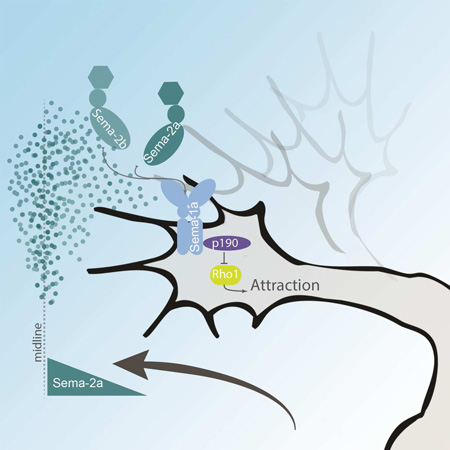
SUMMARY
Commissural axons must cross the midline to form functional midline circuits. In the invertebrate nerve cord and vertebrate spinal cord, midline crossing is mediated in part by Netrin-dependent chemoattraction. Loss of crossing, however, is incomplete in mutants for Netrin or its receptor Frazzled/DCC, suggesting the existence of additional pathways. We identified the transmembrane Semaphorin, Sema-1a, as an important regulator of midline crossing in the Drosophila CNS. We show that in response to the secreted Semaphorins Sema-2a and Sema-2b, Sema-1a functions as a receptor to promote crossing independently of Netrin. In contrast to other examples of reverse signaling where Sema1a triggers repulsion through activation of Rho in response to Plexin binding, in commissural neurons Sema-1a acts independently of Plexins to inhibit Rho to promote attraction to the midline. These findings suggest that Sema-1a reverse signaling can elicit distinct axonal responses depending on differential engagement of distinct ligands and signaling effectors.
Keywords: Axon Guidance, Midline, Semaphorin, Drosophila, Reverse Signaling, Netrin, DCC, chemoattraction, growth cone
Graphical abstract
eTOC BLURB
In bilaterally symmetric animals, assembling precise neural circuits at the midline is critical for the coordination of the two sides of the body. Hernandez-Fleming et al. now uncover an important role for Sema1a reverse signaling in controlling midline axon crossing.
INTRODUCTION
The ability to coordinate the right and left sides of the body relies on neural circuits at the midline. Disruptions to these circuits during development often result in an inability to coordinate movement. For the majority of midline circuits, appropriate circuit formation requires axons to cross the midline. Netrin and its attractive receptor DCC, or Frazzled (Fra) in Drosophila, are highly conserved guidance factors known to promote midline crossing (Harris et al., 1996; Kennedy et al., 1994; Kolodziej et al., 1996; Neuhaus-Follini and Bashaw, 2015a; Serafini et al., 1994). Mutations in DCC are associated with movement disorders in zebrafish, mice and humans (Jain et al., 2014; Rabe Bernhardt et al., 2012; Srour et al., 2010). Despite the conserved role of Netrin and Fra/DCC in midline axon guidance, many axons still cross the midline in netrinAB double mutants (hereafter referred to as NetAB) and fra mutants in Drosophila, suggesting that additional pathways promote midline crossing (Kolodziej et al., 1996; Mitchell et al., 1996). Studies in vertebrate systems have identified additional factors contributing to midline crossing, such as Shh/Boc (Charron et al., 2003), VEGF/Flk1(Ruiz de Almodovar et al., 2010), and Sema/Plexin (Nawabi et al., 2010; Zou et al., 2000). Unfortunately, redundancies in both ligands and receptors have led to ambiguous results when trying to discern molecular mechanisms from mutant phenotypes (Charoy et al., 2012; Delloye-Bourgeois et al., 2015; Hernandez-Enriquez et al., 2015; Parra and Zou, 2010; Sloan et al., 2015). In order to identify additional pathways in a more tractable system, we developed a genetic modifier screen where Fra signaling is specifically reduced in a small subset of commissural neurons in the Drosophila embryo (O’Donnell and Bashaw, 2013a) and identified the transmembrane Semaphorin, Sema-1a, as an important regulator of midline crossing. Semaphorin/Plexin signaling is highly conserved and has been shown to play many roles within the nervous system. In vertebrates, the Sema/Plexin family of signaling molecules is large and diverse; while in Drosophila, Semas and Plexins constitute a fairly small family. There are five Semaphorins identified in Drosophila and only two Plexins. Semas are divided into two classes: transmembrane (Sema-1a, Sema-1b and Sema-5c) or secreted (Sema-2a and Sema-2b) (Pasterkamp, 2012). Neither Sema-1b nor Sema-5c show neural expression in the developing CNS (Khare et al., 2000). The transmembrane Semas bind Plexin A (PlexA), while Plexin B (PlexB) binds the secreted Semas (Ayoob et al., 2006; Winberg et al., 1998). In the fly, Sema-1a is known to act as a repulsive/de-adhesive signal during motor axon guidance (Jeong et al., 2012; Yu et al., 1998; Yu et al., 2000). A role within the CNS, however, is not surprising since the expression patterns of Sema-1a and PlexA both appear to be pan-neural and the longitudinal connectives within the CNS show defects in both sema-1a and plexA mutants (Kolodkin et al., 1993; Winberg et al., 1998). Still, a role for Sema-1a in commissure formation has never been explored. In vertebrates, secreted Semas act at the midline to repel crossing axons from the floor plate (Jongbloets and Pasterkamp, 2014; Nawabi et al., 2010; Zou et al., 2000). The expression pattern of Sema-1a, however, precludes a similar function in fly. Intriguingly, a growing body of evidence has demonstrated that Sema-1a can signal in both a forward direction as a ligand and in reverse as a receptor. Sema-1a reverse signaling can be engaged by PlexA binding, as observed in the visual system and the giant fiber circuit, or potentially through indirect interactions with other secreted Semas as suggested in the olfactory system (Cafferty et al., 2006; Godenschwege et al., 2006; Komiyama et al., 2007; Pecot et al., 2013; Sweeney et al., 2011). During the guidance of Drosophila motor axons, Sema-1a appears to act independently of Plexin and the ligand is not known (Jeong et al., 2012).
In this study, we find that Sema-1a promotes midline crossing independently of Netrin/Frazzled chemoattraction. Sema-1a functions cell autonomously in commissural neurons to promote midline crossing. A region of Sema-1a’s cytodomain previously shown to bind Pebble and RhoGAPp190 is required for Sema-1a to promote midline crossing, and we find that RhoGAPp190 and the downregulation of Rho1 are likely to be important for midline crossing. Surprisingly, Sema-1a’s canonical binding partner, PlexA, does not contribute to Sema-1a’s pro-crossing function. Instead, the secreted Sema2s act as signaling cues. Taken together, these data are consistent with a model where Sema-1a mediates midline crossing through an attractive/adhesive mechanism via RhoGAPp190 in response to secreted Semas.
RESULTS
A genetic screen identifies Sema-1a as a factor that promotes midline crossing
In order to identify molecules that function to promote midline crossing, we performed a genetic screen using a truncated Fra receptor (FraΔC) lacking its cytoplasmic domain, that functions as a dominant negative (Garbe et al., 2007). By specifically expressing FraΔC in a small subset of commissural neurons, the eagle neurons, we were able to establish a highly sensitized background. The eagle neurons are grouped into two clusters per hemisegment, the EGs and EWs. Approximately ten EG neurons project their axons through the anterior commissure, while only three EW neurons project their axons through the posterior commissure (Higashijima et al., 1996)(Figure 1A). In NetAB mutants or fra mutants, the EW neurons show a marked decrease in midline crossing, while the EG neurons are unaffected (Garbe et al., 2007). In fra mutants the EW axons fail to cross the midline in 34% of abdominal segments, and expressing FraΔC specifically in the eagle neurons of an otherwise wild-type embryo results in a similar phenotype (Figure 1B, C, G). We screened large deficiencies covering a majority of the second chromosome and identified dominant enhancers of the FraΔC crossing defects. This approach allowed us to identify subtle crossing defects in heterozygous embryos, and thus avoid potential complications from early gene requirement.
Figure 1. Sema-1a is a positive regulator of midline crossing.
(A–F) Stage 15–16 embryos of the indicated genotypes carrying eg-GAL4 and UAS-CD8 GFP transgenes, stained with anti-HRP (magenta) and anti-GFP (green) antibodies. Anti-GFP labels cell bodies and axons of the eagle neurons (EG and EW). Anterior is up in all images. Scale bar represents 15µm (F). Dotted lines indicate segment boundaries. EG neurons project through the anterior commissure of each segment while EW neurons project through the posterior commissure. Arrowheads indicate segments with non-crossing EW axons and asterisks indicate rescued EW crosses. (A) EW neurons cross in the posterior commissure in 100% of segments in wild-type embryos (starred arrowhead). (B) In fra mutants EW neurons fail to cross in 27% of segments (arrowheads). (C) Expression of UAS-FraΔC selectively in eagle neurons produces a Fra-like phenotype where EW neurons fail to cross in 32% of segments. (D) Heterozygosity for sema-1a dominantly enhances the EW crossing defects in a FraΔC background to 64%. (E) Complete loss of sema-1a further enhances the EW crossing defect to 99% of segments. (F) EW crossing defects in the sema-1a null expressing FraΔC are robustly rescued (99% vs. 24%) when UAS Sema-1a is expressed in eagle neurons. (G) Quantification of EW midline crossing defects in the genotypes shown in (B–F) as well as sema-1a null mutants. Df(2L)ED623 is a chromosomal deficiency containing sema-1a. Data are presented as mean+SEM. n, number of embryos scored for each genotype. Significance was assessed by multiple comparisons using ANOVA (****p<0.0001).
A deficiency on the second chromosome, DF(2L)ED623, enhances the FraΔC phenotype to 49% (Figure 1G). The enhancer activity in this interval was genetically mapped to Sema-1a and a null allele, sema-1aP1, fully recapitulates the enhanced EW defects (Figure 1D, G). Sema-1a mutants alone show no appreciable crossing defects in eagle neurons; however, in the FraΔC screening background, eagle neuron crossing defects are dose dependent and are strongly enhanced when both copies of sema-1a are removed (Figure 1E, G). Furthermore, this mutant phenotype can be rescued when full-length Sema-1a (Sema-1aFL) is restored selectively in the eagle neurons (Figure 1F, G). Close examination of the resulting phenotypes demonstrates that eagle neurons continue to grow ipsilaterally and fail to turn towards the midline. Axons do not exit the CNS or misroute to the anterior commissure. This phenotype suggests that Sema-1a does not mediate axon growth; rather, Sema-1a is required for the medial turn towards the midline. In order to validate the effects of sema-1a seen in the screen, we analyzed the genetic interaction between sema-1a heterozygotes and fra hypomorphs. Loss of one copy of sema-1a leads to an enhancement of EW neuron crossing defects in multiple hypomorphic backgrounds (Figure S1). This result further supports an endogenous role for sema-1a in promoting midline crossing.
Sema-1a promotes midline crossing independently of Netrin/Fra chemoattraction
To test whether Sema-1a functions together with, or independently of, Netrin/Fra chemoattraction, we examined genetic interactions between sema-1a and fra or NetAB mutants. Reduced midline crossing can be readily observed when the entire axon scaffold is stained with anti-HRP antibodies. In wild-type embryos, thick anterior and posterior commissures form in each segment (Figure 2A). Both NetAB and fra null mutants display mild crossing defects, which are observed as thin or occasionally missing commissures (Figure 2B and Figure S1). Sema-1a null mutants, however, show no significant crossing defects in either the axon scaffold or in eagle neurons (Figure 1G and Figure 2C). If sema-1a were functioning in an independent pathway to promote midline crossing, we would expect the loss of sema-1a to enhance the mild crossing defects seen in fra and NetAB mutants. While embryos heterozygous for both fra and sema-1a display no defects, the double mutants exhibit a striking enhancement in crossing defects compared to fra single mutants (total defects: sema-1a, fra = 92% vs. fra = 40%; Figure 2D and E) as well an increase in the number of missing commissures (missing: sema-1a, fra =68% vs. fra=10%; Figure 2D and E). This phenotype suggests a broad role for Sema-1a in commissural guidance and analysis of commissural neuron subsets further supports this idea. Crossing defects are seen in POXN and sema2b-τmyc neurons, but the defects in eagle neurons are by far the most severe (data not shown and Figure S2). Additionally, these defects can only be rescued when both Sema-1a and Fra are co-expressed in sema-1a, fra double mutants indicating they function cooperatively to promote midline crossing (Figure S2). The majority of sema-1a, fra double mutant embryos are nearly commissureless, and these defects can be directly attributed to the loss of sema-1a since the axon scaffold defects in double mutants can be robustly rescued with pan-neural expression of Sema-1aFL (total defects: 56%, missing: 25%; Figure 2F). Importantly, this rescue is only partial since the embryos are still mutant for fra. Furthermore, this dramatic double mutant phenotype is not specific to sema-1a, fra double mutants, as it is nearly identical to the phenotype of NetAB; sema-1a double mutants (total defects: NetAB, sema-1a = 71% vs. NetAB = 25%), again with the strongest increase in the number of missing commissures (missing: NetAB, sema-1a = 48% vs. NetAB = 6%; Figure S1).
Figure 2. Sema-1a promotes midline crossing independent of Netrin/fra.
(A–F) Stage 16 embryos of the indicated genotypes stained with anti-HRP. Arrowheads indicate thin/defective commissures, arrows indicate missing commissures and asterisks indicate rescued commissures. Scale bar represents 15µm (F). (A) Thick anterior and posterior commissures are formed as axons cross the midline in every segment. (B) frazzled (fra3/fra4) mutants show thin (29%) and occasionally missing commissures (10%). (C) sema-1a mutants show no obvious signs of commissural defects. (D) Embryos heterozygous for both sema-1a and fra appear wild-type. (E) fra, sema-1a double mutants show a 68% loss of commissures. (F) Pan-neural expression of Sema-1a partially rescues these defects, and reduces missing commissures to 25%. (G) Quantification of commissural defects as absent (black bar), thin/defective (dark gray) or wild-type (light grey) in the genotypes shown in (A–F). Data are represented as mean+SEM. n, number of embryos scored for each genotype. Significance was assessed by multiple comparisons using ANOVA (****p<0.0001).
To further support the argument that sema-1a acts independently of the Netrin/Fra pathway, we analyzed dominant genetic interactions in commissural neurons. The crossing defects in both fra or NetAB mutants are significantly increased when a single copy of sema-1a is removed (Figure S1). These data demonstrate that Sema-1a must function independently of Netrin/Fra chemoattraction. We also explored the possibility that the effect of sema-1a on midline crossing could be due to up-regulation of Robo1 repulsion. We found that loss of sema-1a did not result in changes in Robo1 protein expression, nor does loss of sema-1a show genetic interaction with slit or robo mutants (Figure S3). Taken together, this evidence suggests that Sema-1a acts independently of Netrin/Fra and is unlikely to exert its pro-crossing effect through regulation of midline repulsion.
Sema-1a is endogenously expressed in eagle commissural neurons during midline crossing
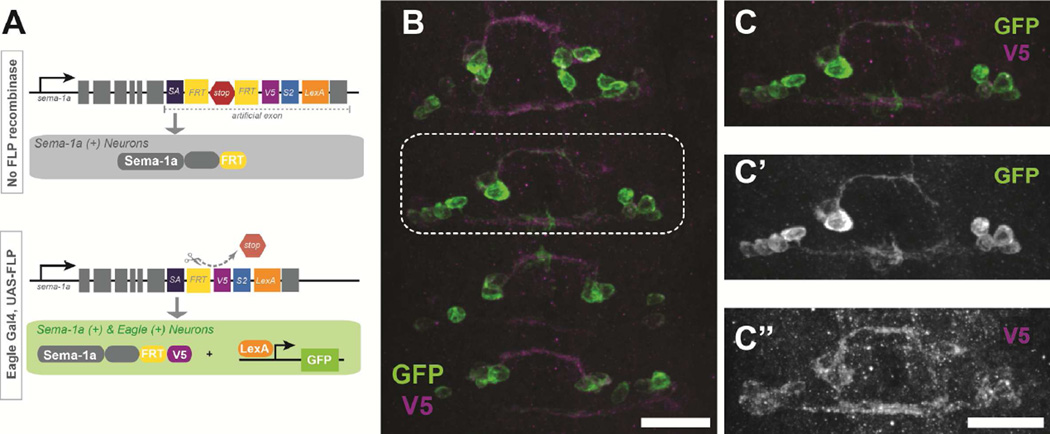
Previously published expression data suggests that Sema-1a is expressed pan-neurally and that Sema-1a protein can be detected throughout the ventral nerve cord including in axon commissures (Kolodkin et al., 1993; Yu et al., 1998). Our initial findings, specifically the pan-neural rescue of the sema-1a, fra double mutant, would suggest that Sema-1a is required in neurons to promote midline crossing. However, it is still unclear in which neurons Sema-1a is acting to promote midline crossing, since it could function in the commissural neurons themselves or in surrounding neurons. To address this question, we first wanted to know if Sema-1a is endogenously expressed in eagle neurons. Antibody staining and in situ hybridization techniques suggested expression in eagle neurons, but due to the broad expression of Sema-1a, we are unable to adequately resolve individual neurons (data not shown). To definitively distinguish endogenous Sema-1a expression in a tissue specific manner, we took advantage of a fly line developed in the Zipursky lab that allows sparse labeling of endogenous Sema-1a (Pecot et al., 2013). Pecot and colleagues generated an artificial exon in the endogenous locus carrying a conditional genetic tracer that allows us to visualize both the cells that express Sema-1a and the Sema-1a protein itself (Pecot et al., 2013). This dual visualization is achieved by the co-expression of a V5-tagged Sema-1a and a LexA transcription factor, which are restricted from expression by a stop cassette flanked by FRT sites (Figure 3A). Thus, tissue specific expression of FLP recombinase excises the stop cassette, allowing visualization of endogenous Sema-1a expression only in the tissue of interest. Expression of FLP in eagle neurons resulted in mosaic expression during the time of midline crossing (Figure 3B). This sparse labeling allowed us to capture endogenous sema-1a expression at single cell resolution. Assessments across multiple embryos indicate that Sema-1a is indeed endogenously expressed in all eagle neurons, including the EW cluster (Figure 3C’). Visualization of Sema-1a protein using the V5 tag reveals a punctate pattern on cell bodies and strong labeling of the axons during midline crossing (Figure 3C”). Interestingly, we see no reductions in Sema-1a expression post-crossing (data not shown).
Figure 3. Sema-1a protein is expressed on Eg axons.
An artificial exon inserted into the endogenous locus for sema-1a allows for tissue specific labeling of endogenous Sema-1a expression. (A) Schematic of sparse labeling strategy adapted from Pecot, et al. 2013. In the presence of FLP recombinase, Sema-1a becomes tagged with a V5 epitope and LexA driven membrane bound GFP labels Sema-1a positive cells. (B–C) An early stage 15 embryo carrying the artificial exon, egGal4, UAS-FLP and LexAop-myrGFP stained with anti-GFP (green) and anti-V5 (magenta) antibodies. (B) Eagle neurons endogenously express Sema-1a during midline crossing. Scale bar represents 15µm (C) Magnification of the boxed region in B. (C’) GFP only staining shows two EW axons crossing the midline (C”) V5 staining reveals that Sema-1a protein is expressed throughout the growing axon. Scale bar represents 15µm
Sema-1a functions cell autonomously and its cytoplasmic domain is required for midline crossing
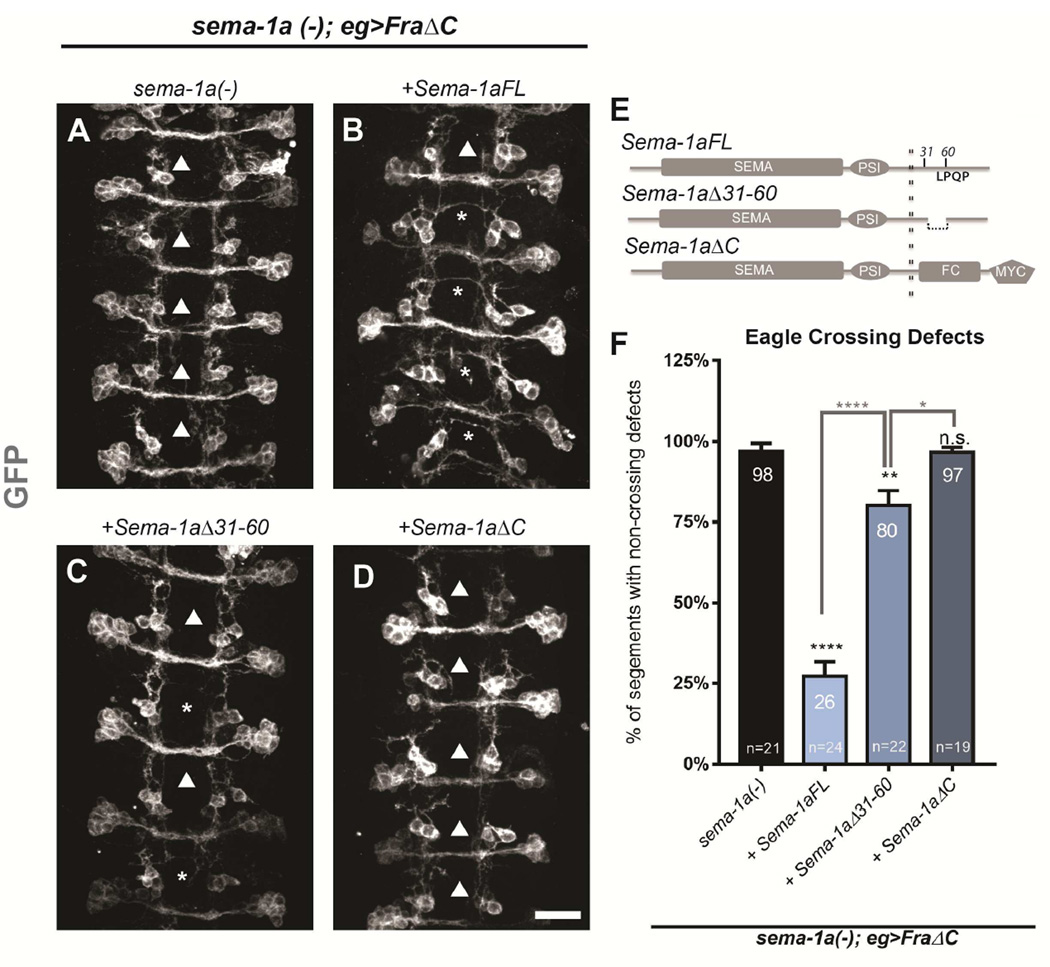
Given that Sema-1a is expressed in commissural neurons and appears to function in neurons to promote crossing, we wanted to explore if Sema-1a functions as a receptor in this context. To determine if Sema-1a promotes midline crossing through reverse signaling, we tested if Sema-1a’s cytoplasmic domain is required. To address cell autonomy without introducing non-autonomous “follower effects,” we used a sema-1a mutant expressing the dominant negative Fra receptor (FraΔC) in the eagle neurons. These embryos display the same level of defects in the eagle neurons as sema-1a, fra double mutants, while the rest of the CNS appears largely wild-type. We compared the ability of full-length and two truncated Sema-1a transgenes to rescue crossing defects in this genetic background. These transgenes are targeted to the same genomic locus and are expressed at comparable levels. All three transgenes are capable of rescuing forward signaling yet only the full-length transgene is able to rescue reverse signaling (Jeong et al., 2012). When full-length Sema-1a (UAS-Sema-1aFL) is restored in this background, eagle neuron crossing defects are reduced from 98% to 26% (Figure 4 and Figure 1), suggesting a cell autonomous requirement. Furthermore, the truncated Sema-1a transgene (UAS-Sema-1aΔC) completely fails to rescue, suggesting that the cytoplasmic domain is required and that Sema-1a likely mediates midline crossing through reverse signaling. To further determine the region within the cytoplasmic domain that is necessary for midline crossing, we tested a third transgene (UAS-Sema-1aΔ31–60) carrying a small deletion within the cytoplasmic domain, which removes amino acids 31–60. This region includes the binding site for downstream effectors of Sema-1a reverse signaling in motor neurons and was demonstrated to physically interact with two opposing regulators of the small GTPase Rho1 (Jeong et al., 2012). Expression of this transgene results in a dramatically reduced rescue, implicating this region in midline crossing and further supporting the conclusion that Sema-1a promotes midline crossing through reverse signaling (Figure 4). Although Sema-1aΔ31–60 does produce a small but significant reduction in crossing defects, it does not rescue crossing nearly as well as the full-length transgene.
Figure 4. Sema-1a expression rescues midline crossing cell autonomously.
(A–D) Stage 15–16 embryos of the indicated genotypes carrying eg-GAL4, UAS-FraΔC and UAS-CD8 GFP transgenes, stained with anti-GFP (green) antibodies to reveal cell bodies and axons of the eagle neurons (EG and EW). Arrowheads indicate segments with non-crossing EW axons and asterisks indicate rescued EW crosses. Scale bar represents 15µm (D). (A) In sema-1a null embryos expressing FraΔC, EW axons fail to cross the midline in 98% of segments (arrowheads). (B) Expression of a full-length Sema-1a transgene strongly rescues these defects (asterisk), with only 26% non-crossing (arrowheads). (C) In contrast, a Sema-1a transgene lacking a small region of the cytoplasmic domain (from aa31–60) rescued the defects to a much lesser extent (80%). (D) Expression of a Sema-1a transgene lacking its entire cytoplasmic domain does not significantly rescue crossing defects. (E) Diagram of transgenic rescue constructs with relevant cytoplasmic regions labeled. (F) Quantification of EW midline crossing defects in the genotypes shown in (A–D). Data are represented as mean+SEM. n, number of embryos scored for each genotype. Significance was assessed by multiple comparisons using ANOVA (****p<0.0001).
These findings in the eagle neurons are consistent with the pan-neural rescue of the sema-1a, fra double mutants. When we pan-neurally express these Sema-1a transgenes we get a similar rescue profile where Sema-1a-FL leads to a strong yet partial rescue, Sema-1aΔ31–60 produces a blunted rescue, and Sema-1aΔC completely fails to rescue (Figure S4). Notably, Sema-1aΔC does rescue forward signaling in other systems (Godenschwege et al., 2002; Jeong et al., 2012). If forward signaling were contributing to midline crossing directly, then we would expect a partial rescue with the Sema-1aΔC transgenes, yet this is not what we see. These data indicate that Sema-1a promotes midline crossing through reverse signaling since it functions cell autonomously and its cytoplasmic domain is required. The results with the small cytoplasmic deletion also point to specific binding partners that may be important for mediating the downstream pathway involved in Sema-1a dependent midline crossing.
RhoGAPp190 and the negative regulation of Rho1 are required for Sema1a-mediated midline crossing
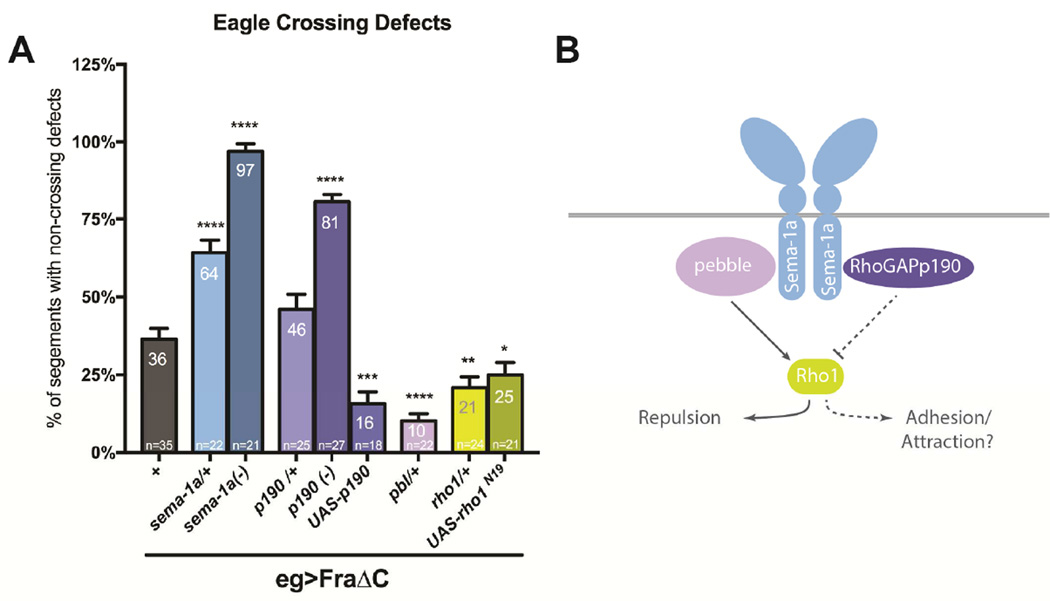
Pebble RhoGEF (Pbl) and RhoGAPp190 (p190) have been identified as effectors of Sema-1a reverse signaling in Drosophila motor neurons (Jeong et al., 2012). Both proteins bind the cytoplasmic region of Sema-1a, and both mutants display distinct defects in motor axon guidance. To investigate the roles of Pbl and p190 in midline crossing, we examined their genetic interactions with sema-1a and fra. Pbl and p190 are known to exert opposing effects on the actin cytoskeleton through regulation of the small GTPase, Rho1. Pebble positively regulates Rho1 and is proposed to function in concert with Sema-1a to produce a repulsive/de-adhesive response in motor neurons (Jeong et al., 2012), while RhoGAPp190 acts as a negative regulator of Rho1 and has been demonstrated to promote adhesion and branch stability (Billuart et al., 2001; Jeong et al., 2012). To investigate if these effectors modulate midline crossing downstream of Sema-1a, we examined whether heterozygosity for pbl or p190 mutations dominantly enhance crossing defects in the sensitized FraΔC background. Heterozygosity for p190 does not significantly enhance crossing defects (46%; Figure 5); however p190 zygotic null mutants produce a dramatic increase in crossing defects, similar to sema-1a nulls in the same background (81%; Figure 5). Like sema-1a mutants, p190 mutants exhibit no eagle axon crossing defects on their own. The enhancement in the screening background is significantly weaker than what is observed in the sema-1a nulls, suggesting the possibility that other downstream effectors may also be involved. In contrast, heterozygosity for pbl did not result in an enhancement of crossing defects. Instead, it suppressed these defects to 10% (Figure 5). We were unable to test pbl null mutants since pbl is required for cytokinesis, but we were able to evaluate their shared downstream target, rho1 (Prokopenko et al., 1999). Reductions in rho1 lead to a similar suppression as pbl, where only 21% of eagle neurons fail to cross the midline. Additionally, expression of a dominant negative Rho1 transgene also suppresses crossing defects. Finally, overexpression of p190 reduces the number of defects seen in FraΔC background to 16% of abdominal segments (Figure 5). Taken together, these results are consistent with the hypothesis that Sema-1a promotes midline crossing through RhoGAPp190 and the down regulation of Rho1.
Figure 5. RhoGAPp190 but not Pebble significantly enhances crossing defects in the FraΔC background.
(A) Quantification of EW midline crossing defects in the FraΔC screening background. Heterozygosity for RhoGAPp190 does not show a significant enhancement in crossing defects, however, RhoGAPp190 nulls do strongly enhance these defects (81%). pebble heterozygotes significantly suppressed these defects (10%). In addition, heterozygosity for rho1 or expression of a Rho1 dominant negative also suppresses the FraΔC phenotype. Data are represented as mean+SEM. n, number of embryos scored for each genotype. Significance was assessed by multiple comparisons using ANOVA (****p<0.0001, ***p<0.001, **p<0.01, *p<0.05). (B) Model of functional responses of Sema-1a reverse signaling through its downstream effectors.
The secreted Semas function to promote midline crossing
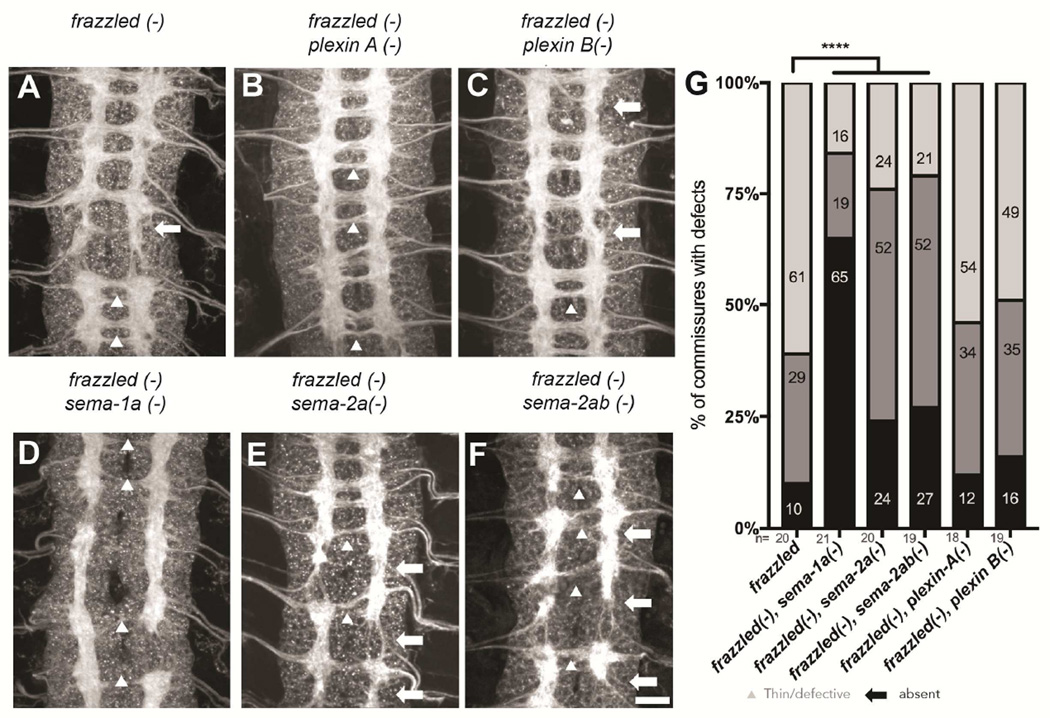
In order to better understand the cellular mechanism of Sema-1a-mediated midline crossing, we next sought to determine which, if any, of the known extra-cellular binding partners of Sema-1a might act as a ligand for reverse signaling in commissural neurons. We would expect that any component of the Sema-1a mediated midline crossing pathway should phenocopy the strong sema-1a, fra double mutant phenotype. Importantly, embryos lacking both fra and plexA or plexB fail to phenocopy sema-1a, fra double mutants, and the crossing defects are not significantly different from fra mutants alone (Figure 6). These results strongly suggest that Plexins are not contributing to Sema1a-dependent midline crossing. Furthermore, multiple alleles of plexA fail to enhance eagle crossing defects in the screening background (Figure S5) or in frazzled mutants (data not shown). In contrast, fra, sema-2a double mutants exhibit defects that resemble sema-1a, fra double mutants, and total defects are significantly enhanced compared to fra single mutants. Although total crossing defects are comparable between the sema-2a, fra double mutants and the sema-1a, fra double mutants, there is a distinct shift in the profile of these defects. The majority of defects identified in fra, sema-2a double mutants are thin/defective commissures while sema-1a, fra double mutants primarily exhibit absent commissures (Figure 6).
Figure 6. Sema-2a significantly enhances crossing defects in frazzled mutants while plexins do not.
(A–F) Stage 16 embryos of the indicated genotypes stained with anti-HRP antibodies. Arrowheads indicate thin/defective commissures and arrows indicate missing commissures. Scale bar represents 15µm (F). (A) fra (fra3/fra4) mutants show thin (10%) and occasionally missing commissures (29%). (B) fra; plexinA (plexAEY16548/plexAEY16548) double mutants resemble fra single mutants with 12% absent, 32% thin/defective and 54% wild-type commissures. (C) fra; plexin B (plexBKG00878/plexBKG00878) double mutants also show no significant enhancement of the fra single mutants with 16% absent, 35% thin/defective and 49% wild-type commissures. (D) Embryos mutant for sema-1a and fra display severe commissural defects. (E) Loss of sema-2a significantly worsens the crossing defects of fra single mutants with 24% absent, 52% thin/defective and only 24% wild-type commissures. (F) Triple mutants lacking fra, sema2a and sema-2b are not significantly different from the fra, sema-2a double mutants (G) Quantification of commissural defects as absent (black bar), thin/defective (dark gray) or wild-type (light grey) in the genotypes shown in (A–F). Data are represented as mean+SEM. n, number of embryos scored for each genotype. Significance was assessed by multiple comparisons using ANOVA (****p<0.0001).
One reason why the fra, sema-2a double mutants may fail to fully recapitulate the sema-1a, fra double mutants may be because of compensation by the other secreted semaphorin, Sema-2b. Sema-2a and Sema-2b show 70% amino acid identity and have been demonstrated to function redundantly in certain tissues (Sweeney et al., 2011; Wu et al., 2011). The secreted Semas are both expressed in the developing nerve cord at the time of commissure formation and both proteins are found to decorate the anterior and posterior commissures (Figure S5)(Emerson et al., 2013; Kolodkin et al., 1993; Wu et al., 2011). Sema-2a, however, displays a distinct enrichment at the midline (Figure S5) (Kolodkin et al., 1993; Wu et al., 2011). To test for a contribution of Sema-2b, we generated fra, sema-2a, sema-2b triple mutants. However, commissural defects in these triple mutants are not significantly different from those seen in the fra, sema-2a double mutants (Figure 6). Because it is difficult to capture subtle changes in commissural defects when examining the entire axon scaffold with HRP, we also evaluated fra, sema-2b double mutants in eagle neurons. We see a clear enhancement of crossing defects when sema-2b is lost (50%) compared to fra single mutants (27%). This enhancement is not as strong as the enhancement seen in fra, sema-2a double mutants (75%) (Figure S6). The fra, sema2ab triple mutants display defects similar to the double mutants (58%). Since the triple mutant fails to recapitulate the sema-1a, fra double mutant, it is likely that the secreted Semas are not the only upstream factors in the Sema-1a pathway.
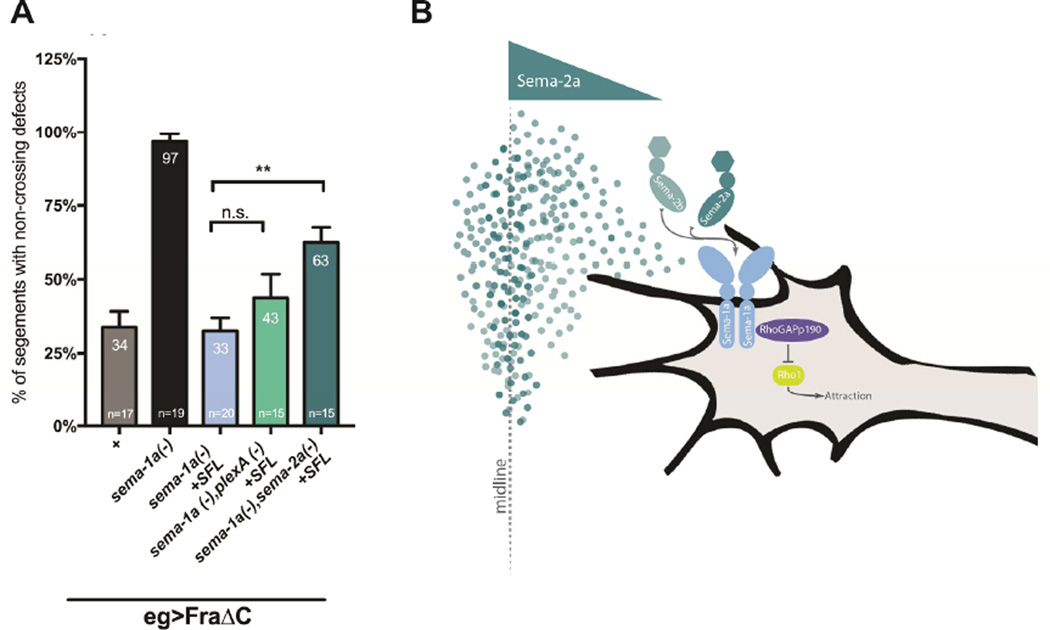
In order to more directly assess if Sema-1a mediates midline crossing in a PlexA or Sema-2 dependent manner, we examined the ability of UAS Sema-1a to rescue sema-1a-dependent crossing defects in the absence of either plexA or sema-2a. If either gene is a required component of the Sema-1a pathway, the ability of UAS Sema-1a to rescue should be suppressed when plexA or sema-2a are also mutant. Therefore, we evaluated the degree of rescue when Sema-1a is expressed in a sema-1a;;plexA double mutant with FraΔC in eagle neurons. Sema-1a is still able to rescue crossing in the absence of plexA, strongly arguing that Sema-1a mediated midline crossing is PlexA independent. However, Sema-1a is not able to rescue to the same extent in the absence of Sema-2a (Figure 7). These data indicate that Sema-2a, and not PlexA, contributes to the Sema-1a mediated midline crossing pathway.
Figure 7. Sema-2a is required for Sema-1a mediated midline crossing.
(A) Histogram quantifies EW midline crossing defects in sema-1a null mutants carrying the transgenes for egGal4 and UAS-FraΔC. This background shows strong EW crossing defects (97%) that can be rescue cell autonomously when full length Sema-1a is expressed selectively in eagle neurons (33%). In the absence of plexA this rescue is not significantly reduced (43%). However, loss of sema-2a significantly suppresses this rescue and embryos still exhibit severe crossing defects (63%) suggesting that sema-2a is required for sema-1a mediated midline crossing. Data are represented as mean+SEM. n, number of embryos scored for each genotype. Significance was assessed by multiple comparisons using ANOVA (**p<0.01). (B) Model of Sema-1a mediated midline crossing.
DISCUSSION
These data demonstrate that Sema-1a represents an important pathway for promoting midline crossing. We find that Sema-1a not only functions as a receptor to promote midline crossing, but it does so independently of its canonical binding partner PlexA. Our genetic data suggest that the secreted Semas represent components of the Sema-1a ligand in this context. Furthermore, the spatial distribution of these components, as well as the known roles of the downstream effectors, suggest this Sema-1a signaling pathway results in an attractive or adhesive response, rather than the repulsive response that is typically associated with Sema/Plexin signaling. In most systems where Sema-1a reverse signaling has been identified, forward signaling has also been found to function. This bidirectional signaling has made it difficult to uncouple the two signaling cascades and determine the mechanism of Sema-1a reverse signaling. We find that specific genetic manipulations at the ventral midline allow us to establish a system where the two pathways can be more clearly separated. In this way, we can begin to define the Sema-1a reverse signaling contribution to midline crossing.
Sema-1a promotes midline crossing
Sema-1a has never before been associated with midline crossing since the null mutants alone show no commissural defects. Eagle neurons in sema-1a mutants also show no significant reduction in crossing (Figure 1). The effect of Sema-1a loss of function is only apparent when the major attractive pathway of Netrin/Fra signaling is removed. We observed this interaction in a number of different backgrounds, first with the Fra dominant negative (FraΔC), as well as with the fra and NetAB mutants, and then most dramatically with the sema-1a, fra or NetAB; sema-1a double mutants. Our lab previously uncovered a Netrin-independent role for Fra as well as a role for Robo2 in promoting midline crossing (Evans et al., 2015; Neuhaus-Follini and Bashaw, 2015b; Yang et al., 2009). Both of these pathways function by negatively regulating Robo1 repulsion at the midline. In order to understand how redundant/ convergent these pathways may be, we further explored the interactions between Sema-1a and known midline pathways. Genetic interactions clearly reveal an independent function between Fra/Netrin chemoattraction and Sema-1a. Genetic interactions with robo1, slit double heterozygotes suggest that Sema-1a is unlikely to function as another anti-repulsive mechanism (Figure S3). Additionally, Robo1 protein expression does not appear to be upregulated in sema-1a mutants (Figure S3). Taken together, our observations indicate that Sema-1a promotes midline crossing independently of known pathways.
Sema-1a mediates midline crossing through reverse signaling in commissural neurons
Reverse signaling through transmembrane Semas has been demonstrated in both invertebrates and vertebrates, where the class 6 Semas show a particular similarity with Drosophila Sema-1a. The role of Sema6D in endocardial cell migration was the first in vivo demonstration of reverse signaling in vertebrates (Toyofuku et al., 2004). More recently, studies of semaphorin reverse signaling in neurons have revealed that class 6 Semas may have roles in axon guidance. For example, a recent study in chick demonstrated that Sema6B functions as a receptor in post-crossing commissural neurons potentially by promoting an outgrowth response (Andermatt et al., 2014). Evidence of a more instructional role for reverse signaling was found in a subset of On direction-selective ganglion cells (OnDSGCs). Here, Sema6A mediates axonal targeting to the accessory optic system (AOS) through an attractive response to Plexin A2 and A4 (Sun et al., 2015). Although it is clear that the capability of transmembrane Semas to signal in reverse and function as axon guidance receptors is highly conserved, it had not been previously known whether Sema reverse signaling contributes directly to midline crossing.
RhoGAPp190 mediates Sema-1a reverse signaling to promote midline crossing
In the majority of cases, Semaphorin reverse signaling promotes repulsive guidance in response to Plexin receptors, yet there are attractive signaling outputs and binding partners as well. Two classes of neurons in the Drosophila visual system, the laminar neurons and the photoreceptors were both found to employ Sema-1a reverse signaling and both bound the canonical binding partner PlexA; however, the laminar neurons exhibit a repulsive response to PlexA, while the photoreceptors show an adhesive response (Cafferty et al., 2006; Hsieh et al., 2014; Pecot et al., 2013). The discovery of competitive downstream effectors (Pbl and RhoGAPp190) with opposing effects on Rho1 begins to explain how Sema-1a reverse signaling could have multiple, and even opposite outputs. It seems likely that there are additional factors that modulate the activity of these Pbl and P190 and impact the ultimate axonal response. For instance, Src family kinases lead to inhibition of p190 activity by phosphorylating p190 within the GTP binding domain (Billuart et al., 2001; Brouns et al., 2001; Roof et al., 2000). Indeed, we have found that src kinases antagonize midline crossing in a Netrin/Frazzled independent fashion (O’Donnell and Bashaw, 2013b), suggesting src may modulate Sema-1a reverse signaling through its effect on P190.
Interestingly, the Sema-1a mediated adhesive response uncovered in the photoreceptors is also dependent on the down regulation of Rho1 (Hsieh et al., 2014). However, in the context of photoreceptor axon guidance, adhesion is mediated by FasII, which is not expressed in the commissural eagle neurons. The implication of p190 as a downstream effector in the context of Sema-1a mediated midline crossing is intriguing since it represents an alternative output for Sema-1a reverse signaling. While Pbl mediates repulsion/defasciculation and target recognition in the motor neurons, p190 is thought to control fasciculation by antagonizing Pbl activity. p190 has been shown to stabilize branches and promote adhesion in other systems, but negative regulation of Rho1 may also promote attraction (Billuart et al., 2001; Ng and Luo, 2004; O’Donnell et al., 2009). Complete loss of p190 in the screening background leads to defects that are similar to complete loss of sema-1a, yet significantly less severe (Figure 5). This may indicate the contribution of additional downstream effectors. While the cytoplasmic region between amino acids 31–60 of Sema-1a provides the binding site for Pbl and p190, it also includes a putative Enabled (Ena) binding site (LPQP). This Ena binding site is required for Sema-1a reverse signaling in the giant fiber (Godenschwege et al., 2002); however, Ena does not appear to contribute Sema-1a’s activity to promote midline crossing (Figure S7). It is likely that additional context specific signaling effectors await discovery.
The secreted Sema2s function as attractive/ adhesive ligands for Sema-1a mediated midline crossing
The genetic interactions we tested implicate the secreted Sema-2s as the potential signaling partners for Sema-1a in mediating midline crossing. Sweeney et al. clearly demonstrate that the Sema-1a ectodomain selectively binds to tissue where Sema-2a is overexpressed, yet evidence for a direct physical interaction is still lacking (Sweeney et al., 2011). Although this interaction is unlikely to be direct, we show that Sema-1a requires Sema-2a to rescue midline crossing (Figure 7). While an interaction between the Sema-2s and Sema-1a reverse signaling was initially proposed in the olfactory system, we show the first genetic evidence to directly link the Sema-2s to a Sema-1a function. Furthermore, the double mutant phenotypes with fra demonstrate that the secreted Semas are required for axons to cross the midline (Figure 6 and Figure S6). Surprisingly, compound mutants with fra also revealed that the removal of sema-2a and sema-2b together (fra, sema-2ab) fails to fully recapitulate the loss of sema-1a (sema-1a, fra) in the eagle neurons or the CNS as a whole (Figure 6 and Figure S6). This may suggest the possibility of additional upstream signaling components and a potentially more complex function for Sema-1a reverse signaling. We used the FraΔC screen to test all the identified Semas and Plexins (including Sema-1b and Sema-5c; data not shown and Figure S7) yet failed to uncover additional enhancers, suggesting that additional factors await discovery. We propose a model where the secreted Sema2s act as attractive cues to promote midline crossing as the simplest interpretation of the observed phenotypes. The medial expression of the secreted Sema2s, in particular Sema-2a, suggests that they signal directional information rather than promote permissive adhesion.
While we demonstrate a role for Sema-1a reverse signaling in pre-crossing commissural axons, forward signaling is important for the formation of longitudinal tracts post-crossing (Jeong et al., 2012; Terman and Kolodkin, 2004; Yang and Terman, 2012; Yu et al., 1998). The midline, as an intermediate target, may offer a unique context for the shift between forward and reverse signaling. Further investigation to uncover regulatory components of the Sema-1a reverse signaling pathway would prove illuminating in understanding how these distinct outputs are achieved.
EXPERIMENTAL PROCEDURES
Genetics
The following Drosophila mutant alleles were used: fra3, fra4, fra6, NetAB, egMZ360 (eg-GAL4), slit2, robo-1GA285. The following stocks were from Bloomington: sema-1aP1, plexin A EY16548, plexin BKG00878, pbl2, and Rho172F. The following stocks were gifts from A. Kolodkin: sema-2aB65, sema-2bC4, sema-2abA15, p1902 and the PlexinA and Sema2b Bac transgenes. The sema-1a artificial exon was a gift from L. Zipursky. The following transgenes were used: UAS-FraΔC, UAS-sema-1aFL, UAS-sema-1aΔ31–60, UAS-sema-1aECFC, UAS-FLP Recombinase, UAS-26XLexAopmyrGFP, UAS-mycp190, and UAS-RhoN19. GAL4 drivers used were elav-GAL4 and eg-GAL4. All crosses were carried out at 25°C. Embryos were genotyped using balancer chromosomes carrying lacZ markers or by the presence of epitope-tagged transgenes. See Supplemental Table 1 for a complete list of genotypes for all of the figures.
Immunofluorescence and imaging
Dechorionated, formaldehyde-fixed, methanol devitellinized embryos were fluorescently stained as previously described (Bashaw, 2010b). Live-dissected embryos were stained as previously described (Bashaw, 2010a). The following primary antibodies were used: mouse anti-1D4/FasII [Developmental Studies Hybridoma Bank (DSHB); 1:100], mouse anti-Beta gal [DSHB; 1:150], mouse anti-Robo [DSHB; 1:50], mouse anti-Myc [DSHB (9E10); 1:500] rabbit anti-GFP [Invitrogen #A11122); 1:500], mouse mAb anti-V5 [Serotec; 1:200], Mouse anti-HA [Covance (16B12) 1:250], Mouse anti-Sema2a [DSHB 1:10], Alexa647-conjugated goat anti-HRP [1:500, Jackson Immunoresearch (#123-605-021); 1:500]. Cyanine 3-conjugated goat anti-rabbit [Jackson; 1:1000], Alexa488-conjugated goat anti-mouse [Molecular Probes; 1:500] were used as secondary antibodies. Images were acquired using a spinning disk confocal system (PerkinElmer) built on a Nikon Ti-U inverted microscope using a Nikon OFN25 60X or 40X objective with a Hamamatsu C10600-10B CCD camera and Yokogawa CSU-10 scanner head with Volocity imaging software. Images were processed using ImageJ.
Phenotypic Quantification
For a detailed description of the quantification methods, please see the supplemental experimental procedures.
Statistical Analysis
All statistical analysis was performed in GraphPad. Comparisons were made between genotypes using ANOVA. For multiple comparisons, significance was assessed by using a Bonferroni correction.
Supplementary Material
Highlights.
Sema-1a functions independently of Netrin to promote midline crossing
Sema-1a mediates midline crossing through reverse signaling in commissural neurons
RhoGAPp190 and the negative regulation of Rho1 is required for midline crossing
The Secreted Sema2s function as attractive or adhesive midline cues
Acknowledgments
We would like to thank members of the Bashaw lab past and present for their constructive discussions throughout this work. In particular, we thank Jonathan Levin for help conducting the primary screen and Celine Santiago for help constructing Supplemental Figure 5. We thank Alex Kolodkin, Liqun Luo and Larry Zipursky for providing countless reagents.
Funding
MHF was supported by NIH T32 GM 7517-34, T32 HD007516-15, and F31 NSO86340-01. This work was supported by NSF Grant IOS-1355181, and NIH Grants R01NS-046333 and R01NS-054739 to G.J.B.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions
MHF, GJB, Conception and design, Acquisition of data, Analysis and interpretation of data, drafting or revising the article. ER, Acquisition of data, Analysis and interpretation of data.
References
- Andermatt I, Wilson NH, Bergmann T, Mauti O, Gesemann M, Sockanathan S, Stoeckli ET. Semaphorin 6B acts as a receptor in post-crossing commissural axon guidance. Development. 2014;141:3709–3720. doi: 10.1242/dev.112185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayoob JC, Terman JR, Kolodkin AL. Drosophila Plexin B is a Sema-2a receptor required for axon guidance. Development. 2006;133:2125–2135. doi: 10.1242/dev.02380. [DOI] [PubMed] [Google Scholar]
- Bashaw GJ. Live dissection and surface labeling of proteins in Drosophila embryos. Cold Spring Harb Protoc. 2010a;2010 doi: 10.1101/pdb.prot5504. db prot5504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashaw GJ. Visualizing axons in the Drosophila central nervous system using immunohistochemistry and immunofluorescence. Cold Spring Harb Protoc. 2010b;2010 doi: 10.1101/pdb.prot5503. db prot5503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billuart P, Winter CG, Maresh A, Zhao X, Luo L. Regulating axon branch stability: the role of p190 RhoGAP in repressing a retraction signaling pathway. Cell. 2001;107:195–207. doi: 10.1016/s0092-8674(01)00522-0. [DOI] [PubMed] [Google Scholar]
- Brouns MR, Matheson SF, Settleman J. p190 RhoGAP is the principal Src substrate in brain and regulates axon outgrowth, guidance and fasciculation. Nature cell biology. 2001;3:361–367. doi: 10.1038/35070042. [DOI] [PubMed] [Google Scholar]
- Cafferty P, Yu L, Long H, Rao Y. Semaphorin-1a functions as a guidance receptor in the Drosophila visual system. J Neurosci. 2006;26:3999–4003. doi: 10.1523/JNEUROSCI.3845-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charoy C, Nawabi H, Reynaud F, Derrington E, Bozon M, Wright K, Falk J, Helmbacher F, Kindbeiter K, Castellani V. gdnf activates midline repulsion by Semaphorin3B via NCAM during commissural axon guidance. Neuron. 2012;75:1051–1066. doi: 10.1016/j.neuron.2012.08.021. [DOI] [PubMed] [Google Scholar]
- Charron F, Stein E, Jeong J, McMahon AP, Tessier-Lavigne M. The morphogen sonic hedgehog is an axonal chemoattractant that collaborates with netrin-1 in midline axon guidance. Cell. 2003;113:11–23. doi: 10.1016/s0092-8674(03)00199-5. [DOI] [PubMed] [Google Scholar]
- Delloye-Bourgeois C, Jacquier A, Charoy C, Reynaud F, Nawabi H, Thoinet K, Kindbeiter K, Yoshida Y, Zagar Y, Kong Y, et al. PlexinA1 is a new Slit receptor and mediates axon guidance function of Slit C-terminal fragments. Nat Neurosci. 2015;18:36–45. doi: 10.1038/nn.3893. [DOI] [PubMed] [Google Scholar]
- Emerson MM, Long JB, Van Vactor D. Drosophila semaphorin2b is required for the axon guidance of a subset of embryonic neurons. Developmental dynamics : an official publication of the American Association of Anatomists. 2013;242:861–873. doi: 10.1002/dvdy.23979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans TA, Santiago C, Arbeille E, Bashaw GJ. Robo2 acts in trans to inhibit Slit-Robo1 repulsion in pre-crossing commissural axons. eLife. 2015;4:e08407. doi: 10.7554/eLife.08407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbe DS, O’Donnell M, Bashaw GJ. Cytoplasmic domain requirements for Frazzled-mediated attractive axon turning at the Drosophila midline. Development. 2007;134:4325–4334. doi: 10.1242/dev.012872. [DOI] [PubMed] [Google Scholar]
- Godenschwege TA, Hu H, Shan-Crofts X, Goodman CS, Murphey RK. Bidirectional signaling by Semaphorin 1a during central synapse formation in Drosophila. Nat Neurosci. 2002;5:1294–1301. doi: 10.1038/nn976. [DOI] [PubMed] [Google Scholar]
- Godenschwege TA, Kristiansen LV, Uthaman SB, Hortsch M, Murphey RK. A conserved role for Drosophila Neuroglian and human L1-CAM in central-synapse formation. Curr Biol. 2006;16:12–23. doi: 10.1016/j.cub.2005.11.062. [DOI] [PubMed] [Google Scholar]
- Harris R, Sabatelli LM, Seeger MA. Guidance cues at the Drosophila CNS midline: identification and characterization of two Drosophila Netrin/UNC-6 homologs. Neuron. 1996;17:217–228. doi: 10.1016/s0896-6273(00)80154-3. [DOI] [PubMed] [Google Scholar]
- Hernandez-Enriquez B, Wu Z, Martinez E, Olsen O, Kaprielian Z, Maness PF, Yoshida Y, Tessier-Lavigne M, Tran TS. Floor plate-derived neuropilin-2 functions as a secreted semaphorin sink to facilitate commissural axon midline crossing. Genes Dev. 2015;29:2617–2632. doi: 10.1101/gad.268086.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashijima S, Shishido E, Matsuzaki M, Saigo K. eagle, a member of the steroid receptor gene superfamily, is expressed in a subset of neuroblasts and regulates the fate of their putative progeny in the Drosophila CNS. Development. 1996;122:527–536. doi: 10.1242/dev.122.2.527. [DOI] [PubMed] [Google Scholar]
- Hsieh HH, Chang WT, Yu L, Rao Y. Control of axon-axon attraction by Semaphorin reverse signaling. Proc Natl Acad Sci U S A. 2014;111:11383–11388. doi: 10.1073/pnas.1321433111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain RA, Bell H, Lim A, Chien CB, Granato M. Mirror movement-like defects in startle behavior of zebrafish dcc mutants are caused by aberrant midline guidance of identified descending hindbrain neurons. J Neurosci. 2014;34:2898–2909. doi: 10.1523/JNEUROSCI.2420-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong S, Juhaszova K, Kolodkin AL. The Control of semaphorin-1a-mediated reverse signaling by opposing pebble and RhoGAPp190 functions in drosophila. Neuron. 2012;76:721–734. doi: 10.1016/j.neuron.2012.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongbloets BC, Pasterkamp RJ. Semaphorin signalling during development. Development. 2014;141:3292–3297. doi: 10.1242/dev.105544. [DOI] [PubMed] [Google Scholar]
- Kennedy TE, Serafini T, de la Torre JR, Tessier-Lavigne M. Netrins are diffusible chemotropic factors for commissural axons in the embryonic spinal cord. Cell. 1994;78:425–435. doi: 10.1016/0092-8674(94)90421-9. [DOI] [PubMed] [Google Scholar]
- Khare N, Fascetti N, DaRocha S, Chiquet-Ehrismann R, Baumgartner S. Expression patterns of two new members of the Semaphorin family in Drosophila suggest early functions during embryogenesis. Mech Dev. 2000;91:393–397. doi: 10.1016/s0925-4773(99)00297-x. [DOI] [PubMed] [Google Scholar]
- Kolodkin AL, Matthes DJ, Goodman CS. The semaphorin genes encode a family of transmembrane and secreted growth cone guidance molecules. Cell. 1993;75:1389–1399. doi: 10.1016/0092-8674(93)90625-z. [DOI] [PubMed] [Google Scholar]
- Kolodziej PA, Timpe LC, Mitchell KJ, Fried SR, Goodman CS, Jan LY, Jan YN. frazzled encodes a Drosophila member of the DCC immunoglobulin subfamily and is required for CNS and motor axon guidance. Cell. 1996;87:197–204. doi: 10.1016/s0092-8674(00)81338-0. [DOI] [PubMed] [Google Scholar]
- Komiyama T, Sweeney LB, Schuldiner O, Garcia KC, Luo L. Graded expression of semaphorin-1a cell-autonomously directs dendritic targeting of olfactory projection neurons. Cell. 2007;128:399–410. doi: 10.1016/j.cell.2006.12.028. [DOI] [PubMed] [Google Scholar]
- Mitchell KJ, Doyle JL, Serafini T, Kennedy TE, Tessier-Lavigne M, Goodman CS, Dickson BJ. Genetic analysis of Netrin genes in Drosophila: Netrins guide CNS commissural axons and peripheral motor axons. Neuron. 1996;17:203–215. doi: 10.1016/s0896-6273(00)80153-1. [DOI] [PubMed] [Google Scholar]
- Nawabi H, Briancon-Marjollet A, Clark C, Sanyas I, Takamatsu H, Okuno T, Kumanogoh A, Bozon M, Takeshima K, Yoshida Y, et al. A midline switch of receptor processing regulates commissural axon guidance in vertebrates. Genes Dev. 2010;24:396–410. doi: 10.1101/gad.542510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhaus-Follini A, Bashaw GJ. Crossing the embryonic midline: molecular mechanisms regulating axon responsiveness at an intermediate target. Wiley interdisciplinary reviews Developmental biology. 2015a doi: 10.1002/wdev.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhaus-Follini A, Bashaw GJ. The Intracellular Domain of the Frazzled/DCC Receptor Is a Transcription Factor Required for Commissural Axon Guidance. Neuron. 2015b;87:751–763. doi: 10.1016/j.neuron.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng J, Luo L. Rho GTPases regulate axon growth through convergent and divergent signaling pathways. Neuron. 2004;44:779–793. doi: 10.1016/j.neuron.2004.11.014. [DOI] [PubMed] [Google Scholar]
- O’Donnell M, Chance RK, Bashaw GJ. Axon growth and guidance: receptor regulation and signal transduction. Annu Rev Neurosci. 2009;32:383–412. doi: 10.1146/annurev.neuro.051508.135614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell MP, Bashaw GJ. Distinct functional domains of the Abelson tyrosine kinase control axon guidance responses to Netrin and Slit to regulate the assembly of neural circuits. Development. 2013a;140:2724–2733. doi: 10.1242/dev.093831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell MP, Bashaw GJ. Src Inhibits Midline Axon Crossing Independent of Frazzled/Deleted in Colorectal Carcinoma (DCC) Receptor Tyrosine Phosphorylation. J Neurosci. 2013b;33:305–314. doi: 10.1523/JNEUROSCI.2756-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parra LM, Zou Y. Sonic hedgehog induces response of commissural axons to Semaphorin repulsion during midline crossing. Nat Neurosci. 2010;13:29–35. doi: 10.1038/nn.2457. [DOI] [PubMed] [Google Scholar]
- Pasterkamp RJ. Getting neural circuits into shape with semaphorins. Nat Rev Neurosci. 2012;13:605–618. doi: 10.1038/nrn3302. [DOI] [PubMed] [Google Scholar]
- Pecot MY, Tadros W, Nern A, Bader M, Chen Y, Zipursky SL. Multiple interactions control synaptic layer specificity in the Drosophila visual system. Neuron. 2013;77:299–310. doi: 10.1016/j.neuron.2012.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokopenko SN, Brumby A, O’Keefe L, Prior L, He Y, Saint R, Bellen HJ. A putative exchange factor for Rho1 GTPase is required for initiation of cytokinesis in Drosophila. Genes Dev. 1999;13:2301–2314. doi: 10.1101/gad.13.17.2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabe Bernhardt N, Memic F, Gezelius H, Thiebes AL, Vallstedt A, Kullander K. DCC mediated axon guidance of spinal interneurons is essential for normal locomotor central pattern generator function. Dev Biol. 2012;366:279–289. doi: 10.1016/j.ydbio.2012.03.017. [DOI] [PubMed] [Google Scholar]
- Roof RW, Dukes BD, Chang JH, Parsons SJ. Phosphorylation of the p190 RhoGAP N-terminal domain by c-Src results in a loss of GTP binding activity. FEBS letters. 2000;472:117–121. doi: 10.1016/s0014-5793(00)01439-3. [DOI] [PubMed] [Google Scholar]
- Ruiz de Almodovar C, Coulon C, Salin PA, Knevels E, Chounlamountri N, Poesen K, Hermans K, Lambrechts D, Van Geyte K, Dhondt J, et al. Matrix-binding vascular endothelial growth factor (VEGF) isoforms guide granule cell migration in the cerebellum via VEGF receptor Flk1. J Neurosci. 2010;30:15052–15066. doi: 10.1523/JNEUROSCI.0477-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafini T, Kennedy TE, Galko MJ, Mirzayan C, Jessell TM, Tessier-Lavigne M. The netrins define a family of axon outgrowth-promoting proteins homologous to C. elegans UNC-6. Cell. 1994;78:409–424. doi: 10.1016/0092-8674(94)90420-0. [DOI] [PubMed] [Google Scholar]
- Sloan TF, Qasaimeh MA, Juncker D, Yam PT, Charron F. Integration of shallow gradients of Shh and Netrin-1 guides commissural axons. PLoS biology. 2015;13:e1002119. doi: 10.1371/journal.pbio.1002119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srour M, Riviere JB, Pham JM, Dube MP, Girard S, Morin S, Dion PA, Asselin G, Rochefort D, Hince P, et al. Mutations in DCC cause congenital mirror movements. Science. 2010;328:592. doi: 10.1126/science.1186463. [DOI] [PubMed] [Google Scholar]
- Sun LO, Brady CM, Cahill H, Al-Khindi T, Sakuta H, Dhande OS, Noda M, Huberman AD, Nathans J, Kolodkin AL. Functional assembly of accessory optic system circuitry critical for compensatory eye movements. Neuron. 2015;86:971–984. doi: 10.1016/j.neuron.2015.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney LB, Chou YH, Wu Z, Joo W, Komiyama T, Potter CJ, Kolodkin AL, Garcia KC, Luo L. Secreted semaphorins from degenerating larval ORN axons direct adult projection neuron dendrite targeting. Neuron. 2011;72:734–747. doi: 10.1016/j.neuron.2011.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terman JR, Kolodkin AL. Nervy links protein kinase a to plexin-mediated semaphorin repulsion. Science. 2004;303:1204–1207. doi: 10.1126/science.1092121. [DOI] [PubMed] [Google Scholar]
- Toyofuku T, Zhang H, Kumanogoh A, Takegahara N, Yabuki M, Harada K, Hori M, Kikutani H. Guidance of myocardial patterning in cardiac development by Sema6D reverse signalling. Nature cell biology. 2004;6:1204–1211. doi: 10.1038/ncb1193. [DOI] [PubMed] [Google Scholar]
- Winberg ML, Noordermeer JN, Tamagnone L, Comoglio PM, Spriggs MK, Tessier-Lavigne M, Goodman CS. Plexin A is a neuronal semaphorin receptor that controls axon guidance. Cell. 1998;95:903–916. doi: 10.1016/s0092-8674(00)81715-8. [DOI] [PubMed] [Google Scholar]
- Wu Z, Sweeney LB, Ayoob JC, Chak K, Andreone BJ, Ohyama T, Kerr R, Luo L, Zlatic M, Kolodkin AL. A combinatorial semaphorin code instructs the initial steps of sensory circuit assembly in the Drosophila CNS. Neuron. 2011;70:281–298. doi: 10.1016/j.neuron.2011.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Garbe DS, Bashaw GJ. A frazzled/DCC-dependent transcriptional switch regulates midline axon guidance. Science. 2009;324:944–947. doi: 10.1126/science.1171320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T, Terman JR. 14-3-3epsilon couples protein kinase A to semaphorin signaling and silences plexin RasGAP-mediated axonal repulsion. Neuron. 2012;74:108–121. doi: 10.1016/j.neuron.2011.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu HH, Araj HH, Ralls SA, Kolodkin AL. The transmembrane Semaphorin Sema I is required in Drosophila for embryonic motor and CNS axon guidance. Neuron. 1998;20:207–220. doi: 10.1016/s0896-6273(00)80450-x. [DOI] [PubMed] [Google Scholar]
- Yu HH, Huang AS, Kolodkin AL. Semaphorin-1a acts in concert with the cell adhesion molecules fasciclin II and connectin to regulate axon fasciculation in Drosophila. Genetics. 2000;156:723–731. doi: 10.1093/genetics/156.2.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Y, Stoeckli E, Chen H, Tessier-Lavigne M. Squeezing axons out of the gray matter: a role for slit and semaphorin proteins from midline and ventral spinal cord. Cell. 2000;102:363–375. doi: 10.1016/s0092-8674(00)00041-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.