Abstract
Childhood poverty is a risk factor for poorer cognitive performance during childhood and adulthood. While evidence linking childhood poverty and memory deficits in adulthood has been accumulating, underlying neural mechanisms are unknown. To investigate neurobiological links between childhood poverty and adult memory performance, we used functional magnetic resonance imaging (fMRI) during a visuospatial memory task in healthy young adults with varying income levels during childhood. Participants were assessed at age 9 and followed through young adulthood to assess income and related factors. During adulthood, participants completed a visuospatial memory task while undergoing MRI scanning. Patterns of neural activation, as well as memory recognition for items, were assessed to examine links between brain function and memory performance as it relates to childhood income. Our findings revealed associations between item recognition, childhood income level, and hippocampal activation. Specifically, the association between hippocampal activation and recognition accuracy varied as a function of childhood poverty, with positive associations at higher income levels, and negative associations at lower income levels. These prospective findings confirm previous retrospective results detailing deleterious effects of childhood poverty on adult memory performance. In addition, for the first time, we identify novel neurophysiological correlates of these deficits localized to hippocampus activation.
Keywords: Visuospatial memory, Hippocampus, fMRI, Childhood poverty
1. Introduction
Childhood poverty is a risk factor for problems in cognition (Adler et al., 2012, Bradley and Corwyn, 2002, Hackman et al., 2010, Hackman and Farah, 2009), which likely contribute to robust income achievement gaps, deficits in math and reading, increased school drop-out, and decreased graduation rates among the poor (Brooks-Gunn and Duncan, 1997, Duncan, 2012). The impact of poverty is complex and linked to various environmental risk factors, including parenting, school, and neighborhood quality (Brooks-Gunn and Duncan, 1997), that often co-occur. Living in poverty is more stressful, which can have detrimental effects on cognitive development (Evans, 2003) and working memory (Evans and Schamberg, 2009). Thus, poverty-related factors may impact brain function and cognitive development. Since these factors both co-occur and interact, it is very difficult to isolate single factors that lead to developmental differences from the overall effects of the complex construct of poverty as a whole, both experimentally and conceptually.
Deficits in memory encoding and recognition are hallmarks of poverty effects in children (Farah et al., 2006, Noble et al., 2007) and adults (Herrmann and Guadagno, 1997). The association between family income and memory performance is mediated, in part, by chronic stress exposure (Evans and Schamberg, 2009, Evans, 2003). Findings linking poverty and its associated risk factors to cognitive impairment, including poorer memory (Lipina and Posner, 2012, Raizada and Kishiyama, 2010), highlight the potentially key role of the hippocampus. The hippocampus plays both a central role in memory (Scoviille and Milner, 1957, Jarrard, 1993, Vann and Albasser, 2011, Lavenex and Lavenex, 2009) and is sensitive to chronic stress during early development (McEwen and Magarinos, 2001, Meaney et al., 1991, Lupien et al., 2009). Previous studies demonstrate that hippocampus might be especially vulnerable to early poverty, as adverse life events occurring in childhood (∼age 8–9) are associated with altered hippocampal development, potentially influencing mental health symptoms in adulthood (Schalinski et al., 2016). Some Hanson et al., 2011, Hanson et al., 2014, Luby et al., 2012, Luby et al., 2013), but not all studies (Hanson et al., 2013), link specific poverty-associated factors (e.g. maternal support, environmental stress) to altered hippocampus, amygdala, and cortical brain volume. Much remains to be learned about complex interactions between poverty, hippocampal function and memory performance.
There is strong evidence linking stronger recruitment of hippocampus with better memory recall (Wong et al., 2013, Bergmann et al., 2016), and visuospatial memory tasks are most commonly used in this context (De Rover et al., 2011, Wong et al., 2013, Longoni et al., 2013). Thus, we hypothesized that childhood income might impact both hippocampal recruitment and visuospatial memory performance in young adults that experienced low income as children. One possibility is that the relationship between childhood income and memory recognition is directly mediated by hippocampal function, but it is also possible that childhood income moderates the relationship between hippocampal function and memory performance, altering the “normal” relationship between the two. We therefore set out to further assess how childhood income-related variability impacts hippocampal function during visuospatial memory performance in adults. This is one of the first prospective longitudinal studies examining links between brain function and cognitive abilities associated with income. We used an existing, prospective longitudinal cohort to test the following hypotheses: (1) Childhood income will positively relate to visuospatial memory performance; (2) Visuospatial memory performance will be reflected in hippocampal activation; and (3) The association between memory recognition and hippocampal activation will differ across income levels.
2. Materials and methods
2.1. Subjects
Fifty-four adults from age 20 to 27 (M = 23.72, SD = 1.31) participated in this study within the context of an ongoing, larger longitudinal study examining associations between income and child development (Kim et al., 2015, Kim et al., 2013, Javanbakht et al., 2015, Evans, 2003, Sripada et al., 2014, Evans et al., 2016, Liberzon et al., 2015). Eleven of the 54 participants did not have data on at least one of our measurements of interest (e.g. income to need ratio in adulthood), and thus were not included in the analyses. This resulted in a total of 43 participants with data on all measures. For the sample reported here, 54% were male and 91% were Caucasian. Poverty was defined according to US census calculations using income to need ratios at age 9. An income to need ratio below 1.0 is typically considered below the poverty line, with the average American household reporting an income to need ratio around 2.0. In our sample, income to need ratios at age 9 ranged from 0.16 to 4.30 (M = 1.75, SD = 1.11), with an equal number of subjects raised below (N = 26) and above (N = 28) the poverty line. Income to needs ratios in adulthood were reported from 0.29 to 9.11 (M = 2.82, SD = 2.21). For the analyses reported here, we used the earliest available data point in this cohort (income to needs at age 9), to examine links between childhood income specifically and adult cognitive abilities. All participants were right handed, had no prior or current treatment for psychiatric disorders, neurological conditions, or MRI contraindications.
2.2. Procedures
All of the procedures were approved by the Institutional Review Boards of Cornell University, the University of Michigan, and the Veterans Affairs Ann Arbor Healthcare System. Written consent was obtained from all subjects. MRI scanning was performed with a Philips 3-T Achieva X-series MRI (Philips Medical Systems). T1-weighted anatomical images (FOV = 256 × 256 mm, slice thickness = 1 mm, 0 mm gap) were completed for slice localization, Talairach transformation, and coregistration. Gradient echo blood oxygen level dependent (BOLD) scans (contiguous axial slices; TR/TE = 2000/30 ms, flip angle = 90°, FOV = 220 × 220 mm, slice thickness = 3 mm3, 0 mm gap, 42 slices) were completed to assess brain function during tasks. E-prime v2.0 was used to present stimuli and record responses (Psychology Software Tools, Pittsburgh, PA). Participants viewed stimuli through MRI-compatible liquid crystal display goggles (NordicNeuroLabs http://www.nordicneurolab.com) and responded to stimuli using an MRI-compatible button box.
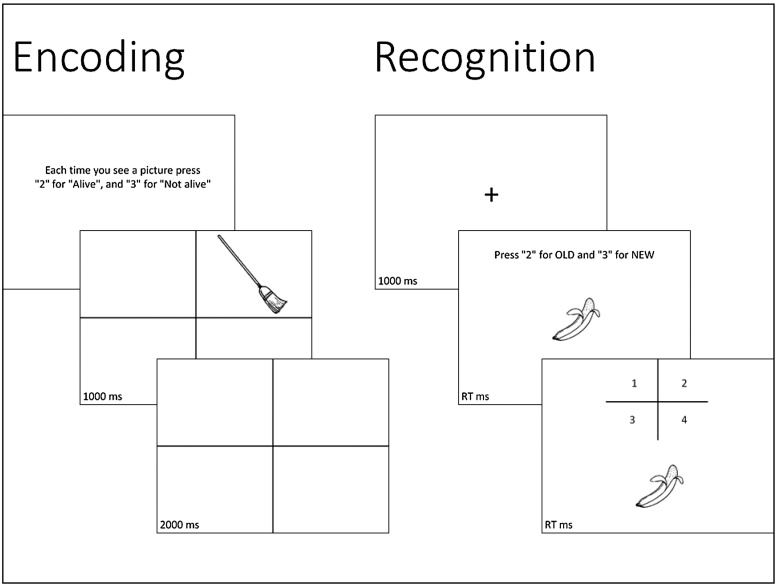
During BOLD scanning, participants completed a two-part visuospatial memory task involving an encoding phase and a recognition phase. During encoding, participants viewed 92 line drawings in one of four quadrants of the screen depicting common objects, animals, and plants. They had to indicate whether each image was “alive” or “not alive”. Each image was on the screen for 1000 ms plus the response time of the participant (not to exceed an additional 2000 ms). Inter-stimulus intervals (ISIs) were jittered 3000–7000 ms. During recognition testing (starting 10–15 min following the end of encoding), participants were presented with 59 images previously viewed and 45 new images in random order. They had to indicate whether the image was “old” or “new”. For “old” images, participants would also indicate where it was initially presented (top left, top right, bottom left, bottom right). Images were presented until the participant made their response(s). ISIs were 1000 ms in duration. The sequence of encoding and recall was repeated, resulting in 2 runs for each portion of the task. Reaction time and accuracy were measured on all trials (Fig. 1).
Fig. 1.
Example trials from Encoding and Recognition.
2.3. Data scoring and analysis
Signal detection, a method that accounts for response bias to provide a more precise measure of accuracy, was used for item recall (Stanislaw and Todorov, 1999). Participant responses were identified as hits (image old and responded “old”), misses (image old and responded “new”), false alarms (image new and responded “old”) and correct rejections (image new and responded “new”). D-Prime (d’) was calculated for each subject: . Greater values for d’ indicate better accuracy. We also calculated a measure of response bias (C): . Positive values for C indicate a propensity to respond “new”, while negative values indicate a propensity to respond “old”. For both formulas, H = hit rate and F = false alarm rate. Analyses were conducted using IBM SPSS (v. 21) to examine associations between income, d’, and brain function during encoding and recognition.
MRI data processing and analysis were performed using the statistical parametric mapping extension for MATLAB (SPM8; www.fil.ion.ucl.ac.uk/spm). Images were motion corrected, slice-time corrected, realigned to the first scan in each run, co-registered with the T1 structural image, normalized to the Montreal Neurological Institute (MNI) template brain, resampled to 3 mm3 voxels and smoothed with a 5 mm3 kernel. Motion parameters for all six planes (x, y, z, roll, pitch, yaw) were examined, and any run with greater than 3 mm of motion in any direction was discarded before analysis. Two runs were excluded from analysis due to excessive motion. All 6 motion parameters were entered as nuisance regressors. Maximum motion per run ranged from 0.16–2.98 mm (M = 0.65, SD = 0.59). Motion was not associated with childhood income (p = 0.58). MRI analyses were conducted in three steps, as described below. This method was conducted for encoding and recognition portions of the task separately.
Step 1. First, we ran a whole brain analysis to examine regions of activation associated with visuospatial memory across all our subjects, irrespective of accuracy on the task or income levels. We included all trial types in this contrast (modeled this as all trials minus the implicit baseline) to identify activation associated with visuospatial memory in general. We included all subjects’ data, and consistent with prior studies, this contrast yielded hippocampus which was our a priori region of interest, based on childhood poverty literature. We also observed activation in additional regions associated with visuospatial memory, task performance and salience processing (Talairach and Tournoux, 1988) (See Table 1). Activation clusters in our ROI – hippocampus, were small volume corrected and clusters that met FWE SVC criteria at p < 0.05 were identified as significant peaks. As expected, we identified significant peaks in hippocampus during both encoding and recognition.
Table 1.
Significant regions of activation during A) Encoding and B) Recognition (k = 10 contiguous voxels, alpha level = 0.001uncorr). Abbreviated regions include anterior cingulate cortex (ACC), dorsal ACC (dACC), rostral ACC (rACC), middle frontal gyrus (MFG), posterior cingulate cortex (PCC), prefrontal cortex (PFC) dorsolateral PFC (dlPFC), inferior frontal gyrus (IFG), orbitofrontal cortex (OFC).
| A) Encoding | |||
|---|---|---|---|
| All Trials – Baseline | |||
| Region | x, y, z | z | k |
| Insula | −39, −4, 10 | 7.07 | 185 |
| dlPFC | −36, −25, 52 | 12.5 | 848 |
| Hippocampus | −33, −28, −14 | 4.13 | 16 |
| MFG | −24, 32, 43 | −5.62 | 96 |
| dACC | −3, 8, 49 | 9.91 | 415 |
| PCC | 3, −37, 43 | −7.54 | 1391 |
| Visual Cortex | 6, −76, 1 | 12.73 | 2539 |
| rACC | 9, 50, −8 | −6.73 | 767 |
| rACC | 24, 32, 37 | −5.50 | 185 |
| B) Recognition | |||
|---|---|---|---|
| All Trials – Baseline | |||
| Region | x, y, z | z | k |
| dlPFC | −45, 5, 37 | 10.24 | 513 |
| Insula | −39, −4, 7 | 5.07 | 179 |
| Thalamus | −18, −31, 1 | 7.59 | 308 |
| PCC | −15, −70, 52 | −9.04 | 872 |
| rACC | −12, 47, −5 | −5.19 | 508 |
| dACC | −6, 11, 46 | 11.76 | 282 |
| Visual cortex | −3, −91, 4 | 9.57 | 3243 |
| Hippocampus | 33, −13, −11 | 4.08 | 41 |
Step 2. Next, to specifically examine the relationships between childhood income, hippocampal activation, and memory performance, we extracted beta weights from a 10 mm sphere around the significant peaks in hippocampus for each individual subject, as defined in step 1, and ran a regression in SPSS with extracted hippocampal beta weight, childhood income, and their interaction as predictors of task accuracy (d’). This analysis controlled for other variables that were found to be correlated with childhood income, including verbal IQ (PPVT score) and adult income levels. For a similar approach, see Whittle et al., 2016. All regressors were mean centered and examined for outliers prior to analysis. Univariate outliers were defined as any value exceeding 3 standard deviations of the mean for that measure, and we examined Mahalanobis distance to screen for multivariate outliers. None of our data points met this criteria, thus all points of measurement were included in the analyses.
Step 3. Finally, as an exploratory analysis, we submitted each of the other regions associated with visuospatial memory identified in step 1 to the regression analysis described in step 2. This included activation in rostral anterior cingulate cortex (rACC), dorsal anterior cingulate cortex (dACC), insula, thalamus, visual cortex, posterior cingulate cortex (PCC), and dorsolateral prefrontal cortex (dlPFC; see Table 1).
3. Results
3.1. Descriptive data
Across all participants, as expected, reported income was higher in adulthood as compared to childhood (t(42) = 3.4, p = 0.001). Income to needs ratio at age 9 was positively correlated with income to needs ratio in adulthood (r = 0.32, p = 0.04), such that lower income at age 9 predicted lower income levels in adulthood. Income to needs ratio at age 9 was also positively correlated with PPVT score (Dunn and Dunn, 2007), such that those with lower childhood income levels had lower verbal IQ scores (r = 0.28, p = 0.04). Thus, we controlled for adult income to needs ratio and PPVT scores in all analyses. Income at age 9 was not correlated with item recognition (d’), response bias (C), or reaction time (ps > 0.09). RT was correlated with d’, such that faster reaction times predicted better item recognition (r = 0.30, p = 0.03).
3.2. Neural activation during encoding
During encoding, (task minus baseline contrast, p < 0.05 FWE SVC) a significant activation cluster was identified in the hippocampus (x, y, z = −33, −28, −14). Activation in this region was not related to income or recognition accuracy (d’), and the income x hippocampal activation interaction on recognition accuracy was not significant (ps > 0.09). Activation in other brain regions associated with the Encoding task (Table 1a) were also not significantly related to income or recognition accuracy (ps > 0.06).
3.3. Neural activation during recognition
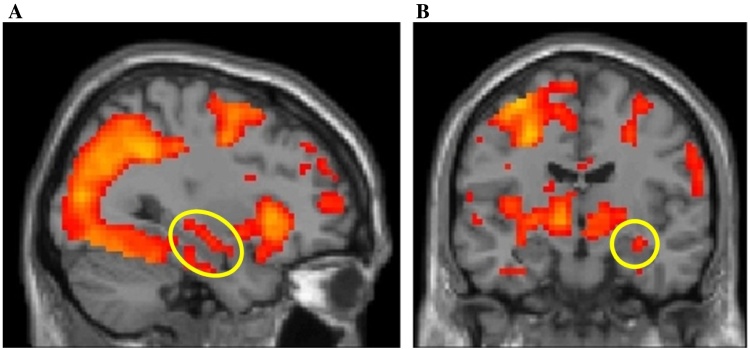
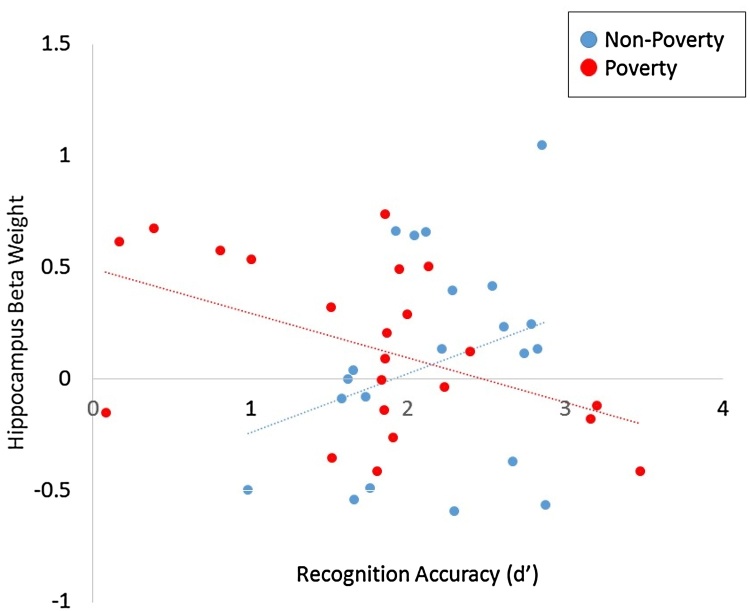
During recognition (task minus baseline contrast, p < 0.05 FWE SVC) a significant activation cluster was identified in the hippocampus, as expected (x, y, z = 33, −13, −11; Fig. 2). The association between childhood income and hippocampal activation was at the cutoff for significance, B = −1.00, t = −2.0, p = 0.05. There was no significant association between recognition accuracy and hippocampal activation, B = −0.42, t = −1.2, p = 0.25, thus the mediation model could not be formally tested, as the proposed mediator (hippocampal activation) was not associated with the proposed outcome (recognition accuracy). However, the interaction between childhood income and hippocampal activation on recognition accuracy (d’) was significant, B = 0.96, t = 2.6, p = 0.01, suggesting that the association between hippocampus activation and recognition accuracy differs across levels of childhood income. Specifically, as income increased, the association between hippocampal activation and accuracy became stronger and more positive, while as income decreased, the association between hippocampal activation and accuracy became more negative (Fig. 3). Adult income and verbal IQ measured on the PPVT, which were entered as nuisance covariates in this analysis, were not associated with hippocampal activation (p’s > 0.69).
Fig. 2.
Neural activation during recognition. Activation in the circled hippocampal region of interest was extracted for further analysis.
Fig. 3.
Results of regression analysis demonstrating variations in the association between accuracy and hippocampal activation across income levels. For illustrative purposes, we divided participants into two groups (non-poverty, poverty) based on the continuous income measure, to graph the income x hippocampal function interaction on d’. The non-poverty group (blue dots) represent adults with no history of poverty, while the poverty group (red dots) represent those who reported living below the poverty line at age 9.)
Activation in other brain regions associated with the Recognition task (Table 1b) were explored, to determine whether patterns of activation were associated with income or task performance. Activations in the task-related regions, including rACC, dACC, insula, thalamus, visual cortex, PCC, and dlPFC, were not associated with income or recognition accuracy.
3.4. Hippocampal volume
We repeated the regression analysis described above to examine relationships between hippocampal volume (total, right, and left), childhood income to need ratio, and performance on the visuospatial memory task (d’). No relationships were observed between our variables of interest and hippocampal volume (p’s > 0.21).
4. Discussion
The purpose of this study was to investigate neural mechanisms underlying deficits in visuospatial memory in young adults with a history of childhood poverty. While the relationship between childhood income and visuospatial memory performance mirrored previous reports (Evans and Schamberg, 2009, Herrmann and Guadagno, 1997) of adults with a history of childhood poverty performing more poorly on a visuospatial recognition task, this relationship did not reach significance in our sample. This is not entirely unexpected, as our study involving complex neuroimaging included less subjects than the studies that examined cognitive functions only.
The interaction we observed between childhood income and hippocampal function on visuospatial memory performance reveals a novel and interesting link between hippocampal function in association with visuospatial memory performance and history of childhood poverty. We demonstrate here that toward higher levels of childhood income, there was an expected positive association between hippocampal activation and memory performance. Conversely, toward lower levels of childhood income, the association between memory performance and hippocampal activation was negative (i.e. more activation was associated with poorer memory performance). We did not detect an association between neural activation in the hippocampus during encoding and performance on the subsequent recognition task, across all the subjects, in contrast to a previous report of hippocampal engagement in both information encoding and recognition (Wong et al., 2013).
These findings suggest a possible “disconnect” between hippocampal activation as observed on fMRI and performance on a visuospatial recognition task in adults with a history of poverty. One relatively straight forward explanation of our findings is that while increased activation in the higher childhood income participants reflects effective activation of hippocampus (i.e. stronger activation leads to better performance), activation in the lower childhood income participants reflects effort associated with difficulty that is not leading to improved performance. These results link previous findings, which separately documented associations between poverty and poorer memory performance (Farah et al., 2006, Noble et al., 2007), and poverty and hippocampal structure (Hanson et al., 2011). These findings are also consistent with data from the animal literature that rodents raised in conditions to model poverty had less capacity for plasticity in hippocampus, which was related to poorer performance on memory and learning tasks (Hackman et al., 2010).
Exploratory analysis aimed to identify activation in other brain regions involved in encoding and recognition, associated with income or recognition accuracy. In our sample, multiple brain regions previously implicated in memory processes, like prefrontal cortex, inferior frontal gyrus, and visual cortex (Preston and Eichenbaum, 2013, Wong et al., 2013) were activated during encoding and recognition tasks, but none of these regions were associated with childhood income or recognition accuracy.
There are important limitations of this study. Like the majority of studies examining early life risk factors, our study reveals links and associations, and should not be interpreted as evidence of causation. While the prospective and objective nature of the assessments confer confidence in our findings, they do not exclude the possibility of additional unaccounted factors contributing to the observed associations. Because poverty is a complex construct, encompassing multiple interacting variables like parental education, parenting style, school and home environment, nutrition, etc., it was not possible to isolate a single specific mechanism that influences brain function and cognitive performance. Mediation models may assist with this in the future, but our sample size provided limited power for this type of analysis. It is important to note here, that examining effects of isolated variables might not constitute the single best strategy either, since the interaction between multiple factors within the construct of childhood poverty might be the most salient contributing factor (Evans, 2003). In addition, we used a “convenience sample” from an existing longitudinal prospective cohort that had been followed up already for 15 years, and income was only measured at four year intervals beginning at age 9. Therefore, we chose the earliest possible data point available for this cohort. Although income in our sample and similar samples tends to remain stable over time (Evans and Schamberg, 2009), we are unable to provide direct evidence of early childhood poverty prior to age 9. We did not examine performance on other (non-visuospatial) memory tasks or other cognitive tasks, and do not know whether deficits in performance and/or the related differences in brain function would extend to other tasks. In addition, since subjects were tested only once as young adults, we were unable to investigate whether the reported deficits would persist into later adulthood.
Despite the limitations, this is the first study to our knowledge to document differences in the associations between brain function and visuospatial memory performance in healthy young adults as a function of childhood income levels. We used prospective data collection over the course of a longitudinal study, which is a significant advantage over previous studies using retrospective data. Our real time data collection allowed for assessment of income at multiple time points across development. Although it has long been known that poverty is related to a number of negative outcomes in adulthood, this is the first study demonstrating associations between visuospatial memory deficits associated with differences in hippocampal function during encoding and recognition, occurring independent of current adult income. Given prior reports of hippocampal recruitment associated with better memory performance (Wong et al., 2013, Bergmann et al., 2016), these results suggest that early experiences of poverty set disadvantaged children on a trajectory of altered neurological functioning that, among other things, may result in compromised memory later in life. Future investigations will need to examine specific mechanisms underlying the links between poverty, neural function, and cognitive performance.
Conflict of interest
The authors declare no competing financial interests.
Funding and disclosure
This research was funded by the NIH Grand Opportunities (GO) Grant (RC2MD004767), the W. T. Grant foundation and the John D. and Catherine T. Mac Arthur Foundation Network on Socioeconomic Status and Health, and the Centers for Disease Control & Prevention Award (U49/CE002099) via the University of Michigan Injury Center.
References
- Adler N., Bush N.R., Pantell M.S. Rigor, vigor, and the study of health disparities. Proc. Natl. Acad. Sci. U. S. A. 2012:17154–17159. doi: 10.1073/pnas.1121399109. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3477386&tool=pmcentrez&rendertype=abstract (Accessed August 21, 2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann H.C. Neural substrates of successful working memory and long-term memory formation in a relational spatial memory task. Cogn. Process. 2016 doi: 10.1007/s10339-016-0772-7. http://www.ncbi.nlm.nih.gov/pubmed/27350001 Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley R.H., Corwyn R.F. Socioeconomic status and child development. Annu. Rev. Psychol. 2002;53:371–399. doi: 10.1146/annurev.psych.53.100901.135233. [DOI] [PubMed] [Google Scholar]
- Brooks-Gunn J., Duncan G.J. The effects of poverty on children. Future Child. 1997;7(2):55–71. http://www.ncbi.nlm.nih.gov/pubmed/24864002 Available at: [PubMed] [Google Scholar]
- De Rover M. Hippocampal dysfunction in patients with mild cognitive impairment: a functional neuroimaging study of a visuospatial paired associates learning task. Neuropsychologia. 2011;49(7):2060–2070. doi: 10.1016/j.neuropsychologia.2011.03.037. Available at: http://www.ncbi.nlm.nih.gov/pubmed/21477602 (Accessed June 7, 2014) [DOI] [PubMed] [Google Scholar]
- Duncan G.J. Give us this day our daily breadth. Child Dev. 2012;83(1):6–15. doi: 10.1111/j.1467-8624.2011.01679.x. Available at: http://www.ncbi.nlm.nih.gov/pubmed/22277003 (Accessed September 10, 2014) [DOI] [PubMed] [Google Scholar]
- Dunn, L.M., Dunn, D.M., 2007. Peabody Picture Vocabulary Test, Fourth Edition.
- Evans G.W., Schamberg M.a. Childhood poverty, chronic stress, and adult working memory. Proc. Natl. Acad. Sci. U. S. A. 2009;106(16):6545–6549. doi: 10.1073/pnas.0811910106. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2662958&tool=pmcentrez&rendertype=abstract (Accessed June 2, 2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans G.W. Childhood cumulative risk exposure and adult amygdala volume and function. J. Neurosci. Res. 2016;94(6):535–543. doi: 10.1002/jnr.23681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans G.W. A multimethodological analysis of cumulative risk and allostatic load among rural children. Dev. Psychol. 2003;39(5):924–933. doi: 10.1037/0012-1649.39.5.924. Available at: 10.1037/0012-1649.39.5.924 (Accessed June 2, 2014) [DOI] [PubMed] [Google Scholar]
- Farah M.J. Childhood poverty: specific associations with neurocognitive development. Brain Res. 2006;1110(1):166–174. doi: 10.1016/j.brainres.2006.06.072. Available at: http://www.ncbi.nlm.nih.gov/pubmed/16879809 (Accessed May 26, 2014) [DOI] [PubMed] [Google Scholar]
- Hackman D.A., Farah M.J. Socioeconomic status and the developing brain. Trends Cognit. Sci. 2009;13(2):65–73. doi: 10.1016/j.tics.2008.11.003. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3575682&tool=pmcentrez&rendertype=abstract (Accessed August 23, 2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackman D.A., Farah M.J., Meaney M.J. Socioeconomic status and the brain: mechanistic insights from human and animal research. Nat. Rev. Neurosci. 2010;11(9):651–659. doi: 10.1038/nrn2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson J.L. Association between income and the hippocampus. PLoS One. 2011;6(5):e18712. doi: 10.1371/journal.pone.0018712. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3087752&tool=pmcentrez&rendertype=abstract (Accessed June 3, 2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson J.L. Family poverty affects the rate of human infant brain growth. PLoS One. 2013;8(12) doi: 10.1371/journal.pone.0080954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson J.L. Behavioral problems after early life stress: contributions of the hippocampus and amygdala. Biol. Psychiatry. 2014:1–9. doi: 10.1016/j.biopsych.2014.04.020. Available at: http://www.ncbi.nlm.nih.gov/pubmed/24993057 (Accessed July 9, 2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann D., Guadagno M.A.N.N. Memory performance and socio-economic status. Appl. Cognit. Psychol. 1997;11:113–120. [Google Scholar]
- Jarrard L.E. On the role of the hippocampus in learning and memory in the rat. Behav. Neural Biol. 1993;60(1):9–26. doi: 10.1016/0163-1047(93)90664-4. http://www.ncbi.nlm.nih.gov/pubmed/8216164 Available at: [DOI] [PubMed] [Google Scholar]
- Javanbakht A. Childhood poverty predicts adult amygdala and frontal activity and connectivity in response to emotional faces. Front. Behav. Neurosci. 2015;9(June):1–8. doi: 10.3389/fnbeh.2015.00154. http://journal.frontiersin.org/Article/10.3389/fnbeh.2015.00154/abstract Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P. Effects of childhood poverty and chronic stress on emotion regulatory brain function in adulthood. Proc. Natl. Acad. Sci. U. S. A. 2013;110(46):18442–18447. doi: 10.1073/pnas.1308240110. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3831978&tool=pmcentrez&rendertype=abstract Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P., Ho S.S., Evans G.W., Liberzon I., Swain J.E. Childhood social inequalities influences neural processes in young adult caregiving. Dev. Psychobiol. 2015;57(8):948–960. doi: 10.1002/dev.21325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavenex P.B., Lavenex P. Spatial memory and the monkey hippocampus: not all space is created equal. Hippocampus. 2009;19(1):8–19. doi: 10.1002/hipo.20485. Available at: http://www.ncbi.nlm.nih.gov/pubmed/18727046 (Accessed June 27, 2014) [DOI] [PubMed] [Google Scholar]
- Liberzon I. Childhood poverty and recruitment of adult emotion regulatory neurocircuitry. Soc. Cognit. Affect. Neurosci. 2015:1–11. doi: 10.1093/scan/nsv045. http://scan.oxfordjournals.org/lookup/doi/10.1093/scan/nsv045 Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipina S.J., Posner M.I. The impact of poverty on the development of brain networks. Front. Hum. Neurosci. 2012;6(August):238. doi: 10.3389/fnhum.2012.00238. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3421156&tool=pmcentrez&rendertype=abstract (Accessed August 23, 2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longoni G. Deficits in memory and visuospatial learning correlate with regional hippocampal atrophy in MS. Brain Struct. Funct. 2013:1–10. doi: 10.1007/s00429-013-0665-9. Available at: http://www.ncbi.nlm.nih.gov/pubmed/24189776 (Accessed June 18, 2014) [DOI] [PubMed] [Google Scholar]
- Luby J.L. Maternal support in early childhood predicts larger hippocampal volumes at school age. Proc. Natl. Acad. Sci. U. S. A. 2012;109(8):2854–2859. doi: 10.1073/pnas.1118003109. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3286943&tool=pmcentrez&rendertype=abstract (Accessed July 12, 2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby J. The effects of poverty on childhood brain development: the mediating effect of caregiving and stressful life events. JAMA Pediatrics. 2013;167(12):1135–1142. doi: 10.1001/jamapediatrics.2013.3139. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4001721&tool=pmcentrez&rendertype=abstract (Accessed July 15, 2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien S.J. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat. Rev. Neurosci. 2009;10(6):434–445. doi: 10.1038/nrn2639. Available at: http://www.ncbi.nlm.nih.gov/pubmed/19401723 (Accessed July 9, 2014) [DOI] [PubMed] [Google Scholar]
- McEwen B.S., Magarinos A.M. Stress and hippocampal plasticity: implications for the pathophysiology of affective disorders. Hum. Psychopharmacol. 2001;16(S1):S7–S19. doi: 10.1002/hup.266. http://www.ncbi.nlm.nih.gov/pubmed/12404531 Available at: [DOI] [PubMed] [Google Scholar]
- Meaney M.J. The effects of neonatal handling on the development of the adrenocortical response to stress: implications for neuropathology and cognitive deficits in later life. Psychoneuroendocrinology. 1991;16(1–3):85–103. doi: 10.1016/0306-4530(91)90072-2. http://www.ncbi.nlm.nih.gov/pubmed/1961847 Available at: [DOI] [PubMed] [Google Scholar]
- Noble K.G., McCandliss B.D., Farah M.J. Socioeconomic gradients predict individual differences in neurocognitive abilities. Dev. Sci. 2007;10(4):464–480. doi: 10.1111/j.1467-7687.2007.00600.x. Available at: http://www.ncbi.nlm.nih.gov/pubmed/17552936 (Accessed July 10, 2014) [DOI] [PubMed] [Google Scholar]
- Preston A.R., Eichenbaum H. Interplay of hippocampus and prefrontal cortex in memory. Curr. Biol. 2013;23(17):R764–R773. doi: 10.1016/j.cub.2013.05.041. Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raizada R.D.S., Kishiyama M.M. Effects of socioeconomic status on brain development, and how cognitive neuroscience may contribute to levelling the playing field. Front. Hum. Neurosci. 2010;4(February):3. doi: 10.3389/neuro.09.003.2010. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2820392&tool=pmcentrez&rendertype=abstract (Accessed July 17, 2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schalinski I. Type and timing of adverse childhood experiences differentially affect severity of PTSD, dissociative and depressive symptoms in adult inpatients. BMC Psychiatry. 2016;16(1):295. doi: 10.1186/s12888-016-1004-5. http://bmcpsychiatry.biomedcentral.com/articles/10.1186/s12888-016-1004-5 Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scoviille W.B., Milner B. Loss of recent memory after bilateral hippocampal lesions. J. Neurol. Neurosurg. Psychiatry. 1957;20:11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripada R.K. Childhood poverty and stress reactivity are associated with aberrant functional connectivity in default mode network. Neuropsychopharmacology. 2014;39(9):2244–2251. doi: 10.1038/npp.2014.75. Available at: http://www.ncbi.nlm.nih.gov/pubmed/24675708 (Accessed September 11, 2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanislaw H., Todorov N. Calculation of signal detection theory measures. Behav. Res. Methods Instrum. Comput. 1999;31(1):137–149. doi: 10.3758/bf03207704. [DOI] [PubMed] [Google Scholar]
- Vann S.D., Albasser M.M. Hippocampus and neocortex: recognition and spatial memory. Curr. Opin. Neurobiol. 2011;21(3):440–445. doi: 10.1016/j.conb.2011.02.002. Available at: http://www.ncbi.nlm.nih.gov/pubmed/21353527 (Accessed May 26, 2014) [DOI] [PubMed] [Google Scholar]
- Whittle S., Liu K., Bastin C., Harrison B.J., Davey C.G. Neurodevelopmental correlates of proneness to guilt and shame in adolescence and early adulthood. Dev. Cognit. Neurosci. 2016;19:51–57. doi: 10.1016/j.dcn.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong J.X., de Chastelaine M., Rugg M.D. Comparison of the neural correlates of encoding item–item and item-context associations. Front. Hum. Neurosci. 2013;7(August):436. doi: 10.3389/fnhum.2013.00436. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3743067&tool=pmcentrez&rendertype=abstract (Accessed August 28, 2014) [DOI] [PMC free article] [PubMed] [Google Scholar]