Abstract
In the adult mammalian brain, GABAergic neurotransmission provides the majority of synaptic inhibition that balances glutamatergic excitatory drive and thereby controls neuronal output. It is generally accepted that synaptogenesis is initiated through highly specific protein–protein interactions mediated by membrane proteins expressed in developing presynaptic terminals and postsynaptic membranes. Accumulating studies have uncovered a number of membrane proteins that regulate different aspects of GABAergic synapse development. In this review, we summarize recent advances in understanding of GABAergic synapse development with a focus on postsynaptic membrane molecules, including receptors, synaptogenic cell adhesion molecules and immunoglobulin superfamily proteins.
1. Introduction
Chemical synapses are specialized asymmetric intercellular junctions that are essential for rapid communication between neurons. In the mammalian brain, there are hundreds of trillions of synapses that connect individual neurons into neural circuits that give rise to behavior and cognition. The formation of these synapses is a multi-step process. Initial contact between presynaptic terminals and postsynaptic somata or dendrites is conducted by trans-synaptic adhesive interactions, which induce specialization of synaptic structures through recruitment of pre- and post-synaptic specific components. Nascent synaptic connections are then selected through a highly regulated validation and elimination process, and undergo activity-dependent refinement to become mature functional synapses. When synapse development goes awry, neural circuit function becomes impaired, and devastating neurological and psychiatric disorders can occur (Cline, 2005; Dudek, 2009; Lisman, 2012; Rubenstein, 2010; Sheng et al., 2012; Yizhar et al., 2011). Thus, understanding the molecular and cellular mechanisms regulating synapse formation and maturation in the developing brain will not only provide important insights into how neural circuits assemble and function, but will also shed the light on molecular processes involved in pathogenesis of many developmental brain illnesses.
Based on the neurotransmitters that they release, chemical synapses are either excitatory or inhibitory. In the central nervous system (CNS), glutamate is the principle excitatory neurotransmitter acting on three types of ionotropic glutamate receptors (AMPA receptors, NMDA receptors and Kainate receptors) to mediate fast excitatory synaptic transmission and induce depolarization in post-synaptic neurons (Traynelis et al., 2010). On the other hand, GABA is the chief inhibitory neurotransmitter in the mature CNS and exerts its action through GABAA-type ionotropic receptors (GABAARs) to achieve fast synaptic inhibition (Ben-Ari et al., 2007). GABAergic inhibition balances glutamatergic excitatory drive and controls neuronal excitability, integration and output. This synaptic excitation and inhibition balance (E/I balance) is important for proper neural circuit function (Akerman and Cline, 2006; Berg et al., 2007; Cline, 2005; Dorrn et al., 2010; Liu, 2004; Maffei et al., 2004; Sun et al., 2010; Tao and Poo, 2005; Wehr and Zador, 2003; Yizhar et al., 2011), and dysregulation of balanced E/I development has been proposed as a potential mechanism for a number of neural development disorders, such as epilepsy, autism and schizophrenia (Cline, 2005; Dudek, 2009; Lisman, 2012; Rubenstein, 2010; Yizhar et al., 2011).
Although the molecular and cellular mechanisms for glutamatergic synapse development have been extensively studied over the last three decades, much less is known about the regulation of GABAergic synapse formation. Developmentally GABAergic synapses are often formed before the appearance of glutamatergic contacts (Ben-Ari et al., 2007; Ben-Ari et al., 1997; Chen and Kriegstein, 2015; Deng et al., 2007; Tyzio et al., 1999). Live imaging experiments show that initial GABAergic synapse formation does not involve dendritic or axonal protrusions unlike glutamatergic synapse formation, indicating different mechanisms for the regulation of inhibitory and excitatory synaptogenesis (Wierenga et al., 2008). Supporting this, accumulating evidence has demonstrated that GABAergic synapse development is critically regulated by a set of membrane molecules specifically expressed at inhibitory synapses (Lee et al., 2013; Mishra et al., 2014; Pettem et al., 2013b; Poulopoulos et al., 2009; Takahashi et al., 2012; Varoqueaux et al., 2004; Woo et al., 2013; Yim et al., 2013). In addition, recent studies have revealed novel functions of NMDA receptors (NMDARs) and GABAARs in the regulation of GABAergic synaptogenesis (Fuchs et al., 2013; Gu et al., 2016). Here we review the molecular mechanisms for the regulation of GABAergic synapse development focusing on the role of the postsynaptic membrane proteins (Fig. 1 and Table 1), in which there has been significant progress over the last several years. Other molecules critical for GABAergic synapse formation have been discussed in two excellent recent reviews (Ko et al., 2015; Kuzirian and Paradis, 2011), and thus are not included in the current review.
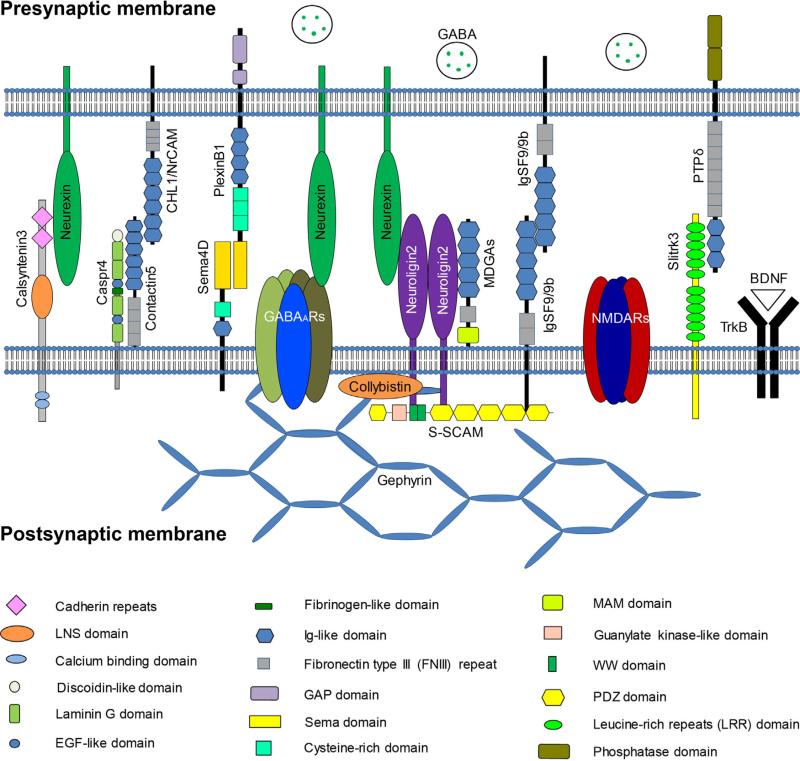
Fig. 1.
Schematic of postsynaptic membrane proteins and their binding partners important for GABAergic synapse development.
Caspr4, contactin-associated transmembrane protein 4; CHL1, close homolog of L1; NrCAM, neuronal cell adhesion molecule; Sema4D, semaphorin 4D; GABAARs, GABAA receptors; S-SCAM, synaptic scaffolding molecule; MDGAs, MAM domain-containing GPI anchor proteins; IgSF9, Immunoglobulin superfamily 9; IgSF9b, Immunoglobulin superfamily 9b; NMDARs, NMDA receptors; Slitrk3, Slit- and Trk-like 3; PTPδ, protein tyrosine phosphatase δ. TrkB, Tropomyosin-related kinase B; BDNF, brain-derived neurotropic factor. LNS, laminin-α, neurexin, sex hormone-binding globulin domain; EGF, epidermal growth factor; Ig-like, Immunoglobulin-like; GAP, GTPase activating protein; MAM, memprin, A5 protein, receptor protein tyrosine phosphatase mu; WW, tryptophan (W).
Table 1.
Functions of postsynaptic membrane proteins in GABAergic synapse development. NMDARs play a critical role in the regulation of GABAergic synapse formation through a signaling pathway involving the C0 domain of the GluN1 subunit. GABAARs regulate GABAergic synapse development likely through a direct interaction with the presynaptic adhesion molecule neurexin. Neuroligin2 is a synaptogenic molecule and functions in GABAergic synapse formation via binding to presynaptic neurexin and postsynaptic adhesion, anchorage or signaling proteins, such as MDGAs, gephyrin, collybistin and S-SCAM. Slitrk3 is a synaptogenic molecule specific to GABAergic synapse development and binds to presynaptic PTPδ in order to direct synapse differentiation. Calsyntenin3 can induce both excitatory and inhibitory synapse formation and its synaptogenic activity requires presynaptic neurexin. IgSF9/9b are transmembrane IgSFs and can mediate hemophilic interactions to promote GABAergic synapse development. MDGAs are GPI-anchored proteins and negatively regulate GABAergic synapse development by interfering with the neuroligin2-neurexin interaction. Contactin5, through interactions with cell adhesion molecules CHL1, NrCAM and Caspr4, functions in formation of the precise axoaxonic GABAergic synapses in the spinal cord. Sema4D can rapidly promote GABAergic synapse formation through its receptors, PlexinB1. TrkB and its ligand, BDNF, regulate both formation and maturation of GABAergic synapses.
| Name | Classification | Binding partners | Functions in inhibitory synapse development |
|---|---|---|---|
| NMDARs | Ionotropic receptor Ligand-gated ion channel |
N-methyl-D-aspartate (NMDA)/Glutamate/glycine/D-serine/Calmodulin |

|
| GABAARs | Ionotropic receptor Ligand-gated ion channel |
γ-aminobutyric acid (GABA)/ Neurexins |

|
| Neuroligin2 | Cell adhesion molecule (Type I) | Neurexins/MDGAs/Gephyrin/Collybistin/S-SCAM |
|
| Slitrk3 | Cell adhesion molecule (Type I) | PTPδ |
|
| Calsyntenin3 | Cell adhesion molecule (Type I) | Neurexins |
|
| IgSF9/9b | Cell adhesion molecule (Type I) | IgSF9/9b/S-SCAM |

|
| MDGAs | Cell adhesion molecule (GPI-anchored) | Neuroligin2 |

|
| Contactin5 | Cell adhesion molecule (GPI-anchored) | CHL1/NrCAM/Caspr4 |

|
| Sema4D | Semaphorin family protein | PlexinB1 |

|
| TrkB | Neurotrophin receptor | BDNF |

|
 Positively regulate GABAergic synaptogenesis.
Positively regulate GABAergic synaptogenesis.  Negatively regulate GABAergic synaptogenesis.
Negatively regulate GABAergic synaptogenesis.
2. Receptors
Ligand-gated ion channels not only mediate fast synaptic transmission in the brain, but also often play a signaling or structural role in the regulation of synapse development. Recent studies have shown that signaling via NMDARs and GABAARs is important for GABAergic synapse development (Arama et al., 2015; Chattopadhyaya et al., 2007; Gu et al., 2016).
2.1. NMDA receptors
NMDARs are a subclass of ionotropic glutamate receptors highly enriched at glutamatergic synapses in the adult brain. While the role of NMDARs in mature neurons is well established, their function in embryonic and early developing neurons is less clear. Both RT-PCR (reverse transcription-polymerase chain reaction) and in situ hybridization experiments have shown that GluN1, an obligatory NMDAR subunit, is detectable in embryonic day 14 (E14) cortical neurons in rodents (Akazawa et al., 1994; Bennett et al., 2006; Campusano et al., 2005; Monyer et al., 1994; Watanabe et al., 1992); moreover, functional NMDARs are measurable through whole-cell patch clamp recording and calcium imaging in E14-E17 cortical neurons (Behar et al., 1999; Blanton and Kriegstein, 1992; Hirasawa et al., 2003; LoTurco et al., 1991). A growing body of evidence has shown that neuronal activity in general and NMDAR activity in particular can accelerate GABAergic synapse maturation (Aamodt et al., 2000; Gonzalez-Forero et al., 2005; Harris et al., 1995; Henneberger et al., 2005; Huang, 2009; Kilman et al., 2002; Lin et al., 2008; Lu et al., 2013; Memo et al., 1991; Nusser et al., 1998; Otis et al., 1994; Rutherford et al., 1997; Wang et al., 1998; Zhu et al., 1995).
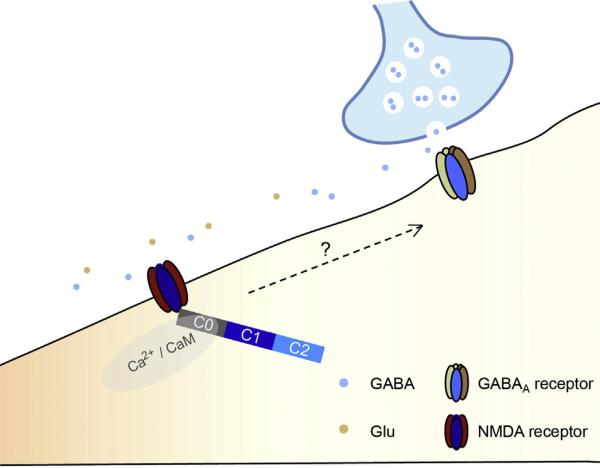
Our recent work has revealed a necessary role of NMDARs in GABAergic synapse formation in early developing neurons (Fig. 2). Specifically, our data demonstrate that single-cell genetic deletion of functional NMDARs in embryonic hippocampal progenitor neurons strongly impairs GABAergic synapse development (Gu et al., 2016). On the other hand, enhancing NMDAR activity augments GABAergic synaptic transmission in immature neurons both in vitro and in vivo (Gu et al., 2016). At the cellular level, loss of NMDARs leads to impairment of both pre- and post-synaptic specializations of GABAergic synapses, suggesting that NMDARs are critical for orchestrating GABAergic synapse assembly (Gu et al., 2016). Interestingly, our data show that NMDAR-dependent GABAergic synapse formation requires the Ca2+-dependent binding of calmodulin (CaM) to the C0 domain of the GluN1 subunit (Gu et al., 2016). These data indicate that the NMDAR functions as an upstream molecule essential for GABAergic synapse formation in early developing hippocampal neurons.
Fig. 2.
NMDARs play an important role in early development of GABAergic synapses. NMDARs regulate GABAergic synaptogenesis via signaling through the calmodulin binding motif in the C0 domain of the NMDAR GluN1 subunit (Gu et al., 2016). Currently functional interaction between NMDAR signaling and GABAergic synaptogenesis machinery remains unclear.
It is worth noting that NMDARs, which have a high affinity for glutamate (Traynelis et al., 2010), are tonically activated by ambient glutamate in the developing brain before the formation of glutamatergic synapses (Blanton and Kriegstein, 1992; Leinekugel et al., 1997; LoTurco et al., 1991), which leads to Ca2+ influx into the neuron. Activation of NMDARs in developing neurons is facilitated by GABAARs, which are detectable in neurons as early as E12-14 and can be activated by ambient GABA in the developing brain (Ben-Ari et al., 2007, 1997). While GABA is the principal inhibitory transmitter in the adult brain, it is excitatory in embryonic and developing neurons due to higher intracellular Cl− concentration, and thus its activity provides the depolarization necessary for NMDAR activation through the removal of the Mg2+ blockade (Ben-Ari et al., 2007; Cherubini et al., 1991). Therefore, a possible scenario is that in early developing neurons the depolarizing drive provided by GABAAR activity facilitates NMDAR activation, which in turn, through signaling via the Ca2+ dependent CaM binding to the C0 domain of the NMDAR GluN1 subunit, regulates GABAergic synapse development (Gu et al., 2016). Additionally, it has been shown that NMDAR activity can regulate GABAergic synapse maturation and plasticity through calcineurin, CaMKII, nitric oxide synthase, or glia secreted factors (Bannai et al., 2009; Diniz et al., 2014; Flores et al., 2015; Marsden et al., 2010; Muir et al., 2010; Nugent et al., 2007; Petrini et al., 2014). The precise roles of these signaling pathways in NMDAR-dependent GABAergic synapse formation remain to be determined, though some studies have revealed intriguing possibilities. A recent study demonstrated that transforming growth factor beta 1 (TGF-β1) released from astrocytes can induce GABAergic synapse formation in cortical neurons. Importantly, TGF-β1 induction of GABAergic synapses depends on NMDAR activity and CaMKII signaling, supporting a potential mechanism for NMDAR-dependent GABAergic development (Diniz et al., 2014). It is currently unclear whether NMDARs functionally interact with inhibitory synapse specific synaptogenic cell adhesion molecules (CAMs), such as Neuroligin2 and Slitrk3 (Graf et al., 2004; Scheiffele et al., 2000; Takahashi et al., 2012; Yim et al., 2013), to control GABAergic synapse formation. Nevertheless, the strong localization of NMDARs at developing GABAergic synapses in the immature brain provides neuroanatomical evidence for a collaborative role of NMDARs and GABAergic synaptogenesis machinery in the regulation of GABAergic synapse formation (Cserep et al., 2012; Gundersen et al., 2004; Szabadits et al., 2011). Future work in our laboratory will focus on understanding the molecular and cellular mechanisms underlying NMDAR-dependent GABAergic synapse development, which will provide important insights into signaling pathways involved in the initiation of GABAergic synaptogenesis.
2.2. GABAA receptors
GABAARs are heteropentameric ligand-gated chloride channels assembled from over 19 subunits (Fritschy and Panzanelli, 2014). In the brain, the vast majority of synaptic GABAARs contain α, β and γ2 subunits with a 2:2:1 stoichiometry (Fritschy and Panzanelli, 2014). Early studies in GABAAR subunit knockout (KO) mice have indicated an important role for GABAARs in GABAergic synapse development. Genetic deletion of the α1 subunit abolished GABAergic synaptic transmission in mature cerebellar Purkinje neurons (Fritschy and Panzanelli, 2006; Patrizi et al., 2008). Interestingly, detailed analysis reveals an intricate mechanism underlying the loss of GABAergic synaptic transmission. It was found that in α1 subunit KO mice, the α3 subunit was transiently expressed in Purkinje neurons during development, leading to the assembly of functional GABAARs and the initial formation of GABAergic synapses between stellate interneurons and Purkinje cells. However, subsequent decline of α3 subunit expression caused a loss of GABAergic synaptic transmission in Purkinje cells. Consequently, initially formed inhibitory synapses were impaired and instead mismatched, non-functional synapses were formed between stellate cell axons with Purkinje dendritic spines, suggesting an important role of GABAARs in functional maturation of GABAergic synapses (Fritschy et al., 2006; Patrizi et al., 2008). In addition, in hippocampal CA1 pyramidal neurons of α2 subunit KO mice, a prominent proportion of synaptic clusters of the major scaffolding protein gephyrin are lost (Fritschy et al., 2012). In hippocampal primary neuron cultures, acute knockdown of the γ2 subunit, which is essential for GABAAR synaptic targeting (Essrich et al., 1998), strongly reduces GABAergic inner-vation (Li et al., 2005). Similarly, in a γ2 subunit knockdown mouse line, there are significantly fewer GABAergic synapses on dendrites of cortical pyramidal neurons and cerebellar Purkinje cells (Frola et al., 2013). On the other hand, increased expression of GABAARs promotes GABAergic synapse formation (Jacob et al., 2009). More recently, in a neuron-heterologous cell co-culture system, GABAARs can initiate the formation of functional synaptic contacts in a subunit-dependent manner (Brown et al., 2016; Fuchs et al., 2013). Taken together, these data support a structural role for GABAARs in GABAergic synapse development.
In addition to a structural role, GABAAR activity is important in GABAergic synapse development. Early studies have indicated that GABAergic innervation is important for proper clustering of postsynaptic GABAARs (Christie et al., 2002; Rao et al., 2000). In basket interneurons in the mouse visual cortex, knockdown of the glutamate decarboxylase 67 (GAD67), which is a rate-limiting enzyme for GABA synthesis, causes significant deficits in perisomatic GABAergic synapse formation onto cortical pyramidal neurons (Chattopadhyaya et al., 2007). Furthermore, the effect of GAD67 knockdown on GABAergic synapse development can be partially rescued by the GABAAR agonist, diazepam, suggesting that postsynaptic GABAAR activity is important for GABAergic synapse formation (Chattopadhyaya et al., 2007). Similarly, it has been reported that GABAAR activity regulates synapse formation between developing medium spiny neurons (Arama et al., 2015). Currently, the mechanisms underlying the regulation of GABAergic synapse development by GABAARs remain unclear. It has been reported that GABAARs directly interact with presynaptic adhesion molecules, neurexins (Zhang et al., 2010). Thus, it is plausible that the GABAAR may exert its effect on GABAergic synapse development through engaging synaptic adhesion molecules.
3. Synaptogenic cell adhesion molecules
Synaptogenic cell adhesion molecules are a class of cell adhesion molecules that induce and organize synapse formation (Siddiqui and Craig, 2011). A widely used assay to test whether a cell adhesion molecule is synaptogenic utilizes co-cultures of neurons with non-neuronal cells expressing the candidate molecule (Scheiffele et al., 2000). The candidate molecule is considered to be synaptogenic if it can induce synapse formation onto the nonneuronal cells. Currently, there are synaptogenic cell adhesion molecules, including neuroligin2, slitrk3 and calsyntenin3, which have been identified to induce GABAergic synapse formation.
3.1. Neuroligin2
Neuroligins (NLGNs) are a family of synaptic cell adhesion molecules consisting of four members: NLGN1–4 (Craig and Kang, 2007; Sudhof, 2008). These are single-passing transmembrane proteins sharing similar domain organizations with a short intracellular domain that contains a PDZ domain binding motif and a gephyrin interacting sequence, as well as a large acetylcholinesterase-like extracellular domain that interacts with presynaptic neurexins to mediate adhesive interactions for synapse development (Bemben et al., 2015). Among the four members, NLGN2 is highly enriched at inhibitory synapses (Graf et al., 2004; Levinson et al., 2005; Varoqueaux et al., 2004), likely due to its ability to interact with two inhibitory synapse proteins, gephyrin and collybistin (Poulopoulos et al., 2009). Early clues to the role of NLGN2 in synapse development were discovered through observations that NLGN2 expressed in nonneuronal cells was capable of inducing presynaptic differentiations in contacting axons, suggesting a synaptogenic function for NLGN2 (Scheiffele et al., 2000). Subsequently, gain-of-function and loss-of-function experiments in hippocampal primary neuron cultures demonstrate that NLGN2 is both necessary and sufficient for GABAergic synapse development. Indeed, GABAergic synapse density is increased by NLGN2 overexpression and decreased by RNAi-mediated NLGN2 knockdown (Chih et al., 2005; Chubykin et al., 2007; Futai et al., 2013; Graf et al., 2004). In addition, gene KO studies demonstrate that NLGN2 is required for GABAergic synapse development in the hippocampus in vivo (Poulopoulos et al., 2009). Specifically, in NLGN2 KOs, GABAergic synaptic transmission is reduced to half of control levels in hippocampal CA1 pyramidal neurons (Poulopoulos et al., 2009). Interestingly, the deficits are limited to perisomatic GABAergic synapses, as GABAergic synapse density is reduced in somatic, but not the dendritic region of CA1 pyramidal neurons in NLGN2 KOs (Blundell et al., 2009; Poulopoulos et al., 2009).
The emerging picture appears to be that NLGN2 plays a region-specific role in GABAergic synapse development. While NLGN2 is required for GABAergic synapse formation at the perisomatic region in hippocampal pyramidal neurons (Poulopoulos et al., 2009), it is not necessary for GABAergic synaptogenesis in brainstem and cerebellar Purkinje cells (Poulopoulos et al., 2009; Varoqueaux et al., 2006; Zhang et al., 2015). In brainstem neurons, genetic deletion of NLGN2 leads to a substantial reduction of both GABAergic and glycinergic transmission, but not inhibitory synapse density (Poulopoulos et al., 2009; Varoqueaux et al., 2006), suggesting that NLGN2 regulates functional maturation, but not initial formation of GABAergic synapses within the brainstem. Similarly, NLGN2 is required for the establishment of inhibitory synaptic transmission, but not synapse formation per se in cerebellar Purkinje cells (Zhang et al., 2015). These data highlight the cell type specific role of NLGN2 in initial formation and functional maturation of inhibitory synapses and suggest diverse mechanisms for inhibitory synaptogenesis across the brain.
Recently, a growing number of molecules that are important in the regulation of GABAergic synapse development and function have been found to interact with NLGN2, including gephyrin, collybistin, synaptic scaffolding molecule (S-SCAM) MAGI2 (membrane-associated guanylate kinase with inverted organization), and MAM domain-containing glycophosphatidylinositol (GPI)-anchored proteins (MDGAs) (Lee et al., 2013; Pettem et al., 2013b; Poulopoulos et al., 2009; Sumita et al., 2007; Woo et al., 2013). The NLGN2-gephyrin interaction is negatively regulated by peptidyl-prolyl cis–trans isomerase Pin1 that controls synaptic abundance of NLGN2 and GABAergic synapse strength (Antonelli et al., 2014). Furthermore, a recent proteomic study to characterize NLGN2 complexes in mouse brain reveals additional inhibitory synapse proteins associated with NLGN2 (Kang et al., 2014), further supporting the role of NLGN2 as a central component of GABAergic synapses in organizing synapse development and functional maturation.
Gephyrin
Gephyrin is a multifunctional, evolutionally conserved cytosolic protein required for molybdenum cofactor synthesis in nonneuronal cells and it acts as an anchorage protein for postsynaptic clustering of glycine receptors and GABAARs at inhibitory synapses in neurons (Fritschy et al., 2008; Tretter et al., 2012; Tyagarajan and Fritschy, 2014). At inhibitory postsynapses, gephyrin forms a supermolecular hexagonal structure that binds to receptors, synaptic adhesion proteins, cytoskeleton proteins and synaptic enzymes, thereby serving as a central organizer for signal transduction at inhibitory synapses (Fritschy et al., 2008; Tretter et al., 2012; Tyagarajan and Fritschy, 2014).
Gephyrin was originally identified in glycine receptor complexes in the spinal cord through affinity purification, and is essential for postsynaptic clustering of glycine receptors (Feng et al., 1998; Kirsch et al., 1993; Levi et al., 2004; Pfeiffer et al., 1982). Subsequently it was found to colocalize and interact with GABAARs in neurons (Essrich et al., 1998; Giustetto et al., 1998; Maric et al., 2011; Nakamura et al., 2016; Sassoe-Pognetto et al., 1995; Sassoe-Pognetto et al., 2000; Tretter et al., 2008). The gephyrin-GABAAR interaction plays a role in receptor clustering at synapses, as evidenced by reduced GABAAR targeting to synapses in neurons lacking gephyrin (Essrich et al., 1998; Fischer et al., 2000; Kneussel et al., 1999; Levi et al., 2004; Yu et al., 2007). These data also indicate that there are gephyrin-independent mechanisms existing in neurons for scaffolding GABAARs at synapses. Although there is evidence for the involvement of dystrophin in GABAAR clustering in neurons, dystrophin is unlikely responsible for gephyrin-independent anchorage of receptors at developing synapses. Indeed, while GABAAR clusters are reduced in hippocampal neurons in adult dystrophin KO mice (Knuesel et al., 1999), dystrophin expression is not detectable until postnatal two weeks and thus unlikely contributes to receptor anchorage at the early developing synapses (Knuesel et al., 2000). Thus, gephyrin-independent mechanisms for clustering of GABAARs at developing synapses remain to be determined. Future work toward a more complete understanding how GABAARs are anchored at synapses will provide important insights to the molecular mechanisms underlying the development and maturation of GABAergic synapses.
Collybistin
Clustering of gephyrin at developing synapses is an important step for GABAergic synapse establishment (Fritschy et al., 2008; Tretter et al., 2012; Tyagarajan and Fritschy, 2014). There are several well-characterized molecular processes that regulate gephyrin clustering at inhibitory synapses, including posttranslational modifications and protein–protein interactions with GABAergic synaptic components (Dejanovic et al., 2014; Tretter et al., 2012; Tyagarajan and Fritschy, 2014). One of the gephyrin binding proteins that is particularly important for GABAergic synaptogenesis is collybistin, a brain-specific guanine nucleotide exchange factor (GEF) for small GTPase Cdc42 (Harvey et al., 2004; Kins et al., 2000; Papadopoulos et al., 2008; Tyagarajan et al., 2011). In both heterologous cells and neurons, collybistin is capable of inducing submembrane clustering of gephyrin (Chiou et al., 2011; Harvey et al., 2004; Kins et al., 2000; Tyagarajan et al., 2011), likely through collybistin's pleckstrin-homology (PH) domain that binds to membranes (Kalscheuer et al., 2009; Reddy-Alla et al., 2010). Collybistin KO mice display considerably reduced synaptic clustering of both gephyrin and GABAARs, in addition to severely impaired GABAergic synaptic transmission (Papadopoulos et al., 2008; Papadopoulos et al., 2007). Thus, collybistin controls GABAergic synaptogenesis through the regulation of gephyrin and GABAARs clustering at developing synapses.
Neuroligin2-gephyrin-collybistin complex
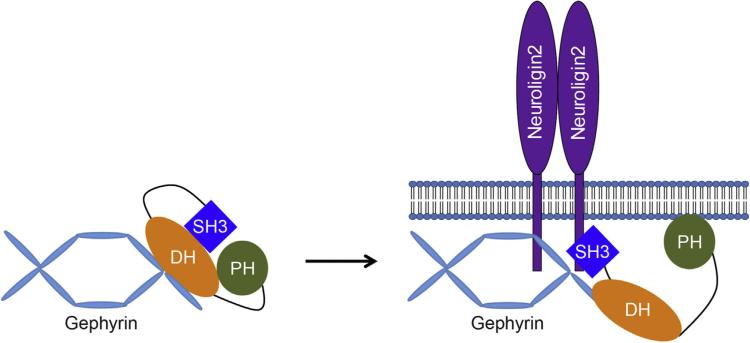
In the brain, the vast majority of collybistin contains an N-terminal src-homology 3 (SH3) domain (Harvey et al., 2004; Soykan et al., 2014), which binds intramolecularly to the dbl-homology (DH)/PH domains and keeps collybistin in a closed, inactive state (Soykan et al., 2014). Indeed, collybistin isoforms lacking the SH3 domain, although expressed at low levels in the brain (Soykan et al., 2014), are intrinsically active in clustering gephyrin at the plasma membrane. In contrast, collybistin variants that carry the SH3 domain colocalize with intracellular gephyrin but do not traffic to the plasma membrane in nonneuronal cells (Harvey et al., 2004; Kins et al., 2000), likely due to impaired accessibility of the PH domain for lipid binding (Reddy-Alla et al., 2010; Soykan et al., 2014). Several neuronal proteins, including NLGN2 (Poulopoulos et al., 2009; Soykan et al., 2014), neuroligin 4 (NLGN4) (Hoon et al., 2011), small GTPase TC10 (Mayer et al., 2013), and the GABAAR α2 subunit (Saiepour et al., 2010), have been reported to regulate the intramolecular interactions between the SH3 and DH/PH domains and thus control the activity of collybistin in gephyrin recruitment and GABAergic synapse formation. Among these, NLGN2-dependent regulation of collybistin has been best characterized. The carboxyl (C)-terminus of NLGN2 can bind to both gephyrin and collybistin (Fig. 3) (Poulopoulos et al., 2009; Soykan et al., 2014). In the absence of NLGN2, collybistin remains in the closed state that interacts with gephyrin in the intracellular locations and can not recruit gephyrin to the plasma membrane for initial formation of GABAergic synapses (Poulopoulos et al., 2009; Soykan et al., 2014). Binding of NLGN2 to the collybistin DH domain disrupts the intramolecular interaction and consequently exposes its PH domain for plasma membrane tethering, leading to collybistin/gephyrin clustering at the plasma membrane (Poulopoulos et al., 2009; Soykan et al., 2014). Moreover, NLGN2 binding to both gephyrin and collybistin contributes to stabilization of collybistin/gephyrin complexes at the plasma membrane, facilitating postsynaptic differentiation of developing GABAergic synapses (Poulopoulos et al., 2009; Soykan et al., 2014). Taken together, these data highlight an important role of NLGN2-mediated postsynaptic signaling in the regulation of GABAergic synapse development.
Fig. 3.
A model for the role of NLGN2 in the regulation of the collybistin intramolecular interaction and postsynaptic accumulation of gephyrin and collybistin.
The intramolecular interaction between the SH3 domain and DH/PH domain of collybistin keeps collybistin in a closed conformation. In this closed state, collybistin can bind to intracellular gephyrin but has a weak ability to promote gephyrin clustering at the plasma membrane. Binding of NLGN2 to the collybistin SH3 domain disrupts the intramolecular interaction, exposes the PH domain for membrane bindings and induces large, stable gephyrin clustering at the plasma membrane (Poulopoulos et al., 2009; Soykan et al., 2014). SH3, src-homology 3; DH, dbl-homology; PH, pleckstrin-homology.
3.2. Slitrk3
As noted before, data from NLGN2 KO mice show that although NLGN2 is important for GABAergic synapse development in hippocampal CA1 neurons, it is only critical for a subset of GABAergic synapses at the perisomatic region (Poulopoulos et al., 2009). This suggests that there are other synaptic adhesion proteins responsible for inhibitory synapse formation during development. The Slit- and Trk-like (Slitrk) family consists of six postsynaptic membrane proteins, each containing two leucine-rich repeat clusters (LRR) in the extracellular region that mediates the trans-synaptic adhesion interaction with presynaptic receptors, protein tyrosine phosphatases (PTPs) (Takahashi et al., 2012; Um et al., 2014a; Yim et al., 2013). Among the six Slitrks, Slitrk3 has recently been identified to be a postsynaptic adhesion molecule specific for inhibitory synapse development (Takahashi et al., 2012; Yim et al., 2013). Expression of Slitrk3 in non-neuronal cells can induce GABAergic, but not glutamatergic, presynaptic differentiation of contacting axons (Takahashi et al., 2012; Yim et al., 2013). Correspondingly, overexpression of Slitrk3 in hippocampal neurons promotes GABAergic, but not glutamatergic, synapse formation (Takahashi et al., 2012; Yim et al., 2013). Importantly, shRNA-mediated knockdown of Slitrk3 strongly reduces synaptic clustering of vesicular GABA transporter (vGAT) and gephyrin, the pre- and post-synaptic markers of inhibitory synapses (Takahashi et al., 2012; Yim et al., 2013). Gene KO data further confirm that Slitrk3 is required for GABAergic synapse development in vivo, as both GABAergic synapse density and inhibitory synaptic transmission are impaired in hippocampal CA1 neurons from Slitrk3 KOs (Takahashi et al., 2012). Mechanistically, Slitrk3 binds to presynaptic transmembrane protein PTPδ to control the coordinated development of GABAergic pre- and post-synaptic specializations (Takahashi et al., 2012; Yim et al., 2013). Collectively, these data demonstrate that Slitrk3 acts as an alternative synaptogenic adhesion molecule, specific for inhibitory synapse development in the brain.
The identification of Slitrk3 and NLGN2 as synaptogenic molecules specific to inhibitory synapses is a major advance in understanding the molecular mechanisms for GABAergic synapse development; but it also raises important new questions. In the hippocampus, principle pyramidal neurons are innervated by at least 21 distinct subclasses of interneurons that make functionally diverse GABAergic synapses onto pyramidal neurons in a domain specific manner (Klausberger and Somogyi, 2008). Are NLGN2 and Slitrk3 responsible for the formation of all GABAergic synapses in hippocampal neurons or are each of them preferentially involved in the development of a subpopulation of GABAergic synapses? Do NLGN2 and Slitrk3 operate in parallel to direct development of different types of GABAergic synapse or they function in different stages during the development of the same synapse? How is trafficking of NLGN2 and Slitrk3 to the developing neuronal surface regulated to induce formation of nascent synapses? Do they exert their synaptogenic function by different mechanisms? The answers to these questions will not only deepen our understanding of these synaptic adhesion molecules, but will also help us understand how the vast array of inhibitory synapses is generated in the brain.
3.3. Calsyntenin3
Calsyntenins, also known as alcadeins, are a subfamily of synaptic adhesion molecules that belong to the cadherin superfamily. Calsyntenins are type I transmembrane proteins, which consist of extracellular domains containing two cadherin repeats and a laminin-a, neurexin, sex hormone-binding globulin (LNS) domain, and an intracellular calcium binding domain. Calsyntenin1 was originally identified in spinal-cord neurons (Vogt et al., 2001). Subsequently, calsyntenin2 and 3 were discovered based on sequence homology (Hintsch et al., 2002). Among the three members, calsyntenin1 is expressed throughout the body; in contrast, calsyntenin2 and 3 are highly enriched in the brain. Immunolabelling and subcellular fractionation reveal that all calsyntenins are localized at synapses (Um et al., 2014b; Vogt et al., 2001).
Recent studies have identified calsyntenin3, but not calsyntenin1 and 2, as a potent synaptogenic protein that can induce excitatory and inhibitory presynaptic differentiation in co-culture assays (Pettem et al., 2013a; Um et al., 2014b). Consistently, both GABAergic and glutamatergic synapse densities in the hippocampal CA1 region are reduced in calsyntenin3 KO mice (Pettem et al., 2013a), confirming the synaptogenic function of calsyntenin 3 in vivo. Interestingly, acute knockdown of calsyntenin1, 2 or 3 individually in hippocampal neuron cultures does not change synapse density (Um et al., 2014b). Only simultaneous knockdown of all three calsyntenins decreases GABAergic, but not glutamatergic, synapse densities, suggesting a functional redundancy of calsyntenins in this experimental preparation (Um et al., 2014b). Similarly, knockdown of calsyntenin1–3 in vivo in cortical neurons impairs inhibitory, but not excitatory synaptic transmission (Um et al., 2014b). Currently, it is unclear whether and how calsyntenins functionally substitute for each other, since only calsyntenin3 is synaptogenic (Pettem et al., 2013a; Um et al., 2014b). It also remains unclear what accounts for the difference of synaptic phenotypes in neurons expressing shRNAs vs neurons from calsyntenin3 KOs (Pettem et al., 2013a; Um et al., 2014b). Mechanistically, synaptogenic function of calsyntenin3 requires presynaptic neurexins (Lu et al., 2014; Pettem et al., 2013a; Um et al., 2014b). Future work will be needed to reveal the molecular mechanisms by which calsyntenin3 regulates GABAergic synapse development. In addition, calsyntenin3 is expressed at high levels in a subset of interneurons (Hintsch et al., 2002; Pettem et al., 2013a). It will be interesting to determine whether calsyntenin3 is a key synaptogenic molecule for GABAergic synapse formation in interneurons.
4. Immunoglobulin superfamily (IgSF) proteins
The Immunoglobulin superfamily (IgSF) consists of a large group of proteins characterized by the presence of Ig domains. Ig domains are important protein interaction motifs that are often found in membrane proteins involved in molecule recognition/binding and intercellular interactions (Barclay, 2003). In the brain, many IgSFs contribute to synaptic adhesive interactions, which are important for the formation, maturation and function of synapses (Shen and Scheiffele, 2010). Recent advances have identified several Ig family proteins that play a key role in the regulation of GABAergic synapse formation and maturation.
4.1. IgSF9 and IgSF9b
IgSF9 and IgSF9b, are evolutionarily conserved Ig superfamily (IgSF) type I transmembrane adhesion molecules (Hansen and Walmod, 2013). In rodents, IgSF9 and IgSF9b share similar domain organization, containing five Ig domains and two fibronectin III (FNIII) domains in the extracellular amino (N)-terminus, and a PDZ-binding motif in the intracellular C-terminus. Both IgSF9 and IgSF9b are strongly expressed in the developing brain and are preferentially localized to dendritic regions (Hansen and Walmod, 2013; Shi et al., 2004).
4.1.1. IgSF9
IgSF9, also called dendrite arborization and synapse maturation 1 (Dasm1), was initially identified through a homology search based on a Drosophila IgSF molecule, Turtle (Shi et al., 2004). RNAi-mediated knockdown and overexpression of dominant-negative mutants in cultured hippocampal neurons showed that IgSF9 regulates dendritic arborization and excitatory synaptic maturation (Shi et al., 2004). However, recent in vivo experiments in IgSF9 KO mice have shown normal dendrite, spine and excitatory synapse development in the hippocampus (Litwack et al., 2004; Mishra et al., 2008). Importantly, the previously observed phenotype following IgSF9 knockdown is also observed when the knockdown is performed in neurons prepared from IgSF9 null mice, suggesting that an off-target effect may explain RNAi-induced deficits (Mishra et al., 2008). Instead, a strong reduction in GABAergic synapse density and impaired inhibitory synaptic transmission are observed in hippocampal neurons prepared from IgSF9 KO mice, indicating a role of IgSF9 in GABAergic synapse development (Mishra et al., 2014). Furthermore, knock-in mice that express a mutant IgSF9 lacking the cytoplasmic domain have normal inhibitory synaptic transmission, suggesting that IgSF9 regulates inhibitory synapse development via ectodomain-mediated interactions, rather than acting through its intracellular domain (Mishra et al., 2014). Interestingly, IgSF9 can mediate homophilic adhesion, but is not a synaptogenic molecule, as it fails to induce GABAergic synapse differentiation in a neuron-heterologous cell co-culture assay (Mishra et al., 2014). Thus, it is likely that IgSF9 functions through other synaptogenic molecules to establish new GABAergic synapses or is involved in stabilization of preexisting synapses.
4.1.2. IgSF9b
IgSF9b is a close homolog of IgSF9 in the mammalian brain and was recently found to be associated with major depressive disorder (Shyn et al., 2011). Interestingly, IgSF9b is highly enriched in GABAergic interneurons with weak expression in pyramidal cells in the hippocampus (Woo et al., 2013). Similar to IgSF9, IgSF9b binds homophilically, but is not sufficient to induce synapse formation (Woo et al., 2013). In primary hippocampal neuronal cultures, IgSF9b forms punctal subcellular structures that colocalize with GABAergic, but not glutamatergic, markers in interneurons, suggesting that it is a synaptic adhesion molecule specific to inhibitory synapses (Woo et al., 2013). In interneurons in hippocampal cultures expressing an shRNA to down regulate IgSF9b expression, GABAergic synapse density and inhibitory synaptic transmission are reduced (Woo et al., 2013), demonstrating a role of IgSF9b in the regulation of GABAergic synapse development. Mechanistically, IgSF9b directly interacts with PDZ domain 4 and 5 of MAGI2, a member of S-SCAM localized at inhibitory synapses (Woo et al., 2013). A previous study demonstrated that MAGI2 binds to NLGN2 through its PDZ1 (Sumita et al., 2007), suggesting that MAGI2 can simultaneously bind to both IgSF9b and NLGN2 acting as a bridge protein. Indeed, binding assay in heterologous cells and brain lysates show that the three proteins can form a ternary complex (Woo et al., 2013). Furthermore, MAGI2 knockdown suppresses colocalization of IgSF9b and NLGN2 at inhibitory synapses and decreases the number of GABAergic synapses (Woo et al., 2013), indicating that IgSF9b may indirectly regulate GABAergic synapse development through NLGN2. The data that IgSF9b can form trans-synaptic homophilic interaction as well as postsynaptic interactions with MAGI2 and NLGN2 suggest an IgSF9b-based trans-synaptic macromolecular complex in interneurons important for GABAergic synapse formation and stabilization.
4.2. MDGA
MDGAs are Ig superfamily adhesion molecules. They consist of two members (MDGA1 and 2), each composed of six Ig domains, a fibronectin III domain, a memprin, A5 protein, receptor protein tyrosine phosphatase mu (MAM) domain, and a GPI anchor that attaches the protein to the plasma membrane (Litwack et al., 2004). MDGAs are highly expressed in developing neurons and have been implicated in brain development (Ishikawa et al., 2011; Joset et al., 2011; Lee et al., 2013; Litwack et al., 2004; Takeuchi and O'Leary, 2006). Recently, two elegant studies demonstrated that MDGAs are important in the regulation of GABAergic synapse development (Lee et al., 2013; Pettem et al., 2013b). In cultured hippocampal neurons, overexpression of MDGA1 decreases GABAergic, but not glutamatergic, synapse density; and shRNA-mediated gene knockdown enhances GABAergic synapse formation, indicating that MDGAs negatively regulate GABAergic synaptogenesis (Lee et al., 2013; Pettem et al., 2013b). Interestingly, MDGA1 is not synaptogenic on their own, but acts through NLGN2 to induce GABAergic development (Lee et al., 2013; Pettem et al., 2013b). Cell-based binding assays demonstrate that NLGN2, but not NLGN1 or 3, interacts in cis with MDGA Ig domains with a nanomolar affinity in an extracellular Ca2+-dependent manner (Lee et al., 2013; Pettem et al., 2013b). This MDGA-NLGN2 interaction inhibits the synaptogenic activity of NLGN2 by blocking NLGN2 from binding to its presynaptic receptor, neurexin (Lee et al., 2013; Pettem et al., 2013b). Currently, it remains unclear how the MDGA-NLGN2 interaction is regulated during development. As NLGN2 is one of the major synaptogenic molecules for GABAergic synapse formation, understanding the dynamic regulation of the MDGA-NLGN2 interaction during development will provide key insights into molecular cascades underlying initial GABAergic synapse assembly.
4.3. Contactins
Contactins (CNTNs) are a subfamily of the Ig superfamily of neural cell-adhesion molecules (Ig-CAMs), consisting of six members (CNTN1-6) (Zuko et al., 2013). CNTNs are non-transmembrane, GPI-anchored proteins attached to the extracellular half of the plasma membrane. An important feature of CNTNs is their six extracellular Ig domains and four fibronection type III (FNIII) domains. These Ig domains have been shown to mediate interactions with receptor protein tyrosine phosphatases (RPTPs) and L1 family cell adhesion molecules (Zuko et al., 2013). Evidence from several lines of investigation has shown that CNTNs are generally involved in development of neuronal axons, dendrites and synapses across many types of neurons in the brain (Zuko et al., 2013).
Among the six members, CNTN1 and CNTN5 have been implicated in GABAergic synapse development. In the cerebellar cortex, CNTN1 is localized at both Golgi cell axons and granule cell dendrites (Chen et al., 2011). Genetic deletion of CNTN1 causes a significant reduction of GABAergic synapse number between Golgi cells and granule cells, showing a critical role of CNTN1 in GABAergic synaptogenesis (Chen et al., 2011). Trafficking of CNTN1 to the plasma membrane of granule cell dendrites is regulated by Tropomyosin-related kinase B (TrkB), which is also important for GABAergic synapse formation and maturation (Chen et al., 2011). Currently it remains unclear how CNTN1 regulates GABAergic synapse development. Possible mechanisms include trans-synaptic adhesion interactions with PTPs and extracellular matric proteins (Bouyain and Watkins, 2010; Michele and Faissner, 2009; Peles et al., 1995).
Another recent study reveals a critical role of CNTN5 in the development of GABAergic synapses. In the spinal cord, one type of GABAergic interneuron makes prominent GABAergic contacts with proprioceptive sensory neuron axonal terminals (Hughes et al., 2005). These high-density axoaxonic GABAergic synapses are important for selective filtering of afferent input and information processing at these synapses (Betley et al., 2009). It was found that CNTN5 is specifically expressed in sensory neurons (Ashrafi et al., 2014). Importantly, genetic deletion of CNTN5 causes a significant reduction of axoaxonic GABAergic synapses, showing an important role of CNTN5 in the development of this type of GABAergic synapses (Ashrafi et al., 2014). At sensory neuron terminals, CNTN5 forms a complex with contactin-associated transmembrane protein 4 (Caspr4), and the CNTN5-Caspr4 complexes interact with the presynaptic L1 Ig family proteins, including close homolog of L1 (CHL1) and neuronal CAM (NrCAM), suggesting that CNTN5 may function through both cis- and trans-synaptic adhesion interactions to regulate axoaxonic GABAergic synapse development (Ashrafi et al., 2014). Indeed, genetic inactivation of these Ig proteins, either alone or in combination as double KOs, produces similar deficits of axoaxonic GABAergic synapse density (Ashrafi et al., 2014), indicating that these Ig family adhesion molecules act through a common pathway to regulate GABAergic synaptogenesis. Currently, it remains to be determined whether CNTNs-mediated GABAergic synapse development described here represents a broad mechanism for GABAergic synaptogenesis across the brain. It will be important to analyze GABAergic synapses in other brain areas in CNTN1 and CNTN5 KO mice.
5. Other postsynaptic membrane proteins important for GABAergic synapse development
5.1. Sema4D
The semaphorins are a large family of membrane-associated and secreted proteins, and are characterized by the presence of a cycteine-rich sema domain that is important for signal transduction (Pasterkamp, 2012). Semaphorins were originally described as axon guidance molecules critical for development of the nervous system (Pasterkamp, 2012). Recent studies have expanded on this and have demonstrated that a subclass of semaphorins, semaphorin 4D (Sema4D), is critical for GABAergic synapse development. In an RNAi screening of hippocampal neuronal cultures to search for molecules important for synapse development, Sema4D was found to specifically regulate GABAergic synapse development (Paradis et al., 2007). Indeed, knockdown Sema4D leads to a decrease in the density of GABAergic synapses, but not glutamatergic synapses (Paradis et al., 2007). In addition, Sema4D is sufficient to promote GABAergic synapse development, as treatment of cultured hippocampal neurons with purified extracellular domain of Sema4D can rapidly induce new GABAergic synapse formation (Kuzirian et al., 2013). Mechanistically, Sema4D signals through binding to its receptor, PlexinB1, to regulate GABAergic synaptogenesis, as the effect of Sema4D treatment in synapse development is lost in neurons prepared from PlexinB1 KO mice (Kuzirian et al., 2013; Raissi et al., 2013). Structurally, Sema4D is a transmembrane protein, which – once targeted to the plasma membrane – can undergo metalloproteinase-mediated cleavage to release its extracellular domain for signaling (Basile et al., 2007). Interestingly, although Sema4D can be proteolytically cleaved from the neuronal surface, the membrane-bound form localized at postsynaptic membranes is important for GABAergic synapse formation, suggesting a mechanism for the regulation of Sema4D signaling (Raissi et al., 2013). Currently, the functional relationship between Sema4D and other known GABAergic synaptogenic molecules, such as NLGN2 and Slitrk3, remains unclear. Does Sema4D function upstream or downstream of these molecules? Alternately, does Sema4D represent a parallel signaling pathway for GABAergic synapse formation independent of NLGN2 and/or Slitrk3? The answer to these questions will likely reveal functional interactions between different membrane molecules important for GABAergic synapse development and clarify the developmental origin of many types of GABAergic synapses in the brain.
5.2. TrkB
TrkB belongs to a family of three receptor tyrosine kinases and plays an important role in development and function of the CNS (Huang and Reichardt, 2003). The influence of TrkB on the development of GABAergic synapses was first revealed in a conditional knockout line in which TrkB was genetically deleted in cerebellar neurons (Rico et al., 2002). Morphological analysis shows that there is a marked reduction in the density of GABAergic synapses onto cerebellar granule cells with no change in the number of glutamatergic synapses in the cerebellum (Chen et al., 2011; Rico et al., 2002). Specifically, loss of TrkB receptors leads to strong reductions in the densities of GABAergic presynaptic markers, GAD65/67 and vGAT as well as the postsynaptic marker, gephyrin (Chen et al., 2011). Interestingly, NLGN2 puncta are not reduced in TrkB knockout cerebellar granule cells, suggesting that TrkB may function downstream of NLGN2-mediated formation/maturation of GABAergic synapses (Chen et al., 2011). Alternatively, TrkB-mediated signaling may represent a different pathway for GABAergic synaptogenesis. By using cerebellar cell-type specific Cre lines, it was further demonstrated that TrkB in both presynaptic Golgi cells and postsynaptic granule cells are important for GABAergic synapse development (Chen et al., 2011). In further support, activation of TrkB by its ligand, brain-derived neurotropic factor (BDNF), promotes GABAergic synapse development, while gene inactivation of BDNF suppresses GABAergic synapse development (Bao et al., 1999; Huang et al., 1999; Kohara et al., 2007; Marty et al., 2000; Seil and Drake-Baumann, 2000; Vicario-Abejon et al., 1998). In addition, BDNF-mediated activation of TrkB is important for activity-dependent regulation of GABAergic synapse development (Hong et al., 2008; Seil and Drake-Baumann, 2000). Mechanistically, the regulation of GABAergic synapse development by TrkB requires CNTN1 and the TrkB C-terminal subdomain that mediates the TrkB interaction with phospholipase C-γ1 (PLC-γ1) (Chen et al., 2011). It is worth noting that TrkB plays a critical role in the regulation of CNTN1 trafficking to the plasma membrane, showing a functional interplay between TrkB-mediated signaling and IgSF protein-mediated adhesion (Chen et al., 2011). As has been discussed before (i.e., the MDGA-NLGN2 interaction), such cross-talk by different membrane proteins that are implicated in GABAergic synapse development probably represents a common mechanism for GABAergic synaptogenesis.
6. Conclusions and perspectives
In the brain, synaptogenesis is initiated through signaling mediated by cell surface membrane proteins expressed at developing presynaptic terminals and postsynaptic membranes. Recent advances have revealed a growing number of membrane molecules unique to GABAergic synapse formation. Exciting progress has been made in understanding the biochemical pathways underlying the function of these membrane proteins in GABAergic synapse development (Gu et al., 2016; Lee et al., 2013; Pettem et al., 2013b; Poulopoulos et al., 2009; Soykan et al., 2014; Takahashi et al., 2012; Yim et al., 2013). An excellent example is NLGN2 whereby an intricate tripartite interaction between NLGN2, gephyrin and collybistin governs GABAergic synapse development in hippocampal neurons (Poulopoulos et al., 2009; Soykan et al., 2014). It will continue to be important to determine the molecular mechanisms underlying the regulation of GABAergic synaptogenesis by these membrane proteins. In addition, it will be equally important to identify which of these molecules are implicated in each of the various steps of GABAergic synapse development, including initial contact, stabilization of nascent connections, and functional maturation of developing synapses. As dysregulation of GABAergic synapse development and function has been implicated in many brain disorders, the precise mechanism for regulating GABAergic synapse development by membrane proteins represents significant progress in understanding the developmental origin of neural inhibition in the brain. This will also generate novel knowledge for designing effective therapeutic reagents targeting GABAergic synapses for intervening and treating neurological and neuropsychiatric diseases.
A remarkable feature of the inhibitory synapse is its enormous diversity. Indeed, pyramidal neurons in the hippocampal CA1 region receive functionally distinct GABAergic inputs from more than twenty types of interneurons (Klausberger and Somogyi, 2008). In addition, many of these GABAergic afferents make domain specific synaptic contacts on pyramidal neurons to provide dynamic control of pyramidal neuron activity in a highly spatially and temporally controlled manner, and such control is critical for proper neural circuit function in the brain (Klausberger and Somogyi, 2008). How are the domain-specific inhibitory synaptic connections established during development? What are the roles of the membrane proteins we discussed above in the formation of highly precise synaptic contacts in a spatially defined manner? Recent advances have demonstrated that Ig superfamily cell adhesion proteins play critical roles in establishing domain-specific GABAergic connections (Ango et al., 2004; Ashrafi et al., 2014; Saghatelyan et al., 2004). In addition, a recent elegant study has shown that the transcription factor NPAS4 (neuronal PAS domain protein 4) regulates activity-dependent, domain-specific formation of synaptic inhibition in mouse hippocampal neurons (Bloodgood et al., 2013), suggesting an unexplored functional interaction between transcription and synaptic adhesion molecules for input-specific GABAergic synaptogenesis. In the future it will be imperative to determine the proteins and signaling pathways underlying the domain-specific formation of inhibitory synapses. This will require the identification of proteins uniquely expressed at presynaptic terminals of different classes of interneurons and at the postsynaptic membranes of the receiving cells, as well as the relevant genetic programs during development. Defining the molecular mechanisms for input-specific development of GABAergic synapses will not only provide a deeper understanding of GABAergic synaptogenesis in the brain, but also enable us to precisely manipulate specific inhibitory input for clinical interventions against mental illnesses.
Acknowledgements
This work was supported by the NIH, NINDS Intramural Research Program (W.L). We thank Sarah Albani for critical comments on the manuscript.
References
- Aamodt SM, Shi J, Colonnese MT, Veras W, Constantine-Paton M. Chronic NMDA exposure accelerates development of GABAergic inhibition in the superior colliculus. J. Neurophysiol. 2000;83:1580–1591. doi: 10.1152/jn.2000.83.3.1580. [DOI] [PubMed] [Google Scholar]
- Akazawa C, Shigemoto R, Bessho Y, Nakanishi S, Mizuno N. Differential expression of five N-methyl-D-aspartate receptor subunit mRNAs in the cerebellum of developing and adult rats. J. Comp. Neurol. 1994;347:150–160. doi: 10.1002/cne.903470112. [DOI] [PubMed] [Google Scholar]
- Akerman CJ, Cline HT. Depolarizing GABAergic conductances regulate the balance of excitation to inhibition in the developing retinotectal circuit in vivo. J. Neurosci. 2006;26:5117–5130. doi: 10.1523/JNEUROSCI.0319-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ango F, di Cristo G, Higashiyama H, Bennett V, Wu P, Huang ZJ. Ankyrin-based subcellular gradient of neurofascin an immunoglobulin family protein, directs GABAergic innervation at purkinje axon initial segment. Cell. 2004;119:257–272. doi: 10.1016/j.cell.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Antonelli R, Pizzarelli R, Pedroni A, Fritschy JM, Del Sal G, Cherubini E, Zacchi P. Pin1-dependent signalling negatively affects GABAergic transmission by modulating neuroligin2/gephyrin interaction. Nat. Commun. 2014;5:5066. doi: 10.1038/ncomms6066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arama J, Abitbol K, Goffin D, Fuchs C, Sihra TS, Thomson AM, Jovanovic JN. GABAA receptor activity shapes the formation of inhibitory synapses between developing medium spiny neurons. Front. Cell. Neurosci. 2015;9:290. doi: 10.3389/fncel.2015.00290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashrafi S, Betley JN, Comer JD, Brenner-Morton S, Bar V, Shimoda Y, Watanabe K, Peles E, Jessell TM, Kaltschmidt JA. Neuronal Ig/Caspr recognition promotes the formation of axoaxonic synapses in mouse spinal cord. Neuron. 2014;81:120–129. doi: 10.1016/j.neuron.2013.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannai H, Levi S, Schweizer C, Inoue T, Launey T, Racine V, Sibarita JB, Mikoshiba K, Triller A. Activity-dependent tuning of inhibitory neurotransmission based on GABAAR diffusion dynamics. Neuron. 2009;62:670–682. doi: 10.1016/j.neuron.2009.04.023. [DOI] [PubMed] [Google Scholar]
- Bao S, Chen L, Qiao X, Thompson RF. Transgenic brain-derived neurotrophic factor modulates a developing cerebellar inhibitory synapse. Learn. Mem. 1999;6:276–283. [PMC free article] [PubMed] [Google Scholar]
- Barclay AN. Membrane proteins with immunoglobulin-like domains—a master superfamily of interaction molecules. Semin. Immunol. 2003;15:215–223. doi: 10.1016/s1044-5323(03)00047-2. [DOI] [PubMed] [Google Scholar]
- Basile JR, Holmbeck K, Bugge TH, Gutkind JS. MT1-MMP controls tumor-induced angiogenesis through the release of semaphorin 4D. J. Biol. Chem. 2007;282:6899–6905. doi: 10.1074/jbc.M609570200. [DOI] [PubMed] [Google Scholar]
- Behar TN, Scott CA, Greene CL, Wen X, Smith SV, Maric D, Liu QY, Colton CA, Barker JL. Glutamate acting at NMDA receptors stimulates embryonic cortical neuronal migration. J. Neurosci. 1999;19:4449–4461. doi: 10.1523/JNEUROSCI.19-11-04449.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bemben MA, Nguyen QA, Wang T, Li Y, Nicoll RA, Roche KW. Autism-associated mutation inhibits protein kinase C-mediated neuroligin-4X enhancement of excitatory synapses. Proc. Natl. Acad. Sci. U. S. A. 2015;112:2551–2556. doi: 10.1073/pnas.1500501112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y, Khazipov R, Leinekugel X, Caillard O, Gaiarsa JL. GABAA, NMDA and AMPA receptors: a developmentally regulated ‘menage a trois’. Trends Neurosci. 1997;20:523–529. doi: 10.1016/s0166-2236(97)01147-8. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Gaiarsa JL, Tyzio R, Khazipov R. GABA: a pioneer transmitter that excites immature neurons and generates primitive oscillations. Physiol. Rev. 2007;87:1215–1284. doi: 10.1152/physrev.00017.2006. [DOI] [PubMed] [Google Scholar]
- Bennett GD, Moser K, Chaudoin T, Rosenquist TH. The expression of the NR1-subunit of the NMDA receptor during mouse and early chicken development. Reprod. Toxicol. 2006;22:536–541. doi: 10.1016/j.reprotox.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Berg RW, Alaburda A, Hounsgaard J. Balanced inhibition and excitation drive spike activity in spinal half-centers. Science. 2007;315:390–393. doi: 10.1126/science.1134960. [DOI] [PubMed] [Google Scholar]
- Betley JN, Wright CV, Kawaguchi Y, Erdelyi F, Szabo G, Jessell TM, Kaltschmidt JA. Stringent specificity in the construction of a GABAergic presynaptic inhibitory circuit. Cell. 2009;139:161–174. doi: 10.1016/j.cell.2009.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanton MG, Kriegstein AR. Properties of amino acid neurotransmitter receptors of embryonic cortical neurons when activated by exogenous and endogenous agonists. J. Neurophysiol. 1992;67:1185–1200. doi: 10.1152/jn.1992.67.5.1185. [DOI] [PubMed] [Google Scholar]
- Bloodgood BL, Sharma N, Browne HA, Trepman AZ, Greenberg ME. The activity-dependent transcription factor NPAS4 regulates domain-specific inhibition. Nature. 2013;503:121–125. doi: 10.1038/nature12743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blundell J, Tabuchi K, Bolliger MF, Blaiss CA, Brose N, Liu X, Sudhof TC, Powell CM. Increased anxiety-like behavior in mice lacking the inhibitory synapse cell adhesion molecule neuroligin 2. Genes Brain Behav. 2009;8:114–126. doi: 10.1111/j.1601-183X.2008.00455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouyain S, Watkins DJ. The protein tyrosine phosphatases PTPRZ and PTPRG bind to distinct members of the contactin family of neural recognition molecules. Proc. Natl. Acad. Sci. U. S. A. 2010;107:2443–2448. doi: 10.1073/pnas.0911235107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown LE, Nicholson MW, Arama JE, Mercer A, Thomson AM, Jovanovic JN. Gamma-aminobutyric acid type a (GABAA) receptor subunits play a direct structural role in synaptic contact formation via their N-terminal extracellular domains. J. Biol. Chem. 2016;291:13926–13942. doi: 10.1074/jbc.M116.714790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campusano JM, Andres ME, Magendzo K, Abarca J, Tapia-Arancibia L, Bustos G. Novel alternative splicing predicts a truncated isoform of the NMDA receptor subunit 1 (NR1) in embryonic rat brain. Neurochem. Res. 2005;30:567–576. doi: 10.1007/s11064-005-2691-3. [DOI] [PubMed] [Google Scholar]
- Chattopadhyaya B, Di Cristo G, Wu CZ, Knott G, Kuhlman S, Fu Y, Palmiter RD, Huang ZJ. GAD67-mediated GABA synthesis and signaling regulate inhibitory synaptic innervation in the visual cortex. Neuron. 2007;54:889–903. doi: 10.1016/j.neuron.2007.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Kriegstein AR. A GABAergic projection from the zona incerta to cortex promotes cortical neuron development. Science. 2015;350:554–558. doi: 10.1126/science.aac6472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen AI, Nguyen CN, Copenhagen DR, Badurek S, Minichiello L, Ranscht B, Reichardt LF. TrkB (tropomyosin-related kinase B) controls the assembly and maintenance of GABAergic synapses in the cerebellar cortex. J. Neurosci. 2011;31:2769–2780. doi: 10.1523/JNEUROSCI.4991-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherubini E, Gaiarsa JL, Ben-Ari Y. GABA: an excitatory transmitter in early postnatal life. Trends Neurosci. 1991;14:515–519. doi: 10.1016/0166-2236(91)90003-d. [DOI] [PubMed] [Google Scholar]
- Chih B, Engelman H, Scheiffele P. Control of excitatory and inhibitory synapse formation by neuroligins. Science. 2005;307:1324–1328. doi: 10.1126/science.1107470. [DOI] [PubMed] [Google Scholar]
- Chiou TT, Bonhomme B, Jin H, Miralles CP, Xiao H, Fu Z, Harvey RJ, Harvey K, Vicini S, De Blas AL. Differential regulation of the postsynaptic clustering of gamma-aminobutyric acid type A (GABAA) receptors by collybistin isoforms. J. Biol. Chem. 2011;286:22456–22468. doi: 10.1074/jbc.M111.236190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie SB, Miralles CP, De Blas AL. GABAergic innervation organizes synaptic and extrasynaptic GABAA receptor clustering in cultured hippocampal neurons. J. Neurosci. 2002;22:684–697. doi: 10.1523/JNEUROSCI.22-03-00684.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chubykin AA, Atasoy D, Etherton MR, Brose N, Kavalali ET, Gibson JR, Sudhof TC. Activity-dependent validation of excitatory versus inhibitory synapses by neuroligin-1 versus neuroligin-2. Neuron. 2007;54:919–931. doi: 10.1016/j.neuron.2007.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline H. Synaptogenesis: a balancing act between excitation and inhibition. Curr. Biol.: CB. 2005;15:R203–205. doi: 10.1016/j.cub.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Craig AM, Kang Y. Neurexin-neuroligin signaling in synapse development. Curr. Opin. Neurobiol. 2007;17:43–52. doi: 10.1016/j.conb.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cserep C, Szabadits E, Szonyi A, Watanabe M, Freund TF, Nyiri G. NMDA receptors in GABAergic synapses during postnatal development. PLoS One. 2012;7:e37753. doi: 10.1371/journal.pone.0037753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejanovic B, Semtner M, Ebert S, Lamkemeyer T, Neuser F, Luscher B, Meier JC, Schwarz G. Palmitoylation of gephyrin controls receptor clustering and plasticity of GABAergic synapses. PLoS Biol. 2014;12:e1001908. doi: 10.1371/journal.pbio.1001908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng L, Yao J, Fang C, Dong N, Luscher B, Chen G. Sequential postsynaptic maturation governs the temporal order of GABAergic and glutamatergic synaptogenesis in rat embryonic cultures. J. Neurosci. 2007;27:10860–10869. doi: 10.1523/JNEUROSCI.2744-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diniz LP, Tortelli V, Garcia MN, Araujo AP, Melo HM, Silva GS, Felice FG, Alves-Leon SV, Souza JM, Romao LF, Castro NG, Gomes FC. Astrocyte transforming growth factor beta 1 promotes inhibitory synapse formation via CaM kinase II signaling. Glia. 2014;62:1917–1931. doi: 10.1002/glia.22713. [DOI] [PubMed] [Google Scholar]
- Dorrn AL, Yuan K, Barker AJ, Schreiner CE, Froemke RC. Developmental sensory experience balances cortical excitation and inhibition. Nature. 2010;465:932–936. doi: 10.1038/nature09119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudek FE. Epileptogenesis: a new twist on the balance of excitation and inhibition. Epilepsy Curr./Am. Epilepsy Soc. 2009;9:174–176. doi: 10.1111/j.1535-7511.2009.01334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essrich C, Lorez M, Benson JA, Fritschy JM, Luscher B. Postsynaptic clustering of major GABAA receptor subtypes requires the gamma 2 subunit and gephyrin. Nat. Neurosci. 1998;1:563–571. doi: 10.1038/2798. [DOI] [PubMed] [Google Scholar]
- Feng G, Tintrup H, Kirsch J, Nichol MC, Kuhse J, Betz H, Sanes JR. Dual requirement for gephyrin in glycine receptor clustering and molybdoenzyme activity. Science. 1998;282:1321–1324. doi: 10.1126/science.282.5392.1321. [DOI] [PubMed] [Google Scholar]
- Fischer F, Kneussel M, Tintrup H, Haverkamp S, Rauen T, Betz H, Wassle H. Reduced synaptic clustering of GABA and glycine receptors in the retina of the gephyrin null mutant mouse. J. Comp. Neurol. 2000;427:634–648. doi: 10.1002/1096-9861(20001127)427:4<634::aid-cne10>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Flores CE, Nikonenko I, Mendez P, Fritschy JM, Tyagarajan SK, Muller D. Activity-dependent inhibitory synapse remodeling through gephyrin phosphorylation. Proc. Natl. Acad. Sci. U. S. A. 2015;112:E65–72. doi: 10.1073/pnas.1411170112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritschy JM, Panzanelli P. Molecular and synaptic organization of GABAA receptors in the cerebellum: effects of targeted subunit gene deletions. Cerebellum. 2006;5:275–285. doi: 10.1080/14734220600962805. [DOI] [PubMed] [Google Scholar]
- Fritschy JM, Panzanelli P. GABAA receptors and plasticity of inhibitory neurotransmission in the central nervous system. Eur. J. Neurosci. 2014;39:1845–1865. doi: 10.1111/ejn.12534. [DOI] [PubMed] [Google Scholar]
- Fritschy JM, Panzanelli P, Kralic JE, Vogt KE, Sassoe-Pognetto M. Differential dependence of axo-dendritic and axo-somatic GABAergic synapses on GABAA receptors containing the alpha1 subunit in Purkinje cells. J. Neurosci. 2006;26:3245–3255. doi: 10.1523/JNEUROSCI.5118-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritschy JM, Harvey RJ, Schwarz G. Gephyrin: where do we stand where do we go? Trends Neurosci. 2008;31:257–264. doi: 10.1016/j.tins.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Fritschy JM, Panzanelli P, Tyagarajan SK. Molecular and functional heterogeneity of GABAergic synapses. Cell. Mol. Life Sci.: CMLS. 2012;69:2485–2499. doi: 10.1007/s00018-012-0926-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frola E, Patrizi A, Goetz T, Medrihan L, Petrini EM, Barberis A, Wulff P, Wisden W, Sassoe-Pognetto M. Synaptic competition sculpts the development of GABAergic axo-dendritic but not perisomatic synapses. PLoS One. 2013;8:e56311. doi: 10.1371/journal.pone.0056311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs C, Abitbol K, Burden JJ, Mercer A, Brown L, Iball J, Anne Stephenson F, Thomson AM, Jovanovic JN. GABA(A) receptors can initiate the formation of functional inhibitory GABAergic synapses. Eur. J. Neurosci. 2013;38:3146–3158. doi: 10.1111/ejn.12331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futai K, Doty CD, Baek B, Ryu J, Sheng M. Specific trans-synaptic interaction with inhibitory interneuronal neurexin underlies differential ability of neuroligins to induce functional inhibitory synapses. J. Neurosci. 2013;33:3612–3623. doi: 10.1523/JNEUROSCI.1811-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giustetto M, Kirsch J, Fritschy JM, Cantino D, Sassoe-Pognetto M. Localization of the clustering protein gephyrin at GABAergic synapses in the main olfactory bulb of the rat. J. Comp. Neurol. 1998;395:231–244. doi: 10.1002/(sici)1096-9861(19980601)395:2<231::aid-cne7>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Forero D, Pastor AM, Geiman EJ, Benitez-Temino B, Alvarez FJ. Regulation of gephyrin cluster size and inhibitory synaptic currents on Renshaw cells by motor axon excitatory inputs. J. Neurosci. 2005;25:417–429. doi: 10.1523/JNEUROSCI.3725-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf ER, Zhang X, Jin SX, Linhoff MW, Craig AM. Neurexins induce differentiation of GABA and glutamate postsynaptic specializations via neuroligins. Cell. 2004;119:1013–1026. doi: 10.1016/j.cell.2004.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X, Zhou L, Lu W. An NMDA receptor-dependent mechanism underlies inhibitory synapse development. Cell Rep. 2016;14:471–478. doi: 10.1016/j.celrep.2015.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen V, Holten AT, Storm-Mathisen J. GABAergic synapses in hippocampus exocytose aspartate on to NMDA receptors: quantitative immunogold evidence for co-transmission. Mol. Cell. Neurosci. 2004;26:156–165. doi: 10.1016/j.mcn.2004.01.017. [DOI] [PubMed] [Google Scholar]
- Hansen M, Walmod PS. IGSF9 family proteins. Neurochem. Res. 2013;38:1236–1251. doi: 10.1007/s11064-013-0999-y. [DOI] [PubMed] [Google Scholar]
- Harris BT, Costa E, Grayson DR. Exposure of neuronal cultures to K+ depolarization or to N-methyl-D-aspartate increases the transcription of genes encoding the alpha 1 and alpha 5 GABAA receptor subunits, brain research. Mol. Brain Res. 1995;28:338–342. doi: 10.1016/0169-328x(94)00240-f. [DOI] [PubMed] [Google Scholar]
- Harvey K, Duguid IC, Alldred MJ, Beatty SE, Ward H, Keep NH, Lingenfelter SE, Pearce BR, Lundgren J, Owen MJ, Smart TG, Luscher B, Rees MI, Harvey RJ. The GDP-GTP exchange factor collybistin: an essential determinant of neuronal gephyrin clustering. J. Neurosci. 2004;24:5816–5826. doi: 10.1523/JNEUROSCI.1184-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henneberger C, Juttner R, Schmidt SA, Walter J, Meier JC, Rothe T, Grantyn R. GluR- and TrkB-mediated maturation of GABA receptor function during the period of eye opening. Eur. J. Neurosci. 2005;21:431–440. doi: 10.1111/j.1460-9568.2005.03869.x. [DOI] [PubMed] [Google Scholar]
- Hintsch G, Zurlinden A, Meskenaite V, Steuble M, Fink-Widmer K, Kinter J, Sonderegger P. The calsyntenins—a family of postsynaptic membrane proteins with distinct neuronal expression patterns. Mol. Cell. Neurosci. 2002;21:393–409. doi: 10.1006/mcne.2002.1181. [DOI] [PubMed] [Google Scholar]
- Hirasawa T, Wada H, Kohsaka S, Uchino S. Inhibition of NMDA receptors induces delayed neuronal maturation and sustained proliferation of progenitor cells during neocortical development. J. Neurosci. Res. 2003;74:676–687. doi: 10.1002/jnr.10795. [DOI] [PubMed] [Google Scholar]
- Hong EJ, McCord AE, Greenberg ME. A biological function for the neuronal activity-dependent component of Bdnf transcription in the development of cortical inhibition. Neuron. 2008;60:610–624. doi: 10.1016/j.neuron.2008.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoon M, Soykan T, Falkenburger B, Hammer M, Patrizi A, Schmidt KF, Sassoe-Pognetto M, Lowel S, Moser T, Taschenberger H, Brose N, Varoqueaux F. Neuroligin-4 is localized to glycinergic postsynapses and regulates inhibition in the retina. Proc. Natl. Acad. Sci. U. S. A. 2011;108:3053–3058. doi: 10.1073/pnas.1006946108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. Trk receptors: roles in neuronal signal transduction. Annu. Rev. Biochem. 2003;72:609–642. doi: 10.1146/annurev.biochem.72.121801.161629. [DOI] [PubMed] [Google Scholar]
- Huang ZJ, Kirkwood A, Pizzorusso T, Porciatti V, Morales B, Bear MF, Maffei L, Tonegawa S. BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell. 1999;98:739–755. doi: 10.1016/s0092-8674(00)81509-3. [DOI] [PubMed] [Google Scholar]
- Huang ZJ. Activity-dependent development of inhibitory synapses and innervation pattern: role of GABA signalling and beyond. J. Physiol. 2009;587:1881–1888. doi: 10.1113/jphysiol.2008.168211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes DI, Mackie M, Nagy GG, Riddell JS, Maxwell DJ, Szabo G, Erdelyi F, Veress G, Szucs P, Antal M, Todd AJ. P boutons in lamina IX of the rodent spinal cord express high levels of glutamic acid decarboxylase-65 and originate from cells in deep medial dorsal horn. Proc. Natl. Acad. Sci. U. S. A. 2005;102:9038–9043. doi: 10.1073/pnas.0503646102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa T, Gotoh N, Murayama C, Abe T, Iwashita M, Matsuzaki F, Suzuki T, Yamamoto T. IgSF molecule MDGA1 is involved in radial migration and positioning of a subset of cortical upper-layer neurons. Dev. Dyn. 2011;240:96–107. doi: 10.1002/dvdy.22496. [DOI] [PubMed] [Google Scholar]
- Jacob TC, Wan Q, Vithlani M, Saliba RS, Succol F, Pangalos MN, Moss SJ. GABA(A) receptor membrane trafficking regulates spine maturity. Proc. Natl. Acad. Sci. U. S. A. 2009;106:12500–12505. doi: 10.1073/pnas.0903943106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joset P, Wacker A, Babey R, Ingold EA, Andermatt I, Stoeckli ET, Gesemann M. Rostral growth of commissural axons requires the cell adhesion molecule MDGA2. Neural Dev. 2011;6:22. doi: 10.1186/1749-8104-6-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalscheuer VM, Musante L, Fang C, Hoffmann K, Fuchs C, Carta E, Deas E, Venkateswarlu K, Menzel C, Ullmann R, Tommerup N, Dalpra L, Tzschach A, Selicorni A, Luscher B, Ropers HH, Harvey K, Harvey RJ. A balanced chromosomal translocation disrupting ARHGEF9 is associated with epilepsy, anxiety, aggression, and mental retardation. Hum. Mutat. 2009;30:61–68. doi: 10.1002/humu.20814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Y, Ge Y, Cassidy RM, Lam V, Luo L, Moon KM, Lewis R, Molday RS, Wong RO, Foster LJ, Craig AM. A combined transgenic proteomic analysis and regulated trafficking of neuroligin-2. J. Biol. Chem. 2014;289:29350–29364. doi: 10.1074/jbc.M114.549279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilman V, van Rossum MC, Turrigiano GG. Activity deprivation reduces miniature IPSC amplitude by decreasing the number of postsynaptic GABA(A) receptors clustered at neocortical synapses. J. Neurosci. 2002;22:1328–1337. doi: 10.1523/JNEUROSCI.22-04-01328.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kins S, Betz H, Kirsch J. Collybistin a newly identified brain-specific GEF, induces submembrane clustering of gephyrin. Nat. Neurosci. 2000;3:22–29. doi: 10.1038/71096. [DOI] [PubMed] [Google Scholar]
- Kirsch J, Wolters I, Triller A, Betz H. Gephyrin antisense oligonucleotides prevent glycine receptor clustering in spinal neurons. Nature. 1993;366:745–748. doi: 10.1038/366745a0. [DOI] [PubMed] [Google Scholar]
- Klausberger T, Somogyi P. Neuronal diversity and temporal dynamics: the unity of hippocampal circuit operations. Science. 2008;321:53–57. doi: 10.1126/science.1149381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kneussel M, Brandstatter JH, Laube B, Stahl S, Muller U, Betz H. Loss of postsynaptic GABA(A) receptor clustering in gephyrin-deficient mice. J. Neurosci. 1999;19:9289–9297. doi: 10.1523/JNEUROSCI.19-21-09289.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knuesel I, Mastrocola M, Zuellig RA, Bornhauser B, Schaub MC, Fritschy JM. Short communication: altered synaptic clustering of GABAA receptors in mice lacking dystrophin (mdx mice). Eur. J. Neurosci. 1999;11:4457–4462. doi: 10.1046/j.1460-9568.1999.00887.x. [DOI] [PubMed] [Google Scholar]
- Knuesel I, Bornhauser BC, Zuellig RA, Heller F, Schaub MC, Fritschy JM. Differential expression of utrophin and dystrophin in CNS neurons: an in situ hybridization and immunohistochemical study. J. Comp. Neurol. 2000;422:594–611. doi: 10.1002/1096-9861(20000710)422:4<594::aid-cne8>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Ko J, Choii G, Um JW. The balancing act of GABAergic synapse organizers. Trends Mol. Med. 2015;21:256–268. doi: 10.1016/j.molmed.2015.01.004. [DOI] [PubMed] [Google Scholar]
- Kohara K, Yasuda H, Huang Y, Adachi N, Sohya K, Tsumoto T. A local reduction in cortical GABAergic synapses after a loss of endogenous brain-derived neurotrophic factor, as revealed by single-cell gene knock-out method. J. Neurosci. 2007;27:7234–7244. doi: 10.1523/JNEUROSCI.1943-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzirian MS, Paradis S. Emerging themes in GABAergic synapse development. Prog. Neurobiol. 2011;95:68–87. doi: 10.1016/j.pneurobio.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzirian MS, Moore AR, Staudenmaier EK, Friedel RH, Paradis S. The class 4 semaphorin Sema4D promotes the rapid assembly of GABAergic synapses in rodent hippocampus. J. Neurosci. 2013;33:8961–8973. doi: 10.1523/JNEUROSCI.0989-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K, Kim Y, Lee SJ, Qiang Y, Lee D, Lee HW, Kim H, Je HS, Sudhof TC, Ko J. MDGAs interact selectively with neuroligin-2 but not other neuroligins to regulate inhibitory synapse development. Proc. Natl. Acad. Sci. U. S. A. 2013;110:336–341. doi: 10.1073/pnas.1219987110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinekugel X, Medina I, Khalilov I, Ben-Ari Y, Khazipov R. Ca2+ oscillations mediated by the synergistic excitatory actions of GABA(A) and NMDA receptors in the neonatal hippocampus. Neuron. 1997;18:243–255. doi: 10.1016/s0896-6273(00)80265-2. [DOI] [PubMed] [Google Scholar]
- Levi S, Logan SM, Tovar KR, Craig AM. Gephyrin is critical for glycine receptor clustering but not for the formation of functional GABAergic synapses in hippocampal neurons. J. Neurosci. 2004;24:207–217. doi: 10.1523/JNEUROSCI.1661-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson JN, Chery N, Huang K, Wong TP, Gerrow K, Kang R, Prange O, Wang YT, El-Husseini A. Neuroligins mediate excitatory and inhibitory synapse formation: involvement of PSD-95 and neurexin-1beta in neuroligin-induced synaptic specificity. J. Biol. Chem. 2005;280:17312–17319. doi: 10.1074/jbc.M413812200. [DOI] [PubMed] [Google Scholar]
- Li RW, Yu W, Christie S, Miralles CP, Bai J, Loturco JJ, De Blas AL. Disruption of postsynaptic GABA receptor clusters leads to decreased GABAergic innervation of pyramidal neurons. J. Neurochem. 2005;95:756–770. doi: 10.1111/j.1471-4159.2005.03426.x. [DOI] [PubMed] [Google Scholar]
- Lin Y, Bloodgood BL, Hauser JL, Lapan AD, Koon AC, Kim TK, Hu LS, Malik AN, Greenberg ME. Activity-dependent regulation of inhibitory synapse development by Npas4. Nature. 2008;455:1198–1204. doi: 10.1038/nature07319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J. Excitation, inhibition, local oscillations, or large-scale loops: what causes the symptoms of schizophrenia? Curr. Opin. Neurobiol. 2012;22:537–544. doi: 10.1016/j.conb.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litwack ED, Babey R, Buser R, Gesemann M, O’Leary DD. Identification and characterization of two novel brain-derived immunoglobulin superfamily members with a unique structural organization. Mol. Cell. Neurosci. 2004;25:263–274. doi: 10.1016/j.mcn.2003.10.016. [DOI] [PubMed] [Google Scholar]
- Liu G. Local structural balance and functional interaction of excitatory and inhibitory synapses in hippocampal dendrites. Nat. Neurosci. 2004;7:373–379. doi: 10.1038/nn1206. [DOI] [PubMed] [Google Scholar]
- LoTurco JJ, Blanton MG, Kriegstein AR. Initial expression and endogenous activation of NMDA channels in early neocortical development. J. Neurosci. 1991;11:792–799. doi: 10.1523/JNEUROSCI.11-03-00792.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W, Bushong EA, Shih TP, Ellisman MH, Nicoll RA. The cell-autonomous role of excitatory synaptic transmission in the regulation of neuronal structure and function. Neuron. 2013;78:433–439. doi: 10.1016/j.neuron.2013.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z, Wang Y, Chen F, Tong H, Reddy MV, Luo L, Seshadrinathan S, Zhang L, Holthauzen LM, Craig AM, Ren G, Rudenko G. Calsyntenin-3 molecular architecture and interaction with neurexin 1alpha. J. Biol. Chem. 2014;289:34530–34542. doi: 10.1074/jbc.M114.606806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffei A, Nelson SB, Turrigiano GG. Selective reconfiguration of layer 4 visual cortical circuitry by visual deprivation. Nat. Neurosci. 2004;7:1353–1359. doi: 10.1038/nn1351. [DOI] [PubMed] [Google Scholar]
- Maric HM, Mukherjee J, Tretter V, Moss SJ, Schindelin H. Gephyrin-mediated gamma-aminobutyric acid type A and glycine receptor clustering relies on a common binding site. J. Biol. Chem. 2011;286:42105–42114. doi: 10.1074/jbc.M111.303412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden KC, Shemesh A, Bayer KU, Carroll RC. Selective translocation of Ca2+/calmodulin protein kinase IIalpha (CaMKIIalpha) to inhibitory synapses. Proc. Natl. Acad. Sci. U. S. A. 2010;107:20559–20564. doi: 10.1073/pnas.1010346107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty S, Wehrle R, Sotelo C. Neuronal activity and brain-derived neurotrophic factor regulate the density of inhibitory synapses in organotypic slice cultures of postnatal hippocampus. J. Neurosci. 2000;20:8087–8095. doi: 10.1523/JNEUROSCI.20-21-08087.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer S, Kumar R, Jaiswal M, Soykan T, Ahmadian MR, Brose N, Betz H, Rhee JS, Papadopoulos T. Collybistin activation by GTP-TC10 enhances postsynaptic gephyrin clustering and hippocampal GABAergic neurotransmission. Proc. Natl. Acad. Sci. U. S. A. 2013;110:20795–20800. doi: 10.1073/pnas.1309078110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memo M, Bovolin P, Costa E, Grayson DR. Regulation of gamma-aminobutyric acidA receptor subunit expression by activation of N-methyl-D-aspartate-selective glutamate receptors. Mol. Pharmacol. 1991;39:599–603. [PubMed] [Google Scholar]
- Michele M, Faissner A. Tenascin-C stimulates contactin-dependent neurite outgrowth via activation of phospholipase C. Mol. Cell. Neurosci. 2009;41:397–408. doi: 10.1016/j.mcn.2009.04.004. [DOI] [PubMed] [Google Scholar]
- Mishra A, Knerr B, Paixao S, Kramer ER, Klein R. The protein dendrite arborization and synapse maturation 1 (Dasm-1) is dispensable for dendrite arborization. Mol. Cell. Biol. 2008;28:2782–2791. doi: 10.1128/MCB.02102-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra A, Traut MH, Becker L, Klopstock T, Stein V, Klein R. Genetic evidence for the adhesion protein IgSF9/Dasm1 to regulate inhibitory synapse development independent of its intracellular domain. J. Neurosci. 2014;34:4187–4199. doi: 10.1523/JNEUROSCI.3671-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12:529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Muir J, Arancibia-Carcamo IL, MacAskill AF, Smith KR, Griffin LD, Kittler JT. NMDA receptors regulate GABAA receptor lateral mobility and clustering at inhibitory synapses through serine 327 on the gamma2 subunit. Proc. Natl. Acad. Sci. U. S. A. 2010;107:16679–16684. doi: 10.1073/pnas.1000589107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y, Morrow DH, Modgil A, Huyghe D, Deeb TZ, Lumb MJ, Davies PA, Moss SJ. Proteomic characterization of inhibitory synapses using a novel pHluorin-tagged GABAAR alpha2 subunit knock-in mouse. J. Biol. Chem. 2016;291:12394–12407. doi: 10.1074/jbc.M116.724443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent FS, Penick EC, Kauer JA. Opioids block long-term potentiation of inhibitory synapses. Nature. 2007;446:1086–1090. doi: 10.1038/nature05726. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Hajos N, Somogyi P, Mody I. Increased number of synaptic GABA(A) receptors underlies potentiation at hippocampal inhibitory synapses. Nature. 1998;395:172–177. doi: 10.1038/25999. [DOI] [PubMed] [Google Scholar]
- Otis TS, De Koninck Y, Mody I. Lasting potentiation of inhibition is associated with an increased number of gamma-aminobutyric acid type A receptors activated during miniature inhibitory postsynaptic currents. Proc. Natl. Acad. Sci. U. S. A. 1994;91:7698–7702. doi: 10.1073/pnas.91.16.7698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos T, Korte M, Eulenburg V, Kubota H, Retiounskaia M, Harvey RJ, Harvey K, O’Sullivan GA, Laube B, Hulsmann S, Geiger JR, Betz H. Impaired GABAergic transmission and altered hippocampal synaptic plasticity in collybistin-deficient mice. EMBO J. 2007;26:3888–3899. doi: 10.1038/sj.emboj.7601819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos T, Eulenburg V, Reddy-Alla S, Mansuy IM, Li Y, Betz H. Collybistin is required for both the formation and maintenance of GABAergic postsynapses in the hippocampus. Mol. Cell. Neurosci. 2008;39:161–169. doi: 10.1016/j.mcn.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Paradis S, Harrar DB, Lin Y, Koon AC, Hauser JL, Griffith EC, Zhu L, Brass LF, Chen C, Greenberg ME. An RNAi-based approach identifies molecules required for glutamatergic and GABAergic synapse development. Neuron. 2007;53:217–232. doi: 10.1016/j.neuron.2006.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasterkamp RJ. Getting neural circuits into shape with semaphorins. Nat. Rev. Neurosci. 2012;13:605–618. doi: 10.1038/nrn3302. [DOI] [PubMed] [Google Scholar]
- Patrizi A, Scelfo B, Viltono L, Briatore F, Fukaya M, Watanabe M, Strata P, Varoqueaux F, Brose N, Fritschy JM, Sassoe-Pognetto M. Synapse formation and clustering of neuroligin-2 in the absence of GABAA receptors. Proc. Natl. Acad. Sci. U. S. A. 2008;105:13151–13156. doi: 10.1073/pnas.0802390105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peles E, Nativ M, Campbell PL, Sakurai T, Martinez R, Lev S, Clary DO, Schilling J, Barnea G, Plowman GD, Grumet M, Schlessinger J. The carbonic anhydrase domain of receptor tyrosine phosphatase beta is a functional ligand for the axonal cell recognition molecule contactin. Cell. 1995;82:251–260. doi: 10.1016/0092-8674(95)90312-7. [DOI] [PubMed] [Google Scholar]
- Petrini EM, Ravasenga T, Hausrat TJ, Iurilli G, Olcese U, Racine V, Sibarita JB, Jacob TC, Moss SJ, Benfenati F, Medini P, Kneussel M, Barberis A. Synaptic recruitment of gephyrin regulates surface GABAA receptor dynamics for the expression of inhibitory LTP. Nat. Commun. 2014;5:3921. doi: 10.1038/ncomms4921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettem KL, Yokomaku D, Luo L, Linhoff MW, Prasad T, Connor SA, Siddiqui TJ, Kawabe H, Chen F, Zhang L, Rudenko G, Wang YT, Brose N, Craig AM. The specific alpha-neurexin interactor calsyntenin-3 promotes excitatory and inhibitory synapse development. Neuron. 2013a;80:113–128. doi: 10.1016/j.neuron.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettem KL, Yokomaku D, Takahashi H, Ge Y, Craig AM. Interaction between autism-linked MDGAs and neuroligins suppresses inhibitory synapse development. J. Cell Biol. 2013b;200:321–336. doi: 10.1083/jcb.201206028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer F, Graham D, Betz H. Purification by affinity chromatography of the glycine receptor of rat spinal cord. J. Biol. Chem. 1982;257:9389–9393. [PubMed] [Google Scholar]
- Poulopoulos A, Aramuni G, Meyer G, Soykan T, Hoon M, Papadopoulos T, Zhang M, Paarmann I, Fuchs C, Harvey K, Jedlicka P, Schwarzacher SW, Betz H, Harvey RJ, Brose N, Zhang W, Varoqueaux F. Neuroligin 2 drives postsynaptic assembly at perisomatic inhibitory synapses through gephyrin and collybistin. Neuron. 2009;63:628–642. doi: 10.1016/j.neuron.2009.08.023. [DOI] [PubMed] [Google Scholar]
- Raissi AJ, Staudenmaier EK, David S, Hu L, Paradis S. Sema4D localizes to synapses and regulates GABAergic synapse development as a membrane-bound molecule in the mammalian hippocampus. Mol. Cell. Neurosci. 2013;57:23–32. doi: 10.1016/j.mcn.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao A, Cha EM, Craig AM. Mismatched appositions of presynaptic and postsynaptic components in isolated hippocampal neurons. J. Neurosci. 2000;20:8344–8353. doi: 10.1523/JNEUROSCI.20-22-08344.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy-Alla S, Schmitt B, Birkenfeld J, Eulenburg V, Dutertre S, Bohringer C, Gotz M, Betz H, Papadopoulos T. PH-domain-driven targeting of collybistin but not Cdc42 activation is required for synaptic gephyrin clustering. Eur. J. Neurosci. 2010;31:1173–1184. doi: 10.1111/j.1460-9568.2010.07149.x. [DOI] [PubMed] [Google Scholar]
- Rico B, Xu B, Reichardt LF. TrkB receptor signaling is required for establishment of GABAergic synapses in the cerebellum. Nat. Neurosci. 2002;5:225–233. doi: 10.1038/nn808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein JL. Three hypotheses for developmental defects that may underlie some forms of autism spectrum disorder. Curr. Opin. Neurol. 2010;23:118–123. doi: 10.1097/WCO.0b013e328336eb13. [DOI] [PubMed] [Google Scholar]
- Rutherford LC, DeWan A, Lauer HM, Turrigiano GG. Brain-derived neurotrophic factor mediates the activity-dependent regulation of inhibition in neocortical cultures. J. Neurosci. 1997;17:4527–4535. doi: 10.1523/JNEUROSCI.17-12-04527.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saghatelyan AK, Nikonenko AG, Sun M, Rolf B, Putthoff P, Kutsche M, Bartsch U, Dityatev A, Schachner M. Reduced GABAergic transmission and number of hippocampal perisomatic inhibitory synapses in juvenile mice deficient in the neural cell adhesion molecule L1. Mol. Cell. Neurosci. 2004;26:191–203. doi: 10.1016/j.mcn.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Saiepour L, Fuchs C, Patrizi A, Sassoe-Pognetto M, Harvey RJ, Harvey K. Complex role of collybistin and gephyrin in GABAA receptor clustering. J. Biol. Chem. 2010;285:29623–29631. doi: 10.1074/jbc.M110.121368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassoe-Pognetto M, Kirsch J, Grunert U, Greferath U, Fritschy JM, Mohler H, Betz H, Wassle H. Colocalization of gephyrin and GABAA-receptor subunits in the rat retina. J. Comp. Neurol. 1995;357:1–14. doi: 10.1002/cne.903570102. [DOI] [PubMed] [Google Scholar]
- Sassoe-Pognetto M, Panzanelli P, Sieghart W, Fritschy JM. Colocalization of multiple GABA(A) receptor subtypes with gephyrin at postsynaptic sites. J. Comp. Neurol. 2000;420:481–498. doi: 10.1002/(sici)1096-9861(20000515)420:4<481::aid-cne6>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Scheiffele P, Fan J, Choih J, Fetter R, Serafini T. Neuroligin expressed in nonneuronal cells triggers presynaptic development in contacting axons. Cell. 2000;101:657–669. doi: 10.1016/s0092-8674(00)80877-6. [DOI] [PubMed] [Google Scholar]
- Seil FJ, Drake-Baumann R. TrkB receptor ligands promote activity-dependent inhibitory synaptogenesis. J. Neurosci. 2000;20:5367–5373. doi: 10.1523/JNEUROSCI.20-14-05367.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen K, Scheiffele P. Genetics and cell biology of building specific synaptic connectivity. Annu. Rev. Neurosci. 2010;33:473–507. doi: 10.1146/annurev.neuro.051508.135302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng M, Sabatini BL, Sudhof TC. Synapses and Alzheimer’s disease. Cold Spring Harb. Perspect. Biol. 2012;4 doi: 10.1101/cshperspect.a005777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi SH, Cox DN, Wang D, Jan LY, Jan YN. Control of dendrite arborization by an Ig family member, dendrite arborization and synapse maturation 1 (Dasm1). Proc. Natl. Acad. Sci. U. S. A. 2004;101:13341–13345. doi: 10.1073/pnas.0405370101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyn SI, Shi J, Kraft JB, Potash JB, Knowles JA, Weissman MM, Garriock HA, Yokoyama JS, McGrath PJ, Peters EJ, Scheftner WA, Coryell W, Lawson WB, Jancic D, Gejman PV, Sanders AR, Holmans P, Slager SL, Levinson DF, Hamilton SP. Novel loci for major depression identified by genome-wide association study of sequenced treatment alternatives to relieve depression and meta-analysis of three studies. Mol. Psychiatry. 2011;16:202–215. doi: 10.1038/mp.2009.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui TJ, Craig AM. Synaptic organizing complexes. Curr. Opin. Neurobiol. 2011;21:132–143. doi: 10.1016/j.conb.2010.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soykan T, Schneeberger D, Tria G, Buechner C, Bader N, Svergun D, Tessmer I, Poulopoulos A, Papadopoulos T, Varoqueaux F, Schindelin H, Brose N. A conformational switch in collybistin determines the differentiation of inhibitory postsynapses. EMBO J. 2014;33:2113–2133. doi: 10.15252/embj.201488143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudhof TC. Neuroligins and neurexins link synaptic function to cognitive disease. Nature. 2008;455:903–911. doi: 10.1038/nature07456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumita K, Sato Y, Iida J, Kawata A, Hamano M, Hirabayashi S, Ohno K, Peles E, Hata Y. Synaptic scaffolding molecule (S-SCAM) membrane-associated guanylate kinase with inverted organization (MAGI)-2 is associated with cell adhesion molecules at inhibitory synapses in rat hippocampal neurons. J. Neurochem. 2007;100:154–166. doi: 10.1111/j.1471-4159.2006.04170.x. [DOI] [PubMed] [Google Scholar]
- Sun YJ, Wu GK, Liu BH, Li P, Zhou M, Xiao Z, Tao HW, Zhang LI. Fine-tuning of pre-balanced excitation and inhibition during auditory cortical development. Nature. 2010;465:927–931. doi: 10.1038/nature09079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabadits E, Cserep C, Szonyi A, Fukazawa Y, Shigemoto R, Watanabe M, Itohara S, Freund TF, Nyiri G. NMDA receptors in hippocampal GABAergic synapses and their role in nitric oxide signaling. J. Neurosci. 2011;31:5893–5904. doi: 10.1523/JNEUROSCI.5938-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H, Katayama K, Sohya K, Miyamoto H, Prasad T, Matsumoto Y, Ota M, Yasuda H, Tsumoto T, Aruga J, Craig AM. Selective control of inhibitory synapse development by Slitrk3-PTPdelta trans-synaptic interaction. Nat. Neurosci. 2012;15(389–398):S381–S382. doi: 10.1038/nn.3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi A, O'Leary DD. Radial migration of superficial layer cortical neurons controlled by novel Ig cell adhesion molecule MDGA1. J. Neurosci. 2006;26:4460–4464. doi: 10.1523/JNEUROSCI.4935-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao HW, Poo MM. Activity-dependent matching of excitatory and inhibitory inputs during refinement of visual receptive fields. Neuron. 2005;45:829–836. doi: 10.1016/j.neuron.2005.01.046. [DOI] [PubMed] [Google Scholar]
- Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, Hansen KB, Yuan H, Myers SJ, Dingledine R. Glutamate receptor ion channels: structure, regulation, and function. Pharmacol. Rev. 2010;62:405–496. doi: 10.1124/pr.109.002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tretter V, Jacob TC, Mukherjee J, Fritschy JM, Pangalos MN, Moss SJ. The clustering of GABA(A) receptor subtypes at inhibitory synapses is facilitated via the direct binding of receptor alpha 2 subunits to gephyrin. J. Neurosci. 2008;28:1356–1365. doi: 10.1523/JNEUROSCI.5050-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tretter V, Mukherjee J, Maric HM, Schindelin H, Sieghart W, Moss SJ. Gephyrin, the enigmatic organizer at GABAergic synapses. Front. Cell. Neurosci. 2012;6:23. doi: 10.3389/fncel.2012.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyagarajan SK, Fritschy JM. Gephyrin: a master regulator of neuronal function? Nat. Rev. Neurosci. 2014;15:141–156. doi: 10.1038/nrn3670. [DOI] [PubMed] [Google Scholar]
- Tyagarajan SK, Ghosh H, Harvey K, Fritschy JM. Collybistin splice variants differentially interact with gephyrin and Cdc42 to regulate gephyrin clustering at GABAergic synapses. J. Cell Sci. 2011;124:2786–2796. doi: 10.1242/jcs.086199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyzio R, Represa A, Jorquera I, Ben-Ari Y, Gozlan H, Aniksztejn L. The establishment of GABAergic and glutamatergic synapses on CA1 pyramidal neurons is sequential and correlates with the development of the apical dendrite. J. Neurosci. 1999;19:10372–10382. doi: 10.1523/JNEUROSCI.19-23-10372.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Um JW, Kim KH, Park BS, Choi Y, Kim D, Kim CY, Kim SJ, Kim M, Ko JS, Lee SG, Choii G, Nam J, Heo WD, Kim E, Lee JO, Ko J, Kim HM. Structural basis for LAR-RPTP/Slitrk complex-mediated synaptic adhesion. Nat. Commun. 2014a;5:5423. doi: 10.1038/ncomms6423. [DOI] [PubMed] [Google Scholar]
- Um JW, Pramanik G, Ko JS, Song MY, Lee D, Kim H, Park KS, Sudhof TC, Tabuchi K, Ko J. Calsyntenins function as synaptogenic adhesion molecules in concert with neurexins. Cell Rep. 2014b;6:1096–1109. doi: 10.1016/j.celrep.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varoqueaux F, Jamain S, Brose N. Neuroligin 2 is exclusively localized to inhibitory synapses. Eur. J. Cell Biol. 2004;83:449–456. doi: 10.1078/0171-9335-00410. [DOI] [PubMed] [Google Scholar]
- Varoqueaux F, Aramuni G, Rawson RL, Mohrmann R, Missler M, Gottmann K, Zhang W, Sudhof TC, Brose N. Neuroligins determine synapse maturation and function. Neuron. 2006;51:741–754. doi: 10.1016/j.neuron.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Vicario-Abejon C, Collin C, McKay RD, Segal M. Neurotrophins induce formation of functional excitatory and inhibitory synapses between cultured hippocampal neurons. J. Neurosci. 1998;18:7256–7271. doi: 10.1523/JNEUROSCI.18-18-07256.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt L, Schrimpf SP, Meskenaite V, Frischknecht R, Kinter J, Leone DP, Ziegler U, Sonderegger P. Calsyntenin-1, a proteolytically processed postsynaptic membrane protein with a cytoplasmic calcium-binding domain. Mol. Cell. Neurosci. 2001;17:151–166. doi: 10.1006/mcne.2000.0937. [DOI] [PubMed] [Google Scholar]
- Wang XH, Zhu WJ, Corsi L, Ikonomovic S, Paljug WR, Vicini S, Grayson DR. Chronic dizocilpine (MK-801) reversibly delays GABA(A) receptor maturation in cerebellar granule neurons in vitro. J. Neurochem. 1998;71:693–704. doi: 10.1046/j.1471-4159.1998.71020693.x. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Inoue Y, Sakimura K, Mishina M. Developmental changes in distribution of NMDA receptor channel subunit mRNAs. Neuroreport. 1992;3:1138–1140. doi: 10.1097/00001756-199212000-00027. [DOI] [PubMed] [Google Scholar]
- Wehr M, Zador AM. Balanced inhibition underlies tuning and sharpens spike timing in auditory cortex. Nature. 2003;426:442–446. doi: 10.1038/nature02116. [DOI] [PubMed] [Google Scholar]
- Wierenga CJ, Becker N, Bonhoeffer T. GABAergic synapses are formed without the involvement of dendritic protrusions. Nat. Neurosci. 2008;11:1044–1052. doi: 10.1038/nn.2180. [DOI] [PubMed] [Google Scholar]
- Woo J, Kwon SK, Nam J, Choi S, Takahashi H, Krueger D, Park J, Lee Y, Bae JY, Lee D, Ko J, Kim H, Kim MH, Bae YC, Chang S, Craig AM, Kim E. The adhesion protein IgSF9b is coupled to neuroligin 2 via S-SCAM to promote inhibitory synapse development. J. Cell Biol. 2013;201:929–944. doi: 10.1083/jcb.201209132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yim YS, Kwon Y, Nam J, Yoon HI, Lee K, Kim DG, Kim E, Kim CH, Ko J. Slitrks control excitatory and inhibitory synapse formation with LAR receptor protein tyrosine phosphatases. Proc. Natl. Acad. Sci. U. S. A. 2013;110:4057–4062. doi: 10.1073/pnas.1209881110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yizhar O, Fenno LE, Prigge M, Schneider F, Davidson TJ, O'Shea DJ, Sohal VS, Goshen I, Finkelstein J, Paz JT, Stehfest K, Fudim R, Ramakrishnan C, Huguenard JR, Hegemann P, Deisseroth K. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature. 2011;477:171–178. doi: 10.1038/nature10360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, Jiang M, Miralles CP, Li RW, Chen G, de Blas AL. Gephyrin clustering is required for the stability of GABAergic synapses. Mol. Cell. Neurosci. 2007;36:484–500. doi: 10.1016/j.mcn.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Atasoy D, Arac D, Yang X, Fucillo MV, Robison AJ, Ko J, Brunger AT, Sudhof TC. Neurexins physically and functionally interact with GABA(A) receptors. Neuron. 2010;66:403–416. doi: 10.1016/j.neuron.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Chen LY, Liu X, Maxeiner S, Lee SJ, Gokce O, Sudhof TC. Neuroligins sculpt cerebellar purkinje-cell circuits by differential control of distinct classes of synapses. Neuron. 2015;87:781–796. doi: 10.1016/j.neuron.2015.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu WJ, Vicini S, Harris BT, Grayson DR. NMDA-mediated modulation of gamma-aminobutyric acid type A receptor function in cerebellar granule neurons. J. Neurosci. 1995;15:7692–7701. doi: 10.1523/JNEUROSCI.15-11-07692.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuko A, Kleijer KT, Oguro-Ando A, Kas MJ, van Daalen E, van der Zwaag B, Burbach JP. Contactins in the neurobiology of autism. Eur. J. Pharmacol. 2013;719:63–74. doi: 10.1016/j.ejphar.2013.07.016. [DOI] [PubMed] [Google Scholar]