Abstract
Background
Deep sternal wound infection (DSWI) is a devastating complication that increases morbidity and mortality in cardiac surgery patients. Vancomycin is often administered intravenously for antibiotic prophylaxis in cardiac surgery. Many cardiac surgeons also apply vancomycin paste topically to the sternal edges. We examined the effect of vancomycin paste upon the incidence of DSWI in patients undergoing elective cardiac surgery.
Methods
We performed a single institution, retrospective medical record review of all patients from 2003 to 2015 who underwent CABG, valve or CABG/valve surgery. We derived the Society for Thoracic Surgeons (STS) DSWI risk index for each patient and performed a systematic review of operative, pharmacy, microbiology and discharge records to identify patients that developed DSWI. Multivariate analyses were used to identify predictors of DSWI in this cohort and to quantify the effect of vancomycin paste.
Results
14,492 patients were examined, of whom 136 patients developed DSWI, resulting in an overall incidence of 0.9%. After multivariate analysis, body mass index, NYHA class and STS DSWI risk index remained statistically significant and associated with DSWI. Although the incidence of DSWI decreased over time, the use of vancomycin paste was not associated with a reduced incidence of DSWI.
Conclusions
There was a marked decrease in the incidence of DSWI over the study period, concurrent with institutional implementation of revised STS antibiotic dosing guidelines in 2007 and other strategies. However, the application of vancomycin paste to the sternal edges of patients undergoing cardiac surgery was not associated with a reduced risk of DSWI.
Sternal wound infection is a rare but serious event after cardiac surgery (1, 2). Surgical site infection (SSI) accounts for 14%–16% of all nosocomial infections in hospitalized patients (3). SSIs are associated with increased morbidity and mortality due to prolonged hospitalization, extended antibiotic administration and additional surgical interventions. Furthermore, it has been estimated that patients with sternal wound infections, total perioperative costs are up to 2.8 times greater than patients with an uncomplicated post-operative course (1).
Over the past several decades there has been a substantial focus on infection control with several quality improvement and patient safety protocols being implemented in an attempt to minimize the occurrence of SSI (4). In 2007, the Society of Thoracic Surgeons (STS) recommended the addition of a glycopeptide, vancomycin, to standard beta-lactam coverage in patients with a high risk of staphylococcal infection undergoing cardiac surgery (5). In addition to routinely administered intravenous antibiotics, vancomycin paste applied directly to the sternal edges following sternotomy, has been used as an adjunct to further reduce the incidence of DSWI. There have been a limited number of studies to date that investigate whether the application of vancomycin paste reduces the incidence of sternal wound infection (6–8).
We hypothesized that the use of vancomycin paste is associated with a reduced frequency of DSWI. To explore this hypothesis, we investigated the incidence of DSWI at our institution in patients undergoing cardiac surgery with sternotomy over a twelve-year period, to determine whether the application of vancomycin paste is associated with the prevention of DSWI.
PATIENTS AND METHODS
Patient Populations
From the medical records of an urban Massachusetts Hospital (Brigham and Women's Hospital, Boston, MA) and with Institutional Review Board approval, all adults (≥ 18 years) undergoing primary and reoperation CABG, valve or valve/CABG only surgery between 01/01/2003 – 06/30/2015 were identified. Text searches and individual review of the local STS database, discharge summaries, surgical records, microbiology reports and transthoracic and transesophageal echocardiogram reports were reviewed. Patients with previous history of infective endocarditis, congenital heart disease other than bicuspid aortic valve, ventricular assist devices and cardiac transplant were excluded from further analysis. Long-term mortality data were obtained from routine institutional follow-up protocols, from our internal research data repository, and the Massachusetts Department of Public Health and the U.S. Social Security Death Indices.
We utilized variables from the STS database to determine the STS-derived estimated risk index of DSWI in our patient population (9–12). We also collected demographic, pharmacy, microbiology and operative records to identify which patients received vancomycin paste and to obtain additional covariates. Vancomycin paste was only prepared by mixing 5 or 10 grams of powdered vancomycin with a small amount of sterile water. This paste was applied directly to the sternal edges at the beginning and the end of the case in an effort to reduce bone marrow bleeding. All diagnoses of DSWI were reconfirmed by independent manual chart review by two investigators.
Definitions
We defined DSWI as an infection involving the muscle, bone and/or mediastinum that occurred within 90 days of surgery, required operative intervention (incision and drainage or re-exploration), had positive cultures if obtained and the patient was not previously on antibiotics at the time of sampling, and received antibiotic treatment beyond routine perioperative prophylaxis (13). This definition is consistent with the STS database classification of post-operative deep sternal infection with the caveat that we specified a 90-day time period from initial surgery compared to the STS definition that utilizes a 30-day time period (13, 14). Bedside procedures were not included as part of the definition of DSWIs as they usually reflected only superficial infection.
Patient variables analyzed included age, gender, race, weight, body mass index (BMI), NYHA Class, presence of diabetes, baseline creatinine, dialysis, peripheral artery disease, previous myocardial infarction, operation type, intra-aortic balloon pump (IABP) and bypass time. These variables were defined in strict accordance to the STS Adult Cardiac Database Data Specifications (14). We also examined the effect of the attending Surgeon and the year of operation to identify institutional factors that increased the risk of DSWI.
Statistical Methods
Categorical variables were summarized by frequency and analyzed by χ2 and Fisher Exact tests. Comparisons of potential clinical predictors of DSWI, including age, estimated STS DSWI risk, vancomycin paste use and surgeon, amongst others against the occurrence of DSWI were performed. Variables associated with DSWI or the use of vancomycin paste (p value < 0.1), were entered into a backward stepwise logistic regression model. Variables with p < 0.05 were retained while forcing age, STS DSWI risk and the use of vancomycin paste into the model. Model fit was assessed using the log-likelihood goodness of fit test. Statistical significance was assigned for two-tailed p values less than 0.05. Statistical analysis was performed with JMP version 12.0 (SAS Institute, Cary, NC).
RESULTS
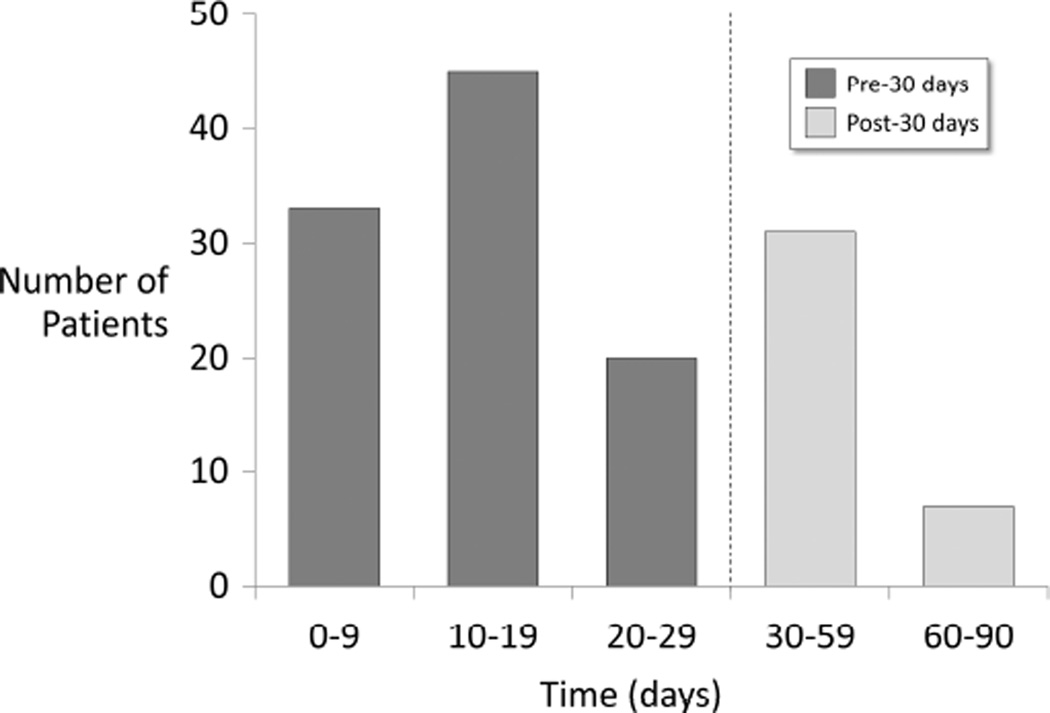
Of the 14,492 patients identified, DSWI occurred in 136 patients (0.9%). Demographic and patient characteristics of the study population are summarized in Table 1. The median calculated STS DSWI risk for the study population was 0.34% (95% CI 0.12 – 0.92%). Vancomycin paste use generally increased over the study period (Table 1). In addition, there were statistically significant differences in use of vancomycin paste by age, gender, race, BMI and other patient and operative characteristics. Without accounting for confounding factors, patients receiving vancomycin paste were not less likely to suffer from DSWI than those that did not receive vancomycin paste (0.8% vs. 1.0%, P=0.10). DSWI occurred at a median of 17 days (10–90% range 6 – 68 days) with 32% of DSWIs occurring more than 30 days after surgery (Figure 1).
Table 1.
Demographics of study population categorized by use of vancomycin paste.
| Study population (N=14,492) |
Vancomycin Paste Used (N=4,997) |
Vancomycin Paste Not Used (N=9,495) |
P Value * | ||
|---|---|---|---|---|---|
| Demographics | |||||
| Age | |||||
| < 40 | 409 (2.8%) | 130 (2.6%) | 279 (2.9%) | ||
| 40–49 | 1024 (7.1%) | 318 (6.4%) | 706 (7.4%) | ||
| 50–59 | 2573 (17.8%) | 852 (17.0%) | 1721 (18.1%) | 0.028 | |
| 60–69 | 3854 (26.6%) | 1354 (27.1%) | 2500 (26.3%) | ||
| 70–79 | 4261 (29.4%) | 1522 (30.5%) | 2739 (28.9%) | ||
| >=80 | 2371 (16.4%) | 821 (16.4%) | 1550 (16.3%) | ||
| Year | |||||
| 2003–2004 | 2323 (16.0%) | 46 (0.9%) | 2277 (24.0%) | ||
| 2005–2006 | 2403 (16.6%) | 689 (13.8%) | 1714 (18.1%) | ||
| 2007–2008 | 2307 (15.9%) | 849 (17.0%) | 1458 (15.4%) | <0.0001 | |
| 2009–2010 | 2380 (16.4%) | 737 (14.7%) | 1643 (17.3%) | ||
| 2011–2012 | 2224 (15.3%) | 1184 (23.7%) | 1040 (10.9%) | ||
| 2013–2015 | 2855 (19.7%) | 1492 (29.9%) | 1363 (14.3%) | ||
| Gender (Female) | 4981 (34.4%) | 1800 (36.0%) | 3181 (33.5%) | 0.002 | |
| Race (Caucasian) | 13689 (94.4%) | 4757 (95.2%) | 8932 (94.1%) | 0.011 | |
| Height (cm) | 170 (155–183) | 173 (157–183) | 0.0001 | ||
| Weight (kg) | 82 (60–107) | 82 (60–107) | 81 (60–107) | 0.043 | |
| BMI (kg/m2) | 27 (22–36) | 28 (22–36) | 27 (22–36) | <0.001 | |
| BMI >30 kg/m2 | 4542 (31%) | 1666 (33.3%) | 2876 (30.3%) | 0.0002 | |
| Medical History | |||||
| Diabetes | 3849 (26.6%) | 1356 (27.1%) | 2493 (26.3%) | 0.25 | |
| Hypertension | 10336 (71.3%) | 3680 (73.6%) | 6656 (70.1%) | <0.0001 | |
| Chronic lung disease | 2068 (14.3%) | 756 (15.1%) | 1312 (13.2%) | 0.033 | |
| Creatinine (mg/dL) | 1.0 (0.7 – 1.5) | 1.0 (0.7 – 1.5) | 1.0 (0.7 – 1.5) | 0.13 | |
| Creatinine > 1.5mg/dL | 1328 (9.2%) | 428 (8.6%) | 900 (9.5%) | 0.07 | |
| Preoperative dialysis | 199 (1.4%) | 77 (1.5%) | 122 (1.3%) | 0.21 | |
| Postoperative renal failure | 877 (6.1%) | 272 (5.4%) | 605 (6.4%) | 0.025 | |
| Peripheral Artery Disease | 1898 (13.1%) | 615 (12.3%) | 1283 (13.5%) | 0.040 | |
| Previous MI | 3537 (24.4%) | 1229 (24.6%) | 2308 (24.3%) | 0.70 | |
| Prior cardiac surgery | 1296 (8.9%) | 457 (9.1%) | 839 (8.8%) | 0.54 | |
| NYHA Class | |||||
| I | 2374 (16.4%) | 708 (14.6%) | 1666 (17.5%) | ||
| II | 6868 (47.4%) | 2487 (49.8%) | 4381 (46.1%) | <0.0001 | |
| III | 4634 (32.0% | 1640 (32.8%) | 2994 (31.6%) | ||
| IV | 616 (4.3%) | 162 (3.2%) | 454 (4.8%) | ||
| STS DSWI Risk Index (%) | 0.34% (0.12% – 0.92%) |
0.33% (0.11% – 0.92%) | 0.34% (0.11% – 0.90%) | 0.020 | |
| STS DSWI Risk Index (%) | |||||
| <0.5% | 9983 (68.9%) | 3394 (67.9%) | 6585 (69.3%) | 0.16 | |
| 0.5 – 1.0% | 3383 (23%) | 1189 (23.8%) | 2189 (23.1%) | ||
| >1.0% | 1136 (8%) | 414 (8.3%) | 721 (7.6%) | ||
| Operation | |||||
| Operative status | |||||
| Elective | 9601 (66.3%) | 3435 (68.8%) | 6166 (64.9%) | ||
| Urgent | 4480 (30.9%) | 1395 (27.9%) | 3085 (32.5%) | <0.0001 | |
| Emergent or salvage | 411 (2.8%) | 167 (3.3%) | 244 (2.6%) | ||
| Operation type | 879 (6.1%) | ||||
| CABG | 5304 (36.6%) | 1809 (36.2%) | 3495 (36.8%) | ||
| Valve | 6404 (44.2%) | 2178 (43.6%) | 4226 (44.5%) | 0.085 | |
| CABG/Valve | 2784 (19.2%) | 1010 (20.2%) | 1774 (18.7%) | ||
| IABP | 783 (5.4%) | 251 (5.0%) | 534 (5.6%) | 0.13 | |
| Bypass Time (min.) | 119 (71–221) | 126 (72–234) | 116 (71–214) | <0.0001 | |
| Bypass time | |||||
| <120 min | 6808 (50.0%) | 2129 (45.1%) | 4682 (53%) | <0.0001 | |
| 120 – 180 min | 4247 (31.3%) | 1531 (32.4%) | 2719 (31%) | ||
| >180 min | 2556 (18.8%) | 1060 (22.5%) | 1498 (17%) | ||
| Open chest | 147 (1.0%) | 58 (1.2%) | 89 (0.9%) | 0.21 | |
| Outcomes | |||||
| DSWI (outcome) | 136 (0.9%) | 38 (0.8%) | 98 (1.0%) | 0.10 | |
| Mortality | |||||
| 0–5 days | 166 (1.1%) | 57 (1.1%) | 109 (1.1%) | 0.97 | |
| 0–1 year | 897 (6.2%) | 314 (6.3%) | 584 (6.2%) | 0.75 | |
| 0–5 years | 2197 (15.2%) | 707 (14.2%) | 1490 (15.7%) | 0.013 | |
Confidence intervals are 10th and 90th percentiles.
Comparison of patients who received vancomycin paste and those who did not.
Figure 1.
Time of onset of deep sternal would infection over the 90-day postoperative period.
Patients who suffered a DSWI had higher BMI, worse NYHA class, and were more likely to have diabetes, chronic lung, renal and peripheral artery disease with a higher median calculated STS DSWI risk (0.54%; 95% CI 0.16 – 1.5%) than patients who did not suffer a DSWI (0.33%; 95% CI 0.11 – 0.90%; P<0.0001, Table 2). They were more likely to undergo urgent and CABG surgery with longer operative times. Patients with a DSWI had increased mortality over the following one (16.9% vs. 6.1%; P<0.0001) and five years (30.2% vs. 15%; P<0.0001). DSWI incidence was not associated with attending surgeon.
Table 2.
Demographics of study population categorized by occurrence of DSWI.
| DSWI (N=136) |
No DSWI (N=14,356) |
P Value | ||
|---|---|---|---|---|
| Demographics | ||||
| Age | ||||
| < 40 | 1 (0.7%) | 408 (2.8%) | 0.50 | |
| 40–49 | 10 (7.4%) | 1014 (7.1%) | ||
| 50–59 | 28 (20.6%) | 2545 (17.7%) | ||
| 60–69 | 35 (25.7%) | 3819 (26.6%) | ||
| 70–79 | 43 (31.6%) | 4218 (29.4%) | ||
| >=80 | 19 (14.0%) | 2352 (16.4%) | ||
| Year | ||||
| 2003–2004 | 45 (33.1%) | 2278 (15.9%) | ||
| 2005–2006 | 27 (19.9%) | 2376 (16.5%) | ||
| 2007–2008 | 23 (16.9%) | 2284 (15.9%) | <0.0001 | |
| 2009–2010 | 26 (19.1%) | 2354 (16.4%) | ||
| 2011–2012 | 8 (5.9%) | 2216 (15.4%) | ||
| 2013–2015 | 7 (5.2%) | 2848 (19.8%) | ||
| Gender (Female) | 45 (33.0%) | 4936 (34.4%) | 0.75 | |
| Race (Caucasian) | 129 (94.9%) | 13560 (94.5%) | 0.63 | |
| Height (cm) | 173 (155–183) | 173 (157–183) | 0.92 | |
| Weight (kg) | 88 (63–110) | 81 (60–107) | 0.0008 | |
| BMI (kg/m2) | 30 (22–39) | 27 (22–36) | 0.0034 | |
| BMI >30 kg/m2 | 65 (47.8%) | 4477 (31.2%) | <0.0001 | |
| Medical History | ||||
| Diabetes | 57 (40.4%) | 3792 (26.4%) | 0.0001 | |
| Hypertension | 103 (75.7%) | 10233 (71.2%) | 0.25 | |
| Chronic lung disease | 28 (20.6%) | 2040 (14.2%) | 0.045 | |
| Creatinine (mg/dL) | 1.1 (0.8 – 1.8) | 1.0 (0.7 – 1.5) | 0.050 | |
| Creatinine > 1.5mg/dL | 21 (15.4%) | 1307 (9.1%) | 0.019 | |
| Preoperative dialysis | 6 (4.4%) | 193 (1.3%) | 0.015 | |
| Postoperative renal failure | 10 (7.4%) | 867 (6.0%) | 0.53 | |
| Peripheral artery disease | 26 (19.1%) | 1872 (13.0%) | 0.048 | |
| Previous MI | 47 (34.6%) | 3490 (24.3%) | 0.008 | |
| Prior cardiac surgery | 13 (9.6%) | 1283 (8.9%) | 0.80 | |
| NYHA Class | ||||
| I | 25 (18.4%) | 2349 (16.3%) | ||
| II | 54 (39.7%) | 6814 (47.6%) | 0.002 | |
| III | 41 (30.2%) | 4593 (32.0%) | ||
| IV | 16 (11.8%) | 600 (4.2%) | ||
| STS DSWI Risk Index (%) | 0.54% (0.16% – 1.5%) | 0.33% (0.11% – 0.90%) | <0.0001 | |
| STS DSWI Risk Index (%) | ||||
| <0.5% | 62 (45.6%) | 9917 (69.1%) | <0.0001 | |
| 0.5 – 1.0% | 50 (36.8%) | 3328 (23.2%) | ||
| >1.0% | 24 (17.7%) | 1111 (7.7%) | ||
| Operation | ||||
| Operative status | ||||
| Elective | 70 (51.5%) | 9531 (66.4%) | ||
| Urgent | 56 (41.2%) | 4424 (30.8%) | 0.0008 | |
| Emergent or salvage | 10 (6.9%) | 401 (2.8%) | ||
| Operation type: | ||||
| CABG | 65 (47.8%) | 5239 (36.4%) | ||
| Valve | 35 (25.7%) | 6369 (19.4%) | <0.0001 | |
| CABG/Valve | 36 (26.5%) | 2748 (44.3%) | ||
| IABP | 17 (2.2%) | 768 (5.3%) | 0.0008 | |
| Bypass Time (min.) | 130 (81–255) | 119 (71–220) | 0.016 | |
| Bypass time | ||||
| <120 min | 49 (39.5%) | 6759 (50.1%) | 0.056 | |
| 120 – 240 min | 45 (36.3%) | 4202 (31.2%) | ||
| >240 min | 30 (24.2%) | 2526 (18.7%) | ||
| Open chest after surgery | 2 (1.5%) | 145 (1.0%) | 0.62 | |
| Vancomycin Paste Use | 38 (27.9%) | 4959 (34.5%) | 0.10 | |
| Outcomes | ||||
| Mortality | ||||
| 0–5 days | 0 (0%) | 166 (1.2%) | 0.07 | |
| 0–1 year | 23 (16.9%) | 874 (6.1%) | <0.0001 | |
| 0–5 years | 41 (30.2%) | 2156 (15.0%) | <0.0001 | |
As both vancomycin use and DSWI varied by year and several other variables, we constructed a multivariable logistic regression model of DSWI. We forced the use of vancomycin paste into a multivariable model (Table 3) and after stringent multivariate analysis, year of operation, BMI >30 kg/m2, NYHA class IV and STS DSWI risk index remained associated with the development of DSWI, while vancomycin paste application was not (OR = 1.2; 95% CI 0.79 – 1.82; P=0.40). Model fit assessed using the likelihood ratio goodness of fit test indicated good model fit (χ2 =111.6 P=0.76, Table 3).
Table 3.
Multivariate model of deep sternal wound infection (N=14,492; AUC=0.731). Log likelihood goodness of fit testing yielded P=0.76 indicating good model fit.
| OR | 95% CI | Multivariate model P value |
||
|---|---|---|---|---|
| Year | ||||
| 2003–2004 | 1 | |||
| 2005–2006 | 0.56 | (0.34 – 0.93) | ||
| 2007–2008 | 0.49 | (0.29 – 0.84) | <0.0001 | |
| 2009–2010 | 0.56 | (0.34 – 0.94) | ||
| 2011–2012 | 0.18 | (0.08 – 0.41) | ||
| 2013–2015 | 0.12 | (0.05 – 0.28) | ||
| BMI > 30 kg/m2 | 1.72 | (1.21 – 2.46) | 0.003 | |
| NYHA Class IV | 1.85 | (1.08 – 3.18) | 0.026 | |
| STS DSWI Risk Index (%) | ||||
| < 0.5% | 1 | |||
| 0.5–1.0% | 1.96 | (1.33 – 2.88) | 0.0002 | |
| > 1.0% | 2.48 | (1.51 – 4.10) | ||
| Vancomycin Paste Use | 1.20 | (0.79 – 1.82) | 0.40 |
COMMENT
We tested the hypothesis that the application of vancomycin paste to sternal edges after sternotomy reduced the incidence of DSWI in cardiac surgery patients. The main finding of this study is that vancomycin paste did not reduce the incidence of DSWI after accounting for STS DSWI risk index and other covariates. These findings are in contradistinction to prior literature, including a randomized, but unblinded trial of vancomycin paste in 416 patients undergoing cardiac surgery where a reduction in DSWI was reported (8). Further, a single surgeon case series using historical controls, observed a lower incidence of DSWI after the use of vancomycin in a calcium-thrombin, and platelet-rich plasma vehicle (6). In a case series of 532 low DSWI-risk patients undergoing coronary artery bypass grafting were subjected to several changes in clinical practice thought to reduce DSWI risk, along with use of vancomycin paste, and did not observe any DSWI (7). An additional case series of 1,020 patients, all receiving vancomycin paste, observed an incidence of DSWI of 0.45%, but failed to reference any control group (15). Comparison of our study with this prior literature reveals several factors that may cause these differences. These include smaller comparison groups, failure to adjust for important covariates, different follow-up periods and the use of different substrate preparations of vancomycin paste.
Our study also has limitations that may importantly impact its findings. We performed a single center, retrospective review and had limited data on specifics about administration of systemic perioperative antibiotics. The frequency of DSWI declined over the study period with a notable decrease in the incidence between 2009 and 2010 (1.5% to 0.7%), probably due to implementation of updated STS antibiotic prophylaxis guidelines for cardiac surgery (5) and quality improvement campaigns for appropriate and timely administration of perioperative antibiotics, adoption of perioperative glucose management protocols (16), and patient-specific sternal closure techniques including double wires and sternal bands (17, 18). In addition, not all surgeons used vancomycin paste and there were changes in practice of the study period; however, we did not observe a relationship between individual surgeon and incidence of DSWI.
There are known risk factors for DSWI including diabetes, renal failure and other measures of clinical state. To account for these important covariates, we used the local STS database to determine the calculated STS DSWI risk for each patient (10–12), observing the STS DSWI risk to be significantly higher in patients that developed DSWI. As the DSWI risk index was developed from data prior to the 2007 STS antibiotic prophylaxis guidelines (5), we examined the value of including other potential covariates upon improvement in multivariate model performance for DSWI. After multivariate analysis including age and STS DSWI risk index, two risk factors, BMI and NYHA class, retained significance despite being accounted for in the STS DSWI risk index. This observation suggests that development of a new STS DSWI risk index utilizing data from after the publication of the STS DSWI risk index is warranted.
The STS defines DSWI by the occurrence within a 30-day post-operative time period (13). In the absence of implanted material the CDC also defines DSWI as infection occurring within 30 days after the operation; however, if an implant, including sternal wires, is in place then CDC definition extends this period to one year (4). The STS does not make a distinction regarding placement of an implant or not. Because we observed 32% of DSWI occurring more than 30 days after surgery, we extended our definition to 90 days post-operatively. Nine additional patients suffered sternal wound infection between 90–365 days postoperatively, but were not classified as DSWI. We believe that consideration should be made to extend the definition of DSWI to 90 days post-operatively to better capture the true incidence of DSWI and that concordance between the STS and CDC definitions is desirable.
In summary, our results demonstrate that the application of vancomycin paste does not reduce the frequency of DSWI. It is possible that vancomycin in a biologically degradable substrate may provide benefit for prevention of DSWI by allowing more effective delivery of vancomycin. Further, although the use of vancomycin paste is not supported for the prevention of DSWI, it may still be a useful adjunct to reduce bleeding. Although DSWI is a rare event, it has a profound impact on morbidity, mortality and overall cost. Further prospective, randomized and blinded trials will be necessary to identify whether certain high-risk populations, such as those with obesity, diabetes, renal insufficiency or advanced heart failure would benefit from vancomycin paste application or adjusted antibiotic dosing regimens during CPB. These trials should consider the substrate used for delivery of vancomycin.
ABBREVIATIONS
- BMI
Body Mass Index
- CDC
Center for Disease Control and Prevention
- CPB
Cardiopulmonary bypass
- DSWI
Deep Sternal Wound Infection
- IABP
Intra-aortic balloon pump
- MIC
Minimum Inhibitory Concentration
- NYHA
New York Heart Association
- SSI
Surgical Site Infection
- STS
Society for Thoracic Surgeons
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Loop FD, Lytle BW, Cosgrove DM, Mahfood S, McHenry MC, Goormastic M, et al. J. Maxwell Chamberlain Memorial Paper. Sternal Wound Complications after Isolated Coronary Artery Bypass Grafting: Early and Late Mortality, Morbidity, and Cost of Care. Ann Thorac Surg. 1990 Feb;49(2):179–186. doi: 10.1016/0003-4975(90)90136-t. [DOI] [PubMed] [Google Scholar]
- 2.Mu Y, Edwards JR, Horan TC, Berrios-Torres SI, Fridkin SK. Improving Risk-Adjusted Measures of Surgical Site Infection for the National Healthcare Safety Network. Infect Control Hosp Epidemiol. 2011 Oct;32(10):970–986. doi: 10.1086/662016. [DOI] [PubMed] [Google Scholar]
- 3.Emori TG, Gaynes RP. An Overview of Nosocomial Infections, Including the Role of the Microbiology Laboratory. Clin Microbiol Rev. 1993 Oct;6(4):428–442. doi: 10.1128/cmr.6.4.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR. Guideline for Prevention of Surgical Site Infection, 1999. Centers for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee. Am J Infect Control. 1999 Apr;27(2):97–132. [PubMed] [Google Scholar]
- 5.Engelman R, Shahian D, Shemin R, Guy TS, Bratzler D, Edwards F, et al. The Society of Thoracic Surgeons Practice Guideline Series: Antibiotic Prophylaxis in Cardiac Surgery, Part II: Antibiotic Choice. Ann Thorac Surg. 2007 Apr;83(4):1569–1576. doi: 10.1016/j.athoracsur.2006.09.046. [DOI] [PubMed] [Google Scholar]
- 6.Hamman BL, Stout LY, Theologes TT, Sass DM, da Graca B, Filardo G. Relation between Topical Application of Platelet-Rich Plasma and Vancomycin and Severe Deep Sternal Wound Infections after a First Median Sternotomy. Am J Cardiol. 2014 Apr 15;113(8):1415–1419. doi: 10.1016/j.amjcard.2013.12.046. [DOI] [PubMed] [Google Scholar]
- 7.Kieser TM, Rose MS, Aluthman U, Montgomery M, Louie T, Belenkie I. Toward Zero: Deep Sternal Wound Infection after 1001 Consecutive Coronary Artery Bypass Procedures Using Arterial Grafts: Implications for Diabetic Patients. J Thorac Cardiovasc Surg. 2014 Nov;148(5):1887–1895. doi: 10.1016/j.jtcvs.2014.02.022. [DOI] [PubMed] [Google Scholar]
- 8.Vander Salm TJ, Okike ON, Pasque MK, Pezzella AT, Lew R, Traina V, et al. Reduction of Sternal Infection by Application of Topical Vancomycin. J Thorac Cardiovasc Surg. 1989 Oct;98(4):618–622. [PubMed] [Google Scholar]
- 9.Shahian DM, Edwards FH. The Society of Thoracic Surgeons 2008 Cardiac Surgery Risk Models: Introduction. Ann Thorac Surg. 2009 Jul;88(1 Suppl):S1. doi: 10.1016/j.athoracsur.2009.05.054. [DOI] [PubMed] [Google Scholar]
- 10.Shahian DM, O'Brien SM, Filardo G, Ferraris VA, Haan CK, Rich JB, et al. The Society of Thoracic Surgeons 2008 Cardiac Surgery Risk Models: Part 1--Coronary Artery Bypass Grafting Surgery. Ann Thorac Surg. 2009 Jul;88(1 Suppl):S2–S22. doi: 10.1016/j.athoracsur.2009.05.053. [DOI] [PubMed] [Google Scholar]
- 11.O'Brien SM, Shahian DM, Filardo G, Ferraris VA, Haan CK, Rich JB, et al. The Society of Thoracic Surgeons 2008 Cardiac Surgery Risk Models: Part 2--Isolated Valve Surgery. Ann Thorac Surg. 2009 Jul;88(1 Suppl):S23–S42. doi: 10.1016/j.athoracsur.2009.05.056. [DOI] [PubMed] [Google Scholar]
- 12.Shahian DM, O'Brien SM, Filardo G, Ferraris VA, Haan CK, Rich JB, et al. The Society of Thoracic Surgeons 2008 Cardiac Surgery Risk Models: Part 3--Valve Plus Coronary Artery Bypass Grafting Surgery. Ann Thorac Surg. 2009 Jul;88(1 Suppl):S43–S62. doi: 10.1016/j.athoracsur.2009.05.055. [DOI] [PubMed] [Google Scholar]
- 13.Shahian DM, Jacobs JP, Edwards FH, Brennan JM, Dokholyan RS, Prager RL, et al. The Society of Thoracic Surgeons National Database. Heart. 2013 Oct;99(20):1494–1501. doi: 10.1136/heartjnl-2012-303456. [DOI] [PubMed] [Google Scholar]
- 14.STS Adult Cardiac Surgery Database Data Collection Forms and Specifications. Version 2.73 [Google Scholar]
- 15.Arruda MV, Braile DM, Joaquim MR, Suzuki FA, Alves RH. The Use of the Vancomycin Paste for Sternal Hemostasis and Mediastinitis Prophylaxis. Rev Bras Cir Cardiovasc. 2008 Jan-Mar;23(1):35–39. doi: 10.1590/s0102-76382008000100007. [DOI] [PubMed] [Google Scholar]
- 16.Furnary AP, Cheek DB, Holmes SC, Howell WL, Kelly SP. Achieving Tight Glycemic Control in the Operating Room: Lessons Learned from 12 Years in the Trenches of a Paradigm Shift in Anesthetic Care. Semin Thorac Cardiovasc Surg. 2006 Winter;18(4):339–345. doi: 10.1053/j.semtcvs.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 17.Franco S, Herrera AM, Atehortua M, Velez L, Botero J, Jaramillo JS, et al. Use of Steel Bands in Sternotomy Closure: Implications in High-Risk Cardiac Surgical Population. Interact Cardiovasc Thorac Surg. 2009 Feb;8(2):200–205. doi: 10.1510/icvts.2008.188136. [DOI] [PubMed] [Google Scholar]
- 18.Shaikhrezai K, Robertson FL, Anderson SE, Slight RD, Brackenbury ET. Does the Number of Wires Used to Close a Sternotomy Have an Impact on Deep Sternal Wound Infection? Interact Cardiovasc Thorac Surg. 2012 Aug;15(2):219–222. doi: 10.1093/icvts/ivs200. [DOI] [PMC free article] [PubMed] [Google Scholar]