Abstract
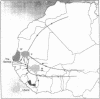
The HLA genes are the most polymorphic coding loci known in humans. DRB-DQA-DQB gene polymorphism was investigated by Taq I restriction fragment length polymorphism analysis in more than 700 West Africans and found to be almost twice as extensive in West Africans as in North European Caucasians. This finding indicates that Africans comprise the oldest and genetically most diverse human population and supports the hypothesis of the occurrence of a population bottleneck in the emergence of the White race. As in Caucasians, less than one-third of possible cis-encoded DQA-DQB combinations were encountered, indicating constraints on the pairing of DQ alpha and beta polypeptides. Heterozygote advantage (i.e., positive selection) was found for DRB, DQA, and DQB alleles as well as for DQA-DQB combinations. However, in West Africans as well as in North Europeans the observed frequencies of DRB-DQA-DQB homozygotes were close to neutrality expectations. Although the hypothesis that HLA polymorphism is maintained by parasite-driven overdominant selection is attractive, there is little evidence to support that view. We propose instead that one of the forces maintaining a low frequency of HLA homozygotes might be a decreased likelihood of potentially autoreactive T-cell clones escaping thymic selection in HLA heterozygotes. This would be consistent with the central role of HLA molecules as self/non-self discriminators.
Full text
PDF
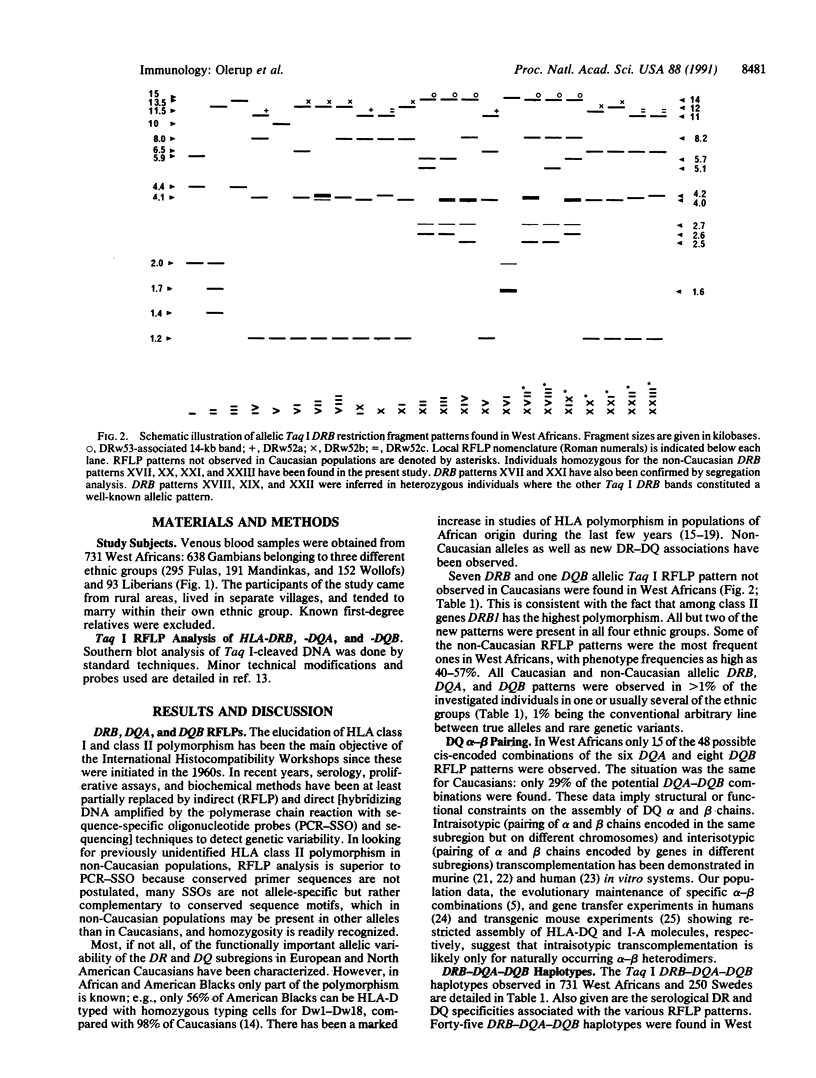
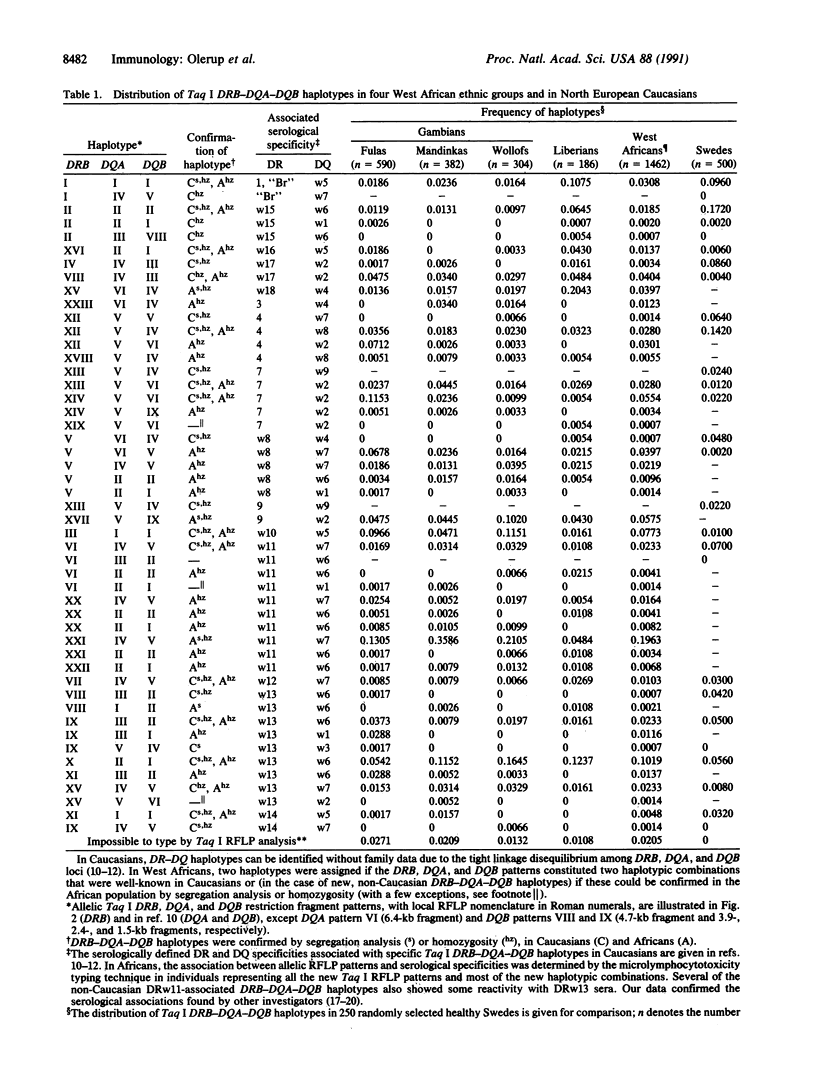

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bidwell J. L., Bidwell E. A., Savage D. A., Middleton D., Klouda P. T., Bradley B. A. A DNA-RFLP typing system that positively identifies serologically well-defined and ill-defined HLA-DR and DQ alleles, including DRw10. Transplantation. 1988 Mar;45(3):640–646. doi: 10.1097/00007890-198803000-00027. [DOI] [PubMed] [Google Scholar]
- Blackman M., Kappler J., Marrack P. The role of the T cell receptor in positive and negative selection of developing T cells. Science. 1990 Jun 15;248(4961):1335–1341. doi: 10.1126/science.1972592. [DOI] [PubMed] [Google Scholar]
- Bodmer W. F. Evolutionary significance of the HL-A system. Nature. 1972 May 19;237(5351):139–passim. doi: 10.1038/237139a0. [DOI] [PubMed] [Google Scholar]
- Cann R. L., Stoneking M., Wilson A. C. Mitochondrial DNA and human evolution. Nature. 1987 Jan 1;325(6099):31–36. doi: 10.1038/325031a0. [DOI] [PubMed] [Google Scholar]
- Carlsson B., Wallin J., Böhme J., Möller E. HLA-DR-DQ haplotypes defined by restriction fragment analysis. Correlation to serology. Hum Immunol. 1987 Oct;20(2):95–113. doi: 10.1016/0198-8859(87)90025-5. [DOI] [PubMed] [Google Scholar]
- Cavalli-Sforza L. L., Piazza A., Menozzi P., Mountain J. Reconstruction of human evolution: bringing together genetic, archaeological, and linguistic data. Proc Natl Acad Sci U S A. 1988 Aug;85(16):6002–6006. doi: 10.1073/pnas.85.16.6002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke B., Kirby D. R. Maintenance of histocompatibility polymorphisms. Nature. 1966 Aug 27;211(5052):999–1000. doi: 10.1038/211999a0. [DOI] [PubMed] [Google Scholar]
- Doherty P. C., Zinkernagel R. M. A biological role for the major histocompatibility antigens. Lancet. 1975 Jun 28;1(7922):1406–1409. doi: 10.1016/s0140-6736(75)92610-0. [DOI] [PubMed] [Google Scholar]
- Doherty P. C., Zinkernagel R. M. Enhanced immunological surveillance in mice heterozygous at the H-2 gene complex. Nature. 1975 Jul 3;256(5512):50–52. doi: 10.1038/256050a0. [DOI] [PubMed] [Google Scholar]
- Figueroa F., Günther E., Klein J. MHC polymorphism pre-dating speciation. Nature. 1988 Sep 15;335(6187):265–267. doi: 10.1038/335265a0. [DOI] [PubMed] [Google Scholar]
- Germain R. N., Bentley D. M., Quill H. Influence of allelic polymorphism on the assembly and surface expression of class II MHC (Ia) molecules. Cell. 1985 Nov;43(1):233–242. doi: 10.1016/0092-8674(85)90028-5. [DOI] [PubMed] [Google Scholar]
- Giles R. C., DeMars R., Chang C. C., Capra J. D. Allelic polymorphism and transassociation of molecules encoded by the HLA-DQ subregion. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1776–1780. doi: 10.1073/pnas.82.6.1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson K., Wiman K., Emmoth E., Larhammar D., Böhme J., Hyldig-Nielsen J. J., Ronne H., Peterson P. A., Rask L. Mutations and selection in the generation of class II histocompatibility antigen polymorphism. EMBO J. 1984 Jul;3(7):1655–1661. doi: 10.1002/j.1460-2075.1984.tb02026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyllensten U. B., Lashkari D., Erlich H. A. Allelic diversification at the class II DQB locus of the mammalian major histocompatibility complex. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1835–1839. doi: 10.1073/pnas.87.5.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes A. L., Nei M. Nucleotide substitution at major histocompatibility complex class II loci: evidence for overdominant selection. Proc Natl Acad Sci U S A. 1989 Feb;86(3):958–962. doi: 10.1073/pnas.86.3.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes A. L., Nei M. Pattern of nucleotide substitution at major histocompatibility complex class I loci reveals overdominant selection. Nature. 1988 Sep 8;335(6186):167–170. doi: 10.1038/335167a0. [DOI] [PubMed] [Google Scholar]
- Jenkins D., Fletcher J., Mijovic C., Bradwell A. R., Barnett A. H. Analysis of MHC class II DNA polymorphisms in Negroid subjects. Mol Immunol. 1990 Mar;27(3):297–302. doi: 10.1016/0161-5890(90)90143-n. [DOI] [PubMed] [Google Scholar]
- Johnson A. H., Rosen-Bronson S., Hurley C. K. Heterogeneity of the HLA-D region in American blacks. Transplant Proc. 1989 Dec;21(6):3872–3873. [PubMed] [Google Scholar]
- Kwok W. W., Thurtle P., Nepom G. T. A genetically controlled pairing anomaly between HLA-DQ alpha and HLA-DQ beta chains. J Immunol. 1989 Dec 1;143(11):3598–3601. [PubMed] [Google Scholar]
- Lawlor D. A., Ward F. E., Ennis P. D., Jackson A. P., Parham P. HLA-A and B polymorphisms predate the divergence of humans and chimpanzees. Nature. 1988 Sep 15;335(6187):268–271. doi: 10.1038/335268a0. [DOI] [PubMed] [Google Scholar]
- Le Meur M., Waltzinger C., Gerlinger P., Benoist C., Mathis D. Restricted assembly of MHC class II molecules in transgenic mice. J Immunol. 1989 Jan 1;142(1):323–327. [PubMed] [Google Scholar]
- Lee K. W., Hurley C. K., Hartzman R., Johnson A. H. The complexity of DRw6 and DR5 haplotypes in American blacks demonstrated by serology, cellular typing, and restriction fragment length polymorphism analysis. Hum Immunol. 1990 Nov;29(3):202–219. doi: 10.1016/0198-8859(90)90115-6. [DOI] [PubMed] [Google Scholar]
- Martell R. W., Oudshoorn M., Arendse B., du Toit E. D. Polymorphism of DRw52 and its association with DRw11 and DRw12 in South African blacks (Negroes) and individuals of mixed ancestry (Cape coloreds). Hum Immunol. 1990 May;28(1):32–38. doi: 10.1016/0198-8859(90)90100-4. [DOI] [PubMed] [Google Scholar]
- Martell R. W., Oudshoorn M., May R. M., du Toit E. D. Restriction fragment length polymorphism of HLA-DRw53 detected in South African blacks and individuals of mixed ancestry. Hum Immunol. 1989 Dec;26(4):237–244. doi: 10.1016/0198-8859(89)90002-5. [DOI] [PubMed] [Google Scholar]
- McConnell T. J., Talbot W. S., McIndoe R. A., Wakeland E. K. The origin of MHC class II gene polymorphism within the genus Mus. Nature. 1988 Apr 14;332(6165):651–654. doi: 10.1038/332651a0. [DOI] [PubMed] [Google Scholar]
- Olerup O., Hillert J., Fredrikson S., Olsson T., Kam-Hansen S., Möller E., Carlsson B., Wallin J. Primarily chronic progressive and relapsing/remitting multiple sclerosis: two immunogenetically distinct disease entities. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7113–7117. doi: 10.1073/pnas.86.18.7113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oudshoorn M., du Toit E. D., Taljaard D. G., MacGregor K. J. A study of the HLA-Dw determinants and their relationship to DR and DQ antigens in three South African population groups: South African Caucasoids, South African Negroes, and Cape coloureds. Hum Immunol. 1986 Nov;17(3):273–287. doi: 10.1016/0198-8859(86)90279-x. [DOI] [PubMed] [Google Scholar]
- Riley E. M., Olerup O., Troye-Blomberg M. The immune recognition of malaria antigens. Parasitol Today. 1991 Jan;7(1):5–11. doi: 10.1016/0169-4758(91)90076-z. [DOI] [PubMed] [Google Scholar]
- Sant A. J., Braunstein N. S., Germain R. N. Predominant role of amino-terminal sequences in dictating efficiency of class II major histocompatibility complex alpha beta dimer expression. Proc Natl Acad Sci U S A. 1987 Nov;84(22):8065–8069. doi: 10.1073/pnas.84.22.8065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringer C. B., Andrews P. Genetic and fossil evidence for the origin of modern humans. Science. 1988 Mar 11;239(4845):1263–1268. doi: 10.1126/science.3125610. [DOI] [PubMed] [Google Scholar]
- Takahata N., Nei M. Allelic genealogy under overdominant and frequency-dependent selection and polymorphism of major histocompatibility complex loci. Genetics. 1990 Apr;124(4):967–978. doi: 10.1093/genetics/124.4.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wake C. T., Long E. O., Mach B. Allelic polymorphism and complexity of the genes for HLA-DR beta-chains--direct analysis by DNA-DNA hybridization. Nature. 1982 Nov 25;300(5890):372–374. doi: 10.1038/300372a0. [DOI] [PubMed] [Google Scholar]
- Yamazaki K., Boyse E. A., Miké V., Thaler H. T., Mathieson B. J., Abbott J., Boyse J., Zayas Z. A., Thomas L. Control of mating preferences in mice by genes in the major histocompatibility complex. J Exp Med. 1976 Nov 2;144(5):1324–1335. doi: 10.1084/jem.144.5.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuhki N., O'Brien S. J. DNA variation of the mammalian major histocompatibility complex reflects genomic diversity and population history. Proc Natl Acad Sci U S A. 1990 Jan;87(2):836–840. doi: 10.1073/pnas.87.2.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- du Toit E. D., MacGregor K. J., Taljaard D. G., Oudshoorn M. HLA-A, B, C, DR and DQ polymorphisms in three South African population groups: South African Negroes, Cape Coloureds and South African Caucasoids. Tissue Antigens. 1988 Mar;31(3):109–125. doi: 10.1111/j.1399-0039.1988.tb02072.x. [DOI] [PubMed] [Google Scholar]