Abstract
Experimental models of Alzheimer’s disease (AD) are critical to gaining a better understanding of pathogenesis and to assess the potential of novel therapeutic approaches. The most commonly used experimental animal models are transgenic mice that overexpress human genes associated with familial AD (FAD) that result in the formation of amyloid plaques. However, AD is defined by the presence and interplay of both amyloid plaques and neurofibrillary tangle pathology. The track record of success in AD clinical trials thus far has been very poor. In part, this high failure rate has been related to the premature translation of highly successful results in animal models that mirror only limited aspects of AD pathology to humans. A greater understanding of the strengths and weakness of each of the various models and the use of more than one model to evaluate potential therapies would help enhance the success of therapy translation from preclinical studies to patients. In this review we summarize the pathological features and limitations of the major experimental models of AD including transgenic mice, transgenic rats, various physiological models of sporadic AD and in vitro human cell culture models.
Introduction
Experimental models are essential to further understand AD pathogenesis and to perform preclinical testing of novel therapeutics. To date, the vast majority of experimental models are animal models, almost exclusively consisting of transgenic mice that express human genes that result in the formation of amyloid plaques (by expression of human APP alone or in combination with human PSEN1) and neurofibrillary tangles (by expression of human MAPT)[14, 34, 117, 172, 173]. Other models have included invertebrate animals such as Drosophila melanogaster and Caenorhabditis elegans, as well as vertebrates such as zebrafish; however, given these models’ greater distance from human physiology they are less extensively used [13, 53, 104]. Since the development of the first transgenic mouse model with substantial amyloid plaque burden in 1995[42], there has been a proliferation of new transgenic models, each with a different phenotype of AD-associated pathology[34, 117, 173]. The development of transgenic models offered much promise about the understanding of AD pathogenesis, allowing questions to be answered that were previously impossible to examine in humans. Accordingly, the number of studies using AD transgenic models rapidly increased. However, questions have been increasingly raised about the validity of relying on the available transgenic models, particularly in light of the very high failure rate of clinical trials of AD therapeutics (of ~99.6%), many of which were successful in preclinical testing using these animal models [6, 27, 139]. These results highlight the often overlooked fact that these animal models do not have AD, they only recapitulate specific pathological features, most commonly in a non-physiological manner designed to allow for efficient experiments. The majority of animal models (both transgenic and physiological models) develop only the amyloid accumulation that defines AD. This often (but not always) results in specific memory-associated cognitive impairments. Importantly however, these models often lack the widespread presence of other pathological features that define AD including neuronal loss and most importantly, neurofibrillary tangle development. This lack of additional AD associated pathology could at least partly account for the lack of translation between preclinical and clinical trials [6], although there have also been a few clinical trial failures for approaches not initially tested in transgenic models[69]. As such, it is important to have a good understanding of the exact neuropathology present in each model, particularly regarding how well this correlates with human AD, so that results can be interpreted more accurately and the likelihood of translation to human studies can increase. Results generated from experimental models can be exceptionally informative about specific aspects of AD if researchers are aware of the limitations associated with each model. Therefore, in this review we will discuss our understanding of the pathogenesis of AD and the features and limitations of the major experimental models of AD that reflect this pathology, including transgenic mice, transgenic rats, physiological models of sporadic AD, invertebrate animals and in vitro human cell culture models.
AD neuropathology
AD is a complex, multi-factorial disease, and one that appears to be unique to humans. The age of onset, rate of progression and the development of pathology are highly variable between patients. AD is defined in the brain by pathological accumulation of amyloid β (Aβ) into extracellular plaques in the brain parenchyma and in the vasculature (known as congophilic amyloid angiopathy [CAA]), and abnormally phosphorylated tau that accumulates intraneuronally forming neurofibrillary tangles (NFTs) [102, 136]. Pathological aggregation of Aβ and phosphorylated tau occurs in a sequential process; small numbers of monomers first aggregate into oligomers intraneuronally, which then continue to aggregate into the fibrils observed in amyloid plaques and NFTs [136, 144]. It is suggested that oligomers are the most neurotoxic species in AD as levels of these species correlate much better with cognitive symptoms than presence of plaques or NFTs [166]. Amyloid plaques primarily consist of aggregated Aβ. The most abundant forms of Aβ are Aβ1–40 and Aβ1–42, but other important Aβ species include Aβ1–38, Aβ1–43 and Aβ with post-translational modifications such as AβN3pE (N-terminally truncated Aβ with a pyroglutamate modification), pAβ (Aβ with phosphorylated serine at position 8 or 26) and Aβ5-x (N-terminally truncated Aβ)[144]. The presence and amount of these different Aβ species is important because each species has a different rate of aggregation and they preferentially form different aggregated species, some more toxic than others. For example pAβ has been shown to promote oligomer formation and propagation, and its presence has been used in the biochemical staging of amyloid deposits [84, 122, 158]. Aβ is a cleavage product of amyloid precursor protein (APP). APP is initially cleaved by BACE1 and then cleaved by γ-secretase (a protease composed of presenilin-1, nicastrin, APH-1 and PEN-2) to release monomeric Aβ. In AD, either increased production of Aβ and/or production of more aggregation prone species of Aβ (in case of FAD) or impaired clearance of Aβ (in the case of sporadic AD [sAD]) results in Aβ accumulation in the brain[144]. Extensive evidence indicates this process initially occurs intraneuronally, predominately in synapses [50, 166]. This accumulation results in the aggregation of Aβ into soluble Aβ oligomers, which are considered to be the most toxic Aβ species[166], which then aggregate into fibrillar amyloid in the parenchyma (plaques) and blood vessels (CAA).
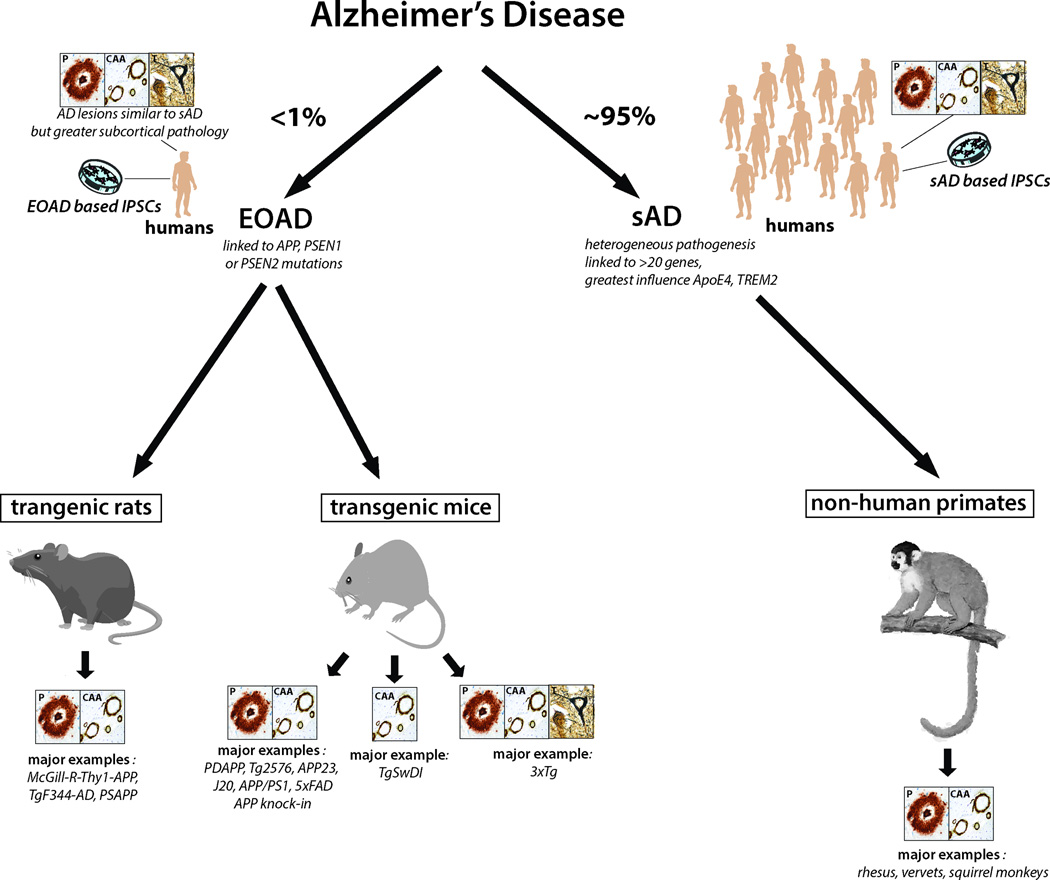
There are many environmental and genetic factors that have been shown to increase risk for AD, but understanding the interplay between these risk factors and their individual contribution to the etiology of AD is an ongoing process. AD is characterized as either familial early-onset (EOAD; <5% of all AD patients, with onset at <65yrs) or sporadic late-onset (sAD; onset >65yrs). Autosomal dominant mutations in presenilin 1, presenilin 2 (PSEN1 and PSEN2) or the amyloid precursor protein (APP) account for only 5–10% of all EOAD cases (~1% of all AD cases), leaving the cause of the majority of EOAD unexplained [17, 52, 170]. sAD afflicts >95% of patients with AD and is related to both genetic and environmental factors [7, 52, 74, 76](see Figure 1). Genome-wide association studies (GWAS) have identified over 20 loci that confer increased risk for sAD, including genes involved in innate immunity, cholesterol metabolism and synaptic/neuronal membrane function, suggesting that the pathogenesis of sAD is quite heterogeneous [28, 52, 75]. The strongest identified genetic risk factor for sAD is the inheritance of the apolipoprotein (apo) E4 allele, the protein product of which influences the aggregation and clearance of brain Aβ [63, 115]. Rare variants of another gene that encodes the triggering receptor expressed on myeloid cells 2 (TREM2) have been reported as a significant risk factor for sAD, with an odds ratio similar to apoE4[162]. Familial AD (FAD) results in an earlier age of onset and different neuropathological and clinical features compared to sAD [80, 127, 147, 156]. The exact phenotype for individual FAD cases varies widely and depends on the mutation present. FAD shows disproportionate subcortical Aβ42 accumulation, associated with enhanced striatal tau pathology [147]. The latter may be responsible for the enhanced prevalence of atypical clinical symptoms in FAD such as prominent myoclonus, dysarthria and extrapyramidal symptoms [147]. In addition, FAD shows significantly different development of associated neuropathology such as TDP-43 and argyrophilic grain disease compared to sAD; in the ADNI sAD cohort the latter two pathologies occurred in about 20% of subjects, while in the FAD DIAN cohort these pathologies were absent [18]. These differences between sAD and FAD may impact the translatability of therapeutic findings in transgenic mouse models that are largely based on over-expression of APP and PSEN1 containing FAD linked mutations.
Figure 1. Schematic of the major animal models of Alzheimer’s disease.
Less than 1% of AD cases are early onset familial Alzheimer’s disease (EOAD) cases that are caused by autosomal dominant mutations in APP, PSEN1 or PSEN2. However, all major transgenic rodent models express these mutated forms of APP and PS1. The best animal models available of sAD are non-human primates. The consistent presence of the types of neuropathology present in each model is shown in the boxes; P: plaques; CAA; congophilic amyloid angiopathy; T: neurofibrillary tangles. We did not consider the presence of pre-tangle pathology in these animal models sufficient to indicate the presence of neurofibrillary tangle pathology. As such, only 3xTg mice express all 3 pathological hallmarks of AD. The specific types animal models included in each category are examples of the most common animal models currently used in AD research.
The current consensus guidelines for neuropathological evaluation of AD are ranked on three parameters (Amyloid, Braak and CERAD staging) to obtain an “ABC” score that quantifies both neuritic plaques and NFT pathology[65, 99]. There have been many clinicopathological studies that have correlated amyloid plaques with cognitive deficits in AD, with an emerging picture that the strongest correlation exists in the earliest stages of the disease and this association greatly weakens as NFTs and neocortical degeneration become more widespread [9, 102, 103, 157, 160, 169]. On the other hand, numerous studies have documented a strong link between neocortical NFTs and cognitive loss[26, 46, 48, 97, 123, 160, 169]. This data is consistent with the amyloid cascade hypothesis that suggests that Aβ plaques /Aβ oligomers kindle widespread tau/NFT pathology, with the latter representing the more direct cause of neuronal and synaptic loss that underlie the clinical disease[144]. However, in this scenario accumulation of Aβ pathology only has a prominent role in the preclinical and MCI stages of AD, while tau pathology is already prominent in early clinical AD. Therefore therapeutic approaches that have been shown to be successful only in Aβ models of AD, would only have the possible expectation of influencing the trajectory of AD pathology in the preclinical or MCI stages of the disease. For potential effects in established AD, a therapy would have to be shown to reduce pathology in models of AD with both Aβ and tau pathology. A further complication is that there is extensive evidence showing medial temporal tauopathy predates Aβ deposition [15, 25, 35], presumably via independent mechanisms, highlighting the need for successful AD therapeutic approaches to directly address tau pathology. In the past, these facts have often not been taken into account in the translation of studies from AD models to patients [171, 172].
The clinical diagnosis of AD is currently based on decline in specific areas of cognition and a positive result on AD biomarker assays including amyloid and/or tau PET; as well as, Aβ and tau levels in the CSF, which directly or indirectly reflect the changes that are used for the neuropathological criteria for AD [8, 67, 81, 144]. Definitive diagnosis of AD still depends on postmortem neuropathological assessment of both amyloid plaques and tau pathology, which are present together in very few AD models.
Transgenic mouse models
The vast majority of animal models used in AD research are transgenic mice. Wild-type mouse APP (695 isoform) has 97% sequence homology with human APP. Importantly, sequence differences between mice and humans include 3 amino acids within the Aβ sequence (R5G, Y10F and H13R)[155, 174]. These differences impair Aβ aggregation and prevent the formation of amyloid plaques in wild-type mice. Therefore, expression of human APP is necessary for the formation of amyloid plaques in mice. Initial transgenic models expressed wild-type human APP in mice, however while these transgenic mice had increased Aβ production, they failed to consistently show extensive AD associated neuropathology [14, 34, 117, 172, 173]. In contrast, expression of human APP containing mutations associated with FAD resulted in consistent plaque pathology and varying amounts of consequent downstream AD-associated pathological features. Multiple transgenic strains have been generated and the exact phenotype for each transgenic strain strongly depends on the FAD mutation, the promoter used and the background mouse strain. Since the vast majority of AD transgenic models have pathology that is dependent on the expression of FAD mutations and most AD clinical trials are conducted in sAD patients, in whom AD pathogenesis has significant distinctions from FAD, this represents one stumbling block for the translatability of success in these models. The neuropathology and associated cognitive impairments for the transgenic mouse strains most commonly used in AD research are detailed in Table 1. It should be noted that the degree to which each model is characterized in terms of the sensitivity of the cognitive testing performed, amount of tau related pathology and the extent of synaptic pathology (demonstrated by ultrastructural studies and/or electrophysiology) greatly varies, making absolute comparisons between models difficult.
Table 1.
Most common transgenic models of AD (references in table are included in electronic supplementary material).
| Model | Mutation | Plaques | CAA | Amyloid PTMs |
NFTs | Synapses | Neuro- degeneration |
Gliosis | Cognitive Impairment |
Other pathology |
|---|---|---|---|---|---|---|---|---|---|---|
| Mice | ||||||||||
| PDAPP [24] |
APPV717F | 8M: Cx, HIP Diffuse and cored[24, 39] |
From 12M[72] |
AβpE3: from 12M[15] pAβ: NE |
None | ↓ SYN and MAP2 density[24 ] 4–5M: ↓ LTP[48] |
18M: None[39] |
Plaque- associated reactive astrocytes, general microgliosis[24, 52] |
MWM: 4–6M[31] FC: 11M[27] |
Aβo: NE |
| Tg2576 [36] |
APPK670N,M671L | 7–8M: focal plaques in Cx[45] 11–13M: Cx, HIP, CB[36] Diffuse and cored |
From 12– 14M[21, 22] |
AβpE3: very minor species, in SUB at 14M[21, 45, 84] pAβ: NE |
None | 4M: ↓ LTP[41] Unaltered SYN and MAP2[38, 41] 4M: ↓ Dendritic spine density[41 ] |
None[38, 41] | Plaque associated astro- and microgliosis from 12M[4, 20, 38] |
MWM: 5–6M[1, 50] FC: 5M[16] |
Soluble Aβo: highest levels at 3M, decreased at 15M[56] |
| APP23[75] | APPK670N,M671L | 6M: rare plaques in Cx and SUB[75] 12M: Cx, HIP, THAL, AMYG[76] Majority fibrillar |
From 12M[76] |
AβpE3: from 11M, very minor species[2, 70] pAβ: from 11M[2] |
None[75] | 24M: Unaltered SYN[8] 24M: Unaltered LTP[63] |
14–18M: ↓ neurons in CA1, no change in Cx[9] |
Fibrillar plaque associated astro- and microgliosis[74 , 75] General astrocytosis[8, 76] |
MWM: 3M[80] | Aβo: at 5M[62] |
| J20[55] | APPK670N,M671L APPV717F |
5–7M: diffuse plaques in HIP 10M: plaques HIP, Cx[55] |
From 11M[77] |
AβpE3: from 8M[21] pAβ: NE |
None | 3–4M: ↓ PSD95[35] 3–6M: ↓ LTP[66] 5M: ↓ SYN[3] |
3M: ↓ neurons in CA1, no change in CA3[83] |
General astrogliosis in HIP from 3M and microgliosis in HIP from 6M[83] |
MWM: 3–4M[11] FC: 3M[69] |
1M: punctate Aβ in HIP Aβo: at 9M[83] |
| APP/PS1 [61] |
APPK670N,M671L PSEN1L166P |
1.5M: Cx 3M: Cx, HIP 3–5M: THAL, Striatum, Brain stem Majority fibrillar[61 ] |
8M: rare, large pial vessels[61] |
NE | None | 3–4M: ↓ plaque- associated dendritic spines[6] 8M: ↓ LTP[26] 4M: unaltered SYN[18] |
17M: ↓ neurons in DG, no change in Cx, CA1[64] |
Plaque associated astro- and microgliosis[61] |
MWM: 7M[73] RAM: 8M[61] |
iAβ: none[61] Aβo: NE |
| APP/PS1 [33] |
APPK670N,M671L PSEN1M146L |
6M: Cx, HIP, all fibrillar 12M: THAL, Striatum, brain stem, all diffuse[28 ] |
18M: pial surface and leptome ninges of CB[21] |
AβpE3: in focal plaques at 18M[21] pAβ: NE |
None | 3–4M: ↓ LTP[79] SYN: NE |
22M: ↓ neurons in CA1[65] |
Plaque- associated astrogliosis at 6M [28, 33] Plaque associated microgliosis at 9–12M[28] |
MWM: 3– 6M[79], but inconsistent[34] FC: 5M[16] Y-maze: 3M[33] |
Aβo: synaptic and plaque- associated [68] |
| APPswe/ PS1dE9 [42] |
APPK670N,M671L PSEN1ΔE9 |
4M: HIP and Cx[25] |
6M: leptome ninges[25] |
AβpE3: from 14M, in plaques and CAA[21] pAβ: punctate from 2M[47] |
None | 8M: ↓ LTP[53] 24M: ↓ SYN[37] |
24M: ↓ neurons in HIP[37] |
12–14M: general and plaque associated astro-and microgliosis[43] |
MWM: 7M[17] FC: 6M[13] |
Aβo: 8M[82] |
| TgSwDI [14] |
APPK670N,M671L APPE693Q APPD694N |
3M: SUB, HIP, Cx 6M: OB, THAL 12M: throughou t brain All diffuse, around vessels[14 , 86] |
3M: SUB, thalamus [86] 12M: meninge s[54] |
AβpE3: in CAA in SUB, thalamus, CA1 at 6M; in Cx at 12M[21] pAβ: NE |
None | NE | 12M: ↓ cholinergic neurons[19] |
6M: CAA- associated astro- and microgliosis[54] |
BM: 3M[85] | Aβo: from 3M[19] |
| APP E693Δ- Tg[78] |
APPE693Δ | None[78] | None[78] | NE | None[78] | 8M: ↓ SYN in HIP 8M: ↓ LTP[78] |
24M: ↓ neurons in CA3[78] |
Microgliosis at 12M Astrogliosis at 18M[78] |
MWM: 8M[78] | iAβ: 8M in HIP, Cx Aβo: 8M[78] |
| APP knock- inNL-G-F[67] |
APPK670N,M671L APPE693G APPI716F |
2M: Cx 4M: HIP, subcortica l[67] |
NE | NE | None[67] | ↓ plaque associated SYN and PSD95[67] |
NE | General and plaque associated astro- and microgliosis from 2– 6M[67] |
Y-maze: 6M[67] | Aβo: NE |
| 3xTg[58] | APPK670N,M671L PSEN1M146V MAPTP301L |
6–12M: Cx, HIP[58] Diffuse and compact[58] |
18M[71] | Aβ5-x from 18M in HIP in plaques[29] AβpE3: minimal at 14– 25M[21] pAβ: NE |
12M: First in CA1, later in Cx[58] |
6M: ↓ LTP[58] 8M: ↓ SYN in CA3, DG and Cx[7] 8M: ↓ MAP2 in CA1, DG, Cx[7] |
12M: ↓ neurons in Cx[81] |
General astro- and microgliosis in CA1 at 7M[10] |
MWM: 6M[5] FC: 6M[5] |
iAβ: 3–4M in Cx and 6M in CA1[58] Aβo: 6M[59] |
| 5xFAD[57] | APPK670N,M671L APPV717I APPV716V PSEN1M146L PSEN1L286V |
2M: SUB, Cx With age: HIP, OB, THAL, brain stem, none in CB[57] |
None at 3M or 6M[86] |
Aβ5-x from 12M in plaques[29] AβpE3: from 3–4M in plaques in Cx and HIP[21, 44] pAβ: |
None[57] | 4M: ↓ SYN[57] 6M: ↓ LTP[46] and PSD95 9M: ↓ syntaxin and PSD95[57] |
9M: focal ↓ neurons in SUB and Cx[57] |
General and plaque associated astro- and microgliosis from 2M[57] |
Y-maze: 4– 5M[57] BM: 6M[86] FC: 5–6M[46, 60] |
iAβ: 1.5M in SUB and Cx[57],↓ with age[44] Aβo: 3M[86] |
| Rats | ||||||||||
| McGill-R- Thy1- APP[49] |
APPK670N,M671L APPV717F |
6–9M: SUB, ECx 13M: HIP, Cx 20M: throughou t brain[32, 49] Diffuse and compact[32] |
None | NE | NE | NE | 12M: none[23] 18M: ↓ neurons in SUB[32] |
Plaque- associated microgliosis[49] 6M: General Astrogliosis[30] |
MWM: 3M[23, 49] FC: 3M[40] Y-maze: 6M[23] |
Plaque associated dystrophic neurites[49] iAβ: 1 week in HIP and Cx[49] Aβo: HIP and Cx[49] |
| TgF344- AD[12] |
APPK670N,M671L PSEN1ΔE9 |
6M: none 16M: HIP, Cx, striatum, CB Diffuse and compact[12] |
16M: HIP and Cx, striatum, CB[12] |
NE | Present at 16M[12] |
NE | 16M: ↓ neurons in Cx and HIP[12] |
General and plaque associated astro- and microgliosis from 6M[12] |
BM: 15M[12] | iAβ: 16M Aβo: 6M in Cx and HIP[12] |
| PSAPP[51] | APPK670N,M671L APPV717F PSEN1M146V |
13M: HIP, Cx, OB, THAL, hypothala mus, none in CB or brain stem Majority diffuse[51 ] |
None[51] | NE | None[51] | 7M: ↓ LTP 22M: unaltered SYN and PSD95[51] |
None | General astrogliosis in HIP and WM plaque associated astro- and microgliosis[51] |
MWM: 7M[51] | Aβo: NE |
HIP: hippocampus; Cx: cortex; CB: cerebellum; ECx: entorhinal cortex; WM: white matter; THAL: thalamus; AβpE3: pyroglutamate Aβ; pAβ: Aβ phosphorylated at serine 8; SYN: synaptophysin; MWM: Morris water maze; FC: Contextual fear conditioning; BM: Barnes maze; NE: not examined; iAβ: intraneuronal Aβ; Aβo: Aβ oligomers
Transgenic mice expressing human APP and PSEN1 with FAD mutations
The initial transgenic mouse models developed expressed APP with an individual FAD mutation. The first example of such models was the PDAPP mouse, which expressed human APP with the Indiana mutation (APPV717F) driven by the PDGF-β promoter, which caused dramatic over-expression (>10-fold) of APP[42]. This resulted in pathology associated with human AD including plaque formation in the cortex and hippocampus, CAA, gliosis, synaptic impairment and cognitive impairment (Table 1). The generation of the Tg2576 mouse model closely followed. Tg2576 mice expressed human APP with the double Swedish mutation (APPK670N/M671L) driven by the PrP promoter, which also resulted in significant over-expression of APP (>5-fold) [62]. Tg2576 mice developed plaques in the frontal, temporal and entorhinal cortices, hippocampus and cerebellum. In addition, CAA, synaptic impairment, gliosis and memory impairment was also present (Table 1). APP23 mice also express APPK670N/M671L; these mice contrast with Tg2576 mice through expression of the APP751 isoform driven by the Thy1 promoter (in comparison to the APP695 isoform driven by the PrP promoter expressed in Tg2576 mice)[151]. APP23 mice have more pronounced CAA, immediately form compact plaques in comparison to the predominantly diffuse plaques found in Tg2576 mice, and have localized neurodegeneration that is not seen in the Tg2576 mice (Table 1;[152]). These differences are despite similar expression levels of the APP transgene, showing that the promoter and APP isoform can greatly influence the type and time-course of AD associated neuropathology in transgenic models.
It was then discovered that expressing multiple FAD associated mutations at once resulted in transgenic mice with more severe pathology that developed at a younger age. This was observed in mice expressing multiple APP FAD mutations, such as in the J20 mouse that expressed both the Swedish and Indiana mutations[100], or more commonly, if APP and PSEN1 FAD mutations were expressed together (referred to as APP/PS1 transgenic mice). Various APP/PS1 transgenic mouse models have been developed and are commonly used in AD research. The specific phenotype of each model varies and depends on the specific FAD mutations and the promoter used (most common models are detailed in Table 1). For example, expression of APPK670N/M671L and PS1L166P results in very early plaque formation beginning at approximately 6 weeks[118], while expression of APPK670N/M671L and PS1M146L results in later plaque formation at approximately 6 months[58]. The most extreme APP/PS1 mouse model that is widely used is the 5xFAD model; these mice express the Swedish (APPK670N/M671L), London (APPV717I) and Florida (APPI716V) APP mutations and the PS1M146L and PS1L286V mutations [107]. The expression of five FAD mutations results in very early intraneuronal Aβ accumulation at 6 weeks, followed closely by plaque formation at 2 months.
Overall, the general features of transgenic mice expressing human APP, with or without human PSEN1, are robust plaque formation, particularly in brain regions typically rich in plaques in AD such as the cortex and hippocampus. All have plaque associated gliosis, similar to that in AD, and the majority have localized pathology associated with synaptic impairment such as decreased long-term potentiation and decreased levels of synaptic markers such as synaptophysin. They all have evidence of cognitive impairment, particularly in spatial memory tasks. However, it is important to note that the timing of cognitive impairment develops much earlier than in AD; typically coinciding with the onset of plaque development in transgenic mice in comparison to decades after plaque development in humans. One major limitation of these transgenic mouse models is the lack of the widespread neurodegeneration and regional brain atrophy that occurs in AD. While there is evidence for minor neurodegeneration in most of these mouse models, it only occurred in very old animals and was localized to very specific brain regions. The other major limitation of these mouse models was that while some showed evidence of localized hyperphosphylated tau that may represent “pretangles”[107, 118, 151, 159], none developed neurofibrillary tangles.
Transgenic mice expressing tau
Wild-type mouse tau does not develop neurofibrillary tangles. This is likely due to the sequence differences between mouse and human tau (share only 88% sequence homology) and the fact that adult mice only express 4R isoforms, not a mixture of 3R and 4R isoforms that are present in humans. Importantly, expression of all 6 isoforms of human tau only results in tangle formation in mice lacking endogenous tau, showing that endogenous mouse tau inhibits the aggregation of human tau [2]. In contrast, NFTs readily form in transgenic mice that express human tau containing mutations associated with FTLD; the most commonly used models being those that express 4R tau with P301L or P301S mutations[49, 91, 92, 135, 177]. These mice develop NFTs, neurodegeneration, atrophy and motor deficits. The necessity of these mutations for NFT development is an obvious limitation of these transgenic mouse models, as these mutations are not associated with AD in humans and the development of mutated tau may influence its toxicity or interaction with Aβ in a way that is not representative of what occurs in AD. Furthermore, over-expression of mutated tau results in significant motor deficits that are not seen in AD and interfere with cognitive testing.
Transgenic mice with both plaques and tangles
A limited number of studies have reported the development of animal models that display both plaques and tangles [10, 51, 91, 109, 121]. These models rely on concurrent expression of mutated forms of APP, MAPT and occasionally also PSEN1 or PSEN2 to drive plaque and tangle formation in the same model. However, the consistent and abundant expression of both plaques and tangles has proven troublesome, and development of both plaques and tangles is typically not observed until old age in these models. Of all of the models reported, only the 3xTg mouse model has been widely used in AD studies and is considered the most complete transgenic mouse model of AD pathology available[108]. 3xTg mice first develop intraneuronal Aβ at 3–4 months, followed by plaque development at approximately 6 months in the cortex and hippocampus. NFTs form at approximately 12 months, initially in CA1 and then in the cortex; however, they are much less extensive compared to AD tissue (see Figure 2). Mice also have minor, localized neurodegeneration, evidence of synaptic impairment and cognitive deficits from 6 months (Table 1). However, 3xTg mice are still limited by the production of mutated Aβ and tau that is not representative of that in sAD and is highly over-expressed in a non-physiological manner. Furthermore, widespread presence of plaques and tangles are typically not observed until old age in these mice and even then the pathology is less then typically seen in AD (see Figure 2).
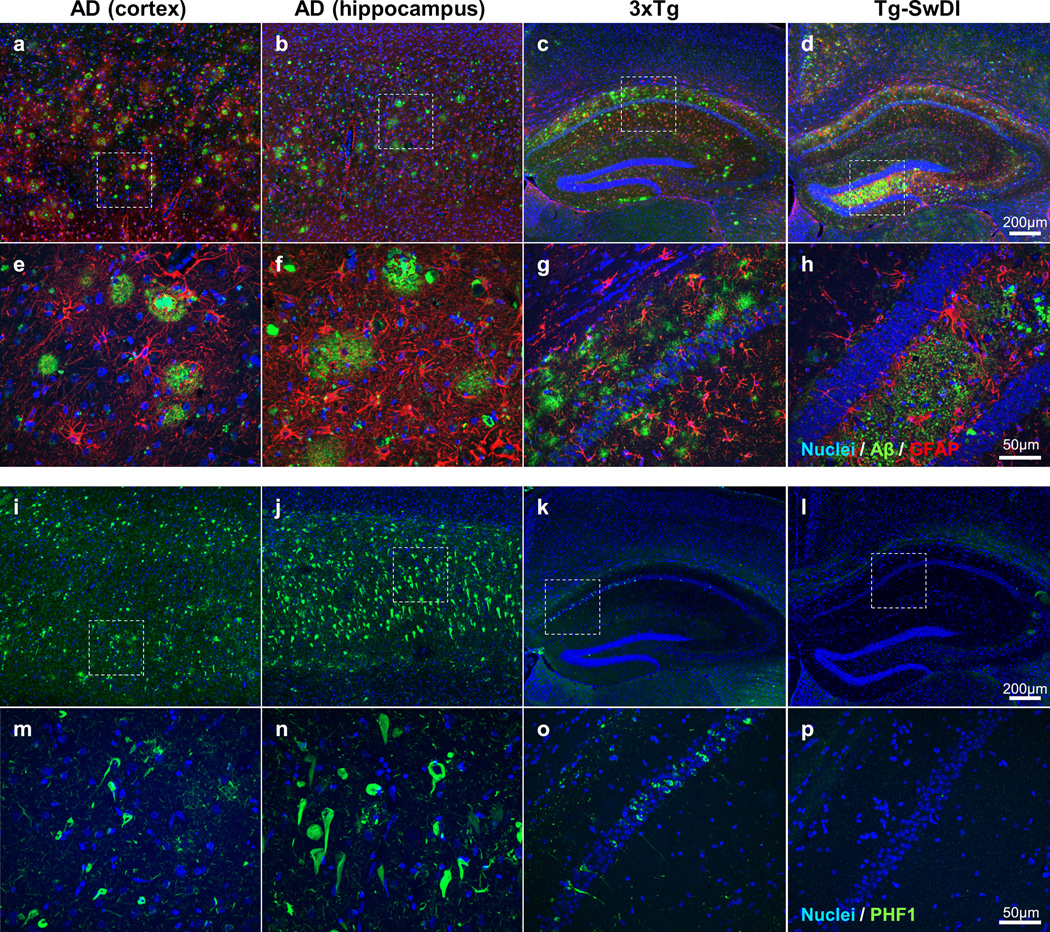
Figure 2. Neuropathological differences between humans with AD and transgenic mouse models of AD.
Fluorescent immunohistochemistry was performed on formalin-fixed paraffin embedded brain sections using the same conditions for human and mouse tissue to highlight species differences. Immunostaining in the human AD cortex (a, e, I, m), human AD hippocampus (b, f, j, n), 3xTg mouse (28 months old) hippocampus and cortex (c, g, k, o), and Tg-SwDI mouse(16 months old) hippocampus and cortex (d, h, l, p) is shown. a–h: shows immunohistochemistry for Aβ (green; labelled using a combination of 4G8 and 6E10 antibodies) and astrocytes (red; labelled using GFAP). i–p: shows immunohistochemistry for phosphorylated tau (green; labelled using PHF1). All sections were counterstained with Hoechst to label nuclei. a–d and i–l show the differences in distribution of Aβ, astrocytes and phosphorylated tau at low magnification throughout the hippocampus and cortex (scale bar for all = 200 µm). e–h and m–p show the differences in morphology of plaques and neurofibrillary tangles at higher magnification (scale bar for all = 50 µm) of the areas outlined by a box in a–d and i–l. The most obvious species differences include the preferential presence of plaques in the cortex in humans (a) in comparison to 3xTg (c) or TgSwDI mice (d), the presence of both extensive numbers of cored and diffuse plaques in humans (e), but not in mice (g, h), and the greater density of neurofibrillary tangles in humans (i, j) in comparison to 3xTg mice (k). As expected, there were no neurofibrillary tangles present in Tg-SwDI mice (l, p).
Unique transgenic mouse models useful for AD research
A number of transgenic mouse models have been developed that are particularly good at replicating a specific pathological feature of AD. For example, the Tg-SwDI transgenic mouse model is a particularly good model of CAA [29]. This model expresses Swedish (APPK670N/M671L), Dutch (APPE693Q) and Iowa (APPD694N) APP FAD mutations. The Dutch and Iowa mutations are associated with hereditary cerebral hemorrhage with amyloidosis (HCHWA), where there is extensive CAA with more limited plaque pathology[73]. Tg-SwDI mice develop robust accumulation of fibrillar vascular Aβ and less prominent diffuse parenchymal plaques, starting at 3 months of age [29](see Figure 2). CAA is mainly present in capillaries, in contrast to the prominent arteriolar CAA in AD. Tg-SwDI mice also have localized neurodegeneration of cholinergic neurons and cognitive impairment (Table 1). Testing the ability of therapeutic approaches to reduce vascular amyloid deposits without complication is of particular importance. In the on-going passive immunization AD clinical trials a major complication has been vasogenic edema (or encephalitis) with/or without hemorrhage (termed amyloid-related imaging abnormalities with edema (ARIA-E) or with hemorrhage (ARIA-H) [116, 132, 133, 171, 172]. ARIAs are also a major issue in the recently reported aducanumab trial (affecting 55% of patients in the high-dose and APOEε4 carriers arm, associated with a 35% patient drop-out rate due to the development of this side effect)[136, 145, 171]. Hence developing a therapy that is effective against CAA without inducing vasogenic edema/encephalitis is of critical importance [116, 132, 133, 136, 144, 172]. Hence the preclinical testing of therapeutic approaches in models with extensive CAA (which virtually all individuals with AD and about a third of aged cognitively normal individuals have) [68, 179] and showing that it does not induce microhemorrhages is of importance. The APP E693Δ-Tg model expresses the Osaka (APPE693Δ) mutation, which results in a unique phenotype of significantly increased expression of Aβ oligomers, synaptic impairment and cognitive impairments from 8 months of age, but no plaque or tau pathology formation [159]. This allows the opportunity to examine the pathological effects of Aβ oligomers and/or the effect of therapeutics specifically on Aβ oligomers, which are thought to be the most toxic Aβ species[79]. The major benefit of these two mouse models is that they replicate specific pathological features of AD more robustly than other models. The limitation of these models is that they do not replicate all features of AD and therefore cannot be used as a complete model of AD.
Knock-in mouse models
The most recently developed transgenic mouse models that replicate AD associated pathology are the knock-in mice. These mice are considered to be a much more physiological model of AD as they are designed to avoid the confounding effects of APP over-expression present in all other transgenic mouse models by humanizing mouse Aβ and knocking in specific APP FAD mutations. As a result, knock-in mice have the same expression of APP and AICD as wild-type mice and APP expression occurs in a physiological manner in the correct brain regions and cell types. Similar to other transgenic mouse models, the timing of pathology depends on the mutations expressed. For example, knock-in of the Swedish, London and Dutch mutations only results in the development of plaques if bred onto a PS1M146V knock-in background [93]. In contrast, knock-in of Swedish and Iberian mutations results in plaque development beginning at 6 months, and gliosis, synaptic alterations and memory impairment from 18 months [129]. Additional knock-in of the Arctic mutation into these mice results in more rapid pathology development including plaque development beginning at 2 months that is more widespread throughout the brain and memory impairment from 6 months [129]. While these transgenic mice represent a significant step forward in the generation of more physiological transgenic models, it still must be acknowledged that they are models of FAD and not sAD and that pathology only develops after knock-in of a combination of specific multiple FAD mutations.
Transgenic rat models
A smaller number of transgenic rat models of AD have also been developed. Transgenic rats have a number of potential advantages over transgenic mice; they are more similar to humans in their physiological, morphological and genetic characteristics, their larger brain makes CSF collection, electrophysiology and imaging easier and they have a richer behavioral phenotype, making more complex behavioral testing possible [32]. Three transgenic rat models have been well characterized in the literature [24, 90, 95] and the specific AD associated neuropathological features of each model are outlined in Table 1. Transgenic rats have a similar phenotype and limitations as transgenic mice; expression of multiple FAD mutations accelerates the development of pathology. The distribution, extent and localization of APP expression is dependent on the promoter used. All models have robust amyloid plaque expression (albeit at lower levels than in transgenic mice) and interestingly, TgF344-AD rats have NFTs [24], despite expression of only endogenous rat tau, not human tau. This is likely due to the greater similarities between rat tau and human tau, in that there are also 6 isoforms of endogenous rat tau. All rat models have some degree of cognitive impairment; however, the degree of impairment has only been extensively characterized in the McGill-R-Thy1-APP rats [90]. In sum, transgenic rats are potentially useful in AD research and offer specific advantages over transgenic mice; however the comparatively minimal use of these models means that greater characterization needs to be done to properly determine their suitability as models of AD.
Physiological models
Two of the major limitations of transgenic rodent models is that they model FAD and not sAD and that the pathology development in these models is typically non-physiological. Finding a naturally occurring model of AD is appealing because they would more accurately represent changes that occur in sAD. Multiple species naturally develop neuropathological features similar to those seen in AD brain, and their potential as naturally occurring models of sAD has been examined. The most commonly used species that display neuropathology similar to AD are discussed below.
Non-human primates
The species with the most well characterized AD neuropathological features are non-human primates. The advantages of using non-human primates to model AD include their biological proximity to humans, behavioral complexity, large brains that are favorable for imaging studies or CSF collection and a natural accumulation of Aβ that has 100% sequence homology with human Aβ[16, 19, 57]. There have been relatively few AD studies that have characterized AD pathology in great apes (chimpanzees, gorillas and orangutans) because of their long lifespan and ethical concerns of using great apes for research studies. Great apes accumulate Aβ in the brain, resulting in the development of amyloid plaques and CAA in aged animals [43, 44, 78, 112, 113, 124]. Plaques are predominantly diffuse and less abundant than that found in human AD. Typically, great apes have more prevalent CAA, which is more likely to contain fibrillar Aβ than plaques. Despite very high sequence homology between great ape and human tau (100% and 99.5% sequence homology between human tau and chimpanzee or gorilla tau respectively), tauopathy is rare. Focal neurons and glia containing phosphorylated tau have been observed in gorillas, but NFTs and tau positive dystrophic neurites are not present [112]. Great apes are capable of forming NFTs [124], but this is a rare event that has only been observed in one chimpanzee studied. It is likely that the presence of additional AD associated risk factors (stroke, high cholesterol and obesity) contributed to NFT formation in this case. Also, memory impairments appear to be mild; appearing more similar to typical age-related memory decline, rather than the extensive cognitive decline seen in AD[57].
Many more studies have been done using old world monkeys (e.g. rhesus monkeys, cynomolgus monkeys, baboons and vervets). The majority of studies have used rhesus monkeys. Again, there is 100% sequence homology between human and rhesus monkey Aβ. Aβ levels accumulate with age, reaching similar levels in the cortex to that observed in human AD, and there is often more Aβ42 than Aβ40 [126]. Plaques are typically found in rhesus monkeys that are older than 25 years and they have a similar distribution that that observed in humans; more being present in the cortex than the hippocampus [54, 56, 96, 134, 146, 150, 164, 165]. In contrast to great apes, parenchymal plaques are more prevalent than CAA in rhesus monkeys, with CAA present in approximately one third of aged rhesus monkeys [164, 165]. The majority of plaques are diffuse; only approximately 20% contained fibrillar Aβ [134, 146]. Minor neuronal loss is observed immediately around compact plaques; however, there is no evidence of widespread neuronal loss, even in brain regions with a high plaque load [146]. It is noteworthy that there is considerable variation in plaque pathology between animals. The two largest studies examining the presence of plaques in rhesus monkeys found that approximately 40% of aged animals (25–31 years) did not have any evidence of plaques or CAA after death of natural causes [164, 165]. However, this may be due to death prior to plaque formation as 100% of the smaller number of very old animals studied (33–39 years) did have plaques. Rhesus monkeys do not have tauopathy, despite a high sequence homology between human and rhesus monkey tau. Interestingly, aged baboons show heavy, but highly localized tauopathy in the hippocampus, which increases with age and is observed in 90% of animals over the age of 26 years [142]. NFTs are not observed in other brain regions, including regions containing plaques and CAA such as the cortex. Aβ deposition is considered to be mild-to-moderate in baboons and there is no apparent relationship between plaques and tangles. A limited number of studies have also been done examining AD associated neuropathology in vervets. Vervets live to approximately 30 years in captivity and have evidence of Aβ deposition, gliosis and neuronal dystrophy with age [72, 88, 89]. Amyloid deposition is first observed at approximately 15 years, and appears first in the vasculature prior to parenchymal plaques. Both diffuse and compact plaques are present and AβN3pE is present in newly developed CAA and plaques at a ratio of approximately 1:1 with general Aβ [39]. Neuritic plaques can be observed, some with phosphorylated tau immunoreactive dystrophic neurites [88]. No NFTs are present. Similar to other non-human primates, there is considerable inter-animal variation in the presence of pathology in vervets.
New world monkeys also naturally develop neuropathology similar to that in AD, the most well studied being squirrel monkeys. Squirrel monkeys have extensive Aβ accumulation after 12 years of age, primarily in the form of CAA in arterioles and capillaries [22, 36, 167, 168]. The prominence of CAA in squirrel monkeys makes this model particularly appropriate for the evaluation of whether a therapeutic approach might be associated with ARIA as a complication in patients [140, 141]. Plaques are also present, which can be either diffuse or compact and are typically smaller than plaques in human AD. Plaques and capillary CAA contain both Aβ40 and Aβ42, while arteriolar CAA primarily contains Aβ40. Aβ deposition is mostly observed in the cortex and amygdala with little deposition in the hippocampus. A recent mass spectrometry study showed that squirrel monkeys have all major Aβ species that are present in the human brain (including Aβ1–40, Aβ1–42, Aβ1–34, Aβ4–40, Aβ4–42, AβN3pE and oxidized Aβ)[125]. While squirrel monkey Aβ also formed SDS stable dimers and trimers similar to humans, these oligomers likely have a different tertiary or quaternary structure from human species [125]. Minimal phosphorylated tau is observed in occasional neurons, but no NFTs are present, even in aged animals [36].
AD associated neuropathology has also been characterized in grey mouse lemurs, which have also been used AD preclinical trials [70, 161]. The maximum lifespan of these prosimians is 18 years in captivity. Plaques have been observed in grey mouse lemurs that are as young as 8 years old and both diffuse and compact plaques can be observed, predominantly in the cortex[11]. Plaques are more commonly observed than CAA and plaques predominantly consist of Aβ42, while CAA consists of both Aβ40 and Aβ42 [98]. Grey mouse lemurs have accumulation of intraneuronal phosphorylated tau, which increases with age, however this is predominantly observed in the cortex and not in the hippocampus (unlike in AD) [12, 47, 83]. Cortical atrophy is observed a subpopulation of animals aged over 3 years[31, 83], which correlates with age-associated cognitive decline[114].
In sum, non-human primates typically have age related Aβ pathology, but tauopathy is rare and/or very limited. Based on previous studies the rhesus monkey is the most practical non-human primate model to study AD because it is so well characterized and the squirrel monkey is the best available non-human primate model to study CAA.
Other physiological models
Other species naturally develop AD associated pathology with age, the most well characterized examples being dogs and the guinea pig relative Octodon degu. Aged dogs have the same Aβ sequence as humans and they develop plaques and CAA starting at 8–9 years of age[137, 143, 148]. Plaques first develop in the prefrontal cortex and later in the temporal and occipital cortices, following a similar, but not identical, pattern to humans. However, these plaques differ from those in human AD as they are primarily diffuse, and therefore may represent an earlier stage of plaque development. A limited number of compact plaques are evident in a small number of aged dogs. AβN3pE is present in a subpopulation of plaques. Other neuropathological features present in aged dogs include cortical atrophy, declined ratio of CSF Aβ42:40, increased Aβ oligomers, and presence of oxidative damage and mitochondrial dysfunction [16]. NFTs are typically not observed; however, pretangles and possible NFTs have been observed in a very limited number of aged, demented dogs [137, 148]. In addition, synaptosomes from demented dogs contain increased total and phosphorylated tau than non-demented dogs, suggesting that cognitive impairment in aged dogs may result from synaptic impairment [148]. A battery of canine-specific cognitive tests have been developed, which show that aged dogs can develop deficits in complex learning tasks, executive function, spatial learning and attention, and memory, and the extent of cognitive decline has been correlated with Aβ deposition in some, but not all, studies[30]. The combination of measurable cognitive decline, AD associated neuropathology, and 3–4 year window of pathology prior to death have resulted in aged dogs being used in numerous preclinical therapeutic studies [30]. However, limitations include lack of NFTs, lack of compact plaques, long lifespan and the lack of consistent pathology in all animals.
Octodon degu have a high sequence homology with human Aβ (has a single amino acid substitution). Some studies have found that Octodon degu have intracellular and extracellular accumulation of Aβ, plaques at old ages, intracellular tau accumulation, astrocytosis, synaptic changes and memory impairment that correlates with increased levels of oligomers (reviewed in [16, 131]). However this pathology appears to be inconsistent as other studies do report any AD associated pathology in aged animals [149].
In sum, physiological models represent the best available models of sAD. However, there are still scientific and practical limitations that prevent widespread use of these models. For example, the best models have long lifespans and pathology can be variable between individual animals, meaning that experiments can be expensive and time-consuming and selection of animals for preclinical testing may be difficult. Furthermore, cognitive testing is less standardized and can be difficult to do. Finally, despite greater sequence homology with human tau, very few physiological models have evidence of tauopathy and none have widespread presence of NFTs similar to that in AD.
Cell culture models
The use of experimental models derived from human tissue bypasses concerns associated with confounding effects due to species differences. However, one of the major limitations associated with generating representative adult human cell-based experimental models is the lack of available, quality post-mortem tissue. The development of induced pluripotent stem cells (iPSCs) addresses this limitation [153]. iPSCs have now been generated from multiple human donor cell types including fibroblasts, blood cells and urine derived epithelial cells. Multiple groups have characterized iPSC lines from donor cells from FAD and sAD patients, which show increased production of Aβ, particularly Aβ42, and tau hyperphosphorylation in comparison to iPSCs derived from age-matched non-demented controls [66, 82, 101, 175]. Some iPSC lines also have evidence of additional AD-associated pathology such as increased activation of GSK3β [66], increased number of large endosomes [66], and accumulation of intraneuronal Aβ oligomers [82].
The limitations associated with using human cell-culture models include the lack of standardized protocols used to generate and maintain these cell lines, the potential that epigenetic modifications present in donor cells may be maintained after reprogramming, and the phenotype variation present in individual iPSC lines due to inter-patient variation. Another complication is that these cell lines may have to be aged in order for an AD-associated phenotype to develop and this can be technically difficult to achieve when using differentiated neurons. Some of these limitations will likely be overcome as these cell lines are more thoroughly characterized in future studies.
An additional concern is that cell culture models do not accurately represent the complex environment that is found in the brain, which includes complex interactions between neurons and the presence other cell types besides neurons (e.g. glia) that are likely to have a very important role in the development of AD. This concern is being partly addressed through the development of 3D cell culture models. These can either be produced through the use of a scaffold (such as hydrogel or Matrigel), which allows more physiological interactions between neurons and glia in 3 dimensions, or through scaffold-free models where cells develop as a 3D organoid [23, 119]. It was recently shown that development of a 3D culture of human neural stem cells transfected with APPK670N/M671L/V717I and PS1ΔE9 in Matrigel scaffolding resulted in the extracellular aggregation of Aβ into plaques and the intracellular aggregation of tau in dystrophic neurites and the cell soma [23, 77]. This is the first time that plaques and tangles been replicated in vitro.
It is important to note that the majority of these cell culture models have been generated from FAD donor cells and it will be necessary to increase the number of sAD lines available going forward to compare the different phenotypes between FAD and sAD. This is important because previous studies have shown that specific FAD mutations are associated with specific iPSC phenotypes and therefore sAD iPSC lines are likely to differ further.
Drosophila, C. Elegans and zebrafish as AD models
Invertebrate animal models (such as Drosophila, C. Elegans) and lower order animal models (such as zebrafish) have also been used in some AD research studies. The use of such animals in AD research is limited by the lack of genetic homology with humans due to the much more simplified genetic make-up in these lower order animals. Furthermore, their nervous system and behavior lack the complexity seen in humans, making comparisons with human disease very difficult. Drosophila, C. Elegans and zebrafish have been confirmed to express orthologues of some of the genes that are essential in AD pathology (such as APP, PSEN1, MAPT and BACE1); however, the presence of these orthologues and the genetic similarity to human genes varies between species. Overall, the sequence homology in genes of interest in AD is minimal between invertebrates and humans and these invertebrate orthologues often lack regions of these genes that are important in AD pathophysiology. The most notable example is the lack of Aβ in Drosophila and C. Elegans [1, 38]. Therefore, invertebrates cannot be considered to model AD without genetic manipulation to express human transgenes of APP, Aβ and/or tau. One of the main advantages of using invertebrates is the ease of genetic manipulation and multiple transgenic lines expressing human APP, Aβ and tau have been developed for each species [1, 38, 111]. Other general advantages of using invertebrate models include easy handling, low cost and short life span of animals. Given these advantages, several groups have used transgenic invertebrate models in high-throughput genetic or drug screens. For example, this approach has proven successful in identifying modifiers of tau toxicity using Drosophila as a model [53]. However, it must be noted that results from such studies must still be interpreted with caution and confirmed using more relevant animal models because of the vast differences between humans and invertebrates, most importantly the lack of conserved functional pathways and the lack of important interactors/mediators involved in the downstream response of expressed human genes.
Factors to consider when choosing the best model
There are many available models of AD pathology, each with their own benefits and limitations. It is exceptionally important to acknowledge that none of the available models replicate all features of human AD, and therefore cannot be considered to be representative models of AD as a complete disease. However, the use of the animal models that are currently available can provide the means to answer vital questions about AD pathophysiology that cannot be answered using humans as long as one has very good knowledge of the selected model and its intrinsic limitations to ensure the interpretation of experimental results can be translated to human AD. What we believe to be the most important factors to consider when using experimental models in AD are discussed below.
Very few models have both plaques and tangles, particularly ones that develop physiologically. The presence of both plaques and tangles is required for diagnosis of AD and how the complex interaction between plaques and tangles affects the development of AD is still being determined. It is evident that crosstalk between Aβ and tau can significantly influence toxicity; increased Aβ production results in NFT formation in FAD and Down Syndrome, while there is also evidence to show that tau increases Aβ-associated toxicity (particularly synaptotoxcity), suggesting that the presence of both pathological features are important to replicate the toxicity that occurs in human AD [106]. Therefore, it is particularly important to determine the effect of a new therapeutic on both plaques and tangles, ideally in a model that contains both so that the pathological effect of the crosstalk between the two can be addressed.
It is difficult to interpret downstream pathological changes in animal models that have non-physiological expression of Aβ and tau. It must be considered that downstream pathology may be artifacts that result from overexpression of APP, PS1 or tau, or from other APP cleavage products besides Aβ (eg N-APP, APP C-terminal fragments, AICD). These additional APP cleavage products are also capable of causing toxicity independent of Aβ [45, 105]. Furthermore, APP overexpression was recently suggested to be the underlying cause of two prominent AD phenotypes, rather than a downstream response to Aβ as was initially suggested based on studies using transgenic mice, calling into question whether this may also be the case for other interpreted examples of downstream AD pathology observed in transgenic mouse models [130]. The issue of non-physiological over-expression of APP or tau can be addressed by using knock-in mouse models, which have physiological expression of humanized endogenous mouse proteins. The additional toxic effects of APP cleavage products besides Aβ is more technically challenging to address, however the use of viral vectors to induce expression of specific isoforms of Aβ in rodent brains have shown promise and could complement the use of transgenic animal models[33, 87, 120].
It must also be considered that endogenous rodent proteins and/or protein pathways may react differently in response to non-physiological expression of specific human proteins and as such, downstream effects cannot be assumed to also occur in humans. The most obvious example comes from results from animal models solely expressing human PS1 with FAD mutations. Despite some mutations in PS1 causing the earliest onset of FAD in humans, sole expression of human PS1 with FAD mutations doesn’t result development of plaques in transgenic mice [138, 154], showing that the response of endogenous mouse proteins to human PS1 is different from that in humans. Furthermore, it is likely that the lack of NFT development in mouse models that overexpress Aβ is due to the endogenous differences between mouse and human tau. An elegant study supporting this hypothesis showed that crossing the APP E693Δ-Tg model with wild-type human tau mice resulted in robust formation of NFTs, which never developed in mice with endogenous mouse tau [163]. These are just two examples of instances where the downstream effects of the human protein expressed in transgenic mice differs from what would occur in humans because of endogenous protein differences, supporting the concept that downstream pathological effects (or lack thereof) should be interpreted carefully.
Endogenous species differences between rodents and humans affect the cleavage and biochemistry of human Aβ in transgenic rodents. For example, plaque cores from transgenic mice are much more soluble than those in human AD, which has been suggested to result from the lack of Aβ post-translational modifications in transgenic mice (such as N-terminal degradation, isomerization, racemization, pyrogluamyl formation and oxidation) [37, 71, 85]. This is an important factor to consider as this increased solubility could contribute to amyloid clearing drugs working much better in transgenic mice than humans. In addition, the mouse background strain can result in altered cleavage of the APP C-terminus. For example, transgenic mice expressing human APP on the C57BL6 background produce much less of the CT99 and CT83 fragments that are most prominent in humans [37, 64], which potentially complicates the translation of results from studies testing β- or γ-secretase targeting therapeutics in these transgenic mice to humans. Interestingly, species differences also appear to influence Aβ biochemistry and deposition in physiological models. Despite being biologically closest to humans, even non-human primates display important differences in Aβ biochemistry. Non-human primates have similar Aβ species in the brain as humans, including the presence of common post-translationally modified species, however it has been suggested that Aβ may form different aggregates to humans that results in altered immunoreactivity to common Aβ antibodies (despite sequence homology) and prevents PIB binding[125, 126].
Transgenic animal models represent partial models of FAD and not sAD. Much more research in humans is necessary to determine the similarities and differences between FAD and sAD. Currently it is known that the distribution of Aβ and tau accumulation is different in FAD and sAD, with more present in subcortical regions in FAD [147]. There is also more grey matter atrophy in subcortical regions in FAD [20], and atypical cognitive symptoms are more likely to be present in FAD [147]. Furthermore, despite similarities between FAD and sAD, the underlying cause of the two subtypes of AD are very different; FAD directly resulting from Aβ over-expression and sAD likely resulting from multiple factors that contribute to poor clearance of Aβ from the brain. More studies are needed to further elucidate the differences between sAD and FAD in humans because it is possible that the lack of translation between preclinical studies and human studies is because of inherent differences between FAD and sAD, suggesting that potentially these therapeutics that worked very well in preclinical studies could be better translated in clinical trials of FAD patients and/or Down syndrome (DS) subjects (where there is overexpression of APP)[55]. If this is the case, then it will be essential to develop new models that are more representative of sAD, so that the effect of novel therapeutics in sAD can be tested more accurately.
Genetic studies have identified multiple loci that convey increased risk for sAD. It will be important for future studies to determine how these genetic risk factors contribute to AD associated pathology, and whether this is replicated in animal models of the disease. Studies examining the role of ApoE4, which is the strongest identified genetic risk factor linked to sAD, have suggested that this may be more complex than first anticipated in animal models due to species differences. Transgenic mouse studies confirmed that ApoE was necessary for the formation of fibrillar amyloid plaques and CAA [4, 5, 41, 61], however they also identified important differences between mouse and human ApoE. Expression of mouse ApoE resulted in greater plaque formation than expression of human ApoE, and mouse ApoE preferentially promoted the formation of parenchymal plaques, while human ApoE promoted the formation of CAA [59, 60, 94]. This is further complicated by the fact that expression of different isoforms of human ApoE in transgenic mice results in different levels of plaque and CAA burden with apoE4 expression enhancing amyloid deposition compared to apoE3 or apoE2 [3, 21, 40, 59, 86, 178]. Ultimately, this raises the concern that other human transgenes of interest (e.g. other loci identified in GWAS studies) may also have to be co-expressed in AD transgenic models in order to replicate the protein interactions that occur in AD. This is particularly important to consider when testing therapeutics that target these interactions. The latter has been critical in the development and preclinical testing of therapeutic approaches that target the interaction between apoE and Aβ [110, 128, 176].
The most prevalent symptom of AD in humans is cognitive impairment. While the majority of animal models show some degree of cognitive impairment, the type and the timing of this impairment must be carefully considered, particularly in preclinical studies. As mentioned above, cognitive impairment occurs at a different stage of pathology development in transgenic mouse and rat models in comparison to humans; occurring at or before the onset of plaque development in rodents and many decades after plaque development in humans. In contrast, initial studies show that more physiological knock-in mouse models develop cognitive impairment many months after plaque development [129], which is more similar to humans. This raises the question of whether the process that mediates cognitive impairment in transgenic animal models is the same as the one that mediates cognitive impairment in humans.
Conclusion
Careful examination of neuropathology and cognitive impairment in multiple species, including those closest to humans, shows that AD is a uniquely human disease. The very poor success rate of ~99.6% with AD targeting clinical trials can in part be explained by the premature translation of successful pathology reduction in transgenic mice to humans [6, 27, 139]. Therefore, the gold standard should be to perform research using human tissue whenever possible. The consistent lack of translation between animal models and human studies has resulted in the development of more human-centric approaches. Many of these approaches are still being developed and fully characterized, however they offer great potential. For example, initial drug screening and patient stratification for clinical trials could be performed using human cell culture models (such as iPSCs), disease pathogenesis could be better examined using ‘omics approaches that allow genome- or proteome-wide screening for altered networks during disease, and expanded development of neuroimaging approaches could provide essential information about disease progression in humans.
Animal models have the obvious advantage of providing the option to do preclinical testing in vivo, allowing the testing of general toxicity of new therapeutics and providing a system in which cognitive testing can be done. New knock-in mouse models are potentially more representative and physiological models of AD; however, they still need to be further validated in future studies. Non-human primates offer the unique advantages of greater genetic similarity to humans and a more physiological relevant development of pathology that better resembles that in found in sAD compared to transgenic models, but studies are limited by availability, costs, time until onset of phenotype and the inconsistent presence of pathology in all animals. New human cell culture models have the advantage of allowing high-throughput screening of novel therapeutics directly using human cells; however these models obviously cannot replace in vivo models for preclinical testing. Therefore, going forward it will be necessary to perform preclinical testing in multiple animal models that each exemplifies a unique aspect of AD pathology, until a more complete and physiological animal model of sAD is available to ensure greater translation of preclinical results to human clinical trials.
Supplementary Material
Acknowledgments
This manuscript was supported by NIH grants: NS073502 and AG08051. We thank Geoffrey Pires for his assistance with figure preparation.
References
- 1.Alexander AG, Marfil V, Li C. Use of Caenorhabditis elegans as a model to study Alzheimer's disease and other neurodegenerative diseases. Front Genet. 2014;5:279. doi: 10.3389/fgene.2014.00279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andorfer C, Kress Y, Espinoza M, de SR, Tucker KL, Barde YA, Duff K, Davies P. Hyperphosphorylation and aggregation of tau in mice expressing normal human tau isoforms. J Neurochem. 2003;86:582–590. doi: 10.1046/j.1471-4159.2003.01879.x. [DOI] [PubMed] [Google Scholar]
- 3.Bales KR, Liu F, Wu S, Lin S, Koger D, DeLong C, Hansen JC, Sullivan PM, Paul SM. Human APOE isoform-dependent effects on brain beta-amyloid levels in PDAPP transgenic mice. J Neurosci. 2009;29:6771–6779. doi: 10.1523/JNEUROSCI.0887-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bales KR, Verina T, Cummins DJ, Du Y, Dodel RC, Saura J, Fishman CE, DeLong CA, Piccardo P, Petegnief V, et al. Apolipoprotein E is essential for amyloid deposition in the APPV717F transgenic mouse model of Alzheimer's disease. PNAS. 1999;96:15233–15238. doi: 10.1073/pnas.96.26.15233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bales KR, Verina T, Dodel RC, Du YS, Altstiel L, Bender M, Hyslop P, Johnstone EM, Little SP, Cummins DJ, et al. Lack of apolipoprotein E dramatically reduces amyloid β-peptide deposition. Nature Gen. 1997;17:263–264. doi: 10.1038/ng1197-263. [DOI] [PubMed] [Google Scholar]
- 6.Banik A, Brown RE, Bamburg J, Lahiri DK, Khurana D, Friedland RP, Chen W, Ding Y, Mudher A, Padjen AL, et al. Translation of Pre-Clinical Studies into Successful Clinical Trials for Alzheimer's Disease: What are the Roadblocks and How Can They Be Overcome? J Alzheimers Dis. 2015;47:815–843. doi: 10.3233/JAD-150136. [DOI] [PubMed] [Google Scholar]
- 7.Bertram L, Tanzi RE. The genetics of Alzheimer's disease. Prog Mol Biol Transl Sci. 2012;107:79–100. doi: 10.1016/B978-0-12-385883-2.00008-4. [DOI] [PubMed] [Google Scholar]
- 8.Blennow K, Mattsson N, Scholl M, Hansson O, Zetterberg H. Amyloid biomarkers in Alzheimer's disease. Trends Pharmacol Sci. 2015;36:297–309. doi: 10.1016/j.tips.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 9.Blessed G, Tomlinson BE. The association between quantitative measures of dementia and senile change in the grey matter of elderly subjects. British Journal of Psychiatry. 1968;114:797–811. doi: 10.1192/bjp.114.512.797. [DOI] [PubMed] [Google Scholar]
- 10.Bolmont T, Clavaguera F, Meyer-Luehmann M, Herzig MC, Radde R, Staufenbiel M, Lewis J, Hutton M, Tolnay M, Jucker M. Induction of tau pathology by intracerebral infusion of amyloid-beta -containing brain extract and by amyloid-beta deposition in APP x Tau transgenic mice. Am J Pathol. 2007;171:2012–2020. doi: 10.2353/ajpath.2007.070403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bons N, Mestre N, Ritchie K, Petter A, Podlisny M, Selkoe D. Identification of amyloid beta protein in the brain of the small, short-lived lemurian primate Microcebus murinus. Neurobiol Aging. 1994;15:215–220. doi: 10.1016/0197-4580(94)90115-5. [DOI] [PubMed] [Google Scholar]
- 12.Bons N, Rieger F, Prudhomme D, Fisher A, Krause KH. Microcebus murinus: a useful primate model for human cerebral aging and Alzheimer's disease? Genes Brain Behav. 2006;5:120–130. doi: 10.1111/j.1601-183X.2005.00149.x. [DOI] [PubMed] [Google Scholar]
- 13.Bouleau S, Tricoire H. Drosophila models of Alzheimer's disease: advances, limits, and perspectives. J Alzheimers Dis. 2015;45:1015–1038. doi: 10.3233/JAD-142802. [DOI] [PubMed] [Google Scholar]
- 14.Boutajangout A, Wisniewski T. Tau-based therapeutic approaches for Alzheimer's Disease - a mini-review. Gerontology. 2014;60:381–385. doi: 10.1159/000358875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Braak H, Braak E. Neuropathological staging of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 16.Braidy N, Poljak A, Jayasena T, Mansour H, Inestrosa NC, Sachdev PS. Accelerating Alzheimer's research through 'natural' animal models. Curr Opin Psychiatry. 2015;28:155–164. doi: 10.1097/YCO.0000000000000137. [DOI] [PubMed] [Google Scholar]
- 17.Cacace R, Sleegers K, Van Broeckhoven C. Molecular genetics of early-onset Alzheimer's disease revisited. Alzheimers Dement. 2016;12:733–748. doi: 10.1016/j.jalz.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 18.Cairns NJ, Perrin RJ, Franklin EE, Carter D, Vincent B, Xie M, Bateman RJ, Benzinger T, Friedrichsen K, Brooks WS, et al. Neuropathologic assessment of participants in two multi-center longitudinal observational studies: the Alzheimer Disease Neuroimaging Initiative (ADNI) and the Dominantly Inherited Alzheimer Network (DIAN) Neuropathology. 2015;35:390–400. doi: 10.1111/neup.12205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Camus S, Ko WK, Pioli E, Bezard E. Why bother using non-human primate models of cognitive disorders in translational research? Neurobiol Learn Mem. 2015;124:123–129. doi: 10.1016/j.nlm.2015.06.012. [DOI] [PubMed] [Google Scholar]
- 20.Cash DM, Ridgway GR, Liang Y, Ryan NS, Kinnunen KM, Yeatman T, Malone IB, Benzinger TL, Jack CR, Jr, Thompson PM, et al. The pattern of atrophy in familial Alzheimer disease: volumetric MRI results from the DIAN study. Neurology. 2013;81:1425–1433. doi: 10.1212/WNL.0b013e3182a841c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Castellano JM, Kim J, Stewart FR, Jiang H, DeMattos RB, Patterson BW, Fagan AM, Morris JC, Mawuenyega KG, Cruchaga C, et al. Human apoE isoforms differentially regulate brain amyloid-beta peptide clearance. Sci Transl Med. 2011;3:89ra57. doi: 10.1126/scitranslmed.3002156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chambers JK, Kuribayashi H, Ikeda S, Une Y. Distribution of neprilysin and deposit patterns of Abeta subtypes in the brains of aged squirrel monkeys (Saimiri sciureus) Amyloid. 2010;17:75–82. doi: 10.3109/13506129.2010.483119. [DOI] [PubMed] [Google Scholar]
- 23.Choi SH, Kim YH, Hebisch M, Sliwinski C, Lee S, D'Avanzo C, Chen H, Hooli B, Asselin C, Muffat J, et al. A three-dimensional human neural cell culture model of Alzheimer's disease. Nature. 2014;515:274–278. doi: 10.1038/nature13800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cohen RM, Rezai-Zadeh K, Weitz TM, Rentsendorj A, Gate D, Spivak I, Bholat Y, Vasilevko V, Glabe CG, Breunig JJ, et al. A transgenic Alzheimer rat with plaques, tau pathology, behavioral impairment, oligomeric abeta, and frank neuronal loss. J Neurosci. 2013;33:6245–6256. doi: 10.1523/JNEUROSCI.3672-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crary JF, Trojanowski JQ, Schneider JA, Abisambra JF, Alafuzoff I, Arnold SE, Atterns J, Beach TG, Cairns NJ, Dickson DW, et al. Primary age-related tauopathy (PART): a common pathology associated with human aging. Acta Neuropathol. 2014;128:755–766. doi: 10.1007/s00401-014-1349-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cummings BJ, Pike CJ, Shankle R, Cotman CW. Beta-amyloid deposition and other measures of neuropathology predict cognitive status in Alzheimer's disease. Neurobiol Aging. 1996;17:921–933. doi: 10.1016/s0197-4580(96)00170-4. [DOI] [PubMed] [Google Scholar]
- 27.Cummings JL, Morstorf T, Zhong K. Alzheimer's disease drug-development pipeline: few candidates, frequent failures. Alzheimers Res Ther. 2014;6:37. doi: 10.1186/alzrt269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cuyvers E, Sleegers K. Genetic variations underlying Alzheimer's disease: evidence from genome-wide association studies and beyond. Lancet Neurol. 2016;15:857–868. doi: 10.1016/S1474-4422(16)00127-7. [DOI] [PubMed] [Google Scholar]
- 29.Davis J, Xu F, Deane R, Romanov G, Previti ML, Zeigler K, Zlokovic BV, van Nostrand WE. Early-onset and robust cerebral microvascular accumulation of amyloid beta-protein in transgenic mice expressing low levels of a vasculotropic Dutch/Iowa mutant form of amyloid beta-protein precursor. J Biol Chem. 2004;279:20296–20306. doi: 10.1074/jbc.M312946200. [DOI] [PubMed] [Google Scholar]
- 30.Davis PR, Head E. Prevention approaches in a preclinical canine model of Alzheimer's disease: benefits and challenges. Front Pharmacol. 2014;5:47. doi: 10.3389/fphar.2014.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dhenain M, Michot JL, Privat N, Picq JL, Boller F, Duyckaerts C, Volk A. MRI description of cerebral atrophy in mouse lemur primates. Neurobiol Aging. 2000;21:81–88. doi: 10.1016/s0197-4580(00)00098-1. [DOI] [PubMed] [Google Scholar]
- 32.Do Carmo S, Cuello AC. Modeling Alzheimer's disease in transgenic rats. Mol Neurodegener. 2013;8:37. doi: 10.1186/1750-1326-8-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Drummond ES, Muhling J, Martins RN, Wijaya LK, Ehlert EM, Harvey AR. Pathology associated with AAV mediated expression of beta amyloid or C100 in adult mouse hippocampus and cerebellum. PLoS One. 2013;8:e59166. doi: 10.1371/journal.pone.0059166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dujardin S, Colin M, Buee L. Invited review: Animal models of tauopathies and their implications for research/translation into the clinic. Neuropathol Appl Neurobiol. 2015;41:59–80. doi: 10.1111/nan.12200. [DOI] [PubMed] [Google Scholar]
- 35.Duyckaerts C. Tau pathology in children and young adults: can you still be unconditionally baptist? Acta Neuropathol. 2011;121:145–147. doi: 10.1007/s00401-010-0794-7. [DOI] [PubMed] [Google Scholar]
- 36.Elfenbein HA, Rosen RF, Stephens SL, Switzer RC, Smith Y, Pare J, Mehta PD, Warzok R, Walker LC. Cerebral beta-amyloid angiopathy in aged squirrel monkeys. Histol Histopathol. 2007;22:155–167. doi: 10.14670/HH-22.155. [DOI] [PubMed] [Google Scholar]
- 37.Esh C, Patton L, Kalback W, Kokjohn TA, Lopez J, Brune D, Newell AJ, Beach T, Schenk D, Games D, et al. Altered APP processing in PDAPP (Val717 --> Phe) transgenic mice yields extended-length Abeta peptides. Biochemistry. 2005;44:13807–13819. doi: 10.1021/bi051213+. [DOI] [PubMed] [Google Scholar]
- 38.Fernandez-Funez P, de Mena L, Rincon-Limas DE. Modeling the complex pathology of Alzheimer's disease in Drosophila. Exp Neurol. 2015;274:58–71. doi: 10.1016/j.expneurol.2015.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Frost JL, Le KX, Cynis H, Ekpo E, Kleinschmidt M, Palmour RM, Ervin FR, Snigdha S, Cotman CW, Saido TC, et al. Pyroglutamate-3 amyloid-beta deposition in the brains of humans, non-human primates, canines, and Alzheimer disease-like transgenic mouse models. Am J Pathol. 2013;183:369–381. doi: 10.1016/j.ajpath.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fryer JD, Simmons K, Parsadanian M, Bales KR, Paul SM, Sullivan PM, Holtzman DM. Human apolipoprotein E4 alters the amyloid-beta 40:42 ratio and promotes the formation of cerebral amyloid angiopathy in an amyloid precursor protein transgenic model. J Neurosci. 2005;25:2803–2810. doi: 10.1523/JNEUROSCI.5170-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fryer JD, Taylor JW, DeMattos RB, Bales KR, Paul SM, Parsadanian M, Holtzman DM. Apolipoprotein E markedly facilitates age-dependent cerebral amyloid angiopathy and spontaneous hemorrhage in amyloid precursor protein Transgenic mice. Journal of Neuroscience. 2003;23:7889–7896. doi: 10.1523/JNEUROSCI.23-21-07889.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Games D, Adams D, Alessandrini R, Barbour R, Berthelette P, Blackwell C, Carr T, Clemens J, Donaldson T, Gillespie F, et al. Alzheimer-type neuropathology in transgenic mice overexpressing V717F b-amyloid precursor protein. Nature. 1995;373:523–527. doi: 10.1038/373523a0. [DOI] [PubMed] [Google Scholar]
- 43.Gearing M, Rebeck GW, Hyman BT, Tigges J, Mirra SS. Neuropathology and apolipoprotein E profile of aged chimpanzees: implications for Alzheimer disease. Proc Natl Acad Sci U S A. 1994;91:9382–9386. doi: 10.1073/pnas.91.20.9382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gearing M, Tigges J, Mori H, Mirra SS. beta-Amyloid (A beta) deposition in the brains of aged orangutans. Neurobiol Aging. 1997;18:139–146. doi: 10.1016/s0197-4580(97)00012-2. [DOI] [PubMed] [Google Scholar]
- 45.Ghosal K, Vogt DL, Liang M, Shen Y, Lamb BT, Pimplikar SW. Alzheimer's disease-like pathological features in transgenic mice expressing the APP intracellular domain. Proc Natl Acad Sci U S A. 2009;106:18367–18372. doi: 10.1073/pnas.0907652106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Giannakopoulos P, Kovari E, Gold G, von Gunten A, Hof PR, Bouras C. Pathological substrates of cognitive decline in Alzheimer's disease. Front Neurol Neurosci. 2009;24:20–29. doi: 10.1159/000197881. [DOI] [PubMed] [Google Scholar]
- 47.Giannakopoulos P, Silhol S, Jallageas V, Mallet J, Bons N, Bouras C, Delaère P. Quantitative analysis of tau protein-immunoreactive accumulations and b amyloid protein deposits in the cerebral cortex of the mouse lemur, Microcebus murinus. Acta Neuropathol. 1997;94:131–139. doi: 10.1007/s004010050684. [DOI] [PubMed] [Google Scholar]
- 48.Gold G, Bouras C, Kovari E, Canuto A, Glaria BG, Malky A, Hof PR, Michel JP, Giannakopoulos P. Clinical validity of Braak neuropathological staging in the oldest-old. Acta Neuropathol. 2000;99:579–582. doi: 10.1007/s004010051163. discussion 583-574. [DOI] [PubMed] [Google Scholar]
- 49.Gotz J, Ittner LM. Animal models of Alzheimer's disease and frontotemporal dementia. Nat Rev Neurosci. 2008;9:532–544. doi: 10.1038/nrn2420. [DOI] [PubMed] [Google Scholar]
- 50.Gouras GK, Tampellini D, Takahashi RH, Capetillo-Zarate E. Intraneuronal beta-amyloid accumulation and synapse pathology in Alzheimer's disease. Acta Neuropathol. 2010;119:523–541. doi: 10.1007/s00401-010-0679-9. Doi 10.1007/s00401-010-0679-9 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grueninger F, Bohrmann B, Czech C, Ballard TM, Frey JR, Weidensteiner C, von Kienlin M, Ozmen L. Phosphorylation of Tau at S422 is enhanced by Abeta in TauPS2APP triple transgenic mice. Neurobiol Dis. 2010;37:294–306. doi: 10.1016/j.nbd.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 52.Guerreiro R, Hardy J. Genetics of Alzheimer's Disease. Neurotherapeutics. 2014;11:432–437. doi: 10.1007/s13311-014-0295-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hannan SB, Drager NM, Rasse TM, Voigt A, Jahn TR. Cellular and molecular modifier pathways in tauopathies: the big picture from screening invertebrate models. J Neurochem. 2016;137:12–25. doi: 10.1111/jnc.13532. [DOI] [PubMed] [Google Scholar]
- 54.Hartig W, Bruckner G, Schmidt C, Brauer K, Bodewitz G, Turner JD, Bigl V. Co-localization of beta-amyloid peptides, apolipoprotein E and glial markers in senile plaques in the prefrontal cortex of old rhesus monkeys. Brain Res. 1997;751:315–322. doi: 10.1016/s0006-8993(96)01423-0. [DOI] [PubMed] [Google Scholar]
- 55.Hartley D, Blumenthal T, Carrillo M, DiPaolo G, Esralew L, Gardiner K, Granholm AC, Iqbal K, Krams M, Lemere CA, et al. Down syndrome and Alzheimer's Disease: common pathways, common goals. Alzheimer's and Dementia. 2015;11:700–709. doi: 10.1016/j.jalz.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Heilbroner PL, Kemper TL. The cytoarchitectonic distribution of senile plaques in three aged monkeys. Acta Neuropathol. 1990;81:60–65. doi: 10.1007/BF00662638. [DOI] [PubMed] [Google Scholar]
- 57.Heuer E, Rosen RF, Cintron A, Walker LC. Nonhuman primate models of Alzheimer-like cerebral proteopathy. Curr Pharm Des. 2012;18:1159–1169. doi: 10.2174/138161212799315885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Holcomb L, Gordon MN, McGowan E, Yu X, Benkovic S, Jantzen P, Saad WK, Mueller R, Morgan D, Sanders S, et al. Accelerated Alzheimer-type phenotype in transgenic mice carrying both mutant amyloid precursor protein and presenilin 1 transgenes. Nature Medicine. 1998;4:97–100. doi: 10.1038/nm0198-097. [DOI] [PubMed] [Google Scholar]
- 59.Holtzman DM, Bales KR, Tenkova T, Fagan AM, Parsadanian M, Sartorius LJ, Mackey B, Olney J, McKeel D, Wozniak D, et al. Apolipoprotein E isoform-dependent amyloid deposition and neuritic degeneration in a mouse model of Alzheimer's disease. PNAS. 2000;97:2892–2897. doi: 10.1073/pnas.050004797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Holtzman DM, Bales KR, Wu S, Bhat P, Parsadanian M, Fagan AM, Chang LK, Sun Y, Paul SM. Expression of human apolipoprotein E reduces amyloid-beta deposition in a mouse model of Alzheimer's disease. Journal of Clinical Investigation. 1999;103:R15–R21. doi: 10.1172/JCI6179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Holtzman DM, Fagan AM, Mackey B, Tenkova T, Sartorius L, Paul SM, Bales KR, Hsiao Ashe K, Irizarry MC, Hyman BT. Apolipoprotein E facilitates neuritic and cerebrovascular plaque formation in an Alzheimer's disease model. Ann Neurol. 2000;47:739–747. [PubMed] [Google Scholar]
- 62.Hsiao KK, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G. Correlative memory deficits, Aβ elevation and amyloid plaques in transgenic mice. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- 63.Huang Y, Mahley RW. Apolipoprotein E: structure and function in lipid metabolism, neurobiology, and Alzheimer's diseases. Neurobiol Dis. 2014;72(Pt A):3–12. doi: 10.1016/j.nbd.2014.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hunter JM, Bowers WJ, Maarouf CL, Mastrangelo MA, Daugs ID, Kokjohn TA, Kalback WM, Luehrs DC, Valla J, Beach TG, et al. Biochemical and morphological characterization of the AbetaPP/PS/tau triple transgenic mouse model and its relevance to sporadic Alzheimer's disease. J Alzheimers Dis. 2011;27:361–376. doi: 10.3233/JAD-2011-110608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hyman BT, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Carrillo MC, Dickson DW, Duyckaerts C, Frosch MP, Masliah E, et al. National Institute on Aging-Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease. Alzheimers Dement. 2012;8:1–13. doi: 10.1016/j.jalz.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Israel MA, Yuan SH, Bardy C, Reyna SM, Mu Y, Herrera C, Hefferan MP, Van Gorp S, Nazor KL, Boscolo FS, et al. Probing sporadic and familial Alzheimer's disease using induced pluripotent stem cells. Nature. 2012;482:216–220. doi: 10.1038/nature10821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jack CR, Jr, Albert MS, Knopman DS, McKhann GM, Sperling RA, Carrillo MC, Thies B, Phelps CH. Introduction to the recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:257–262. doi: 10.1016/j.jalz.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jellinger KA. Alzheimer disease and cerebrovascular pathology: an update. J Neural Transm. 2002;109:813–836. doi: 10.1007/s007020200068. [DOI] [PubMed] [Google Scholar]
- 69.Jones RW. Dimebon disappointment. Alzheimers Res Ther. 2010;2:25. doi: 10.1186/alzrt49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Joseph-Mathurin N, Dorieux O, Trouche SG, Boutajangout A, Kraska A, Fontes P, Verdier JM, Sigurdsson EM, Mestre-Frances N, Dhenain M. Amyloid beta immunization worsens iron deposits in the choroid plexus and cerebral microbleeds. Neurobiol Aging. 2013;34:2613–2622. doi: 10.1016/j.neurobiolaging.2013.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kalback W, Watson MD, Kokjohn TA, Kuo YM, Weiss N, Luehrs DC, Lopez J, Brune D, Sisodia SS, Staufenbiel M, et al. APP transgenic mice Tg2576 accumulate Abeta peptides that are distinct from the chemically modified and insoluble peptides deposited in Alzheimer's disease senile plaques. Biochemistry. 2002;41:922–928. doi: 10.1021/bi015685+. [DOI] [PubMed] [Google Scholar]
- 72.Kalinin S, Willard SL, Shively CA, Kaplan JR, Register TC, Jorgensen MJ, Polak PE, Rubinstein I, Feinstein DL. Development of amyloid burden in African Green monkeys. Neurobiol Aging. 2013;34:2361–2369. doi: 10.1016/j.neurobiolaging.2013.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kamp JA, Moursel LG, Haan J, Terwindt GM, Lesnik Oberstein SA, van Duinen SG, van Roon-Mom WM. Amyloid beta in hereditary cerebral hemorrhage with amyloidosis-Dutch type. Rev Neurosci. 2014;25:641–651. doi: 10.1515/revneuro-2014-0008. [DOI] [PubMed] [Google Scholar]
- 74.Karch CM, Cruchaga C, Goate AM. Alzheimer's disease genetics: from the bench to the clinic. Neuron. 2014;83:11–26. doi: 10.1016/j.neuron.2014.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Karch CM, Goate AM. Alzheimer's disease risk genes and mechanisms of disease pathogenesis. Biol Psychiatry. 2015;77:43–51. doi: 10.1016/j.biopsych.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kim DH, Yeo SH, Park JM, Choi JY, Lee TH, Park SY, Ock MS, Eo J, Kim HS, Cha HJ. Genetic markers for diagnosis and pathogenesis of Alzheimer's disease. Gene. 2014;545:185–193. doi: 10.1016/j.gene.2014.05.031. [DOI] [PubMed] [Google Scholar]
- 77.Kim YH, Choi SH, D'Avanzo C, Hebisch M, Sliwinski C, Bylykbashi E, Washicosky KJ, Klee JB, Brustle O, Tanzi RE, et al. A 3D human neural cell culture system for modeling Alzheimer's disease. Nat Protoc. 2015;10:985–1006. doi: 10.1038/nprot.2015.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kimura N, Nakamura S, Goto N, Narushima E, Hara I, Shichiri S, Saitou K, Nose M, Hayashi T, Kawamura S, et al. Senile plaques in an aged western lowland gorilla. Exp Anim. 2001;50:77–81. doi: 10.1538/expanim.50.77. [DOI] [PubMed] [Google Scholar]
- 79.Knight EM, Kim SH, Kottwitz JC, Hatami A, Albay R, Suzuki A, Lublin A, Alberini CM, Klein WL, Szabo P, et al. Effective anti-Alzheimer Abeta therapy involves depletion of specific Abeta oligomer subtypes. Neurol Neuroimmunol Neuroinflamm. 2016;3:e237. doi: 10.1212/NXI.0000000000000237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Knopman DS. Is dominantly inherited Alzheimer disease a clone of sporadic Alzheimer disease? Neurology. 2015;85:750–751. doi: 10.1212/WNL.0000000000001897. [DOI] [PubMed] [Google Scholar]
- 81.Knopman DS, Jack CR, Jr, Lundt ES, Weigand SD, Vemuri P, Lowe VJ, Kantarci K, Gunter JL, Senjem ML, Mielke MM, et al. Evolution of neurodegeneration-imaging biomarkers from clinically normal to dementia in the Alzheimer disease spectrum. Neurobiol Aging. 2016;46:32–42. doi: 10.1016/j.neurobiolaging.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kondo T, Asai M, Tsukita K, Kutoku Y, Ohsawa Y, Sunada Y, Imamura K, Egawa N, Yahata N, Okita K, et al. Modeling Alzheimer's disease with iPSCs reveals stress phenotypes associated with intracellular Abeta and differential drug responsiveness. Cell Stem Cell. 2013;12:487–496. doi: 10.1016/j.stem.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 83.Kraska A, Dorieux O, Picq JL, Petit F, Bourrin E, Chenu E, Volk A, Perret M, Hantraye P, Mestre-Frances N, et al. Age-associated cerebral atrophy in mouse lemur primates. Neurobiol Aging. 2011;32:894–906. doi: 10.1016/j.neurobiolaging.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kumar S, Rezaei-Ghaleh N, Terwel D, Thal DR, Richard M, Hoch M, Mc Donald JM, Wullner U, Glebov K, Heneka MT, et al. Extracellular phosphorylation of the amyloid beta-peptide promotes formation of toxic aggregates during the pathogenesis of Alzheimer's disease. EMBO J. 2011;30:2255–2265. doi: 10.1038/emboj.2011.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kuo YM, Kokjohn TA, Beach TG, Sue LI, Brune D, Lopez JC, Kalback WM, Abramowski D, Sturchler-Pierrat C, Staufenbiel M, et al. Comparitive analysis of amyloid-beta chemical structure and amyloid plaque morphology of transgenic mouse and Alzheimer's disease brains. JBC. 2001;276:12991–12998. doi: 10.1074/jbc.M007859200. [DOI] [PubMed] [Google Scholar]
- 86.Kuszczyk MA, Sanchez S, Pankiewicz J, Kim J, Duszczyk M, Guridi M, Asuni AA, Sullivan PM, Holtzman DM, Sadowski MJ. Blocking the Interaction between Apolipoprotein E and Abeta Reduces Intraneuronal Accumulation of Abeta and Inhibits Synaptic Degeneration. Am J Pathol. 2013;182:1750–1768. doi: 10.1016/j.ajpath.2013.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lawlor PA, Bland RJ, Das P, Price RW, Holloway V, Smithson L, Dicker BL, During MJ, Young D, Golde TE. Novel rat Alzheimer's disease models based on AAV-mediated gene transfer to selectively increase hippocampal Abeta levels. Mol Neurodegener. 2007;2:11. doi: 10.1186/1750-1326-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lemere CA, Beierschmitt A, Iglesias M, Spooner ET, Bloom JK, Leverone JF, Zheng JB, Seabrook TJ, Louard D, Li D, et al. Alzheimer's disease abeta vaccine reduces central nervous system abeta levels in a non-human primate, the Caribbean vervet. Am J Pathol. 2004;165:283–297. doi: 10.1016/s0002-9440(10)63296-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lemere CA, Iglesias M, Spooner ET, Bloom JK, Leverone JF, Li D, Zheng JB, Seabrook TJ, Selkoe D, Ervin FR, et al. Ab immunization in aged vervet monkeys reduces Ab levels in brain and CSF. Soc Neurosci Abst. 2003 Abst 133.5: 5. [Google Scholar]
- 90.Leon WC, Canneva F, Partridge V, Allard S, Ferretti MT, DeWilde A, Vercauteren F, Atifeh R, Ducatenzeiler A, Klein W, et al. A novel transgenic rat model with a full Alzheimer's-like amyloid pathology displays pre-plaque intracellular amyloid-beta-associated cognitive impairment. J Alzheimers Dis. 2010;20:113–126. doi: 10.3233/JAD-2010-1349. [DOI] [PubMed] [Google Scholar]
- 91.Lewis J, Dickson D, Lin WL, Chisholm L, Corral A, Jones G, Yen SH, Sahara N, Skipper L, Yager D, et al. Enhanced neurofibrillary degeneration in transgenic mice expressing mutant tau and APP. Science. 2001;293:1487–1491. doi: 10.1126/science.1058189. [DOI] [PubMed] [Google Scholar]
- 92.Lewis J, McGowan E, Rockwood J, Melrose H, Nacharaju P, Van Slegtenhorst M, Gwinn-Hardy K, Murphy MP, Baker M, Yu X, et al. Neurofibrillary tangles, amyotrophy and progressive motor disturbance in mice expressing mutant (P301L) tau protein. Nat Genet. 2000;25:402–405. doi: 10.1038/78078. [DOI] [PubMed] [Google Scholar]
- 93.Li H, Guo Q, Inoue T, Polito VA, Tabuchi K, Hammer RE, Pautler RG, Taffet GE, Zheng H. Vascular and parenchymal amyloid pathology in an Alzheimer disease knock-in mouse model: interplay with cerebral blood flow. Mol Neurodegener. 2014;9:28. doi: 10.1186/1750-1326-9-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liao F, Zhang TJ, Jiang H, Lefton KB, Robinson GO, Vassar R, Sullivan PM, Holtzman DM. Murine versus human apolipoprotein E4: differential facilitation of and co-localization in cerebral amyloid angiopathy and amyloid plaques in APP transgenic mouse models. Acta Neuropathol Commun. 2015;3:70. doi: 10.1186/s40478-015-0250-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Liu L, Orozco IJ, Planel E, Wen Y, Bretteville A, Krishnamurthy P, Wang L, Herman M, Figueroa H, Yu WH, et al. A transgenic rat that develops Alzheimer's disease-like amyloid pathology, deficits in synaptic plasticity and cognitive impairment. Neurobiol Dis. 2008;31:46–57. doi: 10.1016/j.nbd.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Martin LJ, Pardo CA, Cork LC, Price DL. Synaptic pathology and glial responses to neuronal injury precede the formation of senile plaques and amyloid deposits in the aging cerebral cortex. Am J Pathol. 1994;145:1358–1381. [PMC free article] [PubMed] [Google Scholar]
- 97.McKee AC, Kosik KS, Kowall NW. Neuritic pathology and dementia in Alzheimer's disease. Ann Neurol. 1991;30:156–165. doi: 10.1002/ana.410300206. [DOI] [PubMed] [Google Scholar]
- 98.Mestre-Frances N, Keller E, Calenda A, Barelli H, Checler F, Bons N. Immunohistochemical analysis of cerebral cortical and vascular lesions in the primate Microcebus murinus reveal distinct amyloid beta1–42 and beta1–40 immunoreactivity profiles. Neurobiol Dis. 2000;7:1–8. doi: 10.1006/nbdi.1999.0270. [DOI] [PubMed] [Google Scholar]
- 99.Montine TJ, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Dickson DW, Duyckaerts C, Frosch MP, Masliah E, Mirra SS, et al. National Institute on Aging-Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease: a practical approach. Acta Neuropathol. 2012;123:1–11. doi: 10.1007/s00401-011-0910-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mucke L, Masliah E, Yu GQ, Mallory M, Rockenstein EM, Tatsuno G, Hu K, Kholodenko D, Johnson-Wood K, McConlogue L. High-level neuronal expression of abeta 1–42 in wild-type human amyloid protein precursor transgenic mice: synaptotoxicity without plaque formation. J Neurosci. 2000;20:4050–4058. doi: 10.1523/JNEUROSCI.20-11-04050.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Muratore CR, Rice HC, Srikanth P, Callahan DG, Shin T, Benjamin LN, Walsh DM, Selkoe DJ, Young-Pearse TL. The familial Alzheimer's disease APPV717I mutation alters APP processing and Tau expression in iPSC-derived neurons. Hum Mol Genet. 2014;23:3523–3536. doi: 10.1093/hmg/ddu064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nelson PT, Alafuzoff I, Bigio EH, Bouras C, Braak H, Cairns N, Davies P, Tredici KD, Duyckaerts C, Frosch MP, et al. Correlation of Alzheimer's disease neuropathologic changes with cognitive status: a review of the literature. JNEN. 2012;71:362–381. doi: 10.1097/NEN.0b013e31825018f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nelson PT, Braak H, Markesbery WR. Neuropathology and cognitive impairment in Alzheimer disease: a complex but coherent relationship. J Neuropathol Exp Neurol. 2009;68:1–14. doi: 10.1097/NEN.0b013e3181919a48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Newman M, Ebrahimie E, Lardelli M. Using the zebrafish model for Alzheimer's disease research. Front Genet. 2014;5:189. doi: 10.3389/fgene.2014.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nikolaev A, McLaughlin T, O'Leary DD, Tessier-Lavigne M. APP binds DR6 to trigger axon pruning and neuron death via distinct caspases. Nature. 2009;457:981–989. doi: 10.1038/nature07767. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 106.Nisbet RM, Polanco JC, Ittner LM, Gotz J. Tau aggregation and its interplay with amyloid-beta. Acta Neuropathol. 2015;129:207–220. doi: 10.1007/s00401-014-1371-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Oakley H, Cole SL, Logan S, Maus E, Shao P, Craft J, Guillozet-Bongaarts A, Ohno M, Disterhoft J, Van EL, et al. Intraneuronal beta-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer's disease mutations: potential factors in amyloid plaque formation. J Neurosci. 2006;26:10129–10140. doi: 10.1523/JNEUROSCI.1202-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Oddo S, Caccamo A, Kitazawa M, Tseng BP, LaFerla FM. Amyloid deposition precedes tangle formation in a triple transgenic model of Alzheimer's disease. Neurobiol Aging. 2003;24:1063–1070. doi: 10.1016/j.neurobiolaging.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 109.Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, Metherate R, Mattson MP, Akbari Y, LaFerla FM. Triple-transgenic model of Alzheimer's disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron. 2003;39:409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- 110.Pankiewicz JE, Guridi M, Kim J, Asuni AA, Sanchez S, Sullivan PM, Holtzman DM, Sadowski MJ. Blocking the apoE/Abeta interaction ameliorates Abeta-related pathology in APOE epsilon2 and epsilon4 targeted replacement Alzheimer model mice. Acta Neuropathol Commun. 2014;2:75. doi: 10.1186/s40478-014-0075-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Paquet D, Bhat R, Sydow A, Mandelkow EM, Berg S, Hellberg S, Falting J, Distel M, Koster RW, Schmid B, et al. A zebrafish model of tauopathy allows in vivo imaging of neuronal cell death and drug evaluation. J Clin Invest. 2009;119:1382–1395. doi: 10.1172/JCI37537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Perez SE, Raghanti MA, Hof PR, Kramer L, Ikonomovic MD, Lacor PN, Erwin JM, Sherwood CC, Mufson EJ. Alzheimer's disease pathology in the neocortex and hippocampus of the western lowland gorilla (Gorilla gorilla gorilla) J Comp Neurol. 2013;521:4318–4338. doi: 10.1002/cne.23428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Perez SE, Sherwood CC, Cranfield MR, Erwin JM, Mudakikwa A, Hof PR, Mufson EJ. Early Alzheimer's disease-type pathology in the frontal cortex of wild mountain gorillas (Gorilla beringei beringei) Neurobiol Aging. 2016;39:195–201. doi: 10.1016/j.neurobiolaging.2015.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Picq JL, Aujard F, Volk A, Dhenain M. Age-related cerebral atrophy in nonhuman primates predicts cognitive impairments. Neurobiol Aging. 2012;33:1096–1109. doi: 10.1016/j.neurobiolaging.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Potter H, Wisniewski T. Apolipoprotein E: essential catalyst of the Alzheimer amyloid cascade. Int J Alz Dis. 2012;2012:489428. doi: 10.1155/2012/489428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Prins ND, Visser PJ, Scheltens P. Can novel therapeutics halt the amyloid cascade? Alzheimer's Research & Therapy. 2010;2:5. doi: 10.1186/alzrt28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Puzzo D, Gulisano W, Palmeri A, Arancio O. Rodent models for Alzheimer's disease drug discovery. Expert Opin Drug Discov. 2015;10:703–711. doi: 10.1517/17460441.2015.1041913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Radde R, Bolmont T, Kaeser SA, Coomaraswamy J, Lindau D, Stoltze L, Calhoun ME, Jaggi F, Wolburg H, Gengler S, et al. Abeta42-driven cerebral amyloidosis in transgenic mice reveals early and robust pathology. EMBO Rep. 2006;7:940–946. doi: 10.1038/sj.embor.7400784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Raja WK, Mungenast AE, Lin YT, Ko T, Abdurrob F, Seo J, Tsai LH. Self-Organizing 3D Human Neural Tissue Derived from Induced Pluripotent Stem Cells Recapitulate Alzheimer's Disease Phenotypes. PLoS One. 2016;11:e0161969. doi: 10.1371/journal.pone.0161969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rebeck GW, Hoe HS, Moussa CE. Beta-amyloid1–42 gene transfer model exhibits intraneuronal amyloid, gliosis, tau phosphorylation, and neuronal loss. J Biol Chem. 2010;285:7440–7446. doi: 10.1074/jbc.M109.083915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ribe EM, Perez M, Puig B, Gich I, Lim F, Cuadrado M, Sesma T, Catena S, Sanchez B, Nieto M, et al. Accelerated amyloid deposition, neurofibrillary degeneration and neuronal loss in double mutant APP/tau transgenic mice. Neurobiol Dis. 2005;20:814–822. doi: 10.1016/j.nbd.2005.05.027. [DOI] [PubMed] [Google Scholar]
- 122.Rijal UA, Kosterin I, Kumar S, Von Arnim CA, Yamaguchi H, Fandrich M, Walter J, Thal DR. Biochemical stages of amyloid-beta peptide aggregation and accumulation in the human brain and their association with symptomatic and pathologically preclinical Alzheimer's disease. Brain. 2014;137:887–903. doi: 10.1093/brain/awt362. [DOI] [PubMed] [Google Scholar]
- 123.Robinson JL, Geser F, Corrada MM, Berlau DJ, Arnold SE, Lee VM, Kawas CH, Trojanowski JQ. Neocortical and hippocampal amyloid-beta and tau measures associate with dementia in the oldest-old. Brain. 2011;134:3708–3715. doi: 10.1093/brain/awr308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rosen RF, Farberg AS, Gearing M, Dooyema J, Long PM, Anderson DC, Davis-Turak J, Coppola G, Geschwind DH, Pare JF, et al. Tauopathy with paired helical filaments in an aged chimpanzee. J Comp Neurol. 2008;509:259–270. doi: 10.1002/cne.21744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rosen RF, Tomidokoro Y, Farberg AS, Dooyema J, Ciliax B, Preuss TM, Neubert TA, Ghiso JA, LeVine H, 3rd, Walker LC. Comparative pathobiology of beta-amyloid and the unique susceptibility of humans to Alzheimer's disease. Neurobiol Aging. 2016;44:185–196. doi: 10.1016/j.neurobiolaging.2016.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rosen RF, Walker LC, Levine H., 3rd PIB binding in aged primate brain: enrichment of high-affinity sites in humans with Alzheimer's disease. Neurobiol Aging. 2011;32:223–234. doi: 10.1016/j.neurobiolaging.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ryman DC, Acosta-Baena N, Aisen PS, Bird T, Danek A, Fox NC, Goate A, Frommelt P, Ghetti B, Langbaum JB, et al. Symptom onset in autosomal dominant Alzheimer disease: a systematic review and meta-analysis. Neurology. 2014;83:253–260. doi: 10.1212/WNL.0000000000000596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sadowski M, Pankiewicz J, Scholtzova H, Mehta P, Prelli F, Quartermain D, Wisniewski T. Blocking the apolipoproteinE/Amyloid b interaction reduces the parenchymal and vascular amyloid-b deposition and prevents memory deficit in AD transgenic mice. PNAS. 2006;103:18787–18792. doi: 10.1073/pnas.0604011103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Saito T, Matsuba Y, Mihira N, Takano J, Nilsson P, Itohara S, Iwata N, Saido TC. Single App knock-in mouse models of Alzheimer's disease. Nat Neurosci. 2014;17:661–663. doi: 10.1038/nn.3697. [DOI] [PubMed] [Google Scholar]
- 130.Saito T, Matsuba Y, Yamazaki N, Hashimoto S, Saido TC. Calpain Activation in Alzheimer's Model Mice Is an Artifact of APP and Presenilin Overexpression. J Neurosci. 2016;36:9933–9936. doi: 10.1523/JNEUROSCI.1907-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Salazar C, Valdivia G, Ardiles AO, Ewer J, Palacios AG. Genetic variants associated with neurodegenerative Alzheimer disease in natural models. Biol Res. 2016;49:14. doi: 10.1186/s40659-016-0072-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Salloway S, Sperling R, Fox NC, Blennow K, Klunk W, Raskind M, Sabbagh M, Honig LS, Porsteinsson AP, Ferris S, et al. Two phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer's disease. N Engl J Med. 2014;370:322–333. doi: 10.1056/NEJMoa1304839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Salloway S, Sperling R, Gilman S, Fox NC, Blennow K, Raskind M, Sabbagh M, Honig LS, Doody R, Van Dyck CH, et al. A phase 2 multiple ascending dose trial of bapineuzumab in mild to moderate Alzheimer disease. Neurology. 2009;73:2061–2070. doi: 10.1212/WNL.0b013e3181c67808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sani S, Traul D, Klink A, Niaraki N, Gonzalo-Ruiz A, Wu CK, Geula C. Distribution, progression and chemical composition of cortical amyloid-beta deposits in aged rhesus monkeys: similarities to the human. Acta Neuropathol. 2003;105:145–156. doi: 10.1007/s00401-002-0626-5. [DOI] [PubMed] [Google Scholar]
- 135.Santacruz K, Lewis J, Spires T, Paulson J, Kotilinek L, Ingelsson M, Guimaraes A, DeTure M, Ramsden M, McGowan E, et al. Tau suppression in a neurodegenerative mouse model improves memory function. Science. 2005;309:476–481. doi: 10.1126/science.1113694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Scheltens P, Blennow K, Breteler MM, de Strooper B, Frisoni GB, Salloway S, Van der Flier WM. Alzheimer's disease. Lancet. 2016;388:505–517. doi: 10.1016/S0140-6736(15)01124-1. [DOI] [PubMed] [Google Scholar]
- 137.Schmidt F, Boltze J, Jager C, Hofmann S, Willems N, Seeger J, Hartig W, Stolzing A. Detection and Quantification of beta-Amyloid, Pyroglutamyl Abeta, and Tau in Aged Canines. J Neuropathol Exp Neurol. 2015;74:912–923. doi: 10.1097/NEN.0000000000000230. [DOI] [PubMed] [Google Scholar]
- 138.Schneider I, Reverse D, Dewachter I, Ris L, Caluwaerts N, Kuiperi C, Gilis M, Geerts H, Kretzschmar H, Godaux E, et al. Mutant presenilins disturb neuronal calcium homeostasis in the brain of transgenic mice, decreasing the threshold for excitotoxicity and facilitating long-term potentiation. J Biol Chem. 2001;276:11539–11544. doi: 10.1074/jbc.M010977200. [DOI] [PubMed] [Google Scholar]
- 139.Schneider LS, Mangialasche F, Andreasen N, Feldman H, Giacobini E, Jones R, Mantua V, Mecocci P, Pani L, Winblad B, et al. Clinical trials and late-stage drug development for Alzheimer's disease: an appraisal from 1984 to 2014. J Intern Med. 2014;275:251–283. doi: 10.1111/joim.12191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Scholtzova H, Nehete P, Nehete BP, Mallory M, Cho EH, Holmes A, Park J, Wren MS, Pardington P, Gupta G, et al. Toll-like receptor 9 stimulation via CpG ODN in a non-human primate model of sporadic cerebral amyloid angiopathy. Alz Dementia. 2015;11:P618. [Google Scholar]
- 141.Scholtzova H, Williams L, Nehete P, Sabado R, Holmes A, Wisniewski T. Innate immunity stimulation via TLR9 in a non-human primate model of sporadic cerebral amyloid angiopathy. Alz Dementia. 2013;9:p508. [Google Scholar]
- 142.Schultz C, Hubbard GB, Rub U, Braak E, Braak H. Age-related progression of tau pathology in brains of baboons. Neurobiol Aging. 2000;21:905–912. doi: 10.1016/s0197-4580(00)00176-7. [DOI] [PubMed] [Google Scholar]
- 143.Schutt T, Helboe L, Pedersen LO, Waldemar G, Berendt M, Pedersen JT. Dogs with Cognitive Dysfunction as a Spontaneous Model for Early Alzheimer's Disease: A Translational Study of Neuropathological and Inflammatory Markers. J Alzheimers Dis. 2016;52:433–449. doi: 10.3233/JAD-151085. [DOI] [PubMed] [Google Scholar]
- 144.Selkoe DJ, Hardy J. The amyloid hypothesis of Alzheimer's disease at 25 years. EMBO Mol Med. 2016;8:595–608. doi: 10.15252/emmm.201606210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sevigny J, Chiao P, Bussiere T, Weinreb PH, Williams L, Maier M, Dunstan R, Salloway S, Chen T, Ling Y, et al. The antibody aducanumab reduces Abeta plaques in Alzheimer's disease. Nature. 2016;537:50–56. doi: 10.1038/nature19323. [DOI] [PubMed] [Google Scholar]
- 146.Shah P, Lal N, Leung E, Traul DE, Gonzalo-Ruiz A, Geula C. Neuronal and axonal loss are selectively linked to fibrillar amyloid-{beta} within plaques of the aged primate cerebral cortex. Am J Pathol. 2010;177:325–333. doi: 10.2353/ajpath.2010.090937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Shinohara M, Fujioka S, Murray ME, Wojtas A, Baker M, Rovelet-Lecrux A, Rademakers R, Das P, Parisi JE, Graff-Radford NR, et al. Regional distribution of synaptic markers and APP correlate with distinct clinicopathological features in sporadic and familial Alzheimer's disease. Brain. 2014;137:1533–1549. doi: 10.1093/brain/awu046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Smolek T, Madari A, Farbakova J, Kandrac O, Jadhav S, Cente M, Brezovakova V, Novak M, Zilka N. Tau hyperphosphorylation in synaptosomes and neuroinflammation are associated with canine cognitive impairment. J Comp Neurol. 2016;524:874–895. doi: 10.1002/cne.23877. [DOI] [PubMed] [Google Scholar]
- 149.Steffen J, Krohn M, Paarmann K, Schwitlick C, Bruning T, Marreiros R, Muller-Schiffmann A, Korth C, Braun K, Pahnke J. Revisiting rodent models: Octodon degus as Alzheimer's disease model? Acta Neuropathol Commun. 2016;4:91. doi: 10.1186/s40478-016-0363-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Struble RG, Price DL, Jr, Cork LC, Price DL. Senile plaques in cortex of aged normal monkeys. Brain Res. 1985;361:267–275. doi: 10.1016/0006-8993(85)91298-3. [DOI] [PubMed] [Google Scholar]
- 151.Sturchler-Pierrat C, Abramowski D, Duke M, Wiederhold KH, Mistl C, Rothacher S, Ledermann B, Bürki K, Frey P, Paganetti PA, et al. Two amyloid precursor protein transgenic mouse models with Alzheimer disease-like pathology. Proc Natl Acad Sci USA. 1997;94:13287–13292. doi: 10.1073/pnas.94.24.13287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sturchler-Pierrat C, Staufenbiel M. Pathogenic mechanisms of Alzheimer's disease analyzed in the APP23 transgenic mouse model. Ann N Y Acad Sci. 2000;920:134–139. doi: 10.1111/j.1749-6632.2000.tb06915.x. [DOI] [PubMed] [Google Scholar]
- 153.Takahashi K, Okita K, Nakagawa M, Yamanaka S. Induction of pluripotent stem cells from fibroblast cultures. Nat Protoc. 2007;2:3081–3089. doi: 10.1038/nprot.2007.418. [DOI] [PubMed] [Google Scholar]
- 154.Takeuchi A, Irizarry MC, Duff K, Saido T, Hsiao Ashe K, Hasegawa H, Mann DM, Hyman BT, Iwatsubo T. Age-related amyloid b deposition in transgenic mice overexpressing both Alzheimer mutant presenilin 1 and amyloid b precursor protein swedish mutant is not associated with global neuronal loss. AJP. 2000;157:331–339. doi: 10.1016/s0002-9440(10)64544-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Tanzi RE, Gusella JF, Watkins PC, Bruns GA, St.George Hyslop P, VanKeuren ML, Patterson D, Pagan S, Kurnit DM, Neve RL. Amyloid b-protein gene: cDNA, mRNA distribution and genetic linkage near the Alzheimer's locus. Science. 1987;235:880–884. doi: 10.1126/science.2949367. [DOI] [PubMed] [Google Scholar]
- 156.Tellechea P, Pujol N, Esteve-Belloch P, Echeveste B, Garcia-Eulate MR, Arbizu J, Riverol M. Early- and late-onset Alzheimer disease: Are they the same entity? Neurologia. 2015 doi: 10.1016/j.nrl.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 157.Thal DR, Rub U, Orantes M, Braak H. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology. 2002;58:1791–1800. doi: 10.1212/wnl.58.12.1791. [DOI] [PubMed] [Google Scholar]
- 158.Thal DR, Walter J, Saido TC, Fandrich M. Neuropathology and biochemistry of Abeta and its aggregates in Alzheimer's disease. Acta Neuropathol. 2015;129:167–182. doi: 10.1007/s00401-014-1375-y. [DOI] [PubMed] [Google Scholar]
- 159.Tomiyama T, Matsuyama S, Iso H, Umeda T, Takuma H, Ohnishi K, Ishibashi K, Teraoka R, Sakama N, Yamashita T, et al. A mouse model of amyloid b oligomers: their contribution to synaptic alteration, abnormal tau phosphorylation, glial activation, and neuronal loss in vivo. J Neurosci. 2010;30:4845–4856. doi: 10.1523/JNEUROSCI.5825-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Tomlinson BE, Blessed G, Roth M. Observations on the brains of demented old people. J Neurol Sci. 1970;11:205–242. doi: 10.1016/0022-510x(70)90063-8. [DOI] [PubMed] [Google Scholar]
- 161.Trouche SG, Asuni A, Rouland S, Wisniewski T, Frangione B, Verdier JM, Sigurdsson EM, Mestre-Frances N. Antibody response and plasma Aβ1–40 in young Microcebus Murinus primates immunized with Aβ1–42 and its derivatives. Vaccine. 2009;27:957–964. doi: 10.1016/j.vaccine.2008.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ulrich JD, Holtzman DM. TREM2 Function in Alzheimer's Disease and Neurodegeneration. ACS Chem Neurosci. 2016;7:420–427. doi: 10.1021/acschemneuro.5b00313. [DOI] [PubMed] [Google Scholar]
- 163.Umeda T, Maekawa S, Kimura T, Takashima A, Tomiyama T, Mori H. Neurofibrillary tangle formation by introducing wild-type human tau into APP transgenic mice. Acta Neuropathol. 2014;127:685–698. doi: 10.1007/s00401-014-1259-1. [DOI] [PubMed] [Google Scholar]
- 164.Uno H, Alsum PB, Dong S, Richardson R, Zimbric ML, Thieme CS, Houser WD. Cerebral amyloid angiopathy and plaques, and visceral amyloidosis in aged macaques. Neurobiol Aging. 1996;17:275–281. doi: 10.1016/0197-4580(95)02063-2. [DOI] [PubMed] [Google Scholar]
- 165.Uno H, Walker LC. The age of biosenescence and the incidence of cerebral beta-amyloidosis in aged captive rhesus monkeys. Ann N Y Acad Sci. 1993;695:232–235. doi: 10.1111/j.1749-6632.1993.tb23058.x. [DOI] [PubMed] [Google Scholar]
- 166.Viola KL, Klein WL. Amyloid beta oligomers in Alzheimer's disease pathogenesis, treatment, and diagnosis. Acta Neuropathol. 2015;129:183–206. doi: 10.1007/s00401-015-1386-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Walker LC, Kitt CA, Schwam E, Buckwald B, Garcia F, Sepinwall J, Price DL. Senile plaques in aged squirrel monkeys. Neurobiol Aging. 1987;8:291–296. doi: 10.1016/0197-4580(87)90067-4. [DOI] [PubMed] [Google Scholar]
- 168.Walker LC, Masters C, Beyreuther K, Price DL. Amyloid in the brains of aged squirrel monkeys. Acta Neuropathol. 1990;80:381–387. doi: 10.1007/BF00307691. [DOI] [PubMed] [Google Scholar]
- 169.Webster SJ, Bachstetter AD, Nelson PT, Schmitt FA, Van Eldik LJ. Using mice to model Alzheimer's dementia: an overview of the clinical disease and the preclinical behavioral changes in 10 mouse models. Front Genet. 2014;5:88. doi: 10.3389/fgene.2014.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Wingo TS, Lah JJ, Levey AI, Cutler DJ. Autosomal recessive causes likely in early-onset Alzheimer disease. Arch Neurol. 2012;69:59–64. doi: 10.1001/archneurol.2011.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wisniewski T, Drummond E. Developing Therapeutic Vaccines Against Alzheimer's Disease. Expert Rev Vaccines. 2016;15:401–415. doi: 10.1586/14760584.2016.1121815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Wisniewski T, Goni F. Immunotherapeutic Approaches for Alzheimer's Disease. Neuron. 2015;85:1162–1176. doi: 10.1016/j.neuron.2014.12.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Wisniewski T, Sigurdsson EM. Murine models of Alzheimer's disease and their use in developing immunotherapies. Biochim Biophys Acta Mol Basis Dis. 2010;1802:847–859. doi: 10.1016/j.bbadis.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Xu G, Ran Y, Fromholt SE, Fu C, Yachnis AT, Golde TE, Borchelt DR. Murine Abeta over-production produces diffuse and compact Alzheimer-type amyloid deposits. Acta Neuropathol Commun. 2015;3:72. doi: 10.1186/s40478-015-0252-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Yagi T, Ito D, Okada Y, Akamatsu W, Nihei Y, Yoshizaki T, Yamanaka S, Okano H, Suzuki N. Modeling familial Alzheimer's disease with induced pluripotent stem cells. Hum Mol Genet. 2011;20:4530–4539. doi: 10.1093/hmg/ddr394. [DOI] [PubMed] [Google Scholar]
- 176.Yamazaki Y, Painter MM, Bu G, Kanekiyo T. Apolipoprotein E as a Therapeutic Target in Alzheimer's Disease: A Review of Basic Research and Clinical Evidence. CNS Drugs. 2016;30:773–789. doi: 10.1007/s40263-016-0361-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Yoshiyama Y, Higuchi M, Zhang B, Huang SM, Iwata N, Saido TC, Maeda J, Suhara T, Trojanowski JQ, Lee VM. Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron. 2007;53:337–351. doi: 10.1016/j.neuron.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 178.Youmans KL, Tai LM, Nwabuisi-Heath E, Jungbauer L, Kanekiyo T, Gan M, Kim J, Eimer WA, Estus S, Rebeck GW, et al. APOE4-specific changes in Abeta accumulation in a new transgenic mouse model of Alzheimer disease. J Biol Chem. 2012;287:41774–41786. doi: 10.1074/jbc.M112.407957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Zhang-Nunes SX, Maat-Schieman ML, Van Duinen SG, Roos RA, Frosch MP, Greenberg SM. The cerebral beta-amyloid angiopathies: hereditary and sporadic. Brain Pathol. 2006;16:30–39. doi: 10.1111/j.1750-3639.2006.tb00559.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.