Abstract
Objective
To determine the factors associated with longitudinal patient-reported dysphagia as measured by the MD Anderson Dysphagia Inventory (MDADI) in locoregionally advanced oropharyngeal carcinoma (OPC) survivors treated with split-field intensity modulated radiotherapy (IMRT).
Methods
A retrospective analysis combined data from three single-institution clinical trials for stage III/IV head and neck carcinoma. According to trial protocols, patients had prospectively-collected MDADI at baseline, 6, 12, and 24 months after treatment. OPC patients with baseline and at least one post-treatment MDADI were included. Longitudinal analysis was completed with multivariate linear mixed effects modeling.
Results
116 patients met inclusion criteria. Mean baseline MDADI composite was 88.3, dropping to 73.8 at 6 months, and rising to 78.6 and 83.3 by 12 and 24 months, respectively (compared to baseline, all p<0.0001). Tumor stage and smoking status were significant predictors of longitudinal MDADI composite scores. Patients with T1, T2, and T3 tumors had 15.9 (p=0.0001), 10.9 (p=0.0049), and 7.5 (p=0.0615), respectively, higher mean MDADI composite than those with T4 tumors, and current smokers had a 9.4 (p=0.0007) lower mean MDADI composite than never smokers.
Conclusion
Patients report clinically-meaningful dysphagia early after split-field IMRT for locoregionally advanced OPC that remains apparent 6 months after treatment. MDADI scores recover slowly thereafter, but remain depressed at 24 months compared to baseline. Higher tumor stage and smoking status are important markers of patient-reported function through the course of treatment, suggesting these are important groups for heightened surveillance and more intensive interventions to optimize swallowing outcomes.
Keywords: MDADI, oropharyngeal carcinoma, IMRT, dysphagia, patient-reported outcomes
Introduction
It is now well-recognized that human papilloma virus (HPV)-associated oropharyngeal carcinoma (OPC) is occurring at an unprecedented rate. Patients with this distinct subtype of head and neck cancer (HNC) have a relatively favorable prognosis, and so are living longer [1-5]. This has created a situation for healthcare teams managing HNC patients that necessitates careful consideration of treatment endpoints that includes not only survival measures, but also survivor function. Central to these efforts rests the ability to accurately characterize longitudinal outcomes that adversely impact the quality of life (QOL) and health of OPC survivors [6-8]. Chief among these functional outcomes is dysphagia, a common but still poorly understood long-term toxicity with potentially devastating QOL effects and secondary morbidity [8-11].
The MD Anderson Dysphagia Inventory (MDADI) represents the most widely employed patient-reported outcome (PRO) metric for the assessment of swallowing through questions directed at the emotional, functional, and physical impact of patient-perceived function [12-15]. A number of investigators have described cross-sectional MDADI outcomes in heterogeneous HNC survivors [13, 16]. Fewer have characterized MDADI changes longitudinally among OPC patients, and these assessments typically follow patients through only the first year or less either due to follow-up limitations or based on the notion that all measureable recovery has occurred by this time [11, 17, 18]. OPC-specific MDADI reports beyond the first year of survivorship are severely limited by small sample size or cross-sectional design [16, 19, 20]. Empirical evidence suggests that continued gains in patient-reported function may occur as the follow-up interval lengthens beyond one year though few studies address this topic and no OPC-specific cohorts include the MDADI as an outcome measure [6, 10]. Though evidence is very limited regarding predictors of MDADI scores at individual time points and/or longitudinally, patient age, tumor site and stage, radiation dose, and pre-treatment MDADI are among factors reported [11, 21]. Given the increasing utilization of PRO metrics such as MDADI from baseline through treatment and into survivorship, the aim of this study is to present longitudinal MDADI results in locally advanced OPC survivors treated with intensity modulated radiotherapy (IMRT) and to assess for clinical predictors of patient-reported swallowing function through two years of follow-up.
Methods
Patient selection and variables
This IRB-approved analysis combined data from three single-institution clinical trials for stage III/IV head and neck carcinoma. According to trial protocols, patients had prospectively-collected MDADI at baseline (i.e. prior to treatment), 6, 12, and 24 months after treatment. Patients treated with intensity modulated radiotherapy for primary squamous cell carcinoma of the oropharynx with baseline and at least one post-treatment MDADI were sampled from trial databases. Consolidated Standards of Reporting Trials – Patient-Reported Outcomes (CONSORT-PRO) criteria are summarized in Table 1. Exclusion criteria were recurrence of disease preceding post-treatment MDADI intervals and surgical management of the primary site in excess of surgical endoscopy with biopsy and/or diagnostic tonsillectomy.
Table 1.
CONSORT-PRO Objectives and Methods
| Objective | Characterize trajectories and predictors of longitudinal patient-reported swallowing outcomes using the MDADI among locallyadvanced OPC patients treated non-surgically |
| Trial Designs | Three phase II non-surgical therapeutic clinical trials for locoregionally advanced HNSCC (1: induction PCC trial, 2: adaptive IMRT trial, 3: randomized induction PCC v C-TPF trial) |
| Participants | OPC; baseline and at least one post-treatment MDADI |
| Intervention | Primary split-field IMRT with induction and/or concurrent chemotherapy |
| Outcome | MDADI composite score difference at 6, 12, and 24 months compared to baseline (secondary outcome measures from trials) |
| Statistical methods | MDADI reports from all available time points (baseline, 6, 12, 24 months) were examined with linear mixed effect multivariate models without imputation of missing data. |
Abbreviations: CONSORT-PRO, Consolidated Standards of Reporting Trials - Patient-Reported Outcomes; MDADI, MD Anderson Dysphagia Inventory; OPC, oropharyngeal carcinoma; HNSCC, head and neck squamous cell carcinoma; T, tumor; N, node; IMRT, intensiy modulated radiation therapy
Treatment
All included patients received definitive, split-field IMRT with systemic therapy. 32 patients were treated on an induction paclitaxel, cetuximab, and carboplatin (PCC) trial, 65 patients on an induction PCC vs cetuximab, docetaxel, cisplatin, and fluorouracil (C-TPF) trial, and the remaining 19 on an adaptive-IMRT trial. Trial details and clinical reports have been published elsewhere, and are briefly reviewed below.
Induction PCC trial
Patients were given an initial loading dose of cetuximab 400 mg/m2 followed by 6 weekly cycles of cetuximab 250 mg/m2, paclitaxel 135 mg/m2, and carboplatin area under the curve (AUC) 2. Risk-based definitive IMRT commenced 2 to 3 weeks after induction therapy. Radiation target volumes and local therapy assignments were based on pre-induction primary tumor staging: radiation as a single modality for T1-T2 and concurrent chemoradiation (cisplatin 100mg/m2 on days 1 and 22, weekly cisplatin 30mg/m2, or weekly carboplatin AUC 2) for T3-T4. Gross disease and margin were administered a dose of 66 Gy in 30 fractions for T1 disease and 72 Gy in 40 to 42 fractions with a concomitant boost fractionation schedule for patients with T2-4 tumors. All radiation schedules were planned for 6 weeks of therapy. Neck dissection was recommended for residual adenopathy after completion of chemoradiotherapy.
Induction PCC vs C-TPF Trial
Patients were randomized 1:1 to receive either PCC per dosing detailed above or the C-TPF regimen consisting of a loading dose of cetuximab 400 mg/m2, cetuximab 250 mg/m2 weeks 2, 4, 5, 7, 8, and 3 cycles of every 3 weeks docetaxel 75 mg/m2 and cisplatin 100 mg/m2 on day 1 with 5-fluorouracil (5-FU) 700 mg/m2/day continuous infusion cisplatin 100 mg/m2 on days 1-4. After 2-4 weeks, patients then went on to definitive local radiotherapy or chemoradiotherapy (cisplatin 40 mg/m2 or carboplatin AUC 2) on the basis of pre-induction stage and HPV-status.
Adaptive IMRT trial
A combined image-guided radiotherapy and adaptive re-planning paradigm was used for IMRT delivery. Clinical target volume 1 (CTV1) was treated to 66 to 70 Gy in 30 to 33 daily fractions. Adaptive re-planning was performed for all patients on trial at least once based on daily CT-on-rails images. Systemic therapy was delivered concurrently in 95% of patients, most commonly weekly cisplatin 30mg/m2.
MDADI
The MDADI is a 20-item questionnaire initially developed and validated in a diverse group of head and neck cancer patients to measure patient-reported quality of life with respect to their swallowing function [12]. It includes one question on global function and 19 additional questions related to the emotional, functional, and physical aspects of swallowing. Patient responses are categorized on a 5-point Likert scale (strongly agree, agree, no opinion, disagree, strongly disagree) and converted numerically from 1 (strongly agree) to 5 (strongly disagree). Scores can then be summarized by taking either the global item, the average of the remaining 19 items, or the average of the subscale items, and multiplying by 20, to yield a global, composite, or subscale score, respectively, on a scale from 20 (worst patient-reported function) to 100 (best patient-reported function). For this analysis, composite and subscale scores were analyzed to provide a representation of both overall and domain-specific patient-reported swallowing function, respectively. Furthermore, composite scores were categorized for ease of interpretation such that a score ≥80 represented “optimal” patient-reported swallowing function, <80 but ≥60 represented “adequate”, and <60 “poor”.
Clinical Variables
A review of the electronic medical records of all eligible patients was completed to compile clinical variables of interest, including age at presentation, sex, smoking history, HPV- and p16-status, cancer stage, primary tumor location, therapeutic combination and radiation/systemic therapy dosing, and need for post-treatment neck dissection.
Statistical analysis
Patient, tumor, and treatment variables were summarized by descriptive statistics, including means (ranges) and counts (percentages). Comparisons of composite and subscale scores between individual time points were accomplished by paired t-tests. Longitudinal analyses used linear mixed effects modeling with mean MDADI composite score characterized by a linear (or first degree) spline basis and knot locations fixed at the middle observed acquisition times (6 and 12 months after treatment). Covariates exhibiting significant association with MDADI score trajectories were identified initially using univariate analysis. For each MDADI score, covariates that attained a p-value <0.20 were subsequently considered for multiple regression analysis using the linear mixed effects model. Sensitivity analysis was performed to control for the potential effect of differential radiation dosing in the final model. To assess for multicollinearity, the variance inflation factor (VIF) was calculated. A p-value <0.05 conferred statistical significance. Final results are reported using the set of covariates that attained statistical significance in multiple regression analysis. Statistical analyses were performed using STATA v14.0 (StataCorp LP, College Station, TX) and SAS 9.4 (SAS Institute INC, Cary, NC) data analysis software.
Results
Patients and Treatment
One hundred sixteen patients were included in the analysis. See Table 2 for demographic and tumor specifics. 61.2% of patients had base of tongue primary tumors and 35.3% were stage T3 or T4. HPV and/or p16 status was known as positive in 66.4% of patients. Among the 29 patients with unknown HPV and p16 status, 17 (58.6%) were never smokers. 83.6% of patients underwent induction and 56% received concurrent chemotherapy. 39.7% received induction followed by concurrent therapy. All patients received at least 66 Gy irradiation with 69% treated to 70 Gy or more. 19.8% of patients had a post-treatment neck dissection for concern of residual regional disease.
Table 2.
Patient characteristics (n = 116)
| Variable | n (%)pvalue* | |
|---|---|---|
| Age, mean (range) | 56.5 (39-77)ns | |
| Sex | Male | 94 (81.0)e |
| Female | 22 (19.0)− | |
| Primary Site | Base of Tongue | 71 (61.2)ns |
| Tonsil | 45 (38.8)− | |
| Tumor stage | T1 | 28 (24.1)c,e,f,p |
| T2 | 47 (40.5)c,e,f,p | |
| T3 | 30 (25.9)ns | |
| T4 | 11 (9.5)− | |
| Nodal stage | N0/1/2a | 11 (9.5)ns |
| N2b | 49 (42.2)ns | |
| N2c | 43 (37.1)c,e,p | |
| N3 | 13 (11.2)− | |
| HPV/p16 status | p16pos &/OR HPVpos | 77 (66.4)na |
| p16neg & HPVneg | 1 (0.8)na | |
| P16unknown & HPVneg | 9 (7.8)na | |
| P16unknown & HPVunknown | 29 (25.0)na | |
| Smoking status | Current | 25 (21.6)c,e,f,p |
| Former | 35 (30.2)ns | |
| Never | 56 (48.3)− | |
| Pack years, mean (range) | All patients | 14.8 (0-132)c,f |
| p16unknown & HPVunknown | 8.1 (0-45)− | |
| Treatment | Induction + Concurrent/IMRT | 46 (39.7)p |
| Concurrent/IMRT | 19 (16.4)ns | |
| Induction + IMRT | 51 (44.0)− | |
| IMRT Dosing | 66Gy | 36 (31.0)ns |
| ≥70Gy | 80 (69.0)− | |
| Post-treatment neck dissection | No | 93 (80.2)ns |
| Yes | 23 (19.8)− | |
Abbreviations: T, tumor; N, node; HPV, human papillomavirus; IMRT, intensity modulated radiotherapy; Gy, gray.
Superscripts:
Univariate longitudinal mixed effects mode with linear spline for MDADI composite and subscale scores, p-value <0.05 is significant; ns, not significant
composite p-value<0.05
emotional subscale p-value<0.05
functional subscale p-value<0.05
physical subscale p-value<0.05.
MDADI Composite and Subgroup Results
Mean baseline MDADI composite score was 88.3 (76% optimal, 19% adequate, 5% poor), dropping to 73.8 at 6 months (34% optimal, 53% adequate, 13% poor), and rising to 78.6 and 83.3 by 12 (45% optimal, 42% adequate, 13% poor) and 24 months (61% optimal, 32% adequate, 7% poor), respectively, relative to baseline (Figure 1). MDADI composite at 24-months remained significantly depressed compared to baseline (p<0.0001). All other pairwise comparisons between time points are also listed in Figure 1. 33%, 28%, and 17% of patients experienced a 20 point MDADI composite drop (Δ20) compared to baseline at 6, 12, and 24 months, respectively. No covariates except the MDADI score at the preceding interval were statistically associated with Δ20 patients (data not shown). The overall composite trajectory was mirrored in subscale scores (emotional, functional, and physical) with the greatest initial decrement and least recovery in the physical domain (Figure 2). All 24-month subscale results remained statistically depressed compared to baseline (emotional p=0.008, functional p=0.006, physical p<0.0001).
Figure 1.
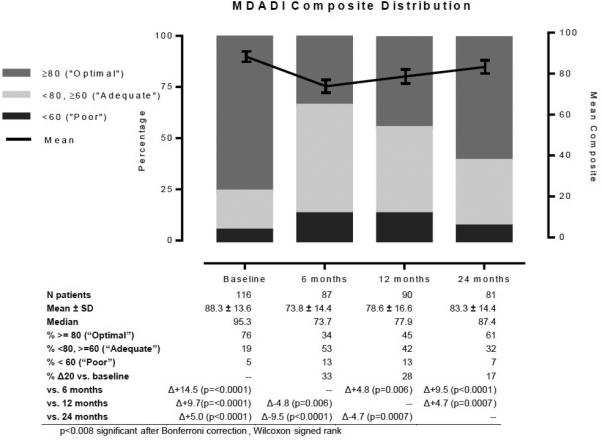
MDADI Composite Trend and Classification
Figure 2.
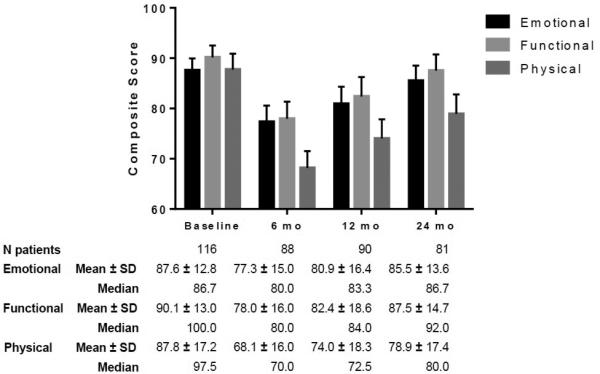
MDADI Subscale Trend
Multivariate Longitudinal Analysis
Tumor and nodal stage, smoking status, and pack-years were significant covariates (p<0.05) in univariate analysis of MDADI composite score (Table 2). MDADI subscale covariate analysis produced similar results. Significant independent associations with MDADI composite score trajectory were identified for tumor stage and smoking status in multivariable analysis (Table 3). Patients with T1, T2, and T3 tumors obtained an estimated 15.9 (p=0.0001), 10.9 (p=0.0049), and 7.5 (p=0.0615), respectively, higher mean MDADI composite score at any given time in the longitudinal model than those with T4 tumors (Figure 3). Sensitivity analysis controlling for radiation dose found nearly identical results by tumor stage. The highest VIF between radiation dose and tumor stage was less than 4. Current smokers demonstrated an estimated 9.4 (p=0.0007) lower mean MDADI composite score than never smokers (Figure 4). Similar trends were also observed for smoking and tumor stage with MDADI subscale scores in the longitudinal model (Table 3).
Table 3.
Multivariate longitudinal mixed effects model with linear spline for MDADI Composite and Subscale Score Estimates
| Composite | Emotional | Functional | Physical | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Estimate | p-value | Estimate | p-value | Estimate | p-value | Estimate | p-value | ||
| Smoking status | Current | −9.404 | 0.0007 | −8.393 | 0.0014 | −10.67 | <.0001 | −7.382 | 0.0213 |
| Former | 0.3642 | 0.8827 | 0.8945 | 0.7007 | −1.838 | 0.4451 | 1.6885 | 0.5611 | |
| Never | 0 | - | 0 | - | 0 | - | 0 | - | |
| Tumor stage | T1 | 15.942 | 0.0001 | 14.462 | 0.0003 | 13.029 | 0.0013 | 16.49 | 0.0007 |
| T2 | 10.927 | 0.0049 | 11.831 | 0.0013 | 8.6327 | 0.0215 | 8.4275 | 0.0631 | |
| T3 | 7.4851 | 0.0615 | 8.1539 | 0.0313 | 5.2651 | 0.175 | 5.4127 | 0.2445 | |
| T4 | 0 | - | 0 | - | 0 | - | 0 | - | |
Figure 3.
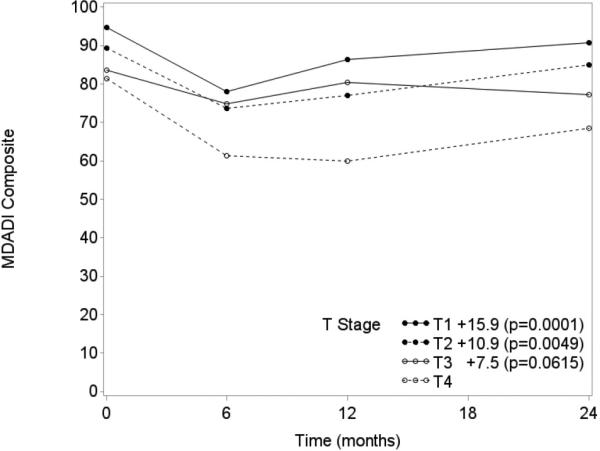
MDADI Composite Trend by Tumor Stage
Figure 4.
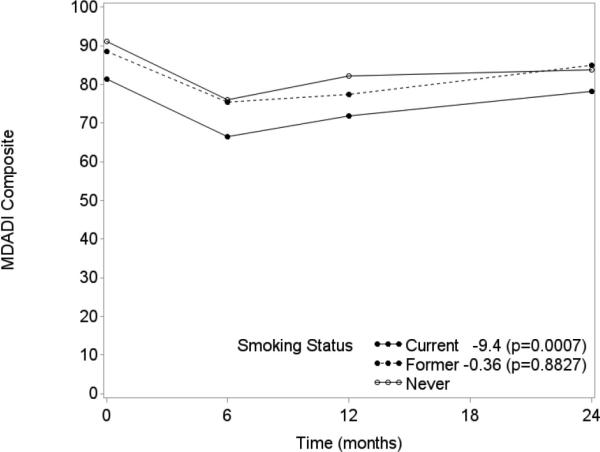
MDADI Composite Trend by Smoking Status
Discussion
Composite MDADI scores are “optimal” (MDADI>80) in over 75% of patients prior to treatment. Over the course of the post-treatment interval, patients expectedly communicate the greatest functional decrement at the first follow-up time point (6 months in our schema) but a large degree of patients (>90%) report recovery of “adequate” or “optimal” function (MDADI>60) by 24 months. Nevertheless, 17% of patients reported a persistently depressed MDADI composite (Δ20) at 24 months compared to baseline after split-field IMRT with systemic therapy for locoregionally advanced stage OPC. Despite this observation, 88% of these patients with a depressed score still reported “adequate” function, while the increment in “poor” function was only 2%. On longitudinal analysis, baseline tumor stage and smoking status are the strongest predictors of patient-reported swallowing over time, even after assessing for multicollinearity with and potential differences in radiation dose by tumor stage.
Patients with larger primary tumors have worse baseline and longitudinal function in many published reports based on diverse measures of swallowing function such as videofluoroscopy (e.g., Penetration-Aspiration Scale scores), validated overall quality of life metrics, and measures of oral intake among others [6, 22-24]. Physiologically, it is intuitive given that a larger tumor is more likely to involve the normal structures responsible for deglutition such as the intrinsic and extrinsic tongue musculature and/or the pharyngeal constrictors. Advanced stage primary tumors also require larger clinical target volumes making it unavoidable to overlap adjacent swallowing critical structures with therapeutic radiation doses, leading to adverse remodeling without regeneration of pre-cancer anatomy. A recent publication demonstrated that a difference in MDADI composite score of 10 points is statistically associated with clinical meaningful between-group differences in swallowing such as feeding tube use, aspiration, oral diet, and PSS-HN diet levels [25]. This provides valuable clinical context and a translation of our patient-reported differences in composite scores between tumor stages, particularly T1 or T2 compared to T4, into endpoints that are more easily grasped by patients and providers alike. Pre-treatment tumor size is unfortunately not a factor that can be modified but this information is useful for patient counseling on expected functional outcomes. Moreover, presentation of longitudinal MDADI results specifically stratified by tumor stage with the inclusion of baseline status may allow for more appropriate comparison of MDADI results reported from surgical trials or series, which often involve highly selected patients with smaller volume primary tumors.
The significance of smoking status as a marker of poorer longitudinal function may be a clinical surrogate for HPV-status, particularly since HPV- and/or p16-status could not be meaningfully accounted for in this analysis due to unknown data in roughly 1/3 of patients. Several publications have recently presented data on the association of improved quality or life and functional outcomes with HPV- and/or p16-associated OPC [26, 27]. Unfortunately, these studies do not adequately control for patient and treatment covariates that may also influence their endpoints and are statistically different between groups of interest, so it remains unclear if improved outcomes are secondary to disease's viral etiology or rather as a consequence of better baseline swallowing function due to smaller primary tumors and lack of associated comorbidities. In this study, it is possible that poorer patient-reported swallowing among smokers may actually reflect poorer physiologic and/or functional outcomes secondary to smoking-related hypoxia within the swallowing structures at risk or the influence of smoking-related comorbidities in a similar manner that these factors seem to influence disease specific and overall survival outcomes [2, 28-31]. We found no difference in longitudinal composite scores between never and former smokers. However, current smokers reported a composite score 9.4 points worse than never smokers at any point in time. Although the duration of smoking cessation in former smokers was not analyzed in this study, these data provide additional support for the importance of smoking cessation and additional evidence to bolster the argument that current smokers do worse through measures of both disease and functional outcomes.
Published reports on the trend of MDADI subscale scores through treatment are limited in both HNC overall and OPC patients in particular. Cartmill et al. presented longitudinal results on 12 oropharynx patients through two years after completion of non-surgical treatment [20]. Their finding of a decline in all subscales with the physical domain as the most affected and only subscale persistently depressed by 2 years compared to baseline is supported by our study. Our MDADI findings with regard to the physical subscale are also supported by a study from Kanatas et al., which found advanced stage oropharynx survivors have the greatest impairment in the physical rather than social-emotional subscale of the University of Washington Quality of Life Questionnaire [32]. These findings suggest that patients learn to cope with the emotional and functional impact of their cancer and treatment despite persistent physical limitations that continue at least into the early years of survivorship.
Age, tumor location, nodal status, treatment combination, radiation dosing, and need for post-treatment neck dissection did not significantly influence MDADI composite or subscale scores in this longitudinal model. Other studies have nicely demonstrated the impact of radiation therapy technique, unilateral vs bilateral radiation, and use of chemotherapy on patient-reported outcomes including the MDADI [15, 33]. The null associations between these factors and MDADI results in our study represent a counterpoint to some of these prior reports that can in large part be attributed to a reasonably homogenous cohort with regard to radiotherapy technique and disease site. Eligibility criteria and treatment paradigms of the parent trials led to a fairly homogenous age of participants, bilateral neck irradiation in all patients, and a narrow radiation dose range. While there was heterogeneity in the combined regimens of biologic and chemotherapeutic agents by induction and/or concurrent means, their impact on dysphagia is unclear. These MDADI data were intentionally presented apart from physiologic tests such as videofluoroscopy or other provider-based assessments of swallowing function to allow for comprehensive presentation and discussion of a broad range of MDADI results as a marker of patient-reported functional outcome results. Nevertheless, as medicine moves increasingly towards shared decision making and patient-centered care, the perspectives of patients must be considered in the context of clinician-derived measures to provide a comprehensive representation of outcomes such as swallowing function [34, 35].
Conclusions
Overall, patients report clinically-meaningful perceived dysphagia early after modern split-field IMRT for locoregionally advanced OPC that remains apparent 6 months after treatment. While MDADI scores recover slowly thereafter, they remain depressed at 24 months compared to baseline suggesting only partial recovery of perceived swallowing function. The physical subscale most strongly influences the overall MDADI composite trend. Analysis of this large cohort demonstrates that higher tumor stage and smoking status are important predictors of patient-reported function through the course of treatment, suggesting that still refined interventions to maximize functional outcomes and minimize persistently depressed patient-reported swallowing are particularly needed in patients meeting these criteria.
Acknowledgments
Conflicts of Interest and Financial Disclosures: Dr. Fuller received/receives grant and/or salary support from: the National Institutes of Health/National Cancer Institute's Paul Calabresi Clinical Oncology Award Program (K12 CA088084-06) and Clinician Scientist Loan Repayment Program (L30 CA136381-02); the SWOG/Hope Foundation Dr. Charles A. Coltman, Jr., Fellowship in Clinical Trials; a General Electric Healthcare/MD Anderson Center for Advanced Biomedical Imaging In-Kind Award; an Elekta AB/MD Anderson Department of Radiation Oncology Seed Grant; the Center for Radiation Oncology Research at MD Anderson Cancer Center; and the MD Anderson Institutional Research Grant Program. Dr. Hutcheson receives grant support from the MD Anderson Institutional Research Grant Program and the National Cancer Institute (R03 CA188162). Drs. Lai, Hutcheson, and Fuller receive grant support from the National Institute of Dental and Craniofacial Research (1R56DE025248-01). Dr. Garden is a consultant for Galera Therapeutics. Statistical analyses for this work was supported in part by National Institutes of Health Cancer Center Support (Core) Grant CA016672 to The University of Texas MD Anderson Cancer Center. These listed funders/supporters played no role in the study design, collection, interpretation of data, manuscript writing, or decision to submit the report for publication.
References
- 1.Fakhry C, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100(4):261–9. doi: 10.1093/jnci/djn011. [DOI] [PubMed] [Google Scholar]
- 2.Ang KK, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363(1):24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang SH, et al. Natural course of distant metastases following radiotherapy or chemoradiotherapy in HPV-related oropharyngeal cancer. Oral Oncology. 2013;49(1):79–85. doi: 10.1016/j.oraloncology.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 4.Wang MB, et al. HPV-Positive Oropharyngeal Carcinoma: A Systematic Review of Treatment and Prognosis. Otolaryngology - Head and Neck Surgery (United States) 2015;153(5):758–769. doi: 10.1177/0194599815592157. [DOI] [PubMed] [Google Scholar]
- 5.Massarelli E, Ferrarotto R, Glisson BS. New strategies in human papillomavirus- related oropharynx cancer: Effecting advances in treatment for a growing epidemic. Clinical Cancer Research. 2015;21(17):3821–3828. doi: 10.1158/1078-0432.CCR-14-1329. [DOI] [PubMed] [Google Scholar]
- 6.Hunter KU, et al. Toxicities affecting quality of life after chemo-IMRT of oropharyngeal cancer: Prospective study of patient-reported, observer-rated, and objective outcomes. International Journal of Radiation Oncology Biology Physics. 2013;85(4):935–940. doi: 10.1016/j.ijrobp.2012.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vainshtein JM, et al. Long-term quality of life after swallowing and salivary-sparing chemo-intensity modulated radiation therapy in survivors of human papillomavirus- related oropharyngeal cancer. Int J Radiat Oncol Biol Phys. 2015;91(5):925–33. doi: 10.1016/j.ijrobp.2014.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Metcalfe CW, Lowe D, Rogers SN. What patients consider important: Temporal variations by early and late stage oral, oropharyngeal and laryngeal subsites. Journal of Cranio-Maxillofacial Surgery. 2014;42(5):641–647. doi: 10.1016/j.jcms.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 9.Roe JWG, et al. Patient-reported outcomes following parotid-sparing intensity- modulated radiotherapy for head and neck cancer. How important is dysphagia? Oral Oncology. 2014;50(12):1182–1187. doi: 10.1016/j.oraloncology.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 10.Feng FY, et al. Intensity-modulated chemoradiotherapy aiming to reduce dysphagia in patients with oropharyngeal cancer: Clinical and functional results. Journal of Clinical Oncology. 2010;28(16):2732–2738. doi: 10.1200/JCO.2009.24.6199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilson JA, Carding PN, Patterson JM. Dysphagia after nonsurgical head and neck cancer treatment: patients' perspectives. Otolaryngol Head Neck Surg. 2011;145(5):767–71. doi: 10.1177/0194599811414506. [DOI] [PubMed] [Google Scholar]
- 12.Chen AY, et al. The development and validation of a dysphagia-specific quality-of-life questionnaire for patients with head and neck cancer: the M. D. Anderson dysphagia inventory. Arch Otolaryngol Head Neck Surg. 2001;127(7):870–6. [PubMed] [Google Scholar]
- 13.Gillespie MB, et al. Swallowing-related quality of life after head and neck cancer treatment. Laryngoscope. 2004;114(8 I):1362–1367. doi: 10.1097/00005537-200408000-00008. [DOI] [PubMed] [Google Scholar]
- 14.Yang CJ, et al. Pretreatment Dysphagia Inventory and videofluorographic swallowing study as prognostic indicators of early survival outcomes in head and neck cancer. Cancer. 2015;121(10):1588–98. doi: 10.1002/cncr.29245. [DOI] [PubMed] [Google Scholar]
- 15.Spencer CR, et al. Eliminating radiotherapy to the contralateral retropharyngeal and high level ii lymph nodes in head and neck squamous cell carcinoma is safe and improves quality of life. Cancer. 2014;120(24):3994–4002. doi: 10.1002/cncr.28938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roe JW, et al. Patient-reported outcomes following parotid-sparing intensity- modulated radiotherapy for head and neck cancer. How important is dysphagia? Oral Oncol. 2014;50(12):1182–7. doi: 10.1016/j.oraloncology.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 17.Sinclair CF, et al. Patient-perceived and objective functional outcomes following transoral robotic surgery for early oropharyngeal carcinoma. Arch Otolaryngol Head Neck Surg. 2011;137(11):1112–6. doi: 10.1001/archoto.2011.172. [DOI] [PubMed] [Google Scholar]
- 18.O'Hara J, et al. Transoral laser microsurgery±adjuvant therapy versus chemoradiotherapy for stage III and IVA oropharyngeal squamous cell carcinoma: Preliminary comparison of early swallowing outcomes. Head and Neck. 2015 doi: 10.1002/hed.23790. [DOI] [PubMed] [Google Scholar]
- 19.Prestwich RJD, et al. Long-term swallow function after chemoradiotherapy for oropharyngeal cancer: The influence of a prophylactic gastrostomy or reactive nasogastric tube. Clinical Oncology. 2014;26(2):103–109. doi: 10.1016/j.clon.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 20.Cartmill B, et al. Long-term functional outcomes and patient perspective following altered fractionation radiotherapy with concomitant boost for oropharyngeal cancer. Dysphagia. 2012;27(4):481–490. doi: 10.1007/s00455-012-9394-0. [DOI] [PubMed] [Google Scholar]
- 21.Dwivedi RC, et al. An exploratory study of the influence of clinico-demographic variables on swallowing and swallowing-related quality of life in a cohort of oral and oropharyngeal cancer patients treated with primary surgery. European Archives of Oto-Rhino-Laryngology. 2012;269(4):1233–1239. doi: 10.1007/s00405-011-1756-y. [DOI] [PubMed] [Google Scholar]
- 22.Hunter KU, et al. Aspiration pneumonia after chemo-intensity-modulated radiation therapy of oropharyngeal carcinoma and its clinical and dysphagia-related predictors. Head and Neck. 2014;36(1):120–125. doi: 10.1002/hed.23275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rinkel RN, et al. Prevalence of swallowing and speech problems in daily life after chemoradiation for head and neck cancer based on cut-off scores of the patient-reported outcome measures SWAL-QOL and SHI. Eur Arch Otorhinolaryngol. 2015 doi: 10.1007/s00405-015-3680-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Starmer H, et al. Pretreatment swallowing assessment in head and neck cancer patients. Laryngoscope. 2011;121(6):1208–11. doi: 10.1002/lary.21800. [DOI] [PubMed] [Google Scholar]
- 25.Hutcheson KA, et al. What is a clinically relevant difference in MDADI scores between groups of head and neck cancer patients? The Laryngoscope. 2015 doi: 10.1002/lary.25778. n/a-n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Naik M, et al. It is not just IMRT: Human papillomavirus related oropharynx squamous cell carcinoma is associated with better swallowing outcomes after definitive chemoradiotherapy. Oral Oncology. 2015;51(8):800–804. doi: 10.1016/j.oraloncology.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 27.Maxwell JH, et al. Quality of life in head and neck cancer patients: Impact of HPV and primary treatment modality. Laryngoscope. 2014;124(7):1592–1597. doi: 10.1002/lary.24508. [DOI] [PubMed] [Google Scholar]
- 28.Purkey MT, et al. Predictors of aspiration pneumonia following radiotherapy for head and neck cancer. Ann Otol Rhinol Laryngol. 2009;118(11):811–6. [PubMed] [Google Scholar]
- 29.Gillison ML, et al. Tobacco smoking and increased risk of death and progression for patients with p16-positive and p16-negative oropharyngeal cancer. J Clin Oncol. 2012;30(17):2102–11. doi: 10.1200/JCO.2011.38.4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O'Sullivan B, et al. Deintensification candidate subgroups in human papillomavirus- related oropharyngeal cancer according to minimal risk of distant metastasis. J Clin Oncol. 2013;31(5):543–50. doi: 10.1200/JCO.2012.44.0164. [DOI] [PubMed] [Google Scholar]
- 31.Huang SH, et al. Refining American joint committee on cancer/union for international cancer control TNM stage and prognostic groups for human papillomavirus-related oropharyngeal carcinomas. Journal of Clinical Oncology. 2015;33(8):836–845. doi: 10.1200/JCO.2014.58.6412. [DOI] [PubMed] [Google Scholar]
- 32.Kanatas A, et al. Issues patients would like to discuss at their review consultation: Variation by early and late stage oral, oropharyngeal and laryngeal subsites. European Archives of Oto-Rhino-Laryngology. 2013;270(3):1067–1074. doi: 10.1007/s00405-012-2092-6. [DOI] [PubMed] [Google Scholar]
- 33.Al-Mamgani A, et al. A prospective evaluation of patient-reported quality-of-life after (chemo)radiation for oropharyngeal cancer: Which patients are at risk of significant quality-of-life deterioration? Radiotherapy and Oncology. 2013;106(3):359–363. doi: 10.1016/j.radonc.2012.12.014. [DOI] [PubMed] [Google Scholar]
- 34.Caudell JJ, et al. Factors associated with long-term dysphagia after definitive radiotherapy for locally advanced head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2009;73(2):410–5. doi: 10.1016/j.ijrobp.2008.04.048. [DOI] [PubMed] [Google Scholar]
- 35.Hutcheson KA, Lewin JS. Functional outcomes after chemoradiotherapy of laryngeal and pharyngeal cancers. Curr Oncol Rep. 2012;14(2):158–65. doi: 10.1007/s11912-012-0216-1. [DOI] [PMC free article] [PubMed] [Google Scholar]


