Abstract
The formation of crystal aggregates, one of the critical processes in kidney stone pathogenesis, involves interactions between crystals (predominantly calcium oxalate monohydrate, COM) and urinary constituents (e.g. proteins), which serve as an adhesive “glue” between crystals in stones. To develop a better understanding of the protein-crystal interactions that lead to crystal aggregation, we have measured the effect of model proteins on bulk COM crystal properties as well as their adsorption on crystal surfaces using three synthetic polyanions: poly(aspartic acid) (polyD), poly(glutamic acid) (polyE), and poly(acrylic acid) (polyAA). These anionic macromolecules reduced the amount of COM crystal aggregation in bulk solution to an extent similar to that observed for mixture of proteins from normal urine, with little difference between the polymers. In contrast, the polymers exhibited differences in measures of COM crystal growth. Polycations such as poly(arginine) (polyR) and poly(lysine) (polyK) reduced aggregation weakly and exerted negligible effects on crystal growth. All polyions were found to associate with COM crystal surfaces, as evidenced by changes in the zeta potential of COM crystals in electrophoretic mobility measurements. On the other hand, COM aggregation and possibly growth can be promoted by many binary mixtures of polycations and polyanions, which appeared to be mediated by polymer aggregate formation rather than loss of crystal charge stabilization. Similarly, crystal aggregation promotion behavior can be driven by forming aggregates of weakly charged polyanions, like Tamm-Horsfall Protein, suggesting that polymer (protein) aggregation may play a critical role in stone formation. Sensitivity of polyanion–COM crystal surface interactions to the chemical composition of polymer side groups were demonstrated by large differences in crystal aggregation behavior between polyD and polyE, which correlated with atomic force microscopy (AFM) measurements of growth inhibition on various COM surfaces and chemical force microscopy (CFM) measurements of unbinding forces between COM crystal surfaces and AFM tips decorated with either carboxylate or amidinium moieties (mimicking polyanion and polyR side chains, respectively). The lack of strong interaction for polyE at the COM (100) surface compared to polyD appeared to be the critical difference. Finally, the simultaneous presence of polyanions and polycations appeared to alter the ability of polycations to mediate unbinding forces in CFM and promote crystal growth. In summary, polyanions strongly associated with COM surfaces and influenced crystallization, while polycations did not, though important differences were observed based on the physicochemical properties of polyanions. Observations suggest that COM aggregation with both polyanion–polycation mixtures and weakly charged polyanions is promoted by polymer aggregate formation, which plays a critical role in bridging crystal surfaces.
Keywords: kidney stone, calcium oxalate, atomic force microscopy, adhesion, aggregation, polyelectrolyte
1.0 Introduction
Characterizing the role of urinary macromolecules has been a major focus of stone research for many decades, with numerous reviews being published on this topic[1–4]. Beginning with the first identification of macromolecules as major components in stone matrix[5], most research has been centered on proteins, which account for ca. 65% of the matrix mass; however, glycosaminoglycans and other polysaccharides found in both urine and stone matrix also have received attention[6–9]. Many homeopathic medicines from various plant products and extracts are thought to contain polysaccharide inhibitors of stone formation[10–12]. Macromolecules such as RNA and DNA are also present in stones, albeit at very low levels, and consequently have attracted little attention. Supramolecular assemblies, such as lipid bilayers that comprise cell membranes, also can function in a manner similar to that of macromolecules, as they contain interfaces with functional groups that collectively can interact with crystal or stone surfaces. The existence of well-defined binding moieties in each of these examples suggests a general model for the role of macromolecules in stone pathogenesis, which constitutes a major knowledge gap in the mechanism of stone formation.
The discussion herein is restricted to stones containing calcium oxalate (CaOx) as the principal crystalline component of these microcrystalline aggregates. Approximately 75% of stones analyzed contain crystals of calcium oxalate monohydrate (COM), calcium oxalate dihydrate (COD), or mixtures of these two phases. Most CaOx stone formers have no clear metabolic derangement, suggesting that macromolecules play a predominant role in CaOx stone formation. While calcium phosphate crystals in various phases (i.e., hydroxyapatite, basic calcium phosphate, and brushite) are frequently found in stones as a minor component, they less commonly comprise the majority of the crystalline fraction, and frequently they are the major component only in association with an underlying disease process. Likewise, crystalline phases of uric acid, struvite (magnesium ammonium phosphate) and L-cystine are commonly found in stones only in association with specific physiologic derangements. In these cases, macromolecules may be less important in triggering formation of these stones.
Proteins and other organic components coat nearly every surface of each microcrystal in CaOx stones[13], though the total organic content is typically only 2 – 3% by weight[14–16]. The most recent research employing proteomic methods has shown that hundreds of proteins are found in CaOx stones and that many of the same proteins are present in other stone types[17–24]. The matrix component tends to be a larger fraction of the total stone mass in other stone types, (Neil Mandel, private communication) but generally little is known about differences between matrix components in disparate stone types. Consequently, the emphasis herein on macromolecular interactions with CaOx is relevant to the most common stone, but the generalizability to other stone types is uncertain.
Kidney stone formation is a form of biomineralization, which is ubiquitous in nature. A common process in biomineralization is the controlling interaction between macromolecules and crystal surfaces, which is thought to be facilitated by multivalent ions that bridge opposing charge groups between proteins and ions in the crystal lattice in commonly formed calcium-based crystals[25, 26]. Notably, proteins influence calcification in living systems, such as the formation of mollusk shells, human bone, and vascular plaque (e.g. atherosclerosis)[27–29]. In the case of kidney stones, interactions between urinary constituents and CaOx crystals may influence one or more critical processes in stone pathogenesis, including crystal nucleation, growth, aggregation, and attachment of crystals and/or aggregates to epithelial surfaces of the kidney[30]. A variety of urinary constituents have emerged as possible inhibitors of crystal aggregation and cell attachment, particularly anionic proteins and glycosaminoglycans[31–34]. Yet urinary macromolecules also are reported to promote COM aggregation and/or attachment to epithelial cells in some studies[35–37]. Alteration of phospholipid layers in cell models that facilitate crystal adhesion is another manifestation of macromolecular interactions[38]. These divergent roles of naturally occurring macromolecules have been reviewed on multiple occasions[17, 39], but the number of proteins contributing to the process complicates further interpretation of data.
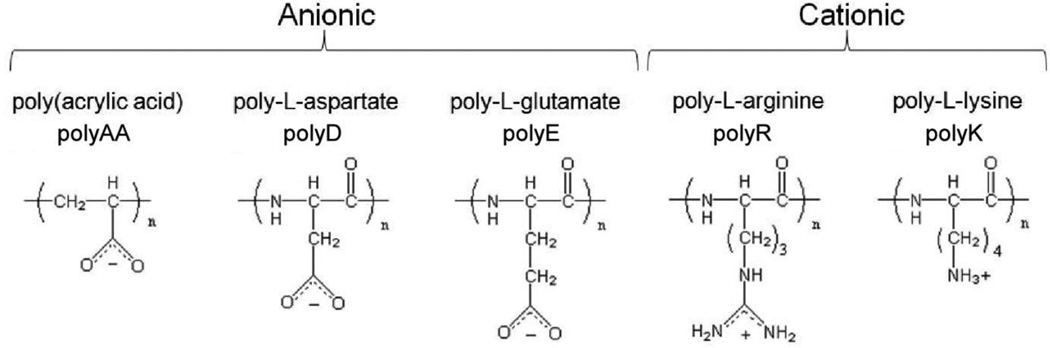
This review will focus on reports that have explored the fundamental processes underlying the formation of microcrystalline CaOx aggregates that constitute stones, including results from previously unreported experiments that create a framework for understanding stone formation. Recognizing that most natural proteins reported to inhibit COM crystallization contain a high proportion of aspartic and glutamic acid residues (up to 40 %)[40], we have investigated extensively the anionic polymers illustrated in Figure 1. In vitro studies reveal that polyelectrolytes with carboxylate functionalities interact strongly with COM surfaces and exert influences similar to urinary proteins from normal urine on calcium oxalate crystallization processes[41–43]. Polyanionic protein adsorption from solution onto COM crystal surfaces can inhibit stone formation by altering crystal habit, inhibiting growth, and directing CaOx crystallization toward COD; a thermodynamically less stable crystal structure that appears to be less active in stone formation[43, 44]. On the other hand, polyanion interactions with COM surfaces can promote processes associated with stone formation by facilitating COM crystal attachment to cellular surfaces of the kidney[45–47], or inducing COM aggregation through self-association (e.g., polyanion aggregation)[48, 49] or by other polymer aggregation processes (e.g., formation of polyanion-polycation aggregates)[49]. Conversely, few examples of cationic macromolecules interacting strongly with COM crystals have been reported[50], though they play a critical role in polyanion-polycation aggregate formation which induces COM aggregation[49]. Also, cationic side chains appear to be critical to crystal growth acceleration, as observed with some amphiphilic proteins[51]. Collectively, in vitro studies suggest that both polymer-polymer and polymer-crystal interactions are important to stone formation.
Fig. 1.
Anionic and cationic polyelectrolytes mimic the amino acid residues of common urinary proteins. Putative inhibitors of COM stone formation are rich in aspartate and glutamate amino acids, which differ by one methylene group. Poly(acrylic acid) (polyAA) is a synthetic polymer containing carboxylic acid side chains placed even closer to the polymer backbone than observed in polyD and polyE. In vivo studies suggest urinary proteins rich in cationic amino acids, similar to arginine (polyR) and lysine (polyK), are not implicated in stone formation (i.e., histone), though they are observed in stone matrix.
2.0 Experimental Methods
2.1 Materials
Calcium oxalate monohydrate (COM) crystals were prepared using HEPES (sodium salt, 99 %, Acros Organics) and the following reagents from Sigma Aldrich: sodium oxalate (Na2C2O4, ≥ 99.5 %), calcium chloride dihydrate (CaCl2·2H2O, ≥ 99 %), and sodium chloride (NaCl, ≥ 99 %). The polyelectrolytes used in these studies were purchased from Sigma Aldrich (multiple different batches with various molecular weights): polyAA partial sodium salt, polyD sodium salt, polyE sodium salt, polyK hydrobromide, and polyR hydrochloride. Cantilevers employed in atomic force microscopy (AFM) studies were modified using 11-mercaptoundecanoic acid (95 %) and mercaptoethylguanidine hemisulfate salt from Sigma Aldrich. All reagents were used as received without further purification.
2.2 Calcium Oxalate Monohydrate Crystallization
COM crystals for AFM analyses were synthesized by a procedure designed to maximize the (100) surface. A 20 mL solution of composition 1.0 mM CaCl2: 1.0 mM Na2C2O4: 150 mM NaCl was prepared in a glass vial by first dissolving NaCl in deionized water, then adding 2 mL of 10 mM CaCl2 while stirring. The sample vial was sealed and placed in an oven at 60°C for one hour prior to adding 2 mL of 10 mM Na2C2O4 dropwise, after which the sample was returned to the oven for a minimum of 48 hours to allow complete crystallization of COM. Detailed procedures used to generate COM crystals with large (010) and (12-1) faces are reported in a previous paper[52].
COM crystal “seeds” for aggregation assays were synthesized by mixing 10 mM stock solutions of CaCl2 and Na2C2O4 at a rate of 1 mL/min (total volume = 4 L) followed by stirring for one week at room temperature. Crystals were allowed to settle by gravity, wherein the supernatant was aspirated and crystals were recovered by low speed centrifugation. The crystals were washed twice with methanol and dried in air at 65°C. The monohydrate crystal structure was confirmed using powder X-ray diffraction (courtesy of Neil Mandel, Mandel International Stone and Molecular Analysis Center, Milwaukee, WI). A 1.5 mg/mL stock solution of crystal seeds was prepared by suspending the dry crystals in a buffer solution (10 mM HEPES and 150 mM NaCl, pH 7.5), which was continuously stirred for 3 weeks prior to use.
2.3 AFM Chemical Force Microscopy Measurements
AFM measurements were performed with an Asylum Research MFP-3D Stand Alone instrument (Santa Barbara, CA). Silicon nitride cantilevers (Veeco Probes, NP model) were used for all chemical force microscopy (CFM) measurements. The cantilever spring constant was measured by the thermal method[53], which yielded values within ± 50% of those reported by the manufacturer (0.12 N/m). Functional groups were immobilized on AFM tips using an established procedure[52] whereby the tip surface was coated with a 5-nm chromium layer followed by 30-nm of gold. Tips were immediately immersed in 1 mM thiol solutions (in ethanol) using 11-mercaptoundecanoic acid, SH(CH2)10COO−, and mercaptoethylguanidine, SH(CH2)2NHC(NH2)2+, to functionalize AFM tips with carboxylate and amidinium groups, respectively.
All CFM measurements were performed in a 0.11 mM calcium oxalate (CaOx) solution. The (100) surfaces of COM crystals were first imaged to locate suitable areas for force measurements. Tip damage was minimized by lightly engaging the tip and imaging in tapping mode at a low scan rate (1.0 Hz). The unbinding force was measured in contact mode with a deflection set point of 20 nm and a retraction velocity of 6 µm/sec. A 5 × 5 µm2 area on the crystal surface was randomly sampled, measuring 10 force curves at ca. 25 different locations. The liquid cell was frequently replenished with fresh CaOx solution, allowing 15 min for the laser deflection signal to equilibrate after each liquid injection. The above procedures were repeated on different surface areas to generate more than 1200 individual force pull-off curves. Data were converted to force histograms and the average force was computed with a normal distribution (labeled FCaOx for interactions of modified tips with the bare COM (100) surface).
The unbinding force was measured in the presence of polyelectrolytes by injecting 0.11 mM CaOx solutions containing 5 µg/mL polymer into the sample cell and allowing 1.5 hours for equilibration. AFM measurements were performed using the same protocol. The unbinding force between modified tips and COM (100) surfaces in the presence of polyelectrolytes (labeled Fadditive) was calculated from an average of 1200 or more pull-off curves.
2.4 COM-Polyelectrolyte Aggregation Assays
COM crystal aggregation was assessed as previously described[49]. Metastable solutions of 0.25 mM CaOx in HEPES buffer (HB: 150mM NaCl and 10mM HEPES, pH 7.5) were incubated with or without polyelectrolyte(s) for 15 minutes at 37°C. COM seed crystals were added to the buffer solution (1.5 mg/mL seeds in HB) and the slurry was mixed for 1 hour. The particle size was measured (see Section 2.6) to assess the level of aggregation in the absence and presence of polyelectrolyte. In studies of oppositely-charged polyelectrolyte mixtures, the polycation was added first to the COM slurry followed by the polyanion, allowing 1 hour of incubation prior to analysis. The relative change in particle diameter (RD) was used as an assessment of particle aggregation,
| (1) |
where RD > 1 implies aggregation and RD < 1 disaggregation.
2.5 Polyelectrolyte Aggregation Assays
Serial dilutions of polycation and polyanion mixtures were prepared in separate 96 well plates (total volume = 0.2 mL) with compositions of 0.25 mM CaOx in HB (i.e. high salt) or 0.075 mM CaOx in 10 mM HEPES at pH 7.5 (i.e. low salt). The CaOx concentrations represented equivalent levels of relative supersaturation at the two different salt concentrations based on calculations using SPEC96, a speciation program developed by George Nancollas (used with permission). Turbidimetric (λ630, PowerWave XS, Biotek) and particle size analyses were performed at room temperature allowing a 15 minute equilibration period following the addition of polyelectrolyte(s).
2.6 Particle Size Distribution Analysis
COM crystal slurries with and without the addition of polyelectrolyte were characterized using an Accusizer 780 (Particle Sizing Systems, Santa Barbara, CA). This instrument is equipped with a 10-mL sampling syringe that measured duplicate 4-mL samplings in the summation mode (0.5–500 µm) for each analysis. Particle size was immediately assessed on COM crystal aggregation assay samples by adding 100–300 µL aliquots into 15 mL sizing buffer (0.225 mM CaOx, HB).
2.7 Zeta Potential Measurements
The zeta potential was measured with a NICOMP 380/ZLS (Particle Sizing Systems, Santa Barbara, CA) using a dynamic light scattering method to determine the electrophoretic mobility. Samples were placed in plastic cuvettes equipped with a platinum electrode. A reference power spectrum was taken without an applied electric field, and then a second time under constant electric field to obtain the sample spectrum. Built-in software was used to calculate the Doppler shift (i.e. change in frequency between the reference and sample spectra) to obtain the electrophoretic mobility,
| (2) |
where U is the electrophoretic mobility (µm/sec), Δν is the Doppler frequency shift, λo is the laser wavelength, n is the solution refractive index, θ is the scattering angle, and E is the applied electrical field (V/cm). The zeta potential (ζ) is calculated by the Smoluchowski equation:
| (3) |
where η is the solution viscosity (Poise) and ε is the dielectric constant of water.
Samples were prepared for zeta potential measurement by placing 750 µg of COM crystal slurries in a cuvette containing 3 mL of saturated calcium oxalate and NaCl. Polyelectrolyte(s) was added to the slurry, allowing 1 hour for adsorption to crystal surfaces. In studies of binary polyelectrolyte mixtures, polycations were added first and allowed to equilibrate for 1 hour prior to the addition of polyanions (though we observed that the order of mixing had no detectable effect on the outcome of the experiment).
3.0 Results and Discussion
3.1 Polymer-Polymer Interactions
Most proteins are molecularly dispersed (i.e., dissolved) in urine, which is a complex aqueous solution. Generally speaking, proteins are stabilized in solution by the presence of hydrophilic (particularly ionic) amino acids and post-translational modifications. In solution, these proteins may be more compact than fully ionic polymers due to the presence of a substantial fraction of hydrophobic amino acids that promote folded (secondary and tertiary) structures. Polymer-polymer interactions that are important to stone formation are long-range (length scale commensurate with the size of proteins or longer) and can promote macromolecular (e.g., protein) aggregation. While certain proteins are capable of forming stable aggregates of discrete size (containing only a few individual proteins molecules), aggregation can occur on a much larger scale leading to phase separation. Mixing nearly equal amounts of highly anionic proteins with highly cationic proteins provides a classic example of this behavior.[54] Indeed, such a mixture was reported to provoke COM crystal aggregation, although the existence of a polymer aggregate phase was not recognized at the time[49]. An example of protein-induced COM aggregation was reported for the protein Tamm-Horsfall protein THP, which is a weak electrolyte that has the propensity to form large aggregates via hydrophobic forces. Prior studies have shown that COM intercrystalline aggregation is promoted under conditions of moderate THP aggregate adsorption on crystal surfaces[48], thus providing a context for conflicting observations in prior literature. Indeed, these findings highlight the sensitivity of polymer-crystal interactions to polymer aggregation behavior.
Numerous experimental and theoretical studies related to the self-assembly of oppositely-charged polyelectrolytes have appeared owing to their applications in medicine, biotechnology, photonics, and catalysis, among others[55]. A significant body of work has been published on polymer layer-by-layer (LbL) assembly[56], wherein multicomponent structures are constructed via interactions that include (but are not limited to) electrostatics, hydrogen-bonding, covalent bonding, hydrophobic interactions, hybridization, and/or biological recognition[56–59]. The association of oppositely-charged polymers in solution leading to phase separation has been investigated extensively as a function of polymer chain length as well as the pH and ionic strength of solutions, including conditions that are far from physiological relevance[54]. Prior studies have revealed that polyanion/polycation mixtures form a separate phase (detectable by turbidity) containing a high concentration of macromolecular (protein) ion pairs with substantial water content, even from solutions containing very low polymer concentrations (< 0.1% or < 1µg/mL) and moderate salt concentrations (0.05 to 0.5 M NaCl). Polymer condensation depletes the solution of both polycations and polyanions through a process akin to polymer induced liquid phase (PILP) formation described by Gower, et al[60]. The PILP process has been extensively investigated in biomineralization systems. While polyanion-polycation pairing is strongly favored even at lower concentrations, detection of aggregates under such conditions is often problematic. At high (non-physiologic) salt concentration, polymer aggregates redisperse (or redissolve) when the small ions reach concentrations adequate to screen the long-range ionic interactions driving polymer aggregation. In the two phase region, microscopic droplets of the polymer aggregate phase can coalesce, analogous to the behavior of oil-water mixtures. Polymer coalescence can take minutes or longer at low droplet concentrations. It is expected that a separated phase will form more readily as polymer or protein chains become longer; and aggregate formation will be maximized when the number of opposing charges on oppositely charged chains are nearly identical. Aggregation still occurs in mixtures with unequal quantities of oppositely-charged proteins (e.g., it has been reported with as little as 10% of the minor component)[54]. The presence of these aggregates in non-stoichiometric polyanion-polycation mixtures caused changes in COM nucleation, aggregation, and growth rate[61].
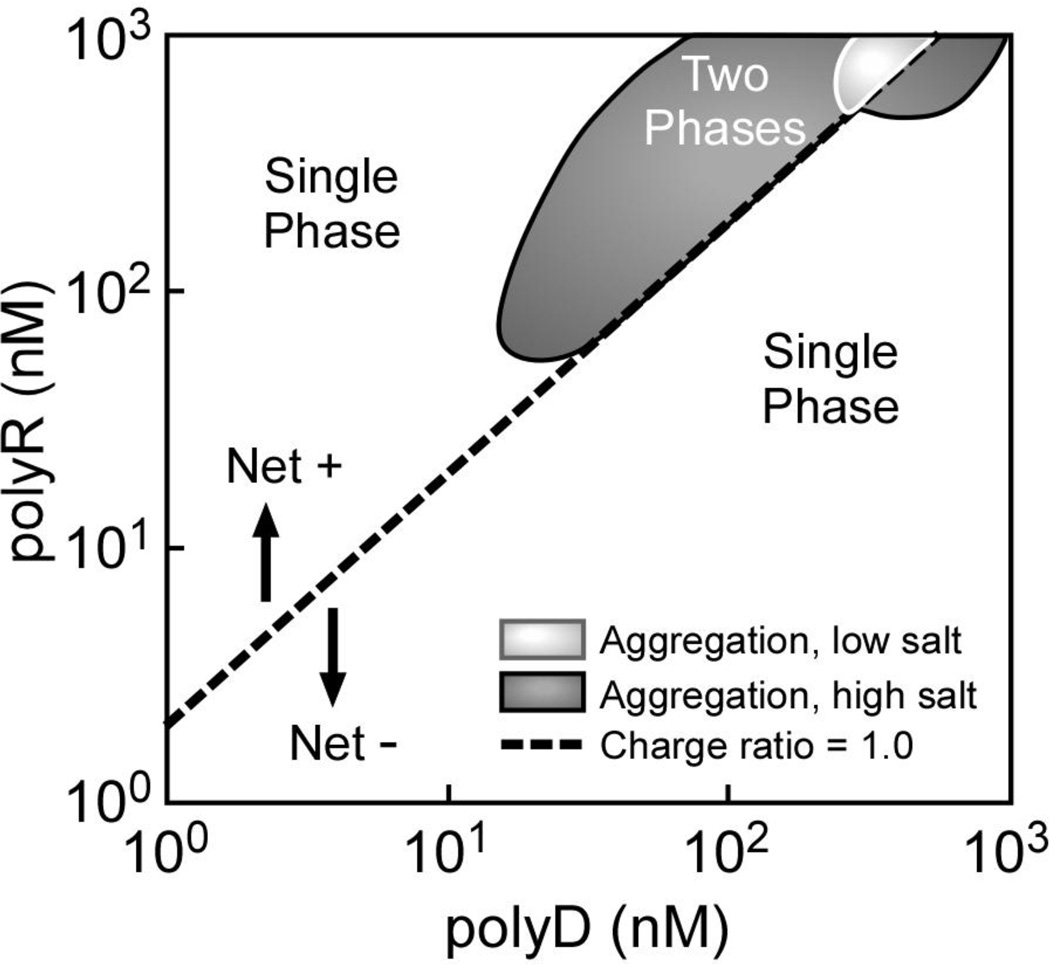
A representative example of polyelectrolyte phase behavior is illustrated in Figure 2 for mixtures of polyR and polyD, which is pertinent to protein-crystal interaction studies discussed below. The formation of polymer aggregates (presence of a second phase) was detected by increased turbidity in mixtures containing nearly equal amounts of polyR and polyD at higher polymer concentrations. Note that the two phase region expanded to lower polymer concentrations at physiologic salt concentrations compared to low salt conditions, similar to previous studies[54]. The dashed line in Figure 2 denotes a neutral +/− charge ratio corresponding to a 1:1 polyD-polyR mixture (per monomer basis, assuming complete ionization). While polyelectrolyte aggregation was expected to occur near the line of zero charge, producing a symmetric two-phase region around that line, the observed two-phase region was clearly shifted to polycation-rich solutions. The explanation for the observed displacement from the equal charge line remains uncertain, though it may reflect either less complete ionization of the polycations compared to the polyanions or a greater amount of impurity in the former. To this end, ion chromatography was used to probe the purity of different lots of polyD and polyR by measuring the counterion concentrations (Na+ for polyD and Cl− or Br− for polyR). These experiments revealed Na+ concentrations at about 95% of the expected value for polyD based on the weight of polymer added, but only 80% of the expected value for Cl- or Br- in polyR samples; consistent with the offset to polycation-rich conditions observed in Figure 2.
Fig. 2.
Phase diagram for polyR-polyD mixtures at low salt and 150 mM NaCl. The molecular weight of polyR and poly D were 26 and 36 kDa, respectively. Dashed line represents composition with equal numbers of anions and cations (calculated). Cloud point determinations were based on using a particle sizing instrument as a detector. Increased turbidity was observed at higher polymer concentrations in mixtures containing nearly equal amounts of polyR and polyD indicating the formation of polymer aggregates; a second, concentrated polymer phase, in these dilute solutions. Note that the two phase region is observed at lower total polymer concentrations in the presence of 150mM NaCl compared with no added salt.
The observed phase behavior can be explained on the basis of electrostatic forces and screening of charges on the polymer chains by the surrounding electrolytes. For example, charge sites on a polyanion chain would experience electrostatic repulsive forces with respect to other anionic sites on the same polymer chain, which favors stretching of the chain to increase the distance between proximal charges, thereby reducing electrostatic repulsion. Likewise, this chain would repel other anionic chains in the solution to remain dispersed. A positive charge on a cationic polymer (or protein) introduced into this environment would experience electrostatic attractive forces with the anionic polymers, leading to ion pairing interactions that will concomitantly reduce the net repulsive forces between anionic moieties on polyanions. The fact that a polycation contains many charges that interact simultaneously with the polyanion leads to an amplification of these attractive forces that increases with the chain length (molecular weight), strongly favoring pairing of polycations with polyanions to form large aggregates that ultimately constitute a separate phase.[54] Added salt(s) screens electrostatic interactions, reducing attractive and repulsive intra/inter-polymer interactions, though long-range interactions remain sufficiently strong at physiologic salt concentrations to allow polymer aggregate formation and phase separation. At salt concentrations of 1M or higher, the electrolytes screen all but very short-range charge interactions, eliminating the amplification of attractive forces between oppositely-charged polymers that derive from longer range interactions, causing the polymer aggregates to redissolve. These conditions are outside the range of normal urine physiology, but the general concepts of electrostatic screening are important to understanding the solution behavior of weakly-charged polyelectrolytes, such as THP.
Polyelectrolytes are stabilized in aqueous solution by the presence of charged groups. For instance, urinary proteins (i.e., weakly-charged polyelectrolytes) are predominantly anionic. The globular or tertiary structures (i.e., chain folding motifs) of many proteins are a consequence of an entropic driving force (notably, the removal of hydrophobic amino acids from contact with water to minimize the free energy). The presence of dissolved salts generally reduces the solubility of urinary proteins, because the weaker long-range electrostatic repulsive forces are more easily overwhelmed. Coalescence (aggregation) of these proteins is dominated by hydrophobic interactions and van der Waals forces, but the accumulation of charge with protein aggregation may reach a critical limit that prevents further addition of proteins beyond a finite size. This behavior is illustrated by the aggregation of desialylated THP[48] in 150 mM NaCl (physiologic saline) wherein the formation of stable aggregates of finite size was independent of THP concentration. Conversely, the same protein remained molecularly dispersed (dissolved) at low salt concentrations. Identical behavior was observed for native THP, but at much higher salt concentration. Notably, native THP remained molecularly dispersed in 150 mM NaCl, but phase separates from solution (and other dissolved proteins) by aggregation at ca. 0.5 M NaCl. Aggregates of either native or desialylated THP induced COM crystal aggregation, while their molecularly-dispersed forms prevented crystal aggregation, as discussed below. Careful scrutiny of experimental conditions in many discordant results for THP[32, 62–67] suggests that the presence or absence of THP aggregates was likely an unrecognized variable in these experimental results.
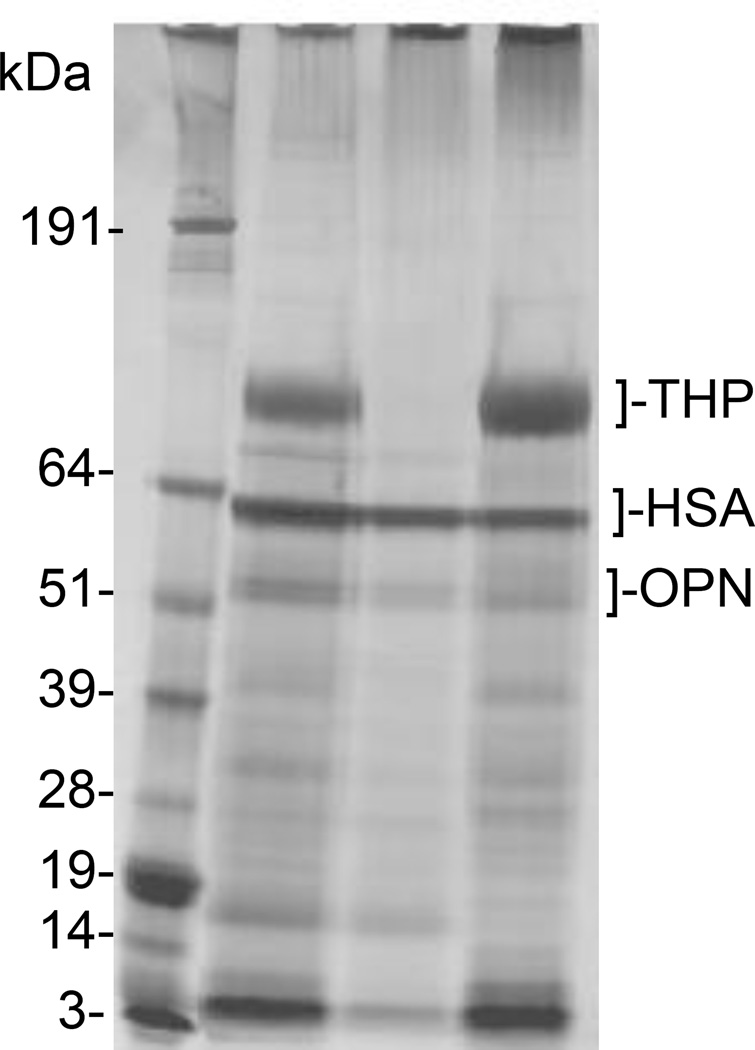
It seems likely that protein aggregate formation in urine environments observed for THP can be generalized to other proteins owing to their limited solubility in water and the amphiphilic nature of an aggregate phase to accommodate both ionic and hydrophobic moieties in typical proteins. The most direct example is illustrated in Figure 3, where 1-dimensional sodium dodecyl sulfate (SDS) polyacrylamide gel electrophoresis data are depicted for normal urine macromolecules in lane 2, macromolecules remaining in the supernatant fluid (lane 3), and macromolecules included in the protein aggregate formed by adding polyR to urine (lane 4). Elution positions are indicated for THP and two additional common urinary proteins, human serum albumin (HSA) and osteopontin (OPN). Clearly, most urine proteins were included in the aggregate phase, despite the fact that only OPN can reasonably be described as a strong polyanion that would dominate interactions with polyR. For the case of weakly-charged polyelectrolytes (e.g., THP), this tendency was exemplified in a Far Western blot, for which desialylated THP exhibited a strong affinity for most urine proteins on an SDS-PAGE gel; however, native THP did not bind to any of these proteins, presumably reflecting the absence of stabilizing charge groups in desialylated THP.
Fig. 3.
SDS-PAGE analysis with silver staining reveals bands for normal urine macromolecules (lane 2), protein aggregates induced by adding polyR to the urinary protein mixture (lane 4), and the fraction of proteins remaining in solution after separation of the protein aggregates with polyR (lane 3). Most proteins appear in the aggregate phase, with many common to both the aggregate phase and supernatant fluid, and a few enriched in the supernatant fluid. Molecular weight markers are shown in lane 1.
3.2 Protein-Crystal Interactions
Molecular Weight Effects
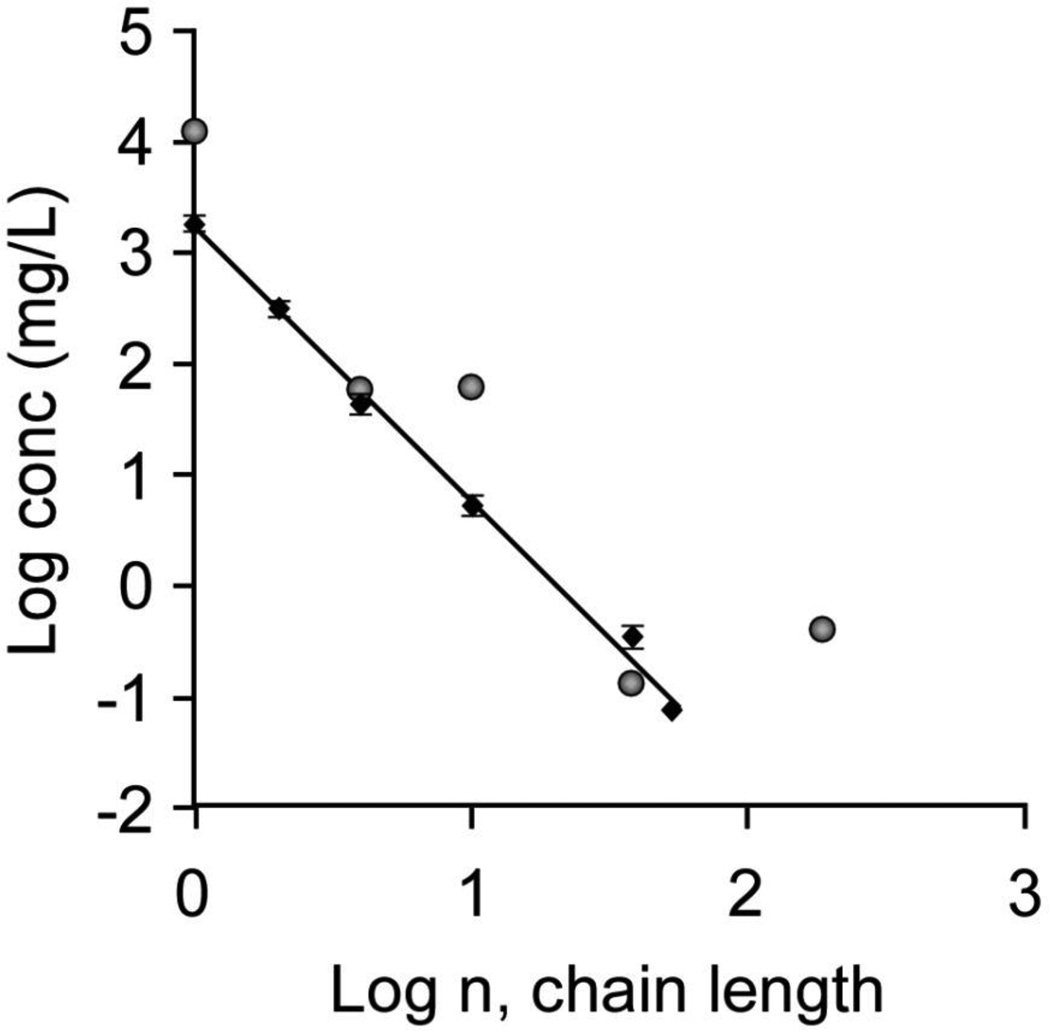
The polymeric nature of proteins is an important consideration for protein interactions with CaOx crystal surfaces, as the number of ionic side chains in polyelectrolytes strongly influences their interactions with crystal surfaces and, subsequently, their efficacy as crystal growth inhibitors. We have explored the molecular weight dependence for CaOx crystal growth rate inhibition using a series of molecular weights of polyD, which has been used frequently as a model polymer for anionic urinary proteins, such as OPN. As illustrated in Figure 4, the concentration of polyD required to achieve equivalent inhibition of crystallization processes is reduced by about 10,000 fold when comparing a protein containing 60 amino acid residues with the amino acid monomers acting independently. Similar behavior was observed when comparing aggregation inhibition of COM seed crystals (Figure 4, diamonds) or the ability to direct crystallization to COD rather than the thermodynamically more stable COM phase (Figure 4, circles), both correlating with significant COM growth rate inhibition (>90%)[61, 68].
Fig. 4.
Polymer molecular weight effects on the inhibition of COM aggregation or COD formation are illustrated in this log-log plot of polymer concentration (conc) vs degree of polymerization (n, chain length) for polyD. The diamonds show the concentration of polyD required at each molecular weight to achieve RD = 0.9 in the aggregation assay. The circles show the concentration of polyD at each molecular weight required to achieve 50% COD formation as a measure of growth rate inhibition of COM.
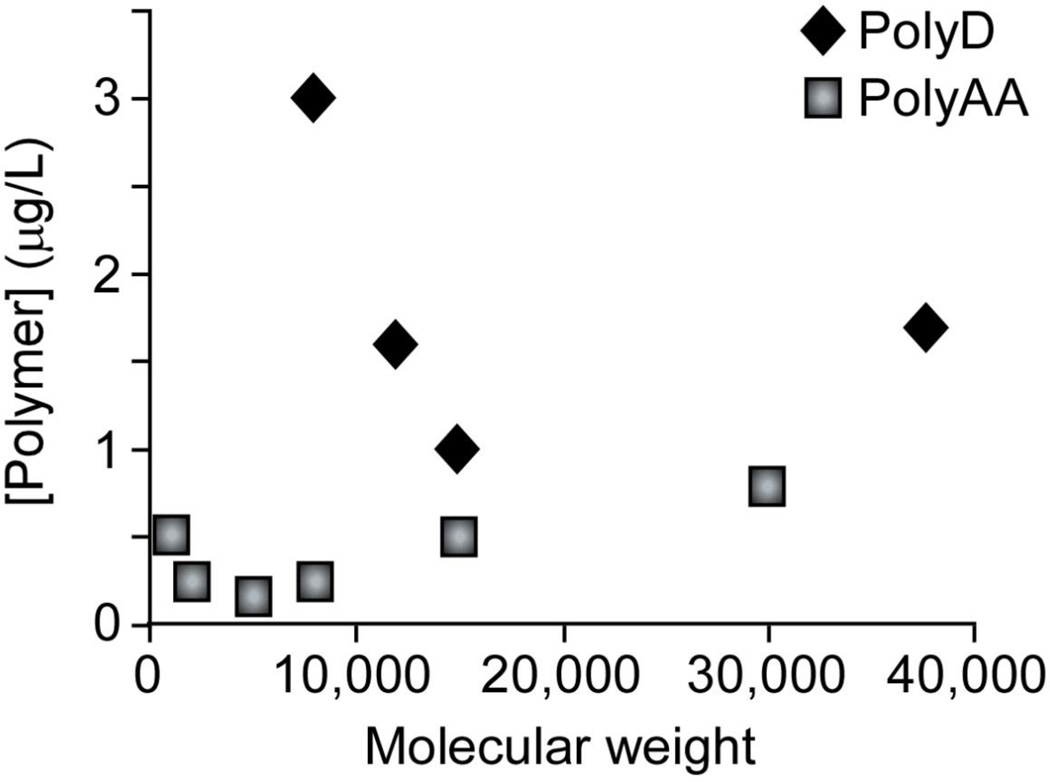
Critical molecular weights for polyD and polyAA in CaOx growth inhibition can be defined as the molecular weight where the minimum mass concentration (number of monomer units) of the polyanion is required to achieve the same level of inhibition in testing a series of molecular weights. As shown in Figure 5, the polymer concentration required to obtain 50% COD in a spontaneous nucleation assay for various molecular weights of polyAA (squares) and polyD (diamonds) reveals a critical molecular weight for each polymer (ca. 5 kDa for polyAA and 15 kDa for polyD). Notably, polyAA appears to be a more efficient inhibitor of CaOx crystallization, as smaller quantities and shorter chains were required to reach the same level of inhibition. Similar characteristics were observed for the inhibition of CaCO3 formation by polyAA, which has been examined for its prevention of CaCO3 scale formation in water pipes[69]. These studies imply that polymers smaller than a critical size (that is, monomers or small oligomers) were much less efficient inhibitors, as multiple molecules were required to bind simultaneously to provide enough carboxylate moieties at a crystal growth site to equal the effect of a single, sufficiently large polymer that can effectively block the entire growth site. Still larger polymers offer no greater benefit than the critical size polymer, because at least one chain must bind to block a growth site and additional monomers beyond the amount required to block an active growth site increase the mass concentration without increasing inhibitory properties.
Fig. 5.
Polymer concentration required to induce COD formation as a function of molecular weight in spontaneous nucleation experiments. Diamonds show data for a polyD molecular weight series. Squares show data for a polyAA molecular weight series. Minimum mass concentrations were noted at ca. 5 kDa for polyAA and ca. 15 kDa for polyD, indicating maximal inhibitor effectiveness for these molecular weights.
Polymer Chemical Structure Effects
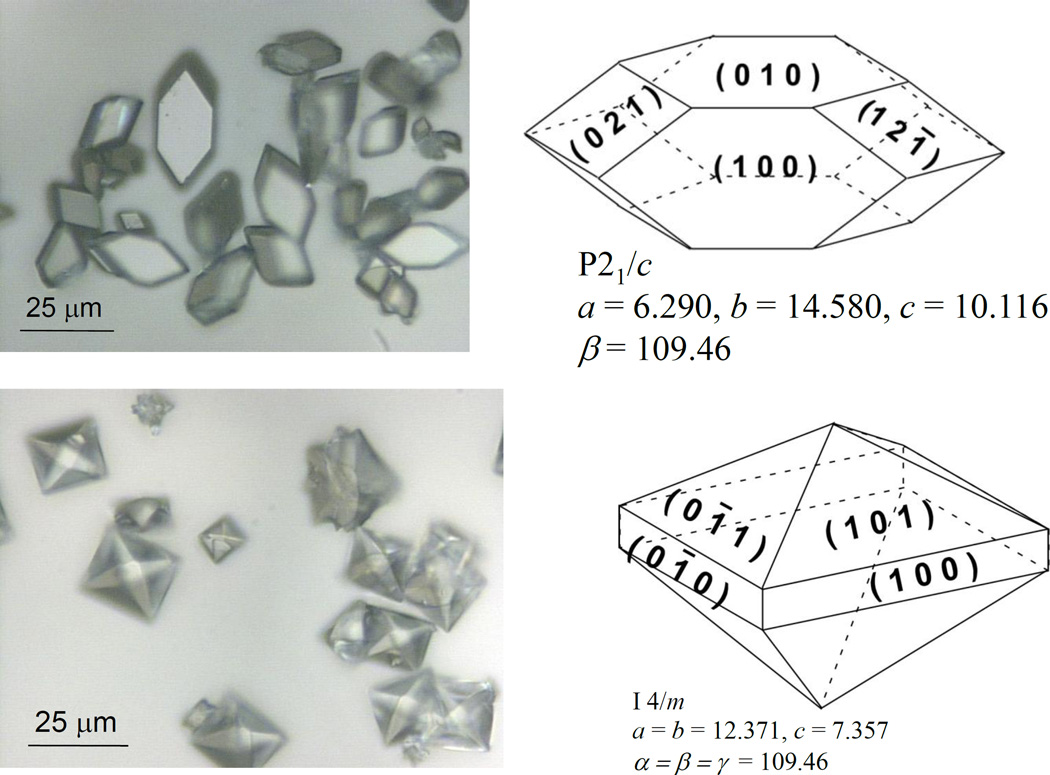
Defining the crystallographic faces of CaOx crystals is an important first step in examining CaOx crystal interactions, since the arrangement and orientation of surface ions differs between different faces of COM and COD crystals (Figure 6). For instance, we have observed preferred polymer interactions at different crystal faces[30]. In prior chemical force microscopy (CFM) studies, we have measured the unbinding force of chemically functionalized atomic force microscopy (AFM) tips with specific CaOx crystal surfaces. Gold-coated AFM tips were modified with either 11-mercaptoundecanoic acid or mercaptoethylguanidine to mimic aspartic acid/glutamic acid (carboxylic acid, –COO−) or arginine (amidinium, –C(NH2)2+) side chains, respectively. The COM (100) face (large hexagonal surface) demonstrated the strongest unbinding force, while the COD (101) face (large triangular surface) demonstrated the weakest unbinding force[30]. The unbinding force was correlated with the surface ion (Ca+2 or Ox−2) density at each face, and the clinical observation that COM crystals are more common in stones than COD[44, 70, 71] is consistent with the fact that the largest faces on COM crystals are most adhesive, while the largest faces on COD are least adhesive toward common protein functional groups[72].
Fig. 6.
Miller indices for major faces of COM (upper) and COD (lower) crystals are indicated in the schematic drawings in the adjacent photomicrographs, along with their space group and unit cell dimensions.
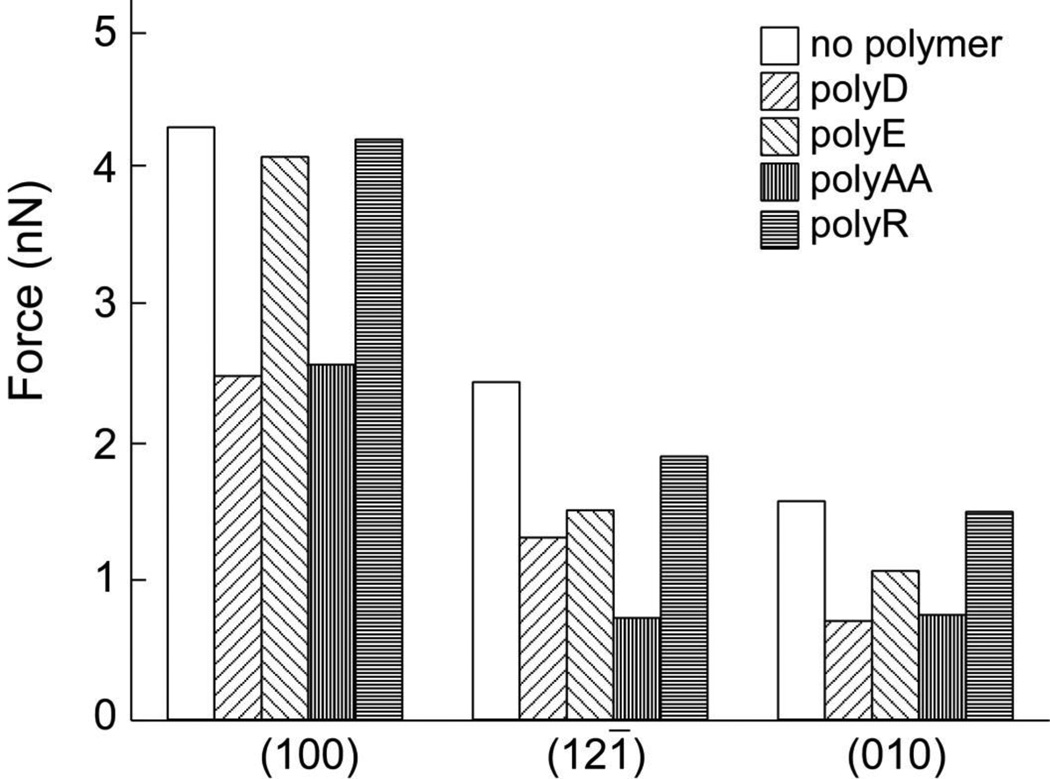
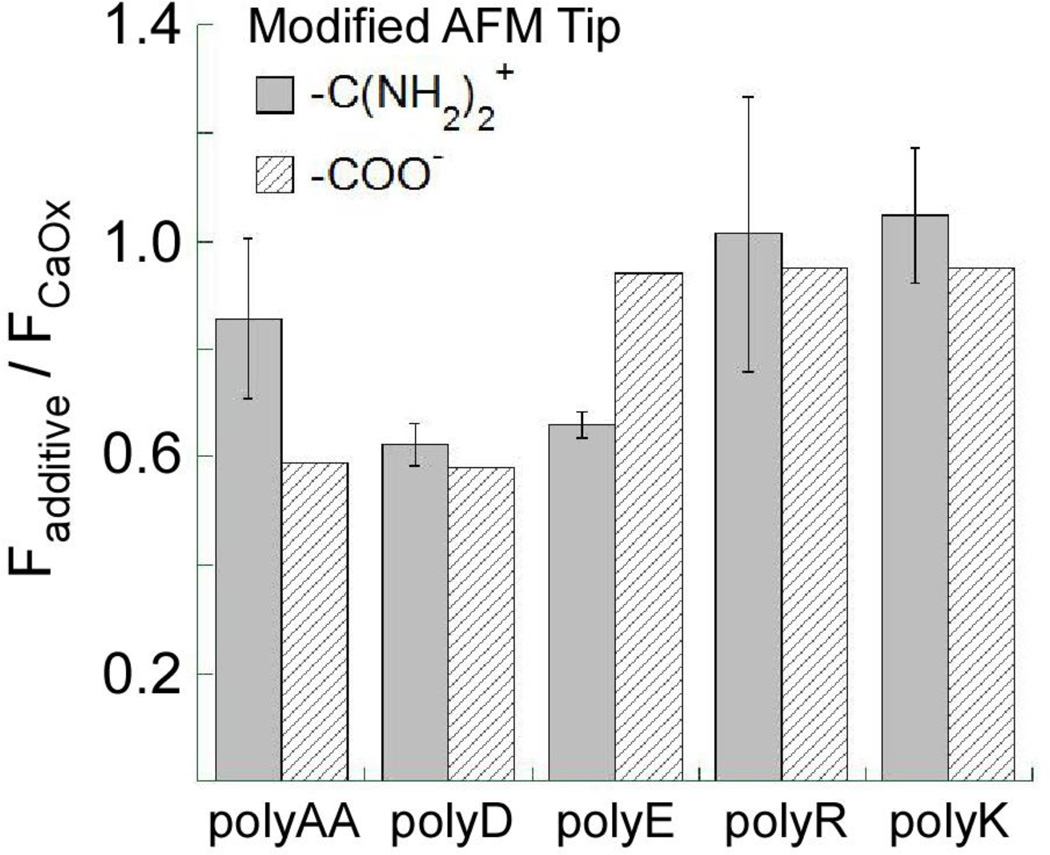
Similarly, the influence of anionic polymers or urinary proteins at CaOx crystal surfaces can be observed by comparing their effects on CFM measurements of unbinding force at each surface. As shown in Figure 7[73], three anionic polyelectrolytes generally reduce the unbinding force for the carboxylic acid functionalized tip at each of the three principal surfaces of COM crystals (except that polyE exerted negligible effect on the unbinding force at the (100) face). These data suggest that polyanions generally bind strongly to COM faces, interfering with tip-crystal binding, though the weaker interaction of polyE with the (100) surface indicates some chemical specificity in this interaction. Likewise, CFM measurements with amidinium modified tips yielded similar results (see Figure 8), though in this case, polyAA demonstrated reduced effectiveness in reducing unbinding force at COM (100) compared to polyD and polyE.
Fig. 7.
Chemical force microscopy measurements of the unbinding force between a carboxylic acid functionalized AFM tip and various COM crystal faces in 150 mM NaCl solution. Measurements were performed with and without various polypeptide additives at polypeptide concentrations of 5µg/mL. (Reprinted with permission from Reference 73)
Fig. 8.
Relative unbinding force measurements at the COM (100) surface with amidinium and carboxylic acid functionalized AFM tips in solutions contained various polyelectrolytes. The relative unbinding force was calculated as the ratio of the force measured in the presence of polyelectrolyte, Fadditive, to the force measured on the bare COM (100) surface in the absence of additive, FCaOx
In situ AFM measurements of COM surfaces growing in the presence of polymeric additives revealed distinct differences in the anisotropic growth rates of the three prominent COM surfaces: (100), (010), and (12-1) faces[50]. Notably, polyE reduced growth along the [010] direction (i.e. analogous to the effects of urinary proteins, such as OPN[74, 75]), while polyD and polyAA inhibited COM growth along the [100] direction. PolyAA was the most potent inhibitor of layered growth in all measured directions on both faces. PolyE exhibited weak growth inhibition on the (100) face, but was more potent than polyD on the (010) face, consistent with CFM measurements[50].
Differences in chemical functional group interactions with crystal surfaces at the molecular level correlate with reported differences in bulk crystallization properties, such as the phase and morphology of spontaneously nucleated CaOx crystals. Whereas polyD and polyAA promoted COD crystallization, polyE induced a dumbbell aggregate of COM at higher (physiologically relevant) concentrations.[50, 68] Polyanions with carboxylic acid residues strongly inhibited COM growth, with inhibition efficacy decreasing in the order polyAA > polyD > polyE[61]. While these polymers demonstrated roughly equivalent effectiveness at preventing aggregation of COM seed crystals at near equilibrium conditions, where all promoted disaggregation[49, 61], their effects in supersaturated media differed. PolyD prevented COM aggregation that is normally observed under supersaturated (growth) conditions in a constant composition growth assay, which has been defined as growth-related aggregation, while polyE promoted this process[61]. PolyAA was not tested in this system, because it interfered with the function of the calcium sensitive electrode in the constant composition assay. Collectively, these observations indicated that polyanions generally bind to COM and inhibit growth and aggregation processes, but that small differences in chemical structure between polyD, polyE, and polyAA side groups were associated with differences in both face selectivity and bulk crystallization processes. These differences have important ramifications in polyelectrolyte mixture studies described herein.
Admittedly, native proteins are more complicated structures compared with model polyelectrolytes, as the former contain many different amino acids (e.g., combinations of D and E residues), additional anionic sites (e.g., phosphorylation and glycosylation), and more complex secondary/tertiary structure. While experiments with native anionic urinary proteins tend to support the inhibitory role of polyanions on COM crystallization processes[76], some unanticipated results have been reported. For example, the unbinding force measured at the (100) surface in the presence of OPN was larger than the control[52], but preferential binding on the (010) surface was implicated by the kinetic inhibition of growth hillock formation[77]. Since OPN contains predominantly D residues (25% compared to 15% E residues), a reduced unbinding force at the (100) surface would have been anticipated with OPN binding. Similarly, its binding preference for the (010) surface is surprising. Several in vivo studies have associated OPN with promotion of stone formation, principally through its ability to simultaneously bind to CaOx crystal and cell surfaces[45]. Obviously, other chemical structural details must play a role in native protein interactions with COM crystals and stones. Other native proteins, like serum albumin and THP, exhibit much weaker interactions with CaOx crystals, though they still inhibit crystal growth and aggregation as long as they remain in solution as molecularly dispersed species. Conversely, proteins can function as crystal growth promoters. One recent report[17] described COM growth promotion by proteins extracted from stone matrix and purified by ion exchange chromatography and molecular sieving, though SDS-PAGE analyses suggested that some of these isolates contained more than one protein. Another report by Farmanesh et al. described similar growth promotion for two proteins (lysozyme and lactoferrin) as well as peptide fragments of lysozyme containing both anionic and cationic residues[51].
The role of non-ionic amino acids must also be considered. Indeed, most protein structures are dominated by non-ionic amino acids, which typically show no apparent effect on crystallization. Experiments examining segments of native protein structures have shown that the inhibitory properties were enhanced by increasing the number of anionic side chains in a segment, generally by adjusted post-translational modifications such as phosphorylation[78–81]. Similar work has been reported for model peptides containing different numbers/sequencing of aspartic acid residues, with increased inhibition of COM growth for increasing acid content[82]. The non-ionic amino acids apparently have little effect on protein-crystal interactions beyond dilution of the effect of the strongly interacting anionic groups or spatial separation of charged groups along the primary sequence, though one publication showed the hydrogen bonding, non-ionic amino acids like serine could enhance surface interactions compared to other non-ionic amino acids[83]. Also, hydrophobic interactions at crystal surfaces have been suggested to play an important role in growth acceleration based on data in calcite (calcium carbonate)[84]. While modeling the complexity of the mixture of hundreds of urinary proteins is beyond our current level of understanding of protein-crystal interactions, numerous studies have revealed that the mixture of proteins from normal individuals inhibits growth and aggregation of COM crystals, consistent with net anionic character of the protein mixtures[85]. In our experiments, the behavior of the mixture of urinary proteins in various bulk crystallization studies was best modeled using polyD as a biomimetic polyanion.
Prior studies indicate that polycations, such as polyR and polyK, are ineffective inhibitors of COM crystal growth in bulk measurements. This is somewhat surprising given that CFM measurements reveal a similar strength of adhesive interaction for both amidinium and carboxylic acid functionalized AFM tips to CaOx crystal surfaces[52]. These polycations weakly disaggregated COM seed crystals compared to polyanions under near equilibrium conditions, and they exhibited little or no effect on growth, aggregation, or phase selectivity[61], although weak growth promotion by proteins and peptides with cationic residues in the presence of nearby anionic residues has been reported[51]. Moreover, no discernible effect of these cationic polymers on COM morphology was observed in spontaneous nucleation experiments. Interestingly, the presence of polyR in solution did not affect the unbinding force for a carboxylic acid functionalized tip at the COM (100) surface (see Figure 7), suggesting that interactions between polycations and COM surfaces are weak compared to those exhibited by carboxylic acid groups. These observations were consistent with CFM measurements performed with amidinium functionalized tips (Figure 8), wherein polyanions reduced the unbinding force and polycations had no effect. Differences were observed in unbinding force measurements dependent on chemical structure of polymer side groups. Notably, polyD and polyE exhibited similar reduction in unbinding force, while polyAA had a less pronounced effect with amidinium functionalized tips. In contrast, the carboxylic acid functionalized tip data showed polyAA and polyD to be similarly effective at reducing unbinding force, while polyE was ineffective.
Zeta potential (ζ) measurements in the presence of polyelectrolytes (Table 1) reveal that these “model proteins” associate with the COM surface and affect surface charge. The zeta potential of COM crystals in the absence of polyelectrolytes (control sample) was −14 mV in both low salt (0.15 mM CaCl2 and Na2Ox) and high salt (0.2 mM CaCl2 and Na2Ox with 150 mM NaCl) buffers. In the presence of polyanions at 5 µg/mL concentration, COM crystals maintain a negative charge, but the magnitude of ζ decreases as poly AA > poly D > control > poly E, which is similar to the trend of decreasing charge density of acid groups per monomer in each polyelectrolyte. The high salt condition (mimicking physiologic saline) appeared to make the surface more strongly negative for each polyanion, though the differences in charge were close to the observed measurement error of ±2 mV. Conversely, the polycations, polyR and polyK, at 5 µg/mL concentration shifted the net charge to postive with similar magnitude and little dependence on added salt concentration. Evidently, polycation association with COM was sufficiently strong for the polymer to reside within the diffuse double layer or hydrodynamic sphere (the thin layer of solvent and ions adjacent to the particle surface that moves with the particle in the presence of an electric field) surrounding the crystal, despite the relative lack of influence on other crystallization processes.
Table 1.
Zeta potential of COM crystals in homopolymer solutions.
| Polyelectrolyte | ζ (mV) low salt |
ζ (mV) high salt |
|---|---|---|
| Control | −14 | −14 |
| PolyD | −19 | −23 |
| PolyE | −8 | −11 |
| PolyAA | −32 | −33 |
| PolyR | +10 | +13 |
| PolyK | +14 | +11 |
Collectively, our observations show that polyanions (i) exhibit very strong binding interactions at COM surfaces, (ii) modify the effective surface charge to a value dependent on the individual adsorbate, and (iii) generally operate as inhibitors in all aspects of crystallization. Differences in polyanion-crystal interactions, particularly between polyD and polyE, demonstrate nuances sensitive to the chemical structure of adsorbates. On the other hand, polycations weakly interact with COM surfaces and have much less influence on crystallization, though they associate with COM to a sufficient level to alter surface charge. Classical colloidal chemistry posits that a mixture of polycations with negatively-charged particles (e.g., COM crystals) in suspension should induce aggregation[86]. Since crystal aggregation is such a critical component of stone formation, the connection between surface charge and aggregation was studied more carefully.
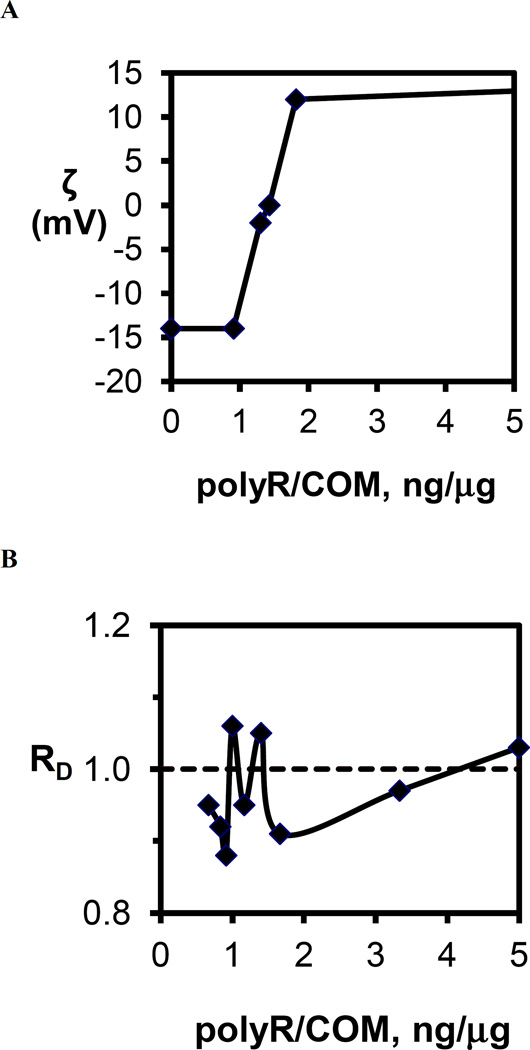
Systematic studies of polyR effect on COM surface charge revealed an increase in ζ with increasing polyR concentration, and effective COM charge neutralization (ζ = 0 mV) was achieved at about 1.43 ng polyR/µg COM (Figure 9A). As polycation coverage on COM surfaces increased further, ζ continued to rise rapidly to the apparent saturation value of ζ = +13 mV (see Table 1). Bulk COM aggregation behavior as a function of polyR concentration was explored carefully in the region of ζ = 0 mV. The loss of surface charge stabilization at ζ = 0 mV should allow polymer strands bound to crystal surfaces to bridge adjacent particles and thereby form a gel or aggregate with near neutral surface charge, but these aggregates should dissipate at lower or higher polymer concentrations where like-charged particles would electrostatically repel one another and stabilize the suspension of particles in solution. Yet, no aggregation was observed with mixtures of polyR and COM crystals (Figure 9B). Evidently, polycations bind with sufficient strength to COM surfaces to migrate with crystals (within the so-called double layer surrounding crystal surfaces) and alter the effective surface charge from negative to positive. This association of polycations, however, is not strong enough to form bridging interactions or shield the surfaces from either the addition of more calcium and oxalate ions to the crystal lattice or adhesive interactions from other macromolecules (or chemically-modified AFM tips). Conversely, polyanions apparently bind strongly to COM and form bridging interactions, but they clearly inhibit aggregation. This latter outcome may simply be the result of charge stabilization of COM particles, but bridging interactions could also be limited by the relatively short polymer chain lengths (nm) compared to crystal sizes (µm), as well as the fact that polyanions have been shown to completely condense on crystal surfaces, leaving no exposed loops or trains in solution[87–92].
Fig. 9.
(A) Zeta potential of COM crystals in 150mM NaCl as a function of polyR to COM crystal mass ratio expressed as (ng polyR/µg COM). The approximately sigmoidal dependence displays a sharp transition from no effect (ζ= −14 mV) at 1ng/µg to nearly the saturation limit (ζ= 12mV) at 2 ng/µg. (B) RD from aggregation assays as a function of polyR to COM crystal mass ratio. Multiple samples were tested in the region of zero surface charge finding no evidence for induction of COM crystal aggregation by the addition of the polycation, polyR. The dashed line indicates the RD value for the control (no polyR added) experiment. The observed deviations from the control are in the range in the uncertainty for this measurement indicating that charge neutralization was not sufficient to trigger COM particle aggregation.
Effects of Protein Aggregation
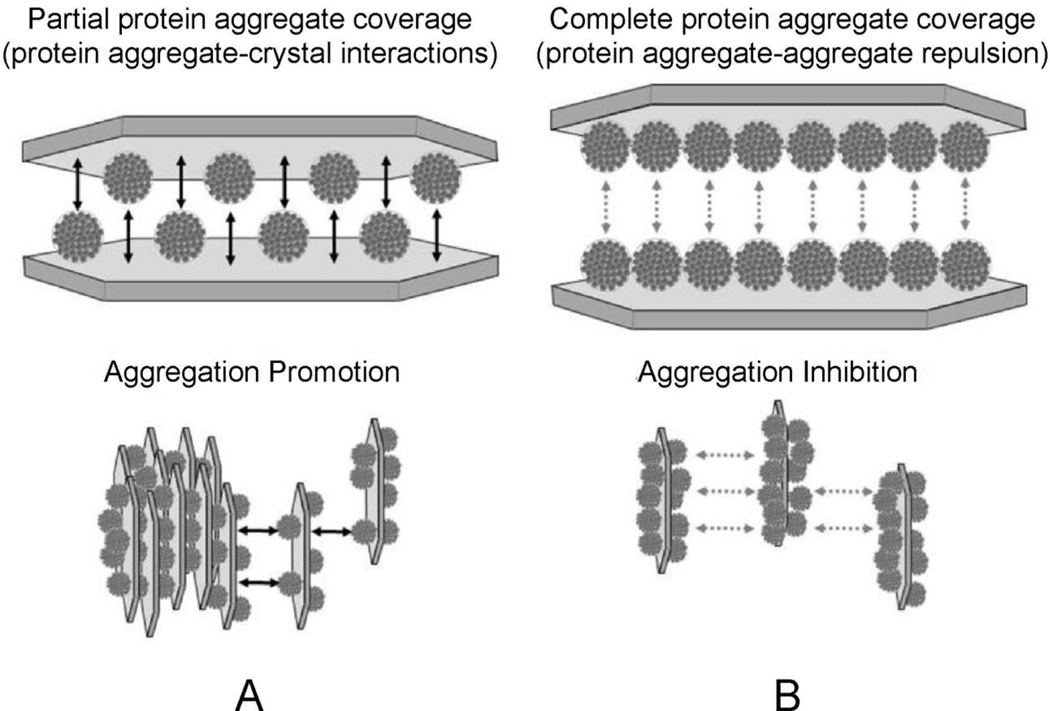
Investigations with THP offered a clear picture of protein aggregate-COM crystal interaction, because THP aggregate particles maintained a stable size for timescales longer than a typical experiment (e.g., a few hours). Data obtained using different ratios of THP aggregate to COM crystal mass yielded variations in COM aggregate formation can be described by a simple adsorption model. These calculations revealed that COM aggregation was maximized at a low fractional surface coverage with protein aggregate (about 30%), such that a protein aggregate bound to the surface of one crystal could find and bind to an exposed surface on an adjacent crystal to form a bridge, as illustrated in Figure 10. At higher fractional surface coverage, the presence of adsorbed THP aggregates on the opposing crystal surface frustrates crystal aggregation, since the protein aggregates do not readily stick to one another[48]. The fact that native THP molecules also could induce COM aggregation at higher salt concentration (where the native protein forms aggregates) corroborated a critical role for protein-protein interactions in COM aggregation. Similar studies under growth conditions have not been performed with desialylated THP, although we would anticipate that the THP aggregates would demonstrate less effective inhibition of growth processes compared to the same concentration of molecularly dispersed protein, by virtue of a reduced number of free protein particles in solution. A similarity in the phase behavior of most urine proteins was implied through the strong interactions between the desialylated THP and other urine proteins in Far Western blots[48]. The possibility of THP aggregate formation was not considered in many early studies, which not surprisingly showed quite varied behaviors for THP-crystal interactions, as summarized in prior reviews of this topic[1, 39, 93].
Fig. 10.
Diagram illustrating a proposed coverage-dependency of desialylated-THP-COM aggregation. The COM “seed” surface is depicted as a single crystal for illustrative purposes, with the characteristic elongated hexagonal morphology of COM crystals (see Figure 6). Adsorption is likely to occur on the (100) surface, which accounts for the largest surface area of single crystals, and is likely the predominantly surface area of COM seeds. Two cases are illustrated here for adsorption of aggregates to COM at (A) partial coverage (50 %) and (B) complete coverage (100 %). The former promotes COM aggregation though crystal-protein-crystal bridging interactions, while at some threshold coverage (illustrated here as a surface completely covered with aggregates) repulsion between protein aggregates on opposing crystal surfaces offsets the attractive interactions resulting from any remaining crystal-protein-crystal bridges. (Reprinted with permission from Reference 47)
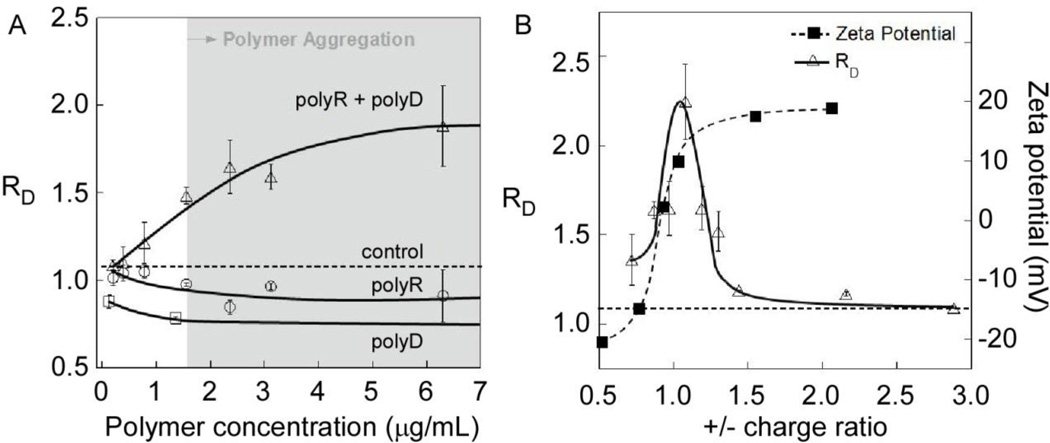
While we initially reported COM aggregation using a nearly equimolar mixture of polyD and polyR[49], subsequent experiments have described the phase behavior over a broader range of mixing ratios, polyion compositions, and polyion concentrations (Figure 11). At near equimolar concentrations of oppositely-charge polyelectrolytes, the relative particle diameter (RD) of COM crystals in the presence of polyR-polyD mixtures increased with increasing polymer concentration, reaching statistical significance at about the polymer phase boundary, indicated by the shaded area in Figure 11A. The data for each polyelectrolyte component separately revealed that these polyions caused disaggregation in the absence of a polymer with opposite charge (i.e., RD < 1.1). This phenomenon was more pronounced for polyD, where a significant reduction in RD observed at low polyD concentrations reached a limiting value of 0.8 (at 1.5 µg/mL) with increasing polyD concentration (tested up to 12 µg/mL). In solutions containing polyR, RD was indistinguishable from the control at low concentrations and reached a limiting value of 0.9 at polyR concentrations above 2 µg/mL.
Fig. 11.
COM aggregation in solutions containing polyR and polyD at varying polymer concentration and charge ratio. (A) Changes in relative diameter of COM seeds in binary and homopolymer solutions of polyR (26 kDa) and polyD (36 kDa) as a function of polymer concentration. Concentrations for mixed polymers refer to polyR concentration, where polyD was added to maintain a 1:1 monomer ratio. (B) Left axis: relative diameters (RD, Eq. 1) of COM crystals with polyR(+)/polyD(−) mixtures in a solution of composition 0.25 mM CaOx:150 mM NaCl:10 mM HEPES at pH 7.5. Varying amounts of polyR (15 – 270 nM) were added to seeded COM solutions containing 49 nM polyD. Right axis: zeta potential of COM-polyelectrolyte particles. Solid lines are interpolated and horizontal dashed lines at RD = 1.1 refer to control measurements obtained in the absence of polyelectrolyte.
Induction of COM aggregation was clearly dependent on aggregation of oppositely-charged polymers leading to phase separation, since neither polymer produced this affect alone. Experiments with THP aggregates demonstrated that the extent of COM aggregation is dependent on the ratio of protein aggregate surface area to COM surface area. Since polyR-polyD aggregates initially formed as very small particles (submicron dimensions) that coalesced gradually to form a discrete phase, the ratio of polymer aggregate to COM crystal surface area changed with reaction time. Consequently, the extent of crystal aggregation was influenced by the temporal nature of this coalescence process, a kinetic component that has not been fully characterized. Duplicating these results quantitatively will depend on precisely matching mixing conditions, although the qualitative features can be easily reproduced.
The change in RD with varying proportions of polyR and polyD is illustrated in Figure 11B. The solutions were prepared with constant polyD concentration and varied amounts of polyR to achieve charge ratios ranging from 0.5 (polyanion-rich) to 3.0 (polycation-rich). The maximum COM aggregate size (RD = 2.2) occurred near COM charge neutrality (polyR/polyD = 1.0), although it is slightly shifted to polycation-rich conditions (i.e., positive surface charge). These observations are analogous to those reported for other oppositely-charged polyelectrolytes, such as DNA-polycation interpolyelectrolyte complexes (IPECs), which exhibit enhanced rates of aggregate formation at equimolar compositions[94]. Surprisingly, the transition in ζ for COM crystals in these mixtures follows an expected sigmoidal transition from negative to positive charge that crosses 0 mV at polyanion rich conditions, thus COM aggregation was apparently not mediated by loss of surface charge stabilization. At polyion charge ratios above or below the maximum, RD decreased rapidly toward the value of the control (RD = 1.1), though we note that either polymer acting independently would cause RD to fall below the control value. Consequently, polyR-polyD aggregates appeared to induce crystal aggregation even in the presence of a surplus of either polyanions or polycations.
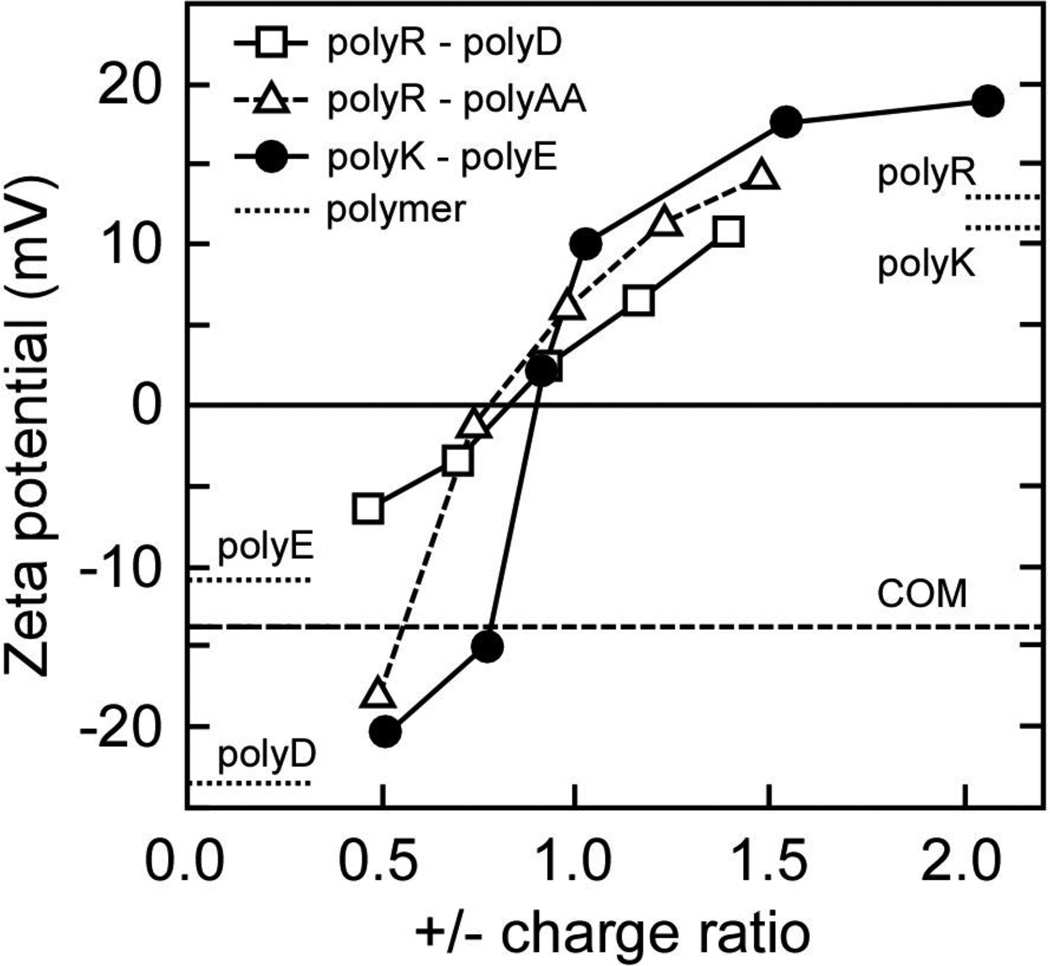
The influence of polyanion/polycation mixtures on COM surface charge and aggregation was explored using other conveniently obtained polyanions and polycations. The zeta potential of COM crystals was measured in solutions containing various pairings of polycations and polyanions mixed in different proportions (Figure 12). These binary mixtures all exhibited similar trends, where ζ ranged from about −10 mV in polyanion-rich solutions to +10 mV in polycation-rich solutions. COM charge neutralization occurred at polyelectrolyte charge ratios between 0.7 and 0.9, suggesting that net zero charge occurred in polyanion rich mixtures for all of these combinations. COM aggregation assays were performed using each of these polycation-polyanion mixtures at equimolar conditions within the two-phase region. The results shown in Table 2 revealed that the choice of polycation had little effect on COM aggregation promotion, while the polyanion selection had a marked effect. Promotion of COM aggregation was observed when either polyD or polyAA was combined with either polyK or polyR, while all mixtures containing polyE resulted in COM disaggregation (RD < 1.1). The lack of polyE interaction at the (100) surface observed in CFM studies is likely correlated with this result. The (100) surface is normally the largest face on COM, and the bridging interactions between crystals must include polymer binding to each crystal; a process that appears to be driven by the polyanion component. Thus, polyE, which does not bind strongly to (100), cannot be part of the bridging interaction required for aggregation, even though polyE will form polymer aggregates with polycations. Conversely, polyAA and polyD were equally effective at inducing COM aggregation, and both demonstrated strong interactions at the (100) surface, as well as frustrating the addition of CaOx growth units to the crystal (growth inhibition).
Fig. 12.
Zeta potential of COM crystals in solutions containing polyelectrolyte mixtures. Polycations (polyR, polyK) and polyanions (polyAA, polyD, polyE) were combined in varying charge ratios. The zeta potential of COM surfaces in the absence of polyelectrolyte (dashed line; control) is −14 mV. The point of zero charge for COM-polyelectrolyte complexes occurs at a polycation to polyanion electrolyte charge ratios below unity, suggesting that the presence of polyanions enhances polycation adsorption at COM crystal surfaces.
Table 2.
COM aggregation in 1:1 mixtures of oppositely-charged polyelectrolytes.
| Polymer Mixtures † | |||
|---|---|---|---|
| Cation | Anion | ||
| polyR | polyD | 1.9 ± 0.2 | |
| polyK | polyD | 1.7 ± 0.2 | |
| polyR | polyAA | 1.42 ± 0.05 | |
| polyR | polyE | 0.93 ± 0.04 | |
| polyK | polyE | 0.83 ± 0.05 | |
0.25 mM CaOx: 150 mM NaCl: 10 mM HEPES, pH 7
In these studies of mixed polyelectrolytes, the positive ζ values for COM crystals in polycation-rich solutions appeared to be higher than those observed with either polycation alone, suggesting a cooperative action with polyanions that promoted polycation association with COM surfaces. CFM measurements were performed in solutions containing different proportions of polyD and polyR at a total polyelectrolyte concentration of 5 µg/mL (Figure 13), to better characterize this effect. Unbinding force in CFM measurements were obtained in solutions containing polycation and polyanion mixtures at 1:2, 1:1, and 2:1 mass ratios, corresponding to 33, 50, and 67 wt% polyR. The results were compared to homopolymer solutions of polyD and polyR (0 and 100 wt% polyR respectively; see also Figure 8). A monotonic increase in unbinding force was observed for the carboxylic acid-modified AFM tip with decreasing polyD content, suggesting that increasing the polyR fraction effectively reduced the surface coverage with polyD. The amidinium-modified AFM tip appeared to bind even more weakly to the COM (100) surface in the presence of polyR/polyD mixtures than it did in the presence of polyD alone; however, in the presence of polyR alone, the unbinding force was no different than the unmodified COM surface. This pattern suggests that polyD promotes the association of polyR at or near the crystal surface, presumably through polyD surface interactions, such that it can interfere with the binding of an amidinium-modified tip. Once polyD is removed (i.e., 100% polyR solution), polyR is no longer bound to the crystal surfaces with sufficient strength to affect the unbinding force, though ζ measurements indicate that polyR is still associated with the surface (within the diffuse double layer).
Fig. 13.
Relative unbinding force between AFM tips modified with amidinium (-C(NH2)+) and carboxylic acid (-COO−) moieties and the COM (100) surface in the solutions containing various mixtures of polyR and polyD. Measurements were performed in 0.11 mM CaOx solutions with a total polyelectrolyte concentration of 5 µg/mL (polyR + polyD) in each experiment.
Data from bulk solution measurements for a similar series of mixtures were previously published[61] and showed that the polyD-rich mixture was nearly identical to the control (no polyelectrolyte added) with respect to COM crystal aggregation promotion. Yet, this results was still was quite different from polyD alone where disaggregation was observed. On the other hand, COM growth rate inhibition measurements were similar between this mixture and polyD alone, indicating that the polymer aggregates present were more strongly involved in crystal aggregation processes than in growth. On the other hand, equimolar (charge neutral) and polyR-rich mixtures apparently blocked growth related aggregation and shifted the predominant growth mechanism to secondary nucleation in the constant composition experiments[61], along with promoting aggregation of COM seed crystals in saturated CaOx solutions[49]. The polyR-rich condition also appeared to increase crystal growth rates compared to the control, which may be analogous to reports of growth acceleration by selected native protein structures containing both anionic and cationic side chains[51].
4.0 Conclusions
In summary, polyanions (e.g., polyD, polyAA, and poly E), native urinary proteins (e.g., THP and OPN), and the mixtures of macromolecules isolated from normal urine, bind strongly to crystal surfaces and generally inhibit COM nucleation, growth, and aggregation. CFM measurements of unbinding force demonstrated that polyanions could block the adhesion of AFM tips chemically modified with both amidinium (cationic) and carboxylic acid (anion) terminal groups, suggesting that these adsorbed polymers mask the crystal surface. Interfacial and bulk crystal assays revealed distinct differences in polyelectrolyte-COM interaction due to differences in polyanion chemical structure (polyD vs polyE) that strongly influenced interactions at selected COM faces, but also manifested as observable differences in bulk crystallization, particularly in aggregation behavior. Conversely, polycations were attracted to the COM crystal surface, presumably through electrostatic attractions that were insufficient to lead to strong binding, and thus resulted in little to no effect on bulk crystallization processes or CFM unbinding force measurements. When polycations were combined with polyanions, the polycation-polyanion aggregates that formed could compete with the polyanion-crystal interactions leading to promotion of crystal aggregation in some cases, and promotion of nucleation and growth in others. The effect of the polymer aggregates was strongly dependent on the binding characteristics of the polyanion component, as exemplified by the differences in COM aggregation behavior between polymer aggregates formed with polyD vs polyE. Studies of THP confirmed the connection between the need for polyanion interactions at the crystal surface and polymer aggregate formation to induce aggregation of COM crystals, suggesting that the only important role for polycations in crystal aggregation is the promotion of polymer aggregation.
Model polyelectrolytes selected for this study contain functional groups that mimic the moieties of urinary constituents in the organic matrix of human kidney stones, and limited experimental data in crystallization assays have demonstrated similar behaviors to native proteins. The mixture of urinary macromolecules may be too complex to be approximated by single model polyelectrolytes; however, the complexity of this mixture argues against a dominant role for an individual component, given that all proteins contain the same amino acids, and therefore the same ionic side chains in various proportions. Experiments reported or discussed here have illustrated a range of behaviors that suggest pathways to stone formation based on viewing the average properties of the mixture as a critical parameter. In particular, these experiments have illustrated the important role that polymer aggregates are likely to play in CaOx crystal growth inhibition and the promotion of crystal aggregation, which do not appear to depend on unique protein confirmations (secondary or tertiary structures). More detailed studies to develop molecular-level knowledge of polyelectrolyte binding to COM surfaces will clearly be required; however, signatures of protein aggregation, possibly involving many different proteins simultaneously, should be sought in studies of urine proteins and stone matrix and are likely to provide further insight into the critical processes governing stone formation.
Acknowledgments
We gratefully acknowledge the financial support provided by the Department of Veterans Affairs through their Research Career Development and Merit Review Programs (RCD and MR 9305 - JAW) and in part with resources and the use of facilities at the Clement J. Zablocki Department of Veterans Affairs Medical Center, Milwaukee, WI. Funding was also obtained through parts of several grants from the National Institutes of Health/National Institute for Diabetes, Digestive, and Kidney Diseases (DK 68551, 74741, and 82550 - JAW). Additional financial support was provided by the Medical College of Wisconsin Jacob Lemann Endowment Grant, the Materials Research Science and Engineering Center (MRSEC) program of the National Science Foundation under Award Number DMR-1420073 (MDW), the Welch Foundation under Award Number E-1794 (JDR), and the National Science Foundation under Award Number 1207441 (JDR). We also gratefully acknowledge the technical support from MIS. MAC (Mandel International Stone and Molecular Analysis Center), Milwaukee, WI, for crystal analysis through Dr. Neil Mandel, as well as particular contributions from Dr. William Zachowicz to ζ-potential measurements and Samuel Cohen to polyanion-polycation aggregation experiments.
Footnotes
Compliance with Ethical Standards:
Human studies: The participating patient in this study was recruited with informed consent to an established study under VA IRB approval (VA-IRB protocol: 9305-01P). All procedures performed in these studies were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments.
Ethical approval: None of the authors has any conflicts of interest to report.
References
- 1.Aggarwal KP, et al. Nephrolithiasis: molecular mechanism of renal stone formation and the critical role played by modulators. Biomed Research International. 2013 doi: 10.1155/2013/292953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaiser ET, Bock SC. Protein inhibitors of crystal-growth. J Urology. 1989;141(3):750–752. doi: 10.1016/s0022-5347(17)41001-9. [DOI] [PubMed] [Google Scholar]
- 3.Kumar V, Lieske JC. Protein regulation of intrarenal crystallization. Curr Opin Nephrol Hy. 2006;15(4):374–380. doi: 10.1097/01.mnh.0000232877.12599.f4. [DOI] [PubMed] [Google Scholar]
- 4.Ryall RL. Glycosaminoglycans, proteins, and stone formation: Adult themes and child's play. Pediatr Nephrol. 1996;10(5):656–666. doi: 10.1007/s004670050185. [DOI] [PubMed] [Google Scholar]
- 5.Boyce WH, Garvey FK. The amount and nature of the organic matrix in urinary calculi - a review. J Urology. 1956;76(3):213–227. doi: 10.1016/S0022-5347(17)66686-2. [DOI] [PubMed] [Google Scholar]
- 6.Gohel MDI, Shum DKY, Tam PC. Electrophoretic separation and characterization of urinary glycosaminoglycans and their roles in urolithiasis. Carbohyd Res. 2007;342(1):79–86. doi: 10.1016/j.carres.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 7.Khan SR, Kok DJ. Modulators of urinary stone formation. Front Biosci. 2004;9:1450–1482. doi: 10.2741/1347. [DOI] [PubMed] [Google Scholar]
- 8.Sallis JD. Glycosaminoglycans as inhibitor of stone formation. Mineral and Electrolyte Metabolism. 1987;13(4):273–277. [PubMed] [Google Scholar]
- 9.Verkoelen CF. Crystal retention in renal stone disease: A crucial role for the glycosaminoglycan hyaluronan? J Am Soc Nephrol. 2006;17(6):1673–1687. doi: 10.1681/ASN.2006010088. [DOI] [PubMed] [Google Scholar]
- 10.Jariwalla RJ. Rice-bran products: Phytonutrients with potential applications in preventive and clinical medicine. Drug Exp Clin Res. 2001;27(1):17–26. [PubMed] [Google Scholar]
- 11.Miyaoka R, Monga M. Use of Traditional Chinese Medicine in the Management of Urinary Stone Disease. International Braz J Urol. 2009;35(4):396–405. doi: 10.1590/s1677-55382009000400002. [DOI] [PubMed] [Google Scholar]
- 12.Nasim MJ, et al. Gist of medicinal plants of Pakistan having ethnobotanical evidendes to crush renal calculi (kidney stones) Acta Pol Pharm. 2014;71(1):3–10. [PubMed] [Google Scholar]
- 13.Khan SR, Hackett RL. Crystal-Matrix Relationships in Experimentally Induced Urinary Calcium-Oxalate Monohydrate Crystals, an Ultrastructural-Study. Calcified Tissue Int. 1987;41(3):157–163. doi: 10.1007/BF02563796. [DOI] [PubMed] [Google Scholar]
- 14.Boyce WH. Organic matrix of human urinary concretions. Am J Med. 1968;45(5):673–683. doi: 10.1016/0002-9343(68)90203-9. [DOI] [PubMed] [Google Scholar]
- 15.Boyce WH, King JS. 1. Some special aspects of metabolic dysfunction - present concepts concerning origin of matrix and stones. Ann NY Acad Sci. 1963;104(2):563–578. doi: 10.1111/j.1749-6632.1963.tb17693.x. [DOI] [PubMed] [Google Scholar]
- 16.Robertson WG, Peacock M, Nordin BEC. Activity products in stone-forming and non-stone-forming urine. Clin Sci. 1968;34(3):579–594. [PubMed] [Google Scholar]
- 17.Aggarwal KP, et al. Peeping into Human Renal Calcium Oxalate Stone Matrix: Characterization of Novel Proteins Involved in the Intricate Mechanism of Urolithiasis. Plos One. 2013;8(7):e69916. doi: 10.1371/journal.pone.0069916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aggarwal KP, et al. 2D map of proteins from human renal stone matrix and evaluation of their effect on oxalate induced renal tubular epithelial cell injury. International Braz J Urol. 2013;39(1):128–136. doi: 10.1590/S1677-5538.IBJU.2013.01.16. [DOI] [PubMed] [Google Scholar]
- 19.Boonla C, et al. Inflammatory and fibrotic proteins proteomically identified as key protein constituents in urine and stone matrix of patients with kidney calculi. Clin Chim Acta. 2014;429:81–89. doi: 10.1016/j.cca.2013.11.036. [DOI] [PubMed] [Google Scholar]
- 20.Canales BK, et al. Proteome of Human Calcium Kidney Stones. Urology. 2010;76(4):1017.e13–1020.e13. doi: 10.1016/j.urology.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 21.Jou YC, et al. Proteomic Study of Renal Uric Acid Stone. Urology. 2012;80(2):260–266. doi: 10.1016/j.urology.2012.02.019. [DOI] [PubMed] [Google Scholar]
- 22.Kaneko K, et al. Comparison of matrix proteins in different types of urinary stone by proteomic analysis using liquid chromatography-tandem mass spectrometry. Int J Urol. 2012;19(8):765–772. doi: 10.1111/j.1442-2042.2012.03005.x. [DOI] [PubMed] [Google Scholar]
- 23.Wu JQ, Gao YH. Physiological conditions can be reflected in human urine proteome and metabolome. Expert Rev Proteomic. 2015;12(6):623–636. doi: 10.1586/14789450.2015.1094380. [DOI] [PubMed] [Google Scholar]
- 24.Okumura N, et al. Diversity in Protein Profiles of Individual Calcium Oxalate Kidney Stones. Plos One. 2013;8(7):e68624. doi: 10.1371/journal.pone.0068624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Solis FJ. Phase diagram of dilute polyelectrolytes: Collapse and redissolution by association of counterions and co-ions. J Chem Phys. 2002;117(19):9009–9015. [Google Scholar]
- 26.Manning GS. Molecular Theory of Polyelectrolyte Solutions with Applications to Electrostatic Properties of Polynucleotides. Q Rev Biophys. 1978;11(2):179–246. doi: 10.1017/s0033583500002031. [DOI] [PubMed] [Google Scholar]
- 27.McKee MD, Nanci A. Osteopontin at mineralized tissue interfaces in bone, teeth, and osseointegrated implants: Ultrastructural distribution and implications for mineralized tissue formation, turnover, and repair. Microsc Res Techniq. 1996;33(2):141–164. doi: 10.1002/(SICI)1097-0029(19960201)33:2<141::AID-JEMT5>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 28.Paloian NJ, Giachelli CM. A current understanding of vascular calcification in CKD. Am J Physiol-Renal. 2014;307(8):F891–F900. doi: 10.1152/ajprenal.00163.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weiner S, Addadi L. Acidic macromolecules of mineralized tissues - the controllers of crystal-formation. Trends Biochem Sci. 1991;16(7):252–256. doi: 10.1016/0968-0004(91)90098-g. [DOI] [PubMed] [Google Scholar]
- 30.Wesson JA, Ward MD. Pathological biomineralization of kidney stones. Elements. 2007;3:415–421. [Google Scholar]
- 31.Grover PK, Ryall RL. Inhibition of calcium oxalate crystal growth and aggregation by prothrombin and its fragments in vitro - Relationship between protein structure and inhibitory activity. Eur J Biochem. 1999;263(1):50–56. doi: 10.1046/j.1432-1327.1999.00448.x. [DOI] [PubMed] [Google Scholar]
- 32.Hess B, Nakagawa Y, Coe FL. Inhibition of calcium-oxalate monohydrate crystal aggregation by urine proteins. Am J Physiol. 1989;257(1):F99–F106. doi: 10.1152/ajprenal.1989.257.1.F99. [DOI] [PubMed] [Google Scholar]
- 33.Lieske JC, Huang E, Toback FG. Regulation of renal epithelial cell affinity for calcium oxalate monohydrate crystals. Am J Physiol-Renal. 2000;278(1):F130–F137. doi: 10.1152/ajprenal.2000.278.1.F130. [DOI] [PubMed] [Google Scholar]
- 34.Springmann KE, et al. Effects of human-urine on aggregation of calcium-oxalate crystals. J Urology. 1986;135(1):69–71. doi: 10.1016/s0022-5347(17)45520-0. [DOI] [PubMed] [Google Scholar]
- 35.Christmas KG, et al. Aggregation and dispersion characteristics of calcium oxalate monohydrate: Effect of urinary species. J Colloid Interf Sci. 2002;256(1):168–174. doi: 10.1006/jcis.2002.8283. [DOI] [PubMed] [Google Scholar]
- 36.Govindaraj A, Selvam R. Increased calcium oxalate crystal nucleation and aggregation by peroxidized protein of human kidney stone matrix and renal cells. Urol Res. 2001;29(3):194–198. doi: 10.1007/s002400100177. [DOI] [PubMed] [Google Scholar]
- 37.Grases F, Gil JJ, Conte A. Glycosaminoglycans - inhibition of calcium-oxalate crystalline growth and promotion of crystal aggregation. Colloid Surface. 1989;36(1):29–38. [Google Scholar]
- 38.Bigelow MW, et al. Surface exposure of phosphatidylserine increases calcium oxalate crystal attachment to IMCD cells. Am J Physiol-Renal. 1997;41(1):F55–F62. doi: 10.1152/ajprenal.1997.272.1.F55. [DOI] [PubMed] [Google Scholar]
- 39.Worcester EM. Inhibitors of stone formation. Semin Nephrol. 1996;16(5):474–486. [PubMed] [Google Scholar]
- 40.Shiraga H, et al. Inhibition of calcium-oxalate crystal-growth invitro by uropontin - another member of the aspartic acid-rich protein superfamily. P Natl Acad Sci USA. 1992;89(1):426–430. doi: 10.1073/pnas.89.1.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ito H, Coe FL. Acidic peptide and polyribonucleotide crystal-growth inhibitors in human urine. Am J Physiol. 1977;233(5):F455–F463. doi: 10.1152/ajprenal.1977.233.5.F455. [DOI] [PubMed] [Google Scholar]
- 42.Worcester EM, et al. The calcium-oxalate crystal growth inhibitor protein produced by mouse kidney cortical-cells in culture is osteopontin. J Bone Miner Res. 1992;7(9):1029–1036. doi: 10.1002/jbmr.5650070905. [DOI] [PubMed] [Google Scholar]
- 43.Wesson JA, Worcester E. Formation of hydrated calcium oxalates in the presence of poly-L-aspartic acid. Scanning Microscopy. 1996;10(2):415–423. [PubMed] [Google Scholar]
- 44.Prien EL. Crystallographic analysis of urinary calculi - A 23 year survey study. J Urology. 1963;89(6):917–924. doi: 10.1016/S0022-5347(17)64673-1. [DOI] [PubMed] [Google Scholar]
- 45.Kohri K, et al. Biomolecular mechanism of urinary stone formation involving osteopontin. Urol Res. 2012;40(6):623–637. doi: 10.1007/s00240-012-0514-y. [DOI] [PubMed] [Google Scholar]
- 46.Kumar V, et al. Annexin II is present on renal epithelial cells and binds calcium oxalate monohydrate crystals. J Am Soc Nephrol. 2003;14(2):289–297. doi: 10.1097/01.asn.0000046030.24938.0a. [DOI] [PubMed] [Google Scholar]
- 47.Sorokina EA, Wesson JA, Kleinman JG. An acidic peptide sequence of nucleolin-related protein can mediate the attachment of calcium oxalate to renal tubule cells. J Am Soc Nephrol. 2004;15(8):2057–2065. doi: 10.1097/01.ASN.0000133024.83256.C8. [DOI] [PubMed] [Google Scholar]
- 48.Pragasam V, et al. Calcium oxalate monohydrate aggregation is induced by desialylated Tamm-Horsfall protein. Urol Res. 2011;39:269–282. doi: 10.1007/s00240-010-0353-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wesson JA, et al. Regulation by macromolecules of calcium oxalate crystal aggregation in stone formers. Urol Res. 2005;33(3):206–212. doi: 10.1007/s00240-004-0455-1. [DOI] [PubMed] [Google Scholar]
- 50.Jung T, et al. Probing crystallization of calcium oxalate monohydrate and the role of macromolecule additives with in situ atomic force microscopy. Langmuir. 2004;20(20):8587–8596. doi: 10.1021/la0488755. [DOI] [PubMed] [Google Scholar]
- 51.Farmanesh S, et al. Natural promoters of calcium oxalate monohydrate crystallization. J Am Chem Soc. 2014;136(36):12648–12657. doi: 10.1021/ja505402r. [DOI] [PubMed] [Google Scholar]
- 52.Sheng XX, et al. Adhesion at calcium oxalate crystal surfaces and the effect of urinary constituents. P Natl Acad Sci USA. 2005;102(2):267–272. doi: 10.1073/pnas.0406835101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hutter JL, Bechhoefer J. Calibration of atomic-force microscope tips. Rev Sci Instrum. 1993;64(7):1868–1873. [Google Scholar]
- 54.Priftis D, Tirrell M. Phase behaviour and complex coacervation of aqueous polypeptide solutions. Soft Matter. 2012;8(36):9396–9405. [Google Scholar]
- 55.Johnston APR, et al. Layer-by-layer engineered capsules and their applications. Curr Opin Colloid In. 2006;11(4):203–209. [Google Scholar]
- 56.Decher G. Fuzzy nanoassemblies: Toward layered polymeric multicomposites. Science. 1997;277(5330):1232–1237. [Google Scholar]
- 57.Tang ZY, et al. Biomedical applications of layer-by-layer assembly: From biomimetics to tissue engineering. Adv Mater. 2006;18(24):3203–3224. [Google Scholar]
- 58.Lowack K, Helm CA. Molecular mechanisms controlling the self-assembly process of polyelectrolyte multilayers. Macromolecules. 1998;31(3):823–833. [Google Scholar]
- 59.Decher G, Hong JD, Schmitt J. Buildup of ultrathin multilayer films by a self-assembly process. 3. consecutively alternating adsorption of anionic and cationic polyelectrolytes on charged surfaces. Thin Solid Films. 1992;210(1–2):831–835. [Google Scholar]
- 60.Gower LB. Biomimetic model systems for investigating the amorphous precursor pathway and its role in biomineralization. Chem Rev. 2008;108(11):4551–4627. doi: 10.1021/cr800443h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kolbach-Mandel A, Kleinman J, Wesson J. Exploring calcium oxalate crystallization: a constant composition approach. Urolithiasis. 2015;43(5):397–409. doi: 10.1007/s00240-015-0781-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Benkovic J, et al. Effect of Tamm-Horsfall protein on calcium-oxalate precipitation. Eur J Clin Chem Clin. 1995;33(10):705–710. doi: 10.1515/cclm.1995.33.10.705. [DOI] [PubMed] [Google Scholar]
- 63.Hallson PC, Rose GA. Uromucoids and urinary stone formation. Lancet. 1979;1(8124):1000–1002. doi: 10.1016/s0140-6736(79)92755-7. [DOI] [PubMed] [Google Scholar]
- 64.Hess B, et al. Molecular abnormality of Tamm-Horsfall glycoprotein in calcium-oxalate nephrolithiasis. Am J Physiol. 1991;260(4):F569–F578. doi: 10.1152/ajprenal.1991.260.4.F569. [DOI] [PubMed] [Google Scholar]
- 65.Mo L, et al. Renal calcinosis and stone formation in mice lacking osteopontin, Tamm-Horsfall protein, or both. Am J Physiol-Renal. 2007;293(6):F1935–F1943. doi: 10.1152/ajprenal.00383.2007. [DOI] [PubMed] [Google Scholar]
- 66.Scurr DS, Robertson WG. Modifiers of calcium-oxalate crystallization found in urine. 3. Studies on the role of Tamm-Horsfall mucoprotein and of ionic-strength. J Urology. 1986;136(2):505–507. doi: 10.1016/s0022-5347(17)44931-7. [DOI] [PubMed] [Google Scholar]
- 67.Ronco P, et al. Physiopathologic aspects of Tamm-Horsfall protein: a phylogenetically conserved marker of the thick ascending limb of Henle's loop. Advances in Nephrology from the Necker Hospital. 1987;16:231–249. [PubMed] [Google Scholar]
- 68.Wesson JA, Worcester EM, Kleinman JG. Role of anionic proteins in kidney stone formation: Interaction between model anionic polypeptides and calcium oxalate crystals. J Urology. 2000;163(4):1343–1348. doi: 10.1016/s0022-5347(05)67775-0. [DOI] [PubMed] [Google Scholar]
- 69.Amjad Z, Koutsoukos PG. Impact of Surfactants on the Performance of "Green Additives" as Calcium Carbonate Inhibitors for Aqueous Systems. Tenside Surfact Det. 2014;51(1):40–45. [Google Scholar]
- 70.Mandel NS, Mandel GS. Urinary-tract stone disease in the United-States veteran population. 2. Geographical analysis of variations in composition. J Urology. 1989;142(6):1516–1521. doi: 10.1016/s0022-5347(17)39145-0. [DOI] [PubMed] [Google Scholar]
- 71.Schubert G. Stone analysis. Urol Res. 2006;34(2):146–150. doi: 10.1007/s00240-005-0028-y. [DOI] [PubMed] [Google Scholar]
- 72.Wesson JA, Ward MD. Role of crystal surface adhesion in kidney stone disease. Curr Opin Nephrol Hy. 2006;15(4):386–393. doi: 10.1097/01.mnh.0000232879.50716.6f. [DOI] [PubMed] [Google Scholar]
- 73.Sheng X. Dissertation. MN: University of Minnesota-Minneapolis; 2004. Face-specific molecular adhesion and binding to calcium oxalate monohydrate: implication for kidney stone disease; p. 127. [Google Scholar]
- 74.Wesson JA, et al. Osteopontin is a critical inhibitor of calcium oxalate crystal formation and retention in renal tubules. J Am Soc Nephrol. 2003;14(1):139–147. doi: 10.1097/01.asn.0000040593.93815.9d. [DOI] [PubMed] [Google Scholar]
- 75.Wesson JA, et al. Osteopontin in nephrolithiasis. J Am Soc Nephrol. 2002;13:574A–575A. [Google Scholar]
- 76.Farmanesh S, et al. Specificity of growth inhibitors and their cooperative effects in calcium oxalate monohydrate crystallization. J Am Chem Soc. 2014;136:367–376. doi: 10.1021/ja410623q. [DOI] [PubMed] [Google Scholar]
- 77.De Yoreo JJ, Qiu SR, Hoyer JR. Molecular modulation of calcium oxalate crystallization. Am J Physiol-Renal. 2006;291(6):F1123–F1131. doi: 10.1152/ajprenal.00136.2006. [DOI] [PubMed] [Google Scholar]
- 78.Wang LJ, et al. Phosphorylation of osteopontin is required for inhibition of calcium oxalate crystallization. J Phys Chem B. 2008;112(30):9151–9157. doi: 10.1021/jp804282u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Grohe B, et al. Control of calcium oxalate crystal growth by face-specific adsorption of an osteopontin phosphopeptide. J Am Chem Soc. 2007;129(48):14946–14951. doi: 10.1021/ja0745613. [DOI] [PubMed] [Google Scholar]
- 80.Hunter GK, et al. Role of Phosphate Groups in Inhibition of Calcium Oxalate Crystal Growth by Osteopontin. Cells Tissues Organs. 2009;189(1–4):44–50. doi: 10.1159/000151430. [DOI] [PubMed] [Google Scholar]
- 81.O'Young J, et al. Phosphorylation of osteopontin peptides mediates adsorption to and incorporation into calcium oxalate crystals. Cells Tissues Organs. 2009;189(1–4):51–55. doi: 10.1159/000151724. [DOI] [PubMed] [Google Scholar]
- 82.Farmanesh S, et al. High-throughput platform for design and screening of peptides as inhibitors of calcium oxalate monohydrate crystallization. J Crystal Growth. 2013;373:13–19. [Google Scholar]
- 83.Wang LJ, et al. Modulation of calcium oxalate crystallization by linear aspartic acid-rich peptides. Langmuir. 2006;22(17):7279–7285. doi: 10.1021/la060897z. [DOI] [PubMed] [Google Scholar]
- 84.Chen CL, et al. Tuning calcite morphology and growth acceleration by a rational design of highly stable protein-mimetics. Scientific Reports. 2014;4:6266. doi: 10.1038/srep06266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Anderson NG, Anderson NL, Tollaksen SL. Proteins of human-urine. 1. Concentration and analysis by 2-dimensional electrophoresis. Clin Chem. 1979;25(7):1199–1210. [PubMed] [Google Scholar]
- 86.Hiemenz PC, Rajagopalan R. Principles of colloid and surface chemistry. New York: Marcel Dekker; 1997. p. xix.p. 650. 3rd, rev. and expanded/ed. [Google Scholar]
- 87.Bohmer MR, Evers OA, Scheutjens J. Weak polyelectrolytes between 2 surfaces - adsorption and stabilization. Macromolecules. 1990;23(8):2288–2301. [Google Scholar]
- 88.Cheng H, et al. Polynucleotide adsorption to negatively charged surfaces in divalent salt solutions. Biophys J. 2006;90(4):1164–1174. doi: 10.1529/biophysj.105.070649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hansma HG, et al. Reproducible imaging and dissection of plasmid DNA under liquid with the atomic force microscope. Science. 1992;256(5060):1180–1184. doi: 10.1126/science.256.5060.1180. [DOI] [PubMed] [Google Scholar]
- 90.Pastre D, et al. Adsorption of DNA to mica mediated by divalent counterions: A theoretical and experimental study. Biophys J. 2003;85(4):2507–2518. doi: 10.1016/s0006-3495(03)74673-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Scheutjens J, Fleer GJ. Statistical theory of the adsorption of interacting chain molecules. 2. Train, loop, and tail size distribution. J Phys Chem. 1980;84(2):178–190. [Google Scholar]
- 92.Vanderschee HA, Lyklema J. A lattice theory of polyelectrolyte adsorption. J Phys Chem. 1984;88(26):6661–6667. [Google Scholar]
- 93.Hess B. Tamm-Horsfall glycoprotein and calcium nephrolithiasis. Miner Electrol Metab. 1994;20(6):393–398. [PubMed] [Google Scholar]
- 94.Kabanov AV, Kabanov VA. Interpolyelectrolyte and block ionomer complexes for gene delivery: Physicochemical aspects. Adv Drug Deliver Rev. 1998;30(1–3):49–60. doi: 10.1016/s0169-409x(97)00106-3. [DOI] [PubMed] [Google Scholar]