Abstract
Trabecular bone score (TBS) has been proposed as a dual-energy X-ray absorptiometry (DXA) derived measure of underlying quality of trabecular bone; however, TBS is not considered valid for those with body mass index (BMI) >37 kg/m2. Our objective was to determine the association between TBS and lumbar spine (trabecular) volumetric BMD (LS-VBMD) and to examine whether the association varied by BMI and body composition among older men below this clinical threshold. We used regression models to study 3479 men age ≥65 years enrolled in the Osteoporotic Fractures in Men (MrOS) study who had TBS from spine DXA scans, LS-VBMD from central quantitative computed tomography, measures of trunk fat and lean mass from DXA, and BMI <37kg/m2. TBS was categorized as normal (n = 925), partially degraded (n = 1747), and degraded (n = 807). TBS was inversely related to BMI, trunk fat mass, and trunk lean mass (all p< 0.001). The relationship between TBS and LS-VBMD was nonlinear with magnitude of effect (slope of regression line using standardized variables) ranging from 0.07 (95% CI, −0.02 to 0.15) among those with degraded TBS up to 0.71 (95% CI, 0.54 to 0.89) among those with normal TBS. The relationship was still nonlinear after adjusting for age, clinical site, and either BMI, trunk lean mass, or trunk fat mass. The magnitude of effect relating TBS and LS-VBMD also decreased with increasing BMI (interaction, p = 0.090) and increasing trunk lean mass (interaction, p = 0.001), but not with increasing trunk fat mass (interaction, p = 0.224). In summary, the strength of the association between TBS and LS-VBMD among older men was variable and dependent on BMI and body composition, particularly trunk lean mass. The clinical utility of TBS among older men may be somewhat limited among men with high BMI or high trunk lean mass.
Keywords: Osteoporosis, Trabecular Bone Score, Body Mass Index, Bone Mineral Density
Introduction
A real BMD has been the standard tool for use in the diagnosis of osteoporosis as well as an essential component for the computation of fracture risk, but the measure has some limitations. Since areal BMD is a two-dimensional projection, it cannot fully separate cortical and trabecular bone and is confounded by bone size, because larger bones with the same volumetric density will have higher areal BMD than smaller bones. The areal BMD measure itself also does not assess local variation in density that might be attributable to structural features of the cortices and large gaps in trabecular microarchitecture, which is crucial to structural integrity and resistance to fracture. Other imaging modalities including central quantitative computed tomography (QCT) provide a more detailed distribution of the trabecular and cortical bone, as well as microarchitecture of the bone; however, such imaging is limited by its availability outside research centers and its higher radiation dose and cost.
Trabecular bone score (TBS) has been proposed as a novel and readily obtained secondary measure of bone quality that might improve fracture risk prediction when used in conjunction with areal lumbar spine or hip bone mineral density (BMD) measured by dual-energy X-ray absorptiometry (DXA).(1) TBS is a measure of grayscale texture in the underlying DXA image. TBS is not a direct measure of bone architecture because it is derived from a two-dimensional image of a three-dimensional structure. Central to the interpretation is that a dense trabecular network in the three-dimensional bone will project to a two-dimensional grayscale image with few apparent gaps; ie, an image with a large number of evenly distributed small pixel-to-pixel variations.(2) A compromised trabecular network with large gaps would project to a two-dimensional grayscale image where there is less averaging introduced by the projection and hence the gaps would still be apparent in the two-dimensional image.
TBS has been reported to predict incident hip and major osteoporotic fractures in several studies, independent of areal BMD and other clinical risk factors.(3–5) We reported that lower TBS is associated with a higher risk of major osteoporotic and hip fractures among older U.S. men, albeit with a risk score of modest magnitude.(6) Although ex vivo studies have suggested that TBS is related to several bone parameters as assessed by micro–computed tomography (μCT),(1) it remains unclear which of the parameters is the factor underlying TBS, because the parameters are all highly correlated. Moreover, TBS measurements may be limited by the modification of the textural analysis by soft tissue external to the spine, as shown in a study assessing the effect of simulated soft tissue on the TBS score of a spine phantom.(7) Furthermore, some human studies have noted that body mass index (BMI) is negatively correlated with TBS,(8–10) whereas BMI has been shown to be positively associated with lumbar spine (trabecular) volumetric BMD (LS-VBMD) on QCT.(11) These reports suggest a more complex and indirect association between TBS and QCT parameters than demonstrated in the ex vivo studies. Hence, the complex relationships between BMI, TBS, and LS-VBMD remain unclear.
Our first objective was to determine the overall association between TBS and LS-VBMD among older men, and our second objective was to determine whether measures of body size and composition (BMI, trunk fat mass, trunk lean mass) modify the associations of TBS with LS-VBMD.
Subjects and Methods
From 2000 to 2002, the Osteoporotic Fractures in Men (MrOS) study enrolled 5994 ambulatory men aged 65 years and older living in one of six U.S. metropolitan areas.(12,13) The baseline clinical exam included height (Harpenden stadiometer), weight (balance beam or electronic scale), and DXA scans. BMI was calculated from measured height and weight using the formula BMI=weight (kg)/height (m)2. A subcohort of 3683 men included a sequential selection of white participants and all nonwhite participants who also had central QCT scans.(14) The study sample for this analysis included 3479 men who had TBS from DXA, compartmental volumetric BMD (assessed by lumbar spine QCT), and BMI <37 kg/m2 at baseline (see Fig. 1). Further details concerning study cohort and methods have been published elsewhere.(13) All participants gave written informed consent; the study was conducted in accord with the Helsinki Declaration, and ethics approval was granted through review boards at the respective study centers.
Fig. 1.
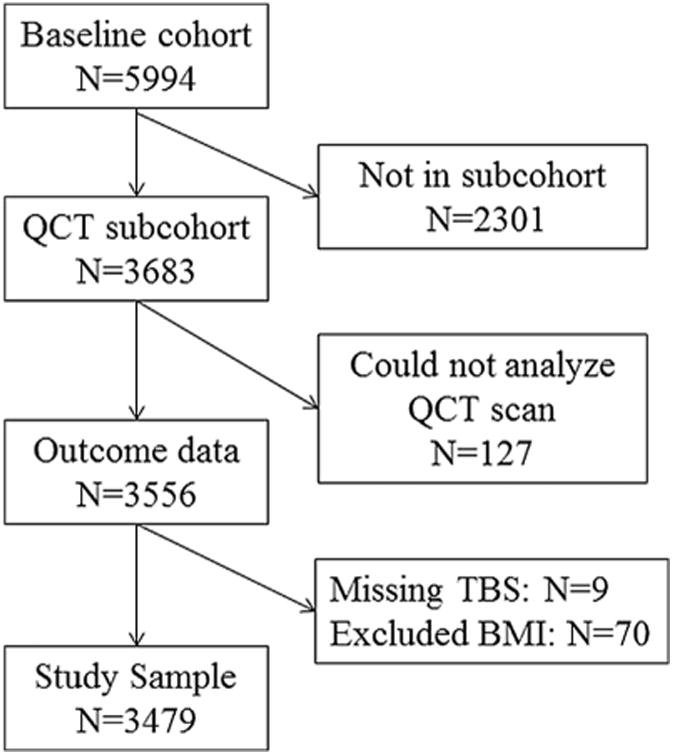
Study flowchart.
Measurement of BMD, trunk fat mass, trunk lean mass, and TBS
Participants had spine, hip, and whole-body scans using QDR 4500 fan-beam densitometers (Hologic, Bedford, MA, USA). Fat mass and lean mass (total and by skeletal site) were obtained using the whole-body scans. The study used standardized procedures for position and scanning and certification of DXA operators to ensure DXA reproducibility. A set of whole-body, spine, hip, and linearity phantoms were circulated between centers and measured to derive cross-calibration equations; however, variation between centers was within acceptable limits and no corrections were required. In addition to BMI, other predictors of interest were DXA-derived fat mass and lean mass at the trunk.
Lumbar spine DXA images were anonymized and then scored using Med-Imaps Software version 2.1 (Pessac, France). The final TBS score had inclusion/exclusion of vertebra L1–L4 concordant with the inclusion/exclusion criteria of the original BMD assessments. As recommended by a working group of TBS experts, TBS scores were grouped into three categories indicating the quality of the bone microarchitecture according to clinically relevant thresholds (degraded [≤1.2], partially degraded [>1.2 to <1.35], and normal [≥1.35]).(2)
Bone parameters derived from QCT
Central QCT scans were done at each center using a standard protocol and sent to a central repository at University of California, San Francisco, for further image analysis.(15) Scans of the lumbar vertebra L1 and L2 were obtained at settings of 120 kVp, 150 mA, 1-mm slice thickness, and 512×512 matrix size in spiral reconstruction mode. Calibration standards with known hydroxyapatite concentrations (150, 75, and 0mg/cm3) (Image Analysis Inc., Columbia, KY, USA) were included for each the participant scan and all measures were converted from Hounsfield units (HU) to equivalent concentration (g/cm3) of calcium hydroxyapatite. The trabecular LS-VBMD was calculated from the region of trabecular bone (10-mm slice) in the vertebral mid-centrum.
Statistical methods
We used ANOVA and pairwise t tests for between TBS category comparisons of continuous variables and chi square tests for between category comparisons of categorical variables. We supplemented this initial assessment of the association between TBS and other variables with linear regression models and TBS as outcome. Because these associations were nonlinear, we used cubic spline terms (five knots at the 5th, 25th, 50th, 75th, and 95th percentiles) to provide the best fit for the estimated model R-squared. The cubic spline model had model R-squared 0.1152 (compared with 0.0902 for the model with linear term only and 0.1135 for the model with a linear and squared term). For the main analysis we ran a series of regression models with lumbar spine LS-VBMD as outcome. The first model included only TBS, whereas the second model included TBS, BMI, and a TBS-BMI interaction term. Two additional models were run with trunk lean mass or trunk fat mass in place of BMI in the second model. The association between TBS and LS-VBMD was again nonlinear so we used cubic spline terms (five knots, TBS score 1.05, 1.15, 1.25, 1.35, and 1.45) for main effect and interactions to provide a best model fit. We further compared models with and without interactions with the likelihood ratio test. We then summarized the resulting spline models graphically by showing TBS versus the predicted values of LS-VBMD overall and by quintile of BMI, quintile of trunk lean mass, and quintile of trunk fat mass. We included all interaction terms for the models regardless of test values for consistency and to visually compare models with and without statistically significant interactions. In view of the interactions, we performed a secondary analysis assessing the distribution of TBS, LS-VBMD, and Spearman correlations stratified by quintile of BMI, trunk lean mass, and trunk fat mass. Finally, we ran a stratified analysis adjusted for age and clinical site within each TBS clinical category using only linear terms and no interaction, noting that the violation of linearity and effect modification were less evident within each of the three categories of TBS score. To get an estimate of the magnitude of effect we reparameterized all the variables to have study sample means of zero and SDs of 1. All analysis was performed using SAS version 9.3 (SAS Institute, Inc., Cary, NC, USA) or Stata Version 12 (Stata Corp., College Station, TX, USA).
Results
Table 1 displays the baseline characteristics of the study sample stratified by clinical TBS category. The study sample included 925 (26.5%) men with normal TBS, 1747 (50.2%) men with partially degraded TBS, and 807 (23.2%) men with degraded TBS. Men with partially degraded TBS had higher BMI, higher fat mass, higher lean mass, and lower LS-VBMD than those with normal TBS scores. These differences were also observed when comparing men with degraded TBS to those with partially degraded or normal TBS scores. Those in the degraded TBS category were substantially more likely to be in the top quintile versus the bottom quintile of BMI, trunk lean mass, and trunk fat mass. Those in the normal TBS category were substantially more likely to be in the bottom quintile versus the top quintile of BMI, trunk lean mass, and trunk fat mass.
Table 1. Baseline Characteristics of Eligible Participants Stratified by TBS Category.
| Characteristics | TBS category (n= 3479) | ||
|---|---|---|---|
|
| |||
| Degraded (TBS=0.74 to 1.20) (n=807) | Partially degraded (TBS=1.21 to 1.34) (n= 1747) | Normal (TBS=1.35 to 1.63) (n=925) | |
| Age (years), mean ± SD | 73.3 ±5.6 | 74.0 ± 6.0 | 73.3 ±5.9 |
| BMI (kg/m2), mean ± SD | 29.6 ±3.5 | 26.8 ± 3.1 | 25.6 ±2.9 |
| Trunk lean mass (kg), mean±SD | 59.2 ±7.4 | 56.1 ±6.9 | 55.4 ±6.3 |
| Trunk fat mass (kg), mean±SD | 26.3 ±6.7 | 20.8 ± 5.9 | 17.9 ±5.3 |
| LS-VBMD (g/cm3), mean ± SD | 0.096 ± 0.034 | 0.106 ±0.036 | 0.128 ±0.040 |
| BMI, quintile 1 (17.2–24.2 kg/m2), n (%) | 45 (5.6) | 351 (20.1) | 300 (32.4) |
| BMI, quintile 5 (30.0–37.0 kg/m2), n (%) | 354 (43.9) | 274 (15.7) | 67 (7.2) |
| Trunk lean mass, quintile 1 (15.6–25.6 kg), n (%) | 97 (12.1) | 386 (22.2) | 209 (22.7) |
| Trunk lean mass, quintile 5 (31.7–43.3 kg), n (%) | 257 (32.1) | 315 (18.1) | 120 (13.0) |
| Trunk fat mass, quintile 1 (1.8–8.6 kg), n (%) | 36 (4.5) | 327 (18.8) | 329 (35.7) |
| Trunk fat mass, quintile 5 (15.3–27.2 kg), n (%) | 381 (47.6) | 259 (14.9) | 52 (5.6) |
Between-group differences p < 0.001 for all variables (ANOVA and pairwise t tests for continuous variable and chi squared for categorical variables). TBS= trabecular bone score.
We also assessed the associations between BMI, lean trunk mass, and fat trunk mass with TBS using continuous variables (with spline terms). Increasing BMI, trunk fat mass, and trunk lean mass were all related to lower TBS (p < 0.001 for all associations). The BMI model accounted for 23.0% of the variation in TBS; the trunk fat mass model accounted for 27.4%of the variation in TBS; and the trunk lean mass model accounted for only 5.6% of the variation in TBS.
Figure 2 shows the univariate relationship between TBS scores and LS-VBMD. Higher TBS scores were associated with the same or higher LS-VBMD and there was a strong attenuation of magnitude of effect (or slope) with decreasing TBS score, especially for those men with degraded TBS, for whom the curve was nearly flat. Using a stratified analysis, the magnitude of effect was 0.71 (95% CI, 0.54 to 0.89) among those with normal TBS, 0.28 (95% CI, 0.16 to 0.41) among those with partially degraded TBS, and 0.07 (95% CI, –0.02 to 0.15) among those with degraded TBS.
Fig. 2.
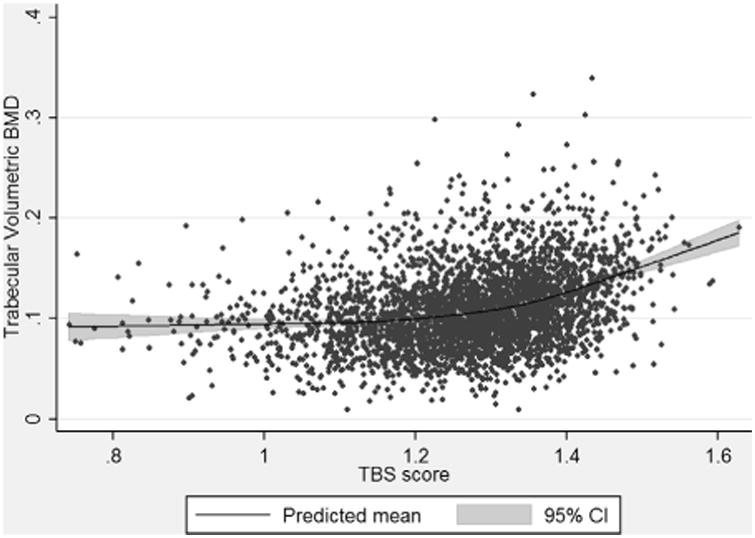
The association between TBS and lumbar spine trabecular volumetric BMD. TBS = trabecular bone score.
The relationships between TBS scores and LS-VBMD stratified by BMI quintiles are shown in Fig. 3A. Within each quintile of BMI, higher TBS scores were associated with the same or higher LS-VBMD, but with some attenuation of magnitude of effect with decreasing TBS score. Increasing BMI quintile was associated with a higher predicted LS-VBMD for any given TBS score. We also observed a likely attenuation of magnitude of effect with increasing BMI quintile (p = 0.090 for interaction terms). Due to these interrelationships, the predicted LS-VBMD of men with degraded TBS in the highest BMI quintile was similar to that of men with partially degraded TBS in lower BMI quintiles.
Fig. 3.
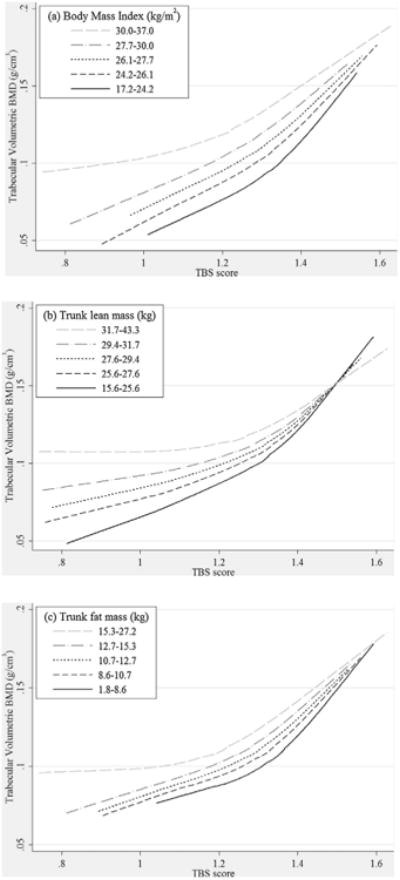
The association between TBS and lumbar spine trabecular volumetric BMD stratified by (A) body mass index quintile, (B) trunk lean mass quintile, and (C) trunk fat mass quintile. TBS=trabecular bone score.
The relationships between TBS scores and LS-VBMD stratified by trunk lean mass quintiles are shown in Fig. 3B. Within each quintile of trunk lean mass, higher TBS scores were associated with the same or higher LS-VBMD, but with some attenuation of magnitude of effect with decreasing TBS score. Increasing trunk lean mass was associated with a higher predicted LS-VBMD for those with lower TBS scores, but a similar predicted LS-VBMD for those with higher TBS scores. We also observed an attenuation of magnitude of effect with increasing trunk lean mass quintile (p = 0.001 for interaction terms). Due to these interrelationships, the predicted LS-VBMD of men with degraded TBS in the highest lean mass quintile was similar to that of men with partially degraded TBS in lower trunk lean mass quintiles.
The relationships between TBS scores and LS-VBMD stratified by trunk fat mass quintiles are shown in Fig. 3C. Within each quintile of trunk fat mass, higher TBS scores were associated with the same or higher LS-VBMD, but with some attenuation of magnitude of effect with decreasing TBS score. Increasing trunk fat mass quintile was associated with a slightly higher predicted LS-VBMD for any given TBS score. There was no statistically significant difference (p=0.224) in magnitude of effect with increasing fat mass quintile.
Table 2 shows the median and interquartile range of TBS and LS-VBMD as well as the Spearman correlation between TBS and LS-VBMD stratified by quintile of BMI, trunk lean mass, and trunk fat mass. Increasing BMI quintile was associated with lower TBS score, but higher LS-VBMD. In addition, Increasing BMI quintile was associated with a widening of the interquartile range of TBS score, whereas the interquartile range of LS-VBMD was similar across quintiles of BMI. Finally, increasing BMI quintile was associated with a lower Spearman correlation between TBS and LS-VBMD. These relationships were similar when trunk lean mass or trunk fat mass quintiles were substituted for BMI quintiles in the analyses.
Table 2. Median and IQR of TBS and LS-VBMD and the Spearman Correlation Between TBS and LS-VBMD Stratified by Quintile of BMI, Trunk Lean Mass, and Trunk Fat Mass.
| TBS score median (IQR) | LS-VBMD median (IQR) | TBS versus LS-VBMD Spearman correlation (95% CI) | |
|---|---|---|---|
| BMI quintile (kg/m2) | |||
| 17.2–24.2 | 1.34 (1.28–1.39) | 0.095 (0.073–0.120) | 0.47 (0.41–0.53) |
| 24.2–26.1 | 1.31 (1.25–1.37) | 0.102 (0.080–0.127) | 0.46 (0.40–0.53) |
| 26.1–27.7 | 1.29 (1.23–1.36) | 0.105 (0.086–0.129) | 0.46 (0.40–0.52) |
| 27.7–30.0 | 1.27 (1.19–1.34) | 0.112 (0.088–0.136) | 0.44 (0.38–0.50) |
| 30.0–37.0 | 1.20 (1.10–1.27) | 0.115 (0.091–0.142) | 0.38 (0.32–0.45) |
| Trunk lean mass quintile (kg) | |||
| 15.6–25.6 | 1.30 (1.24–1.37) | 0.097 (0.075–0.123) | 0.43 (0.37–0.49) |
| 25.6–27.6 | 1.31 (1.24–1.37) | 0.105 (0.084–0.129) | 0.38 (0.31–0.44) |
| 27.6–29.4 | 1.30 (1.23–1.36) | 0.103 (0.083–0.129) | 0.36 (0.29–0.43) |
| 29.4–31.7 | 1.27 (1.18–1.34) | 0.108 (0.087–0.133) | 0.36 (0.30–0.43) |
| 31.7–43.3 | 1.24 (1.15–1.32) | 0.114 (0.090–0.139) | 0.29 (0.22–0.36) |
| Trunk fat mass quintile (kg) | |||
| 1.8–8.6 | 1.35 (1.29–1.40) | 0.105 (0.082–0.133) | 0.41 (0.35–0.48) |
| 8.6–10.7 | 1.32 (1.26–1.37) | 0.104 (0.082–0.130) | 0.41 (0.35–0.48) |
| 10.7–12.7 | 1.29 (1.22–1.35) | 0.105 (0.082–0.128) | 0.35 (0.29–0.42) |
| 12.7–15.3 | 1.27 (1.19–1.33) | 0.109 (0.085–0.133) | 0.43 (0.36–0.49) |
| 15.3–27.2 | 1.18 (1.10–1.26) | 0.105 (0.086–0.130) | 0.29 (0.23–0.36) |
IQR=interquartile range; TBS =trabecular bone score; LS-VBMD=lumbar spine (trabecular) volumetric BMD.
Table 3 shows the regression coefficients from age and clinical-site adjusted models stratified by clinical category (normal, partially degraded, degraded). The relationships were qualitatively similar to the graphical depictions of the nonlinear relationships. TBS was a strong independent predictor of LS-VBMD among those with normal TBS after separately adjusting for each of the three secondary parameters (BMI, trunk fat mass, and trunk lean mass) with magnitude of effects ranging from 0.67 to 0.73. The effects sizes were lower in magnitude (range, 0.30 to 0.47) among those with partially degraded TBS and were even smaller in magnitude among those with degraded TBS (range, 0.16 to 0.28). Higher BMI, higher trunk fat mass, and higher trunk lean mass were associated with higher LS-VBMD in all categories of TBS and the confidence intervals excluded the null in all instances with the exception of lean trunk mass among men with normal TBS.
Table 3. Associations Between TBS and LS-VBMD: Adjusted for BMI, Trunk Fat Mass, or Trunk Lean Mass and Stratified by TBS Category.
| TBS category (n= 3479)
|
|||
|---|---|---|---|
| Degraded (TBS=0.74–1.20) (n=807) | Partially degraded (TBS=1.21–1.34) (n =1747) | Normal (TBS= 1.35–1.63) (n = 925) | |
| Model 1 | |||
| TBS | 0.29 (0.20–0.37) | 0.47 (0.36–0.58) | 0.73 (0.56–0.90) |
| BMI | 0.34 (0.28–0.40) | 0.36 (0.32–0.41) | 0.29 (0.21–0.36) |
| Model 2 | |||
| TBS | 0.19 (0.11–0.28) | 0.39 (0.27–0.51) | 0.72 (0.55–0.89) |
| Trunk fat mass | 0.16 (0.10–0.22) | 0.20 (0.15–0.25) | 0.18 (0.10–0.26) |
| Model 3 | |||
| TBS | 0.16 (0.08–0.24) | 0.31 (0.19–0.42) | 0.67 (0.50–0.85) |
| Trunk lean mass | 0.22 (0.17–0.28) | 0.16 (0.11–0.20) | 0.05 (–0.02 to 0.12) |
Values are regression coefficients (95% confidence interval). The measure of association was the standardized magnitude of effect; ie, beta coefficient from regression models using standardized variables for independent and dependent variables. All regression models were adjusted for age and clinical site. TBS= trabecular bone score; LS-VBMD=lumbar spine (trabecular) volumetric BMD.
Discussion
Our study confirmed that TBS is positively associated with LS-VBMD among older men, but the association is not linear; the magnitude of effect is attenuated with lower (ie, degraded) values of TBS. We also note that the distribution (median and interquartile range) of TBS varied by BMI, trunk lean mass, and trunk fat mass with those in the highest quintile of the respective body size or composition variable having the lowest median TBS along with the widest interquartile range. Our findings suggest that the waning of the association of TBS with LS-VBMD at lower levels of TBS is due to in large part to increasing prevalence of higher BMI among men with lower TBS together with a countervailing association of higher LS-VBMD among men with higher BMI.
There are several possible reasons for the nonlinear association of TBS and LS-VBMD. First, TBS is a priori limited by the DXA image modality. Because DXA lumbar spine images are two-dimensional images, they include a superposition of vertebral body, posterior elements, cortical bone, and trabecular bone, and are impacted by the amount of soft tissue. BMI is indicative of large body size, and this can affect the DXA image and subsequently TBS. In particular, increasing soft tissue thickness introduces image noise. Analytically, any source of image noise will lower the initial slope of the variogram, and thus alter the TBS algorithm. Adjustments have been made to the TBS algorithm (D. Hans, personal communication) to try to account for any effect increased body mass may have on accuracy of TBS measurements; however, TBS measures are still not considered valid among those with a BMI higher than 37 kg/m2. Winzenrieth and colleagues(16) performed simulations whereby DXA-like images were constructed from CT images and with these images showed that mathematically constructed Gaussian noise shifted the TBS scores downward while it preserved the rank order of the test samples. This observed downward shift of scores has been experimentally replicated for DXA-machine–derived TBS scores using spine phantoms and simulated soft tissue.(7) It is not clear whether even correctly specified adjustments, which recalibrate the TBS score to reflect the bone architecture in the absence of interceding tissue, will improve the loss of precision and increased variation introduced by extraskeletal mass (whether it is lean mass or fat mass).
Initial studies were quite favorable in demonstrating the relationship between TBS and bone strength parameters. In particular, using disarticulated vertebra, TBS was shown to be a strong predictor of many trabecular microarchitecture parameters including bone volume fraction, connectivity, trabecular spacing, trabecular thickness, and trabecular number.(1) Silva and colleagues(2) reported strong correlations of spine TBS and high-resolution QCT bone microarchitecture parameters of the radius among postmenopausal women with primary hyperthyroidism, whereas Muschitz and colleagues(17) reported strong correlations between spine TBS and calculated mCT bone microarchitecture parameters of transiliac bone biopsies of men and premenopausal women with idiopathic osteoporosis. However, none of these studies examined whether or not the associations of TBS with microarchitectural parameters vary by BMI, lean tissue mass, or fat tissue mass.
We hypothesized that fat and lean tissue might have differential effects on the relationships between TBS and trabecular LS-VBMD. We found some underlying similarity in that both in higher trunk lean mass and trunk fat mass were both associated with higher LS-VBMD. However, we found substantial effect modification between TBS and trunk lean mass that was not present for fat mass; ie, the association of lean mass and LS-VBMD varied by TBS (attenuation with increased TBS). The effect modification works in both directions, hence the association between TBS and LS-VBMD weakens (consistent with loss of TBS measurement accuracy) as trunk lean mass increases. This raises the question as to whether or not the association between TBS and incident fractures also becomes weaker as BMI or trunk lean mass increase; we suspect that this would need to be addressed in large meta-analyses such as the FRAX cohorts because of the requisite sample size and fracture outcomes necessary to assess potential interactions.
There are several possible explanations for the slightly different effect of lean versus fat tissue, including variability in distribution of tissue or in the attenuation of the X-rays. The calculation of TBS depends on the estimation of skeletal mass adjusted for the interceding tissue. If the interceding tissue is itself heterogeneous, this heterogeneity when superimposed onto the spine two-dimensional image likely introduces more noise in the pixel-by-pixel grayscale transitions, thereby potentially weakening the associations of TBS with true trabecular bone parameters such as LS-VBMD and trabecular microarchitectural parameters. Another possibility relates to the amount of tissue, because the amount of lean trunk mass is on average double that of the average lean fat mass. Finally, the use of BMI to correct for nonskeletal tissue might be suboptimal because BMI does not distinguish lean versus fat tissue, nor does it take into account tissue location. As a consequence, the recalibration of TBS according to BMI might yield differential effects when considering trunk lean mass versus trunk fat mass and may introduce effect modification and/or calibration errors when considering these variables separately.
Our study has several strengths. Foremost is the systematic assessment of TBS, BMI, trunk fat mass, trunk lean mass, and LS-VBMD in a large sample of community-dwelling older men, with a sufficient number of men to study variation across the full clinical range of these variables. In particular, unlike others, the present study is sufficiently powered to detect both nonlinear relationships and interactions between variables that may not be detected in other smaller studies. A limitation of the present study is that the observed findings might be particular to both the population studied (relatively healthy older U.S. men) as well as the particular DXA machine (Hologic QDR 4500) and software version used in MrOS. The correlation between TBS and BMI does vary significantly by study. Kim and colleagues(18) reported a positive correlation 44(r=0.10) between TBS and BMI in a study of 1474 postmenopausal Korean women (Lunar DXA); Bazzocchi and colleagues(19) reported no correlation in a study of 250 Italian men and women (Lunar DXA); Sritara and colleagues(9) reported a weak negative correlation in a study of 848 Thai men and women (Hologic DXA); and Romagnoli and colleagues(20) reported a negative correlation in a study of 87 Italian men (Hologic DXA). Finally, McCloskey and colleagues(21) performed a large meta-analysis of studies of men and women of different ethnic origins (both Hologic and Lunar DXA), and reported an overall weaker negative correlation between TBS and BMI (r=−0.16) than what we have found in the MrOS study population. We would not expect the same findings for contexts in which TBS was uncorrelated or positively correlated with BMI.
In summary, we found that the strength of the association between TBS and trabecular LS-VBMD among older men was variable and dependent on BMI and body composition (particularly the component of trunk lean mass). The clinical utility of TBS among older men may be limited because of underestimation of bone strength parameters for those with high BMI or high trunk lean mass. Additional large studies with sufficient power to detect interactions would be required to assess whether or not the association between TBS and incident fractures also varies by BMI or trunk lean mass.
Acknowledgments
This work was supported by a grant from the National Institutes of Health (NIH) (primarily under grant number R21 AG046571). The Osteoporotic Fractures in Men (MrOS) Study is supported by NIH funding. The following institutes provide support: the National Institute on Aging (NIA), the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Center for Advancing Translational Sciences (NCATS), and NIH Roadmap for Medical Research under the following grant numbers: U01 AG027810, U01 AG042124, U01 AG042139, U01 AG042140, U01 AG042143, U01 AG042145, U01 AG042168, U01 AR066160, and UL1 TR000128. This article is the result of work supported with resources and use of facilities of the Minneapolis VA Health Care System.
Footnotes
Disclosures: All authors state that they have no conflicts of interest.
Authors' roles: Study concept and design: KEE, ESO, and JTS. Data collection: KEE, ESO, and EBC. Data analysis and interpretation: LL, TNV, KEE, BCT, and JTS. Drafting manuscript: LL. Critical review and final approval of manuscript content: TNV, KEE, BCT, PMC, AVS, DCB, ESO, NEL, EBC, and JTS. LL and TNV performed the statistical analyses and are independent of any commercial funder. They had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analyses.
References
- 1.Hans D, Barthe N, Boutroy S, Pothuaud L, Winzenrieth R, Krieg MA. Correlations between trabecular bone score, measured using anteroposterior dual-energy X-ray absorptiometry acquisition, and 3-dimensional parameters of bone microarchitecture: an experimental study on human cadaver vertebrae. J Clin Densitom. 2011;14:302–12. doi: 10.1016/j.jocd.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 2.Silva BC, Leslie WD, Resch H, et al. Trabecular bone score: a noninvasive analytical method based upon the DXA image. J Bone Miner Res. 2014;29:518–30. doi: 10.1002/jbmr.2176. [DOI] [PubMed] [Google Scholar]
- 3.McCloskey EV, Oden A, Harvey NC, et al. Adjusting fracture probability by trabecular bone score. Calcif Tissue Int. 2015;96:500–9. doi: 10.1007/s00223-015-9980-x. [DOI] [PubMed] [Google Scholar]
- 4.Hans D, Goertzen AL, Krieg MA, Leslie WD. Bone microarchitecture assessed by TBS predicts osteoporotic fractures independent of bone density: the Manitoba study. J Bone Miner Res. 2011;26:2762–9. doi: 10.1002/jbmr.499. [DOI] [PubMed] [Google Scholar]
- 5.Briot K, Paternotte S, Kolta S, et al. Added value of trabecular bone score to bone mineral density for prediction of osteoporotic fractures in postmenopausal women: the OPUS study. Bone. 2013;57:232–6. doi: 10.1016/j.bone.2013.07.040. [DOI] [PubMed] [Google Scholar]
- 6.Schousboe JT, Vo T, Taylor BC, et al. Prediction of incident major osteoporotic and hip fractures by trabecular bone score (TBS) and prevalent radiographic vertebral fracture in older men. J Bone Miner Res. 2016 Mar;31(3):690–7. doi: 10.1002/jbmr.2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amnuaywattakorn S, Sritara C, Utamakul C, et al. Simulated increased soft tissue thickness artefactually decreases trabecular bone score: a phantom study. BMC Musculoskelet Disord. 2016;17:17. doi: 10.1186/s12891-016-0886-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leslie WD, Krieg MA, Hans D. Clinical factors associated with trabecular bone score. J Clin Densitom. 2013;16:374–9. doi: 10.1016/j.jocd.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 9.Sritara C, Thakkinstian A, Ongphiphadhanakul B, et al. Age-adjusted dual X-ray absorptiometry-derived trabecular bone score curve for the lumbar spine in Thai females and males. J Clin Densitom Forthcoming. doi: 10.1016/j.jocd.2015.05.068. Eub 2015 Jun 19. [DOI] [PubMed] [Google Scholar]
- 10.Leib E, Winzenrieth R, Aubry-Rozier B, Hans D. Vertebral microarchitecture and fragility fracture in men: a TBS study. Bone. 2014;62:51–5. doi: 10.1016/j.bone.2013.12.015. [DOI] [PubMed] [Google Scholar]
- 11.Evans AL, Paggiosi MA, Eastell R, Walsh JS. Bone density, microstructure and strength in obese and normal weight men and women in younger and older adulthood. J Bone Miner Res. 2015;30:920–8. doi: 10.1002/jbmr.2407. [DOI] [PubMed] [Google Scholar]
- 12.Blank JB, Cawthon PM, Carrion-Petersen ML, et al. Overview of recruitment for the osteoporotic fractures in men study (MrOS) Contemp Clin Trials. 2005;26:557–68. doi: 10.1016/j.cct.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 13.Orwoll E, Blank JB, Barrett-Connor E, et al. Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study—a large observational study of the determinants of fracture in older men. Contemp Clin Trials. 2005;26:569–85. doi: 10.1016/j.cct.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 14.Cauley JA, Blackwell T, Zmuda JM, et al. Correlates of trabecular and cortical volumetric bone mineral density at the femoral neck and lumbar spine: the Osteoporotic Fractures in Men study (MrOS) J Bone Miner Res. 2010;25:1958–71. doi: 10.1002/jbmr.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lang TF, Li J, Harris ST, Genant HK. Assessment of vertebral bone mineral density using volumetric quantitative CT. J Comput Assist Tomogr. 1999;23:130–7. doi: 10.1097/00004728-199901000-00027. [DOI] [PubMed] [Google Scholar]
- 16.Winzenrieth R, Michelet F, Hans D. Three-dimensional (3D) microarchitecture correlations with 2D projection image gray-level variations assessed by trabecular bone score using high-resolution computed tomographic acquisitions: effects of resolution and noise. J Clin Densitom. 2013;16:287–96. doi: 10.1016/j.jocd.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 17.Muschitz C, Kocijan R, Haschka J, et al. TBS reflects trabecular microarchitecture in premenopausal women and men with idiopathic osteoporosis and low-traumatic fractures. Bone. 2015;79:259–66. doi: 10.1016/j.bone.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 18.Kim JH, Choi HJ, Ku EJ, et al. Regional body fat depots differently affect bone microarchitecture in postmenopausal Korean women. Osteoporos Int. 2016 Mar;27(3):1161–8. doi: 10.1007/s00198-015-3329-1. [DOI] [PubMed] [Google Scholar]
- 19.Bazzocchi A, Ponti F, Diano D, et al. Trabecular bone score in healthy ageing. Br J Radiol. 2015;88:20140865. doi: 10.1259/bjr.20140865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Romagnoli E, Lubrano C, Carnevale V, et al. Assessment of trabecular bone score (TBS) in overweight/obese men: effect of metabolic and anthropometric factors. Endocrine Forthcoming. doi: 10.1007/s12020-016-0857-1. Epub 2016 Jan 27. [DOI] [PubMed] [Google Scholar]
- 21.McCloskey EV, Odén A, Harvey NC, et al. A meta-analysis of trabecular bone score in fracture risk prediction and its relationship to FRAX. J Bone Miner Res. 2016 May;31(5):940–8. doi: 10.1002/jbmr.2734. [DOI] [PubMed] [Google Scholar]


