Abstract
Background
The purpose of this study is to determine the outcome and performance of bovine pericardial valves in the pulmonary position.
Methods
This is a retrospective review of all patients with congenital heart disease who had pulmonary valve replacement using a bovine pericardial valve from 2002 to 2009 at a single institution.
Results
There were 73 consecutive patients, with a median age of 17.3 years (range, 2.1 to 64.4). Their diagnosis was tetralogy of Fallot (n = 47), pulmonary stenosis (n = 11), or other (n = 15). Sixty-nine patients had 91 previous surgical procedures. The mean time from last surgery was 19.9 ± 11.6 years. Forty-three patients had concomitant surgical procedures. There were no perioperative deaths. Clinical follow-up was available in 68 patients (93%). There were no late deaths, and all patients were in New York Heart Association functional class I during a median follow-up period of 2.6 years (range, 0.2 to 8.0). One patient had endocarditis necessitating valve removal 2 years after surgery. Freedom from pulmonary valve reoperation was 100%, 97.7%, and 97.7% at 1, 3, and 5 years, respectively (95% confidence interval: 93.2% to 100%). Mean pulmonary valve gradient at follow-up was 19 ± 14 mm Hg. Degree of pulmonary insufficiency was less than moderate in 62 patients, moderate in 4, and more than moderate in 2. Freedom from moderate-severe or severe pulmonary insufficiency was 97.7%, 89.1%, and 89.1% at 1, 3, and 5 years, respectively (5-year 95% confidence interval: 77.0% to 100%).
Conclusions
Pulmonary valve replacement using a bovine pericardial valve can be accomplished with low perioperative morbidity and favorable midterm outcomes. Further follow-up is necessary to evaluate the long-term performance of bovine pericardial valves in the pulmonary position.
Surgical management of cyanotic congenital heart disease (CHD) such as tetralogy of Fallot and pulmonary stenosis includes relief of right ventricular (RV) outflow tract obstruction using techniques that may result in significant pulmonary insufficiency (PI). Although PI is initially well tolerated, it can eventually lead to progressive RV dilation and RV failure, manifested by exercise intolerance, arrhythmias, and sudden death [1, 2]. Pulmonary valve replacement has been used to prevent and reverse the deterioration of RV function in patients who have significant PI late after CHD surgery [3–8]. Although the early results of this strategy are promising, less is known about the mid- and long-term outcomes of pulmonary valve replacement on patient’s symptoms, RV size, and function [3, 7, 9].
Moreover, there is still controversy regarding the optimal valve in the pulmonary position. Bioprosthetic valves perform well hemodynamically, but are prone to structural degeneration that results in multiple reoperations. Mechanical valves lead to a persistent need for anticoagulation therapy, and despite some positive reports in the literature, have generally been associated with pulmonary thromboembolic complications [10, 11]. In our institution we have utilized bovine pericardial valves for pulmonary valve replacement because of the superior long-term outcomes of these valves in the aortic and mitral positions and because of the promising early results with their use in the pulmonary position [12, 13, 14].
The purpose of this study was to review our experience and to better characterize the outcome and performance of bovine pericardial valves in the pulmonary position.
Material and Methods
Study Design
Between April 2002 and December 2009, all patients who had pulmonary valve replacement with a bovine pericardial valve were identified using the divisional pediatric cardiothoracic surgery database at the University of California, San Francisco. The study was approved and monitored by the Institutional Review Board, and owing to its retrospective nature, the need for patient consent was waived.
Medical records were reviewed, and the following data were retrieved and analyzed: basic demographic data; clinical presentation and medical and surgical histories; intraoperative data including associated surgical procedures; postoperative outcomes including immediate and late complications; follow-up data collected from the last clinic visit; and echocardiographic data obtained before surgery, at hospital discharge, and during last follow-up.
Statistical Analysis
Data are expressed as frequencies, as mean plus or minus one standard deviation, and as median with ranges. Estimate of survival and freedom from pulmonary valve reoperation were computed using the Kaplan-Meier method. For subgroup analyses, Student’s t test was used to compare the parametric values, and the Mann-Whitney U test was used to compare the nonparametric values. Stat Mate III software (ATMS Co, Tokyo, Japan) was used for statistical analysis. A probability value less than 0.05 was considered significant for all analyses.
Results
Patient Characteristics
There were 73 consecutive patients who had pulmonary valve replacement using a bovine pericardial valve (44 male and 29 female). The median age at the time of valve insertion was 17.3 years (range, 2.1 to 64.3), and the median weight was 54.3 kg (range, 12.2 to 154 kg). The CHD diagnosis included tetralogy of Fallot (n = 47), pulmonary stenosis (n = 11), pulmonary atresia (n = 7; 4 with intact septum and 3 with a ventricular septal defect) and other diagnoses (n = 8; Table 1).
Table 1.
Congenital Heart Disease Diagnosis
| Diagnosis | Number of Patients |
|---|---|
| Tetralogy of Fallot | 47 |
| With left pulmonary artery originating from ascending aorta | 1 |
| Congenital pulmonary stenosis | 11 |
| Pulmonary atresia with intact ventricular septum | 4 |
| Pulmonary atresia with ventricular septal defect | 3 |
| Double-outlet right ventricle | 2 |
| Transposition of the great arteries | 1 |
| Absent pulmonary valve syndrome | 1 |
| Coarctation of the aorta complex, with ventricular septal defect | 1 |
| Pulmonary valve dysplasia | 1 |
| Truncus arteriosus | 1 |
| Ventricular septal defect with pulmonary stenosis | 1 |
Sixty-nine patients (95%) had 91 previous surgical procedures: tetralogy of Fallot repair (n = 47), RV outflow tract reconstruction (n = 12), systemic to pulmonary artery shunt (n = 10), pulmonary valvotomy (n = 10), pulmonary atresia/ventricular septal defect repair (n = 3), and other procedures (n = 9). In these 69 patients, the RV outflow tract was reconstructed with the use of a transannular patch in 50, whereas 14 patients had either pulmonary valvotomy or valve-sparing tetralogy of Fallot repair. Three patients had RV to pulmonary artery homograft placement. One patient had a pulmonary valvotomy, followed by pulmonary valve insertion that failed 7 years later. One patient had subarterial ventricular septal defect repair that resulted in PI. Fourteen patients (19%) had 15 previous catheter-based interventions, including 9 branch or peripheral pulmonary artery stent placements, 2 catheter ablations, 2 pulmonary valve perforations using radiofrequency ablation, and 2 other procedures (Table 2).
Table 2.
Previous Surgical and Catheter-Based Interventionsa
| Previous Interventions | Number of Patients |
|---|---|
| Surgery | |
| Tetralogy of Fallot repair | 47 |
| Right ventricular outflow tract reconstruction | 12 |
| Systemic to pulmonary artery shunt | 10 |
| Pulmonary valvotomy | 10 |
| Pulmonary atresia/ventricular septal defect repair | 3 |
| Double-outlet right ventricle repair | 2 |
| Ventricular septal defect (subarterial type) closure | 1 |
| Arterial switch operation | 1 |
| Supravalvular pulmonary stenosis repair | 1 |
| Pulmonary valve replacement | 1 |
| Truncus arteriosus repair | 1 |
| Brock procedure | 1 |
| Coarctation of the aorta repair (thoracotomy) | 1 |
| Catheter-based intervention | |
| Branch or peripheral pulmonary artery stent placement | 9 |
| Catheter ablation for right ventricular arrhythmia focus | 2 |
| Radiofrequency pulmonary valve perforation | 2 |
| Balloon pulmonary valve dilatation | 1 |
| Automated implantable cardioverter-defibrillator placement | 1 |
Some patients had more than one intervention.
The mean time interval from the last surgical intervention on the RV outflow tract was 19.9 ± 11.6 years and was shorter for patients who had an RV to pulmonary artery conduit or previous pulmonary valve insertion when compared with the patients who had a transannular patch reconstruction (10.5 ± 5.5 versus 19.6 ± 10.5 years, p = 0.03) or pulmonary valvotomy/valve-sparing tetralogy of Fallot repair (10.5 ± 5.5 versus 24.5 ± 14.7 years, p = 0.01). Fifty-three patients (73%) were symptomatic; exercise intolerance was present in 28 patients (38%), shortness of breath in 25 patients (34%), palpitations in 13 patients (18%), chest pain in 9 patients (12%), syncope in 5 patients (7%), and arrhythmia in 4 patients (5%; ventricular fibrillation in 2 patients, frequent premature ventricular contraction in 1 patient, and atrial fibrillation in 1 patient).
Twenty-four patients were in New York Heart Association (NYHA) functional class I, 41 patients were in NYHA class II, 6 patients were in NYHA class III, and 2 patients were in NYHA class IV. The mean QRS duration was 143 ± 31 ms, and was longer in the patients who were in worse NYHA functional class (136 ± 34 ms in classes I and II versus 158 ± 23 in classes III and IV, p = 0.05). On preoperative echocardiogram, all patients had evidence of severe PI and RV dilation or dysfunction. The mean RV outflow tract pressure gradient was 17 ± 17 mm Hg. Three patients had moderate and 2 patients had severe tricuspid regurgitation. Preoperative cardiac magnetic resonance imaging (MRI) was available in 41 patients (56%). The mean pulmonary regurgitant fraction was 47.1% ± 13.2%, the mean RV end-diastolic volume was 180 ± 56 mL/m2, and the RV ejection fraction was 45.0% ± 9.1%. Fourteen patients had qualitatively moderate or severe RV dysfunction on echocardiogram or MRI evidence of RV ejection fraction less than 40%.
Operative Intervention
The indications for pulmonary valve replacement were as follows: symptoms of shortness of breath, exercise intolerance, chest pain or syncope; evidence of progressive RV dilation or worsening RV function by echocardiogram or cardiac MRI; development or progression of tricuspid insufficiency; and new development of arrhythmia or evidence of progressive lengthening of the QRS complex.
The operation was performed through a median sternotomy. Cardiopulmonary bypass was established using aortic and bicaval cannulation and mild hypothermia. All operations were performed under continuous monitoring with transesophageal echocardiography to rule out the presence of air in the left heart. If there were no intracardiac shunts, the operation was performed on a beating heart. If an intracardiac shunt was found or if there was need for concomitant intracardiac procedures, the heart was arrested using intermittent doses of cold blood cardioplegia. The transannular patch or previous RV to pulmonary artery conduit was opened longitudinally, and all calcifications were debrided as appropriate. Residual pulmonary valve tissue and obstructing muscle bundles were removed.
The posterior one third of the bovine pericardial valve was sutured to the pulmonary annulus with interrupted mattress polypropylene sutures. A bovine pericardial patch was sutured to the main pulmonary artery and the RV outflow tract opening to create a roof. The anterior part of the valve’s sewing ring was sutured to the patch with continuous polypropylene suture to avoid perivalvular leaks. All patients were treated with aspirin for 3 to 6 months.
The median size of bioprosthesis implanted was 25 mm (range, 19 to 29 mm). Forty-three patients (59%) had a total of 68 concomitant surgical procedures: atrial septal defect closure (n = 24), RV outflow tract muscle resection or plication or both (n = 15), pulmonary arterioplasty (n = 11), cryoablation of the infundibular septum (n = 6), right atrial Maze procedure (n = 3), and other procedures (n = 9). Of the patients who had cryoablation of the infundibular septum, 3 had evidence of an arrhythmogenic focus by intraoperative mapping; and in 3 who had history of ventricular tachycardia, the cryoablation was performed empirically. In 4 patients who had previously placed pulmonary artery stents and no gradient across them, the stents were left in place. Of 5 patients who had in-stent stenosis, 1 patient had intraoperative balloon dilation and 4 patients had removal of stent material with concomitant anterior patch pulmonary arterioplasty.
The mean cardiopulmonary bypass time was 74.8 ± 30.2 minutes. The mean aortic crossclamp time for the 35 patients who required cardioplegic arrest was 50.2 ± 27.8 minutes.
Early Results
There were no early deaths. One patient required a brief period of extracorporeal life support due to refractory supraventricular tachyarrhythmia leading to hemodynamic instability, and recovered without adverse effects. Six additional patients had postoperative arrhythmias. One patient with a history of previously implanted cardioverter-defibrillator had ventricular fibrillation and was successfully cardioverted without adverse effects. One patient who had chronic preoperative atrial fibrillation had paroxysmal atrial tachycardia and multiple premature ventricular contractions and underwent planned cardioverter-defibrillator placement. One patient had a short run of nonsustained ventricular tachycardia, 1 patient had ventricular bigeminy, 1 patient had atrial fibrillation, and 1 patient had atrial flutter. All were treated medically without further complications. The mean preoperative QRS duration of patients who had postoperative arrhythmia was longer than the QRS duration of the patients who did not, but this difference was not statistically significant (157 ± 20 ms versus 140 ± 32 ms, p = 0.08).
Two patients had postpericardiotomy syndrome that required surgical drainage. One patient required reoperation for bleeding, and 1 patient had hemidiaphragm paralysis but did not require further treatment. The mean mechanical ventilation time was 8.4 ± 6.7 hours, the mean intensive care unit stay was 3.2 ± 1.4 days, and the mean hospital stay was 6.9 ± 3.9 days.
Late Results
Follow-up was available for 68 patients (93%) with a median follow-up of 2.6 years (range, 0.2 to 8.0). There were no late deaths during the follow-up period. One patient had valve explantation and RV outflow tract reconstruction with a homograft due to infectious endocarditis with vegetations 2 years after pulmonary valve replacement.
Three patients had planned placement of implantable cardioverter-defibrillator at the same admission with pulmonary valve replacement, and 2 other patients required placement of implantable cardioverter-defibrillator at a later date. The preoperative mean QRS duration and mean end-diastolic RV volume were not significantly different between the patients who required implantable cardioverter defibrillator placement and those who did not (163 ± 24 ms versus 140 ± 32 ms, p = 0.13; and 211 ± 46 mL/m2 versus 179 ± 57 mL/m2, respectively; p = 0.34). Two patients had peripheral pulmonary artery stent placement, and 1 patient underwent catheter ablation for atrial fibrillation. Of the 4 patients who presented with preoperative arrhythmia, 3 had resolution of their arrhythmia during the follow-up period. One patient (with preoperative implantable cardioverter-defibrillator) had persistent episodes of ventricular tachyarrhythmia successfully treated by the defibrillator.
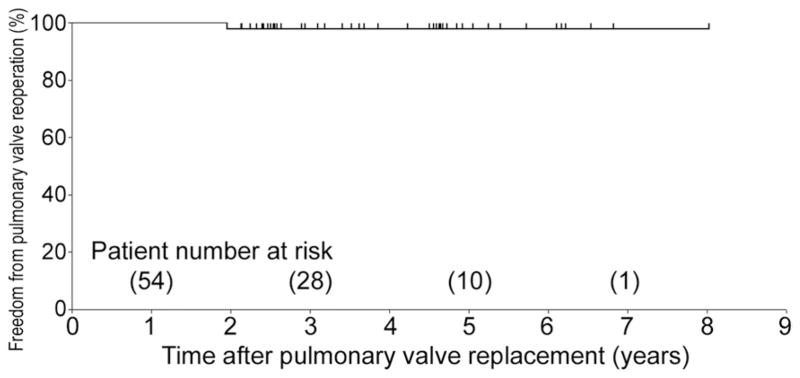
All patients were in the NYHA class I on follow-up. There was significant improvement in NYHA class for the whole cohort after pulmonary valve replacement (p < 0.001). Of the 14 patients who had more than moderate RV dysfunction preoperatively, late echocardiographic information was available for 12 patients. Of these, 8 patients had normal RV function, 2 patients had mildly reduced RV function, and 2 had moderately depressed RV function. Of the 5 patients who had more than moderate tricuspid valve insufficiency, the degree of insufficiency improved after pulmonary valve insertion in 2 patients and remained unchanged in 3 patients. There was a stabilization of the mean QRS duration after pulmonary valve replacement (142.5 ± 31.5 ms versus 139.1 ± 28.0 ms, p = 0.54). The actuarial survival estimate was 100% at 1, 3, and 5 years. The freedom from pulmonary valve reoperation was 100%, 97.7%, and 97.7% at 1, 3, and 5 years, respectively (5-year 95% confidence interval: 93.2% to 100%; Fig 1).
Fig 1.
Freedom from valve-related reoperation after pulmonary valve replacement using a bovine pericardial valve.
Bovine Pericardial Valve Function
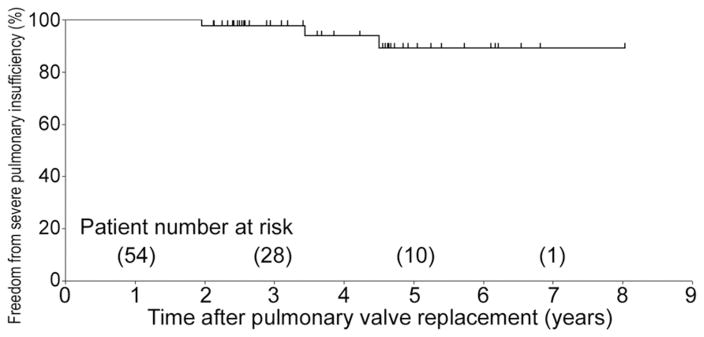
Follow-up echocardiogram showed that the mean pulmonary valve gradient was 19 ± 14 mm Hg. The degree of PI was none in 14 patients (22%), trace in 14 patients (22%), mild in 25 patients (38%), mild to moderate in 6 patients (9%), moderate in 4 patients (6%), and moderate to severe in 2 patients (3%). Freedom from moderate-severe or severe PI was 100%, 97.7%, and 89.1% at 1, 3, and 5 years, respectively (5-year 95% confidence interval: 77.0% to 100%; Fig 2).
Fig 2.
Freedom from severe pulmonary insufficiency after pulmonary valve replacement using a bovine pericardial valve.
Comment
The detrimental effect of chronic PI on RV function is increasingly being recognized. Many studies have demonstrated an association between the severity of PI and RV dilation, RV dysfunction, tricuspid insufficiency, development of heart failure symptoms, increased incidence of atrial and ventricular arrhythmias, prolongation of the QRS, and sudden death [1, 2, 15]. During the last decade, pulmonary valve replacement has widely been adopted as the management strategy of choice for CHD patients with chronic PI, and resulted in improvement of symptoms, a decrease in RV size, stabilization of QRS, and a decrease in the incidence of atrial and ventricular arrhythmias [3, 6–9]. The improvement of patient’s symptoms and the stabilization of QRS were also observed in our cohort.
The optimal timing of pulmonary valve insertion is still controversial [15, 16]. One has to balance the need for valve insertion to prevent progression of the detrimental effects of PI against the limited life expectancy of all biological valves that leads to multiple reoperations. The development of symptoms is a clear indication for intervention in our practice and for others [3, 11, 15, 17]. However, defining the most appropriate timing of valve replacement in the asymptomatic patient is still a work in progress. Therrien and associates [15] studied 55 adults who underwent pulmonary valve insertion late after tetralogy of Fallot repair using radionuclide angiogram and found that, although there was significant symptomatic improvement in all patients, only patients who had preserved preoperative RV ejection fraction had postoperative improvement of the RV function. In addition, it was shown that prolongation of QRS duration more than 180 ms was predictive of ventricular tachycardia and sudden death, and that pulmonary valve replacement led to stabilization of the QRS duration and to a decrease in the incidence of arrhythmias [2, 15]. For the asymptomatic patient, one should monitor the RV size/function and the QRS duration and intervene when there is evidence of progressive RV dilation and gradual increase in the QRS duration, and certainly before deterioration of the RV function or prolongation of QRS to more than 180 ms.
Cardiac MRI has in recent years been established as the diagnostic study of choice to help determine the appropriate timing for valve insertion in patients with chronic PI [7, 8, 16]. On echocardiogram, the unusual shape of the RV and the unpredictable way it dilates, which can be quite asymmetric in case of a large ventriculotomy and a big outflow patch, make volumetric analysis difficult. The geometric assumptions that are used to calculate ejection fraction and to estimate the RV size are quite inaccurate in these cases. In contrast, MRI allows for complete and direct visualization as well as reproducible assessment of RV function and size. Therrien and associates [16] reported that, although there was a marked decrease in the RV size in patients with corrected preoperative RV end-diastolic volume of more than 170 mL/m2 and end-systolic volume of more than 85 mL/m2, none of these patients had normalization of the RV size. The patients who had preoperative MRIs in our institution had similar end-diastolic volumes. In our institution, cardiac MRI became a standard part of the preoperative evaluation of patients with chronic PI in the last 3 years, explaining the low percentage of cardiac MRIs in our cohort. We expect that as MRI use expands, it will result in referral of more asymptomatic patients with end-diastolic volumes significantly less than 170 mL/m2.
The optimal choice of pulmonary valve is still controversial. Homografts are available in small sizes and can be used in children. Although homografts have initially good hemodynamic performance, they calcify over time and become insufficient [10]. Mechanical valves require anticoagulation therapy, and despite some positive reports, have been associated with pulmonary thromboembolic complications [11]. Stented xenograft valves are the most commonly employed bioprosthetic valves in the pulmonary position. Kanter and associates [4] reported their experience with stented porcine valves in the pulmonary position and found that the actuarial freedom from reoperation was 100% at 8 years. Fiore and associates [10] found, however, that the use of a porcine valve was associated with a high RV to pulmonary artery gradient during a follow-up period of 20 months.
Allen and colleagues [14] reported excellent early outcomes using bovine pericardial valves in the pulmonary position alone or as part of a RV to pulmonary artery conduit created using a Gore-Tex patch (W. L. Gore & Associates, Flagstaff, AZ) with 100% freedom from reoperation at 5 years. Fiore and colleagues [10] also showed promising early outcomes using the bovine pericardial valve, with 92% of freedom from reoperation at 5 years. Late valve dysfunction was lower in the bovine pericardial valve when compared with porcine valves and homografts (5.5% versus 19% versus 54%, respectively; p < 0.05) [10].
Another potential advantage of the bovine pericardial valve is that it may deteriorate by combined stenosis and regurgitation, rather than by regurgitation alone. In this patient population, the stenosis may be trophic to the RV, especially if it is mild or moderate and does not progress rapidly. Conversely, insufficiency alone may reproduce the RV dilation that the patient suffered from before the pulmonary valve replacement. The performance, rate, and mode of structural degeneration of the bovine pericardial valves in the pulmonary position need to be key areas of interest as we continue to follow this cohort of patients.
In our cohort, pulmonary valve replacement using a bovine pericardial valve was accomplished with low morbidity and no perioperative mortality. The early and midterm clinical outcomes are favorable with stabilization of the QRS duration and possible improvement in the RV function. The freedom from reoperation of 97.7% at 5 years is comparable to that reported in the literature. Moreover, the hemodynamic performance of this valve was excellent at midterm: the mean gradient was 18.9 mm Hg, and 89% of the patients had freedom from more than moderate PI. Five patients required additional interventions for arrhythmia or peripheral pulmonary stenosis. All patients are doing well and have no cardiac-related symptoms.
The main limitation of this study is that it represents a retrospective review of a single center’s experience with a limited follow-up period. The study extends across a period during which the indications for and preoperative workup of pulmonary valve replacement have changed, and some of the results (for example, the percentage of patients with preoperative MRI) reflect this change.
In summary, pulmonary valve replacement using bovine pericardial valve can be accomplished with low perioperative mortality and morbidity, and favorable midterm clinical outcomes and excellent midterm valve function. Longer follow-up is necessary to further evaluate the performance of the bovine pericardial valves in the pulmonary position.
References
- 1.Frigiola A, Redington AN, Cullen S, Vogel M. Pulmonary regurgitation is an important determinant of right ventricular contractile dysfunction in patients with surgically repaired tetralogy of Fallot. Circulation. 2004;110(Suppl 1):II153–7. doi: 10.1161/01.CIR.0000138397.60956.c2. [DOI] [PubMed] [Google Scholar]
- 2.Gatzoulis MA, Balaji S, Webber SA, et al. Risk factors for arrhythmia and sudden cardiac death late after repair of tetralogy of Fallot: a multicentre study. Lancet. 2000;356:975–81. doi: 10.1016/S0140-6736(00)02714-8. [DOI] [PubMed] [Google Scholar]
- 3.Discigil B, Dearani JA, Puga FJ, et al. Late pulmonary valve replacement after repair of tetralogy of Fallot. J Thorac Cardiovasc Surg. 2001;121:344–51. doi: 10.1067/mtc.2001.111209. [DOI] [PubMed] [Google Scholar]
- 4.Kanter KR, Budde JM, Parks WJ, et al. One hundred pulmonary valve replacements in children after relief of right ventricular outflow tract obstruction. Ann Thorac Surg. 2002;73:1801–6. doi: 10.1016/s0003-4975(02)03568-3. [DOI] [PubMed] [Google Scholar]
- 5.Kogon BE, Patel M, Pernetz M, McConnell M, Book W. Late pulmonary valve replacement in congenital heart disease patients without original congenital pulmonary valve pathology. Pediatr Cardiol. 2010;31:74–9. doi: 10.1007/s00246-009-9574-3. [DOI] [PubMed] [Google Scholar]
- 6.Therrien J, Siu SC, Harris L, et al. Impact of pulmonary valve replacement on arrhythmia propensity late after repair of tetralogy of Fallot. Circulation. 2001;103:2489–94. doi: 10.1161/01.cir.103.20.2489. [DOI] [PubMed] [Google Scholar]
- 7.van Straten A, Vliegen HW, Hazekamp MG, et al. Right ventricular function after pulmonary valve replacement in patients with tetralogy of Fallot. Radiology. 2004;233:824–9. doi: 10.1148/radiol.2333030804. [DOI] [PubMed] [Google Scholar]
- 8.Vliegen HW, van Straten A, de Roos A, et al. Magnetic resonance imaging to assess the hemodynamic effects of pulmonary valve replacement in adults late after repair of tetralogy of Fallot. Circulation. 2002;106:1703–7. doi: 10.1161/01.cir.0000030995.59403.f8. [DOI] [PubMed] [Google Scholar]
- 9.Adamson L, Vohra HA, Haw MP. Does pulmonary valve replacement post repair of tetralogy of Fallot improve right ventricular function? Interact Cardiovasc Thorac Surg. 2009;9:520–7. doi: 10.1510/icvts.2009.211011. [DOI] [PubMed] [Google Scholar]
- 10.Fiore AC, Rodefeld M, Turrentine M, et al. Pulmonary valve replacement: a comparison of three biological valves. Ann Thorac Surg. 2008;85:1712–8. doi: 10.1016/j.athoracsur.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 11.Waterbolk TW, Hoendermis ES, den Hamer IJ, Ebels T. Pulmonary valve replacement with a mechanical prosthesis. Promising results of 28 procedures in patients with congenital heart disease. Eur J Cardiothorac Surg. 2006;30:28–32. doi: 10.1016/j.ejcts.2006.02.069. [DOI] [PubMed] [Google Scholar]
- 12.Pellerin M, Mihaileanu S, Couëtil JP, et al. Carpentier-Edwards pericardial bioprosthesis in aortic position: long-term follow-up 1980 to 1994. Ann Thorac Surg. 1995;60(Suppl):292–5. doi: 10.1016/0003-4975(95)00225-a. [DOI] [PubMed] [Google Scholar]
- 13.Eric Jamieson WR, Marchand MA, Pelletier CL, et al. Structural valve deterioration in mitral replacement surgery: comparison of Carpentier-Edwards supra-annular porcine and Perimount pericardial bioprostheses. J Thorac Cardiovasc Surg. 1999;118:297–304. doi: 10.1016/S0022-5223(99)70220-5. [DOI] [PubMed] [Google Scholar]
- 14.Allen BS, El-Zein C, Cuneo B, Cava JP, Barth MJ, Ilbawi MN. Pericardial tissue valves and Gore-Tex conduits as an alternative for right ventricular outflow tract replacement in children. Ann Thorac Surg. 2002;74:771–7. doi: 10.1016/s0003-4975(02)03767-0. [DOI] [PubMed] [Google Scholar]
- 15.Therrien J, Siu SC, McLaughlin PR, Liu PP, Williams WG, Webb GD. Pulmonary valve replacement in adults late after repair of tetralogy of fallot: are we operating too late? J Am Coll Cardiol. 2000;36:1670–5. doi: 10.1016/s0735-1097(00)00930-x. [DOI] [PubMed] [Google Scholar]
- 16.Therrien J, Provost Y, Merchant N, Williams W, Colman J, Webb G. Optimal timing for pulmonary valve replacement in adults after tetralogy of Fallot repair. Am J Cardiol. 2005;95:779–82. doi: 10.1016/j.amjcard.2004.11.037. [DOI] [PubMed] [Google Scholar]
- 17.Morales DL, Braud BE, DiBardino DJ, et al. Perimount bovine pericardial valve to restore pulmonary valve competence late after right ventricular outflow tract repair. Congenital Heart Dis. 2007;2:115–20. doi: 10.1111/j.1747-0803.2007.00083.x. [DOI] [PubMed] [Google Scholar]