resolving in concert. Tissue sections revealed deep tissue invasion and blood vessel involvement by fungal hyphae, strongly suggesting that the lesions were caused by hematogenous spread of the fungus from a primary site in the lung rather than by extrinsic contamination, direct inoculation, or secondary opportunistic colonization. While necrosis was observed in the lesions of both patients, little cellular reaction to the presence of fungal hyphae was noted.
The isolates from these leukemia patients were similar in their inability to grow at 37°C. In our case and that of Henke et al. (3), primary isolation on Sabouraud glucose agar recovered several colonies of the mould on plates incubated at 26 to 30°C, but no growth was found on plates incubated at 35 or 37°C. Henke et al. (3) reported a 6.5% conidial germination rate after 72 h at 35°C for their patient isolate, but the isolate did not grow at 37°C. An insect-associated control strain failed to germinate. The two isolates that we tested, one clinical and one environmental, did not grow at 35°C. Ability to grow at 35 to 37°C is considered a requirement for human pathogenicity, but these two cases suggest that Beauveria species may have the potential to grow in tissues of the profoundly immunocompromised host. In contrast, animal studies have demonstrated that B. bassiana can survive in animal organs without becoming invasive. In a survey of the mycobiota of small mammals, B. bassiana was one of the commonest fungi cultured from lungs without evidence of tissue invasion (1). Similarly, B. bassiana was noninvasive when inoculated intramuscularly into normal mice (7).
Our isolate was identified as B. bassiana by the characteristic clusters of basally swollen conidiogenous cells and the shape of the conidia, by its profile in the BIOLOG system, and by a high ITS similarity to published sequences of B. bassiana and its teleomorph C. bassiana. Henke et al. (3) suggested that ITS region sequences of their case isolate were closest to those of isolates deposited under the name Botrytis tenella, which has long been considered a synonym of B. bassiana. Their analysis uncovered greater variation than expected among the isolates of Beauveria examined, leading to uncertainty as to whether some species formerly considered as synonyms may need reevaluation. However, the sequences from their case isolate (AJ457169 and AJ457170) are deposited under the name B. bassiana.
B. bassiana is a well known and widely dispersed insect pathogen. Some strains have been developed for use as biological insecticides, and these products have been approved since 1999 by the U.S. Environmental Protection Agency for use on all food and feed crops. At work in her garden center, our patient used a combined insecticide and fungicide that did not contain B. bassiana. However, it is possible that she was exposed to the fungus, either from the soil or from organic pesticides, while living in an agricultural area. B. bassiana is a cosmopolitan fungus found in soil and many other substrates. The fungus is occasionally detected in air sampling (8), and Henke et al. (3) suggested that their patient was initially infected by airborne conidia. In an experimental study, pneumonitis was induced in rodents exposed to airborne Beauveria spores (11).
Although the evidence concerning the pathogenicity of B. bassiana in the present case is weakened by the isolate's failure to grow at 35°C and recovery of the fungus from only one site, it is strengthened by the histopathology revealing extensive soft tissue infiltration and invasion of blood vessels and by similarities with the case reported by Henke et al. (3) of invasive Beauveria infection in a leukemia patient.
Nucleotide sequence accession number.
The sequence of the nuclear ribosomal ITS region from the case isolate was deposited in GenBank with the accession number AY513236.
Acknowledgments
We thank Maria Johnston, Department of Microbiology, Dunedin Public Hospital, New Zealand, and Ben Wilson, Department of Biological Sciences, University of Alberta, Edmonton, Canada. Special appreciation is expressed to Connie Fe C. Gibas, Microfungus Collection, University of Alberta, for assistance with sequencing the case isolate.
Financial assistance to L. Sigler from the Natural Sciences and Engineering Research Council of Canada is gratefully acknowledged.
REFERENCES
- 1.Carmichael, J. W. 1961. Fungi from Alberta rodents. Mycopathol. Mycol. Appl. 14:9-135. [DOI] [PubMed] [Google Scholar]
- 2.De Hoog, G. S., J. Guarro, J. Gene, and M. J. Figueras. 2000. Atlas of clinical fungi. Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands.
- 3.Henke, M. O., G. S. de Hoog, U. Gross, G. Zimmermann, D. Kraemer, and M. Weig. 2002. Human deep tissue infection with an entomopathogenic Beauveria species. J. Clin. Microbiol. 40:2698-2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kisla, T. A., A. Cu-Unjieng, L. Sigler, and J. Sugar. 2000. Medical management of Beauveria bassiana keratitis. Cornea 19:405-406. [DOI] [PubMed] [Google Scholar]
- 5.Low, C. D., P. R. Badenoch., and D. J. Coster. 1997. Beauveria bassiana keratitis cured by deep lamellar dissection. Cornea 16:698-699. [PubMed] [Google Scholar]
- 6.Sachs, S. W., J. Baum, and C. Mies. 1985. Beauveria bassiana keratitis. Br. J. Ophthalmol. 69:548-550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Semalulu, S. S., J. M. MacPherson, H. B. Scheifer, G. G. Khachatourians. 1992. Pathogenicity of Beauveria bassiana in mice. Zentbl. Vetmed. Reihe B 39:81-90. [DOI] [PubMed] [Google Scholar]
- 8.Sigler, L., and A. Flis. 1998. Catalogue of the University of Alberta Microfungus Collection and Herbarium (UAMH). University of Alberta, Edmonton, Canada.
- 9.Sigler, L. 2003. Miscellaneous opportunistic fungi: Microascaceae and other ascomycetes, hyphomycetes, coelomycetes and basidiomycetes, p. 637-676. In D. H. Howard (ed.), Pathogenic fungi in humans and animals. Marcel Dekker, New York, N.Y.
- 10.Sigler, L., and P. E. Verweij. 2003. Aspergillus, Fusarium, and other opportunistic moniliaceous fungi, p 1726-1760. In P. R. Murray, E. J. Baron, J. H. Jorgensen, M. A. Pfaller, and R. H. Yolken (ed.), Manual of clinical microbiology, 8th ed. ASM Press, Washington, D.C.
- 11.Song, J. Y. 1989. Experimental study on farmer's lung-like lesions caused by Beauveria bassiana. Zhonghua Bing Li Xue Za Zhi 18:111-114. [PubMed] [Google Scholar]