Abstract
Objective
Numerous studies show that depressive symptoms measured at a single assessment predict greater future stroke risk. Longer term symptom patterns, such as variability across repeated measures or worst symptom level, might better reflect adverse aspects of depression than a single measurement. This prospective study compared five approaches to operationalizing depressive symptoms at annual assessments as predictors of stroke incidence.
Design
Cohort followed for incident stroke over an average of 6.4 years.
Setting
The Adult Changes in Thought cohort follows initially cognitively-intact, community-dwelling older adults from a population base defined by membership in Group Health, a Seattle-based nonprofit healthcare organization.
Participants
3,524 individuals aged 65 and older.
Measurements
We identified 665 incident strokes using ICD codes. We considered both baseline Centers for Epidemiologic Study – Depression scale (CES-D) score and, using a moving window of three most recent annual CES-D measurements, we compared most recent, maximum, average, and intra-individual variability of CES-D scores as predictors of subsequent stroke using Cox proportional hazards models.
Results
Greater maximum (HR=1.18; 95% CI=1.07–1.30), average (HR=1.20; 95% CI=1.05–1.36), and intra-individual variability (HR=1.15; 95% CI=1.06–1.24) in CES-D were each associated with elevated stroke risk, independent of sociodemographics, cardiovascular risks, cognition, and daily functioning. Neither baseline nor most recent CES-D was associated with stroke. In a combined model, intra-individual variability in CES-D predicted stroke, but average CES-D did not.
Conclusions
Capturing the dynamic nature of depression is relevant in assessing stroke risk. Fluctuating depressive symptoms may reflect a prodrome of reduced cerebrovascular integrity.
Keywords: depression, variability, cerebrovascular, elderly
Objective
Depression has a cumulative lifetime incidence of 16% in the general population(1) and is associated with increased risk of stroke.(2–3) Depression may increase stroke risk via neuroendocrine(4) and/or immunological(5) dysregulation, poor health behaviors,(6–8) the development of hypertension and diabetes,(9–10) and/or antidepressant medication use.(11–13) Studies examining the association between depression and stroke demonstrate substantial heterogeneity,(2–3) which may, in part, reflect differences in how or when depressive symptoms were measured. Many studies consider only baseline score on a depression instrument, such as the Centers for Epidemiologic Studies-Depression scale (CES-D). However, this approach provides only a snapshot of depressive symptoms at a relatively arbitrary point in time (typically, study enrollment). More comprehensive operationalization of depressive symptoms as a dynamic construct may offer more precise estimates of the association between depressive symptoms and stroke risk, as well as mechanistic insights into this association. For example, instability in depressive symptoms across repeated assessments may reflect evolving cerebrovascular disease and presage elevations in stroke risk independently of average depressive symptoms.
In addition to baseline level, this prospective study thus investigated four other approaches to operationalizing depressive symptoms based on a moving window of the three most recent assessments: proximal level, maximum level across the three visits, average level across the three visits, and intra-individual variability across the three visits (Figure 1). We evaluated associations between each operationalization and stroke incidence. We hypothesized that (a) average level of and (b) intra-individual variability in depressive symptoms would be most strongly associated with incident stroke because they reflect cumulative exposure to depressive symptoms and mood dysregulation, respectively. Specifically, average level of depressive symptoms potentially indexes the accumulation of biological (e.g., neuroendocrine, inflammatory) and behavioral (e.g., low physical activity, smoking) factors related to depression that have been shown to increase stroke risk.(4–8). Intra-individual variability potentially indexes mood dysregulation, which has been shown to predict a variety of poor health outcomes such as mortality(14) and self-reported chronic illness.(15) Mood dysregulation may lead to more frequent engagement of the hypothalamic-pituitary-adrenal (HPA) axis in response to daily stressors, which predicts stroke onset and severity in humans and animal models.(16) In addition, intra-individual variability in other behavioral markers have been linked to cerebrovascular abnormalities (e.g., white matter hyperintensities).(17) We also hypothesized that baseline and proximal levels would be least associated with risk of stroke given that these single time-point variables capture fewer aspects of the dynamic psychological construct and are less reliable because they draw on less information.
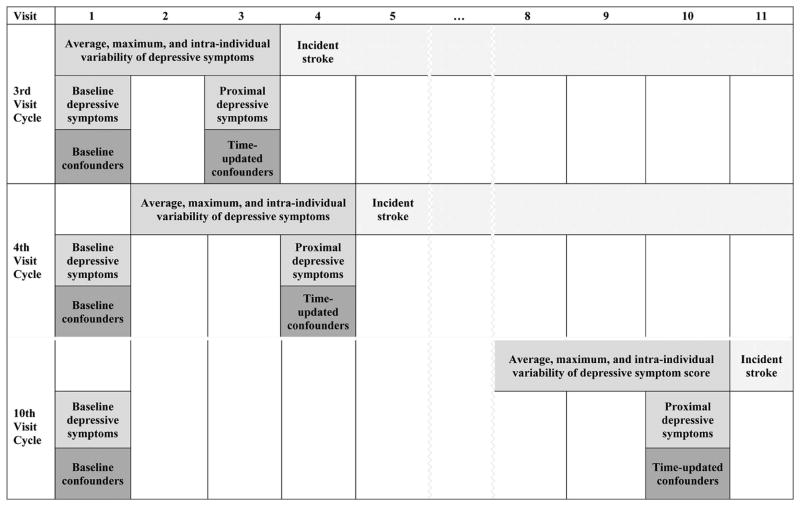
Figure 1.
Timing of assessments of depressive symptoms, confounders, and stroke. The moving window of the three most recent assessments is illustrated for three example visits: visit 3, visit 4, and visit 10. Baseline CES-D was a time-invariant variable. All other CES-D variables were time-varying: proximal CES-D, average CES-D over the three most recent visits, maximum CES-D over the three most recent visits, and intra-individual variability in CES-D over the three most recent visits. Baseline confounders were age, sex, race, and education. Time-varying confounders were marital status, self-rated health, activities of daily living, exercise, cognitive status, body mass index, and Framingham Risk Score.
Methods
Data
Participants were drawn from the Adult Changes in Thought (ACT) cohort, which has been described in detail elsewhere.(18–19) In brief, ACT enrolled cognitively-intact, community dwelling adults aged 65 and above in 1994–1996 (N=2,581) and 2001–2003 (N=881) from a population base defined by membership in Group Health, a Seattle-based nonprofit healthcare organization. Continuous enrollment of 10–15 new participants per month using the same methods began in 2005. The data considered here were obtained from all participants who had not withdrawn from the study as of the September 30, 2012 data freeze, at which time there were N=4,131 participants. Informed consent was obtained from all participants. Demographic, medical history, and functional status was assessed via in-person interviews every two years. Study information is also linked to medical records and pharmacy files from Group Health. ACT data together with Group Health data were used to identify prevalent and incident stroke in the current study. Exclusion criteria included history of stroke (ICD code or self-report) or transient ischemic attack (self-report) at the initial ACT study visit. Inclusion criteria included the presence of at least one CES-D score over the follow-up period and availability of covariate data. Participants were censored after dropping out of the ACT cohort, even if information was available in Group Health medical records. The final analytic sample included 3,524 individuals assessed at up to 11 visits (mean = 4.08 visits) over up to 18 years (mean = 6.39 years). Of these participants, 466 were evaluated at only one visit, 662 were evaluated at 2 visits, and 2,223 were evaluated at three or more visits. The participants evaluated at three or more visits were assessed at an average of 5.53 visits over 10.74 years, with a mean inter-visit interval of 2.48 years.
Measures
The primary outcome was first incident stroke (of any type), identified from ICD-9 codes included in the Center for Medicare and Medicaid Services’ Chronic Condition Data Warehouse algorithm (430, 431, 433.01, 433.11, 433.21, 433.31, 433.81, 433.91, 434.00, 434.01, 434.10, 434.11, 434.90, 434.91, 435.0, 435.1, 435.3, 435.8, 435.9, 436, 997.02) in inpatient and outpatient medical records.(20–23) If the principal diagnosis for the billing occasion indicated rehabilitation services (V57.xx), or the visit included ICD codes indicating a head injury (800–804.9 or 850–854.1), then the event was not classified as incident stroke, regardless of other ICD codes. If the diagnosis was based on outpatient services, the event was only classified as a stroke if there were at least two stroke-related records within a 12-month period.
The primary exposure was depressive symptoms, assessed annually with the 10-item Centers for Epidemiologic Studies-Depression scale (CES-D; 24), which can range from 0 to 30. Five variables based on CES-D score were computed: baseline score (i.e., CES-D at first available ACT study visit), CES-D score at most recent visit, maximum CES-D score over the three most recent visits, average CES-D score over the three most recent visits, and intra-individual variability in CES-D scores (i.e., standard deviation) over the three most recent visits. Baseline score was a time-invariant variable. All other CES-D variables were time-varying. Maximum, average, and intra-individual variability were computed using data from the three most recent study visits, as opposed to all study visits in order to create time-varying variables that capture the dynamic nature of depressive symptoms. Variables computed over the entire study period would have been static variables that reflect a different period of exposure for different participants because participants entered the study at different, relatively arbitrary points of time between age 65 and 101 and had different lengths of follow-up.
Figure 1 illustrates the moving window of the three most recent assessments used to derive the different operationalizations of CES-D at different study visits. Note that three of the depressive symptom operationalizations (i.e., maximum CES-D, average CES-D, and intra-individual variability in CES-D) required participants to have been evaluated at least three visits. Because 1,301 of the 3,524 participants were only evaluated once or twice, analyses involving these three CES-D operationalizations only included 2,223 participants. Sensitivity analyses were conducted to determine whether results from analyses involving baseline CES-D and proximal CES-D were different in this subset of participants with more follow-up data.
Covariates included age (continuous), female sex, race (White versus all other), years of education (continuous), marital status (married or living with a partner versus all other), self-rated health (continuous, 5-point scale from excellent to poor), activities of daily living (continuous, count of self-reported limitations in six activities), exercise (self-report of at least 15 minutes of exercise three times a week or more), cognitive status (continuous, Cognitive Assessment Screening Instrument score derived using item response theory; 25), body mass index (continuous), and ten-year Framingham Risk Score (FRS, continuous).(26) Components of the FRS (i.e., presence of type II diabetes mellitus, current smoking, systolic blood pressure, diagnoses of heart disease, atrial fibrillation, and left ventricular hypertrophy) were obtained from medical records or survey responses. Age, sex, race, and education were measured at baseline. All other covariates were measured at the most recent prior interview (see Figure 1).
Statistical Analysis
Descriptive statistics and correlations were computed using SPSS version 22. We computed correlations among the five variables that reflected alternative operationalizations of CES-D using data from all observations. Survival analysis with time-varying confounders was conducted in Mplus version 7 using Cox regression. Survival time was entered as months until stroke or last assessment, and all models adjusted for baseline age, in addition to the other covariates. An examination of all possible interactions of the predictors and a function of survival time indicated that the assumption of proportional hazards was tenable. Initially, we ran five separate models. In each model, one of the five CES-D variables was included, along with covariates. Because the standard deviation of CES-D scores is dependent on absolute level of depressive symptoms, we also ran a combined model that included both intra-individual variability in CES-D scores across three most recent visits and the mean CES-D score across those same visits, along with covariates. This combined model separated the relative predictive value of fluctuations in depressive symptoms from that of persistent level of depressive symptoms. Sensitivity analyses tested whether results changed if the distributions of the CES-D variables were normalized through logarithmic transformation.
Results
At baseline, most cohort members were in their 8th decade of life, white, and well-educated (Table 1). Over the follow-up period of up to 11 visits (mean = 4.1 visits) over up to 18 years (mean = 6.4 years), 665 (19%) individuals developed incident stroke. Means, standard deviations, and inter-correlations among the five CES-D variables (across all participants and visits) are shown in Table 2. The range of baseline CES-D scores spanned the entire range of the scale, from 0 to 30, though only 9% of the sample obtained a score of at least 10, which indicates the presence of a depressive disorder.(20) Over the course of the 18-year follow-up period, 21% of the sample obtained at least one CES-D score of 10 or greater.
Table 1.
Baseline characteristics of the ACT sample (N=3,736)
| Mean (SD) or % | |
|---|---|
| Age | 74.3 (6.5) |
| Sex, % female | 58.2 |
| Race, % White | 91.6 |
| Education (years) | 14.8 (3.2) |
| Marital status, % married | 55.2 |
| Self-rated health (1–5) | 2.5 (0.9) |
| Activities of daily living (0–6) | 0.3 (0.8) |
| Exercise, % yes | 70.6 |
| Cognitive Assessment Screening Instrument (IRT scoring) | 0.3 (0.7) |
| Body Mass Index | 27.4 (4.9) |
| Framingham Risk Score (0–100) | 8.7 (7.9) |
Table 2.
Means, standard deviations, and inter-correlations (Pearson’s r) of alternative operationalizations of CES-D variables
| Mean | SD | 1 | 2 | 3 | 4 | |
|---|---|---|---|---|---|---|
| 1. Baseline | 3.71 | 4.21 | - | |||
| 2. Proximal | 4.01 | 4.39 | 0.62 | - | ||
| 3. Average | 3.52 | 3.30 | 0.65 | 0.83 | - | |
| 4. Maximum | 5.57 | 4.56 | 0.60 | 0.80 | 0.94 | - |
| 5. Variability | 2.05 | 1.75 | 0.36 | 0.54 | 0.60 | 0.83 |
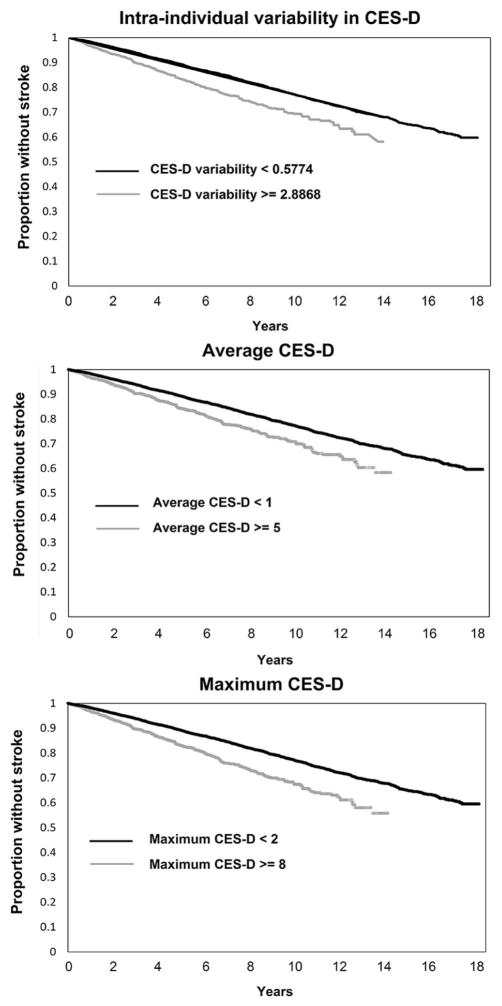
Table 3 displays standardized results from separate models adjusted for age, sex, race, education, marital status, self-rated health, activities of daily living, exercise, cognitive status, body mass index and FRS. Incident stroke risk was predicted by: average CES-D score over the three most recent visits, maximum CES-D score over the three most recent visits, and intra-individual variability in CES-D scores over the three most recent visits. Neither baseline CES-D score nor most recent CES-D score was associated with incident stroke independent of covariates. Figure 2 displays rates of incident stroke as a function of these three variables, broken into highest and lowest quartiles.
Table 3.
Standardized results from separate Cox regression models predicting incident stroke (N=3,736)
| Hazard ratio | 95% CI | p | |
|---|---|---|---|
| Baseline CES-D | 1.00 | 0.97 to 1.03 | 0.956 |
| Proximal CES-D | 1.02 | 0.97 to 1.08 | 0.411 |
| Average CES-Da | 1.20 | 1.05 to 1.36 | 0.006 |
| Maximum CES-Da | 1.18 | 1.07 to 1.30 | 0.002 |
| CES-D variabilitya | 1.15 | 1.06 to 1.24 | <0.001 |
Note. All models controlled for age, sex, race, education, marital status, self-rated health, activities of daily living, exercise, cognitive status, body mass index, and Framingham Risk Score. P-values were from Wald chi-square tests, df = 1.
Computed over the three most recent assessments
Figure 2.
Kaplan-Meier curves from three separate models estimating the association between (A) intra-individual variability in CES-D over three most recent visits, (B) average CES-D over three most recent visits, and (C) maximum CES-D over three most recent visits and incident stroke independent of all covariates. Within each panel, the continuous CES-D variable was split into highest and lowest quartiles for visualization purposes.
Because intra-individual variability in depressive symptoms (i.e., standard deviation of CES-D scores across the three most recent visits) is dependent on absolute level of depressive symptoms (i.e., mean CES-D score across the three most recent visits), an additional model was estimated to determine whether intra-individual variability in depressive symptoms independently predicted incident stroke. This model included both intra-individual variability in CES-D and average CES-D, along with covariates. In this combined model, intra-individual variability in CES-D remained a significant predictor of incident stroke (standardized HR=1.11; 95% CI = 1.00 to 1.22; Wald chi-square (df = 1) = 4.28; p = 0.039), independent of average CES-D (standardized HR=1.11; 95% CI = 0.94 to 1.31; Wald chi-square df = 1) = 1.40; (p = 0.236) and all covariates. No substantive changes were noted in sensitivity analyses involving transformed CES-D variables or conducted within the subset of 2,223 participants were participated in at least three visits.
Conclusions
This study confirmed the association between depressive symptoms and increased risk of stroke, independent of sociodemographic characteristics, cardiovascular risk factors, cognition, and daily functioning. We found an association when we operationalized self-reported depressive symptoms as: average level of symptoms across several visits, maximum level of symptoms across several visits, or intra-individual variability in symptoms across several visits. These findings suggest that approaches to operationalizing depressive symptoms that capture the dynamic nature of depression over time may be most relevant in assessing stroke risk. In contrast, depressive symptoms measured at a single time-point (i.e., at study baseline or at the most recent prior visit) did not predict incident stroke independent of covariates. The results are consistent with recent conceptualizations of depression as neither state-like nor trait-like, but rather as exhibiting both state-like and trait-like components.(27) Indeed, in a previous longitudinal study using the CES-D, the magnitude of state variation was comparable to that of trait variation.(28) Thus, it is important to measure depressive symptoms at more than one time point because symptoms fluctuate over time.(29)
Among the variables capturing depressive symptom severity (i.e., baseline, proximal, average, and maximum CES-D levels), average level across recent study visits was most strongly associated with stroke risk. Compared to baseline, proximal, and maximum CES-D levels, this operationalization of depression better reflects a level of symptoms that is persistent over time, as opposed to transient or episodic. Thus, results from the current study suggest that the chronicity of depressive symptoms may be particularly salient when evaluating stroke risk. For example, clinicians may obtain additional, meaningful information by considering both current and prior scores on depression instruments. These results are consistent with cognitive aging research suggesting that average depressive symptoms over multiple visits may be a stronger predictor of cognitive dysfunction and decline than baseline or concurrent depressive symptoms.(30)
The association between depressive symptom level and incident stroke may be causal. For example, persistently depressed individuals may be more likely to engage in poor health behaviors that increase stroke risk independent of the cardiovascular risk factors included in our models (e.g., exercise, body mass index, Framingham Risk Score).(6–8) An alternative, non-causal and non-mutually exclusive explanation is that higher levels of depressive symptoms reflect subclinical cerebrovascular disease (e.g., reduced cerebral blood flow, disconnection by white matter lesions, disruptions in neurotransmitter pathways due to proinflammatory cytokines).(31) All of these disease processes could increase vulnerability to stroke. It should be noted that the severity of depressive symptoms in the current sample was relatively low, and mild elevations on depressive symptom questionnaires can reflect factors other than clinical depression, such as medical illness and responses to stressors. These factors may increase stroke risk through mechanisms unrelated to clinical depression.
Intra-individual variability in depressive symptoms across recent visits was also significantly associated with stroke risk and was a better predictor than the average level of depressive symptoms over the same visits in a combined model. In the current study, intra-individual variability was calculated across three visits over a five-year period. Therefore, this variable likely captures both short-term (e.g., day-to-day) emotional reactivity as well as longer-term (e.g., year-to-year) changes in mood. Thus, the relatively long inter-visit interval in this study (i.e., 2.5 years) is not ideal for indexing short-term emotional reactivity and limits interpretation of results. Nonetheless, it is relevant to note that increased reactivity to stressors may lead to more frequent engagement of the HPA axis, which is known to influence stroke risk and severity.(16) Indeed, treatment with a glucocorticoid receptor antagonist prevented the negative effects of stress on ischemic disease in mice.(32) Longer-term changes in mood may represent an early prodrome of cerebrovascular abnormalities that eventually culminate in stroke. For example, older adults with new-onset depression have more vascular risk factors and MRI changes suggesting subtle cerebrovascular disease than older adults with early-onset depression.(33–34) Variability in depressive symptoms may also be a marker of fluctuations in blood pressure, which could herald incident stroke.(35)
The observation that increased intra-individual variability in a behavioral marker signifies a neurological prodrome has been extensively studied in the cognitive literature, where greater intra-individual variability in cognitive performance (both within a single test session and over the course of several years) precedes the clinical onset of neurodegenerative and other neurological diseases, particularly those affecting the frontal lobes.(36) Demonstrations of the prognostic significance of intra-individual variability in depressive symptoms are just beginning to emerge in the literature. For example, in a study of 160 older adults with macular degeneration, variability in scores on the self-report Geriatric Depression Scale over a six-month period was associated with informant-reported cognitive decline three years later, independent of age, education, and baseline level of depressive symptoms.(37) Of note, population-based studies with standard measurement intervals may be more likely to detect the clinical significance of intra-individual variability in a disease marker than clinic-based studies in which measurements are time-locked to clinical events. For example, in the population-based ACT study, variability in renal function, but not absolute level of renal function, was found to be a risk factor for dementia.(38) Together, these results support the clinical utility of regular screenings.
A limitation of this study is its generalizability, as the sample was older and predominantly white. Another limitation is that we did not adjust for antidepressant use or compliance, both of which are affected by depressive symptom severity and persistence, and therefore may partially mediate the link between depressive symptoms and stroke. Although antidepressant medication might also be a time-varying confounder, the magnitude of effect of antidepressants on symptomology would have to be very large to plausibly introduce substantial bias in the current study. Nonetheless, exploring the role of antidepressant medication in the association between depression and stroke should be a priority for future research. While analyses did not control for non-antidepressant medications, which could plausibly influence both depressive symptoms and stroke risk, confounding by these medications is an unlikely explanation for the current results. This study included an unusually comprehensive set of covariates that included self-rated health, cognitive and functional abilities, and Framingham Risk Scores. In particular, the Framingham Risk Score is likely to capture much of the variance in cardiovascular health that could be influenced by non-antidepressant medications. It should also be noted that the primary outcome of the current study was first incident stroke of any type, as the ACT cohort is too small to support separate analyses of ischemic and hemorrhagic stroke.
Another limitation of this study is that depression evaluation was limited to the CES-D, and no diagnostic criteria were applied. Therefore, the results of this study are only generalizable to depressive symptoms measured with this commonly-used questionnaire, and not to clinical disorders. However, the finding that higher scores on a depressive symptom questionnaire are independently predictive of future stroke is important even if the underlying mechanism(s) is found to be unrelated to clinical depression. Similarly, this study does not take into account participants’ lifetime history of depressive disorders or records of hospitalizations for depressive disorders, which would have provided important information about lifetime exposure.
Strengths of this study include the large sample size, extensive longitudinal follow-up, and the use of medical records to verify incident stroke and objectively measure several, time-varying confounders. Most importantly, no prior research has examined the link between within-person variability of depressive symptoms and subsequent stroke risk, or systematically compared alternative operationalizations of symptoms. Importantly, by exploring multiple approaches to operationalizing depressive systems, we have identified a novel predictor of stroke.
In conclusion, this study underscores the imperative for characterizing the dynamic nature of depression in the context of stroke risk assessment. Depressive symptoms measured at a single point in time may be less likely to predict incident stroke. Further, intra-individual variability in depressive symptoms across several visits may be a better predictor of stroke risk than level of depressive symptoms. Future studies should explore whether the predictive accuracy of commonly-used stroke risk calculators could be improved with the addition of information on level, chronicity, and/or variability of depressive symptoms. In addition, future studies are needed to explore causal versus non-causal mechanisms linking these variables to stroke risk.
Acknowledgments
This work was supported by the Advanced Psychometrics Methods in Cognitive Aging Research (R13 AG030995), the National Institutes of Health (K99 AG047963, F31 HL112613, T32 MH017119, P50 AG05136, K23 AG046377, T32 NS007153, T32 MH 020004, U01 AG006781), and by the Yerby Postdoctoral Fellowship Program. The authors acknowledge Edward D. Huey, M.D. for his valuable comments on a draft of this manuscript.
Footnotes
Conflicts of Interest: No disclosures to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kessler RC, Berglund P, Demler O, et al. National comorbidity survey replication. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) JAMA. 2003;289:3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- 2.Pan A, Sun Q, Okereke OI, et al. Depression and Risk of Stroke Morbidity and Mortality. JAMA. 2011;306:1241–1249. doi: 10.1001/jama.2011.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dong J-Y, Zhang Y-H, Tong J, et al. Depression and risk of stroke a meta-analysis of prospective studies. Stroke. 2012;43:32–37. doi: 10.1161/STROKEAHA.111.630871. [DOI] [PubMed] [Google Scholar]
- 4.Musselman DL, Evans DL, Nemeroff CB. The relationship of depression to cardiovascular disease: epidemiology, biology, and treatment. Arch Gen Psychiatry. 1998;55:580–592. doi: 10.1001/archpsyc.55.7.580. [DOI] [PubMed] [Google Scholar]
- 5.Shimbo D, Chaplin W, Crossman D, et al. Role of depression and inflammation in incident coronary heart disease events. Am J Cardiol. 2005;96:1016–1021. doi: 10.1016/j.amjcard.2005.05.064. [DOI] [PubMed] [Google Scholar]
- 6.Anda RF, Williamson DF, Escobedo LG, et al. Depression and the dynamics of smoking. A national perspective. JAMA. 1990;264:1541–1545. [PubMed] [Google Scholar]
- 7.Camacho TC, Roberts RE, Lazarus NB, et al. Physical activity and depression: Evidence from the Alameda County Study. Am J Epidemiol. 1991;134:220–231. doi: 10.1093/oxfordjournals.aje.a116074. [DOI] [PubMed] [Google Scholar]
- 8.Strine TW, Mokdad AH, Dube SR, et al. The association of depression and anxiety with obesity and unhealthy behaviors among community-dwelling US adults. Gen Hosp Psychiatry. 2008;30:127–137. doi: 10.1016/j.genhosppsych.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 9.Jonas BS, Franks P, Ingram DD. Are symptoms of anxiety and depression risk factors for hypertension? Longitudinal evidence from the national Health and Nutrition Examination Survey I Epidemiologic Follow-up Study. Arch Fam Med. 1997;6:43–49. doi: 10.1001/archfami.6.1.43. [DOI] [PubMed] [Google Scholar]
- 10.Mezuk B, Eaton WW, Albrecht S, et al. Depression and type 2 diabetes over the lifespan: a meta-analysis. Diabetes Care. 2008;31:2383–2390. doi: 10.2337/dc08-0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Y, Guo JJ, Li H, Wulsin L, et al. Risk of cerebrovascular events associated with antidepressant use in patients with depression: A population-based, nested case-control study. Ann Pharmacother. 2008;42:177–184. doi: 10.1345/aph.1K369. [DOI] [PubMed] [Google Scholar]
- 12.Smoller JW, Allison M, Cochrane BB, et al. Antidepressant use and risk of incident cardiovascular morbidity and mortality among postmenopausal women in the Women’s Health Initiative study. Arch Intern Med. 2009;169:2128–2139. doi: 10.1001/archinternmed.2009.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Glassman AH, Roose SP, Bigger JT., Jr The safety of tricyclic antidepressants in cardiac patients. Risk-benefit reconsidered. JAMA. 1993;269:2673–2675. [PubMed] [Google Scholar]
- 14.Mroczek DK, Stawski RS, Turiano NA, et al. Emotional reactivity and mortality: longitudinal findings from the VA Normative Aging Study. J Gerontol B: Psych Sci Soc Sci. 2013;70:398–406. doi: 10.1093/geronb/gbt107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Piazza JR, Charles ST, Sliwinski MJ, Mogle J, Almeida DM. Affective reactivity to daily stressors and long-term risk of reporting a chronic physical health condition. Ann Behav Med. 2013;45:110–120. doi: 10.1007/s12160-012-9423-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Craft TKS, DeVries AC. Vulnerability to stroke: implications of perinatal programming of the hypothalamic-pituitary-adrenal axis. Front Behav Neurosci. 2009;3:54. doi: 10.3389/neuro.08.054.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bunce DJ, Anstey KJ, Christensen H, et al. White matter hyperintensities and within-person variability in community-dwelling adults aged 60 to 64 years. Neuropsychologia. 2007;45:2009–2015. doi: 10.1016/j.neuropsychologia.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 18.Kukull WA, Higdon R, Bowen JD, et al. Dementia and alzheimer disease incidence: A prospective cohort study. Archives of Neurology. 2002;59:1737–1746. doi: 10.1001/archneur.59.11.1737. [DOI] [PubMed] [Google Scholar]
- 19.Wang L, Van Belle G, Kukull WB, et al. Predictors of Functional Change: A Longitudinal Study of Nondemented People Aged 65 and Older. J Am Geriatr Soc. 2002;50:1525–1534. doi: 10.1046/j.1532-5415.2002.50408.x. [DOI] [PubMed] [Google Scholar]
- 20.Benesch C, Witter DM, Wilder AL, et al. Inaccuracy of the ICD-9 CM in identifying the diagnosis of ischemic cerebrovascular disease. Neurology. 1998;50:306. doi: 10.1212/wnl.49.3.660. [DOI] [PubMed] [Google Scholar]
- 21.Birman Deych E, Waterman AD, Yan Y, et al. Accuracy of ICD-9-CM codes for identifying cardiovascular and stroke risk factors. Medical Care. 2005;43:480–485. doi: 10.1097/01.mlr.0000160417.39497.a9. [DOI] [PubMed] [Google Scholar]
- 22.Goldstein LB. Accuracy of ICD-9-CM coding for the identification of patients with acute ischemic stroke. Effect of modifier codes Stroke. 1998;29:1602–1604. doi: 10.1161/01.str.29.8.1602. [DOI] [PubMed] [Google Scholar]
- 23.Tirschwell DL, Longstreth WT. Validating administrative data in stroke research. Stroke. 2002;33:2465–2470. doi: 10.1161/01.str.0000032240.28636.bd. [DOI] [PubMed] [Google Scholar]
- 24.Andresen EM, Carter WB, Malmgren JA, Patrick DL. Screening for depression in well older adults. Am J Preventive Med. 1994;10:77–84. [PubMed] [Google Scholar]
- 25.Teng EL, Hasegawa K, Homma A, et al. The Cognitive Abilities Screening Instrument (CASI): a practical test for cross-cultural epidemiological studies of dementia. Int Psychogeriatr. 1994;6:45–58. doi: 10.1017/s1041610294001602. [DOI] [PubMed] [Google Scholar]
- 26.D’Agostino RB, Wolf PA, Belanger AJ, et al. Stroke risk profile: adjustment for antihypertensive medication. The Framingham Study Stroke. 1994;25:40–43. doi: 10.1161/01.str.25.1.40. [DOI] [PubMed] [Google Scholar]
- 27.Davey A, Halverson CF, Jr, Zonderman AB, et al. Change in depressive symptoms in the Baltimore Longitudinal Study of Aging. J Gerontol Series B: Psych Sci. 2004;59B:P270–P277. doi: 10.1093/geronb/59.6.p270. [DOI] [PubMed] [Google Scholar]
- 28.Nesselroade JR, Featherman DL. Intraindividual variability in older adults’ depression scores: Some implications for developmental theory and longitudinal research. In: Magnusson D, Berman L, Rudinger G, Torestad B, editors. Stability and change: Methods and models for data treatment. London: Cambridge University Press; 1991. pp. 47–66. [Google Scholar]
- 29.McDougall GJ, Jr, Morgan S, Vaughan PW. Sixteen-month evaluation of depressive symptomatology in older adults. Arch Psychiatr Nursing. 2012;26:e13–e21. doi: 10.1016/j.apnu.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dotson VM, Resnick SM, Zonderman AB. Differential association of concurrent, baseline, and average depressive symptoms with cognitive decline in older adults. Am J Geriatr Psychiatry. 2008;16:318–330. doi: 10.1097/JGP.0b013e3181662a9c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taylor WD, Aizenstein HJ, Alexopoulos GS. The vascular depression hypothesis: mechanisms linking vascular disease with depression. Mol Psychiatry. 2013;18:63–974. doi: 10.1038/mp.2013.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sugo N, Hurn PD, Morahan MB, et al. Social stress exacerbates focal cerebral ischemia in mice. Stroke. 2002;33:1660–1664. doi: 10.1161/01.str.0000016967.76805.bf. [DOI] [PubMed] [Google Scholar]
- 33.Hickie I, Scott E, Naismith S, et al. Late-onset depression: genetic, vascular and clinical contributions. Psychol Med. 2001;31:1403–1412. doi: 10.1017/s0033291701004731. [DOI] [PubMed] [Google Scholar]
- 34.Krishnan KR. Biological risk factors in late life depression. Biol Psychiatry. 2002;52:185–192. doi: 10.1016/s0006-3223(02)01349-5. [DOI] [PubMed] [Google Scholar]
- 35.Muntner P, Whittle J, Lynch AI, et al. Visit-to-visit variability of blood pressure and coronary heart disease, stroke, heart failure, and mortality: A cohort study. Ann Intern Med. 2015;163:329–338. doi: 10.7326/M14-2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.MacDonald SW, Li SC, Backman L. Neural underpinnings of within-person variability in cognitive functioning. Psychol Aging. 2009;24:792–808. doi: 10.1037/a0017798. [DOI] [PubMed] [Google Scholar]
- 37.Rovner BW, Casten RJ, Leiby BE. Variability in depressive symptoms predicts cognitive decline in age-related macular degeneration. Am J Geriatr Psychiatry. 2009;17:574–581. doi: 10.1097/jgp.0b013e31819a7f46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O’Hare AM, Walker R, Haneuse S, et al. Relationship between longitudinal measures of renal function and onset of dementia in a community cohort of older adults. J Am Geriatr Soc. 2012;60:2215–2222. doi: 10.1111/j.1532-5415.2012.04238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]