Abstract
The use of photodynamic therapy (PDT) in the treatment of brain cancer has produced exciting results in clinical trials over the past decade. PDT is based on the concept that a photosensitizer exposed to a specific light wavelength produces the predominant cytotoxic agent, to destroy tumor cells. However, delivering an efficient light source to the brain tumor site is still a challenge. The light source should be delivered by placing external optical fibers into the brain at the time of surgical debulking of the tumor. Consequently, there exists the need for a minimally invasive treatment for brain cancer PDT. In this study, we investigated an attractive non-invasive option on glioma cell line by using Tb3+-doped LaF3 scintillating nanoparticles (LaF3:Tb) in combination with photosensitizer, meso-tetra(4-carboxyphenyl)porphyrin (MTCP), followed by activation with soft X-ray (80 kVp). Scintillating LaF3:Tb nanoparticles, with sizes of approximately 25 nm, were fabricated. The particles have a good dispersibility in aqueous solution and possess high biocompatibility. However, significant cytotoxicity was observed in the glioma cells while the LaF3:Tb nanoparticles with MTCP were exposed under X-ray irradiation. The study has demonstrated a proof of concept as a non-invasive way to treat brain cancer in the future.
Keywords: Non-invasive, Photodynamic therapy, LaF3:Tb, X-ray, Brain cancer
Background
Malignant gliomas are the most common type of primary brain tumors; the survival rate is about 2 years for patients with grade III tumors and 1 year for those with grade IV tumors, and the average life expectancy at 5 years is not higher than 5% in Taiwan [1, 2]. Because malignant gliomas are located in billions of interacted neurons and physiologic barriers, especially the blood-brain barrier (BBB), which protects infiltrating glioma cells from the effect of chemotherapeutic agents, this causes gliomas to be difficult to treat. Despite the advances in conventional approaches, including surgery, radiotherapy, and chemotherapy, the effectiveness of treatment in these patients remains limited. Many of the current treatments in malignant gliomas have inadequate drug delivery and cause damage to healthy brain tissue [3].
Photodynamic therapy (PDT) is based on the concept of proceeding through the activation of photosensitizer by a specific light wavelength (620–690 nm) to produce the predominant cytotoxic agent, such as free radicals and singlet oxygen (1O2). The use of PDT in the treatment of brain tumors has produced exciting results in clinical trials over the past decade [4]. PDT is expected to be the breakthrough for the treatment of malignant glioma because it has selective cytotoxicity to target infiltrating malignant brain tumor cells and induces a cytotoxic reaction only in the light-exposed areas. Nevertheless, the limited penetration range of light causes the assessment of the light distribution and tumoricidal effects of PDT inside the brain to be difficult [5, 6]. To ensure adequate dispersion of light to the area of brain tumors, two strategies that use fiber optic devices could be carried out, the usage of which is determined by the size, stage, and localization of tumor. First, interstitial PDT is a method by stereotactically inserting optical fibers and filling the tumor cavity with a light-diffusing medium, such as lipid solution, to spread the light evenly throughout the tumor cavity. Second, an intraoperative of the balloon irradiator in a resected tumor cavity after an invasive craniotomy could be used [4, 7]. However, all of these treatments require the external optical fibers be placed within the brain tumors. Consequently, there exists the need for a minimally invasive brain cancer PDT.
An attractive non-invasive option is to use scintillating nanoparticles with photosensitizer through X-ray irradiation to enable the light source to reach a higher tissue penetration depth in the range of 8–14 cm [6]. This approach is based on the concept that scintillating nanoparticles, such as Tb3+-doped LaF3 crystal (LaF3:Tb), can locally convert X-ray into light and the emitted luminescences are able to activate the photosensitizers on the mechanism of fluorescence resonance energy transfer (FRET), further resulting in activating photosensitizer to induce 1O2 for cancer therapy [8]. The conversion of X-ray into fluorescence emission by LaF3:Tb is based on the mechanism that Tb3+ ions exhibit the transitions resulting mainly from the excited level, 5D4, down to the lower levels, 7Fj (j = 6–3), and can be accompanied by the photoluminescence properties as Tb3+ doped in low vibrational energy and high resistivity properties of LaF3 host material [9, 10]. LaF3:Tb has demonstrated luminescence at 487, 542, 582, and 620 nm under the excitation of X-ray [10]. Upon X-ray irradiation, photosensitizers are activated by photons emitted from LaF3:Tb nanoparticles while the absorption band of photosensitizers and the emission band of scintillation nanoparticles overlap. It has been reported that approximately 56.7% of energy can be transferred from X-ray to the adjacent photosensitizers via LaF3:Tb nanoparticles [11].
X-ray-excited PDT, based on scintillating nanoparticles, was first introduced by Chen and Zhang [12] in 2006 and recently several studies have demonstrated this effect into proof of concept [8, 13–15]. For future clinical applications, the photosensitizers can be loaded onto nanoparticles, which can lead to a more direct and specific localization of the photosensitizer to the brain tumor sites and increase the efficiency and selectivity in treatment. Another aspect of this approach is the treatments using nanoparticles are regarded as one of the most promising approaches to transport photosensitizers across the barriers of BBB as well as in combination of PDT with radiotherapy for brain cancer treatment [16, 17]. More importantly, X-ray not only can penetrate the tissue much deeper than the laser light source but also can extend the popularity of PDT to resource-limited hospitals because the X-ray system is widely used in the clinic for both diagnosis and therapy.
Here we demonstrate a proof of concept as a non-invasive PDT on glioma cell line (9L) by the treatment of soft X-ray (180 kVp) and photosensitizer, meso-tetra(4-carboxyphenyl)porphyrin (MTCP), employing scintillating nanoparticles. Although scintillating nanoparticles have been studied in PDT [13, 15, 18], to the best of the authors’ knowledge, the non-invasive PDT concept of using scintillating nanoparticles in brain cancer cells has not been described.
Methods
Synthesis of LaF3:Tb Nanoparticles
The aqueous-dispersible LaF3:Tb nanoparticles were synthesized by a modified wet chemical precipitation method according to Liu et al. [10]. Three major components, La(NO3)3·6H2O, TbCl3·6H2O, and NH4F solutions, were purchased from Sigma-Aldrich. Briefly, 4.3 mmol La(NO3)3·6H2O and 1.1 mmol TbCl3·6H2O were dissolved in 150 ml of de-ionized water, followed by 58.4 mmol of NH4F solution with a volume of 46 ml added dropwise to the complex solution. The reaction was stirred for 2 h at room temperature. Finally, the ultimate solution was centrifuged, washed with de-ionized water three times, and stored at 4 °C until use.
Characterization of LaF3:Tb Nanoparticles
The morphology of particles was observed by dropping onto a copper grid using a transmission electron microscopy (TEM; Hitachi H-7100, Japan). Energy-dispersive X-ray spectroscopy (EDX) system attached to TEM was used to analyze the composition of ions in particles. X-ray diffraction (XRD; Geiger Flex, Rigaku) was utilized to identify the crystalline phase composition using Cu Kα radiation (λ = 0.15406 nm) with the potential at 30 kV and the current at 20 mA. The lattice parameters (a-axis and c-axis) were calculated from the major reflection peaks, (111), (300), (113), and (302), with the equation in the hexagonal crystal system: 1/d 2 = 4/3{(h 2 + hk + k 2)/a 2} + (l 2/c 2), where h, k, and l are Miller’s indices and d is the interplanar spacing [19]. The fluorescence emission characteristics of LaF3:Tb were measured using the fluorescence spectrometer (F-7000 FL, Hitachi) with excitation at 260 nm.
Cell Viability
The viability of LaF3:Tb particles on the fibroblast cell line (3T3) was evaluated by cell proliferation reagent (WST-1, Roche). 3T3 cells cultivated in Dulbecco’s modified Eagle’s medium with high glucose (DMEM, Sigma-Aldrich) supplemented with 10% fetal bovine serum (FBS) were seeded in a 96-well petri dish (3000 cells/well) and kept in a humidified environment with 5% CO2 at 37 °C overnight. Then, cells were exposed to different concentrations of LaF3:Tb particles, followed by 4 h of incubation period. Later, the media was replaced with fresh media and further incubated for another 24 or 72 h. After that, cells were rinsed once for WST-1 assay. The cells incubated with 100 μl fresh medium containing 10% WST-1 reagent for 2 h were measured by the absorbance at 450 nm. Positive controls were cells exposed to 1% Triton X-100 solution.
In Vitro Effect of LaF3:Tb
9L glioma cells grown in DMEM media supplemented with 10% FBS and 100 units/ml of penicillin were seeded in 96-well plates (5000 cells/well) and cultured overnight. Then, cells received the treatment of mixed solution of LaF3:Tb (1 mg/ml) with MTCP (0.5 mg/ml) for 4 h (n = 5). Subsequently, they were washed with phosphate-buffered saline (PBS) twice and then exposed to portable X-ray systems (PX-80M, PoYe, Taiwan) for 1 min. The X-ray source was set at 10 mA and 80 kVp with 50 cm of exposed distance from generator to sample. The effect was evaluated after cell incubation for 24 h and analyzed by WST-1 assay (Roche) according to the manufacturer’s protocol. Cells treated with PBS were used as control groups. All values were presented as mean ± standard deviation (SD) in quintet repeat. Statistical analysis was performed using Student’s t test. Values of p < 0.05 were considered as statistically significant.
Results and Discussion
Materials Characterization
The study shows that the LaF3:Tb nanoparticles can potentially be activated by soft X-ray and used to activate PDT as a promising treatment of glioma cells. LaF3:Tb is formed by the self-recrystallization that the aggregative assemblies of La3+ and F− precursors tended to form which is a crystallographic orientation under hydrothermal process. Meanwhile, the hydrothermal can lead the Tb3+ ions to substitute the lattice of La3+ in LaF3 crystallite [8]. TEM images revealed the particles were fabricated uniformly in size with hexagon-like shape. The size of the particles was about 25 nm with a little agglomeration (Fig. 1a). The nanopores (about 3–5 nm) were observed homogeneously distributed on the surface of particles due to the restrictions at the interface of mismatched lattices during the self-recrystallization [8]. Within a single nanoparticle, lattice spacing value was measured to be 0.31 nm, corresponding to the d-spacing of the (111) plane in the hexagonal LaF3 crystal (Fig. 1b). The XRD also showed the similar pattern belonging to a hexagonal structure of LaF3 crystals (JCPDS standard card no. 32-483), and no extra peaks were observed in the spectrum; however, peaks were slightly shifted to larger angles (Fig. 1c), referring that the particles mainly comprised Tb3+-doped LaF3 particles. The calculated lattice parameters of LaF3:Tb (a = b = 7.0866 nm and c = 7.2198 nm) were smaller than those of the LaF3 crystal (a = b = 7.1871 nm and c = 7.3501 nm), which can be attributed to the smaller radius of the Tb3+ ion (92.3 pm) in comparison to the La3+ ion (103.2 pm) [20]. Additionally, the EDX also clearly showed the composition of La, F, and Tb ions in particles, further proving the substitution of Tb3+ ions in LaF3 crystalline (Fig. 1d). Cu was detected in the spectrum because LaF3:Tb particles were dropped onto the TEM support film, the copper grids, under the detection of EDX.
Fig. 1.
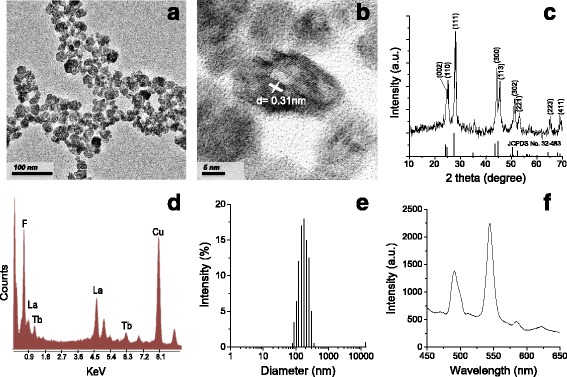
Characterization of LaF3:Tb particles. a TEM images; b crystal lattice planes; c XRD pattern and with standard data quote from JCPDS file no. 32-483; d EDX spectrum; e hydrodynamic size distribution of particles suspended in culture medium supplemented with 10% FBS; and f photoluminescence spectrum of particles obtained in water using an excitation wavelength of 260 nm
In order to be admitted into the biomedical area, it is an important issue to take the fabrication of water-dispersible nanoparticles into consideration. In this study, LaF3:Tb nanoparticles can be well dispersed in aqueous solution (polydispersity index = 0.137). The hydrodynamic size of particles is approximately 157.3 nm (Fig. 1e), which is around eightfold greater than the physical diameter (Fig. 1a). The discrepancy is reasonable because it resulted from the presence of clumping and included hydration layers of water on particles when the particles are in an aqueous solution.
Fluorescence emission spectra of LaF3:Tb can be measured under UV or X-ray excitation, which can excite LaF3:Tb nanoparticles to almost the same emission peaks [14]. Upon excitation with a wavelength of 260 nm, a fluorescence of LaF3:Tb was clearly observed at four typical emissions peaks (480–510, 525–560, 575–590, and 615–630 nm) due to the absorption energy level of Tb3+ ions from 4f to 5d (Fig. 1f). The dominant green band around 540 nm can be caused by the 5D4 to 7Fj (j = 6–3) transitions of Tb3+ [21]. Overall, the results demonstrate that the LaF3:Tb nanoparticle could be used in biological applications and regulate photosensitizer activation by X-ray.
In Vitro Effect of LaF3:Tb Nanoparticles
For in vitro study, the biocompatibility of LaF3:Tb nanoparticles is a concern. Here we assessed the effects of nanoparticles on viability of cells by use of WST-1 assay (Fig. 2). The viability of cultured fibroblast cells (3T3) to LaF3:Tb was determined using various concentrations. The results showed LaF3:Tb nanoparticles have a good biocompatibility and the cytotoxicity effect was not obviously implied as it can be seen on closer inspection within 10 mg/ml.
Fig. 2.

The viability of LaF3:Tb nanoparticles. LaF3:Tb nanoparticles assessed in 3T3 cells were determined using various concentrations by use of the WST-1 assay. Cells exposed to 1% Triton X-100 solution were regarded as positive control. Values are mean ± SD in triplicate
Because of the encouraging results from the viability assay, we further studied the impact of X-ray on LaF3:Tb nanoparticles at a concentration of 1 mg/ml with photosensitizers (LaF3:Tb-MTCP). In consideration of the exact spectrum match of the spectral LaF3:Tb’s emission and the photosensitizer’s absorption to achieve a high FRET efficiency, MTCP was chosen to be combined with LaF3:Tb. MTCP has demonstrated that the absorption spectrum overlaps well with the emission band (543 nm) of LaF3:Tb nanoparticles [11]. The results, examined in rat glioblastoma 9L cell line, showed the cell viability was decreased in the control group (PBS) when cells were exposed to soft X-ray (Fig. 3). Although it is widely accepted that malignant glioma is one of the most radioresistant tumor types, cells can be sensitive to a low radiation dose because their repair mechanisms are not induced [22]. Importantly, when LaF3:Tb-MTCP groups were excited by X-ray, the cell viability significantly reduced from 77 to 28% rather than the decrease in the control groups (p < 0.01). The cell viability of LaF3:Tb-MTCP in a dark place (77%) might be due to a mild toxicity caused by MTCP. However, a significant decrease of viability in LaF3:Tb-MTCP was mainly due to the excited LaF3:Tb nanoparticles because they can transfer the X-ray energy to MTCP and induce 1O2 generation to destruct the tumor, whereas the energy transfer has not been found in X-ray-excited MTCP [11]. Indeed, we may not escape the possibility of cytotoxicity (photoelectric and Compton effects) induced by X-ray on LaF3:Tb nanoparticles; however, this effect generally only happens in high-energy excitation (more than 500 keV) [23].
Fig. 3.
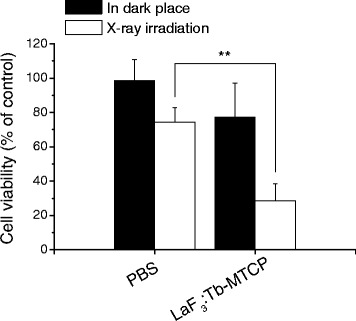
The impact of LaF3:Tb-MTCP nanoparticles with X-ray irradiation. The particles of LaF3:Tb-MTCP were assessed in 9L glioma cells. The X-ray source was set at 10 mA and 80 kVp with 50 cm of exposed distance from generator to sample. The effect was evaluated after cell incubation for 24 h and analyzed by WST-1 assay. Values are mean ± SD for quintet repeat. **p < 0.01 vs. control by Student’s t test
In this study, MTCP was adsorbed onto the LaF3:Tb surface by simply mixing the LaF3:Tb particles with MTCP (data not shown). It has been proved by Liu et al. [11] that MTCP can be spontaneously adsorbed onto the LaF3:Tb surface due to an electrostatic interaction between the positively charged LaF3:Tb, from unsaturated surface Tb3+ atoms, and the deprotonated carboxylate groups of MTCP at neutral pH [14, 24]. Although the treated solution might contain some free MTCP, the efficient energy transfer can only occur from LaF3:Tb nanoparticles to MTCP and induce 1O2 generation if they are situated in close proximity.
Although almost all nanoparticles do not efficiently overcome the BBB to brain tumor sites, some exceptions have been reported in recent years [25–28]. Wu et al. [26] employed SiO2 nanoparticles, which are 15 nm in physical diameter and 156 nm of hydrodynamic size, and the study showed that the particles can majorly accumulate in the olfactory bulb, striatum, and hippocampus through intranasal instillation. Additionally, Hirschberg’s laboratory [29, 30] has investigated the advantage of using monocytes and macrophage as cell-based delivery vehicles to ingest large payloads of nanoparticles such as gold nanoparticles or superparamagnetic iron oxide nanoparticles. Thus, we believe LaF3:Tb nanoparticles in the future with appropriate design or delivering route can efficiently overcome the BBB for non-invasive PDT in brain cancer treatment.
Conclusions
According to our preliminary finding, LaF3:Tb nanoparticles could find biological applications, for they have been obtained in nanoscale (approximately 25 nm in physical size), water-dispersible, and with high biocompatibility. However, it shows cytotoxicity on the 9L glioma cell line only when nanoparticles with photosensitizers are exposed under the X-ray exposure. Thus, we believe scintillating nanoparticles in combination with X-ray could be a potential approach for non-invasive PDT in brain cancer for future clinical applications, even though an ideal scintillating nanoparticle that processes the energy transfer from X-ray to photosensitizers efficiently will still be an important issue for practical applications. We will further investigate the in vivo study in the following research.
Acknowledgements
The authors would like to thank Dr. Jian-Yuan Huang for the support of particles preparation and thank Ms. Abigail L. Magee and Ms. Yu-Wen Fang for grammatical corrections.
Funding
The study was financially supported by the Japan Society for the Promotion of Science (JSPS) and the Ministry of Science and Technology (Taiwan), through projects P16356 and MOST1042811B002065, respectively.
Authors’ Contributions
Both FHL and NH, as the corresponding authors, have made a great contribution to manuscript direction and experimental design. MHC and YJJ carried out all experimental tests as well as drafted and revised the manuscript. SKW has offered a useful model for in vitro analysis. YSC, as a medical professional, has proposed an idea and played a consultant role in the medical application. All authors have read and approved the final manuscript.
Competing Interests
The authors declare that they have no competing interests.
Abbreviations
- BBB
Blood-brain barrier
- DMEM
Dulbecco’s modified Eagle’s medium
- EDX
Energy-dispersive X-ray spectroscopy
- FBS
Fetal bovine serum
- FRET
Fluorescence resonance energy transfer
- LaF3:Tb
Tb3+-doped LaF3 scintillating nanoparticles
- MTCP
Meso-tetra(4-carboxyphenyl)porphyrin
- PBS
Phosphate-buffered saline
- PDT
Photodynamic therapy
- SD
Standard deviation
- TEM
Transmission electron microscopy
- WST-1
Cell proliferation reagent
- XRD
X-ray diffraction
Contributor Information
Nobutaka Hanagata, Phone: +81 29 860 4774, Email: hanagata.nobutaka@nims.go.jp.
Feng-Huei Lin, Phone: +886 2 2732 0443, Email: double@ntu.edu.tw.
References
- 1.Huang YC, Wei KC, Chang CH, Yang JT, Ho JT, Shen CC, Su CF, Cho DY, Ma HI, Lin JW, et al. A retrospective survey of patients with malignant gliomas treated in the neuro-oncological care system under the Universal National Health Insurance program in Taiwan. J Clin Neurosci. 2011;18:784–788. doi: 10.1016/j.jocn.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 2.Laws ER, Parney IF, Huang W, Anderson F, Morris AM, Asher A, Lillehei KO, Bernstein M, Brem H, Sloan A, et al. Survival following surgery and prognostic factors for recently diagnosed malignant glioma: data from the Glioma Outcomes Project. J Neurosurg. 2003;99:467–473. doi: 10.3171/jns.2003.99.3.0467. [DOI] [PubMed] [Google Scholar]
- 3.Tigli Aydin RS, Kaynak G, Gumusderelioglu M. Salinomycin encapsulated nanoparticles as a targeting vehicle for glioblastoma cells. J Biomed Mater Res A. 2016;104:455–464. doi: 10.1002/jbm.a.35591. [DOI] [PubMed] [Google Scholar]
- 4.Muller PJ, Wilson BC. Photodynamic therapy of brain tumors—a work in progress. Lasers Surg Med. 2006;38:384–389. doi: 10.1002/lsm.20338. [DOI] [PubMed] [Google Scholar]
- 5.Robertson CA, Evans DH, Abrahamse H. Photodynamic therapy (PDT): a short review on cellular mechanisms and cancer research applications for PDT. J Photochem Photobiol B. 2009;96:1–8. doi: 10.1016/j.jphotobiol.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 6.Klein S, Dell’Arciprete ML, Wegmann M, Distel LV, Neuhuber W, Gonzalez MC, Kryschi C. Oxidized silicon nanoparticles for radiosensitization of cancer and tissue cells. Biochem Biophys Res Commun. 2013;434:217–222. doi: 10.1016/j.bbrc.2013.03.042. [DOI] [PubMed] [Google Scholar]
- 7.Goodell TT, Muller PJ. Photodynamic therapy: a novel treatment for primary brain malignancy. J Neurosci Nurs. 2001;33:296–300. doi: 10.1097/01376517-200112000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Tang Y, Hu J, Elmenoufy AH, Yang X. Highly efficient FRET system capable of deep photodynamic therapy established on x-ray excited mesoporous LaF3:Tb scintillating nanoparticles. ACS Appl Mater Interfaces. 2015;7:12261–12269. doi: 10.1021/acsami.5b03067. [DOI] [PubMed] [Google Scholar]
- 9.Xia F, Liu S, Wang Y, Mao J, Li X, Wang Y, Chen G. Fast and intense green emission of Tb3+ in borosilicate glass modified by Cu+ Sci Rep. 2015;5:15387. doi: 10.1038/srep15387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu Y, Chen W, Wang S, Joly AG, Westcott S, Woo BK. X-ray luminescence of LaF3:Tb3+ and LaF3:Ce3+, Tb3+ water-soluble nanoparticles. J Appl Physics. 2008;103:06315. [Google Scholar]
- 11.Liu Y, Chen W, Wang S, Joly AG. Investigation of water-soluble x-ray luminescence nanoparticles for photodynamic activation. Appl Phys Lett. 2008;92:043901. doi: 10.1063/1.2835701. [DOI] [Google Scholar]
- 12.Chen W, Zhang J. Using nanoparticles to enable simultaneous radiation and photodynamic therapies for cancer treatment. J Nanosci Nanotechnol. 2006;6:1159–1166. doi: 10.1166/jnn.2006.327. [DOI] [PubMed] [Google Scholar]
- 13.Clement S, Deng W, Camilleri E, Wilson BC, Goldys EM. X-ray induced singlet oxygen generation by nanoparticle-photosensitizer conjugates for photodynamic therapy: determination of singlet oxygen quantum yield. Sci Rep. 2016;6:19954. doi: 10.1038/srep19954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elmenoufy AH, Tang Y, Hu J, Xu H, Yang X. A novel deep photodynamic therapy modality combined with CT imaging established via X-ray stimulated silica-modified lanthanide scintillating nanoparticles. Chem Commun. 2015;51:12247–12250. doi: 10.1039/C5CC04135J. [DOI] [PubMed] [Google Scholar]
- 15.Chen H, Wang GD, Chuang YJ, Zhen Z, Chen X, Biddinger P, Hao Z, Liu F, Shen B, Pan Z, Xie J. Nanoscintillator-mediated X-ray inducible photodynamic therapy for in vivo cancer treatment. Nano Lett. 2015;15:2249–2256. doi: 10.1021/nl504044p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu J, Gao J, Wei Q. Combination of photodynamic therapy with radiotherapy for cancer treatment. J Nanomater. 2016;2016:8507924. [Google Scholar]
- 17.Pourgholi F, Hajivalili M, Farhad JN, Kafil HS, Yousefi M. Nanoparticles: novel vehicles in treatment of glioblastoma. Biomed Pharmacother. 2016;77:98–107. doi: 10.1016/j.biopha.2015.12.014. [DOI] [PubMed] [Google Scholar]
- 18.Bulin AL, Vasil’ev A, Belsky A, Amans D, Ledoux G, Dujardin C. Modelling energy deposition in nanoscintillators to predict the efficiency of the X-ray-induced photodynamic effect. Nanoscale. 2015;7:5744–5751. doi: 10.1039/C4NR07444K. [DOI] [PubMed] [Google Scholar]
- 19.Aizawa K, Ricard H, Ishiwara H. Lattice parameter control of epitaxially grown hexagonal LaF3 films on GaAs(111) substrates by incorporation of orthorhombic YF3. Jpn J Appl Phys. 1992;31:L508–L510. doi: 10.1143/JJAP.31.L508. [DOI] [Google Scholar]
- 20.Gaurkhede SG, Khandpekar MM, Pati SP, Singh AT. Synthesis of hexagonal LaF3: Nd3+, Sm3+ nano crystals and studies of NLO properties. Nanosystems: Phy Chem Math. 2013;4:241–246. [Google Scholar]
- 21.Seed Ahmed SAA, Ntwaeaborwa OM, Kroon RE. The energy transfer mechanism in Ce, Tb co-doped LaF3 nanoparticles. Curr Appl Phys. 2013;13:1264–1268. doi: 10.1016/j.cap.2013.03.021. [DOI] [Google Scholar]
- 22.Beauchesne PD, Bertrand S, Branche R, Linke SP, Revel R, Dore J-F, Pedeux RM. Human malignant glioma cell lines are sensitive to low radiation doses. Int J Cancer. 2003;105:33–40. doi: 10.1002/ijc.11033. [DOI] [PubMed] [Google Scholar]
- 23.Retif P, Pinel S, Toussaint M, Frochot C, Chouikrat R, Bastogne T, Barberi-Heyob M. Nanoparticles for radiation therapy enhancement: the key parameters. Theranostics. 2015;5:1030–1044. doi: 10.7150/thno.11642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bonneau R, Pottier R, Bagno O, Joussot-Dubien J. pH dependence of singlet oxygen production in aqueous solutions using thiazine dyes as photosensitizers. Photochem Photobiol. 1975;21:159–163. doi: 10.1111/j.1751-1097.1975.tb06646.x. [DOI] [PubMed] [Google Scholar]
- 25.Masserini M. Nanoparticles for brain drug delivery. ISRN Biochem. 2013;2013:238428. doi: 10.1155/2013/238428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu J, Wang C, Sun J, Xue Y. Neurotoxicity of silica nanoparticles: brain localization and dopaminergic neurons damage pathways. ACS Nano. 2011;5:4476–4489. doi: 10.1021/nn103530b. [DOI] [PubMed] [Google Scholar]
- 27.Sousa F, Mandal S, Garrovo C, Astolfo A, Bonifacio A, Latawiec D, Menk RH, Arfelli F, Huewel S, Legname G, et al. Functionalized gold nanoparticles: a detailed in vivo multimodal microscopic brain distribution study. Nanoscale. 2010;2:2826–2834. doi: 10.1039/c0nr00345j. [DOI] [PubMed] [Google Scholar]
- 28.Imperatore R, Carotenuto G, Di Grazia MA, Ferrandino I, Palomba L, Mariotti R, Vitale E, De Nicola S, Longo A, Cristino L. Imidazole-stabilized gold nanoparticles induce neuronal apoptosis: an in vitro and in vivo study. J Biomed Mater Res A. 2015;103:1436–1446. doi: 10.1002/jbm.a.35289. [DOI] [PubMed] [Google Scholar]
- 29.Christie C, Madsen SJ, Peng Q, Hirschberg H. Macrophages as nanoparticle delivery vectors for photothermal therapy of brain tumors. Ther Deliv. 2015;6:371–384. doi: 10.4155/tde.14.121. [DOI] [PubMed] [Google Scholar]
- 30.Baek SK, Makkouk AR, Krasieva T, Sun CH, Madsen SJ, Hirschberg H. Photothermal treatment of glioma; an in vitro study of macrophage-mediated delivery of gold nanoshells. J Neurooncol. 2011;104:439–448. doi: 10.1007/s11060-010-0511-3. [DOI] [PMC free article] [PubMed] [Google Scholar]


