Abstract
Background
Diabetes mellitus (types 1 and 2) leads to secondary complications such as neuropathy, which reduce a patient’s quality of life. Apelin and its receptor, APJ, have been shown to have antinociceptive effects and to decrease blood glucose levels.
Objectives
The present experimental study was conducted in Iran and investigated the role of apelin, which is used to manage type 1 diabetes mellitus, during exercise training.
Materials and Methods
Male Wistar rats (n = 36) were assigned by simple random allocation to six groups (n = 6): non-diabetic (ND), diabetic (D), sedentary non-diabetic (SND), sedentary diabetic (SD), exercise non-diabetic (END), and exercise diabetic (ED). Diabetes was induced by a single subcutaneous injection of streptozotocin (50 mg/kg). Exercise training consisted of treadmill running 60 minutes/day × 5 days/week for 10 weeks. The tail-flick test was used to assess the thermal pain threshold, and an apelin enzyme immunoassay kit was utilized to assess plasma apelin levels.
Results
Plasma apelin level was higher (0.3 vs. 0.1, P < 0.0001) and the tail-flick latency was lower (2.2 vs. 3.8, P < 0.0001) in the D group than in the ND group. After the training program, plasma apelin levels decreased in the exercise groups, and the tail-flick latency increased in the ED group. No correlation was found between apelin blood concentrations and tail-flick latency following the training program in the ED group.
Conclusions
These findings suggest that apelin does not play any significant role in regulating the pain threshold in type 1 diabetes mellitus during exercise training.
Keywords: Apelin Protein, Rat, Pain Threshold, Exercise, Type 1 Diabetes Mellitus
1. Background
Diabetes mellitus has been predicted to potentially affect more than 350 million people worldwide by the year 2030 (1). Both type 1 and type 2 diabetes mellitus are associated with the development of macro- and micro vascular complications such as painful neuropathy. This indicates that hyperglycemia is the primary etiological factor. Thus, the management of hyperglycemia appears to be one of the major therapeutic goals. For instance, the prevention of hyperglycemia and the maintenance of normoglycemia are thought to be of primary importance in reducing the incidence of diabetes-related complications (2).
Many studies have shown that apelin can act as an antinociceptive factor that participates in the potentiation of opioid signaling (3, 4), and its injection can exert antinociceptive effects on the acetic acid-induced mouse visceral pain model (5). In addition, research has indicated that apelin plays an eminent role in energy metabolism. Interestingly, recent studies have shown that apelin lowers plasma glucose via increased glucose utilization in fat and muscle tissues (6), particularly in a type 1 diabetic rat model (7). Further, apelin and its receptor, APJ, are recently identified multifunctional peptides that have important physiological effects on several homeostatic systems, including blood glucose regulation (6).
On the other hand, exercise training has been accepted and is generally recognized as a useful non-pharmacological alternative for the management of type 1 diabetes mellitus. Regular physical activity may maintain chronic glycemic control by stimulating muscle glucose uptake (8), and, thus, it is beneficial in decreasing diabetic complications and positively influencing long-term glycemic control in type 1 diabetes (9). Additionally, it has been reported that exercise significantly improved selected measures of peripheral nerve function and may positively influence the pathological factors associated with neuropathy by promoting microvascular dilatation, reducing oxidative stress, and increasing neurotrophic factors (10). The positive effects of exercise training are associated with beneficial changes in various systems such as antioxidant systems, blood pressure, adipogenesis, and inflammation. Various studies have indicated that the positive effects of exercise on pulmonary hypertension (11, 12) and systemic hypertension (13, 14) could be mediated by regulating the apelin/APJ system. We hypothesize that the effects of aerobic exercise training on pain perception in type 1 diabetes mellitus may be attributed to the regulation of apelin. Furthermore, regular exercise training may have some influence on the plasma apelin level. Sheibani et al. reported that, after aerobic exercise, the plasma levels of apelin decreased (15). In contrast, Kadoglou et al. found that following exercise intervention, there was a significant incremental increase in serum apelin concentrations (16). These findings suggest that more studies are required in this area.
2. Objectives
Considering that, until now, no study has been conducted regarding the effects of aerobic exercise training on plasma apelin levels and its relationship to pain threshold in type 1 diabetes, the present study aimed to investigate the effects of aerobic exercise training on plasma apelin levels and pain threshold in type 1 diabetic rats.
3. Materials and Methods
The present experimental study was conducted in the sports physiology lab of the physical training and sports science faculty at the Bu-Ali Sina university, Hamadan, Iran from May to September 2013.
3.1. Experimental Animals
A total of 36 male Wistar rats (300 ± 30 g) were used in the present study. They were obtained from the Razi Institute, Tehran, Iran. All protocols were strictly adhered to in accordance with the ethical standards of the national research council’s 1996 guide for the care and use of laboratory animals (17) which was approved by the research ethics committee of the Hamadan University of Medical Sciences (Code: WR.RES.1391.68). The rats were randomly classified into six groups (n = 6 in each group): nondiabetic (ND), diabetic (D), sedentary nondiabetic (SND), sedentary diabetic (SD), exercise nondiabetic (END) and exercise diabetic (ED). The rats were housed in plastic cages (3 rats/cage) under a 12-hour light-dark cycle at 22°C and 60% air humidity in a large, air-conditioned room with controlled temperature, lighting, and humidity with free access to water and rodent pellet chow. The sample size in the present study was calculated using the resource equation method.
3.2. Plasma Analysis
Rats were anesthetized by means of an intraperitoneal (ip) injection of ketamine (75 mg/kg) and xylazine (5 mg/kg). Rats’ blood samples (non-fasting) were obtained from the tail vein (1 - 1.5 mL/rat), 3 days after streptozotocin (STZ) treatment for the ND and D groups and 1 day after the last exercise session for the SND, END, SD and ED groups. Blood was collected in ethylenediaminetetraacetic acid (EDTA)-containing tubes and centrifuged at 5,000 rpm for 15 minutes at 4°C. The plasma was separated and frozen at -26°C until analysis. Endogenous plasma apelin was quantified using an Apelin-36 enzyme immunoassay kit (Phoenix Pharmaceuticals, Burlingame, CA, USA) according to the manufacturer’s instructions. This kit detects apelin-36 as well as smaller, biologically active isoforms (i.e., Apelin-36, 13, and 12). Each sample was analyzed in duplicate. Absorbance was recorded at 450 nm using an enzyme-linked immunosorbent assay (ELISA) plate reader (Elisys UNO, HUMAN Gesellschaft fur biochemica und diagnostica mbH, Wiesbaden, Germany), and a standard logarithmic curve was plotted and used to calculate apelin concentration in the plasma samples.
3.3. STZ-Induced Diabetes
After a 1-week acclimatization period, diabetes was induced by a single subcutaneous injection of streptozotocin (STZ) (50 mg/kg), which was dissolved in a citrate buffer solution (0.1 mol/L citric acid, 0.1 mol/L sodium citrate, pH 4.5). A single dose of citrate buffer was injected into the normoglycemic groups. Forty-eight hours after STZ treatment, blood glucose levels were measured to confirm that a hyperglycemic state had been reached. Blood was obtained from a small nick in the tail and measured using a glucometer (Accu-Chek Active, Ireland). Hyperglycemic rats showed blood glucose levels ≥ 300 mg/dL. Plasma glucose levels in the normoglycemic animals remained normal during the study.
3.4. Maximal Exercise Test
Maximum aerobic capacity was computed by the maximal exercise test. First, all rats were submitted to an adaptation period on a rodent treadmill at a speed of 0.3 km/hour for 15 minutes over three consecutive days. Subsequently, they ran individually at an initial speed of 0.3 km/hour with an increase of 0.3 km/hour every 3 minutes.
3.5. Exercise Training Protocol
One week after diabetes induction, animals were submitted to exercise training. Aerobic exercise training, which was designed based on the above description, was performed at low-moderate intensity (~ 50% maximal running speed of the maximal exercise test) for 1 hour per day (8 am to 9 am), 5 days a week for 10 weeks, with a gradual increase in speed from 0.6 to 1.2 km/hour. Initially, the rats ran for 60 minutes every day at 0.6 km/hour for 3 weeks, then 60 minutes at 0.7, 0.8, 0.9, 1.0, and 1.1 km/hour for the fourth, fifth, sixth, seventh, and eighth weeks, respectively. Finally, they ran for 60 minutes at 1.2 km/hour for the last two weeks of the study (ninth and tenth weeks).
3.6. Tail-flick Test
The nociceptive response was evaluated by recording the latency to tail withdrawal in reaction to noxious skin heating by a tail-flick apparatus (Borj Sanat, Tehran, Iran). The thermal intensity of the tail-flick apparatus was set on degree 35, corresponding to a temperature of 50°C. Furthermore, the cut-off time for maximum latencies was set at 10 seconds to avoid tail tissue damage. The location of tail-flick thermal stimulus was 8 cm from the tip of the tail. Baseline latencies were determined for all groups one day after exercise training.
3.7. Statistical Analysis
First, normal distribution of the data was tested and confirmed using the Kolmogorov-Smirnov (K–S) test. Using SPSS version 19 to analyze the collected data, a repeated measures analysis of variance (ANOVA) was run. Additionally, a Pearson product-moment correlation was conducted to determine the association between plasma apelin levels and tail-flick latency. Data are presented as means ± SD, and the significance level was set at P < 0.05. All stages of this study were performed by one researcher, and all measurements and data collection were performed by the same researcher.
4. Results
4.1. Acute Effect of Diabetes on Plasma Apelin and Pain Threshold
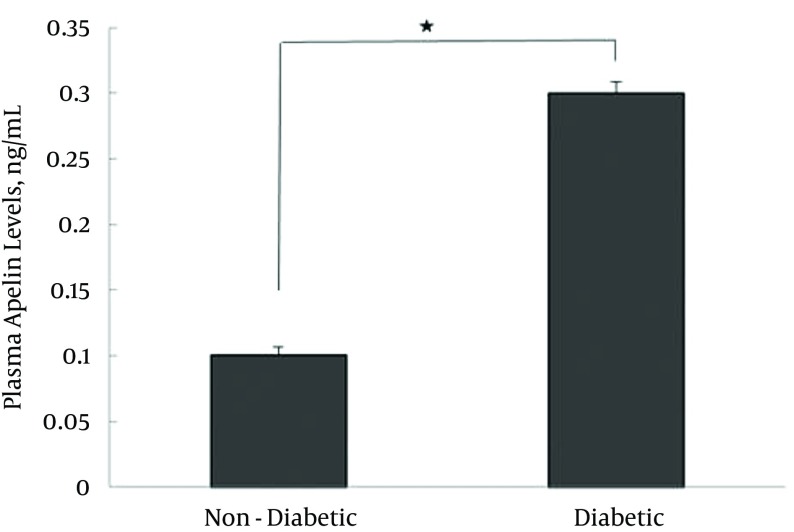
The mean plasma apelin level of the D group (0.3 ± 0.01 ng/mL) was significantly higher than that of the ND group (0.1 ± 0.007 ng/mL, P < 0.0001), the results of which are presented in Table 1 and Figure 1.
Table 1. Plasma Apelin Levels and Tail-Flick Latency in Non-Diabetic and Diabetic Groups (3 Days After STZ Treatment)a.
| Non-Diabetic | Diabetic | T | Df | P Value | |
|---|---|---|---|---|---|
| Apelin levels, ng/mL | 0.1 ± 0.01 | 0.3 ± 0.02 | -16.90 | 10 | 0.000 |
| Tail-Flick latency, S | 3.8 ± 0.45 | 2.2 ± 0.23 | 7.155 | 10 | 0.000 |
aValues are expressed as mean ± SD
Figure 1. Plasma Apelin Levels (3 Days After STZ Treatment) in Diabetic and Non-Diabetic Animals.
Diabetic vs. non-diabetic, N = 6, t = -16.9,* P < 0.0001. Values are expressed as mean ± SD.
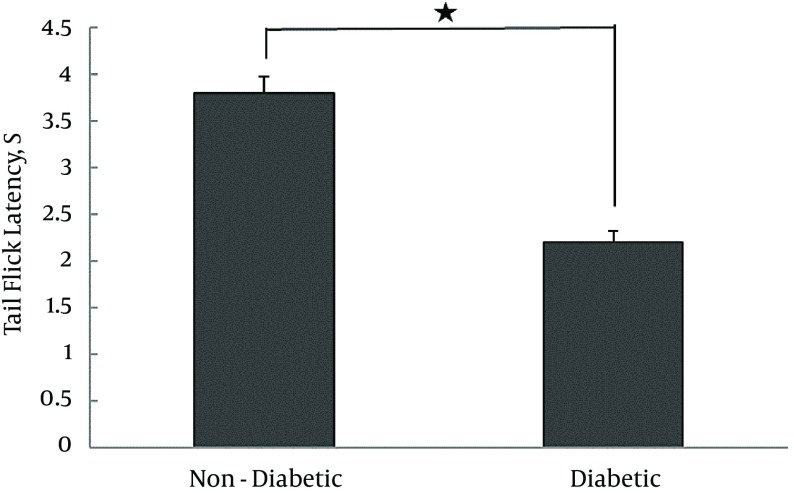
In contrast, the tail-flick latency of diabetic animals (2.2 ± 0.12 seconds) was significantly lower compared to that of their non-diabetic counterparts (3.8 ± 0.18 seconds, P < 0.0001), the results of which are summarized in Table 1 and Figure 2.
Figure 2. Tail-Flick Latency (3 Days After STZ Treatment) in Diabetic and Non-Diabetic Animals.
Diabetic vs. non-diabetic, N = 6, t = 7.155, *P < 0.0001). Values are expressed as mean ± SD.
4.2. Effect of Aerobic Exercise Program on Plasma Apelin Levels
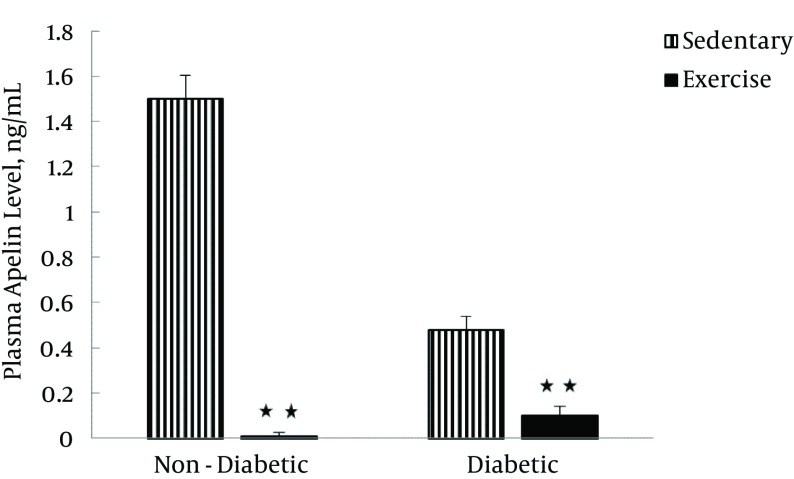
Table 2 shows the plasma apelin levels of four groups before and after the exercise training program. The results of repeated measures ANOVA indicated that exercise training had significant effects on plasma apelin levels (F [1–20] = 296.74, P < 0.0001). Also, diabetes exerted significant effects on plasma apelin levels (F [1–20] = 148.18, P < 0.0001). Finally, the interaction between the exercise training program and diabetes significantly affected plasma apelin levels (F [1–20] = 106.19, P < 0.0001; Figure 3).
Table 2. Plasma Apelin Levels, Tail Flick Latency, and Blood Glucose Levels in Four Groups Before and After the Exercise Training Programa.
| Non-Diabetic | Diabetic | |||
|---|---|---|---|---|
| Sedentary | Exercise | Sedentary | Exercise | |
| Before Exercise Program | ||||
| Apelin Levels, ng/mL | 0.1 ± 0.02 | 0.1 ± 0.08 | 0.3 ± 0.3 | 0.3 ± 0.38 |
| Tail-Flick Latency, S | 3.8 ± 0.45 | 3.8 ± 0.40 | 2.2 ± 0.3 | 2.2 ± 0.27 |
| Glucose Levels, mg/dL | 108 ± 8.5 | 108 ± 7.8 | 371.5 ± 7.8 | 371.5 ± 8.2 |
| After Exercise Program | ||||
| Apelin Levels, ng/mL | 1.5 ± 0.26 | 0.01 ± 0.04 | 0.48 ± 0.05 | 0.1 ± 0.02 |
| Tail-Flick Latency, S | 2.18 ± 0.45 | 3.18 ± 0.21 | 2.6 ± 0.23 | 4.3 ± 0.29 |
| Glucose Levels, mg/dL | 109 ± 5.3 | 85 ± 4.3 | 421 ± 7.3 | 335 ± 5.4 |
aValues are expressed as mean ± SD.
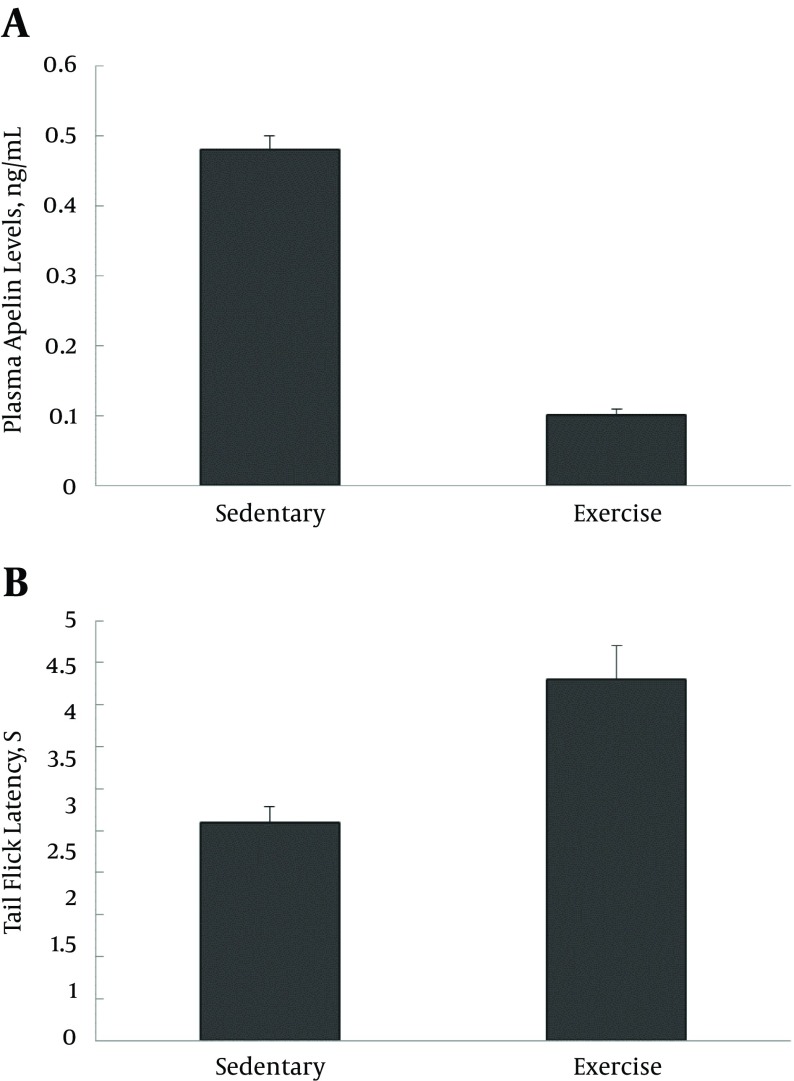
Figure 3. Plasma Apelin Levels in Sedentary (Sedentary Non-Diabetic and Sedentary Diabetic) and exercise (Exercise Non-Diabetic and Exercise Diabetic) Groups, 1 Day After the Last Training Session.
Values are expressed as mean ± SD.
4.3. Effect of Aerobic Exercise Program on Pain Threshold
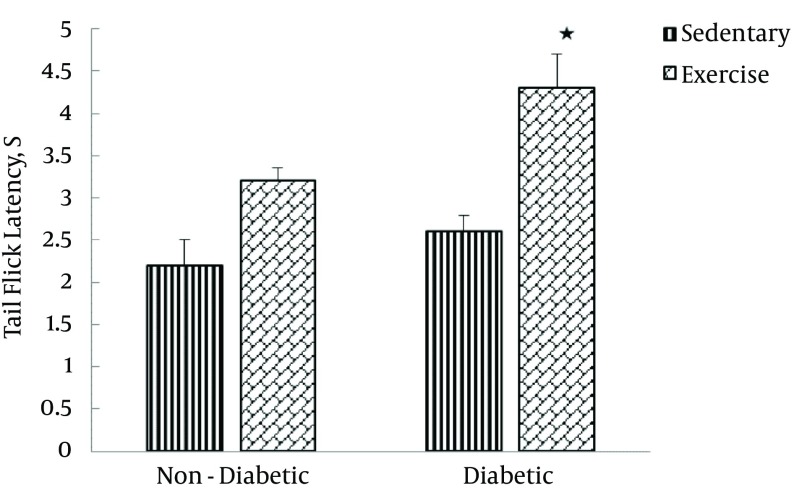
Table 2 also shows the tail-flick latency of four groups before and after the exercise training program. The results of repeated measures ANOVA indicated that the exercise training protocol had no significant effects on tail-flick latency (F [1-20] = 1.69, P = 0.208) while diabetes significantly affected tail-flick latency (F [1–20] = 7.30, P = 0.014). Finally, the interaction between the exercise training program and diabetes significantly exerted its effects on tail-flick latency (F [1–20] = 18.53, P < 0.0001; Figure 4).
Figure 4. Tail-Flick Latency in Sedentary (Sedentary Non-Diabetic and Sedentary Diabetic) and Exercise (Exercise Non-Diabetic and Exercise Diabetic) Groups, 1 Day After the Last Training Session.
Values are expressed as mean ± SD.
No statistically significant correlation was found between the blood apelin levels and the tail-flick latency in ED after the exercise training protocol. It was observed that the decrease in plasma apelin levels coincided with an increase in tail-flick latency in type 1 diabetic rats after the exercise training program (Figure 5A, 5B).
Figure 5. Blood Apelin Levels and the Tail-Flick Latency.
A, Decrease in plasma apelin levels and; B, increase in tail-flick latency in the exercise diabetic group compared with the sedentary diabetic group, at the end of exercise training program (1 day after the last training session). Values are expressed as mean ± SD. (**P < 0.0001).
4.4. Effect of Aerobic Exercise Program on Blood Glucose
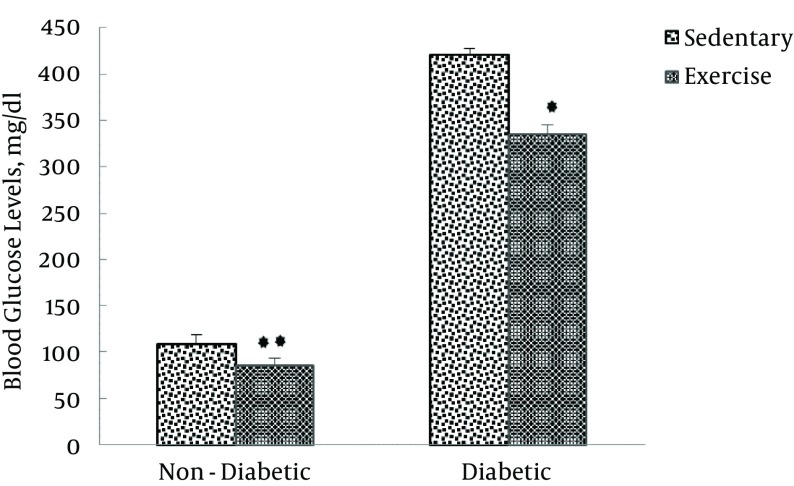
Table 2 also shows that, after the exercise training protocol in non-diabetic groups, blood glucose levels of exercised animals (85 ± 8.4 mg/dL) were significantly lower compared to that of sedentary animals (109 ± 9.4 mg/dL). Regarding the diabetic groups, blood glucose levels of exercised animals (335 ± 2.3 mg/dL) were significantly lower than that of sedentary animals (421 ± 3 mg/dL, P < 0.0001), the results of which are presented in Figure 6.
Figure 6. Blood Glucose Levels in Non-Diabetic (Sedentary and Exercise) and Diabetic (Sedentary and Exercise) Groups, 1 Day After the Last Training Session.
Values are expressed as mean ± SD. (**P < 0.0001).
5. Discussion
The available literature states that the positive effects of exercise on diabetes mellitus are associated with beneficial changes in various systems and or hormone secretions. For instance, the significant effect of exercise on hypertension is mediated by regulating apelin (11-14). This suggests a relationship between the regulation of apelin and the significant effects of exercise training on the management of type 1 diabetes mellitus (T1DM). The present study showed that an aerobic exercise training program lowered the levels of apelin in both healthy and diabetic groups and, in contrast, elevated the tail-flick latency in the diabetic group.
5.1. Effect of Aerobic Exercise Training Program on Plasma Apelin Levels
Several studies have indicated that T1DM influences plasma apelin levels (18, 19). Attempting to fill another gap in the field, the current study investigated plasma apelin concentrations in type 1 diabetic and non-diabetic rats. The findings of the present study showed that plasma apelin levels were higher in type 1 diabetic rats compared with controls. This implies that T1DM increases plasma apelin concentrations. These findings are in agreement with those of Falcao-Pires et al. who evaluated plasma and myocardial apelin concentrations in hypertrophic conditions in the presence or absence of diabetes mellitus. They demonstrated a significant increase in plasma apelin levels and blood glucose levels of diabetic rats (18). The current study’s findings are also confirmed by Meral et al. who found that the circulating apelin levels of children with T1DM significantly increased in comparison with the healthy controls (19).
It is widely known that the STZ-induced diabetic model is characterized by the destruction of pancreatic β-cells and a consequent decrease in circulating insulin levels. Therefore, the present findings might be explained by considering the apelin levels a compensatory mechanism that increases in order to raise glucose utilization in insulin-lacking hyperglycemic conditions in T1DM (18).
In the present study, it was also found that, in spite of a significant reduction in body weight (data not shown), plasma apelin levels significantly increased in diabetic rats. These finding are in contrast to those of Boucher et al. who found that obesity increased plasma apelin levels in concordance with body fat content in association with hyperinsulinemia (20). It is therefore likely that adiposity may not be a major determinant in some conditions and that different mechanisms might be involved in regulating blood apelin concentrations, such as the therapeutic roles of apelin in hyperglycemic conditions of T1DM in certain body organs such as the pancreas (21) and kidneys (22-24). This might be due to the classical model of STZ-induced type 1 diabetes that produces a range of functional and structural changes in rodents. These changes include early development of renal hypertrophy, progressive increases in albuminuria, and changes to the renal ultrastructure, such as mesangial expansion and glomerular basement membrane thickening (2).
In the present study, it was found that the biggest change in plasma apelin levels occurred between the ND group and the SND group (Table 1). This can be justified by taking into consideration that adipose tissue affects apelin synthesis and secretion. These findings are in line with those of Boucher et al. who reported that apelin synthesis and secretion from the adipose tissue were upregulated by insulin. They found that blood apelin levels and their apelin mRNA concentrations in adipocytes increased in concordance with body fat content and hyperinsulinemia (20).
After the exercise training protocol, while it was found that plasma apelin levels decreased in exercise groups, an increase was observed in the sedentary groups. In other words, aerobic exercise training decreased plasma apelin levels in both diabetic (type 1) and the ND subgroups of the exercise group. In general, this result is well supported by other studies, for instance, Sheibani et al. (15) and Kadoglou et al. (16) have found that exercise training may influence plasma apelin levels. Specifically, this result in the present study is in complete agreement with those of Sheibani et al. (15) who indicated that exercise caused a decrease in plasma apelin levels in obese women. Yet, this result was not confirmed by Kadoglou et al. (16) whose study showed that exercise could increase the plasma apelin levels of type 2 diabetic patients.
For this reason, researchers have found it necessary to concentrate on these contrasting results in various studies and to justify this decrease in plasma apelin levels by arguing that exercise training might have exerted some significant effects on glycemic control. With regard to the contrast between the findings of this study and those of Kadoglou et al. (16), it can be argued that the presence of insulin in type 2 diabetic subjects in the latter study and the lack of insulin in the type 1 diabetic model used in the present study might have brought about these different results.
Additionally, the present study showed a significant decrease in both blood glucose levels (Figure 6) and plasma apelin levels (Figure 3) in type 1 diabetic and non-diabetic rats following the exercise training protocol. Similarly, some studies have shown that exercise training increased glucose utilization in muscle tissue and reduced insulin requirements (25).
5.2. Effect of Aerobic Exercise Training Program on Pain Threshold
It was found in this study that the tail-flick latency is lower in type 1 diabetic rats than in controls, indicating that diabetic rats exhibited thermal hyperalgesia. Our findings are well supported by the related literature, which frequently reports that diabetes can increase hypersensitivity to thermal stimulation. For instance, Courteix et al. observed a decrease in thresholds for noxious heat stimuli and the delay for the tail withdrawal which was significantly lower in diabetic rats than in normal rats (26). Similarly, Ohsawa et al. maintained that activation of protein kinase C in the spinal cord caused thermal hyperalgesia and allodynia in diabetic mice (27). Furthermore, the activation of protein kinase C resulted in increasing the release of substance P, which led to thermal hyperalgesia and allodynia. Additionally, Kamei et al. found that STZ-induced diabetic mice showed thermal allodynia and hyperalgesia in the tail-flick test (28, 29).
It was observed that tail-flick latency decreased as apelin concentrations rose in STZ-induced diabetic rats before starting the exercise protocol. This finding is justified by considering the fact that hyperglycemia might affect µ-opioid receptors. In this regard, Chen et al. stated that hyperglycemia is demonstrated to be associated with desensitization or decreased functional expression of the µ-opioid receptor (30) and Lv et al. maintained that the apelin-induced antinociceptive effects were mediated by the µ-opioid receptor (5).
In the present study, it was found that blood sugar levels of the ED group were significantly lower compared to those of the SD group (Figure 6), and hyperalgesia of the ED group was significantly lower than that of the SD group, following the exercise training protocol. Aerobic exercise training had significant effects on glycemic control and hyperalgesia in T1DM. Additionally, it was shown that the exercise training program decreased tail-flick latency in the END group compared to that of the SND group; however, this effect was not statistically significant. A possible explanation is that the exercise intensity used in this study was 50% of maximal aerobic capacity, while 50% intensity of maximal aerobic capacity is not adequate for healthy subjects to show statistically significant changes in pain perception. Higher intensities of exercise (~75% of maximal aerobic capacity) are needed to elicit exercise-induced analgesia to a pain stimulus (31). This finding is well confirmed by several studies showing that hyperglycemia is the primary etiologic factor that can trigger many complications in diabetes mellitus (2, 32, 33), such as hyperalgesia; in addition, patients with type 1 or type 2 diabetes develop secondary complications, the risk of which is related to the duration of diabetes and the degree of glycemic control.
Exercise training has been accepted and generally recommended for the management of T1DM. Physical activity develops and maintains chronic glycemic control by stimulating muscle glucose uptake (8). Exercise also improves the selected measures of peripheral nerve function and glycemic control (10). Rossi et al. have reported that swim training for a long duration reduces thermal hyperalgesia in STZ-induced diabetic female rats (33).
Patients with diabetes frequently have abnormal perceptions of thermal stimuli, called thermal hyperalgesia. Nociceptive sensitivity was assessed using the tail-flick apparatus. The present study found that tail-flick latency obtained from the ED group was significantly different from those of the SD group after a 10-week aerobic exercise training program. In other words, the diabetes-associated hyperalgesia of the ED group was significantly lower than that of the SD group. These findings are not consistent with those of Chen et al. who indicated that treadmill exercise completely prevented thermal hyperalgesia in 4 weeks, whereas after 8 weeks (day 56) the beneficial effects of treadmill running were progressively decreased (34). This inconsistency might be due to the exercise protocol duration, which was longer in the present study than in that of the exercise protocol described by Chen et al. (34). Also, this may be explained by the intensity and duration in each exercise session (25). In addition, these findings are well supported by the related literature about the effects of exercise on pain perception in diabetes mellitus (10, 25, 30, 34).
In the present study, no correlation was found between apelin concentrations and the tail-flick latency in the ED group after the exercise training protocol. To the best of our knowledge, this finding has not been found in the related literature and is the first report about the effects of aerobic exercise training on plasma apelin concentrations and the tail-flick latency in type 1 diabetic animals. Finally, based on our study’s findings, we speculate that apelin does not play any significant role in regulating the pain threshold in T1DM during exercise training.
5.3. Study Limitations
As in other similar studies, we induced diabetes type I through STZ injection, and, while it is better to induce diabetes type I through transgenic rats, it was impossible for us to do so.
Acknowledgments
The authors would like to thank the members of the exercise physiology department for their valuable suggestions and assistance.
Footnotes
Authors’ Contribution:Study concept and design, Ali Heidarianpour and Reza Delavar; acquisition of data, Ali Heidarianpour and Reza Delavar; analysis and interpretation of data, Ali Heidarianpour and Reza Delavar; drafting of the manuscript, Ali Heidarianpour and Reza Delavar; critical revision of the manuscript for important intellectual content, Al Heidarianpour; statistical analysis, Ali Heidarianpour; administrative, technical, and material support, Heidarianpour and Vahidian Rezazadeh; study supervision, Ali Heidarianpour.
Funding/Support:This work was supported by a research grant from Bu-Ali Sina university, Hamadan, Iran.
References
- 1.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27(5):1047–53. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 2.Calcutt NA, Cooper ME, Kern TS, Schmidt AM. Therapies for hyperglycaemia-induced diabetic complications: from animal models to clinical trials. Nat Rev Drug Discov. 2009;8(5):417–29. doi: 10.1038/nrd2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Befort K, Filliol D, Darcq E, Ghate A, Matifas A, Lardenois A, et al. Gene expression is altered in the lateral hypothalamus upon activation of the mu opioid receptor. Ann N Y Acad Sci. 2008;1129:175–84. doi: 10.1196/annals.1417.028. [DOI] [PubMed] [Google Scholar]
- 4.Xu N, Wang H, Fan L, Chen Q. Supraspinal administration of apelin-13 induces antinociception via the opioid receptor in mice. Peptides. 2009;30(6):1153–7. doi: 10.1016/j.peptides.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 5.Lv SY, Qin YJ, Wang NB, Yang YJ, Chen Q. Supraspinal antinociceptive effect of apelin-13 in a mouse visceral pain model. Peptides. 2012;37(1):165–70. doi: 10.1016/j.peptides.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 6.Dray C, Knauf C, Daviaud D, Waget A, Boucher J, Buleon M, et al. Apelin stimulates glucose utilization in normal and obese insulin-resistant mice. Cell Metab. 2008;8(5):437–45. doi: 10.1016/j.cmet.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 7.Shehata MI, Ahmed SA, Gomaa RS, Abulmeaty MM. Effect of apelin on insulin resistance, beta cell function and lipid profile in healthy and diabetic rat models. Zagazig J. 2015;19(1) [Google Scholar]
- 8.Tonoli C, Heyman E, Roelands B, Buyse L, Cheung SS, Berthoin S, et al. Effects of different types of acute and chronic (training) exercise on glycaemic control in type 1 diabetes mellitus: a meta-analysis. Sports Med. 2012;42(12):1059–80. doi: 10.2165/11635380-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 9.Souto DL, de Miranda MP. Physical excercises on glycemic control in type 1 diabetes mellitus. Nutricion Hospitalaria. 2011;26(3):425–9. doi: 10.1590/S0212-16112011000300001. [DOI] [PubMed] [Google Scholar]
- 10.Kluding PM, Pasnoor M, Singh R, Jernigan S, Farmer K, Rucker J, et al. The effect of exercise on neuropathic symptoms, nerve function, and cutaneous innervation in people with diabetic peripheral neuropathy. J Diabetes Complications. 2012;26(5):424–9. doi: 10.1016/j.jdiacomp.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andersen CU, Hilberg O, Mellemkjaer S, Nielsen-Kudsk JE, Simonsen U. Apelin and pulmonary hypertension. Pulm Circ. 2011;1(3):334–46. doi: 10.4103/2045-8932.87299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen S, Xue F, Jin HL, Chen L, Chen Y, Wang GF, et al. [Effect of swimming exercise on the expression of apelin and its receptor in pulmonary tissues of rats with hypoxic pulmonary hypertension]. Zhongguo ying yong sheng li xue za zhi= Zhongguo yingyong shenglixue zazhi= Chinese journal of applied physiology. 2012;28(1):5–8. [PubMed] [Google Scholar]
- 13.Mahmoody SA, Gharakhanlou R, Roshan VD, Hedayati M. Individual and concomitant effects of cardioprotective programs on cardiac apelinergic system and oxidative state in L-NAME-induced hypertension. Clin Exp Hypertens. 2013;35(1):20–7. doi: 10.3109/10641963.2012.685536. [DOI] [PubMed] [Google Scholar]
- 14.Zhang J, Ren CX, Qi YF, Lou LX, Chen L, Zhang LK, et al. Exercise training promotes expression of apelin and APJ of cardiovascular tissues in spontaneously hypertensive rats. Life Sci. 2006;79(12):1153–9. doi: 10.1016/j.lfs.2006.03.040. [DOI] [PubMed] [Google Scholar]
- 15.Sheibani S, Hanachi P, Refahiat MA. Effect of Aerobic Exercise on Serum Concentration of Apelin, TNFalpha and Insulin in Obese Women. Iran J Basic Med Sci. 2012;15(6):1196–201. [PMC free article] [PubMed] [Google Scholar]
- 16.Kadoglou NPE, Vrabas IS, Kapelouzou A, Lampropoulos S, Sailer N, Kostakis A, et al. The impact of aerobic exercise training on novel adipokines, apelin and ghrelin, in patients with type 2 diabetes. Med Sci Monit Basic Res. 2012;18(5):CR290–5. doi: 10.12659/MSM.882734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National research council.. Guide for the care and use of laboratory animals. Institute of laboratory animal resources: 1996. Available from: http://www.nap.edu/readingroom/books/labrats. [Google Scholar]
- 18.Falcao-Pires I, Goncalves N, Gavina C, Pinho S, Teixeira T, Moura C, et al. Correlation between plasma levels of apelin and myocardial hypertrophy in rats and humans: possible target for treatment? Expert Opin Ther Targets. 2010;14(3):231–41. doi: 10.1517/14728220903485685. [DOI] [PubMed] [Google Scholar]
- 19.Meral C, Tascilar E, Karademir F, Tanju IA, Cekmez F, Ipcioglu OM, et al. Elevated plasma levels of apelin in children with type 1 diabetes mellitus. J Pediatr Endocrinol Metab. 2010;23(5):497–502. doi: 10.1515/jpem.2010.081. [DOI] [PubMed] [Google Scholar]
- 20.Boucher J, Masri B, Daviaud D, Gesta S, Guigne C, Mazzucotelli A, et al. Apelin, a newly identified adipokine up-regulated by insulin and obesity. Endocrinology. 2005;146(4):1764–71. doi: 10.1210/en.2004-1427. [DOI] [PubMed] [Google Scholar]
- 21.Chen H, Zheng C, Zhang X, Li J, Li J, Zheng L, et al. Apelin alleviates diabetes-associated endoplasmic reticulum stress in the pancreas of Akita mice. Peptides. 2011;32(8):1634–9. doi: 10.1016/j.peptides.2011.06.025. [DOI] [PubMed] [Google Scholar]
- 22.Day RT, Cavaglieri RC, Feliers D. Apelin retards the progression of diabetic nephropathy. Am J Physiol Renal Physiol. 2013;304(6):F788–800. doi: 10.1152/ajprenal.00306.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hus-Citharel A, Bouby N, Frugiere A, Bodineau L, Gasc JM, Llorens-Cortes C. Effect of apelin on glomerular hemodynamic function in the rat kidney. Kidney Int. 2008;74(4):486–94. doi: 10.1038/ki.2008.199. [DOI] [PubMed] [Google Scholar]
- 24.Nishida M, Hamaoka K. The Apelin–APJ system: Its role in renal physiology and potential therapeutic applications for renal disease. [Google Scholar]
- 25.Chimen M, Kennedy A, Nirantharakumar K, Pang TT, Andrews R, Narendran P. What are the health benefits of physical activity in type 1 diabetes mellitus? A literature review. Diabetologia. 2012;55(3):542–51. doi: 10.1007/s00125-011-2403-2. [DOI] [PubMed] [Google Scholar]
- 26.Courteix C, Eschalier A, Lavarenne J. Streptozocin-induced diabetic rats: behavioural evidence for a model of chronic pain. Pain. 1993;53(1):81–8. doi: 10.1016/0304-3959(93)90059-x. [DOI] [PubMed] [Google Scholar]
- 27.Ohsawa M, Kamei J. Possible involvement of spinal protein kinase C in thermal allodynia and hyperalgesia in diabetic mice. European Journal of Pharmacology. 1999;372(3):221–8. doi: 10.1016/s0014-2999(99)00228-9. [DOI] [PubMed] [Google Scholar]
- 28.Kamei J, Zushida K. Effect of mexiletine on thermal allodynia and hyperalgesia in diabetic mice. Jpn J Pharmacol. 2000;84(1):89–92. doi: 10.1254/jjp.84.89. [DOI] [PubMed] [Google Scholar]
- 29.Kamei J, Zushida K, Morita K, Sasaki M, Tanaka S. Role of vanilloid VR1 receptor in thermal allodynia and hyperalgesia in diabetic mice. Eur J Pharmacol Discipline . 2001;422(1-3):83–6. doi: 10.1016/s0014-2999(01)01059-7. [DOI] [PubMed] [Google Scholar]
- 30.Chen S, Sweigart KL, Lakoski JM, Pan H. Functional μ Opioid Receptors Are Reduced in the Spinal Cord Dorsal Horn of Diabetic Rats. Anesthesiology. 2002;97(6):1602–8. doi: 10.1097/00000542-200212000-00037. [DOI] [PubMed] [Google Scholar]
- 31.Hoffman MD, Shepanski MA, Ruble SB, Valic Z, Buckwalter JB, Clifford PS. Intensity and duration threshold for aerobic exercise-induced analgesia to pressure pain. Arch Phys Med Rehabil. 2004;85(7):1183–7. doi: 10.1016/j.apmr.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 32.Heidarianpour A, Hajizadeh S, Khoshbaten A, Niaki AG, Bigdili MR, Pourkhalili K. Effects of chronic exercise on endothelial dysfunction and insulin signaling of cutaneous microvascular in streptozotocin-induced diabetic rats. Eur J Cardiovasc Prev Rehabil. 2007;14(6):746–52. doi: 10.1097/HJR.0b013e32817ed02f. [DOI] [PubMed] [Google Scholar]
- 33.Rossi DM, Valenti VE, Navega MT. Exercise training attenuates acute hyperalgesia in streptozotocin-induced diabetic female rats. Clinics (Sao Paulo). 2011;66(9):1615–9. doi: 10.1590/S1807-59322011000900019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen YW, Hsieh PL, Chen YC, Hung CH, Cheng JT. Physical exercise induces excess hsp72 expression and delays the development of hyperalgesia and allodynia in painful diabetic neuropathy rats. Anesth Analg. 2013;116(2):482–90. doi: 10.1213/ANE.0b013e318274e4a0. [DOI] [PubMed] [Google Scholar]