Abstract
Background
Adolescents with type 1 diabetes are at increased risk for deteriorating glycemic control, poor quality of life, and depressive symptoms. Stress and coping are related to these outcomes in adolescents with diabetes, yet few studies have examined these constructs longitudinally.
Purpose
To describe stress and coping in adolescents with type 1 diabetes and to examine coping strategies as predictors of adolescent adjustment (i.e., depressive symptoms, quality of life) and glycemic control.
Methods
Adolescents with type 1 diabetes completed measures of diabetes-related stress, coping, symptoms of depression, and quality of life at baseline, 6 months, and 12 months. Data on glycemic control were collected from adolescents’ medical charts.
Results
Adolescents’ use of primary control coping (e.g, problem solving) and secondary control engagement coping (e.g., positive thinking) strategies predicted significantly fewer problems with quality of life and fewer depressive symptoms over time. In contrast, the use of disengagement coping strategies (e.g., avoidance) predicted more problems with quality of life and depressive symptoms. Coping was not a significant predictor of glycemic control. Coping mediated the effects of diabetes-related stress on depressive symptoms and quality of life.
Conclusions
The ways in which adolescents with type 1 diabetes cope with diabetes-related stress predict quality of life and symptoms of depression but not glycemic control. Through the use of screening to identify adolescent’ diabetes-related stress and targeted interventions to improve coping responses, there is potential to improve outcomes.
Keywords: Coping, Type 1 Diabetes, Adolescents, Quality of Life, Depression, Stress
Type 1 diabetes is one of the most common chronic health conditions in youth, with over 18,000 new cases diagnosed each year, and the prevalence is increasing (1). The recommended intensive treatment regimen is often burdensome for adolescents, requiring frequent daily blood glucose checks, multiple insulin injections/boluses, consistent monitoring of carbohydrate intake and exercise, and checking urine for ketones when necessary (2). As children transition into adolescence, they are at risk for deteriorating glycemic control (3); a recent national study found that only 17% of adolescents were meeting recommended targets (4). In addition, youth with type 1 diabetes are at risk for elevated levels of depressive symptoms (5), and poorer quality of life (3). The negative effects of chronic stress on physical and mental health are well known (6), but stress in adolescents with type 1 diabetes is unique in that it may have both a direct, physiological effect on glycemic control, and an indirect effect through its impact on self-management. Thus, how adolescents respond to diabetes-related stress may serve as risk or protective factors for adjustment.
Adolescents report diabetes-related stress in a number of areas, including feeling different from peers, feeling guilty about high or low blood sugar levels, burnout with daily diabetes care, and parents nagging (7). A recent study found that adolescents’ reports of diabetes-related stress – especially stress related to parents (e.g., parental nagging and criticism) and diet (e.g., not being able to snack when I want) – were related to poorer glycemic control (8). While some of these stressors are controllable (e.g., bringing supplies to treat low blood sugar), others are largely uncontrollable for adolescents (e.g., parents nagging, feeling different from peers). The ways in which adolescents cope with diabetes-related stress have been linked with depressive symptoms, adherence, and glycemic control (9–11). More recently, a longitudinal study of coping in adolescents with type 1 diabetes found a cyclical relationship between glycemic control, psychological symptoms, and coping; greater use of active coping predicted better glycemic control, but poorer glycemic control and psychological symptoms at time 1 predicted greater use of withdrawal coping at time 2, which, in turn, resulted in greater symptoms and poorer glycemic control at time 3 and 4 (12). These studies support that how adolescents cope with diabetes-related stress may mediate the effects of stress on outcomes. Much of the previous research on coping has used an approach/avoidance distinction, which may limit our ability to improve outcomes, as these categories contain overlapping constructs (13). For example, a strategy such as problem solving, which is typically categorized as “active/approach” coping may not be effective in dealing with an uncontrollable stressor. A strategy such as distraction, which is often categorized as “avoidance” coping may actually be a more adaptive way to handle an uncontrollable stressor. On the other hand, a strategy such as physical aggression, categorized as “active/approach,” is typically associated with poorer outcomes. In contrast, a control-based model of coping has been empirically tested, and shown to predict outcomes in pediatric populations (14). In this model, primary control engagement coping strategies are defined as attempts directed toward influencing events or conditions (e.g., problem solving) or directly regulating one’s emotions (e.g., emotional expression). Secondary control engagement coping strategies are defined as efforts to adapt to the stressor or one’s response to it (e.g., cognitive restructuring, distraction). Finally, disengagement coping strategies are defined as attempts to avoid the stressor or one’s response to it (e.g., withdrawal, avoidance) (15). The most adaptive coping strategies are those that match the controllability of the stressor. For example, in studies of pediatric cancer, a largely uncontrollable stressor, the use of secondary control coping strategies, such as acceptance and distraction, may be more adaptive than the use of primary control coping strategies, such as problem-solving (16). One of the few studies to use a control-based model of coping in type 1 diabetes found that primary control coping was related to better quality of life and glycemic control, and this relationship was mediated through increased self-management (17). To our knowledge, however, there have been no longitudinal studies in which the effect of control-based coping strategies on outcomes over time in adolescents with type 1 diabetes has been examined.
The purpose of the current study is to describe stress and coping in adolescents with type 1 diabetes and to determine which coping strategies predict adolescent adjustment. Given that the type of stress may influence coping, diabetes-related stressors are described in adolescents. Next, the ways in which adolescents cope with diabetes stress were examined as predictors of psychosocial adjustment (depressive symptoms, quality of life) and glycemic control over time. Based on the literature, it was hypothesized that greater use of engagement coping strategies (primary control and secondary control coping) and less use of disengagement coping strategies at baseline would be related to fewer symptoms of depression, fewer problems with quality of life, and better glycemic control over 12 months. Finally, coping was tested as a mediator of the effect of stress on outcomes over time.
Method
Participants
Adolescents were eligible for the study if they were between the ages of 10–16 years, were able to speak and read English, and had been diagnosed with type 1 diabetes for at least six months. This age range was chosen because it encompasses a developmental stage when children become more responsible for diabetes management, and 10 is the age at which regular screening for depression is recommended (18)., and children in the second decade of life (ages 10–19 years) are considered adolescents (19). We chose to focus on mothers, as they are typically the caregivers primarily responsible for diabetes care, and mothers report higher levels of diabetes-related distress than fathers (20). The majority of the sample was White, non-Hispanic (74.3%), 45% were female, and the mean age was 12.8 ± 2.1. Duration of diabetes ranged from 0–14 years (mean duration = 5.1 ± 3.7 years), and the majority were using continuous subcutaneous insulin infusions (82.9%). The mean A1C level was 7.6 ± 1.1%.
Procedure
In line with the protocol approved by the University’s Institutional Review Board, the study was described to mothers and adolescents during a routine visit to the outpatient diabetes clinic. If they were eligible, the mother and adolescent scheduled a visit to the laboratory, where, after giving consent/assent, they completed a set of questionnaires and were compensated for their time. Of the 394 families approached, 99 were ineligible, 118 refused (most common reasons were time and distance), 60 expressed interest but were unable to schedule a visit, and 117 completed data. Clinical information (e.g., glycosylated hemoglobin A1C) was collected from the adolescents’ medical records. To allow for longitudinal tests of mediation (21), mothers and adolescents (117 dyads) completed questionnaires again during regularly scheduled clinic visits 6 months (n = 105) and 12 months later (n = 99). There were no significant differences in age, sex, family income, duration of diabetes, or therapy type (pump vs. injections) between participants who completed follow-up data and those who did not.
Measures
Mothers completed a demographics questionnaire and provided clinical information regarding the child’s date of diagnosis and type of insulin therapy (pump vs. injections).
Adolescents’ diabetes-related stress and coping was measured with the Responses to Stress Questionnaire, Type 1 Diabetes Version (RSQ, 15, 17). Adolescents reported how often they experienced 10 types of diabetes-related stress over the past 6 months (e.g., taking care of diabetes, feeling different from peers), to yield a Total Stress Score (ranging from 0–30, higher scores indicate greater diabetes-related stress). An item asked about perceived control, “Select the response that shows how much control you think you have over these problems” with responses ranging from 1 (none) to 4 (total). Adolescents then completed 57 items asking how they respond to these stressors. Confirmatory factor analyses support three separate coping factors on the RSQ: primary control engagement coping (problem solving, emotional modulation, emotional expression); secondary control engagement coping (positive thinking, cognitive restructuring, acceptance, distraction); and disengagement coping (avoidance, denial, wishful thinking) (15, 22). These factors have shown convergent and discriminant validity with other coping measures and physiological measures of stress reactivity (23). Internal consistency for the present study was 0.74 for total stress at time 1. For primary control coping, internal consistency was 0.78 at time 1, 0.78 at time 2, and 0.85 at time 3. For secondary control coping, internal consistency was 0.78 at time 1, 0.74 at time 2, and 0.80 at time 3. For disengagement coping, internal consistency was 0.80 at time 1, 0.78 at time 2, and 0.81 at time 3. To control for response bias and individual differences in base rates of item endorsement, proportion scores (i.e., type of coping in relation to total coping) were created by dividing each factor score by the total score (24).
Adolescents’ quality of life was measured with the Pediatric Quality of Life Inventory, Diabetes Module (PedsQL, 25). Scaled scores range from 0–100, and higher scores reflect better quality of life. Internal consistency for the current sample was 0.87 at time 1, 0.92 at time 2, and 0.89 at time 3.
Adolescent depressive symptoms were measured with the Child Depression Inventory (CDI, 26). The CDI consists of 27 items, with scores ranging from 0–54; higher scores indicate higher current levels of depression. Scores ≥ 13 suggest clinical levels of depression, and adolescents in the current study who scored at or above this cutoff were evaluated for depression by a licensed clinical psychologist, with referrals made as needed. Internal consistency for the current sample was excellent: 0.90 at time 1, 0.89 at time 2, and 0.93 at time 3.
Glycemic control was assessed by A1C, which provides objective measure of average blood glucose values over the most recent 8–12 weeks; it is routinely measured quarterly in patients with type 1 diabetes. Maintaining an A1C of <7.5% is recommended for children and adolescents (2). Analyses were performed using the Bayer Diagnostics DCA2000® machine, which provides results in 6 minutes on a fingerstick blood sample (normal range = 4.2–6.3%). The reliability of this method is high, and control checks are typically run every two weeks. A1C values were collected from adolescents’ medical charts at the clinic visit closest to the completion of questionnaire data.
Data Analyses
First, descriptive statistics were conducted to describe diabetes-related stress. Next, bivariate correlations were conducted to examine associations between stress, coping, psychological adjustment (i.e., symptoms of depression, quality of life), and glycemic control over time. Linear regression models were fitted to assess the association of the variables of interest (primary and secondary control coping, disengagement coping) with the outcomes of glycemic control (A1C) and quality of life (PedsQL) and depressive symptoms (CDI score), adjusting for pre-selected covariates (child sex, child age, family income, marital status, child race, therapy type and duration of diabetes). The Huber-White sandwich estimator was used for the above models accounting for repeated measures at time (1, 2, 3). We used odds ratios (odds of a higher response relative to a lower response) to summarize results.
The three time points of the longitudinal design allows for true tests of mediation (21). To test our final hypothesis, that coping would mediate the effects of diabetes-related stress on adolescent adjustment, the indirect effect of stress on each outcome was tested (i.e. QOL, depressive symptoms, and glycemic control) through coping using the RMediation package, which provides confidence intervals for mediated effects, or the product of two regression coefficients. Mediation is said to occur if the confidence interval does not include zero (27). Testing mediation, or indirect effects, in this way is thought to be more powerful than more traditional tests of mediation, and it is most suitable for smaller sample sizes (28). If coping variables (measured at time 2) were significantly associated with diabetes-related stress (at time 1) and adolescent adjustment (A1C, CDI, or PedsQL at time 3), the coping variables were considered potential mediators.
Results
Adolescent Stress
All of the adolescents in our sample reported some diabetes-related stress; as seen in Table 1, scores ranged from 2–23, with a mean total stress score of 11.3 (± 4.6). The most commonly endorsed stressor was diabetes care, selected by 83% of the adolescents in our sample as being “somewhat” or “very” stressful. Other commonly selected stressors were “others asking about my pump/injections/meter” and “parents bug me about taking care of myself.” Adolescents perceived fairly high control over diabetes stress (mean = 3.3 out of 4). In terms of depressive symptoms, approximately 9% of the adolescents scored above the clinical cutoff (CDI score ≥ 13) at baseline and 6 months, and 15% at 12 months. Adolescents reported generally good quality of life; mean score on the PedsQL was 83.7.
Table 1.
Frequency and Mean Levels of Diabetes-Related Stressors
| Diabetes-Related Stressor | Not at All/A Little n (%) |
Somewhat/Very n (%) |
Mean (SD) |
|---|---|---|---|
| Feeling different from other kids | 91 (78) | 25 (22) | 1.03 (0.76) |
| Dealing with diabetes care | 20 (17) | 95 (83) | 2.38 (0.85) |
| Feeling guilty or upset about “bad” numbers | 82 (71) | 34 (29) | 1.14 (0.78) |
| Don’t know how to tell others about my diabetes | 94 (82) | 21 (18) | 0.75 (0.90) |
| Others ask about my pump/injections/monitor | 65 (57) | 50 (43) | 1.38 (0.90) |
| Parents bug me about taking care of myself | 57 (50) | 58 (50) | 1.61 (1.12) |
| Missing school due to clinic visits | 104 (90) | 12 (10) | 0.58 (0.70) |
| Seeing my family worry about me | 84 (72) | 32 (28) | 1.09 (0.86) |
| Teachers/coaches/nurses didn’t understand diabetes | 95 (84) | 18 (16) | 0.65 (0.89) |
| Diabetes got in the way of personal goals | 93 (80) | 23 (20) | 0.75 (0.91) |
As seen in Table 2, cross-sectional correlations indicate that higher levels of diabetes-related stress were associated with less use of primary control coping (r = −.25, p = .013) and secondary control coping (r = −.42, p < .001), and greater use of disengagement coping (r = .21, p = .024) at baseline. Diabetes-related stress was also associated with all three types of coping 6 months later (all p < .01) Additionally, higher levels of diabetes-related stress was also associated with significantly poorer quality of life (r = −.22,), greater symptoms of depression (r =.26), and poorer glycemic control (r = ..29) 12 months later, all p < .05. Perceived control over diabetes-related stress was related to greater use of primary and secondary control coping (r = .25, .43, respectively) and less use of disengagement coping (r = −.27) at baseline, and to significantly higher levels of secondary control coping (r = .30) and lower levels of disengagement coping (r = −.20) 6 months later.
Table 2.
Descriptive Statistics and Bivariate Correlations among Stress, Coping, and Outcomes
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Stress T1 | -- | ||||||||||
| M = 11.26 (4.65) | |||||||||||
| 2. Controllability T1 | −.37*** | -- | |||||||||
| M = 3.34 (.74) | |||||||||||
| 3. Primary Control T1 | −.25** | .25* | -- | ||||||||
| M = .19 (.04) | |||||||||||
| 4. Secondary Control T1 | −.42*** | .43*** | .20* | -- | |||||||
| M = .27 (.05) | |||||||||||
| 5. Disengagement T1 | .21* | −.26* | −.49*** | −.43*** | -- | ||||||
| M = .14 (03) | |||||||||||
| 6. Primary Control T2 | −.27** | .16 | .61*** | .32*** | −.44*** | -- | |||||
| M = .20 (.04) | |||||||||||
| 7. Secondary Control T2 | −.47*** | .30** | .22* | .62*** | −.27** | .41*** | -- | ||||
| M = .28 (.05) | |||||||||||
| 8. Disengagement T2 | .29** | −.20* | −.37*** | −.40*** | .64*** | −.66*** | −.53*** | -- | |||
| M = .14 (.03) | |||||||||||
| 9. PedsQL T3 | −.22* | .15 | .07 | .30*** | .06 | .18 | .39** | −.03 | -- | ||
| M = 85.73 (12.52) | |||||||||||
| 10. CDI T3 | .26* | −.23* | −.25* | −.42*** | .18 | −.23* | −.37** | .14 | −.79*** | -- | |
| M = 4.82 (6.92) | |||||||||||
| 11. HbA1c T3 | .29** | −.32*** | −.11 | −.18 | .08 | −.18 | −.31** | −.12 | −.33*** | .38*** | -- |
| M = 7.92 (1.24) |
Note. Primary Control, Secondary Control, and Disengagement Coping were reported by adolescents on the Responses to Stress Questionnaire.
PedsQL = Diabetes-Specific Pediatric Quality of Life. CDI = Children’s Depression Inventory. T1 = Baseline data, T2 = 6 month data, T3 = 12 month data.
Adolescent Coping as a Predictor of Psychosocial and Diabetes-Related Outcomes
After adjusting for covariates, adolescents’ use of primary control coping strategies predicted significantly higher quality of life (3.53, 95% CI [1.68, 5.37], p < .001) and fewer symptoms of depression over time (−2.62, 95% CI [−3.57, −1.67], p < .001). Similarly, adolescents’ use of secondary control coping strategies predicted higher quality of life (4.71, 95% CI [2.75, 6.67], p < .001) and fewer depressive symptoms over time (−3.09, 95% CI [−4.03, −2.15], p < .001. In contrast, adolescents’ use of disengagement coping strategies predicted significantly greater depressive symptoms over time [1.87, 95% CI [0.51, 3.22], p = .007), but disengagement coping was not a significant predictor of quality of life [−0.65, 95% CI [−2.96, 1.66], p = .582). Coping was not a significant predictor of glycemic control.
Coping as a Mediator of Stress and Adjustment
Following the statistical guidelines of MacKinnon and colleagues (2002), secondary control coping met the conditions as a possible mediator of the effect of stress on glycemic control (A1C), as it was significantly associated with both diabetes stress at baseline and glycemic control at 12 months. Linear regression analyses were conducted to determine the regression coefficients (unstandardized B) and standardized error for each of the proposed pathways, adjusting for baseline A1C, perceived controllability, and other covariates. Table 3 presents the overall model, including the semi-partial correlations (sr2), indicating the unique contribution of each variable. The overall model was significant, predicting 41% of the variance in A1C at time 3, but secondary control coping was not a significant predictor in the final mode. Thus, the indirect effect of stress on glycemic control through secondary control coping was not significant, with an indirect effect estimate = .017 (SE = .009), 95% CI [−.002, .035].
Table 3.
Summary of Regression Model Predicting Adolescents’ Glycemic Control over 12 Months
| Full Model: F(76, 9) = 7.67*** | ||
|---|---|---|
| Variable | β | sr2 |
| Step 1: R2 change = .41*** | ||
| Baseline HbA1c | .60*** | .54 |
| Child Sex | .02 | .02 |
| Child Age | .06 | .05 |
| Income | −.06 | −.04 |
| Marital Status | −.07 | −.05 |
| Race/Ethnicity | .01 | .01 |
| Step 2: R2 change = .05* | ||
| Baseline HbA1c | .58*** | .51 |
| Child Sex | .04 | .04 |
| Child Age | .07 | .06 |
| Income | −.02 | −.02 |
| Marital Status | −.04 | −.03 |
| Race/Ethnicity | .02 | .02 |
| Stress T1 | .06 | .06 |
| Controllability T1 | −.21* | −.18 |
| Step 3: R2 change = .02 | ||
| Baseline HbA1c | .56*** | .50 |
| Child Sex | .05 | .05 |
| Child Age | .07 | .07 |
| Income | −.03 | −.02 |
| Marital Status | −.02 | −.02 |
| Race/Ethnicity | .02 | .02 |
| Stress T1 | .03 | .02 |
| Controllability T1 | −.18 | −.16 |
| Secondary Control Coping T2 | −.14 | −.13 |
| Model Adjusted R2 = .41 | ||
Note. T1 = baseline, T2 = 6 month data.
p <.05.
p < .01.
p < .001.
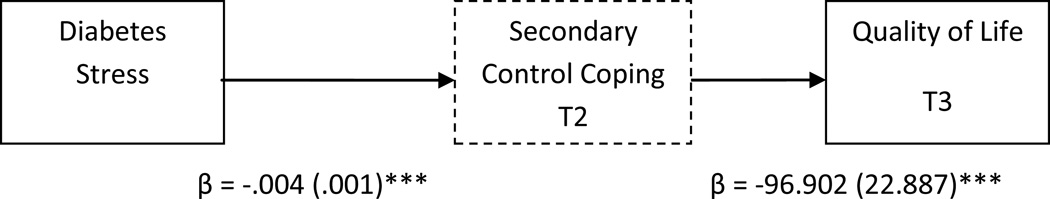
Secondary control coping also met the conditions as a possible mediator of the effect of stress on quality of life (PedsQL). Table 4 presents the overall model, including the semi-partial correlations (sr2), indicating the unique contribution of each variable. After adjusting for baseline quality of life, perceived controllability, and other covariates, the overall model was significant, predicting 31% of the variance in quality of life at time 3. Secondary control coping was a significant partial mediator of the effect of stress on quality of life (see Figure 1), with an indirect effect estimate of −0.39 (SE = .14), 95% CI [−.68, −.16], explaining 15% of the variance in quality of life.
Table 4.
Summary of Regression Model Predicting Adolescents’ Quality of Life over 12 Months
| Full Model: F(72, 9) = 4.94*** | ||
|---|---|---|
| Variable | β | sr2 |
| Step 1: R2 change = .22** | ||
| Baseline PedsQL | .36*** | .35 |
| Child Sex | .04 | .04 |
| Child Age | −.19 | −.18 |
| Income | .13 | .10 |
| Marital Status | .06 | .05 |
| Race/Ethnicity | .01 | .01 |
| Step 2: R2 change = .01 | ||
| Baseline PedsQL | .36** | .29 |
| Child Sex | .05 | .05 |
| Child Age | −.18 | −.17 |
| Income | .12 | .10 |
| Marital Status | .17 | .06 |
| Race/Ethnicity | .03 | .02 |
| Stress T1 | −.07 | −.05 |
| Controllability T1 | −.09 | −.08 |
| Step 3: R2 change = .15*** | ||
| Baseline PedsQL | .29* | .24 |
| Child Sex | −.01 | −.01 |
| Child Age | −.22* | −.21 |
| Income | .10 | .08 |
| Marital Status | .01 | .01 |
| Race/Ethnicity | −.01 | −.01 |
| Stress T1 | .04 | .03 |
| Controllability T1 | −.13 | −.11 |
| Secondary Control Coping T2 | .45*** | .39 |
| Model Adjusted R2 = .31 | ||
Note. PesQL = Pediatric Quality of Life; T1 = baseline; T2 = 6 month data.
p <.05.
p < .01.
p < .001.
Figure 1.
Secondary Control Coping (measured at time 2) as a mediator of diabetes-related stress (measured at time 1) and adolescent quality of life (measured at time 3). Unstandardized coefficients estimates and standard errors are presented for each step in this pathway, after adjusting for covariates.
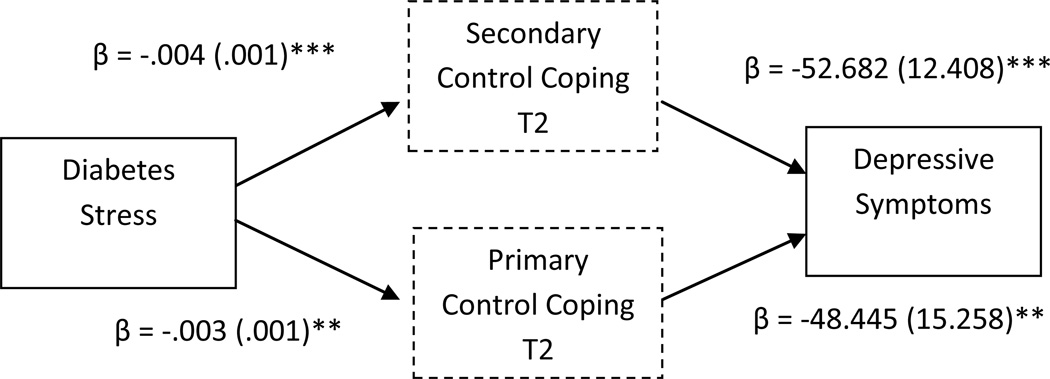
Finally, primary and secondary control coping met the conditions as possible mediators of the effect of stress on depressive symptoms (CDI). After adjusting for baseline levels of depression (CDI), perceived controllability, and other covariates, the overall model was significant (see Table 5), predicting 49% of the variance in depressive symptoms at time 3. The addition of coping to the model explained 29% of the variance. Primary control coping emerged as a significant partial mediator, with an indirect effect estimate of 0.15 (SE = .07), 95% CI [.034, .299]. Secondary control coping also emerged as a significant partial mediator, with an indirect effect estimate of .21 (SE = .07), 95% CI [.085, .371] (see Figure 2).
Table 5.
Summary of Regression Model Predicting Adolescents’ Depressive Symptoms over 12 Months
| Full Model: F(68, 10) = 8.53*** | ||
|---|---|---|
| Variable | β | sr2 |
| Step 1: R2 change = .26*** | ||
| Baseline CDI | .35*** | .34 |
| Child Sex | .09 | .09 |
| Child Age | .24* | .24 |
| Income | −.19 | −.16 |
| Marital Status | −.01 | −.01 |
| Race/Ethnicity | .01 | .01 |
| Step 2: R2 change = .01 | ||
| Baseline CDI | .29* | .23 |
| Child Sex | .09 | .09 |
| Child Age | .24* | .23 |
| Income | −.18 | −.15 |
| Marital Status | −.02 | −.01 |
| Race/Ethnicity | .02 | .02 |
| Stress T1 | .06 | .05 |
| Controllability T1 | −.06 | −.05 |
| Step 3: R2 change = .29*** | ||
| Baseline CDI | .34** | .26 |
| Child Sex | .18 | .17 |
| Child Age | .35*** | .33 |
| Income | −.19* | −.16 |
| Marital Status | .08 | .07 |
| Race/Ethnicity | .03 | .03 |
| Stress T1 | −.19 | −.14 |
| Controllability T1 | .07 | .05 |
| Primary Control Coping | −.32** | −.26 |
| Secondary Control Coping T2 | −.43*** | −.34 |
| Model Adjusted R2 = .49 | ||
Note. CDI = Children’s Depression Inventory; T1 = baseline; T2 = 6 month data.
p <.05.
p < .01.
p < .001.
Figure 2.
Primary and Secondary Control Coping (measured at time 2) as mediators of diabetes-related stress (measured at time 1) and adolescent depressive symptoms (measured at time 3). Unstandardized coefficients estimates and standard errors are presented for each step in this pathway, after adjusting for covariates.
Discussion
The current study is the first to use a control-based model of coping in a prospective study of adolescents with type 1 diabetes. Similar to previous research (8), adolescents with type 1 diabetes in our sample experienced stress related to daily diabetes care, others asking about diabetes, and parents reminding them to care for themselves. This diabetes-related stress was related to glycemic control, depressive symptoms, and quality of life over time. Further, the ways in which adolescents coped with diabetes-related stress predicted psychosocial adjustment (depression, quality of life) but not glycemic control. Finally, secondary control coping partially mediated the effects of stress on adolescents’ quality of life and primary and secondary control coping mediated the effects of stress on depressive symptoms, explaining a substantial percentage of the variance in these outcomes (15% and 29%, respectively). These findings have important implications for future research and practice.
First, coping may help to predict the relationship between diabetes-related stress and outcomes (8), and this model of coping captures important control-based differences. Specifically, greater use of primary control coping strategies (e.g., problem solving) and secondary control coping strategies (e.g., acceptance, distraction) predicted significantly fewer symptoms of depression and fewer problems with quality of life 12 months later. While some aspects of diabetes are controllable (e.g., choosing what to eat, checking blood sugar), others are not (e.g., people asking about diabetes, feeling different from peers). By acknowledging this unpredictability and encouraging adolescents to use secondary control coping strategies (e.g., acceptance, distraction) to deal with this type of stress, providers may help to reduce some of the frustration and discouragement often seen in adolescents with type 1 diabetes. It is also important to note that higher levels of stress predicted lower use of these adaptive coping strategies, suggesting that higher levels of stress may compromise adolescents’ ability to use primary and secondary control strategies, as compared to disengagement strategies, which require less cognitive effort (29).
In addition, secondary control coping mitigated the effects of stress on depressive symptoms and quality of life. Quality of life is an important outcome to consider – a recent national study (3) found that youth with diabetes are at risk for poorer quality of life, which can predict deteriorating diabetes care. Further, the risk for depressive symptoms is higher among adolescents with type 1 and type 2 diabetes, and depressive symptoms have also been linked with deteriorating glycemic control (30, 31). Thus, teaching adolescents to use effective coping strategies may reduce their risk for problems with quality of life and depression, and ultimately, deteriorating glycemic control. Given that age was significantly associated with greater depressive symptoms and poorer quality of life, it may also be important to teach these strategies in early adolescence, to prevent later problems.
The current study may also inform interventions to improve outcomes in youth with type 1 diabetes. For example, Grey and colleagues developed and tested a Coping Skills Training (CST) intervention that improved both glycemic control and quality of life in adolescents with type 1 diabetes (32). Yet, more recently, randomized controlled trials with young adolescents (age 11–14) found that a diabetes education intervention had similar or better effects than a CST program on glycemic control and adherence (33, 34). The earlier studies of CST may have had stronger effects because they included role-playing to help adolescents deal with some of the uncontrollable aspects (e.g., feeling different from peers), while much of the work on coping skills training has focused on the more controllable aspects of diabetes (e.g., problem-solving with parents). Findings from the current study suggest that more emphasis is needed on helping adolescents to identify and effectively cope with the more uncontrollable aspects of diabetes as well, Secondary control coping strategies have been shown to be more adaptive for dealing with uncontrollable stressors, such as cancer (35) or parental depression (36). These strategies, such as acceptance, positive thinking, and distraction, can be taught (37), and increased use of these strategies has been related to better psychosocial outcomes, such as fewer symptoms of depression and externalizing symptoms in youth (38). However, as seen in our sample, adolescents are less likely to use these adaptive strategies under greater levels of stress, and therefore CST interventions may be strengthened by including opportunities to practice these strategies in relatively low-stress environments.
The current study supports that diabetes-related stress is experienced by most adolescents with type 1 diabetes, and how they respond to it has important implications for both physiological and psychosocial adjustment. Given the findings that stress predicted adolescents’ coping 6 months later, it may be that higher levels of stress make it difficult for adolescents to use the more cognitively demanding strategies, such as cognitive reappraisal. In a previous study of coping in youth with type 1 diabetes, low-income and minority youth were more likely to use disengagement coping strategies and less likely to use primary and secondary control strategies (17). Thus, these youth may need extra support and reinforcement to use the most adaptive strategies to cope with diabetes-related stress.
Unlike previous studies in which greater use of primary control coping and active coping was related to better glycemic control (e.g., 12, 17), coping did not predict glycemic control in the multivariate analysis. This difference may be explained by the fact that the current sample was in relatively good glycemic control (mean A1C was 7.6%/60 mmol/mol in our sample), and therefore there was less variability in glycemic control. Our findings suggest that an increased focus on secondary control coping strategies, including distraction, acceptance, positive thinking, may have a stronger impact on depressive symptoms and quality of life than on glycemic control, but effects of coping on glycemic control may be more apparent in a sample with greater variation in glycemic control. Alternatively, adolescents may experience other sources of stress (e.g., school, family) as more salient than diabetes-related stress (39).
Limitations
While a major strength of the study is the longitudinal design, it is still possible that problems with depression or quality of life preceded coping. Further, our sample was in relatively good glycemic control, which may limit the generalizability of our findings. Similarly, the families in our sample had fairly high socioeconomic status and income, which may also affect generalizability. Further, the majority of participants endorsed fairly low levels of most of the stressors, suggesting that this may be a particularly resilient sample. Further studies are needed to replicate these findings in a more diverse sample. In addition, future studies may examine whether coping moderates, or buffers, the effects of stress. Finally, data on diabetes self-management is needed, as it is likely to play an important role in glycemic control.
Conclusions
The current study confirms the need to support adolescents’ efforts to cope effectively with diabetes-related stress. Adolescents’ coping has been related to diabetes management (17), suggesting that future studies should examine coping as a predictor of adherence to treatment. Further, while interventions have been developed to improve coping in youth with type 1 diabetes, it is important for investigators to measure the coping skills they are teaching. For example, in Grey and colleagues’ CST intervention, improvements in primary and secondary control coping were found to mediate changes in quality of life (40). Results highlight the need to identify sources of stress and support effective coping strategies for adolescents with type 1 diabetes, which may help to reduce symptoms of depression and improve quality of life.
Acknowledgments
This research was supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases [K23 DK088454] and the National Center for Research Resources (UL1 RR024139]. The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official view of NIH.
Footnotes
Conflict of Interest
The authors have no conflicts of interest to report.
Ethical Adherence
The current study was approved by the Institutional Review Board, and informed consent (and assent) was obtained for all study participants.
References
- 1.Hamman RF, Bell RA, Dabelea D, et al. The SEARCH for Diabetes in Youth Study: Rationale, Findings, and Future Directions. Diabetes Care. 2014;37:3336–3344. doi: 10.2337/dc14-0574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Diabetes Association. Standards of medical care in diabetes - 2016. Diabetes Care. 2016;39:S1–S106. [Google Scholar]
- 3.Hood KK, Beavers DP, Yi-Frazier J, et al. Psychosocial Burden and Glycemic Control During the First 6 Years of Diabetes: Results From the SEARCH for Diabetes in Youth Study. J Adolesc Health. 2014;55:498–504. doi: 10.1016/j.jadohealth.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller KM, Foster NC, Beck RW, et al. Current state of type 1 diabetes treatment in the U.S.: Updated data from the T1D Exchange clinic registry. Diabetes Care. 2015;38:971–978. doi: 10.2337/dc15-0078. [DOI] [PubMed] [Google Scholar]
- 5.Lawrence JM, Standiford DA, Loots B, et al. Prevalence and Correlates of Depressed Mood Among Youth With Diabetes: The SEARCH for Diabetes in Youth Study. Pediatrics. 2006;117(4):1348–1358. doi: 10.1542/peds.2005-1398. [DOI] [PubMed] [Google Scholar]
- 6.Juster RP, McEwen BS, Lupien SJ. Allostatic load biomarkers of chronic stress and impact on health and cognition. Neurosci Biobehav Rev. 2010;35:2–16. doi: 10.1016/j.neubiorev.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 7.Davidson M, Penney EA, Muller B, et al. Stressors and self-care challenges faced by adolescents living with type 1 diabetes. Appl Nurs Res. 2004;17:72–80. doi: 10.1016/j.apnr.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 8.Delamater AM, Patino-Fernandez AM, Smith KE, et al. Measurement of diabetes stress in older children and adolescents with type 1 diabetes mellitus. Pediatr Diab. 2013;14:50–56. doi: 10.1111/j.1399-5448.2012.00894.x. [DOI] [PubMed] [Google Scholar]
- 9.Delamater AM, Kurtz SM, Bubb J, et al. Stress and coping in relation to metabolic control of adolescents with type 1 diabetes. J Devel Behav Pediatr. 1987;8:136–140. [PubMed] [Google Scholar]
- 10.Graue M, Wentzel-Larsen T, Bru E, et al. The coping styles of adolescents with type 1 diabetes are associated with degree of metabolic control. Diabetes Care. 2004;27:1313–1317. doi: 10.2337/diacare.27.6.1313. [DOI] [PubMed] [Google Scholar]
- 11.Reid GJ, Dubow EF, Carey TC, et al. Contribution of coping to medical adjustment and treatment responsibility among children and adolescents wtih diabetes. Devel Behav Pediatr. 1994;15:327–335. [PubMed] [Google Scholar]
- 12.Luyckx K, Seiffge-Krenke I, Hampson S. Glycemic control, coping, and internalizing and externalizing symptoms in adolescents with type 1 diabetes: A cross-lagged, longitudinal approach. Diabetes Care. 2010;33:1424–1429. doi: 10.2337/dc09-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Skinner EA, Edge K, Altman J, et al. Searching for the structure of coping: A review and critique of the category systems for classifying ways of coping. Psychol Bull. 2003;129(2):216–269. doi: 10.1037/0033-2909.129.2.216. [DOI] [PubMed] [Google Scholar]
- 14.Compas BE, Jaser SS, Dunn MJ, et al. Coping with chronic illness in children and adolescents. Ann Rev Clin Psychol. 2012;8:455–480. doi: 10.1146/annurev-clinpsy-032511-143108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Connor-Smith JK, Compas BE, Wadsworth ME, et al. Responses to stress in adolescence: Measurement of coping and involuntary stress responses. J Consult Clin Psychol. 2000;68:976–992. [PubMed] [Google Scholar]
- 16.Compas BE, Desjardins L, Vannatta K, et al. Children and adolescents coping with cancer: self- and parent reports of coping and anxiety/depression. Health Psychol. 2014;33:853–861. doi: 10.1037/hea0000083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jaser SS, Faulkner MS, Whittemore R, et al. Coping, self-management, and adaptation in adolescents with type 1 diabetes. Ann Behav Med. 2012;43:311–319. doi: 10.1007/s12160-012-9343-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Silverstein JH, Klingensmith G, Copeland K, et al. Care of children and adolescents with type 1 diabetes. Diabetes Care. 2005;28:184–212. doi: 10.2337/diacare.28.1.186. [DOI] [PubMed] [Google Scholar]
- 19.Lerner RM, Steinberg L. Handbook of Adolescent Psychology. 2nd. Hoboken, NJ: John Wiley & Sons; 2004. [Google Scholar]
- 20.Whittemore R, Jaser S, Chao A, et al. Psychological experience of parents of children with type 1 diabetes: a systematic mixed-studies review. Diabetes Educ. 2012;38(4):562–579. doi: 10.1177/0145721712445216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maxwell SE, Cole DA. Bias in cross-sectional analyses of longitudinal mediation. Psychol Methods. 2007;12:23–44. doi: 10.1037/1082-989X.12.1.23. [DOI] [PubMed] [Google Scholar]
- 22.Compas BE, Boyer MC, Stanger C, et al. Latent variable analysis of coping, anxiety/depression, and somatic symptoms in adolescents with chronic pain. J Consult Clin Psychol. 2006;56:1132–1142. doi: 10.1037/0022-006X.74.6.1132. [DOI] [PubMed] [Google Scholar]
- 23.Dufton L, Dunn MJ, Slosky LS, et al. Self-reported and laboratory-based responses to stress in children with recurrent abdominal pain and anxiety. J Pediatr Psychol. 2011;36:95–105. doi: 10.1093/jpepsy/jsq070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vitaliano PP, Maiuro RD, Russo J, et al. Raw versus relative scores in the assessment of coping strategies. J Behav Med. 1987;10:1–18. doi: 10.1007/BF00845124. [DOI] [PubMed] [Google Scholar]
- 25.Varni JW, Burwinkle TM, Jacobs JR, et al. The PedsQL in type 1 and type 2 diabetes: Reliability and validity of the Pediatric Quality of Life Inventory Generic Core Scales and Type 1 Diabetes Module. Diabetes Care. 2003;26:631–637. doi: 10.2337/diacare.26.3.631. [DOI] [PubMed] [Google Scholar]
- 26.Kovacs M. The Children's Depression Inventory (CDI) Psychopharm Bull. 1985;21:995–998. [PubMed] [Google Scholar]
- 27.Tofighi D, MacKinnon DP. RMediation: An R package for mediation analysis confidence intervals. Behav Res Methods. 2011;43:692–700. doi: 10.3758/s13428-011-0076-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.MacKinnon DP, Lockwood CM, Hoffman JM, et al. A comparison of methods to test mediation and other intervening variable effects. Psychol Methods. 2002;7:83–104. doi: 10.1037/1082-989x.7.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robinson KE, Pearson MM, Cannistraci CJ, et al. Functional neuroimaging of working memory in survivors of childhood brain tumors and healthy children: Associations with coping and psychosocial outcomes. Child Neuropsychol. 2015;21:779–802. doi: 10.1080/09297049.2014.924492. [DOI] [PubMed] [Google Scholar]
- 30.Hassan K, Loar R, Anderson BJ, et al. The role of socioeconomic status, depression, quality of life, and glycemic control in type 1 diabetes mellitus. J Pediatr. 2006;149:526–531. doi: 10.1016/j.jpeds.2006.05.039. [DOI] [PubMed] [Google Scholar]
- 31.McGrady ME, Laffel L, Drotar D, et al. Depressive symptoms and glycemic control in adolescents with type 1 diabetes: mediational role of blood glucose monitoring. Diabetes Care. 2009;32:804–806. doi: 10.2337/dc08-2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grey M, Boland EA, Davidson M, et al. Coping skills training for youth with diabetes mellitus has long-lasting effects on metabolic control and quality of life. J Pediatr. 2000;137:107–113. doi: 10.1067/mpd.2000.106568. [DOI] [PubMed] [Google Scholar]
- 33.Holmes CS, Chen R, Mackey E, et al. Randomized clinical trial of clinic-integrated, low-intensity treatment to prevent deterioration of disease care in adolescents with type 1 diabetes. Diabetes Care. 2014;37:1535–1543. doi: 10.2337/dc13-1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grey M, Whittemore R, Jeon S, et al. Internet psycho-education programs improve outcomes in youth with type 1 diabetes. Diabetes Care. 2013;36:2475–2482. doi: 10.2337/dc12-2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Compas BE, Desjardins L, Vannatta K, et al. Children and adolescents coping with cancer: self- and parent reports of coping and anxiety/depression. Health Psychol. 2014;33:853–861. doi: 10.1037/hea0000083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jaser SS, Langrock AM, Keller G, et al. Coping with the stress of parental depression II: adolescent and parent reports of coping and adjustment. J Clin Child Adolesc Psychol. 2005;34:193–205. doi: 10.1207/s15374424jccp3401_18. [DOI] [PubMed] [Google Scholar]
- 37.Compas BE, Forehand R, Keller G, et al. Randomized Controlled Trial of a Family Cognitive-Behavioral Preventive Intervention for Children of Depressed Parents. J Consult Clin Psychol. 2009;77:1007–1020. doi: 10.1037/a0016930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Compas BE, Champion JE, Forehand R, et al. Coping and parenting: Mediators of 12-month outcomes of a family group cognitive-behavioral preventive intervention with families of depressed parents. J Consult Clin Psychol. 2010;78:623–634. doi: 10.1037/a0020459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chao A, Minges KE, Park C, et al. General life and diabetes-related stressors in early adolescents with type 1 diabetes. J Pediatr Health Care. doi: 10.1016/j.pedhc.2015.06.005. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jaser SS, Whittemore R, Chao A, et al. Mediators of 12-month outcomes of two Internet interventions for youth with type 1 diabetes. J Pediatr Psychol. 2014;39:306–315. doi: 10.1093/jpepsy/jst081. [DOI] [PMC free article] [PubMed] [Google Scholar]