Abstract
Oxidized low-density lipoprotein (OxLDL), which contains hundreds of different oxidized lipid molecules, is a hallmark of hyperlipidemia and atherosclerosis. The same oxidized lipids found in OxLDL are also formed in apoptotic cells, and are present in tissues as well as in the circulation under pathological conditions. In many disease contexts, oxidized lipids constitute damage signals, or patterns, that activate pattern-recognition receptors and significantly contribute to inflammation. This article reviews recent discoveries and emerging trends in the field of oxidized lipids and regulation of inflammation, focusing on oxidation products of polyunsaturated fatty acids esterified into cholesteryl esters and phospholipids. The paper highlights context-dependent activation and biased agonism of TLR4 and the NLRP3 inflammasome, among other signaling pathways activated by oxidized lipids.
Keywords: oxidized lipid, inflammation, toll-like receptor, inflammasome
Complexity of OxLDL
A PubMed search for oxidized low-density lipoprotein (OxLDL) yields between 6,000 –9,000 papers, depending on spelling. These publications refer to OxLDL as a complex lipoprotein consisting of roughly 600 molecules of unesterified (free) cholesterol (FC), 1600 cholesteryl esters (CE), 700 phospholipids (PL), 185 triglycerides (TG), and one molecule of apolipoprotein B-100 (apoB). CE, PL and TG are all esters incorporating one, two or three fatty acyl chains, respectively. Among the variety of saturated and non-saturated fatty acyls, linoleic (LA), arachidonic (AA), and docosahexaenoic (DHA) acids are common polyunsaturated fatty acyls (PUFA) in these esters.
Cholesterol and PUFA are susceptible to enzymatic and free radical oxidation, producing numerous - on the scale of hundreds - oxidative products. Furthermore, OxCE and OxPL can covalently modify apoB and other proteins; can be hydrolyzed by lipases to produce oxidized free fatty acids; and can decompose to produce highly reactive end products, like malondialdehyde (MDA) or 4-hydroxy-2-nonenal (4-HNE), which in turn covalently modify proteins and some phospholipids [1]. This partial list underscores the complex composition of OxLDL.
Evidently, all of these products are unlikely to occur in one OxLDL particle simultaneously, which would otherwise require a prolonged incubation of native LDL with ions of transition metals, e.g., copper. Examples of a more “physiologic” models of OxLDL are made from LDL in vitro with cells overexpressing 15-lipoxygenase (15-LO), incubated with myeloperoxidase, hemoglobin, or free radical generators [2, 3]. The important fact is that regardless of the in vitro model, many of the oxidized lipid species found in OxLDL have also been found in human blood, atherosclerotic lesions, inflamed lung, multiple sclerosis brain, and rheumatoid joints - to mention only a few from the long list of pathological conditions and associated tissues [4–7].
Part of the reason for this widespread occurrence of oxidized lipids and their covalent adducts to proteins is that they arise not only in LDL (and HDL) as a consequence of an oxidative insult, but also in cells, intracellularly and on the cell surface, both under physiological conditions and particularly under stress and during apoptosis.
The DAMPs concept
Oxidized lipids and lipoproteins exert profound biological effects, both homeostatic and adverse, depending on duration and tissue context. This is often explained as due to the formation of damage-associated molecular patterns (DAMPs) arising from the oxidative damage of lipids and lipoproteins [4, 8]. Host-derived DAMPs share common structural motifs with microbial pathogen-associated molecular patterns (PAMPs) and/or activate the same pattern-recognition receptors (PRRs) present on immune and vascular cells.
The phosphocholine (PC) epitope is one such example. PC is the most common headgroup of PLs and is not recognized by any PRR in native LDL or on the surface of viable cells. However, an exposed PC of OxPL, such as POVPC [1-palmitoyl-2-(5-oxovaleroyl)-sn-glycero-3-phosphocholine], in OxLDL and on apoptotic cells is a major ligand that mediates the binding of OxLDL to CD36 and scavenger receptor class B type I (SR-B1). CD36 recognizes the PC on both free POVPC and POVPC covalently linked to apoB, as does the soluble PRR natural antibody E06/T15 [9–12]. These properties make host-derived PC in OxPL, but not in non-oxidized PL, a DAMP. The same PC group is an exposed moiety on the cell wall polysaccharide of common infectious pathogens, including pneumococci, which makes bacterial PC a PAMP. Immunizing Ldlr−/− mice with Streptococcus pneumoniae raised E06/T15 titers and protected mice from diet-induced atherosclerosis, the pathology in which OxPL plays a major role [13].
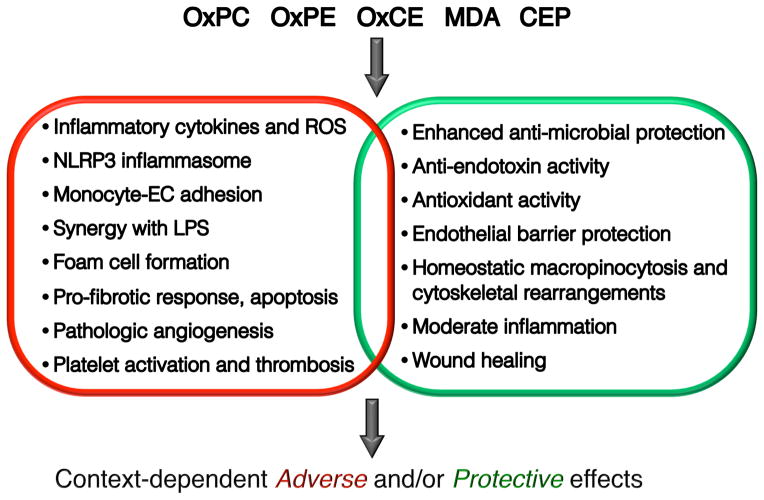
The concept of DAMPs and PAMPs activating the same PRRs implies that the end result of this activation would be similar, if not the same. However, this is not always the case. In the mechanisms described below, we will emphasize similarities, but also the differences and the bias in PRR activation by DAMPs as compared to PAMPs. We will also describe both adverse and protective effects of oxidized lipids occurring in different tissue and pathophysiological contexts (Fig. 1).
Figure 1. Context-dependent adverse and protective effects of oxidized lipids.
This chart summarizes, in an abbreviated format, major adverse and protective effects of key oxidized lipids reviewed in this article. It also emphasizes that a specific cellular response to oxidized lipids can have adverse and/or protective effects, depending on the tissue and pathology context. OxPC, oxidized phosphatidylcholine; OxPE, oxidized phosphatidylethanolamine; OxCE, oxidized cholesteryl ester; MDA, malondialdehyde [including MDA derivatives, such as MAA (malondialdehyde-acetaldehyde)]; CEP, 2-(ω-carboxyethyl)pyrrole [oxidation end product of DHA (docosahexaenoic acid)].
Biased activation of TLR4 by OxCE and OxPE vs. LPS in macrophages
Oxidized cholesteryl esters (OxCEs) have been identified as active components in the OxLDL produced by LDL incubation with cells expressing 15-LO [14–16]. Unlike an OxLDL oxidized with Cu2+, the 15-LO-modified, minimally oxidized LDL (mmLDL) does not contain advanced lipid oxidation products and does not bind to CD36. In particular, cholesteryl (9,11)-epidioxy-15-hydroperoxy-(5Z,13E)-prostadienoate (abbreviated as BEP-CE for the presence of bicyclic endoperoxide and hydroperoxide groups) in mmLDL is produced by 15-LO oxidation of cholesteryl arachidonate. BEP-CE stimulate macrophages to accumulate lipid via macropinocytosis and to express inflammatory cytokines, among other effects [14–16]. BEP-CE and other OxCEs have been detected in human blood and atherosclerotic lesions [16, 17]. mmLDL binds to CD14 [18], a receptor that recognizes bacterial lipopolysaccharide (LPS) and presents it to toll-like receptor-4 (TLR4)/lymphocyte antigen 96 (MD-2). Unlike wild type macrophages, Tlr4 mutant and Tlr4−/− primary macrophages fail to produce inflammatory cytokines in response to mmLDL or OxCE [15, 16, 18]. OxCE also induces MD-2 recruitment to TLR4 and TLR4 dimerization [16].
In the TLR4/MD-2 heterodimer, MD-2 is an LPS-binding receptor, which provides a hydrophobic pocket for five of the six saturated fatty acyl chains of LPS. The sixth fatty acyl forms a hydrophobic interaction with the TLR4 of a different TLR4/MD-2 pair. Furthermore, the phosphate groups of LPS contribute to the assembly of a TLR4/MD-2 tetramer by forming ionic interactions with a cluster of positively charged residues in TLR4 and MD-2 [19]. In this LPS-induced receptor complex, the intracellular toll/interleukin 1 receptor (TIR) homology domains of two molecules of TLR4 dimerize and recruit adaptor molecules, which initiates signaling cascades, eventually resulting in robust inflammatory responses [19]. The cholesterol molecule has congruent architecture to dock into the MD-2 binding pocket. Its hydrocarbon chain, together with the steroid, form an elongated hydrophobic structure that may dock in the hydrophobic pocket of MD-2. In addition, a hydroxyl group linked to the other side of the steroid may stabilize cholesterol at the positively charged entrance to the pocket. Indeed, experimental evidence demonstrates that MD-2 binds cholesterol, and in fact, MD-2 in human plasma and in mouse atherosclerotic lesions are associated with unesterified cholesterol [20]. It is unlikely that unesterified cholesterol binding to MD-2 activates TLR4. However, both LPS and, importantly, OxCE-modified bovine serum albumin (BSA) compete with cholesterol for MD-2 binding [20]. It is therefore plausible to suggest that the polyoxygenated fatty acyl chain in BEP-CE and/or components of OxCE-protein conjugates provide additional interaction surfaces, which, in combination with cholesterol anchoring in the MD-2 hydrophobic pocket, contribute to OxCE-induced TLR4 dimerization, which was observed experimentally [16].
The MD-2 recognition and TLR4 signaling also mediate biological effects of oxidized phosphatidylethanolamine (OxPE) [21]. The authors studied TLR4/MD-2 activation by extracellular vesicles (EVs) secreted by 15-LO expressing cells and identified a 15-LO oxidation product of a rare PE, 1,2-diarachidonoyl-sn-glycero-3-phosphoethanolamine, as a molecule capable of TLR4 activation. Molecular modeling suggested that 15-H(p)ETE-PE docks in the MD-2 binding pocket together with a resident fatty acid. The hydroxylated sn-2 chain, protruding from the MD-2 binding pocket, then interacts with the hydrophobic patch of the TLR4 ectodomain [21]. In agreement, 15-H(p)ETE-PE stimulated TLR4-dependent IL-6 and IL-4 secretion from macrophages.
Remarkably, despite the fact that both OxCE and OxPE bind to MD-2 and stimulate TLR4 dimerization, downstream TLR4 signaling induced by OxCE and OxPE differs from that induced by LPS. Myeloid differentiation primary response 88 (MyD88) is a TLR4 adaptor that mediates the majority of LPS effects at the plasma membrane and initiates a robust, often excessive, NF-κB-dependent inflammatory cytokine response [22]. In contrast, spleen tyrosine kinase (SYK) has been identified as a kinase, which is recruited to TLR4 and mediates the majority of mmLDL- and OxCE-induced effects in macrophages. Those effects were characterized by a modest AP-1 dependent cytokine production, but more profound cytoskeletal responses, macropinocytosis-associated intracellular lipid accumulation, and foam cell formation [15, 23, 24]. EV-associated OxPE, in addition to IL-6, induced secretion of the anti-inflammatory IL-4 [21]. One can conclude that at low levels OxCE and OxPE activation of TLR4 results in a more homeostatic response, in contrast to the acute and excessive inflammatory response to LPS. However, under conditions of increased and chronic production of OxCE and OxPE, such as in hyperlipidemia, low-grade but prolonged response would result in chronic inflammation and promote atherosclerosis. In agreement, the SYK inhibitor fostamatinib, administered at the beginning and during feeding Ldlr−/− or Apoe−/− mice a high-cholesterol diet (HCD), reduced monocytosis, vascular inflammation and atherosclerotic lesion size [25, 26]. However, fostamatinib, administered to Apoe−/− mice 8 weeks after the start of HCD feeding, failed to prevent progression of established plaques [26]. In part, this could be explained by degradation of early oxidation products like BEP-CE and diminished role of the TLR4/SYK pathway in advanced atherosclerotic plaques.
In addition to the pattern-recognition character of TLR4, the differences between LPS-vs. OxCE- or OxPE-mediated TLR4 responses attest to the TLR4 functional selectivity, often termed “biased agonism”. This concept helps explain the instances when different ligands of the same receptor preferentially activate one signaling pathway over the other, which infers the multidimensionality of receptor signaling.
Inflammatory effects of OxPL in endothelial cells
The vascular endothelium not only serves as a permeable barrier, but also proactively responds to many physiological and pathophysiological events in the circulation and the vessel wall. Healthy endothelium exerts its functions through regulation of vascular tone and anticoagulant/fibrinolytic effects. Endothelial cells (ECs) can also serve as sentinels that sense PAMPs [27]. As introduced in the previous section, OxLDL can promote macrophage lipid accumulation and foam cell formation. Ample evidence indicates that OxLDL also plays a central role in endothelial dysfunction leading to atherosclerosis [28].
Initially, OxPLs, present in OxLDL, were identified as strong endothelial activators [29]. POVPC, introduced in the earlier section, is only one of many products of oxidation of PAPC [1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine], a common PL. Other products include but not limited to PEIPC [1-palmitoyl-2-(5,6-epoxyisoprostanoyl)-sn-glycero-3-phosphocholine], PGPC [1-palmitoyl-2-glutaryl-sn-glycero-3-phosphocholine], and KOdiA-PC [1-palmitoyl-2-(5-keto-6-octene-dioyl)-sn-glycero-3-phosphocholine]. These and other OxPLs have been used individually or more often as a mixture of oxidized PAPC (OxPAPC) in many in vitro and in vivo studies. High levels of OxPL are found in hyperlipidemic human plasma and in atherosclerotic lesions [17, 30, 31], suggesting the involvement of OxPL in vascular impairment. Treatment of cultured ECs with OxPAPC increases the expression of the CS-1 segment of fibronectin, MCP-1, and IL-8 [32, 33]. OxPAPC effects are mediated by a number of cell surface receptors, including CD36, SR-B1, TLR2, EP2, PAF receptor and TMEM30a, but OxPAPC does not activate TLR4 [8, 28, 34–37]. Although CD36 is a major scavenger receptor for OxPAPC, CD36 is abundant in macrophages but not in ECs, which suggests that signaling pathways elicited by OxPAPC and the consequent gene expression profile in ECs may be distinct from those in macrophages. This was addressed in a study in which primary ECs obtained from 149 individuals were activated by OxPAPC [38]. Among other findings, this work has demonstrated that individual variations in heme oxygenase-1 (HO-1) expression largely determined the architecture of signaling networks activated by OxPAPC. Inflammatory effects of OxPAPC in ECs are counteracted by NOTCH1, which itself is suppressed by circulating OxPL [39]. In addition to ligand/receptor binding, OxPAPC also affects EC membrane stiffness by depleting membrane cholesterol [40].
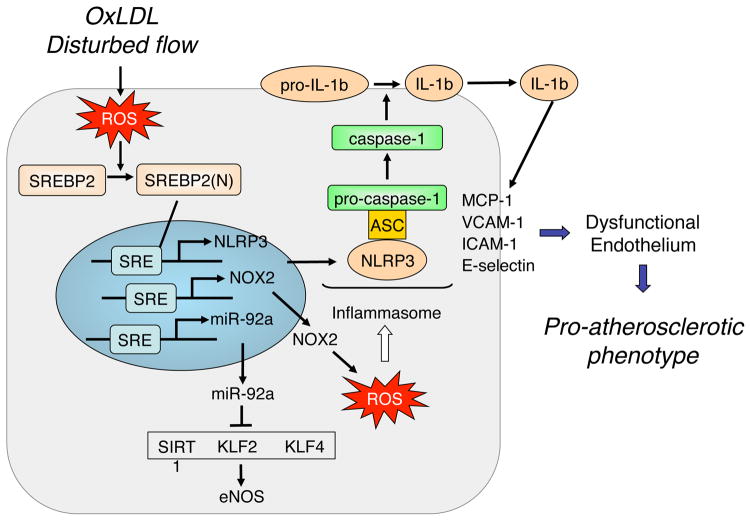
The inflammasome is a major component of innate immunity originally defined in macrophages [41, 42]. Acting as PAMPs and DAMPs, multiple infectious pathogens and molecules arising under the conditions of sterile inflammation induce the NLRP3 inflammasome. Upon activation, there is subsequent cleavage and activation of caspase-1 and release of IL-1β/IL-18 by monocytes/macrophages [43, 44]. A prevalent hypothesis of inflammasome induction involves a two-step mechanism. In macrophages, Signal 1 involves the TLR/NF-κB-regulated transcriptional upregulation of NLRP3 and pro-IL-1β/pro-IL-18. Signal 2 stimuli, including exogenous ATP, K+ efflux, and ROS, induce the assembly of the inflammasome and hence the activation of caspase-1 for the proteolytic cleavage of pro-IL-1β/pro-IL-18 [43, 44]. The NLRP3 inflammasome induction is implicated in atherosclerosis. Ldlr−/− mice receiving bone-marrow-derived monocytes/macrophages lacking the core component proteins of inflammasome (i.e., NLRP3, ASC, or IL-1β) are resistant to atherosclerosis [44]. The uptake of OxLDL by CD36 induces NLRP3 inflammasome in macrophages, which is impaired in Apoe−/− Cd36−/− mice. These mice also exhibited less atherosclerosis [45]. Although information on NLRP3 inflammasome in ECs is scant, recent studies reveal that pro-inflammatory conditions with elevated oxidative stress can also elicit the inflammasome-related innate immune response in ECs (Fig. 2). Mechanical stimuli, such as disturbed flow, induce the NLRP3 inflammasome in cultured ECs [46]. In vivo, NLRP3 inflammasome is induced in the intima of the aortic arch, an area of disturbed blood flow-induced elevated oxidative burden, in C57BL/6 mice. It appears that angiotensin II, OxLDL and OxPAPC all induce NLRP3 inflammasome in ECs, which largely depends on the increased oxidative stress [47, 48].
Figure 2. NLRP3 inflammasome induction in ECs.
Acting as DAMPs, OxLDL, OxPL, and disturbed flow, increase oxidative stress in the endothelium leading to the induction of SREBP2 promoting the downstream transcriptional activation of NLRP3, NOX2, and miR-92a. Consequently, the inflammasome component proteins (e.g., NLRP3) and oxidative burden in ECs are increased, which result in the induction of the NLRP3 inflammasome. As a result of elevated levels of IL-1β, the innate immune response in ECs is augmented, exemplified by the increased expression of MCP-1, VCAM-1, ICAM-1, and E-selectin. The SREBP2-induced miR-92a targets SIRT1, KLF2, and KLF4, which leads to the dysfunctional endothelium. Collectively, oxidized lipids, acting via the NLRP3 inflammasome, contribute to an atherogenic phenotype of ECs.
Sterol regulatory element binding protein 2 (SREBP2) plays a canonical role in cholesterol homeostasis through its transcriptional regulation of genes involved in cholesterol biosynthesis and LDL uptake [49]. Similar to the NLRP3 inflammasome, various oxidative insults including OxPAPC activate SREBP2 in ECs [46]. Functioning as a transcription factor, the cleaved form of SREBP2, namely the N-terminus of SREBP2 [SREBP2(N)], transactivates NLRP3 and NOX2 genes. With respect to the two-step mechanism of NLRP3 inflammasome activation, SREBP2 transactivation of NLRP3 and NOX2 with the ensuing increased ROS could be viewed as respective Signals 1 and 2 in ECs. The hierarchical role of SREBP2 in inducing the inflammasome in the endothelium in relation to atherosclerosis is demonstrated in Apoe−/− mice with EC-specific overexpression of SREBP2(N), which have augmented atherosclerosis when compared to control Apoe−/− mice [47]. Consistently, aberrant levels of cleaved SREBP and associated increase in expression of NLRP3, ASC, and IL-1β are evident in the aorta of diabetic and atherosclerotic pigs [50].
Protective and adaptive effects of OxPL
These convincing results from various laboratories showing pro-inflammatory effects of OxPL uncover only one layer in OxPL-mediated biological functions. In the context of inflammasome, a recent study has demonstrated an immunoregulatory effect of OxPAPC [36]. Using dendritic cells as a model system, this work showed that OxPAPC and LPS bind to distinct domains of caspase-11, which results in different and synergistic inflammasome-dependent activities. While both OxPAPC and LPS induce the caspase-11-dependent IL-1β release, only LPS induces pyroptosis. The authors hypothesized that OxPAPC primes inflammasome activation to hyper-activate dendritic cells and increase T cell activation, resulting in enhanced antimicrobial protection. OxPAPC primed only dendritic cells but not macrophages [36]. This study is a good example of the innate immune system adaptation to decode the complexity of PAMP and DAMP signals and to respond selectively to microbial and sterile inflammatory stimuli [51]. In an interesting turn, NF-κB (which is not activated by OxPAPC [37, 52]) has been shown to restrict inflammasome activation via p62/SQSTM1-dependent autophagy of damaged mitochondria, thereby limiting the ROS for Signal 2 of NLRP3 inflammasome activation [53]. This new mechanism suggests that NF-κB is not only a strong positive regulator of inflammation, but also orchestrates a self-limiting host response to allow for resolution of inflammation and tissue repair. Thus, among many factors, OxPAPC plays a prominent role in temporal and tissue-specific, context-dependent regulation of NLRP3 inflammasome, leading to complex outcomes in vascular inflammation.
There is also compelling evidence that OxPAPC inhibits the interaction of LPS with lipopolysaccharide binding protein (LBP), CD14 and MD-2 [37, 54] and thereby prevents TLR4-mediated inflammatory signaling and expression of inflammatory cytokines. In LPS-injected mice, the co-administration of OxPAPC inhibited inflammation and protected mice from lethal endotoxin shock [37, 55]. Individual components of OxPAPC and other OxPLs have the anti-endotoxin activity at concentrations close to those found in blood, as measured by HPLC/MS. In contrast, pro-inflammatory effects of OxPLs required concentrations exceeding the physiologic range in the circulation [55, 56] but likely present under the conditions of hyperlipidemia and in the environment of atherosclerotic lesions.
The anti-inflammatory effects of OxPLs are regulated in part by the transcription factor nuclear factor (erythroid-derived 2)-like 2 (Nrf2), which is a well-recognized target of OxPAPC. Components of OxPAPC induce a covalent modification of cysteine residues in Keap-1, which disrupts the Keap-1-Nrf2 interaction [57]. As a result, Nrf2 avoids Keap-1-dependent degradation, translocates to the nucleus and activates expression of genes whose promoters contain the antioxidant response element. These genes, including HO-1, NQO1, GCL and glutathione-S-transferases, promote a protective, antioxidant response [58]. This response to OxPAPC has been documented both in vitro and in vivo [59–61]. Some cyclopentenone and isoprostaine OxPAPC components, which activate Nrf2, mimic structurally related pro-resolving eicosanoids and exert robust anti-inflammatory activity [62, 63]. OxPAPC is among a limited number of biological molecules that are capable of protecting the integrity of the endothelial barrier in the inflamed lung [64, 65]. Tellingly, synthetic OxPL analogs, Lecinoxoids, reduced atherosclerosis and inhibited nonalcoholic steatohepatitis and liver fibrosis without affecting steatosis in mouse models [66–68].
It is well documented that phagocytosis of apoptotic cells, often called efferocytosis, does not lead to an inflammatory response and maintains immunologic self-tolerance. An interesting work [69] has identified OxPE generated by 12/15-LO as a key factor in this process. Unlike bone marrow-derived macrophages, resident macrophages derived from the yolk sac express high levels of 12/15-LO. OxPE on the surface of resident macrophages sequester soluble receptors for apoptotic cells, such as milk fat globule-EGF 8 protein (MFGE8), thus preventing uptake of apoptotic cells by inflammatory monocytes. Alox15−/− (gene encoding 12/15-LO) mice have enhanced uptake of apoptotic cells by inflammatory monocytes and activation of T cells specific for apoptotic cell-derived self-antigens, thus disrupting self-tolerance and resulting in a lupus-like autoimmune disease.
Thus, compelling data (only partially summarized in this section because of space limitations) suggest that at physiologic concentrations, OxPLs restrict excessive inflammation and/or provide protection against acute oxidative stress, microbial infection or similar insults, as well as mediate fundamental homeostatic functions.
Inflammatory effects of lipid oxidation end products
So far, we described biological effects of esterified PUFA oxidative products, OxPL and OxCE, which exert either adverse or protective effects depending on the pathology and tissue context, as well as engage in biased activation of innate immune receptors. Noticeably, in addition to oxidized fatty acyls, oxysterols play important roles in macrophage and EC biology [70, 71] (Box 1). As the esterified oxidized PUFA decompose to produce so-called oxidation end products, namely, short carbon chain aldehydes and ketones, they become uniformly proinflammatory. Even the end products derived from the dietician’s favorite, ω-3 fatty acid DHA, possess strong inflammatory properties. Yet, depending on the tissue and the tissue damage context, these inflammatory effects may have either adverse or beneficial consequences [72].
Box 1. Oxysterols and inflammation.
Free cholesterol can be oxidized via enzymatic reactions, catalyzed by cytochrome P450 family enzymes, and by ROS. Oxysterols, found both intracellularly and in OxLDL, induce a large number of profound cellular responses. For example, 7-ketocholesterol, a product of ROS-mediated cholesterol oxidation in OxLDL, regulates endothelial biomechanics and induces expression of inflammatory genes [70, 71]; enzymatically produced 25-hydroxycholesterol (25-OHC), among other oxysterols, activates the anti-inflammatory liver X receptor and other nuclear receptors and regulates function of insulin-induced gene 1. As is the case with oxidized PUFA products, oxysterols mediate both pro- and anti- inflammatory responses. For instance, depending on the cell type and/or a specific microbial agonist of TLRs, 25-OHC promotes or inhibits cellular inflammatory responses [71].
MDA and 4-HNE are classic examples of PUFA oxidation end products, which are highly reactive and capable of covalent modification of proteins and lipids. As recently reviewed [73], MDA and its derivatives are proinflammatory and are involved in the pathogenesis of age-related macular degeneration (AMD), atherosclerosis, lung inflammation and liver fibrosis, among other pathologies. MDA gives rise to more complex lipid motifs, such as a highly reactive and immunogenic 4-methyl-1,4-dihydropyridine-3,5-dicarbaldehyde, also termed malondialdehyde-acetaldehyde (MAA). As oxidation-produced DAMPs, MDA epitopes are recognized by multiple arcs of innate immunity, including the scavenger receptor SR-A1, the Fcγ receptor CD16, complement factors H and C3a, stabilin-1, and a number of natural antibodies [73–77]. Immunization of mice with MAA-modified LDL was atheroprotective [78]. Thus, the balance of inflammatory responses to MDA epitopes and the pathways that block MDA or clear it from tissues and the circulation contribute to the progression and outcomes of many chronic pathophysiological conditions.
Oxidation of DHA esterified into a glycerophosphocholine specifically produces 4-hydroxy-7-oxohept-5-enoic acid (HOHA). In turn, HOHA reacts with protein ε-amino groups or with a primary amino group of PE and produces 2-(ω-carboxyethyl)pyrrole (CEP) [79, 80]. CEP-modified proteins have been detected in the retina of patients with AMD [79], during wound healing and in vascularized tumors [81], in atherosclerotic lesions and in hyperlipidemic plasma [82]. TLR2 has been identified as a receptor on endothelial cells that recognizes the CEP modification and mediates cellular activation [81]. The CEP-activated TLR2 in EC signals through MyD88 [81], and in macrophages CEP induces polarization into an M1-like, inflammatory phenotype inducing the expression of iNOS, IL-1β, TNFα and IL-12 [83]. CEP adducts activate platelets to induce granule secretion and aggregation, and in vivo thrombosis, with CEP-proteins signaling via TLR9 and CEP-PE conjugates via TLR2 [84]. The CEP-protein/TLR9/MyD88/IRAK1 pathway primarily results in PI3K/AKT2 and SRC activation, whereas the CEP-PE/TLR2/MyD88/IRAK4 pathway involves TRAF6, SFKs, SYK, and PLCγ2, which leads to activation of platelet integrins [80, 84]. Importantly, macrophages regulate tissue levels of CEP via a TLR2/CD36 clearance mechanism [82].
Similar to OxCE and OxPL biological effects, CEP modifications of proteins and PE contribute to both pathological mechanisms in AMD, sickle cell anemia, excessive platelet aggregation and atherosclerosis [79, 80, 83–85], and to protective processes such as wound healing [81]. Thus, the tissue and pathology context as well as the balance between the mechanisms producing or clearing CEP determine its role in disease progression. These findings, related to CEP biology, highlight the complexity of underlying processes and the importance of considering all factors contributing to specific pathology.
Concluding remarks and future perspectives
The DAMPs concept helped interpret many experimental observations of proinflammatory and atherogenic effects of oxidized lipids and lipoproteins. However, a growing body of evidence suggests that the biology of oxidized lipids is more complex than merely mimicking inflammatory effects of PAMPs. Covalent modifications of proteins and lipids by reactive oxidized lipids, competition with PAMPs for receptor binding, biased agonism of receptor activation, many anti-inflammatory, adaptive and protective effects of oxidized lipids, in addition to their proinflammatory effects, would depend on specific pathology and tissue milieu. Noticeably, many of the effects reviewed herein have both beneficial and adverse effects. On one hand, the increased expression of proinflammatory cytokines in response to OxPL could represent a physiological trigger of sterile inflammation for tissue repair. If OxPL-induced inflammation is sustained and not resolved, the oxidized lipids and end products then become adverse. One translational implication of the sustained exposure to OxPL is exemplified by a recent study showing that subjects with elevated Lp(a), the prominent carrier of OxPL in plasma, have increased arterial inflammation and enhanced peripheral blood mononuclear cells trafficking to the arterial wall [86].
In summary, the multifaceted events all point to nuanced and context-dependent responses to oxidized lipids, which necessitate a new, big data-driven framework for understanding oxidized lipid biology. In the Outstanding Questions box, we outline several possible directions of future work, which will address unresolved mechanistic questions and help understand contributions of oxidized lipids to the pathogenesis of human disease.
Outstanding Questions.
Reactive oxidized lipids covalently modify proteins and other lipids. Does this occur at random or is there selectivity? For example, considering cholesterol binds to MD-2, do OxCE isoketals preferentially modify MD-2 and/or TLR4? Does HOHA target specific proteins to form CEP adducts?
Oxidized lipids exert both pro- and anti-inflammatory effects on endothelial cells. How does this affect endothelial biology in the hyperlipidemic milieu? Does this support the “response-to-injury” hypothesis of atherosclerosis?
Are mechanisms and functional consequences of inflammasome induction in macrophages different from those in endothelial cells? Do macrophage and endothelial responses synergize or counter each other and how does this affect the vessel wall as a whole?
Can oxidized lipid- and lipoprotein-regulated gene expression in vascular system in relation to atherosclerosis be studied by big data approaches (e.g., lipidomics, genomics, epigenetics, proteomics)? In return, will data from these system level experiments provide non-biased, global, and comprehensive information on vascular biology in health and disease? Will this advance precision medicine based treatment of cardiovascular diseases?
Trends.
Lipoprotein and intracellular lipid oxidation is a common pathophysiological response to oxidative stress and hyperlipidemia.
Oxidized lipids and lipoproteins, acting as DAMPs, activate pattern-recognition receptors and induce transcription factor- and NLRP3 inflammasome-mediated immune responses in macrophages and endothelial cells. Such induction can have either adverse or protective effects depending on time, tissue, and pathophysiological context.
Innate immune responses to oxidized lipid DAMPs differ from those to microbial PAMPs, representing biased agonism of pattern-recognition receptors.
Retention of oxidized lipoproteins in the vessel wall synergizes with injury to the endothelium to promote atherogenesis. These data effectively merge the “response-to-retention” and “response-to-injury” hypotheses.
Acknowledgments
Research in authors’ laboratories is supported by grants HL124174, HL055798, HL088093 (Y.I.M.) and HL125643, HL089940 (J.Y-J.S) from the National Institutes of Health. The authors wish to thank Ms. Marcy Martin for her help in preparing the manuscript. We apologize to the authors whose work could not be cited due to space constrains.
GLOSSARY
- Biased agonism
also known as “functional selectivity” referring to the binding of different ligands to the same receptor but activating distinct signaling pathways
- Damage-associated molecular patterns (DAMPs)
The term was introduced by analogy to PAMPs and it refers to host-derived biomolecules, which upon modification, often oxidation, become agonists to the same pattern-recognition receptors that are activated by PAMPs
- Inflammasome
a key component of the innate immune system. Induced and activated by PAMPs and DAMPs, the inflammasome component proteins NLRP3, ASC, and pro-caspase-1 form a high-order complex, leading to the sequential cleavage/activation of caspase-1 and IL-1β
- Oxidized cholesteryl ester (OxCE)
often created by oxidation of cholesteryl arachidonate with 15-lipoxygenase or a free radical generator. For the purpose of this article, OxCE refers to CE molecules with an oxidized PUFA acyl chain, but not oxidized cholesterol
- Oxidized low-density lipoprotein (OxLDL)
An in vitro model of OxLDL is often achieved by incubating native LDL with Cu2+. This robust free radical oxidation reaction yields many OxPL, OxCE, oxysterol, and end oxidation products. Other methods to oxidize LDL can produce a more limited set of oxidation products. The presence of many of the same oxidized lipid species, as generated in OxLDL, in human plasma and atherosclerotic lesions supports the importance of the OxLDL model
- Oxidized 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine (OxPAPC)
often created by exposure of PAPC to air, is a common model of oxidized phospholipids, consisting of tens of oxidative products, some of which are described in this review
- Pathogen-associated molecular patterns (PAMPs)
The concept of PAMPs helps explain how structurally different molecules of different pathogens activate the same pattern-recognition receptors of the innate immunity, such as toll-like and scavenger receptors
- Sterol regulatory element-binding protein 2 (SREBP2)
is a transcription factor governing cholesterol homeostasis by transactivating genes that regulate cholesterol biosynthesis and lipoprotein uptake. Additionally, SREBP2 is involved in innate immune response by activating genes involved in inflammatory response, including NLRP3
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Esterbauer H, et al. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 1991;11(1):81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 2.Miller YI, et al. Lipoprotein modification and macrophage uptake: Role of pathologic cholesterol transport in atherogenesis. Subcellular Biochemistry. 2010;51:229–251. doi: 10.1007/978-90-481-8622-8_8. [DOI] [PubMed] [Google Scholar]
- 3.Tsimikas S, Miller YI. Oxidative modification of lipoproteins: Mechanisms, role in inflammation and potential clinical applications in cardiovascular disease. Curr Pharm Des. 2011;17:27–37. doi: 10.2174/138161211795049831. [DOI] [PubMed] [Google Scholar]
- 4.Miller YI, et al. Oxidation-specific epitopes are danger associated molecular patterns recognized by pattern recognition receptors of innate immunity. Circ Res. 2011;108:235–248. doi: 10.1161/CIRCRESAHA.110.223875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller YI, Tsimikas S. Oxidation-specific epitopes as targets for biotheranostic applications in humans: biomarkers, molecular imaging and therapeutics. Curr Opin Lipidol. 2013;24(5):426–37. doi: 10.1097/MOL.0b013e328364e85a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schuh C, et al. Oxidative tissue injury in multiple sclerosis is only partly reflected in experimental disease models. Acta Neuropathol. 2014;128(2):247–66. doi: 10.1007/s00401-014-1263-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Imai Y, et al. Identification of Oxidative Stress and Toll-like Receptor 4 Signaling as a Key Pathway of Acute Lung Injury. Cell. 2008;133(2):235–249. doi: 10.1016/j.cell.2008.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Binder CJ, et al. Innate sensing of oxidation-specific epitopes in health and disease. Nat Rev Immunol. 2016;16(8):485–97. doi: 10.1038/nri.2016.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horkko S, et al. Monoclonal autoantibodies specific for oxidized phospholipids or oxidized phospholipid-protein adducts inhibit macrophage uptake of oxidized low-density lipoproteins. J Clin Invest. 1999;103(1):117–128. doi: 10.1172/JCI4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shaw PX, et al. Natural antibodies with the T15 idiotype may act in atherosclerosis, apoptotic clearance, and protective immunity. J Clin Invest. 2000;105(12):1731–1740. doi: 10.1172/JCI8472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gillotte-Taylor K, et al. Scavenger receptor class B type I as a receptor for oxidized low density lipoprotein. J Lipid Res. 2001;42(9):1474–1482. [PubMed] [Google Scholar]
- 12.Boullier A, et al. Phosphocholine as a pattern recognition ligand for CD36. J Lipid Res. 2005;46(5):969–976. doi: 10.1194/jlr.M400496-JLR200. [DOI] [PubMed] [Google Scholar]
- 13.Binder CJ, et al. Pneumococcal vaccination decreases atherosclerotic lesion formation: molecular mimicry between Streptococcus pneumoniae and oxidized LDL. Nat Med. 2003;9(6):736–743. doi: 10.1038/nm876. [DOI] [PubMed] [Google Scholar]
- 14.Harkewicz R, et al. Cholesteryl ester hydroperoxides are biologically active components of minimally oxidized LDL. J Biol Chem. 2008;283(16):10241–10251. doi: 10.1074/jbc.M709006200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choi SH, et al. Lipoprotein accumulation in macrophages via toll-like receptor-4-dependent fluid phase uptake. Circ Res. 2009;104:1355–1363. doi: 10.1161/CIRCRESAHA.108.192880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choi SH, et al. Polyoxygenated cholesterol ester hydroperoxide activates TLR4 and SYK dependent signaling in macrophages. PLoS One. 2013;8(12):e83145. doi: 10.1371/journal.pone.0083145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ravandi A, et al. Release and capture of bioactive oxidized phospholipids and oxidized cholesteryl esters during percutaneous coronary and peripheral arterial interventions in humans. J Am Coll Cardiol. 2014;63(19):1961–71. doi: 10.1016/j.jacc.2014.01.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller YI, et al. Minimally modified LDL binds to CD14, induces macrophage spreading via TLR4/MD-2, and inhibits phagocytosis of apoptotic cells. J Biol Chem. 2003;278(3):1561–1568. doi: 10.1074/jbc.M209634200. [DOI] [PubMed] [Google Scholar]
- 19.Park BS, et al. The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature. 2009;458(7242):1191–1195. doi: 10.1038/nature07830. [DOI] [PubMed] [Google Scholar]
- 20.Choi SH, et al. MD-2 binds cholesterol. Biochem Biophys Res Commun. 2016;470(4):877–80. doi: 10.1016/j.bbrc.2016.01.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mancek-Keber M, et al. Toll-like receptor 4 senses oxidative stress mediated by the oxidation of phospholipids in extracellular vesicles. Sci Signal. 2015;8(381):ra60. doi: 10.1126/scisignal.2005860. [DOI] [PubMed] [Google Scholar]
- 22.Kenny EF, O’Neill LAJ. Signalling adaptors used by Toll-like receptors: An update. Cytokine. 2008;43(3):342–349. doi: 10.1016/j.cyto.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 23.Bae YS, et al. Macrophages Generate Reactive Oxygen Species in Response to Minimally Oxidized Low-Density Lipoprotein: Toll-Like Receptor 4- and Spleen Tyrosine Kinase-Dependent Activation of NADPH Oxidase 2. Circ Res. 2009;104(2):210–218. doi: 10.1161/CIRCRESAHA.108.181040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choi SH, et al. Spleen Tyrosine Kinase Regulates AP-1 Dependent Transcriptional Response to Minimally Oxidized LDL. PLoS One. 2012;7(2):e32378. doi: 10.1371/journal.pone.0032378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hilgendorf I, et al. The Oral Spleen Tyrosine Kinase Inhibitor Fostamatinib Attenuates Inflammation and Atherogenesis in Low-Density Lipoprotein Receptor-Deficient Mice. Arterioscler Thromb Vasc Biol. 2011;31(9):1991–1999. doi: 10.1161/ATVBAHA.111.230847. [DOI] [PubMed] [Google Scholar]
- 26.Lindau A, et al. Atheroprotection through SYK inhibition fails in established disease when local macrophage proliferation dominates lesion progression. Basic Res Cardiol. 2016;111(2):20. doi: 10.1007/s00395-016-0535-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Andonegui G. Mice that exclusively express TLR4 on endothelial cells can efficiently clear a lethal systemic Gram-negative bacterial infection. The Journal of Clinical Investigation. 2009;119(7):1921–1930. doi: 10.1172/JCI36411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee S, et al. Role of Phospholipid Oxidation Products in Atherosclerosis. Circ Res. 2012;111(6):778–799. doi: 10.1161/CIRCRESAHA.111.256859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Navab M, et al. Thematic review series: The Pathogenesis of Atherosclerosis The oxidation hypothesis of atherogenesis: the role of oxidized phospholipids and HDL. J Lipid Res. 2004;45(6):993–1007. doi: 10.1194/jlr.R400001-JLR200. [DOI] [PubMed] [Google Scholar]
- 30.Byun YS, et al. Relationship of oxidized phospholipids on apolipoprotein B-100 to cardiovascular outcomes in patients treated with intensive versus moderate atorvastatin therapy: the TNT trial. J Am Coll Cardiol. 2015;65(13):1286–95. doi: 10.1016/j.jacc.2015.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Capoulade R, et al. Oxidized Phospholipids, Lipoprotein(a), and Progression of Calcific Aortic Valve Stenosis. J Am Coll Cardiol. 2015;66(11):1236–46. doi: 10.1016/j.jacc.2015.07.020. [DOI] [PubMed] [Google Scholar]
- 32.Yeh M, et al. Increased transcription of IL-8 in endothelial cells is differentially regulated by TNF-alpha and oxidized phospholipids. Arterioscler Thromb Vasc Biol. 2001;21(10):1585–1591. doi: 10.1161/hq1001.097027. [DOI] [PubMed] [Google Scholar]
- 33.Lee H, et al. Role for peroxisome proliferator-activated receptor alpha in oxidized phospholipid-induced synthesis of monocyte chemotactic protein-1 and interleukin-8 by endothelial cells. Circ Res. 2000;87(6):516–21. doi: 10.1161/01.res.87.6.516. [DOI] [PubMed] [Google Scholar]
- 34.Li R, et al. Identification of Prostaglandin E2 Receptor Subtype 2 As a Receptor Activated by OxPAPC. Circ Res. 2006;98(5):642–650. doi: 10.1161/01.RES.0000207394.39249.fc. [DOI] [PubMed] [Google Scholar]
- 35.Chen R, et al. Human TMEM30a promotes uptake of antitumor and bioactive choline phospholipids into mammalian cells. J Immunol. 2011;186(5):3215–25. doi: 10.4049/jimmunol.1002710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zanoni I, et al. An endogenous caspase-11 ligand elicits interleukin-1 release from living dendritic cells. Science. 2016;352(6290):1232–6. doi: 10.1126/science.aaf3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bochkov VN, et al. Protective role of phospholipid oxidation products in endotoxin-induced tissue damage. Nature. 2002;419(6902):77–81. doi: 10.1038/nature01023. [DOI] [PubMed] [Google Scholar]
- 38.Romanoski CE, et al. Network for activation of human endothelial cells by oxidized phospholipids: a critical role of heme oxygenase 1. Circ Res. 2011;109(5):e27–41. doi: 10.1161/CIRCRESAHA.111.241869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Briot A, et al. Endothelial NOTCH1 is suppressed by circulating lipids and antagonizes inflammation during atherosclerosis. J Exp Med. 2015;212(12):2147–63. doi: 10.1084/jem.20150603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yeh M, et al. Role for Sterol Regulatory Element-Binding Protein in Activation of Endothelial Cells by Phospholipid Oxidation Products. Circ Res. 2004;95(8):780–788. doi: 10.1161/01.RES.0000146030.53089.18. [DOI] [PubMed] [Google Scholar]
- 41.Davis BK, et al. The inflammasome NLRs in immunity, inflammation, and associated diseases. Annu Rev Immunol. 2011;29:707–35. doi: 10.1146/annurev-immunol-031210-101405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Latz E, et al. Activation and regulation of the inflammasomes. Nat Rev Immunol. 2013;13(6):397–411. doi: 10.1038/nri3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rajamaki K, et al. Cholesterol crystals activate the NLRP3 inflammasome in human macrophages: a novel link between cholesterol metabolism and inflammation. PLoS One. 2010;5(7):e11765. doi: 10.1371/journal.pone.0011765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Duewell P, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464(7293):1357–1361. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sheedy FJ, et al. CD36 coordinates NLRP3 inflammasome activation by facilitating intracellular nucleation of soluble ligands into particulate ligands in sterile inflammation. Nat Immunol. 2013;14(8):812–20. doi: 10.1038/ni.2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xiao H, et al. Sterol Regulatory Element Binding Protein 2 Activation of NLRP3 Inflammasome in Endothelium Mediates Hemodynamic-Induced Atherosclerosis Susceptibility. Circulation. 2013;128(6):632–42. doi: 10.1161/CIRCULATIONAHA.113.002714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen Z, et al. Oxidative stress activates endothelial innate immunity via sterol regulatory element binding protein 2 (SREBP2) transactivation of microRNA-92a. Circulation. 2015;131(9):805–14. doi: 10.1161/CIRCULATIONAHA.114.013675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yin Y, et al. Early hyperlipidemia promotes endothelial activation via a caspase-1-sirtuin 1 pathway. Arterioscler Thromb Vasc Biol. 2015;35(4):804–16. doi: 10.1161/ATVBAHA.115.305282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brown MS, Goldstein JL. The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell. 1997;89(3):331–340. doi: 10.1016/s0092-8674(00)80213-5. [DOI] [PubMed] [Google Scholar]
- 50.Li Y, et al. Activation of sterol regulatory element binding protein and NLRP3 inflammasome in atherosclerotic lesion development in diabetic pigs. PLoS One. 2013;8(6):e67532. doi: 10.1371/journal.pone.0067532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Napier BA, Monack DM. A lipid arsenal to control inflammation. Science. 2016;352(6290):1173–4. doi: 10.1126/science.aag0366. [DOI] [PubMed] [Google Scholar]
- 52.Bochkov VN, et al. Oxidized phospholipids stimulate tissue factor expression in human endothelial cells via activation of ERK/EGR-1 and Ca(++)/NFAT. Blood. 2002;99(1):199–206. doi: 10.1182/blood.v99.1.199. [DOI] [PubMed] [Google Scholar]
- 53.Zhong Z, et al. NF-kappaB Restricts Inflammasome Activation via Elimination of Damaged Mitochondria. Cell. 2016;164(5):896–910. doi: 10.1016/j.cell.2015.12.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Erridge C, et al. Oxidized phospholipid inhibition of toll-like receptor (TLR) signaling is restricted to TLR2 and TLR4: roles for CD14, LPS-binding protein, and MD2 as targets for specificity of inhibition. J Biol Chem. 2008;283(36):24748–59. doi: 10.1074/jbc.M800352200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Oskolkova OV, et al. Oxidized phospholipids are more potent antagonists of lipopolysaccharide than inducers of inflammation. J Immunol. 2010;185(12):7706–12. doi: 10.4049/jimmunol.0903594. [DOI] [PubMed] [Google Scholar]
- 56.von Schlieffen E, et al. Multi-Hit Inhibition of Circulating and Cell-Associated Components of the Toll-Like Receptor 4 Pathway by Oxidized Phospholipids. Arterioscler Thromb Vasc Biol. 2009;29(3):356–362. doi: 10.1161/ATVBAHA.108.173799. [DOI] [PubMed] [Google Scholar]
- 57.Espinosa-Diez C, et al. Antioxidant responses and cellular adjustments to oxidative stress. Redox Biol. 2015;6:183–97. doi: 10.1016/j.redox.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Al-Sawaf O, et al. Nrf2 in health and disease: current and future clinical implications. Clin Sci (Lond) 2015;129(12):989–99. doi: 10.1042/CS20150436. [DOI] [PubMed] [Google Scholar]
- 59.Kadl A, et al. Identification of a Novel Macrophage Phenotype That Develops in Response to Atherogenic Phospholipids via Nrf2. Circ Res. 2010;107(6):737–746. doi: 10.1161/CIRCRESAHA.109.215715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jyrkkanen HK, et al. Nrf2 regulates antioxidant gene expression evoked by oxidized phospholipids in endothelial cells and murine arteries in vivo. Circ Res. 2008;103(1):e1–9. doi: 10.1161/CIRCRESAHA.108.176883. [DOI] [PubMed] [Google Scholar]
- 61.Furnkranz A, et al. Oxidized phospholipids trigger atherogenic inflammation in murine arteries. Arterioscler Thromb Vasc Biol. 2005;25(3):633–8. doi: 10.1161/01.ATV.0000153106.03644.a0. [DOI] [PubMed] [Google Scholar]
- 62.Bretscher P, et al. Phospholipid oxidation generates potent anti-inflammatory lipid mediators that mimic structurally related pro-resolving eicosanoids by activating Nrf2. EMBO Mol Med. 2015;7(5):593–607. doi: 10.15252/emmm.201404702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Friedli O, Freigang S. Cyclopentenone-containing oxidized phospholipids and their isoprostanes as pro-resolving mediators of inflammation. Biochim Biophys Acta. 2016 doi: 10.1016/j.bbalip.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 64.Meliton AY, et al. Oxidized phospholipids protect against lung injury and endothelial barrier dysfunction caused by heat-inactivated Staphylococcus aureus. Am J Physiol Lung Cell Mol Physiol. 2015;308(6):L550–62. doi: 10.1152/ajplung.00248.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Birukova AA, et al. Dual role of vinculin in barrier-disruptive and barrier-enhancing endothelial cell responses. Cell Signal. 2016;28(6):541–51. doi: 10.1016/j.cellsig.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Feige E, et al. Inhibition of monocyte chemotaxis by VB-201, a small molecule lecinoxoid, hinders atherosclerosis development in ApoE(−)/(−) mice. Atherosclerosis. 2013;229(2):430–9. doi: 10.1016/j.atherosclerosis.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 67.Mendel I, et al. VB-201, an oxidized phospholipid small molecule, inhibits CD14- and Toll-like receptor-2-dependent innate cell activation and constrains atherosclerosis. Clin Exp Immunol. 2014;175(1):126–37. doi: 10.1111/cei.12212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mendel I, et al. Treatment with Oxidized Phospholipids Directly Inhibits Nonalcoholic Steatohepatitis and Liver Fibrosis Without Affecting Steatosis. Dig Dis Sci. 2016;61(9):2545–53. doi: 10.1007/s10620-016-4159-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Uderhardt S, et al. 12/15-Lipoxygenase Orchestrates the Clearance of Apoptotic Cells and Maintains Immunologic Tolerance. Immunity. doi: 10.1016/j.immuni.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 70.Oh MJ, et al. Oxidized LDL signals through Rho-GTPase to induce endothelial cell stiffening and promote capillary formation. J Lipid Res. 2016;57(5):791–808. doi: 10.1194/jlr.M062539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Guillemot-Legris O, et al. Oxysterols in Metabolic Syndrome: From Bystander Molecules to Bioactive Lipids. Trends Mol Med. 2016;22(7):594–614. doi: 10.1016/j.molmed.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 72.Yakubenko VP, Byzova TV. Biological and pathophysiological roles of end-products of DHA oxidation. Biochim Biophys Acta. 2016 doi: 10.1016/j.bbalip.2016.09.022. [DOI] [PMC free article] [PubMed]
- 73.Busch CJ, Binder CJ. Malondialdehyde epitopes as mediators of sterile inflammation. Biochim Biophys Acta. 2016 doi: 10.1016/j.bbalip.2016.06.016. [DOI] [PubMed] [Google Scholar]
- 74.Weismann D, et al. Complement factor H binds malondialdehyde epitopes and protects from oxidative stress. Nature. 2011;478(7367):76–81. doi: 10.1038/nature10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Veneskoski M, et al. Specific recognition of malondialdehyde and malondialdehyde acetaldehyde adducts on oxidized LDL and apoptotic cells by complement anaphylatoxin C3a. Free Radic Biol Med. 2011;51(4):834–43. doi: 10.1016/j.freeradbiomed.2011.05.029. [DOI] [PubMed] [Google Scholar]
- 76.Zhu X, et al. Scavenger receptor function of mouse Fcgamma receptor III contributes to progression of atherosclerosis in apolipoprotein E hyperlipidemic mice. J Immunol. 2014;193(5):2483–95. doi: 10.4049/jimmunol.1303075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rantakari P, et al. Stabilin-1 expression defines a subset of macrophages that mediate tissue homeostasis and prevent fibrosis in chronic liver injury. Proc Natl Acad Sci U S A. 2016;113(33):9298–303. doi: 10.1073/pnas.1604780113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gonen A, et al. Atheroprotective immunization with malondialdehyde-modified LDL is hapten specific and dependent on advanced MDA adducts: implications for development of an atheroprotective vaccine. J Lipid Res. 2014;55(10):2137–55. doi: 10.1194/jlr.M053256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gu X, et al. Carboxyethylpyrrole protein adducts and autoantibodies, biomarkers for age-related macular degeneration. J Biol Chem. 2003;278(43):42027–35. doi: 10.1074/jbc.M305460200. [DOI] [PubMed] [Google Scholar]
- 80.Biswas S, et al. Novel phosphatidylethanolamine derivatives accumulate in circulation in hyperlipidemic ApoE−/− mice and activate platelets via TLR2. Blood. 2016;127(21):2618–29. doi: 10.1182/blood-2015-08-664300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.West XZ, et al. Oxidative stress induces angiogenesis by activating TLR2 with novel endogenous ligands. Nature. 2010 doi: 10.1038/nature09421. advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kim YW, et al. Receptor-Mediated Mechanism Controlling Tissue Levels of Bioactive Lipid Oxidation Products. Circ Res. 2015;117(4):321–32. doi: 10.1161/CIRCRESAHA.117.305925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cruz-Guilloty F, et al. T cells and macrophages responding to oxidative damage cooperate in pathogenesis of a mouse model of age-related macular degeneration. PLoS One. 2014;9(2):e88201. doi: 10.1371/journal.pone.0088201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Panigrahi S, et al. Engagement of platelet toll-like receptor 9 by novel endogenous ligands promotes platelet hyperreactivity and thrombosis. Circ Res. 2013;112(1):103–12. doi: 10.1161/CIRCRESAHA.112.274241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Guo J, et al. Efficient Quantitative Analysis of Carboxyalkylpyrrole Ethanolamine Phospholipids: Elevated Levels in Sickle Cell Disease Blood. Chem Res Toxicol. 2016;29(7):1187–97. doi: 10.1021/acs.chemrestox.6b00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.van der Valk FM, et al. Oxidized Phospholipids on Lipoprotein(a) Elicit Arterial Wall Inflammation and an Inflammatory Monocyte Response in Humans. Circulation. 2016;134(8):611–24. doi: 10.1161/CIRCULATIONAHA.116.020838. [DOI] [PMC free article] [PubMed] [Google Scholar]