Abstract
Heart failure (HF) is a common disease with increased risk for mortality and morbidity among patients with type 2 diabetes mellitus (T2DM). Optimal glycemic control in this patient population is challenging as many available therapies can potentially exacerbate symptoms of HF. Empagliflozin is one in a novel class of agents, the sodium glucose co-transporter 2 (SGLT2) inhibitors, that lowers blood glucose by increasing urinary glucose excretion and improves glycemic control and lowers body weight and blood pressure. In the recent EMPA-REG OUTCOME trial, empagliflozin was shown to improve cardiovascular outcomes in patients with T2DM and established cardiovascular risk where it reduced HF hospitalizations and cardiovascular death, with a consistent benefit among patients both with and without baseline HF. Here, we review the empagliflozin data on HF outcomes and discuss potential mechanisms for its benefits in HF with a focus on the potentially significant impact that empagliflozin may have on the care of patients with T2DM and HF in the future.
Keywords: empagliflozin, heart failure, SGLT2 inhibitor, type 2 diabetes mellitus
Introduction
Heart failure (HF) is a common and deadly disease with an estimated prevalence of more than 5.7 million in the United States.(1) HF contributes to 1 in every 9 deaths in the United States and there is an estimated 5 year survival of 50% at the time of diagnosis.(1) HF is a frequent comorbidity among patients with type 2 diabetes mellitus (T2DM), especially among older adults (~22% in patients ≥65 years) (2), and may be an important mediator of left ventricular systolic and diastolic dysfunction leading to HF.,(3) Furthermore, a diagnosis of T2DM carries an adverse prognosis among those with systolic HF and has been shown to increase risk for all-cause mortality and cardiovascular death in both ischemic and non-ischemic etiologies of HF.(4)
The optimal management of T2DM in HF remains a challenge due to multiple factors. Some anti-hyperglycemic therapies such as insulin and thiazolidinediones promote weight gain and fluid retention thereby potentially counteracting the beneficial effects of glycemic control on HF. Therapies such as the thiazolidinediones and saxagliptin, a dipepitdyl-peptidase 4 (DPP-4) inhibitor, have been associated with signals of increased risk of HF compared with standard care, resulting in product-label cautionary language modifications required by the FDA.(5, 6) Furthermore, until recently, there have been no anti-hyperglycemic therapies for T2DM shown to modify HF outcomes.
Recently, a novel class of medications, the sodium glucose co-transporter 2 (SGLT2) inhibitors, have been approved for management of glycemic control in T2DM. One medication in particular, empagliflozin, has recently been shown to improve both glycemic control and cardiovascular outcomes, a first for a glucose lowering therapy in the modern area.(7) Here, we review the mechanism of action of empagliflozin, summarize current data focused on HF outcomes, highlight important safety issues pertinent to the HF population, and consider the potential future impact of empagliflozin on the growing population of patients with T2DM and with or at risk for HF.
Mechanism of Action
Glucose is freely filtered into the urine at the glomerulus and reabsorbed by both SGLT proteins 1 and 2 located in the proximal tubule of the kidney. SGLT2 transports approximately 90% of filtered glucose back into the systemic circulation by coupling glucose transport to the electrochemical sodium gradient,(8) while SGLT1 reabsorbs the remaining 10% under normal physiologic conditions.(9) Empagliflozin, as well as other SGLT2 inhibitors presently in clinical use (canagliflozin and dapagliflozin), selectively inhibit SGLT2, which decreases the renal tubular threshold for glycosuria and increases urinary excretion of glucose, thereby reducing blood glucose independent of insulin. This unique mechanism of action avoids many of the limitations of other anti-hyperglycemic agents such as weight gain and hypoglycemia that occur through augmented insulin secretion.
Since sodium is co-transported with glucose, inhibition of SGLT2 also causes a small natriuresis in addition to the osmotic diuresis resulting from increased urinary glucose excretion. This results in an increase of 107 ml to 450 ml of urine output per day.(10) Furthermore, there may also be greater increases in hemoglobin and hematocrit concentrations among empagliflozin treated groups compared with placebo or active controls. These changes appear to be a class effect with all SGLT2 inhibitors, although a recent systematic review found that empagliflozin demonstrated the largest increase in hematocrit.(11) Whether these changes are related to volume contraction leading to hemoconcentration or off-target effects such as erythropoietin stimulation and increased red cell mass remains to be determined.
The pharmacodynamics of empagliflozin were evaluated in a phase I study of healthy adults. This study demonstrated that over the first 24 hours, glucose reabsorption was inhibited by 40% on average across doses studied, with a graded association with increasing dose up to 60% inhibition of glucose reabsorption up to a dose of 100 mg, with no further increase at higher doses.(12) In patients with T2DM, this equates to excretion of urinary glucose ranging from 78 to 90 grams per day, depending on the dose and on circulating concentrations of glucose.(13) In both patients with T2DM and healthy subjects, empagliflozin has similar pharmacokinetic properties. It is rapidly absorbed orally reaching peak levels in 1.5 hours with 78% bioavailability.(14) Once absorbed, it is metabolized in the liver by glucuronidation into three conjugates, with each metabolite consisting of less than 10% of the drug in circulation.(14) The half-life of empagliflozin is approximately 12.4 hours. The drug is primarily excreted by the kidney and in the feces as mostly unaltered drug. None of the pharmacodynamics or pharmacokinetics properties of empagliflozin have been specifically studied in patients with HF.
Clinical Outcomes
There are six phase III, randomized, controlled clinical trials demonstrating the efficacy of empagliflozin at lowering glycosylated hemoglobin (HbA1c) as monotherapy or add-on to existing diabetes therapies.(15–20) None of these trials specifically included or excluded patients with HF; however, only the EMPA-REG PIO(18) trial had a pre-specified data collection and analysis for signs and symptoms of HF and edema. The proportion of participants with HF at baseline was not reported. In this study, patients with HbA1c ≥ 7 and ≤10% were randomized to treatment with once daily empagliflozin (10 mg or 25 mg) or placebo as add-on therapy to pioglitazone ± metformin for 24 weeks. At 6 weeks post-randomization, there was no increase in the frequency of edema or HF in patients receiving empagliflozin and pioglitazone compared with placebo. Peripheral edema was reported in two patients receiving placebo and one receiving empagliflozin 25 mg; one patient receiving empagliflozin 10mg reported symptoms of HF.
In 2015, the results of the EMPA-REG OUTCOME trial were published in the New England Journal of Medicine demonstrating the beneficial effects of empagliflozin on cardiovascular morbidity and mortality.(21) This multicenter, randomized, double-blind, placebo-controlled trial evaluated 7028 patients with T2DM and established cardiovascular risk randomized to either one of two doses of empagliflozin (10 mg or 25 mg daily) or placebo. Cardiovascular risk was defined as the presence of at least one of the following: history of myocardial infarction or stroke, coronary artery disease with documented evidence of unstable angina, ischemia by non-invasive testing, or coronary angiography/revascularization, and peripheral arterial disease. Pooled analyses of the two empagliflozin dose groups compared with placebo showed a 14% reduction in the primary composite outcome of 3-point major adverse cardiovascular events (MACE): cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke over a median treatment period of 2.6 years and median follow up period of 3.1 years. In addition, the empagliflozin treated group vs placebo also demonstrated risk reduction in death from cardiovascular causes (HR 0.62, 95% CI 0.49–0.77), death from any cause (HR 0.68, CI 95% 0.57–0.82), and hospitalization for HF (HR 0.65, CI 95% 0.50–0.85). The beneficial effects of empagliflozin on cardiovascular outcomes emerged early, as early as 1 month and within 3–6 months of initiation of therapy, and was sustained throughout the observation period.
Given the robust and somewhat surprising beneficial effects on HF and cardiovascular death, the authors further investigated HF outcomes in all the participants and subgroups including those with and without baseline HF.(7) The majority of the study participants were male and white; only 5% of the participants were black. The prevalence of coronary artery disease, stroke, and stage III or worse chronic kidney disease (CKD) was 76%, 24%, and 26%, respectively. The mean body mass index (BMI) of the study cohort was 30.6 kg/m2. Approximately 10% of the overall study cohort had pre-existing HF. The majority of participants were taking angiotensin converting enzyme inhibitors, angiotensin receptor blockers, and beta blockers at baseline.
The findings of the analysis are summarized in the Table. There was a 34% relative risk reduction (RRR) in the pre-specified composite outcome of cardiovascular death or hospitalization for HF in the empagliflozin vs placebo treated group (HR 0.66, 95% CI 0.55–0.79). This was consistent between doses and across subgroups of age, race, and kidney function, use of cardiovascular or anti-hyperglycemic medications, and over a wide range of outcomes. Investigator reported HF, defined by a standardized Medical Dictionary for Regulatory Activities (MedDRA) query encompassing a group of HF related search terms, and investigator reported HF meeting criteria for serious adverse events were also significantly lower with empagliflozin than with placebo (30% and 31% RRR, respectively). The proportion of patients who had introduction of loop diuretics during follow-up was significantly lower in the empagliflozin treated group compared with placebo (8.6% vs. 13.3%). Approximately 43% of all the participants were using loop diuretics at baseline, and comprised a larger group than those with reported prior HF; nevertheless, the benefits of empagliflozin on 3-point MACE and cardiovascular death were consistent whether or not participants were using loop diuretics at baseline.(7)
Table.
Summary of heart failure outcomes in the EMPA-REG OUTCOME trial
| Outcome | Outcomes in all patients with and without heart failure at baseline | |||
|---|---|---|---|---|
| Placebo (N = 2333) | Empagliflozin (N = 4687) | HR (95% CI) | P-value | |
| n (%) | n (%) | |||
| Heart failure hospitalization or cardiovascular death | 198 (8.5) | 265 (5.7) | 0.66 (0.55–0.79) | <0.001 |
| Hospitalization for or death from heart failure | 194 (4.5) | 129 (2.8) | 0.61 (0.47–0.79) | <0.001 |
| Hospitalization for heart failure | 95 (4.1) | 126 (2.7) | 0.65 (0.50–0.85) | 0.002 |
| Investigator-reported heart failure | 143 (6.1) | 204 (4.4) | 0.70 (0.56–0.87) | 0.001 |
| Investigator-reported serious heart failure | 136 (5.8) | 192 (4.1) | 0.69 (0.55–0.86) | 0.001 |
| All-cause mortality | 925 (39.6) | 1725 (36.8) | 0.89 (0.82–0.96) | 0.003 |
| Outcome | Outcomes in patients with heart failure at baseline | ||
|---|---|---|---|
| Placebo (N = 244) | Empagliflozin (N = 462) | HR (95% CI) | |
| n (%) | n (%) | ||
| Heart failure hospitalization or cardiovascular death | 49 (20.1) | 75 (16.2) | 0.72 ( 0.50–1.04) |
| Hospitalization for heart failure | 30 (12.3) | 48 (10.4) | 0.75 (0.48–1.19) |
| Cardiovascular death | 27 (11.1) | 38 (8.2) | 0.71 (0.43–1.16) |
| All-cause mortality | 35 (14.3) | 56 (12.1) | 0.79 (0.52–1.20) |
| Outcome | Outcomes in patients not on loop diuretics at baseline | ||||
|---|---|---|---|---|---|
| Placebo n with event/n |
% | Empagliflozin n with event/n |
% | HR (95% CI) | |
| Introduction of loop diuretics | 262/1969 | 13.3 | 340/3962 | 8.6 | 0.62 (0.53–0.73) |
| Hospitalization for heart failure or introduction of loop diuretics | 313/2013 | 15.5 | 411/4027 | 10.2 | 0.63 (0.54–0.73) |
| Heart failure hospitalization or cardiovascular death or introduction of loop diuretics | 399/2044 | 19.5 | 532/4066 | 13.1 | 0.64 (0.56–0.73) |
CI, confidence interval; HR, hazard ratio
When results were examined among patients stratified by baseline HF status, as expected, the rates of HF hospitalization, cardiovascular death, and all-cause mortality were much higher (2–6 fold) in patients with HF at baseline compared with those without HF. However, the risk reduction seen with empagliflozin was consistent regardless of baseline HF status. Among patients with baseline HF treated with empagliflozin, non-significant relative risk reductions were seen for HF hospitalization (25%), cardiovascular death (29%), and all-cause mortality (21%). The lack of statistical significance was likely due to the small number of patients with HF at baseline in the study (n = 706, ~10% overall). Body weight and blood pressure were both lower among empagliflozin treated patients compared with placebo both with and without HF at baseline. Although patients with baseline HF weighed ~6 kg more than those without HF, empagliflozin reduced weight by ~2 kg in both groups. Decreases in systolic blood pressure and increases in hematocrit with empagliflozin treatment were consistent with prior clinical trial data and similar by HF status at baseline.
Mechanisms for Cardiovascular Benefit in Heart Failure
The exact mechanisms by which empagliflozin improves HF outcomes are not fully understood. The lack of benefit in EMPA-REG OUTCOME on atherosclerotic outcomes suggests that empagliflozin effects are not mediated through reduction or prevention of atherothrombotic events such as myocardial infarction or stroke. Assessments of left ventricular function and B-type natriuretic peptide were not performed in EMPA-REG OUTCOME so changes in these important prognostic markers in HF cannot be directly linked to the beneficial effects of empagliflozin on HF outcomes. Furthermore, because HF and mortality outcome curves diverged early in treatment (<6 months and as early as 1 month), it is unlikely that the benefit is entirely attributable to improved glycemic control.
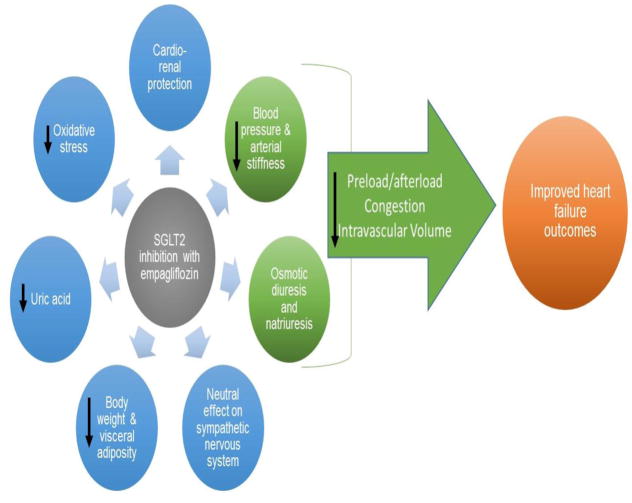
Several mechanisms have been postulated (Figure) including effects on osmotic diuresis and natriuresis contributing to blood pressure lowering without a compensatory increase in sympathetic nervous system activation (22), decreased arterial stiffness and vascular resistance (23), improvements in weight and visceral adiposity (24), decreases in uric acid and oxidative stress (25, 26), off-target effects on lipid metabolism, and a shift in myocardial fuel energetics (27). A recent theory suggests that SGLT2 inhibition may improve myocardial work efficiency based on a shift in fuel energetics with myocardial substrate metabolism switching away from free fatty acids and towards ketones, which are a more energy efficient fuel. The resulting improvement in myocardial metabolic efficiency could translate into long term benefit as it relates to HF. Increases in hemoglobin and hematocrit related to SGLT2 inhibitor treatment have also been observed in multiple studies and may play a role. For example, in EMPA REG OUTCOME, the mean change from baseline in hemoglobin and hematocrit among those treated with empagliflozin 25 mg was 0.8 ± 1.3 g/dL and 5.0 ± 5.3 %, respectively, compared with -0.1 ± 1.2 g/dL and 0.9 ± 4.7 % with placebo (21). Whether these changes reflect early and sustained plasma volume reduction (as seen in other studies of SGLT2 inhibitors) or if they represent off-target effects such as stimulation of the erythropoietin axis, as well as the clinical relevance of such changes, remains to be determined. A recent analysis from EMPA REG OUTCOME revealed that change in hemoglobin/hematocrit was strongly associated with both improved HF and risk of death.
Figure 1.
Potential Mechanisms for Benefit in Heart Failure Outcomes With Empagliflozin
Since hypertension is a major risk factor for both systolic and diastolic HF and also a modifiable target, the effects of empagliflozin on blood pressure may be an important mediator of the observed benefit in HF. In one study using 24 hour ambulatory monitors, the empagliflozin treated arm vs placebo had a statistically significant reduction in systolic blood pressure by 3.44 to 4.16 mmHg (10 mg and 25 mg doses respectively) at 12 weeks and a 1.36 to 1.72 mmHg reduction in diastolic blood pressure.(28) In another study also demonstrating blood pressure effects, patients with type 1 diabetes taking empagliflozin were found to have reduced arterial stiffness as measured by radial, carotid and aortic waveforms.(22) The effects on blood pressure and arterial stiffness were hypothesized to be due to weight loss, smooth muscle relaxation in response to a negative sodium balance, and improved glycemic control. The blood pressure lowering effect of empagliflozin may account for some of the cardiovascular benefits as blood pressure lowering has been consistently shown to prevent cardiovascular events (including HF) in patients with T2DM.(29) This potential vasodilator effect, although mild, can also have a beneficial effect in those with HF to improve hemodynamics and may contribute to reduced hospitalizations in those treated with empagliflozin.(30)
Beneficial effects on intracardiac filling pressures may also contribute to the observed HF benefits seen with empagliflozin. Lowering of these pressures and prevention of recurrence with diuretics is a key component of treatment in patients with HF. The diuretic effect of empagliflozin, although not as robust as traditional diuretics, can be seen with the small increase in hematocrit and urea. Although the diuretic effect may be mild, those treated with empagliflozin had a lower incidence of edema compared with placebo.(31) Empagliflozin use in HF could help to prevent elevated intracardiac filling pressures and clinical decompensation as well as ameliorate symptoms of pulmonary congestion.
The mild diuretic effect of empagliflozin may also have a beneficial effect on the cardiac-kidney axis. Hyperfiltration is an early kidney hemodynamic abnormality seen in T2DM that increases risk for development of diabetic nephropathy. In the native state, SGLT2 reabsorbs glucose and sodium back into the circulation and distal sodium delivery to the macular densa is reduced. The juxtaglomerular apparatus senses this as a low volume stimulus causing an afferent renal vasodilatory response. This tubuloglomerular feedback system results in hyperfiltration. Empagliflozin reduces sodium reabsorption in the proximal tubule resulting in increased sodium delivery to the juxtaglomerular apparatus and has been shown to decrease renal hyperfiltration.(32) Empagliflozin has also been shown to decrease albuminuria in patients with T2DM and CKD.(33) Recently, prespecified renal outcomes of the EMPA-REG trial have been published.(34) Pooled analyses of the two empagliflozin dose groups compared with placebo showed a 38% relative risk reduction of the prespecified composite microvascular outcome: initiation of retinal photocoagulation, vitreous hemorrhage, diabetes-related blindness, or incident or worsening nephropathy. This reduction was primarily driven by the difference in the renal component, in which the group treated with empagliflozin as compared with placebo had a reduction in incident or worsening nephropathy, progression to macroalbuminuria, and initiation of renal replacement therapy. The addition of empagliflozin appears to have an impact on slowing the progression of renal disease as well as lowering risk of clinical renal events. When further analyzing this by prespecified subgroups of those with GFR >60 mL/min/1.73 m2 compared to those with a GFR of 30–60 mL/min/1.73 m2, both groups had similar benefits in cardiovascular outcomes and HF outcomes with no differences in adverse events. This renal protective effect may contribute to improved heart failure outcomes as renal impairment has been associated with worse outcomes in heart failure.(35) Moreover, potential benefits could also be mediated through improved interactions in those with cardiorenal syndrome.(36)
The weight loss seen in empagliflozin is also significant. The inhibition of SGLT2 causes an estimated excretion of 60–100g/day of glucose which results in a loss of 240–400 kcal/day. This result is a clinical weight loss of approximately 1.8 kg, as well as reductions in waist circumference and indices of total and visceral adiposity.(24) Visceral adiposity has been associated with adverse remodeling of the left ventricle and deranged hemodynamics such as lower cardiac output and increased systemic vascular resistance.(37) The benefit of empagliflozin on HF outcomes therefore could also be contributed to by these changes in body composition, although it is not likely not the primary mechanism. The decrease in body weight and effective circulating volume has the potential to cause an increased activation of the sympathetic nervous system. Clinically this is important, as high sympathetic nervous system activity is associated with increased mortality in patients with HF.(38) With the use of empagliflozin, there is no evidence of an adverse effect on the sympathetic nervous system as there are no changes in measured levels of plasma noradrenalin and adrenalin levels or changes in evidence of increased heart rate variability.(22)
Increased levels of uric acid have been linked to an increase risk in incident HF and adverse outcomes in patients with HF.(39) Empagliflozin has also been found to decrease levels of uric acid.(21) As empagliflozin increases the delivery of glucose to the distal nephron, facilitated glucose transporter member 9 (GLUT9), a urate transporter secretes urate into the urine in exchange for glucose.(25) Oxidative stress is another important pathologic pathway that is increasingly recognized as a contributor in the progression of HF. An excess of reactive oxidation species leads to adverse cardiac remodeling, impairment of myocardial contractility and adverse interactions with the metabolism of nitric oxide which is important in endothelial function.(40) In a 4 week trial, empagliflozin was found to reduce markers of oxidative stress which may also contribute to the benefits in HF seen with empagliflozin.(26)
Given the early and significant beneficial effect seen on HF outcomes with empagliflozin, the most likely mechanistic hypothesis favors the hemodynamic effects of combined diuresis and natriuresis with concomitant blood pressure lowering culminating in an overall vasodilator effect. This probably results in decreased preload and afterload which leads to less congestive symptoms of heart failure and fewer subsequent hospitalizations. Beneficial effects on the cardiorenal axis likely also play an important role in this hemodynamic construct.
Safety and Adverse Events in Heart Failure
There do not appear to be any additional safety concerns in the ~10% of patients with baseline HF in the EMPA-REG OUTCOME study. There is a higher incidence of genital mycotic infections in the empagliflozin treated group in which the risk appears higher in women and uncircumcised men, though these events seem to be reasonably easily treated, uncommonly recur even in patients who continue the medication, and rarely lead to drug discontinuation.(21) Empagliflozin also has a low incidence of hypoglycemic events due to its insulin independent mechanism, but with a possible increased risk of ketoacidosis that presents atypically with mildly elevated glucose levels.
In patients with HF at baseline treated with empagliflozin in EMPA-REG OUTCOME, there was a higher proportion of patients with adverse events leading to drug discontinuation compared to those treated with empagliflozin without baseline HF. However, this effect was likely driven by the condition of HF, as among all patients with HF at baseline, the proportion of adverse events and adverse events leading to discontinuation did not differ between treatment groups.(31) Volume depletion is a concern with empagliflozin due to the osmotic diuresis and natriuresis effects of the drug. Combined with the blood pressure lowering effect of empagliflozin, there is the potential to cause symptomatic hypotension. Although there has not been a higher incidence of these events with empagliflozin treatment compared with placebo reported, in a systematic review evaluating the class effect of SGLT2 inhibitors there was a slight increased risk for volume depletion.(41) Prescribing information suggests there may be an increased risk of symptomatic hypotension or volume depletion in patients with renal impairment, elderly or lower baseline systolic blood pressure so caution is advised when initiating empagliflozin in this population.
Kidney dysfunction in the setting of HF is a common clinical scenario as up to 50% of patients with acute or chronic HF have some degree of CKD.(36) As empagliflozin has to be filtered to reach the proximal tubule to have a clinical effect, some degree of kidney function must be present. When used in patients with stage 2 or 3 CKD, empagliflozin had similar adverse event rates when compared with placebo. It is notable though that in those with worse baseline renal function, the glucose lowering effect is attenuated with an increased risk of worsening renal function, volume depletion and urinary tract infection.(33) For this reason, empagliflozin remains restricted to patients with a glomerular filtration rate (GFR) >45 mL/min/1.73 m2. The recently published EMPA-REG renal outcomes do not show a significant difference in adverse events in those with GFR 30–60 mL/min/1.73 m2 and demonstrated that empagliflozin may have a renal protective effect.(34) Thus the restriction may be lowered to a GFR > 30 mL/min/1.73 m2 in the future. The effectiveness of empagliflozin has not been specifically evaluated in patients with HF and CKD, but the same recommendations to avoid use among those with CKD 4 or higher likely still applies due to the increased risk of adverse events and likelihood of decreased glucose lowering effect.
Diuretic therapy is a standard treatment in HF so concurrent use with empagliflozin could potentially be a concern regarding overdiuresis. A randomized, open label cross-over study evaluated for potential drug-drug interactions between empagliflozin and hydrochlorothiazide or torsemide.(42) Administration of empagliflozin with torsemide or hydrochlorothiazide did not demonstrate any change in the pharmacokinetic profile of any drug, and no adverse or serious adverse events were reported. One limitation in this study though is that the dose of torsemide used was 5 mg daily, which may not be generalizable to the HF population as frequently higher doses of diuretics are used.
Recently, there has been a growing concern over an increased risk for developing diabetic ketoacidosis related to use of SGLT2 inhibitors (including empagliflozin) in the literature and media. A recent search of the FDA Adverse Event Reporting System (FAERS) database identified 20 case of ketoacidosis in patients treated with SGLT2 inhibitors from March 2013 to June 2014.(43) Factors identified as having possibly triggered the episodes included concurrent illness, reduced oral intake, and reduced insulin dose.(44) It is not currently known whether the risk for developing diabetic ketoacidosis related to SGLT2 inhibitors is greater in patients with heart failure. Further research is being directed toward identifying which patients are at greatest risk for this side effect and toward developing strategies to minimize the risk to patients.
Another recent FDA warning was issued regarding an increased risk for bone fractures associated with decreased bone mineral density and osteoporosis with SGLT2 inhibitor use.(45) SGLT2 inhibitors are thought to increase serum phosphate concentrations with the potential to adversely affect bone metabolism.(46) Although adverse effects on bone turnover have not been reported with empagliflozin, the potential for osteoporosis associated with use of these agents and the consequences of reduced bone strength in heart failure patients should be carefully considered.
Conclusions and Future Directions
Empagliflozin is a paradigm shifting medication for T2DM management and the first to show benefits in cardiovascular outcomes in the context of a dedicated CV outcomes trial in regards to the composite outcome of cardiovascular death, nonfatal myocardial infarction and nonfatal stroke. The robust efficacy in reduction in HF hospitalizations or cardiovascular death is consistent across multiple subgroups of patients and among those with or without HF at baseline. These benefits may be mediated through the effects of empagliflozin on blood pressure, arterial stiffness, body weight, fat distribution, uric acid or oxidative stress. The effect of diuresis and natriuresis is unique to empagliflozin and SGLT2 inhibitor class of medications in comparison to other therapies available for the treatment of T2DM.
Further study is needed in dedicated HF populations with pre-specified HF assessments to examine the effects of empagliflozin on left ventricular structure and function, hemodynamics, New York Heart Association class, and to determine if differences exist between HF with preserved or reduced ejection fraction. Incorporating measures of cardiac function and biomarkers such as natriuretic peptides into future studies of empagliflozin will help elucidate its effects on markers of HF severity and response to treatment. Studies combining empagliflozin with anti-hyperglycemic therapies with known risk for HF, such as the thiazolidinediones and saxagliptin, will clarify if empagliflozin can mitigate the adverse HF related effects of other anti-hyperglycemic therapies. Long term data are also needed to determine if the HF and cardiovascular benefits are sustained or if any adverse effects may arise late in treatment. It is also unknown whether the benefits seen with empagliflozin are unique to the drug or if there is a class effect. Trials examining the efficacy of other FDA approved and emerging SGLT2 inhibitors in the population with diabetes and chronic heart failure (Safety of canagliflozin in diabetic patients with chronic heart failure: randomized, non-inferiority trial [CANDLE], UMIN000017669 (47); and Dapagliflozin Effect on Symptoms and Biomarkers in Diabetes Patients With Heart Failure [DEFINE-HF], NCT02653482) are currently ongoing. Finally, in the wake of demonstration of CVD benefit across these trials, debate is ongoing as to whether clinicians should prescribe these medications for the primary purpose of HF prevention, rather than focusing on glycemic control per se.
Acknowledgments
Funding Sources
Dr. Neeland is supported by grant K23DK106520 from the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institute of Health and by the Dedman Family Scholarship in Clinical Care from UT Southwestern.
Abbreviations
- CKD
chronic kidney disease
- HF
heart failure
- SGLT2
sodium glucose co-transporter 2
- T2DM
type 2 diabetes mellitus
Footnotes
Relationship with Industry
DP and NDAR have nothing to disclose. IJN has received research support from Boehringer-Ingelheim, the manufacturer of empagliflozin. DKM reports personal fees for clinical trial leadership from Boehringer-Ingelheim, Janssen Research and Development LLC, Merck Sharp and Dohme, Lilly USA, Novo Nordisk, GlaxoSmithKline, Takeda Pharmaceuticals North America, AstraZeneca, Lexicon, Eisai; and personal consulting fees from Sanofi-Aventis Group, Merck Sharp and Dohme, Novo Nordisk, Lilly USA and Regeneron.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation. 2016;133(4):e38–e360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 2.Bertoni AG, Hundley WG, Massing MW, Bonds DE, Burke GL, Goff DC., Jr Heart failure prevalence, incidence, and mortality in the elderly with diabetes. Diabetes Care. 2004;27(3):699–703. doi: 10.2337/diacare.27.3.699. [DOI] [PubMed] [Google Scholar]
- 3.Devereux RB, Roman MJ, Paranicas M, O’Grady MJ, Lee ET, Welty TK, et al. Impact of diabetes on cardiac structure and function: the strong heart study. Circulation. 2000;101(19):2271–6. doi: 10.1161/01.cir.101.19.2271. [DOI] [PubMed] [Google Scholar]
- 4.Cubbon RM, Adams B, Rajwani A, Mercer BN, Patel PA, Gherardi G, et al. Diabetes mellitus is associated with adverse prognosis in chronic heart failure of ischaemic and non-ischaemic aetiology. Diab Vasc Dis Res. 2013;10(4):330–6. doi: 10.1177/1479164112471064. [DOI] [PubMed] [Google Scholar]
- 5.Lago RM, Singh PP, Nesto RW. Congestive heart failure and cardiovascular death in patients with prediabetes and type 2 diabetes given thiazolidinediones: a meta-analysis of randomised clinical trials. Lancet. 2007;370(9593):1129–36. doi: 10.1016/S0140-6736(07)61514-1. [DOI] [PubMed] [Google Scholar]
- 6.Scirica BM, Braunwald E, Raz I, Cavender MA, Morrow DA, Jarolim P, et al. Heart failure, saxagliptin, and diabetes mellitus: observations from the SAVOR-TIMI 53 randomized trial. Circulation. 2014;130(18):1579–88. doi: 10.1161/CIRCULATIONAHA.114.010389. [DOI] [PubMed] [Google Scholar]
- 7.Fitchett D, Zinman B, Wanner C, Lachin JM, Hantel S, Salsali A, et al. Heart failure outcomes with empagliflozin in patients with type 2 diabetes at high cardiovascular risk: results of the EMPA-REG OUTCOME(R) trial. Eur Heart J. 2016;37(19):1526–34. doi: 10.1093/eurheartj/ehv728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hediger MA, Rhoads DB. Molecular physiology of sodium-glucose cotransporters. Physiol Rev. 1994;74(4):993–1026. doi: 10.1152/physrev.1994.74.4.993. [DOI] [PubMed] [Google Scholar]
- 9.Vallon V, Platt KA, Cunard R, Schroth J, Whaley J, Thomson SC, et al. SGLT2 mediates glucose reabsorption in the early proximal tubule. J Am Soc Nephrol. 2011;22(1):104–12. doi: 10.1681/ASN.2010030246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.List JF, Woo V, Morales E, Tang W, Fiedorek FT. Sodium-glucose cotransport inhibition with dapagliflozin in type 2 diabetes. Diabetes Care. 2009;32(4):650–7. doi: 10.2337/dc08-1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baker WL, Smyth LR, Riche DM, Bourret EM, Chamberlin KW, White WB. Effects of sodium-glucose co-transporter 2 inhibitors on blood pressure: a systematic review and meta-analysis. J Am Soc Hypertens. 2014;8(4):262–75. e9. doi: 10.1016/j.jash.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 12.Seman L, Macha S, Nehmiz G, Simons G, Ren B, Pinnetti S, et al. Empagliflozin (BI 10773), a Potent and Selective SGLT2 Inhibitor, Induces Dose-Dependent Glucosuria in Healthy Subjects. Clin Pharmacol Drug Dev. 2013;2(2):152–61. doi: 10.1002/cpdd.16. [DOI] [PubMed] [Google Scholar]
- 13.Heise T, Seman L, Macha S, Jones P, Marquart A, Pinnetti S, et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of multiple rising doses of empagliflozin in patients with type 2 diabetes mellitus. Diabetes Ther. 2013;4(2):331–45. doi: 10.1007/s13300-013-0030-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boehringer-Ingelheim. [Accessed July 26, 2016];Jardiance Prescribing Information. 2016 cited 2016 April 16. Available from: http://docs.boehringer-ingelheim.com/Prescribing%20Information/PIs/Jardiance/jardiance.pdf.
- 15.Roden M, Weng J, Eilbracht J, Delafont B, Kim G, Woerle HJ, et al. Empagliflozin monotherapy with sitagliptin as an active comparator in patients with type 2 diabetes: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Diabetes Endocrinol. 2013;1(3):208–19. doi: 10.1016/S2213-8587(13)70084-6. [DOI] [PubMed] [Google Scholar]
- 16.Haring HU, Merker L, Seewaldt-Becker E, Weimer M, Meinicke T, Broedl UC, et al. Empagliflozin as add-on to metformin in patients with type 2 diabetes: a 24-week, randomized, double-blind, placebo-controlled trial. Diabetes Care. 2014;37(6):1650–9. doi: 10.2337/dc13-2105. [DOI] [PubMed] [Google Scholar]
- 17.Haring HU, Merker L, Seewaldt-Becker E, Weimer M, Meinicke T, Woerle HJ, et al. Empagliflozin as add-on to metformin plus sulfonylurea in patients with type 2 diabetes: a 24-week, randomized, double-blind, placebo-controlled trial. Diabetes Care. 2013;36(11):3396–404. doi: 10.2337/dc12-2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kovacs CS, Seshiah V, Swallow R, Jones R, Rattunde H, Woerle HJ, et al. Empagliflozin improves glycaemic and weight control as add-on therapy to pioglitazone or pioglitazone plus metformin in patients with type 2 diabetes: a 24-week, randomized, placebo-controlled trial. Diabetes Obes Metab. 2014;16(2):147–58. doi: 10.1111/dom.12188. [DOI] [PubMed] [Google Scholar]
- 19.Rosenstock J, Jelaska A, Frappin G, Salsali A, Kim G, Woerle HJ, et al. Improved glucose control with weight loss, lower insulin doses, and no increased hypoglycemia with empagliflozin added to titrated multiple daily injections of insulin in obese inadequately controlled type 2 diabetes. Diabetes Care. 2014;37(7):1815–23. doi: 10.2337/dc13-3055. [DOI] [PubMed] [Google Scholar]
- 20.Ridderstrale M, Andersen KR, Zeller C, Kim G, Woerle HJ, Broedl UC. Comparison of empagliflozin and glimepiride as add-on to metformin in patients with type 2 diabetes: a 104-week randomised, active-controlled, double-blind, phase 3 trial. Lancet Diabetes Endocrinol. 2014;2(9):691–700. doi: 10.1016/S2213-8587(14)70120-2. [DOI] [PubMed] [Google Scholar]
- 21.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med. 2015;373(22):2117–28. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 22.Cherney DZ, Perkins BA, Soleymanlou N, Har R, Fagan N, Johansen OE, et al. The effect of empagliflozin on arterial stiffness and heart rate variability in subjects with uncomplicated type 1 diabetes mellitus. Cardiovasc Diabetol. 2014;13:28. doi: 10.1186/1475-2840-13-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chilton R, Tikkanen I, Cannon CP, Crowe S, Woerle HJ, Broedl UC, et al. Effects of empagliflozin on blood pressure and markers of arterial stiffness and vascular resistance in patients with type 2 diabetes. Diabetes Obes Metab. 2015;17(12):1180–93. doi: 10.1111/dom.12572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neeland IJ, McGuire DK, Chilton R, Crowe S, Lund SS, Woerle HJ, et al. Empagliflozin reduces body weight and indices of adipose distribution in patients with type 2 diabetes mellitus. Diab Vasc Dis Res. 2016;13(2):119–26. doi: 10.1177/1479164115616901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheeseman C. Solute carrier family 2, member 9 and uric acid homeostasis. Curr Opin Nephrol Hypertens. 2009;18(5):428–32. doi: 10.1097/MNH.0b013e32832ee3de. [DOI] [PubMed] [Google Scholar]
- 26.Nishimura R, Tanaka Y, Koiwai K, Inoue K, Hach T, Salsali A, et al. Effect of empagliflozin monotherapy on postprandial glucose and 24-hour glucose variability in Japanese patients with type 2 diabetes mellitus: a randomized, double-blind, placebo-controlled, 4-week study. Cardiovasc Diabetol. 2015;14:11. doi: 10.1186/s12933-014-0169-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mudaliar S, Alloju S, Henry RR. Can a Shift in Fuel Energetics Explain the Beneficial Cardiorenal Outcomes in the EMPA-REG OUTCOME Study? A Unifying Hypothesis. Diabetes Care. 2016;39(7):1115–22. doi: 10.2337/dc16-0542. [DOI] [PubMed] [Google Scholar]
- 28.Tikkanen I, Narko K, Zeller C, Green A, Salsali A, Broedl UC, et al. Empagliflozin reduces blood pressure in patients with type 2 diabetes and hypertension. Diabetes Care. 2015;38(3):420–8. doi: 10.2337/dc14-1096. [DOI] [PubMed] [Google Scholar]
- 29.Emdin CA, Rahimi K, Neal B, Callender T, Perkovic V, Patel A. Blood pressure lowering in type 2 diabetes: a systematic review and meta-analysis. JAMA. 2015;313(6):603–15. doi: 10.1001/jama.2014.18574. [DOI] [PubMed] [Google Scholar]
- 30.Cohn JN, Archibald DG, Ziesche S, Franciosa JA, Harston WE, Tristani FE, et al. Effect of vasodilator therapy on mortality in chronic congestive heart failure. Results of a Veterans Administration Cooperative Study. N Engl J Med. 1986;314(24):1547–52. doi: 10.1056/NEJM198606123142404. [DOI] [PubMed] [Google Scholar]
- 31.Fitchett D, Zinman B, Wanner C, Lachin JM, Hantel S, Salsali A, et al. Heart failure outcomes with empagliflozin in patients with type 2 diabetes at high cardiovascular risk: results of the EMPA-REG OUTCOME(R) trial. Eur Heart J. 2016 doi: 10.1093/eurheartj/ehv728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cherney DZ, Perkins BA, Soleymanlou N, Maione M, Lai V, Lee A, et al. Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation. 2014;129(5):587–97. doi: 10.1161/CIRCULATIONAHA.113.005081. [DOI] [PubMed] [Google Scholar]
- 33.Barnett AH, Mithal A, Manassie J, Jones R, Rattunde H, Woerle HJ, et al. Efficacy and safety of empagliflozin added to existing antidiabetes treatment in patients with type 2 diabetes and chronic kidney disease: a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2014;2(5):369–84. doi: 10.1016/S2213-8587(13)70208-0. [DOI] [PubMed] [Google Scholar]
- 34.Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, Mattheus M, et al. Empagliflozin and Progression of Kidney Disease in Type 2 Diabetes. N Engl J Med. 2016 doi: 10.1056/NEJMoa1515920. [DOI] [PubMed] [Google Scholar]
- 35.Damman K, Valente MA, Voors AA, O’Connor CM, van Veldhuisen DJ, Hillege HL. Renal impairment, worsening renal function, and outcome in patients with heart failure: an updated meta-analysis. Eur Heart J. 2014;35(7):455–69. doi: 10.1093/eurheartj/eht386. [DOI] [PubMed] [Google Scholar]
- 36.Damman K, Testani JM. The kidney in heart failure: an update. Eur Heart J. 2015;36(23):1437–44. doi: 10.1093/eurheartj/ehv010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Neeland IJ, Gupta S, Ayers CR, Turer AT, Rame JE, Das SR, et al. Relation of regional fat distribution to left ventricular structure and function. Circ Cardiovasc Imaging. 2013;6(5):800–7. doi: 10.1161/CIRCIMAGING.113.000532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaye DM, Lefkovits J, Jennings GL, Bergin P, Broughton A, Esler MD. Adverse consequences of high sympathetic nervous activity in the failing human heart. J Am Coll Cardiol. 1995;26(5):1257–63. doi: 10.1016/0735-1097(95)00332-0. [DOI] [PubMed] [Google Scholar]
- 39.Huang H, Huang B, Li Y, Huang Y, Li J, Yao H, et al. Uric acid and risk of heart failure: a systematic review and meta-analysis. Eur J Heart Fail. 2014;16(1):15–24. doi: 10.1093/eurjhf/hft132. [DOI] [PubMed] [Google Scholar]
- 40.Tsutsui H, Kinugawa S, Matsushima S. Oxidative stress and heart failure. Am J Physiol Heart Circ Physiol. 2011;301(6):H2181–90. doi: 10.1152/ajpheart.00554.2011. [DOI] [PubMed] [Google Scholar]
- 41.Wu JH, Foote C, Blomster J, Toyama T, Perkovic V, Sundstrom J, et al. Effects of sodium-glucose cotransporter-2 inhibitors on cardiovascular events, death, and major safety outcomes in adults with type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2016;4(5):411–9. doi: 10.1016/S2213-8587(16)00052-8. [DOI] [PubMed] [Google Scholar]
- 42.Heise T, Mattheus M, Woerle HJ, Broedl UC, Macha S. Assessing pharmacokinetic interactions between the sodium glucose cotransporter 2 inhibitor empagliflozin and hydrochlorothiazide or torasemide in patients with type 2 diabetes mellitus: a randomized, open-label, crossover study. Clin Ther. 2015;37(4):793–803. doi: 10.1016/j.clinthera.2014.12.018. [DOI] [PubMed] [Google Scholar]
- 43.FDA Drug Safety Communication. [Accessed July 26, 2016];FDA warns that SGLT2 inhibitors for diabetes may result in a serious condition of too much acid in the blood. 2015 Aug 13; Available from: http://www.fda.gov/Drugs/DrugSafety/ucm446845.htm.
- 44.Taylor SI, Blau JE, Rother KI. SGLT2 Inhibitors May Predispose to Ketoacidosis. J Clin Endocrinol Metab. 2015;100(8):2849–52. doi: 10.1210/jc.2015-1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. [Accessed July 26, 2016];Invokana and Invokamet (canagliflozin): Drug Safety Communication - New Information on Bone Fracture Risk and Decreased Bone Mineral Density. Available from: http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm461876.htm.
- 46.Taylor SI, Blau JE, Rother KI. Possible adverse effects of SGLT2 inhibitors on bone. Lancet Diabetes Endocrinol. 2015;3(1):8–10. doi: 10.1016/S2213-8587(14)70227-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tanaka A, Inoue T, Kitakaze M, Oyama J, Sata M, Taguchi I, et al. Rationale and design of a randomized trial to test the safety and non-inferiority of canagliflozin in patients with diabetes with chronic heart failure: the CANDLE trial. Cardiovasc Diabetol. 2016;15(1):57. doi: 10.1186/s12933-016-0381-x. [DOI] [PMC free article] [PubMed] [Google Scholar]