Abstract
Adolescent ethanol exposure increases risky choice and alters corticotropin releasing factor (CRF) systems in adulthood. The impact of stress on risky choice after adolescent intermittent ethanol (AIE) exposure is not known. We investigated time-specific effects of AIE vapor exposure during early adolescence on risky choice after stress or no stress in adulthood. Male Wistar rats were exposed to air or AIE vapor on postnatal days 28–42 (adolescence) and were exposed to 10 days of social defeat or no stress on postnatal days 172–181 (adulthood). Risky choice was assessed in the probability discounting task under baseline conditions and after days 1 and 10 of social defeat. CRF and CRF receptor 1 (CRFR1) mRNA levels were assessed in the prefrontal cortex (PFC) and the central nucleus of the amygdala (CeA) 24 h post-stress to evaluate persistent effects of stress on the brain. AIE exposure had no effect on risky choice either at baseline or after social defeat. Additionally, neither acute nor chronic social defeat affected risky choice in air-exposed rats. In the PFC, chronic social defeat selectively decreased CRF mRNA levels in air-exposed rats and increased CRFR1 mRNA levels in all rats. AIE exposure increased CRF mRNA levels in the CeA with no effect of social stress. Our results indicate no effect of ethanol exposure via vapor during early adolescence on risky choice, while our previous findings indicated that AIE exposure via gavage affected risky choice. Both AIE exposure and social defeat altered CRF and CRFR1 mRNA levels in the brain.
Keywords: Adolescent ethanol, Probability discounting, Risky decision making, Social defeat, CRF, CRFR1
1. Introduction
Extensive neurodevelopment occurs during adolescence, including in prefrontal cortical brain regions involved in the evaluation of risks and rewards [1,2]. Alcohol exposure may interfere with this ongoing neuromaturation, leading to increased risk taking in adulthood. Studies in humans have shown that high school students [3], university undergraduates [4,5] and young adults [6] with a history of heavy alcohol use make more risky decisions compared to abstainers and moderate users. Rodent studies have demonstrated that adolescent ethanol exposure increases risk-based choices in the probability discounting task [7–12].
Stress and stress hormones are also known to promote different types of risky decision making in humans [13–16]. In rats, exposure to restraint stress combined with foot-shock impaired flexible decision making [17]. Acute restraint stress disrupted cost/benefit decision making in rats, although the suboptimal decision-making pattern may not be completely disadvantageous in this task [18]. In the delayed-discounting task, exposure to pharmacological stress impaired flexibility in impulsive decision making [19], while restraint stress had no effect on impulsive decision making [18]. These findings suggest that the effects of acute stress on risky choice depend on the form of decision making. Knowledge of the effects of stress on risk-based decision making and the long-term effects alcohol exposure during adolescence on adult risky choice in response to stress are currently lacking.
Exposure to stress results in the release of corticotropin releasing factor (CRF) from the paraventricular nucleus (PVN) of the hypothalamus and the subsequent synthesis and release of glu-cocorticoids from the adrenal cortex. The release of CRF from the PVN is regulated by negative feedback loops involving brain structures affected by adolescent ethanol exposure, including the prefrontal cortex (PFC) and amygdala [20]. Previous studies demonstrated that adult rats with a history of adolescent ethanol exposure had decreased CRF expressing neurons in the amygdala [21,22]. Notably, the PFC and amygdala are both involved in risky choice in the probability discounting task [23,24]. We hypothesized here that dysregulation in the CRF system in the PFC and the central nucleus of the amygdala (CeA) induced by adolescent ethanol exposure would be associated with risky choice in the probability discounting task under baseline and stressful conditions in adulthood.
The present studies were conducted to evaluate the long-term effects of adolescent intermittent ethanol (AIE) vapor exposure on adult risky choice behavior in the probability discounting task. Our previous work demonstrated that AIE exposure via intragastric gavage throughout adolescence (postnatal days 28–53, PND) increased risky choice in adult rats [7]. However, recent studies suggested that there may be distinct consequences of ethanol exposure in early versus late adolescence, PND 25–45 and PND 45–65, respectively [25]. In the present studies, rats were exposed to AIE during early adolescence to further characterize time-specific effects of AIE exposure on risky choice. In addition, the present studies extended our work to AIE exposure via ethanol vapor. Although the ethanol vapor exposure procedure lacks face validity, it has several important advantages. Specifically, ethanol vapor inhalation is a non-invasive procedure that allows for precise control of the dose, duration, and pattern of ethanol exposure, as well as maintenance of stable blood ethanol concentrations for extended periods of time in the presence of normal body weight regulation and general ingestive behavior [26,27]. Importantly, in contrast to the intragastric gavage procedure [28,29], our previous work showed that the vapor exposure procedure did not change plasma adrenocorticotropic hormone and corticosterone levels in control rats that did not receive ethanol, suggesting the absence of a physiological stress response to the vapor procedure [30]. In the present studies, we used the identical ethanol vapor exposure procedure and the same equipment as in our previous work. Thus, the use of vapor exposure limits the potentially confounding effects of stress experienced during AIE exposure on risky choice under baseline conditions and in response to stress during adulthood. We showed previously that the AIE vapor exposure used in the present studies altered the adult CRF system in the hypothalamic-pituitary-adrenal axis that regulates the stress response [31,32]. Therefore, we also investigated the effects of acute and chronic social defeat stress in AIE vapor- and air-exposed rats. The social defeat procedure, consisting of confrontations with an aggressive conspecific, is often used as an ecological stressor in rodents. Social defeat has been shown to produce depression-like behavior [33], but its effects on risky choice have not been investigated previously. Finally, we assessed CRF and CRF receptor type 1 (CRFR1) mRNA levels in the PFC and CeA after chronic social defeat because these brain areas are involved in responsiveness to social stress [34].
2. Methods
2.1. Subjects
Wistar male rats (n = 46, Charles River, Raleigh, NC) arrived at the Salk Institute vivarium on postnatal day (PND) 15 in groups of 6 male pups together with a nursing female. Pups were weaned on postnatal day 21 (PND 21) and pair-housed in a humidity- and temperature-controlled vivarium on a 12 h/12 h reverse light/dark cycle. Food and water were available ad libitum. Adolescent rats were exposed to AIE vapor (AIE-exposed rats) or air (air-exposed control rats), then transferred to the University of California San Diego (UCSD) for behavioral testing (see below). Upon arrival at UCSD, rats underwent the required 42 day quarantine period. After release from quarantine, rats were pair-housed in a humidity- and temperature-controlled vivarium on a 12 h/12 h reverse light/dark cycle. Rats were food restricted during behavioral training and testing throughout the experiment (PND106-182), and received 18 g of chow per day in addition to food pellets earned in the behavioral session. Water was available ad libitum. All of the procedures were conducted in accordance with the guidelines of the American Association for the Accreditation of Laboratory Animal Care and the National Research Council’s Guide for Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at UCSD and Salk Institute.
2.2. AIE vapor exposure
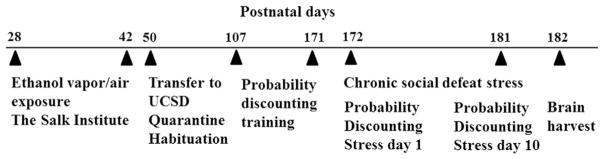
A timeline of the experimental events, including AIE exposure, behavioral testing, chronic social defeat exposure and brain sample collection, is presented in Fig. 1.
Fig. 1.
Timeline of experimental design showing the sequence of AIE exposure, training and testing in the probability discounting task, exposure to social defeat and brain sample collection across the lifespan of the rats. See text for details.
The vapor chamber system that was used in this study was provided by La Jolla Alcohol Research, Inc. (La Jolla, CA, USA, http://www.ljari.com) and has been described in detail previously [32]. Briefly, rats were exposed to alcohol vapor or air (control rats) for 6 h/day from PND 28–42. After each exposure, rats were returned to the vivarium in a clean cage. Blood ethanol concentrations (BECs) were sampled from different groups of rats throughout AIE vapor exposure. BECs were 212.96 ± 8.65 mg/dl (PND 28; AIE day 1), 221.33 ± 5.73 mg/dl (PND 30, AIE day 3), 285.68 ± 5.57 (PND 34, AIE day 7), 325.80 ± 2.93 mg/dl (PND 37, AIE day 10) and 311.28 ± 6.65 mg/dl (PND 42, AIE day 14).
2.3. Probability discounting procedure
Adult rats (PND 107–171) were trained and tested in the probability discounting task as described elsewhere [7]. Briefly, each session consisted of five blocks. The probability that the large reward (four pellets) would be delivered decreased across successive blocks within a session (1.0, 0.5, 0.25, 0.125, and 0.0625). The small reward (one food pellet) probability was always 1.0. Sixteen forced trials (8 large risky, 8 small safe), where only one alternative was available, were conducted at the beginning of each block. The 16 forced trials were followed by 10 choice trials, in which the rats were presented with both side alternatives, and a response to either of these resulted in the consequence specific to that alternative: either one food pellet with 100% certainty or four food pellets according to the probability that was active in that block. The rats were tested in the probability discounting procedure 5 days per week until preference in all free choice blocks was stable (<10% variability in the proportion of large reward selections across the last five consecutive sessions). It took approximately 33 daily sessions to train rats in this procedure.
2.4. Social defeat procedure
After stable performance in the probability discounting task was achieved, the rats (PND 172–181) were exposed to chronic social defeat stress for 10 consecutive days. Details of the social defeat procedure have been described elsewhere [35]. Briefly, both AIE-exposed and air-exposed rats were divided into stressed and non-stressed sub-groups with equal risky response probabilities. The stress-exposed rats were housed in a separate compartment within the cage of a reproductive pair of rats and any pre-weanling pups for 24 h. Each day, the female and pups were removed from the cage and the barrier separating the intruder rat from the male resident rat was removed. The ensuing physical interaction between the rats lasted for 3 min, or until the intruder rat adopted a supine defeat position for 3 consecutive seconds. On days 1 (acute stress) and 10 (chronic stress) of social defeat, the rats were immediately tested in the probability discounting task and subsequently returned to the compartment within a different resident rat’s home cage. On days 2–9 of stress exposure, rats were returned to the compartment within a different resident rat’s home cage immediately after social defeat. The resident-intruder pairings were alternated daily.
2.5. Brain sample collection
After the final social defeat stress exposure and behavioral testing, the rats were left for 24 h in the protective compartment of a resident rat’s home cage before being humanly euthanized. Brains were removed and snap frozen and the PFC and CeA were dissected with 19-gauge punches taken from 2 mm brain slices for analysis of CRF and CRFR1 mRNA levels.
2.6. Quantitative real-time PCR (qPCR)
DNase-treated RNA was isolated by “RNeasy Mini Kit” (Qiagen, Hilden, Germany) from the dissected PFC and CeA regions. cDNA was synthesized using “High Capacity cDNA Reverse Transcription” kit (Life Technologies, CA, USA). For this study, actb (ID: Rn00667869 m1) was used as house-keeping gene (average% control ΔΔct calculation for CRF and CRFR1 for each treatment group). To examine the expression of CRF and CRFR1 mRNA levels, TaqMan qPCR was performed (Applied Biosystems, CA, USA). The TaqMan probes and primers used were: actb (ID: Rn00667869 m1), crh (ID: Rn01462137 m1), and crhr1 (ID: Rn00578611 m1). Threshold cycle (Ct) values were measured for each primer and were compared using statistical analysis.
2.7. Statistical methods
All of the group data were subjected to univariate analysis of variance (ANOVA) using SPSS 18 (SPSS, Chicago, IL). Significant main and interaction effects were followed by t-tests using a Šidák adjustment for multiple comparisons. For repeated-measures analyses, Mauchly’s test of sphericity of the covariance matrix was applied. When the sphericity assumption was violated, the degrees of freedom for any term that involved that factor were adjusted to more conservative values by applying the Huynh-Feldt correction. We report the uncorrected degrees of freedom. The level of significance was set to 0.05.
The number of trials completed (130 trials per session) was compared using a 3-way mixed ANOVA with AIE and Stress as between-subjects factors and Condition (Baseline, Stress Day 1, Stress Day 10) as the within subjects factor. However, not all rats completed the session during stress days 1 and 10, leading to missing values for risky response probability in the final session block. In repeated measures ANOVAs, a subject with a single missing value is excluded from the entire analysis. Thus, comparisons of risky response probabilities across blocks during baseline and stress days 1 and 10 were performed separately to restrict the problem of small sample sizes to as few comparisons as possible. First, 3-way mixed ANOVAs (Block × Stress × AIE) were performed separately for each of the 3 conditions (Baseline, Stress Day 1, Stress Day 10) with AIE and Stress as the between-subjects factors and Block as the within-subjects factor. Second, 3-way mixed ANOVAs (Condition × AIE × Stress) were performed for each block with AIE and Stress as the between-subjects factors and Condition as the within-subjects factor.
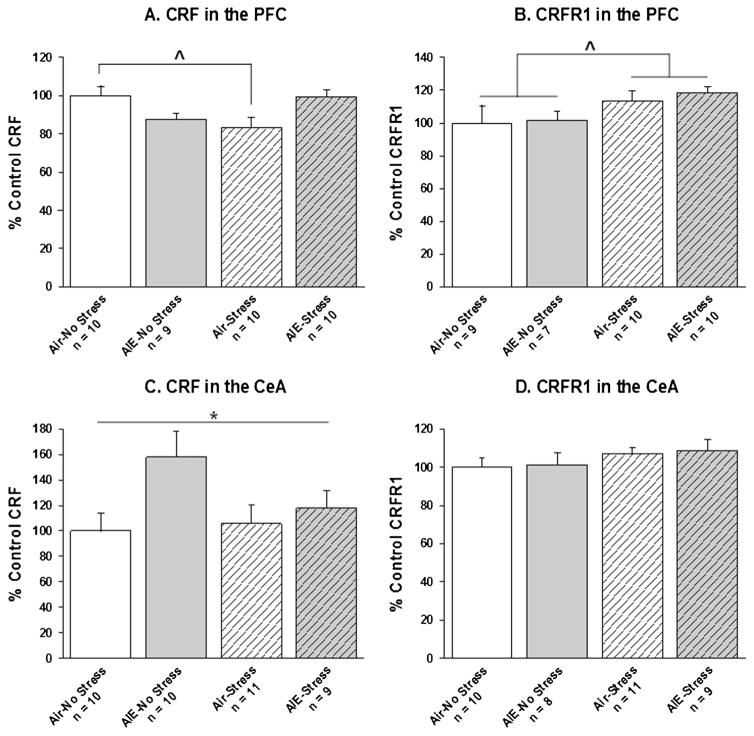
Data on CRF/CRFR1 mRNA levels are expressed as percent of control (i.e., air-no stress experimental group). Between-subjects 2-way ANOVAs were conducted with AIE and Stress as the factors for CRF and CRFR1 in the PFC and CeA. For the CRF results, some brain samples were not viable in some regions, leading to varying sample sizes across the brain regions examined. Additionally, an observation was considered an outlier if it differed from the group mean by more than 2 standard deviations. Outliers were removed separately for CRF and CRFR1 in each brain region. Final sample sizes for each group in each analyses are depicted in Fig. 3.
Fig. 3.
CRF and CRFR1 mRNA levels in the prefrontal cortex (PFC) and the central nucleus of the amygdala (CeA). Data are expressed as% control rats (i.e., air-no stress group). Caret denotes a significant difference between air-stress and air-no stress experimental groups (A: ^, p < 0.05) and a significant main effect of Stress exposure (B: ^, p < 0.05) in the ANOVA. Asterisk denotes a significant main effect of AIE exposure (C: *, p < 0.05).
3. Results
3.1. Trials completed
There were significant main effects of Condition (F2,84 = 6.04, p < 0.01), Stress (F1,42 = 7.27, p < 0.05) and a Condition × Stress interaction (F2,84 = 3.98, p < 0.05). There was no effect of AIE exposure on the number of trials completed. Post hoc tests revealed that stress-exposed rats completed significantly fewer trials on the first (111.45 ± 4.68) and tenth days (113.73 ± 5.81) of stress than during baseline (127.17 ± 1.34). For non-stressed rats, there were no differences in number of trials completed on baseline (127.42 ± 1.27), stress day 1 (128.79 ± 0.68) and stress day 10 (119.96 ± 3.05) (data not shown).
3.2. Risky response probabilities
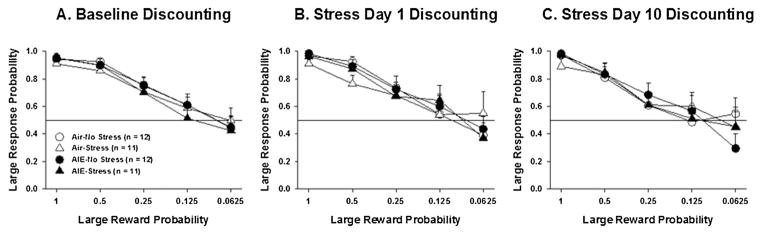
There was a significant main effect of Block during baseline (F4,168 = 86.21, p < 0.001; Fig. 2A), on the first day of stress exposure (F4,120 = 29.19, p < 0.001; Fig. 2B), and on the tenth day of stress exposure (F4,104 = 28.56, p < 0.001; Fig. 2C). Post hoc tests revealed that the probability of responding for the larger reward decreased as the probability of receiving the larger reward also decreased. However, there were no effects of AIE exposure or Stress on preference for the large risky reward either at baseline, Day 1 of stress, or Day 10 of stress.
Fig. 2.
Preference for the large risky reward. Large response probability as a function of large reward probability across blocks of trials during baseline (A) and on stress day 1 (B) and stress day 10 (C) for air-exposed and AIE vapor-exposed rats. All data are expressed as the mean ± SEM. Caret depicts a main effect of Stress exposure in the ANOVA (^, p < 0.05).
Independent 3-way ANOVAs were performed in each block. There was no effect of AIE exposure or Stress in any block. In blocks 1, 2, 4 and 5 there was no effect of Condition. In Block 3 there was a significant main effect of Condition (F2,78 = 3.97, p < 0.05). Post hoc tests revealed that preference for the large reward in block three was greater during baseline (0.72) than during Stress Session 10 (0.63) for all groups of rats with no differences attributable to AIE or stress exposure.
3.3. CRF and CRFR1 mRNA analyses
In the PFC, there was a significant AIE × Stress interaction on CRF mRNA levels (F1,35 = 11.07, p < 0.01). Post hoc tests revealed that in air-exposed rats, stress significantly reduced CRF mRNA levels in the PFC relative to no stress (Fig. 3A). In contrast, in AIE-exposed rats, there was no difference between stress and no stress on CRF mRNA levels in the PFC. Independent of AIE exposure, stress-exposed rats had significantly higher CRFR1 mRNA levels in the PFC than non-stressed rats (main effect of Stress; F1,32 = 4.86, p < 0.05; Fig. 3B).
In the CeA, AIE-exposed rats had significantly higher CRF mRNA levels, independent of stress (main effect of AIE exposure; F1,36 = 4.54, p < 0.05; Fig. 3C). There was no effect of AIE or Stress on CRFR1 gene expression in the CeA (Fig. 3D).
4. Discussion
The results of the present studies demonstrated that exposure to ethanol vapor during early adolescence had no effect on risky choice in the probability discounting task in adulthood. Regardless of AIE exposure, neither acute nor chronic social defeat stress had any effect on risky choice. However, both acute and chronic social stress suppressed rats’ behavior, as indicated by fewer trials completed after social defeat. In the PFC, stress exposure increased CRFR1 mRNA levels in both AIE-exposed and air-exposed rats and decreased CRF mRNA levels in air-exposed rats only. In the CeA, AIE-exposure increased CRF mRNA levels independent of stress exposure.
AIE exposure via vapor during early adolescence (PND 28–42) did not increase risky choice in the probability discounting task, contrary to what we had predicted. This result is in contrast to our previous work [7] and work from other laboratories that showed increased risky decision making in rats exposed to ethanol for extended periods (PND 28–57) during adolescence [8–12]. A recent study reported impaired attentional and executive performance after ethanol exposure during late but not early adolescence [36], suggesting that deficits in some higher order cognitive functions may only emerge after ethanol exposure during late adolescence. Therefore, a critical window for AIE exposure to produce increases in risky choice may be either late adolescence or extended exposure throughout the entire adolescent period.
The absence of any effect of AIE exposure on risk-based decision-making in the present study may have resulted from differences in severity of AIE exposure and/or route of ethanol administration across studies. For example, BECs in the present study (AIE vapor: 200–300 mg/dl) were lower than BECs in our earlier study (AIE gavage: 300–500 mg/dl) [37]. However, others have reported increased risky choice in adulthood after self-administration of ethanol-containing gelatin during adolescence, resulting in much lower BECs [8–12]. Thus, differences in the severity of AIE exposure are unlikely to account for the different results in the present study and in previously published work [7]. Further, administration of ethanol via intragastric gavage triggers hormonal stress responses [38]. There is growing evidence that exposure to stressors in adolescence impairs brain development, behavioral responses to drugs and alcohol, and cognitive performance in adulthood [39,40]. Therefore, the stress of ethanol administration via gavage during adolescence may have contributed to the increased risky choice observed in our previous work [37]. On the other hand, adult rats exposed to stress-free voluntary ethanol self-administration throughout adolescence exhibited increased risky choice [8–12]. These findings better support the importance of the time of adolescent ethanol exposure on risky choice discussed above rather than the effects of stress associated with ethanol administration. It is also possible that the lack of effects on risky choice is a limitation of the ethanol vapor model. For example, AIE vapor exposure produced inconsistent effects on adult ethanol self-administration resulting in either no effect [41] or increased ethanol consumption [42] in adult rats. Thus, further research is needed to determine if the age of subjects during AIE vapor exposure contributed to the lack of effects on risky choice in the present study or if the vapor procedure per se is not sufficient to alter risky choice.
Other experimental factors may also contribute to the absence of the effects of AIE exposure on risky choice. For example, all animals performed above chance for risk preference and showed shallow discounting curves suggesting that the animals were at ceiling for risk preference. In addition, the probability discounting task requires extensive training and the lack of effects of AIE exposure on risky choice may have resulted from the possibility that rats were engaged in habitual responding during testing.
Exposure to acute or chronic social defeat stress suppressed general responding in the probability discounting task as reflected by decreased number of trials completed. However, neither acute, nor chronic social defeat stress affected risky choice. In the probability discounting task, rats were trained for extended periods prior to stress exposure. Therefore, similar to the AIE results described above, lack of an effect of social stress on risky choice may be attributed to the fact that decision making in this task was habitual in well-trained rats. Consistent with this notion, exposure to stress [43–46] or stress hormones [47,48] has been shown to impair flexible decision making, while leaving habitual response strategies unaffected. Furthermore, decision making is not a unitary construct and stress exposure may have different effects on different decision-making tasks. For example, restraint stress had no effect on delayed discounting but impaired performance on an effort-based discounting task [18]. Finally, stress from transportation between institutes may have affected the adult response to social stress. However, this possibility is also unlikely because physiological and behavioral signs of acute stress from transportation typically dissipate within a week [49,50], and the rats in this study were fully acclimatized before behavioral training and testing began.
Findings on the long-term effects of AIE exposure on the CRF system remain limited. Alcohol exposure is known to attenuate the HPA response to stress [51]. Furthermore, blunted HPA axis responses to stress have been reported after adolescent ethanol exposure [31,32]. In the present studies, AIE exposure increased CRF mRNA levels in the CeA regardless of stress exposure, although the effect appeared to be more pronounced in non-stressed AIE-exposed rats (post hoc comparisons were not performed because there was no significant AIE × Stress interaction effect in the ANOVA). In contrast, immunohistochemical studies reported decreased CRF cell counts in the CeA [21] and decreased CRF density with no change in CRF cell counts [22] in adult rats allowed to self-administer ethanol during adolescence. Interestingly, while CRF-like immunoreactivity in the amygdala was decreased immediately after ethanol withdrawal in adult rats, CRF-like immunoreactivity in the amygdala was increased after 6 weeks of ethanol withdrawal in adult rats [52]. These findings suggest that increased CRF in the CeA in the present results may emerge only after an extended period of abstinence following AIE exposure.
The PFC is particularly sensitive to the damaging effects of chronic stress. Changes in dendritic plasticity in the PFC were observed after one week of stress exposure, while several weeks of stress exposure were required before dendritic changes were observed in the hippocampus [53]. In agreement with this finding, there was no effect of 10 days of social defeat exposure on CRF or CRFR1 mRNA levels in the CeA, while in the PFC, social defeat increased CRFR1 mRNA levels in both AIE-exposed and air-exposed rats and decreased CRF mRNA levels air-exposed rats only. Similarly, exposure to acute restraint stress increased CRFR1 mRNA levels in the PFC, but also increased CRF mRNA levels in the PFC [54]. It is important to note that in the present study, brains were collected 24 h after the final stress exposure to detect persistent effects of stress, while in earlier work, brains were collected 3 h after stress exposure and reflect immediate effects of stress [54]. Therefore, decreased CRF mRNA levels in the PFC may only emerge 24 h after stress exposure, and the immediate effects of stress on CRF mRNA levels in the CeA may have already dissipated. In addition, infection after injury could contribute to the CRFR1 results in the PFC because immune activation/infection potentiates HPA response [55,56]. However, this outcome is unlikely because in the present work, resident/intruder pairings were alternated to counterbalance aggressiveness toward any one intruder. The rats exposed to social defeat received only minor physical injuries during the exposure to the resident, as rats were separated from the resident immediately upon showing a defeated supine posture or after a maximum of 3 min.
5. Conclusions
In summary, our results indicate no effect of ethanol vapor exposure during early adolescence on risky choice in the probability discounting task. Together with previous findings in the literature [7–12], the present results are significant because they highlight the potential importance of age of ethanol exposure to produce the long-term increases in risky choice after either extended or late-adolescence ethanol exposure. Alternatively, the lack of effects in the present study may suggest that AIE exposure via vapor may not be sufficient to alter risky choice during adulthood. In the PFC, chronic social defeat selectively decreased CRF mRNA levels in air-exposed rats and increased CRFR1 mRNA levels in all rats. AIE exposure increased CRF mRNA levels in the CeA with no effect of social stress. Both AIE exposure and chronic social defeat stress produced alterations in CRF and CRFR1 mRNA levels in the brain in the absence of changes in risky choice, suggesting that the risky choice aspect of multifaceted decision making may not be affected by social stress and/or may not depend on CRF system functioning. Alternatively, differential patterns of CRF expression suggest separate effects of social defeat stress and AIE exposure on CRF function and further research is needed to determine under which experimental conditions these effects can be revealed behaviorally.
HIGHLIGHTS.
Ethanol vapor exposure during early adolescence had no effect on adult risky choice.
Acute or chronic social defeat stress had no effect of risky choice.
Both AIE exposure and social defeat altered CRF and CRFR1 mRNA levels in the brain.
Risky choice may not be affected by social stress and may not depend on CRF system.
Acknowledgments
This work was supported by NIH grant: U01-AA019970-NADIA (AM) and U01-AA019973-NADIA (SL). The NIH had no role in the study design, data collection, data analysis, data interpretation, writing of the report, or decision to submit the article for publication.
We would like to thank Mr. Brian Kwan for excellent technical help with the social defeat experiments, and Joonho Hong and Sejin Bae for their excellent help with qPCR analyses.
Footnotes
Conflict of interest
AM has received contract research support from Forest Laboratories and Astra-Zeneca, and honoraria/consulting fees from AbbVie during the past 3 years. The remaining authors report no financial conflicts of interest. There are no actual or potential financial conflicts of interest.
References
- 1.Ernst M, Pine DS, Hardin M. Triadic model of the neurobiology of motivated behavior in adolescence. Psychol Med. 2006;36:299–312. doi: 10.1017/S0033291705005891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lenroot RK, Giedd JN. Brain development in children and adolescents: insights from anatomical magnetic resonance imaging. Neurosci Biobehav Rev. 2006;30:718–729. doi: 10.1016/j.neubiorev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 3.Xiao L, Bechara A, Grenard LJ, Stacy WA, Palmer P, Wei Y, et al. Affective decision-making predictive of Chinese adolescent drinking behaviors. J Int Neuropsychol Soc. 2009;15:547–557. doi: 10.1017/S1355617709090808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goudriaan AE, Grekin ER, Sher KJ. Decision making and binge drinking: a longitudinal study. Alcohol Clin Exp Res. 2007;31:928–938. doi: 10.1111/j.1530-0277.2007.00378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gullo MJ, Dawe S, McHugh MJ. Impulsivity and Adolescent Substance Use: From Self-report Measures to Neuroimaging and Beyond. In: Bardo MT, Fishbein DH, Milich R, editors. Inhibitory Control and Drug Abuse Prevention. Springer; New York, New York, NY: 2011. pp. 161–175. [Google Scholar]
- 6.Cantrell H, Finn PR, Rickert ME, Lucas J. Decision making in alcohol dependence: insensitivity to future consequences and comorbid disinhibitory psychopathology. Alcohol Clin Exp Res. 2008;32:1398–1407. doi: 10.1111/j.1530-0277.2008.00714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boutros N, Semenova S, Liu W, Crews FT, Markou A. Adolescent intermittent ethanol exposure is associated with increased risky choice and decreased dopaminergic and cholinergic neuron markers in adult rats. Int J Neuropsychopharmacol. 2014;18 doi: 10.1093/ijnp/pyu003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clark JJ, Nasrallah NA, Hart AS, Collins AL, Bernstein IL, Phillips PE. Altered risk-based decision making following adolescent alcohol use results from an imbalance in reinforcement learning in rats. PLoS One. 2012;7:e37357. doi: 10.1371/journal.pone.0037357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McMurray MS, Amodeo LR, Roitman JD. Effects of voluntary alcohol intake on risk preference and behavioral flexibility during rat adolescence. PLoS One. 2014;9:e100697. doi: 10.1371/journal.pone.0100697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nasrallah NA, Yang TW, Bernstein IL. Long-term risk preference and suboptimal decision making following adolescent alcohol use. Proc Natl Acad Sci U S A. 2009;106:17600–17604. doi: 10.1073/pnas.0906629106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nasrallah NA, Clark JJ, Collins AL, Akers CA, Phillips PE, Bernstein IL. Risk preference following adolescent alcohol use is associated with corrupted encoding of costs but not rewards by mesolimbic dopamine. Proc Natl Acad Sci U S A. 2011;108:5466–5471. doi: 10.1073/pnas.1017732108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schindler AG, Tsutsui KT, Clark JJ. Chronic alcohol intake during adolescence, but not adulthood, promotes persistent deficits in risk-based decision making. Alcohol Clin Exp Res. 2014;38:1622–1629. doi: 10.1111/acer.12404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buckert M, Schwieren C, Kudielka BM, Fiebach CJ. Acute stress affects risk taking but not ambiguity aversion. Front Neurosci. 2014;8:82. doi: 10.3389/fnins.2014.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miu AC, Heilman RM, Houser D. Anxiety impairs decision-making: psychophysiological evidence from an Iowa Gambling Task. Biol Psychol. 2008;77:353–358. doi: 10.1016/j.biopsycho.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 15.Porcelli AJ, Delgado MR. Acute stress modulates risk taking in financial decision making. Psychol Sci. 2009;20:278–283. doi: 10.1111/j.1467-9280.2009.02288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Putman P, Antypa N, Crysovergi P, van der Does WA. Exogenous cortisol acutely influences motivated decision making in healthy young men. Psychopharmacology (Berl) 2010;208:257–263. doi: 10.1007/s00213-009-1725-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Graham LK, Yoon T, Kim JJ. Stress impairs optimal behavior in a water foraging choice task in rats. Learn Mem. 2010;17:1–4. doi: 10.1101/lm.1605510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shafiei N, Gray M, Viau V, Floresco SB. Acute stress induces selective alterations in cost/benefit decision-making. Neuropsychopharmacology. 2012;37:2194–2209. doi: 10.1038/npp.2012.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schwager AL, Haack AK, Taha SA. Impaired flexibility in decision making in rats after administration of the pharmacological stressor yohimbine. Psychopharmacology (Berl) 2014;231:3941–3952. doi: 10.1007/s00213-014-3529-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herman JP. Neural pathways of stress integration: relevance to alcohol abuse. Alcohol Res Curr Rev. 2012;34:441–447. doi: 10.35946/arcr.v34.4.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gilpin NW, Karanikas CA, Richardson HN. Adolescent binge drinking leads to changes in alcohol drinking, anxiety, and amygdalar corticotropin releasing factor cells in adulthood in male rats. PLoS One. 2012;7:e31466. doi: 10.1371/journal.pone.0031466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wills TA, Knapp DJ, Overstreet DH, Breese GR. Interactions of stress and CRF in ethanol-withdrawal induced anxiety in adolescent and adult rats. Alcohol Clin Exp Res. 2010;34:1603–1612. doi: 10.1111/j.1530-0277.2010.01245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.St Onge JR, Floresco SB. Prefrontal cortical contribution to risk-based decision making. Cereb Cortex. 2010;20:1816–1828. doi: 10.1093/cercor/bhp250. [DOI] [PubMed] [Google Scholar]
- 24.Ghods-Sharifi S, St Onge JR, Floresco SB. Fundamental contribution by the basolateral amygdala to different forms of decision making. J Neurosci. 2009;29:5251–5259. doi: 10.1523/JNEUROSCI.0315-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spear LP. Adolescent alcohol exposure: are there separable vulnerable periods within adolescence? Physiol Behav. 2015 doi: 10.1016/j.physbeh.2015.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gilpin NW, Richardson HN, Cole M, Koob GF. Vapor inhalation of alcohol in rats. Curr Protoc Neurosci. 2008;Chapter 9(Unit 9):29. doi: 10.1002/0471142301.ns0929s44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rogers J, Wiener SG, Bloom FE. Long-term ethanol administration methods for rats: advantages of inhalation over intubation or liquid diets. Behav Neural Biol. 1979;27:466–486. doi: 10.1016/s0163-1047(79)92061-2. [DOI] [PubMed] [Google Scholar]
- 28.Balcombe JP, Barnard ND, Sandusky C. Laboratory routines cause animal stress. Contemp Top Lab Anim Sci. 2004;43:42–51. [PubMed] [Google Scholar]
- 29.Walker MK, Boberg JR, Walsh MT, Wolf V, Trujillo A, Duke MS, et al. A less stressful alternative to oral gavage for pharmacological and toxicological studies in mice. Toxicol Appl Pharmacol. 2012;260:65–69. doi: 10.1016/j.taap.2012.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee S, Schmidt D, Tilders F, Cole M, Smith A, Rivier C. Prolonged exposure to intermittent alcohol vapors blunts hypothalamic responsiveness to immune and non-immune signals. Alcohol Clin Exp Res. 2000;24:110–122. [PubMed] [Google Scholar]
- 31.Allen CD, Rivier CL, Lee SY. Adolescent alcohol exposure alters the central brain circuits known to regulate the stress response. Neuroscience. 2011;182:162–168. doi: 10.1016/j.neuroscience.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Logrip ML, Rivier C, Lau C, Im S, Vaughan J, Lee S. Adolescent alcohol exposure alters the rat adult hypothalamic-pituitary-adrenal axis responsiveness in a sex-specific manner. Neuroscience. 2013;235:174–186. doi: 10.1016/j.neuroscience.2012.12.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Der-Avakian A, Mazei-Robison MS, Kesby JP, Nestler EJ, Markou A. Enduring deficits in brain reward function after chronic social defeat in rats: susceptibility, resilience, and antidepressant response. Biol Psychiatry. 2014;76:542–549. doi: 10.1016/j.biopsych.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Backstrom T, Winberg S. Central corticotropin releasing factor and social stress. Front Neurosci. 2013;7:117. doi: 10.3389/fnins.2013.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Der-Avakian A, Markou A. Withdrawal from chronic exposure to amphetamine, but not nicotine, leads to an immediate and enduring deficit in motivated behavior without affecting social interaction in rats. Behav Pharmacol. 2010;21:359–368. doi: 10.1097/FBP.0b013e32833c7cc8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sanchez-Roige S, Pena-Oliver Y, Ripley TL, Stephens DN. Repeated ethanol exposure during early and late adolescence: double dissociation of effects on waiting and choice impulsivity. Alcohol Clin Exp Res. 2014;38:2579–2589. doi: 10.1111/acer.12535. [DOI] [PubMed] [Google Scholar]
- 37.Boutros N, Semenova S, Markou A. Adolescent intermittent ethanol exposure diminishes anhedonia during ethanol withdrawal in adulthood. Eur Neuropsychopharmacol. 2014;24:856–864. doi: 10.1016/j.euroneuro.2014.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bonnichsen M, Dragsted N, Hansen AK. The welfare impact of gavaging laboratory rats. Anim Welfare. 2005;14:223–227. [Google Scholar]
- 39.Isgor C, Kabbaj M, Akil H, Watson SJ. Delayed effects of chronic variable stress during peripubertal-juvenile period on hippocampal morphology and on cognitive and stress axis functions in rats. Hippocampus. 2004;14:636–648. doi: 10.1002/hipo.10207. [DOI] [PubMed] [Google Scholar]
- 40.McCormick CM, Mathews IZ. HPA function in adolescence: role of sex hormones in its regulation and the enduring consequences of exposure to stressors. Pharmacol Biochem Behav. 2007;86:220–233. doi: 10.1016/j.pbb.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 41.Slawecki CJ, Betancourt M. Effects of adolescent ethanol exposure on ethanol consumption in adult rats. Alcohol (Fayetteville, NY) 2002;26:23–30. doi: 10.1016/s0741-8329(01)00192-6. [DOI] [PubMed] [Google Scholar]
- 42.Gass JT, Glen WB, Jr, McGonigal JT, Trantham-Davidson H, Lopez MF, Randall PK, et al. Adolescent alcohol exposure reduces behavioral flexibility, promotes disinhibition, and increases resistance to extinction of ethanol self-administration in adulthood. Neuropsychopharmacology. 2014;39:2570–2583. doi: 10.1038/npp.2014.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Braun S, Hauber W. Acute stressor effects on goal-directed action in rats. Learn Mem. 2013;20:700–709. doi: 10.1101/lm.032987.113. [DOI] [PubMed] [Google Scholar]
- 44.Dias-Ferreira E, Sousa JC, Melo I, Morgado P, Mesquita AR, Cerqueira JJ, et al. Chronic stress causes frontostriatal reorganization and affects decision-making. Science. 2009;325:621–625. doi: 10.1126/science.1171203. [DOI] [PubMed] [Google Scholar]
- 45.Elliott AE, Packard MG. Intra-amygdala anxiogenic drug infusion prior to retrieval biases rats towards the use of habit memory. Neurobiol Learn Mem. 2008;90:616–623. doi: 10.1016/j.nlm.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 46.Liston C, Miller MM, Goldwater DS, Radley JJ, Rocher AB, Hof PR, et al. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J Neurosci. 2006;26:7870–7874. doi: 10.1523/JNEUROSCI.1184-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cerqueira JJ, Pego JM, Taipa R, Bessa JM, Almeida OF, Sousa N. Morphological correlates of corticosteroid-induced changes in prefrontal cortex-dependent behaviors. J Neurosci. 2005;25:7792–7800. doi: 10.1523/JNEUROSCI.1598-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Koot S, Baars A, Hesseling P, van den Bos R, Joels M. Time-dependent effects of corticosterone on reward-based decision-making in a rodent model of the iowa gambling task. Neuropharmacology. 2013;70:306–315. doi: 10.1016/j.neuropharm.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 49.Obernier JA, Baldwin RL. Establishing an appropriate period of acclimatization following transportation of laboratory animals. ILAR J. 2006;47:364–369. doi: 10.1093/ilar.47.4.364. [DOI] [PubMed] [Google Scholar]
- 50.Tuli JS, Smith JA, Morton DB. Stress measurements in mice after transportation. Lab Anim. 1995;29:132–138. doi: 10.1258/002367795780740249. [DOI] [PubMed] [Google Scholar]
- 51.Spanagel R, Noori HR, Heilig M. Stress and alcohol interactions: animal studies and clinical significance. Trends Neurosci. 2014;37:219–227. doi: 10.1016/j.tins.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 52.Zorrilla EP, Valdez GR, Weiss F. Changes in levels of regional CRF-like-immunoreactivity and plasma corticosterone during protracted drug withdrawal in dependent rats. Psychopharmacology (Berl) 2001;158:374–381. doi: 10.1007/s002130100773. [DOI] [PubMed] [Google Scholar]
- 53.Arnsten AF. Stress signalling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci. 2009;10:410–422. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Meng QY, Chen XN, Tong DL, Zhou JN. Stress and glucocorticoids regulated corticotropin releasing factor in rat prefrontal cortex. Mol Cell Endocrinol. 2011;342:54–63. doi: 10.1016/j.mce.2011.05.035. [DOI] [PubMed] [Google Scholar]
- 55.Leonard BE, HPA The, and immune axes in stress: the involvement of the serotonergic system. Eur Psychiatry. 2005;20(Suppl 3):S302–6. doi: 10.1016/s0924-9338(05)80180-4. [DOI] [PubMed] [Google Scholar]
- 56.Black PH. Stress and the inflammatory response: a review of neurogenic inflammation. Brain Behav Immun. 2002;16:622–653. doi: 10.1016/s0889-1591(02)00021-1. [DOI] [PubMed] [Google Scholar]