Abstract
Objective
To measure the volume of the endolymph drainage system in temporal bone specimens with Meniere’s disease, as compared with specimens with endolymphatic hydrops without vestibular symptoms and with nondiseased specimens
Study Design
Comparative human temporal bone analysis
Methods
We generated 3-dimensional models of the vestibular aqueduct, endolymphatic sinus and duct, and intratemporal portion of the endolymphatic sac and calculated the volume of those structures. We also measured the internal and external aperture of the vestibular aqueduct, as well as the opening (if present) of the utriculoendolymphatic (Bast’s) valve and compared the measurements in our 3 study groups.
Results
The volume of the vestibular aqueduct and of the endolymphatic sinus, duct, and intratemporal endolymphatic sac was significantly lower in the Meniere’s disease group than in the endolymphatic hydrops group (P<0.05). The external aperture of the vestibular aqueduct was also smaller in the Meniere’s disease group. Bast’s valve was open only in some specimens in the Meniere’s disease group.
Conclusion
In temporal bones with Meniere’s disease, the volume of the vestibular aqueduct, endolymphatic duct, and intratemporal endolymphatic sac was lower, and the external aperture of the vestibular aqueduct was smaller as compared with bones from donors who had endolymphatic hydrops without vestibular symptoms and with nondiseased bones. The open status of the Bast’s valve in the Meniere’s group could be secondary to higher retrograde endolymph pressures caused by smaller drainage systems. These anatomic findings could correlate with the reason that some patients with hydrops develop clinical symptoms, while others do not.
Keywords: Histopathology, endolymphatic hydrops, Meniere’s disease, endolymphatic duct, endolymphatic sac, endolymphatic sinus, vestibular aqueduct, utriculoendolymphatic valve, Bast’s valve, human temporal bone, volume, 3D reconstruction, volume
INTRODUCTION
Meniere’s disease is an inner ear disorder characterized by episodic vertigo, tinnitus, and aural fullness.1 Yamakawa2 and Hallpike and Cairns3 were the first authors to report dilation of the scala media of the cochlea, with displacement of Reissner’s membrane into the scala vestibuli. Endolymphatic hydrops is considered to be the most descriptive feature of the disease, supported by several human temporal bone and imaging studies.1,4–9 Nevertheless, the assumption that endolymphatic hydrops is the direct cause of the symptoms has been questioned, because hydrops could be a coincidental finding in asymptomatic patients.1,5,6,9–15 Furthermore, a review that analyzed 541 hydropic temporal bones from 276 deceased donors with Meniere’s disease concluded that hydrops, as an isolated factor, is insufficient to cause Meniere’s disease.14
Hydrops may reflect the changes in the anatomy of the membranous labyrinth as a consequence of overaccumulation of endolymph.3 Impaired absorption of endolymph is regarded as the main cause of endolymphatic hydrops. The endolymphatic duct and sac are considered responsible for the absorption, acting as active ion-transporting structures.1,16,17,18 Many theories have attempted to explain the origin of the symptoms of hydrops, but none of those has been universally accepted. Moreover, no irrefutable theory explains why some patients with endolymphatic hydrops develop symptoms, while others do not.
To our knowledge, no previous study has performed a 3-dimensional (3D) comparative analysis of the endolymph drainage system in temporal bones from donors who had Meniere’s disease, as compared with specimens from donors who had endolymphatic hydrops without vestibular symptoms and with nondiseased specimens. The purpose of our study was to compare anatomic findings in those 3 groups by measuring the volume of the vestibular aqueduct, endolymphatic sinus and duct, and intratemporal portion of the endolymphatic sac; the size of the internal and external aperture of the vestibular aqueduct; and the opening (if present) of the utriculoendolymphatic valve (also known as Bast’s valve).
MATERIALS AND METHODS
Specimens
In this study, we analyzed human temporal bones in 3 groups - Meniere’s disease group, endolymphatic hydrops group, and a nondiseased control group. The Meniere’s disease group included bones with signs of endolymphatic hydrops from donors who had clinical symptoms matching the diagnostic criteria of Meniere’s disease proposed by the American Academy of Otolaryngology–Head and Neck Surgery (AAO-HNS).19 We excluded specimens with neoplastic growths or otosclerosis foci involving the vestibular aqueduct or the endolymphatic sac, previous otologic surgery, and processing or removal artifacts. The final Meniere’s disease group included 16 temporal bones from 16 donors (10 men and 6 women); their mean age was 70.18±13.24 years (range, 45 to 89 years).
The endolymphatic hydrops group included 16 temporal bones from 16 donors (13 men and 3 women) who had histologic signs of endolymphatic hydrops that did not meet the diagnostic criteria for Meniere’s disease, age-matched to the Meniere’s disease group; their mean age was 66.43±10.99 years (range, 45 to 85 years).
We also included, as a control group, 16 nondiseased temporal bones from 14 donors (11 men and 5 women); their mean age was 63.11±10.83 years (range, 41 to 80 years).
All temporal bones had been previously harvested during autopsy, fixed in 10% buffered formalin, decalcified with ethylenediaminetetraacetic acid (EDTA), dehydrated in graded concentrations of alcohol, and embedded in celloidin. Each temporal bone was serially sectioned in the horizontal plane at a thickness of 20 μm. Every 10th section was stained with hematoxylin and eosin, and then mounted on a glass slide.
Vestibular aqueduct, endolymphatic sinus and duct, and endolymphatic sac
To measure the volume of the vestibular aqueduct, endolymphatic sinus and duct, and intratemporal portion of the endolymphatic sac, we scrutinized the horizontal sections of the specimens containing the vestibular organ of the inner ear. Measuring the extratemporal portion of the endolymphatic sac was not feasible using our study’s methodology; this portion of the sac is composed by layers of dura mater, and temporal bone removal and processing damage the architecture of that structure.
Using a high-resolution scanner (PathScan Enabler IV, Meyer Instruments, Houston, TX, USA), we scanned the temporal bone slides and uploaded them to a personal computer. On each slide, we labeled the following areas: (1) the bony volume of the vestibular aqueduct, (2) the lumen of the endolymphatic sinus and duct, and (3) the bony volume of the intratemporal endolymphatic sac. To generate our 3D reconstruction model for measuring volume, we used Amira software (Amira 3D Software for Life Sciences, FEI, Hillsboro, OR) (Fig. 1).
Fig. 1.
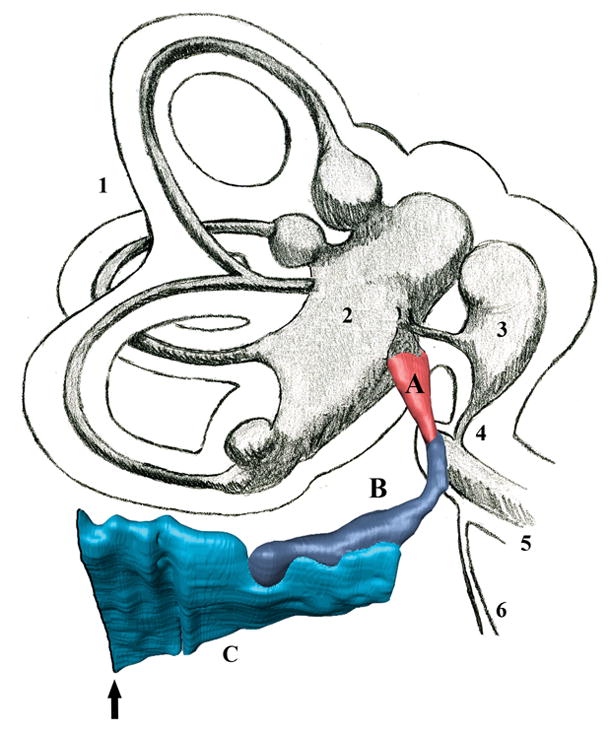
A 3D model generated to calculate the volume of the endolymph drainage system compartments, with a schematic representation of the vestibular system. A= endolymphatic sinus; B = vestibular aqueduct and endolymphatic duct; C= intratemporal portion of the endolymphatic sac; Arrow = external aperture of the vestibular aqueduct; 1 = semicircular canals; 2 = utricle; 3 = saccule; 4 = ductus reuniens; 5 = basal turn of the cochlea; 6 = cochlear aqueduct.
Internal and external aperture of aqueduct; opening of Bast’s valve
We measured the internal and external apertures of the vestibular aqueduct, as well as the opening (if present) of Bast’s valve. The opening of Bast’s valve was only considered for analysis in temporal bones with intact utricular membranes. Using a light microscope (Nikon Eclipse E400, Nikon, Japan), we scrutinized every stained slide containing those structures, under 40x magnification. In our analysis, we included the widest measurement of each of the apertures and of the opening of Bast’s valve.
Anatomic boundaries
We defined the vestibular aqueduct as the bony canal extending from the medial wall of the vestibule, running parallel to the common crus, to the posterior fossa. We defined the internal aperture as the proximal opening of the vestibular aqueduct into the medial wall of the vestibule; and the external aperture, as the site where the vestibular aqueduct opens in the foveate fossa.
We defined the endolymphatic sinus as the fusiform membranous structure located in a groove on the posteromedial surface of the vestibule, formed by the confluence of the utricular and saccular ducts. We defined the endolymphatic duct as the tubular membranous structure that starts from the end of the endolymphatic sinus, running through the vestibular aqueduct to the endolymphatic sac. Our measurements of both the endolymphatic sinus and the endolymphatic duct included only the lumen of those structures (not any fibrous, granulation, or soft tissue, and not any exudates).
We defined the intratemporal endolymphatic sac as the membranous structure housed in the vestibular aqueduct; we scrutinized it from its transition point with the endolymphatic duct until the external opening of the vestibular aqueduct. With a light microscope, it is not possible to clearly state the exact transition area between the endolymphatic duct and the endolymphatic sac20–22; thus, in order to ensure consistency among our temporal bone specimens, we defined the beginning of the endolymphatic sac by the first slide containing the crista ampullaris of the posterior semicircular canal. Our calculation of the size of the endolymphatic sac was based on our analysis of its bony volume.
We defined Bast’s valve, located in the anteroinferior wall of the utricle at the opening of the utricular duct, as the slit-shaped structure in between 2 lips: the outer lip, consisting of a central core of loosely woven fibrocytes covered by cuboidal epithelium, and the inner lip, a membrane histologically identical to the utricular wall. We calculated the length by the widest distance between the 2 highest points of both the inner and the outer lips.
Statistical analysis
To compare the results, we used the nonparametric Mann-Whitney U test (SPSS 23.0 software for Windows, SPSS Inc., Chicago, IL). All results were expressed as the mean±standard deviation (SD). Findings were considered statistically significant when the P value was less than 0.05.
RESULTS
Using the criteria proposed by Paparella et al.6 for the severity of endolymphatic hydrops, we histologically classified the temporal bone specimens in the Meniere’s disease group and in the endolymphatic hydrops group. Of the 16 specimens in the Meniere’s disease group, we found slight hydrops in 4 (25%), moderate hydrops in 1 (6.25%), and profound hydrops in 11 (68.75%). In contrast, of the 16 specimens in the endolymphatic hydrops group, we found slight hydrops in 9 (56.25%), moderate hydrops in 5 (31.25%), and profound hydrops in only 1 (6.25%).
Vestibular aqueduct, endolymphatic sinus and duct, and endolymphatic sac
In the Meniere’s disease group, the volume of the vestibular aqueduct, endolymphatic duct, and intratemporal portion of the endolymphatic sac was significantly lower, as compared with both the endolymphatic hydrops group (Fig. 2; Fig. 3) and the nondiseased group (Tables 1 and 2). In the endolymphatic hydrops group, we found no differences as compared with the nondiseased group in the volume of any of those structures. Between the 3 groups, the difference in the volume of the endolymphatic sinus (Fig. 4) was not statistically significant (P>0.05) (Table 2).
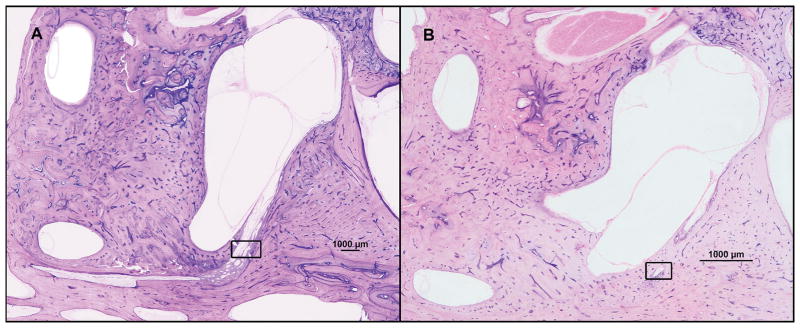
Fig. 2.
Two representative horizontal sections of human temporal bones, showing the vestibular aqueduct and endolymphatic duct in two of our study groups (hematoxylin and eosin; 2x). A = endolymphatic hydrops; B = Meniere’s disease. Squared area: vestibular aqueduct and endolymphatic duct.
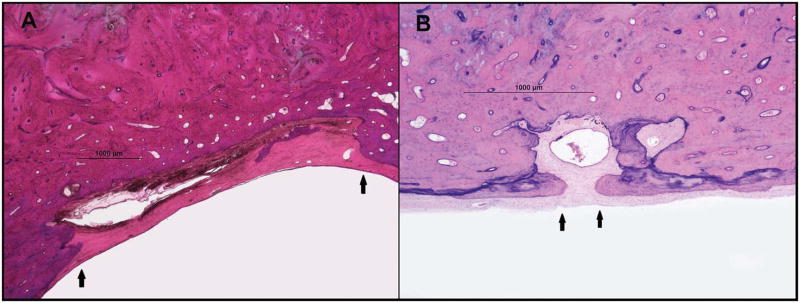
Fig. 3.
Two representative horizontal sections of human temporal bones, showing the endolymphatic sac and the external aperture of the vestibular aqueduct in two of our study groups (hematoxylin and eosin; 4x). A = endolymphatic hydrops; B = Meniere’s disease. Arrows: bony edges of the external aperture of the vestibular aqueduct.
Table 1.
Measurements of endolymphatic structures, by group
| Structure | Meniere’s disease group | Endolymphatic hydrops group | Nondiseased group | |
|---|---|---|---|---|
| Mean volume (mm3) | Vestibular aqueduct | 2.85 | 7.93 | 7.93 |
| Endolymphatic sinus | 0.29 | 0.26 | 0.34 | |
| Endolymphatic duct | 0.34 | 0.40 | 0.48 | |
| Intratemporal portion of endolymphatic sac | 1.95 | 6.21 | 5.97 | |
| Mean width of the opening (mm) | Internal aperture | 0.35 | 0.42 | 0.38 |
| External aperture | 3.95 | 8.18 | 7.28 | |
| Bast’s valve | 0.21 | - | - |
Table 2.
P values for between-group comparisons
| Meniere’s disease group vs. Endolymphatic hydrops group | Meniere’s disease group vs. Nondiseased group | Endolymphatic hydrops group vs. Nondiseased group | |
|---|---|---|---|
| Vestibular aqueduct | 0.000* | 0.001* | 1.00 |
| Endolymphatic sinus | 0.384 | 0.555 | 0.190 |
| Endolymphatic duct | 0.015* | 0.009* | 0.328 |
| Intratemporal portion of endolymphatic sac | 0.001* | 0.004* | 0.547 |
| Internal aperture | 0.439 | 0.380 | 0.925 |
| External aperture | 0.001* | 0.008* | 0.621 |
| Bast’s valve | - | - | - |
statistically significant
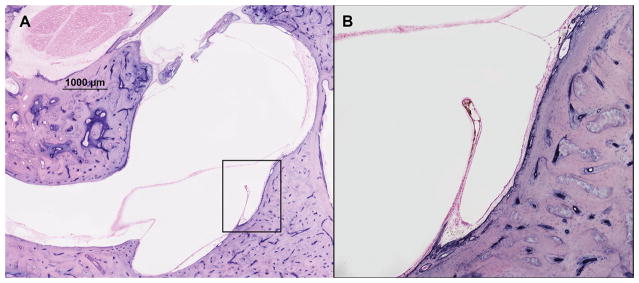
Fig. 4.
Two representative horizontal sections of human temporal bones, showing the endolymphatic sinus and Bast’s valve in a specimen from a deceased donor who had Meniere’s disease (hematoxylin and eosin). A = 2x magnification; B = 10x magnification
In specimens with profound hydrops (in the Meniere’s disease group and the endolymphatic hydrops group combined), the mean volume of the vestibular aqueduct (2.50 mm3), endolymphatic duct (0.30 mm3), and intratemporal portion of the endolymphatic sac (1.92 mm3) was smaller than in specimens with moderate or slight hydrops (vestibular aqueduct: 3.97 mm3; endolymphatic duct: 0.76 mm3; and intratemporal portion of the endolymphatic sac: 0.47 mm3) (P>0.05).
Internal and external aperture of aqueduct; opening of Bast’s valve
In the Meniere’s disease group, the mean size of the external aperture of the vestibular aqueduct was smaller than in both the endolymphatic hydrops group (P=0.001) and the nondiseased group (P=0.008) (Table 2). Between the 3 groups, the difference in the size of the internal aperture of the vestibular aqueduct was not statistically significant (P>0.05) (Table 2).
The status (open or closed) of the Bast’s valve was evaluated in 11 (68.75%) of the specimens in the Meniere’s disease group; 8 (50%), the endolymphatic hydrops group; and 7 (43.75%), the nondiseased group. Of those 11 specimens in the Meniere’s disease group, the valve was closed in 6 (54.54%) of them; mean width of the opening in the 5 specimens with open valve was 0.21±0.17 mm (range, 0.053 to 0.42 mm) (Table 1; Fig. 4). In all 8 of the measurable specimens in the endolymphatic hydrops group, and in all 7 of the measurable specimens in the nondiseased group, the valve was closed.
DISCUSSION
We studied the endolymphatic sinus and duct, as well as the intratemporal portion of the endolymphatic sac, because of extensive evidence of their role in the absorption of endolymph.4,23,24 Several ion homeostasis mechanisms have been identified in the endolymphatic sac, such as active Na+/K+-ATPase, aquaporins, and adrenocorticosteroid receptors.25,26 Furthermore, some studies created endolymphatic hydrops by injecting vasopressin in animals by stimulating the aquaporin receptors present in the endolymphatic sac.27 However, this artificially induced hydrops went into remission within a few days, suggesting that, without any other concomitant change, the excess endolymph was absorbed over time. Thus, one or more additional cofactors are required before hydrops persists and clinical symptoms occur.
In 2 of our study groups—the endolymphatic hydrops group and the nondiseased group—the volume and openings of the selected structures were similar. However, in the Meniere’s disease group, the external opening of the vestibular aqueduct was smaller, and the volume of the vestibular aqueduct, endolymphatic duct, and intratemporal portion of the endolymphatic sac was significantly lower—findings that suggest at least partial obstruction of the endolymph drainage.
Several previous human temporal bone and imaging studies have reported similar findings in their Meniere’s population (as compared with nondiseased controls), including hypoplasia of the vestibular aqueduct; narrowing of the lumen of the endolymphatic duct; hypodevelopment of Trautmann’s triangle; the relationship of the position of the posterior fossa dural plate to the endolymphatic sac; aberrant (lateral) displacement of the lateral venous sinus; and smaller external apertures of the aqueduct.1,5,6,23,28–33 Given these anatomic differences, high-resolution MRI and CT scans could become valuable diagnostic tools in the evaluation of selected patients, especially those with atypical presentations of Meniere’s disease.
One hypothesis regarding why smaller endolymphatic compartments could cause symptoms is based on the principle of “functional reserve.” Smaller vestibular aqueducts and smaller endolymphatic ducts and sacs (smaller because of developmental, environmental, infectious, or even genetic issues) could be complicating factors in the advent of abnormalities in endolymph homeostasis.1,4–8,30 Patients with larger compartments can likely deal more adequately with an overload of endolymph. Foster and Breeze34 described hydropic ears as intermittent Starling resistor to blood flow within the inner ear—the increased pressure in the endolymphatic system of Meniere’s patients could surpass the perfusion pressure of the inner ear, causing fluctuations in its blood supply. In individuals with a high functional reserve, even high endolymph pressures would not exceed the critical perfusion pressure. Thus, the anatomic changes in patients with Meniere’s disease could create an environment of low “functional reserve,” preventing the endolymph drainage system from dealing with disturbances in fluid homeostasis and leading to clinical symptoms.1,4–8,30 In addition, we found that the subgroup in our study with profound hydrops had smaller vestibular aqueducts and smaller endolymphatic ducts and sacs (as compared with the subgroups with moderate or slight hydrops). Thus, the size of the duct or sac correlates with the function of the sac and fluid homeostasis in the inner ear.23
The endolymphatic sinus and Bast’s valve are considered possible compensation mechanisms to a retrograde increase in the volume of endolymph.17,35 In our study, the valve was open in 54% of the temporal bone specimens in the Meniere’s disease group; in contrast, none of the valves were open in the other 2 groups (the endolymphatic hydrops group and the nondiseased group). The reported anatomic changes in the Meniere’s disease group could increase the retrograde pressure of endolymph, causing Bast’s valve to open.
Bast’s valve might be responsible for protecting the pars superior from collapsing, in the event of decreased volume or any ruptures in the membranous labyrinth structures.36 Collapse of those walls would interfere with the movements of the cupulae of the canals and of the otolithic membrane, preventing those structures from working properly.36 It is also possible that, if the endolymphatic duct is narrowed or occluded, the overflow causing the valve to open can dislocate the crista of the structures in the pars superior, leading to vertigo.35,36 As Meniere’s progresses and further impairs the absorptive capacity of the sac, the valve could remain persistently open; if so, with no protective mechanism against fluctuations in the pressure of endolymph, the sensorial epithelia could be more vulnerable to deleterious changes.36,37
We found no difference in the volume of the endolymphatic sinus in our 3 study groups. Previous studies have hypothesized that this structure might also have an active role in endolymph volume regulation, working in a one-way fashion as a mechanic valve.18,38 But in our Meniere’s disease group, we were not able to demonstrate clear distention of the endolymphatic sinus; other types of volume receptors may be involved in the endolymphatic sinus.
In summary, we found that the volume and size of several structures responsible for endolymph drainage were significantly decreased in temporal bones with Meniere’s disease (as compared with specimens from donors who had endolymphatic hydrops without vestibular symptoms and with nondiseased specimens). These finds could correlate with both the degree of endolymphatic hydrops and the development of clinical symptoms in patients with Meniere’s disease.
CONCLUSION
Our findings demonstrate that temporal bones of patients with Meniere’s disease had lower volume of the vestibular aqueduct, endolymphatic duct, and intratemporal portion of the endolymphatic sac, and smaller external aperture of the vestibular aqueduct as compared specimens with endolymphatic hydrops without vestibular symptoms and with nondiseased specimens. The open status of the Bast’s valve in the Meniere’s group could be secondary to higher retrograde endolymph pressures caused by smaller drainage systems. These anatomic differences may relate to the cause of clinical symptoms in Meniere’s disease.
Acknowledgments
Source of Funding: This project was funded by the National Institute on Deafness and Other Communication Disorders (NIDCD) of the U.S. National Institutes of Health (NIH), grant U24 DC011968; the International Hearing Foundation; the Starkey Hearing Foundation; and the Lions 5M Hearing Foundation
We are grateful to all study participants for their contributions; to Ms. Mary E. Knatterud, PhD, for critically reviewing the manuscript; and to Grace Sinae Park, B.S., for great technical support. We also thank our supporters: National Institute on Deafness and Other Communication Disorders (NIDCD) of the U.S. National Institutes of Health (NIH); the International Hearing Foundation; the Starkey Hearing Foundation; and the Lions 5M Hearing Foundation.
Footnotes
Conflict of interest: None
Financial disclosures: None
Level of evidence: N/A
References
- 1.Paparella MM. Pathogenesis and pathophysiology of Meniere’s disease. Acta Otolaryngol Suppl. 1991;485:26–35. doi: 10.3109/00016489109128041. [DOI] [PubMed] [Google Scholar]
- 2.Yamakawa K. Hearing organ of a patient who showed Meniere’s symptoms. J Otolaryngol Soc Jpn. 1938;44:2310–2312. [Google Scholar]
- 3.Hallpike CS, Cairns H. Observations on the pathology of Meniere’s syndrome: (Section of Otology) Proc R Soc Med. 1938;31:1317–1336. doi: 10.1177/003591573803101112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kimura RS. Membranous hydrops in the inner ear of the guinea pig after the obliteration of the endolymphatic sac. Pract Otorhinolaryngol. 1965;27:343–354. [Google Scholar]
- 5.Paparella MM. Pathogenesis of Meniere’s disease and Meniere’s syndrome. Acta Otolaryngol Suppl. 1984;406:10–25. doi: 10.3109/00016488309122996. [DOI] [PubMed] [Google Scholar]
- 6.Paparella MM. Pathology of Meniere’s disease. Ann Otol Rhinol Laryngol Suppl. 1984;112:31–35. doi: 10.1177/00034894840930s406. [DOI] [PubMed] [Google Scholar]
- 7.Paparella MM, Djalilian HR. Etiology, pathophysiology of symptoms, and pathogenesis of Meniere’s disease. Otolaryngol Clin North Am. 2002;35:529–545. vi. doi: 10.1016/s0030-6665(02)00019-1. [DOI] [PubMed] [Google Scholar]
- 8.Salvinelli F, Greco F, Trivelli M, Linthicum FH., Jr Meniere’s disease. Histopathological changes: a post mortem study on temporal bones. Eur Rev Med Pharmacol Sci. 1999;3:189–193. [PubMed] [Google Scholar]
- 9.Sajjadi H, Paparella MM. Meniere’s disease. Lancet. 372:406–414. doi: 10.1016/S0140-6736(08)61161-7. [DOI] [PubMed] [Google Scholar]
- 10.Rauch SD, Merchant SN, Thedinger BA. Meniere’s syndrome and endolymphatic hydrops. Double-blind temporal bone study. Ann Otol Rhinol Laryngol. 1989;98:873–883. doi: 10.1177/000348948909801108. [DOI] [PubMed] [Google Scholar]
- 11.Schuknecht HF, Gulya AJ. Endolymphatic hydrops. An overview and classification. Ann Otol Rhinol Laryngol Suppl. 1983;106:1–20. doi: 10.1177/00034894830920s501. [DOI] [PubMed] [Google Scholar]
- 12.Wackym PA. Histopathologic findings in Meniere’s disease. Otolaryngol Head Neck Surg. 1995;112:90–100. doi: 10.1016/S0194-59989570307-1. [DOI] [PubMed] [Google Scholar]
- 13.Merchant SN, Adams JC, Nadol JB., Jr Pathophysiology of Meniere’s syndrome: are symptoms caused by endolymphatic hydrops? Otol Neurotol. 2005;26:74–81. doi: 10.1097/00129492-200501000-00013. [DOI] [PubMed] [Google Scholar]
- 14.Foster CA, Breeze RE. Endolymphatic hydrops in Meniere’s disease: cause, consequence, or epiphenomenon? Otol Neurotol. 2013;34:1210–1214. doi: 10.1097/MAO.0b013e31829e83df. [DOI] [PubMed] [Google Scholar]
- 15.Fraysse BG, Alonso A, House WF. Meniere’s disease and endolymphatic hydrops: clinical-histopathological correlations. Ann Otol Rhinol Laryngol Suppl. 1980;89:2–22. doi: 10.1177/00034894800896s201. [DOI] [PubMed] [Google Scholar]
- 16.Sperling NM, Paparella MM, Yoon TH, Zelterman D. Symptomatic versus asymptomatic endolymphatic hydrops: a histopathologic comparison. Laryngoscope. 1993;103:277–285. doi: 10.1288/00005537-199303000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Kimura RS, Schuknecht HF, Ota CY. Blockage of the cochlear aqueduct. Acta Otolaryngol. 1974;77:1–12. doi: 10.3109/00016487409124591. [DOI] [PubMed] [Google Scholar]
- 18.Salt AN, Rask-Andersen H. Responses of the endolymphatic sac to perilymphatic injections and withdrawals: evidence for the presence of a one-way valve. Hear Res. 2004;191:90–100. doi: 10.1016/j.heares.2003.12.018. [DOI] [PubMed] [Google Scholar]
- 19.Committee on Hearing and Equilibrium guidelines for the diagnosis and evaluation of therapy in Meniere’s disease. American Academy of Otolaryngology–Head and Neck Foundation, Inc. Otolaryngol Head Neck Surg. 1995;113:181–185. doi: 10.1016/S0194-5998(95)70102-8. [DOI] [PubMed] [Google Scholar]
- 20.Dahlmann A, von During M. The endolymphatic duct and sac of the rat: a histological, ultrastructural, and immunocytochemical investigation. Cell Tissue Res. 1995;282:277–289. doi: 10.1007/BF00319118. [DOI] [PubMed] [Google Scholar]
- 21.Lundquist PG. Aspects on endolymphatic sac morphology and function. Arch Otorhinolaryngol. 1976;212:231–240. doi: 10.1007/BF00453671. [DOI] [PubMed] [Google Scholar]
- 22.Shambaugh GE, Jr, Clemis JD, Arenberg IK. Endolymphatic duct and sac in Meniere’s disease. Arch Otolaryngol. 1969;89:816–825. doi: 10.1001/archotol.1969.00770020818006. [DOI] [PubMed] [Google Scholar]
- 23.Nadol J. Pathogenesis of Meniere’s syndrome. In: Harris J, editor. Meniere’s disease. Kugler publications; The Hague: 1999. pp. 73–79. [Google Scholar]
- 24.Naito T. Experimental studies on Meniere’s disease. Otorhinolaryngol Soc Jpn Tokyo. 1950;53:19–20. [Google Scholar]
- 25.Kumagami H, Loewenheim H, Beitz E, et al. The effect of anti-diuretic hormone on the endolymphatic sac of the inner ear. Pflugers Arch. 1998;436:970–975. doi: 10.1007/s004240050731. [DOI] [PubMed] [Google Scholar]
- 26.Wackym PA, Glasscock ME, 3rd, Linthicum FH, Jr, Friberg U, Rask-Andersen H. Immunohistochemical localization of Na+, K+-ATPase in the human endolymphatic sac. Arch Otorhinolaryngol. 1988;245:221–223. doi: 10.1007/BF00463931. [DOI] [PubMed] [Google Scholar]
- 27.Katagiri Y, Takumida M, Hirakawa K, Anniko M. Long-term administration of vasopressin can cause Meniere’s disease in mice. Acta Otolaryngol. 2014;134:990–1004. doi: 10.3109/00016489.2014.902989. [DOI] [PubMed] [Google Scholar]
- 28.Shimizu S, Cureoglu S, Yoda S, Suzuki M, Paparella MM. Blockage of longitudinal flow in Meniere’s disease: A human temporal bone study. Acta Otolaryngol. 2011;131:263–268. doi: 10.3109/00016489.2010.532155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hebbar GK, Rask-Andersen H, Linthicum FH., Jr Three-dimensional analysis of 61 human endolymphatic ducts and sacs in ears with and without Meniere’s disease. Ann Otol Rhinol Laryngol. 1991;100:219–225. doi: 10.1177/000348949110000310. [DOI] [PubMed] [Google Scholar]
- 30.Ikeda M, Sando I. Endolymphatic duct and sac in patients with Meniere’s disease. A temporal bone histopathological study. Ann Otol Rhinol Laryngol. 1984;93:540–546. doi: 10.1177/000348948409300603. [DOI] [PubMed] [Google Scholar]
- 31.Liu F, Huang W, Wang Z, et al. Noninvasive evaluation of endolymphatic space in healthy volunteers using magnetic resonance imaging. Acta Otolaryngol. 2011;131:247–257. doi: 10.3109/00016489.2010.524938. [DOI] [PubMed] [Google Scholar]
- 32.Sennaroglu L, Yilmazer C, Basaran F, Sennaroglu G, Gursel B. Relationship of vestibular aqueduct and inner ear pressure in Meniere’s disease and the normal population. Laryngoscope. 2001;111:1625–1630. doi: 10.1097/00005537-200109000-00025. [DOI] [PubMed] [Google Scholar]
- 33.Tanioka H, Kaga H, Zusho H, Araki T, Sasaki Y. MR of the endolymphatic duct and sac: findings in Meniere disease. AJNR Am J Neuroradiol. 1997;18:45–51. [PMC free article] [PubMed] [Google Scholar]
- 34.Foster CA, Breeze RE. The Meniere attack: an ischemia/reperfusion disorder of inner ear sensory tissues. Med Hypotheses. 2013;81:1108–1115. doi: 10.1016/j.mehy.2013.10.015. [DOI] [PubMed] [Google Scholar]
- 35.Konishi S. The ductus reuniens and utriculo-endolymphatic valve in the presence of endolymphatic hydrops in guinea-pigs. Journal of laryngology and otology. 1977;91:1033–1045. doi: 10.1017/s0022215100084747. [DOI] [PubMed] [Google Scholar]
- 36.Schuknecht HF, Belal AA. The utriculo-endolymphatic valve: its functional significance. Journal of laryngology and otology. 1975;89:985–996. doi: 10.1017/s0022215100081305. [DOI] [PubMed] [Google Scholar]
- 37.Hofman R, Segenhout JM, Buytaert JA, Dirckx JJ, Wit HP. Morphology and function of Bast’s valve: additional insight in its functioning using 3D-reconstruction. Eur Arch Otorhinolaryngol. 2008;265:153–157. doi: 10.1007/s00405-007-0424-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wit HP. Does the endolymphatic sinus function as a one-way valve? Hear Res. 2007;224:115–116. doi: 10.1016/j.heares.2006.10.003. [DOI] [PubMed] [Google Scholar]