Abstract
We describe the synthesis of analogs of XHE-III-74, a selective α4β3γ2 GABAAR ligand, shown to relax airway smooth muscle ex vivo and reduce airway hyperresponsiveness in a murine asthma model. To improve properties of this compound as an asthma therapeutic, a series of analogs with a deuterated methoxy group in place of methoxy group at C-8 position was evaluated for isotope effects in preclinical assays; including microsomal stability, cytotoxicity, and sensorimotor impairment. The deuterated compounds were equally or more metabolically stable than the corresponding non-deuterated analogs and increased sensorimotor impairment was observed for some deuterated compounds. Thioesters were more cytotoxic in comparison to other carboxylic acid derivatives of this compound series. The most promising compound 16 identified from the in vitro screens also strongly inhibits smooth muscle constriction in ex vivo guinea pig tracheal rings. Smooth muscle relaxation, determined by reduction of airway hyperresponsiveness with a murine ovalbumin sensitized and challenged model, showed that 16 was efficacious at low methacholine concentrations. However, this effect was limited due to suboptimal pharmacokinetics of 16. Based on these findings, further analogs of XHE-III-74 will be investigated to improve in vivo metabolic stability while retaining the efficacy at lung tissues involved in asthma pathology.
Keywords: XHE-III-74, deuterated compounds, asthma, GABAA receptor, airway hyperresponsiveness, airway smooth muscle
Introduction
Asthma is a major healthcare challenge, effecting an estimated 300 million people globally.[1] Over $56 billion in asthma-related healthcare expenses occur in the United States annually.[2] Moreover, asthma accounts for the majority of missed school/work days, doctor and emergency room visits, and patient hospitalizations in young persons.[1–3] Consequently, asthma continues to be a significant healthcare burden in terms of morbidity, productivity, and medical costs. Beta 2-adrenergic agonists and inhaled corticosteroids (ICs) are the most often prescribed treatments for the acute and chronic management of asthma. Both agents present efficacy, compliance, and adverse side effect concerns.[4–8] Hence, there is an unmet need for asthma therapies with novel mechanisms of action to better control diseases with decreased adverse side effects.
The GABAA receptor (GABAAR) is a ligand-gated chloride ion channel best known for its role in central nervous system (CNS) inhibitory neurotransmission. GABAARs are heteropentameric receptors mainly comprised of combinations of 19 different subunits (α1–6, β1–3, γ1–3, δ, ε, π, θ, ρ1–3). Classical GABAAR consist of two α, two β and one “tertiary” subunit (γ, δ, ε, θ, or π).[9, 10] The receptor subunits have been identified in airway smooth muscle, airway epithelium, and inflammatory cells, and their ligand-mediated activation has been shown to reduce immune response measures and reduce airway hyperresponsiveness (ex vivo and in vivo).[11–16] In these studies, GABA dose-dependently reduced IL-12 and IL-6 production in LPS stimulated macrophages.[15] GABA and muscimol also inhibited anti-CD3 and antigen specific T cell proliferation.[17] Honokiol, a GABAAR agonist, reduced cardinal features of the asthma-like phenotype including inflammation (reduced airway eosinophilia), mucous cell metaplasia, collagen deposition, and airway hyperresponsiveness in an acute and chronic ovalbumin-induced murine asthma models.[18] However, nonselective GABAAR activation is associated with unwanted CNS effects[19] and increased mucous production.[16, 20, 21] To preclude these side effects in the treatment of asthma, subtype-selective GABAAR ligands were identified, especially those exhibiting preferential efficacy at α4/α5β3γ2 GABAAR.[22–25] In these studies, α4β3γ2 GABAAR selective compounds CMD-45 and XHE-III-74 relaxed pre-contracted airway smooth muscle. In addition, XHE-III-74 reduced airway resistance in a murine house dust mite model of asthma. The ethyl ester of XHE-III-74 reduced airway hyperresponsiveness in a murine ovalbumin sensitized and challenged (S/C) asthma model, whereas XHE-III-74A, the corresponding carboxylic acid, significantly reduced airway eosinophilia[26] and reduced IL-2 production following PMA/PHA activation of Jurkat cells. Furthermore, XHE-III-74A exhibited no CNS effects because its negative charge precluded brain penetration.
Our previous studies showed that the C-8 methoxy group of XHE-II-74 (herein indicated as C-7) is essential for α4β3γ2 GABAAR selectivity. [27]To improve the metabolic stability of this group, we herein describe the synthesis of compounds bearing a deuterated form (-OCD3) because the C-D bond is stronger than the C-H bond. We further report the synthesis and characterization of a series XHE-III-74 analogs additionally bearing different ester, thioester, and amide functionalities at the C-3 position to identify compounds with high metabolic stability, absence of CNS effects, low toxicity, and efficacy in a murine asthma model.
Results
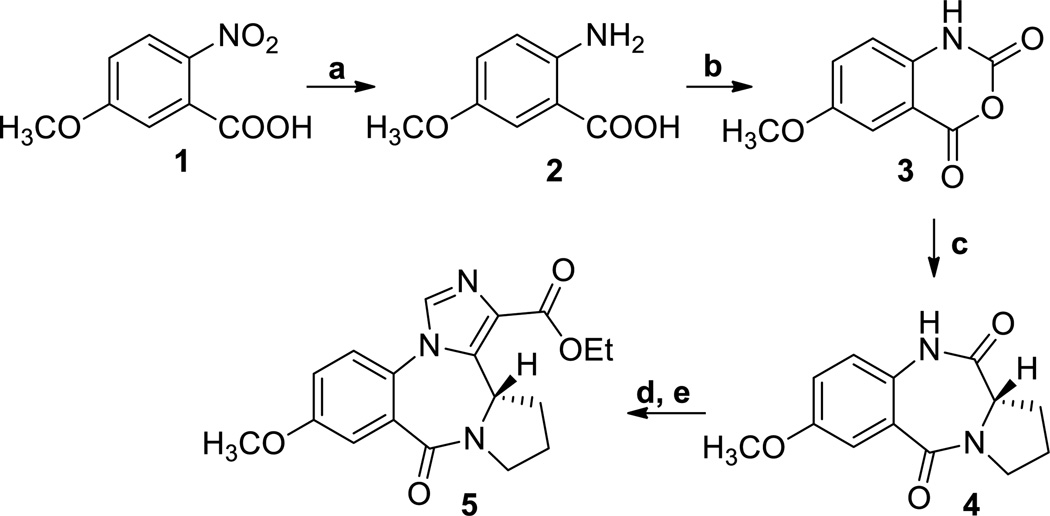
In agreement with the earlier route of Fryer and Gu[27] [28]the synthesis of XHE-III-74 (7) started with the synthesis of 5-methoxyanthranilic acid 2 (Scheme 1) from 5-methoxy-2-nitrobenzoic acid 1 by hydrogenation.
Scheme 1. Synthesis of XHE-III-74 EE.
a. H2, Pd/C, EtOAc, rt, 8 h, 97%; b. triphosgene, HCl/H2O, rt, 4h, 89%; c. L-proline, DMSO, 160 °C, 2h, 96%; d. t-BuOK, (EtO)2POCl, THF, −20 °C to rt, 4 h; e. t-BuOK, CNCH2CO2Et, −20 °C to rt, 8h, 60%.
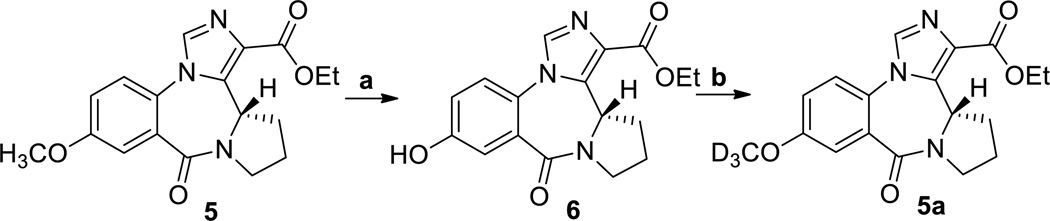
The aniline 2 was converted into isotoic anhydride 3 with triphosgene. Anhydride 3 was heated with L-proline in DMSO to generate the corresponding benzodiazepine 4. This compound was converted into the imidazobenzodiazepine, XHE-III-74 ethyl ester 5. The introduction of the OCD3 group was achieved using the demethylation-alkylation sequence as depicted in Scheme 2.
Scheme 2.
a. AlCl3, ethanethiol, CH2Cl2, rt, 24 h, 84%; b. Cs2CO3, CD3I, CH2Cl2, rt, 18 h, 86%.
As shown in Scheme 2, ester 5 was demethylated at C-8 using aluminum chloride and ethanethiol in methylene chloride at room temperature to furnish the corresponding phenol 6. The phenol was re-alkylated with deuterated iodomethane (CD3I) using Cs2CO3 in methylene chloride at room temperature in excellent yield producing the OCD3 analog 5a.
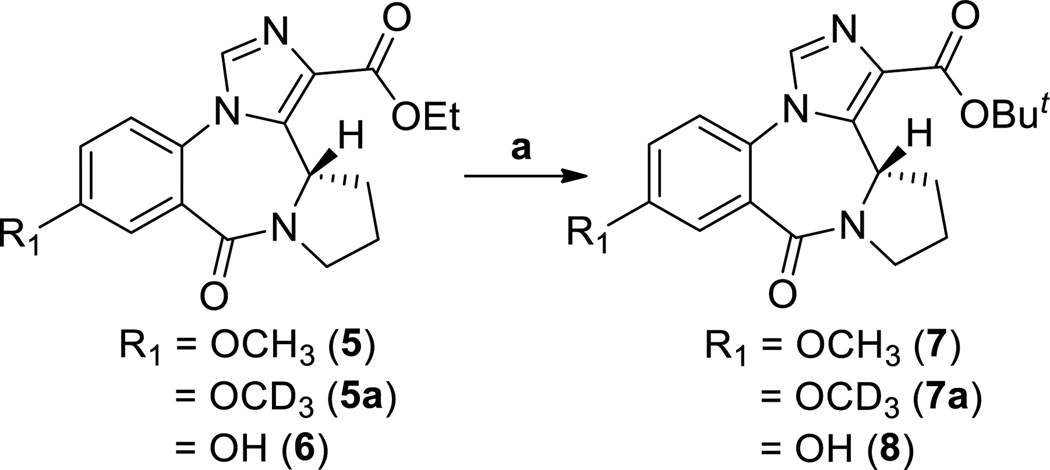
The esters 5, 5a and 6 were converted into the corresponding tert-butyl analogs 7 (XHE-III-74), 7a and 8, respectively, via trans-esterification with lithium in the presence of tert-butanol in THF at 50 °C (Scheme 3).
Scheme 3.
a. Li rod pieces, t-BuOH, THF, 50 °C, 30 min, 65–67%.
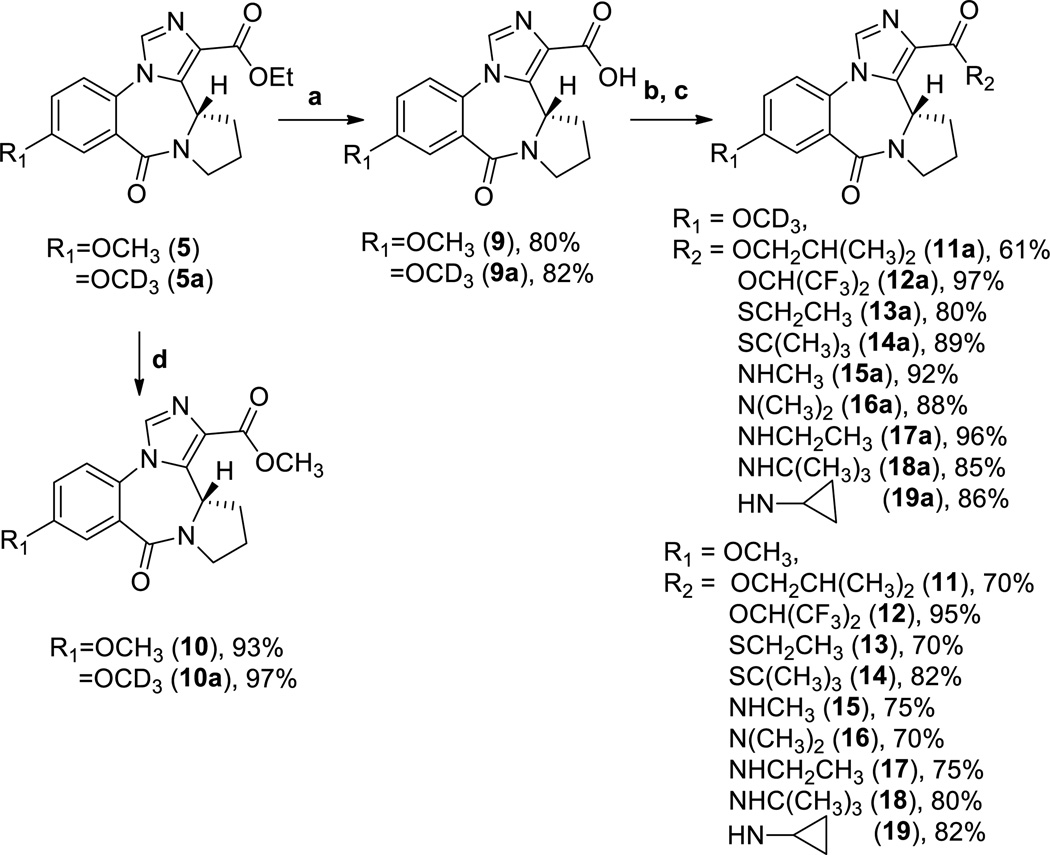
For generation of the other ester and amide analogs of XHE-III-74 (7), a three step protocol was used (Scheme 4).
Scheme 4.
a. NaOH, H2O, EtOH, 50 °C, 15 min; 1N HCl; b. CH2Cl2, SOCl2, 52 °C, 1h; c. CH2Cl2, corresponding amine, thiol, or alcohol, Et3N, d. CH3OH, NaOMe, reflux, 1 h.
The ethyl esters 5 and 5a were saponified to give the corresponding acids 9 and 9a. The subsequent reactions with thionyl chloride in methylene chloride yielded the corresponding acid chlorides, which in turn were converted to esters, thioesters, and amides (11, 11a–19, and 19a). The methyl esters 10 and 10a were formed in the presence of NaOMe in methanol.
Initially, human and mouse liver microsomal stability was investigated to identify metabolically labile compounds and deuterated compounds that are more stable than their non-deuterated counterparts. The results are summarized in Table 1.
Table 1.
In vitro liver microsomal stability of XHE-III-74 analogs.
| Entry | Compound | Microsomal stability (human) % remaining after 1 houra |
Microsomal stability (mouse) % remaining after 1 houra |
|---|---|---|---|
| 1 | 5 | 99.1 ± 0.1 | 85.4 ± 0.3 |
| 2 | 5a | 92.8 ± 0.3 | 85.5 ± 0.3 |
| 3 | 6 | 91.7 ± 0.2 | 91.1 ± 0.1 |
| 4 | 7 | 92.1 ± 1.0 | 16.2 ± 0.2 |
| 5 | 7a | 91.9 ± 0.4 | 17.6 ± 0.2 |
| 6 | 8 | 90.6 ± 0.2 | 46.6 ± 0.3 |
| 7 | 9 | 56.1 ± 0.5 | 52.9 ± 0.5 |
| 8 | 9a | 84.6 ± 0.3 | 84.6 ± 0.2 |
| 9 | 10 | 89.7 ± 0.2 | 70.3 ± 0.2 |
| 10 | 10a | 92.9 ± 0.2 | 85.9 ± 0.2 |
| 11 | 11 | 77.8 ± 0.2 | 13.4 ± 0.9 |
| 12 | 11a | 78.7 ± 0.2 | 22.4 ± 0.3 |
| 13 | 12 | -b | -b |
| 14 | 12a | 77.1 ± 0.2 | 62.5 ± 0.3 |
| 15 | 13 | -b | -b |
| 16 | 13a | -b | -b |
| 17 | 14 | -b | -b |
| 18 | 14a | -b | -b |
| 19 | 15 | 93.8 ± 0.3 | 54.3 ± 0.2 |
| 20 | 15a | 94.9 ± 0.2 | 56.8 ± 0.3 |
| 21 | 16 | 92.4 ± 0.2 | 82.5 ± 0.3 |
| 22 | 16a | 96.3 ± 0.3 | 83.1 ± 0.2 |
| 23 | 17 | 90.7 ± 0.3 | 50.1 ± 0.2 |
| 24 | 17a | 93.5 ± 0.2 | 94.8 ± 0.2 |
| 25 | 18 | 92.8 ± 0.5 | 4.2 ± 0.2 |
| 26 | 18a | 95.6 ± 0.2 | 9.4 ± 0.4 |
| 27 | 19 | 79.9 ± 0.2 | 91.3 ± 0.2 |
| 28 | 19a | 88.1 ± 0.2 | 94.1 ± 0.2 |
Half-life, intrinsic clearance and metabolic rates calculated for each experiment are shown in Supporting Information.
Compound was not soluble at 10 µM in PBS with 1% DMSO. Data were acquired by two independent experiments carried out in triplet.
The parent compound XHE-III-74 (7) was metabolized rapidly by mouse liver microsomes with 16.2% remaining after 1 h (Table 1, Entry 4). The corresponding half-life was less than 24 min. However, 7 was stable in the presence of human liver microsomes, similar to the majority of compounds investigated. Less than 80% of the parent was observed after 1 hour for compounds 9, 11, 11a, 12a, and 19 (Table 1, Entries 7, 11, 12, 14 and 27). For 9 and 19, stability of the deuterated analog (9a and 19a) is significantly increased (Table 1, Entries 7, 8, 27, and 28). A smaller number of compounds were stable in the presence of mouse liver microsomes for 1 hour. The most stable compounds (as judged by less than 20% loss at 1 h) were 5, 5a, 6, 9a, 10a, 16, 16a, 17a, 19, and 19a (Table 1, Entries 1, 2, 3, 8, 10, 21, 22, 24, 27, and 28). All compounds that exhibited good stability in mouse microsomes were also stable in the presence of human liver microsomes. Importantly, different metabolic rates for deuterated and non-deuterated compounds in the presence of mouse liver microsomes were observed for 9, 10, 11, and 17 and their corresponding deuterated analogs (Table 1, Entries 7–12, 23, 24). Further characterization of these compounds included the determination of their cytotoxicity using three different cell lines; HEK293 kidney cells, HepG2 liver cells, and BEAS2B lung epithelial cells (Table 2).
Table 2.
In vitro cytotoxicity of XHE-III-74 analogs
| Entry | Compound | Toxicity in HEK293 (Kidney) LD50 (µM)1 |
Toxicity in HEPG2 (Liver) LD50 (µM)1 |
Toxicity in BEAS 2B (Lung) LD50 (µM)1 |
|---|---|---|---|---|
| 1 | 5 | >400 | >400 | >400 |
| 2 | 5a | >400 | >400 | >400 |
| 3 | 6 | >400 | >400 | >400 |
| 4 | 7 | >100 | >400 | >200 |
| 5 | 7a | >100 | >400 | >200 |
| 6 | 8 | >400 | >400 | >400 |
| 7 | 9 | >400 | >400 | >400 |
| 8 | 9a | >400 | >400 | >400 |
| 9 | 10 | >400 | >400 | >400 |
| 10 | 10a | >400 | >400 | >400 |
| 11 | 11 | >100 | >200 | >200 |
| 12 | 11a | >100 | >200 | >200 |
| 13 | 12 | >200 | >400 | >400 |
| 14 | 12a | >200 | >400 | >400 |
| 15 | 13 | >100 | >100 | >100 |
| 16 | 13a | >100 | >100 | >100 |
| 17 | 14 | 18.8 ± 2.4 | >100 | >100 |
| 18 | 14a | 16.8 ± 2.0 | >100 | >100 |
| 19 | 15 | >100 | >400 | >400 |
| 20 | 15a | >100 | >400 | >400 |
| 21 | 16 | >400 | >400 | >400 |
| 22 | 16a | >400 | >400 | >400 |
| 23 | 17 | >400 | >400 | >400 |
| 24 | 17a | >400 | >400 | >400 |
| 25 | 18 | >200 | >400 | >400 |
| 26 | 18a | >200 | >400 | >400 |
| 27 | 19 | >200 | >400 | >400 |
| 28 | 19a | >200 | >400 | >400 |
Compounds were incubated at different concentrations with specified cells for 48 hours, followed by detection of viability using Cell-Titer Glo (Promega). The results were normalized using DMSO (negative) and 3-dibutylamino-1-(4-hexyl-phenyl)-propan-1-one (150 µM in DMSO final concentration, positive. Data were acquired by three independent experiments carried out in quadruplet.
Most of the compounds exhibited no major toxicity at the concentrations tested. The compounds with the most pronounced cytotoxicity were thioesters 13, 13a, 14, and 14a (Table 2, Entries 15–18). Among the compounds identified as stable in human and mouse liver microsomes, only 19 and 19a showed some toxicity in HEK293 cells (Table 2, Entries 27 and 28).
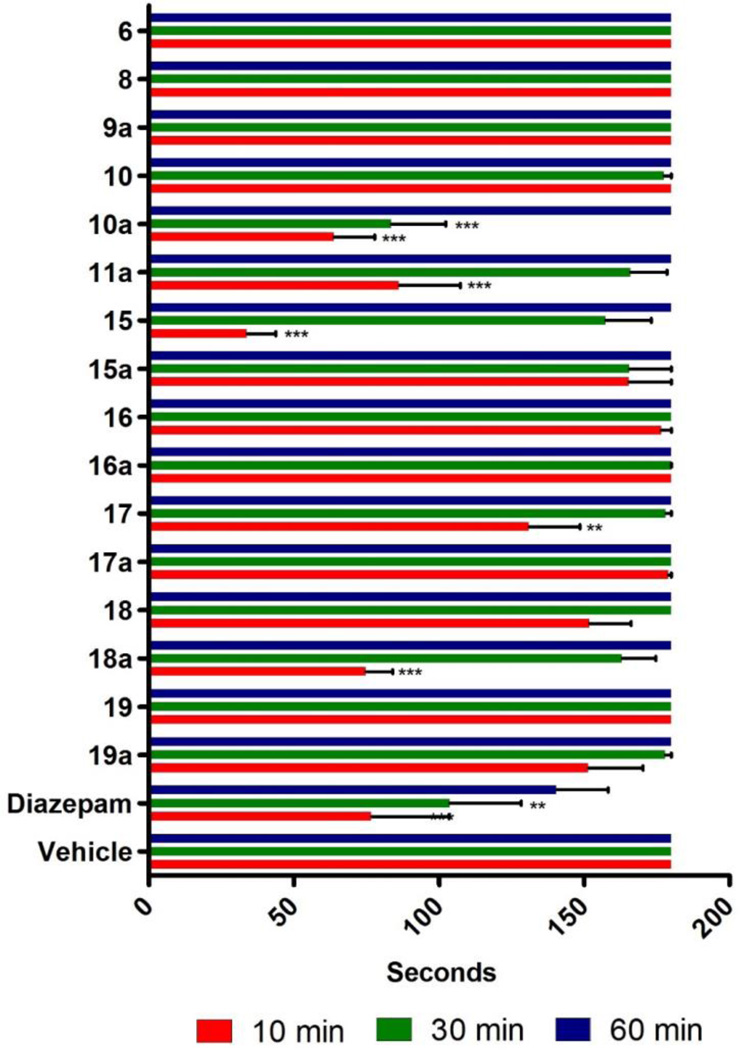
Next, possible adverse CNS sensorimotor effects were evaluated using a rotarod apparatus (Figure 1).
Figure 1.
Effect of compounds on sensorimotor coordination. Swiss Webster mice were tested on a rotarod at 15 rpm for 3 min at 10, 30, and 60 min following compound exposure. Mice (N = 10) received a single i.p. injection of test compound (40 mg/kg), diazepam (5 mg/kg), or vehicle (50% PBS, 40% propylene glycol, 10% DMSO). The time of fall was recorded if it occurred prior to 3 min. Data are expressed as mean ± SEM (N = 10). ** (p<.01) or *** (p <0.001) significance compared to vehicle-treated mice. Compounds 11, 12, 12a, 13, 13a, 14, 14a did not dissolve in the vehicle.
Swiss Webster mice were injected i.p. with 40 mg/kg of the indicated compound. The sensorimotor test was carried out after 10, 30 and 60 minutes. All compounds, including the diazepam positive control, showed the greatest impairment of sensorimotor steadiness at 10 minutes, followed by 30 and 60 minutes. The compounds that caused the most severe motor impairment were 10a, 11a, 15 and 18a. In addition, some of the compounds 11, 12, 12a, 13, 13a, 14 and 14a were not soluble in the vehicle (50% PBS, 40% propylene glycol, 10% DMSO) and could not be tested. Similar rotarod data were published previously for 5 and 9, showing that 5 weakly induced sensorimotor impairment whereas 9 did not, likely due to the inability of the acid to penetrate the blood brain barrier.[26] Compound 7 (XHE-III-74) induced sensorimotor impairment in rats at 15 mg/kg (unpublished results). Taken together, compounds in this series that are stable in the presence of human and mouse liver microsomes and exhibited neither cytotoxicity nor sensorimotor impairment are 6, 9a, 16, 16a, and 17a.
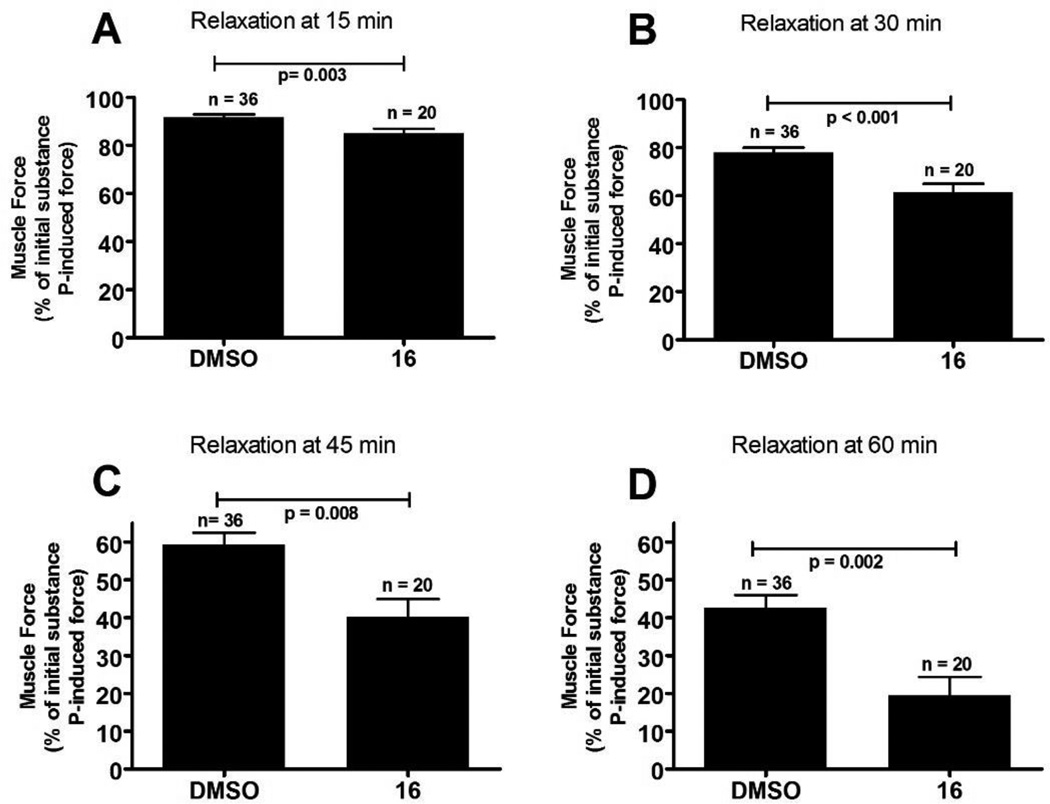
At this point 16/16a showed the most promising performance of the nondeuterated/deuterated pairs of compounds. We confirmed α4β3γ2 GABAAR subtype selectivity of 16 by comparing its GABA induced current potentiation with the α1β3γ2 GABAAR (see supporting information). At a concentration of 1 µM 16, 161% potentiation was observed for the α1β3γ2 GABAAR in contrast to 319% potentiation for the α4β3γ2 GABAAR in the presence of a GABA EC20 concentration. To investigate if 16, like other analogs of XHE-III-74, exhibits the ability to relax airway smooth muscle, we carried out an ex vivo assay employing tracheal rings pre-contracted with substance P (Figure 2).
Figure 2. Airway smooth muscle contractile force in guinea pig tracheal rings.
Tracheal rings were contracted with 1 µM substance P and then treated with 50 µM of 16 (or the vehicle control 0.1% DMSO). The percent of remaining contractile force was measured at various time points and expressed as a percent of the initial substance P induced contractile force. N and p-values are given for each condition.
Results show that 16 reduced the contractile force of substance P in guinea pig tracheal rings over a period of one hour. The highest significance was observed after 30 minutes with a p<0.001. Thus, 16 is able to relax airway smooth muscle consistent with other analogs of XHE-III-74.[25, 26]
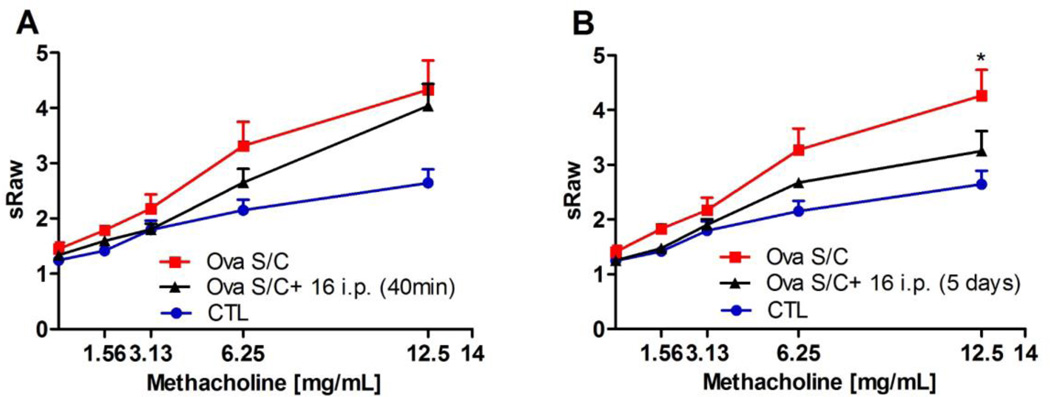
We then investigated if 16 could reduce airway hyperresponsiveness in a mouse asthma model. To establish asthma-like disease, male BALB/c mice were sensitized with three i.p. injections of ovalbumin (2 mg/kg/d emulsified in 2 mg of alum on days 0, 7 and 14, in a total volume of 100 µL), followed by intra-nasal challenge (1.6 mg/kg/d ovalbumin for 5 days on days 23–27). Control mice were sensitized with ovalbumin but challenged with saline.[26] Separate groups of ovalbumin (S/C) BALB/c mice received a single 40 mg/kg i.p. dose of 16 forty minutes before the measurement or twice daily 40 mg/kg of 16 i.p. for a duration of five days during the ovalbumin challenge period (Figure 3, A and B).
Figure 3. Effect of 16 on airway hyperresponsiveness.
Specific airway resistance (sRAW) to increasing doses of methacholine measured by DSI's Buxco® FinePointe non-invasive airway mechanics instrument. (A) BALB/c mice were administered 40 mg/kg of 16 single i.p. injection 40 minutes prior to analysis; (B) administration of 16 at 40 mg/kg i.p. injections daily for 5 days. Data represent mean ± SEM from 4–7 mice in each group. * indicates p < 0.05 significance compared to vehicle-treated mice.
For animals treated with a single dose of 16, no statistical significant specific airway resistance (sRAW) differences in comparison to the vehicle-treated group were observed at any of the methacholine concentrations tested. Importantly, significant reduction in sRAW (p<0.05) was observed at the 12.5 mg/ml methacholine challenge for animals given 16 over a 5 day treatment course. In light of the observed partial in vivo efficacy of 16 in reducing airway hyperresponsiveness, we were prompted to carry out a pharmacokinetic study to investigate its stability in vivo (Figure 4).
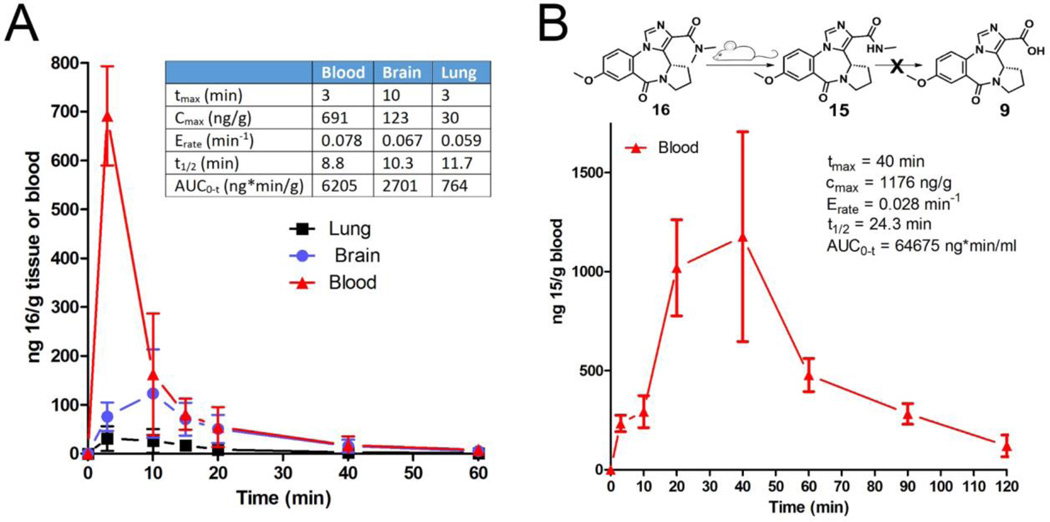
Figure 4. Pharmacokinetic analysis.
A) Concentration changes of compound 16 in mouse brain, lung, and blood over time when given as a 5 mg/kg, i.p. injection. B) Quantification of metabolite 15 in mouse blood at indicated time points.
Following an i.p. dose of 5 mg/kg, 16 was quantified in blood, brain, and lung at different time points (Figure 4, A). Compound 16 reached maximum absorption within minutes (tmax = 3 minutes) but also cleared very rapidly, with a half-life of 8.8 minutes. The overall exposure of 16 (AUC = 6205 ng*min/ml) was not very high. Markedly less distribution of 16 was observed in brain and lung. Compound 16 did penetrate the blood brain barrier but reached only a very low Cmax of 123 ng/g. The distribution in the lung was even less pronounced with an AUC of 764 ng*min/ml in comparison to AUC = 6205 ng*min/ml in blood. We investigated the presence of two likely metabolites of 16, namely 15 (formed by N-demethylation) and 9 (formed by saponification). Compound 15 was observed in substantial amounts in blood after only 3 minutes after injection (Figure 4, B). The peak blood concentration was higher than the parent compound and occurred later, as expected for a metabolite. Overall, the distribution of 15 was superior to 16 with an AUC of 64675 ng*min/ml. Compound 9 however, which was shown previously to reduce airway hyperresponsiveness, was not detected in any blood samples.
Discussion
Esters and amides are common functional groups in many FDA-approved drugs, although a number of such compounds are prodrugs that rely on endogenous enzymes such as peptidases to be activated. XHE-III-74 (7), has a tert-butyl ester function shown to be labile in the presence of mouse liver microsomes, while relatively stable in human liver microsomes. Nevertheless, XHE-III-74 reduces airway hyperresponsiveness when given by aerosol delivery in mice.[25] Thus, the stability of XHE-III-74 is sufficient in mice when administered directly to the target organ. Other compounds with limited half-lives in the presence of mouse liver microsomes are 18 and 18a bearing a tert-butyl amide, 8 bearing a tert-butyl ester, and 11, 11a bearing a isobutyl ester. Thus, we observed that esters and amides with longer and more branched carbon chains are metabolically less stable than their short carbon chain analogs. Another aspect of this SAR is the comparison of deuterated and non-deuterated compounds. Overall, we found a trend that deuterated compounds are equally or more stable than their corresponding non-deuterated analogs. This effect was very pronounced for acids 9 and 9a, probably because a hydrolysable carboxylic ester or amide group inherent to other analogs in this series is missing. Thus, for 9 and 9a, oxidation of the methoxy group (deuterated or non-deuterated) to the corresponding hemiacetal followed by hydrolysis might be the rate-determining step of metabolism. This specific route of metabolism can be species dependent. For instance, 17 and 17a have similar stability in human liver microsomes, however in the presence of mouse liver microsomes non-deuterated compound 17 is notably less stable. Therefore, we confirmed that small molecule metabolism is highly species dependent and involves many different metabolic reactions that are structure dependent.
The cytotoxicity of XHE-III-74 analogs was not very pronounced. The analysis showed that 50% of all compounds have LD50 values greater than 400 µM for the sensitive HEK293 kidney cells. For the BEAS2B lung cells, 71% of the compounds were not toxic. The compounds with the highest toxicity were thioesters 14 and 14a. Interestingly, the deuterium effect was less pronounced for cytotoxicity, although small differences were observed (Supporting Information). Sensorimotor impairment is another important screen for unwanted side effects in a possible asthma drug. Among the different GABAAR in the CNS, the α1β3γ2 GABAAR subtype is known to mediate sedation, which in turn compromises sensorimotor skills.[29] Thus, compounds with high efficacy toward this receptor subtype are expected to induce sedation in vivo, as seen for diazepam.[30] However, due to the unique scaffold of XHE-III-74 and its analogs, low efficacy towards α1β3γ2 GABAAR and high efficacy towards α4β3γ2 GABAAR was shown, e.g. XHE-III-74 (7),[25] XHE-III-74EE (5),[30] and XHE-III-74A (9).[26] Only a few of the compounds described herein induced sensorimotor effects comparable to diazepam, however at concentrations that were eight times greater. At 40 mg/kg deuterated compounds in comparison to non-deuterated compounds are more likely to impair sensorimotor abilities. This effect correlated with their better metabolic stability. For instance, 30% of 10 was metabolically converted after one hour, whereas only 15% of deuterated 10a was converted during the same time. Accordingly, 10 did not induce any sensorimotor impairment in contrast to 10a. Thus, increasing the half-life of a drug candidate using deuterium amplified not only the therapeutic effect but also potentiated possible side effects. The only compounds investigated herein that were stable in mouse and human liver microsomes and did not induce sensorimotor impairment were compounds 6, 9a, 16, 16a, and 17a.
Compound 16, like other compounds in this series e.g. 5, 7, and 9,[26] significantly reduced airway muscle constriction caused by substance P. Airway smooth muscle relaxation is an important hallmark of an efficacious asthma treatment and compound 16 mediated this effect within 15 minutes. In vivo, using ovalbumin S/C mice, 16 reduced airway hyperresponsiveness at high methacholine challenge when given chronically. Similar effects were reported for 5 when given repeatedly over five days.26 Both compounds (5 and 16) are metabolically stable in vitro and non-toxic, however, their effect was less pronounced in vivo probably due to subpharmacological concentrations. Ethyl ester 5 was shown to be absorbed and cleared slower with a t1/2 of 16.3 min instead of 8.8 minutes in comparison to dimethyl amide 16. Using the same dose, the AUC for lung exposure decreased from 4516 ng*min/ml for 5 to 764 ng*min/ml for 16. Finally, it was shown that 5 metabolized to 9 probably by mouse esterases and compound 9 decreased airway eosinophilia.[26] In contrast, compound 16 metabolized to 15 (demethylation) but no hydrolysis yielding 9 was detected. Thus, the incremental weaker pharmacodynamic effects of 16 is in part mediated by its short half-life and the inability to form the anti-inflammatory compound 9.
It can be concluded that the unique scaffold of GABAAR subtype-selective XHE-III-74 analogs is responsible for negligible inhibition of sensorimotor skills in mice in contrast to the non-selective GABAAR modulator diazepam. Similarities of compounds derived from both scaffolds include the absence of cytotoxicity. We confirmed that deuterated compounds were equally or more resistant to metabolism when compared with their non-deuterated counterparts. However, metabolic stability in the presence of liver microsomes in vitro did not predict rapid clearance of lead compound 16 in vivo. Therefore, significant relaxation of airway smooth muscle induced by 16 ex vivo was only partially recapitulated in vivo by measuring airway hyperresponsiveness. To overcome the shortcomings of 16, carboxylic ester and amide bioisosteres will be investigated in the future to identify new compounds with improved pharmacokinetics and better activity in murine models of asthma.
Experimental section
Chemistry
5-Methoxyanthranilic acid (2)
A solution of 2-nitro-5-methoxybenzoic acid 1 (60.3 g, 306 mmol) in EtOAc (1.5 L) was degassed under reduced pressure and refilled with argon (repeated 3 times). Palladium (10% w/w on carbon, 3.9g, 1.2 mol%) was added to the above solution. The reaction flask was evacuated under reduced pressure and refilled with H2 from a balloon (repeated 3–4 times to make sure that the solution was saturated with H2). The reaction mixture was stirred at rt for 6 h. After the completion of the reaction (TLC, silica gel), the solution was filtered over a bed of celite to remove the Pd. The solids were washed with ethyl acetate. The solvent was removed under reduced pressure to yield acid 2 as a yellow solid in 97% (49.7 g) yield: M.p = 147–149 °C; 1H NMR (300 MHz, CDCl3) δ 3.66 (s, 3H), 6.71 (d, 1H, J = 9.0 Hz), 6.94 (dd, 1H, J = 9.0 Hz, 3.0 Hz), 7.19 (d, 1H, J = 3.0 Hz), 8.40 (bs, 2H). The spectral data matched the reported values[27]. This material was employed directly in the next step.
5-Methoxyisotoic anhydride (3)
The 5-methoxyanthranilic acid 2 (30 g, 179 mmol) was dissolved in a mixture of H2O (1.2 L) and conc. HCl (15 mL), and this was followed by the addition of triphosgene (63.8 g, 215 mmol). The contents were stirred at rt for 3–4 h until the completion of the reaction (TLC, silica gel). A white solid precipitated from the solution after completion. The solids were collected by filtration and washed with H2O (4 L). The solids were dried under vacuum to give pure anhydride 3 in 89% (30.7 g) yield: M.p = 237–239 °C; 1H NMR (300 MHz, CDCl3) δ 3.65 (s, 3H), 7.11 (d, 1H, J = 8.9 Hz), 7.35 (dd, 1H, J = 8.9 Hz, 2.7 Hz), 7.19 (d, 1H, J = 2.7 Hz), 11.61 (bs, 1H). The spectral data were identical with the reported values[27]. This material was employed directly in the next step. Triphosgene is toxic; care must be exercised.
(S)-2,3-Dihydro-7-methoxy-1H-pyrrolo[2,1-c][1,4]benzodiazepine-5,11(10H,11aH)-dione (4)
A mixture of 5-methoxyisotoic anhydride 3 (30.7 g, 158.9 mmol) and L-proline (20.2 g, 174.8 mmol) in dry DMSO (300 mL) was heated with stirring at 160 °C for 2 h. The white turbid reaction mixture which resulted, became a clear brown solution as the temperature was increased above 80 °C. After the completion of the reaction on examination by TLC (silica gel), the solution was cooled to rt. The mixture was poured into 250 mL of ice water to yield benzodiazepine 4 as a white solid. The solids were collected by vacuum filtration and washed with ice cold water (2 × 50 mL). The filtrate was extracted with ethyl acetate and the solvent was removed under reduced pressure to yield solid diazepine 4. The combined solids were dried in a vacuum oven at 80 °C for 4 h. The yield of 4 was 96% (37.9 g): M.p = 214–216 °C; [α]D25= +444.40 (c 1%, in CH2Cl2); 1H NMR (300 MHz, CDCl3) δ 1.82–2.01 (m, 3H), 2.50–2.51 (m, 1H), 3.40–3.49 (m, 1H), 3.56–3.63 (m, 1H), 3.78 (s, 3H), 4.07–4.10 (m, 1H), 7.07 (d, 1H, J = 8.7 Hz), 7.13 (dd, 1H, J = 8.7 Hz, 3.0 Hz), 7.26 (d, 1H, J = 3.0 Hz), 10.30 (bs, 1H). The spectral data were identical to the reported values[27]. This material was employed directly in the next step.
(S)-Ethyl-7-methoxy-9-oxo-11,12,13,13a-tetrahydro-9H-benzo[e]imidazoα]pyrrolo[1,2-a][1,4]diazepine-1-carboxylate (5)
A well dried reaction flask was evacuated completely and flushed with argon. The flask was then charged with diazepine 4 (37 g, 150.2 mmol) in dry THF (800 mL). The turbid solution was cooled to −20 °C. Potassium tert-butoxide (33.7 g, 300.4 mmol) was added to the flask and the solution was stirred at rt for 1 h. Diethyl chlorophosphate (43.4 mL, 300.4 mmol) was then slowly added to the reaction mixture at −20 °C and it was allowed to stir at rt over a period of 2–3 h. The cloudy reaction mixture became a clear golden brown solution. After complete consumption of the starting material 4 (TLC), the reaction was cooled to −20 °C, afterwhich ethyl isocyanoacetate (32.8 mL, 300.4 mmol) and potassium tert-butoxide (33.7 g, 300.4 mmol) were added. The reaction mixture was stirred at rt for 8 h. The reaction was quenched with a saturated aq solution of NaHCO3 (80 mL). The THF was removed under reduced pressure and the aq layer was extracted with CH2Cl2 (300 mL × 3). The combined organic layer was separated and washed with brine (400 mL) and dried (Na2SO4). The CH2Cl2 was removed under reduced pressure and the dark brown pasty liquid residue, which resulted, was washed with ether to yield the crude ethyl ester 5. This crude solid was recrystallized from CH2Cl2 to yield ethyl ester 5 as a pure white solid in 60% (30.85 g) yield: M.p = 195–197 °C; (Lit. report: 175–176 °C)[27]; [α]D25= +18.00 (c 0.5%, in CH2Cl2); 1H NMR (300 MHz, CDCl3) δ 1.44 (t, 3H, J = 7.2 Hz), 2.15–2.31 (m, 3H), 3.49–3.61 (m, 2H), 3.75–3.85 (m, 1H), 3.91 (s, 3H), 4.41 (q, 2H, J = 7.2 Hz), 4.75 (d, 1H, J = 6.9 Hz), 7.15 (dd, 1H, J = 8.9 Hz, 3.0 Hz), 7.32 (d, 1H, J = 8.9 Hz), 7.59 (d, 1H, J = 3.0 Hz), 7.82 (s, 1H); 13C NMR (75 MHz, CDCl3) δ 14.3, 24.3, 28.4, 46.6, 53.4, 55.8, 61.2, 114.5, 119.7, 124.6, 126.0, 127.3, 130.6, 135.8, 137.6, 159.4, 162.6, 163.7; HRMS (ESI) (M+Na)+, calcd. for C18H19N3O4Na 364.1273; Found 364.1279. The spectral data were identical to the reported values[27]. This material was employed directly in the next step.
(S)-Ethyl-7-hydroxy-9-oxo-11,12,13,13a-tetrahydro-9H-benzo[e]imidazoα]pyrrolo[1,2-a][1,4]diazepine-1-carboxylate (6)
In an oven dried round bottom flask, dry CH2Cl2 (50 ml) was added and cooled to 0 °C. Then AlCl3 (3 g, 22.8 mmol) and ethanethiol (4.5 ml, 60.8 mmol) were added to the above flask slowly at 0 °C. The ice bath was removed and the reaction was allowed to warm up to rt. After the AlCl3 dissolved completely, ester 5 (2.6 g, 7.62 mmol) was added to the mixture at rt and it was stirred for 24 h under Ar. The reaction time will vary with the scale of the reaction. After completion of the reaction (TLC, silica gel), the solution was poured onto ice and was acidified using an aq 2N HCl solution. The solution was extracted 7 times with CH2Cl2 and 4 times with EtOAc separately. Since the product was soluble in water, the extraction procedure was carried out until there was no more product observed in the aqueous layer (TLC, silica gel). The combined organic layer was washed with brine and dried (Na2SO4). The solvent was removed under reduced pressure and the residue was purified by flash column chromatography on [silica gel, 4% MeOH in CH2Cl2] to furnish the phenolic ethyl ester 6 as a solid (2.1 g) in 84% yield: M.p = >260 °C (decomp.); 1H NMR (300 MHz, CDCl3) δ 1.44 (t, 3H, J = 7.1 Hz), 2.19–2.42 (m, 3H), 3.55–3.64 (m, 2H), 3.81–3.89 (m, 1H), 4.42 (q, 2H, J = 7.1 Hz), 4.82 (d, 1H, J = 7.3 Hz), 7.13 (dd, 1H, J = 8.7 Hz, 2.6 Hz), 7.27–7.31 (m, 1H), 7.85 (s, 1H), 7.91 (d, 1H, J = 2.6 Hz), 9.22 (s, 1H); 13C NMR (75 MHz, CDCl3) δ 14.3, 24.4, 28.4, 46.9, 53.8, 61.2, 117.5, 120.8, 124.9, 125.2, 127.7, 129.5, 136.0, 137.2, 157.6, 162.8, 164.6; HRMS (ESI) (M+H)+, calcd. for C17H18N3O4 328.1292; Found 328.1293.
(S)-Ethyl-7-(2H3)-methoxy-9-oxo-11,12,13,13a-tetrahydro-9H-benzo[e]imidazoα]pyrrolo-[1,2-a][1,4]diazepine-1-carboxylate (5a)
To a solution of phenol 6 (1.5 g, 4.6 mmol) in CH2Cl2 (30 mL), Cs2CO3 (3 g, 9.2 mmol) was added and the mixture stirred at rt for 30 min. Then CD3I (2.3 ml, 36.8 mmol) was added slowly and the reaction mixture was stirred at rt for 24 h. After completion of the reaction the mixture was quenched with cold water and extracted with CH2Cl2. The combined organic layer was washed with brine and dried (Na2SO4). The solvent was removed under reduced pressure and the residue was purified by flash column chromatography [silica gel, 2% CH3OH in CH2Cl2] to furnish ester 5a as a solid (1.36 g) in 86% yield: M.p = 195–196 °C; [α]D25= +20.00 (c 0.5%, in CH2Cl2); 1H NMR (300 MHz, CDCl3) δ 1.46 (t, 3H, J = 7.1 Hz), 2.17–2.36 (m, 3H), 3.54–3.63 (m, 2H), 3.77–3.84 (m, 1H), 4.43 (q, 2H, J = 7.1 Hz), 4.77 (d, 1H, J = 7.3 Hz), 7.16 (dd, 1H, J = 8.8 Hz, 2.8 Hz), 7.33 (d, 1H, J = 8.8 Hz), 7.60 (d, 1H, J = 2.8 Hz), 7.82 (s, 1H); 13C NMR (75 MHz, CDCl3) δ 14.3, 24.3, 28.3, 46.5, 53.4, 61.1, 114.4, 119.7, 124.5, 126.0, 127.6, 130.5, 135.8, 137.6, 159.3, 162.8, 163.7; HRMS (ESI) (M+H)+, calcd. for C18H172H3N3O4 345.1637; Found 345.1635. The 13C-D signal was not observed due to long relaxation time, line broadening, reduced NOE effect and spin-spin coupling.
(S)-tert-Butyl-7-methoxy-9-oxo-11,12,13,13a-tetrahydro-9H-benzo[e]imidazo[5,1-c]pyrrolo[1,2-a][1,4]diazepine-1-carboxylate (7)
A flame dried round bottom flask was charged with dry THF (30 mL) and lithium rod (excess, cut into small pieces) was added. Dry tert-butanol (2.6 mL, 27.1 mmol) was added to the above flask at rt and the mixture which resulted was heated to 45–50 °C under Ar until the tert-butanol reacted completely. This freshly prepared lithium tert-butoxide solution was transferred carefully with a cannula to another flame dried round bottom flask charged with ester 5 (1.0 g, 2.71 mmol) and stirred at 50 °C under Ar for 30 min. After the completion of the reaction (TLC, silica gel), the flask was cooled to rt and the THF removed under reduced pressure. Ice water (10 mL) was added to the residue and it was then extracted with EtOAc. The organic layer was washed with water (2 × 10 mL) and brine (15 mL). The solvent was removed under reduced pressure and the residue was purified by flash column chromatography [silica gel, EtOAc/hexane (7:3)] to yield tert-butyl ester (XHE-III-74) 7 as a solid (0.72 g) in 67% yield: M.p = 115–117 °C (119–121 °C)[27]; [α]D25= +36.00 (c 0.5%, in CH2Cl2); 1H NMR (300 MHz, CDCl3) δ 1.63 (s, 9H), 2.14–2.28 (m, 3H), 3.47–3.61(m, 2H), 3.74–3.81 (m, 1H), 3.90 (s, 3H), 4.73 (d, 1H, J = 6.9 Hz), 7.14 (dd, 1H, J = 8.8 Hz, 3.0 Hz), 7.30 (d, 1H, J = 8.8 Hz), 7.57 (d, 1H, J = 3.0 Hz), 7.83 (s, 1H); 13C NMR (75 MHz, CDCl3) δ 24.3, 28.2, 28.3, 46.7, 53.4, 55.9, 81.9, 114.5, 119.7, 124.6, 126.1, 128.9, 130.5, 135.6, 136.5, 159.4, 162.2, 163.8; HRMS (ESI) (M+Na)+, calcd. for C20H23N3O4Na 392.1586; Found 392.1574. The spectral data were identical to the reported values[27].
(S)-7-Methoxy-9-oxo-11,12,13,13a-tetrahydro-9H-benzo[e]imidazo[5,1-c]pyrrolo[1,2-a][1,4]di-azepine-1-carboxylic acid (9)
The ester 5 (2.12 g, 6.21 mmol) was dissolved in a mixture of EtOH (4 mL) and H2O (3 mL) after which solid NaOH (1.2 g, 31.0 mmol) was added to the solution. This reaction mixture was heated to 50 °C for 15 min and the EtOH was removed under reduced pressure. The remaining aq solution was stirred at 0 °C for 10 min and then conc HCl was added dropwise to the solution until the pH was 3–4 (pH paper). A pale yellow precipitate which formed was left in the solution and the mixture was allowed to stir at rt for 2 h. The precipitate was collected by filtration, washed with cold water (2 × 5 mL) and the aq layer also was allowed to stand at rt for 10 h to obtain additional solid 9. The combined solids were dried in a vacuum oven at 80 °C for 7 h to get pure acid 9 in 80 % yield: M.p = 210–211 °C; [α]D25= +8.00 (c 0.25%, in CH3OH); 1H NMR (300 MHz, DMSO) δ 2.03–2.16 (m, 3H), 3.50–3.63 (m, 3H), 3.87 (s, 3H), 4.84 (d, 1H, J = 7.5 Hz), 7.31 (dd, 1H, J = 8.9 Hz, 3.0 Hz), 7.41 (d, 1H, J = 3.0 Hz), 7.63 (d, 1H, J = 8.9 Hz), 8.21 (s, 1H); 13C NMR (75 MHz, DMSO) δ 24.9, 28.7, 47.3, 53.6, 56.8, 115.3, 119.9, 126.7, 127.0, 128.6, 131.0, 137.4, 137.5, 159.5, 164.0, 165.3; HRMS (ESI) (M+H)+, calcd. for C16H16N3O4 314.1141; Found 314.1141. The spectral data were identical to the reported values[27]. This material was employed directly in the next step.
(S)-Methyl-7-methoxy-9-oxo-11,12,13,13a-tetrahydro-9H-benzo[e]imidazo[5,1-c]pyrrolo[1,2-a][1,4]diazepine-1-carboxylate (10)
To an oven dried two neck round bottom flask, ester 5 (0.30 g, 0.87 mmol) was added in dry methanol (10 mL) and then NaOMe (0.2g, 3.48 mmol) was added to the solution. The mixture was heated to reflux until the reaction was complete (~1h, confirmed by TLC, silica gel). Then the reaction mixture was quenched with an ice cold aq NaHCO3 solution. The solvent was removed under reduced pressure and the residue was dissolved in water and extracted with EtOAc (2× 20 mL). The combined organic layer was then washed with brine and dried (Na2SO4). The solvent was removed under reduced pressure and the residue was purified by flash column chromatography [silica gel, EtOAc/hexane (1:1)] to yield pure methyl ester 10 as a solid in (0.286 g) 93% yield: M.p = 180–182 °C; [α]D25= +8.00 (c 0.25%, in CH2Cl2); 1H NMR (300 MHz, CDCl3) δ 2.16–2.35 (m, 3H), 3.52–3.61 (m, 2H), 3.75–3.81 (m, 1H), 3.91 (s, 3H), 3.94 (s, 3H), 4.75 (d, 1H, J = 6.9 Hz), 7.16 (dd, 1H, J = 8.7 Hz, 2.7 Hz), 7.34 (d, 1H, J = 8.7 Hz), 7.59 (d, 1H, J = 2.7 Hz), 7.88 (s, 1H); 13C NMR (75 MHz, CDCl3) δ 24.4, 28.4, 46.6, 52.2, 53.4, 55.9, 114.5, 119.8, 124.7, 125.9, 126.9, 130.6, 135.9, 137.9, 159.5, 162.9, 163.7. HRMS (ESI) (M+Na)+, calcd. for C17H17N3O4Na 350.1117; Found 350.1125.
General method for the synthesis of esters and amides (11, 11a–19, and 19a)
A mixture of acid (9 or 9a, 0.32 mmol) individually, thionyl chloride (5.12 mmol) and dry CH2Cl2 (8 mL) was added to an oven dried round bottomed flask under argon. This suspension was allowed to reflux at 52 °C (the outside oil bath temperature was at 60 °C) for 1 h under an atmosphere of argon. The solution became a clear yellow color. The absence of the starting material was confirmed by the examination by TLC (silica gel). The organic solvent and excess thionyl chloride were removed under reduced pressure. This evaporation was repeated a couple of times with dry CH2Cl2 (5 mL) to remove excess thionyl chloride and any HCl. The yellow residue, which was obtained, was dissolved in dry CH2Cl2 (10 mL) and cooled to 0 °C for 10 min under argon. The appropriate nucleophile (alcohol/thiol/amine, 3.2 mmol), followed by Et3N (1.6 mmol) was added to the reaction mixture at 0°C and the mixture was then allowed to warm to rt and stirred for 2–7 h. After the completion of the reaction (TLC, silica gel), the solvent was removed under reduced pressure. The residue was quenched with ice cold water (5 mL) and extracted with CH2Cl2 (8 mL × 2). The combined organic layer was washed with brine (5 mL). The solvent was removed under reduced pressure and the residue was purified by flash column chromatography (silica gel) to yield the corresponding pure esters, thioesters and amides whose properties are depicted below and as well as in the SI. Note: We observed in a control experiment that the mixture of dichloromethane plus thionyl chloride boils at 52 °C.
(S)-Isobutyl-7-methoxy-9-oxo-11,12,13,13a-tetrahydro-9H-benzo[e]imidazo[5,1-c]pyrrolo[1,2-a][1,4]diazepine-1-carboxylate (11)
The isobutyl ester 11 was prepared from acid 9 following the general procedure with dry isobutanol as the nucleophile. The crude residue was purified by flash column chromatography [silica gel, EtOAc/hexane (7:3)] to yield pure isobutyl ester 11 as a solid in 70% yield: M.p = 126–127 °C (104–106 °C)[27]; 1H NMR (300 MHz, CDCl3) δ 1.04 (d, 6H, J = 6.7 Hz), 2.13–2.37 (m, 4H), 3.49–3.63 (m, 2H), 3.76–3.84 (m, 1H), 3.92 (s, 3H), 4.14 (d, 2H, J = 6.9 Hz), 4.76 (d, 1H, J = 6.9 Hz), 7.17 (dd, 1H, J= 8.8 Hz, 2.9 Hz), 7.33 (d, 1H, J = 8.8 Hz), 7.60 (d, 1H, J = 2.9 Hz), 7.82 (s, 1H); 13C NMR (75 MHz, CDCl3) δ 19.3, 24.4, 27.8, 28.4, 46.6, 53.5, 55.9, 71.2, 114.5, 119.8, 124.6, 126.2, 127.8, 130.6, 135.9, 137.6, 159.4, 163.1, 163.8. HRMS (ESI) (M+Na)+, calcd. for C20H23N3O4Na 392.1586; Found 392.1594. The spectral data were identical to the reported values[27].
Biology
Experimental animals
5–10 week old male BALB/c and Swiss Webster mice (Charles River Laboratory, WIL, MA) and adult (425–450 g) male Hartley guinea pigs (Charles River Laboratory, WIL, MA) were used for the experiments. The animals were housed under specific pathogen-free conditions, under standard conditions of humidity, temperature and a controlled 12 h light and dark cycle and had free access to food and water. All animal experiments were in compliance with the University of Wisconsin, Milwaukee or Columbia University Institutional Animal Care and Use Committees (IACUC).
Microsomal stability assay and cytotoxicity assay
Rotarod assay
Swiss Webster mice were trained to maintain balance at a constant speed of 15 rpm on the rotarod apparatus (Omnitech Electronics Inc., Nova Scotia, Canada) until mice could perform for three minutes at three consecutive time points. Separate groups of mice received intraperitoneal (i.p.) injections of vehicle (10% DMSO, 40% propylene glycol and 50% PBS) or test compounds. Diazepam was used as a positive control compound (5 mg/kg) in an approximate volume of 100 µl. Ten minutes after each injection, mice were placed on the rotarod for three minutes. A fail was assigned for each mouse that fell from the rotarod prior to 3 minutes. Mice were rested two to three days before administration of another dose or a different compound.
Guinea pig airway smooth muscle organ bath
Guinea pigs were anesthetized with intraperitoneal pentobarbital (100 mg/kg). Their tracheas were then surgically removed and transected into cross-sectional sections containing two cartilaginous rings as described previously.26 The epithelium was removed with cotton swabs and the rings were suspended by two silk threads in 4 ml jacketed organ baths (Radnoti Glass Technology). One thread was attached to a Grass FT03 force transducer (Grass-Telefactor) coupled via Biopac hardware to a computer with Acknowledge 7.3.3 software (Biopac Systems) for continuous digital recording of muscle tension. The rings were bathed in 4 mL of KH buffer solution (composition in mM: 118 NaCl, 5.6 KCl, 0.5 CaCl2, 0.2 MgSO4, 25 NaHCO3, 1.3 NaH2PO4, 5.6 d-glucose) with 10 µM indomethacin (DMSO vehicle final concentration of 0.01%), which was continuously bubbled with 95% O2 and 5% CO2 at pH 7.4, 37 °C. The rings were equilibrated at 1 g of isotonic tension for 1 h with new KH buffer added every 15 min. All rings were precontracted with 10 µM N-vanillylnonanamide (capsaicin analog) and then two cycles of cumulatively increasing concentrations of acetylcholine (0.1–100 µM) with extensive buffer washes between and after those two cycles with resetting of the resting tension to 1.0 g. To eliminate the confounding effects of airway nerves and histamine receptors, tetrodotoxin (1 µM) and pyrilamine (10 µM) were added to the buffer. After a stable baseline tension of 1.0 g was established, tracheal rings were contracted with 1 µM substance P. After the peak contraction was reached, 50 µM of compound 16 (or the vehicle control 0.1% DMSO) was added to each bath. The percentage of initial contraction remaining at 15, 30, 45, and 60 min after compound exposure was compared between groups.
Assessment of Airway hyper-responsiveness
Airway hyper-responsiveness to methacholine in conscious, spontaneously breathing animals was measured by DSI's Buxco® FinePointe Non-Invasive Airway Mechanics (NAM) instrument.[26] Before measurements were taken, mice were acclimated to the chamber for 15 minutes daily for 5 days. The chambers were also calibrated each time before data collection. Briefly, the nasal chamber in combination with the thoracic chamber permits computation of Specific Airway Resistance (sRaw). The FinePointe software computes sRaw with all other ventilatory parameters derived by the NAM analyzer. Mice were exposed to aerosolized PBS (for the baseline measurement) or methacholine (1.56–12.5 mg/mL) for 1 min and readings were taken and averaged for 3 mins after each nebulization. Data obtained were presented as sRaw versus aerosolized methacholine concentration (mg/ml). Data analysis was carried out using 2way ANOVA repeated measures with Bonferroni post-test. Overall p-value was 0.0937 and F = 1.763. The Bonferroni post-test gave significance for 12.5 mg methacholine as indicated in Figure 2.
Pharmacokinetic study
Supplementary Material
Acknowledgments
This work was supported by the University of Wisconsin-Milwaukee, the National Institutes of Health R03DA031090 (L.A.A.), R01NS076517 (J.M.C., L.A.A.), R01HL118561 (J.M.C., L.A.A., C.W.E., D.C.S.), R01MH096463 (J.M.C., L.A.A.), R01GM065281 (C.W.E., G.T.Y., J.M.C.), and R01HL122340 (C.W.E.), as well as the University of Wisconsin Milwaukee Research Foundation (Catalyst grant), the Lynde and Harry Bradley Foundation, the Richard and Ethel Herzfeld Foundation, the Stony Wold-Herbert Fund (G.T.Y.), the Foundation for Anesthesia Education and Research (G.T.Y.). We thank Dr. Beryl R. Forman and Jennifer L. Nemke (Animal Facility at UWM) for their guidance and support.
References
- 1.American_Asthma_Foundation. Impact of asthma. 2016 Available at: americanasthmafoundation.org/impact-asthma. [Google Scholar]
- 2.Barnett SB, Nurmagambetov TA. Costs of asthma in the United States: 2002–2007. J Allergy Clin Immunol. 2011;127:145–152. doi: 10.1016/j.jaci.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 3.Trends in asthma morbidity and mortality. Washington, DC: American Lung Association Epidemiology & Statistics Unit Research and Program Services; 2012. [Google Scholar]
- 4.Cates CJ, Cates MJ. Regular treatment with salmeterol for chronic asthma: serious adverse events. Cochrane Database Syst Rev. 2008 doi: 10.1002/14651858.CD006363.pub2. CD006363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cates CJ, Cates MJ. Regular treatment with formoterol for chronic asthma: serious adverse events. Cochrane Database Syst Rev. 2012;4 doi: 10.1002/14651858.CD006923.pub3. CD006923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cates CJ, Jaeschke R, Schmidt S, Ferrer M. Regular treatment with formoterol and inhaled steroids for chronic asthma: serious adverse events. Cochrane Database Syst Rev. 2013;6 doi: 10.1002/14651858.CD006924.pub3. CD006924. [DOI] [PubMed] [Google Scholar]
- 7.Dahl R. Systemic side effects of inhaled corticosteroids in patients with asthma. Respir Med. 2006;100:1307–1317. doi: 10.1016/j.rmed.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 8.Kelly HW, Sternberg AL, Lescher R, Fuhlbrigge AL, Williams P, Zeiger RS, Raissy HH, Van Natta ML, Tonascia J, Strunk RC, Group CR. Effect of inhaled glucocorticoids in childhood on adult height. N Engl J Med. 2012;367:904–912. doi: 10.1056/NEJMoa1203229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mortensen M, Patel B, Smart TG. GABA Potency at GABA(A) Receptors Found in Synaptic and Extrasynaptic Zones. Front Cell Neurosci. 2011;6:1. doi: 10.3389/fncel.2012.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olsen RW, Sieghart W. International Union of Pharmacology. LXX. Subtypes of gamma-aminobutyric acid(A) receptors: classification on the basis of subunit composition, pharmacology, and function. Update. Pharmacol Rev. 2008;60:243–260. doi: 10.1124/pr.108.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alam S, Laughton DL, Walding A, Wolstenholme AJ. Human peripheral blood mononuclear cells express GABAA receptor subunits. Mol. Immunol. 2006;43:1432–1442. doi: 10.1016/j.molimm.2005.07.025. [DOI] [PubMed] [Google Scholar]
- 12.Bjurstom H, Wang J, Ericsson I, Bengtsson M, Liu Y, Kumar-Mendu S, Issazadeh-Navikas S, Birnir B. GABA, a natural immunomodulator of T lymphocytes. J Neuroimmunol. 2008;205:44–50. doi: 10.1016/j.jneuroim.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 13.Gallos G, Townsend E, Yim P, Virag L, Zhang Y, Xu D, Bacchetta M, Emala CW. Airway epithelium is a predominant source of endogenous airway GABA and contributes to relaxation of airway smooth muscle tone. Am J Physiol Lung Cell Mol Physiol. 2013;304:L191–L197. doi: 10.1152/ajplung.00274.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mizuta K, Xu D, Pan Y, Comas G, Sonett JR, Zhang Y, Panettieri RA, Jr., Yang J, Emala CW., Sr. GABAA receptors are expressed and facilitate relaxation in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2008;294:L1206–L1216. doi: 10.1152/ajplung.00287.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reyes-Garcia MG, Hernandez-Hernandez F, Hernandez-Tellez B, Garcia-Tamayo F. GABA (A) receptor subunits RNA expression in mice peritoneal macrophages modulate their IL-6/IL-12 production. J Neuroimmunol. 2007;188:64–68. doi: 10.1016/j.jneuroim.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 16.Xiang YY, Wang S, Liu M, Hirota JA, Li J, Ju W, Fan Y, Kelly MM, Ye B, Orser B, O'Byrne PM, Inman MD, Yang X, Lu WY. A GABAergic system in airway epithelium is essential for mucus overproduction in asthma. Nat Med. 2007;13:862–867. doi: 10.1038/nm1604. [DOI] [PubMed] [Google Scholar]
- 17.Tian J, Chau C, Hales TG, Kaufman DL. GABA(A) receptors mediate inhibition of T cell responses. J Neuroimmunol. 1999;96:21–28. doi: 10.1016/s0165-5728(98)00264-1. [DOI] [PubMed] [Google Scholar]
- 18.Munroe ME, Businga TR, Kline JN, Bishop GA. Anti-inflammatory effects of the neurotransmitter agonist Honokiol in a mouse model of allergic asthma. J Immunol. 2010;185:5586–5597. doi: 10.4049/jimmunol.1000630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Whiting PJ. GABA-A receptor subtypes in the brain: a paradigm for CNS drug discovery? Drug Discov Today. 2003;8:445–450. doi: 10.1016/s1359-6446(03)02703-x. [DOI] [PubMed] [Google Scholar]
- 20.Fu XW, Wood K, Spindel ER. Prenatal nicotine exposure increases GABA signaling and mucin expression in airway epithelium. Am J Respir Cell Mol Biol. 2011;44:222–229. doi: 10.1165/rcmb.2010-0109OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gundavarapu S, Wilder JA, Mishra NC, Rir-Sima-Ah J, Langley RJ, Singh SP, Saeed AI, Jaramillo RJ, Gott KM, Pena-Philippides JC, Harrod KS, McIntosh JM, Buch S, Sopori ML. Role of nicotinic receptors and acetylcholine in mucous cell metaplasia, hyperplasia, and airway mucus formation in vitro and in vivo. J Allergy Clin Immunol. 2012;130:770–780. e711. doi: 10.1016/j.jaci.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clayton T, Poe MM, Rallapalli S, Biawat P, Savic MM, Rowlett JK, Gallos G, Emala CW, Kaczorowski CC, Stafford DC, Arnold LA, Cook JM. A Review of the Updated Pharmacophore for the Alpha 5 GABA(A) Benzodiazepine Receptor Model. Int J Med Chem. 2015;2015:430248. doi: 10.1155/2015/430248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gallos G, Yim P, Chang S, Zhang Y, Xu D, Cook JM, Gerthoffer WT, Emala CW., Sr. Targeting the restricted alpha-subunit repertoire of airway smooth muscle GABAA receptors augments airway smooth muscle relaxation. Am J Physiol Lung Cell Mol Physiol. 2012;302:L248–L256. doi: 10.1152/ajplung.00131.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gallos G, Yocum GT, Siviski ME, Yim PD, Fu XW, Poe MM, Cook JM, Harrison N, Perez-Zoghbi J, Emala CW., Sr. Selective targeting of the alpha5-subunit of GABAA receptors relaxes airway smooth muscle and inhibits cellular calcium handling. Am J Physiol Lung Cell Mol Physiol. 2015;308:L931–L942. doi: 10.1152/ajplung.00107.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yocum GT, Gallos G, Zhang Y, Jahan R, Stephen MR, Varagic Z, Puthenkalam R, Ernst M, Cook JM, Emala CW. Targeting the gamma-Aminobutyric Acid A Receptor alpha4 Subunit in Airway Smooth Muscle to Alleviate Bronchoconstriction. Am J Respir Cell Mol Biol. 2016;54:546–553. doi: 10.1165/rcmb.2015-0176OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Forkuo GS, Guthrie ML, Yuan NY, Nieman AN, Kodali R, Jahan R, Stephen MR, Yocum GT, Treven M, Poe MM, Li G, Yu OB, Hartzler BD, Zahn NM, Ernst M, Emala CW, Stafford DC, Cook JM, Arnold LA. Development of GABAA Receptor Subtype-Selective Imidazobenzodiazepines as Novel Asthma Treatments. Mol Pharm. 2016;13:2026–2038. doi: 10.1021/acs.molpharmaceut.6b00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li X, Ma C, He X, Yu J, Han D, Zhang C, Atack JR, Cook JM. Studies in search of diazepam-insensitive subtype selective agents for GABA (A)/Bz receptors. Medicinal Chemistry Research. 2002;11:504–537. [Google Scholar]
- 28.Stafford DC, Cook JM, Arnold LA, Emala CW, Gallos G, Stephen MR. Novel GABAA agonists and methods of using to control airway hyperresponsiveness and inflammation in asthma. WO2014047413 A1. 2014
- 29.McKernan RM, Rosahl TW, Reynolds DS, Sur C, Wafford KA, Atack JR, Farrar S, Myers J, Cook G, Ferris P, Garrett L, Bristow L, Marshall G, Macaulay A, Brown N, Howell O, Moore KW, Carling RW, Street LJ, Castro JL, Ragan CI, Dawson GR, Whiting PJ. Sedative but not anxiolytic properties of benzodiazepines are mediated by the GABA(A) receptor alpha1 subtype. Nat Neurosci. 2000;3:587–592. doi: 10.1038/75761. [DOI] [PubMed] [Google Scholar]
- 30.Ramerstorfer J, Furtmuller R, Vogel E, Huck S, Sieghart W. The point mutation gamma 2F77I changes the potency and efficacy of benzodiazepine site ligands in different GABAA receptor subtypes. Eur J Pharmacol. 2010;636:18–27. doi: 10.1016/j.ejphar.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.