Abstract
Mechanosensitive Merkel cells are thought to have finite lifespans, but controversy surrounds the frequency of their replacement and which precursor cells maintain the population. We found by embryonic EdU administration that Merkel cells undergo terminal cell division in late embryogenesis and survive long into adulthood. We also found that new Merkel cells are produced infrequently during normal skin homeostasis and that their numbers do not change during natural or induced hair cycles. In contrast, live imaging and EdU experiments showed that mild mechanical injury produced by skin shaving dramatically increases Merkel cell production. We confirmed with genetic cell ablation and fate-mapping experiments that new touch dome Merkel cells in adult mice arise from touch dome keratinocytes. Together, these independent lines of evidence show that Merkel cells in adult mice are long-lived, are replaced rarely during normal adult skin homeostasis, and that their production can be induced by repeated shaving. These results have profound implications for understanding sensory neurobiology and human diseases such as Merkel cell carcinoma.
Keywords: Somatosensation, Development, Touch, Merkel cell carcinoma
INTRODUCTION
A primary function of mammalian skin is to provide a protective barrier against environmental insults. Trauma to skin cells necessitates their frequent replacement by resident skin progenitors to maintain skin integrity (Levy et al., 2005; Page et al., 2013). These progenitor cells maintain the barrier function of the skin and insure that skin appendages such as hair follicles, sebaceous and sweat glands continue to function (Jensen et al., 2009; Lu et al., 2012). Skin cell turnover occurs on a regular schedule (for instance, as part of the hair cycle) and as needed following injury (Hsu et al., 2011; Jaks et al., 2008). Different progenitor cell populations located in different regions of the skin participate in these processes (Horsley et al., 2006; Ito et al., 2005; Levy et al., 2005; 2007).
Merkel cells are mechanosensitive cells found in mammalian hairy skin, whisker follicles and glabrous (non-hairy) skin of the hands and feet (Halata et al., 2003). Merkel cells are innervated by slowly-adapting type 1 (SA1) afferent neurons, and these Merkel cell-neurite complexes detect certain light touch stimuli (Iggo and Muir, 1969; Johnson and Hsiao, 1992; Johnson and Lamb, 1981; Maricich et al., 2012; 2009). Reported variations in Merkel cell numbers during the hair cycle (Moll et al., 1996; Nakafusa et al., 2006) and genetic lineage tracing studies (Doucet et al., 2013; Van Keymeulen et al., 2009; Wright et al., 2015; Xiao et al., 2015) suggest that, like other skin cells, adult Merkel cells are regularly replaced. However, the frequency of Merkel cell replacement and identities of Merkel cell progenitors remain unclear.
We analyzed Merkel cell lifespan by EdU birthdating studies beginning in embryogenesis, leading to the unexpected discovery that they persisted into late adulthood. This prompted us to perform a multifaceted analysis to investigate the kinetics of Merkel cell production, survival and replacement. Surprisingly, we found that touch dome Merkel cell numbers are constant throughout the hair cycle and that new Merkel cells are infrequently generated during adult skin homeostasis. We repeatedly visualized the same touch domes over many months using confocal microscopy in living adult transgenic mice. Consistent with our EdU birthdating studies, we observed that a significant number of Merkel cells lived for longer than 5 months. Furthermore, we illustrate that large numbers of new Merkel cells were generated only in the setting of Merkel cell loss induced by repeated shaving and confirm that these new Merkel cells arise from touch dome keratinocytes. These data reveal important insights into Merkel cell biology that have potential relevance for understanding peripheral somatosensation and the development of Merkel cell carcinoma.
RESULTS
Embryonic Merkel cells persist into late adulthood and new Merkel cells are rarely made during adult skin homeostasis
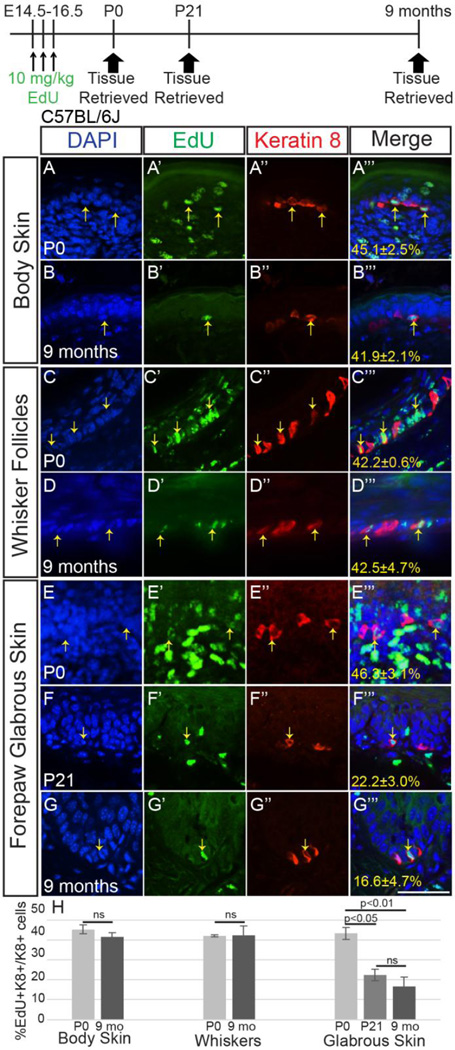
Keratin (K)14-expressing epidermal progenitors generate the first set of murine Merkel cells in late embryogenesis, beginning at embryonic day (E)14.5 and continuing until birth (Wright et al., 2015). However, it is unclear for how long this initial cohort of Merkel cells survives into postnatal life. To quantify the lifespan of these embryonic-born Merkel cells, we employed a birthdating approach using the modified nucleoside 5-ethynyl-2'-deoxyuridine (EdU). Incorporation of modified nucleosides like EdU into DNA occurs during S-phase; cells that become post-mitotic after incorporation retain EdU throughout their lives, while the signal is diluted until it becomes undetectable (~2–5 cell divisions) in cells that continue to divide (Ganusov and De Boer, 2012; Kiel et al., 2007). To determine the lifespan of Merkel cells created during embryogenesis, we administered EdU (10mg/kg) by once daily intraperitoneal injection to pregnant C57Bl/6J female mice at E14.5, E15.5, and E16.5, the ages of peak Merkel cell generation (Wright et al., 2015). We harvested and sectioned skin from progeny mice of the same litters at postnatal day (P)0, P21 and 9 months of age (n=4 mice/age), immunostained for the Merkel cell marker Keratin 8 (K8), visualized EdU, then calculated the percentage of K8+ cells that were also EdU+ (Figure 1). Qualitatively, the robustness of the EdU signal in K8+ cells was similar at all three ages, suggesting that the majority of cells labeled during embryogenesis that survived into adulthood did not continue to divide over time. Percentages of K8+EdU+/K8+ cells did not change between P0 and 9 months of age in back and belly skin or in whisker follicles (p=0.37 and 0.95, t-test; Figure 1A–D’’’, H), nor between P21 and 9 months of age in the glabrous skin of the forepaw (Figure 1F–G’’’, H). A decrease in the forepaw between P0 and P21 was noted, likely secondary to continued production of Merkel cells within the glabrous skin at this age (p=0.003, one-way ANOVA, P0 vs. P21 p<0.05, P0 vs. 9 mo. p<0.01, P21 vs. 9 mo. p>0.05; Figure 1E–F’’’, H). These data indicate that Merkel cells born during embryogenesis survive for at least 9 months after becoming post-mitotic.
Figure 1. K8+ cells born in embryogenesis survive at least 9 months.
Single z-slice confocal images of sectioned back skin (A–B’’’), whisker follicles (C–D’’’) and glabrous forepaw skin (EG’’’) from P0 (A–A’’’, C–C’’’, E–E’’’), P21 (F–F’’’) and 9 month-old (B–B’’’, D–D’’’, G–G’’’) female C57Bl/6J mice that received 10mg/kg EdU at E14.5, 15.5 and 16.5. Tissues were processed for EdU (A’–G’, green) and K8 immunostaining (A’’–G’’, red). Yellow arrows indicate K8+EdU+ cells. Exposure times are similar for all panels. (H) Average percentages (± SEM) of K8+ cells that were EdU+ in each skin region (n=4 mice/age). Scale bar: 50µm.
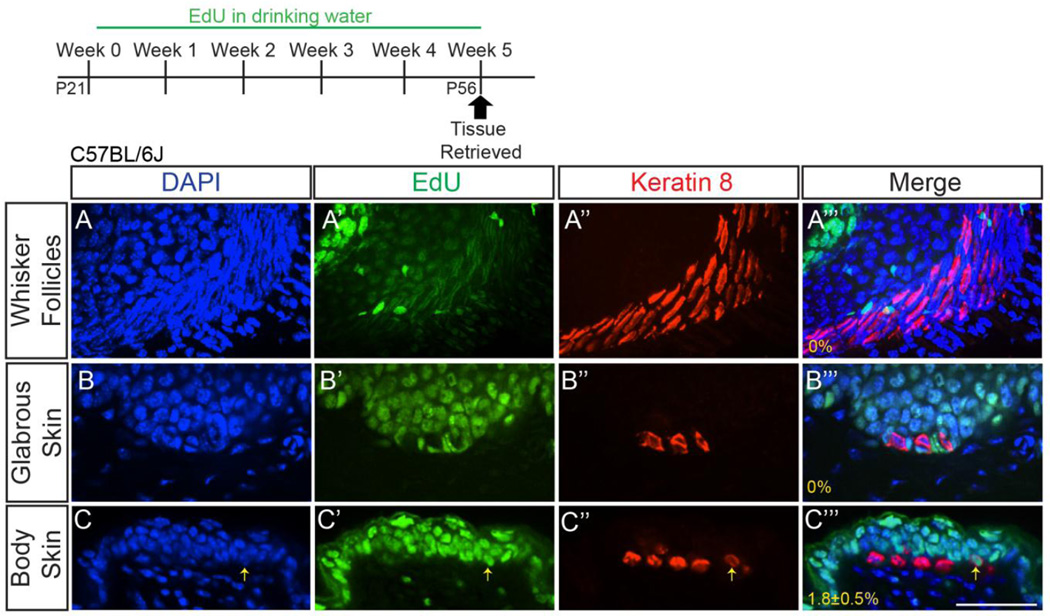
We were surprised to see persistence of embryonic-born Merkel cells out to 9 months of age, as previous lines of evidence suggested that this cell population should have undergone multiple rounds of complete turnover and replacement during this time (Doucet et al., 2013; Nakafusa et al., 2006; Xiao et al., 2015). We re-examined the frequency of Merkel cell production during the first hair cycle (P21–P56), a five week period of time during which approximately 50% of the Merkel cell population would be predicted to have been generated and incorporated into touch domes (Doucet et al., 2013). EdU was administered in the drinking water (0.2mg/mL) to P21 C57Bl/6J female mice for 5 weeks, after which time back and belly skin, whisker follicles, and glabrous skin of the forepaw was retrieved and processed for K8 and EdU (Figure 2A–C’’’; n=3 mice). We verified that EdU exposure did not cause Merkel cell loss, as mice that received EdU and age-matched untreated mice had comparable numbers of K8+ cells/touch dome (20.3±2.5 vs 16.7±0.7, respectively, p=0.41, t-test; n=3 mice/condition; images not shown). No K8+ cells in the whisker follicles or glabrous paw skin were found to have incorporated EdU (whiskers: >250 K8+ cells/mouse; paws: >70 K8+ cells/mouse; Figure 2A–B’’’). In back and belly skin, a very small proportion (1.8±0.5%) of K8+ cells were EdU+ after 5 weeks of EdU exposure (>300 K8+ cells/mouse; Figure 2C–C’’’). Presented images are from back skin, though no difference was seen in the proportion of K8+EdU+/K8+ cells between back and belly skin. Taken together, these data indicate that new Merkel cells are generated and maintained as part of normal skin homeostasis during the first hair cycle, but only very infrequently and only in touch domes.
Figure 2. Few adult Merkel cells are formed, and only within touch domes.
Sectioned whisker follicles (z-stack projection; A–A’’’), glabrous forepaw skin (single z-slice; B–B’’’), and back skin (single z-slice; C–C’’’) from female P56 C57BL/6J mice that received 0.2mg/mL EdU in their drinking water for five weeks. Tissues were processed for EdU (A’, B’, C’; green) and K8 immunostaining (A’’, B’’, C’’; red). Yellow arrow (C–C’’’) indicates a K8+EdU+ cell. Percentages of K8+ cells that were EdU+ are shown (A’’’–C’’’) (n=3 mice). Scale bar: 50µm.
Merkel cell numbers decrease over early postnatal life, remain relatively constant throughout adulthood and do not oscillate with the adult hair cycle
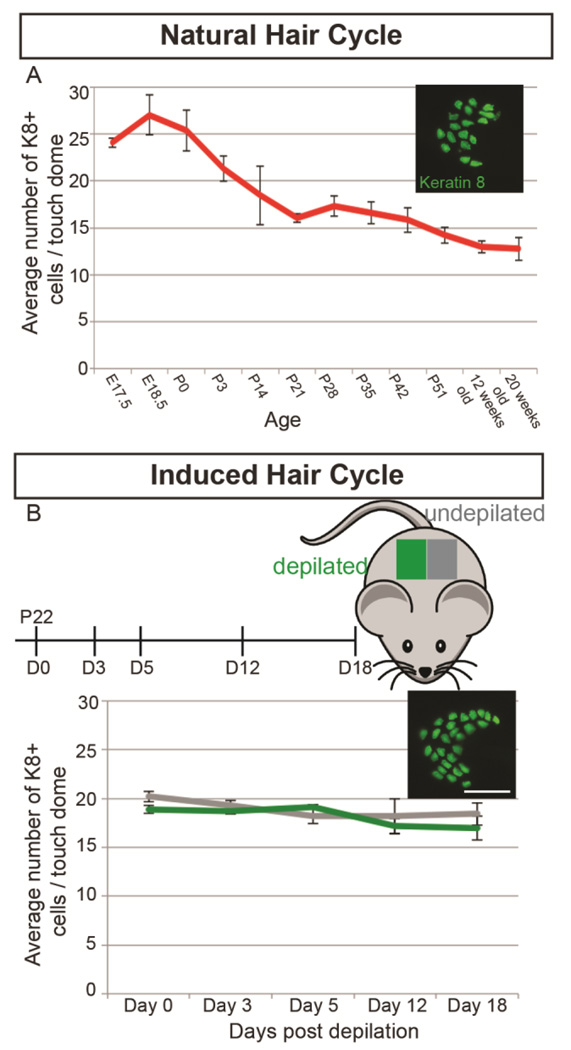
Our data demonstrating the persistence of embryonic-born Merkel cells and infrequent generation of adult-born Merkel cells was seemingly inconsistent with previous data demonstrating oscillations of Merkel cell number with the hair cycle. To examine whether touch dome Merkel cell numbers on a C57Bl/6J background were linked to hair cycle stage, we immunostained wholemount back skin from female mice for the Merkel cell marker K8 during hair follicle morphogenesis (E17–P21), during the first hair cycle (P21–P52) and at times after the second and third hair cycles (12 and 20 weeks of age) (>20 touch domes/mouse, n=3–5 mice/age; Figure 3A). Portions of the skin were sectioned and stained with hematoxylin and eosin (Figure S1A–L) to validate hair cycle stage using published guidelines (Müller-Röver et al., 2001). Average numbers of K8+ cells/touch dome peaked at E18.5, decreased precipitously until P21, and then decreased slightly (~20%) up to 20 weeks of age (one-way ANOVA, F=2.80, p=0.02; Figure 3A). There were no differences in K8+ cell numbers during any stage of the first hair cycle (P21–P51) (one-way ANOVA, F=1.33, p=0.29; Figure S2). These data show that average Merkel cell numbers do not change during natural hair cycles in the back skin of C57Bl/6J mice.
Figure 3. K8+ cell numbers in touch domes decrease over the first 3 weeks of postnatal life but remain constant throughout natural and induced hair cycles.
(A) Average K8+ cell numbers per touch dome at different stages of the natural hair cycle in back skin of C57Bl/6J female mice of varying ages (n=3–5 mice/age). Inset shows skin wholemount immunostained for Keratin 8. One-way ANOVA demonstrates no differences in average K8+ cell number per touch dome during the first hair cycle, (F=1.33, p=0.29). (B) Average K8+ cell numbers per touch dome in depilated (green) vs. undepilated (gray) back skin 0, 3, 5, 12, and 18 days post-depilation (n=3 mice/timepoint). Inset shows whole mount skin immunostained for Keratin 8. Scale bar: 50µm. Two-way ANOVA demonstrates no differences in average K8+ cell number per touch dome between induced and naturally cycling skin at any time (F=0.5848, p=0.7). Bars on graphs are SEMs.
We also tested the influence of hair cycle induction on touch dome Merkel cell numbers. Back skin of P22 female C57Bl/6J mice was shaved and depilated with Surgicream (right side) or left untreated (left side), then harvested 0, 3, 5, 12 or 18 days later. These timepoints were chosen to correlate with noted Merkel cell number changes after hair cycle induction as previously published (Moll et al., 1996). We verified that depilation induced the hair cycle on hematoxylin and eosin-stained skin sections, which showed that shaved/depilated skin entered anagen and transitioned to catagen sooner than untreated skin from the same mice (Figure S1M–V). Wholemount immunostaining revealed no differences in K8+ cell numbers between induced and naturally cycling skin at any time (two-way ANOVA F(4, 20)=0.5848, p=0.7; Figure 3B). These data demonstrate that Merkel cell numbers do not vary with induced hair cycle stage.
In vivo visualization of touch domes demonstrates long Merkel cell lifespan
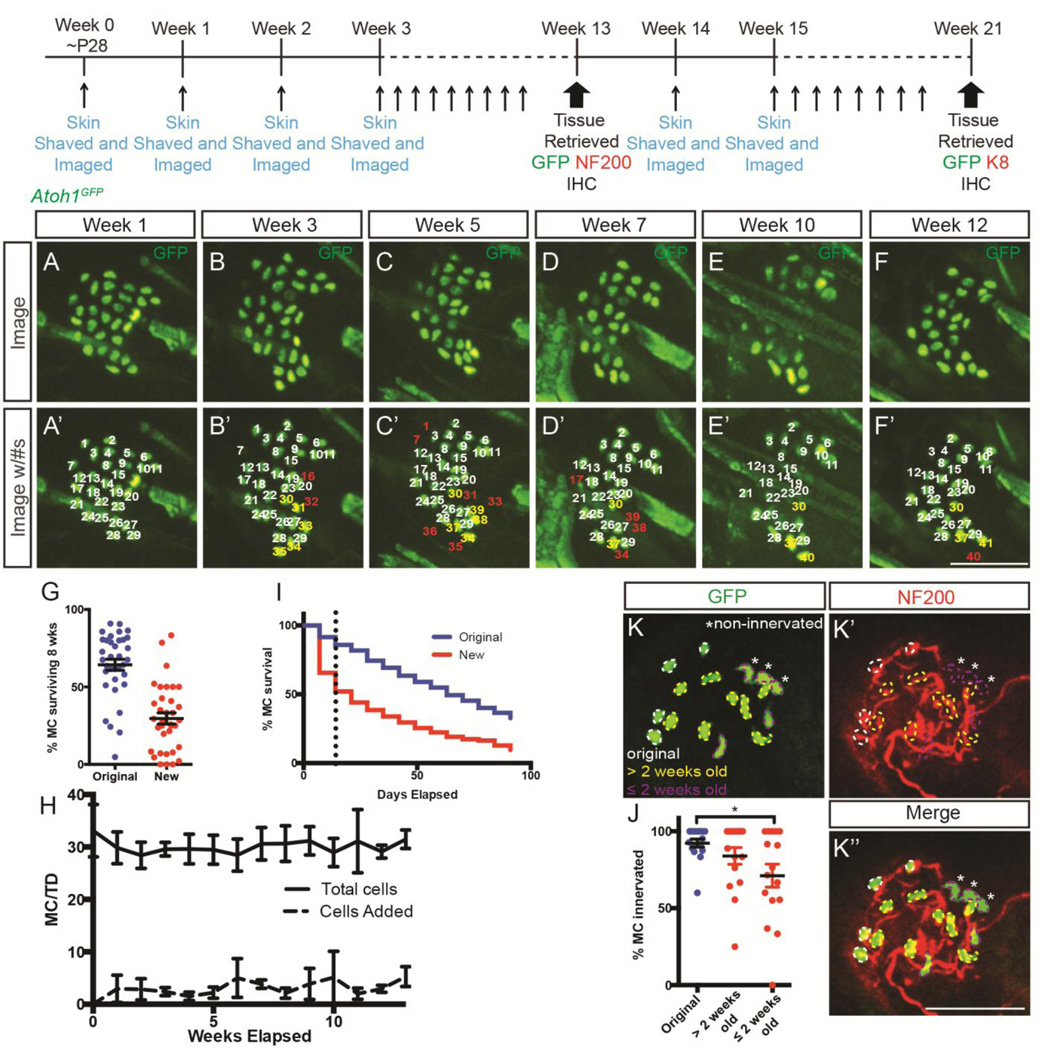
As a final approach to investigate Merkel cell persistence in adulthood, we devised a strategy to repeatedly image the same touch domes in living adult mice over an extended period of time. Young (~P28) Atoh1GFP mice, in which all Merkel cells are GFP+ (Lumpkin et al., 2003), were anesthetized and had the same regions of belly skin shaved once per week with a straight razor to allow repeated visualization of the same touch domes (Figure S3A). A square imaging area was marked with India Ink on the belly skin, permitting mapping and tracing of individual touch dome locations from week to week (Figure S3A). Individual touch domes were identified and mapped from week to week based on their location within the square and proximity to other touch domes. Individual touch domes (7–12/mouse from 4 mice (37 total); n=2,530 total GFP+ cells) were imaged on a spinning disc confocal microscope each week for 13–21 weeks (Figure 4A–F’). GFP+ cells were confined to touch domes and were never seen in the follicular or interfollicular epidermis. Cells were considered “original” Merkel cells if they were observed at the first imaging session (week 0) or “new” if they were observed in subsequent weeks, but not at week 0. While this system did not permit unique labeling of individual cells as they were traced from week to week, their location on the skin, relative proximity to other GFP+ cells and relationship to the central hair follicle allowed us to reproducibly identify and track them over time. We found that during the first 8 weeks (one estimate of Merkel cell lifespan) (Doucet et al., 2013) 64.3±3.6% of original GFP+ cells remained at week 8 (Figure 4G). Furthermore, 52±3.6% and 28±6.54% of original GFP+ cells (n=579 cells from n=4 mice) survived for 13 and 21 weeks, respectively (Figure 4). Touch domes retained much of their original organization over this time, illustrating relative stability of this sensory structure and allowing us to repeatedly identify the same Merkel cells (Figure 4A–F). The persistence of adult Merkel cells is consistent with our EdU pulse-chase experiments (Figure 1) and illustrates the unanticipated longevity of this epidermal cell population.
Figure 4. In vivo imaging of touch domes in Atoh1GFP mice.
Adolescent Atoh1GFP mice were shaved and imaged once weekly to enable tracking of individual touch domes over time. (A–F’) Atoh1GFP+ cells of the same touch dome at weeks 1 (A, A’), 3 (B, B’), 5 (C, C’), 7 (D, D’), 10 (E, E’), and 12 (F, F’) showing endogenous GFP expression. Numbers in (A–F’) indicate individual cells that survived (white), died (red) or that were born (yellow) over these weeks. (G) Percentage of original (blue) and new (red) GFP+ cells surviving for the first 8 weeks of imaging (original) or first 8 weeks following creation (new). Each dot represents one touch dome. (H) Average numbers of GFP+ cells (n=4 mice) per touch dome (solid line; p=0.534, one-way ANOVA) and new cells per touch dome over 13 weeks of imaging (dashed line; p=0.280, one-way ANOVA). (I) Survival curves for original (blue) and new (red) GFP+ cells. In the first two weeks, survival is significantly lower for new cells than for original cells (slope original, −0.828; slope new −3.293; p=0.004) while after two weeks the survival of new and original cells is similar (slope original, −0.532; slope new −0.449; p=0.222) (K–K’’) Back skin immunostained for GFP (K, green) and NF200 (K’, red) showing innervation of original (white outline), >2 weeks old (yellow outline) and ≤ 2 weeks old (purple outline) cells. Non-innervated cells are indicated by asterisks. (J) Percentage of original (blue) and new (red) GFP+ cells surviving >2 weeks (old-new) and <2 week (new-new) contacted by NF200+ nerve terminals at the end of the imaging period. Scale bars: 50µm.
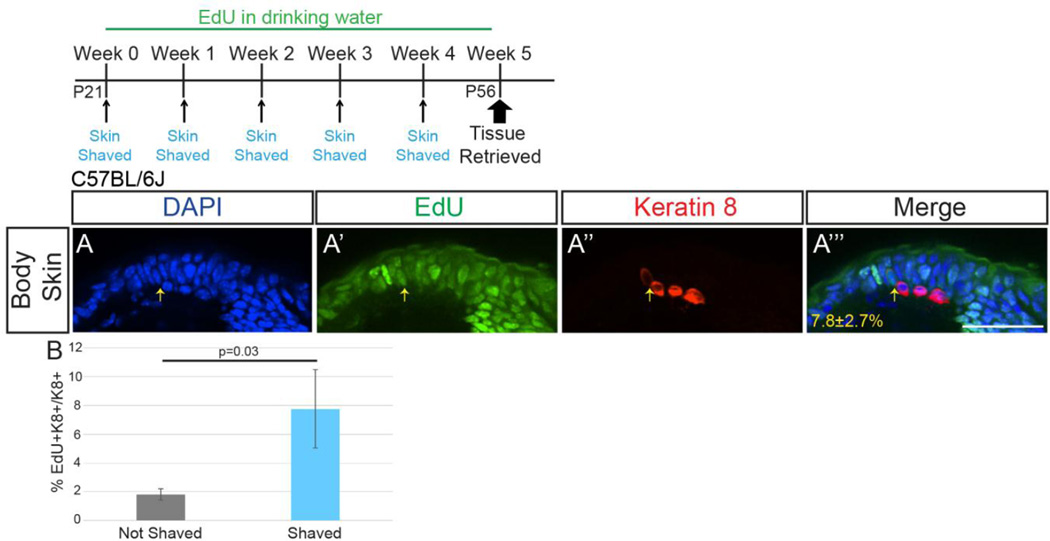
Interestingly, we noted the appearance of new and loss of original GFP+ cells in this model over time that was unaccounted for in our adult chronic EdU administration experiment (Figure 2C–C’’’). We hypothesized that this Merkel cell turnover was somehow linked to the repeated shaving necessary for imaging, being present only in this induced regenerative skin condition. To test this, we repeated the above adult EdU administration experiment (0.2mg/mL in drinking water for 5 weeks) while shaving a section of back and belly skin with a straight razor once weekly for four weeks, as had been done to prepare the skin for live imaging, then harvested skin and stained for K8 and EdU one week after the last shave (P56). K8+EdU+ cells made up 7.8±2.7% of the K8+ population at this time, a 430% increase in Merkel cell production over that seen without shaving (Figure 5; p=0.03, one-tailed t-test; n=2 mice; >150 K8+ cells/mouse). These data suggest that shaving of hairy skin induces cell division that leads to the production of large numbers of new Merkel cells, a process that occurs only rarely during normal skin homeostasis.
Figure 5. More Merkel cells are generated after shaving of skin.
(A) Sectioned back skin (single z-slice) from female P56 C57BL/6J mice that received 0.2mg/mL EdU in their drinking water for five weeks and were shaved once weekly. Tissue was processed for EdU (A’; green) and K8 immunostaining (A’’; red). Yellow arrow indicates a K8+EdU+ cell. Percentage of K8+ cells that were EdU+ shown (A’’’) (n=2 mice). (B) Average percent of K8+EdU+/K8+ cells in back and belly skin of shaved and unshaved mice (n=2–3 mice/condition). Bars on graph are SEMs. Scale bar: 50µm.
We wondered whether the production of new Merkel cells in this skin shaving paradigm led to increased numbers of these cells in touch domes over time. Our live imaging experiments showed that 3.14±0.6 new GFP+ cells per touch dome appeared each week throughout the imaging period, with no clear correlation with mouse age or hair cycle stage as judged by gross skin appearance (Figure S3A). However, average Merkel cell numbers/touch dome remained constant over time (Figure 4H), suggesting that Merkel cell production was offset by Merkel cell loss. New GFP+ cells had a lower median survival (21 days) relative to original GFP+ cells (84 days), and half of all new GFP+ cells disappeared within two weeks of creation (Figure 4I, Figure S4). The slope of the survival curve for new GFP+ cells within their first two weeks was significantly steeper than that of original GFP+ cells during that time (slope original = −0.83±0.1; slope new = −3.35±0.4; p=0.0005, t-test). However, continued survival of new GFP+ cells remaining 3 weeks after their creation was similar to that of original GFP+ cells (slope original = −0.53±0.1; slope new = −0.45±0.1; p=0.4, t-test; Figure 4I, Figure S4). After 21 weeks of imaging, 93% of new GFP+ cells co-expressed the Merkel cell marker K8 (182/195 GFP+K8+/K8+, n=1 mouse) confirming that they were Merkel cells. This quantification was performed on only a single mouse due to resource restrictions and the longevity of this experiment. These data show that new Merkel cells are at increased risk of death following their creation compared to original Merkel cells.
Survival of new Merkel cells is correlated with innervation
Merkel cell survival depends on innervation (English et al., 1983; Nurse et al., 1984; Xiao et al., 2015), so we hypothesized that survival of new GFP+ cells may be related to innervation by SA1 afferents. To test this, we immunostained skin harvested at 13 weeks for NF200 and quantified the number of GFP+ cells innervated by NF200+ endings. For this analysis, we divided GFP+ cells into three groups: 1) original cells, 2) cells >2 weeks old at the time of tissue retrieval and 3) cells arising ≤2 weeks prior to tissue retrieval. We found that fewer GFP+ cells arising ≤2 weeks prior to tissue retrieval (71.1±7.5%) than those >2 weeks old (84.9±5.2%) or original (91.8±2.5%) GFP+ cells were innervated (one-way ANOVA p=0.023; Tukeys post-hoc p=0.026 ≤2 weeks prior to tissue retrieval vs. original; p=0.5 >2 weeks old vs. original; n=255 original and n=323 new cells from n=2 mice; Figure 4J–K’’). These data suggest that prolonged survival of newly-generated Merkel cells is related to innervation status.
New Merkel cells arise from touch dome keratinocyte proliferation
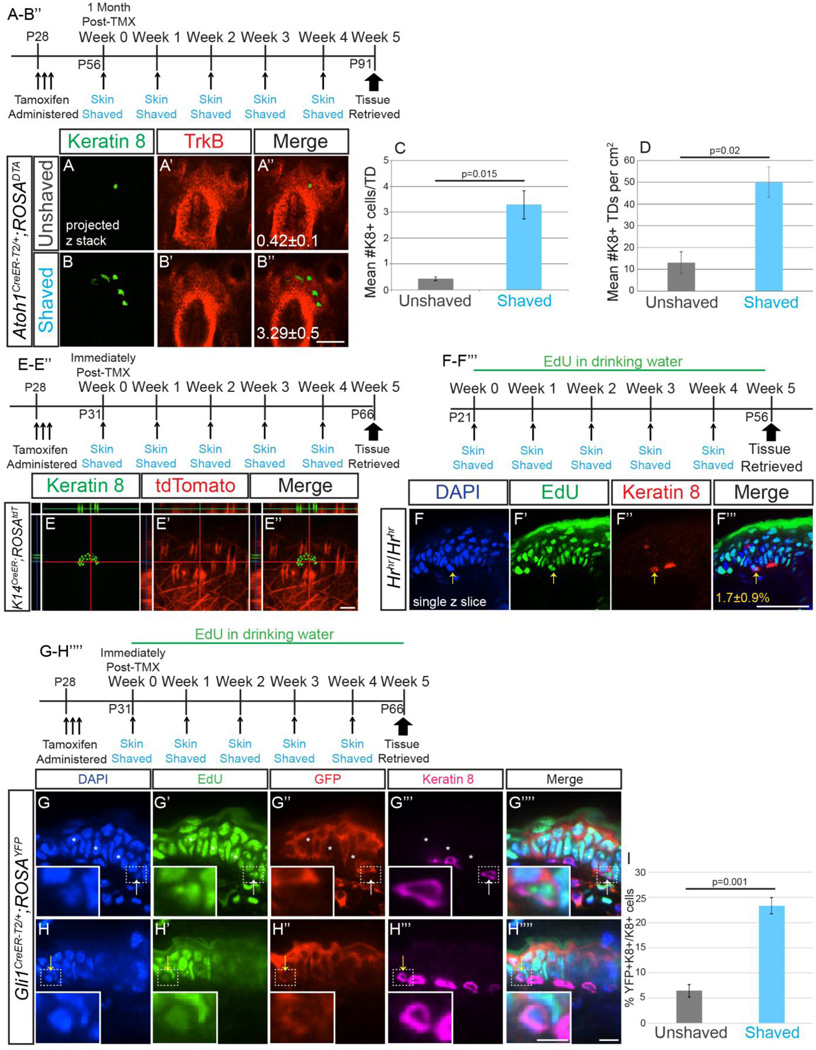
We previously reported that Merkel cells in adult mice derive from an Atoh1+ lineage (Wright et al., 2015). To determine whether new Merkel cell production after repeated shaving required existing Atoh1+ cells, we ablated these cells by administering tamoxifen (250mg/kg for 3 consecutive days) to Atoh1CreER-T2/+; ROSADTA mice, a paradigm in which Merkel cell numbers do not recover even six months post-tamoxifen administration (Wright et al., 2015). Consistent with our previous experiments, 28 days after tamoxifen administration Atoh1CreER-T2/+; ROSADTA mice had 98% fewer K8+ cells per touch dome than Atoh1CreER-T2/+; ROSADTA mice that did not receive tamoxifen (0.42±0.1 vs. 16.5±1.5 K8+ cells/TD; p=0.002, t-test; n=2–4 mice/genotype). However, Atoh1CreER-T2/+; ROSADTA mice (n=3) treated with tamoxifen that had their back and belly skin shaved once per week for four weeks had nearly 8× more Merkel cells than those that were not shaved (3.29±0.5 vs. 0.42±0.1 K8+ cells/TD; p=0.015, t-test; Figure 6A–C). Furthermore, the density of touch domes containing at least one K8+ cell was 4× higher in shaved vs. unshaved Atoh1CreER-T2/+; ROSADTA mice (50.0±7.1 vs. 13±5 per cm2; p=0.02, t-test; Figure 6D). In fact, touch dome density in shaved Atoh1CreER-T2/+; ROSADTA mice was equivalent to that in C57Bl/6J control mice (58±3 per cm2, p=0.38, t-test), indicating that essentially all touch domes in tamoxifen-treated, shaved Atoh1CreER-T2/+; ROSADTA mice can make new Merkel cells, while touch domes in treated, unshaved mice do not. These data demonstrate that neither the Atoh1 lineage nor signals from existing Merkel cells are necessary for the production of new Merkel cells induced by shaving.
Figure 6. New Merkel cells are derived from Gli1+ touch dome keratinocytes, not Atoh1+, K14+ or hair follicle lineages, and production does not require existing Merkel cells.
(A–B’’) Confocal z-stack projections of wholemount back skin from tamoxifen-treated unshaved (A–A’’) or shaved once weekly (B–B’’) Atoh1CreER-T2/+; ROSADTA mice immunostained for K8 (A, B; green) and TrkB (A’, B’; red). Numbers of K8+ cells (A’’, B’’) are shown. (C) Average number of K8+ cells per touch dome in shaved and unshaved back and belly skin of mice (n=2–3 mice/condition) (D) Average number of touch domes containing at least 1 K8+ cell per 1cm2 of back and belly skin (n=2–3 mice/condition). (E–E’’) Single z-slice confocal image of wholemount back skin from tamoxifen-treated K14CreER; ROSAtdTomato mouse shaved once weekly immunostained for K8 (green, E) and imaged for endogenous tdTomato (red, E’). (F–F’’’) Single confocal z-slice of sectioned belly skin from a hairless Hrhr/Hrhr mouse given EdU in drinking water and shaved once weekly for five weeks. Yellow arrow indicates a K8+EdU+ cell. Percentage of K8+EdU+/K8+ cells±SEM (F’’’) are shown (n=3 mice/treatment). (G–H’’’’) Single confocal z-slice of sectioned belly skin from tamoxifen-treated Gli1CreER;ROSAYFP mice shaved once weekly visualized for EdU (green, G’, H’) and immunostained for YFP (red, G’’, H’’) and K8 (magenta, G’’’, H’’’). Insets are of K8+EdU+ cells that are YFP− (G–G’’’’, white arrow) and YFP+ (H–H’’’’, yellow arrow). Asterisks indicate YFP− touch dome keratinocytes. (I) Quantification of the percent of K8+YFP+/K8+ cells in unshaved and shaved GliCreER;ROSAYFP mice. Bars on graph are SEMs. Scale bars: A–F’’’ 50µm; G–H’’’’ 10 µm, insets 5 µm.
We next asked whether K14+ epidermal cells found in interfollicular skin (Vasioukhin et al., 1999) gave rise to new Merkel cells following shaving. To label K14+ cells, we gave tamoxifen (250mg/kg for 3 consecutive days) to P28 K14CreER; ROSAtdTomato mice, then either shaved them or left them untouched for 5 consecutive weeks (n=3 mice/treatment). With this tamoxifen administration paradigm close to 100% of interfollicular epidermal cells demonstrate recombination, while hair follicles and touch dome keratinocytes recombine sparsely or not at all (Ostrowski et al., 2015; Wright et al., 2015). No K8+tdTomato+ cells were seen in either shaved or unshaved skin (>400 K8+ cells from 10 back and 10 belly touch domes per mouse; Figure 6E–E’’), demonstrating that K14+ progenitors do not give rise to new Merkel cells.
To test the contribution of hair follicle progenitors to Merkel cell generation, we administered EdU in the drinking water to Hairless (Hrhr/Hrhr) mice, which lack cycling hair follicles secondary to dysregulated differentiation of hair follicle progenitors (Benavides et al., 2009; Zarach et al., 2004). After shaving their belly skin once weekly for 4 weeks, we found that 1.7±0.9% of K8+ touch dome cells were also EdU+ in Hrhr/Hrhr mice, indicating that hair follicle progenitors are not needed for new Merkel cell generation (n>35 K8+ cells/mouse; n=3 mice; Figure 6F–F’’’).
Finally, to determine whether touch dome keratinocytes were the source of new Merkel cells, we administered tamoxifen (250mg/kg for 3 consecutive days) to Gli1CreER-T2; ROSAYFP mice and shaved their belly skin once weekly for 4 weeks or left them unshaved (n=3 mice/treatment). Tissue was analyzed one week after the last shave (P66) and K8+ cells analyzed for presence of YFP (Figure 6G–H’’’’). With this dosage paradigm, a portion of touch dome keratinocytes remained YFP−, indicating incomplete recombination of the population (Fig. 6G’’). Consistent with data from others (Xiao et al., 2015), we found that only a small proportion of K8+ cells were YFP+ in unshaved skin (6.5±1.3%; Fig. 6I). However, this percentage was much higher in shaved skin (23.3±1.6%, p=0.001, t-test; Fig. 6I), consistent with the observed increase in Merkel cell production after shaving. Notably, the percentage of K8+YFP+/K8+ cells is very close to the average number of new GFP+ cells persisting at 5 weeks of imaging (19.2±2.4%, p=0.24, t-test). Many K8+YFP+ cells were also EdU+, indicating that they arose through proliferation of recombined touch dome keratinocytes (Fig. 6H–H’’’’). These data demonstrate that touch dome keratinocytes give rise to new Merkel cells following skin shaving.
DISCUSSION
Our data provide several lines of evidence demonstrating that Merkel cells are long-lived, with Merkel cells born during embryogenesis surviving until at least 9 months of age in back and belly skin, whisker follicles and glabrous skin (Figure 1). This demonstrates that Merkel cells are by far the longest-lived post-mitotic epidermal cell population in mouse hairy and glabrous skin. We propose that long Merkel cell lifespans are critical for maintaining signaling fidelity between Merkel cells and innervating SA1 afferent fibers. Merkel cell turnover rates similar to those of other skin cells would necessitate repeated reestablishment of Merkel cell-neurite contacts. This would represent an unusual arrangement, as neurons typically form relatively stable contacts with cell populations that do not turn over (for example, other neurons or hair cells of the inner ear), and over time this could degrade the mechanosensory apparatus. Secondly, our data illustrate that while very few Merkel cells are generated during normal homeostasis in the first hair cycle, many more are formed after repeated shaving of the skin. This is a novel response that we hypothesize is secondary to mild superficial wound healing. We confirmed that these new Merkel cells arise from touch dome keratinocytes, as has been previously reported (Doucet et al., 2013; Xiao et al., 2014).
Our data show that touch dome Merkel cell numbers decrease by ~45% between E18.5 and P21 (Figure 3), demonstrating that initial production occurs in excess and is followed by a culling period. We hypothesize that this culling occurs secondary to limiting amounts of trophic factors, likely derived from SA1 afferents. In support of this hypothesis, large scale Merkel cell innervation occurs between E16.5 and birth (Pasche et al., 1990; Vielkind et al., 1995), timing that correlates well with the onset of the decline in Merkel cell numbers. Moreover, skin-derived overexpression of BDNF and NT3 leads to increased numbers of mature and innervated Merkel cells in glabrous and hairy skin, respectively (Albers et al., 1996; Botchkarev et al., 1999; LeMaster et al., 1999). Merkel cells develop normally in NT3-knockout mice but are lost by P14 along with their innervating afferents, suggesting that NT3 is required for the maintenance of Merkel cell neurite-complexes in hairy skin after birth (Airaksinen et al., 1996). Our data also demonstrate that Merkel cell numbers decrease slightly as mice age (Figure 3). This aging-related decline could result from waning trophic support from the nerve, decreased Merkel cell replacement following injury, or a combination of the two. Further experiments are necessary to test the involvement of BDNF and/or NT3 in adult Merkel cell maintenance during skin homeostasis and injury. Either way, declines in Merkel cell numbers could play an important role in the pathophysiology of aging-related somatosensory deficits. Further studies are needed to directly address this possibility.
Unlike previous studies (Moll et al., 1996; Nakafusa et al., 2006), we did not observe changes in touch dome Merkel cell numbers during either natural or induced hair cycles (Figure 3). This conclusion is supported by our live imaging data (Figure 4H) and lack of a significant change in percentage of K8+EdU+ cells in touch domes between P0 and 9 months of age (Figure 1A–B’’’, H). These data provide strong evidence that Merkel cell numbers do not fluctuate during the course of the hair cycle. We believe that the discrepancy between our observations and previously published work is most likely secondary to methodological differences. Our Merkel cell counts at various stages of the natural and induced hair cycles were done in wholemount preparations of skin, thereby insuring that no cells were lost during tissue processing. Analysis of epidermal sheets (Nakafusa et al., 2006) may have led to inaccurate estimates of Merkel cell numbers, as it is our experience that >50% of Merkel cells can adhere to the dermal surface in these preps (unpublished observations). Numbers of Merkel cells that stick to the dermal surface could change during different stages of the hair cycle, leading to the erroneous conclusion that Merkel cell numbers were changing. Likewise, counts done on small amounts of serially-sectioned mouse skin (Moll et al., 1996) could also bias whole Merkel cell quantifications.
Our chronic EdU administration experiments in adult mice showed that 1.8% of touch dome Merkel cells became EdU+ over 5 weeks, but that no new cells were produced in whisker follicles or glabrous skin (Figure 2). Assuming a constant rate of production and survival, approximately 0.36% of touch dome Merkel cells are newly generated each week (1.8%/5 weeks = 0.36%). If this is projected out to 9 months of age (the oldest age that we examined), we would expect that 14% of Merkel cells should be new (0.36%*39 weeks = 14%). Following E14.5–E16.5 EdU administration, we found that 45.1±2.5% and 41.9±2.1% of K8+ cells were EdU+ at P0 and 9 months of age, respectively. Therefore, we would predict that 38.8% of 9 month old K8+ cells would retain EdU+ in the embryonic EdU administration experiment (45.1%*(1–0.14) = 38.8%). This number is close to the observed 41.9%, suggesting that our calculations are likely accurate. However, the difference between the percentages of touch dome K8+ cells that were also EdU+ at P0 and 9 months was not statistically significant, likely because the study was underpowered at n=4 mice/age. Regardless, our data support the conclusion that, in touch domes, there is a very low rate of Merkel cell turnover associated with normal skin homeostasis.
Interestingly, we found that Merkel cell homeostasis differs between touch dome Merkel cells and those that reside in whisker follicles and glabrous skin of the forepaw. Greater than 750 and 220 K8+ cells, respectively, were counted in each of these two locations and no Merkel cell was found to have incorporated EdU during 3–8 weeks of age. We predict that this difference in Merkel cell production is due to the proximity of Merkel cells to the epidermal border, and therefore higher potential exposure to environmental insults. Glabrous skin is much thicker than hairy skin, and Merkel cells of the whisker follicle are very deep to the epidermis, likely providing a protective barrier permitting Merkel cell persistence. The proportion of EdU+ Merkel cells that surround whisker follicles did not decrease between P0 and 9 months of age, consistent with a lack of turnover and replacement. However, Merkel cells of the glabrous skin did decrease between P0 and P21. We have noted that Merkel cells of the paw are generated later in development than Merkel cells of the back and belly skin (Reed-Geaghan et al., 2016), likely explaining this decrease in percentage of cells in early postnatal life. Consistent with a lack of new Merkel cell production in glabrous skin that we noted from chronic EdU exposure from 3–8 weeks of age, the percentage of EdU+ Merkel cells from P21 to 9 months of age is again unchanged.
A serendipitous and surprising finding of our study supported by live imaging and chronic EdU administration experiments is that repeated shaving induces Merkel cell death and creation of new Merkel cells in touch domes (Figs. 2, 5). Genetic deletion of adult Merkel cells alone following tamoxifen administration to adult Atoh1CreER-T2; ROSADTA mice was insufficient to induce new Merkel cell production (Wright et al., 2015) (Figure 6). This is an important finding because it shows that no intrinsic counting mechanism exists to determine when Merkel cell production is required. Rather, we conclude that Merkel cell loss coupled to signals induced by repetitive shaving is necessary for Merkel cell production. We hypothesize that these signals arise in the epidermis following skin injury (Hardy et al., 2003; Lai et al., 2012). The identity and cellular origins of these signals, and what types of skin manipulation/injury are capable of inducing them, require further study.
The existence and identity of the precursor cells that maintain the adult touch dome Merkel cell population has been a source of controversy. Based on fate mapping and conditional deletion studies viewed in light of presumed Merkel cell turnover in adult animals, we recently proposed that Atoh1+ progenitors performed this role (Wright et al., 2015). Our new data force a reconsideration of this interpretation. Because adult Merkel cells express Atoh1 (Lumpkin et al., 2003; Ostrowski et al., 2015), fate mapping in our previous study marked all Merkel cells. Therefore, what we observed previously was undoubtedly the long-term survival of post-mitotic Merkel cells in embryonic and adult mice, not replacement of dying cells by an Atoh1+ progenitor. This explains the very low percentage of Ki67+ Merkel cells that we saw in adult mice (Wright et al., 2015). Of note, our new data do not change the interpretation of our observation that some embryonic Atoh1+ cells multiply, a conclusion substantiated by EdU incorporation and expansion of lineage-traced cell numbers during embryogenesis (Wright et al., 2015). We also showed previously that tamoxifen administration to adult Atoh1CreER-T2; ROSADTA mice, where diphtheria toxin A expression is driven in Atoh1-expressing cells, led to Merkel cell death without subsequent replacement (Wright et al., 2015). Again, we interpreted this finding as evidence that Atoh1+ progenitors maintain the adult Merkel cell population, and that elimination of those cells prevented creation of new Merkel cells in this paradigm. Rather, we likely deleted only post-mitotic cells, and this action alone was insufficient to induce new Merkel cell formation (see below). Furthermore, our fate-mapping and EdU labeling data in K14CreER; ROSAtdTomato and Hrhr/Hrhr mice (Figure 6) show that new Merkel cells do not arise from K14+ cells nor hair follicle progenitors as have been previously proposed (Van Keymeulen et al., 2009). Given these data, the presence of columnar K8+EdU+ cells in the touch dome epithelium following repeated shaving (Figure 5), and our identification of K8+YFP+EdU+ cells in shaved, tamoxifen-treated Gli1CreERT2; ROSAYFP mice (Figure 6), we agree with recent reports (Doucet et al., 2013; Woo et al., 2010; Xiao et al., 2015) suggesting that new Merkel cells arise from K17+/Gli1+ progenitors in touch domes of adult mice.
Our EdU labeling data support the conclusion that new Merkel cells created in the setting of skin injury arise through cell division. However, we did find K8+YFP+ cells in tamoxifen-treated, shaved Gli1CreER-T2;ROSAYFP mice that had not incorporated EdU. This is possibly due to not all proliferating cells being labeled with this EdU administration paradigm, a subpopulation of K8+ cells expressing Gli1, and/or transdifferentiation of Gli1+ cells. We favor the first interpretation, as only 2% of K8+ cells express Gli1 and there has been no evidence of Merkel cell transdifferentiation from other skin cell populations (Xiao et al., 2015).
Our observations may have relevance for understanding genesis of Merkel cell carcinoma (MCC), a rare but aggressive skin cancer. Given similarities in the expression of molecular markers, it is likely that MCC arises from Merkel cell progenitors (Eng et al., 2007; Leonard et al., 2002; Tang and Toker, 1978; Tilling and Moll, 2012). Our data suggest that induction of Merkel cell production following even mild skin wounds may, in combination with Merkel cell polyomavirus infection (Feng et al., 2008; Shuda et al., 2015) and/or UV radiation, provide another “hit” that leads to oncogenesis. Identifying the signaling pathways responsible for Merkel cell progenitor activation could therefore provide insight into the molecular pathways responsible for initiating this devastating cancer.
MATERIALS AND METHODS
Mice
Female C57BL/6J (JAX 000664), Atoh1GFP (JAX 013593;(Lumpkin et al., 2003)), Atoh1CreER-T2 ((Fujiyama et al., 2009)), Hairless (Charles River Crl:SKH1-Hrhr), K14CreER (JAX 005107; (Vasioukhin et al., 1999)), ROSAtdTomato (JAX 007914; (Madisen et al., 2010)), and ROSADTA (JAX 009669; (Voehringer et al., 2008)) mice were maintained in accordance with International Animal Care and Use Committee guidelines at the Children’s Hospital of Pittsburgh of the University of Pittsburgh Medical Center. For embryonic ages, the plug date was designated as E0.5. Mice for live imaging were anesthetized with 100mg/kg ketamine, 10mg/kg xylazine mixture. Embryonic and early postnatal mice were determined to be female by analyzing internal reproductive structures post-mortem.
Tamoxifen and EdU administration
Tamoxifen (Sigma-Aldrich) was dissolved in a 9:1 corn oil/ethanol solution at a 5% concentration. Mice were briefly anesthetized with isoflurane and tamoxifen administered by oral gavage at a dose of 250mg/kg once daily for three consecutive days. For embryonic administration, EdU (Invitrogen) was dissolved in sterile phosphate buffered saline (PBS) at a 10mM concentration and administered by intraperitoneal injection at a dose of 10mg/kg to pregnant females. For adult administration, EdU was dissolved in ddH2O water at a 0.2 mg/ml concentration and provided ad libitum for five weeks.
Tissue processing
Adult mice were euthanized by cervical dislocation, their skin shaved with an electric razor, depilated with Surgicream, and dissected into cold PBS. Embryos were dissected from pregnant dams and decapitated before tissue dissection. Skin processed for immunohistochemistry was fixed in 4% paraformaldehyde for 30–60 minutes (adult tissue) or overnight (whole embryos, P0, and P3 mice) and washed in PBS. Tissue for cryosectioning was cryopreserved in 30% sucrose/PBS.
Histology
Tissue was embedded in optimum cutting temperature (O.C.T.; Thermo Fisher Scientific) and serially sectioned on a cryostat (1950M; Leica) at 25µm. Slides were vacuum dried, rehydrated in PBS, and blocked with 5% normal donkey serum in 0.3%PBS-T (PBS with Triton X-100). EdU was detected with an imaging kit (Click-iT EdU; Invitrogen) and slides pre-treated with 2N HCl for 15 minutes. Slides were incubated overnight in blocking solution containing dilutions of the following primary antibodies: chicken anti-GFP (1:1,000; GFP-1010 Aves Labs), goat anti-TrkB (1:200; AF1494; R&D Systems), rabbit anti-NF200 (Sigma- Aldrich, NF142; 1:500), and rat anti-keratin 8 (1:20; TROMA-1; Developmental Studies Hybridization Bank). After primary antibody incubation, sections were washed and incubated for 30 minutes at room temperature in blocking solution containing the appropriate secondary antibodies obtained from Jackson ImmunoResearch Laboratories, Inc. (1:500): Alexa Fluor 488-conjugated donkey anti-rat, Alexa Fluor 488-conjugated donkey anti-chicken, Cy3-conjugated donkey anti-chicken, Cy3-conjugated donkey anti-goat, Cy3-conjugated donkey anti-rabbit, and/or Cy3-conjugated donkey anti-rat. Sections were stained with the nuclear probe DAPI (1:1000; Thermo Fisher Scientific) to visualize nuclei and mounted in ProLong Gold (Invitrogen). Whole-mount immunostaining was performed on pelts of hairy skin. Fixed skin was dissected, underlying adipose tissue removed, and washed for 5–8 hours in 0.3% PBS-T. Tissue was incubated with primary antibodies for 3 (embryonic skin) or 4 (adult skin) days, washed for 5–8 hours in 0.3% PBS-T, and then incubated with secondary antibodies for 1 (embryonic skin) or 2 (adult skin) days, all at room temperature. Antibodies were diluted in 20% dimethyl sulfoxide/5% normal donkey serum/0.3% PBS-T.
Imaging
Confocal images for live imaging were acquired with spinning-disc confocal imaging system (UltraVIEW VoX; PerkinElmer) utilizing a sensitive EM-CCD camera (C9100-13; Hamamatsu Photonics) allowing for minimal light exposure and photoxicity. The system was coupled to an inverted microscope (Axio Observer; Carl Zeiss) with a C-Apochromat 40×, 1.1 NA water immersion objective. Images were obtained minimizing light exposure and resulting photoxicity and analyzed with the Volocity (Perkin Elmer) Acquisition and Analysis software. Images presented here are maximum intensity projections of a z-series or single axial slices (as noted in the Figures) consisting of 10µm optical slices collected every 0.45µm. For in vivo imaging of Atoh1GFP touch domes, mice were placed on a specially designed platform with their belly skin on a coverslip. A 10× objective with 1.6× optivar was used to capture Z-stacks of 120µm thickness with single images taken every 3µm. Presented images are projections of the entire Z-stack. Mice were repeatedly shaved and imaged once a week for 13–21 weeks, at which time they were sacrificed, tissue retrieved and immunostained for GFP and NF200 (week 13) or GFP and K8 (week 21). Touch domes were identified from week to week based on their location to the square drawn on their bellies and their proximity to other touch domes (Figure S3A). Cells were classified as original or new based on positioning relative to the hair follicle and other cells from week to week. Non-confocal images were acquired with a Leica DM5500B fluorescent microscope using HCX Plan Apochromat 40×, 1.25 NA and HC Plan Apochromat 10×, 0.4 NA objectives, Leica DFC420 camera and Leica Acquisition Software v4.2. Images were cropped and brightness and contrast enhanced for publication quality with Adobe Photoshop and/or Illustrator.
Cell counts
All cell counts were done on the mid-back and belly skin of mice. For K8+ cell counts from E18.5 to 20 weeks of age at least 20 touch domes per mouse were counted for each back and belly (n=3–5 mice/age). Cell counts for K8+EdU+ co-label in C57BL/6J and Hr/Hr mice, K8+tdTomato+ co-label in K14CreER; ROSAtdTomato mice, and K8+YFP+EdU+ co-label in Gli1CreER-T2;ROSAYFP mice were done on single slices of confocal z-stacked images (>100 K8+ cells/tissue/mouse). Statistical tests were students t-test (Excel) or one way ANOVA followed by post-hoc Tukeys multiple comparisons testing (Prism).
Supplementary Material
Cryosectioned hematoxylin and eosin-stained back skin from female C57Bl/6J mice (A–L) and from undepilated (M–Q) or depilated (R–V) regions of adolescent mice. Ages (A–L) or days after depilation (M–V) and hair cycle stages are indicated in the panels. Scale bar: 50µm.
Back skin from female C57Bl/6J mice was wholemount immunostained for Keratin 8 (green). Panels represent different quantified touch domes. Scale bar: 50µm.
(A) Belly skin of mouse #1145 prior to imaging and over 13 weeks showing skin pigment changes suggestive of progression through the hair cycle. Images used to track individual touch domes (marked by unique colors) are shown below for each week. Average Merkel cell numbers per touch dome (B–E) and number of new Merkel cells added (B’–E’) throughout the imaging periods. Gray bars denote anagen. Red graphs are from mouse in panel A. SEMs are shown on each graph.
Survival of original (blue) and new (red) GFP+ cells over the imaging periods (A, B - 21 weeks; C, D – 13 weeks). Slopes for survival curves of original and new cells at <2 and >2 weeks for individual mice are shown within each graph.
Highlights.
Embryonic Merkel cells persist long into adulthood
Average Merkel cell number does not change with the hair cycle
Few Merkel cells are generated in adulthood
Skin shaving induces Merkel cell production
Merkel cells likely arise from proliferation of Gli1+ progenitors
Acknowledgments
We thank members of the Maricich and Fyffe-Maricich labs, Drs. Kathryn Albers, Brian Davis, Sharyl Fyffe-Maricich, H. Richard Koerber, Erin Reed-Geaghan and Sarah Ross for their thoughtful discussions and input. This work was supported by NIH AR059114 (SMM) and the Richard King Mellon Foundation Institute for Pediatric Research (SMM, TAS).
List of Symbols/Abbreviations Used
- DTA
diphtheria toxin subunit A
- E
Embryonic age
- EdU
5-ethynyl-2'-deoxyuridine
- GFP
green fluorescent protein
- K8
Keratin 8
- MCC
Merkel cell carcinoma
- P
Postnatal age in days
- SA1
Slowly adapting type 1 afferent
- YFP
yellow fluorescent protein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This work was performed in Pittsburgh, Pennsylvania, USA.
Author Contributions
MCW and SMM designed the study; MCW, GJL, AMB, ACK, JAH and TAS performed the experiments; MCW, GJL, and SMM analyzed the data; MCW and SMM wrote the manuscript with input from the other authors.
The authors declare no competing financial interests.
REFERENCES
- Airaksinen MS, Koltzenburg M, Lewin GR, Masu Y. Specific Subtypes of Cutaneous Mechanoreceptors Require Neurotrophin-3 Following Peripheral Target Innervation. Neuron. 1996 doi: 10.1016/s0896-6273(00)80047-1. [DOI] [PubMed] [Google Scholar]
- Albers KM, Perrone TN, Goodness TP, Jones ME, Green MA, Davis BM. Cutaneous overexpression of NT-3 increases sensory and sympathetic neuron number and enhances touch dome and hair follicle innervation. J. Cell Biol. 1996;134:487–497. doi: 10.1083/jcb.134.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benavides F, Oberyszyn TM, VanBuskirk AM, Reeve VE, Kusewitt DF. The hairless mouse in skin research. J. Dermatol. Sci. 2009;53:10–18. doi: 10.1016/j.jdermsci.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botchkarev VA, Kief S, Paus R, Moll I. Overexpression of brain-derived neurotrophic factor increases Merkel cell number in murine skin. J. Invest. Dermatol. 1999;113:691–692. doi: 10.1046/j.1523-1747.1999.00725.x. [DOI] [PubMed] [Google Scholar]
- Doucet YS, Woo S-H, Ruiz ME, Owens DM. The touch dome defines an epidermal niche specialized for mechanosensory signaling. Cell Rep. 2013;3:1759–1765. doi: 10.1016/j.celrep.2013.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng TY, Boersma MG, Fuller CD, Goytia V, Jones WE, Joyner M, Nguyen DD. A comprehensive review of the treatment of Merkel cell carcinoma. Am. J. Clin. Oncol. 2007;30:624–636. doi: 10.1097/COC.0b013e318142c882. [DOI] [PubMed] [Google Scholar]
- English KB, Norman D, Horch K. Effects of chronic denervation in type I cutaneous mechanoreceptors (Haarscheiben) The Anatomical Record. 1983 doi: 10.1002/ar.1092070109. [DOI] [PubMed] [Google Scholar]
- Feng H, Shuda M, Chang Y, Moore PS. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science. 2008;319:1096–1100. doi: 10.1126/science.1152586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiyama T, Yamada M, Terao M, Terashima T, Hioki H, Inoue YU, Inoue T, Masuyama N, Obata K, Yanagawa Y, Kawaguchi Y, Nabeshima Y-I, Hoshino M. Inhibitory and excitatory subtypes of cochlear nucleus neurons are defined by distinct bHLH transcription factors, Ptf1a and Atoh1. Development. 2009;136:2049–2058. doi: 10.1242/dev.033480. [DOI] [PubMed] [Google Scholar]
- Ganusov VV, De Boer RJ. A mechanistic model for bromodeoxyuridine dilution naturally explains labelling data of self-renewing T cell populations. J R Soc Interface. 2012 doi: 10.1098/rsif.2012.0617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halata Z, Grim M, Bauman KI. Friedrich Sigmund Merkel and his “Merkel cell,” morphology, development, and physiology: review and new results. Anat Rec A Discov Mol Cell Evol Biol. 2003;271:225–239. doi: 10.1002/ar.a.10029. [DOI] [PubMed] [Google Scholar]
- Hardy MM, Blomme EAG, Lisowski A, Chinn KS, Jones A, Harmon JM, Opsahl A, Ornberg RL, Tripp CS. Selective cyclooxygenase-2 inhibition does not alter keratinocyte wound responses in the mouse epidermis after abrasion. J. Pharmacol. Exp. Ther. 2003;304:959–967. doi: 10.1124/jpet.102.044545. [DOI] [PubMed] [Google Scholar]
- Horsley V, O'Carroll D, Tooze R, Ohinata Y, Saitou M, Obukhanych T, Nussenzweig M, Tarakhovsky A, Fuchs E. Blimp1 defines a progenitor population that governs cellular input to the sebaceous gland. Cell. 2006;126:597–609. doi: 10.1016/j.cell.2006.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu Y-C, Pasolli HA, Fuchs E. Dynamics between stem cells, niche, and progeny in the hair follicle. Cell. 2011;144:92–105. doi: 10.1016/j.cell.2010.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iggo A, Muir AR. The structure and function of a slowly adapting touch corpuscle in hairy skin. J. Physiol. (Lond.) 1969;200:763–796. doi: 10.1113/jphysiol.1969.sp008721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M, Liu Y, Yang Z, Nguyen J, Liang F, Morris RJ, Cotsarelis G. Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat. Med. 2005;11:1351–1354. doi: 10.1038/nm1328. [DOI] [PubMed] [Google Scholar]
- Jaks V, Barker N, Kasper M, van Es JH, Snippert HJ, Clevers H, Toftgård R. Lgr5 marks cycling, yet long-lived, hair follicle stem cells. Nat. Genet. 2008;40:1291–1299. doi: 10.1038/ng.239. [DOI] [PubMed] [Google Scholar]
- Jensen KB, Collins CA, Nascimento E, Tan DW, Frye M, Itami S, Watt FM. Lrig1 Expression Defines a Distinct Multipotent Stem Cell Population in Mammalian Epidermis. Cell Stem Cell. 2009;4:427–439. doi: 10.1016/j.stem.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KO, Hsiao SS. Neural mechanisms of tactual form and texture perception. Annu. Rev. Neurosci. 1992;15:227–250. doi: 10.1146/annurev.ne.15.030192.001303. [DOI] [PubMed] [Google Scholar]
- Johnson KO, Lamb GD. Neural mechanisms of spatial tactile discrimination: neural patterns evoked by braille-like dot patterns in the monkey. J. Physiol. (Lond.) 1981;310:117–144. doi: 10.1113/jphysiol.1981.sp013540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiel MJ, He S, Ashkenazi R, Gentry SN, Teta M, Kushner JA, Jackson TL, Morrison SJ. Haematopoietic stem cells do not asymmetrically segregate chromosomes or retain BrdU. Nature. 2007;449:238–242. doi: 10.1038/nature06115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai Y, Li D, Li C, Muehleisen B, Radek KA, Park HJ, Jiang Z, Li Z, Lei H, Quan Y, Zhang T, Wu Y, Kotol P, Morizane S, Hata TR, Iwatsuki K, Tang C, Gallo RL. The antimicrobial protein REG3A regulates keratinocyte proliferation and differentiation after skin injury. Immunity. 2012;37:74–84. doi: 10.1016/j.immuni.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeMaster AM, Krimm RF, Davis BM, Noel T, Forbes ME, Johnson JE, Albers KM. Overexpression of brain-derived neurotrophic factor enhances sensory innervation and selectively increases neuron number. J. Neurosci. 1999;19:5919–5931. doi: 10.1523/JNEUROSCI.19-14-05919.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard JH, Cook AL, Van Gele M, Boyle GM, Inglis KJ, Speleman F, Sturm RA. Proneural and proneuroendocrine transcription factor expression in cutaneous mechanoreceptor (Merkel) cells and Merkel cell carcinoma. Int. J. Cancer. 2002;101:103–110. doi: 10.1002/ijc.10554. [DOI] [PubMed] [Google Scholar]
- Levy V, Lindon C, Harfe BD, Morgan BA. Distinct stem cell populations regenerate the follicle and interfollicular epidermis. Dev. Cell. 2005;9:855–861. doi: 10.1016/j.devcel.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Levy V, Lindon C, Zheng Y, Harfe BD, Morgan BA. Epidermal stem cells arise from the hair follicle after wounding. The FASEB Journal. 2007 doi: 10.1096/fj.06-6926com. [DOI] [PubMed] [Google Scholar]
- Lu CP, Polak L, Rocha AS, Pasolli HA, Chen S-C, Sharma N, Blanpain C, Fuchs E. Identification of stem cell populations in sweat glands and ducts reveals roles in homeostasis and wound repair. Cell. 2012;150:136–150. doi: 10.1016/j.cell.2012.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumpkin EA, Collisson T, Parab P, Omer-Abdalla A, Haeberle H, Chen P, Doetzlhofer A, White P, Groves A, Segil N, Johnson JE. Math1-driven GFP expression in the developing nervous system of transgenic mice. Gene Expr. Patterns. 2003;3:389–395. doi: 10.1016/s1567-133x(03)00089-9. [DOI] [PubMed] [Google Scholar]
- Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR, Lein ES, Zeng H. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 2010;13:133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maricich SM, Morrison KM, Mathes EL, Brewer BM. Rodents rely on Merkel cells for texture discrimination tasks. J. Neurosci. 2012;32:3296–3300. doi: 10.1523/JNEUROSCI.5307-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maricich SM, Wellnitz SA, Nelson AM, Lesniak DR, Gerling GJ, Lumpkin EA, Zoghbi HY. Merkel cells are essential for light-touch responses. Science. 2009;324:1580–1582. doi: 10.1126/science.1172890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll I, Paus R, Moll R. Merkel cells in mouse skin: intermediate filament pattern, localization, and hair cycle-dependent density. J. Invest. Dermatol. 1996;106:281–286. doi: 10.1111/1523-1747.ep12340714. [DOI] [PubMed] [Google Scholar]
- Müller-Röver S, Handjiski B, van der Veen C, Eichmüller S, Foitzik K, McKay IA, Stenn KS, Paus R. A comprehensive guide for the accurate classification of murine hair follicles in distinct hair cycle stages. J. Invest. Dermatol. 2001;117:3–15. doi: 10.1046/j.0022-202x.2001.01377.x. [DOI] [PubMed] [Google Scholar]
- Nakafusa J, Narisawa Y, Shinogi T, Taira K, Tanaka T, Inoue T, Misago N. Changes in the number of Merkel cells with the hair cycle in hair discs on rat back skin. Br. J. Dermatol. 2006;155:883–889. doi: 10.1111/j.1365-2133.2006.07441.x. [DOI] [PubMed] [Google Scholar]
- Nurse CA, Macintyre L, Diamond J. A quantitative study of the time course of the reduction in Merkel cell number within denervated rat touch domes. Neuroscience. 1984;11:521–533. doi: 10.1016/0306-4522(84)90042-3. [DOI] [PubMed] [Google Scholar]
- Ostrowski SM, Wright MC, Bolock AM, Geng X, Maricich SM. Ectopic Atoh1 expression drives Merkel cell production in embryonic, postnatal and adult mouse epidermis. Development. 2015;142:2533–2544. doi: 10.1242/dev.123141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page ME, Lombard P, Ng F, Göttgens B, Jensen KB. The epidermis comprises autonomous compartments maintained by distinct stem cell populations. Cell Stem Cell. 2013;13:471–482. doi: 10.1016/j.stem.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasche F, Mérot Y, Carraux P, Saurat JH. Relationship between Merkel cells and nerve endings during embryogenesis in the mouse epidermis. J. Invest. Dermatol. 1990;95:247–251. doi: 10.1111/1523-1747.ep12484847. [DOI] [PubMed] [Google Scholar]
- Reed-Geaghan EG, Wright MC, See LA, Adelman PC, Lee KH, Koerber HR, Maricich SM. Merkel Cell-Driven BDNF Signaling Specifies SAI Neuron Molecular and Electrophysiological Phenotypes. J. Neurosci. 2016;36:4362–4376. doi: 10.1523/JNEUROSCI.3781-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuda M, Guastafierro A, Geng X, Shuda Y, Ostrowski SM, Lukianov S, Jenkins FJ, Honda K, Maricich SM, Moore PS, Chang Y. Merkel Cell Polyomavirus Small T Antigen Induces Cancer and Embryonic Merkel Cell Proliferation in a Transgenic Mouse Model. PLoS ONE. 2015;10:e0142329. doi: 10.1371/journal.pone.0142329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang CK, Toker C. Trabecular carcinoma of the skin: an ultrastructural study. Cancer. 1978;42:2311–2321. doi: 10.1002/1097-0142(197811)42:5<2311::aid-cncr2820420531>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Tilling T, Moll I. Which are the cells of origin in merkel cell carcinoma? J Skin Cancer. 2012;2012:680410. doi: 10.1155/2012/680410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Keymeulen A, Mascre G, Youseff KK, Harel I, Michaux C, De Geest N, Szpalski C, Achouri Y, Bloch W, Hassan BA, Blanpain C. Epidermal progenitors give rise to Merkel cells during embryonic development and adult homeostasis. J. Cell Biol. 2009;187:91–100. doi: 10.1083/jcb.200907080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasioukhin V, Degenstein L, Wise B, Fuchs E. The magical touch: genome targeting in epidermal stem cells induced by tamoxifen application to mouse skin. Proc. Natl. Acad. Sci. U.S.A. 1999;96:8551–8556. doi: 10.1073/pnas.96.15.8551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vielkind U, Sebzda MK, Gibson IR, Hardy MH. Dynamics of Merkel cell patterns in developing hair follicles in the dorsal skin of mice, demonstrated by a monoclonal antibody to mouse keratin 8. Acta Anat (Basel) 1995;152:93–109. doi: 10.1159/000147688. [DOI] [PubMed] [Google Scholar]
- Voehringer D, Liang H-E, Locksley RM. Homeostasis and effector function of lymphopenia-induced “memory-like” T cells in constitutively T cell-depleted mice. J. Immunol. 2008;180:4742–4753. doi: 10.4049/jimmunol.180.7.4742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo S-H, Stumpfova M, Jensen UB, Lumpkin EA, Owens DM. Identification of epidermal progenitors for the Merkel cell lineage. Development. 2010;137:3965–3971. doi: 10.1242/dev.055970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright MC, Reed-Geaghan EG, Bolock AM, Fujiyama T, Hoshino M, Maricich SM. Unipotent, Atoh1+ progenitors maintain the Merkel cell population in embryonic and adult mice. J. Cell Biol. 2015;208:367–379. doi: 10.1083/jcb.201407101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y, Thoresen DT, Williams JS, Wang C, Perna J, Petrova R, Brownell I. Neural Hedgehog signaling maintains stem cell renewal in the sensory touch dome epithelium. Proc. Natl. Acad. Sci. U.S.A. 2015;112:7195–7200. doi: 10.1073/pnas.1504177112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y, Williams JS, Brownell I. Merkel cells and touch domes: more than mechanosensory functions? Exp. Dermatol. 2014;23:692–695. doi: 10.1111/exd.12456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarach JM, Beaudoin GMJ, Coulombe PA, Thompson CC. The co-repressor hairless has a role in epithelial cell differentiation in the skin. Development. 2004;131:4189–4200. doi: 10.1242/dev.01303. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Cryosectioned hematoxylin and eosin-stained back skin from female C57Bl/6J mice (A–L) and from undepilated (M–Q) or depilated (R–V) regions of adolescent mice. Ages (A–L) or days after depilation (M–V) and hair cycle stages are indicated in the panels. Scale bar: 50µm.
Back skin from female C57Bl/6J mice was wholemount immunostained for Keratin 8 (green). Panels represent different quantified touch domes. Scale bar: 50µm.
(A) Belly skin of mouse #1145 prior to imaging and over 13 weeks showing skin pigment changes suggestive of progression through the hair cycle. Images used to track individual touch domes (marked by unique colors) are shown below for each week. Average Merkel cell numbers per touch dome (B–E) and number of new Merkel cells added (B’–E’) throughout the imaging periods. Gray bars denote anagen. Red graphs are from mouse in panel A. SEMs are shown on each graph.
Survival of original (blue) and new (red) GFP+ cells over the imaging periods (A, B - 21 weeks; C, D – 13 weeks). Slopes for survival curves of original and new cells at <2 and >2 weeks for individual mice are shown within each graph.