“Prediction is difficult, especially about the future.” - Anonymous
Cardiovascular disease (CVD) is a leading cause of death worldwide and continues to increase in prevalence compared to previous decades, in part because of the aging of the world population (1). Atherosclerotic CVD starts at a very young age and progresses over time allowing sufficient time for screening and early detection of the condition (2). Advances in biomarker research and developments related to CVD over the past 30 years have led to more sensitive screening methods, a greater emphasis on its early detection and diagnosis, and improved treatments resulting in more favorable clinical outcomes in the community (3, 4). However, the use of biomarkers for different purposes in CVD remains an important area of research that has been explored by scientists over the years and many new developments are still underway. Therefore, a detailed description of all CVD biomarkers that are currently being used or investigated for future use in the field of cardiovascular medicine is out of scope for any review article. In the present review, we do not intend to replicate the information from previous exhaustive reviews on biomarkers (5) but highlight key statistical and clinical issues with an emphasis on methods to evaluate the incremental yield of biomarkers, including their clinical utility, a pre-requisite before any putative novel biomarker is utilized in clinical practice. In addition, we will summarize information regarding recent novel heart failure biomarkers in current practice, which are undergoing scrutiny before they can be available for clinical use, and their impact on clinical outcomes.
Biomarker Definition
The National Institute of Health Consortium in 2001 defined a biomarker as a “characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention” (6). Subsequently, in 2009 the American Heart Association outlined the extensive criteria for how newer biomarkers should be evaluated in a standardized fashion before their clinical use can be recommended (7). The characteristics of an ideal biomarker to be used for a given purpose in any disease condition with a special emphasis on CVD are detailed in previous reviews (5, 8).
Biomarker Types
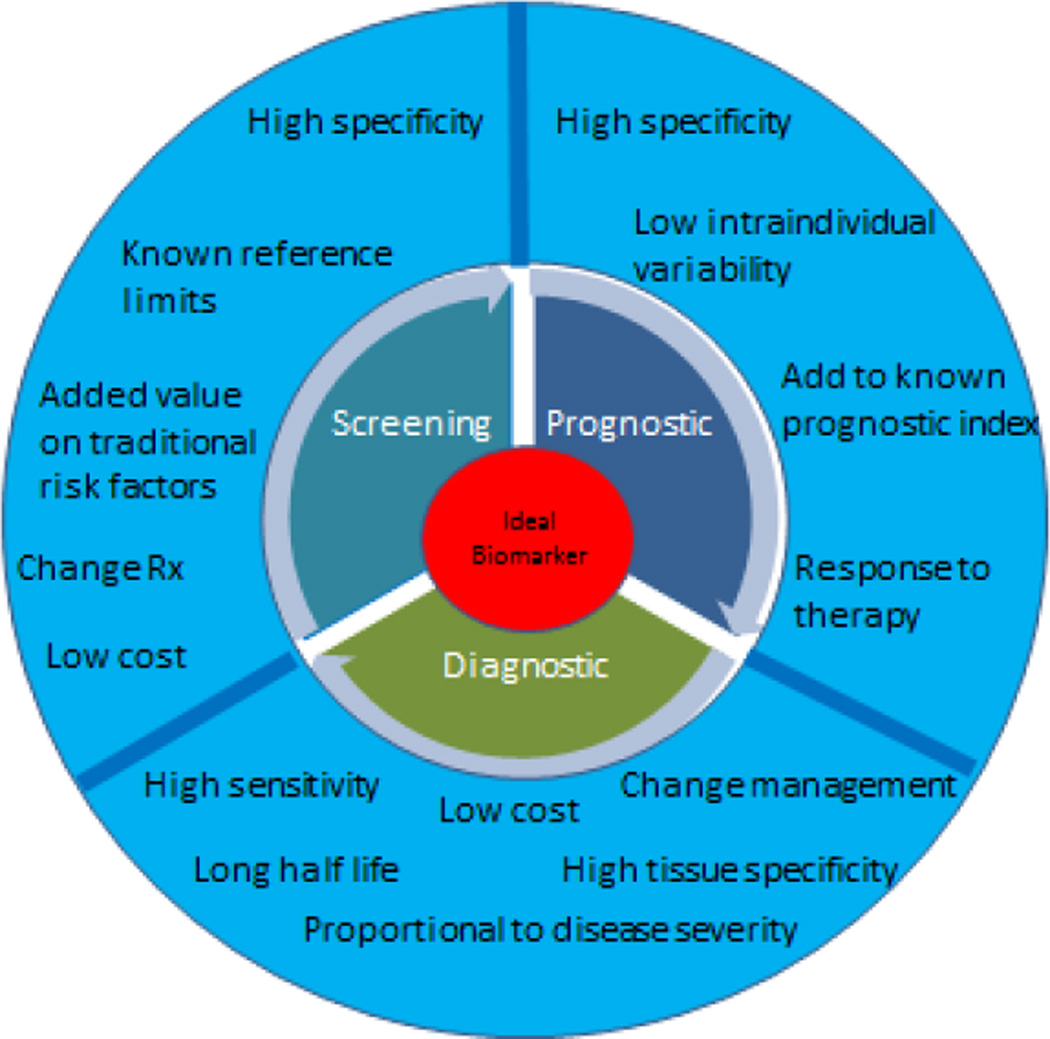
Biomarkers play an important role in the evaluation of disease as well as in the development of drug treatments for disease conditions. In the late phases of drug development, biomarkers can even be helpful in determining the accurate doses for any given drug. In more recent times, biomarkers are being considered as surrogate end points for clinical trials as well. Biomarkers are traditionally classified on the basis of their intended use as screening, diagnostic or prognostic. Desired characteristics of a novel biomarker according to their intended use are also displayed in Figure 1. More recently, there has been a national shift toward development of precision medicine, especially with a focus on development of new cancer drugs. On January 30th 2015, US President Barack Obama introduced in his State of Union address the Precision Medicine Initiative (9) that takes into account individual differences in genes, environment and lifestyle factors, emphasizing more effective and targeted treatment goals (10).
Figure 1.
Ideal characteristics of a biomarker according to their intended use.
From a precision medicine perspective, biomarkers can be classified as prognostic, pharmacodynamic or predictive biomarkers. A prognostic biomarker is one that provides information on the likely course of a disease condition in an untreated individual or in an individual treated with conventional therapies. In contrast, a predictive biomarker is one that can be used to identify individuals who are most likely to respond to a given therapy or that distinguishes candidates who can be considered for specific targeted therapies (11, 12). Thus, predictive biomarkers help to tailor therapy according to the patient’s needs. So far these clinical trial designs based on evaluating a biomarker for prognostic or predictive utility have been limited to the field of oncology; however, other fields of medicine including cardiovascular medicine and infectious diseases have now started adopting these designs as well (13). Lastly, pharmacodynamic biomarkers measure the effect of a drug on the disease state itself. In other words, they represent the change in a target organism in response to the disease and its treatment. For example, changes in circulating natriuretic peptide levels are reflective of heart failure severity and, therefore, blood natriuretic peptide levels are now being proposed as a surrogate endpoint to test the efficacy of drug treatment (14). Similarly, use of statins to reduce serum cholesterol levels is another example where changes in concentration of a biomarker (low density lipoprotein [LDL] cholesterol) is used to guide therapy to reduce the risk of CVD in future. But first, it is imperative to confirm that biomarker levels (natriuretic peptide or LDL cholesterol as examples above) should correlate well with a clinical outcome at individual and population levels.
Biomarker Characteristics- General Principles
Accuracy, precision, high sensitivity and specificity are important characteristics of an ideal biomarker. Before clinical utilization, if a biomarker is to be used for screening or for prognostic purposes, a high specificity (which is expressed as likelihood ratio [LR]) is required (“rule in”). (15) The desirable likelihood ratio for a screening test is typically >10. Whereas, if a biomarker is evaluated for diagnostic purposes, a high sensitivity (LR <0.10) is recommended. Second, it is important to establish reference limits (16) with the understanding that reference limits are influenced by the characteristic of an assay in the group analyzed to derive those limits (17). For instance, blood troponin assays made by several manufacturers are different and have varying reference limits for detection of clinically important vascular events such as an acute myocardial infarction (18). Third, higher discrimination capabilities are necessary for an assay before it can be used clinically (19). Discrimination limits allow separation of abnormal levels from normal levels of a biomarker according to the disease condition studied. More importantly, differentiating between ‘undesirable’ or ‘abnormal’ levels from the ‘levels that require treatment’ is also critical before a biomarker is considered ‘fit’ to be used in clinical practice for a ‘given purpose’. Lastly, calibration is a test to assess the ability of a biomarker to predict risk in a given sample compared to the actual observed risk in the same sample of individuals or in a different population all together. For example, the risk of CVD based on cutoff values of waist circumference or of body mass index among US residents or white individuals is generally higher compared to other ethnicities (especially South Asians) (20–22). Hence, a risk score that includes BMI or waist circumference based on American cohort would require recalibration before it can be applied to individuals with other ethnicities with appropriate adjustment for BMI or waist circumference values which predict higher CVD risk (23). Recalibration is also important to account for differences in baseline absolute risk across different samples (24). Hosmer-Lemeshow goodness-of-fit statistic is a statistical test that is frequently used to examine the model calibration (25).
Assessment of Biomarker Association with Disease
Biomarkers generally represent a biochemical change at a tissue or a body organ level. Therefore, they are associated with a biologic or pathologic process. However, the clinical outcomes from these processes in terms of biomarkers as disease indicators could be different. For example, troponin elevation up to a certain degree can be present in congestive heart failure, pulmonary embolism and more conventionally and classically in acute myocardial ischemia/infarction. Moreover, biomarkers that are intended to be used in clinical practice can be useful if changes in their levels adequately mirror improvement in the disease process itself when the disease is being treated (predictive biomarkers), thereby reflecting an improvement in patient outcome. For example, blood B-type natriuretic peptide (BNP) concentrations increase with worsening heart failure status. Additionally, a clinically useful biomarker should be able to provide meaningful information about prognosis and/or guide clinical decision making and not simply duplicate information that is already available clinically. Derivation and validation to associate a biomarker to a disease process should also be carried out in different subsets of population (26). In general, biomarkers predicting disease risk perform much better in the derivation cohort compared to a validation cohort. Universal biomarker standards have also been proposed according to their intended use for disease diagnosis (27) and prognosis (28).
Added advantage over available biomarkers or risk factors
It is largely believed that no single statistical method can be used alone to test a novel biomarker in an optimal manner for assessing its incremental clinical use. In this context, it is noteworthy that CVD is a disease process that progresses over years from a subclinical state to clinical symptoms, often due to presence of CVD risk factors. Therefore, the prediction of an individual’s CVD risk over a 1–10 year period traditionally involves assessment of CVD risk factors such as individual’s age, gender, baseline levels of systolic and diastolic blood pressure, serum cholesterol, smoking status and history of diabetes. However there are some limitations to using these risk factors alone in a model as prognostic tools for CVD risk prediction (29). Traditionally, any novel CVD biomarker is first examined in a multivariable model that includes all standard risk factors, and the measure of effect size for estimating CVD risk is assessed as hazard ratio or odds ratio. It is important to remember that simply a higher hazard ratio for CVD is not sufficient to confer higher risk associated with a given biomarker. The new biomarker should be able to provide added information about individual’s risk, above and beyond the traditional risk factors at baseline. Appropriate assessment of the incremental yield of a new biomarker for predicting CVD risk requires appropriate evaluation of its discrimination and calibration potential, as explained above. In general, individual circulating biomarkers have thus far failed to improve CVD risk prediction substantially over standard risk factors with higher sensitivity and specificity and major gains in discrimination and calibration (30). Hence, investigators have also explored the possibility of using multiple biomarkers in addition to traditional risk factors to examine individual’s CVD risk. However, using multiple biomarkers to assess individuals’ CVD risk has only shown to modestly improve prediction beyond standard risk factors (31). Multimarker methodology also has some important limitations before implementation. Some of the important considerations before a multimarker risk model is implemented clinically include proper accounting for the inherent correlation among measured biomarkers, evaluating the reproducibility of the model (with bootstrapping techniques or external validation), assessing transportability and applicability to different populations or ethnicities with metrics such as model calibration (as described above). Of note, a multimarker technique has been successfully implemented in some areas in clinical practice such as the MELD (model for end-stage liver disease) score (32).
Statistical assessment of CVD risk related to a new Biomarker
First, it is important to assess the association of biomarker with the disease as explained above. In this respect, biomarker has to be related to the outcome of interest in a statistically significant manner. This is performed by regression models [logistic or Cox(33)]. Unfortunately, statistical significance alone does not imply clinical significance because several weak biomarkers could still be associated with the outcome of interest if examined within larger samples. Therefore, several metrics are used in the context of risk prediction models such as the ability to separate those who will develop the disease from those who will not. Hence, the receiver operating curve (ROC) or the area under the curve (AUC; C-statistic) (34–36) are most widely accepted tool for model discrimination, though with few exceptions when individuals are classified into risk categories (37). Newer statistical methods have been developed to define newer metrics that can help us evaluate the clinical utility of new markers beyond traditional measures such as an increase in the area under the ROC curve or the association of the biomarker with outcomes above and beyond the available standard risk factors. Consequently, reclassification tables or the NRI (net reclassification index) has now been proposed and are being widely used (38). Quantifying improvement of models by NRI analyses requires an objective way to classify risk by categories, therefore meaningful risk categories are needed a priori. Hence, the NRI reclassification tables constructed separately for participants with and without disease events identify the correct movements in categories – upwards for events and downwards for non-events. In other words, it is possible to show how many individuals actually would change categories based on using information from new biomarker after reclassification. Figure 2 displays an example of calculating NRI by using categorical data and reclassifying based on information from new biomarker. However, there are some limitations to these analyses as well. First and by far the most important limitation is that biomarker data have to be analyzed in categories (instead of in a continuous fashion). This requires risk classification examination by categories based on prior studies to guide such risk classification by new biomarkers. Moreover, once the reclassification is performed, relying solely on number or percentage of reclassified subjects could be misleading when examining a new biomarker. Therefore a new method of reclassification has been proposed that includes examining the risk based on discrimination slopes. Each upward movement above the midline from the slope happens for an additional reclassified event and a downward movement for each additional reclassified non-event. By this means, any need for evaluating the disease risk by categories is mitigated. This method is referred to as integrated discrimination improvement or IDI that is a graphical and easily understandable way to reclassify the risk (39, 40).
Figure 2.
Net reclassification index (NRI) calculation
Genetic Biomarkers
Recent genetic studies have shown some consistent loci or genes that are independently associated with higher risk of CVD and with CVD risk factors. A key difference in examining genetic biomarkers compared to other circulating or imaging biomarkers is that genetic markers are present at birth and can be ascertained even prior to birth. Although they are not influenced by environmental factors, gene-environment interactions can sometimes be responsible for development of disease states. Genetic information is also being evaluated to guide drug therapy based on the presence or absence of those markers and their association with outcomes. Several pharmacogenomic assays are now approved by the FDA (41) for clinical use to assess risk of adverse events, mode of drug action and to predict the effect of drug on the disease (42). Changes in the DNA sequence and epigenetic changes resulting in changes to gene expressions and phenotypes have also been associated with CVD traits and disease risk (43). Gene expression studies have been very useful in identifying patterns of cardiac hypertrophy, (44, 45) myocardial infarction, (46) different forms of heart failure (47–50) and even for surveillance of cellular rejection in heart transplant recipients (51).
The classical approach of examining the link between genetic markers and disease outcome has been the linkage approach and association studies. The linkage approach is a family-based approach that utilizes identifying large segments of genome containing millions of DNA bases that are similar among patients with disease of interest within families. Thereafter, further fine mapping identifies a single-gene in those large segments of genome to link with disease. Linkage strategies have been quite effective in mapping single gene disorders with large genetic effects; however they are limited by their design to identify links for polygenic diseases of multifactorial etiology (52). Association strategies differ from linkage strategy because they can be utilized in studying more complex diseases with modest genetic effects. Thus, genome-wide association studies (GWAS), a more recent and popular design that surveys the whole genome to create single nucleotide polymorphism (SNP) maps and databases, have now made it possible to identify clear genetic markers associated with CVD (53–56). The process of examining the strength of genetic markers to assess CVD risk is similarly rigorous as for circulating biomarkers (as above). So far, addition of genetic markers to traditional CVD risk factors marginally improves CVD risk stratification for prognostication (57–59).
Lastly, differences in protein expression from a variety of biological samples such as blood, urine or tissues and the association of such proteins with CVD, (i.e. proteomics) has been explored enormously in the last 2 decades to develop biomarkers (60–62). Large scale databases of cardiac proteins have been created (63) that allow exploration with experimental studies to characterize changes in protein expression and associate them with phenotypes and identify exact physiologic pathways that may help in better assessment of CVD risk (64) and allow further progress in drug discovery and therapeutic approaches in CVD (65–67).
Novel cardiovascular biomarkers under evaluation
There are numerous CVD biomarkers under evaluation and a detailed review is beyond the scope of this review. Several classifications exist currently to classify CVD biomarkers. Most commonly, biomarkers can be grouped based on disease specificity such as biomarkers of heart failure (BNP, N-terminal prohormone of brain natriuretic peptide [NT-proBNP], atrial natriuretic peptide [ANP], ST-2 etc), of atherosclerotic coronary disease (troponin T or I, creatinine phosphokinase-MB etc.), or they can be grouped according to their use such as in acute changes (copeptin, high sensitivity Troponin, galectin-3, ST2) versus in the chronic stage of CVD to estimate prognosis (coronary calcium by CT). Alternatively, CVD biomarkers can be grouped according to the pathologic process they represent, such as inflammation (e.g., C-reactive protein, interleukin 6, Fibrinogen, monocyte chemotactic protein-1, tumor necrosis factor alpha etc) oxidative stress (e.g., isoprostanes), and metabolic (e.g., lipoprotein (a), low-density lipoproteins, high density lipoprotein, ApoB 100, Lipoprotein-associated phospholipase A2, Homocysteine, vitamin D, fibroblast growth factor 23, adiponectin, glycated hemoglobin, haptoglobin etc). In the next section we present some examples of novel biomarkers which are currently being investigated for heart failure and emphasize some of the key concepts influencing their use in clinical practice.
Key Novel Heart Failure Biomarkers
Individual investigators have proposed classification of heart failure biomarkers according to the pathologic process they indicate (68). Previous reviews have described relevant limitations of novel heart failure biomarkers for use as treatment guidance (69) and sex differences when using these biomarkers for clinical use (70). Further consensus statements have recommended establishing a consortium to allow novel biomarkers to be concomitantly analyzed in a pooled sample of randomized clinical trials and hypotheses to be generated for testing each biomarker in biomarker-guided trials (71).
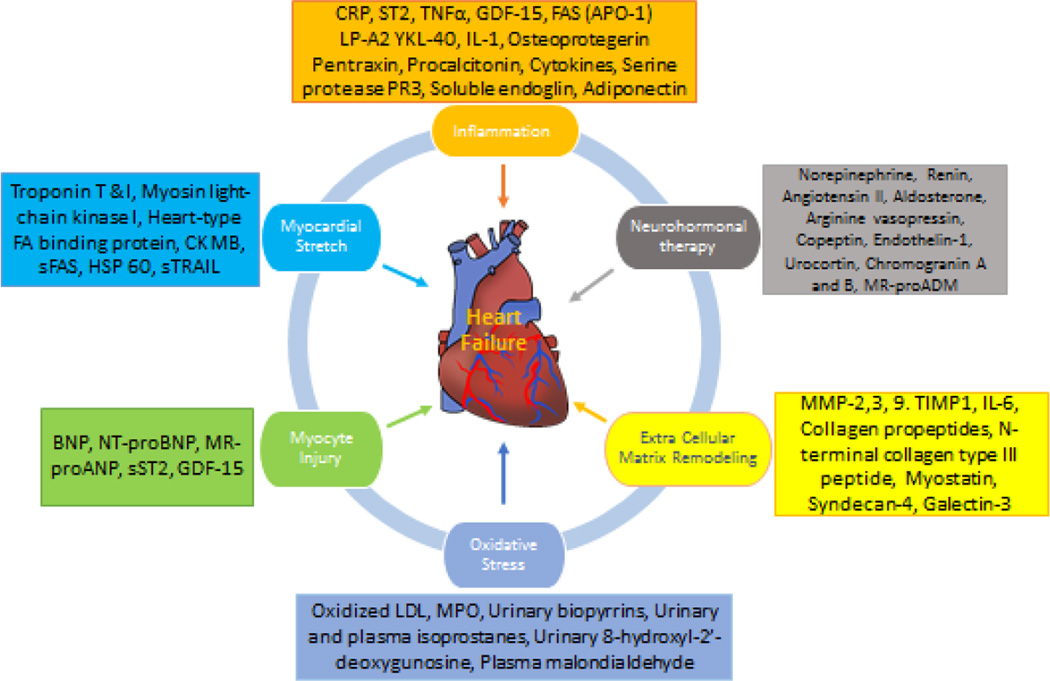
Figure 3 is an attempt to differentiate these heart failure biomarkers according to the pathophysiologic process they are most associated with.
Figure 3.
Classification of heart failure biomarkers according to pathophysiologic processes. (Adapted from Ahmad et al. Nat. Rev. Cardiol. 2012; 9:347–359)
Myocardial stretch leads to production of pro BNP compound that is later broken down into BNP and NT proBNP (inert form). Higher concentration of BNP in the blood of a patient who presents to an emergency room is associated with greater probability of a diagnosis of heart failure. Moreover, higher BNP concentration on admission to the hospital is also associated with greater in-hospital mortality (72). NT proBNP, which is a more stable form of BNP, is also predictive of a diagnosis of heart failure. Medications and other therapies utilized currently to treat heart failure are also known to reduce BNP levels effectively; however with some exceptions (73). It is important to understand, however, that BNP levels are inversely associated with obesity (74, 75), and may also be influenced by presence of kidney disease (76). Circulating ANP on the other hand is overall more unstable in blood compared to BNP or NT proBNP and therefore has a limited use in diagnosis or prognosis. However, a mid-regional ANP, a prohormone isolated from mid region of the molecule (MR-proANP) has shown promise in diagnosis of heart failure in a multinational biomarker study (BACH trial) (77) in acute heart failure patients although its added incremental utility over BNP for diagnostic purposes is yet to be fully proven. So far, the research shows that MR-proANP could be of added advantage for the diagnosis of heart failure in obese, elderly and patients with renal disease when compared to BNP (78). MR-proANP is also being evaluated for prognosis of heart failure as well.
Cardiomyocyte necrosis releases Troponin I or T (cardiac isomers of proteins from troponin-tropomyosin complex) in circulation of an individual and they are typically useful in detection of myocardial ischemia. High sensitive assays of Troponin T and I, however, are also elevated in the blood of patients with severe heart failure and therefore have been appropriately studied for the prediction of heart failure (79, 80) and for prognostication (81–84) in those with established heart failure. Another biomarker that is associated with both ischemic and heart failure is Copeptin, a precursor protein of arginine vasopressin (ADH). Copeptin levels are elevated in the immediate post ischemic period (85) and also correlate with higher risk of death(86) and new-onset heart failure.(87) Some investigators have reported the superiority of copeptin over BNP and NT-proBNP concentrations for predicting death, although the caveat is that these biomarkers are often closely related (88).
Neutrophil gelatinase-associated lipocalin (NGAL) (89), another glycoprotein covalently bound to matrix metaloproteinase-9, is released by renal tubular cells in response to renal inflammation and injury, and it has also shown to offer added prognostic and diagnostic value along with BNP in the GALLANT trial (90). However, subsequent studies with corresponding biomarker data from other heart failure trials did not replicate these findings (91). Therefore, the clinical utility of this biomarker above and beyond other commonly used biomarkers in chronic heart failure patients with renal injury is questionable (92).
Galectin-3 is an exciting biomarker with an important role in development and regulation of cardiac fibrosis and remodeling (93). In patients diagnosed with acute decompensated heart failure, blood Galectin-3 level has shown to be predictive of mortality on short-term follow up. In fact, investigators have also suggested the superiority of galectin-3 (94) or enhanced predictive power for mortality when used along with BNP (95) levels in patients with both preserved and reduced left ventricular ejection fraction (96, 97). Overall, researchers currently underscore the added advantage of using a multimarker approach in heart failure; however, the independent use of Galectin-3 alone in heart failure patients is not as well supported by literature for the prediction of prognosis (93). Other markers of extracellular matrix such as metalloproteins which degrade collagen (MMPs), specific tissue inhibitors of metalloproteins (TIMPs), procollagen type III amino-terminal propeptide (PIIINP), or procollagen type I carboxy terminal peptide (PICP) that have been traditionally related to hypertensive heart disease(98) are currently explored as biomarkers with implications for assessing disease severity, prognosis and response to treatment among patients with heart failure with preserved ejection fraction (HFpEF) (99).
Additionally, higher levels of blood Procalcitonin, an acute phase reactant, have been associated with a greater likelihood of the presence of infection in patients with heart failure. Therefore, procalcitonin can sometimes be useful for excluding infections or pneumonia in patients seen in the emergency room with shortness of breath who are suspected to have a diagnosis of acute on chronic heart failure.
ST-2 is a receptor from interleukin family (IL-33) with two gene forms – soluble (sST2) and transmembrane form (100). Like other biomarkers, blood ST-2 levels are also shown to predict mortality and new onset heart failure (101). Researchers have also examined the predictive ability of ST-2 levels complementary to other traditional risk factors and NT-proBNP levels in ST-elevation myocardial infarction patients (102). It also has a role over traditional heart failure risk factors for determining prognosis (103, 104). These findings have led researchers to explore the use of ST-2 as part of a multimarker approach for assessing the prognosis of patients with heart failure (105).
Mid-regional pro-adernomedullin (MR-proADM) is a stable prohormone fragment of adernomedullin, a vasodilatory peptide, and elevated circulating levels are strongly associated with the presence of chronic heart failure (106–108). MR-proADM has been shown to be superior to both BNP and NT-proBNP in predicting 90-day mortality among patients with dyspnea and heart failure (77).
Growth differentiation factor – 15 – is classified as a biomarker with anti-hypertrophic effects (apoptosis) (109, 110) and investigators have linked the elevated levels of this biomarker to assess prognosis in chronic heart failure patients (111, 112) and among community dwelling adults as a predictor of all-cause mortality, including non-cardiovascular mortality above and beyond the information provided by blood NT-proBNP and C-reactive protein levels (113).
Lastly, small non coding RNAs i.e. micro RNAs(114) are known to play a significant role in regulation of cardiac hypertrophy (e.g. microRNA-133)(115), fibrosis [e.g. microRNA-21 (116) microRNA-29 (117)] and heart failure (118, 119). These associations of microRNA to cardiac hypertrophy and cardiac fibrosis in several studies also makes them an attractive biomarker to guide future heart failure therapy (120–124). One study has even found that various combinations of these microRNAs can potentially be used to differentiate heart failure with preserved ejection fraction from reduced ejection fraction (125). In addition, long non coding RNA (126) which were first detected in blood in 2008, have since also been evaluated by several investigators in relation to heart failure prognosis (127). However, at the present time, concerns regarding variability in measurement of microRNA, added value of both microRNAs and long noncoding RNAs to established heart failure biomarkers, and unclear pathophysiologic role in heart failure or specificity for development of heart failure are undermining their utility to be of clinical use.
Several other novel biomarkers are also being explored as potential heart failure biomarkers, such as osteoprotegerin (128), osteopontin (129), adiponectin (130), neopterin (131), cardiotrophin-1 (132), glycoprotein 130 (133) and red cell width (RDW) (134); however, some of these are yet to be fully investigated in larger, non-selected samples for their association with incident heart failure.
Conclusion
In summary, there are numerous CVD biomarkers that are currently available and that have clinical use as diagnostic, prognostic or predictive biomarkers. Several of these biomarkers have to be vigorously tested to assess their clinical utility across a varying spectrum of patients with atherosclerotic CVD and who have with different comorbidities. The demonstration of the validity and clinical utility of any given biomarker across different sets of patients is essential prior to its routine use in clinical practice. Desirable characteristics of any CVD biomarker include that its measurement should be easy, preferably at point-of-care over a short time period with adequate precision and accuracy, and the demonstration of low intra-individual variability. Biomarker may be able to reflect pathophysiologic process of heart disease, and also may be able to provide meaningful information about prognosis and assist guide clinical decision making without duplicating any information that is already available clinically. Biomarkers evaluating prognostic outcomes should report discrimination, calibration and reclassification in patients by evaluating statistical models with and without the biomarker in order to demonstrate their added value over traditional and other commonly used biomarkers. Genetic biomarkers are at the forefront of being evaluated for added utility and they too need rigorous assessments to evaluate their relations with the CVD and for their added advantage over traditional risk factors. Utilizing biomarkers as surrogate end points for predictive and prognostic values in clinical trials will likely dictate our future of CVD treatment, and will also open up avenues to evaluate biomarkers as possible targets for drug delivery and development.
Acknowledgments
This work was supported by contract NO1 25195 and HHSN268201500001I from the National Health Institute
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: None
References
- 1. [accessed on July 23, 2016]; http://www.who.int/mediacentre/factsheets/fs310/en/
- 2.Balagopal PB, de Ferranti SD, Cook S, Daniels SR, Gidding SS, Hayman LL, et al. Nontraditional risk factors and biomarkers for cardiovascular disease: mechanistic, research, and clinical considerations for youth: a scientific statement from the American Heart Association. Circulation. 2011;123:2749–2769. doi: 10.1161/CIR.0b013e31821c7c64. [DOI] [PubMed] [Google Scholar]
- 3.Goff DC, Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB, Gibbons R, et al. 2013 ACC/AHA Guideline on the Assessment of Cardiovascular Risk: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S49–S73. doi: 10.1161/01.cir.0000437741.48606.98. [DOI] [PubMed] [Google Scholar]
- 4.Thygesen K, Alpert JS, White HD, et al. on behalf of the Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction, TASK FORCE MEMBERS: Chairpersons: Kristian Thygesen (Denmark), Biomarker Group: Allan S. Jaffe CU. Universal Definition of Myocardial Infarction. Circulation. 2007;116:2634–2653. doi: 10.1161/CIRCULATIONAHA.107.187397. [DOI] [PubMed] [Google Scholar]
- 5.Vasan RS. Biomarkers of Cardiovascular Disease: Molecular Basis and Practical Considerations. Circulation. 2006;113:2335–2362. doi: 10.1161/CIRCULATIONAHA.104.482570. [DOI] [PubMed] [Google Scholar]
- 6.Atkinson AJ, Colburn WA, DeGruttola VG, DeMets DL, Downing GJ, Hoth DF, et al. Biomarkers and surrogate endpoints: Preferred definitions and conceptual framework. Clin Pharmacol Ther. 2001;69:89–95. doi: 10.1067/mcp.2001.113989. [DOI] [PubMed] [Google Scholar]
- 7.Hlatky MA, Greenland P, Arnett DK, Ballantyne CM, Criqui MH, Elkind MS, et al. Criteria for evaluation of novel markers of cardiovascular risk: a scientific statement from the American Heart Association. Circulation. 2009;119:2408–2416. doi: 10.1161/CIRCULATIONAHA.109.192278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang TJ. Assessing the Role of Circulating, Genetic, and Imaging Biomarkers in Cardiovascular Risk Prediction. Circulation. 2011;123:551–565. doi: 10.1161/CIRCULATIONAHA.109.912568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. [accessed July 23, 2016]; https://www.whitehouse.gov/precision-medicine.
- 10.Collins FS, Varmus H. A New Initiative on Precision Medicine. N Engl J Med. 2015;372:793–795. doi: 10.1056/NEJMp1500523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.La Thangue NB, Kerr DJ. Predictive biomarkers: a paradigm shift towards personalized cancer medicine. Nat Rev Clin Oncol. 2011;8:587–596. doi: 10.1038/nrclinonc.2011.121. [DOI] [PubMed] [Google Scholar]
- 12.Malottki K, Biswas M, Deeks JJ, Riley RD, Craddock C, Johnson P, et al. Stratified medicine in European Medicines Agency licensing: a systematic review of predictive biomarkers. BMJ Open. 2014:4. doi: 10.1136/bmjopen-2013-004188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tajik P, Zwinderman AH, Mol BW, Bossuyt PM. Trial Designs for Personalizing Cancer Care: A Systematic Review and Classification. Clinical Cancer Research. 2013;19:4578–4588. doi: 10.1158/1078-0432.CCR-12-3722. [DOI] [PubMed] [Google Scholar]
- 14. [accessed July 23, 2016]; https://clinicaltrials.gov/ct2/show/NCT02554890/
- 15.Deeks JJ, Altman DG. Diagnostic tests 4: likelihood ratios. BMJ. 2004;329:168–169. doi: 10.1136/bmj.329.7458.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Solberg HE. International Federation of Clinical Chemistry (IFCC), Scientific Committee, Clinical Section, Expert Panel on Theory of Reference Values, and International Committee for Standardization in Haematology (ICSH), Standing Committee on Reference Values. Approved Recommendation (1986) on the theory of reference values. Part 1. The concept of reference values. J Clin Chem Clin Biochem. 1987;25:337–342. [PubMed] [Google Scholar]
- 17.Lott JA, Mitchell LC, Moeschberger ML, Sutherland DE. Estimation of reference ranges: how many subjects are needed? Clin Chem. 1992;38:648–650. [PubMed] [Google Scholar]
- 18.Morrow DA, Antman EM. Evaluation of High-Sensitivity Assays for Cardiac Troponin. Clin Chem. 2009;55:5–8. doi: 10.1373/clinchem.2008.117218. [DOI] [PubMed] [Google Scholar]
- 19.Sunderman FW. Current Concepts of “Normal Values,” “Reference Values,” and “Discrimination Values” in Clinical Chemistry. Clin Chem. 1975;21:1873–1877. [PubMed] [Google Scholar]
- 20.Gupta M, Singh N, Verma S. South Asians and Cardiovascular Risk: What Clinicians Should Know. Circulation. 2006;113:e924–e929. doi: 10.1161/CIRCULATIONAHA.105.583815. [DOI] [PubMed] [Google Scholar]
- 21.Zeng Q, He Y, Dong S, Zhao X, Chen Z, Song Z, et al. Optimal cut-off values of BMI, waist circumference and waist:height ratio for defining obesity in Chinese adults. British Journal of Nutrition. 2014;112:1735–1744. doi: 10.1017/S0007114514002657. [DOI] [PubMed] [Google Scholar]
- 22.Kaplan RC, Aviles-Santa ML, Parrinello CM, Hanna DB, Jung M, Castaneda SF, et al. Body Mass Index, Sex, and Cardiovascular Disease Risk Factors Among Hispanic/Latino Adults: Hispanic Community Health Study/Study of Latinos. J Am Heart Assoc. 2014;3:e000923. doi: 10.1161/JAHA.114.000923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.D’Agostino RB, Sr, Grundy S, Sullivan LM, Wilson P. Validation of the Framingham coronary heart disease prediction scores: results of a multiple ethnic groups investigation. JAMA. 2001;286:180–187. doi: 10.1001/jama.286.2.180. [DOI] [PubMed] [Google Scholar]
- 24.D’Agostino RB, Sr, Grundy S, Sullivan LM, Wilson P for the CHD Risk Prediction Group. Validation of the framingham coronary heart disease prediction scores: Results of a multiple ethnic groups investigation. JAMA. 2001;286:180–187. doi: 10.1001/jama.286.2.180. [DOI] [PubMed] [Google Scholar]
- 25.Lemeshow STAN, Hosmer DW. A Review of Goodness of fit Statistics for use in the Development of Logistic Regression Models. Am J Epidemiol. 1982;115:92–106. doi: 10.1093/oxfordjournals.aje.a113284. [DOI] [PubMed] [Google Scholar]
- 26.Wade ANGI. Derivation versus validation. Archives of Disease in Childhood. 2000;83:459–460. doi: 10.1136/adc.83.6.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig LM, et al. Towards Complete and Accurate Reporting of Studies of Diagnostic Accuracy: The STARD Initiative. Clin Chem. 2003;49:1–6. doi: 10.1373/49.1.1. [DOI] [PubMed] [Google Scholar]
- 28.McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM. Reporting Recommendations for Tumor Marker Prognostic Studies. J Clin Oncol. 2005;23:9067–9072. doi: 10.1200/JCO.2004.01.0454. [DOI] [PubMed] [Google Scholar]
- 29.Ware JH. The Limitations of Risk Factors as Prognostic Tools. N Engl J Med. 2006;355:2615–2617. doi: 10.1056/NEJMp068249. [DOI] [PubMed] [Google Scholar]
- 30.Kaptoge S, Di Angelantonio E, Pennells L, Wood AM, White IR, Gao P, et al. Emerging Risk Factors Collaboration. C-Reactive Protein, Fibrinogen, and Cardiovascular Disease Prediction. N Engl J Med. 2012;367:1310–1320. doi: 10.1056/NEJMoa1107477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang TJ, Gona P, Larson MG, Tofler GH, Levy D, Newton-Cheh C, et al. Multiple Biomarkers for the Prediction of First Major Cardiovascular Events and Death. N Engl J Med. 2006;355:2631–2639. doi: 10.1056/NEJMoa055373. [DOI] [PubMed] [Google Scholar]
- 32.Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, et al. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464–470. doi: 10.1053/jhep.2001.22172. [DOI] [PubMed] [Google Scholar]
- 33.Cox DR. Regression models and life tables. J R Stat Soc. 1972;34:187–220. [Google Scholar]
- 34.Baker SG. The Central Role of Receiver Operating Characteristic (ROC) Curves in Evaluating Tests for the Early Detection of Cancer. J Natl Cancer Inst. 2003;95:511–515. doi: 10.1093/jnci/95.7.511. [DOI] [PubMed] [Google Scholar]
- 35.Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143:29–36. doi: 10.1148/radiology.143.1.7063747. [DOI] [PubMed] [Google Scholar]
- 36.Pepe MS. An Interpretation for the ROC Curve and Inference Using GLM Procedures. Biometrics. 2000;56:352–359. doi: 10.1111/j.0006-341x.2000.00352.x. [DOI] [PubMed] [Google Scholar]
- 37.Cook NR. Use and Misuse of the Receiver Operating Characteristic Curve in Risk Prediction. Circulation. 2007;115:928–935. doi: 10.1161/CIRCULATIONAHA.106.672402. [DOI] [PubMed] [Google Scholar]
- 38.Pencina MJ, D’Agostino RB, Sr, D’Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172. doi: 10.1002/sim.2929. [DOI] [PubMed] [Google Scholar]
- 39.Pencina MJ, D’Agostino RB, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Statist Med. 2011;30:11–21. doi: 10.1002/sim.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Steyerberg EW, Vickers AJ, Cook NR, Gerds T, Gonen M, Obuchowski N, et al. Assessing the performance of prediction models: a framework for traditional and novel measures. Epidemiology. 2010;21:128–138. doi: 10.1097/EDE.0b013e3181c30fb2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. [accessed July 23, 2016]; http://www.fda.gov/drugs/scienceresearch/researchareas/pharmacogenetics/ucm083378.htm/
- 42.Roden DM. Cardiovascular pharmacogenomics: current status and future directions. J Hum Genet. 2015 doi: 10.1038/jhg.2015.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Calore M, De Windt LJ, Rampazzo A. Genetics meets epigenetics: Genetic variants that modulate noncoding RNA in cardiovascular diseases. J Mol Cell Cardiol. 2015;89(Pt A):27–34. doi: 10.1016/j.yjmcc.2015.10.028. [DOI] [PubMed] [Google Scholar]
- 44.Friddle CJ, Koga T, Rubin EM, Bristow J. Expression profiling reveals distinct sets of genes altered during induction and regression of cardiac hypertrophy. Proc Natl Acad Sci. 2000;97:6745–6750. doi: 10.1073/pnas.100127897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sheehy SP, Huang S, Parker KK. Time-Warped Comparison of Gene Expression in Adaptive and Maladaptive Cardiac Hypertrophy. Circ Cardiovasc Genet. 2009;2:116–124. doi: 10.1161/CIRCGENETICS.108.806935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stanton LW, Garrard LJ, Damm D, Garrick BL, Lam A, Kapoun AM, et al. Altered Patterns of Gene Expression in Response to Myocardial Infarction. Circ. Res. 2000;86:939–945. doi: 10.1161/01.res.86.9.939. [DOI] [PubMed] [Google Scholar]
- 47.Kittleson MM, Minhas KM, Irizarry RA, Ye SQ, Edness G, Breton E, et al. Gene expression analysis of ischemic and nonischemic cardiomyopathy: shared and distinct genes in the development of heart failure. Physiol Genomics. 2005;21:299–307. doi: 10.1152/physiolgenomics.00255.2004. [DOI] [PubMed] [Google Scholar]
- 48.Yang J, Moravec CS, Sussman MA, DiPaola NR, Fu D, Hawthorn L, et al. Decreased SLIM1 Expression and Increased Gelsolin Expression in Failing Human Hearts Measured by High-Density Oligonucleotide Arrays. Circulation. 2000;102:3046–3052. doi: 10.1161/01.cir.102.25.3046. [DOI] [PubMed] [Google Scholar]
- 49.Kittleson MM, Ye SQ, Irizarry RA, Minhas KM, Edness G, Conte JV, et al. Identification of a Gene Expression Profile That Differentiates Between Ischemic and Nonischemic Cardiomyopathy. Circulation. 2004;110:3444–3451. doi: 10.1161/01.CIR.0000148178.19465.11. [DOI] [PubMed] [Google Scholar]
- 50.Hwang JJ, Allen PD, Tseng GC, Lam CW, Fananapazir L, Dzau VJ, et al. Microarray gene expression profiles in dilated and hypertrophic cardiomyopathic end-stage heart failure. Physiol Genomics. 2002;10:31–44. doi: 10.1152/physiolgenomics.00122.2001. [DOI] [PubMed] [Google Scholar]
- 51.Pham MX, Teuteberg JJ, Kfoury AG, Starling RC, Deng MC, Cappola TP, et al. Gene-Expression Profiling for Rejection Surveillance after Cardiac Transplantation. N Engl J Med. 2010;362:1890–1900. doi: 10.1056/NEJMoa0912965. [DOI] [PubMed] [Google Scholar]
- 52.Genetic dissection of complex traits. Science. 1994;265:2037. doi: 10.1126/science.8091226. [DOI] [PubMed] [Google Scholar]
- 53.Hirschhorn JN. Genomewide Association Studies GÇö Illuminating Biologic Pathways. N Engl J Med. 2009;360:1699–1701. doi: 10.1056/NEJMp0808934. [DOI] [PubMed] [Google Scholar]
- 54.Samani NJ, Erdmann J, Hall AS, Hengstenberg C, Mangino M, Mayer B, et al. Genomewide Association Analysis of Coronary Artery Disease. N Engl J Med. 2007;357:443–453. doi: 10.1056/NEJMoa072366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Genome-wide association of early-onset myocardial infarction with single nucleotide polymorphisms and copy number variants. Nat Genet. 2009;41:334–341. doi: 10.1038/ng.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hardy J, Singleton A. Genomewide Association Studies and Human Disease. N Engl J Med. 2009;360:1759–1768. doi: 10.1056/NEJMra0808700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Paynter NP, Chasman DI, Buring JE, Shiffman D, Cook NR, Ridker PM. Cardiovascular Disease Risk Prediction With and Without Knowledge of Genetic Variation at Chromosome 9p21.3. Ann Intern Med. 2009;150:65–72. doi: 10.7326/0003-4819-150-2-200901200-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ganesh SK, Arnett DK, Assimes TL, Basson CT, Chakravarti A, Ellinor PT, et al. Genetics and Genomics for the Prevention and Treatment of Cardiovascular Disease: Update: A Scientific Statement From the American Heart Association. Circulation. 2013;128:2813–2851. doi: 10.1161/01.cir.0000437913.98912.1d. [DOI] [PubMed] [Google Scholar]
- 59.he CARD. A comprehensive 1000 Genomes-based genome-wide association meta-analysis of coronary artery disease. Nat Genet. 2015;47:1121–1130. doi: 10.1038/ng.3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McGregor E, Dunn MJ. Proteomics of the Heart: Unraveling Disease. Circ Res. 2006;98:309–321. doi: 10.1161/01.RES.0000201280.20709.26. [DOI] [PubMed] [Google Scholar]
- 61.Ray S, Reddy PJ, Choudhary S, Raghu D, Srivastava S. Emerging nanoproteomics approaches for disease biomarker detection: A current perspective. J Proteomics. 2011;74:2660–2681. doi: 10.1016/j.jprot.2011.04.027. [DOI] [PubMed] [Google Scholar]
- 62.Wilson PWF. Progressing From Risk Factors to Omics. Circulation: Circ Cardiovasc Genet. 2008;1:141–146. doi: 10.1161/CIRCGENETICS.108.815605. [DOI] [PubMed] [Google Scholar]
- 63. [Accessed July 19, 2016]; http://userpage.chemie.fu-berlin.de/~pleiss/
- 64.Ronsein GE, Pamir N, von Haller PD, Kim DS, Oda MN, Jarvik GP, et al. Parallel reaction monitoring (PRM) and selected reaction monitoring (SRM) exhibit comparable linearity, dynamic range and precision for targeted quantitative HDL proteomics. J Proteomics. 2015;113:388–399. doi: 10.1016/j.jprot.2014.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Napoli C, Zullo A, Picascia A, Infante T, Mancini FP. Recent advances in proteomic technologies applied to cardiovascular disease. J Cell Biochem. 2013;114:7–20. doi: 10.1002/jcb.24307. [DOI] [PubMed] [Google Scholar]
- 66.Yao C, Chen BH, Joehanes R, Otlu B, Zhang X, Liu C, et al. Integromic Analysis of Genetic Variation and Gene Expression Identifies Networks for Cardiovascular Disease Phenotypes. Circulation. 2015;131:536–549. doi: 10.1161/CIRCULATIONAHA.114.010696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Huan T, Rong J, Tanriverdi K, Meng Q, Bhattacharya A, McManus DD, et al. Dissecting the Roles of MicroRNAs in Coronary Heart Disease via Integrative Genomic Analyses. Arterioscler Thromb Vasc Biol. 2015;35:1011–1021. doi: 10.1161/ATVBAHA.114.305176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Braunwald E. Biomarkers in Heart Failure. N Engl J Med. 2008;358:2148–2159. doi: 10.1056/NEJMra0800239. [DOI] [PubMed] [Google Scholar]
- 69.Maisel A. Biomonitoring and Biomarker-Guided Therapy: The Next Step in Heart Failure and Biomarker Research. J Am Coll Cardiol. 2011;58:1890–1892. doi: 10.1016/j.jacc.2011.07.032. [DOI] [PubMed] [Google Scholar]
- 70.Daniels LB, Maisel AS. Cardiovascular biomarkers and sex: the case for women. Nat Rev Cardiol. 2015;12:588–596. doi: 10.1038/nrcardio.2015.105. [DOI] [PubMed] [Google Scholar]
- 71.Ahmad T, Fiuzat M, Pencina MJ, Geller NL, Zannad F, Cleland JGF, et al. Charting a Roadmap for Heart Failure Biomarker Studies. JACC: Heart Fail. 2014;2:477–488. doi: 10.1016/j.jchf.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fonarow GC, Peacock WF, Phillips CO, Givertz MM, Lopatin M. Admission B-Type Natriuretic Peptide Levels and In-Hospital Mortality in Acute Decompensated Heart Failure. J Am Coll Cardiol. 2007;49:1943–1950. doi: 10.1016/j.jacc.2007.02.037. [DOI] [PubMed] [Google Scholar]
- 73.McMurray JJV, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, et al. Angiotensin+Neprilysin Inhibition versus Enalapril in Heart Failure. N Engl J Med. 2014;371:993–1004. doi: 10.1056/NEJMoa1409077. [DOI] [PubMed] [Google Scholar]
- 74.Cheng S, Fox CS, Larson MG, Massaro JM, McCabe EL, Khan AM, et al. Relation of Visceral Adiposity to Circulating Natriuretic Peptides in Ambulatory Individuals. Am J Cardiol. 108:979–984. doi: 10.1016/j.amjcard.2011.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fox ER, Musani SK, Bidulescu A, Nagarajarao HS, Samdarshi TE, Gebreab SY, et al. Relation of Obesity to Circulating B-Type Natriuretic Peptide Concentrations in Blacks: The Jackson Heart Study. Circulation. 2011;124:1021–1027. doi: 10.1161/CIRCULATIONAHA.110.991943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Takase H, Dohi Y. Kidney function crucially affects B-type natriuretic peptide (BNP), N-terminal proBNP and their relationship. Eur J Clin Invest. 2014;44:303–308. doi: 10.1111/eci.12234. [DOI] [PubMed] [Google Scholar]
- 77.Maisel A, Mueller C, Nowak R, Peacock WF, Landsberg JW, Ponikowski P, et al. Mid-region pro-hormone markers for diagnosis and prognosis in acute dyspnea: results from the BACH (Biomarkers in Acute Heart Failure) trial. J Am Coll Cardiol. 2010;55:2062–2076. doi: 10.1016/j.jacc.2010.02.025. [DOI] [PubMed] [Google Scholar]
- 78.Khan SQ, Dhillon O, Kelly D, Squire IB, Struck J, Quinn P, et al. Plasma N-terminal B-Type natriuretic peptide as an indicator of long-term survival after acute myocardial infarction: comparison with plasma midregional pro-atrial natriuretic peptide: the LAMP (Leicester Acute Myocardial Infarction Peptide) study. J Am Coll Cardiol. 2008;51:1857–1864. doi: 10.1016/j.jacc.2008.01.041. [DOI] [PubMed] [Google Scholar]
- 79.deFilippi CR, de Lemos JA, Christenson RH. ASsociation of serial measures of cardiac troponin t using a sensitive assay with incident heart failure and cardiovascular mortality in older adults. JAMA. 2010;304:2494–2502. doi: 10.1001/jama.2010.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Saunders JT, Nambi V, de Lemos JA, Chambless LE, Virani SS, Boerwinkle E, et al. Cardiac Troponin T Measured by a Highly Sensitive Assay Predicts Coronary Heart Disease, Heart Failure, and Mortality in the Atherosclerosis Risk in Communities Study. Circulation. 2011;123:1367–1376. doi: 10.1161/CIRCULATIONAHA.110.005264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Horwich TB, Patel J, MacLellan WR, Fonarow GC. Cardiac Troponin I Is Associated With Impaired Hemodynamics, Progressive Left Ventricular Dysfunction, and Increased Mortality Rates in Advanced Heart Failure. Circulation. 2003;108:833–838. doi: 10.1161/01.CIR.0000084543.79097.34. [DOI] [PubMed] [Google Scholar]
- 82.Masson S, Anand I, Favero C, Barlera S, Vago T, Bertocchi F, et al. Serial Measurement of Cardiac Troponin T Using a Highly Sensitive Assay in Patients With Chronic Heart Failure: Data From 2 Large Randomized Clinical Trials. Circulation. 2012;125:280–288. doi: 10.1161/CIRCULATIONAHA.111.044149. [DOI] [PubMed] [Google Scholar]
- 83.Peacock WF, De Marco T, Fonarow GC, Diercks D, Wynne J, Apple FS, et al. Cardiac Troponin and Outcome in Acute Heart Failure. N Engl J Med. 2008;358:2117–2126. doi: 10.1056/NEJMoa0706824. [DOI] [PubMed] [Google Scholar]
- 84.Xue Y, Clopton P, Peacock WF, Maisel AS. Serial changes in high-sensitive troponin I predict outcome in patients with decompensated heart failure. Eur J Heart Fail. 2011;13:37–42. doi: 10.1093/eurjhf/hfq210. [DOI] [PubMed] [Google Scholar]
- 85.Khan SQ, Dhillon OS, O’Brien RJ, Struck J, Quinn PA, Morgenthaler NG, et al. C-Terminal Provasopressin (Copeptin) as a Novel and Prognostic Marker in Acute Myocardial Infarction: Leicester Acute Myocardial Infarction Peptide (LAMP) Study. Circulation. 2007;115:2103–2110. doi: 10.1161/CIRCULATIONAHA.106.685503. [DOI] [PubMed] [Google Scholar]
- 86.Maisel A, Xue Y, Shah K, Mueller C, Nowak R, Peacock WF, et al. Increased 90-Day Mortality in Patients With Acute Heart Failure With Elevated Copeptin: Secondary Results From the Biomarkers in Acute Heart Failure (BACH) Study. Circ Heart Fail. 2011;4:613–620. doi: 10.1161/CIRCHEARTFAILURE.110.960096. [DOI] [PubMed] [Google Scholar]
- 87.Kelly D, Squire IB, Khan SQ, Quinn P, Struck J, Morgenthaler NG, et al. C-Terminal Provasopressin (Copeptin) is Associated With Left Ventricular Dysfunction, Remodeling, and Clinical Heart Failure in Survivors of Myocardial Infarction. J Card Fail. 2008;14:739–745. doi: 10.1016/j.cardfail.2008.07.231. [DOI] [PubMed] [Google Scholar]
- 88.Neuhold S, Huelsmann M, Strunk G, Stoiser B, Struck J, Morgenthaler NG, et al. Comparison of Copeptin, B-Type Natriuretic Peptide, and Amino-Terminal Pro-B-Type Natriuretic Peptide in Patients With Chronic Heart Failure: Prediction of Death at Different Stages of the Disease. J Am Coll Cardiol. 2008;52:266–272. doi: 10.1016/j.jacc.2008.03.050. [DOI] [PubMed] [Google Scholar]
- 89.Kjeldsen L, Johnsen AH, Sengelov H, Borregaard N. Isolation and primary structure of NGAL, a novel protein associated with human neutrophil gelatinase. J Biol Chem. 1993;268:10425–10432. [PubMed] [Google Scholar]
- 90.Maisel AS, Mueller C, Fitzgerald R, Brikhan R, Hiestand BC, Iqbal N, et al. Prognostic utility of plasma neutrophil gelatinase-associated lipocalin in patients with acute heart failure: The NGAL EvaLuation Along with B-type NaTriuretic Peptide in acutely decompensated heart failure (GALLANT) trial. Eur J Heart Fail. 2011;13:846–851. doi: 10.1093/eurjhf/hfr087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nymo SH, Ueland T, Askevold ET, Flo TH, Kjekshus J, Hulthe J, et al. The association between neutrophil gelatinase-associated lipocalin and clinical outcome in chronic heart failure: results from CORONA. J Intern Med. 2012;271:436–443. doi: 10.1111/j.1365-2796.2011.02503.x. [DOI] [PubMed] [Google Scholar]
- 92.Shrestha K, Borowski AG, Troughton RW, Thomas JD, Klein AL, Tang WHW. Renal Dysfunction Is a Stronger Determinant of Systemic Neutrophil Gelatinase Associated Lipocalin Levels Than Myocardial Dysfunction in Systolic Heart Failure. J Card Fail. 2011;17:472–478. doi: 10.1016/j.cardfail.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Srivatsan V, George M, Shanmugam E. Utility of galectin-3 as a prognostic biomarker in heart failure: where do we stand? Eur J Prev Cardiol. 2015 Sep;22(9):1096–1110. doi: 10.1177/2047487314552797. [DOI] [PubMed] [Google Scholar]
- 94.van Kimmenade RR, Januzzi JL, Jr, Ellinor PT, Sharma UC, Bakker JA, Low AF, et al. Utility of Amino-Terminal Pro-Brain Natriuretic Peptide, Galectin-3, and Apelin for the Evaluation of Patients With Acute Heart Failure. J Am Coll Cardiol. 2006;48:1217–1224. doi: 10.1016/j.jacc.2006.03.061. [DOI] [PubMed] [Google Scholar]
- 95.de Boer RA, Lok DJA, Jaarsma T, van der Meer P, Voors AA, Hillege HL, et al. Predictive value of plasma galectin-3 levels in heart failure with reduced and preserved ejection fraction. Ann Med. 2010;43:60–68. doi: 10.3109/07853890.2010.538080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shah RV, Chen-Tournoux AA, Picard MH, van Kimmenade RRJ, Januzzi JL. Galectin-3, cardiac structure and function, and long-term mortality in patients with acutely decompensated heart failure. Eur J Heart Fail. 2010;12:826–832. doi: 10.1093/eurjhf/hfq091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Edelmann F, Holzendorf V, Wachter R, Nolte K, Schmidt AG, Kraigher-Krainer E, et al. Galectin-3 in patients with heart failure with preserved ejection fraction: results from the Aldo-DHF trial. Eur J Heart Fail. 2014 doi: 10.1002/ejhf.203. n/a. [DOI] [PubMed] [Google Scholar]
- 98.Dhingra R, Pencina MJ, Schrader P, Wang TJ, Levy D, Pencina K, et al. Relations of matrix remodeling biomarkers to blood pressure progression and incidence of hypertension in the community. Circulation. 2009;119:1101–1107. doi: 10.1161/CIRCULATIONAHA.108.821769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.O’Meara E, de Denus S, Rouleau JL, Desai A. Circulating Biomarkers in Patients with Heart Failure and Preserved Ejection Fraction. Curr Heart Fail Rep. 2013;10:350–358. doi: 10.1007/s11897-013-0160-x. [DOI] [PubMed] [Google Scholar]
- 100.Sanada S, Hakuno D, Higgins LJ, Schreiter ER, McKenzie ANJ, Lee RT. IL-33 and ST2 comprise a critical biomechanically induced and cardioprotective signaling system. J Clin Invest. 2007;117:1538–1549. doi: 10.1172/JCI30634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shimpo M, Morrow DA, Weinberg EO, Sabatine MS, Murphy SA, Antman EM, et al. Serum Levels of the Interleukin-1 Receptor Family Member ST2 Predict Mortality and Clinical Outcome in Acute Myocardial Infarction. Circulation. 2004;109:2186–2190. doi: 10.1161/01.CIR.0000127958.21003.5A. [DOI] [PubMed] [Google Scholar]
- 102.Sabatine MS, Morrow DA, Higgins LJ, MacGillivray C, Guo W, Bode C, et al. Complementary Roles for Biomarkers of Biomechanical Strain ST2 and N-Terminal Prohormone B-Type Natriuretic Peptide in Patients With ST-Elevation Myocardial Infarction. Circulation. 2008;117:1936–1944. doi: 10.1161/CIRCULATIONAHA.107.728022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Januzzi JL, Jr, Peacock WF, Maisel AS, Chae CU, Jesse RL, Baggish AL, et al. Measurement of the Interleukin Family Member ST2 in Patients With Acute Dyspnea: Results From the PRIDE (Pro-Brain Natriuretic Peptide Investigation of Dyspnea in the Emergency Department) Study. J Am Coll Cardiol. 2007;50:607–613. doi: 10.1016/j.jacc.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 104.Shah RV, Chen-Tournoux AA, Picard MH, van Kimmenade RRJ, Januzzi JL. Serum Levels of the Interleukin-1 Receptor Family Member ST2, Cardiac Structure and Function, and Long-Term Mortality in Patients With Acute Dyspnea. Circ Heart Fail. 2009;2:311–319. doi: 10.1161/CIRCHEARTFAILURE.108.833707. [DOI] [PubMed] [Google Scholar]
- 105.Pascual-Figal DA, Manzano-Fernandez S, Boronat M, Casas T, Garrido IP, Bonaque JC, et al. Soluble ST2, high-sensitivity troponin T- and N-terminal pro-B-type natriuretic peptide: complementary role for risk stratification in acutely decompensated heart failure. Eur J Heart Fail. 2011;13:718–725. doi: 10.1093/eurjhf/hfr047. [DOI] [PubMed] [Google Scholar]
- 106.Jougasaki M, Wei CM, McKinley LJ, Burnett JC., Jr Elevation of circulating and ventricular adrenomedullin in human congestive heart failure. Circulation. 1995;92:286–289. doi: 10.1161/01.cir.92.3.286. [DOI] [PubMed] [Google Scholar]
- 107.Nishikimi T, Saito Y, Kitamura K, Ishimitsu T, Eto T, Kangawa K, et al. Increased plasma levels of adrenomedullin in patients with heart failure. J Am Coll Cardiol. 1995;26:1424–1431. doi: 10.1016/0735-1097(95)00338-X. [DOI] [PubMed] [Google Scholar]
- 108.Jougasaki M, Rodeheffer RJ, Redfield MM, Yamamoto K, Wei CM, McKinley LJ, et al. Cardiac secretion of adrenomedullin in human heart failure. J Clin Invest. 1996;97:2370–2376. doi: 10.1172/JCI118680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Schlittenhardt D, Schober A, Strelau J, Bonaterra G, Schmiedt W, Unsicker K, et al. Involvement of growth differentiation factor-15/macrophage inhibitory cytokine-1 (GDF-15/MIC-1) in oxLDL-induced apoptosis of human macrophages in vitro and in arteriosclerotic lesions. Cell Tissue Res. 2004;318:325–333. doi: 10.1007/s00441-004-0986-3. [DOI] [PubMed] [Google Scholar]
- 110.Xu J, Kimball TR, Lorenz JN, Brown DA, Bauskin AR, Klevitsky R, et al. GDF15/MIC-1 Functions As a Protective and Antihypertrophic Factor Released From the Myocardium in Association With SMAD Protein Activation. Circ Res. 2006;98:342–350. doi: 10.1161/01.RES.0000202804.84885.d0. [DOI] [PubMed] [Google Scholar]
- 111.Kempf T, Horn-Wichmann R+, Brabant G, Peter T, Allhoff T, Klein G, et al. Circulating Concentrations of Growth-Differentiation Factor 15 in Apparently Healthy Elderly Individuals and Patients with Chronic Heart Failure as Assessed by a New Immunoradiometric Sandwich Assay. Clin Chem. 2007;53:284–291. doi: 10.1373/clinchem.2006.076828. [DOI] [PubMed] [Google Scholar]
- 112.Kempf T, von Haehling S, Peter T, Allhoff T, Cicoira M, Doehner W, et al. Prognostic Utility of Growth Differentiation Factor-15 in Patients With Chronic Heart Failure. J Am Coll Cardiol. 2007;50:1054–1060. doi: 10.1016/j.jacc.2007.04.091. [DOI] [PubMed] [Google Scholar]
- 113.Daniels LB, Clopton P, Laughlin GA, Maisel AS, Barrett-Connor E. Growth-Differentiation Factor-15 Is a Robust, Independent Predictor of 11-Year Mortality Risk in Community-Dwelling Older Adults: The Rancho Bernardo Study. Circulation. 2011;123:2101–2110. doi: 10.1161/CIRCULATIONAHA.110.979740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet. 2004;5:522–531. doi: 10.1038/nrg1379. [DOI] [PubMed] [Google Scholar]
- 115.Care A, Catalucci D, Felicetti F, Bonci D, Addario A, Gallo P, et al. MicroRNA-133 controls cardiac hypertrophy. Nat Med. 2007;13:613–618. doi: 10.1038/nm1582. [DOI] [PubMed] [Google Scholar]
- 116.Thum T, Gross C, Fiedler J, Fischer T, Kissler S, Bussen M, et al. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature. 2008;456:980–984. doi: 10.1038/nature07511. [DOI] [PubMed] [Google Scholar]
- 117.van Rooij E, Sutherland LB, Thatcher JE, DiMaio JM, Naseem RH, Marshall WS, et al. Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proc Natl Acad Sci. 2008;105:13027–13032. doi: 10.1073/pnas.0805038105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tijsen AJ, Pinto YM, Creemers EE. Non-cardiomyocyte microRNAs in heart failure. Cardiovasc Res. 2012;93:573–582. doi: 10.1093/cvr/cvr344. [DOI] [PubMed] [Google Scholar]
- 119.Melman YF, Shah R, Das S. MicroRNAs in Heart Failure: Is the Picture Becoming Less miRky? Circ Heart Fail. 2014;7:203–214. doi: 10.1161/CIRCHEARTFAILURE.113.000266. [DOI] [PubMed] [Google Scholar]
- 120.Melman YF, Shah R, Danielson K, Xiao J, Simonson B, Barth A, et al. Circulating MicroRNA-30d Is Associated With Response to Cardiac Resynchronization Therapy in Heart Failure and Regulates Cardiomyocyte Apoptosis: A Translational Pilot Study. Circulation. 2015;131:2202–2216. doi: 10.1161/CIRCULATIONAHA.114.013220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ovchinnikova ES, Schmitter D, Vegter EL, ter Maaten JM, Valente MAE, Liu LCY, et al. Signature of circulating microRNAs in patients with acute heart failure. Eur J Heart Fail. 2016;18:414–423. doi: 10.1002/ejhf.332. [DOI] [PubMed] [Google Scholar]
- 122.Thum T, Galuppo P, Wolf C, Fiedler J, Kneitz S, van Laake LW, et al. MicroRNAs in the Human Heart: A Clue to Fetal Gene Reprogramming in Heart Failure. Circulation. 2007;116:258–267. doi: 10.1161/CIRCULATIONAHA.107.687947. [DOI] [PubMed] [Google Scholar]
- 123.Vegter EL, van der Meer P, De Windt LJ, Pinto YM, Voors AA. MicroRNAs in heart failure: from biomarker to target for therapy. Eur J Heart Fail. 2016;18:457–468. doi: 10.1002/ejhf.495. [DOI] [PubMed] [Google Scholar]
- 124.Akat KM, Moore-McGriff D, Morozov P, Brown M, Gogakos T, Correa Da Rosa J, et al. Comparative RNA-sequencing analysis of myocardial and circulating small RNAs in human heart failure and their utility as biomarkers. Proc Natl Acad Sci. 2014;111:11151–11156. doi: 10.1073/pnas.1401724111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Watson CJ, Gupta SK, O’Connell E, Thum S, Glezeva N, Fendrich J, et al. MicroRNA signatures differentiate preserved from reduced ejection fraction heart failure. Eur J Heart Fail. 2015;17:405–415. doi: 10.1002/ejhf.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mercer TR, Dinger ME, Mattick JS. Long non-coding RNAs: insights into functions. Nat Rev Genet. 2009;10:155–159. doi: 10.1038/nrg2521. [DOI] [PubMed] [Google Scholar]
- 127.Kumarswamy R, Bauters C, Volkmann I, Maury F, Fetisch J, Holzmann A, et al. Circulating Long Noncoding RNA, LIPCAR, Predicts Survival in Patients With Heart Failure. Circ Res. 2014;114:1569–1575. doi: 10.1161/CIRCRESAHA.114.303915. [DOI] [PubMed] [Google Scholar]
- 128.Ueland T, Jemtland R, Godang K, Kjekshus J, Hognestad A, Omland T, et al. Prognostic value of osteoprotegerin in heart failure after acute myocardial infarction. J Am Coll Cardiol. 2004;44:1970–1976. doi: 10.1016/j.jacc.2004.06.076. [DOI] [PubMed] [Google Scholar]
- 129.Bjerre M, Pedersen SH, Mogelvang R, Lindberg S, Jensen JS, Galatius S, et al. High osteopontin levels predict long-term outcome after STEMI and primary percutaneous coronary intervention. Eur J Prev Cardiol. 2013;20:922–929. doi: 10.1177/2047487313487083. [DOI] [PubMed] [Google Scholar]
- 130.Beatty AL, Zhang MH, Ku IA, Na B, Schiller NB, Whooley MA. Adiponectin is associated with increased mortality and heart failure in patients with stable ischemic heart disease: Data from the Heart and Soul Study. Atherosclerosis. 2012;220:587–592. doi: 10.1016/j.atherosclerosis.2011.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Nazer B, Ray KK, Sloan S, Scirica B, Morrow DA, Cannon CP, et al. Prognostic utility of neopterin and risk of heart failure hospitalization after an acute coronary syndrome. Eur Heart J. 2011;32:1390–1397. doi: 10.1093/eurheartj/ehr032. [DOI] [PubMed] [Google Scholar]
- 132.Zolk O, Ng LL, O’Brien RJ, Weyand M, Eschenhagen T. Augmented Expression of Cardiotrophin-1 in Failing Human Hearts Is Accompanied by Diminished Glycoprotein 130 Receptor Protein Abundance. Circulation. 2002;106:1442–1446. doi: 10.1161/01.cir.0000033117.39335.df. [DOI] [PubMed] [Google Scholar]
- 133.Askevold ET, Nymo S, Ueland T, Gravning J+, Wergeland R, Kjekshus J, et al. Soluble Glycoprotein 130 Predicts Fatal Outcomes in Chronic Heart Failure: Analysis From the Controlled Rosuvastatin Multinational Trial in Heart Failure (CORONA) Circ: Heart Fail. 2013;6:91–98. doi: 10.1161/CIRCHEARTFAILURE.112.972653. [DOI] [PubMed] [Google Scholar]
- 134.Tonelli M, Sacks F, Arnold M, Moye L, Davis B, Pfeffer M, et al. Relation Between Red Blood Cell Distribution Width and Cardiovascular Event Rate in People With Coronary Disease. Circulation. 2008;117:163–168. doi: 10.1161/CIRCULATIONAHA.107.727545. [DOI] [PubMed] [Google Scholar]