Abstract
Background
Regular physical activity is important for improving and maintaining health, but sedentary behavior is difficult to change. Providing objective, real-time feedback on physical activity with wearable motion-sensing technologies (activity monitors) may be a promising, scalable strategy to increase physical activity or decrease weight.
Purpose
We synthesized the literature on the use of wearable activity monitors for improving physical activity and weight-related outcomes and evaluated moderating factors that may have an impact on effectiveness.
Methods
We searched five databases from January 2000 to January 2015 for peer-reviewed, English-language randomized controlled trials among adults. Random-effects models were used to produce standardized mean differences (SMDs) for physical activity outcomes and mean differences (MDs) for weight outcomes. Heterogeneity was measured with I2.
Results
Fourteen trials (2,972 total participants) met eligibility criteria; accelerometers were used in all trials. Twelve trials examined accelerometer interventions for increasing physical activity. A small significant effect was found for increasing physical activity (SMD 0.26; 95% CI 0.04 to 0.49; I2=64.7%). Intervention duration was the only moderator found to significantly explain high heterogeneity for physical activity. Eleven trials examined effects of accelerometer interventions on weight. Pooled estimates showed a small significant effect for weight loss (MD −1.65 kg; 95% CI −3.03 to −0.28; I2=81%), and no moderators were significant.
Conclusions
Accelerometers demonstrated small positive effects on physical activity and weight loss. The small sample sizes with moderate to high heterogeneity in the current studies limit the conclusions that may be drawn. Future studies should focus on how best to integrate accelerometers with other strategies to increase physical activity and weight loss.
Keywords: Accelerometers, Weight loss, Physical activity
INTRODUCTION
Participation in regular physical activity is associated with a wide range of mental and physical health benefits. Patients with diabetes, obesity, or musculoskeletal disease, in particular, derive significant benefits from regular physical activity, including favorable effects on blood pressure, lipid profiles, joint pain, weight control, body composition, and psychological well-being [1]. Despite proven benefits and widespread public health and clinical calls to increase physical activity, sedentary behavior has proven difficult to change.
Self-monitoring is a key behavioral strategy to increase an individual’s physical activity, and objective self-monitoring is considered the gold standard [2]. Pedometers are tools that can be used for objective self-monitoring and are designed to detect ambulatory activity to provide a simple estimate of physical activity volume. These devices provide several positive characteristics including simplicity, affordability, validity, and reliability, and they have been successfully implemented into physical activity and weight loss studies [3]. Pedometer usage has been associated with significant increases in physical activity and significant decreases in both body mass index, blood pressure [4] and weight loss, with interventions of longer duration leading to greater weight loss than shorter duration programs [5]. Pedometers continue to be widely used to monitor daily ambulation activity, as a tool for prescribing increased mobility (e.g., daily step targets), and for motivating individuals to increase their activity level [6,7] [8]. However, pedometers have limitations, such as producing step-count inaccuracies in overweight and obese populations and those with slower ambulation [9,10], as well as and the inability to capture exercise intensity [3]. Newer activity monitoring technologies, such as accelerometers, offer advantages over pedometers. These include the potential to detect lateral and vertical movements and measure the intensity of physical activity [6]. In addition, activity monitors used by consumers and researchers now have extensive feedback loops. These feedback loops provide real-time data to the wearer via computer programs and mobile applications that allow for tailoring intervention content [11–13]. Further, some devices provide an option to relay information to a third party such as family, friends, or clinicians. The ability to transmit data to patients’ physicians and healthcare teams makes these devices attractive for clinical applications, although this capability is in its infancy of implementation and evaluation [11].
Newer, direct-to-consumer activity tracking devices are rarely examined as intervention tools. We are unaware of any systematic reviews that have quantitatively described outcomes using devices such as accelerometers. Furthermore, factors that may moderate the effects of these newer self-monitoring technologies remain to be explored. Thus, the objectives of this literature synthesis were to (1) determine the effectiveness of newer activity monitoring technologies for increasing physical activity and decreasing body weight outcomes and (2) describe factors that impact the effectiveness of such technologies (i.e., chronic disease status, location where the device is worn on the body, the device’s role in the overall intervention approach, and duration of the intervention).
METHODS
We followed a standard protocol for this review and conducted it in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) statement [14]. Each step was pilot-tested to train and calibrate study investigators. The PROSPERO registration number is CRD42015017343. This review is part of a larger report for the U.S. Veterans Health Administration’s Evidence-based Synthesis Program to investigate existing evidence on wearable activity devices.
Data Sources and Search Strategy
We searched MEDLINE (via PubMed), Embase, CINAHL, SPORTDiscus, and Cochrane CENTRAL from January 1, 2000, to January 6, 2015 (Appendix). We used Medical Subject Heading (MeSH) terms and selected free-text terms for wearable activity monitors and for outcomes of interest (e.g., movement, exercise therapy, physical fitness) along with validated search terms for study designs of interest [15]. We also reviewed the bibliographies of included trials and systematic reviews [16–22] for missed publications. All citations were imported into two electronic databases (for referencing, EndNote® Version X5, Thomson Reuters, Philadelphia, PA; for data abstraction, DistillerSR; Evidence Partners Inc., Manotick, ON, Canada).
Inclusion Criteria and Screening
To be included, studies had to (1) include adults ≥18 years of age, (2) use a wearable activity monitor not described as a pedometer (i.e., measures vertical acceleration movement and provides objective feedback to the user), (3) report changes in the outcomes of physical activity or weight, (4) be a randomized controlled trial (RCT) with a total sample size >20 participants and outcomes ≥3 months, and (5) be published in an English-language peer-reviewed journal. Studies were excluded if they were not a population of interest, did not include an outcome of interest, used pedometers only, or used activity monitors that do not provide feedback to the wearer.
Two trained investigators screened titles and abstracts for relevance to the objectives of the study. Full-text articles identified by either investigator as potentially relevant were retrieved for further review and examined by two investigators against the eligibility criteria. Disagreements were resolved by discussion or by a third investigator. In addition, trials with three or more arms were examined for appropriateness of all arms for inclusion.
Data Abstraction
Data from included trials were abstracted into a customized database by a trained investigator and confirmed by a second investigator. Disagreements were resolved by consensus or by obtaining a third investigator’s opinion when consensus could not be reached. Data elements included date of publication, sample size, population characteristics (e.g., chronic medical illness status, sex, age), and descriptors to assess applicability, quality elements, and outcomes. Key intervention characteristics abstracted were the type of activity monitor (e.g., brand, location worn on body), type of adjunctive intervention (e.g., behavioral weight management strategies, physical activity education), and duration and frequency of intervention.
Risk of Bias
We used key quality criteria described in the Cochrane Collaboration Risk of Bias Tool to assess risk of bias in each included study [23]. The tool evaluates 6 different domains across 7 questions: (1) selection bias (i.e., adequacy of random-sequence generation, allocation concealment); (2) performance bias for each outcome (i.e., knowledge of allocated intervention by participants and study personnel that could introduce bias); (3) detection bias for each outcome (i.e., knowledge of allocated intervention by outcome assessors); (4) attrition bias (i.e., amount, nature, or handling of incomplete outcome data); (5) reporting bias (i.e., selective outcome reporting); (6) other bias (e.g., differences in relation to baseline measures, reliable primary outcomes, protection against contamination).
We evaluated each domain as low, unclear, or high risk of bias. The overall score of low risk of bias required selection bias related to random sequencing and allocation concealment; performance bias; and detection bias to be scored “low risk” with no other important concerns. For performance bias and detection bias, studies did not need to blind study personnel and participants to receive a low risk of bias if outcome measurement was not likely to be influenced by lack of blinding. A judgment of unclear risk of bias was assigned if 1 or 2 domains were scored “not clear” or “not done.” Studies judged to be high risk of bias had more than 2 domains scored “not clear” or “not done.”
Data Synthesis
When meta-analysis was feasible, we computed summary estimates of effect. We aggregated outcomes when there were at least three studies with the same outcome. Continuous outcomes were analyzed using standardized mean differences (SMDs) for physical activity outcomes and mean differences (MDs) for weight outcomes in a random-effects model with the Knapp-Hartung [24] correction to confidence intervals. The method we used to interpret the SMD as an effect size is as follows: small effect size, SMD=0.2; medium, SMD=0.5; and large, SMD≥0.8 [25]. We evaluated for statistical heterogeneity using visual inspection of forest plots and the I2 statistic. We assessed for potential publication bias by comparing registered clinical trials in ClinicalTrials.gov with published literature. All quantitative analyses were performed in R (R Foundation for Statistical Computing, Vienna, Austria) with the metafor package [26].
We conducted analyses separately for interventions versus inactive controls (e.g., waitlist, usual care) and interventions versus active comparators (e.g., group weight loss, counseling). Three trials had more than one intervention arm [27–29]. Two of these trials compared different adjunctive interventions to continuous monitoring via accelerometers [27,28]. For these two studies, we selected the intervention with the less intensive adjuncts (e.g., monthly counseling vs. weekly counseling). The third trial tested the impact of continuous versus intermittent accelerometer feedback [29]. For this study, we selected the comparisons between continuous accelerometer use and control because this was the type of accelerometer use evaluated in all other studies.
If a quantitative synthesis was not feasible (due to less than 3 studies in a subgroup), we analyzed the data qualitatively. We gave more weight to the evidence from higher quality studies with more precise estimates of effect. We focused on documenting and identifying patterns of the intervention across outcome categories. We analyzed potential reasons for inconsistency in treatment effects across studies by evaluating differences in the study population, intervention, comparator, and outcome definitions.
Moderator Effects
We explored potential sources of heterogeneity, including characteristics of the population operationalized as overweight/obese/sedentary, older adults, healthy volunteers, and those with other chronic medical illnesses (e.g., diabetes), and the intervention duration in weeks, and location on the body where the device is worn. We aimed to assess the differential impact of type of adjunctive interventions (e.g., behavioral weight management intervention, physical activity education, goal-setting) as a source of potential heterogeneity. Because type and quantity of adjunctive interventions varied greatly from study to study, we operationalized this moderator as the role of the wearable activity monitor in the overall intervention (i.e., major vs. minor component). To be categorized as a major component of the intervention, the wearable activity monitor needed to be the central motivational enhancement strategy intended to improve the primary outcome of the study. Other adjunctive interventions might be included but played a minor role in enhancing physical activity. To be categorized as a minor component, the wearable activity device needed to be an integrated part of a suite of other motivation enhancement interventions, such as a structured exercise program, behavioral counseling, or disease self-management techniques. Two independent investigators categorized the role of the device, and another investigator reconciled any discrepancies.
RESULTS
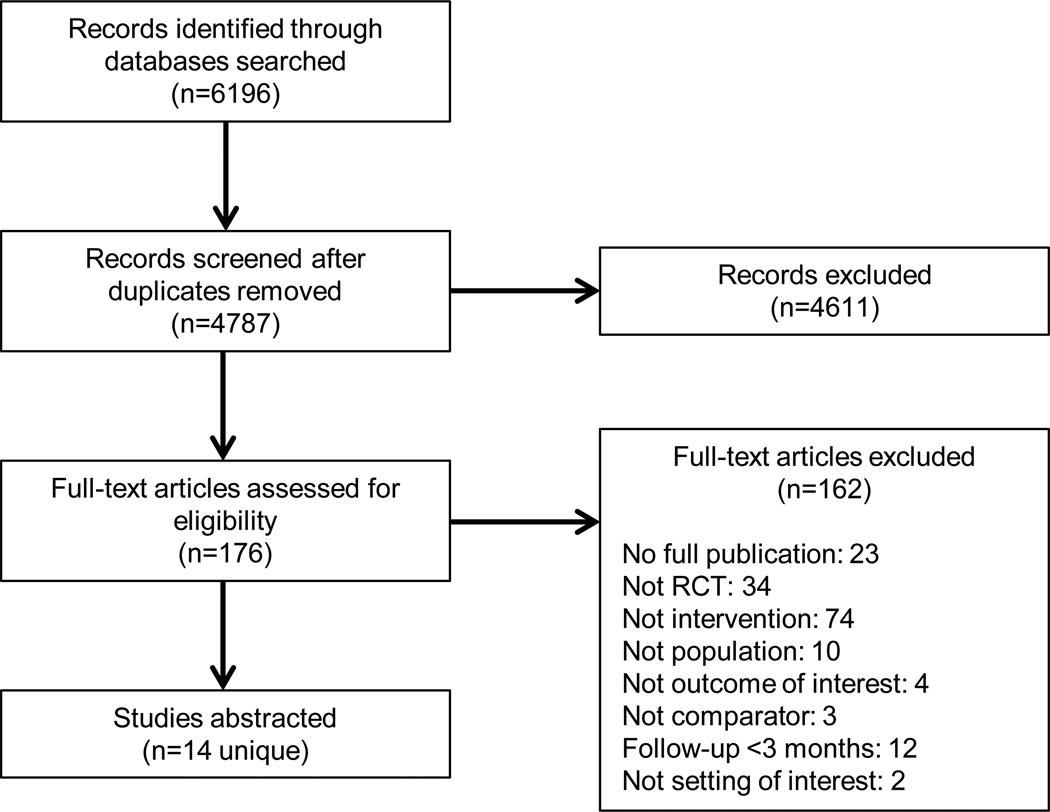
Our search identified 6,196 citations and, after removing duplicates, we screened 4,787 titles and abstracts for eligibility criteria, leaving 176 citations for full-text review. In total, 14 trials met eligibility criteria (Figure 1). Across these, women were 62.5% of the population; median age was 49.7 years (range 28.7 to 79.8 years); and the intervention duration ranged from 12 to 52 weeks. Only 4 trials reported race. The majority of trials were conducted in the United States (n=8), and study sizes ranged from 20 to 544 participants (median n=62), with the majority of studies (n=8) randomizing fewer than 70 participants.
Figure 1.
Literature flow diagram
Table 1 summarizes the study characteristics of the 14 included studies. Twelve studies reported on outcomes related to physical activity [29–40] and 11 on outcomes related to weight [27–29,31–37,39]. Four trials were conducted with older adults; five with overweight, obese, or sedentary adults; three with participants with a chronic medical illness (e.g., chronic obstructive pulmonary disease, metabolic disease, diabetes); and two with healthy volunteers. The device—usually worn on the waist (n=8 trials)—was a major component of the intervention in nine trials and a minor component in five trials. While we searched broadly for wearable non-pedometer devices, all identified trials used some form of accelerometer-based motion-sensing technology. Comparators were active in 3 trials and inactive in 11 trials. The number of planned interactions with participants in the accelerometer interventions ranged from none to 52 weekly contacts. Trials used a wide variety of adjunctive interventions in conjunction with accelerometers, including intensive diet, weight, and physical activity behavioral counseling; tailored written feedback; and web-based supportive educational modules. A search of ClinicalTrials.gov identified one completed but unpublished trial (NCT00544245) that appeared to meet our inclusion criteria, which suggests little potential for publication bias.
Table 1.
Description of included studies
| Study | Population (N) |
Device Name |
Intervention (weeks duration) |
Comparator | Outcome |
|---|---|---|---|---|---|
| Greene, 2013 [35] |
Healthy volunteers (513) |
Not Reported |
Access to online social network + continuous accelerometer use and feedback Duration: 24 weeks |
Inactive: Printed lifestyle guidelines on diet and exercise |
PA Weight |
| Koizumi, 2009 [38] |
Older adults (68) |
Kenz Lifecorder |
Accelerometer with feedback + goal- setting Duration: 12 weeks |
Inactive: 12 weeks of blinded accelerometer use |
PA |
| Luley, 2014 [27] |
Chronic medical illness (184) |
Aipermoti on 440 |
3-arm study (2 interventional) Intervention arm 1: 1-time instruction on iet and physical activity + accelerometer use + 52 weekly individual letters with feedback. Intervention arm 2: Instruction on diet and physical activity + accelerometer use + 12 monthly counseling calls Duration: 52 weeks |
Inactive: 1- time, 1-hour session consisting of diet education, diet regimen, and physical activity education |
Weight |
| Nicklas, 2014 [31] |
Older adults (48) |
Lifecorder PlusVR tri-axial |
Weight loss intervention that included hypocaloric diet (2 prepared meals a day) + 4 days/week supervised exercise + self-regulatory wearing an accelerometer, documenting activity, and 6 weekly session of behavioral counseling Duration: 20 weeks |
Active: 5-month weight loss intervention consisting of diet education and physical activity education, structured exercise and in-person counseling |
PA Weight |
| Paschali, 2005 [40] |
Chronic medical illness (30) |
BioTrainer | Continuous accelerometer use and feedback + 4 monthly in-person exercise behavioral counseling sessions + workbook Duration: 12 weeks |
Active: Accelerometer use with 4 monthly in- person counseling, walking plan, education, behavioral self- management, goal-setting, and monitoring |
PA |
| Polzien, 2007 [29] |
Overweight/ obese and/or sedentary (57) |
SenseWe ar Pro Armband |
Intervention arm 1: Continuous technology-based behavioral weight control program: individualized counseling sessions + continuous accelerometer use and feedback. Intervention arm 2: Intermittent technology-based behavioral weight control program: individualized counseling sessions + 3 weeks of accelerometer use and feedback Duration: 12 weeks |
Active: 7 in- person individualized counseling sessions consisting of diet education, diet regimen (1200 to 1500 kcal/day; dietary fat <20% of total energy intake), physical activity education, and weight goal- setting |
PA Weight |
| Reijonsaa ri, 2012 [37] |
Healthy volunteers (544) |
Uni-axial | 12 months of continuous accelerometer use and feedback + access to telephone counseling (frequency not defined) Duration: 52 weeks |
Inactive: Physical exams explanation and information on physical activity and occupational healthcare |
PA Weight |
| Shrestha, 2013 [36] |
Overweight/ obese and/or sedentary (28) |
Polar FA20 |
1-time 1.5-hour lifestyle instruction + 6 months of continuous accelerometer use and feedback Duration: 24 weeks |
Inactive: Self- directed exercise and/or U.S. Army physical training |
PA Weight |
| Shuger, 2011 [28] |
Overweight/ obese and/or sedentary (197) |
BodyMedi a SenseWe ar Armband |
Intervention arm 1: 14 group weight loss sessions + 6 phone calls + workbook. Intervention arm 2: Accelerometer alone: Continuous accelerometer use and feedback Intervention arm 3: Continuous accelerometer use and feedback + 14 group weight loss sessions + 6 individual phone calls + workbook Duration: 36 weeks |
Inactive: Self- directed weight loss manual with diet education, physical activity education, and weight goal- setting |
Weight |
| Slootmak er, 2009 [39] |
Overweight/ obese and/or sedentary (102) |
PAM (model AM101) |
3 months of continuous accelerometer use + tailored physical activity feedback and motivational tips via web-based portal Duration: 12 weeks |
Inactive: A single written brochure with brief physical activity recommendatio ns |
PA Weight |
| Tabak, 2014 [30] |
Chronic medical illness (29) |
Activity Coach |
Web-based exercise program, accelerometer-based activity senor and motivational messaging, COPD self-management module, and as needed web-portal teleconsultation Duration: 36 weeks |
Inactive: Usual care |
PA |
| Thompso n, 2014 [32] |
Overweight/ obese and/or sedentary (20) |
GRUVE triaxial |
12 weeks of continuous accelerometer use and feedback + weekly brief counseling sessions on increasing activity + treadmill desk Duration: 12 weeks |
Inactive: 12 weeks of blinded accelerometer use |
PA Weight |
| Thompso n, 2014 [34] |
Older adults (49) |
FitBit | 24 weeks of continuous accelerometer use and feedback + weekly brief telephone counseling sessions focused on accelerometer feedback + in-person counseling. Duration: 12 weeks |
Inactive: 24 weeks of blinded accelerometer use |
PA Weight |
| Wijsman, 2013 [33] |
Older adults (235) |
DirectLife | 12 weeks of continuous accelerometer use and feedback + personal website + personal e-coach who gives updates on activity status and advice via web portal Duration: 12 weeks |
Inactive: 3- month waitlist control |
PA Weight |
Abbreviations: COPD=chronic obstructive pulmonary disease; PA=physical activity
Physical Activity
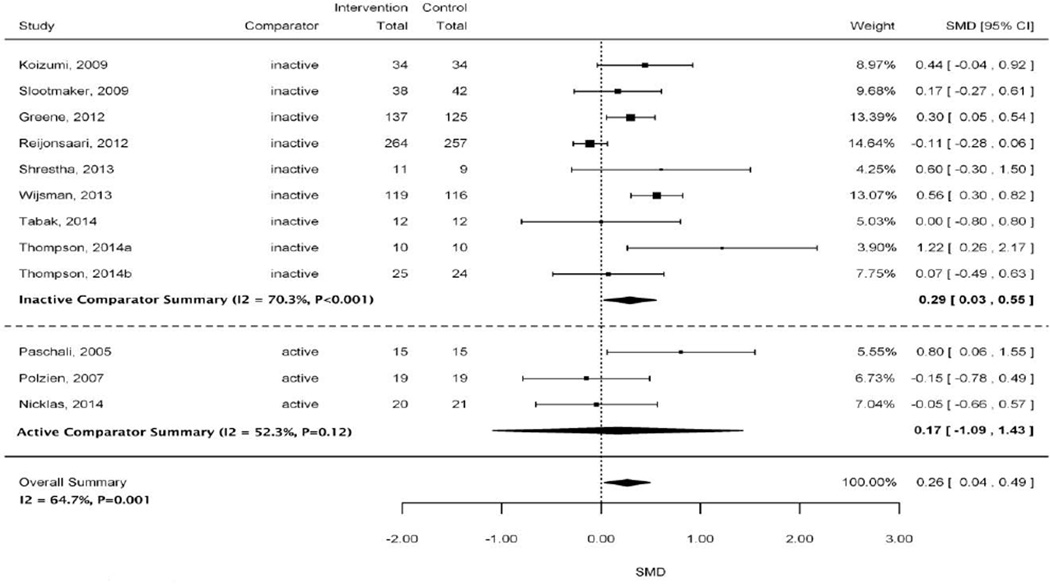
Figure 2 shows the effect of interventions that used accelerometers on physical activity with an overall pooled estimate and stratified pooled estimates by inactive and active comparator subgroups. The overall pooled estimate indicated a small, statistically significant effect for interventions using accelerometers to increase physical activity (SMD 0.26; 95% CI 0.04 to 0.49) with a high amount of heterogeneity (I2=64.7%). A similar small effect was found for interventions using accelerometers to increase physical activity when compared with an inactive comparator (SMD 0.29; 95% CI 0.03 to 0.55). This summary estimate had high heterogeneity (I2=70.3%). A small positive overall effect was also observed for accelerometer devices when compared with an active comparator, but this estimate was not statistically significant (SMD 0.17; 95% CI −1.09 to 1.43; moderate heterogeneity I2=52.3%).
Figure 2.
Forest plot of studies included in physical activity meta-analysis stratified by active and inactive comparators
Abbreviations: CI=confidence interval; SMD=standardized mean difference
Weight Loss
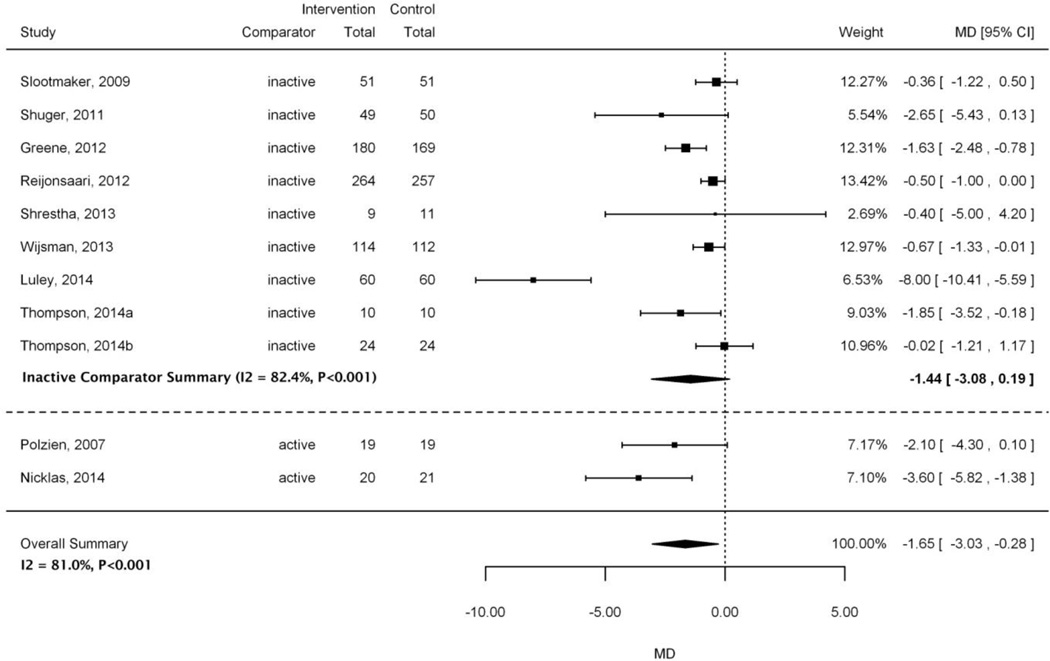
Figure 3 shows the pooled effect of the accelerometer interventions on weight loss across the 11 included trials and stratified estimates by inactive and active comparator subgroups. Overall, the pooled estimate showed a small significant effect for weight loss (MD −1.65 kg; 95% CI −3.03 to −0.28; I2=81%). Compared with inactive controls, the impact of accelerometer interventions on weight loss was similar to that observed in the overall summary estimate (MD −1.44 vs. −1.65 kg, respectively). However, the inactive pooled estimate was not statistically significant. Both the stratified and overall summary estimates displayed high heterogeneity as assessed by I2 values >80%.
Figure 3.
Forest plot of studies included in weight loss meta-analysis stratified by active and inactive comparators
Abbreviations: CI=confidence interval; MD=mean difference
Two small trials judged to be at high risk of bias compared accelerometer interventions with active comparators [29,31]. While both trials demonstrated a positive trend of weight loss (MD −3.60 to −2.10), only one study [31] was statistically significant. In that study, the accelerometer was judged to play a minor role and was paired with adjunctive interventions consisting of structured and supervised exercise training, meal preparation twice daily, and behavior counseling delivered over 5 months. In the study that was not statistically significant, the accelerometer played a major role, but the intervention was only 12 weeks [29].
Moderators
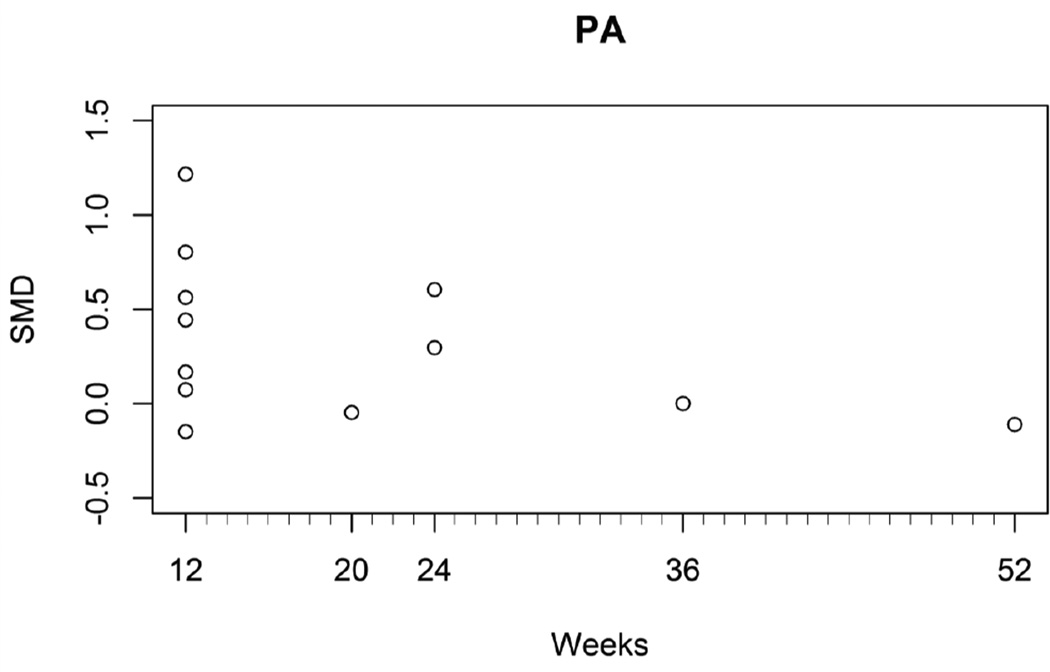
We examined several moderators (i.e., population characteristics, device location, major vs. minor role of accelerometer, intervention duration) as potentials sources to explain significant heterogeneity (Table 2). Only one study characteristic, intervention duration in weeks, was associated with a very small, negative effect on physical activity (moderator p=0.02; SMD −0.013; 95% CI −0.023 to −0.002) with low heterogeneity (I2=21.1%, p=0.24) (Figure 4). This effect was not observed for the outcome of weight loss (moderator p=0.18; MD −0.06; 95% CI −0.15 to 0.03).
Table 2.
Subgroup analyses by population, location of accelerometer, and intervention role
| Population |
||||||
|---|---|---|---|---|---|---|
| Outcome | Overweight/Obese/ Sedentary |
Older Adults | Healthy Volunteers | Chronic Medical Illnesses | ||
| 4 studies | 4 studies | 2 studies | 2 studies | |||
| Physical activity | SMD 0.35 (95% CI −0.52 to 1.22) |
SMD 0.34 (95% CI −0.11 to 0.80) |
SMD range −0.11 to 0.30 | SMD range 0.00 to 0.80 | ||
| 5 studies | 3 studies | 2 studies | 1 study | |||
| Weight | MD −1.22 kg (95% CI −2.48 to 0.02) |
MD −1.08 kg (95% CI, −5.22 to 3.06) |
MD range −1.63 to −0.50 kg | MD −8.00 kg (95% CI −10.41 to −5.59) |
||
| Location of Accelerometer | ||||||
| Waist | Arm | Wrist | Multisite | |||
| 7 studies | 2 studies | 1 study | 1 study | |||
| Physical activity | SMD 0.24 (95% CI −0.15 to 0.63) |
SMD range −0.15 to 0.60 | SMD 0.56 (95% CI 0.30 to 0.82) |
SMD 0.00 (95% CI −0.80 to 0.80) |
||
| 6 studies | 3 studies | 1 study | No studies | |||
| Weight | MD −2.01 (95% CI −4.99 to 0.97) |
MD −2.08 (95% CI −4.13 to −0.02) |
MD −0.67 (95% CI −1.33 to −0.01) |
|||
| Role of Accelerometer | ||||||
| Major Role | Minor Role | |||||
| Physical activity | SMD 0.26 (95% CI −0.02 to 0.54) |
SMD 0.28 (95% CI −0.43 to 1.00) |
||||
| Weight | MD −1.47 kg (95% CI −3.47 to 0.53) |
MD −1.99 kg (95% CI −4.10 to 0.12) |
||||
Abbreviations: CI=confidence interval; MD=mean difference; SMD=standardized mean difference
Figure 4.
Relationship between weeks of physical activity intervention duration and standardized mean difference
Abbreviations: PA=physical activity; SMD=standardized mean difference
Risk of Bias
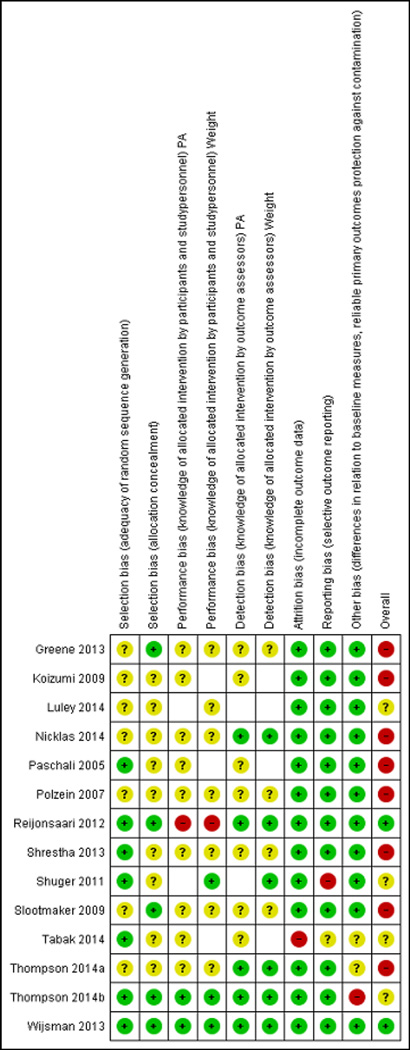
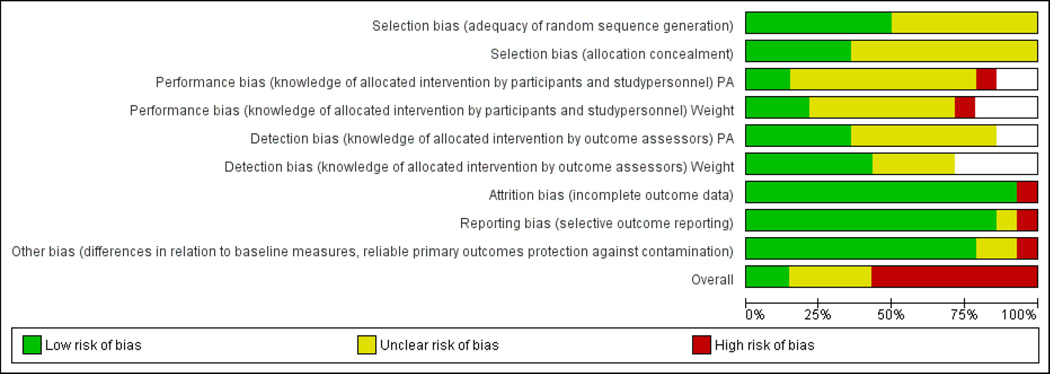
Figure 5 provides the risk of bias with our judgments for each individual domain per study, and Figure 6 provides the risk of bias with our judgments about each risk of bias item presented as total percentages across all included studies. The majority of studies (8 of 14 [57.1%]) were judged to be at high risk of bias, 4 (28.6%) were at unclear risk of bias, and only 2 studies (14.3%) were judged to be at low risk of bias. For risk of selection bias, 7 of the 14 trials (50.0%) did not give details about the method for generating the random sequence, resulting in a rating of unclear risk of bias. For the majority of trials (9 of 14 [64.3%]), there was an unclear risk of bias due to inadequate detail about allocation concealment provided by authors. In 9 of 12 trials (75.0%) involving the outcome of physical activity and 7 of 12 trials (58.3%) with the outcome of weight change, there is unclear risk of bias due to knowledge of the allocated intervention by study personnel (i.e., performance bias). In 7 of 12 trials (58.3%) involving the outcome of physical activity and 4 of 12 trials (30.8%) with the outcome of weight change, there is unclear risk of bias due to knowledge of the allocated intervention by the outcome assessor (i.e., detection bias). The majority of trials (13 of 14 [92.9%]) reported complete outcome data that included information on attrition and exclusions from analysis.
Figure 5.
Risk of bias summary: review authors’ judgments about risk of bias item for each included study
Green=low risk of bias; Yellow=unclear risk of bias; Red=high risk of bias; White=not reported Abbreviation: PA=physical activity
Figure 6.
Risk of bias as a percentage of all included studies
White=not reported
Abbreviation: PA=physical activity
DISCUSSION
We systematically reviewed the literature on the effectiveness of newer wearable technology devices for increasing physical activity levels or decreasing body weight. We identified 14 trials that met our inclusion criteria. All included studies were published in the last 10 years, indicating the relatively new use of motion-sensing technologies in studies aimed at promoting physical activity or weight loss. Although we broadly searched for wearable motion sensing technologies, no included studies used types of technologies other than accelerometers (e.g., GPS, hand gesture, eye gesture, hand swipe). In addition, all of the studies used activity data captured directly from the activity monitor device rather than from a secondary or passive activity monitor such as a smartphone.
The use of pedometers has been found to produce significant and potentially clinically relevant changes in physical activity and weight [4,5]. However, due to improvements in technology to provide more accurate measurement of movement and enhancements in delivery of feedback to participants, accelerometers are increasing in popularity. We found that interventions that integrate accelerometers produced relatively small clinical improvements in contrast to earlier finding of pedometers that reported more robust clinical improvements [5,41,42]. The differences between findings on pedometers and accelerometers are likely multifactorial and due, in part, to differences in accuracy of movement between pedometers and accelerometers, the role of devices in the overall intervention approaches, and variations in study design and risk of bias across studies.
The significant increase in physical activity levels and a significant decrease in body weight we report here were muted when accelerometer interventions were compared with more robust active comparators than with inactive controls. These main effect analyses, however, had substantial clinical and statistical heterogeneity. The variability is likely due to a combination of factors related to underlying differences in populations, comparators, interventions, and study quality issues. Further, there were substantial differences in types of outcome measures used in the physical activity studies (e.g., steps/day, hours/day, minutes/week, metabolic values), which led to challenges in conducting and interpreting the pooled estimates. Efforts to standardize collection and reporting are needed to improve interpretability across studies.
We sought to explore statistical heterogeneity in intervention effects on both physical activity and weight by multiple single factors (i.e., recruited populations, role of device relative to other motivational enhancement strategies, location of device as a proxy for ease of use, duration of intervention). Only duration of intervention was a statistically significant moderator in our analyses. Two previous systematic reviews [4,5] on pedometer-based walking programs found longer duration studies produced greater weight loss, which contrasts with our results finding no significant moderator effect with intervention duration. However, our results indicate that shorter duration programs produced a larger effect on increasing physical activity compared to longer duration programs. Several factors may influence our finding on intervention duration, including participant adherence to use of the accelerometers and the role of the accelerometer among the variety of adjunctive interventions used in these studies. Further, the majority of our included studies had an intervention duration of 12 to 24 weeks, with only a few studies reporting greater durations, which may limit our ability to fully examine the effect of studies with longer durations. However, this finding is indirectly supported by studies of supervised exercise [43,44]. In general, these studies have reported that outcomes may be influenced by an optimal and unknown intervention duration “sweet spot” that may also depend on intensity and frequency. It is also plausible that after the novelty of accelerometers wears off, so may the potential motivating effects. This reasoning is consistent with what others have found; more than half of individuals who purchase a wearable activity monitor stop using it and, of these, one-third stop use in the first months [45]. Our qualitative analyses also identified aspects that may explain the substantial variability among studies. In general, interventions that capitalized on the self-monitoring and tailored activity device-driven feedback capabilities were associated with greater decreases in weight loss. Effects were even greater when these strategies were paired with behavioral counseling focused on device feedback. The same qualitative finding was not consistently seen for greater increases in physical activity with better implementation of device-driven feedback and self-monitoring functionality.
Our review identified several gaps in the literature. We found no head-to-head comparisons between accelerometers and pedometers for our outcomes. Thus, it is unclear whether accelerometers, either independently or when coupled with a variety of adjunctive interventions, improve physical activity or weight loss over pedometers. We identified only three studies among patients with chronic medical illnesses and five studies among those who are overweight, obese, or sedentary. Use and effectiveness of accelerometers may differ among participants who are motivated to use these devices to achieve different goals; for example, those who are trying to increase physical activity to reduce pain from osteoarthritis compared with participants whose goal is to lose weight. Because of the diversity of adjunctive interventions across included trials, our review was unable to provide guidance on the optimal adjunctive interventions needed to enhance functionally of accelerometers in motivating behavior change. Our results support diminished effects of accelerometers over time. Future research should measure how often participants wear accelerometers and how participants interact with their generated data to explore facilitators and barriers to sustained interaction with these devices. Furthermore, we did not find any studies that sought to integrate physical activity data from wearable accelerometers into patients’ medical records to facilitate ongoing primary care and chronic disease management. Such research could be of real value to clinicians and policymakers. Also, the increasing inclusion of accelerometers directly into smartphones warrants clinical trials that evaluate the effectiveness of this technology.
Our review has a number of strengths, including a protocol-driven design, a comprehensive search, and careful quality assessment. Our review—and the literature—have limitations: the number of studies is small; many had design limitations (8 of 14 were judged to be at high risk of bias); the range of interventions evaluated was diverse; and the number and reporting of studies precluded any analyses of variability in accelerometers by more than one variable at a time. Our review was limited to English-language publications, but the likelihood of identifying relevant data unavailable from English-language sources is low. The small sample sizes of most included trials and the populations recruited also limited our findings. While we conducted subgroup analysis to explore multiple single factors that may contribute to heterogeneity, the observed heterogeneity is likely attributable to a combination of factors or to some that were unmeasured by our work or available in the current literature. The overall low number and small size of trials per outcome precluded us from conducting multivariable analyses.
CONCLUSION
To our knowledge, our review is the first quantitative synthesis of newer wearable activity devices and their potential effects on increasing physical activity and weight loss. We found that the use of accelerometers produces small positive effects on physical activity and weight. The small sample sizes with moderate to high heterogeneity in the current studies limit the conclusions that may be drawn. It is important to note that we were not able to isolate the individual impact of accelerometers as a standalone strategy to promote weight loss or increase physical activity because all included studies contained some level of adjunctive intervention. Future studies should focus on how best to integrate accelerometers with other strategies to increase physical activity and weight loss.
Acknowledgments
The authors would like to thank Liz Wing, MA, for assistance with manuscript preparation. Ms. Wing is an employee of the Duke Clinical Research Institute, Durham, NC, and received no compensation for her work apart from her usual salary.
APPENDIX
| Database: MEDLINE (via PubMed) | ||
|---|---|---|
| Search date: 01/06/15 | ||
| Set | Search Terms | Results |
| 1 | “Accelerometry”[Mesh] OR “Magnetometry”[Mesh] OR “Motor Activity/instrumentation”[Mesh] OR fitness track*[tiab] OR activity track*[tiab] OR fitness monitor*[tiab] OR gps[tiab] OR “global positioning”[tiab] OR activity monitor*[tiab] OR motion sens*[tiab] OR accelerometer[tiab] OR accelerometers[tiab] OR accelerometry[tiab] OR gyroscope[tiab] OR gyroscopic[tiab] OR gyroscopes[tiab] OR actograph[tiab] OR actographic[tiab] OR actography[tiab] OR actographs[tiab] OR wearable system[tiab] OR wearable systems[tiab] OR wearable sensor[tiab] OR wearable sensors[tiab] OR ((step[tiab] OR steps[tiab]) AND (counting[tiab] OR counted[tiab] OR counter[tiab] OR counters[tiab] OR count[tiab])) OR actigraph[tiab] OR (basis[tiab] AND peak[tiab]) OR “bowflex boost”[tiab] OR “fit link”[tiab] OR (misfit[tiab] AND shine[tiab]) OR (polar[tiab] AND loop[tiab]) OR bodybugg[tiab] OR bodymedia[tiab] OR fitbit[tiab] OR fitbug[tiab] OR fuelband[tiab] OR garmin[tiab] OR gowear[tiab] OR gruve[tiab] OR ibitz[tiab] OR iqua[tiab] OR lumo[tiab] OR motoactiv[tiab] OR runtastic[tiab] OR scosche[tiab] OR smartband[tiab] OR striiv[tiab] OR tomtom[tiab] OR vivofit[tiab] OR vivosmart[tiab] OR wahoo[tiab] OR wakemate[tiab] OR withings[tiab] |
52,751 |
| 2 | “Movement”[Mesh] OR “Exercise Movement Techniques”[Mesh] OR “Exercise Therapy”[Mesh] OR “Physical Fitness”[Mesh] OR “Physical Endurance”[Mesh] OR “Physical Exertion”[Mesh] OR fitness[tiab] OR activity[tiab] OR active[tiab] OR walk*[tiab] OR run*[tiab] OR step[tiab] OR steps[tiab] OR exercise[tiab] OR move*[tiab] |
3,555,057 |
| 3 | (randomized controlled trial[pt] OR controlled clinical trial[pt] OR randomized[tiab] OR randomised[tiab] OR randomization[tiab] OR randomisation[tiab] OR randomly[tiab] OR trial[tiab] OR groups[tiab]) NOT (Editorial[ptyp] OR Letter[ptyp] OR Case Reports[ptyp] OR Comment[ptyp]) |
2,079,904 |
| 4 | #1 AND #2 AND #3 | 4858 |
| 5 | #4 NOT (“Child”[Mesh] NOT “Adult”[Mesh]) | 4355 |
| 6 | #5, English, 2000 – present | 3506 |
| Database: Embase | ||
| Search date: 01/06/15 | ||
| Set | Search Terms | Results |
| 1 | ‘accelerometry’/exp OR ‘magnetometry’/exp OR (fitness NEAR/2 track*):ab,ti OR (activity NEAR/2 track*):ab,ti OR (fitness NEAR/2 monitor*):ab,ti OR gps:ab,ti OR ‘global positioning’:ab,ti OR (activity NEAR/2 monitor):ab,ti OR (motion NEAR/2 sens*):ab,ti OR accelerometer:ab,ti OR accelerometers:ab,ti OR accelerometry:ab,ti OR gyroscope:ab,ti OR gyroscopic:ab,ti OR gyroscopes:ab,ti OR actograph:ab,ti OR actographic:ab,ti OR actography:ab,ti OR actographs:ab,ti OR ‘wearable system’:ab,ti OR ‘wearable systems’:ab,ti OR ‘wearable sensor’:ab,ti OR ‘wearable sensors’:ab,ti OR ((step OR steps):ab,ti AND (counting OR counted OR counter OR counters OR count):ab,ti) OR actigraph:ab,ti OR (basis NEAR/3 peak):ab,ti,df OR ‘bowflex boost’:ab,ti,df OR ‘fit link’:ab,ti,df OR (misfit NEAR/3 shine):ab,ti,df OR (polar NEAR/3 loop):ab,ti,df OR bodybugg:ab,ti,df OR bodymedia:ab,ti,df OR fitbit:ab,ti,df OR fitbug:ab,ti,df OR fuelband:ab,ti,df OR garmin:ab,ti,df OR gowear:ab,ti,df OR gruve:ab,ti,df OR ibitz:ab,ti,df OR iqua:ab,ti,df OR lumo:ab,ti,df OR motoactiv:ab,ti,df OR runtastic:ab,ti,df OR scosche:ab,ti,df OR smartband:ab,ti,df OR striiv:ab,ti,df OR tomtom:ab,ti,df OR vivofit:ab,ti,df OR vivosmart:ab,ti,df OR wahoo:ab,ti,df OR wakemate:ab,ti,df OR withings:ab,ti,df |
45,316 |
| 2 | ‘movement (physiology)’/exp OR ‘physical activity, capacity and performance’/exp OR ‘kinesiotherapy’/exp OR ‘fitness’/exp OR fitness:ab,ti OR activity:ab,ti OR active:ab,ti OR walk*:ab,ti OR run*:ab,ti OR step:ab,ti OR steps:ab,ti OR exercise:ab,ti OR move*:ab,ti |
4,564,954 |
| 3 | (‘randomized controlled trial’/exp OR ‘crossover procedure’/exp OR ‘double blind procedure’/exp OR ‘single blind procedure’/exp OR random*:ab,ti OR factorial*:ab,ti OR crossover*:ab,ti OR (cross NEAR/1 over*):ab,ti OR (doubl* NEAR/1 blind*):ab,ti OR (singl* NEAR/1 blind*):ab,ti OR assign*:ab,ti OR allocat*:ab,ti OR volunteer*:ab,ti) NOT (‘case report’/exp OR ‘case study’/exp OR ‘editorial’/exp OR ‘letter’/exp OR ‘note’/exp) |
1,431,100 |
| 4 | #1 AND #2 AND #3 | 3250 |
| 5 | #4 NOT (‘child’/exp NOT ‘adult’/exp) | 2888 |
| 6 | #5 AND [embase]/lim NOT [medline]/lim | 1051 |
| 7 | #6, Limits: English, 2000- | 988 |
| Database: CINAHL | ||
| Search date: 01/06/15 | ||
| Set | Search Terms | Results |
| 1 | (MH “Accelerometry”) OR (MH “Magnetics+”) OR TI ( “fitness track*” or “activity track*” or “fitness monitor*” or gps or “global positioning” or “activity monitor*” or “motion sens*” or accelerometer or accelerometers or accelerometry or gyroscope or gyroscopic or gyroscopes or actograph or actographic or actography or actographs or “wearable system” or “wearable systems” or “wearable sensor” or “wearable sensors” or ((step or steps) and (counting or counted or counter or counters or count)) or actigraph or (basis and peak) or “bowflex boost” or “fit link” or (misfit and shine) or (polar and loop) or bodybugg or bodymedia or fitbit or fitbug or fuelband or garmin or gowear or gruve or ibitz or iqua or lumo or motoactiv or runtastic or scosche or smartband or striiv or tomtom or vivofit or vivosmart or wahoo or wakemate or withings ) OR AB ( “fitness track*” or “activity track*” or “fitness monitor*” or gps or “global positioning” or “activity monitor*” or “motion sens*” or accelerometer or accelerometers or accelerometry or gyroscope or gyroscopic or gyroscopes or actograph or actographic or actography or actographs or “wearable system” or “wearable systems” or “wearable sensor” or “wearable sensors” or ((step or steps) and (counting or counted or counter or counters or count)) or actigraph or (basis and peak) or “bowflex boost” or “fit link” or (misfit and shine) or (polar and loop) or bodybugg or bodymedia or fitbit or fitbug or fuelband or garmin or gowear or gruve or ibitz or iqua or lumo or motoactiv or runtastic or scosche or smartband or striiv or tomtom or vivofit or vivosmart or wahoo or wakemate or withings ) |
14,089 |
| 2 | (MH “Movement+”) OR (MH “Exercise+”) OR (MH “Therapeutic Exercise+”) OR (MH “Physical Activity”) OR (MH “Physical Fitness+”) OR (MH “Exertion+”) OR TI ( OR fitness OR activity OR active OR walk* OR run* OR step OR steps OR exercise OR move* ) OR AB ( OR fitness OR activity OR active OR walk* OR run* OR step OR steps OR exercise OR move* ) |
361,653 |
| 3 | (MH “Treatment Outcomes+”) OR randomized OR PT clinical trial | 317,587 |
| 4 | #1 AND #2 AND #3 | 636 |
| 5 | #4, English, 2000- | 602 |
| Database: SPORTDiscus | ||
| Search date: 01/06/15 | ||
| Set | Search Terms | Results |
| 1 | DE “ACCELEROMETERS” OR TI ( “fitness track*” or “activity track*” or “fitness monitor*” or gps or “global positioning” or “activity monitor*” or “motion sens*” or accelerometer or accelerometers or accelerometry or gyroscope or gyroscopic or gyroscopes or actograph or actographic or actography or actographs or “wearable system” or “wearable systems” or “wearable sensor” or “wearable sensors” or ((step or steps) and (counting or counted or counter or counters or count)) or actigraph or (basis and peak) or “bowflex boost” or “fit link” or (misfit and shine) or (polar and loop) or bodybugg or bodymedia or fitbit or fitbug or fuelband or garmin or gowear or gruve or ibitz or iqua or lumo or motoactiv or runtastic or scosche or smartband or striiv or tomtom or vivofit or vivosmart or wahoo or wakemate or withings ) OR AB ( “fitness track*” or “activity track*” or “fitness monitor*” or gps or “global positioning” or “activity monitor*” or “motion sens*” or accelerometer or accelerometers or accelerometry or gyroscope or gyroscopic or gyroscopes or actograph or actographic or actography or actographs or “wearable system” or “wearable systems” or “wearable sensor” or “wearable sensors” or ((step or steps) and (counting or counted or counter or counters or count)) or actigraph or (basis and peak) or “bowflex boost” or “fit link” or (misfit and shine) or (polar and loop) or bodybugg or bodymedia or fitbit or fitbug or fuelband or garmin or gowear or gruve or ibitz or iqua or lumo or motoactiv or runtastic or scosche or smartband or striiv or tomtom or vivofit or vivosmart or wahoo or wakemate or withings ) |
6204 |
| 2 | (random* OR trial) | 56299 |
| 3 | #1 AND #2 | 639 |
| 4 | #3, English, 2000-, Academic Journals | 543 |
| Database: Cochrane CENTRAL | ||
| Search date: 01/06/15 | ||
| Set | Search Terms | Results |
| 1 | [mh Accelerometry] OR [mh Magnetometry] | 341 |
| 2 | “fitness track*”:ab,ti or “activity track*”:ab,ti or “fitness monitor*”:ab,ti or gps:ab,ti or “global positioning”:ab,ti or “activity monitor*”:ab,ti or “motion sens*”:ab,ti or accelerometer:ab,ti or accelerometers:ab,ti or accelerometry:ab,ti or gyroscope:ab,ti or gyroscopic:ab,ti or gyroscopes:ab,ti or actograph:ab,ti or actographic:ab,ti or actography:ab,ti or actographs:ab,ti or “wearable system”:ab,ti or “wearable systems”:ab,ti or “wearable sensor”:ab,ti or “wearable sensors”:ab,ti or ((step:ab,ti or steps:ab,ti) and (counting:ab,ti or counted:ab,ti or counter:ab,ti or counters:ab,ti or count:ab,ti)) or actigraph:ab,ti or (basis:ab,ti and peak:ab,ti) or “bowflex boost”:ab,ti or “fit link”:ab,ti or (misfit:ab,ti and shine:ab,ti) or (polar:ab,ti and loop:ab,ti) or bodybugg:ab,ti or bodymedia:ab,ti or fitbit:ab,ti or fitbug:ab,ti or fuelband:ab,ti or garmin:ab,ti or gowear:ab,ti or gruve:ab,ti or ibitz:ab,ti or iqua:ab,ti or lumo:ab,ti or motoactiv:ab,ti or runtastic:ab,ti or scosche:ab,ti or smartband:ab,ti or striiv:ab,ti or tomtom:ab,ti or vivofit:ab,ti or vivosmart:ab,ti or wahoo:ab,ti or wakemate:ab,ti or withings:ab,ti |
2945 |
| 3 | #1 OR #2 | 3204 |
| 4 | [mh “Movement”] OR [mh “Exercise Movement Techniques”] OR [mh “Exercise Therapy”] OR [mh “Physical Fitness”] OR [mh “Physical Endurance”] OR [mh “Physical Exertion”] |
29,268 |
| 5 | fitness:ab,ti OR activity:ab,ti OR active:ab,ti OR walk*:ab,ti OR run*:ab,ti OR step:ab,ti OR steps:ab,ti OR exercise:ab,ti OR move*:ab,ti |
134,034 |
| 6 | #4 OR #5 | 140,071 |
| Set | Search Terms | Results |
| 7 | #3 AND #6 | 1630 |
| 8 | #5, 2000 – present, In Trials | 1281 |
Footnotes
CONFLICT OF INTEREST STATEMENT
This report is based on research conducted by the Evidence-based Synthesis Program (ESP) Center located at the Durham VA Medical Center, Durham, NC, funded by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Quality Enhancement Research Initiative. The findings and conclusions in this document are those of the author(s) who are responsible for its contents; the findings and conclusions do not necessarily represent the views of the Department of Veterans Affairs or the United States government. Therefore, no statement in this article should be construed as an official position of the Department of Veterans Affairs.
No investigators have any affiliations or financial involvement (eg, employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties) that conflict with material presented in the report.
REFERENCES
- 1.U.S. Department of Health and Human Services. Physical Activity and Health: A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion; 1996. [Accessed June 23, 2015]. www.cdc.gov/nccdphp/sgr/contents.htm. [Google Scholar]
- 2.Burke LE, Wang J, Sevick MA. Self-monitoring in weight loss: a systematic review of the literature. J Am Diet Assoc. 2011;111:92–102. doi: 10.1016/j.jada.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tudor-Locke C, Lutes L. Why do pedometers work? A reflection upon the factors related to successfully increasing physical activity. Sports Med. 2009;39:981–993. doi: 10.2165/11319600-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 4.Bravata DM, Smith-Spangler C, Sundaram V, et al. Using pedometers to increase physical activity and improve health: a systematic review. JAMA. 2007;298:2296–2304. doi: 10.1001/jama.298.19.2296. [DOI] [PubMed] [Google Scholar]
- 5.Richardson CR, Newton TL, Abraham JJ, Sen A, Jimbo M, Swartz AM. A meta-analysis of pedometer-based walking interventions and weight loss. Ann Fam Med. 2008;6:69–77. doi: 10.1370/afm.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bassett DR, Jr, Dinesh J. Use of pedometers and accelerometers in clinical populations: validity and reliability issues. Physical Therapy Reviews. 2010;15:135–142. [Google Scholar]
- 7.Tudor-Locke C. Taking steps toward increasing physical activity: using pedometers to measure and motivate. [Accessed June 23, 2015];President’s Council on Physical Fitness and Sports Research Digest. 2002 Jun;(17) Series 3 www.presidentschallenge.org/informed/digest/docs/200206digest.pdf.
- 8.Swift DL, Dover SE, Nevels TR, et al. The intervention composed of aerobic training and non-exercise physical activity (I-CAN) study: Rationale, design and methods. Contemp Clin Trials. 2015;45:435–442. doi: 10.1016/j.cct.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 9.Le Masurier GC, Tudor-Locke C. Comparison of pedometer and accelerometer accuracy under controlled conditions. Medicine & Science in Sports & Exercise. 2003;35:867–871. doi: 10.1249/01.MSS.0000064996.63632.10. [DOI] [PubMed] [Google Scholar]
- 10.Tyo BM, Fitzhugh EC, Bassett DR, Jr, John D, Feito Y, Thompson DL. Effects of body mass index and step rate on pedometer error in a free-living environment. Med Sci Sports Exerc. 2011;43:350–356. doi: 10.1249/MSS.0b013e3181e9b133. [DOI] [PubMed] [Google Scholar]
- 11.Gonzalez C, Herrero P, Cubero JM, et al. PREDIRCAM eHealth platform for individualized telemedical assistance for lifestyle modification in the treatment of obesity, diabetes, and cardiometabolic risk prevention: a pilot study (PREDIRCAM 1) J Diabetes Sci Technol. 2013;7:888–897. doi: 10.1177/193229681300700411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lyons EJ, Lewis ZH, Mayrsohn BG, Rowland JL. Behavior change techniques implemented in electronic lifestyle activity monitors: a systematic content analysis. J Med Internet Res. 2014;16:e192. doi: 10.2196/jmir.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Troiano RP, Berrigan D, Dodd KW, Masse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40:181–188. doi: 10.1249/mss.0b013e31815a51b3. [DOI] [PubMed] [Google Scholar]
- 14.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. W264. [DOI] [PubMed] [Google Scholar]
- 15.Lefebvre C, Manheimer E, Glanville J. Higgins JPT, Green GreenS, editors. Chapter 6: Searching for studies. [Accessed July 22, 2014];Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration. 2011 www.cochrane-handbook.org.
- 16.Baker PR, Francis DP, Soares J, Weightman AL, Foster C. Community wide interventions for increasing physical activity. Cochrane Database Syst Rev. 2015;1:Cd008366. doi: 10.1002/14651858.CD008366.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mansi S, Milosavljevic S, Baxter GD, Tumilty S, Hendrick P. A systematic review of studies using pedometers as an intervention for musculoskeletal diseases. BMC Musculoskelet Disord. 2014;15:231. doi: 10.1186/1471-2474-15-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mays RJ, Rogers RK, Hiatt WR, Regensteiner JG. Community walking programs for treatment of peripheral artery disease. J Vasc Surg. 2013;58:1678–1687. doi: 10.1016/j.jvs.2013.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vaes AW, Cheung A, Atakhorrami M, et al. Effect of ‘activity monitor-based’ counseling on physical activity and health-related outcomes in patients with chronic diseases: A systematic review and meta-analysis. Ann Med. 2013;45:397–412. doi: 10.3109/07853890.2013.810891. [DOI] [PubMed] [Google Scholar]
- 20.De Bourdeaudhuij I, Van Dyck D, De Meester F, et al. Built environment, physical activity, and obesity. Int J Obes (Lond) 2011;35:S149. [Google Scholar]
- 21.Bort-Roig J, Gilson N, Puig-Ribera A, Contreras R, Trost S. Measuring and Influencing Physical Activity with Smartphone Technology: A Systematic Review. Sports Med. 2014;44:671–686. doi: 10.1007/s40279-014-0142-5. [DOI] [PubMed] [Google Scholar]
- 22.Bellet RN, Adams L, Morris NR. The 6-minute walk test in outpatient cardiac rehabilitation: validity, reliability and responsiveness—a systematic review. Physiotherapy. 2012;98:277–287. doi: 10.1016/j.physio.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 23.Higgins JPT, Altman DG, Sterne JAC. Higgins JPT, SGreen S, editors. Chapter 8: Assessing risk of bias in included studies. [Accessed June 23, 2015];Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration. 2011 www.cochrane-handbook.org.
- 24.Cornell JE, Mulrow CD, Localio R, et al. Random-effects meta-analysis of inconsistent effects: a time for change. Ann Intern Med. 2014;160:267–270. doi: 10.7326/M13-2886. [DOI] [PubMed] [Google Scholar]
- 25.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd. Hillsdale, NJ: Lawrence Earlbaum Associates; 1988. [Google Scholar]
- 26.Viechtbauer W. Conducting meta-analyses in R with the metafor package. Journal of Statistical Software. 2010;36:1–48. [Google Scholar]
- 27.Luley C, Blaik A, Gotz A, et al. Weight loss by telemonitoring of nutrition and physical activity in patients with metabolic syndrome for 1 year. J Am Coll Nutr. 2014;33:363–374. doi: 10.1080/07315724.2013.875437. [DOI] [PubMed] [Google Scholar]
- 28.Shuger SL, Barry VW, Sui X, et al. Electronic feedback in a diet- and physical activity-based lifestyle intervention for weight loss: a randomized controlled trial. Int J Behav Nutr Phys Act. 2011;8:41. doi: 10.1186/1479-5868-8-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Polzien KM, Jakicic JM, Tate DF, Otto AD. The efficacy of a technology-based system in a short-term behavioral weight loss intervention. Obesity (Silver Spring) 2007;15:825–830. doi: 10.1038/oby.2007.584. [DOI] [PubMed] [Google Scholar]
- 30.Tabak M, Brusse-Keizer M, van der Valk P, Hermens H, Vollenbroek-Hutten M. A telehealth program for self-management of COPD exacerbations and promotion of an active lifestyle: a pilot randomized controlled trial. Int J Chron Obstruct Pulmon Dis. 2014;9:935–944. doi: 10.2147/COPD.S60179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nicklas BJ, Gaukstern JE, Beavers KM, Newman JC, Leng X, Rejeski WJ. Self-monitoring of spontaneous physical activity and sedentary behavior to prevent weight regain in older adults. Obesity (Silver Spring) 2014;22:1406–1412. doi: 10.1002/oby.20732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thompson WG, Koepp GA, Levine JA. Increasing physician activity with treadmill desks. Work. 2014;48:47–51. doi: 10.3233/WOR-131708. [DOI] [PubMed] [Google Scholar]
- 33.Wijsman CA, Westendorp RG, Verhagen EA, et al. Effects of a web-based intervention on physical activity and metabolism in older adults: randomized controlled trial. J Med Internet Res. 2013;15:e233. doi: 10.2196/jmir.2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thompson WG, Kuhle CL, Koepp GA, McCrady-Spitzer SK, Levine JA. “Go4Life” exercise counseling, accelerometer feedback, and activity levels in older people. Arch Gerontol Geriatr. 2014;58:314–319. doi: 10.1016/j.archger.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 35.Greene J, Sacks R, Piniewski B, Kil D, Hahn JS. The impact of an online social network with wireless monitoring devices on physical activity and weight loss. J Prim Care Community Health. 2013;4:189–194. doi: 10.1177/2150131912469546. [DOI] [PubMed] [Google Scholar]
- 36.Shrestha M, Combest T, Fonda SJ, Alfonso A, Guerrero A. Effect of an accelerometer on body weight and fitness in overweight and obese active duty soldiers. Mil Med. 2013;178:82–87. doi: 10.7205/milmed-d-12-00275. [DOI] [PubMed] [Google Scholar]
- 37.Reijonsaari K, Vehtari A, Kahilakoski OP, van Mechelen W, Aro T, Taimela S. The effectiveness of physical activity monitoring and distance counseling in an occupational setting - results from a randomized controlled trial (CoAct) BMC Public Health. 2012;12:344. doi: 10.1186/1471-2458-12-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koizumi D, Rogers NL, Rogers ME, Islam MM, Kusunoki M, Takeshima N. Efficacy of an accelerometer-guided physical activity intervention in community-dwelling older women. J Phys Act Health. 2009;6:467–474. doi: 10.1123/jpah.6.4.467. [DOI] [PubMed] [Google Scholar]
- 39.Slootmaker SM, Chinapaw MJ, Schuit AJ, Seidell JC, Van Mechelen W. Feasibility and effectiveness of online physical activity advice based on a personal activity monitor: randomized controlled trial. J Med Internet Res. 2009;11:e27. doi: 10.2196/jmir.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paschali AA, Goodrick GK, Kalantzi-Azizi A, Papadatou D, Balasubramanyam A. Accelerometer feedback to promote physical activity in adults with type 2 diabetes: a pilot study. Percept Mot Skills. 2005;100:61–68. doi: 10.2466/pms.100.1.61-68. [DOI] [PubMed] [Google Scholar]
- 41.Heesch KC, Dinger MK, McClary KR, Rice KR. Experiences of women in a minimal contact pedometer-based intervention: a qualitative study. Women Health. 2005;41:97–116. doi: 10.1300/J013v41n02_07. [DOI] [PubMed] [Google Scholar]
- 42.Tudor-Locke C, Myers AM, Rodger NW. Formative evaluation of the First Step Program: a practical intervention to increase daily physical activity. Can J Diabetes Care. 2000;47(1):23–28. [Google Scholar]
- 43.Marcus BH, Williams DM, Dubbert PM, et al. Physical activity intervention studies: what we know and what we need to know: a scientific statement from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism (Subcommittee on Physical Activity); Council on Cardiovascular Disease in the Young; and the Interdisciplinary Working Group on Quality of Care and Outcomes Research. Circulation. 2006;114:2739–2752. doi: 10.1161/CIRCULATIONAHA.106.179683. [DOI] [PubMed] [Google Scholar]
- 44.Linke SE, Gallo LC, Norman GJ. Attrition adherence rates of sustained vs. intermittent exercise interventions. Ann Behav Med. 2011;42:197–209. doi: 10.1007/s12160-011-9279-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nielsen Newswire. [Accessed July 2, 2015];Are consumers really interested in wearing tech on their sleeves? 2014 Mar 20; www.nielsen.com/us/en/insights/news/2014/tech-styles-are-consumers-really-interested-in-wearing-tech-on-their-sleeves.html.