Abstract
Background
Malnutrition, which is associated with increased medical complications in older hospitalized patients, can be attenuated by providing nutritional supplements.
Objective
This study evaluates the cost effectiveness of a specialized oral nutritional supplement (ONS) in malnourished older hospitalized patients.
Methods
We conducted an economic evaluation alongside a multicenter, randomized, controlled clinical trial (NOURISH Study). The target population was malnourished older hospitalized patients in the USA. We used 90-day (base case) and lifetime (sensitivity analysis) time horizons. The study compared a nutrient-dense ONS, containing high protein and β-hydroxy-β-methylbutyrate to placebo. Outcomes included health-care costs, measured as the product of resource use and per unit cost; quality-adjusted life-years (QALYs) (90-day time horizon); life-years (LYs) saved (lifetime time horizon); and the incremental cost-effectiveness ratio (ICER). All costs were inflated to 2015 US dollars.
Results
In the base-case analysis, 90-day treatment group costs averaged US$22,506 per person, compared to US$22,133 for the control group. Treatment group patients gained 0.011 more QALYs than control group subjects, reflecting the treatment group’s significantly greater probability of survival through 90 days’ follow-up, as reported by the clinical trial. Hence, the 90-day follow-up period ICER was US$33,818/QALY. Assuming a lifetime time horizon, estimated treatment group life expectancy exceeded control group life expectancy by 0.71 years. Hence, the lifetime ICER was US$524/LY. The follow-up period for the trial was relatively short. Some of the patients were lost to follow-up, thus reducing collection of health-care utilization data during the clinical trial.
Conclusion
Our findings suggest that the investigative ONS cost-effectively extends the lives of malnourished hospitalized patients.
Key Points for Decision Makers
| Malnutrition is associated with increased health-care utilization, costs, and mortality. The results of our study suggest an opportunity to improve the health and survival of malnourished older hospitalized patients at a low marginal cost. |
| During the 90-day study period, the target nutrition therapy improved health at a cost of no more than US$34,000 per quality-adjusted life years. When extending the time horizon to patients’ entire lifetime, the intervention would cost only US$524 per life-year saved. |
| A shift by payers towards value-based purchasing may encourage health-care professionals and providers to further examine interventions that deliver cost-effective results. |
Introduction
Malnutrition is a prevalent but under recognized problem in hospitalized patients. Up to 50 % of patients may be malnourished at hospital admission [1]. Moreover, decreased appetite, decreased food intake, and dietary disruptions caused by medical procedures all tend to cause a patient’s nutrition status to deteriorate during hospitalization [2]. Malnutrition is most prevalent in older adults, a population that commonly has multiple comorbidities and that accounts for more than 30 % of hospital admissions and half of total hospital expenditures in the USA [3, 4].
The clinical implications of malnutrition at hospital admission are substantial, as the condition is associated with increased medical complications and infections [5–7]. Malnutrition also has important economic ramifications, as it is associated with longer length of stay, delayed recovery, and increased risks of readmission and mortality [7, 8]. Patients with malnutrition or with poorer nutritional status also have significantly higher costs [9, 10].
Malnutrition and its effects can be attenuated by identifying, screening and providing a nutritional intervention to this patient population. A systemic review and meta-analysis by Cawood et al. reported that the use of multi-nutrient, high protein oral nutritional supplements (ONS) is associated with improved outcomes in a range of patient populations in a variety of health-care settings [11]. They concluded that high protein ONS (providing ≥20 % total energy from protein) confers clinical, nutritional and functional benefits and that there is little evidence that it suppresses normal food intake. In a subsequent meta-analysis, Stratton et al. found that use of ONS significantly reduced hospital readmissions during a 2- to 12-month period following discharge [12]. The review also found that most observed benefits accrued to older individuals with a variety of conditions following hospitalization.
A recent randomized, controlled study (NOURISH Study) reported that early initiation and sustained use of a nutrient-dense ONS significantly decreased post-hospitalization mortality rates in a population of malnourished older adults hospitalized for congestive heart failure (CHF), acute myocardial infarction (AMI), pneumonia, or chronic obstructive pulmonary disease (COPD) (4.8 vs. 9.7 %; p = 0.018) [13]. A key question is whether this adjunctive therapy is cost effective. The following analysis addresses this question using subject-level data from the clinical trial. We report results for the trial period (extending 90 days’ post-hospital discharge) in terms of cost per quality-adjusted life-year (QALY) saved, a metric that accounts for both longevity and quality of life. We also project cost effectiveness in terms of cost per life-year saved over a lifetime.
Materials and Methods
We estimated the cost effectiveness of the investigative treatment evaluated in the NOURISH trial. NOURISH was a prospective, randomized, double-blind study (clinicaltrials.gov NCT01626742) evaluating the effect of specialized ONS in 622 elderly (≥65 years of age) patients hospitalized with CHF, AMI, pneumonia or COPD in the USA. A total of 313 patients received the investigative ONS and 309 received the placebo product. The mean age of the study population was 78 years. The mean body mass index (BMI) was about 24 kg/m2. Per enrollment criteria, all patients were functionally independent at the study admission; patients from nursing homes or residential facilities were excluded. Only malnourished patients were included in the study, with a Subjective Global Assessment (SGA) class of B (moderate or suspected malnutrition) or C (severe malnutrition). More details about the study population and compliance information have been described elsewhere [13].
The investigative product was a nutrient-dense, ready-to-drink liquid ONS containing a high concentration of protein and beta-hydroxy-beta-methylbutyrate. The placebo product was also a ready-to-drink liquid, containing carbohydrate and vitamin C. Subjects were given designated products twice a day, from the time of enrollment during hospitalization to 90 days after discharge. The study tracked health-care utilization, quality of life and survival over the course of the study period. Cost effectiveness is the incremental cost incurred by subjects in the trial treatment arm (compared to controls) divided by the accrued incremental health benefits, measured in terms of either added survival (life-years) or added QALYs.
We conducted two cost-effectiveness analyses from a US health-care system perspective. The horizon for one analysis matched the follow-up period for the underlying clinical trial and therefore extended through the index hospitalization and concluded at the end of 90 days’ post-discharge. That analysis used QALYs to measure added benefits. As recommended by guidelines for conducting cost-effectiveness analyses, we also developed a lifetime horizon based on the clinical trial results and other sources [14]. This lifetime horizon analysis used life years in place of QALYs because no quality-of-life information was collected beyond the 90-day post-discharge trial follow-up period. The population was the intention-to-treat group from the clinical trial. We included costs and benefits pertinent to the health-care payer perspective. All costs were inflated to 2015 US dollars using the Consumer Price Index [15].
Ninety-Day Post-Discharge Analysis
Costs for this time horizon have four components: (1) the investigative treatment; (2) hospitalization costs prior to discharge; (3) readmission hospitalizations post-discharge; and (4) other health-care consumption post-discharge.
Intervention Costs
We estimated costs for the investigative treatment (component 1) as the product of the trial-reported number of servings consumed per subject and the price per serving. We assumed the unit price for the investigative treatment is US$3.00 per serving. We did not include placebo costs in this analysis.
Hospital Costs Prior to Discharge
We estimated costs for the index hospitalization (component 2) as the product of the trial-reported length of stay for each subject and a per-day average cost for the subject’s primary admission Clinical Classifications Software (CCS) diagnosis, as calculated using cost-per-day values reported by the Healthcare Cost and Utilization Project (HCUPNet) database (in particular, HCUP’s total population cost for the indicated diagnosis divided by the product of the number of discharges for that diagnosis and the HCUP-reported average length of stay per discharge for that diagnosis) [16]. Per the trial inclusion criteria, the primary index hospitalization diagnosis for all subjects was one of the following: CHF, AMI, pneumonia, or COPD. Finally, for NOURISH subjects without a recorded length of stay, we used the HCUPNet estimate for cost-per-stay for the subject’s diagnosis.
Readmission Costs
We estimated costs for hospital readmissions (component 3) in the same way, although for the readmissions, we did not restrict diagnoses to a particular list of conditions. For example, using the HCUPNet database, we were able to calculate an average daily cost of US$1871 for asthma. When the length of stay was two days, total costs of a readmission for asthma were US$3742. Because HCUPNet does not report cost-per-day estimates for all readmission diagnoses, we could not in all cases compute costs as the product of the length of stay and cost-per-day. When cost-per-day from HCUPNet was unavailable (2.5 % of cases), we estimated the cost using cost-per-day or cost-per-stay estimates taken from the literature. For example, if the patient was readmitted with fall within 90-day post-discharge, we estimated total costs using cost-per-day estimate from Roudsari et al. [17]. Observation days were not counted as readmissions.
Other Health-Care Costs
We calculated costs for outpatient care following discharge (component 4). For patients discharged to their home, the trial data reported visits to the hospital emergency room (ER), hospital outpatient setting, primary-care doctor, medical specialists, urgent-care setting, and other medical-care settings. Cost estimates come from the 2012 Medical Panel Expenditure Survey (MEPS) database and the published literature (Table 1) [18, 19]. Rehabilitation and nursing-home costs were calculated separately as follows.
Table 1.
Average cost per patient per unit
| Type of visit or service | Average unit costa (US$) |
|---|---|
| Hospital emergency room | 975 |
| Hospital clinic or outpatient department | 814 |
| Primary care physician | 156 |
| Medical specialist | 223 |
| Other medical care | 194 |
| Urgent care | 176 |
| Medication | 81 |
aUnit cost is cost per visit for all items in this table, except for medication. For medication, the unit cost is the average amount charged for one prescription. Sources: For all entries in this table except for urgent care, cost estimates come from the 2012 Medical Panel Expenditure Survey (MEPS) database [18]. For urgent care, costs come from Mehrotra et al. [19]
Rehabilitation Costs
For subjects discharged from the hospital directly to an inpatient rehabilitation facility, we summed inpatient costs (the product of length of stay and a cost-per-day of US$1361) and outpatient costs (the product of number of outpatient visits and cost-per outpatient visit of US$194 [18–20]. We were not able to identify outpatient rehabilitation costs from the MEPS and therefore, we assumed that this cost was the same as the average cost for other health-care visits. Here, we assumed: (1) length of inpatient stay was the difference between the hospital discharge dates and rehabilitation discharge dates; and (2) the “number of visits” corresponded to outpatient visits made subsequent to discharge from the rehabilitation facility. When missing data ruled out calculation of the inpatient and outpatient rehabilitation costs (16.3 % of patients), we instead included a cost of US$18,109 [20], which corresponds to the average cost of an inpatient stay in a rehabilitation facility. For all subjects not discharged to an inpatient rehabilitation facility, we assumed that the cost incurred amounts to the number of outpatient visits times the per visit cost of US$194.
Long-Term Care (Nursing Home) Costs
For subjects discharged to a long-term care facility for whom we had the needed information, we estimated cost as the product of length of stay and a cost-per-day of US$359 [20]. For subjects discharged to a long-term care facility lost to follow-up and with missing length of stay we assumed a cost-per-stay of US$9691 [20].
Utility Weight
Finally, we estimated quality-adjusted survival through the end of the trial’s follow-up period (90 days’ post discharge). In general, the number of QALYs accrued over an entire year ranges from 0.0 to 1.0, depending on an individual’s preference-weighted quality of life, which reflects factors such as level of discomfort and diminished function. The number of QALYs accrued per year (the health condition’s “utility weight”) is close to 1.0 if a health condition has a small adverse impact on quality of life. On the other hand, a grave health condition (e.g. being in a vegetative state) considered equal to being dead would have a utility weight of 0. We estimated QALYs accrued for each subject as the interpolated area under the utility weight curve. The clinical trial reported two utility weight estimates at each time point (discharge, 30, 60, and 90 days’ post-discharge): the 36-Item Short Form Health Survey (SF-36) estimated utility weight, and the EuroQOL five dimensions with three levels of problems (EQ-5D-3L) utility weight.
Lifetime Horizon Analysis
Since no cost information was available for subjects after the conclusion of the follow-up period, which ended at 90 days’ post-discharge, we assumed that costs incurred after this time were independent of the subject’s treatment arm. As a result, the difference in lifetime costs is the same as the difference in costs through 90 days’ post-discharge and therefore, costs were not discounted. In short, we used the same incremental cost estimates for the lifetime analysis as we used for the 90-day post-discharge analysis.
Because utility information was not available for the period beyond the end of follow-up, we measured benefits as life-years survived post-discharge. We did not discount life-years because of the growing controversy relating to discounting nonmonetary health benefits [21, 22]. Clinical trial data informed estimates of survival through the end of follow-up. To estimate post-discharge survival, we used projections reported by Cho et al. because those projections are population based, and are conditioned on health status, as well as on age and gender [23]. Cho et al. reported specific survival estimates for COPD and CHF, which we used for subjects with those conditions. They also reported survival for subjects with low/medium severity comorbidities (in contrast to either no comorbidities or “high” severity comorbidities). For AMI and for pneumonia, we used survival estimates for the “low/medium” comorbidity category described by Cho et al.
Results
Ninety-Day Post-Discharge Analysis
Costs
The 313 subjects in the treatment arm of the clinical trial consumed a total of 29,481 servings of the investigative nutritional supplement, or an average of 94.19 servings per person (cost: US$283 per person).
Table 2 details hospital length of stay and costs prior to discharge. Treatment group length of stay and per-person costs are lower than corresponding values for the control group for CHF and COPD patients, but higher for AMI and pneumonia patients. Length of stay and costs averaged across all four health conditions are 1–2 % higher for the treatment group than for the control group. The average cost per treatment group subject was US$13,017, compared to US$12,863 on average for the control group subjects.
Table 2.
Hospital length of stay and health-care costs of initial hospitalizations
| Cost per daya (US$) | Control group | Treatment group | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Total days | Total cost (US$) | Subjects | Cost per subject (US$) | Total days | Total cost (US$) | Subjects | Cost per subject (US$) | ||
| CHF | 2232 | 517 | 1154,129 | 78 | 14,797 | 454 | 1,013,490 | 79 | 12,829 |
| AMI | 4318 | 98 | 423,163 | 25 | 16,927 | 138 | 595,882 | 30 | 19,863 |
| Pneumonia | 1953 | 621 | 1,213,000 | 100 | 12,130 | 669 | 1,306,759 | 95 | 13,755 |
| COPD | 1868 | 634 | 1,184,345 | 106 | 11,173 | 620 | 1,158,193 | 109 | 10,626 |
| Total | 1870 | 3,974,638 | 309 | 12,863 | 1881 | 4,074,324 | 313 | 13,107 | |
CHF congestive heart failure, AMI acute myocardial infarction, COPD chronic obstructive pulmonary disease
aCost estimates come from the 2012 Healthcare Cost and Utilization Project (HCUP) database [16]
bDifferences between the control and treatment groups did not achieve statistical significance (p < 0.05)
Readmission costs averaged US$4687 per patient in the treatment group, compared to US$4739 in the control group. Of the 80 readmitted patients in the treatment group (25.6 % of treatment group patients), 15 experienced a second readmission, six experienced a third readmission, and one experienced a fourth readmission. Length of stay over all readmissions for these 80 patients averaged 8.36 days, or 1.87 days per treatment group patient (including those with no readmissions). For the control group, there were 81 readmitted patients (26.2 % of control group patients), with 15 experiencing a second readmission, and two experiencing a third readmission. For this group, the length of stay over all readmissions averaged 7.92 days, or 1.84 days per control group patient (including those with no readmissions).
Table 3 details costs for other care. The cost per subject is similar, although generally slightly higher for the treatment group than for the control group. On the other hand, the control group’s long-term care facility costs exceed corresponding treatment group costs. Because of the long-term care facility costs, total per-subject costs for the control group slightly exceeded corresponding costs for the treatment group (US$4532 vs. US$4519 per subject).
Table 3.
Other health-care costs during the 90-day post-discharge period
| Unit cost (US$) | Control group (N = 309) | Treatment group (N = 313) | |||||
|---|---|---|---|---|---|---|---|
| Cohort total number | Cohort total cost (US$) | Cost per subject (US$) | Cohort total number | Cohort total cost (US$) | Cost per subject (US$) | ||
| Hospital emergency room visits | 975a | 87 | 84,809 | 274 | 78 | 76,035 | 243 |
| Hospital outpatient visits | 814a | 79 | 64,311 | 208 | 87 | 70,823 | 226 |
| Primary care doctor visits | 156a | 408 | 63,839 | 207 | 483 | 75,574 | 241 |
| Medical specialist visits | 223a | 340 | 75,949 | 246 | 323 | 72,152 | 231 |
| Urgent care visits | 176a | 4 | 705 | 2 | 9 | 1585 | 5 |
| Other medical care visits | 194a | 41 | 7942 | 26 | 63 | 12,203 | 39 |
| Prescriptions | 81a | 4191 | 337,711 | 1093 | 4477 | 360,757 | 1153 |
| Rehabilitation | |||||||
| Inpatient day | 1361b | 341 | 464,301 | 1503 | 350 | 476,556 | 1523 |
| Outpatient visits | 194c | 167 | 32,348 | 105 | 308 | 59,659 | 191 |
| Inpatient stay | 18,109b | 4 | 72,437 | 234 | 4 | 72,437 | 231 |
| Long-term care facility | |||||||
| Length of stay (days) | 359b | 195 | 69,993 | 227 | 165 | 59,225 | 189 |
| Stays | 9691b | 13 | 125,989 | 408 | 8 | 7532 | 248 |
| Total | 1,400,332 | 4532 | 1,414,537 | 4519 | |||
aSources: For all entries in this table except for urgent care, cost estimates come from the 2012 Medical Panel Expenditure Survey (MEPS) database [17]. For urgent care, costs come from Mehrotra et al. [19]
bCost estimate comes from Grabowski [20]
cCost estimate comes from the 2012 Medical Panel Expenditure Survey (MEPS) database [18]
Summary of Costs
Per-subject treatment group costs included: (1) US$283 for the investigational supplement; (2) US$13,017 for the index hospitalization; (3) US$4687 for readmissions; and (4) US$4519 for other medical care. The total average cost per treatment group subject amounted to US$22,506. Per subject control group costs included: (1) no cost for the placebo; (2) US$12,863 for the index hospitalization; (3) US$4739 for readmissions; and (4) US$4532 for other medical care. The total average cost per control group subject amounted to US$22,133. The incremental cost of the treatment is therefore estimated to be US$372 per subject.
Health Benefits
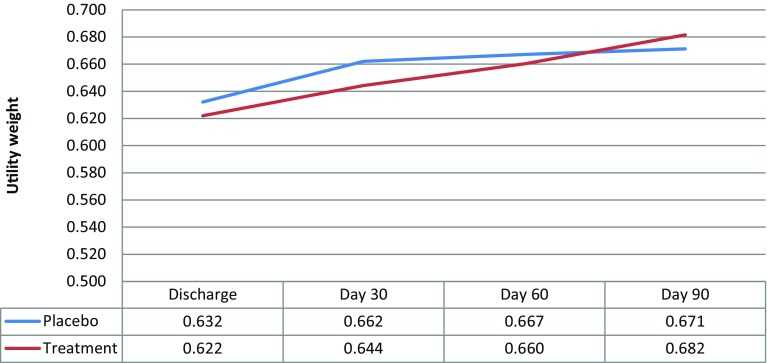
During the first 90 days’ post discharge, treatment group mortality was 4.8 %, corresponding to an average survival of 87.4 days. The control group had a mortality rate of 9.7 %, corresponding to an average survival of 83.9 days, or 3.5 days less than average survival for the treatment group (0.0096 life-years). The mortality rate was significantly lower with the investigative ONS relative to placebo [13]. Based on the SF-36 utility weights, as shown in Fig. 1, the treatment group accrued an average of 0.140 QALYs during the 90 days’ post-discharge, compared to 0.129 QALYs on average for the control group (difference of 0.011 QALYs). Using the EQ-5D instrument, the estimated QALY gain for the treatment group averaged 0.164 QALYs, versus a gain of 0.167 QALYs for the control group (a gain of 0.003 QALYs more for the control group compared to the treatment group).
Fig. 1.
Utility weights based on the SF-36, as recorded at discharge, and 30-, 60-, and 90-days’ post-discharge
Cost Effectiveness
Limiting attention to the treatment cost alone (US$283 per subject) and using the SF-36 results (0.011 QALY gain for the treatment group compared to the control group), the cost effectiveness of the intervention based on the first 90 days’ post-discharge is US$25,727 per QALY (US$29,479 per life-year). Including all incremental costs (US$372 per treated subject) resulted in a cost-effectiveness ratio of US$33,818 per QALY. The EQ-5D results suggest that the treatment group experienced slightly lower quality of life (0.003 QALYs) over the study period.
Lifetime Horizon Analysis
Costs
Because we assume that costs incurred after the first 90 days’ post-discharge do not depend on treatment, we assume that incremental costs for the treatment group are zero after the 90-day cut-off. Hence, the incremental costs for this analysis are the same as they are for the 90-day post-discharge analysis.
Health Benefits
Among treatment group subjects, post-discharge survival averaged an estimated 8.77 years, or 0.71 years more than the average 8.06 years of survival for the control group.
Cost Effectiveness
Limiting attention to the treatment cost alone, the treatment cost effectiveness was US$398 per life-year gained. Including all costs resulted in a cost-effectiveness ratio of US$524 per life-year gained.
Discussion
Our cost-effectiveness analysis of the NOURISH study is consistent with other smaller studies that have shown oral nutritional supplements to be cost effective over the short term (3 months) [24, 25]. Moreover, although previous studies have reported that ONS improves a range of health outcomes, the NOURISH study is the first to show a statistically significant impact on survival in older hospitalized patients [13].
The economic assessment described in this paper suggests that the nutritional therapy for older, malnourished hospitalized patients can be highly cost effective. Even when the analysis conservatively accounts only for health benefits accrued within the first 90 days’ post-discharge, this specialized ONS therapy improved health at a cost of no more than US$34,000 per QALY (based on SF-36 results), a value below (more favorable than) the commonly cited benchmark of US$50,000 to US$100,000 per QALY [26]. Although costs may vary by region and facility, the difference in costs between the two arms should vary much less. That is because many costs in this analysis show up in both arms of the trial. Even more importantly, a major component of cost differences is the nutritional supplement, and because it is a nationally distributed product, its costs should not vary substantially across regions.
In contrast to findings based on the SF-36, quality-of-life measurements based on the EQ-5D suggest accrual of slightly more QALYs per person in the control group, compared to the treatment group, during the first 90 days’ post-hospital discharge. The EQ-5D may not have sufficient granularity to characterize shifts in quality of life in the context of the NOURISH trial. For example, the EQ-5D-derived utility weight was identical across at least two time points for approximately 40 % of all subjects.
Our 90-day post-discharge analysis limits attention to the first 90 days following hospital discharge because that is the extent of the empirical follow-up recorded in the NOURISH trial. Extending the analysis time horizon by making reasonable assumptions about survival gains after that period is recommended by health-economic methodology guidelines [27] and is appropriate when evaluating an intervention that has an impact on patient survival. We estimated that if the cohort were followed for their entire lifetime, the intervention would cost US$524 per life-year saved. Conservatively assuming that over each life-year, a patient may gain 0.6 QALYs (i.e. assuming the individual’s quality of life is severely compromised), and yields a cost-per-QALY ratio of less than US$1000 per QALY gained. This result compares favorably to a number of well-accepted health-care interventions, including treatment of atrial fibrillation in 70-year-olds with warfarin (US$2573 per QALY), dialysis for critically ill 60-year-old men with kidney injury (US$5590 per QALY), influenza vaccinations for the US population aged over 50 (US$8053/QALY), and aspirin for the prevention of CHD in 65-year-old women with moderate risk (US$16,122/QALY) [28–31].
The post-discharge analysis also implies that the survival advantage associated with treatment quickly outweighs the quality-of-life decrements for treatment suggested by the EQ-5D data (see above). In particular, the nearly 2-fold higher mortality rate in the control group (9.7 %) compared to the treatment group (4.8 %), along with an assumed QALY weight of 0.6 implies an expected annual 4.7 % QALY gain for each subject in the treatment group. At that rate, the survival advantage outweighs the 90-day post-discharge quality-of-life decrement (0.003 QALYs) in just over one additional month beyond the 90-day post-discharge time period.
Although the use of randomized controlled trial data provides a robust basis for assessing the value conferred by nutritional supplements, the trial data had limitations for the purpose of assessing economic value. First, survival data were only available for 90 days’ post-discharge. Our current effort addressed the limited follow-up by using published survival estimates that account for baseline age and comorbidity. As noted, even without follow-up, our results suggest this specialized ONS is cost effective. Nonetheless, it would be useful to assess the extent to which the extrapolation we used is consistent with the trial population’s experience. Future research may be needed to investigate effect of ONS on patients in other health-care facilities.
Second, there were limitations in the collection of health-care utilization data in the clinical trial. For example, for some subjects, the number of days spent at rehabilitation facilities was not clear or was missing. That uncertainty reflects lack of clarity regarding the nature of some visits – in particular, whether the visits were inpatient stays or outpatient visits. Because there is no reason to believe that the intervention systematically influenced the introduction of this uncertainty, we do not believe that our conclusions have been substantively affected. Nonetheless, mitigating this source of uncertainty would improve future studies. In addition, we did not collect staff and care-giver time to provide assistance to promote consumption. However, subjects in the treatment group consumed similar number of servings per subject (94 servings) compared to subjects in the control group (96 servings) and therefore, we assumed costs of staff and care-giver time were also similar between two groups.
Third, we used mean estimates for quality-of-life (SF-36) and health-care utilization even though these outcomes did not in many cases differ statistically between the two trial groups. Setting aside the statistical significance criterion prevents this analysis from excluding information about differences between the two trial arms that are important but fail to meet the conventional p < 0.05 criterion that has been criticized because of its arbitrary nature [32]. In any case, restricting ourselves to costs and clinical benefits that differ statistically across the two trial arms (the increased survival duration of 3.5 days in the treatment group and the added US$283 cost of the nutritional supplement) yields a cost-effectiveness ratio of US$29,500 per life-year gained. That result is similar to and even somewhat more favorable than the base-case result we report.
Finally, the generalizability of our results is limited. The study only included patients with four medical conditions and was mainly based on the US health-care system. Findings therefore may not be directly applicable to other settings or countries. A number of factors, such as population characteristics and different payment systems, could influence relevance to other settings. Future research is needed to assess the cost effectiveness of this therapy in other countries.
Although up to 50 % of hospitalized patients are at risk of malnutrition, few patients received nutrition intervention during hospitalization [1, 33–39]. Data from a 11-year retrospective study reported ONS use was less than 2 % [38]. The Canadian Malnutrition Task Force cohort study also reported that only 7 % hospitalized patients received nutrition support during the first week of hospitalization [39]. A recent survey of hospital clinicians found “opportunities to improve education around a nationally standardized approach to nutrition assessment, as well as the need for increasing clinician participation in the nutrition care process [40]”. All current guidelines recommend use of ONS for patients who are at risk for malnutrition [41, 42]. The results of our study suggest an opportunity to improve the health and survival of these patients at a low marginal cost. Few interventions that have been studied achieve health gains less expensively, particularly in this population. A shift by payers, including the Centers for Medicare and Medicaid Services, towards value-based purchasing may encourage health-care professionals and providers to further examine interventions that deliver cost-effective results.
Author contribution
YZ, JTC, and SG contributed to study conception and design, data analysis, drafting and revision of the article, and final approval of the article. ML and JN contributed to study conception and design, drafting and revision of the article, and final approval of the article. PJN contributed to drafting and revision of the article and final approval of the article.
Compliance with ethical standards
The protocol received approval from the appropriate site Institutional Review Board and has been performed in accordance with the ethical standards of the Declaration of Helsinki. All patients provided written informed consent.
Funding source
The study was funded by Abbott Nutrition. Researchers at Tufts Medical Center retained full control over question formulation, study selection, data extraction, data analyses, and interpretation of results.
COI statement
YZ, JTC, and PJN report receiving grant funding from Abbott Nutrition. SG, ML, JN report employment, including stock ownership/options, by Abbott Nutrition.
References
- 1.Somanchi M, Tao X, Mullin GE. The facilitated early enteral and dietary management effectiveness trial in hospitalized patients with malnutrition. JPEN J Parenter Enteral Nutr. 2011;35(2):209–216. doi: 10.1177/0148607110392234. [DOI] [PubMed] [Google Scholar]
- 2.DeFrances CJ, Lucas CA, Buie VC, Golosinskiy A. 2006 National Hospital Discharge Survey. Natl Health Stat Rep. 2008;5:1–20. [PubMed] [Google Scholar]
- 3.Agarwal E, Miller M, Yaxley A, Isenring E. Malnutrition in the elderly: a narrative review. Maturitas. 2013;76:296–302. doi: 10.1016/j.maturitas.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 4.Corish CA, Kennedy NP. Protein-energy undernutrition in hospital in-patients. Br J Nutr. 2000;83(6):575–591. doi: 10.1017/S000711450000074X. [DOI] [PubMed] [Google Scholar]
- 5.Chandra RK. Nutrition and the immune system: an introduction. Am J Clin Nutr. 1997;66(2):460S–463S. doi: 10.1093/ajcn/66.2.460S. [DOI] [PubMed] [Google Scholar]
- 6.Fry DE, Pine M, Jones BL, Meimban RJ. Patient characteristics and the occurrence of never events. Arch Surg. 2010;145(2):148–151. doi: 10.1001/archsurg.2009.277. [DOI] [PubMed] [Google Scholar]
- 7.Lim SL, Ong KC, Chan YH, Loke WC, Ferguson M, Daniels L. Malnutrition and its impact on cost of hospitalization, length of stay, readmission and 3-year mortality. Clin Nutr. 2012;31(3):345–350. doi: 10.1016/j.clnu.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 8.Allaudeen N, Vidyarthi A, Maselli J, Auerbach A. Redefining readmission risk factors for general medicine patients. J Hosp Med. 2011;6(2):54–60. doi: 10.1002/jhm.805. [DOI] [PubMed] [Google Scholar]
- 9.Braunschweig C, Gomez S, Sheean PM. Impact of declines in nutritional status on outcomes in adult patients hospitalized for more than 7 days. J Am Diet Assoc. 2000;100(11):1316–22 (quiz 13 23–4). [DOI] [PubMed]
- 10.Corkins MR, Guenter P, DiMaria-Ghalili RA, Jensen GL, Malone A, Miller S, et al. Malnutrition diagnoses in hospitalized patients: United States, 2010. JPEN J Parenter Enteral Nutr. 2014;38(2):186–195. doi: 10.1177/0148607113512154. [DOI] [PubMed] [Google Scholar]
- 11.Cawood AL, Elia M, Stratton RJ. Systematic review and meta-analysis of the effects of high protein oral nutritional supplements. Ageing Res Rev. 2012;11(2):278–296. doi: 10.1016/j.arr.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 12.Stratton RJ, Hebuterne X, Elia M. A systematic review and meta-analysis of the impact of oral nutritional supplements on hospital readmissions. Ageing Res Rev. 2013;12(4):884–897. doi: 10.1016/j.arr.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 13.Deutz N, Matheson EM, Matarese LE, Luo M, Baggs GE, Nelson JL, et al. Readmission and mortality in malnourished, older, hospitalized adults treated with a specialized oral nutritional supplement: a randomized clinical trial. Clin Nutr. 2016;35(1):18–26. doi: 10.1016/j.clnu.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 14.Ramsey S, Willke R, Briggs A, Brown R, Buxton M, Chawla A, et al. Good research practices for cost-effectiveness analysis alongside clinical trials: the ISPOR RCT-CEA Task Force report. Value Health. 2005;8(5):521–533. doi: 10.1111/j.1524-4733.2005.00045.x. [DOI] [PubMed] [Google Scholar]
- 15.Consumer Price Index. US Bureau of Labor Statistics. 2015. Available: http://www.bls.gov/cpi/. Accessed 9 June 2015.
- 16.HCUPnet. Healthcare Cost and Utilization Project (HCUP). 2012. Rockville: Agency for Healthcare Research and Quality. Available: http://hcupnet.ahrq.gov/. Accessed 9 June 2015.
- 17.Roudsari BS, Ebel BE, Corso PS, Molinari NA, Koepsell TD. The acute medical care costs of fall-related injuries among the U.S. older adults. Injury. 2005;36(11):1316–1322. doi: 10.1016/j.injury.2005.05.024. [DOI] [PubMed] [Google Scholar]
- 18.Medical Expenditure Panel Survey (MEPS). Rockville: Agency for Healthcare Research and Quality; 2012. [cited 2015 June 9]. Available from: http://meps.ahrq.gov/mepsweb/.
- 19.Mehrotra A, Liu H, Adams J, Wang MC, Lave J, Thygeson NM, et al. The costs and quality of care for three common illnesses at retail clinics as compared to other medical settings. Ann Intern Med. 2009;151(5):321–328. doi: 10.7326/0003-4819-151-5-200909010-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grabowski DC. Post-acute and long-term care: a primer on services, expenditures and Payment methods U.S. Department of Health and Human Services 2010. Available: http://aspe.hhs.gov/daltcp/reports/2010/paltc.pdf. Accessed 9 June 2015.
- 21.Hillman AL, Kim MS. Economic decision making in healthcare. A standard approach to discounting health outcomes. Pharmaco. Economics. 1995;7(3):198–205. doi: 10.2165/00019053-199507030-00003. [DOI] [PubMed] [Google Scholar]
- 22.Severens JL, Milne RJ. Discounting health outcomes in economic evaluation: the ongoing debate. Value Health. 2004;7(4):397–401. doi: 10.1111/j.1524-4733.2004.74002.x. [DOI] [PubMed] [Google Scholar]
- 23.Cho H, Klabunde CN, Yabroff KR, Wang Z, Meekins A, Lansdorp-Vogelaar I, et al. Comorbidity-adjusted life expectancy: a new tool to inform recommendations for optimal screening strategies. Ann Intern Med. 2013;159(10):667–676. doi: 10.7326/0003-4819-159-10-201311190-00005. [DOI] [PubMed] [Google Scholar]
- 24.Neelemaat F, Bosmans JE, Thijs A, Seidell JC, van Bokhorst-de van der Schueren MA. Oral nutritional support in malnourished elderly decreases functional limitations with no extra costs. Clinical nutrition. 2012;31(2):183–190. doi: 10.1016/j.clnu.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 25.Norman K, Pirlich M, Smoliner C, Kilbert A, Schulzke JD, Ockenga J, et al. Cost-effectiveness of a 3-month intervention with oral nutritional supplements in disease-related malnutrition: a randomised controlled pilot study. Eur J Clin Nutr. 2011;65(6):735–742. doi: 10.1038/ejcn.2011.31. [DOI] [PubMed] [Google Scholar]
- 26.Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness–the curious resilience of the US$50,000-per-QALY threshold. N Engl J Med. 2014;371(9):796–797. doi: 10.1056/NEJMp1405158. [DOI] [PubMed] [Google Scholar]
- 27.Gold MR. Cost-effectiveness in health and medicine. New York: Oxford University Press; 1996. [Google Scholar]
- 28.O’Brien CL, Gage BF. Costs and effectiveness of ximelagatran for stroke prophylaxis in chronic atrial fibrillation. JAMA. 2005;293(6):699–706. doi: 10.1001/jama.293.6.699. [DOI] [PubMed] [Google Scholar]
- 29.Desai AA, Baras J, Berk BB, Nakajima A, Garber AM, Owens D, et al. Management of acute kidney injury in the intensive care unit: a cost-effectiveness analysis of daily vs alternate-day hemodialysis. Arch Intern Med. 2008;168(16):1761–1767. doi: 10.1001/archinte.168.16.1761. [DOI] [PubMed] [Google Scholar]
- 30.Maciosek MV, Solberg LI, Coffield AB, Edwards NM, Goodman MJ. Influenza vaccination health impact and cost effectiveness among adults aged 50 to 64 and 65 and older. Am J Prev Med. 2006;31(1):72–79. doi: 10.1016/j.amepre.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 31.Pignone M, Earnshaw S, Pletcher MJ, Tice JA. Aspirin for the primary prevention of cardiovascular disease in women: a cost-utility analysis. Arch Intern Med. 2007;167(3):290–295. doi: 10.1001/archinte.167.3.290. [DOI] [PubMed] [Google Scholar]
- 32.Nuzzo R. Scientific method: statistical errors. Nature. 2014;506(7487):150–152. doi: 10.1038/506150a. [DOI] [PubMed] [Google Scholar]
- 33.Pirlich M, Schutz T, Norman K, Gastell S, Lubke HJ, Bischoff SC, et al. The German hospital malnutrition study. Clin Nutr. 2006;25(4):563–572. doi: 10.1016/j.clnu.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 34.Bauer JD, Isenring E, Torma J, Horsley P, Martineau J. Nutritional status of patients who have fallen in an acute care setting. J Hum Nutr Diet. 2007;20(6):558–564. doi: 10.1111/j.1365-277X.2007.00832.x. [DOI] [PubMed] [Google Scholar]
- 35.Naber TH, Schermer T, de Bree A, Nusteling K, Eggink L, Kruimel JW, et al. Prevalence of malnutrition in nonsurgical hospitalized patients and its association with disease complications. Am J Clin Nutr. 1997;66(5):1232–1239. doi: 10.1093/ajcn/66.5.1232. [DOI] [PubMed] [Google Scholar]
- 36.McWhirter JP, Pennington CR. Incidence and recognition of malnutrition in hospital. BMJ. 1994;308(6934):945–948. doi: 10.1136/bmj.308.6934.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Planas M, Audivert S, Perez-Portabella C, Burgos R, Puiggros C, Casanelles JM, et al. Nutritional status among adult patients admitted to an university-affiliated hospital in Spain at the time of genoma. Clin Nutr. 2004;23(5):1016–1024. doi: 10.1016/j.clnu.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 38.Philipson TJ, Snider JT, Lakdawalla DN, Stryckman B, Goldman DP. Impact of oral nutritional supplementation on hospital outcomes. Am J Manag Care. 2013;19(2):121–128. [PubMed] [Google Scholar]
- 39.Allard JP, Keller H, Jeejeebhoy KN, Laporte M, Duerksen DR, Gramlich L, et al. Malnutrition at hospital admission-contributors and effect on length of stay: a prospective cohort study from the Canadian malnutrition task force. JPEN J Parenter Enteral Nutr. 2015;40(4):487–497. doi: 10.1177/0148607114567902. [DOI] [PubMed] [Google Scholar]
- 40.Patel V, Romano M, Corkins MR, DiMaria-Ghalili RA, Earthman C, Malone A, et al. Nutrition screening and assessment in hospitalized patients: a survey of current practice in the United States. Nutr Clin Pract. 2014;29(4):483–490. doi: 10.1177/0884533614535446. [DOI] [PubMed] [Google Scholar]
- 41.Volkert D, Berner YN, Berry E, Cederholm T, Coti Bertrand P, Milne A, et al. ESPEN guidelines on enteral nutrition: geriatrics. Clin Nutr. 2006;25(2):330–360. doi: 10.1016/j.clnu.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 42.Mueller C, Compher C, Ellen DM, American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.) Board of Directors A.S.P.E.N. clinical guidelines: Nutrition screening, assessment, and intervention in adults. JPEN J Parenter Enteral Nutr. 2011;35(1):16–24. doi: 10.1177/0148607110389335. [DOI] [PubMed] [Google Scholar]