Abstract
Background
Subtotal or total colectomy or proctocolectomy with permanent ileostomy (TC-PI) may be a treatment option for medically refractory colonic Crohn's disease (CD).
Aim
To perform a systematic review and meta-analysis to evaluate the rate, risk factors and outcomes of CD recurrence after TC-PI.
Methods
In a systematic review ending March 31, 2016, we identified 18 cohort studies (1438 adults) who underwent TC-PI for colonic CD (median follow-up, 7.4 years; interquartile range, 5.3-9.0). We estimated pooled rates (with 95% confidence interval [CI]) of clinical and surgical recurrence, and risk factors for disease recurrence.
Results
On meta-analysis, the risk of clinical recurrence after TC-PI was 28.0% (95% CI, 21.7-35.3; 14 studies, 260/1004 patients), with a 5- and 10-year median cumulative rate of 23.5% (range, 7-35) and 40% (range, 11-60), respectively. The risk of surgical recurrence was 16.0% (95% CI, 11.1-22.7; 10 studies; 183/1092 patients), with a 5- and 10-year median cumulative rate of 10% (range, 3-29) and 18.5% (range, 14-34), respectively. The risk of clinical and surgical recurrence in patients without ileal disease at baseline was 11.5% (95% CI, 7.7-16.8) and 10.4% (95% CI, 4.5-22.5), respectively. History of ileal disease was associated with 3.2 times higher risk of disease recurrence (RR, 3.2; 95% CI, 1.8-5.6). Other inconsistent risk factors for disease recurrence were penetrating disease and young age at disease onset.
Conclusions
Small bowel clinical recurrence occurs in about 28% of patients after TC-PI for colonic CD. Disease recurrence risk is 3.2 times higher in patients with history of ileal disease, and continued medical therapy may be advisable in this population. In patients without ileal inflammation at surgery, continued endoscopic surveillance may identify asymptomatic disease recurrence to guide therapy.
Keywords: Recurrence, ileostomy, Crohn's disease
Introduction
Despite significant advances in the treatment of Crohn's disease (CD), nearly 50% of patients require surgery within 10 years of their diagnosis, and an additional 30% require surgery at some point in their lifetime (1-3). Surgery is rarely curative for CD, and most patients experience post-surgical disease recurrence, along a continuum of endoscopic, clinical and surgical recurrence. The course of CD after surgery is difficult to predict, however, the risk of clinical and/or surgical recurrence can be stratified and mitigated through the early use of colonoscopy and prophylactic medical therapy among at-risk individuals (4-7). However, the majority of these data have focused on CD patients undergoing limited surgical resection with ileocolonic anastomosis (4-8).
For patients with extensive colonic or severe perianal CD refractory to medical therapy, for whom limited resection with primary anastomosis may not be an option, subtotal or total colectomy or proctocolectomy with permanent ileostomy (TC-PI) may be considered. In an early population-based study from North America of patients with CD undergoing surgery, approximately 10% patients underwent TC-PI (1, 9-11). However, there is limited data on the rate of recurrence of active CD in the small bowel. Data from luminal CD suggests that despite significant changes in disease behaviour over time, disease extent largely remains stable with progression seen only in 6.5% of patients (1, 12), so it is conceivable that risk of post-surgical disease recurrence in the small bowel would be low in these patients undergoing a permanent ileostomy. Risk factors, both modifiable and non-modifiable, associated with small bowel recurrence are also not well understood; knowledge of these may inform management strategies including use of prophylactic pharmacological therapy, and periodic endoscopic surveillance to diagnose and treat asymptomatic endoscopic recurrence.
Hence, to better understand the natural history of CD after TC-PI, we synthesized published data through a systematic review with meta-analysis, and evaluated the rate of small bowel recurrence and stoma-related complications after TC-PI. Furthermore, we evaluated risk factors associated with disease recurrence. These data would be very informative in a shared-decision making process before and after surgical resection, and allow for an optimization of post-operative surveillance strategies among these individuals.
Methods
This systematic review followed the preferred reporting items for systematic reviews and meta-analysis (PRISMA) standards, and followed an a priori published protocol (registered at the International Prospective Register of Systematic Reviews, PROSPERO CRD42016039310).
Study Selection
Studies were included if they were: (a) cohort studies, case series and randomized controlled trials in (b) patients (adult or paediatric) with CD (c) who underwent TC-PI for known colonic CD (with or without known small bowel disease), and reported (d) rate of small bowel disease recurrence (clinical, defined as symptoms due to recurrence of CD in the small bowel; surgical, defined as presence of active CD in small bowel necessitating surgical resection) and/or risk factors associated with disease recurrence, (e) with a minimum of one year of follow-up after TC-PI. While we acknowledge that there are differences in types of colectomy, for this study, subtotal colectomy and total colectomy and proctocolectomy, with permanent ileostomy were considered equivalent.
We excluded the following studies: (a) case-control or cross-sectional studies; (b) studies with insufficient follow-up on the fate of TC-PI (i.e., only report occurrence of TC-PI, but not outcomes with regard to small bowel recurrence); (c) studies on outcomes of temporary fecal diversion through diverting ileostomy or colostomy; and (d) studies exclusively in patients with active small bowel disease at time of TC-PI. In the case of multiple studies from the same cohort, we included data from the most recent comprehensive report.
Search Strategy
We conducted a comprehensive search of multiple electronic databases from inception to August 14, 2015, in adults with no language restrictions; this search was updated on March 31, 2016. The databases included: Ovid Medline, Ovid EMBASE, Ovid Cochrane Central Register of Controlled Trials, Ovid Cochrane Database of Systematic Reviews, Web of Science, and Scopus. The search strategy was designed and conducted by an experienced medical librarian with input from the study's investigators, using controlled vocabulary supplemented with keywords, for studies on recurrence after TC-PI in CD (see details in Supplementary Appendix). In addition, conference abstracts (Digestive Disease Week, United European Gastroenterology Week, American College of Gastroenterology annual meeting, Advances in Inflammatory Bowel Diseases meeting organized by the Crohn's and Colitis Foundation of America, and European Crohn's and Colitis Organization annual meeting) from 2012 to 2015, as well as bibliography of the selected articles and review articles on the topic were manually searched for additional studies, with no language restrictions. Two reviewers (PSD and PM) independently assessed the title and abstract of studies identified in the primary search for inclusion, and the full text of remaining articles were examined to determine whether they met inclusion criteria. Any discrepancy in article selection was resolved by consensus, and in discussion with a third reviewer (SS).
Data Abstraction and Quality Assessment
Data on study-, patient-, surgery-related characteristics, as well as outcomes of interest from included studies were abstracted onto a standardized data collection form (MF, PM). Any discrepancies were addressed by a joint re-evaluation of the original article. The methodological quality of studies was assessed using National Institute of Clinical Excellence (NICE) quality assessment for case series checklist.
Outcomes Assessed
The primary outcome measures were the proportion of patients with (a) clinical recurrence (as defined in individual studies) and (b) surgical recurrence (recurrence requiring resection) of CD after TC-PI, at last reported follow-up. In addition, we evaluated the proportion of patients undergoing surgery due to stoma-related complications. To specifically estimate risk of recurrence in patients with purely colonic disease without known small bowel disease, we performed a sensitivity analysis restricting to cohorts in which none of the patients had known ileal disease at time of TC-PI. In order to assess differences in rates of primary outcome in the pre-biologic (before 1998) and biologic era (after 1998), we compared weighted pooled rates in studies published in the corresponding period.
We, qualitatively and quantitatively (if feasible and reported in >2 studies), identified demographic, clinical and treatment-related factors associated with recurrence of CD in the small bowel.
Statistical Analysis
We used the random-effects model described by DerSimonian and Laird to calculate pooled rates (and 95% confidence interval [CI]) of clinical recurrence, surgical recurrence and stoma-related complications (13). In addition, to estimate 5- and 10-year cumulative rates of clinical and surgical recurrence of CD, we reported median (and range). To identify risk factors associated with recurrence, we pooled maximally adjusted risk estimate (odds ratio or relative risk; to account for confounding variables), where reported, using random-effects model. Specifically, to compare risk of recurrence in patients with and without known ileal disease at time of TC-PI, we calculated pooled unadjusted RR using raw events from studies which reported this. We assessed heterogeneity between study-specific estimates using the inconsistency index (I2), and used cut-offs of <30%, 30%-59%, 60%-75% and >75% to suggest low, moderate, substantial and considerable heterogeneity, respectively (14). Small study effects were assessed qualitatively using funnel plot asymmetry and quantitatively using the Egger's regression test (15). All analysis was performed using Comprehensive Meta-Analysis (CMA) version 2 (Biostat, Englewood, NJ).
Results
Of 2017 unique studies identified using our search strategy, 17 studies met our inclusion criteria (10, 11, 16-28, 30, 31). In addition, we identified one abstracts from conference proceedings (29) and hence, included a total of 18 studies for quantitative synthesis. Figure 1 shows the schematic diagram of study selection.
Figure 1. Study selection flow chart.
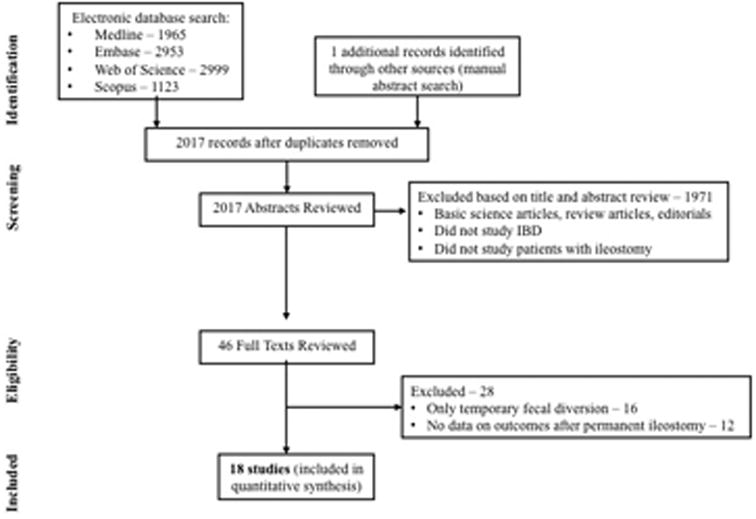
Characteristics of Included Studies
Table 1 describes the characteristics of included studies. The median number of patients was 72 (range, 14-182) and median follow-up was 7.4 years (range, 1.2-15). Studies were performed and reported over a wide time period, with period of patient recruitment from 1934-2007, and period of publication from 1972-2014. While no studies were performed exclusively in pediatric patients, six studies reported lower range of age <18 years. Overall, ten studies were performed exclusively in the pre-biologic era (i.e., all patients were operated and analysed before the availability of anti-TNF agents in 1998), one study exclusively in the biologic era and three studies in the overlapping period (i.e., some patients were operated before availability of anti-TNF agents in 1998 and others after 1998). Among the eight studies that explicitly reported definition of clinical recurrence (10, 17, 23, 25, 27-29, 31), two didn't (17, 29) required confirmation of disease activity (through endoscopy and/or imaging) in patients with new onset gastrointestinal symptoms. Six studies were performed in North America (11, 16, 19, 23, 24, 31) and 12 in Europe (10, 17, 18, 20-22, 25-30). Of note, three studies were performed at a single centre (16, 23, 24), of which two reported clinical recurrence over a non-overlapping period, and one reported surgical recurrence; hence, all three studies contributed to the analyses.
Table 1. Characteristics of included studies.
| Authors | Location | Study Period, median follow-up (y) after TC-PI | Number of patients with TC-PI | Proportion of patients with known small bowel disease at time of surgery (%) | Definition of clinical recurrence | 5- and 10-year risk of clinical recurrence | 5- and 10-year risk of surgical recurrence |
|---|---|---|---|---|---|---|---|
| Korelitz, 1972 | New York, USA | 1940-1970, NR | 67 | NR | NR | - | - |
| Steinberg, 1974 | Birmingham, UK | 1942-1973, 9y | 73 | 52 | New-onset symptoms | 20%, NR | - |
| Weterman, 1976 | Leuven, Belgium | 1934-1972, 8.3y | 16 | NR | NR | - | - |
| Vender, 1979 | Connecticut, USA | 1958-1974, 8.8y | 34 | 62 | NR | 27%, 40% | - |
| Goligher, 1985 | Leeds, UK | 1959-1984, 15y | 162 | NR | NR | NR, 60% | - |
| Ritchie, 1990 | London, UK | 1952-1988, NR | 182 | NR | NR | 7%, 11% | - |
| Harling, 1991 | Copenhagen, Denmark | 1964-1989, 7.7y | 84 | 0 | NR | - | 29%, NR |
| Heimann, 1993 | New York, USA | 1976-1989, 3y | 37 | NR | New-onset symptoms, confirmed by imaging or endoscopy | - | - |
| Ho, 1995 | New York, USA | 1952-1984, 6.8y | 182 | 64 | NR | - | NR, 34% |
| Yamamoto, 2000 | Birmingham, UK | 1958-1997, 18.6y | 103 | 36 | New-onset symptoms, confirmed by imaging, | - | 13%, 17% |
| Ecker, 2001 | Homburg, Germany | NR, 5.4y | 92 | NR | NR | - | 3%, 14% |
| Bernell, 2001 | Huddinge, Sweden | 1955-1989, 12.9y | 89 | NR | New-onset symptoms, confirmed by imaging, endoscopy or need for re-initiation of IBD-related therapy | 24%, 37% | - |
| Fichera, 2005 | Illinois, USA | 1985-2003, 1.2y | 76 | 0 | NR | - | 9%, 20% |
| Onali, 2009 | Rome, Italy | 2001-2007 | 14 | NR | New-onset symptoms, confirmed by imaging, endoscopy | - | - |
| Amiot, 2011 | Paris, France | 1990-2005 | 55 | 58 | New-onset symptoms, confirmed by imaging, endoscopy or need for re-initiation of IBD-related therapy | 27%, 40% | 10%, NR |
| Koriche, 2012 | Paris, France | 1973-2010 | 83 | 54 | New-onset symptoms, with need for re-initiation or escalation of IBD-related therapy | - | - |
| Leal-Valdivieso, 2012 | Barcelona, Spain | - | 16 | 37 | NR | - | - |
| Lopez, 2014 | Massachusetts, USA | NR, 1.6y | 73 | 35 | New-onset symptoms, confirmed by imaging, endoscopy or histology | 35%, NR | - |
Abbreviations: NR, not reported; y, years
Overall, the studies were at moderate risk of bias, 17 were performed in referral centres (10, 11, 16-26, 28-31) and one in population (27), seventeen were retrospective (10, 16-31) and one prospective (11). Supplementary Table 1 details the quality of these included studies according to NICE criteria.
Clinical Recurrence after Total Colectomy with Permanent Ileostomy
Fourteen studies reported rates of CD recurrence after TC-PI in 1004 patients (10, 16-21, 23, 25, 27, 28-31). On meta-analysis, the rate of clinical recurrence was 28% (95% CI, 21.7-35.3; 260/1004 patients), with considerable heterogeneity (I2=80%) (Figure 2). Median 5- (6 studies) and 10-year (5 studies) cumulative rates of clinical recurrence were 23.5% (range, 7-35) and 40% (range, 11-60), respectively. When restricting the analysis to patients without history of ileal disease, the rate of clinical recurrence was lower at 11.5% (95% CI, 7.7-16.8; n=2 studies). Among three studies reporting time to recurrence, the median time to recurrence ranged from 1 to 5.6 years (28-30). In six studies reporting the location of recurrence in 132 patients, 81% patients experienced ileal, 3% jejunal, 2% upper gastrointestinal tract and 14% extensive small bowel recurrence (10, 17, 20, 21, 25, 31).
Figure 2.
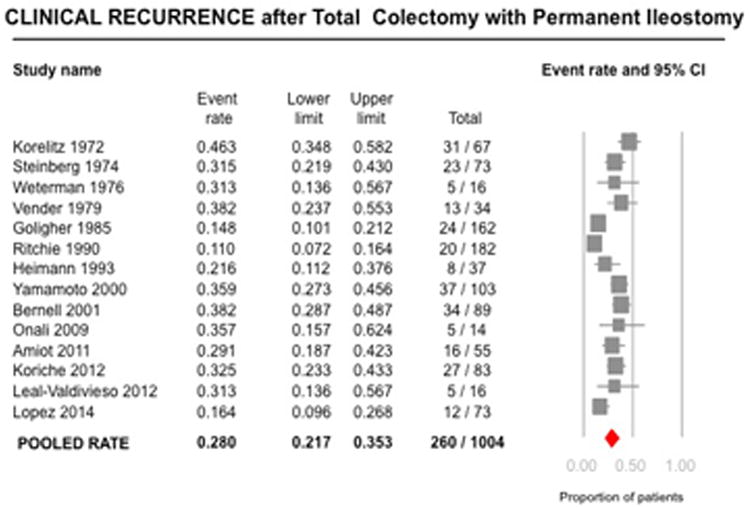
Pooled summary rate (and 95% confidence interval) of clinical recurrence after total colectomy and ileostomy in patients with Crohn's disease, using random effects model, based on 14 studies with 1004 patients.
Rates of clinical recurrence were similar among studies in the pre-biologic and biologic era (published before 1998 vs. after 1998: 26.9% vs. 30.0%, p=0.64). No single study contributed significantly to heterogeneity, based on visual inspection of the forest plots. Cumulative meta-analysis revealed that the rates of recurrence were higher in studies published before 1990 and stable after that (Supplementary Figure 1). Due to considerable heterogeneity, tests for small study effects were unreliable.
Surgical Recurrence after Total Colectomy with Permanent Ileostomy
Ten studies including 1092 patients evaluated the risk for reoperation due to recurrence (10-11, 17, 20-22, 24-26, 29). On meta-analysis, the rate of surgical recurrence was 16.0% (95% CI, 11.1-22.7; 183/1092 patients), with considerable heterogeneity (I2=84%) (Figure 3). Median 5- (5 studies) and 10-year (4 studies) cumulative rates of surgical recurrence were 10% (range, 3-29) and 18.5% (range, 14-34), respectively. Among the three studies reporting time to recurrence, the median time to surgical recurrence ranged from 2.4 to 9.6 years (10, 25, 29.). When restricting the analysis to patients without documented ileal disease at time of TC-PI, rate of surgical recurrence was lower at 10.4% (95% CI, 4.5-22.5; n=5 studies).
Figure 3.
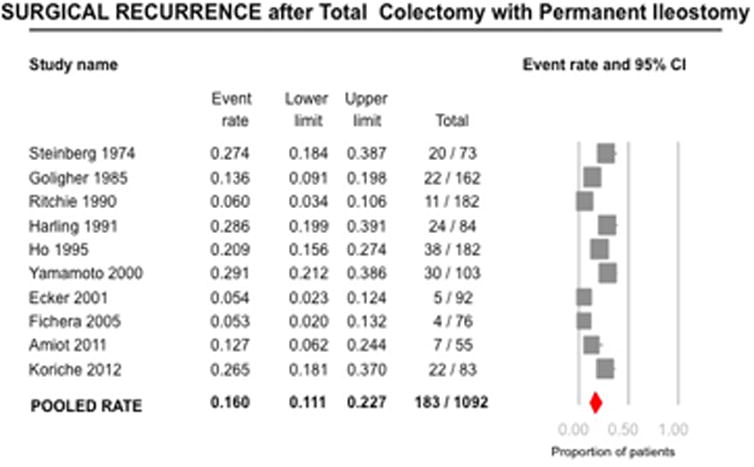
Pooled summary rate (and 95% confidence interval) of surgical recurrence after total colectomy and ileostomy in patients with Crohn's disease, using random effects model, based on 10 studies with 1092 patients.
Rates of surgical recurrence were similar among studies in the pre-biologic and biologic era (published before 1998 vs. after 1998: 19.3% vs. 10.8%, p=0.22). No single study contributed significantly to heterogeneity, based on visual inspection of the forest plots. Cumulative meta-analysis revealed that the rates of surgical recurrence were higher in studies published before 1990, and stable after that. Due to considerable heterogeneity, tests for small study effects were unreliable.
Surgery for Stoma-related Complications
Seven studies reported the rate of stoma-related complications requiring surgery after TC-PI in 701 patients (10, 17, 19, 20, 24-26). On meta-analysis, the rate of re-operation was 15.6% (95% CI, 8.5-27.0; 108/701 patients), with considerable heterogeneity (I2=78%) (Supplementary Figure 2). In two studies that reported the indication for reoperation, the most common reasons were: skin ulcerations (45%), stoma retraction (21%), stenosis (10%), prolapse (10%), hernia (6%) and small bowel obstruction due to adhesions (6%) (10, 26).
Risk Factors Associated with Recurrence of Crohn's Disease
Ten studies evaluated factors associated with clinical and/or surgical recurrence. Given the continuum of clinical and surgical recurrence, for evaluation of risk factors for recurrence of CD, these outcomes were taken in a hierarchical order. The most consistent risk factor associated with recurrence of CD was the presence of small bowel disease at time of TC-PI. On meta-analysis of three studies that reported recurrence rates in patients with and without ileal disease at baseline (19, 24, 29), presence of ileal disease at time of TC-PI was associated with 3.2 times higher risk of disease recurrence (RR, 3.2; 95% CI, 1.8-5.6) (Supplementary Figure 3).
No other clinical or demographic factors were consistently associated with increased risk of disease recurrence. In two studies, young age at CD disease onset (<18y: HR for clinical recurrence, 2.9) and at time of surgery (<30y: HR for surgical recurrence, 2.6) was associated with increased risk of disease recurrence (25, 31). Complications of CD phenotype leading to TC-PI (fistulas, abscesses and/or perianal disease) was also associated with increased risk of disease recurrence in three out of four studies where this was studied (10, 19, 27, 31). Similarly, the use of biologic therapy prior to TC-PI (perhaps as a marker of severe disease) was also associated with increased risk of clinical recurrence (HR, 3.7) (31). Smoking was not associated with increased risk of recurrence of CD after TC-PI in one study (10).
Only two studies reported on postoperative preventive therapies (10, 30). Amiot et al reported that none of the patients were administered prophylactic therapy after (TC-PI), and 13/16 with established clinical recurrence after TC-PI were treated with medications. Leal-Valdivieso et al reported that of 16 patients treated with TC-PI, 7 were treated with mesalamine and 3 with thiopurines for prevention of recurrence; of note, in their cohort, 37% had known ileal disease at time of TC-PI. However, the potential impact of these therapies on prevention of recurrence was not reported.
Discussion
In this systematic review and meta-analysis of 18 cohort studies of 1438 patients who underwent TC-PI for refractory colonic CD, we made several key observations for clinical practice. First, about 1/3rd of patients may develop clinical, and about 16% may develop surgical recurrence after TC-PI for colonic CD. Second, history of ileal disease is a consistent risk factor associated with a 3-fold increase in risk of disease recurrence. Additionally, disease complications as fistula or abscess and/or perianal complications and use of biologics prior to TC-PI, and young age at disease onset or surgery may be risk factors for disease recurrence.
TC-PI is perhaps the last therapeutic option for patients with medically refractory colonic and/or perianal CD. Additionally, temporary fecal diversion with diverting ileostomy (with concurrent medical therapy) is sometimes considered in patients with refractory perianal and/or colonic CD with the intention of restoring bowel continuity at a later time with better disease control. However, almost 2/3rd of such patients may not undergo restoration of bowel continuity and may end up with ‘permanent’ diverting ileostomy, with or without completion colectomy (32). Despite the psychological and social impact of definitive ileostomy, TC-PI may restore quality of life and permit return to social activities in patients with uncontrolled active disease (33).
While there is considerable literature on rates, risk factors and outcomes of disease recurrence after ileocolonic resection with primary anastomosis (7), there is limited literature on outcomes of patients with permanent ileostomy. Though traditionally disease location in CD is believed to be stable over time, we observed that about 1/3rd of patients may develop clinical recurrence of CD in the small bowel over a median 7 year follow-up, with varying extent. While this rate is considerably smaller than rates after primary ileocolonic anastomosis in which patients experience near-universal endoscopic recurrence and over 50% may develop clinical recurrence by 5 years, rate of CD recurrence after TC-PI is high enough that it cannot be ignored, and TC-PI should not be labelled as a ‘curative’ procedure in these patients (4-8).
Guidelines suggest early postoperative prophylaxis strategy and/or routine endoscopic monitoring in patients with surgically induced remission through ileocolonic resection and primary anastomosis based on a multitude of putative risk factors for disease recurrence (34, 35). However, there is paucity of literature on approach to management of CD after TC-PI. Through our review, we identified presence of prior ileal disease as a consistent risk factor for disease recurrence. This is probably akin to endoscopic recurrence in the neoterminal ileum being a strong predictor of future clinical and surgical recurrence in patients with ileocolonic anastomosis (36). In fact, Chongthammakun et al observed that patients with Rutgeerts score >0 in the end-ileostomy had 8-fold higher risk of surgical recurrence and 5-fold higher risk of needing IBD-related medications than patients without evidence of subclinical endoscopic disease (37). In these patients, it may be reasonable to initiate postoperative prophylactic therapy after TC-PI. Other risk factors include penetrating and/or perianal complications, young age at disease onset and/or surgery were inconsistently associated with increased risk of recurrence of CD after TC-PI, and in patients with these, periodic endoscopic monitoring of end ileostomy may be considered.
There is limited data on the effectiveness of pharmacological therapy in preventing and treating recurrence of CD in the small bowel after TC-PI as this population is typically excluded from clinical trials. In the included studies, only two studies reported on postoperative prophylactic therapy, and neither of these studies reported on the impact of preventive therapy on the risk of recurrence (10, 30). Four studies reported on the effectiveness of pharmacological therapies after established clinical recurrence, and of 85 patients treated, 27 responded to medical management (10, 16, 25, 30). However, it is important to note that most studies were conducted in the prebiologic era. With the ever expanding therapeutic options for management of CD, it is likely that most patients with recurrence of CD in the small bowel would be amenable to medical management in the current era.
The strengths of this systematic review include: (a) comprehensive and systematic literature search with well-defined inclusion criteria; (b) assessment of multiple clinically relevant short- and long-term outcomes of TC-PI in the management of refractory colonic and/or perianal CD; (c) sub-group and cumulative meta-analysis sensitivity analyses to evaluate changes in long-term outcomes with the advent and use of biologic agents; and (d) systematic assessment of factors associated with long-term outcomes of temporary FD.
There are several limitations in our study. First, the meta-analysis was based on retrospective observational studies performed at tertiary referral centres with inherent selection bias. These studies were conducted over a wide time period, over which the endoscopic/radiologic assessment and pharmacological management of CD has evolved. Second, while there was complete follow-up for most studies, long-term follow-up was not uniformly available. There were probably unmeasured confounding factors influencing decisions on management after TC-PI, accounting for variations in practice and hence, considerable heterogeneity between studies. Third, definition of clinical recurrence was not available for most of the studies and varied when available. There was very limited data on perianal recurrence after TC-PI. Finally, risk factors for CD recurrence were not consistently studied and reported. Smoking status, the most consistent risk factor for recurrence after ileocaecal resection, couldn't be evaluated here because of paucity of available data. We did not attempt to contact study investigators for additional unpublished data.
In conclusion, TC-PI for medically refractory colonic and/or perianal CD is not curative, and about 1/3rd of patients experience clinical recurrence of CD in the small bowel; approximately 16% may experience severe recurrence requiring surgery. Patients with known subclinical ileal disease, presence of disease-related complications (like penetrating and/or perianal disease), failure of biologic therapy prior to TC-PI, may be at high risk of recurrence of CD after TC-PI, and may be considered for continued pharmacological prophylaxis and/or periodic endoscopic monitoring of end ileostomy.
Supplementary Material
Acknowledgments
Dr. Fumery is supported by the French Society of Gastroenterology (SNFGE, bourse Robert Tournut). Dr. Dulai is supported by the NIDDK training grant 5T32DK007202. Dr. Singh is supported by the NIH/NLM training grant T15LM011271 and the American College of Gastroenterology Junior Faculty Development Award and Crohn's and Colitis Foundation of American Career Development Award.
Footnotes
Disclosures: None of the authors have any relevant financial disclosures.
- Study concept and design: MF, PSD, SS
- Acquisition of data: MF, PSD, PM, AMF, SS
- Analysis and interpretation of data: MF, PSD, PM, SS
- Drafting of the manuscript: MF, PSD, SS
- Critical revision of the manuscript for important intellectual content: PM, AFM, SR, WJS
- Approval of the final manuscript: MF, PSD, PM, AFM, SR, WJS, SS
- Guarantor of the article: SS
Systematic Review Registration: PROSPERO #CRD42016039310
References
- 1.Peyrin-Biroulet L, Loftus EV, Jr, Colombel JF, Sandborn WJ. The natural history of adult Crohn's disease in population-based cohorts. Am J Gastroenterol. 2010;105:289–97. doi: 10.1038/ajg.2009.579. [DOI] [PubMed] [Google Scholar]
- 2.Cosnes J, Nion-Larmurier I, Beaugerie L, Afchain P, Tiret E, Gendre JP. Impact of the increasing use of immunosuppressants in Crohn's disease on the need for intestinal surgery. Gut. 2005;54:237–41. doi: 10.1136/gut.2004.045294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frolkis AD, Dykeman J, Negrón Me, et al. Risk of surgery for inflammatory bowel diseases has decreased over time: a systematic review and meta-analysis of population-based studies. Gastroenterology. 2013;145:996–1006. doi: 10.1053/j.gastro.2013.07.041. [DOI] [PubMed] [Google Scholar]
- 4.Regueiro M, Feagan BG, Zou B, et al. Infliximab Reduces Endoscopic, but Not Clinical, Recurrence of Crohn's Disease After Ileocolonic Resection. Gastroenterology. 2016;150:1568–78. doi: 10.1053/j.gastro.2016.02.072. [DOI] [PubMed] [Google Scholar]
- 5.Swoger JM, Regueiro M. Evaluation for postoperative recurrence of Crohn disease. Gastroenterol Clin North Am. 2012;41:303–14. doi: 10.1016/j.gtc.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 6.Singh S, Garg SK, Pardi DS, Wang Z, Murad MH, Loftus EV., Jr Comparative efficacy of pharmacologic interventions in preventing relapse of Crohn's disease after surgery: a systematic review and network meta-analysis. Gastroenterology. 2015;148:64–76. doi: 10.1053/j.gastro.2014.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buisson A, Chevaux JB, Allen PB, Bommelaer G, Peyrin-Biroulet L. Review article: the natural history of postoperative Crohn's disease recurrence. Aliment Pharmacol Ther. 2012;35:625–33. doi: 10.1111/j.1365-2036.2012.05002.x. [DOI] [PubMed] [Google Scholar]
- 8.De Cruz P, Kamm MA, Hamilton AL, et al. Crohn's disease management after intestinal resection: a randomised trial. Lancet. 2015;385:1406–17. doi: 10.1016/S0140-6736(14)61908-5. [DOI] [PubMed] [Google Scholar]
- 9.Coscia M, Gentilini L, Laureti S, et al. Risk of permanent stoma in extensive Crohn's colitis: the impact of biological drugs. Colorectal Dis. 2013;15:1115–22. doi: 10.1111/codi.12249. [DOI] [PubMed] [Google Scholar]
- 10.Amiot A, Gornet JM, Baudry C, et al. Crohn's disease recurrence after total proctocolectomy with definitive ileostomy. Dig Liver Dis. 2011;43:698–702. doi: 10.1016/j.dld.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 11.Fichera A, McCormack R, Rubin MA, et al. Long-term outcome of surgically treated Crohn's colitis: a prospective study. Dis Colon Rectum. 2005;48:963–9. doi: 10.1007/s10350-004-0906-3. [DOI] [PubMed] [Google Scholar]
- 12.Panaccione R, Sandborn WJ, Loftus EV, et al. Phenotypic classification of Crohn's disease patients in Olmsted County, Minnesota: application of the Vienna classification. Gastroenterology. 1999;116(Part 2):A810. [Google Scholar]
- 13.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 14.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Egger M, Smith GD, Phillips AN. Meta-analysis: principles and procedures. BMJ. 1997;315:1533–7. doi: 10.1136/bmj.315.7121.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Korelitz BI, Present DH, Alpert LI, Marshak RH, Janowitz HD. Recurrent regional ileitis after ileostomy and colectomy for granulomatous colitis. N Engl J Med. 1972;287:110–5. doi: 10.1056/NEJM197207202870302. [DOI] [PubMed] [Google Scholar]
- 17.Steinberg DM, Allan RN, Thompson H, Brooke BN, Alexander-Williams J, Cooke WT. Excisional surgery with ileostomy for Crohn's colitis with particular reference to factors affecting recurrence. Gut. 1974;15:845–51. doi: 10.1136/gut.15.11.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weterman IT, Peña AS. The long-term prognosis of ileorectal anastomosis and proctocolectomy in Crohn's disease. Scand J Gastroenterol. 1976;11:185–91. [PubMed] [Google Scholar]
- 19.Vender RJ, Rickert RR, Spiro HM. The outlook after total colectomy in patients with Crohn's colitis and ulcerative colitis. J Clin Gastroenterol. 1979;1:209–17. doi: 10.1097/00004836-197909000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Goligher JC. The long-term results of excisional surgery for primary and recurrent Crohn's disease of the large intestine. Dis Colon Rectum. 1985;28:51–5. doi: 10.1007/BF02553908. [DOI] [PubMed] [Google Scholar]
- 21.Ritchie JK. The results of surgery for large bowel Crohn's disease. Ann R Coll Surg Engl. 1990;72:155–7. [PMC free article] [PubMed] [Google Scholar]
- 22.Harling H, Hegnhøj J, Rasmussen TN, Jarnum S. Fate of the rectum after colectomy and ileostomy for Crohn's colitis. Dis Colon Rectum. 1991;34:931–5. doi: 10.1007/BF02049711. [DOI] [PubMed] [Google Scholar]
- 23.Heimann TM, Greenstein AJ, Lewis B, Kaufman D, Heimann DM, Aufses AH., Jr Prediction of early symptomatic recurrence after intestinal resection in Crohn's disease. Ann Surg. 1993;218:294–8. doi: 10.1097/00000658-199309000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ho I, Greenstein AJ, Bodian CA, Janowitz HD. Recurrence of Crohn's disease in end ileostomies. Inflamm Bowel Dis. 1995:173–8. [PubMed] [Google Scholar]
- 25.Yamamoto T, Allan RN, Keighley MR. Audit of single-stage proctocolectomy for Crohn's disease: postoperative complications and recurrence. Dis Colon Rectum. 2000;43:249–56. doi: 10.1007/BF02236990. [DOI] [PubMed] [Google Scholar]
- 26.Ecker KW, Gierend M, Kreissler-Haag D, Feifel G. Reoperations at the ileostomy in Crohn's disease reflect inflammatory activity rather than surgical stoma complications alone. Int J Colorectal Dis. 2001;16:76–80. doi: 10.1007/s003840000279. [DOI] [PubMed] [Google Scholar]
- 27.Bernell O, Lapidus A, Hellers G. Recurrence after colectomy in Crohn's colitis. Dis Colon Rectum. 2001;44:647–54. doi: 10.1007/BF02234559. [DOI] [PubMed] [Google Scholar]
- 28.Onali S, Petruzziello C, Calabrese E, et al. Frequency, pattern, and risk factors of postoperative recurrence of Crohn's disease after resection different from ileo-colonic. J Gastrointest Surg. 2009;13:246–52. doi: 10.1007/s11605-008-0726-1. [DOI] [PubMed] [Google Scholar]
- 29.Koriche D, Salleron J, Gower-Rousseau C, Cortot A, Colombel JF, Zerbib P. Recurrence of Crohn's Disease After Definitive Stoma: A Retrospective Study in 83 Patients. Gastroenterology. 2012;142(S1):S-254–S-255. [Google Scholar]
- 30.Leal-Valdivieso C, Marín I, Mañosa M, et al. Should we monitor Crohn's disease patients for postoperative recurrence after permanent ileostomy? Inflamm Bowel Dis. 2012;18:E196. doi: 10.1002/ibd.21730. [DOI] [PubMed] [Google Scholar]
- 31.Lopez J, Konijeti GG, Nguyen DD, Sauk J, Yajnik V, Ananthakrishnan AN. Natural history of Crohn's disease following total colectomy and end ileostomy. Inflamm Bowel Dis. 2014;20:1236–41. doi: 10.1097/MIB.0000000000000072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singh S, Ding NS, Mathis KL, et al. Systematic review with meta-analysis: faecal diversion for management of perianal Crohn's disease. Aliment Pharmacol Ther. 2015;42:783–92. doi: 10.1111/apt.13356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kasparek MS, Glatzle J, Temeltcheva T, Mueller MH, Koenigsrainer A, Kreis ME. Long-term quality of life in patients with Crohn's disease and perianal fistulas: influence of fecal diversion. Dis Colon Rectum. 2007;50:2067–74. doi: 10.1007/s10350-007-9006-5. [DOI] [PubMed] [Google Scholar]
- 34.Annese V, Daperno M, Rutter MD, et al. European evidence based consensus for endoscopy in inflammatory bowel disease. J Crohns Colitis. 2013;7:982–1018. doi: 10.1016/j.crohns.2013.09.016. [DOI] [PubMed] [Google Scholar]
- 35.Buisson A, Chevaux JB, Bommelaer G, Peyrin-Biroulet L. Diagnosis, prevention and treatment of postoperative Crohn's disease recurrence. Dig Liver Dis. 2012;44:453–60. doi: 10.1016/j.dld.2011.12.018. [DOI] [PubMed] [Google Scholar]
- 36.Rutgeerts P, Geboes K, Vantrappen G, Beyls J, Kerremans R, Hiele M. Predictability of the postoperative course of Crohn's disease. Gastroenterology. 1990;99:956–63. doi: 10.1016/0016-5085(90)90613-6. [DOI] [PubMed] [Google Scholar]
- 37.Chongthammakun V, Lopez R, She B. Correlation of Rutgeerts Score and Postoperative Recurrence of Crohn's Disease in Patients With End Ileostomy. Gastroenterology. 2014;146(S1):S-229. doi: 10.1093/gastro/gow043. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


