Abstract
Rationale
Electrospray mass spectrometry methods for the analysis of phosphatidylcholines (PCs) routinely include ammonium acetate or ammonium formate in the mobile phase. In an effort to justify and optimize the use of these additives, we investigated possible functions of ammonium compounds in the ionization of PCs.
Methods
Because PCs contain a quaternary amine, the role of ammonium in neutralizing the negatively charged phosphate group was investigated by using deuterated ammonium acetate, adjusting the pH, varying the organic solvent composition, and by comparing the additives ammonium acetate, ammonium formate and ammonium bicarbonate. Seven PC standards were measured ranging from lyso 1-palmitoyl-sn-glycero-3-phosphocholine to 1,2-dieicosapentaenoyl-sn-glycero-3-phosphocholine as well as a mixture of PCs in a krill oil dietary supplement.
Results
Under all conditions tested, aqueous acetonitrile provided more abundant formation of protonated PCs than did aqueous methanol. Regardless of the mobile phase composition and electrospray ion source parameters, no [M+NH4]+ ions were detected. Adding deuterated ammonium acetate to the mobile phase failed to form deuterated PCs, indicating that ammonium is not the source of the proton that neutralizes the phosphate negative charge. Instead, water was the source of the proton as deuterated water resulted in the formation of [M+D]+ ions. Addition of organic acids, ammonium formate, ammonium acetate, or ammonium bicarbonate to the mobile phase did not enhance and in most cases suppressed PC ionization.
Conclusions
Ammonium compounds and organic acids can suppress ionization of PCs when using an aqueous acetonitrile mobile phase during electrospray.
Keywords: phosphatidylcholine, ammonium acetate, electrospray, mass spectrometry
Introduction
Phosphatidylcholines (PCs) are phospholipids consisting of a choline head group, phosphoric acid, glycerol, and two fatty acid acyl groups (Figure 1). Important constituents of cell membranes, PCs have critical roles in human health[1] ranging from liver function[2] to signaling pathways.[3] Because krill is rich in PCs containing omega-3 fatty acids, dietary supplements containing krill oil are being marketed as antioxidants with possible cardiovascular health benefits.[4] To support the analysis of PCs in biological systems and dietary supplements, sensitive and accurate analytical methods are required such as those based on electrospray liquid chromatography-mass spectrometry (LC-MS) and LC-tandem mass spectrometry (MS/MS).
Figure 1.
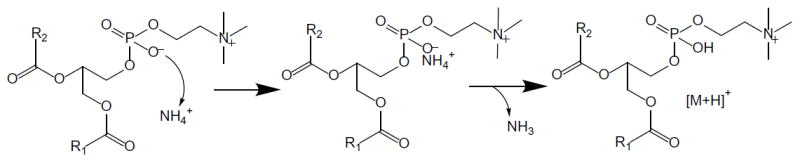
Chemical structure of phosphatidylcholine (PC) and hypothetical role of ammonium in protonation during positive ion electrospray. As indicated in the text, no evidence of [M+NH4]+ formation was obtained.
Numerous electrospray mass spectrometry-based methods have been reported for the analysis of PCs.[5-13] A common practice among these analyses is the inclusion of ammonium compounds (usually ammonium formate or ammonium acetate) in the electrospray mobile phase. Although justification is rarely provided, the addition of ammonium compounds during electrospray has been suggested to minimize PC adducts with sodium or potassium[9] as has been reported for MALDI,[14] and to facilitate the formation of ammonium adducts of lipids such as diacylglycerols and triacylglycerols.[13] However, no ammonium adducts of PCs have been reported.
In this investigation, the effects of ammonium compounds on electrospray ionization of PCs were investigated using MS, LC-MS and LC-MS/MS with a mixture of PC standards, krill oil rich in PCs, and a combination of mobile phases. This investigation is intended to clarify the role of ammonium in the electrospray mobile phase and to enhance the productivity of future electrospray mass spectrometric analyses of PCs.
EXPERIMENTAL
Materials
Except as noted, all standards were purchased from Sigma-Aldrich (St. Louis, MO). The PCs didocosahexaenoyl-sn-glycero-3-phosphocholine (DHA DHA PC), 1,2-dieicosapentaenoyl-sn-glycero-3-phosphocholine (EPA EPA PC), 1-palmitoyl-sn-glycero-3-phosphocholine (palm PC), 19:0 lyso PC 1-nonadecanoyl-2-hydroxy-sn-glycero-3-phosphocholine (nona PC), 18:0 lyso PC 1-stearoyl-2-hydroxy-sn-glycero-3-phosphocholine (stearoyl PC), 18:1 lyso PC 1-oleoyl-2-hydroxy-sn-glycero-3-phosphocholine (oleoyl PC), and 17:0 lyso PC 1-heptadecanoyl-2-hydroxy-sn-glycero-3-phosphocholine (hepta PC) were selected to provide variation in acyl chain length, variation in acyl chain saturation, and mono- (lyso) as well as di-substitution. [d7]-Ammonium acetate and deuterated water were purchased from Cambridge Isotopes Laboratories (Tewksbury, MA).
For the preparation of krill oil, 300 g of frozen ground krill (Euphausia superba) was refluxed with 750 mL of hexane/ethanol (79:21; v/v) for one hour. After filtration, the solvent was removed under vacuum at 70 °C, and the resulting krill oil was stored at -80 °C until analysis. Solvents used during extraction were LCMS grade and were purchased from Honeywell Burdick and Jackson (Morris Plains, NJ). All other solvents were also LCMS grade but were purchased from Thermo Fisher (Waltham, MA).
LC-MS/MS
PC standards were dissolved in acetonitrile (1 μM each), and krill oil was dissolved in chloroform/acetone (60:40; v/v) before dilution using acetonitrile to a final concentration of 1 μg/mL. PCs were analyzed either during infusion using a syringe containing acetonitrile/water (80:20; v/v) or acetonitrile/deuterated water (80:20; v/v) with or without 5 mM ammonium compounds, or after injection (1 μL injection volume) onto a Waters (Milford, MA) Cortecs C18 HPLC column (2.7 μm, 2.1 × 50 mm). During LC-MS and LC-MS/MS, PCs were eluted using an isocratic mobile phase consisting of acetonitrile/water (80:20; v/v) at a flow rate of 0.6 mL/min and detected using positive ion electrospray mass spectrometry. Mobile phases were also tested that contained methanol or acetonitrile with or without 0.1% formic acid, 0.1% acetic acid, 5 mM ammonium formate, 5 mM ammonium acetate, or 5 mM ammonium bicarbonate.
Positive ion electrospray mass spectra, tandem mass spectra and selected reaction monitoring (SRM) data were obtained using a Shimadzu (Kyoto, Japan) LCMS-8060 triple quadrupole mass spectrometer equipped with a Shimadzu Nexera UHPLC system. During SRM, the transition of each PC precursor ion to the class-specific phosphocholine ion of m/z 184[15,16] was monitored as indicated in Table 1. High resolution accurate mass measurements were obtained using a Waters (Milford, MA) quadrupole time-of-flight Synapt mass spectrometer equipped with positive ion electrospray.
Table 1.
Positive ion electrospray selected reaction monitoring (SRM) precursor and product ions used for comparison of mobile phase conditions for PC ionization.
| Precursorion m/z | Production m/z | Dwell time (msec) | Q1 Bias (V) | CE (eV) | Q3 Bias (V) | |
|---|---|---|---|---|---|---|
| EPA EPA PC | 827.1 | 184.1 | 25 | -24 | -36 | -19 |
| Nona PC | 538.2 | 184.1 | 25 | -30 | -35 | -20 |
| Stearoyl PC | 524.2 | 184.1 | 25 | -30 | -35 | -20 |
| Palm PC | 496.2 | 184.1 | 25 | -14 | -26 | -19 |
| Hepta PC | 510.2 | 184.1 | 25 | -30 | -30 | -21 |
| Oleoyl PC | 522.2 | 184.1 | 25 | -30 | -30 | -20 |
| DHA DHA PC | 878.6 | 184.1 | 25 | -20 | -34 | -12 |
RESULTS AND DISCUSSION
When comparing methanol and acetonitrile as mobile phases for the positive ion electrospray mass spectrometric measurement of PCs, acetonitrile provided superior signal-to-noise in all cases (Figure 2). Previous methods for the LC-MS/MS analysis of PCs have reported using either methanol or acetonitrile, although the use of methanol has been more common. The addition of 5 mM ammonium formate to the methanol mobile phase, as has been reported in the literature, increased the PC signals by approximately 5-fold compared with methanol alone, but the PC signal obtained using neat acetonitrile was another 10-fold higher (Figure 2). Because acetonitrile provided far superior electrospray ionization of PCs relative to methanol, all subsequent studies of the effects of ammonium additives on PC ionization contained acetonitrile instead of methanol.
Figure 2.
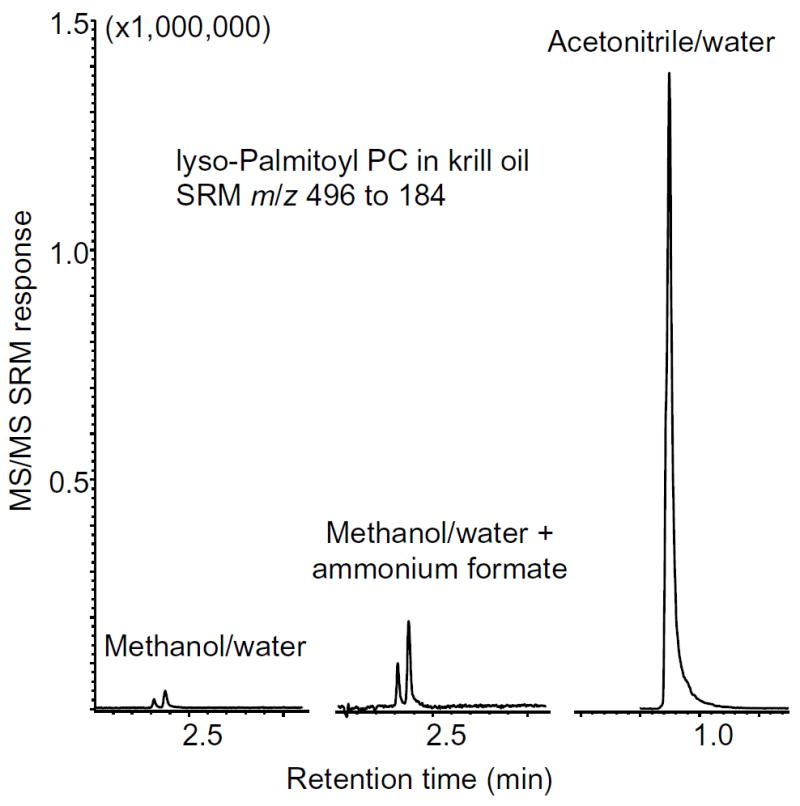
Positive ion electrospray LC-MS/MS analysis of 1 μL of 0.01 mg/mL of krill oil. The detection of lyso palmitoyl PC (SRM of the protonated molecule of m/z 496 forming the abundant fragment ion of m/z 184) is shown to compare effects of methanol/water (80:20; v/v), methanol/water + 5 mM ammonium formate or acetonitrile/water (80:20/v/v) on PC ionization. The two peaks detected in the methanol traces represent separation of the isomeric sn1 and sn2 lyso palmitoyl PCs.
Although PCs contain a fixed positive charge on the choline group, they are zwitterions at physiological pH due to a negatively charged phosphate group (Figure 1). Therefore, an additional positive charge is required for mass spectrometric analysis, and this may be achieved by attaching a proton or another positively charged group that can neutralize the phosphate. For example, Dodbiba, et al.[17] enhanced PC ionization during electrospray by adding dicationic, tricationic, and tetracationic ion pair agents and forming imidazolium, phosphonium or pyrrolidinium adducts. We tested the hypothesis that ammonium adducts of PCs might form during electrospray, as reported for diglycerides and triglycerides,[13] and then eliminate neutral ammonia to form protonated PCs (Figure 1). Despite adjustment of the ionization voltages, ion source temperature and declustering parameters in the electrospray ion source over the widest ranges allowed by the instrument, no ammonium adducts with PCs could be detected (Figure 3). The ammonium compound added to the mobile phase also made no difference to adduct formation, as no ammonium adducts with PC were detected when ammonium formate, ammonium acetate (Figure 3) or ammonium bicarbonate (5 mM) were used.
Figure 3.
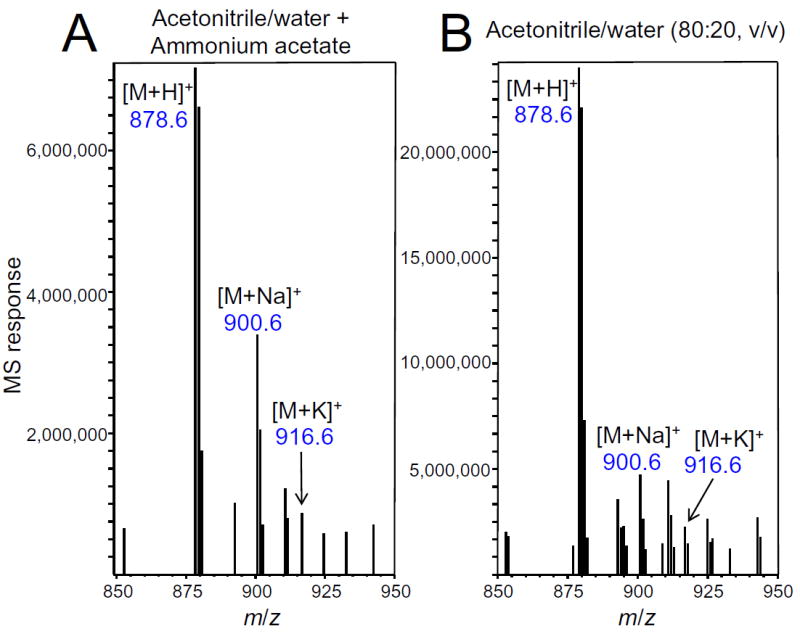
Positive ion electrospray mass spectra of DHA DHA PC in acetonitrile/water (80:20; v/v) containing A) 5 mM ammonium acetate; or B) no ammonium acetate. Protonated PC and adducts with sodium and potassium were detected, but no [M+NH4]+ was observed at m/z 895.6. The addition of ammonium acetate did not eliminate or reduce the relative abundances of [M+Na]+ and [M+K]+ ions. Similar results were obtained when ammonium formate or ammonium bicarbonate was substituted for ammonium acetate (data not shown).
As shown in the positive ion mass spectra of DHA DHA PC in Figure 3, [M+H]+, [M+Na]+ and [M+K]+ ions were observed in the presence or absence of ammonium acetate (5 mM). Despite the expectation that ammonium acetate in the mobile phase might decrease the relative abundances of sodium and potassium adducts,[9] their abundances relative to the protonated molecule did not decrease (Figure 3). When DHA DHA PC was analyzed without ammonium acetate (Figure 3), the ion abundances of all three species, [M+H]+, [M+Na]+ and [M+K]+, were higher (over three-fold higher for the protonated molecule), indicating that ammonium acetate was not selective in suppressing electrospray ionization. Note that regardless of the ion source parameters, the protonated molecule was always the base peak of the PC mass spectrum.
Even though no ammonium adducts were observed, we investigated whether ammonium in the mobile phase might donate a proton as reported for ammonia chemical ionization of PCs.[18] Deuterated ammonium acetate was added to acetonitrile containing DHA DHA PC, and the solution was infused into the electrospray ion source. Instead of observing [M+D]+, only [M+H]+ was detected at m/z 878.6 (Figure 4A). Furthermore, the abundance of the M+1 isotope did not increase in the presence of [d7]-ammonium acetate (Figures 3 and 4). Therefore, ammonium compounds added to the mobile phase not only do not suppress sodium and potassium adducts of PCs, they do not contribute to the formation of [M+H]+ ions during electrospray.
Figure 4.
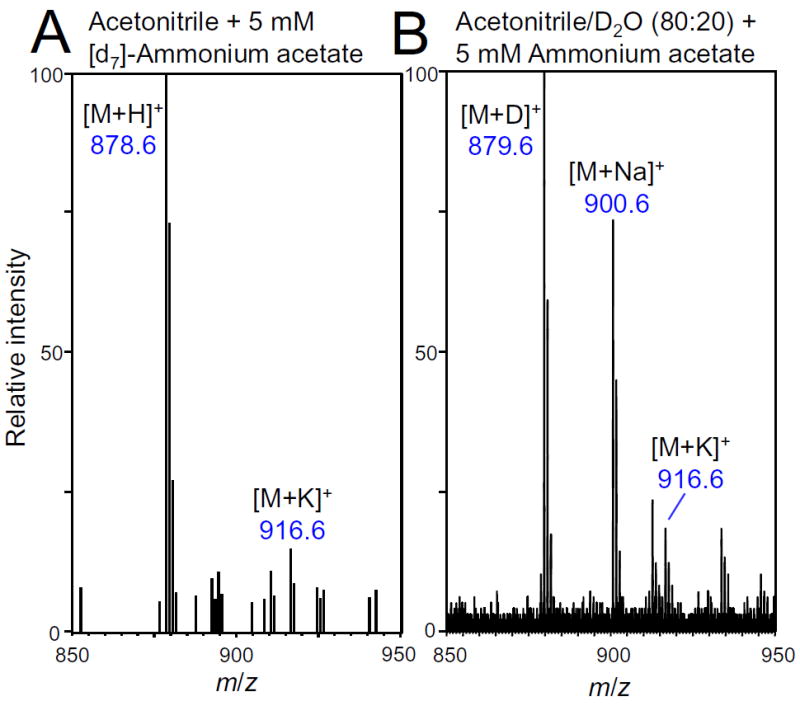
Positive ion electrospray mass spectra of DHA DHA PC infused in A) 100% acetonitrile containing 5 mM [d7]-ammonium acetate; or B) acetonitrile/D2O (80:20; v/v) containing 5 mM ammonium acetate. Note that the formation of [M+D]+ ions at m/z 879.6 occurred only when using D2O, indicating that water and not ammonium donates the proton during the ionization of PCs.
Since the source of hydrogen for the formation of protonated PCs was not ammonium, water in the mobile phase was investigated as an alternate source. DHA DHA PC in acetonitrile/deuterated water (80:20; v/v) was infused into the electrospray source and measured using positive ion electrospray (Figure 4B). The mass of protonated DHA DHA PC was increased by one mass unit (m/z 879.6) due to the incorporation of deuterium from deuterated water (Figure 4B). Therefore instead of ammonium, water was determined to be the source of protons for the ionization of PCs during electrospray mass spectrometry. This is consistent with the lower proton affinity of water (164.8 kcal/mol) relative to that of ammonia (205.0 kcal/mol),[19] which permits the facile transfer of a proton from water (but not from ammonia) to an oxygen of PC.
Using krill oil as a complex source of PCs, we investigated whether acidifying the mobile phase improved the signal-to-noise for the LC-MS/MS measurement of PCs. Instead of enhancing formation of protonated PCs such as EPA EPA PC, the addition of 0.1% acetic acid almost completely suppressed ionization (Figure 5). Similar results were observed for the other PCs in krill oil including DHA DHA PC, EPA EPA PC, palm PC, stearoyl PC, oleoyl PC, and hepta PC (identified by comparison with standards). In place of acetic acid, adding 0.1% formic acid to the mobile phase produced some protonated PCs, but the abundances were also much lower than those obtained using only acetonitrile in water (Figure 5). Therefore, acidification of the mobile phase did not enhance ionization of PCs when using a mobile phase containing aqueous acetonitrile.
Figure 5.
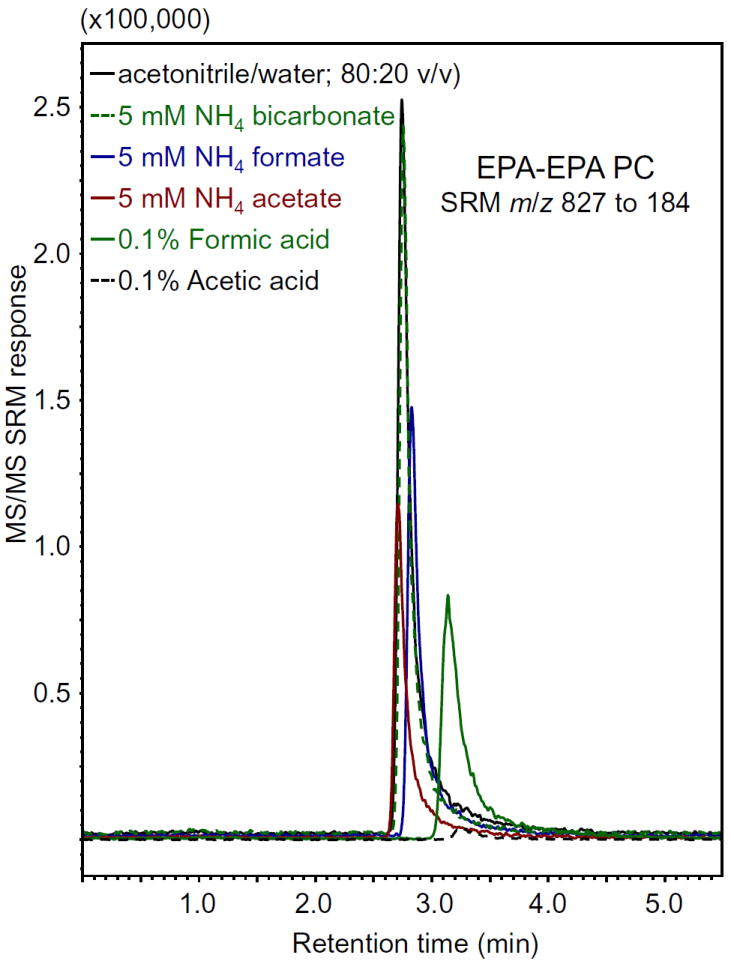
Comparison of mobile phase additives for the measurement of EPA EPA PC in krill oil using positive ion electrospray LC-MS/MS with SRM ([M+H]+ m/z 827.1 to m/z 184). Note that formic acid, acetic acid, ammonium formate, and ammonium acetate were less effective than ammonium bicarbonate, which was nearly equivalent to neat acetonitrile/water (80:20; v/v) as the mobile phase.
To determine how the addition of ammonium compounds affected protonation of PCs, LC-MS/MS of krill oil was carried out using acetonitrile in water with or without 5 mM ammonium formate, ammonium acetate or ammonium bicarbonate (Figure 5). Addition of ammonium formate or ammonium acetate suppressed PC signals approximately 60%. Although no better than aqueous acetonitrile without any additives, addition of 5 mM ammonium bicarbonate to the mobile phase at least did not suppress the LC-MS/MS signals of krill PCs (Figure 5).
To obtain the most intense PC signals, positive ion electrospray should be used with mobile phases containing aqueous acetonitrile without additives such as methanol or ammonium compounds. The inclusion of methanol, organic acids or ammonium compounds in the mobile phase suppress ionization of PCs. Furthermore, addition of ammonium compounds does not selectively reduce the formation of cations with sodium or potassium. If chromatographic constraints require the use of ammonium additives, the use of ammonium bicarbonate is preferred as it suppresses electrospray ionization of PCs the least.
Acknowledgments
We thank Shimadzu Scientific Instruments and Shimadzu Corporation for providing the UHPLC-MS/MS system used during this investigation. This project was funded by the National Institutes of Health grants T32 AT007533 and F31 AT009039 from the Office of the Director and the National Center for Complementary and Integrative Health.
References
- 1.Canty DJ, Zeisel SH. Lecithin and choline in human health and disease. Nutr Rev. 1994;52:327. doi: 10.1111/j.1753-4887.1994.tb01357.x. [DOI] [PubMed] [Google Scholar]
- 2.Kawashima Y, Mizuguchi H, Kozuka H. Modulation by dietary oils and clofibric acid of arachidonic acid content in phosphatidylcholine in liver and kidney of rat: Effects on prostaglandin formation in kidney. Biochim Biophys Acta. 1994;1210:187. doi: 10.1016/0005-2760(94)90120-1. [DOI] [PubMed] [Google Scholar]
- 3.Exton JH. Signaling through phosphatidylcholine breakdown. J Biol Chem. 1990;265:1. [PubMed] [Google Scholar]
- 4.Ulven SM, Holven KB. Comparison of bioavailability of krill oil versus fish oil and health effect. Vas Health Risk Manag. 2015;11:511. doi: 10.2147/VHRM.S85165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim H-Y, Wang T-CL, Ma Y-C. Liquid chromatography/mass spectrometry of phospholipids using electrospray ionization. Anal Chem. 1994;66:3977. doi: 10.1021/ac00094a020. [DOI] [PubMed] [Google Scholar]
- 6.Schwarz E, Prabakaran S, Whitfield P, Major H, Leweke FM, Koethe D, McKenna P, Bahn S. High throughput lipidomic profiling of schizophrenia and bipolar disorder brain tissue reveals alterations of free fatty acids, phosphatidylcholines, and ceramides. J Proteome Res. 2008;7:4266. doi: 10.1021/pr800188y. [DOI] [PubMed] [Google Scholar]
- 7.Sun T, Wetzel SJ, Johnson ME, Surlow BA, Patton-Vogt J. Development and validation of a hydrophilic interaction liquid chromatography–tandem mass spectrometry method for the quantification of lipid-related extracellular metabolites in Saccharomyces cerevisiae. J Chromatogr B. 2012;897:1. doi: 10.1016/j.jchromb.2012.03.034. [DOI] [PubMed] [Google Scholar]
- 8.Choi JM, Kim T-E, Cho J-Y, Lee HJ, Jung BH. Development of lipidomic platform and phosphatidylcholine retention time index for lipid profiling of rosuvastatin treated human plasma. J Chromatogr B. 2014;944:157. doi: 10.1016/j.jchromb.2013.10.029. [DOI] [PubMed] [Google Scholar]
- 9.Abbassi-Ghadi N, Jones EA, Gomez-Romero M, Golf O, Kumar S, Huang J, Kudo H, Goldin RD, Hanna GB, Takats Z. A comparison of DESI-MS and LC-MS for the lipidomic profiling of human cancer tissue. J Am Soc Mass Spectrom. 2016;27:255. doi: 10.1007/s13361-015-1278-8. [DOI] [PubMed] [Google Scholar]
- 10.Losito I, Facchini L, Diomede S, Conte E, Megli FM, Cataldi TR, Palmisano F. Hydrophilic interaction liquid chromatography electrospray ionization-tandem mass spectrometry of a complex mixture of native and oxidized phospholipids. J Chromatogr A. 2015;1422:194. doi: 10.1016/j.chroma.2015.10.023. [DOI] [PubMed] [Google Scholar]
- 11.Schwalbe-Herrmann M, Willmann J, Leibfritz D. Separation of phospholipid classes by hydrophilic interaction chromatography detected by electrospray ionization mass spectrometry. J Chromatogr A. 2010;1217:5179. doi: 10.1016/j.chroma.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 12.Gorrochategui E, Casas J, Pérez-Albaladejo E, Jáuregui O, Porte C, Lacorte S. Characterization of complex lipid mixtures in contaminant exposed JEG-3 cells using liquid chromatography and high-resolution mass spectrometry. Environ Sci Pollut Res Int. 2014;21:11907. doi: 10.1007/s11356-014-3172-5. [DOI] [PubMed] [Google Scholar]
- 13.Sommer U, Herscovitz H, Welty FK, Costello CE. LC-MS-based method for the qualitative and quantitative analysis of complex lipid mixtures. J Lipid Res. 2006;47:804. doi: 10.1194/jlr.M500506-JLR200. [DOI] [PubMed] [Google Scholar]
- 14.Wang H-YJ, Bin Liu C, Wu H-W. A simple desalting method for direct MALDI mass spectrometry profiling of tissue lipids. J Lipid Res. 2011;52:840. doi: 10.1194/jlr.D013060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haroldsen PE, Gaskell SJ. Quantitative analysis of platelet activating factor using fast atom bombardment/tandem mass spectrometry. Biomed Environ Mass Spectrom. 1989;18:439. doi: 10.1002/bms.1200180613. [DOI] [PubMed] [Google Scholar]
- 16.Hsu FF, Turk J. Characterization of phosphatidylinositol, phosphatidylinositol-4-phosphate, and phosphatidylinositol-4,5-bisphosphate by electrospray ionization tandem mass spectrometry: a mechanistic study. J Am Soc Mass Spectrom. 2000;11:986. doi: 10.1016/S1044-0305(00)00172-0. [DOI] [PubMed] [Google Scholar]
- 17.Dodbiba E, Xu C, Payagala T, Wanigasekara E, Moon MH, Armstrong DW. Use of ion pairing reagents for sensitive detection and separation of phospholipids in the positive ion mode LC-ESI-MS. Analyst. 2011;136:1586. doi: 10.1039/c0an00848f. [DOI] [PubMed] [Google Scholar]
- 18.Crawford CG, Plattner RD. Ammonia chemical ionization mass spectrometry of intact diacyl phosphatidylcholine. J Lipid Res. 1983;24:456. [PubMed] [Google Scholar]
- 19.Lias SG, Liebman JF, Levin RD. Evaluated gas phase basicities and proton affinities of molecules; heats of formation of protonated molecules. J Phys Chem Ref Data. 1984;13:695. [Google Scholar]


