Abstract
Background
Sleep is a predictor of infectious illness that may depend on one’s socioeconomic status (SES).
Purpose
This study aimed to investigate the moderating effects of objective and subjective SES on sleep-clinical cold risk link and test whether nasal inflammation serves as a plausible biological pathway.
Methods
This study combined data (n = 732) from three viral challenge studies. Measures of self-reported sleep and objective and subjective measures of SES were obtained. Participants were quarantined and administrated rhinovirus (RV) or influenza virus and monitored over 5 (RV) or 6 (influenza) days for the development of a cold. Symptom severity, including mucus production and nasal clearance time, and levels of nasal cytokines (interleukin (IL)-6 and IL-1β) were measured prior to administration and each day during the quarantined period.
Results
Subjective SES, but not objective SES, moderated associations between shorter sleep duration and increased likelihood of a clinical cold. Compared to ≥8-hour sleepers, ≤6-hour sleepers with low subjective SES were at increased risk for developing a cold (OR = 2.57, 95% CI 1.10–6.02). There was no association between sleep duration and colds in high subjective SES participants. Among infected individuals who reported low subjective SES, shorter sleep duration was associated with greater mucus production. There was no evidence that markers of nasal inflammation mediated the link between sleep duration and cold susceptibility among those reporting low subjective SES.
Conclusion
Subjective SES may reflect an important social factor for understanding vulnerability to and protection against infectious illness among short sleepers.
Keywords: Sleep, Upper respiratory infection, Socioeconomic status, Cytokines
Introduction
Sleep has emerged as a critical factor in the promotion and maintenance of physical health [1, 2]. Poor sleep, as characterized by short sleep duration (e.g., sleeping fewer than 6 h per night), poor sleep efficiency, and poor subjective sleep quality, predicts incidence and severity of a number of chronic medical conditions, including cardiovascular disease, type 2 diabetes [3, 4], and susceptibility to acute infectious illness [5, 6]. While the mechanisms that underlie these associations remain unclear, there is growing appreciation for the influence sleep has on modulating the immune system [7], including immune processes essential to protecting individuals from development of upper respiratory infections. Studies employing partial or total sleep restriction observe consistent changes in aspects of the immune system (e.g., diminished T-cell proliferation [8, 9], a shift away from T-helper cell 1 cytokine production [8, 10], reduced natural killer cell cytotoxicity [11], and activation of inflammatory pathways [12]) that may increase risk for upper respiratory infections. Associations between sleep and immune function have also been shown beyond laboratory settings. For example, prior studies show that shorter sleep duration and poorer sleep efficiency, assessed both objectively using wrist actigraphy and subjectively using sleep diaries, were associated with increased susceptibility to a biologically verified cold following an experimental rhinovirus challenge [5, 6]. These associations were independent of potential confounds, including demographic factors, health behaviors, and psychological variables.
Despite clear links between sleep and immune functioning, substantial individual variability exists and not all short and disrupted sleepers experimentally exposed to rhinovirus develop a clinical cold. One potential moderator of the association of sleep and vulnerability to upper respiratory infection is socioeconomic status (SES). A large body of evidence suggests that the chronic distress and burden related to lack of resources and opportunity create wear and tear on multiple biological systems, including the immune system [13–16]. For example, individuals low in income and educational attainment show enhanced inflammatory activity [17–19] and diminished cell-mediated immunity [20, 21] compared to high SES individuals. Over time, the effects of low SES on immune function may render an individual more immunologically vulnerable to the deleterious effects of poor sleep.
Conversely high SES may serve a protective role in buffering individuals against the negative health outcomes conferred by poor sleep. Poor sleep has been linked to increased sensitivity to stressors, both in the laboratory and in naturalistic settings [22–24]. High SES individuals have greater financial resources and knowledge which enable them to cope more effectively with daily hassles and tensions that would otherwise lead to stress-related decrements in immunity and increased susceptibility to infectious illness. Moreover, high SES individuals may have more flexibility for making up lost sleep during the day [25, 26], which may offset the immunological consequences conferred by lost sleep at night. To date, the role of SES in understanding the link between sleep and infectious illness has not been investigated.
SES can be measured a number of different ways. Objective SES indicators typically include household income, educational attainment, and occupational status. Such markers are strongly related to health outcomes, including measures of immune dysfunction [27, 28]. In addition, one’s subjective SES (i.e., one’s perceived socioeconomic standing relative to a reference, which is often others in their community or country) can be assessed. Like objective indicators of SES, lower subjective SES has been linked to negative health outcomes, with effects often over and above those of objective SES indicators [29–31]. It is possible that either or both may moderate associations between sleep and susceptibility to upper respiratory infection. However, one of the challenges inherent in measuring subjective SES, like other self-report measures, is the possibility that personality characteristics, such as negative affectivity or one’s level of perceived stress, may contribute to both the judgments made about one’s subjective SES and to mediating pathways related to clinical cold susceptibility. As such, it is important to account for the role of these affective processes in testing the moderating effects of SES on relationships between sleep and clinical cold risk.
The goal of this study was to examine whether SES moderated associations between self-reported sleep habits and susceptibility to the common cold. To do this, we combined data from 740 individuals who participated in one of the three previous viral challenge studies using similar procedures. This aggregation across studies provided the statistical power needed to test the moderating effects of SES on the sleep-cold relationship. It was hypothesized that measures of SES would moderate the link between poor sleep and clinical cold risk such that the negative influence of poor sleep on likelihood of developing a cold following viral challenge would be stronger among low SES participants. Further, we explored whether nasal inflammation, which has been shown previously to predict illness severity following respiratory infection [32, 33], may serve as a plausible biological pathway through which sleep may influence the cold susceptibility among low SES individuals.
Methods
Participants
The present analyses combined archival data from the three viral challenge studies (Pittsburgh Cold Study 2 (PCS2), Pittsburgh Mind-Body Center Study (PMBC), Pittsburgh Cold Study 3 (PCS3)) carried out between 1997 and 2011 that followed a common set of procedures (www.commoncoldproject.com). These procedures included a physical exam, standardized questionnaires, and subsequent participation in a viral challenge trial. The total sample consisted of 740 participants (334 in PCS2, 193 in PMBC, and 213 in PCS3). PCS2 was conducted between 1997 and 2001, while PMBC and PCS3 were conducted between 2000 and 2004 and 2007 and 2011, respectively.
Study participants were recruited from the Pittsburgh, Pennsylvania metropolitan area, through study advertisements and were judged by physical examination to be in good health. Each study received institutional review board approval, and written, informed consent was obtained for all study participants. All volunteers underwent medical screenings and were excluded from study eligibility if they had a history of psychiatric illness, asthma, or cardiovascular disorders; had undergone major nasal or otologic surgery; had an abnormal urinalysis, complete blood count, or blood enzymes; were pregnant or currently lactating; tested seropositive for HIV; or were taking regular medications (except birth control).
Study Procedures
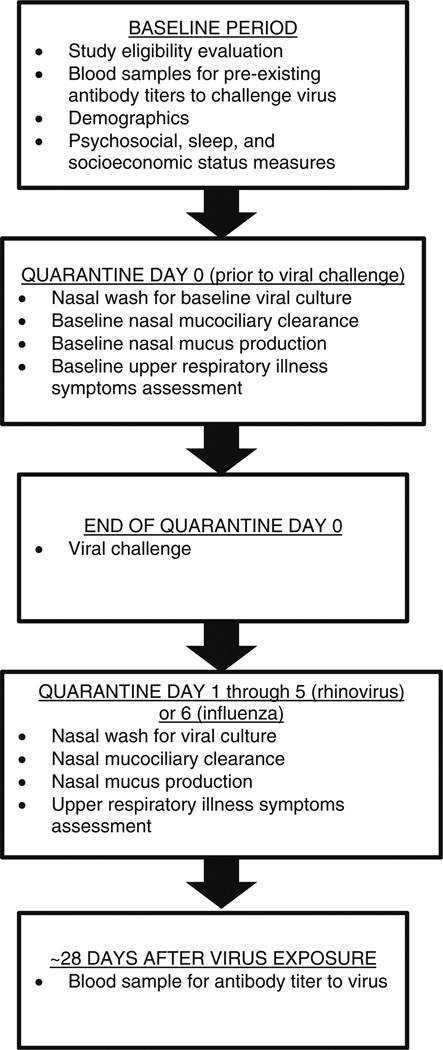
The temporal sequence of the study events is depicted in Fig. 1. Measures of sleep habits, objective and subjective socioeconomic status, perceived stress, and negative affectivity were obtained by self-report during the weeks between the initial screening and the experimental viral challenge. During the first 24 h of quarantine (prior to viral exposure), participants underwent a nasal examination and nasal lavage. Baseline nasal mucociliary clearance and mucus production were also quantified. Participants were excluded at this point if viral pathogens were successfully isolated from nasal wash samples, if they had nasal congestion, nasal discharge, or mucosal edema, or if they reported symptoms of an upper respiratory illness.
Fig. 1.
Temporal sequence of an experimental viral challenge trial
At the end of a 24-h quarantine (day 0), participants received nasal drops containing one of the three viruses: rhinovirus (RV) 39 (n = 593), RV23 (n = 103), or Influenza A/Texas/36/91 (n = 37). Disease expression in all the three viruses is a common cold-like upper respiratory illness. Participants exposed to either of the two rhinoviruses were given 100–300 tissue culture infectious dose50; those exposed to the influenza virus were given a 105 tissue culture infectious dose50.
Following viral challenge, participants were quarantined for an additional 5 (RVs) or 6 (influenza) days. During this period, participants were evaluated daily for cold symptoms, nasal mucociliary clearance, and nasal mucus production. Daily nasal wash samples were obtained, frozen, and later cultured for the challenge virus using standard techniques [34]. A blood draw was obtained approximately 28 days later and assayed for virus-specific antibodies.
Sleep Measures
Measures of self-reported sleep duration, sleep efficiency, and subjective sleep quality over the past month were assessed using validated items derived from the Pittsburgh Sleep Quality Index [35]. In PCS2 and PMBC, self-reported sleep duration (hours) was calculated as follows: (total time in bed minus minutes required to fall asleep (i.e., sleep latency) and minutes of wakefulness during the night)/60. In PCS3, self-reported sleep duration was obtained based on respondent’s report of hours of actual sleep per night. In all three studies, self-reported sleep efficiency was calculated as follows: (sleep duration/total time in bed) × 100. Self-reported subjective sleep quality was obtained by asking participants to rate their sleep quality overall (0=very good, 1=fairly good, 2=fairly bad, 3=very bad).
Objective and Subjective Socioeconomic Status
Educational attainment was the objective indicator of SES used across the three cold studies. Education was assessed by the question, “What is the highest grade or year of school you completed?” There were 18 categories ranging from “no formal education” to “doctoral degree (PhD, MD, EdD, DVM, DDS, JD, etc)” [31]. Based on their response, participants were assigned a number of years of education (e.g., high school education = 12 years, associate degree = 14 years, and a PhD = 20 years). Subjective socioeconomic status was assessed by asking participants to place themselves on a 9-rung ladder (check a rung) based on where they stand compared to other persons in the USA in terms of income, education, and occupation [28]. They were assigned scores ranging from 1 (the lowest rung) to 9 (the highest rung).
Standard Control Variables
Six study variables were obtained at screening and used as covariates in analyses. These included participants’ age, sex (male, female), self-reported race (white/Caucasian, black/African American, other), virus (rhinovirus, influenza), season of trial (spring, summer, fall, winter), and body mass index (BMI, kg/m2). Two additional measures were also included as controls: study (PCS2, PMBC, PCS3) and preexisting immunity (viral-specific antibody titers) to the challenge virus (titers <4 or ≥4).
Perceived Stress and Negative Affectivity
Perceived stress was assessed using the 10-item version of the Perceived Stress Scale [36], which provides a measure of global stress perception over the past month. Neuroticism was assessed as a measure of trait negative affectivity across the three cohorts. In PCS2 and PMBC, neuroticism was assessed using items derived from Goldberg’s adjective scale [37] and was represented by the five (PMBC) or ten (PCS2) highest-loading items. In these studies, internal reliabilities for neuroticism were 0.80–0.85. In PCS3, neuroticism was measured by 10-item Big Five subscale of the International Personality Item Pool [38]. The internal reliability neuroticism was 0.88. To establish equivalency across studies, we computed z-scores for each subscale before including them in analyses.
Infection
Serum antibodies to the challenge virus were obtained from a baseline blood draw. In order to maximize the rate of infection by the virus in PMBC and PCS3, specific levels of serum antibody to the challenge virus were obtained at screening and participants were excluded with titers >4. Antibody titers were not used as a screening variable in PCS2.
Serum antibodies were also obtained approximately 28 days after viral exposure. The results were expressed as reciprocals of the final dilution of serum [39]. Antibody titers were quantified using a microtiter neutralization assay for rhinoviruses and hemagglutination inhibition assay for the influenza virus. Daily nasal lavage samples were also collected and frozen at −80 °C. These samples were later cultured for the virus using standard techniques [39]. Infection was defined as the recovery of the challenge virus on any of the 5 (RVs) or 6 (influenza) post-challenge days or a ≥4-fold increase in the virus-specific serum neutralizing antibody titer measured pre-exposure to 28 days post-exposure.
Signs of Illness
We obtained two objective markers of illness: daily mucus production and nasal mucociliary clearance function. To assess daily mucus production, used tissues were collected each day and sealed in plastic bags [40]. The bags were weighed and the weights of the tissues and bags were subtracted. Nasal clearance function is an objective measure of congestion and measures the ability of the nasal cilia in clearing mucus from the nasal passage to the throat. Nasal clearance function was measured by administering a dye into the anterior area of the nose and calculating the time (minutes) taken for the dye to reach the back of the throat [40].
In order to create baseline-adjusted daily scores for each measure of illness, we subtracted the appropriate baseline score (i.e., from the day before the challenge virus was administered) from each of the 5 (RV) or 6 (influenza) post-challenge daily scores [41]. Negative-adjusted scores were assigned a value of 0. Average nasal clearance function was calculated by summing the respective adjusted daily scores for each measure over all post-challenge days and then dividing by the total number of days. Total mucus weight scores were obtained by summing the adjusted daily scores across the 5 (RVs) or 6 (influenza) post-challenge days. Values for participants infected with the influenza virus (n = 38) were subsequently multiplied by 5/6 to establish equivalency across the two sampling frames.
Clinical Cold
Study participants were considered to have a clinical cold (yes, no) if they were both infected with the challenge virus and met illness criteria. Illness criterion for an objective cold required either a total baseline-adjusted mucus weight of ≥10 g or an average (across all post-challenge days) baseline-adjusted nasal clearance time of ≥7 min [41].
Nasal Cytokines
Nasal wash fluid was recovered from each participant on each of the study days and immediately stored at −70 °C until assay. Nasal wash fluid was assayed (in duplicate) for interleukin (IL)-6 and IL-1β using commercially available enzyme-linked immunosorbent assays (ELISAs; Endogen; PCS2, PCS3) and by Biosource Ten-plex bead Immunoassay (Biosource International, Camarillo, CA; PMBC) following manufacturers’ protocols. Cytokine responses to virus exposure were quantified as the log area under the curve (AUC) of the 5 (or 6) post-exposure days adjusted for baseline (pre-exposure) values. Cytokine data were included only if they were complete for each study day. The final sample included in analyses were n = 512 for IL-6 and n = 511 for IL-1β, respectively.
Statistical Analyses
Data were analyzed using IBM SPSS statistics software, version 23 (SPSS Inc., Chicago, IL). All tests were two-tailed and the criterion for significance was set at p < 0.05. Data were drawn from 740 participants across three cold study cohorts; we analyzed data on 732 individuals for which self-reported sleep data were available. Descriptive statistics are presented as means and standard deviation or percentages. Body mass index (BMI), mucus production, nasal clearance time, and AUC nasal inflammation (IL-6 and IL-1β) were log (base-10) transformed to better approximate a normal distribution. Binary multiple logistic regression analyses were carried out between sleep and cold susceptibility, adjusted for our set of covariates, including age, BMI, and perceived stress which were treated as continuous, while sex, race, virus type, season of trial, pre-challenge antibodies, and study were dummy coded. We report unstandardized regression coefficients with standard errors and probability values as well as odds ratios (OR) with 95% confidence intervals (CI).
Moderation analyses were carried out using logistic regression. To test for interactions with educational attainment and subjective SES, first order centered cross-product terms for sleep variables and the proposed modifier variables were created. Interaction terms were then entered into individual regression equations with the corresponding main effects and control variables. Because measures of objective and subjective SES are often correlated, and to rule out both perceived stress and negative affectivity as a potential third-factor explanations, we fit a regression model that adjusted negative affectivity, perceived stress, the SES indicator not being tested as a moderator, and the respective interaction terms of each of these variables with the sleep variable of interest. Interactions are reported as the unstandardized regression coefficient and standard error as well as ORs with 95% CIs.
To better clarify associations and to provide an estimate of effect size between self-reported sleep duration and cold susceptibility as well as the modifying influence of subjective SES, we fit additional regression models where sleep duration was categorized based on hours and subjective SES was categorized by approximate tertiles (low, medium, high). Here we report OR with 95% CI.
Because levels of nasal cytokines have previously been shown to predict illness expression among individuals infected by experimental inoculation with rhinovirus, we tested whether nasal inflammation (IL-6 and IL-1β) served as a possible mechanism through which the moderated effect of subjective socioeconomic status on sleep duration influences cold susceptibility. To this end, we first carried out separate adjusted linear regression models to assess associations between nasal inflammation and objective markers of illness (mucus weight and nasal clearance time) among infected participants. Next, we fit two regression models predicting likelihood of meeting threshold for illness expression that adjusted for the cytokine of interest and the respective interaction term of that cytokine with self-reported sleep duration.
Results
Sample Characteristics
Table 1 presents descriptive data for all variables involved in the analyses. Of the 732 participants, 545 (74.5%) were infected with the challenge virus and 210 (28.7%) developed a clinical cold as defined by infection and objective cold criterion. As expected, self-reported sleep measures were intercorrelated (sleep quality and sleep duration, r = −0.28, p < 0.001; sleep quality and sleep efficiency, r = −0.35, p < 0.001; and sleep duration and sleep efficiency, r = 0.58, p < 0.001). Education and subjective SES were also positively correlated (r = 0.23, p < 0.001). Greater educational attainment was associated with better sleep efficiency (r = 0.08, p = 0.04) but not sleep duration or sleep quality. Higher subjective SES was significantly associated with better sleep quality (r = −0.09, p = 0.02) and sleep efficiency (r = 0.10, p = 0.009) but not self-reported sleep duration (r = 0.03, p = 0.73).
Table 1.
Sample characteristics (n = 732)
| Variable | Means (standard deviation) or % (n) |
|---|---|
| Age (years) | 31.4 (10.7) |
| Sex (% female) | 48.9 (358) |
| Race | |
| White/Caucasian | 64.8 (474) |
| Black/African American | 30.4 (223) |
| Other | 4.8 (35) |
| Virus type | |
| Rhinovirus (RV)39 | 80.9 (592) |
| RV23 | 14.1 (103) |
| Influenza virus | 5.0 (37) |
| Season of trial | |
| Winter | 17.5 (128) |
| Spring | 46.9 (343) |
| Summer | 17.3 (127) |
| Fall | 18.3 (134) |
| Study cohort | |
| PCS2 (1997–2001) | 45.2 (331) |
| PMBC (2000–2004) | 25.8 (189) |
| PCS3 (2007–2011) | 29.0 (212) |
| Body mass index (kg/m2) | 27.3 (6.4) |
| Pre-challenge antibody levels (titers) | 4.9 (5.6) |
| Educational attainment (years) | 13.8 (2.0) |
| Subjective SES ladder (0-low to 10-high) |
4.4 (1.9) |
| Perceived stress | 14.1 (6.5) |
| Self-reported sleep | |
| Sleep duration (h) | 7.0 (1.7) |
| Sleep efficiency (%) | 88.4 (13.1) |
| Sleep quality (0-very good to 3-very bad) |
0.9 (0.6) |
SES socioeconomic status
Sleep and Susceptibility to the Common Cold
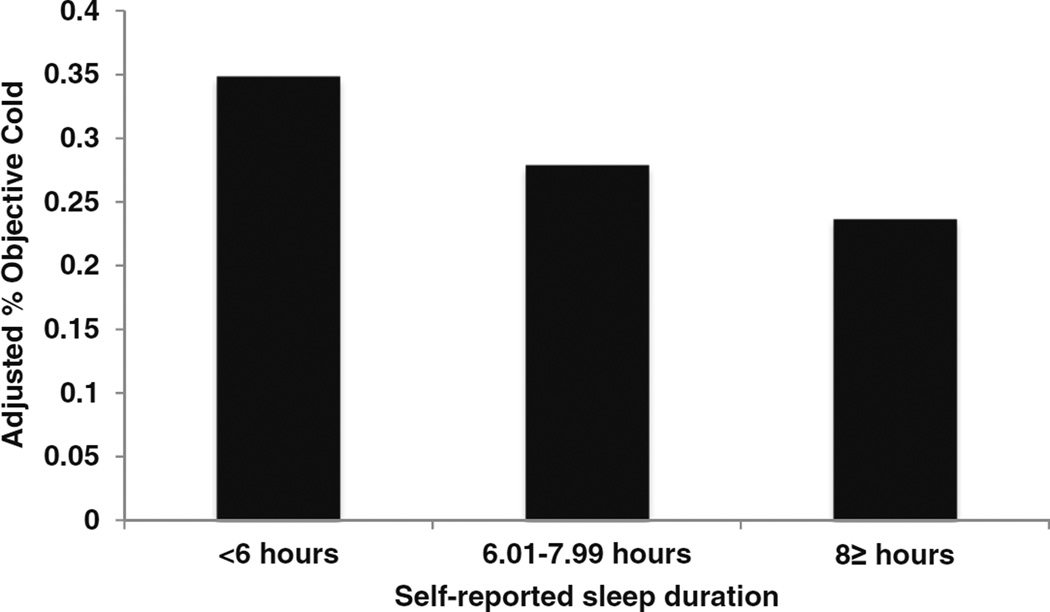
Shorter self-reported sleep duration, modeled as a continuous predictor, was associated with increased susceptibility for the common cold, independent of study covariates including age, sex, race, BMI, perceived stress, pre-challenge antibodies, season of trial, study, and virus type (B = −0.13, SE = 0.06, p = 0.018; OR = 0.88, 95% CI 0.79–0.98). To estimate the effect size, sleep duration was categorized as follows: ≤6 h, n = 201; 6.01–7.99 h, n = 321; and ≥8 h, n = 207 (Fig. 2). Participants who slept 6 or fewer hours were at significantly greater risk of a clinical cold than those sleeping ≥8 h (OR = 1.93, 95% CI 1.20–3.10). A similar elevation in risk, though not statistically significant, was observed in participants sleeping 6.01–7.99 h per night (OR = 1.39, 95% CI 0.90–2.15) compared to ≥8 h sleepers [reference]. Sleep efficiency and sleep quality were not statistically related to clinical cold risk (efficiency: p = 0.20; quality: p = 0.28).
Fig. 2.
Self-reported sleep duration is prospectively associated with the percentage of participants who subsequently developed the cold. The percentage of colds is based on predicted values (adjusted for age, sex, race, body mass index, perceived stress, pre-challenge viral-specific antibody levels, season of trial, virus type, and study)
Because we previously reported on associations between self-reported sleep duration and sleep efficiency using participants included in PMBC [5], we reran our analyses excluding these participants, reducing our sample from 732 to 580 participants. In these analyses, adjusting for study covariates, shorter sleep duration continued to be related to cold susceptibility (B = −0.11, SE = 0.07, p = 0.11; OR = 0.90, 95% CI 0.79–1.02), albeit below statistical significance. Self-reported sleep efficiency and subjective sleep quality were unrelated to cold susceptibility (p > 0.40).
Moderating Effects of Objective and Subjective Socioeconomic Status
In order to investigate whether objective or subjective SES moderated the effects of sleep on cold susceptibility, we carried out separate regression models where either educational attainment or subjective SES was treated as a moderator. Associations between sleep and cold susceptibility did not vary as a function of educational attainment (p > 0.50). In contrast, subjective SES significantly moderated the link between sleep duration and development of a clinical cold (B = 0.08, SE = 0.03, p = 0.007; OR = 1.08, 95% CI 1.02–1.14).
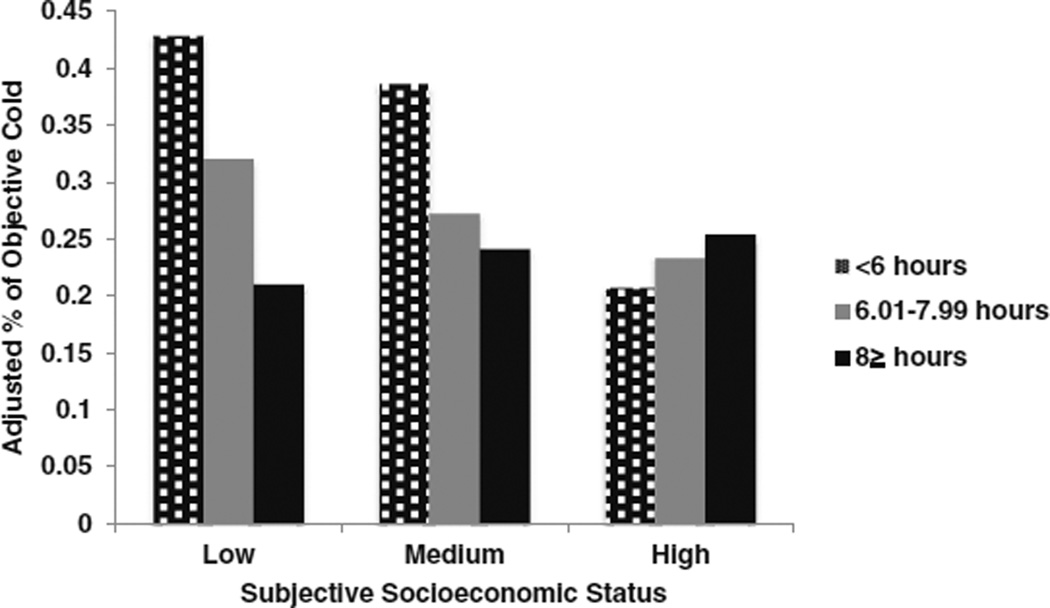
To provide an estimate of effect size for predicting clinical colds, subjective SES was categorized into approximate tertiles (low, n = 256; medium, n = 277; high, n = 194). Participants who were short sleepers (≤6 h) were at increased risk of developing a clinical cold compared to ≥8 h sleepers if they fell in the low (OR = 2.57, 95% CI 1.10–6.02) and to some extent the medium tertile of subjective SES (OR = 1.88, 95%CI 0.85–4.16) but not the high tertile (OR = 1.07, 95%CI 0.40–2.97). Participants sleeping 6.01–7.99 h per night were at no greater clinical cold risk irrespective of subjective SES tertile (Fig. 3). Because the association of self-reported sleep duration with cold susceptibility appeared to be due, in part, to the significant association found with the PMBC sample that was published previously, we reran the moderation analysis excluding the PMBC sample. Here we found that subjective SES continued to moderate the link between self-reported sleep duration and cold susceptibility (B = 0.09, SE = 0.03, p = 0.009; OR = 1.09, 95% CI 1.02–1.16).
Fig. 3.
Subjective socioeconomic status moderates the association between self-reported sleep duration, measured prior to viral exposure, and the percentage of participants who subsequently developed the cold. The percentage of colds is based on predicted values (adjusted for age, sex, race, body mass index, perceived stress, pre-challenge viral-specific antibody levels, season of trial, virus type, and study)
These interaction effects remained significant after adjustment for the main effects of educational attainment, negative affectivity, perceived stress, as well as their respective interactions with sleep duration (educational attainment × sleep duration, negative affectivity × sleep duration, perceived stress × sleep duration) (B = 0.09, SE =0.03, p = 0.003; OR = 1.09, 95% CI 1.03–1.16). There was no evidence that subjective SES moderated associations of either sleep efficiency or subjective sleep quality with cold susceptibility.
The observed elevated risk of developing the cold in participants reporting shorter self-reported sleep duration and lower subjective SES may have been due to increased susceptibility to infection and/or increased illness expression among those infected. In this regard, among infected participants, there was a significant interaction between self-reported sleep duration and subjective SES in predicting likelihood of meeting the illness criteria for mucus production or nasal clearance time (B = 0.06, SE = 0.03, p = 0.03; OR = 1.07, 95% CI 1.01–1.13). Specifically, compared to ≥8-h sleepers, short sleepers (≤6 h) who were also low in subjective SES were significantly more likely to meet illness criteria for illness expression (OR = 2.57, 95% CI 1.10–6.02). Next, separate adjusted linear regression models were computed to better clarify the influence of self-reported sleep duration on mucus production and nasal clearance time among those reporting low subjective SES who were infected with the virus. In this regard, shorter self-reported sleep duration was significantly associated with greater total adjusted mucus production (B = −0.05, SE = 0.03, t(182) = −2.07, p = 0.04; R2 = 0.14, F(11, 182) = 3.79, p < 0.001) but not average nasal clearance time (B = −0.02, SE = 0.02, t(182) = −0.95, p = 0.34; R2 = 0.01, F(11, 182) = 1.16, p= 0.32).
Analyses also revealed that an interaction between sleep duration and subjective SES was significantly related to likelihood of being infected (B = 0.07, SE = 0.03, p = 0.02; OR = 1.08, 95% CI 1.01–1.14); however, a probing of the interaction indicated that ≥8-h sleepers reporting high subjective SES were more likely than 6.01–7.99-h sleepers to become infected (OR = 3.41, 95% CI 1.23–9.49). No such associations were observed among those medium or low in subjective SES. Taken together, it appears that the link between self-reported sleep duration and cold susceptibility among those low in subjective SES is driven primarily through illness expression.
Nasal Inflammation as a Potential Mechanism
Previous studies demonstrate that levels of nasal cytokines positively predict illness expression among infected individuals [32, 33]. Among infected participants adjusted analyses revealed that higher levels of IL-6 and IL-1β were related to greater total adjusted mucus weight (IL-6: B = 0.32, SE = 0.03, t(498) = 11.30, p < 0.001; R2 = 0.25, F(11, 498) = 16.74, p < 0.001; IL-1β: B = 0.12, SE = 0.03, t(497) = 4.34, p < 0.001; R2 = 0.10, F(11, 497) = 5.94, p < 0.001) and longer nasal clearance time (IL-6: B = 0.12, SE = 0.02, t(498) = 5.81, p < 0.001; R2 = 0.07, F(11, 498) = 4.41, p < 0.001; IL-1β:B= 0.06, SE = 0.02, t(497) = 4.34, p = 0.002; R2 = 0.03, F(11, 497) = 2.29, p = 0.01). However, the interaction between self-reported sleep duration and subjective SES was not related to either levels of IL-6 (B = −0.003, SE = 0.01, t(494) = −0.25, p = 0.80; R2 = 0.29, F(13, 494) = 16.60, p < 0.001) or IL-1β (B = −0.004, SE = 0.01, t(493) = −0.29, p = 0.77; R2 = 0.07, F(13, 493) = 4.12, p < 0.001). Further, the interaction effect of sleep duration × subjective SES on likelihood of meeting the illness criteria for mucus production or nasal clearance time remained significant after adjustment for the main effects of nasal cytokines, as well as their respective interactions with sleep duration in separate models (adjusting for IL-6: B = 0.07, SE = 0.03, p = 0.03; OR = 1.07, 95% CI 1.01–1.15; adjusting for IL-1β: B = 0.06, SE = 0.03, p = 0.04; OR = 1.06, 95% CI 1.00–1.13). Accordingly, these data suggest that the nasal cytokines examined do not play a significant role in mediating the effects of sleep duration on cold susceptibility among those low in subjective SES.
Discussion
Shorter self-reported sleep duration was prospectively associated with increased incidence of the common cold following experimental viral challenge. Subjective SES, however, moderated this relationship such that shorter sleep predicted cold incidence in participants who reported low subjective SES but not among those who reported high subjective SES. These findings were independent of potential covariates, including age, sex, race, BMI, perceived stress, pre-challenge antibody levels, season, virus type, study, objective SES (i.e., educational attainment), and negative affectivity.
The prospective association between sleep duration and clinical cold risk is consistent with a previous analysis of a subset of the data used here [5] as well as an analysis where sleep was measured behaviorally using wrist actigraphy [6]. This paper extends those previous findings by showing the moderating influence of subjective SES on the link between sleep and cold susceptibility. While the graded nature of the association of SES and negative health outcomes is well established [28, 29], it is unclear how lower SES confers vulnerability to infectious illness among short sleepers. Chronic stress exposure, which is common among those low in SES [28, 42, 43], may play an important role. Chronic stress is consistently associated with decrements in aspects of the immune system (e.g., T-cell immunity) critical to the adequate protection from infectious agents, including the cold virus [44], and low SES individuals display elevated levels of systemic inflammation [45, 46]. The present findings were independent of perceived stress, as well as its interaction with sleep duration; however, the measure used assessed global stress perception over the past month, which may not reflect the biological cost incurred through longer stress exposures.
Another interpretation of the present findings is that high subjective SES effectively buffers against the negative influences of short sleep on clinical cold risk. Higher SES is associated with greater access to personal (e.g., perceived control) and social resources (e.g., social capital) that may enhance an individual’s ability to cope effectively [47], thus providing some protection against the immune decrements associated with inadequate sleep. Taken together, subjective SES appears to play an influential role in modifying how sleep affects cold susceptibility.
Unlike subjective SES, educational attainment, which served as an objective indicator of SES in our aggregated dataset, failed to moderate associations between sleep and susceptibility to the common cold. There are several explanations for this observation. In general, educational attainment, while easily reported on, serves as a fairly crude measure of one’s status in society. For instance, years of education do not capture the quality of the education received and are insensitive to incongruity with current occupational status and income. Unfortunately, income was not assessed consistently across the three cohorts; household income was measured in two of the cohorts analyzed here (i.e., PMBC and PCS3). While underpowered, a test of the moderating role of household income on the association between sleep duration and cold susceptibility in this restricted sample revealed no significant effect (data not shown).
In contrast to educational attainment, subjective SES provides a more comprehensive assessment of one’s social status that may incorporate multiple aspects of SES (e.g., assets and wealth, living conditions). The subjective SES measure allows individuals to weigh their education, income, and occupational status based on their relevance to their personal social context. Additionally, subjective SES likely taps psychological constructs, such as sense of control [26, 48], which may exert effects on health beyond those captured by objective measures of SES.
What are the biological mechanisms through which shorter self-reported sleep duration may increase susceptibility to the cold among those reporting low subjective SES? There is growing evidence that disturbed sleep, including short sleep duration, is associated with increased levels of inflammation [12, 49, 50]. Excess inflammation, measured locally in nasal secretions, has been shown to partially mediate the psychological factors and subsequent development of a cold following viral challenge [32].We found that elevated levels of local IL-6 and IL-1β were associated with greater illness severity (i.e., greater mucus weight and longer nasal clearance time) in this aggregated infected sample; however, self-reported sleep duration did not interact with subjective SES to account for significant variance in levels of nasal inflammation. Further, statistically controlling for levels of nasal inflammation failed to alter our findings, which suggests that, in this study, nasal inflammation was not a strong pathway. Future studies exploring alternative biological mechanisms previously linked to sleep, low SES, and cold susceptibility are warranted.
One of the clear strengths of this study was our ability to increase the statistical power to test the moderating effects of objective and subjective SES by aggregating data across three prior cold study cohorts. However, this required the inclusion of participants from a cohort (i.e., PMBC) where we previously published an association of self-reported short sleep duration with cold susceptibility [5]. When we restricted our analysis to the two other cohorts (i.e., PCS2 and PCS3), the association between sleep duration and cold susceptibility was present but attenuated below statistical significance. While this may be due to reduced statistical power, it may also reflect differences in sleep measurement employed in the PMBC cohort in the previously published analyses. Specifically, in addition to the retrospective sleep item used in the current analysis, the prior report [5] using PMBC data relied on sleep estimates obtained during daily telephone interviews, which helped ensure timely assessments and likely improved accuracy of nightly sleep by reducing recall bias. Consistent with this, this prior study also documented an association between self-reported sleep efficiency and cold susceptibility [5], which was absent in the current analysis. There are likely a couple of reasons why the present study differs from the prior work. The primary reason is that in order to aggregate across the three cohorts, we relied on retrospective self-report items characterizing sleep over the past month, which is a limitation. In addition, the questions used to estimate self-reported sleep duration differed slightly across studies, which may have introduced measurement error. As such, it is perhaps not surprising that we were unable to fully replicate the prior findings. That said, the moderation effect of subjective SES does persist despite removing the PMBC data, suggesting that the influence of subjective SES is robust in this context.
Infectious illness remains among the top 10 causes of mortality in the USA [51], which emphasizes the public health implications of these findings. Focusing first on sleep, this work provides further compelling evidence that sleep represents a modifiable target for intervention to reduce infectious disease risk. In this regard, several randomized controlled trials targeting sleep, typically through some variant of cognitive-behavioral therapy for insomnia (CBT-I), show promise in improving immune mechanisms (e.g., inflammatory activity) relevant to cold susceptibility [52, 53]. The present findings, however, point out that addressing sleep uniformly across a population may not be helpful. The data suggest low SES participants may be particularly vulnerable to the ill effects of short sleep, which is consistent with the fact that low SES populations shoulder more of the burden of infectious illness than those of higher SES [54, 55].
One of the limitations of this study was our reliance on self-reported sleep measures. However, our prior work indicates similar sleep effects when using behavioral measures (e.g., wrist actigraphy) [6]. From a practical standpoint, it appears that a simple set of questions, such as typical sleep and wake time along with estimates of sleep latency and minutes of wakefulness, appear, with sufficient sample size, to predict cold susceptibility. While there have been recent strides towards integrating social and behavioral factors into routine medical assessments, including electronic medical records [56], sleep has not been included among the list of candidates. Given the robust literature linking sleep and health [2], the integration of sleep assessment into broad medical settings (e.g., primary care), particularly in low income communities, may provide clinically relevant information.
In summary, this study provides the first evidence that low subjective SES confers added vulnerability to infectious illness among self-reported short sleepers exposed to experimental viral challenge. Said another way, high subjective SES buffers the effects of short sleep on upper respiratory infection susceptibility. Although it is recognized that this study does not provide direct evidence of causality, the prospective nature of the sleep and SES measurement prior to the experimental viral challenge eliminates concerns of reverse causality. Future studies employing more varied and comprehensive measures of SES are indicated as well as a need to more extensively test the synergistic nature of sleep-SES link in other models of infectious (e.g., vaccinations) and noninfectious (e.g., cardiovascular risk) disease.
Acknowledgments
The data used for this article were collected by the Laboratory for the Study of Stress, Immunity, and Disease at Carnegie Mellon University under the directorship of Sheldon Cohen, PhD and were accessed via the Common Cold Project (CCP) website (www.commoncoldproject.com). CCP data are made publically available through a grant from the National Center for Complementary and Integrative Health (AT006694); the conduct of the studies was supported by grants from the National Institute of Mental Health (MH50429), the National Heart, Lung, and Blood Institute (HL65111; HL65112), and the National Institute for Allergy and Infectious Diseases (AI066367); secondary support was provided by a grant from the National Institutes of Health to the University of Pittsburgh Clinical and Translational Science Institute (UL1 RR024153 and UL1 RT000005), and supplemental support was provided by John D. and Catherine T. MacArthur Foundation Research Network on Socioeconomic Status & Health. Dr. Prather’s participation was supported by a grant from the National Heart, Lung, & Blood Institute (K08HL112961).
Footnotes
Compliance with Ethical Standards
Authors’ Statement of Conflict of Interest and Adherence to Ethical Standards Dr. Prather is currently a paid consultant for Posit Science. He reports no other potential conflicts of interest. Janicki-Deverts, Adler, Hall, Cohen declare that they have no conflict of interest.
References
- 1.Luyster FS, Strollo PJ, Jr, Zee PC, et al. Sleep: A health imperative. Sleep. 2012;35:727–734. doi: 10.5665/sleep.1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buysse DJ. Sleep health: Can we define it? Does it matter? Sleep. 2014;37:9–17. doi: 10.5665/sleep.3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cappuccio FP, D'Elia L, Strazzullo P, Miller MA. Quantity and quality of sleep and incidence of type 2 diabetes: A systematic review and meta-analysis. Diabetes Care. 2010;33:414–420. doi: 10.2337/dc09-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cappuccio FP, Cooper D, D'Elia L, Strazzullo P, Miller MA. Sleep duration predicts cardiovascular outcomes: A systematic review and meta-analysis of prospective studies. Eur Heart J. 2011;32:1484–1492. doi: 10.1093/eurheartj/ehr007. [DOI] [PubMed] [Google Scholar]
- 5.Cohen S, Doyle WJ, Alper CM, Janicki-Deverts D, Turner RB. Sleep habits and susceptibility to the common cold. Arch Intern Med. 2009;169:62–67. doi: 10.1001/archinternmed.2008.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prather AA, Janicki-Deverts D, Hall MH, Cohen S. Behaviorally assessed sleep and susceptibility to the common cold. Sleep. 2015;38:1353–1359. doi: 10.5665/sleep.4968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Irwin MR. Why sleep is important for health: A psychoneuroimmunology perspective. Annu Rev Psychol. 2015;66:143–172. doi: 10.1146/annurev-psych-010213-115205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Besedovsky L, Lange T, Born J. Sleep and immune function. Pflugers Arch. 2012;463:121–137. doi: 10.1007/s00424-011-1044-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bollinger T, Bollinger A, Skrum L, et al. Sleep-dependent activity of T cells and regulatory T cells. Clin Exp Immunol. 2009;155:231–238. doi: 10.1111/j.1365-2249.2008.03822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dimitrov S, Lange T, Tieken S, Fehm HL, Born J. Sleep associated regulation of T helper 1/T helper 2 cytokine balance in humans. Brain Behav Immun. 2004;18:341–348. doi: 10.1016/j.bbi.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 11.Irwin M, McClintick J, Costlow C, et al. Partial night sleep deprivation reduces natural killer and cellular immune responses in humans. FASEB J. 1996;10:643–653. doi: 10.1096/fasebj.10.5.8621064. [DOI] [PubMed] [Google Scholar]
- 12.Irwin MR, Olmstead R, Carroll JE. Sleep disturbance, sleep duration, and inflammation: A systematic review and meta-analysis of cohort studies and experimental sleep deprivation. Biol Psychiatry. 2016;18:40–52. doi: 10.1016/j.biopsych.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seeman T, Epel E, Gruenewald T, Karlamangla A, McEwen BS. Socio-economic differentials in peripheral biology: Cumulative allostatic load. Ann N Y Acad Sci. 2010;1186:223–239. doi: 10.1111/j.1749-6632.2009.05341.x. [DOI] [PubMed] [Google Scholar]
- 14.Chen E, Miller GE. Socioeconomic status and health: Mediating and moderating factors. Annu Rev Clin Psychol. 2013;9:723–749. doi: 10.1146/annurev-clinpsy-050212-185634. [DOI] [PubMed] [Google Scholar]
- 15.Petersen KL, Marsland AL, Flory J, et al. Community socioeconomic status is associated with circulating interleukin-6 and C-reactive protein. Psychosom Med. 2008;70:646–652. doi: 10.1097/PSY.0b013e31817b8ee4. [DOI] [PubMed] [Google Scholar]
- 16.Steptoe A, Kunz-Ebrecht S, Owen N, et al. Socioeconomic status and stress-related biological responses over the working day. Psychosom Med. 2003;65:461–470. doi: 10.1097/01.psy.0000035717.78650.a1. [DOI] [PubMed] [Google Scholar]
- 17.Pollitt RA, Kaufman JS, Rose KM, et al. Cumulative life course and adult socioeconomic status and markers of inflammation in adulthood. J Epidemiol Community Health. 2008;62:484–491. doi: 10.1136/jech.2006.054106. [DOI] [PubMed] [Google Scholar]
- 18.Friedman EM, Herd P. Income, education, and inflammation: Differential associations in a national probability sample (the MIDUS study) Psychosom Med. 2010;72:290–300. doi: 10.1097/PSY.0b013e3181cfe4c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koster FT, McGregor DD, Mackaness GB. The mediator of cellular immunity. II. Migration of immunologically committed lymphocytes into inflammatory exudates. J Exp Med. 1971;133:400–409. doi: 10.1084/jem.133.2.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fagundes CP, Bennett JM, Alfano CM, et al. Social support and socioeconomic status interact to predict Epstein-Barr virus latency in women awaiting diagnosis or newly diagnosed with breast cancer. Health Psychol. 2012;31:11–19. doi: 10.1037/a0025599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Janicki-Deverts D, Cohen S, Doyle WJ, Marsland AL, Bosch J. Childhood environments and cytomegalovirus serostatus and reactivation in adults. Brain Behav Immun. 2014;40:174–181. doi: 10.1016/j.bbi.2014.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Minkel JD, Banks S, Htaik O, et al. Sleep deprivation and stressors: Evidence for elevated negative affect in response to mild stressors when sleep deprived. Emotion. 2012;12:1015–1020. doi: 10.1037/a0026871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoo SS, Gujar N, Hu P, Jolesz FA, Walker MP. The human emotional brain without sleep—a prefrontal amygdala disconnect. Curr Biol. 2007;17:R877–R878. doi: 10.1016/j.cub.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 24.Franzen PL, Gianaros PJ, Marsland AL, et al. Cardiovascular reactivity to acute psychological stress following sleep deprivation. Psychosom Med. 2011;73:679–682. doi: 10.1097/PSY.0b013e31822ff440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bailis DS, Segall A, Mahon MJ, Chipperfield JG, Dunn EM. Perceived control in relation to socioeconomic and behavioral resources for health. Soc Sci Med. 2001;52:1661–1676. doi: 10.1016/s0277-9536(00)00280-x. [DOI] [PubMed] [Google Scholar]
- 26.Adler N, Snibe AC. The role of psychosocial processes in explaining the gradient between socioeconomic status and health. Curr Dir in Psychol Sci. 2003;12:119–123. [Google Scholar]
- 27.Miller G, Chen E, Cole SW. Health psychology: Developing biologically plausible models linking the social world and physical health. Annu Rev Psychol. 2009;60:501–524. doi: 10.1146/annurev.psych.60.110707.163551. [DOI] [PubMed] [Google Scholar]
- 28.Adler NE, Boyce T, Chesney MA, et al. Socioeconomic status and health. The challenge of the gradient. Am Psychol. 1994;49:15–24. doi: 10.1037//0003-066x.49.1.15. [DOI] [PubMed] [Google Scholar]
- 29.Adler N, Singh-Manoux A, Schwartz J, et al. Social status and health: A comparison of British civil servants in Whitehall-II with European- and African-Americans in CARDIA. Soc Sci Med. 2008;66:1034–1045. doi: 10.1016/j.socscimed.2007.11.031. [DOI] [PubMed] [Google Scholar]
- 30.Adler NE, Epel ES, Castellazzo G, Ickovics JR. Relationship of subjective and objective social status with psychological and physiological functioning: Preliminary data in healthy white women. Health Psychol. 2000;19:586–592. doi: 10.1037//0278-6133.19.6.586. [DOI] [PubMed] [Google Scholar]
- 31.Cohen S, Alper CM, Doyle WJ, et al. Objective and subjective socioeconomic status and susceptibility to the common cold. Health Psychol. 2008;27:268–274. doi: 10.1037/0278-6133.27.2.268. [DOI] [PubMed] [Google Scholar]
- 32.Cohen S, Doyle WJ, Skoner DP. Psychological stress, cytokine production, and severity of upper respiratory illness. Psychosom Med. 1999;61:175–180. doi: 10.1097/00006842-199903000-00009. [DOI] [PubMed] [Google Scholar]
- 33.Doyle WJ, Gentile DA, Cohen S. Emotional style, nasal cytokines, and illness expression after experimental rhinovirus exposure. Brain Behav Immun. 2006;20:175–181. doi: 10.1016/j.bbi.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 34.Gwaltney JM, Jr, Colonno RJ, Hamparian VV, Turner RB. Rhinovirus. In: Schmidt NJ, Emmons RW, editors. Diagnostic procedures for viral, rickettsial and chlamydial infections. Washington, D.C: American Public Health Association; 1989. pp. 579–614. [Google Scholar]
- 35.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 36.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- 37.Goldberg LR. The development of markers for the big-five factor structure. Psychol Assess. 1992:26–42. [Google Scholar]
- 38.Goldberg LR. The intenational personality item pool and the future of public-domain personality measures. J Res Pers. 2006;40:84–96. [Google Scholar]
- 39.Gwaltney JM, Jr, Colonno RI, Hamparian VV, Turner RB. Rhinovirus. In: Schmidt NI, Ernmons RW, editors. Diagnostic procedures for viral, rickettsial, and chlamydial infections. Washington, DC: American Public Healh Association; 1989. pp. 579–614. [Google Scholar]
- 40.Doyle WJ, McBride TP, Skoner DP, et al. A double-blind, placebo-controlled clinical trial of the effect of chlorpheniramine on the response of the nasal airway, middle ear and eustachian tube to provocative rhinovirus challenge. Pediatr Infect Dis J. 1988;7:229–238. doi: 10.1097/00006454-198803000-00033. [DOI] [PubMed] [Google Scholar]
- 41.Cohen S, Doyle WJ, Skoner DP, Rabin BS, Gwaltney JM., Jr Social ties and susceptibility to the common cold. JAMA. 1997;277:1940–1944. [PubMed] [Google Scholar]
- 42.Baum A, Garofalo JP, Yali AM. Socioeconomic status and chronic stress. Does stress account for SES effects on health? Ann N Y Acad Sci. 1999;896:131–144. doi: 10.1111/j.1749-6632.1999.tb08111.x. [DOI] [PubMed] [Google Scholar]
- 43.McEwen BS, Gianaros PJ. Central role of the brain in stress and adaptation: Links to socioeconomic status, health, and disease. Ann N Y Acad Sci. 2010;1186:190–222. doi: 10.1111/j.1749-6632.2009.05331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Segerstrom SC, Miller GE. Psychological stress and the human immune system: A meta-analytic study of 30 years of inquiry. Psychol Bull. 2004;130:601–630. doi: 10.1037/0033-2909.130.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gruenewald TL, Cohen S, Matthews KA, Tracy R, Seeman TE. Association of socioeconomic status with inflammation markers in black and white men and women in the coronary artery risk development in young adults (CARDIA) study. Soc Sci Med. 2009;69:451–459. doi: 10.1016/j.socscimed.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ranjit N, Diez-Roux AV, Shea S, et al. Socioeconomic position, race/ethnicity, and inflammation in the multi-ethnic study of atherosclerosis. Circulation. 2007;116:2383–2390. doi: 10.1161/CIRCULATIONAHA.107.706226. [DOI] [PubMed] [Google Scholar]
- 47.Gallo LC, Matthews KA. Understanding the association between socioeconomic status and physical health: Do negative emotions play a role? Psychol Bull. 2003;129:10–51. doi: 10.1037/0033-2909.129.1.10. [DOI] [PubMed] [Google Scholar]
- 48.Kraus MW, Piff PK, Keltner D. Social class, sense of control, and social explanation. J Pers Soc Psychol. 2009;97:992–1004. doi: 10.1037/a0016357. [DOI] [PubMed] [Google Scholar]
- 49.Prather AA, Epel ES, Cohen BE, Neylan TC, Whooley MA. Gender differences in the prospective associations of self-reported sleep quality with biomarkers of systemic inflammation and coagulation: Findings from the heart and soul study. J Psychiatr Res. 2013;47:1228–1235. doi: 10.1016/j.jpsychires.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Patel SR, Zhu X, Storfer-Isser A, et al. Sleep duration and biomarkers of inflammation. Sleep. 2009;32:200–204. doi: 10.1093/sleep/32.2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Murphy SL, Xu J, Kochanek KD. Deaths: Final data for 2010. Natl Vital Stat Rep. 2013;61:1–117. [PubMed] [Google Scholar]
- 52.Irwin MR, Olmstead R, Carrillo C, et al. Cognitive behavioral therapy vs. tai chi for late life insomnia and inflammatory risk: A randomized controlled comparative efficacy trial. Sleep. 2014;37:1543–1552. doi: 10.5665/sleep.4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Black DS, O'Reilly GA, Olmstead R, Breen EC, Irwin MR. Mindfulness meditation and improvement in sleep quality and daytime impairment among older adults with sleep disturbances: A randomized clinical trial. JAMA Intern Med. 2015;175:494–501. doi: 10.1001/jamainternmed.2014.8081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hotez PJ. Neglected infections of poverty in the United States of America. PLoS Negl Trop Dis. 2008;2:e256. doi: 10.1371/journal.pntd.0000256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bate SL, Dollard SC, Cannon MJ. Cytomegalovirus seroprevalence in the United States: The national health and nutrition examination surveys, 1988–2004. Clin Infect Dis. 2010;50:1439–1447. doi: 10.1086/652438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Capturing social and behavioral domains and measures in electronic health records: Phase 2. Washington D.C.: National Academies Press; 2014. [PubMed] [Google Scholar]