Abstract
Parental availability influences fear expression and learning across species, but the effect of maternal buffering on fear learning in humans is unknown. Here we investigated the effect of maternal availability during fear conditioning in a group of children (ages 8–10) and adolescents (ages 11–13) from a low-income population with a range of trauma exposure. Acoustic startle response data were collected to measure fear-potentiated startle (FPS) in 104 participants. A total of 62 participants were tested with the mother available and 42 when the mother was not in the testing room. We observed that maternal availability during fear conditioning interacted with age to affect FPS discrimination between CS+ and CS–. In line with previous findings suggesting an absence of maternal buffering in adolescents, fear discrimination was affected by maternal availability only in children. Second, we observed that the effect of maternal buffering on FPS discrimination in children was not influenced by maternally reported warmth. In conclusion, we demonstrated that maternal availability improved discrimination in children, regardless of the quality of the relationship. Adolescents discriminated irrespective of maternal status, suggesting that childhood may be a sensitive period for environmental influences on key processes such as learning of danger and safety signals.
Keywords: Fear conditioning, fear-potentiated startle, maternal buffering, maternal warmth, trauma exposure
Introduction
In most altricial species, the availability of a parent during early development is crucial for survival. In addition to providing for the basic needs of the developing infant, the availability of the parent or caregiver can have profound effects on neural and behavioral development (Callaghan & Tottenham, 2016). For instance, it is well recognized that the availability of the mother can significantly decrease or even eliminate fear in the offspring when exposed to stress. This phenomenon, known as maternal buffering, has been shown across species (Kikusui, Winslow, & Mori, 2006). When using maternal separation as a stressor, nonhuman primate studies showed increased behavioral stress responses and cortisol release in the infants. However, lower cortisol levels were observed upon reunion with the mother or when infants had access to auditory or visual stimuli associated with the mother (Bayart, Hayashi, Faull, Barchas, & Levine, 1990; Coe, Mendoza, Smotherman, & Levine, 1978; Levine, Johnson, & Gonzalez, 1985). It is suggested that maternal buffering promotes attachment behavior by reducing fear (Moriceau & Sullivan, 2006) and is therefore thought to be particularly relevant in the context of fear learning. To date, only nonhuman research on maternal buffering of fear learning has been conducted, and further investigation in humans is warranted.
Fear learning promotes the ability to distinguish safety and danger, which is vital for survival (Maren, 2001). Usually, pairing a shock with a neutral odor results in future avoidance of this odor, which was indeed observed in older rat pups (>postnatal day (PN) 10), but not in the young pups (<PN10) (Sullivan, Landers, Yeaman, & Wilson, 2000). Interestingly, maternal presence in the older pups also resulted in an absence of fear learning, though they learned to avoid the odor when the mother was not present (Barr et al., 2009; Moriceau & Sullivan, 2006). This mechanism is thought to prevent infants from learning to fear the mother, even when she may be associated with painful stimulation such as stepping on the neonates, which promotes survival in care-dependent infants (Sullivan et al., 2000). For fear learning to occur, glucocorticoid secretion and subsequent activation of the amygdala are required (Barr et al., 2009; Moriceau & Sullivan, 2006). Indeed, the shift observed around PN10 is associated with the functional emergence of the amygdala, allowing older pups to learn fear in the absence of the mother (Moriceau, Roth, & Sullivan, 2010). Furthermore, the coupling between the amygdala and the prefrontal cortex (PFC) is crucial for fear learning, as PFC activation is thought to inhibit the amygdala, thereby decreasing the fear response (Phelps, Delgado, Nearing, & LeDoux, 2004; Stevens et al., 2013). Although maternal buffering on fear learning has not been examined in humans, a recent fMRI study on fear responses to pictures of mother versus stranger revealed that children showed less right amygdala activation and a negative PFC-amygdala connectivity when viewing pictures of their mother compared to viewing pictures of a stranger (Gee et al., 2014). Furthermore, maternal presence was related to better behavioral regulation in an emotional context (Gee et al., 2014). Maternal presence is thought to provide an important context for fear learning (Sullivan & Perry, 2015); however, it is not known how maternal presence affects fear conditioning and learning of safety cues in humans.
There is emerging evidence that when offspring become more independent from their caregiver, the effect of maternal buffering diminishes. In a recent study, maternal support eliminated the cortisol stress response to a social stressor in children (ages 9–10), but not in adolescents (ages 15–16) (Hostinar, Johnson, & Gunnar, 2015). Previous work has shown that maternal buffering of infant cortisol reactivity to stress was moderated by secure attachment (Nachmias, Gunnar, Mangelsdorf, Parritz, & Buss, 1996). In rats, maternal effects on cortical activity of the infant reduced with age (Sarro, Wilson, & Sullivan, 2014). In the human fMRI study discussed above, the effect of maternal buffering on amygdala activation and amygdala–PFC coupling was only observed in children (<10 years old), whereas in the adolescents (>10 years old), no difference in amygdala reactivity and functional coupling was observed in response to maternal compared to stranger faces (Gee et al., 2014). An increasing body of evidence suggests that brain circuits important for fear learning show a developmental shift from childhood to adolescence (Glenn et al., 2012; Jovanovic et al., 2014), as we see functional and structural changes in the PFC (Gogtay et al., 2004; Spear, 2000), as well as a shift in amygdala–PFC functional connectivity (Gabard-Durnam et al., 2014) in the transition from childhood to adolescence. Although most research on maternal buffering has focused on early developmental stages (Hostinar, Sullivan, & Gunnar, 2014), these findings show the relevance of investigating the differential effects of maternal buffering in childhood and adolescence.
The quality of maternal care is thought to influence the efficacy of maternal buffering (Gunnar, Hostinar, Sanchez, Tottenham, & Sullivan, 2015). A more supportive mother–child relationship was associated with more maternal influence on the amygdala–PFC coupling (Gee et al., 2014), suggesting more efficient maternal buffering. While early studies focused on attachment security between mother in child, which was associated with maternal responsiveness to child’s distress (Nachmias et al., 1996), maternal warmth toward her child may also impact the quality of the mother–child relationship. Maternal warmth, which includes showing affection, positive regard and attentiveness to your child, was positively associated with the child’s well-being, for example, decreased anxious behavior (McCabe, Clark, & Barnett, 1999) and better self-regulation (Eiden, Colder, Edwards, & Leonard, 2009). Moreover, higher maternal warmth is thought to protect against negative factors, such as maternal depression (Brennan, Le Brocque, & Hammen, 2003), maternal intrusiveness (Ispa et al., 2004), but also long-term biological effects of poverty (Chen, Miller, Kobor, & Cole, 2011), and low birth weight (Tully, Arseneault, Caspi, Moffitt, & Morgan, 2004). It is likely that the effect of maternal buffering on fear learning depends on the quality of the mother–child relationship, which may include maternal warmth in addition to secure attachment. On the other hand, the theory of “any kind of mother in a storm” suggests that an infant forms a bond with the caregiver regardless of the quality of care received in order to survive in a harsh environment (Sapolsky, 2009). Therefore, maternal warmth may be an important moderator of the effect of maternal buffering, but its role in resource-poor caregiving environments remains unclear.
In this study, we investigated the effect of maternal availability on fear conditioning in a group of children (ages 8–10) and adolescents (ages 11–13) from a low-income population with a range of trauma exposure. Specifically, we investigated discrimination between danger and safety by comparing fear-potentiated startle (FPS) to the CS+ and the CS−, where better discrimination is reflected by a larger difference between the FPS to the CS+ and the CS−. We hypothesized that maternal availability during fear learning would interact with age to affect FPS; specifically, children would benefit more from maternal availability, while adolescents would not.
Methods
Participants
Study participants were 104 children and adolescents between 8 and 13 years of age (mean = 9.9, SD = 1.6 years) and their mothers recruited from the waiting rooms of primary care clinics in an urban hospital in Atlanta, GA. The participants were recruited as part of an ongoing longitudinal study of trauma exposure in children. A subset of data from this study has been published previously (Jovanovic et al., 2014; Gamwell et al., 2015); however, the current study only included individuals that had (1) complete startle data, (2) available information about maternal presence during the startle testing, and (3) data on maternal warmth. In order to be eligible for the study, child participants had to be between 8 and 13 years of age and willing to participate; exclusion criteria were a diagnosis of autism spectrum disorders, bipolar or psychotic disorders, or cognitive disability. Mothers were excluded if they had current psychosis or a positive urine test for substances of abuse. Prior to their participation, all mothers signed informed consent as well as parental permission for their children, and the children provided study assent approved by the Emory University Institutional Review Board and the Grady Hospital Research Oversight Committee.
Maternal availability
The larger longitudinal study of mother-child dyads involved startle testing of mothers and their children; however, the mothers were not always tested at the same time as their children. For logistical reasons, mothers were frequently tested either at an earlier or later date, depending on the availability of her child and scheduling constraints. There were no other reasons why the mother was absent or present, so the distinction was based only on logistical reasons. On the occasions when the mother was tested at the same time as her child, both were prepped for electrode placement and tested in adjacent sound-attenuated booths used for startle experiments. The child could see the mother prepped for startle testing and going into the booth next to its own. Both sound-attenuated booths were located in the same larger room. Figure 1 shows the two booths which are placed side-by-side; once in the booth, the child did not have visual access to the mother, but was told that she was available throughout the startle test.
Figure 1.
Picture showing the startle testing booths. As shown in the picture, the child and adult booths are side-by-side. When the mother and child were tested at the same time, they were together during the electrode preparation; however, once they entered the booth, the child was out of visual and auditory contact with the mother. The child was told the mother was available.
On the occasions when the mother was not tested on the same day, she was being interviewed in a different room down the hall from her child. The child could see her exit the testing room and go down the hall. Mother and child were reunited after startle testing which lasted approximately 60 min. Of the 104 children, 42 were tested when the mother was absent from the testing room, and 62 were tested with the mother available. Table 1 shows demographic and assessment differences between the two groups of children.
Table 1.
Distribution of sex, trauma exposure, and clinical variables across the Maternal Status and Age Groups.
| Maternal status |
|||||||
|---|---|---|---|---|---|---|---|
| Unavailable |
Available |
||||||
| Age group |
Age group |
||||||
| 8–10 years |
11–13 years |
8–10 years |
11–13 years |
EFFECT |
|||
| n = 14 | n = 28 | n = 36 | n = 26 | AGE | STATUS | INTRX | |
| Sex (% Female) | 64.3% | 50.0% | 52.8% | 53.8% | p = .67 | p = .88 | |
| Child Trauma Exposure, VEX-R (M, SE) | 14.4, 2.4 | 20.7, 1.7 | 11.9, 1.5 | 18.8, 1.7 | p < .01 | p = .24 | p = .88 |
| Child Anxiety, BASC (M, SE) | 49.6, 2.8 | 49.9, 2.0 | 50.9, 1.8 | 50.5, 2.1 | p = .99 | p = .66 | p = .86 |
| Maternal Warmth, PQ (M, SE) | 92.2, 2.4 | 91.0, 1.7 | 92.1, 1.5 | 91.3, 1.7 | p = .57 | p = .96 | p = .91 |
Main effects of maternal availability and age, and interaction effects are listed. The only effect was increased trauma exposure in adolescents compared to children.
VEX-R = Violence Exposure, Revised (child report); BASC = Behavioral Assessment Scale for Children (child report); PQ = Parenting Questionnaire (maternal report).
Clinical assessments
Trauma exposure in children was assessed using the Violence Exposure Scale for Children-Revised (VEX-R; Fox & Leavitt, 1995). The VEX-R is a 22-item cartoon-based self-report interview of children’s lifetime exposure to violence.
To characterize the sample in terms of psychological symptoms and potential diagnoses, the Behavior Assessment System for Children—Second Edition (BASC-2; Reynolds & Kamphaus, 2004) was administered to children. The BASC-2 yields several clinical and adaptive continuous subscale scores normalized by child age and gender in a nonclinical normative sample, as well as categorical cutoffs for clinically significant scores. For the present study, we included the Anxiety, Depression, Attention Problems, and Hyperactivity subscales, as well as the Inattention/Hyperactivity composite scale because these areas are common trauma-related sequelae in children (Barber, Kohl, Kassam-Adams, & Gold, 2014; D’Andrea, Ford, Stolbach, Spinazzola, & van der Kolk, 2012). Test–retest reliability, internal consistency, and convergent validity of the scales are very high (Reynolds & Kamphaus, 2004).
Parental warmth in the mothers was assessed using the Parenting Questionnaire (PQ; McCabe et al., 1999). The PQ is a 50-item parent self-report of parenting practices, including warmth. The measure was derived from the Parenting Dimensions Inventory and the Family Environment Scale. The 22-item warmth subscale has demonstrated good internal consistency (.90) in a sample of African-American participants (McCabe et al., 1999), as well as within the current study sample (.83).
Startle testing
The acoustic startle response data were collected using Biopac MP150 for Windows (Biopac Systems, Inc., Aero Camino, CA). Electromyographic (EMG) data were sampled at 1000 Hz and amplified using the EMG module of the Biopac system. The acquired data were filtered, rectified, and smoothed in MindWare software (MindWare Technologies, Inc) and exported for statistical analyses. EMG activity was recorded from two 5 mm Ag/AgCl electrodes placed over the orbicularis oculi muscle, approximately 1 cm under the pupil and 1 cm below the lateral canthus. A ground electrode was placed behind the ear. The impedances for all participants were less than 6 kilo-ohms. The EMG signal was filtered with low- and high-frequency cutoffs at 28 and 500 Hz, respectively. Startle magnitude was assessed as the peak amplitude of the EMG contraction 20–200 ms following the acoustic stimulus.
Participants were seated in a sound attenuated booth and asked to remain still and look at a computer monitor approximately 1 m in front of them. The startle probe (noise burst) was a 106-dB [A] SPL, 40-ms burst of broadband noise delivered binaurally through headphones. The unconditioned stimulus (US) was an aversive airblast directed to the larynx at an intensity of 50 p.s.i. and 100 ms in duration. This US has been used successfully in our lab to elicit fear-conditioned responses in pediatric populations (Jovanovic et al., 2014). The conditioned stimuli (CSs) were colored shapes presented on a computer monitor using Superlab 4.5 presentation software (Cedrus, Inc.) for 6 s prior to the delivery of the startle probe, and co-terminated with the US 500 ms after the presentation of the startle stimulus. The CS+ was paired with the airblast 100% of the time, and the CS− was never paired with the airblast. The session consisted of three blocks, each with three CS+ trials, three CS− trials, and three noise alone (NA, no CS presented during startle probe) trials, for a total of 27 startle trials. In all phases of the experiment, intertrial intervals will be randomized between 9 and 22 s. A response keypad (Cedrus, Inc.) was incorporated in the experiment: at the beginning and end of each block questions appeared on the screen asking if a shape was followed by an airblast (e.g., “Was the purple triangle followed by an airblast?”). The child was asked to respond to the question be pressing “Yes”, “No”, of “I don’t know”. The same question was asked for each CS. Of the 104 participants, 84 had responses on the keypad; the nonresponders did not differ across age (p = .76) or maternal presence (p = .12) groups. The CS contingencies were counterbalanced across participants.
Data analyses
FPS was indexed by calculating percent potentiation for each CS type, in order to account for individual differences in startle magnitude as well as startle habituation. This value was derived as follows: Percent Startle Potentiation = 100 × (startle magnitude during CS trials – NA startle)/(NA startle). The first hypothesis was tested using a repeated measures analysis of variance (ANOVA) with the within-subject factors of Trial Type (CS+, CS–) and Age Group (2 levels: children, ages 8–10 years, adolescents, ages 11–13 years) and Maternal Status (2 levels: available, unavailable) as between subjects factors. In addition, we calculated a FPS discrimination score by subtracting the percent potentiation to the CS– from the percent potentiation to the CS+. Age Group and Maternal Status were analyzed for the effect on the discrimination score using a univariate ANOVA. Maternal warmth was categorized as high or low using a median split and was used in the secondary analyses of Maternal Status. The second hypothesis was tested using a univariate ANOVA with between groups factors of Maternal Status and Maternal Warmth, and FPS discrimination score as the dependent variable. Degree of child-reported trauma exposure on the VEXR and child sex were used as covariates in the analyses. All analyses were performed using SPSS 20.0 for Windows, and alpha was set to 0.05.
Results
The demographic and assessment data for the children in the Maternal Status (available, unavailable) groups are listed in Table 1. The two groups did not differ in age, sex distribution, child anxiety, depression, attention problems, hyperactivity, combined inattention/hyperactivity, or maternal warmth. Sixteen children crossed the threshold for clinically significant symptoms on one or more subscales of the BASC-2 (4 for Anxiety, 3 for Depression, 6 for Attention Problems, 6 for Hyperactivity, and 2 for combined Inattention/Hyperactivity), but the distribution of clinically significant scores did not differ by group. However, the children that were tested when the mother was absent reported higher rates of exposure to violence. In the secondary analyses, we included this measure as a covariate. We also included sex as a covariate given that we previously found significant sex differences in FPS (Gamwell et al., 2015).
Conditioning was successful in all participants, since startle to the CS+ trials was significantly potentiated compared to startle to the noise alone (no CS) trials, F(1, 100) = 7.70, p = .007. In addition, keypad responses on the US contingency awareness measure (i.e., “was this shape followed by an airblast?”) were significantly higher for the CS+ than CS−, F(1,80) = 44.92, p < .0001. Both age groups showed significant discrimination between CS+ and CS− on this measure (children p = .02, adolescents, p < .001); however, adolescents showed more robust contingency awareness compared to children (Age Group main effect p = .02). Maternal Status, on the other hand, did not affect keypad responses.
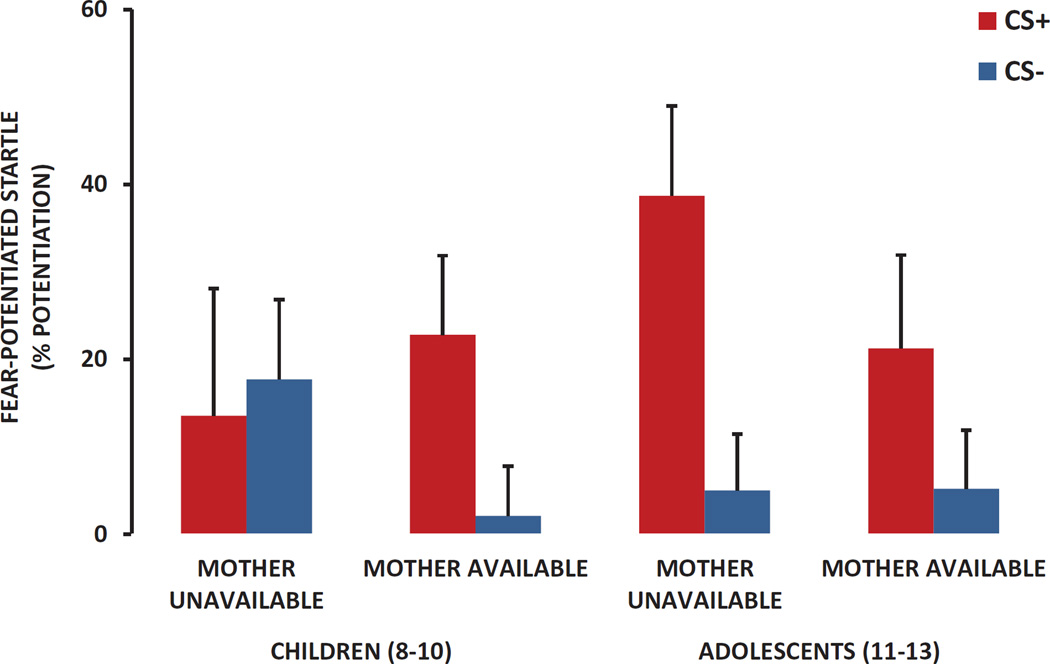
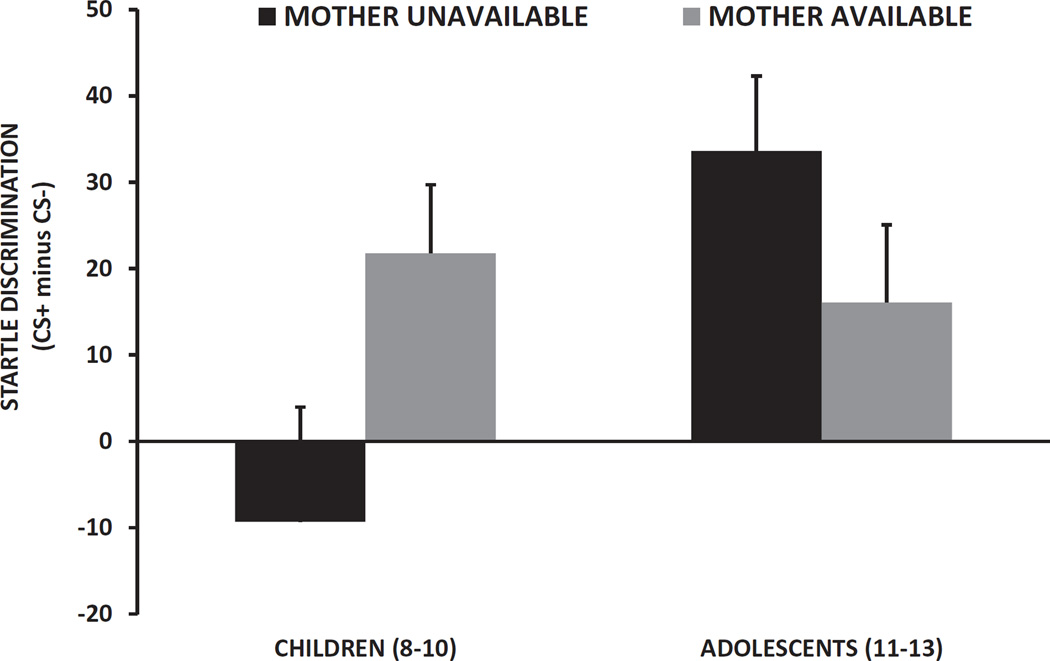
A repeated measures ANOVA of Trial Type (CS+ vs. CS−) on startle potentiation revealed significantly higher FPS to the CS+ compared to the CS−, F(1, 100) = 11.84, p = .001, as well as an interaction effect of Age Group and Maternal Status, F(1, 100) = 4.87, p = .03, see Figure 2. This interaction effect remained significant after covarying for child trauma exposure and sex, p < .03. When we examined the effect of Trial Type within each Age and Maternal Status group separately, the 8–10 year olds whose mothers were unavailable did not show decreased FPS to the CS– compared to the CS+ (p = .60), whereas the same age children did show significant discrimination if their mothers were available, F(1, 35) = 5.61, p = .02. The adolescents showed higher FPS to the CS+ versus CS– in both conditions (mother unavailable, p = .003; mother available, p = .02). We further compared the FPS discrimination score between children whose mothers were present versus absent in each Age Group separately after controlling for child trauma exposure and sex. Again, Maternal Status did not have an impact on fear discrimination in adolescents; however in the younger children, FPS discrimination was greater if the mother was available than when she was not, F(1, 48) = 3.97, p = .05, Figure 3.
Figure 2.
Mean and SE fear-potentiated startle (% potentiation from noise alone) to the reinforced conditioned stimulus (CS+) and nonreinforced conditioned stimulus (CS–) across Age and Maternal Status groups.
Figure 3.
Mean and SE fear-potentiated startle discrimination score (%FPS to CS+ minus % FPS to CS–) across Age and Maternal Status groups.
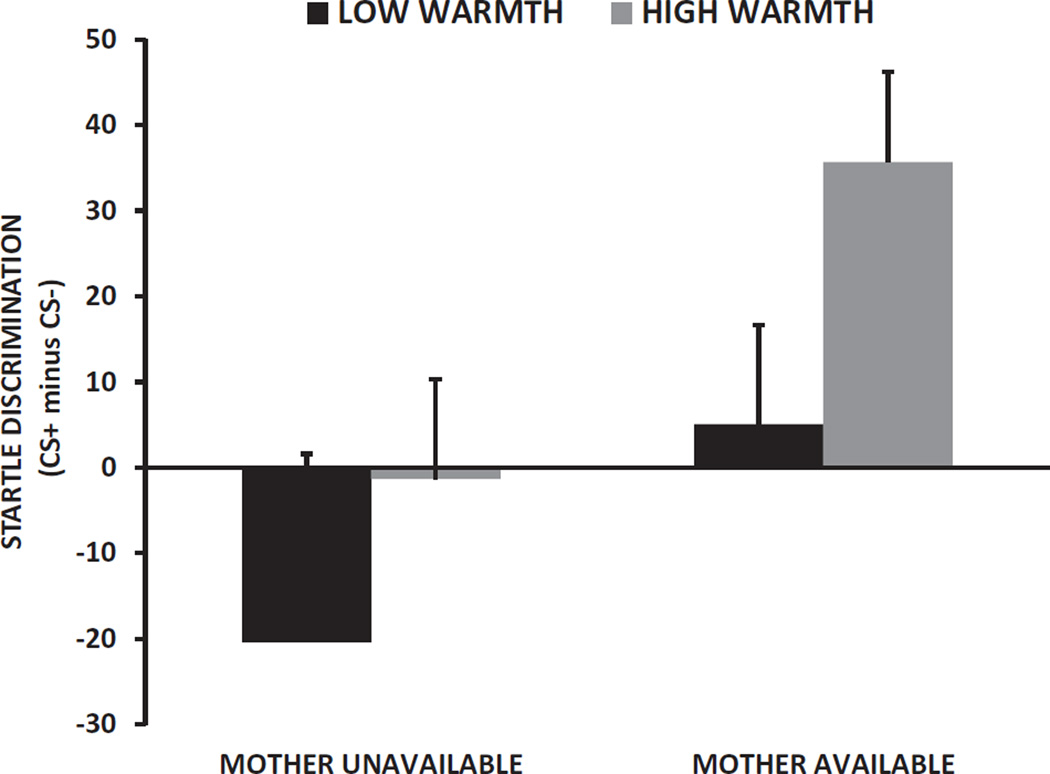
Next we examined the effect of maternally reported warmth (PQ) on the effect of her presence in the 8- to 10-year-old children. We analyzed the FPS discrimination score with Maternal Status (available, unavailable) and Maternal Warmth as between groups factors, controlling for trauma exposure and sex. While we again found that Maternal Status significantly increased discrimination, F(1, 48) = 4.17, p < .05, there were no main or interaction effects with Maternal Warmth, Figure 4. In addition, we performed a hierarchical linear regression of FPS discrimination in the 8- to 10-year-old children with child sex and trauma exposure entered in step 1, Maternal Status entered in step 2, and a continuous measure of Maternal Warmth entered in step 3. Again we found that maternal availability predicted greater FPS discrimination, Fchange (1, 45) = 3.97, p = .05. Maternal warmth had a tendency to increase discrimination, Fchange (1, 46) = 3.54, p = .067, but did not moderate the association between maternal availability and discrimination.
Figure 4.
Mean and SE fear-potentiated startle discrimination score (%FPS to CS+ minus % FPS to CS–) in 8- to 10-year-old children across Maternal Status groups and High and Low Warmth.
Discussion
Here we investigated the effect of maternal availability on fear conditioning in a group of children (ages 8–10) and adolescents (ages 11–13) from a low-income population with a range of trauma exposure. More specifically, we assessed discrimination between danger and safety by comparing FPS to the CS+ and the CS–. We observed that maternal availability in the adjacent startle both (see Figure 1) during fear conditioning interacted with age to affect FPS discrimination. In line with our hypothesis, children did benefit from maternal availability and showed reduced FPS to the CS– compared to the CS+, whereas children did not learn to discriminate danger from safety when the mother was unavailable. On the other hand, adolescents did not benefit from maternal availability; they showed significant discrimination irrespective of maternal status. Second, we investigated whether the effect of maternal buffering on fear conditioning in the 8- to 10-year-old individuals was influenced by maternally reported warmth. In contrast to what we expected, maternally reported warmth did not interact with maternal availability to affect FPS in children. Although maternally reported warmth improved fear learning, this effect was not significant, and maternal availability was a stronger predictor of FPS discrimination.
Our finding that maternal availability influenced fear learning in 8- to 10-year-old children complements previous animal and human studies on maternal buffering. We demonstrated that maternal availability is associated with a reduced fear response (FPS) to the safety cue (CS–) in children. In contrast, in maternal absence children did not learn to distinguish fear and safety cues, resulting in a larger FPS to both the CS+ and CS–. This finding is therefore in line with previous studies that showed an association between maternal presence and reduced cortisol responses, and fear expression in nonhuman primates (Bayart et al., 1990; Coe et al., 1978; Levine et al., 1985), decreased amygdala activation in response to faces, negative amygdala–PFC connectivity, and better behavioral regulation in an emotional context in humans (Gee et al., 2014). Successful discrimination is reflected by fear inhibition to safety signals, that is, decreased FPS to the CS–, for which the output of the amygdala needs to be inhibited by the PFC (Kim & Jung, 2006). Hence, the functional connectivity between the PFC and limbic circuitry is crucial for discrimination (Lissek et al., 2014). The finding by Gee and colleagues (2014) that maternal cues improved PFC–amygdala coupling in children provides a plausible biological explanation for the effect of maternal availability on fear discrimination observed in this study.
In accordance with previous studies (Gee et al., 2014; Hostinar et al., 2015; Sarro et al., 2014), we only observed an effect of maternal buffering in children and not in the adolescents. Maternal buffering is needed to secure the infant’s bond with the caregiver at any cost (Sullivan et al., 2000), conversely, adolescence is a time of increasing independence and reduced effects of maternal buffering likely reflect this change. Increasing independence is paralleled by developmental switches in the role of the amygdala, structural and functional changes in the PFC (Gogtay et al., 2004; Spear, 2000), and increased amygdala–PFC coupling (Gabard-Durnam et al., 2014). Interestingly, the same areas are involved in successful fear conditioning, and accordingly, we found that adolescents, but not children, showed robust fear discrimination irrespective of maternal presence. This developmental switch does not suggest that adolescents or adults do not benefit from social buffering because at these ages peers take on an increasing role in social buffering (Hennessy, Kaiser, & Sachser, 2009; Terranova, Cirulli, & Laviola, 1999); however, a recent study found that peers increased cortisol responses in adolescents during a social stressor (Doom, Doyle, & Gunnar, 2016, this volume). Maternal availability during childhood, however, can have an impact on social buffering later in life. In a study of rhesus macaques, it was shown that nursery-reared, in contrast to mother-reared, adolescents were unable to benefit from social buffering during a stress-provoking challenge (Winslow, Noble, Lyons, Sterk, & Insel, 2003), indicating long-term effects of maternal availability on the potential to benefit from social buffering.
In contrast to what we hypothesized, maternally reported warmth did not impact the effect of maternal buffering in children. In a previous study, a more supportive mother–child relationship was found to increase the maternal influence on the amygdala–PFC coupling (Gee et al., 2014). Although we did observe a positive trend of maternally reported warmth on discrimination learning, which could indicate that a better relationship improves the ability to learn danger and safety signals, this finding did not reach significance, and was inferior to the effect of maternal availability. Specifically, the availability of mothers who reported lower levels of warmth increased discrimination more than the absence of mothers with high levels of warmth. Therefore, our result might be better explained by the theory of “any kind of mother in a storm”, which states that even in cases of low-quality maternal care, infants may benefit from maternal availability (Sapolsky, 2009). Even though our measure of maternally reported warmth was not associated with the ability to learn discrimination during childhood, the quality of the mother–child relationship, including parental responsiveness to child distress, may impact fear learning, and the ability to benefit from social buffering later in life. Future studies should examine the role of maternal warmth along with other aspects of parenting that predict a more supportive mother–child relationship. Furthermore, our ability to interpret the absence of significant findings for maternal warmth may be limited by our use of a self-report measure for maternal warmth because respondents are likely to minimize aspects of parenting that appear socially undesirable (Morsbach & Prinz, 2006). In addition, it is possible that for traumatized parents, self-reported warmth is a less reliable measure than for non-traumatized parents, thereby limiting our ability to observe what could otherwise be an important effect. Nevertheless, a number of studies do support the use of self-reported parental warmth in traumatized parents (e.g., Gonçalves Boeckel, Wagner, & Grassi-Oliveira, 2015; Lavi & Slone, 2012). Still, there is a wide recognition of the importance of social support in maintaining physical and psychological health in at-risk populations (Brewin, Andrews, & Valentine, 2000; Cobb, 1976; Ozbay, Fitterling, Charney, & Southwick, 2008), indicating the relevance for further investigation of the long-term effects of all aspects of the child–mother relationship on the ability to benefit from social buffering.
Results of the current study should be considered along with a few additional limitations. First, because it was a post hoc opportunistic analysis, it was not designed to investigate the effect of maternal availability, and we did not use a standard randomization procedure to assign children to one of the groups. However, the observed maternal status groups were comparable in size and were matched on demographic data. Only the scores for violence exposure were higher in the maternal absence group, but importantly, did not differ within the age groups. Moreover, including exposure to violence as a covariate did not change the results, and it is therefore unlikely that this affected our findings. Second, problems associated with self-report measures may have impacted not only our findings regarding maternal warmth, as discussed previously, but also child self-report of violence exposure and psychological symptoms. Third, we did not measure brain correlates of the effect of maternal buffering on fear conditioning, and future studies using functional neuroimaging could improve our understanding of underlying neural mechanisms. Finally, maternally reported warmth did not influence the effect of maternal availability on current levels of fear discrimination. Yet, the mother–child relationship might impact fear conditioning and the potential to benefit from social buffering in later life. Therefore, it is important to conduct longitudinal studies and follow at-risk children into adolescence, as this is the time when most mental disorders emerge (Lee et al., 2014).
In conclusion, we demonstrated that maternal availability improved fear discrimination in children, regardless of the quality of the relationship. Adolescents showed robust discrimination with and without the mother in the room, suggesting that childhood may be a sensitive period for environmental influences on key processes such as learning about danger and safety. The steep increase of mental disorders during adolescence calls for a better understanding of the more plastic period before age 10, and potential long-term consequences of the mother–child relationships in this period. Although children may benefit from “any kind of mother in the storm”, long-term effects on the ability to benefit from social buffering should be investigated, given its imperative role in maintaining physical and mental health.
Acknowledgments
We thank Allen Graham, Angelo Brown, and the Grady Trauma Project staff for their assistance with participant recruitment and data collection.
Funding
The conference that occasioned this special issue was supported by NSF grant BCS-1439258. This work was supported by National Institute of Health Grants MH100122 (PI, T.J.) and HD071982 (PI, B.B.), the Emory Medical Care Foundation, the Atlanta Clinical Translational Science Institute, the NIH National Centers for Research Resources (M01 RR00039). This work was funded in part by the Brain and Behavior Foundation (formerly NARSAD; T.J.).
Footnotes
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Barber BA, Kohl KL, Kassam-Adams N, Gold JI. Acute stress, depression, and anxiety symptoms among English and Spanish speaking children with recent trauma exposure. Journal of Clinical Psychology in Medical Settings. 2014;21(1):66–71. doi: 10.1007/s10880-013-9382-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr GA, Moriceau S, Shionoya K, Muzny K, Gao P, Wang S, Sullivan RM. Transitions in infant learning are modulated by dopamine in the amygdala. Nature Neuroscience. 2009;12(11):1367–1369. doi: 10.1038/nn.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayart F, Hayashi KT, Faull KF, Barchas JD, Levine S. Influence of maternal proximity on behavioral and physiological responses to separation in infant rhesus monkeys (Macaca mulatta) Behavioral Neuroscience. 1990;104(1):98–107. [PubMed] [Google Scholar]
- Boeckel MG, Wagner A, Grassi-Oliveira R. The effects of intimate partner violence exposure on the maternal bond and PTSD symptoms of children. Journal of Interpersonal Violence. 2015 doi: 10.1177/0886260515587667. Advance online publication. [DOI] [PubMed] [Google Scholar]
- Brennan PA, Le Brocque R, Hammen C. Maternal depression, parent-child relationships, and resilient outcomes in adolescence. Journal of the American Academy of Child & Adolescent Psychiatry. 2003;42(12):1469–1477. doi: 10.1097/00004583-200312000-00014. [DOI] [PubMed] [Google Scholar]
- Brewin CR, Andrews B, Valentine JD. Meta-analysis of risk factors for posttraumatic stress disorder in trauma-exposed adults. Journal of Consulting and Clinical Psychology. 2000;68(5):748–766. doi: 10.1037//0022-006x.68.5.748. [DOI] [PubMed] [Google Scholar]
- Callaghan BL, Tottenham N. The neuro-environmental loop of plasticity: A cross-species analysis of parental effects on emotion circuitry development following typical and adverse caregiving. Neuropsychopharmacology Reviews. 2016;41:163–176. doi: 10.1038/npp.2015.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E, Miller GE, Kobor MS, Cole SW. Maternal warmth buffers the effects of low early-life socioeconomic status on pro-inflammatory signaling in adulthood. Molecular Psychiatry. 2011;16(7):729–737. doi: 10.1038/mp.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb S. Social support as a moderator of life stress. Psychosomatic Medicine. 1976;38(5):300–314. doi: 10.1097/00006842-197609000-00003. [DOI] [PubMed] [Google Scholar]
- Coe CL, Mendoza SP, Smotherman WP, Levine S. Mother-infant attachment in the squirrel monkey: Adrenal response to separation. Behavioral Biology. 1978;22(2):256–263. doi: 10.1016/s0091-6773(78)92305-2. [DOI] [PubMed] [Google Scholar]
- D’Andrea W, Ford J, Stolbach B, Spinazzola J, van der Kolk BA. Understanding interpersonal trauma in children: Why we need a developmentally appropriate trauma diagnosis. American Journal of Orthopsychiatry. 2012;82(2):187–200. doi: 10.1111/j.1939-0025.2012.01154.x. [DOI] [PubMed] [Google Scholar]
- Doom JR, Doyle CM, Gunnar MR. Social stress buffering by friends in childhood and adolescence: Effects on HPA and oxytocin activity. Social Neuroscience. 2016:1–14. doi: 10.1080/17470919.2016.1149095. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiden RD, Colder C, Edwards EP, Leonard KE. A longitudinal study of social competence among children of alcoholic and non-alcoholic parents: Role of parental psychopathology, parental warmth, and self-regulation. Psychology of Addictive Behaviors: Journal of the Society of Psychologists in Addictive Behaviors. 2009;23(1):36–46. doi: 10.1037/a0014839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox NA, Leavitt LA. The Violence Exposure Scale for Children - VEX (preschool version) College Park, MD: Department of Human Development, University of Maryland; 1995. [Google Scholar]
- Gabard-Durnam LJ, Flannery J, Goff B, Gee DG, Humphreys KL, Telzer E, Tottenham N. The development of human amygdala functional connectivity at rest from 4 to 23 years: A cross-sectional study. NeuroImage. 2014;95:193–207. doi: 10.1016/j.neuroimage.2014.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamwell K, Nylocks M, Cross D, Bradley B, Norrholm SD, Jovanovic T. Fear conditioned responses and PTSD symptoms in children: Sex differences in fear-related symptoms. Developmental Psychobiology. 2015;57(7):799–808. doi: 10.1002/dev.21313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee DG, Gabard-Durnam L, Telzer EH, Humphreys KL, Goff B, Shapiro M, Tottenham N. Maternal buffering of human amygdala-prefrontal circuitry during childhood but not during adolescence. Psychological Science. 2014;25(11):2067–2078. doi: 10.1177/0956797614550878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn CR, Klein DN, Lissek S, Britton JC, Pine DS, Hajcak G. The development of fear learning and generalization in 8-13 year-olds. Developmental Psychobiology. 2012;54(7):675–684. doi: 10.1002/dev.20616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(21):8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR, Hostinar CE, Sanchez MM, Tottenham N, Sullivan RM. Parental buffering of fear and stress neurobiology: Reviewing parallels across rodent, monkey, and human models. Social Neuroscience. 2015;10(5):474–478. doi: 10.1080/17470919.2015.1070198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennessy MB, Kaiser S, Sachser N. Social buffering of the stress response: Diversity, mechanisms, and functions. Frontiers in Neuroendocrinology. 2009;30(4):470–482. doi: 10.1016/j.yfrne.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Hostinar CE, Johnson AE, Gunnar MR. Parent support is less effective in buffering cortisol stress reactivity for adolescents compared to children. Developmental Science. 2015;18(2):281–297. doi: 10.1111/desc.12195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostinar CE, Sullivan RM, Gunnar MR. Psychobiological mechanisms underlying the social buffering of the hypothalamic–pituitary–adrenocortical axis: A review of animal models and human studies across development. Psychological Bulletin. 2014;140(1):256–282. doi: 10.1037/a0032671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ispa JM, Fine MA, Halgunseth LC, Harper S, Robinson J, Boyce L, Brady-Smith C. Maternal intrusiveness, maternal warmth, and mother–toddler relationship outcomes: Variations across low-income ethnic and acculturation groups. Child Development. 2004;75(6):1613–1631. doi: 10.1111/j.1467-8624.2004.00806.x. [DOI] [PubMed] [Google Scholar]
- Jovanovic T, Nylocks KM, Gamwell KL, Smith A, Davis TA, Norrholm SD, Bradley B. Development of fear acquisition and extinction in children: Effects of age and anxiety. Neurobiology of Learning and Memory. 2014;113(0):135–142. doi: 10.1016/j.nlm.2013.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikusui T, Winslow JT, Mori Y. Social buffering: Relief from stress and anxiety. Philosophical Transactions of the Royal Society of London B: Biological Sciences. 2006;361(1476):2215–2228. doi: 10.1098/rstb.2006.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Jung MW. Neural circuits and mechanisms involved in Pavlovian fear conditioning: A critical review. Neuroscience & Biobehavioral Reviews. 2006;30(2):188–202. doi: 10.1016/j.neubiorev.2005.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavi I, Slone M. Parental practices and political violence: The protective role of parental warmth and authority-control in Jewish and Arab Israeli children. American Journal of Orthopsychiatry. 2012;82(4):550–561. doi: 10.1111/j.1939-0025.2012.01183.x. [DOI] [PubMed] [Google Scholar]
- Lee FS, Heimer H, Giedd JN, Lein ES, Šestan N, Weinberger DR, Casey BJ. Adolescent mental health—Opportunity and obligation. Science. 2014;346(6209):547–549. doi: 10.1126/science.1260497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine S, Johnson DF, Gonzalez CA. Behavioral and hormonal responses to separation in infant rhesus monkeys and mothers. Behavioral Neuroscience. 1985;99(3):399–410. doi: 10.1037//0735-7044.99.3.399. [DOI] [PubMed] [Google Scholar]
- Lissek S, Bradford DE, Alvarez RP, Burton P, Espensen-Sturges T, Reynolds RC, Grillon C. Neural substrates of classically conditioned fear-generalization in humans: A parametric fMRI study. Social Cognitive and Affective Neuroscience. 2014;9(8):1134–1142. doi: 10.1093/scan/nst096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S. Neurobiology of Pavlovian fear conditioning. Annual Review of Neuroscience. 2001;24(1):897–931. doi: 10.1146/annurev.neuro.24.1.897. [DOI] [PubMed] [Google Scholar]
- McCabe KM, Clark R, Barnett D. Family protective factors among urban African American youth. Journal of Clinical Child Psychology. 1999;28(2):137–150. doi: 10.1207/s15374424jccp2802_2. [DOI] [PubMed] [Google Scholar]
- Moriceau S, Roth TL, Sullivan RM. Rodent model of infant attachment learning and stress. Developmental Psychobiology. 2010;52(7):651–660. doi: 10.1002/dev.20482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S, Sullivan RM. Maternal presence serves as a switch between learning fear and attraction in infancy. Nature Neuroscience. 2006;9(8):1004–1006. doi: 10.1038/nn1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morsbach SK, Prinz RJ. Understanding and improving the validity of self-report of parenting. Clinical Child and Family Psychology Review. 2006;9(1):1–21. doi: 10.1007/s10567-006-0001-5. [DOI] [PubMed] [Google Scholar]
- Nachmias M, Gunnar MR, Mangelsdorf S, Parritz R, Buss KA. Behavioral inhibition and stress reactivity: The moderating role of attachment security. Child Development. 1996;67(2):508–522. [PubMed] [Google Scholar]
- Ozbay F, Fitterling H, Charney D, Southwick S. Social support and resilience to stress across the life span: A neurobiologic framework. Current Psychiatry Reports. 2008;10(4):304–310. doi: 10.1007/s11920-008-0049-7. [DOI] [PubMed] [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans. Neuron. 2004;43(6):897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Reynolds CR, Kamphaus RW. Behavior Assessment System for Children-Second Edition (BASC-2) Bloomington, MN: Pearson; 2004. [Google Scholar]
- Sapolsky R. Any kind of mother in a storm. Nature Neuroscience. 2009;12(11):1355–1356. doi: 10.1038/nn1109-1355. [DOI] [PubMed] [Google Scholar]
- Sarro EC, Wilson DA, Sullivan RM. Maternal regulation of infant brain state. Current Biology: CB. 2014;24(14):1664–1669. doi: 10.1016/j.cub.2014.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neuroscience & Biobehavioral Reviews. 2000;24(4):417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Stevens JS, Jovanovic T, Fani N, Ely TD, Glover EM, Bradley B, Ressler KJ. Disrupted amygdala-prefrontal functional connectivity in civilian women with posttraumatic stress disorder. Journal of Psychiatric Research. 2013;47(10):1469–1478. doi: 10.1016/j.jpsychires.2013.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan RM, Landers M, Yeaman B, Wilson DA. Neurophysiology: Good memories of bad events in infancy. Nature. 2000;407(6800):38–39. doi: 10.1038/35024156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan RM, Perry RE. Mechanisms and functional implications of social buffering in infants: Lessons from animal models. Social Neuroscience. 2015;10(5):500–511. doi: 10.1080/17470919.2015.1087425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terranova M, Cirulli F, Laviola G. Behavioral and hormonal effects of partner familiarity in periadolescent rat pairs upon novelty exposure. Psychoneuroendocrinology. 1999;24(6):639–656. doi: 10.1016/s0306-4530(99)00019-0. [DOI] [PubMed] [Google Scholar]
- Tully LA, Arseneault L, Caspi A, Moffitt TE, Morgan J. Does maternal warmth moderate the effects of birth weight on twins’ attention-deficit/hyperactivity disorder (ADHD) symptoms and low IQ? Journal of Consulting and Clinical Psychology. 2004;72(2):218–226. doi: 10.1037/0022-006X.72.2.218. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Noble PL, Lyons CK, Sterk SM, Insel TR. Rearing effects on cerebrospinal fluid oxytocin concentration and social buffering in rhesus monkeys. Neuropsychopharmacology. 2003;28(5):910–918. doi: 10.1038/sj.npp.1300128. [DOI] [PubMed] [Google Scholar]