Abstract
Background
Studies have shown that HIV infection is associated with an impaired influenza vaccine response. We examined the role of cellular phenotypes and function in influenza vaccine responsiveness in healthy controls and aviremic HIV-infected subjects on antiretroviral treatment (ART).
Methods
16 healthy controls and 26 ART+ aviremic HIV+ subjects were enrolled in the current study. Blood was collected at pre-vaccination (D0), and on days 7–10 (D7) and 14–21 (D14) following the 2013–2014 seasonal influenza vaccine administrations. Subjects were classified as responders if neutralizing titers against H1N1 virus increased ≥ 4-fold at D14 compared to D0. A serial analysis of B and CD4+ T cell frequencies and activation was performed on D0 and D7 by flow cytometry.
Results
9 of 26 (34.6%) HIV-infected individuals and 7 of 16 (43.8%) healthy controls were classified as responders to influenza vaccines. Total B cell apoptosis (annexin V) was increased on D7 post-vaccination in non-responders but not in responders among both controls and HIV+ subjects. Surface CD80 expression on memory B cells and intracellular CD40L expression on memory CD4+ T cells were induced on D7 in responders of controls but not in non-responders. The CD80 and CD40L induction was not demonstrable in HIV-infected subjects regardless of responders and non-responders. Memory CD4+ T cell cycling tended to increase on D7 in the four study groups but did not achieve significance. All the other parameters were indistinguishable between responders and non-responders, regardless of HIV-infection status.
Conclusion
The perturbation of activation and apoptotic induction on B cells or CD4+ T cells after seasonal influenza vaccination in non-responders and HIV-infected subjects may help understand the mechanism of impaired vaccine responsiveness.
Keywords: HIV disease, B cells, immunologic responders and non-responders, influenza vaccine
INTRODUCTION
HIV infection is associated with high incidence of influenza infection [1, 2]. Seasonal influenza vaccination is highly recommended for HIV-1 infected individuals to reduce influenza-related morbidity and mortality [3]. In HIV-infected patients, the antibody responses to influenza vaccination are frequently impaired [4, 5]. Immune suppression, low CD4+ T cell counts, aging, IL-10 promoter polymorphisms, baseline low naïve CD4+ T cells, and IL-28B levels have been associated with poor immune response to influenza vaccination [6–9]. It is clear that antiretroviral treatment (ART)-naïve and viremic patients have impaired influenza vaccine responses to primary and recall immunization [10, 11]. ART treatment does not fully restore seroprotection until higher or multiple doses of vaccine are administered [11–13].
The purpose of this study was to characterize T cell and B cell dysfunction in immunologic responders and non-responders of healthy controls and HIV-infected individuals that may preclude appropriate influenza vaccine response. Understanding the immune perturbations in these individuals can help improve the immunogenicity of vaccine regimens and establish guidelines on repeat doses to improve immune responses. We have a particular interest in evaluating the differential function of T and B cells in responders compared to non-responders at baseline and after vaccination, including cell frequency and count, apoptosis, activation, cycling and cytokine production. In the current study, we found that the induction of B cell apoptosis both in controls and HIV-infected individuals, CD40L induction in memory CD4+ T cells and CD80 induction on memory B cells in healthy controls following vaccination distinguished responders and non-responders.
MATERIALS AND METHODS
Study subjects
16 healthy controls and 26 HIV+ ART-treated aviremic patients (plasma HIV RNA < 50 copies/mL) were studied. Clinical characteristics of participants were shown in table 1. All participants had received the influenza vaccine the previous year and would again during the study year. HIV-infected and HIV-negative volunteers were recruited between June 2013 and March 2014 to receive the 2013–2014 influenza vaccine and to donate blood at 3 time points: day 0 (D0) prior to vaccination, then on day 7–10 (D7), and day 14–21 (D14) post-vaccination. This study was approved by the Medical University of South Carolina IRB (Pro00020606), and all participants provided written informed consent. We previously reported that anti-H1N1 neutralizing antibody response was the predominant viral strain [14]. Therefore vaccine responders were defined as a four-fold increase in neutralization from baseline titers on D14, and non-responders were defined as less than a four-fold increase in neutralization from baseline titers against H1N1 virus [15].
Table 1.
Clinical characteristics and baseline immune parameters
| HIV− responder (n = 7) |
HIV− non-responder (n = 9) |
P value HIV− |
HIV+ responder (n = 9) |
HIV+ non-responder (n = 17) |
P value HIV+ |
|
|---|---|---|---|---|---|---|
| Age | 38 (31–51) | 38 (31–51) | 0.59 | 39 (31–44 | 46 (36–55) | 0.23 |
| Sex (male/female) | 4/3 (57%) | 2/7 (22%) | 0.30 | 6/3 (67%) | 11/6 (65%) | 0.99 |
| CD4+ T cell counts | 722 (485–970) | 759 (459–1010) | > 0.99 | 577 (476–791) | 561 (442–751) | 0.95 |
| Co-infection with HCV | 1 | 1 | ||||
| Infection duration | ||||||
| 2–5 years | 4 | 1 | ||||
| 5–10 years | 1 | 2 | ||||
| > 10 years | 4 | 14 | ||||
| Nadir CD4+ T cell counts | 361 (221–454) | 261 (163–464) | 0.59 |
Data are medians (interquartile ranges)
P value: comparisons of P value between responders and non-responders
Influenza virus strains and vaccination
A/California/7/2009 (H1N1) NIB-74 is an influenza A (H1N1) vaccine strain, derived from A/Christchurch/16/2010, National Institute for Biological Standards and Control (NIBSC; London, UK; code 13/200). A/Victoria/361/2011 (H3N2) NYMCX-223A is an influenza A (H3N2) vaccine strain, derived from A/Texas/50/2012, National Institute for Biological Standards and Control (NIBSC; London, UK; code 13/252). B/Massachusetts/2/2012 (B) NYMC BX-51B is an influenza A (H3N2) vaccine strain, derived from B/Massachusetts/2/2012, National Institute for Biological Standards and Control (NIBSC; London, UK; code 14/106). These viruses were used in the microneutralization assay. The strains for the 2013–2014 seasonal vaccine were H1N1, H3N2, and B type.
Cells
Plasma was collected from EDTA-contained blood, aliquot, and stored at −80°C before use. PBMCs were isolated over a Ficoll-Hypaque cushion (GE, Pittsburgh, PA). Antibodies were incubated with cells at room temperature for 10 minutes. After surface staining, cells were washed and subsequently analyzed by flow cytometry.
Flow cytometry
The fluorochrome-labeled monoclonal antibodies used in this study included: antibodies against CD3-Peridinin Chlorophyll (Percp, Miltenyi Biotec), CD8-APCcy (Biolegend), CD4-phycoerytherin (PE, BD Pharmingen), CD27-APCcy7 (BD Pharmingen), IgD-PEcy7 (Biolegend, San Diego, CA), CD40L-PE (BD Pharmingen), CD19- fluorescein isothiocyanate (FITC, BD Pharmingen), CD80-allophycocyanin (APC, BD Pharmingen), PD1-PE (BD Pharmingen), CD45RO-PEcy7 (BD Pharmingen), ki67-FITC (BD Pharmingen), IFN-γ-FITC (BD Pharmingen), CD38-APC, annexin V-FITC, and isotype control Abs (BD Pharmingen). No annexin V staining was used as a control for gating strategy. All others were gated based on isotypes. Cells were identified by their forward (FSC) and side scatter (SSC) characteristics and were analyzed with a Guava 8HT flow cytometer (Millipore, Billerica, MA).
Microneutralization assay
The detailed method was descripted in our previous study [14]. Briefly, plasma samples were serial diluted and heat-inactivated. Plasma samples were mixed with tissue culture infective doses (TCID50) for the H1N1 strain virus. The cells were cultured and the presence of viral protein was detected by ELISA. The endpoint titer was calculated by the reciprocal of the highest dilution of plasma with an OD value less than X, where X = {(average of V wells) − (average of C wells)}/2 + (average of C wells).
Statistical analysis
Conventional measurements of the central location and dispersion were performed to describe the data, and the differences in continuous measurements between the two groups were compared by the Wilcoxon matched-pairs signed rank test (paired) or Mann-Whitney's U test (unpaired). In the pre-specified hypothesis, we were interested in the comparisons of HIV+ subjects versus HIV− subjects, or vaccine responders versus non-responders; therefore, p-values from comparing the interested group to each of control groups were not adjusted for multiple comparisons [16]. The same approach was applied to the comparisons of immune parameters induced by anti-CD4 IgGs and control antibodies. To explore associations between pairs of continuous variables, Spearman's rank correlation was used. Comparison analysis was performed using SPSS software (version 16.01, Chicago, IL, USA). All tests were 2-sided, and P ≤ 0.05 was considered to denote statistical significance.
RESULTS
B cell parameters pre- and post- vaccination in responders and non-responders among healthy controls and HIV-infected subjects
An individual was considered a responder if he or she had the standard 4-fold or greater increase [15] in D14 versus D0 vaccination microneutralization titer (seroconversion). Of the controls, 7 were responders, and 9 were non-responders (43.75%). Of the HIV+ subjects, 9 were responders, and 17 were non-responders (34.6%). None of the differences in the frequency of responders between the controls (n = 16) and HIV+ subjects (n = 26) was significant (P > 0.05).
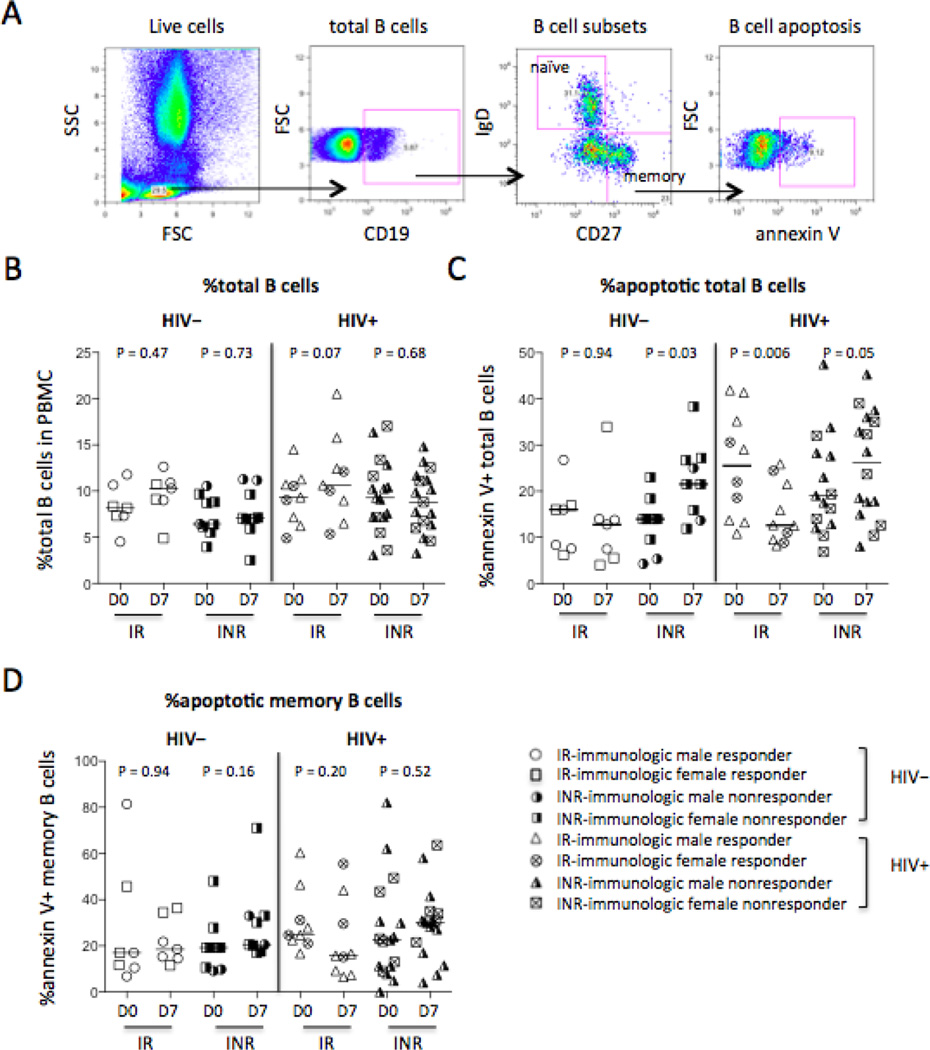
Next, frequencies and apoptosis of B cells were assessed by flow cytometry. Pre- and post-vaccination, the frequencies of total B cells in PBMCs were similar in controls and HIV+ subjects and in responders and non-responders (Fig. 1A–1B). Interestingly, more frequent B cell apoptosis was observed after vaccination in non-responders but not in responders regardless of HIV infection (Fig. 1C). Notably, the frequencies of total B cells in controls and all HIV+ subjects at baseline were similar (P = 0.14, Fig. 1B); but the frequency of annexin V binding among total B cells (P = 0.004, Fig. 1C) but not among memory B cells (P = 0.18, Fig. 1D) was increased at baseline in all HIV+ subjects compared with controls. There was a highly significant decrease in B cell apoptosis in the HIV+ immune responders on D7 compared to D0 (Fig. 1C), implying that B cell apoptotic function may be an important factor in vaccine response in HIV+ subjects. These results suggest that although frequencies of B cells are recovered in HIV+ subjects after ART treatment and viral suppression, B cell function, as measured by annexin V binding, may not be fully recovered.
Figure 1.
B cell frequency and apoptosis in responders and non-responders. Blood samples were tested for surface staining, and PBMCs were tested for apoptosis pre- and post-influenza vaccinations. (A) Representative dot plots display the gating strategy used to assess the percentages of B cells (tB) in PBMCs and the frequencies of B cell apoptosis. (B) The median frequencies of total B cells (CD19+) in PBMCs. The median frequencies of annexin V binding among total B cells (CD19+, C) and memory B cells (mB, CD19+CD27+IgD−, D). IR: immunologic responder; INR: immunologic non-responder.
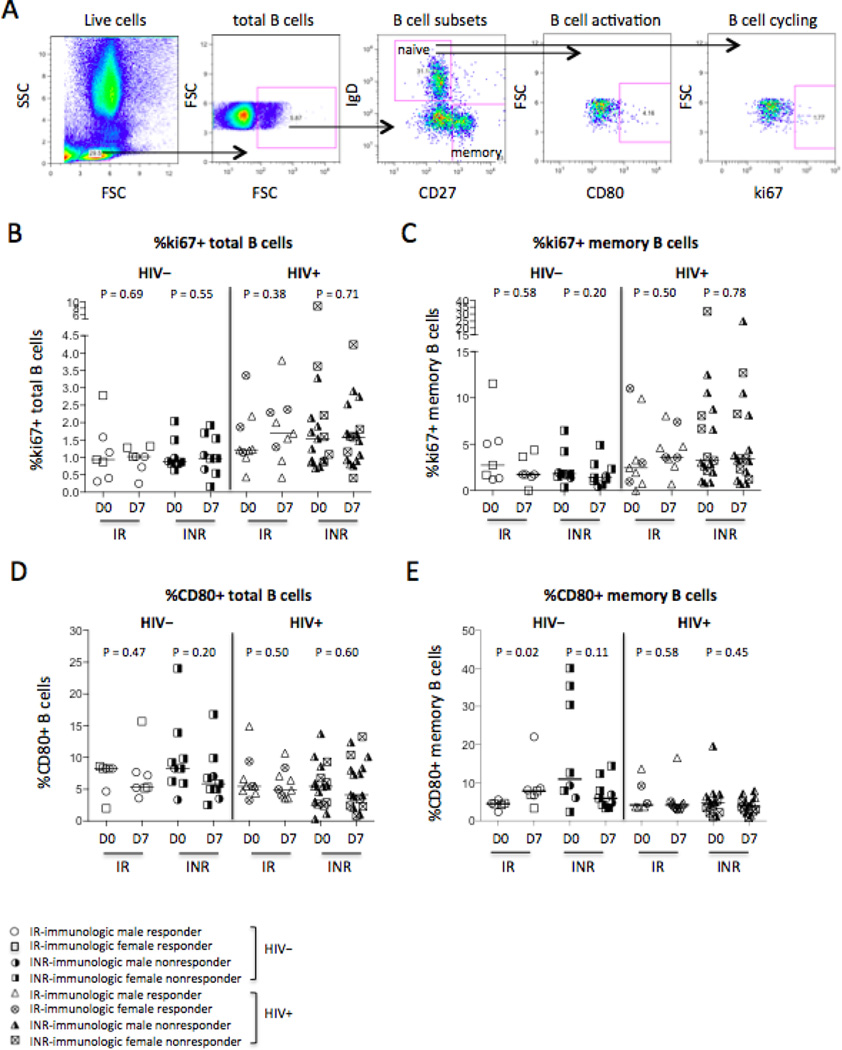
B cell activation and cycling were also assessed in responders and non-responders in controls and HIV+ subjects pre- and post- vaccination (Fig. 2A–2E). Baseline level of ki67 expression in total B cells (P = 0.03, Fig. 2B) but not in memory B cells (P = 0.20, Fig. 2C) was elevated in all HIV+ subjects compared to controls, and CD80 expression on total (P = 0.50, Fig. 2D) and memory (P = 0.10, Fig. 2E) B cells was similar at baseline in both groups. Interestingly, the frequency of CD80+ memory B cells was increased on D7 post-vaccination only in responders of controls, but not in non-responders or in HIV-infected subjects (Fig. 2E). These results suggest that memory B cell activation as reflected by CD80 expression may be important in vaccine recall antigen responses in healthy controls. HIV-infected subjects had impaired CD80 induction on memory B cells despite long-term ART treatment and viral suppression.
Figure 2.
B cell cycling and activation in responders and non-responders. Blood samples pre-and post-influenza vaccinations were tested CD80 for B cell activation and ki67 for B cell cycling. (A) Representative dot plots display the gating strategy used to assess the percentages of ki67+ or CD80+ total B cells or memory B cells. The median frequencies of ki67+ total B cells (B) and memory B cells (C). The median frequencies of CD80+ total B cells (D) and memory B cells (E).
CD4+ T cell parameters pre- and post-vaccination in responders and non-responders of healthy controls and HIV-infected subjects
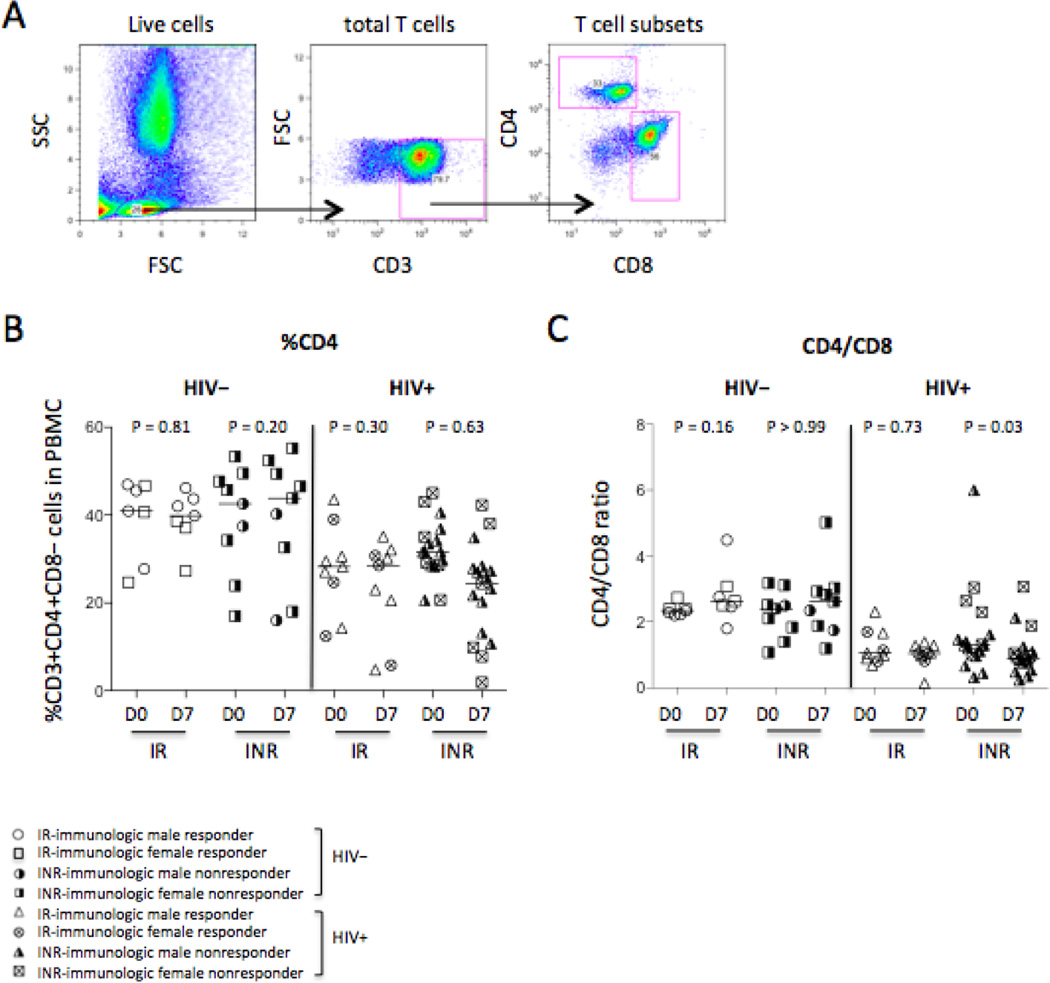
CD4+ T cells are required for B cells to produce antibodies in response to influenza vaccination because influenza vaccine antigens are T cell-dependent antigens [17]. We therefore analyzed the frequency of CD4+ T cells in total CD3+ T cells (Fig. 3A–3B) and the ratio of CD4+ T cells versus CD8+ T cells (Fig. 3A and 3C) on D0 and D7. Interestingly, we observe a decrease in the ratio of CD4+ T cells versus CD8+ T cells in immunologic non-responders of HIV+ subjects on D7 compared to D0 but not in responders and controls (Fig. 3C). However, the p value in figure 3C is not significant on D0 compared to D7 in non-responders of HIV+ subjects if the outlier (CD4/CD8 ratio > 5) was removed from HIV+ INR on D0 (P = 0.07). Baseline frequency of CD4+ T cells (P = 0.002, Fig. 3B) and ratio of CD4+ T cells versus CD8+ T cells (P < 0.0001, Fig. 3C) decreased in all HIV+ subjects compared to healthy controls, but these markers were similar in responders and non-responders (Fig. 3).
Figure 3.
CD4+ T cell distribution. (A) Representative dot plots display the gating strategy used to assess the percentages of CD3+CD4+CD8− T cells in PBMCs and the ratio of CD4+ T cells versus CD8+ T cells. (B) The median frequency of CD3+CD4+CD8− T cells in PBMCs. (C) The median ratio of CD4+ T cells versus CD8+ T cells.
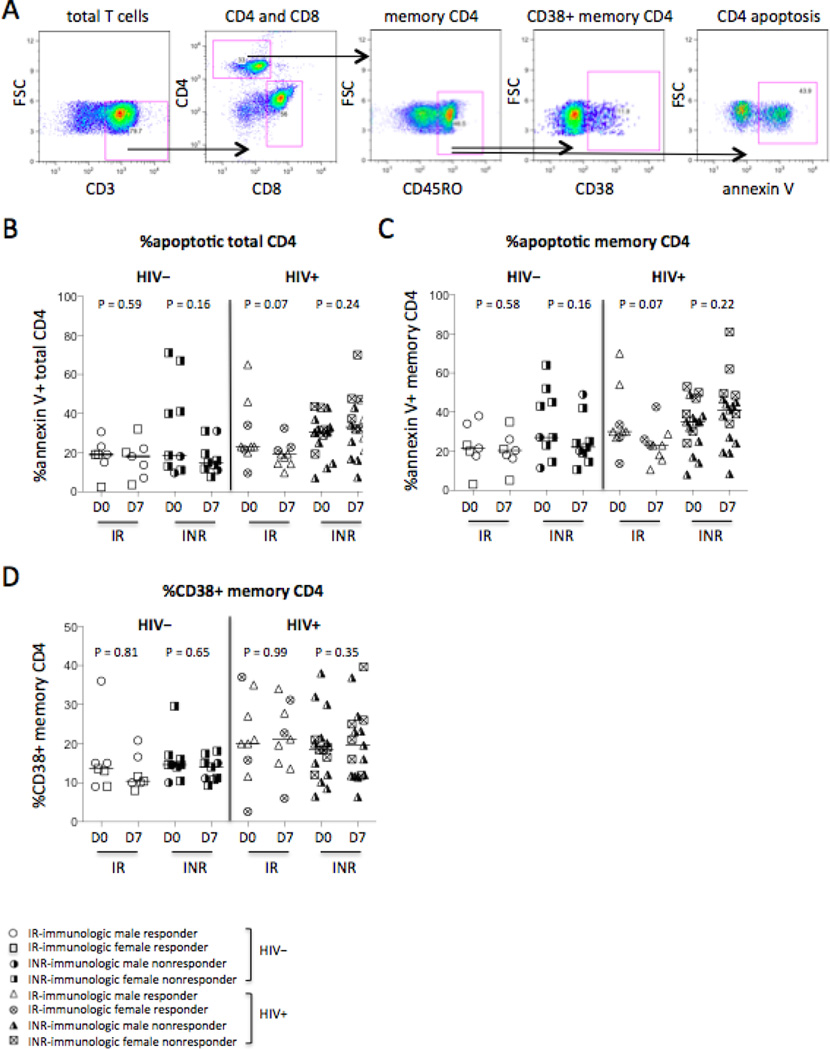
Next, we assessed CD4+ T cell apoptosis and activation that are important immunologic parameters for HIV disease progression [18]. Baseline frequencies of apoptosis on total CD4+ T cells (CD3+CD4+CD8−, P = 0.13) or on memory CD4+ T cells (CD4+CD3+CD8−CD45RO+, P = 0.20) were similar in healthy controls and HIV+ subjects, and were similar on D0 and D7 in the four study groups (Fig. 4A–4C). In HIV disease, CD38 is a recognized nonspecific immunologic parameter of T cell activation [19, 20]. Baseline level of chronic immune activation reflected by the frequency of CD38+ on memory CD4+ T cells tended to increase in all HIV+ subjects compared to healthy controls (P = 0.055, Fig. 4D). Nonetheless, we did not identify differences in these markers between responders and non-responders (Fig. 4). These results suggest that even after long-term ART treatment, CD4+ T cell frequencies and activation were not fully normalized in all patients compared with healthy controls at baseline regardless of immunologic responders and non-responders.
Figure 4.
CD4+ T cell activation and apoptosis. CD38 expression on memory CD4+ T cells was analyzed in fresh blood samples. Annexin V binding on CD4+ T cells was analyzed in fresh isolated PBMC. (A) Representative dot plots display the gating strategy used to assess the percentages of annexin V binding on CD4+ T cell (CD3+CD4+CD8−, tCD4) and memory CD4+ T cell (mCD4, CD3+CD4+CD8−CD45RO+). The median frequencies of annexin V binding on total CD4+ T cells (B) and memory CD4+ T cells (C). (D) The median frequencies of CD38+ memory CD4+ T cells.
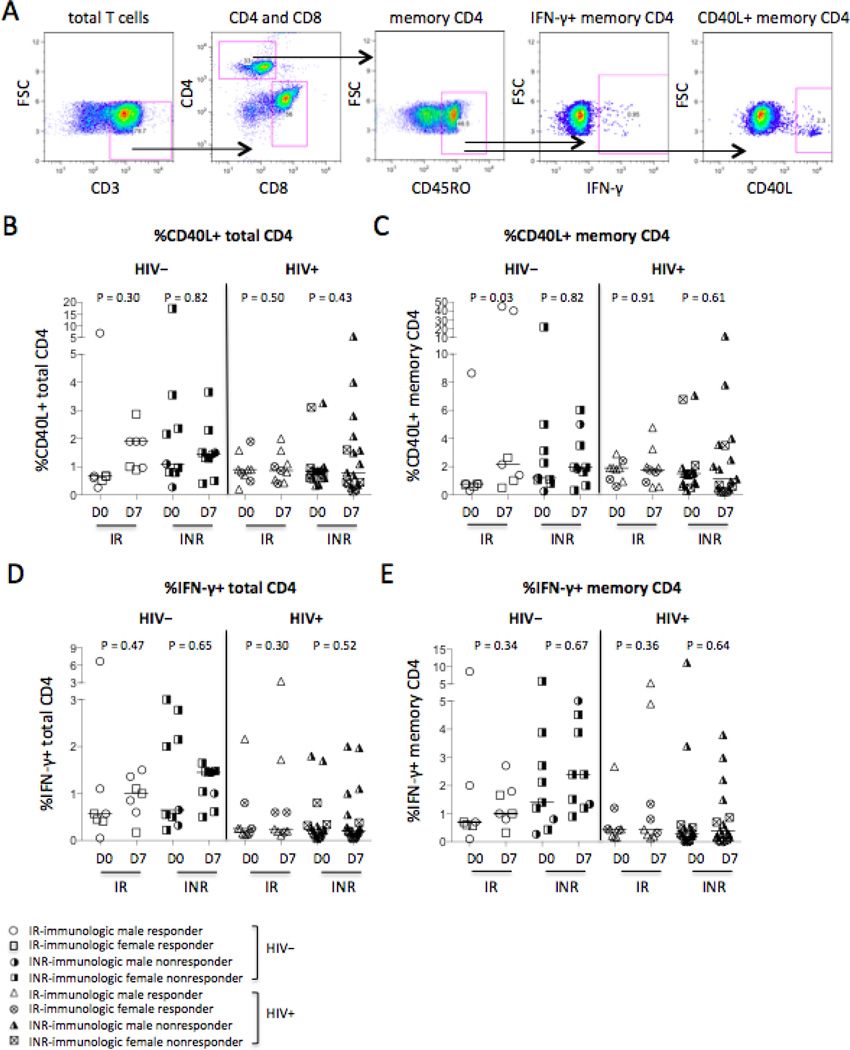
Intracellular CD40L and IFN-γ expression have been reported to be reasonably specific markers of recent T cell receptor engagement in CD4+ T cells [21–23]. To investigate the function of CD4+ T cells in response to vaccination in vivo, the frequencies of intracellular CD40L and IFN-γ-producing CD4+ T cells were compared between controls and HIV-infected subjects, and immunologic responders and non-responders (Fig. 5A–5E). Vaccination increased CD40L expression in memory CD4+ T cells but not total CD4+ T cells on D7 compared to D0 only in responders of controls but not in non-responders or HIV-infected subjects (Fig. 5B–5C). However, if the outliers (%CD40L+CD4/mCD4 > 5%) were removed in HIV− responders, vaccination significantly increased CD40L expression in total CD4+ T cells (P = 0.001, Fig. 5B) but not in memory CD4+ T cells (P = 0.08, Fig. 5C) on D7 compared to D0. The frequencies of IFN-γ-producing CD4+ T cells or memory CD4+ T cells tended to increase in response to vaccination in all healthy controls but not in all HIV+ subjects regardless of responders and non-responders (Fig. 5D–5E). The results were the same if the removed outliers were removed (%IFN-γ+CD4/mCD4 > 5%, Fig. 5D–5E). Baseline levels of CD40L-producing memory CD4+ T cells (P = 0.003) but not total CD4+ T cells (P = 0.58) were increased in HIV+ subjects compared to healthy controls (Fig. 5B–5C), but baseline frequencies of IFN-γ-producing CD4+ T cells (P = 0.001, Fig. 5D) and memory CD4+ T cells (P = 0.001, Fig. 5E) were reduced in all HIV+ subjects compared to all healthy controls. These results suggest that antigen-specific CD4+ T cell function in HIV+ subjects was not fully recovered after ART treatment and viral suppression.
Figure 5.
Intracellular CD40L+ and IFN-γ+ in CD4+ T cells. (A) Representative dot plots display the gating strategy used to assess the percentages of intracellular CD40L+ or IFN-γ+ in CD4+ T cells. The median frequencies of CD40L+ total CD4+ T cells (B) and memory CD4+ T cells (C). The median frequencies of IFN-γ+ total CD4+ T cells (D) and memory CD4+ T cells (E).
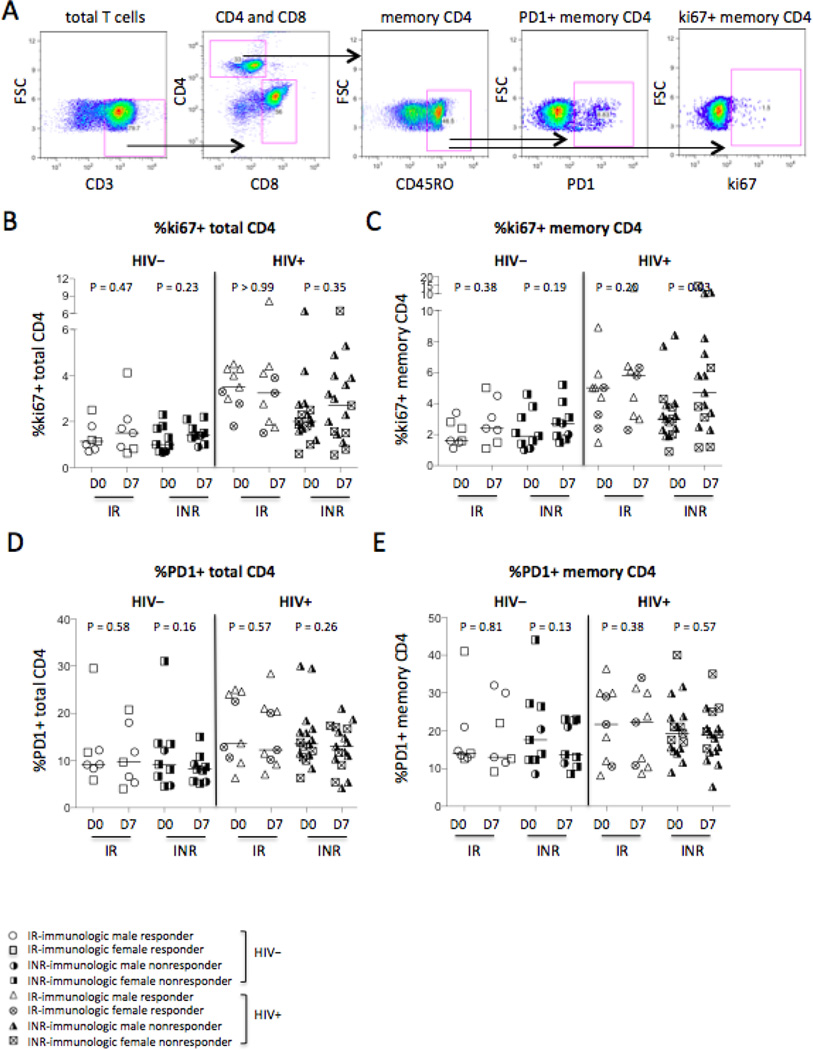
PD-1 is an immunologic parameter related to T cell exhaustion that is linked to impaired T cell function [24]. The intracellular expression of ki67, an immunologic parameter of ongoing or recent cell cycle entry [25], was measured in CD4+ T cells in the current study (Fig. 6A–6E). The frequency of memory CD4+ T cell cycling increased in immunologic non-responders of HIV+ subjects in response to vaccination (P = 0.02, Fig. 6C), and tended to increase in response to vaccination in the other three study groups but did not achieve significant differences regardless of HIV infection status (Fig. 6C). Furthermore, baseline frequencies of ki67-expressing CD4+ T cells (P < 0.001, Fig. 6B) and memory CD4+ T cells (P = 0.003, Fig. 6C) were increased in all HIV+ subjects compared to all healthy controls. Moreover, we found that baseline frequency of PD1+ total CD4+ T cells (P = 0.02, Fig. 6D) but not memory CD4+ T cells (P = 0.34, Fig. 6E) was higher in all HIV+ subjects compared to all healthy controls. Vaccination did not change PD1 expression on CD4+ T cells in any group (Fig. 6D–6E). Altogether, baseline levels of CD4+ T cell activation revealed that CD4+ T cells are activated with heightened expression of ki67, PD1, and CD38, despite long-term ART treatment and viral suppression in the HIV-infected individuals.
Figure 6.
CD4+ T cell cycling and exhaustion. (A) Representative dot plots display the gating strategy used to assess the percentages of intracellular ki67 and surface PD1 expression on CD4+ T cells. The median frequencies of ki67+ CD4+ T cells (B) and memory CD4+ T cells (C). The median frequencies of PD1+ CD4+ T cells (D) and memory CD4+ T cells (E).
In summary, we have observed the differences of CD80 induction on memory B cells and CD40L induction in memory CD4+ T cells by after influenza vaccination in responders of healthy controls. HIV-infected subjects had impaired CD80 induction on memory B cells and CD40L induction on memory CD4+ T cells regardless of responders and non-responders. In addition, we have seen the differences in B cell apoptosis between responders and non-responders, which with vaccination induced-B cell apoptosis in non-responders but not in responders, regardless of HIV-infection status.
DISCUSSION
The aim of this study was to define the predictors of recall immune responses to influenza vaccination in healthy controls and HIV-infected subjects. In the current study, serologic responses to the trivalent 2013–2014 seasonal influenza vaccination were measured by H1N1 virus-specific microneutralization assay, and cellular function was analyzed by flow cytometry in a cohort of healthy controls and aviremic ART+ HIV-infected subjects. To define the cellular function in response to influenza vaccine antigen stimulation in vivo, blood or PBMCs samples were analyzed by frequencies, cell activation, cycling, and cytokine production among CD4+ T cells and B cells, and differences in responders and non-responders of HIV+ and HIV− subjects were compared. Changes in frequency of B cell apoptosis following vaccination distinguish responders and non-responders of HIV+ and HIV− subjects; CD80 on memory B cells and CD40L in memory CD4+ T cells were induced in response to vaccination only in immunologic responders of HIV− subjects but not in HIV+ subjects. When the whole study population was taken into account, regardless of vaccine response, baseline levels of apoptotic B cells, and PD1, cycling, and CD38 expression on CD4+ or memory CD4+ T cells were elevated in HIV+ patients compared with controls.
No significant induction of CD38, PD1 and IFN-γ in total or memory CD4+ T cells and no significant induction of ki67 on total or memory B cells were observed by after vaccinations. Previous studies show that cellular activation was observed on day 1 post-influenza vaccination [26]. Timing of sample collection may impact these observations, and day 7 measurements post-vaccination may be too late to detect the optimal time of some cell activation markers. It was noted that HIV-infected subjects had higher baseline levels of B cell and CD4+ T cell activation; suggesting elevated chronic immune activation in these long-term viral-suppressive ART treated HIV-infected subjects.
CD40L is a specific marker of CD4+ T cells that have been engaged via T cell receptor stimulation [27]. Impaired expression of CD40L in CD4+ T cells has been reported in HIV-infected individuals compared to healthy controls in response to super-antigen staphylococcal enterotoxin B in vitro [28]. In the current study, CD40L was induced in memory CD4+ T cells on day 7 post-vaccination in immunologic responders of controls but not in non-responders and HIV-infected individuals, implying that CD40L may be an important marker for antigen-specific CD4+ T cells in response to influenza vaccination in healthy individuals in vivo.
B7 CD80/CD86 is a costimulatory or activation molecule expressed on B cells that is crucial for IgG production against influenza viral infection [29]. In the current study, CD80 was induced on memory B cells on day 7 post-vaccination in immunologic responders of controls but not in non-responders and HIV-infected individuals. Additionally, vaccine-induced of B cell apoptosis was the marker distinguishing immunologic non-responders from responders regardless of HIV infection status, implying that CD80 induction on B cells may be an important marker for appropriate B cell vaccine response in healthy individuals while B cell apoptosis may imply poor vaccine response in both controls and HIV-infected individuals under viral suppression and ART treatment.
Upon encountering a T cell-dependent antigen such as influenza vaccination, CD4+ T cells are activated to express CD40L to provide help for B cells. B cells then undergo subsequent activation to express CD80, proliferation (ki67) and antibody production. Alternatively, they become anergic or undergo apoptosis and fail to produce sufficient amount of antibodies [30]. B cell receptor-induced mitochondrial dysfunction is a central mechanism for B cell receptor induced cell apoptosis [30], which may account for B cell apoptotic induction by vaccination in immune non-responders in the current study. CD40/CD40L signaling between CD4+ T cells and B cells has been shown to reverse B cell receptor induced apoptosis [31], suggesting a possible mechanism accounting for immune non-responders in healthy control individuals.
The current study suggests that CD40L in memory CD4+ T cells and CD80 on memory B cells may be critical for vaccine response responders among healthy controls, but they are not necessary for vaccine responses responders among HIV-infected individuals, suggesting that they are important immune parameters for vaccine responses in healthy controls. However, baseline frequencies of apoptosis and ki67 expression in total B cells were elevated in aviremic ART-treated HIV+ subjects compared to healthy controls. This may result in B cell perturbations in response to vaccination in HIV+ subjects. Moreover, B cell apoptosis is the only marker to distinguish responders and non-responders regardless HIV infection status. The main limitation to this study is its relatively small sample size that may impact on interpretation of the results and preclude drawing further conclusions. To the best of our knowledge, this is the first reported association of increased B cell apoptosis post-influenza vaccination with vaccine nonresponse. This study may provide some information for vaccine design.
Acknowledgments
This work was supported by NIH grants AI091526, the University of Alabama at Birmingham, Center for AIDS Research (P30 AI027767), the 12th Five Year Research Project of People's Liberation Army (CWS11J160), the International Science and Technology Cooperation Program of China (2011DFR30420), the National High Technology Research and Development Program (863 program 2012AA02A404-7), and National Nature Science Foundation in China (81460258).
Footnotes
The authors declare no competing financial interests.
Reference
- 1.Cohen JP, Macauley C. Susceptibility to influenza A in HIV-positive patients. JAMA. 1989;261:245. doi: 10.1001/jama.1989.03420020097023. [DOI] [PubMed] [Google Scholar]
- 2.Fine AD, Bridges CB, De Guzman AM, Glover L, Zeller B, Wong SJ, et al. Influenza A among patients with human immunodeficiency virus: an outbreak of infection at a residential facility in New York City. Clin Infect Dis. 2001;32:1784–1791. doi: 10.1086/320747. [DOI] [PubMed] [Google Scholar]
- 3.Aberg JA, Gallant JE, Anderson J, Oleske JM, Libman H, Currier JS, et al. Primary care guidelines for the management of persons infected with human immunodeficiency virus: recommendations of the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis. 2004;39:609–629. doi: 10.1086/423390. [DOI] [PubMed] [Google Scholar]
- 4.Tebas P, Frank I, Lewis M, Quinn J, Zifchak L, Thomas A, et al. Poor immunogenicity of the H1N1 2009 vaccine in well controlled HIV-infected individuals. AIDS. 2010;24:2187–2192. doi: 10.1097/QAD.0b013e32833c6d5c. [DOI] [PubMed] [Google Scholar]
- 5.Cagigi A, Nilsson A, Pensieroso S, Chiodi F. Dysfunctional B-cell responses during HIV-1 infection: implication for influenza vaccination and highly active antiretroviral therapy. Lancet Infect Dis. 2010;10:499–503. doi: 10.1016/S1473-3099(10)70117-1. [DOI] [PubMed] [Google Scholar]
- 6.Tang YW, Li H, Wu H, Shyr Y, Edwards KM. Host single-nucleotide polymorphisms and altered responses to inactivated influenza vaccine. J Infect Dis. 2007;196:1021–1025. doi: 10.1086/521370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.George VK, Pallikkuth S, Parmigiani A, Alcaide M, Fischl M, Arheart KL, et al. HIV infection worsens Age-Associated Defects in Antibody Responses to Influenza Vaccine. J Infect Dis. 2015 doi: 10.1093/infdis/jiu840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tan Y, Tamayo P, Nakaya H, Pulendran B, Mesirov JP, Haining WN. Gene signatures related to B-cell proliferation predict influenza vaccine-induced antibody response. Eur J Immunol. 2014;44:285–295. doi: 10.1002/eji.201343657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramirez LA, Daniel A, Frank I, Tebas P, Boyer JD. Seroprotection of HIV-infected subjects after influenza A(H1N1) vaccination is directly associated with baseline frequency of naive T cells. J Infect Dis. 2014;210:646–650. doi: 10.1093/infdis/jiu132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fuller JD, Craven DE, Steger KA, Cox N, Heeren TC, Chernoff D. Influenza vaccination of human immunodeficiency virus (HIV)-infected adults: impact on plasma levels of HIV type 1 RNA and determinants of antibody response. Clin Infect Dis. 1999;28:541–547. doi: 10.1086/515170. [DOI] [PubMed] [Google Scholar]
- 11.Malaspina A, Moir S, Orsega SM, Vasquez J, Miller NJ, Donoghue ET, et al. Compromised B cell responses to influenza vaccination in HIV-infected individuals. J Infect Dis. 2005;191:1442–1450. doi: 10.1086/429298. [DOI] [PubMed] [Google Scholar]
- 12.McKittrick N, Frank I, Jacobson JM, White CJ, Kim D, Kappes R, et al. Improved immunogenicity with high-dose seasonal influenza vaccine in HIV-infected persons: a single-center, parallel, randomized trial. Ann Intern Med. 2013;158:19–26. doi: 10.7326/0003-4819-158-1-201301010-00005. [DOI] [PubMed] [Google Scholar]
- 13.Pathirana RD, Bredholt G, Akselsen PE, Pedersen GK, Cox RJ. A(H1N1)pdm09 vaccination of health care workers: improved immune responses in low responders following revaccination. J Infect Dis. 2012;206:1660–1669. doi: 10.1093/infdis/jis589. [DOI] [PubMed] [Google Scholar]
- 14.Luo Z, Ma L, Zhang L, Martin L, Wan Z, Warth S, et al. Key differences in B cell activation patterns and immune correlates among treated HIV-infected patients versus healthy controls following influenza vaccination. Vaccine. 2016;34:1945–1955. doi: 10.1016/j.vaccine.2015.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bonduelle O, Yahia N, Siberil S, Benhabiles N, Carrat F, Krivine A, et al. Longitudinal and integrative biomodeling of effector and memory immune compartments after inactivated influenza vaccination. J Immunol. 2013;191:623–631. doi: 10.4049/jimmunol.1203483. [DOI] [PubMed] [Google Scholar]
- 16.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1:43–46. [PubMed] [Google Scholar]
- 17.Vigano A, Bricalli D, Trabattoni D, Salvaggio A, Ruzzante S, Barbi M, et al. Immunization with both T cell-dependent and T cell-independent vaccines augments HIV viral load secondarily to stimulation of tumor necrosis factor alpha. AIDS Res Hum Retroviruses. 1998;14:727–734. doi: 10.1089/aid.1998.14.727. [DOI] [PubMed] [Google Scholar]
- 18.Sousa AE, Carneiro J, Meier-Schellersheim M, Grossman Z, Victorino RM. CD4 T cell depletion is linked directly to immune activation in the pathogenesis of HIV-1 and HIV-2 but only indirectly to the viral load. J Immunol. 2002;169:3400–3406. doi: 10.4049/jimmunol.169.6.3400. [DOI] [PubMed] [Google Scholar]
- 19.Boasso A, Hardy AW, Anderson SA, Dolan MJ, Shearer GM. HIV-induced type I interferon and tryptophan catabolism drive T cell dysfunction despite phenotypic activation. PLoS One. 2008;3:e2961. doi: 10.1371/journal.pone.0002961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wursch D, Ormsby CE, Romero-Rodriguez DP, Olvera-Garcia G, Zuniga J, Jiang W, et al. CD38 Expression in a Subset of Memory T Cells Is Independent of Cell Cycling as a Correlate of HIV Disease Progression. Dis Markers. 2016;2016:9510756. doi: 10.1155/2016/9510756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frentsch M, Arbach O, Kirchhoff D, Moewes B, Worm M, Rothe M, et al. Direct access to CD4+ T cells specific for defined antigens according to CD154 expression. Nat Med. 2005;11:1118–1124. doi: 10.1038/nm1292. [DOI] [PubMed] [Google Scholar]
- 22.Boisvert J, Edmondson S, Krummel MF. Immunological synapse formation licenses CD40-CD40L accumulations at T-APC contact sites. J Immunol. 2004;173:3647–3652. doi: 10.4049/jimmunol.173.6.3647. [DOI] [PubMed] [Google Scholar]
- 23.Pahar B, Li J, Rourke T, Miller CJ, McChesney MB. Detection of antigen-specific T cell interferon gamma expression by ELISPOT and cytokine flow cytometry assays in rhesus macaques. J Immunol Methods. 2003;282:103–115. doi: 10.1016/j.jim.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 24.Petrovas C, Price DA, Mattapallil J, Ambrozak DR, Geldmacher C, Cecchinato V, et al. SIV-specific CD8+ T cells express high levels of PD1 and cytokines but have impaired proliferative capacity in acute and chronic SIVmac251 infection. Blood. 2007;110:928–936. doi: 10.1182/blood-2007-01-069112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang W, Younes SA, Funderburg NT, Mudd JC, Espinosa E, Davenport MP, et al. Cycling memory CD4+ T cells in HIV disease have a diverse T cell receptor repertoire and a phenotype consistent with bystander activation. J Virol. 2014;88:5369–5380. doi: 10.1128/JVI.00017-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin YC, Winokur P, Blake A, Wu T, Manischewitz J, King LR, et al. Patients with MS under daclizumab therapy mount normal immune responses to influenza vaccination. Neurol Neuroimmunol Neuroinflamm. 2016;3:e196. doi: 10.1212/NXI.0000000000000196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stuehler C, Nowakowska J, Bernardini C, Topp MS, Battegay M, Passweg J, et al. Multispecific Aspergillus T cells selected by CD137 or CD154 induce protective immune responses against the most relevant mold infections. J Infect Dis. 2015;211:1251–1261. doi: 10.1093/infdis/jiu607. [DOI] [PubMed] [Google Scholar]
- 28.Espinosa E, Ormsby CE, Reyes-Teran G, Asaad R, Sieg SF, Lederman MM. Dissociation of CD154 and cytokine expression patterns in CD38+ CD4+ memory T cells in chronic HIV-1 infection. J Acquir Immune Defic Syndr. 2010;55:439–445. doi: 10.1097/QAI.0b013e3181ef991d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rau FC, Dieter J, Luo Z, Priest SO, Baumgarth N. B7-1/2 (CD80/CD86) direct signaling to B cells enhances IgG secretion. J Immunol. 2009;183:7661–7671. doi: 10.4049/jimmunol.0803783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eeva J, Pelkonen J. Mechanisms of B cell receptor induced apoptosis. Apoptosis. 2004;9:525–531. doi: 10.1023/B:APPT.0000038032.22343.de. [DOI] [PubMed] [Google Scholar]
- 31.Eeva J, Postila V, Matto M, Nuutinen U, Ropponen A, Eray M, et al. Kinetics and signaling requirements of CD40-mediated protection from B cell receptor-induced apoptosis. Eur J Immunol. 2003;33:2783–2791. doi: 10.1002/eji.200324227. [DOI] [PubMed] [Google Scholar]