Abstract
Context
Rapid developments in e-cigarettes, or electronic nicotine delivery systems (ENDS), and the evolution of the overall tobacco product marketplace warrant frequent evaluation of the published literature. The purpose of this article is to report updated findings from a comprehensive review of the published scientific literature on ENDS.
Evidence acquisition
The authors conducted a systematic review of published empirical research literature on ENDS through May 31, 2016, using a detailed search strategy in the PubMed electronic database, expert review, and additional targeted searches. Included studies presented empirical findings and were coded to at least one of nine topics: (1) Product Features; (2) Health Effects; (3) Consumer Perceptions; (4) Patterns of Use; (5) Potential to Induce Dependence; (6) Smoking Cessation; (7) Marketing and Communication; (8) Sales; and (9) Policies; reviews and commentaries were excluded. Data from included studies were extracted by multiple coders (October 2015 to August 2016) into a standardized form and synthesized qualitatively by topic.
Evidence synthesis
There were 686 articles included in this systematic review. The majority of studies assessed patterns of ENDS use and consumer perceptions of ENDS, followed by studies examining health effects of vaping and product features.
Conclusions
Studies indicate that ENDS are increasing in use, particularly among current smokers, pose substantially less harm to smokers than cigarettes, are being used to reduce/quit smoking, and are widely available. More longitudinal studies and controlled trials are needed to evaluate the impact of ENDS on population-level tobacco use and determine the health effects of longer-term vaping.
CONTEXT
Electronic nicotine delivery systems (ENDS), or e-cigarettes, produce an inhaled aerosol instead of smoke, providing an alternative mode of nicotine delivery.1–6 The U.S. Food and Drug Administration’s Center for Tobacco Products finalized a rule to regulate ENDS.7 Thus, there is a pressing need to identify the pathways by which to manage ENDS and how to strike a balance between potential benefits and harms to public health.4,8,9
Reviews have been published on ENDS in general10–21 or on specific topics, including: health effects22–58; impact on smoking cessation29,40–43,45,47,48,51,59–73; product features22,39,41–43,45,47,74–77; potential to induce dependence28,45,47,78,79; consumer perceptions26,48,80,81; patterns of use8,26,43,48,51,82–90; and policies.8,48,91–98 Two conducted a bibliometric review of the ENDS literature86,99 and 46% of the reviews were systematic.11,13,15,16,22–28,33,34,36,39,46,48,53–58,60,61,66,68,70–75,79–82,85,86,88–90,93,97,100–104 The dramatically changing product landscape warrants frequent updates and synthesis of the rapidly growing evidence to inform prudent practice, policy, and regulation.9,105,106 This comprehensive review presents a current synthesis of empirical studies on ENDS across a broad range of topics.
EVIDENCE ACQUISITION
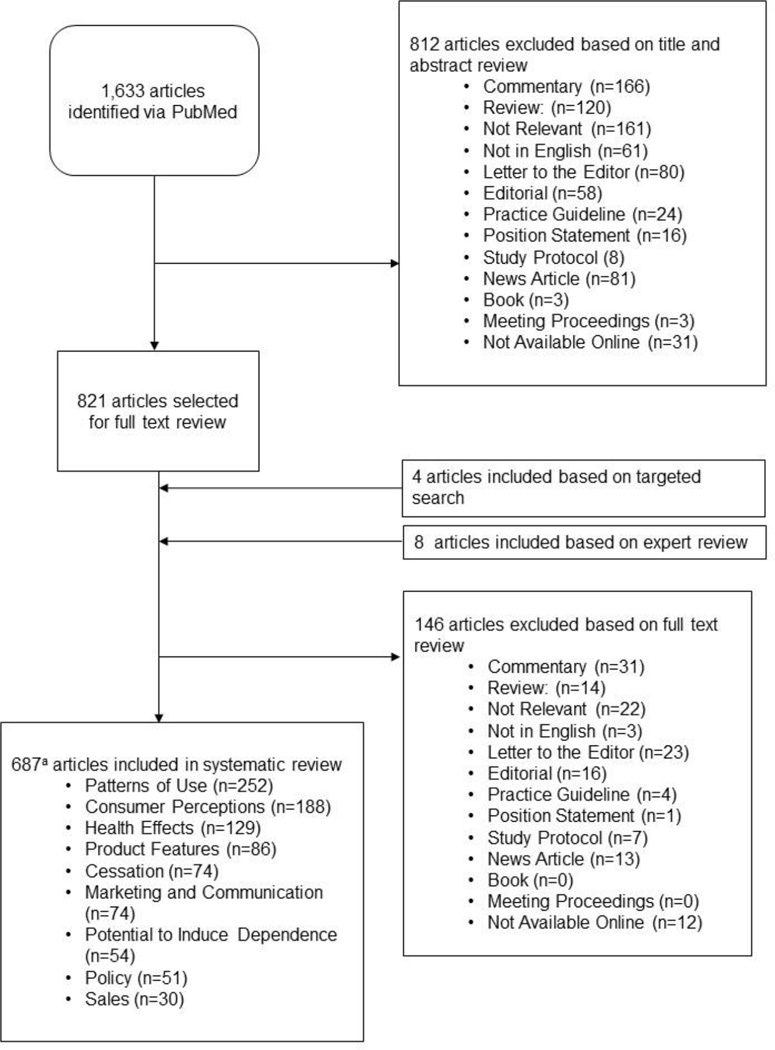
A systematic review of empirical articles on ENDS was conducted via a PubMed search through May 31, 2016 (Appendix Table 1 shows search strategy and eligibility criteria).107 Analyses were conducted from October 2015 to August 2016. Included studies (Figure 1) were catalogued into one or more of the following topics:
Product Features;
Health Effects;
Consumer Perceptions;
Patterns of Use;
Potential to Induce Dependence;
Smoking Cessation;
Marketing and Communication;
Sales; and
Policies.
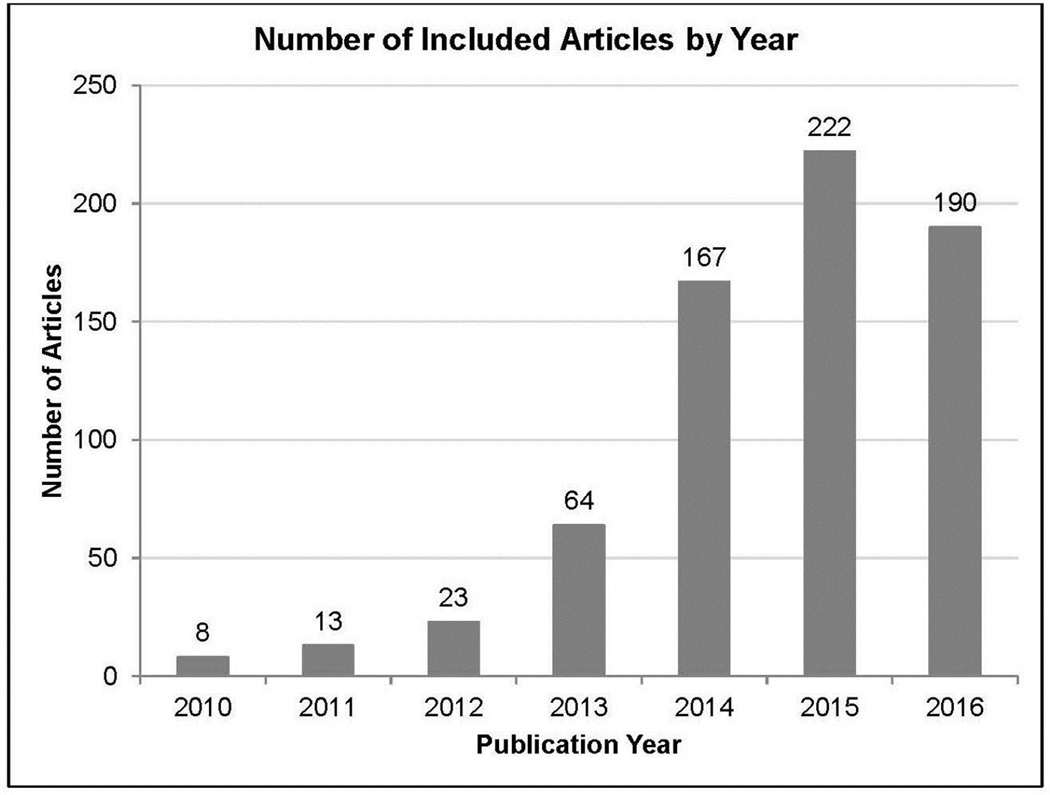
Categories 5 and 6 were added to the original protocol107 to provide information. Study quality is not presented herein, but will appear in future papers. Figure 2 depicts the number of studies published each year. Appendix Table 2 details coding of articles.
Figure 1.
Flowchart of studies included in the ENDS systematic review.
aTotal number of articles combined across categories exceeds the total number of unique articles because many fit into multiple categories.
ENDS, electronic nicotine delivery system
Figure 2.
Number of included articles by year.
a2016 accounts for publications through June 1, 2016.
Terminology
The inhalation of ENDS aerosol is referred to as “vaping,” and the inhalation of the smoke from any combustible tobacco product as “smoking.”
EVIDENCE SYNTHESIS
Of 1,634 articles identified through PubMed, 676 were included in the review (Figure 1). An additional ten studies were included through targeted searches or discussion with experts.
Product Features
There were 75 studies of ENDS products, liquids, and emissions.5,6,108–180 Ten additional studies were published on methods to analyze ENDS liquids and vapor.181–190 One study measured hazardous waste potential of ENDS disposal.191
Product performance and design
Products comprise a cartridge, heating element, and battery.6,112,156,192,193 ENDS are available in three main subtypes: disposable “cigalike,” rechargeable “cigalike,” and rechargeable vaporizers (tank or open systems). Larger ENDS devices (i.e., tank/modified) can produce blood nicotine concentrations approaching those of cigarettes, but with a slower absorption rate; higher blood nicotine levels are more common among experienced vapers.145,192–197
Liquid/vapor analysis
Mainstream and exhaled ENDS vapor contains ultrafine and fine particulate matter at similar sizes to that of smoke.108,113,121,132,139–142,148–151,162,163 Some studies found that the amount of particulate matter produced by ENDS is significantly lower than that found in smoke,113,144,148,149 whereas others found no difference or slightly higher concentrations in ENDS. Because the chemicals in vapor particles differ substantially from those in smoke, it is unclear what these results about size and volume of particulate matter imply about relative harm of ENDS vapor versus smoke.121,132,142,163
The ENDS nicotine content in liquid and vapor varies across manufacturers, devices, cartridges, and puff to puff.110–116,122,124,126,128,129,138,143,147,153,159,160,183,184 Mainstream and exhaled ENDS vapor contains nicotine113,148,150,151 generally at lower levels than in smoke,113,124,136,144,157 or at a level comparable to smoking a low-nicotine cigarette.122 One study suggested nicotine may be detected on surfaces in the home of vapers,127 but another study found no difference in deposited nicotine on surfaces between homes with vapers and homes without smokers.109
Liquids, mainstream, and exhaled ENDS vapor can contain propylene glycol,111,122,125,130,133,148,150,151,184 vegetable glycerin, additives, and flavorings.148,150,151,155 Although several potentially toxic constituents have been measured in some ENDS liquid and vapor, including tobacco-specific nitrosamines, heavy metals, and carbonyls (i.e., formaldehyde, acrolein, aldehydes), there are much fewer total constituents at much lower or trace levels when ENDS are used as intended, in non-“dry puff” conditions, than levels observed in smoke.111,113,117–120,123,125,131,134–138,144,146,148,150,151,154,157–159,161,162
Summary
There is wide variability in nicotine delivery by ENDS brand, product type, and user profile.112,126,153,159 The amount of particulate matter and the chemical composition of vapor are unclear in terms of harms. Some liquids and vapor contain some potentially toxic constituents, but in far fewer numbers and at much lower or trace levels than found in smoke.
Health Effects
There have been 116 articles that examine the impact of vaping on human health142,150,162,169,193,195,197–305 and 13 on animal health.243,306–317 Studies address physiologic and cognitive effects of vaping, adverse events associated with vaping, exposure to secondhand vapor, and cytotoxicity of ENDS. Specific human biomarkers measured are listed in Table 1.
Table 1.
Biomarkers Measured by Outcome Assessed in Published ENDS Studies
| Outcome | Biomarker |
|---|---|
| Nicotine | |
| Pulmonary | |
| Cardiovascular | |
| Cytotoxicity | |
| Other biomarkers |
|
ENDS, Electronic nicotine delivery systems
Physiologic and cognitive effects
Human exposure to some potentially harmful chemicals is significantly lower for ENDS than for cigarettes. Laboratory studies find modest increases in nicotine biomarkers after vaping.169,195,197,203,206,209–211,215,222,223,232,233,239,246,248,264,271,284,300 Vaping has no or minimal impact on other physiologic measures (i.e., exhaled carbon monoxide, complete blood count, body weight),142,202,203,230,237,239,242,257,265,272,274,276,282,285,292,303 with improvements in outcomes seen for smokers switching to ENDS, such as reduced blood pressure, improved lung function, and improved disease symptoms (i.e., asthma and chronic obstructive pulmonary disease).214,230,273,287 Some studies have examined cardiovascular measures associated with vaping,195,197,209,222,223,226,232,246,248,257,273,300,301 with the majority finding an increase in heart rate,195,223,232,246,248,300 but three finding no change after use.209,222,273 Studies measuring cognitive effects of vaping indicate some positive impacts, including improved memory and mood, consistent with a meta-analysis of acute positive benefits of nicotine318; studies also report effects related to withdrawal reversal among smokers.207,208,214
Adverse events
The total of all tobacco/nicotine exposures reported by the National Poison Data System was 1% (n=10,452) of the total calls for household substance single exposures in 2014 (N=1,002,495).266 Within this context, ENDS comprised 29.5% of all tobacco- and nicotine-related calls (n=3,910), up from 14.7% in 2013 (n=1,495). Cigarettes comprised 43.1% (n=5,714) of all tobacco- and nicotine-related calls in 2014 and 57.1% (n=5,817) in 2013.266,319 Between 2012 and 2015, ENDS accounted for 14.2% of the nicotine single exposure calls among children aged ≤5 years, compared with 60.1% from cigarette exposures and 16.4% from other tobacco product exposures.280
The Food and Drug Administration received 35 adverse event reports (respiratory symptoms, eye irritation, headache, nausea, sore throat/irritation, dizziness, racing/irregular heart rate) of passive vapor exposure between January 2012 and December 2014.258 Other studies report the most common adverse events associated with vaping as mouth and throat irritation, nausea, headache, and dry cough.193,199,202,203,214,217,232,240,249,264,265,288,303,304,320 From 2012 to 2015, there were 92 reported overheating/fire/explosion events in the U.S., and about half resulted in injuries (i.e., thermal burns, lacerations, or smoke inhalation).291
Secondhand electronic nicotine delivery system vapor exposure
Three studies have examined the individual health effects among non-smokers/vapers of exposure to secondhand ENDS vapor, one with machine-generated vapor215,216 and two via human-generated vapor.169,236 Two studies found no difference in cotinine levels following vapor and smoke exposures,215,236 whereas one study found nicotine content present in oral fluid from those exposed to vapor was much lower than from those exposed to smoke.169 One study found no differences in pulmonary function following vapor and smoke exposure,215 but white blood cell count, granulocyte count, and lymphocyte count increased significantly following smoke exposure, whereas vapor exposure did not affect complete blood count.216 Secondhand vapor studies to date show that non-users may be exposed to nicotine in ENDS vapor but the level of exposure is low, and exposure to other compounds also appears very low, or at trace or non-detectable levels when compared with secondhand smoke. It is unclear if any levels are sufficient to be of biological concern to humans. More-definitive studies are needed before conclusions about harm can be made.321,322
Cytotoxicity
In cellular studies, exposure to ENDS vapor increased anti-inflammatory processes,275,278 placed oxidative stress on exposed cells,269,279,294,301 and increased cell apoptosis and necrosis.305 Particular ENDS flavors are more cytotoxic than others, but generally most studied ENDS liquids are much less cytotoxic than cigarette smoke extract.213,228,238,250,254,260,295,296,308 In cytotoxicity studies, cinnamon flavor in ENDS was found to be the most toxic (i.e., inhibiting cell survival or inducing cell stress/morphologic change) when comparing flavors.198,247 Most studies find that nicotine levels are not correlated with cytotoxicity.198,213,238,255,260,312 Two studies examined the effect of vegetable glycerin and propylene glycol, frequently found in e-liquids, on various human and animal cells and found that they were not cytotoxic for any cell type.198,297
Animal models
Studies performed in animal models show that exposure to ENDS may have some physiologic effects (i.e., reduced weight, oxidative stress, neurobiological changes),243,306,310,312–317 yet these effects are less substantial than those caused by exposure to cigarettes.243,307,310,312–317 Attempts to generalize from animal to human effects are premature without further research on human samples.
Summary
Studies on the health effects of vaping indicate no or minimal impact on physiologic biomarkers and some possible acute positive effects on cognition and mood regulation. Adverse events reported by vapers are generally mild and resolve, though there have been serious adverse events reported in some cases. There have been a greater number of poison control center calls related to nicotine exposures from ENDS in recent years, including exposures among children. Calls related to cigarette exposures—where smoking is much more prevalent than vaping—remain more common. Secondhand vape studies show that non-users may be exposed to nicotine in vapor, but at lower levels than when exposed to smoke.
Consumer Perceptions
One hundred eighty-eight articles have addressed consumer perceptions of ENDS.145,192–194,214,241,288,299,320,323–505
Awareness
Awareness of ENDS has increased among U.S. adults since 2009,323,339,362,378,384,386,388,391,394,395,440 with 86.4% of the U.S. population aware of these products in 2013.440 Awareness is highest among younger age groups, non-Hispanic white populations, and those with higher income and education.323,370,391,440 Most healthcare providers are aware of ENDS.334,336,377,458 Awareness of ENDS has increased in other countries over time.326,333,337,356,359,369,374,380,381,396,414,451,452,460,475,483,494
Product perceptions
Generally, ENDS are perceived to be less harmful than cigarettes regardless of the respondent’s tobacco use status.192–194,214,288,325–327,332,335,336,338,353,354,361,369,372,374,382,384,389,390,446,448,449,453–457,461–464,467,471–474,476,477,484,485,488,490,492,493,495,502,503 In the U.S. and Great Britain, this belief has eroded over time with more individuals mistakenly believing ENDS are as harmful or more harmful that cigarettes.391,414 Studies suggest that ENDS are also perceived as less addictive than cigarettes,367,375,403,446 tools that may help users reduce or quit smoking,320,327,330,338,357,374,448,453,455,458,461,464,467,481,483,485,486,488,493,495,499 less expensive than cigarettes,335,338,447,455,456,472,499 and more convenient or easier to use than cigarettes.335,338,357,400,499
Common ENDS concerns include the lack of research on long-term use,397,399,436 absence of regulation,397,399 potential harms of use,443,464 potential to undermine other tobacco control measures,399,401,480 and social stigma.299,402,464,467,477
Reasons for use
The most commonly cited reasons for use by vapers include:
to address tobacco craving/withdrawal symptoms194,320,353 as reported by 67%–79% of adults194 and as a smoking-reduction/cessation aid,192,193,214,241,323,324,326,332,337,338,347,350,353,358,369,372,375,376,381,382,389,393,395,421,422,426,432,434,441,444,456,459,461,463,472,482,502 which was reported by 55%–85% of adults in nationally representative samples323,350,395;
to evade smokefree policies or avoid disturbing people with secondhand smoke,192,193,214,323,324,332,337,338,341,345,347,350,353,358,369,372,374,376,387,389,395,421,426,432,434,441,455,456,461,472,478,502 as reported by 45%–85% of adults in nationally representative samples323,350,395; and
because they are perceived as less harmful/less toxic than cigarettes192,194,323,324,326,337,338,341,345–347,350,353,358,372,374,375,382,395,422,434,455,456,459,461,472,502 by 45%–75% of adults in nationally representative samples.323,350,395
Users also report using ENDS because they are less expensive than cigarettes,145,194,332,337,338,341,350,353,358,372,389,395,455,456,459,472,502 for relapse prevention,194,353,372 out of curiosity,341,347,350,358,421,422,432,434,441,444,456,461,476 because they are accessible and convenient,341,366,387,477 for social reasons,341,347,363,387,434,444,461,502,504 and because they taste337,358,426,472,477,496 or smell better than cigarettes.341,353,389,459,461 Flavors are viewed as an attractive characteristic of ENDS,345,433 and are a cited reason for vaping.332,341,350,456,466,477
Summary
Awareness of ENDS is high, and most users report using them to aid in smoking cessation, to evade smokefree policies, or because they are perceived as less harmful. Beliefs that ENDS are less harmful than cigarettes have lessened over time.
Patterns of Use
Two hundred and fifty articles153,192–194,197,203,239,265,271,304,323–326,329,332,333,335–339,341–344,346,348,350,351,353–362,365,367–369,372,374–376,378–381,384,388,390,393–396,404–406,408,414–416,418,420–422,425,426,432,434,436,440,441,444–450,453–456,459–462,465,468,472,475,476,478,488,489,491,494,496–498,500,502,504,506–646 have been published on vaping patterns.
Electronic nicotine delivery system use in the U.S
National cross-sectional data indicate that ENDS ever and past 30–day use in the U.S. has increased over time, particularly among youth from 2010 to 2014 (Table 2). This is also true for studies based outside of the U.S.329,528,543,592,613,647 However, past 30–day ENDS use among U.S. youth did not change significantly from 2014 to 2015, indicating a slowing in prevalence as reported in both the National Youth Tobacco Survey and the Monitoring the Future survey.640,648 The 2014 National Health Interview Survey showed that ever use of ENDS among young adults aged 18–24 years in the U.S. in 2014 was 21.6%, whereas “every day” or “some days” use in the same age group was 5.1%.610,639 These data also show that ever use and “some days” or “every day” use of ENDS among all adults was 12.6% and 3.7%, respectively, in 2014.610,639 Although young adults were significantly more likely to report ever use of ENDS than older adults, they were not more likely than older adults to be using ENDS “some days” or “every day.”610
Table 2.
Prevalence of Ever Use and Past 30-Day Use of ENDS (2011–2015)
| 2011 | 2012 | 2013 | 2014 | 2015 | |
|---|---|---|---|---|---|
| Ever use of ENDS | |||||
| Youth | 3.3%526 | 6.8%394,526 | 8.0%a | 19.8%a | b |
| Middle school students | 1.4%526 | 2.7%526 | 3.0%509 | 10.1%a | b |
| High school students | 4.7%526 | 10.0%526 | 11.9%509 | 27.3%a | b |
| Young adults (aged 18–24) | 6.9%339 | 4.1%339 | 7.8%339 | 21.6%639 | b |
| Adults (aged 18+) | 6.2%339 | 8.1%339,343 | 8.5%339 | 12.6%610–14.1%601 | b |
| Past 30-day use of ENDS | |||||
| Youth | 1.1%526 | 2.1%394,526 | 3.1%a | 9.3%a–13.9%811 | 13.2%648 |
| Middle school students | 0.6%521,526 | 1.1%521,526 | 1.1%509 | 3.9%510 | 5.3%640 |
| High school students | 1.5%521,526 | 2.8%521,526 | 4.5%509 | 13.4%510–17.1%811 | 16.0%640 |
| Young adults (aged 18–24) | c, d | d | 0.9%339 | b | b |
| Adults (aged 18+) | 1.3%339 | d | 1.9%339 | 4.8%601 | b |
Data not reported in recent Centers for Disease Control and Prevention reports or in the published literature but are publicly available.
Data not yet available in the published literature.
Sample size too small to estimate past 30-day ENDS in young adults before 2012–2013.
Annual estimates unavailable; estimates were collapsed over two-year intervals (2010–2011 and 2012–2013) due to small sample size.
ENDS, electronic nicotine delivery systems
Use of ENDS varies by smoking status, with the majority of users being current smokers, followed by former smokers; never smokers are least likely to have tried ENDS.323,333,336–339,342,344,346,348,350,351,354–357,359,361,369,380,381,384,395,396,421,455,461,472,504,527,531,541,543,551,552,561,568,575,577–579,582,584,590 National Health Interview Survey data show that recent former smokers (quit <1 year ago) were more likely to report using ENDS “every day” or “some days” (22.0%) than adults who had never smoked (0.4%), former smokers who had quit for ≥1 year (2.3%), or former smokers who quit cigarettes ≥4 years ago (0.8%).610,639 Studies show the use of ENDS is correlated with use of other tobacco products,325,362,394,468,509,517,520,524,557,558,584,585,621,649 marijuana,325,432,496,512,523,539,559,571,592,593,609,642 and alcohol.325,432,496,497,512,523,539,549,559,563,571,572,584,590,592,593,609,650
Frequency of electronic nicotine delivery system use
There are an increasing number of studies characterizing frequency of ENDS use, with most studies indicating ENDS are used infrequently (i.e., 1–2 days per month or “rarely”) by the majority of users,335,350,381,395,507,590,593,601,643 and more frequent (e.g., daily) use is higher among recent quitters395,610 and current smokers.326,335,374,395,404,422,514,536,541,569 According to National Health Interview Survey data, recent quitters (<1 year) were four times more likely to be daily ENDS users than current smokers (13% vs 3.5%).610 In 2014, the National Youth Tobacco Survey added measures of frequency of use in the past 30 days and found that of the 13.4% of high school students reporting any past 30–day ENDS use, 45.4%% (or 6.1% of the population) had tried ENDS on 1–2 days, 16.2% (2.2% of the population) on 3–5 days, 12.0% (1.6% of the population) on 6–9 days, 10.9% (1.5% of the population) on 10–19 days, 5.8% (0.8% of the population) on 20–29 days, and 9.7% (1.3% of the population) used ENDS all 30 days.510,633 Of the 3.9% of middle school students reporting past 30–day ENDS use, over half (54.5%, or 2.1% of the population) had used ENDS on 1–2 days.510,633
Topography
Ten studies examined ENDS user topography.153,197,239,271,513,538,556,564,580,583 Puff duration197,538 and volume197,239 are higher for ENDS products than for cigarettes, but flow rate197 (among experienced vapers) and puff counts239 (upon initial use in naïve vapers) have been found to be lower than for cigarettes. Higher liquid nicotine concentration has been associated with shorter puffs,271,564 but puff velocity shows no effect on nicotine yield.153 There is significant intersubject variability in puff behavior,583 suggesting more research is needed to determine the impact of user topography on vapor production and nicotine intake.
Summary
Because ENDS is a new and potentially disruptive technology,4 it is expected that ENDS use has increased in all age groups since their introduction and up until at least 2014. The rate of increase among youth has recently slowed or flattened in 2015. ENDS use is most common among current and recent former smokers, whereas prevalence is low among never smokers and long-term former smokers. Most past 30–day ENDS use among youth in the U.S. consists of use on 1–2 days in the past month. Daily ENDS users are most likely to be recent former smokers.
Potential to Induce Dependence
The potential of ENDS to induce dependence is considered to be primarily a function of the products’ ability to quickly deliver a rapid and adequate dose of nicotine to the brain of the user,651 but cigarette smoking is still the most rapid method of nicotine delivery.651,652 Twenty-eight studies have examined nicotine biomarkers resulting from vaping.195–197,199,203,206,209,211,215,221–223,225,232,239,246,300,303,335,529,564,580,615,653–657 Nicotine delivery is dependent on characteristics of ENDS devices and liquids, such as battery size, device type, propylene glycol/vegetable glycerin ratio, and nicotine liquid concentration, as well as individual user differences (i.e., naïve or not naïve).195,196,199,206,246,264,271,300,513,538,564,580,656,657 Five clinical laboratory reports of experienced vapers indicated ten puffs of nicotine-containing ENDS reliably increased plasma nicotine within 5–10 minutes195–197,223,580 in all but one report,580 in which plasma nicotine levels significantly lower and reached a peak more slowly than that achieved with ten puffs from a cigarette. Recent assessments with second-generation devices demonstrate that a plasma nicotine concentration similar to that of cigarettes can be achieved after vaping, dependent upon the user’s puff topography197 or ENDS liquid nicotine concentration.564,580 Whether such levels are routinely achieved by most vapers is unclear.
The immediate reinforcing subjective effects of using a drug that are substantially but not solely a function of dose and speed of nicotine delivery also influence its ability to induce dependence and elicit repeated use.651,652 Twenty-four studies have examined the subjective effects of vaping.193,195–197,199,203,206–209,211,221–223,225,232,239,264,300,303,328,330,335,360,427,429,529,540,653,654,658–660 These studies indicate vaping decreases adverse symptoms related to smoking abstinence (e.g., craving/urges to smoke, irritability)197,199,206–208,222,223,330,360,540,659 and increases ratings of satisfaction/pleasantness.195,197,199,222,223,225,335 Though some smokers find ENDS less reinforcing195 and satisfying than cigarettes,239,344 second-generation devices are more satisfying than first-generation devices.330
There is only one proposed measure of ENDS dependence540,661; however, studies have used many approaches to assessing ENDS dependence and drawing comparisons between products, such as adapting existing scales for cigarette or nicotine dependence or measuring perceived dependence. Approximately one third of former smokers who are daily vapers perceive their dependence on ENDS to be as strong or stronger than their previous dependence on cigarettes.193,360,446,662 These studies suggest that the current class of ENDS products may have significantly lower ability to induce dependence than cigarettes, but are capable of inducing some level of satisfaction and dependence, especially when using second-generation (e.g., tank or mod devices with adequate concentrations of nicotine e-liquid/juice).
Smoking Cessation
A key question regarding ENDS is their potential role in facilitating smoking abstinence or meaningful smoking reduction. Included study designs and outcomes relevant to smoking cessation and vaping are presented in Appendix Table 3.
Four RCTs show that ENDS are effective in helping some adult smokers to quit or to reduce their cigarette consumption.203,653,663–665 In the studies that assessed smoking cessation, rates of cessation in the ENDS study groups were similar to or higher than rates of cessation seen in previous clinical trials of nicotine-replacement therapy (NRT).666 Some prospective studies with loosely defined comparison groups report that vaping may be associated with no change or negative correlations with cessation.323,393,522,667–670 This stands in contrast to other studies with more-precise measures of how ENDS were used (e.g. duration of use, type of device, use specifically for cessation), which suggest that regular, more intensive vaping can facilitate quit attempts and cessation.252,514,516,548 Many longitudinal studies without comparison groups,202,232,241,287,288,409,486,536,574,658,671–675 and cross-sectional studies,193,374,375,398,454,537,588,676–678 suggest that ENDS can help some adult smokers quit or reduce smoking.350,395,555,575,679
The conclusions from the longitudinal323,393,522,555,575,577,667,669,670,679,680 and cross-sectional studies350,395,463,532,555,575,679,681 reporting negative correlations between those who tried ENDS and smoking cessation have serious limitations, including: selection bias (e.g., smokers who quit by using ENDS were excluded from the sample), inadequate measures of exposure (e.g., ever use in one’s lifetime) to test for a cessation indication, and confounders (e.g., smokers who have repeatedly failed to quit are more likely to try ENDS).668,682,683 This is similar to studies of NRT and smoking cessation in which some observational studies showed negative correlations,684,685 whereas >80 RCTs of NRT show strong positive cessation effects.686 Observational studies with more-robust measures of how ENDS were used (e.g., duration of use, type of device, use for cessation) suggest ENDS can facilitate quit attempts and cessation.514,516,548,677 More research—especially independent, high-quality RCTs with appropriate measures and control groups—is needed to further determine whether and how ENDS can be an effective cigarette-cessation or - reduction aids.
Marketing and Communication
There have been 74 articles on the marketing and communication of ENDS products.348,357,370,403,410,424,433,438,451,471,475,477,499,506,629,687–746
Among noncombustible tobacco products, ENDS advertisements are the most widely circulated.699 Individuals aware of ENDS report the most common product exposures are through in-person communications, by seeing them at the point of sale, and through online and TV advertisements.324,357,395,471,475,717–719,735,742 Conventions provide manufacturers an opportunity to promote and introduce new products through free samples, celebrity appearances, and branded merchandise giveaways.732,746 ENDS are promoted heavily online451,695 through ENDS company–sponsored advertisements697 and users’ social media profiles,424 with occurrences on YouTube690,697,706,707,710,722,731,739 and Twitter.691,713,715,725–727,733,743 Youth and young adult exposure to TV advertisements for ENDS has increased since 2011.689,735 Two RCTs712,720 and four cross-sectional studies403,629,723,741 have shown that tobacco marketing exposure may promote ENDS uptake.348,403,712,720 Exposure to industry and ingredient warnings is associated with lower odds of intent to purchase ENDS.714,734,747 Several studies have reported the presence of interior and exterior ENDS advertisements at tobacco retail outlets,702,704,705,729 including one study which found that the ENDS advertisements featured flavored products at the eye level of children.705 The total expenditure for ENDS advertisements across all media channels is increasing annually,748,749 with a 52% increase from 2013 to 2014.724 Blu eCigs led in total advertisement expenditure until Altria’s MarkTen entered the national market in 2014.724 Online ENDS advertisements do not account for a large portion of this financial investment.750 However, this expenditure information is outdated and there is no established method to assess true advertisement expenditures in the current media environment.
Commonly marketed as alternatives to cigarettes,111,708,751–755 ENDS advertisements often make claims, such as being an effective smoking-cessation aid.5,111,555,709,753 The most common claims advertise ENDS as a healthier alternative to cigarettes,111,433,708,709,729,753 and a way to circumvent smoking bans.708,751,752,754 Advertisements also highlight celebrity use to appeal to youth.708,753
Sales
There have been 30 articles addressing the sales of ENDS products.44,398,424,438,471,472,696,701,704,715,729,730,756–774 The ENDS market is expanding701,756 and accessible to consumers through internet vendors472,729,769,771 and in most tobacco outlets.472,704,729,730,758,759 Field observations of tobacco retail outlets have found that more than half of tobacco retailers sell ENDS.438,729,730,762 One study found no significant relationship between retail availability and neighborhood demographics,704 but another study indicated a greater likelihood of ENDS retailers in communities with higher median incomes.758 One cross-sectional survey of adults found that frequent (weekly/daily) vapers were significantly more likely to purchase over the Internet than infrequent (monthly or less) vapers.472 Studies715,763,772 assessing online retailers found inadequate age verification methods, with one study reporting a 93.7% rate of successful youth purchases without age verification.763 ENDS products can be sold at an estimated 200%–400% markup in vape shops.696 Information is limited on the impact of pricing on ENDS sales, with one study indicating that vapers are two to three times more sensitive to price than smokers,757 and several studies finding that ENDS are substitutes for cigarettes as cigarette prices increase.761,770,773
Policy
There have been 51 articles focused on ENDS and policy. Eleven studies described ENDS policies enacted at the local,775,776 state,776–780 and country level781–784; others addressed proposed city policies,785 university policies,786 public and stakeholder opinion of ENDS policies,334,340,343,351,354,357,374,392,398,399,443,452,469,484,485,717,787–794 the unmet research needs of state and community tobacco control practitioners regarding ENDS,795 implementation and enforcement of ENDS policies in hospitals,796 and policies related to vaping on American transit systems.797
Seven studies examined the impact of policies (e.g., smokefree indoor air laws) on interest,798,799 demand,757,800 or use of ENDS.624,801,802 One study found that in the event of a menthol cigarette ban, 15.1% of menthol smokers would switch to menthol ENDS.802 Four studies examined the impact of state-level ENDS policies803,804 and potential areas of ENDS regulation747,805,806 on tobacco use, suggesting that a ban on ENDS may increase demand for cigarettes.
DISCUSSION
This review highlights several major findings. First, ENDS are a heterogeneous and evolving product category, with variation in physical factors that can influence vapor production (e.g., tank style, battery power, temperature). E-liquids contain various combinations of nicotine, flavors, and carriers. These factors affect nicotine delivery, appeal, and ease of product use and underscore the degree to which individual preferences may play a role in use patterns. Second, detectable levels of some potential toxicants have been found in ENDS liquids and vapor, but studies of actual human exposures are few. Although it is difficult to estimate the precise difference in harmful exposure of vaping compared to smoking, experts have concluded that ENDS are substantially less harmful than cigarettes267 and toxicant exposures are derived both from far fewer chemicals and at much lower levels or trace levels, estimated to be one fourth to one ninety-fifth those of cigarette smoke.125,237,321,322 Third, ENDS can produce mild adverse reactions (e.g., irritation, nausea) in some users; poisonings via misuse and unintended exposure have also been documented.266 Fourth, as expected for a novel product, vaping experimentation increased rapidly since being introduced in the U.S.509,510,610,807–809 and abroad,329,369,414,528,543,647,810 particularly among youth, but in 2015 in the U.S. use appears to be leveling off. ENDS uptake trends have coincided with significant reductions in smoking prevalence to record lows among youth and adults.509,510,807–809 The majority of vaping in all age groups occurs among current smokers and recent studies also show that the prevalence of daily vaping is very low in both youth and adults.404,610,633 Fifth, RCTs and population-based studies with more-precise exposure measures show that ENDS are at least as effective as NRT in helping some smokers to quit or reduce their smoking514,516,548,610 and may reach more smokers at scale than NRT.1,321,322 Finally, various ENDS policies (e.g., inclusion in smokefree indoor air) have been implemented in many jurisdictions,779 but there has been little evaluation of the impact of these policies on behavior.
Limitations
A strength of this review is its synthesis of a broad range of study designs and outcomes related to ENDS. Limitations include that eligible studies were restricted to those that are peer reviewed, indexed in PubMed, and available in English language. The authors did not quantify the risk of bias of the evidence given the size and heterogeneity of the sample of included studies. Though bias is possible, the authors used a process for identifying studies categorized under multiple outcomes and regular team communication to reduce bias in data extraction. The summary of studies on patterns of ENDS use is limited to U.S. data; however, a manuscript is in progress to synthesize these studies in depth, including studies conducted in other countries and other topics relevant to vaping patterns (e.g., devices, flavors, and brands).
CONCLUSIONS
There are a number of factors to consider when synthesizing the results. First, ENDS products are highly variable. A standardized method is needed to characterize products with respect to nicotine and toxicant delivery and their potential harms, both relative to smoking and relative to no use (absolute harm). Second, the field lacks consistent definitions of types of users and patterns of use, which complicates the interpretation of research findings. Third, many studies have small sample or cell sizes and employ convenience samples, raising concerns about selection bias, unmeasured confounders, low statistical power, and limited generalizability to draw firm national public health or policy conclusions. Fourth, there exist gaps in the current evidence base, including longitudinal data and data on reasons for vaping and use trajectories (including polytobacco use and use of cessation aids) that may help to explain population impacts and changing trends.8 Fifth, caution must be exercised when drawing conclusions from in vitro, or cellular, studies because effects on cells cannot be readily extrapolated to human harms.
Supplementary Material
Acknowledgments
Financial support for this study was provided by The Schroeder Institute at Truth Initiative, the Robert Wood Johnson Foundation (Grant ID: 72208 and 72390), and a NIH K01 Career Development Award in Tobacco Control Regulatory Research (Principal Investigator, Pearson; 1K01DA037950-01). The funding agreement ensured the authors’ independence in designing the study, interpreting the data, writing, and publishing the report. All authors are employed by Truth Initiative. The authors would like to acknowledge Shyanika Rose, Caroline Cobb, Shari Feirman, Ollie Ganz, and Lyubov Teplitskaya for their help in extracting data for this review.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No financial disclosures were reported by the authors of this paper.
REFERENCES
- 1.Cobb NK, Abrams DB. The FDA, e-cigarettes, and the demise of combusted tobacco. N Engl J Med. 2014;371(16):1469–1471. doi: 10.1056/NEJMp1408448. http://dx.doi.org/10.1056/NEJMp1408448. [DOI] [PubMed] [Google Scholar]
- 2.Fiore MC, Schroeder SA, Baker TB. Smoke, the chief killer--strategies for targeting combustible tobacco use. N Engl J Med. 2014;370(4):297–299. doi: 10.1056/NEJMp1314942. http://dx.doi.org/10.1056/NEJMp1314942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fagerstrom K, Eissenberg T. Dependence on tobacco and nicotine products: a case for product-specific assessment. Nicotine Tob Res. 2012;14(11):1382–1390. doi: 10.1093/ntr/nts007. http://dx.doi.org/10.1093/ntr/nts007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abrams DB. Promise and peril of e-cigarettes: can disruptive technology make cigarettes obsolete? JAMA. 2014;311(2):135–136. doi: 10.1001/jama.2013.285347. http://dx.doi.org/10.1001/jama.2013.285347. [DOI] [PubMed] [Google Scholar]
- 5.Trtchounian A, Williams M, Talbot P. Conventional and electronic cigarettes (ecigarettes) have different smoking characteristics. Nicotine Tob Res. 2010;12(9):905–912. doi: 10.1093/ntr/ntq114. http://dx.doi.org/10.1093/ntr/ntq114. [DOI] [PubMed] [Google Scholar]
- 6.Williams M, Talbot P. Variability among electronic cigarettes in the pressure drop, airflow rate, and aerosol production. Nicotine Tob Res. 2011;13(12):1276–1283. doi: 10.1093/ntr/ntr164. http://dx.doi.org/10.1093/ntr/ntr164. [DOI] [PubMed] [Google Scholar]
- 7.Food and Drug Administration. Deeming Tobacco Products To Be Subject to the Federal Food, Drug, and Cosmetic Act, as Amended by the Family Smoking Prevention and Tobacco Control Act; Restrictions on the Sale and Distribution of Tobacco Products and Required Warning Statements for Tobacco Products. In: Food and Drug Administration H, editor. [Docket No FDA-2014-N-0189]. Vol RIN 0910-AG38. Final Rule ed2016. [PubMed] [Google Scholar]
- 8.Levy DT, Cummings KM, Villanti AC, et al. A framework for evaluating the public health impact of e-cigarettes and other vaporized nicotine products. Addiction. doi: 10.1111/add.13394. In press. Online April 25, 2016. http://dx.doi.org/10.1111/add.13394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Villanti AC, Vargyas EJ, Niaura RS, Beck SE, Pearson JL, Abrams DB. Food and Drug Administration regulation of tobacco: integrating science, law, policy, and advocacy. Am J Public Health. 2011;101(7):1160–1162. doi: 10.2105/AJPH.2011.300229. http://dx.doi.org/10.2105/AJPH.2011.300229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Breland AB, Spindle T, Weaver M, Eissenberg T. Science and electronic cigarettes: current data, future needs. J Addict Med. 2014;8(4):223–233. doi: 10.1097/ADM.0000000000000049. http://dx.doi.org/10.1097/ADM.0000000000000049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grana RA, Ling PM, Benowitz N, Glantz S. Electronic cigarettes. Cardiology patient page. Circulation. 2014;129(19):e490–e492. doi: 10.1161/CIRCULATIONAHA.114.008545. http://dx.doi.org/10.1161/CIRCULATIONAHA.114.008545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hajek P. Electronic cigarettes have a potential for huge public health benefit. BMC Med. 2014;12:225. doi: 10.1186/s12916-014-0225-z. http://dx.doi.org/10.1186/s12916-014-0225-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Palazzolo DL. Electronic Cigarettes and Vaping: A New Challenge in Clinical Medicine and Public Health. A Literature Review. Front Public Health. 2013;1:56. doi: 10.3389/fpubh.2013.00056. http://dx.doi.org/10.3389/fpubh.2013.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Riker CA, Lee K, Darville A, Hahn EJ. E-cigarettes: promise or peril? Nurs Clin North Am. 2012;47(1):159–171. doi: 10.1016/j.cnur.2011.10.002. http://dx.doi.org/10.1016/j.cnur.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 15.Alawsi F, Nour R, Prabhu S. Are e-cigarettes a gateway to smoking or a pathway to quitting? Br Dent J. 2015;219(3):111–115. doi: 10.1038/sj.bdj.2015.591. http://dx.doi.org/10.1038/sj.bdj.2015.591. [DOI] [PubMed] [Google Scholar]
- 16.Born H, Persky M, Kraus DH, Peng R, Amin MR, Branski RC. Electronic Cigarettes: A Primer for Clinicians. Otolaryngol Head Neck Surg. 2015;153(1):5–14. doi: 10.1177/0194599815585752. http://dx.doi.org/10.1177/0194599815585752. [DOI] [PubMed] [Google Scholar]
- 17.Collaco JM, Drummond MB, McGrath-Morrow SA. Electronic cigarette use and exposure in the pediatric population. JAMA Pediatr. 2015;169(2):177–182. doi: 10.1001/jamapediatrics.2014.2898. http://dx.doi.org/10.1001/jamapediatrics.2014.2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Orellana-Barrios MA, Payne D, Mulkey Z, Nugent K. Electronic Cigarettes-A Narrative Review for Clinicians. Am J Med. 2015;128(7):674–681. doi: 10.1016/j.amjmed.2015.01.033. http://dx.doi.org/10.1016/j.amjmed.2015.01.033. [DOI] [PubMed] [Google Scholar]
- 19.Sanford Z, Goebel L. E-cigarettes: an up to date review and discussion of the controversy. W V Med J. 2014;110(4):10–15. [PubMed] [Google Scholar]
- 20.Breland A, Soule E, Lopez A, Ramoa C, El-Hellani A, Eissenberg T. Electronic cigarettes: what are they and what do they do? Ann N Y Acad Sci. doi: 10.1111/nyas.12977. In press. Online January 15, 2016. http://dx.doi.org/10.1111/nyas.12977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Franck C, Filion KB, Kimmelman J, Grad R, Eisenberg MJ. Ethical considerations of e-cigarette use for tobacco harm reduction. Respir Res. 2016;17(1):53. doi: 10.1186/s12931-016-0370-3. http://dx.doi.org/10.1186/s12931-016-0370-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burstyn I. Peering through the mist: systematic review of what the chemistry of contaminants in electronic cigarettes tells us about health risks. BMC Public Health. 2014;14(1):18. doi: 10.1186/1471-2458-14-18. http://dx.doi.org/10.1186/1471-2458-14-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cahn Z, Siegel M. Electronic cigarettes as a harm reduction strategy for tobacco control: a step forward or a repeat of past mistakes? J Public Health Policy. 2011;32(1):16–31. doi: 10.1057/jphp.2010.41. http://dx.doi.org/10.1057/jphp.2010.41. [DOI] [PubMed] [Google Scholar]
- 24.Callahan-Lyon P. Electronic cigarettes: human health effects. Tob Control. 2014;23(Suppl 2):ii36–ii40. doi: 10.1136/tobaccocontrol-2013-051470. http://dx.doi.org/10.1136/tobaccocontrol-2013-051470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang H. Research gaps related to the environmental impacts of electronic cigarettes. Tob Control. 2014;23(Suppl 2):ii54–ii58. doi: 10.1136/tobaccocontrol-2013-051480. http://dx.doi.org/10.1136/tobaccocontrol-2013-051480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Durmowicz EL. The impact of electronic cigarettes on the paediatric population. Tob Control. 2014;23(Suppl 2):ii41–ii46. doi: 10.1136/tobaccocontrol-2013-051468. http://dx.doi.org/10.1136/tobaccocontrol-2013-051468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Farsalinos KE, Polosa R. Safety evaluation and risk assessment of electronic cigarettes as tobacco cigarette substitutes: a systematic review. Ther Adv Drug Saf. 2014;5(2):67–86. doi: 10.1177/2042098614524430. http://dx.doi.org/10.1177/2042098614524430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gualano MR, Passi S, Bert F, La Torre G, Scaioli G, Siliquini R. Electronic cigarettes: assessing the efficacy and the adverse effects through a systematic review of published studies. J Public Health (Oxf) 2015;37(3):488–497. doi: 10.1093/pubmed/fdu055. http://dx.doi.org/10.1093/pubmed/fdu055. [DOI] [PubMed] [Google Scholar]
- 29.Knorst MM, Benedetto IG, Hoffmeister MC, Gazzana MB. The electronic cigarette: the new cigarette of the 21st century? J Bras Pneumol. 2014;40(5):564–572. doi: 10.1590/S1806-37132014000500013. http://dx.doi.org/10.1590/S1806-37132014000500013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lippi G, Favaloro EJ, Meschi T, Mattiuzzi C, Borghi L, Cervellin G. E-cigarettes and cardiovascular risk: beyond science and mysticism. Semin Thromb Hemost. 2014;40(1):60–65. doi: 10.1055/s-0033-1363468. http://dx.doi.org/10.1055/s-0033-1363468. [DOI] [PubMed] [Google Scholar]
- 31.Middlekauff HR, Park J, Moheimani RS. Adverse Effects of Cigarette and Noncigarette Smoke Exposure on the Autonomic Nervous System: Mechanisms and Implications for Cardiovascular Risk. J Am Coll Cardiol. 2014;64(16):1740–1750. doi: 10.1016/j.jacc.2014.06.1201. http://dx.doi.org/10.1016/j.jacc.2014.06.1201. [DOI] [PubMed] [Google Scholar]
- 32.Oh AY, Kacker A. Do electronic cigarettes impart a lower potential disease burden than conventional tobacco cigarettes?: Review on e-cigarette vapor versus tobacco smoke. Laryngoscope. 2014;124(12):2702–2706. doi: 10.1002/lary.24750. http://dx.doi.org/10.1002/lary.24750. [DOI] [PubMed] [Google Scholar]
- 33.Orr MS. Electronic cigarettes in the USA: a summary of available toxicology data and suggestions for the future. Tob Control. 2014;23(Suppl 2):ii18–ii22. doi: 10.1136/tobaccocontrol-2013-051474. http://dx.doi.org/10.1136/tobaccocontrol-2013-051474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pisinger C, Dossing M. A systematic review of health effects of electronic cigarettes. Prev Med. 2014;69c:248–260. doi: 10.1016/j.ypmed.2014.10.009. http://dx.doi.org/10.1016/j.ypmed.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 35.Schivo M, Avdalovic MV, Murin S. Non-cigarette tobacco and the lung. Clin Rev Allergy Immunol. 2014;46(1):34–53. doi: 10.1007/s12016-013-8372-0. http://dx.doi.org/10.1007/s12016-013-8372-0. [DOI] [PubMed] [Google Scholar]
- 36.Schroeder MJ, Hoffman AC. Electronic cigarettes and nicotine clinical pharmacology. Tob Control. 2014;23(Suppl 2):ii30–ii35. doi: 10.1136/tobaccocontrol-2013-051469. http://dx.doi.org/10.1136/tobaccocontrol-2013-051469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith JE. Electronic cigarettes: a safer alternative or potential poison? Home Healthc Nurse. 2014;32(9):532–535. doi: 10.1097/NHH.0000000000000138. http://dx.doi.org/10.1097/NHH.0000000000000138. [DOI] [PubMed] [Google Scholar]
- 38.Suter MA, Mastrobattista J, Sachs M, Aagaard K. Is there evidence for potential harm of electronic cigarette use in pregnancy? Birth Defects Res A Clin Mol Teratol. 2015;103(3):186–195. doi: 10.1002/bdra.23333. http://dx.doi.org/10.1002/bdra.23333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang L, Rudy SF, Cheng JM, Durmowicz EL. Electronic cigarettes: incorporating human factors engineering into risk assessments. Tob Control. 2014;23(Suppl 2):ii47–ii53. doi: 10.1136/tobaccocontrol-2013-051479. http://dx.doi.org/10.1136/tobaccocontrol-2013-051479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bertholon JF, Becquemin MH, Annesi-Maesano I, Dautzenberg B. Electronic Cigarettes: A Short Review. Respiration. 2013;86(5):433–438. doi: 10.1159/000353253. http://dx.doi.org/10.1159/000353253. [DOI] [PubMed] [Google Scholar]
- 41.Cooke A, Fergeson J, Bulkhi A, Casale TB. The Electronic Cigarette: The Good, the Bad, and the Ugly. J Allergy Clin Immunol Pract. 2015;3(4):498–505. doi: 10.1016/j.jaip.2015.05.022. http://dx.doi.org/10.1016/j.jaip.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 42.Drummond MB, Upson D. Electronic cigarettes. Potential harms and benefits. Ann Am Thorac Soc. 2014;11(2):236–242. doi: 10.1513/AnnalsATS.201311-391FR. http://dx.doi.org/10.1513/AnnalsATS.201311-391FR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Etter JF, Bullen C, Flouris AD, Laugesen M, Eissenberg T. Electronic nicotine delivery systems: a research agenda. Tob Control. 2011;20(3):243–248. doi: 10.1136/tc.2010.042168. http://dx.doi.org/10.1136/tc.2010.042168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim AE, Loomis B, Rhodes B, Eggers ME, Liedtke C, Porter L. Identifying e-cigarette vape stores: description of an online search methodology. Tob Control. 2016;25(1):e19–e23. doi: 10.1136/tobaccocontrol-2015-052270. http://dx.doi.org/10.1136/tobaccocontrol-2015-052270. [DOI] [PubMed] [Google Scholar]
- 45.Lopez AA, Eissenberg T. Science and the evolving electronic cigarette. Prev Med. 2015;80:101–106. doi: 10.1016/j.ypmed.2015.07.006. http://dx.doi.org/10.1016/j.ypmed.2015.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meo SA, Al Asiri SA. Effects of electronic cigarette smoking on human health. Eur Rev Med Pharmacol Sci. 2014;18(21):3315–3319. [PubMed] [Google Scholar]
- 47.Nowak D, Gohlke H, Hering T, et al. Position Paper of the German Respiratory Society (DGP) on Electronic Cigarettes (E-Cigarettes) in Cooperation with the following Scientific Societies and Organisations: BVKJ, BdP, DGAUM, DGG, DGIM, DGK, DKG, DGSMP, GPP. Gesundheitswesen. 2015;77(7):508–511. doi: 10.1055/s-0035-1547232. http://dx.doi.org/10.1055/s-0035-1547232. [DOI] [PubMed] [Google Scholar]
- 48.Rahman MA, Hann N, Wilson A, Worrall-Carter L. Electronic cigarettes: patterns of use, health effects, use in smoking cessation and regulatory issues. Tob Induc Dis. 2014;12(1):21. doi: 10.1186/1617-9625-12-21. http://dx.doi.org/10.1186/1617-9625-12-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rowell TR, Tarran R. Will Chronic E-Cigarette Use Cause Lung Disease? Am J Physiol Lung Cell Mol Physiol. 2015;309(12):L1398–L1409. doi: 10.1152/ajplung.00272.2015. http://dx.doi.org/10.1152/ajplung.00272.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weaver M, Breland A, Spindle T, Eissenberg T. Electronic cigarettes: a review of safety and clinical issues. J Addict Med. 2014;8(4):234–240. doi: 10.1097/ADM.0000000000000043. http://dx.doi.org/10.1097/ADM.0000000000000043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Campbell-Heider N, Snow D. Teen Use of Electronic Cigarettes: What Does the Research Tell Us? J Addict Nurs. 2016;27(1):56–61. doi: 10.1097/JAN.0000000000000114. http://dx.doi.org/10.1097/JAN.0000000000000114. [DOI] [PubMed] [Google Scholar]
- 52.Chague F, Rochette L, Gudjoncik A, Cottin Y, Zeller M. Electronic cigarettes and sports: Dangerous liaisons? Int J Cardiol. 2016;215:400–401. doi: 10.1016/j.ijcard.2016.04.048. http://dx.doi.org/10.1016/j.ijcard.2016.04.048. [DOI] [PubMed] [Google Scholar]
- 53.Ioakeimidis N, Vlachopoulos C, Tousoulis D. Efficacy and Safety of Electronic Cigarettes for Smoking Cessation: A Critical Approach. Hellenic J Cardiol. 2016;57(1):1–6. doi: 10.1016/s1109-9666(16)30011-2. http://dx.doi.org/10.1016/S1109-9666(16)30011-2. [DOI] [PubMed] [Google Scholar]
- 54.Marsot A, Simon N. Nicotine and Cotinine Levels With Electronic Cigarette: A Review. Int J Toxicol. 2016;35(2):179–185. doi: 10.1177/1091581815618935. http://dx.doi.org/10.1177/1091581815618935. [DOI] [PubMed] [Google Scholar]
- 55.Nelluri B, Murphy K, Mookadam F, Mookadam M. The current literature regarding the cardiovascular effects of electronic cigarettes. Future Cardiol. 2016;12(2):167–179. doi: 10.2217/fca.15.83. http://dx.doi.org/10.2217/fca.15.83. [DOI] [PubMed] [Google Scholar]
- 56.Polosa R, Campagna D, Sands MF. Counseling patients with asthma and allergy about electronic cigarettes: an evidence-based approach. Ann Allergy Asthma Immunol. 2016;116(2):106–111. doi: 10.1016/j.anai.2015.10.012. http://dx.doi.org/10.1016/j.anai.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 57.Riley HE, Berry-Bibee E, England LJ, Jamieson DJ, Marchbanks PA, Curtis KM. Hormonal contraception among electronic cigarette users and cardiovascular risk: a systematic review. Contraception. 2016;93(3):190–208. doi: 10.1016/j.contraception.2015.11.003. http://dx.doi.org/10.1016/j.contraception.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 58.Zulkifli A, Abidin EZ, Abidin NZ, et al. Electronic cigarettes: a systematic review of available studies on health risk assessment. Rev Environ Health. doi: 10.1515/reveh-2015-0075. In press. Online April 21, 2016. http://dx.doi.org/10.1515/reveh-2015-0075. [DOI] [PubMed] [Google Scholar]
- 59.Do electronic cigarettes help with smoking cessation? Drug Ther Bull. 2014;52(11):126–129. doi: 10.1136/dtb.2014.11.0288. http://dx.doi.org/10.1136/dtb.2014.11.0288. [DOI] [PubMed] [Google Scholar]
- 60.Franck C, Budlovsky T, Windle SB, Filion KB, Eisenberg MJ. Electronic cigarettes in north america: history, use, and implications for smoking cessation. Circulation. 2014;129(19):1945–1952. doi: 10.1161/CIRCULATIONAHA.113.006416. http://dx.doi.org/10.1161/CIRCULATIONAHA.113.006416. [DOI] [PubMed] [Google Scholar]
- 61.Harrell PT, Simmons VN, Correa JB, Padhya TA, Brandon TH. Electronic nicotine delivery systems ("e-cigarettes"): review of safety and smoking cessation efficacy. Otolaryngol Head Neck Surg. 2015;151(3):381–393. doi: 10.1177/0194599814536847. http://dx.doi.org/10.1177/0194599814536847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Meier E, Tackett AP, Wagener TL. Effectiveness of electronic aids for smoking cessation. Curr Cardiovasc Risk Rep. 2013;7(6) doi: 10.1007/s12170-013-0343-8. http://dx.doi.org/10.1007/s12170-013-0343-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Orr KK, Asal NJ. Efficacy of Electronic Cigarettes for Smoking Cessation. Ann Pharmacother. 2014;48(11):1502–1506. doi: 10.1177/1060028014547076. http://dx.doi.org/10.1177/1060028014547076. [DOI] [PubMed] [Google Scholar]
- 64.Bullen C. Electronic cigarettes for smoking cessation. Curr Cardiol Rep. 2014;16(11):538. doi: 10.1007/s11886-014-0538-8. http://dx.doi.org/10.1007/s11886-014-0538-8. [DOI] [PubMed] [Google Scholar]
- 65.Lee AH, Stater BJ, Close L, Rahmati R. Are e-cigarettes effective in smoking cessation? Laryngoscope. 2015;125(4):785–787. doi: 10.1002/lary.24954. http://dx.doi.org/10.1002/lary.24954. [DOI] [PubMed] [Google Scholar]
- 66.McRobbie H, Bullen C, Hartmann-Boyce J, Hajek P. Electronic cigarettes for smoking cessation and reduction. Cochrane Database Syst Rev. 2014;12:Cd010216. doi: 10.1002/14651858.CD010216.pub2. http://dx.doi.org/10.1002/14651858.cd010216.pub2. [DOI] [PubMed] [Google Scholar]
- 67.Odum LE, O'Dell KA, Schepers JS. Electronic cigarettes: do they have a role in smoking cessation? J Pharm Pract. 2012;25(6):611–614. doi: 10.1177/0897190012451909. http://dx.doi.org/10.1177/0897190012451909. [DOI] [PubMed] [Google Scholar]
- 68.Rahman MA, Hann N, Wilson A, Mnatzaganian G, Worrall-Carter L. E-cigarettes and smoking cessation: evidence from a systematic review and meta-analysis. PLoS One. 2015;10(3):e0122544. doi: 10.1371/journal.pone.0122544. http://dx.doi.org/10.1371/journal.pone.0122544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bond K, Nunes N. Electronic Cigarettes for Smoking Cessation. Am Fam Physician. 2016;93(6):492. [PubMed] [Google Scholar]
- 70.Kalkhoran S, Glantz SA. E-cigarettes and smoking cessation in real-world and clinical settings: a systematic review and meta-analysis. Lancet Respir Med. 2016;4(2):116–128. doi: 10.1016/S2213-2600(15)00521-4. http://dx.doi.org/10.1016/S2213-2600(15)00521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Khoudigian S, Devji T, Lytvyn L, Campbell K, Hopkins R, O'Reilly D. The efficacy and short-term effects of electronic cigarettes as a method for smoking cessation: a systematic review and a meta-analysis. Int J Public Health. 2016;61(2):257–267. doi: 10.1007/s00038-016-0786-z. http://dx.doi.org/10.1007/s00038-016-0786-z. [DOI] [PubMed] [Google Scholar]
- 72.Lam C, West A. Are electronic nicotine delivery systems an effective smoking cessation tool? Can J Respir Ther. 2015;51(4):93–98. [PMC free article] [PubMed] [Google Scholar]
- 73.Malas M, van der Tempel J, Schwartz R, et al. Electronic Cigarettes for Smoking Cessation: A Systematic Review. Nicotine Tob Res. 2016;18(10):1926–1936. doi: 10.1093/ntr/ntw119. http://dx.doi.org/10.1093/ntr/ntw119. [DOI] [PubMed] [Google Scholar]
- 74.Brown CJ, Cheng JM. Electronic cigarettes: product characterisation and design considerations. Tob Control. 2014;23(Suppl 2):ii4–ii10. doi: 10.1136/tobaccocontrol-2013-051476. http://dx.doi.org/10.1136/tobaccocontrol-2013-051476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cheng T. Chemical evaluation of electronic cigarettes. Tob Control. 2014;23(Suppl 2):ii11–ii17. doi: 10.1136/tobaccocontrol-2013-051482. http://dx.doi.org/10.1136/tobaccocontrol-2013-051482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bekki K, Uchiyama S, Ohta K, Inaba Y, Nakagome H, Kunugita N. Carbonyl Compounds Generated from Electronic Cigarettes. Int J Environ Res Public Health. 2014;11(11):11192–11200. doi: 10.3390/ijerph111111192. http://dx.doi.org/10.3390/ijerph111111192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jerry JM, Collins GB, Streem D. E-cigarettes: Safe to recommend to patients? Cleve Clin J Med. 2015;82(8):521–526. doi: 10.3949/ccjm.82a.14054. [DOI] [PubMed] [Google Scholar]
- 78.Caponnetto P, Campagna D, Papale G, Russo C, Polosa R. The emerging phenomenon of electronic cigarettes. Expert Rev Respir Med. 2012;6(1):63–74. doi: 10.1586/ers.11.92. http://dx.doi.org/10.1586/ers.11.92. [DOI] [PubMed] [Google Scholar]
- 79.Evans SE, Hoffman AC. Electronic cigarettes: abuse liability, topography and subjective effects. Tob Control. 2014;23(Suppl 2):ii23–ii29. doi: 10.1136/tobaccocontrol-2013-051489. http://dx.doi.org/10.1136/tobaccocontrol-2013-051489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pepper JK, Brewer NT. Electronic nicotine delivery system (electronic cigarette) awareness, use, reactions and beliefs: a systematic review. Tob Control. 2014;23(5):375–384. doi: 10.1136/tobaccocontrol-2013-051122. http://dx.doi.org/10.1136/tobaccocontrol-2013-051122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tomashefski A. The perceived effects of electronic cigarettes on health by adult users: A state of the science systematic literature review. J Am Assoc Nurse Pract. 2016;28(9):510–515. doi: 10.1002/2327-6924.12358. http://dx.doi.org/10.1002/2327-6924.12358. [DOI] [PubMed] [Google Scholar]
- 82.Carroll Chapman SL, Wu LT. E-cigarette prevalence and correlates of use among adolescents versus adults: A review and comparison. J Psychiatr Res. 2014;54:43–54. doi: 10.1016/j.jpsychires.2014.03.005. http://dx.doi.org/10.1016/j.jpsychires.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bauld L, MacKintosh AM, Ford A, McNeill A. E-Cigarette Uptake Amongst UK Youth: Experimentation, but Little or No Regular Use in Nonsmokers. Nicotine Tob Res. 2016;18(1):102–103. doi: 10.1093/ntr/ntv132. http://dx.doi.org/10.1093/ntr/ntv132. [DOI] [PubMed] [Google Scholar]
- 84.Lauterstein D, Hoshino R, Gordon T, Watkins BX, Weitzman M, Zelikoff J. The changing face of tobacco use among United States youth. Curr Drug Abuse Rev. 2014;7(1):29–43. doi: 10.2174/1874473707666141015220110. http://dx.doi.org/10.2174/1874473707666141015220110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Meernik C, Goldstein AO. A critical review of smoking, cessation, relapse and emerging research in pregnancy and post-partum. Br Med Bull. 2015;114(1):135–146. doi: 10.1093/bmb/ldv016. http://dx.doi.org/10.1093/bmb/ldv016. [DOI] [PubMed] [Google Scholar]
- 86.Pepper JK, Eissenberg T. Waterpipes and electronic cigarettes: increasing prevalence and expanding science. Chem Res Toxicol. 2014;27(8):1336–1343. doi: 10.1021/tx500200j. http://dx.doi.org/10.1021/tx500200j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schneider S, Diehl K. Vaping as a Catalyst for Smoking? An Initial Model on the Initiation of Electronic Cigarette Use and the Transition to Tobacco Smoking Among Adolescents. Nicotine Tob Res. 2016;18(5):647–653. doi: 10.1093/ntr/ntv193. http://dx.doi.org/10.1093/ntr/ntv193. [DOI] [PubMed] [Google Scholar]
- 88.Echevarria C, Sinha IP. Heterogeneity in the measurement and reporting of outcomes in studies of electronic cigarette use in adolescents: a systematic analysis of observational studies. Tob Control. doi: 10.1136/tobaccocontrol-2015-052881. In press. Online April 29, 2016. http://dx.doi.org/10.1136/tobaccocontrol-2015-052881. [DOI] [PubMed] [Google Scholar]
- 89.Wang M, Wang JW, Cao SS, Wang HQ, Hu RY. Cigarette Smoking and Electronic Cigarettes Use: A Meta-Analysis. Int J Environ Res Public Health. 2016;13(1) doi: 10.3390/ijerph13010120. http://dx.doi.org/10.3390/ijerph13010120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhong J, Cao S, Gong W, Fei F, Wang M. Electronic Cigarettes Use and Intention to Cigarette Smoking among Never-Smoking Adolescents and Young Adults: A Meta-Analysis. Int J Environ Res Public Health. 2016;13(5) doi: 10.3390/ijerph13050465. http://dx.doi.org/10.3390/ijerph13050465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kadowaki J, Vuolo M, Kelly BC. A review of the current geographic distribution of and debate surrounding electronic cigarette clean air regulations in the United States. Health Place. 2014;31:75–82. doi: 10.1016/j.healthplace.2014.11.003. http://dx.doi.org/10.1016/j.healthplace.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Farsalinos KE, Le Houezec J. Regulation in the face of uncertainty: the evidence on electronic nicotine delivery systems (e-cigarettes) Risk Manag Healthc Policy. 2015;8:157–167. doi: 10.2147/RMHP.S62116. http://dx.doi.org/10.2147/RMHP.S62116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Freiberg MJ. Federal approaches to the regulation of noncigarette tobacco products. Am J Prev Med. 2012;43(5 Suppl 3):S249–S254. doi: 10.1016/j.amepre.2012.07.036. http://dx.doi.org/10.1016/j.amepre.2012.07.036. [DOI] [PubMed] [Google Scholar]
- 94.Kaufman N, Mahoney M. E-cigarettes: policy options and legal issues amidst uncertainty. J Law Med Ethics. 2015;43(Suppl 1):23–26. doi: 10.1111/jlme.12209. http://dx.doi.org/10.1111/jlme.12209. [DOI] [PubMed] [Google Scholar]
- 95.Saitta D, Ferro GA, Polosa R. Achieving appropriate regulations for electronic cigarettes. Ther Adv Chronic Dis. 2014;5(2):50–61. doi: 10.1177/2040622314521271. http://dx.doi.org/10.1177/2040622314521271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Syx E. The case of the electronic cigarette in the EU. Eur J Health Law. 2014;21(2):161–175. doi: 10.1163/15718093-12341312. http://dx.doi.org/10.1163/15718093-12341312. [DOI] [PubMed] [Google Scholar]
- 97.Tremblay MC, Pluye P, Gore G, Granikov V, Filion KB, Eisenberg MJ. Regulation profiles of e-cigarettes in the United States: a critical review with qualitative synthesis. BMC Med. 2015;13(1):130. doi: 10.1186/s12916-015-0370-z. http://dx.doi.org/10.1186/s12916-015-0370-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mainous AG, 3rd, Tanner RJ, Mainous RW, Talbert J. Health Considerations in Regulation and Taxation of Electronic Cigarettes. J Am Board Fam Med. 2015;28(6):802–806. doi: 10.3122/jabfm.2015.06.150114. http://dx.doi.org/10.3122/jabfm.2015.06.150114. [DOI] [PubMed] [Google Scholar]
- 99.Zyoud SH, Al-Jabi SW, Sweileh WM. Worldwide research productivity in the field of electronic cigarette: a bibliometric analysis. BMC Public Health. 2014;14(1):667. doi: 10.1186/1471-2458-14-667. http://dx.doi.org/10.1186/1471-2458-14-667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Caldwell B, Sumner W, Crane J. A systematic review of nicotine by inhalation: is there a role for the inhaled route? Nicotine Tob Res. 2012;14(10):1127–1139. doi: 10.1093/ntr/nts009. http://dx.doi.org/10.1093/ntr/nts009. [DOI] [PubMed] [Google Scholar]
- 101.Hajek P, Etter JF, Benowitz N, Eissenberg T, McRobbie H. Electronic cigarettes: review of use, content, safety, effects on smokers and potential for harm and benefit. Addiction. 2014;109(11):1801–1810. doi: 10.1111/add.12659. http://dx.doi.org/10.1111/add.12659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nowak D, Jorres RA, Ruther T. E-cigarettes--prevention, pulmonary health, and addiction. Dtsch Arztebl Int. 2014;111(20):349–355. doi: 10.3238/arztebl.2014.0349. http://dx.doi.org/10.3238/arztebl.2014.0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Patnode CD, Henderson JT, Thompson JH, Senger CA, Fortmann SP, Whitlock EP. Behavioral Counseling and Pharmacotherapy Interventions for Tobacco Cessation in Adults, Including Pregnant Women: A Review of Reviews for the U.S. Preventive Services Task Force. Ann Intern Med. 2015;163(8):608–621. doi: 10.7326/M15-0171. http://dx.doi.org/10.7326/M15-0171. [DOI] [PubMed] [Google Scholar]
- 104.Tidey JW, Miller ME. Smoking cessation and reduction in people with chronic mental illness. BMJ. 2015;351:h4065. doi: 10.1136/bmj.h4065. http://dx.doi.org/10.1136/bmj.h4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zeller M, Hatsukami D. Strategic Dialogue on Tobacco Harm Reduction G. The Strategic Dialogue on Tobacco Harm Reduction: a vision and blueprint for action in the U.S. Tob Control. 2009;18(4):324–332. doi: 10.1136/tc.2008.027318. http://dx.doi.org/10.1136/tc.2008.027318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.U.S. DHHS. Chapter 15: The Changing Landscape of Tobacco Control -- Current Status and Future Directions from the The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. Atlanta, GA: U.S. DHHS, CDC, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. [Google Scholar]
- 107.Glasser AM, Cobb CO, Teplitskaya L, et al. Electronic nicotine delivery devices, and their impact on health and patterns of tobacco use: a systematic review protocol. BMJ Open. 2015;5(4):e007688. doi: 10.1136/bmjopen-2015-007688. http://dx.doi.org/10.1136/bmjopen-2015-007688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bertholon JF, Becquemin MH, Roy M, et al. Comparison of the aerosol produced by electronic cigarettes with conventional cigarettes and the shisha. Rev Mal Respir. 2013;30(9):752–757. doi: 10.1016/j.rmr.2013.03.003. http://dx.doi.org/10.1016/j.rmr.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 109.Bush D, Goniewicz ML. A pilot study on nicotine residues in houses of electronic cigarette users, tobacco smokers, and non-users of nicotine-containing products. Int J Drug Policy. 2015;26(6):609–611. doi: 10.1016/j.drugpo.2015.03.003. http://dx.doi.org/10.1016/j.drugpo.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cameron JM, Howell DN, White JR, Andrenyak DM, Layton ME, Roll JM. Variable and potentially fatal amounts of nicotine in e-cigarette nicotine solutions. Tob Control. 2014;23(1):77–78. doi: 10.1136/tobaccocontrol-2012-050604. http://dx.doi.org/10.1136/tobaccocontrol-2012-050604. [DOI] [PubMed] [Google Scholar]
- 111.Cheah NP, Chong NW, Tan J, Morsed FA, Yee SK. Electronic nicotine delivery systems: regulatory and safety challenges: Singapore perspective. Tob Control. 2014;23(2):119–125. doi: 10.1136/tobaccocontrol-2012-050483. http://dx.doi.org/10.1136/tobaccocontrol-2012-050483. [DOI] [PubMed] [Google Scholar]
- 112.Cobb NK, Byron MJ, Abrams DB, Shields PG. Novel nicotine delivery systems and public health: the rise of the "e-cigarette". Am J Public Health. 2010;100(12):2340–2342. doi: 10.2105/AJPH.2010.199281. http://dx.doi.org/10.2105/AJPH.2010.199281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Czogala J, Goniewicz ML, Fidelus B, Zielinska-Danch W, Travers MJ, Sobczak A. Secondhand exposure to vapors from electronic cigarettes. Nicotine Tob Res. 2014;16(6):655–662. doi: 10.1093/ntr/ntt203. http://dx.doi.org/10.1093/ntr/ntt203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Davis B, Dang M, Kim J, Talbot P. Nicotine concentrations in electronic cigarette refill and do-it-yourself fluids. Nicotine Tob Res. 2015;17(2):134–141. doi: 10.1093/ntr/ntu080. http://dx.doi.org/10.1093/ntr/ntu080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Davis B, Razo A, Nothnagel E, Chen M, Talbot P. Unexpected nicotine in Do-it-Yourself electronic cigarette flavourings. Tob Control. 2016;25(1):e67–e68. doi: 10.1136/tobaccocontrol-2015-052468. http://dx.doi.org/10.1136/tobaccocontrol-2015-052468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Etter JF, Zather E, Svensson S. Analysis of refill liquids for electronic cigarettes. Addiction. 2013;108(9):1671–1679. doi: 10.1111/add.12235. http://dx.doi.org/10.1111/add.12235. [DOI] [PubMed] [Google Scholar]
- 117.Farsalinos KE, Gillman IG, Melvin MS, et al. Nicotine levels and presence of selected tobacco-derived toxins in tobacco flavoured electronic cigarette refill liquids. Int J Environ Res Public Health. 2015;12(4):3439–3452. doi: 10.3390/ijerph120403439. http://dx.doi.org/10.3390/ijerph120403439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Farsalinos KE, Voudris V, Poulas K. E-cigarettes generate high levels of aldehydes only in 'dry puff' conditions. Addiction. 2015;110(8):1352–1356. doi: 10.1111/add.12942. http://dx.doi.org/10.1111/add.12942. [DOI] [PubMed] [Google Scholar]
- 119.Farsalinos KE, Gillman G, Poulas K, Voudris V. Tobacco-Specific Nitrosamines in Electronic Cigarettes: Comparison between Liquid and Aerosol Levels. Int J Environ Res Public Health. 2015;12(8):9046–9053. doi: 10.3390/ijerph120809046. http://dx.doi.org/10.3390/ijerph120809046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Farsalinos KE, Kistler KA, Gillman G, Voudris V. Evaluation of electronic cigarette liquids and aerosol for the presence of selected inhalation toxins. Nicotine Tob Res. 2015;17(2):168–174. doi: 10.1093/ntr/ntu176. http://dx.doi.org/10.1093/ntr/ntu176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fuoco FC, Buonanno G, Stabile L, Vigo P. Influential parameters on particle concentration and size distribution in the mainstream of e-cigarettes. Environ Pollut. 2014;184:523–529. doi: 10.1016/j.envpol.2013.10.010. http://dx.doi.org/10.1016/j.envpol.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 122.Geiss O, Bianchi I, Barahona F, Barrero-Moreno J. Characterisation of mainstream and passive vapours emitted by selected electronic cigarettes. Int J Hyg Environ Health. 2015;218(1):169–180. doi: 10.1016/j.ijheh.2014.10.001. http://dx.doi.org/10.1016/j.ijheh.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 123.Goel R, Durand E, Trushin N, et al. Highly Reactive Free Radicals in Electronic Cigarette Aerosols. Chem Res Toxicol. 2015;28(9):1675–1677. doi: 10.1021/acs.chemrestox.5b00220. http://dx.doi.org/10.1021/acs.chemrestox.5b00220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Goniewicz ML, Hajek P, McRobbie H. Nicotine content of electronic cigarettes, its release in vapour and its consistency across batches: regulatory implications. Addiction. 2014;109(3):500–507. doi: 10.1111/add.12410. http://dx.doi.org/10.1111/add.12410. [DOI] [PubMed] [Google Scholar]
- 125.Goniewicz ML, Knysak J, Gawron M, et al. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob Control. 2014;23(2):133–139. doi: 10.1136/tobaccocontrol-2012-050859. http://dx.doi.org/10.1136/tobaccocontrol-2012-050859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Goniewicz ML, Kuma T, Gawron M, Knysak J, Kosmider L. Nicotine levels in electronic cigarettes. Nicotine Tob Res. 2013;15(1):158–166. doi: 10.1093/ntr/nts103. http://dx.doi.org/10.1093/ntr/nts103. [DOI] [PubMed] [Google Scholar]
- 127.Goniewicz ML, Lee L. Electronic cigarettes are a source of thirdhand exposure to nicotine. Nicotine Tob Res. 2015;17(2):256–258. doi: 10.1093/ntr/ntu152. http://dx.doi.org/10.1093/ntr/ntu152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Goniewicz ML, Gupta R, Lee YH, et al. Nicotine levels in electronic cigarette refill solutions: A comparative analysis of products from the U.S., Korea, and Poland. Int J Drug Policy. 2015;26(6):583–588. doi: 10.1016/j.drugpo.2015.01.020. http://dx.doi.org/10.1016/j.drugpo.2015.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hadwiger ME, Trehy ML, Ye W, Moore T, Allgire J, Westenberger B. Identification of amino-tadalafil and rimonabant in electronic cigarette products using high pressure liquid chromatography with diode array and tandem mass spectrometric detection. J Chromatogr A. 2010;1217(48):7547–7555. doi: 10.1016/j.chroma.2010.10.018. http://dx.doi.org/10.1016/j.chroma.2010.10.018. [DOI] [PubMed] [Google Scholar]
- 130.Herrington JS, Myers C. Electronic cigarette solutions and resultant aerosol profiles. J Chromatogr A. 2015;1418:192–199. doi: 10.1016/j.chroma.2015.09.034. http://dx.doi.org/10.1016/j.chroma.2015.09.034. [DOI] [PubMed] [Google Scholar]
- 131.Hutzler C, Paschke M, Kruschinski S, Henkler F, Hahn J, Luch A. Chemical hazards present in liquids and vapors of electronic cigarettes. Arch Toxicol. 2014;88(7):1295–1308. doi: 10.1007/s00204-014-1294-7. http://dx.doi.org/10.1007/s00204-014-1294-7. [DOI] [PubMed] [Google Scholar]
- 132.Ingebrethsen BJ, Cole SK, Alderman SL. Electronic cigarette aerosol particle size distribution measurements. Inhal Toxicol. 2012;24(14):976–984. doi: 10.3109/08958378.2012.744781. http://dx.doi.org/10.3109/08958378.2012.744781. [DOI] [PubMed] [Google Scholar]
- 133.Kienhuis AS, Soeteman-Hernandez LG, Bos PM, Cremers HW, Klerx WN, Talhout R. Potential harmful health effects of inhaling nicotine-free shisha-pen vapor: a chemical risk assessment of the main components propylene glycol and glycerol. Tob Induc Dis. 2015;13(1):15. doi: 10.1186/s12971-015-0038-7. http://dx.doi.org/10.1186/s12971-015-0038-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kim HJ, Shin HS. Determination of tobacco-specific nitrosamines in replacement liquids of electronic cigarettes by liquid chromatography-tandem mass spectrometry. J Chromatogr A. 2013;1291:48–55. doi: 10.1016/j.chroma.2013.03.035. http://dx.doi.org/10.1016/j.chroma.2013.03.035. [DOI] [PubMed] [Google Scholar]
- 135.Kosmider L, Sobczak A, Fik M, et al. Carbonyl compounds in electronic cigarette vapors: effects of nicotine solvent and battery output voltage. Nicotine Tob Res. 2014;16(10):1319–1326. doi: 10.1093/ntr/ntu078. http://dx.doi.org/10.1093/ntr/ntu078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Laugesen M. Nicotine and toxicant yield ratings of electronic cigarette brands in New Zealand. N Z Med J. 2015;128(1411):77–82. [PubMed] [Google Scholar]
- 137.Lerner CA, Sundar IK, Watson RM, et al. Environmental health hazards of e-cigarettes and their components: Oxidants and copper in e-cigarette aerosols. Environ Pollut. 2015;198:100–107. doi: 10.1016/j.envpol.2014.12.033. http://dx.doi.org/10.1016/j.envpol.2014.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lisko JG, Tran H, Stanfill SB, Blount BC, Watson CH. Chemical Composition and Evaluation of Nicotine, Tobacco Alkaloids, pH, Selected Flavors in E-Cigarette Cartridges and Refill Solutions. Nicotine Tob Res. 2015;17(10):1270–1278. doi: 10.1093/ntr/ntu279. http://dx.doi.org/10.1093/ntr/ntu279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Long GA. Comparison of select analytes in exhaled aerosol from e-cigarettes with exhaled smoke from a conventional cigarette and exhaled breaths. Int J Environ Res Public Health. 2014;11(11):11177–11191. doi: 10.3390/ijerph111111177. http://dx.doi.org/10.3390/ijerph111111177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Manigrasso M, Buonanno G, Fuoco FC, Stabile L, Avino P. Aerosol deposition doses in the human respiratory tree of electronic cigarette smokers. Environ Pollut. 2015;196:257–267. doi: 10.1016/j.envpol.2014.10.013. http://dx.doi.org/10.1016/j.envpol.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 141.Manigrasso M, Buonanno G, Stabile L, Morawska L, Avino P. Particle doses in the pulmonary lobes of electronic and conventional cigarette users. Environ Pollut. 2015;202:24–31. doi: 10.1016/j.envpol.2015.03.008. http://dx.doi.org/10.1016/j.envpol.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 142.Marini S, Buonanno G, Stabile L, Ficco G. Short-term effects of electronic and tobacco cigarettes on exhaled nitric oxide. Toxicol Appl Pharmacol. 2014;278(1):9–15. doi: 10.1016/j.taap.2014.04.004. http://dx.doi.org/10.1016/j.taap.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 143.Martinez RE, Dhawan S, Sumner W, Williams BJ. On-Line Chemical Composition Analysis of Refillable Electronic Cigarette Aerosol-Measurement of Nicotine and Nicotyrine. Nicotine Tob Res. 2015;17(10):1263–1269. doi: 10.1093/ntr/ntu334. http://dx.doi.org/10.1093/ntr/ntu334. [DOI] [PubMed] [Google Scholar]
- 144.McAuley TR, Hopke PK, Zhao J, Babaian S. Comparison of the effects of e-cigarette vapor and cigarette smoke on indoor air quality. Inhal Toxicol. 2012;24(12):850–857. doi: 10.3109/08958378.2012.724728. http://dx.doi.org/10.3109/08958378.2012.724728. [DOI] [PubMed] [Google Scholar]
- 145.McQueen A, Tower S, Sumner W. Interviews with "vapers": implications for future research with electronic cigarettes. Nicotine Tob Res. 2011;13(9):860–867. doi: 10.1093/ntr/ntr088. http://dx.doi.org/10.1093/ntr/ntr088. [DOI] [PubMed] [Google Scholar]
- 146.Oh JA, Shin HS. Identification and Quantification of Several Contaminated Compounds in Replacement Liquids of Electronic Cigarettes by Gas Chromatography-Mass Spectrometry. J Chromatogr Sci. 2015;53(6):841–848. doi: 10.1093/chromsci/bmu146. http://dx.doi.org/10.1093/chromsci/bmu146. [DOI] [PubMed] [Google Scholar]
- 147.Pagano T, DiFrancesco AG, Smith SB, et al. Determination of Nicotine Content and Delivery in Disposable Electronic Cigarettes Available in the United States by Gas Chromatography-Mass Spectrometry. Nicotine Tob Res. 2016;18(5):700–707. doi: 10.1093/ntr/ntv120. http://dx.doi.org/10.1093/ntr/ntv120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Pellegrino RM, Tinghino B, Mangiaracina G, et al. Electronic cigarettes: an evaluation of exposure to chemicals and fine particulate matter (PM) Ann Ig. 2012;24(4):279–288. [PubMed] [Google Scholar]
- 149.Ruprecht AA, De Marco C, Pozzi P, et al. Comparison between particulate matter and ultrafine particle emission by electronic and normal cigarettes in real-life conditions. Tumori. 2014;100(1):24e–27e. doi: 10.1700/1430.15833. [DOI] [PubMed] [Google Scholar]
- 150.Schober W, Szendrei K, Matzen W, et al. Use of electronic cigarettes (e-cigarettes) impairs indoor air quality and increases FeNO levels of e-cigarette consumers. Int J Hyg Environ Health. 2014;217(6):628–637. doi: 10.1016/j.ijheh.2013.11.003. http://dx.doi.org/10.1016/j.ijheh.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 151.Schripp T, Markewitz D, Uhde E, Salthammer T. Does e-cigarette consumption cause passive vaping? Indoor Air. 2013;23(1):25–31. doi: 10.1111/j.1600-0668.2012.00792.x. http://dx.doi.org/10.1111/j.1600-0668.2012.00792.x. [DOI] [PubMed] [Google Scholar]
- 152.Stepanov I, Fujioka N. Bringing attention to e-cigarette pH as an important element for research and regulation. Tob Control. 2015;24(4):413–414. doi: 10.1136/tobaccocontrol-2014-051540. http://dx.doi.org/10.1136/tobaccocontrol-2014-051540. [DOI] [PubMed] [Google Scholar]
- 153.Talih S, Balhas Z, Eissenberg T, et al. Effects of user puff topography, device voltage, and liquid nicotine concentration on electronic cigarette nicotine yield: measurements and model predictions. Nicotine Tob Res. 2015;17(2):150–157. doi: 10.1093/ntr/ntu174. http://dx.doi.org/10.1093/ntr/ntu174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Talih S, Balhas Z, Salman R, Karaoghlanian N, Shihadeh A. "Direct Dripping": A High-Temperature, High-Formaldehyde Emission Electronic Cigarette Use Method. Nicotine Tob Res. 2016;18(4):453–459. doi: 10.1093/ntr/ntv080. http://dx.doi.org/10.1093/ntr/ntv080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Tierney PA, Karpinski CD, Brown JE, Luo W, Pankow JF. Flavour chemicals in electronic cigarette fluids. Tob Control. 2016;25(1):e10–e15. doi: 10.1136/tobaccocontrol-2014-052175. http://dx.doi.org/10.1136/tobaccocontrol-2014-052175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Trtchounian A, Talbot P. Electronic nicotine delivery systems: is there a need for regulation? Tob Control. 2011;20(1):47–52. doi: 10.1136/tc.2010.037259. http://dx.doi.org/10.1136/tc.2010.037259. [DOI] [PubMed] [Google Scholar]
- 157.Tayyarah R, Long GA. Comparison of select analytes in aerosol from e-cigarettes with smoke from conventional cigarettes and with ambient air. Regul Toxicol Pharmacol. 2014;11(11):11177–11191. doi: 10.1016/j.yrtph.2014.10.010. http://dx.doi.org/10.1016/j.yrtph.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 158.Uchiyama S, Ohta K, Inaba Y, Kunugita N. Determination of Carbonyl Compounds Generated from the E-cigarette Using Coupled Silica Cartridges Impregnated with Hydroquinone and 2,4-Dinitrophenylhydrazine, Followed by High-Performance Liquid Chromatography. Anal Sci. 2013;29(12):1219–1222. doi: 10.2116/analsci.29.1219. http://dx.doi.org/10.2116/analsci.29.1219. [DOI] [PubMed] [Google Scholar]
- 159.Westenberger B. Evaluation of e-cigarettes. St Louis, MO: Food and Drug Administration; 2009. pp. 1–8. [Google Scholar]
- 160.Williams M, Ghai S, Talbot P. Disposable electronic cigarettes and electronic hookahs: evaluation of performance. Nicotine Tob Res. 2015;17(2):201–208. doi: 10.1093/ntr/ntu118. http://dx.doi.org/10.1093/ntr/ntu118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Williams M, To A, Bozhilov K, Talbot P. Strategies to Reduce Tin and Other Metals in Electronic Cigarette Aerosol. PLoS One. 2015;10(9):e0138933. doi: 10.1371/journal.pone.0138933. http://dx.doi.org/10.1371/journal.pone.0138933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Williams M, Villarreal A, Bozhilov K, Lin S, Talbot P. Metal and silicate particles including nanoparticles are present in electronic cigarette cartomizer fluid and aerosol. PLoS One. 2013;8(3):e57987. doi: 10.1371/journal.pone.0057987. http://dx.doi.org/10.1371/journal.pone.0057987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zhang Y, Sumner W, Chen DR. In vitro particle size distributions in electronic and conventional cigarette aerosols suggest comparable deposition patterns. Nicotine Tob Res. 2013;15(2):501–508. doi: 10.1093/ntr/nts165. http://dx.doi.org/10.1093/ntr/nts165. [DOI] [PubMed] [Google Scholar]
- 164.Allen JG, Flanigan SS, LeBlanc M, et al. Flavoring Chemicals in E-Cigarettes: Diacetyl, 2,3-Pentanedione, and Acetoin in a Sample of 51 Products, Including Fruit-, Candy-, and Cocktail-Flavored E-Cigarettes. Environ Health Perspect. 2016;124(6):733–739. doi: 10.1289/ehp.1510185. http://dx.doi.org/10.1289/EHP348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Fernandez E, Ballbe M, Sureda X, Fu M, Salto E, Martinez-Sanchez JM. Particulate Matter from Electronic Cigarettes and Conventional Cigarettes: a Systematic Review and Observational Study. Curr Environ Health Rep. 2015;2(4):423–429. doi: 10.1007/s40572-015-0072-x. http://dx.doi.org/10.1007/s40572-015-0072-x. [DOI] [PubMed] [Google Scholar]
- 166.Han S, Chen H, Zhang X, Liu T, Fu Y. Levels of Selected Groups of Compounds in Refill Solutions for Electronic Cigarettes. Nicotine Tob Res. 2016;18(5):708–714. doi: 10.1093/ntr/ntv189. http://dx.doi.org/10.1093/ntr/ntv189. [DOI] [PubMed] [Google Scholar]
- 167.Farsalinos KE, Yannovits N, Sarri T, Voudris V, Poulas K. Protocol proposal for, and evaluation of, consistency in nicotine delivery from the liquid to the aerosol of electronic cigarettes atomizers: regulatory implications. Addiction. 2016;111(6):1069–1076. doi: 10.1111/add.13299. http://dx.doi.org/10.1111/add.13299. [DOI] [PubMed] [Google Scholar]
- 168.Flora JW, Meruva N, Huang CB, et al. Characterization of potential impurities and degradation products in electronic cigarette formulations and aerosols. Regul Toxicol Pharmacol. 2016;74:1–11. doi: 10.1016/j.yrtph.2015.11.009. http://dx.doi.org/10.1016/j.yrtph.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 169.Gallart-Mateu D, Elbal L, Armenta S, de la Guardia M. Passive exposure to nicotine from e-cigarettes. Talanta. 2016;152:329–334. doi: 10.1016/j.talanta.2016.02.014. http://dx.doi.org/10.1016/j.talanta.2016.02.014. [DOI] [PubMed] [Google Scholar]
- 170.Geiss O, Bianchi I, Barrero-Moreno J. Correlation of volatile carbonyl yields emitted by e-cigarettes with the temperature of the heating coil and the perceived sensorial quality of the generated vapours. Int J Hyg Environ Health. 2016;219(3):268–277. doi: 10.1016/j.ijheh.2016.01.004. http://dx.doi.org/10.1016/j.ijheh.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 171.Gillman IG, Kistler KA, Stewart EW, Paolantonio AR. Effect of variable power levels on the yield of total aerosol mass and formation of aldehydes in e-cigarette aerosols. Regul Toxicol Pharmacol. 2016;75:58–65. doi: 10.1016/j.yrtph.2015.12.019. http://dx.doi.org/10.1016/j.yrtph.2015.12.019. [DOI] [PubMed] [Google Scholar]
- 172.Kosmider L, Sobczak A, Prokopowicz A, et al. Cherry-flavoured electronic cigarettes expose users to the inhalation irritant, benzaldehyde. Thorax. 2016;71(4):376–377. doi: 10.1136/thoraxjnl-2015-207895. http://dx.doi.org/10.1136/thoraxjnl-2015-207895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Maloney JC, Thompson MK, Oldham MJ, et al. Insights from two industrial hygiene pilot e-cigarette passive vaping studies. J Occup Environ Hyg. 2016;13(4):279–287. doi: 10.1080/15459624.2015.1116693. http://dx.doi.org/10.1080/15459624.2015.1116693. [DOI] [PubMed] [Google Scholar]
- 174.Williams M, Villarreal A, Davis B, Talbot P. Comparison of the Performance of Cartomizer Style Electronic Cigarettes from Major Tobacco and Independent Manufacturers. PLoS One. 2016;11(2):e0149251. doi: 10.1371/journal.pone.0149251. http://dx.doi.org/10.1371/journal.pone.0149251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Sosnowski TR, Kramek-Romanowska K. Predicted Deposition of E-Cigarette Aerosol in the Human Lungs. J Aerosol Med Pulm Drug Deliv. 2016;29(3):299–309. doi: 10.1089/jamp.2015.1268. http://dx.doi.org/10.1089/jamp.2015.1268. [DOI] [PubMed] [Google Scholar]
- 176.Soule EK, Maloney SF, Spindle TR, Rudy AK, Hiler MM, Cobb CO. Electronic cigarette use and indoor air quality in a natural setting. Tob Control. doi: 10.1136/tobaccocontrol-2015-052772. In press. Online February 15, 2016. http://dx.doi.org/10.1136/tobaccocontrol-2015-052772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Miao S, Beach ES, Sommer TJ, Zimmerman JB, Jordt SE. High-Intensity Sweeteners in Alternative Tobacco Products. Nicotine Tob Res. 2016;18(11):2169–2173. doi: 10.1093/ntr/ntw141. http://dx.doi.org/10.1093/ntr/ntw141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Peace MR, Baird TR, Smith N, Wolf CE, Poklis JL, Poklis A. Concentration of Nicotine and Glycols in 27 Electronic Cigarette Formulations. J Anal Toxicol. 2016;40(6):403–407. doi: 10.1093/jat/bkw037. http://dx.doi.org/10.1093/jat/bkw037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Mikheev VB, Brinkman MC, Granville CA, Gordon SM, Clark PI. Real-Time Measurement of Electronic Cigarette Aerosol Size Distribution and Metals Content Analysis. Nicotine Tob Res. 2016;18(9):1895–1902. doi: 10.1093/ntr/ntw128. http://dx.doi.org/10.1093/ntr/ntw128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Buettner-Schmidt K, Miller DR, Balasubramanian N. Electronic Cigarette Refill Liquids: Child-Resistant Packaging, Nicotine Content, and Sales to Minors. J Pediatr Nurs. 2016;31(4):373–379. doi: 10.1016/j.pedn.2016.03.019. http://dx.doi.org/10.1016/j.pedn.2016.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.El-Hellani A, El-Hage R, Baalbaki R, et al. Free-Base and Protonated Nicotine in Electronic Cigarette Liquids and Aerosols. Chem Res Toxicol. 2015;28(8):1532–1537. doi: 10.1021/acs.chemrestox.5b00107. http://dx.doi.org/10.1021/acs.chemrestox.5b00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kavvalakis MP, Stivaktakis PD, Tzatzarakis MN, et al. Multicomponent Analysis of Replacement Liquids of Electronic Cigarettes Using Chromatographic Techniques. J Anal Toxicol. 2015;39(4):262–269. doi: 10.1093/jat/bkv002. http://dx.doi.org/10.1093/jat/bkv002. [DOI] [PubMed] [Google Scholar]
- 183.Kubica P, Kot-Wasik A, Wasik A, Namiesnik J. "Dilute & shoot" approach for rapid determination of trace amounts of nicotine in zero-level e-liquids by reversed phase liquid chromatography and hydrophilic interactions liquid chromatography coupled with tandem mass spectrometry-electrospray ionization. J Chromatogr A. 2013;1289:13–18. doi: 10.1016/j.chroma.2013.02.078. http://dx.doi.org/10.1016/j.chroma.2013.02.078. [DOI] [PubMed] [Google Scholar]
- 184.Hahn J, Monakhova YB, Hengen J, et al. Electronic cigarettes: overview of chemical composition and exposure estimation. Tob Induc Dis. 2014;12(1):23. doi: 10.1186/s12971-014-0023-6. http://dx.doi.org/10.1186/s12971-014-0023-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Marco E, Grimalt JO. A rapid method for the chromatographic analysis of volatile organic compounds in exhaled breath of tobacco cigarette and electronic cigarette smokers. J Chromatogr A. 2015;1410:51–59. doi: 10.1016/j.chroma.2015.07.094. http://dx.doi.org/10.1016/j.chroma.2015.07.094. [DOI] [PubMed] [Google Scholar]
- 186.Kubica P, Wasik A, Kot-Wasik A, Namiesnik J. An evaluation of sucrose as a possible contaminant in e-liquids for electronic cigarettes by hydrophilic interaction liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem. 2014;406(13):3013–3018. doi: 10.1007/s00216-014-7690-2. http://dx.doi.org/10.1007/s00216-014-7690-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Garcia-Gomez D, Gaisl T, Barrios-Collado C, Vidal-de-Miguel G, Kohler M, Zenobi R. Real-Time Chemical Analysis of E-Cigarette Aerosols By Means Of Secondary Electrospray Ionization Mass Spectrometry. Chemistry. 2016;22(7):2452–2457. doi: 10.1002/chem.201504450. http://dx.doi.org/10.1002/chem.201504450. [DOI] [PubMed] [Google Scholar]
- 188.Olmedo P, Navas-Acien A, Hess C, Jarmul S, Rule A. A direct method for e-cigarette aerosol sample collection. Environ Res. 2016;149:151–156. doi: 10.1016/j.envres.2016.05.008. http://dx.doi.org/10.1016/j.envres.2016.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Regueiro J, Giri A, Wenzl T. Optimization of a Differential Ion Mobility Spectrometry-Tandem Mass Spectrometry Method for High-Throughput Analysis of Nicotine and Related Compounds: Application to Electronic Cigarette Refill Liquids. Anal Chem. 2016;88(12):6500–6508. doi: 10.1021/acs.analchem.6b01241. http://dx.doi.org/10.1021/acs.analchem.6b01241. [DOI] [PubMed] [Google Scholar]
- 190.Beauval N, Howsam M, Antherieu S, et al. Trace elements in e-liquids - Development and validation of an ICP-MS method for the analysis of electronic cigarette refills. Regul Toxicol Pharmacol. 2016;79:144–148. doi: 10.1016/j.yrtph.2016.03.024. http://dx.doi.org/10.1016/j.yrtph.2016.03.024. [DOI] [PubMed] [Google Scholar]
- 191.Krause MJ, Townsend TG. Hazardous waste status of discarded electronic cigarettes. Waste Manag. 2015;39:57–62. doi: 10.1016/j.wasman.2015.02.005. http://dx.doi.org/10.1016/j.wasman.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 192.Foulds J, Veldheer S, Berg A. Electronic cigarettes (e-cigs): views of aficionados and clinical/public health perspectives. Int J Clin Pract. 2011;65(10):1037–1042. doi: 10.1111/j.1742-1241.2011.02751.x. http://dx.doi.org/10.1111/j.1742-1241.2011.02751.x. [DOI] [PubMed] [Google Scholar]
- 193.Dawkins L, Turner J, Roberts A, Soar K. 'Vaping' profiles and preferences: an online survey of electronic cigarette users. Addiction. 2013;108(6):1115–1125. doi: 10.1111/add.12150. http://dx.doi.org/10.1111/add.12150. [DOI] [PubMed] [Google Scholar]
- 194.Etter JF, Bullen C. Electronic cigarette: users profile, utilization, satisfaction and perceived efficacy. Addiction. 2011;106(11):2017–2028. doi: 10.1111/j.1360-0443.2011.03505.x. http://dx.doi.org/10.1111/j.1360-0443.2011.03505.x. [DOI] [PubMed] [Google Scholar]
- 195.Vansickel AR, Eissenberg T. Electronic cigarettes: effective nicotine delivery after acute administration. Nicotine Tob Res. 2013;15(1):267–270. doi: 10.1093/ntr/ntr316. http://dx.doi.org/10.1093/ntr/ntr316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Farsalinos KE, Spyrou A, Tsimopoulou K, Stefopoulos C, Romagna G, Voudris V. Nicotine absorption from electronic cigarette use: comparison between first and new-generation devices. Sci Rep. 2014;4:4133. doi: 10.1038/srep04133. http://dx.doi.org/10.1038/srep04133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Spindle TR, Breland AB, Karaoghlanian NV, Shihadeh AL, Eissenberg T. Preliminary results of an examination of electronic cigarette user puff topography: the effect of a mouthpiece-based topography measurement device on plasma nicotine and subjective effects. Nicotine Tob Res. 2015;17(2):142–149. doi: 10.1093/ntr/ntu186. http://dx.doi.org/10.1093/ntr/ntu186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Bahl V, Lin S, Xu N, Davis B, Wang YH, Talbot P. Comparison of electronic cigarette refill fluid cytotoxicity using embryonic and adult models. Reprod Toxicol. 2012;34(4):529–537. doi: 10.1016/j.reprotox.2012.08.001. http://dx.doi.org/10.1016/j.reprotox.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 199.Bullen C, McRobbie H, Thornley S, Glover M, Lin R, Laugesen M. Effect of an electronic nicotine delivery device (e cigarette) on desire to smoke and withdrawal, user preferences and nicotine delivery: randomised cross-over trial. Tob Control. 2010;19(2):98–103. doi: 10.1136/tc.2009.031567. http://dx.doi.org/10.1136/tc.2009.031567. [DOI] [PubMed] [Google Scholar]
- 200.Camus M, Gallois C, Marteau P. Ulcerative colitis and electronic cigarette: what's the matter? Am J Gastroenterol. 2014;109(4):608–609. doi: 10.1038/ajg.2013.439. http://dx.doi.org/10.1038/ajg.2013.439. [DOI] [PubMed] [Google Scholar]
- 201.Cantrell FL. Adverse effects of e-cigarette exposures. J Community Health. 2014;39(3):614–616. doi: 10.1007/s10900-013-9807-5. http://dx.doi.org/10.1007/s10900-013-9807-5. [DOI] [PubMed] [Google Scholar]
- 202.Caponnetto P, Auditore R, Russo C, Cappello GC, Polosa R. Impact of an electronic cigarette on smoking reduction and cessation in schizophrenic smokers: a prospective 12-month pilot study. Int J Environ Res Public Health. 2013;10(2):446–461. doi: 10.3390/ijerph10020446. http://dx.doi.org/10.3390/ijerph10020446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Caponnetto P, Campagna D, Cibella F, et al. EffiCiency and Safety of an eLectronic cigAreTte (ECLAT) as tobacco cigarettes substitute: a prospective 12-month randomized control design study. PLoS One. 2013;8(6):e66317. doi: 10.1371/journal.pone.0066317. http://dx.doi.org/10.1371/journal.pone.0066317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Chatham-Stephens K, Law R, Taylor E, et al. Notes from the field: calls to poison centers for exposures to electronic cigarettes--United States, September 2010–February 2014. MMWR Morb Mortal Wkly Rep. 2014;63(13):292–293. [PMC free article] [PubMed] [Google Scholar]
- 205.Chen IL. FDA summary of adverse events on electronic cigarettes. Nicotine Tob Res. 2013;15(2):615–616. doi: 10.1093/ntr/nts145. http://dx.doi.org/10.1093/ntr/nts145. [DOI] [PubMed] [Google Scholar]
- 206.Dawkins L, Corcoran O. Acute electronic cigarette use: nicotine delivery and subjective effects in regular users. Psychopharmacology (Berl) 2014;231(2):401–407. doi: 10.1007/s00213-013-3249-8. http://dx.doi.org/10.1007/s00213-013-3249-8. [DOI] [PubMed] [Google Scholar]
- 207.Dawkins L, Turner J, Crowe E. Nicotine derived from the electronic cigarette improves time-based prospective memory in abstinent smokers. Psychopharmacology (Berl) 2013;227(3):377–384. doi: 10.1007/s00213-013-2983-2. http://dx.doi.org/10.1007/s00213-013-2983-2. [DOI] [PubMed] [Google Scholar]
- 208.Dawkins L, Turner J, Hasna S, Soar K. The electronic-cigarette: effects on desire to smoke, withdrawal symptoms and cognition. Addict Behav. 2012;37(8):970–973. doi: 10.1016/j.addbeh.2012.03.004. http://dx.doi.org/10.1016/j.addbeh.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 209.Eissenberg T. Electronic nicotine delivery devices: ineffective nicotine delivery and craving suppression after acute administration. Tob Control. 2010;19(1):87–88. doi: 10.1136/tc.2009.033498. http://dx.doi.org/10.1136/tc.2009.033498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Etter JF. Levels of saliva cotinine in electronic cigarette users. Addiction. 2014;109(5):825–829. doi: 10.1111/add.12475. http://dx.doi.org/10.1111/add.12475. [DOI] [PubMed] [Google Scholar]
- 211.Etter JF, Bullen C. Saliva cotinine levels in users of electronic cigarettes. Eur Respir J. 2011;38(5):1219–1220. doi: 10.1183/09031936.00066011. http://dx.doi.org/10.1183/09031936.00066011. [DOI] [PubMed] [Google Scholar]
- 212.Farsalinos KE, Romagna G. Chronic idiopathic neutrophilia in a smoker, relieved after smoking cessation with the use of electronic cigarette: a case report. Clin Med Insights Case Rep. 2013;6:15–21. doi: 10.4137/CCRep.S11175. http://dx.doi.org/10.4137/CCRep.S11175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Farsalinos KE, Romagna G, Allifranchini E, et al. Comparison of the cytotoxic potential of cigarette smoke and electronic cigarette vapour extract on cultured myocardial cells. Int J Environ Res Public Health. 2013;10(10):5146–5162. doi: 10.3390/ijerph10105146. http://dx.doi.org/10.3390/ijerph10105146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Farsalinos KE, Romagna G, Tsiapras D, Kyrzopoulos S, Voudris V. Characteristics, perceived side effects and benefits of electronic cigarette use: a worldwide survey of more than 19,000 consumers. Int J Environ Res Public Health. 2014;11(4):4356–4373. doi: 10.3390/ijerph110404356. http://dx.doi.org/10.3390/ijerph110404356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Flouris AD, Chorti MS, Poulianiti KP, et al. Acute impact of active and passive electronic cigarette smoking on serum cotinine and lung function. Inhal Toxicol. 2013;25(2):91–101. doi: 10.3109/08958378.2012.758197. http://dx.doi.org/10.3109/08958378.2012.758197. [DOI] [PubMed] [Google Scholar]
- 216.Flouris AD, Poulianiti KP, Chorti MS, et al. Acute effects of electronic and tobacco cigarette smoking on complete blood count. Food Chem Toxicol. 2012;50(10):3600–3603. doi: 10.1016/j.fct.2012.07.025. http://dx.doi.org/10.1016/j.fct.2012.07.025. [DOI] [PubMed] [Google Scholar]
- 217.Hua M, Alfi M, Talbot P. Health-related effects reported by electronic cigarette users in online forums. J Med Internet Res. 2013;15(4):e59. doi: 10.2196/jmir.2324. http://dx.doi.org/10.2196/jmir.2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.McCauley L, Markin C, Hosmer D. An unexpected consequence of electronic cigarette use. Chest. 2012;141(4):1110–1113. doi: 10.1378/chest.11-1334. http://dx.doi.org/10.1378/chest.11-1334. [DOI] [PubMed] [Google Scholar]
- 219.Thornton SL, Oller L, Sawyer T. Fatal intravenous injection of electronic nicotine delivery system refilling solution. J Med Toxicol. 2014;10(2):202–204. doi: 10.1007/s13181-014-0380-9. http://dx.doi.org/10.1007/s13181-014-0380-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Thota D, Latham E. Case report of electronic cigarettes possibly associated with eosinophilic pneumonitis in a previously healthy active-duty sailor. J Emerg Med. 2014;47(1):15–17. doi: 10.1016/j.jemermed.2013.09.034. http://dx.doi.org/10.1016/j.jemermed.2013.09.034. [DOI] [PubMed] [Google Scholar]
- 221.van Staden SR, Groenewald M, Engelbrecht R, Becker PJ, Hazelhurst LT. Carboxyhaemoglobin levels, health and lifestyle perceptions in smokers converting from tobacco cigarettes to electronic cigarettes. S Afr Med J. 2013;103(11):865–868. doi: 10.7196/samj.6887. http://dx.doi.org/10.7196/samj.6887. [DOI] [PubMed] [Google Scholar]
- 222.Vansickel AR, Cobb CO, Weaver MF, Eissenberg TE. A clinical laboratory model for evaluating the acute effects of electronic •cigarettes•: nicotine delivery profile and cardiovascular and subjective effects. Cancer Epidemiol Biomarkers Prev. 2010;19(8):1945–1953. doi: 10.1158/1055-9965.EPI-10-0288. http://dx.doi.org/10.1158/1055-9965.EPI-10-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Vansickel AR, Weaver MF, Eissenberg T. Clinical laboratory assessment of the abuse liability of an electronic cigarette. Addiction. 2012;107(8):1493–1500. doi: 10.1111/j.1360-0443.2012.03791.x. http://dx.doi.org/10.1111/j.1360-0443.2012.03791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Vardavas CI, Anagnostopoulos N, Kougias M, Evangelopoulou V, Connolly GN, Behrakis PK. Short-term pulmonary effects of using an electronic cigarette: impact on respiratory flow resistance, impedance, and exhaled nitric oxide. Chest. 2012;141(6):1400–1406. doi: 10.1378/chest.11-2443. http://dx.doi.org/10.1378/chest.11-2443. [DOI] [PubMed] [Google Scholar]
- 225.Wagener TL, Meier E, Hale JJ, et al. Pilot investigation of changes in readiness and confidence to quit smoking after E-cigarette experimentation and 1 week of use. Nicotine Tob Res. 2014;16(1):108–114. doi: 10.1093/ntr/ntt138. http://dx.doi.org/10.1093/ntr/ntt138. [DOI] [PubMed] [Google Scholar]
- 226.Farsalinos KE, Tsiapras D, Kyrzopoulos S, Savvopoulou M, Voudris V. Acute effects of using an electronic nicotine-delivery device (electronic cigarette) on myocardial function: comparison with the effects of regular cigarettes. BMC Cardiovasc Disord. 2014;14(1):78. doi: 10.1186/1471-2261-14-78. http://dx.doi.org/10.1186/1471-2261-14-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Tzatzarakis MN, Tsitoglou KI, Chorti MS, et al. Acute and short term impact of active and passive tobacco and electornic cigarette smoking on inflammatory markers. [Abstract] Toxicology Letters. 2013;221S:S86. http://dx.doi.org/10.1016/j.toxlet.2013.05.101. [Google Scholar]
- 228.Cervellati F, Muresan XM, Sticozzi C, et al. Comparative effects between electronic and cigarette smoke in human keratinocytes and epithelial lung cells. Toxicol In Vitro. 2014;28(5):999–1005. doi: 10.1016/j.tiv.2014.04.012. http://dx.doi.org/10.1016/j.tiv.2014.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Vakkalanka JP, Hardison LS, Jr, Holstege CP. Epidemiological trends in electronic cigarette exposures reported to U.S. Poison Centers. Clin Toxicol (Phila) 2014;52(5):542–548. doi: 10.3109/15563650.2014.913176. http://dx.doi.org/10.3109/15563650.2014.913176. [DOI] [PubMed] [Google Scholar]
- 230.Polosa R, Morjaria J, Caponnetto P, et al. Effect of smoking abstinence and reduction in asthmatic smokers switching to electronic cigarettes: evidence for harm reversal. Int J Environ Res Public Health. 2014;11(5):4965–4977. doi: 10.3390/ijerph110504965. http://dx.doi.org/10.3390/ijerph110504965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Behar RZ, Davis B, Wang Y, Bahl V, Lin S, Talbot P. Identification of toxicants in cinnamon-flavored electronic cigarette refill fluids. Toxicol In Vitro. 2014;28(2):198–208. doi: 10.1016/j.tiv.2013.10.006. http://dx.doi.org/10.1016/j.tiv.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 232.Nides MA, Leischow SJ, Bhatter M, Simmons M. Nicotine blood levels and short-term smoking reduction with an electronic nicotine delivery system. Am J Health Behav. 2014;38(2):265–274. doi: 10.5993/AJHB.38.2.12. http://dx.doi.org/10.5993/AJHB.38.2.12. [DOI] [PubMed] [Google Scholar]
- 233.Hajek P, Goniewicz ML, Phillips A, Myers Smith K, West O, McRobbie H. Nicotine intake from electronic cigarettes on initial use and after 4 weeks of regular use. Nicotine Tob Res. 2015;17(2):175–179. doi: 10.1093/ntr/ntu153. http://dx.doi.org/10.1093/ntr/ntu153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Eberlein CK, Frieling H, Kohnlein T, Hillemacher T, Bleich S. Suicide attempt by poisoning using nicotine liquid for use in electronic cigarettes. Am J Psychiatry. 2014;171(8):891. doi: 10.1176/appi.ajp.2014.14030277. http://dx.doi.org/10.1176/appi.ajp.2014.14030277. [DOI] [PubMed] [Google Scholar]
- 235.Schipper EM, de Graaff LC, Koch BC, et al. A New Challenge: Suicide Attempt using Nicotine Fillings for Electronic Cigarettes. Br J Clin Pharmacol. 2014;78(6):1469–1471. doi: 10.1111/bcp.12495. http://dx.doi.org/10.1111/bcp.12495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Ballbe M, Martinez-Sanchez JM, Sureda X, et al. Cigarettes vs. e-cigarettes: Passive exposure at home measured by means of airborne marker and biomarkers. Environ Res. 2014;135c:76–80. doi: 10.1016/j.envres.2014.09.005. http://dx.doi.org/10.1016/j.envres.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 237.Hecht SS, Carmella SG, Kotandeniya D, et al. Evaluation of toxicant and carcinogen metabolites in the urine of e-cigarette users versus cigarette smokers. Nicotine Tob Res. 2015;17(6):704–709. doi: 10.1093/ntr/ntu218. http://dx.doi.org/10.1093/ntr/ntu218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Misra M, Leverette RD, Cooper BT, Bennett MB, Brown SE. Comparative in vitro toxicity profile of electronic and tobacco cigarettes, smokeless tobacco and nicotine replacement therapy products: e-liquids, extracts and collected aerosols. Int J Environ Res Public Health. 2014;11(11):11325–11347. doi: 10.3390/ijerph111111325. http://dx.doi.org/10.3390/ijerph111111325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Norton KJ, June KM, O'Connor RJ. Initial puffing behaviors and subjective responses differ between an electronic nicotine delivery system and traditional cigarettes. Tob Induc Dis. 2014;12(1):17. doi: 10.1186/1617-9625-12-17. http://dx.doi.org/10.1186/1617-9625-12-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Ordonez JE, Kleinschmidt KC, Forrester MB. Electronic cigarette exposures reported to Texas poison centers. Nicotine Tob Res. 2015;17(2):209–211. doi: 10.1093/ntr/ntu223. http://dx.doi.org/10.1093/ntr/ntu223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Polosa R, Caponnetto P, Maglia M, Morjaria JB, Russo C. Success rates with nicotine personal vaporizers: a prospective 6-month pilot study of smokers not intending to quit. BMC Public Health. 2014;14:1159. doi: 10.1186/1471-2458-14-1159. http://dx.doi.org/10.1186/1471-2458-14-1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Popa C. Infrared spectroscopy study of the influence of inhaled vapors/smoke produced by cigarettes of active smokers. J Biomed Opt. 2015;20(5):051003. doi: 10.1117/1.JBO.20.5.051003. http://dx.doi.org/10.1117/1.JBO.20.5.051003. [DOI] [PubMed] [Google Scholar]
- 243.Sussan TE, Gajghate S, Thimmulappa RK, et al. Exposure to electronic cigarettes impairs pulmonary anti-bacterial and anti-viral defenses in a mouse model. PLoS One. 2015;10(2):e0116861. doi: 10.1371/journal.pone.0116861. http://dx.doi.org/10.1371/journal.pone.0116861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Willershausen I, Wolf T, Weyer V, Sader R, Ghanaati S, Willershausen B. Influence of E-smoking liquids on human periodontal ligament fibroblasts. Head Face Med. 2014;10:39. doi: 10.1186/1746-160X-10-39. http://dx.doi.org/10.1186/1746-160X-10-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Wu Q, Jiang D, Minor M, Chu HW. Electronic cigarette liquid increases inflammation and virus infection in primary human airway epithelial cells. PLoS One. 2014;9(9):e108342. doi: 10.1371/journal.pone.0108342. http://dx.doi.org/10.1371/journal.pone.0108342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Yan XS, D'Ruiz C. Effects of using electronic cigarettes on nicotine delivery and cardiovascular function in comparison with regular cigarettes. Regul Toxicol Pharmacol. 2015;71(1):24–34. doi: 10.1016/j.yrtph.2014.11.004. http://dx.doi.org/10.1016/j.yrtph.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 247.Lerner CA, Sundar IK, Yao H, et al. Vapors produced by electronic cigarettes and e-juices with flavorings induce toxicity, oxidative stress, and inflammatory response in lung epithelial cells and in mouse lung. PLoS One. 2015;10(2):e0116732. doi: 10.1371/journal.pone.0116732. http://dx.doi.org/10.1371/journal.pone.0116732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Cooke WH, Pokhrel A, Dowling C, Fogt DL, Rickards CA. Acute inhalation of vaporized nicotine increases arterial pressure in young non-smokers: a pilot study. Clin Auton Res. 2015;25(4):267–270. doi: 10.1007/s10286-015-0304-z. http://dx.doi.org/10.1007/s10286-015-0304-z. [DOI] [PubMed] [Google Scholar]
- 249.Forrester MB. Pediatric Exposures to Electronic Cigarettes Reported to Texas Poison Centers. J Emerg Med. 2015;49(2):136–142. doi: 10.1016/j.jemermed.2014.12.073. http://dx.doi.org/10.1016/j.jemermed.2014.12.073. [DOI] [PubMed] [Google Scholar]
- 250.Husari A, Shihadeh A, Talih S, Hashem Y, El Sabban M, Zaatari G. Acute Exposure to Electronic and Combustible Cigarette Aerosols: Effects in an Animal Model and in Human Alveolar Cells. Nicotine Tob Res. 2016;18(5):613–619. doi: 10.1093/ntr/ntv169. http://dx.doi.org/10.1093/ntr/ntv169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.LoVecchio F, Zoph O. Incidence of electronic cigarette exposures in children skyrockets in Arizona. Am J Emerg Med. 2015;33(6):834–835. doi: 10.1016/j.ajem.2015.02.036. http://dx.doi.org/10.1016/j.ajem.2015.02.036. [DOI] [PubMed] [Google Scholar]
- 252.Manzoli L, Flacco ME, Fiore M, et al. Electronic Cigarettes Efficacy and Safety at 12 Months: Cohort Study. PLoS One. 2015;10(6):e0129443. doi: 10.1371/journal.pone.0129443. http://dx.doi.org/10.1371/journal.pone.0129443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Maridet C, Atge B, Amici JM, Taieb A, Milpied B. The electronic cigarette: the new source of nickel contact allergy of the 21st century? Contact Dermatitis. 2015;73(1):49–50. doi: 10.1111/cod.12373. http://dx.doi.org/10.1111/cod.12373. [DOI] [PubMed] [Google Scholar]
- 254.Neilson L, Mankus C, Thorne D, Jackson G, DeBay J, Meredith C. Development of an in vitro cytotoxicity model for aerosol exposure using 3D reconstructed human airway tissue; application for assessment of e-cigarette aerosol. Toxicol In Vitro. 2015;29(7):1952–1962. doi: 10.1016/j.tiv.2015.05.018. http://dx.doi.org/10.1016/j.tiv.2015.05.018. [DOI] [PubMed] [Google Scholar]
- 255.Sancilio S, Gallorini M, Cataldi A, di Giacomo V. Cytotoxicity and apoptosis induction by e-cigarette fluids in human gingival fibroblasts. Clin Oral Investig. 2016;20(3):477–483. doi: 10.1007/s00784-015-1537-x. http://dx.doi.org/10.1007/s00784-015-1537-x. [DOI] [PubMed] [Google Scholar]
- 256.Schweitzer KS, Chen SX, Law S, et al. Endothelial disruptive proinflammatory effects of nicotine and e-cigarette vapor exposures. Am J Physiol Lung Cell Mol Physiol. 2015;309(2):L175–L187. doi: 10.1152/ajplung.00411.2014. http://dx.doi.org/10.1152/ajplung.00411.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Szoltysek-Boldys I, Sobczak A, Zielinska-Danch W, Barton A, Koszowski B, Kosmider L. Influence of inhaled nicotine source on arterial stiffness. Przegl Lek. 2014;71(11):572–575. [PubMed] [Google Scholar]
- 258.Durmowicz EL, Rudy SF, Chen IL. Electronic cigarettes: analysis of FDA adverse experience reports in non-users. Tob Control. 2016;25(2):242. doi: 10.1136/tobaccocontrol-2015-052235. http://dx.doi.org/10.1136/tobaccocontrol-2015-052235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Gupta S, Gandhi A, Manikonda R. Accidental nicotine liquid ingestion: emerging paediatric problem. Arch Dis Child. 2014;99(12):1149. doi: 10.1136/archdischild-2014-306750. http://dx.doi.org/10.1136/archdischild-2014-306750. [DOI] [PubMed] [Google Scholar]
- 260.Scheffler S, Dieken H, Krischenowski O, Forster C, Branscheid D, Aufderheide M. Evaluation of E-cigarette liquid vapor and mainstream cigarette smoke after direct exposure of primary human bronchial epithelial cells. Int J Environ Res Public Health. 2015;12(4):3915–3925. doi: 10.3390/ijerph120403915. http://dx.doi.org/10.3390/ijerph120403915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Vannier S, Ronziere T, Ferre JC, Lassalle V, Verin M. Reversible cerebral vasoconstriction syndrome triggered by an electronic cigarette: case report. Eur J Neurol. 2015;22(5):e64–e65. doi: 10.1111/ene.12657. http://dx.doi.org/10.1111/ene.12657. [DOI] [PubMed] [Google Scholar]
- 262.Hureaux J, Drouet M, Urban T. A case report of subacute bronchial toxicity induced by an electronic cigarette. Thorax. 2014;69(6):596–597. doi: 10.1136/thoraxjnl-2013-204767. http://dx.doi.org/10.1136/thoraxjnl-2013-204767. [DOI] [PubMed] [Google Scholar]
- 263.Dicpinigaitis PV, Lee Chang A, Dicpinigaitis AJ, Negassa A. Effect of e-Cigarette Use on Cough Reflex Sensitivity. Chest. 2016;149(1):161–165. doi: 10.1378/chest.15-0817. http://dx.doi.org/10.1378/chest.15-0817. [DOI] [PubMed] [Google Scholar]
- 264.D'Ruiz CD, Graff DW, Yan XS. Nicotine delivery, tolerability and reduction of smoking urge in smokers following short-term use of one brand of electronic cigarettes. BMC Public Health. 2015;15(1):991. doi: 10.1186/s12889-015-2349-2. http://dx.doi.org/10.1186/s12889-015-2349-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.McRobbie H, Phillips A, Goniewicz ML, et al. Effects of Switching to Electronic Cigarettes with and without Concurrent Smoking on Exposure to Nicotine, Carbon Monoxide, and Acrolein. Cancer Prev Res (Phila) 2015;8(9):873–878. doi: 10.1158/1940-6207.CAPR-15-0058. http://dx.doi.org/10.1158/1940-6207.CAPR-15-0058. [DOI] [PubMed] [Google Scholar]
- 266.Mowry JB, Spyker DA, Brooks DE, McMillan N, Schauben JL. 2014 Annual Report of the American Association of Poison Control Centers' National Poison Data System (NPDS): 32nd Annual Report. Clin Toxicol (Phila) 2015;53(10):962–1147. doi: 10.3109/15563650.2015.1102927. http://dx.doi.org/10.3109/15563650.2015.1102927. [DOI] [PubMed] [Google Scholar]
- 267.Nutt DJ, Phillips LD, Balfour D, et al. Estimating the harms of nicotine-containing products using the MCDA approach. Eur Addict Res. 2014;20(5):218–225. doi: 10.1159/000360220. http://dx.doi.org/10.1159/000360220. [DOI] [PubMed] [Google Scholar]
- 268.Aug A, Altraja S, Kilk K, Porosk R, Soomets U, Altraja A. E-Cigarette Affects the Metabolome of Primary Normal Human Bronchial Epithelial Cells. PLoS One. 2015;10(11):e0142053. doi: 10.1371/journal.pone.0142053. http://dx.doi.org/10.1371/journal.pone.0142053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Carnevale R, Sciarretta S, Violi F, et al. Acute impact of tobacco versus electronic cigarette smoking on oxidative stress and vascular function. Chest. 2016;150(3):606–612. doi: 10.1016/j.chest.2016.04.012. http://dx.doi.org/10.1016/j.chest.2016.04.012. [DOI] [PubMed] [Google Scholar]
- 270.Chen BC, Bright SB, Trivedi AR, Valento M. Death following intentional ingestion of e-liquid. Clin Toxicol (Phila) 2015;53(9):914–916. doi: 10.3109/15563650.2015.1090579. http://dx.doi.org/10.3109/15563650.2015.1090579. [DOI] [PubMed] [Google Scholar]
- 271.Dawkins LE, Kimber CF, Doig M, Feyerabend C, Corcoran O. Self-titration by experienced e-cigarette users: blood nicotine delivery and subjective effects. Psychopharmacology (Berl) 2016;233(15–16):2933–2941. doi: 10.1007/s00213-016-4338-2. http://dx.doi.org/10.1007/s00213-016-4338-2. [DOI] [PubMed] [Google Scholar]
- 272.Dicpinigaitis PV, Lee Chang A, Dicpinigaitis AJ, Negassa A. Effect of Electronic Cigarette Use on the Urge-to-Cough Sensation. Nicotine Tob Res. 2016;18(8):1763–1765. doi: 10.1093/ntr/ntw021. http://dx.doi.org/10.1093/ntr/ntw021. [DOI] [PubMed] [Google Scholar]
- 273.Farsalinos K, Cibella F, Caponnetto P, et al. Effect of continuous smoking reduction and abstinence on blood pressure and heart rate in smokers switching to electronic cigarettes. Intern Emerg Med. 2016;11(1):85–94. doi: 10.1007/s11739-015-1361-y. http://dx.doi.org/10.1007/s11739-015-1361-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Ferrari M, Zanasi A, Nardi E, et al. Short-term effects of a nicotine-free e-cigarette compared to a traditional cigarette in smokers and non-smokers. BMC Pulm Med. 2015;15:120. doi: 10.1186/s12890-015-0106-z. http://dx.doi.org/10.1186/s12890-015-0106-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Higham A, Rattray NJ, Dewhurst JA, et al. Electronic cigarette exposure triggers neutrophil inflammatory responses. Respir Res. 2016;17(1):56. doi: 10.1186/s12931-016-0368-x. http://dx.doi.org/10.1186/s12931-016-0368-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Hom S, Chen L, Wang T, Ghebrehiwet B, Yin W, Rubenstein DA. Platelet activation, adhesion, inflammation, and aggregation potential are altered in the presence of electronic cigarette extracts of variable nicotine concentrations. Platelets. 2016:1–9. doi: 10.3109/09537104.2016.1158403. http://dx.doi.org/10.3109/09537104.2016.1158403. [DOI] [PubMed] [Google Scholar]
- 277.Howard C. A New Source for Nicotine Exposures in Pediatric Patients: Electronic Cigarettes. J Emerg Nurs. 2016;42(5):451–453. doi: 10.1016/j.jen.2016.03.008. http://dx.doi.org/10.1016/j.jen.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 278.Iskandar AR, Gonzalez-Suarez I, Majeed S, et al. A framework for in vitro systems toxicology assessment of e-liquids. Toxicol Mech Methods. 2016;26(6):389–413. doi: 10.3109/15376516.2016.1170251. http://dx.doi.org/10.3109/15376516.2016.1170251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Ji EH, Sun B, Zhao T, et al. Characterization of Electronic Cigarette Aerosol and Its Induction of Oxidative Stress Response in Oral Keratinocytes. PloS One. 2016;11(5):e0154447. doi: 10.1371/journal.pone.0154447. http://dx.doi.org/10.1371/journal.pone.0154447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Kamboj A, Spiller HA, Casavant MJ, Chounthirath T, Smith GA. Pediatric Exposure to E-Cigarettes, Nicotine, and Tobacco Products in the United States. Pediatrics. 2016;137(6):e20160041. doi: 10.1542/peds.2016-0041. http://dx.doi.org/10.1542/peds.2016-0041. [DOI] [PubMed] [Google Scholar]
- 281.Khairudin MN, Mohd Zahidin AZ, Bastion ML. Front to back ocular injury from a vaping-related explosion. BMJ Case Rep. 2016 doi: 10.1136/bcr-2016-214964. http://dx.doi.org/10.1136/bcr-2016-214964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Kotandeniya D, Carmella SG, Pillsbury ME, Hecht SS. Combined analysis of N'-nitrosonornicotine and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol in the urine of cigarette smokers and e-cigarette users. J Chromatogr B Analyt Technol Biomed Life Sci. 2015;1007:121–126. doi: 10.1016/j.jchromb.2015.10.012. http://dx.doi.org/10.1016/j.jchromb.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Kumetz EA, Hurst ND, Cudnik RJ, Rudinsky SL. Electronic cigarette explosion injuries: A case series. Am J Emerg Med. doi: 10.1016/j.ajem.2016.04.010. In press. Online April 8, 2016. http://dx.doi.org/10.1016/j.ajem.2016.04.010. [DOI] [PubMed] [Google Scholar]
- 284.Maina G, Castagnoli C, Passini V, et al. Transdermal nicotine absorption handling e-cigarette refill liquids. Regul Toxicol Pharmacol. 2016;74:31–33. doi: 10.1016/j.yrtph.2015.11.014. http://dx.doi.org/10.1016/j.yrtph.2015.11.014. [DOI] [PubMed] [Google Scholar]
- 285.Page F, Hamnett N, Wearn C, Hardwicke J, Moiemen N. The acute effects of electronic cigarette smoking on the cutaneous circulation. J Plast Reconstr Aesthet Surg. 2016;69(4):575–577. doi: 10.1016/j.bjps.2015.12.015. http://dx.doi.org/10.1016/j.bjps.2015.12.015. [DOI] [PubMed] [Google Scholar]
- 286.Paley GL, Echalier E, Eck TW, et al. Corneoscleral Laceration and Ocular Burns Caused by Electronic Cigarette Explosions. Cornea. 2016;35(7):1015–1018. doi: 10.1097/ICO.0000000000000881. http://dx.doi.org/10.1097/ICO.0000000000000881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Polosa R, Morjaria JB, Caponnetto P, et al. Persisting long term benefits of smoking abstinence and reduction in asthmatic smokers who have switched to electronic cigarettes. Discov Med. 2016;21(114):99–108. [PubMed] [Google Scholar]
- 288.Pratt SI, Sargent J, Daniels L, Santos MM, Brunette M. Appeal of electronic cigarettes in smokers with serious mental illness. Addict Behav. 2016;59:30–34. doi: 10.1016/j.addbeh.2016.03.009. http://dx.doi.org/10.1016/j.addbeh.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 289.Reuther WJ, Hale B, Matharu J, Blythe JN, Brennan PA. Do you mind if I vape? Immediate effects of electronic cigarettes on perfusion in buccal mucosal tissue - a pilot study. Br J Oral Maxillofac Surg. 2016;54(3):338–341. doi: 10.1016/j.bjoms.2015.12.001. http://dx.doi.org/10.1016/j.bjoms.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 290.Ring Madsen L, Vinther Krarup NH, Bergmann TK, et al. A Cancer That Went Up in Smoke: Pulmonary Reaction to e-Cigarettes Imitating Metastatic Cancer. Chest. 2016;149(3):e65–e67. doi: 10.1016/j.chest.2015.09.003. http://dx.doi.org/10.1016/j.chest.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 291.Rudy SF, Durmowicz EL. Electronic nicotine delivery systems: overheating, fires and explosions. Tob Control. doi: 10.1136/tobaccocontrol-2015-052626. In press. Online March 9, 2016. http://dx.doi.org/10.1136/tobaccocontrol-2015-052626. [DOI] [PubMed] [Google Scholar]
- 292.Russo C, Cibella F, Caponnetto P, et al. Evaluation of Post Cessation Weight Gain in a 1-Year Randomized Smoking Cessation Trial of Electronic Cigarettes. Sci Rep. 2016;6:18763. doi: 10.1038/srep18763. http://dx.doi.org/10.1038/srep18763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Schaal C, Chellappan S. Nicotine-Mediated Regulation of Nicotinic Acetylcholine Receptors in Non-Small Cell Lung Adenocarcinoma by E2F1 and STAT1 Transcription Factors. PloS One. 2016;11(5):e0156451. doi: 10.1371/journal.pone.0156451. http://dx.doi.org/10.1371/journal.pone.0156451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Scheffler S, Dieken H, Krischenowski O, Aufderheide M. Cytotoxic Evaluation of e-Liquid Aerosol using Different Lung-Derived Cell Models. Int J Environ Res Public Health. 2015;12(10):12466–12474. doi: 10.3390/ijerph121012466. http://dx.doi.org/10.3390/ijerph121012466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Shen Y, Wolkowicz MJ, Kotova T, Fan L, Timko MP. Transcriptome sequencing reveals e-cigarette vapor and mainstream-smoke from tobacco cigarettes activate different gene expression profiles in human bronchial epithelial cells. Sci Rep. 2016;6:23984. doi: 10.1038/srep23984. http://dx.doi.org/10.1038/srep23984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Sherwood CL, Boitano S. Airway epithelial cell exposure to distinct e-cigarette liquid flavorings reveals toxicity thresholds and activation of CFTR by the chocolate flavoring 2,5-dimethypyrazine. Respir Res. 2016;17(1):57. doi: 10.1186/s12931-016-0369-9. http://dx.doi.org/10.1186/s12931-016-0369-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Shivalingappa PC, Hole R, Westphal CV, Vij N. Airway Exposure to E-Cigarette Vapors Impairs Autophagy and Induces Aggresome Formation. Antioxid Redox Signal. 2016;24(4):186–204. doi: 10.1089/ars.2015.6367. http://dx.doi.org/10.1089/ars.2015.6367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Sommerfeld K, Lukasik-Glebocka M, Kulza M, et al. Intravenous and oral suicidal e-liquid poisonings with confirmed nicotine and cotinine concentrations. Forensic Sci Int. 2016;262:e15–e20. doi: 10.1016/j.forsciint.2016.03.005. http://dx.doi.org/10.1016/j.forsciint.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 299.Soule EK, Nasim A, Rosas S. Adverse Effects of Electronic Cigarette Use: A Concept Mapping Approach. Nicotine Tob Res. 2015;18(5):678–685. doi: 10.1093/ntr/ntv246. http://dx.doi.org/10.1093/ntr/ntv246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.St Helen G, Havel C, Dempsey DA, Jacob P, 3rd, Benowitz NL. Nicotine delivery, retention and pharmacokinetics from various electronic cigarettes. Addiction. 2016;111(3):535–544. doi: 10.1111/add.13183. http://dx.doi.org/10.1111/add.13183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Teasdale JE, Newby AC, Timpson NJ, Munafo MR, White SJ. Cigarette smoke but not electronic cigarette aerosol activates a stress response in human coronary artery endothelial cells in culture. Drug Alcohol Depend. 2016;163:256–260. doi: 10.1016/j.drugalcdep.2016.04.020. http://dx.doi.org/10.1016/j.drugalcdep.2016.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Valentine GW, Jatlow PI, Coffman M, Nadim H, Gueorguieva R, Sofuoglu M. The effects of alcohol-containing e-cigarettes on young adult smokers. Drug Alcohol Depend. 2016;159:272–276. doi: 10.1016/j.drugalcdep.2015.12.011. http://dx.doi.org/10.1016/j.drugalcdep.2015.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Walele T, Sharma G, Savioz R, Martin C, Williams J. A randomised, crossover study on an electronic vapour product, a nicotine inhalator and a conventional cigarette. Part B Safety and subjective effects. Regul Toxicol Pharmacol. 2016;74:193–199. doi: 10.1016/j.yrtph.2015.12.004. http://dx.doi.org/10.1016/j.yrtph.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 304.Wang MP, Ho SY, Leung LT, Lam TH. Electronic Cigarette Use and Respiratory Symptoms in Chinese Adolescents in Hong Kong. JAMA Pediatr. 2016;170(1):89–91. doi: 10.1001/jamapediatrics.2015.3024. http://dx.doi.org/10.1001/jamapediatrics.2015.3024. [DOI] [PubMed] [Google Scholar]
- 305.Yu V, Rahimy M, Korrapati A, et al. Electronic cigarettes induce DNA strand breaks and cell death independently of nicotine in cell lines. Oral Oncol. 2016;52:58–65. doi: 10.1016/j.oraloncology.2015.10.018. http://dx.doi.org/10.1016/j.oraloncology.2015.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.McGrath-Morrow SA, Hayashi M, Aherrera A, et al. The effects of electronic cigarette emissions on systemic cotinine levels, weight and postnatal lung growth in neonatal mice. PLoS One. 2015;10(2):e0118344. doi: 10.1371/journal.pone.0118344. http://dx.doi.org/10.1371/journal.pone.0118344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Ponzoni L, Moretti M, Sala M, et al. Different physiological and behavioural effects of e-cigarette vapour and cigarette smoke in mice. Eur Neuropsychopharmacol. 2015;25(10):1775–1786. doi: 10.1016/j.euroneuro.2015.06.010. http://dx.doi.org/10.1016/j.euroneuro.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 308.Romagna G, Allifranchini E, Bocchietto E, Todeschi S, Esposito M, Farsalinos KE. Cytotoxicity evaluation of electronic cigarette vapor extract on cultured mammalian fibroblasts (ClearStream-LIFE): comparison with tobacco cigarette smoke extract. Inhal Toxicol. 2013;25(6):354–361. doi: 10.3109/08958378.2013.793439. http://dx.doi.org/10.3109/08958378.2013.793439. [DOI] [PubMed] [Google Scholar]
- 309.Rubenstein DA, Hom S, Ghebrehiwet B, Yin W. Tobacco and e-cigarette products initiate Kupffer cell inflammatory responses. Mol Immunol. 2015;67(1 Pt B):652–660. doi: 10.1016/j.molimm.2015.05.020. http://dx.doi.org/10.1016/j.molimm.2015.05.020. [DOI] [PubMed] [Google Scholar]
- 310.Salturk Z, Cakir C, Sunnetci G, et al. Effects of Electronic Nicotine Delivery System on Larynx: Experimental Study. J Voice. 2015;29(5):560–563. doi: 10.1016/j.jvoice.2014.10.013. http://dx.doi.org/10.1016/j.jvoice.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 311.Smith D, Aherrera A, Lopez A, et al. Adult Behavior in Male Mice Exposed to E-Cigarette Nicotine Vapors during Late Prenatal and Early Postnatal Life. PLoS One. 2015;10(9):e0137953. doi: 10.1371/journal.pone.0137953. http://dx.doi.org/10.1371/journal.pone.0137953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Hwang JH, Lyes M, Sladewski K, et al. Electronic cigarette inhalation alters innate immunity and airway cytokines while increasing the virulence of colonizing bacteria. J Mol Med (Berl) 2016;94(6):667–679. doi: 10.1007/s00109-016-1378-3. http://dx.doi.org/10.1007/s00109-016-1378-3. [DOI] [PubMed] [Google Scholar]
- 313.Lauterstein DE, Tijerina PB, Corbett K, et al. Frontal Cortex Transcriptome Analysis of Mice Exposed to Electronic Cigarettes During Early Life Stages. Int J Environ Res Public Health. 2016;13(4) doi: 10.3390/ijerph13040417. http://dx.doi.org/10.3390/ijerph13040417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.El Golli N, Rahali D, Jrad-Lamine A, et al. Impact of electronic-cigarette refill liquid on rat testis. Toxicol Mech Methods. 2016:1–8. doi: 10.3109/15376516.2016.1163448. http://dx.doi.org/10.3109/15376516.2016.1163448. [DOI] [PubMed] [Google Scholar]
- 315.El Golli N, Dkhili H, Dallagi Y, et al. Comparison between electronic cigarette refill liquid and nicotine on metabolic parameters in rats. Life Sci. 2016;146:131–138. doi: 10.1016/j.lfs.2015.12.049. http://dx.doi.org/10.1016/j.lfs.2015.12.049. [DOI] [PubMed] [Google Scholar]
- 316.Golli NE, Jrad-Lamine A, Neffati H, et al. Impact of e-cigarette refill liquid exposure on rat kidney. Regul Toxicol Pharmacol. 2016;77:109–116. doi: 10.1016/j.yrtph.2016.02.012. http://dx.doi.org/10.1016/j.yrtph.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 317.Panitz D, Swamy H, Nehrke K. A C. elegans model of electronic cigarette use: Physiological effects of e-liquids in nematodes. BMC Pharmacol Toxicol. 2015;16:32. doi: 10.1186/s40360-015-0030-0. http://dx.doi.org/10.1186/s40360-015-0030-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Heishman SJ, Kleykamp BA, Singleton EG. Meta-analysis of the acute effects of nicotine and smoking on human performance. Psychopharmacology (Berl) 2010;210(4):453–469. doi: 10.1007/s00213-010-1848-1. http://dx.doi.org/10.1007/s00213-010-1848-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Mowry JB, Spyker DA, Cantilena LR, Jr, McMillan N, Ford M. 2013 Annual Report of the American Association of Poison Control Centers' National Poison Data System (NPDS): 31st Annual Report. Clin Toxicol (Phila) 2014;52(10):1032–1283. doi: 10.3109/15563650.2014.987397. http://dx.doi.org/10.3109/15563650.2014.987397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Soule EK, Rosas SR, Nasim A. Reasons for electronic cigarette use beyond cigarette smoking cessation: A concept mapping approach. Addict Behav. 2016;56:41–50. doi: 10.1016/j.addbeh.2016.01.008. http://dx.doi.org/10.1016/j.addbeh.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Royal College Physicians. Nicotine without smoke: Tobacco harm reduction. London: 2016. Apr, [Google Scholar]
- 322.McNeill A, Brose LS, Calder R, Hitchman S, Hajek P, McRobbie H. E-cigarettes: an evidence update -- A report commissioned by Public Health England. London, England: Public Health England; 2015. [DOI] [PubMed] [Google Scholar]
- 323.Adkison SE, O'Connor RJ, Bansal-Travers M, et al. Electronic nicotine delivery systems: international tobacco control four-country survey. Am J Prev Med. 2013;44(3):207–215. doi: 10.1016/j.amepre.2012.10.018. http://dx.doi.org/10.1016/j.amepre.2012.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Baumann AW, Kohler C, Kim YI, et al. Differences in Electronic Cigarette Awareness, Use History, and Advertisement Exposure Between Black and White Hospitalized Cigarette Smokers. J Cancer Educ. 2015;30(4):648–654. doi: 10.1007/s13187-014-0767-y. http://dx.doi.org/10.1007/s13187-014-0767-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Berg CJ, Stratton E, Schauer GL, et al. Perceived harm, addictiveness, and social acceptability of tobacco products and marijuana among young adults: marijuana, hookah, and electronic cigarettes win. Subst Use Misuse. 2015;50(1):79–89. doi: 10.3109/10826084.2014.958857. http://dx.doi.org/10.3109/10826084.2014.958857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Brown J, West R, Beard E, Michie S, Shahab L, McNeill A. Prevalence and characteristics of e-cigarette users in Great Britain: Findings from a general population survey of smokers. Addict Behav. 2014;39(6):1120–1125. doi: 10.1016/j.addbeh.2014.03.009. http://dx.doi.org/10.1016/j.addbeh.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Camenga DR, Cavallo DA, Kong G, et al. Adolescents' and Young Adults' Perceptions of Electronic Cigarettes for Smoking Cessation: A Focus Group Study. Nicotine Tob Res. 2015;17(10):1235–1241. doi: 10.1093/ntr/ntv020. http://dx.doi.org/10.1093/ntr/ntv020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Copp SR, Collins JL, Dar R, Barrett SP. The effects of nicotine stimulus and response expectancies on male and female smokers' responses to nicotine-free electronic cigarettes. Addict Behav. 2015;40:144–147. doi: 10.1016/j.addbeh.2014.09.013. http://dx.doi.org/10.1016/j.addbeh.2014.09.013. [DOI] [PubMed] [Google Scholar]
- 329.Czoli CD, Hammond D, White CM. Electronic cigarettes in Canada: prevalence of use and perceptions among youth and young adults. Can J Public Health. 2014;105(2):e97–e102. doi: 10.17269/cjph.105.4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Dawkins L, Kimber C, Puwanesarasa Y, Soar K. First- versus second-generation electronic cigarettes: predictors of choice and effects on urge to smoke and withdrawal symptoms. Addiction. 2015;110(4):669–677. doi: 10.1111/add.12807. http://dx.doi.org/10.1111/add.12807. [DOI] [PubMed] [Google Scholar]
- 331.Farquhar B, Mark K, Terplan M, Chisolm MS. Demystifying Electronic Cigarette Use in Pregnancy. J Addict Med. 2015;9(2):157–158. doi: 10.1097/ADM.0000000000000100. http://dx.doi.org/10.1097/ADM.0000000000000100. [DOI] [PubMed] [Google Scholar]
- 332.Farsalinos KE, Romagna G, Voudris V. Factors associated with dual use of tobacco and electronic cigarettes: A case control study. Int J Drug Policy. 2015;26(6):595–600. doi: 10.1016/j.drugpo.2015.01.006. http://dx.doi.org/10.1016/j.drugpo.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 333.Gravely S, Fong GT, Cummings KM, et al. Awareness, Trial, and Current Use of Electronic Cigarettes in 10 Countries: Findings from the ITC Project. Int J Environ Res Public Health. 2014;11(11):11691–11704. doi: 10.3390/ijerph111111691. http://dx.doi.org/10.3390/ijerph111111691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Haber LA, Ortiz GM. Clearing the air: Inpatient providers' knowledge, perspectives, and experience with electronic cigarettes. J Hosp Med. 2014;9(12):805–807. doi: 10.1002/jhm.2279. http://dx.doi.org/10.1002/jhm.2279. [DOI] [PubMed] [Google Scholar]
- 335.Harrell PT, Marquinez NS, Correa JB, et al. Expectancies for Cigarettes, E-Cigarettes, and Nicotine Replacement Therapies Among E-Cigarette Users (aka Vapers) Nicotine Tob Res. 2015;17(2):193–200. doi: 10.1093/ntr/ntu149. http://dx.doi.org/10.1093/ntr/ntu149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Hendricks PS, Cases MG, Thorne CB, et al. Hospitalized smokers' expectancies for electronic cigarettes versus tobacco cigarettes. Addict Behav. 2015;41:106–111. doi: 10.1016/j.addbeh.2014.09.031. http://dx.doi.org/10.1016/j.addbeh.2014.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Hummel K, Hoving C, Nagelhout GE, et al. Prevalence and reasons for use of electronic cigarettes among smokers: Findings from the International Tobacco Control (ITC) Netherlands Survey. Int J Drug Policy. 2015;26(6):601–608. doi: 10.1016/j.drugpo.2014.12.009. http://dx.doi.org/10.1016/j.drugpo.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 338.Kadimpati S, Nolan M, Warner DO. Attitudes, beliefs, and practices regarding electronic nicotine delivery systems in patients scheduled for elective surgery. Mayo Clinic Proceedings. 2015;90(1):71–76. doi: 10.1016/j.mayocp.2014.11.005. http://dx.doi.org/10.1016/j.mayocp.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 339.King BA, Patel R, Nguyen K, Dube SR. Trends in awareness and use of electronic cigarettes among U.S. adults, 2010–2013. Nicotine Tob Res. 2015;17(2):219–227. doi: 10.1093/ntr/ntu191. http://dx.doi.org/10.1093/ntr/ntu191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Kolar SK, Rogers BG, Hooper MW. Support for Indoor Bans on Electronic Cigarettes among Current and Former Smokers. Int J Environ Res Public Health. 2014;11(12):12174–12189. doi: 10.3390/ijerph111212174. http://dx.doi.org/10.3390/ijerph111212174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Kong G, Morean ME, Cavallo DA, Camenga DR, Krishnan-Sarin S. Reasons for Electronic Cigarette Experimentation and Discontinuation Among Adolescents and Young Adults. Nicotine Tob Res. 2015;17(7):847–854. doi: 10.1093/ntr/ntu257. http://dx.doi.org/10.1093/ntr/ntu257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Krishnan-Sarin S, Morean ME, Camenga DR, Cavallo DA, Kong G. E-cigarette Use Among High School and Middle School Adolescents in Connecticut. Nicotine Tob Res. 2015;17(7):810–818. doi: 10.1093/ntr/ntu243. http://dx.doi.org/10.1093/ntr/ntu243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Majeed BA, Dube SR, Sterling K, Whitney C, Eriksen MP. Opinions about electronic cigarette use in smoke-free areas among u.s. Adults, 2012. Nicotine Tob Res. 2015;17(6):675–681. doi: 10.1093/ntr/ntu235. http://dx.doi.org/10.1093/ntr/ntu235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Martinez-Sanchez JM, Ballbe M, Fu M, et al. Electronic cigarette use among adult population: a cross-sectional study in Barcelona, Spain (2013–2014) BMJ Open. 2014;4(8):e005894. doi: 10.1136/bmjopen-2014-005894. http://dx.doi.org/10.1136/bmjopen-2014-005894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.McDonald EA, Ling PM. One of several 'toys' for smoking: young adult experiences with electronic cigarettes in New York City. Tob Control. 2015;24(6):588–593. doi: 10.1136/tobaccocontrol-2014-051743. http://dx.doi.org/10.1136/tobaccocontrol-2014-051743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Pepper JK, Emery SL, Ribisl KM, Rini CM, Brewer NT. How risky is it to use e-cigarettes? Smokers' beliefs about their health risks from using novel and traditional tobacco products. J Behav Med. 2015;38(2):318–326. doi: 10.1007/s10865-014-9605-2. http://dx.doi.org/10.1007/s10865-014-9605-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Pepper JK, Ribisl KM, Emery SL, Brewer NT. Reasons for starting and stopping electronic cigarette use. Int J Environ Res Public Health. 2014;11(10):10345–10361. doi: 10.3390/ijerph111010345. http://dx.doi.org/10.3390/ijerph111010345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Pokhrel P, Fagan P, Kehl L, Herzog TA. Receptivity to E-cigarette Marketing, Harm Perceptions, and E-cigarette Use. Am J Health Behav. 2015;39(1):121–131. doi: 10.5993/AJHB.39.1.13. http://dx.doi.org/10.5993/AJHB.39.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Popova L, Ling PM. Nonsmokers' responses to new warning labels on smokeless tobacco and electronic cigarettes: an experimental study. BMC Public Health. 2014;14:997. doi: 10.1186/1471-2458-14-997. http://dx.doi.org/10.1186/1471-2458-14-997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Rutten LJ, Blake KD, Agunwamba AA, et al. Use of e-Cigarettes among Current Smokers: Associations among Reasons for Use, Quit Intentions, and Current Tobacco Use. Nicotine Tob Res. 2015;17(10):1228–1234. doi: 10.1093/ntr/ntv003. http://dx.doi.org/10.1093/ntr/ntv003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Sanders-Jackson AN, Tan AS, Bigman CA, Henriksen L. Knowledge About E-Cigarette Constituents and Regulation: Results From a National Survey of U.S. Young Adults. Nicotine Tob Res. 2015;17(10):1247–1254. doi: 10.1093/ntr/ntu276. http://dx.doi.org/10.1093/ntr/ntu276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Shiffman S, Sembower MA, Pillitteri JL, Gerlach KK, Gitchell JG. The Impact of Flavor Descriptors on Nonsmoking Teens' and Adult Smokers' Interest in Electronic Cigarettes. Nicotine Tob Res. 2015;17(10):1255–1262. doi: 10.1093/ntr/ntu333. http://dx.doi.org/10.1093/ntr/ntu333. [DOI] [PubMed] [Google Scholar]
- 353.Tucker JS, Shadel WG, Golinelli D, Ewing B. Alternative Tobacco Product Use and Smoking Cessation Among Homeless Youth in Los Angeles County. Nicotine Tob Res. 2014;16(11):1522–1526. doi: 10.1093/ntr/ntu133. http://dx.doi.org/10.1093/ntr/ntu133. [DOI] [PubMed] [Google Scholar]
- 354.Wackowski OA, Delnevo CD. Smokers' attitudes and support for e-cigarette policies and regulation in the USA. Tob Control. 2015;24(6):543–546. doi: 10.1136/tobaccocontrol-2014-051953. http://dx.doi.org/10.1136/tobaccocontrol-2014-051953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Cardenas VM, Breen PJ, Compadre CM, et al. The smoking habits of the family influence the uptake of e-cigarettes in U.S. children. Ann Epidemiol. 2015;25(1):60–62. doi: 10.1016/j.annepidem.2014.09.013. http://dx.doi.org/10.1016/j.annepidem.2014.09.013. [DOI] [PubMed] [Google Scholar]
- 356.Hamilton HA, Ferrence R, Boak A, et al. Ever Use of Nicotine and Nonnicotine Electronic Cigarettes Among High School Students in Ontario, Canada. Nicotine Tob Res. 2015;17(10):1212–1218. doi: 10.1093/ntr/ntu234. http://dx.doi.org/10.1093/ntr/ntu234. [DOI] [PubMed] [Google Scholar]
- 357.Li J, Newcombe R, Walton D. The use of, and attitudes towards, electronic cigarettes and self-reported exposure to advertising and the product in general. Aust N Z J Public Health. 2014;38(6):524–528. doi: 10.1111/1753-6405.12283. http://dx.doi.org/10.1111/1753-6405.12283. [DOI] [PubMed] [Google Scholar]
- 358.Schmidt L, Reidmohr A, Harwell TS, Helgerson SD. Prevalence and reasons for initiating use of electronic cigarettes among adults in Montana, 2013. Prev Chronic Dis. 2014;11:E204. doi: 10.5888/pcd11.140283. http://dx.doi.org/10.5888/pcd11.140283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Moore GF, Littlecott HJ, Moore L, Ahmed N, Holliday J. E-cigarette use and intentions to smoke among 10–11-year-old never-smokers in Wales. Tob Control. 2016;25(2):147–152. doi: 10.1136/tobaccocontrol-2014-052011. http://dx.doi.org/10.1136/tobaccocontrol-2014-052011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Etter JF. Explaining the effects of electronic cigarettes on craving for tobacco in recent quitters. Drug Alcohol Depend. 2015;148:102–108. doi: 10.1016/j.drugalcdep.2014.12.030. http://dx.doi.org/10.1016/j.drugalcdep.2014.12.030. [DOI] [PubMed] [Google Scholar]
- 361.Ambrose BK, Rostron BL, Johnson SE, et al. Perceptions of the Relative Harm of Cigarettes and E-cigarettes Among U.S. youth. Am J Prev Med. 2014;47(2 Suppl 1):S53–S60. doi: 10.1016/j.amepre.2014.04.016. http://dx.doi.org/10.1016/j.amepre.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Amrock SM, Zakhar J, Zhou S, Weitzman M. Perception of e-cigarette harm and its correlation with use among U.S. adolescents. Nicotine Tob Res. 2015;17(3):330–336. doi: 10.1093/ntr/ntu156. http://dx.doi.org/10.1093/ntr/ntu156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Barbeau AM, Burda J, Siegel M. Perceived efficacy of e-cigarettes versus nicotine replacement therapy among successful e-cigarette users: a qualitative approach. Addict Sci Clin Pract. 2013;8(1):5. doi: 10.1186/1940-0640-8-5. http://dx.doi.org/10.1186/1940-0640-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Berg CJ, Haardoerfer R, Escoffery C, Zheng P, Kegler M. Cigarette users' interest in using or switching to electronic nicotine delivery systems for smokeless tobacco for harm reduction, cessation, or novelty: a cross-sectional survey of U. S. adults. Nicotine Tob Res. 2015;17(2):245–255. doi: 10.1093/ntr/ntu103. http://dx.doi.org/10.1093/ntr/ntu103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Cho JH, Shin E, Moon SS. Electronic-cigarette smoking experience among adolescents. J Adolesc Health. 2011;49(5):542–546. doi: 10.1016/j.jadohealth.2011.08.001. http://dx.doi.org/10.1016/j.jadohealth.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 366.Choi K, Fabian L, Mottey N, Corbett A, Forster J. Young adults' favorable perceptions of snus, dissolvable tobacco products, and electronic cigarettes: findings from a focus group study. Am J Public Health. 2012;102(11):2088–2093. doi: 10.2105/AJPH.2011.300525. http://dx.doi.org/10.2105/AJPH.2011.300525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Choi K, Forster J. Characteristics associated with awareness, perceptions, and use of electronic nicotine delivery systems among young U.S. Midwestern adults. Am J Public Health. 2013;103(3):556–561. doi: 10.2105/AJPH.2012.300947. http://dx.doi.org/10.2105/AJPH.2012.300947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Choi K, Forster JL. Beliefs and experimentation with electronic cigarettes: a prospective analysis among young adults. Am J Prev Med. 2014;46(2):175–178. doi: 10.1016/j.amepre.2013.10.007. http://dx.doi.org/10.1016/j.amepre.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Dockrell M, Morrison R, Bauld L, McNeill A. E-cigarettes: prevalence and attitudes in Great Britain. Nicotine Tob Res. 2013;15(10):1737–1744. doi: 10.1093/ntr/ntt057. http://dx.doi.org/10.1093/ntr/ntt057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Emery SL, Vera L, Huang J, Szczypka G. Wanna know about vaping? Patterns of message exposure, seeking and sharing information about e-cigarettes across media platforms. Tob Control. 2014;23(Suppl 3):iii17–iii25. doi: 10.1136/tobaccocontrol-2014-051648. http://dx.doi.org/10.1136/tobaccocontrol-2014-051648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.England LJ, Anderson BL, Tong VT, et al. Screening practices and attitudes of obstetricians-gynecologists toward new and emerging tobacco products. Am J Obstet Gynecol. 2014;211(6):695. doi: 10.1016/j.ajog.2014.05.041. http://dx.doi.org/10.1016/j.ajog.2014.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Etter JF. Electronic cigarettes: a survey of users. BMC Public Health. 2010;10:231. doi: 10.1186/1471-2458-10-231. http://dx.doi.org/10.1186/1471-2458-10-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Faletau J, Glover M, Nosa V, Pienaar F. Looks like smoking, is it smoking?: Children's perceptions of cigarette-like nicotine delivery systems, smoking and cessation. Harm Reduct J. 2013;10(1):30. doi: 10.1186/1477-7517-10-30. http://dx.doi.org/10.1186/1477-7517-10-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Gallus S, Lugo A, Pacifici R, et al. E-cigarette awareness, use, and harm perceptions in Italy: a national representative survey. Nicotine Tob Res. 2014;16(12):1541–1548. doi: 10.1093/ntr/ntu124. http://dx.doi.org/10.1093/ntr/ntu124. [DOI] [PubMed] [Google Scholar]
- 375.Goniewicz ML, Lingas EO, Hajek P. Patterns of electronic cigarette use and user beliefs about their safety and benefits: an Internet survey. Drug Alcohol Rev. 2013;32(2):133–140. doi: 10.1111/j.1465-3362.2012.00512.x. http://dx.doi.org/10.1111/j.1465-3362.2012.00512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Harrington KF, Hull NC, Akindoju O, et al. Electronic Cigarette Awareness, Use History, and Expected Future Use Among Hospitalized Cigarette Smokers. Nicotine Tob Res. 2014;16(11):1512–1517. doi: 10.1093/ntr/ntu054. http://dx.doi.org/10.1093/ntr/ntu054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Kandra KL, Ranney LM, Lee JG, Goldstein AO. Physicians' attitudes and use of e-cigarettes as cessation devices, North Carolina, 2013. PLoS One. 2014;9(7):e103462. doi: 10.1371/journal.pone.0103462. http://dx.doi.org/10.1371/journal.pone.0103462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.King BA, Alam S, Promoff G, Arrazola R, Dube SR. Awareness and ever-use of electronic cigarettes among U.S. adults, 2010–2011. Nicotine Tob Res. 2013;15(9):1623–1627. doi: 10.1093/ntr/ntt013. http://dx.doi.org/10.1093/ntr/ntt013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Kinnunen JM, Ollila H, El-Amin Sel T, Pere LA, Lindfors PL, Rimpela AH. Awareness and determinants of electronic cigarette use among Finnish adolescents in 2013: a population-based study. Tob Control. 2015;24(e4):e264–e270. doi: 10.1136/tobaccocontrol-2013-051512. http://dx.doi.org/10.1136/tobaccocontrol-2013-051512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Kralikova E, Kubatova S, Truneckova K, Kmetova A, Hajek P. The electronic cigarette: what proportion of smokers have tried it and how many use it regularly? Addiction. 2012;107(8):1528–1529. doi: 10.1111/j.1360-0443.2012.03916.x. http://dx.doi.org/10.1111/j.1360-0443.2012.03916.x. [DOI] [PubMed] [Google Scholar]
- 381.Kralikova E, Novak J, West O, Kmetova A, Hajek P. Do e-Cigarettes Have the Potential to Compete With Conventional Cigarettes?: A Survey of Conventional Cigarette Smokers' Experiences With e-Cigarettes. Chest. 2013;144(5):1609–1614. doi: 10.1378/chest.12-2842. http://dx.doi.org/10.1378/chest.12-2842. [DOI] [PubMed] [Google Scholar]
- 382.Li J, Bullen C, Newcombe R, Walker N, Walton D. The use and acceptability of electronic cigarettes among New Zealand smokers. N Z Med J. 2013;126(1375):48–57. [PubMed] [Google Scholar]
- 383.Myslin M, Zhu SH, Chapman W, Conway M. Using Twitter to examine smoking behavior and perceptions of emerging tobacco products. J Med Internet Res. 2013;15(8):e174. doi: 10.2196/jmir.2534. http://dx.doi.org/10.2196/jmir.2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Pearson JL, Richardson A, Niaura RS, Vallone DM, Abrams DB. e-Cigarette awareness, use, and harm perceptions in U.S. adults. Am J Public Health. 2012;102(9):1758–1766. doi: 10.2105/AJPH.2011.300526. http://dx.doi.org/10.2105/AJPH.2011.300526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Pepper JK, McRee AL, Gilkey MB. Healthcare providers' beliefs and attitudes about electronic cigarettes and preventive counseling for adolescent patients. J Adolesc Health. 2014;54(6):678–683. doi: 10.1016/j.jadohealth.2013.10.001. http://dx.doi.org/10.1016/j.jadohealth.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Pepper JK, Reiter PL, McRee AL, Cameron LD, Gilkey MB, Brewer NT. Adolescent males' awareness of and willingness to try electronic cigarettes. J Adolesc Health. 2013;52(2):144–150. doi: 10.1016/j.jadohealth.2012.09.014. http://dx.doi.org/10.1016/j.jadohealth.2012.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Peters RJ, Jr, Meshack A, Lin MT, Hill M, Abughosh S. The social norms and beliefs of teenage male electronic cigarette use. J Ethn Subst Abuse. 2013;12(4):300–307. doi: 10.1080/15332640.2013.819310. http://dx.doi.org/10.1080/15332640.2013.819310. [DOI] [PubMed] [Google Scholar]
- 388.Regan AK, Promoff G, Dube SR, Arrazola R. Electronic nicotine delivery systems: adult use and awareness of the 'e-cigarette' in the USA. Tob Control. 2013;22(1):19–23. doi: 10.1136/tobaccocontrol-2011-050044. http://dx.doi.org/10.1136/tobaccocontrol-2011-050044. [DOI] [PubMed] [Google Scholar]
- 389.Richardson A, Pearson J, Xiao H, Stalgaitis C, Vallone D. Prevalence, Harm Perceptions, and Reasons for Using Noncombustible Tobacco Products Among Current and Former Smokers. Am J Public Health. 2014;104(8):1437–1444. doi: 10.2105/AJPH.2013.301804. http://dx.doi.org/10.2105/ajph.2013.301804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Sutfin EL, McCoy TP, Morrell HE, Hoeppner BB, Wolfson M. Electronic cigarette use by college students. Drug Alcohol Depend. 2013;131(3):214–221. doi: 10.1016/j.drugalcdep.2013.05.001. http://dx.doi.org/10.1016/j.drugalcdep.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Tan AS, Bigman CA. E-cigarette awareness and perceived harmfulness: prevalence and associations with smoking-cessation outcomes. Am J Prev Med. 2014;47(2):141–149. doi: 10.1016/j.amepre.2014.02.011. http://dx.doi.org/10.1016/j.amepre.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Trumbo CW, Harper R. Use and Perception of Electronic Cigarettes Among College Students. J Am Coll Health. 2013;61(3):149–155. doi: 10.1080/07448481.2013.776052. http://dx.doi.org/10.1080/07448481.2013.776052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Vickerman KA, Carpenter KM, Altman T, Nash CM, Zbikowski SM. Use of electronic cigarettes among state tobacco cessation quitline callers. Nicotine Tob Res. 2013;15(10):1787–1791. doi: 10.1093/ntr/ntt061. http://dx.doi.org/10.1093/ntr/ntt061. [DOI] [PubMed] [Google Scholar]
- 394.Wang B, King BA, Corey CG, Arrazola RA, Johnson SE. Awareness and Use of Non-conventional Tobacco Products Among U.S. Students, 2012. Am J Prev Med. 2014;47(2 Suppl 1):S36–S52. doi: 10.1016/j.amepre.2014.05.003. http://dx.doi.org/10.1016/j.amepre.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Zhu SH, Gamst A, Lee M, Cummins S, Yin L, Zoref L. The Use and Perception of Electronic Cigarettes and Snus among the U.S. Population. PLoS One. 2013;8(10):e79332. doi: 10.1371/journal.pone.0079332. http://dx.doi.org/10.1371/journal.pone.0079332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Yong HH, Borland R, Balmford J, et al. Trends in E-Cigarette Awareness, Trial, and Use Under the Different Regulatory Environments of Australia and the United Kingdom. Nicotine Tob Res. 2015;17(10):1203–1211. doi: 10.1093/ntr/ntu231. http://dx.doi.org/10.1093/ntr/ntu231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Eversman MH. Harm reduction in U.S. tobacco control: Constructions in textual news media. Int J Drug Policy. 2015;26(6):575–582. doi: 10.1016/j.drugpo.2015.01.018. http://dx.doi.org/10.1016/j.drugpo.2015.01.018. [DOI] [PubMed] [Google Scholar]
- 398.Fraser D, Weier M, Keane H, Gartner C. Vapers' perspectives on electronic cigarette regulation in Australia. Int J Drug Policy. 2015;26(6):589–594. doi: 10.1016/j.drugpo.2015.01.019. http://dx.doi.org/10.1016/j.drugpo.2015.01.019. [DOI] [PubMed] [Google Scholar]
- 399.Hiscock R, Goniewicz ML, McEwen A, et al. E-cigarettes: online survey of UK smoking cessation practitioners. Tob Induc Dis. 2014;12(1):13. doi: 10.1186/1617-9625-12-13. http://dx.doi.org/10.1186/1617-9625-12-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Kotecha S, Jawad M, Iliffe S. Knowledge, attitudes and beliefs towards waterpipe tobacco smoking and electronic shisha (e-shisha) among young adults in London: a qualitative analysis. Prim Health Care Res Dev. 2016;17(2):166–174. doi: 10.1017/S1463423615000237. http://dx.doi.org/10.1017/S1463423615000237. [DOI] [PubMed] [Google Scholar]
- 401.Rooke C, Cunningham-Burley S, Amos A. Smokers' and ex-smokers' understanding of electronic cigarettes: a qualitative study. Tob Control. 25(e1):e60–e66. doi: 10.1136/tobaccocontrol-2014-052151. http://dx.doi.org/10.1136/tobaccocontrol-2014-052151. [DOI] [PubMed] [Google Scholar]
- 402.Tan AS, Bigman CA, Mello S, Sanders-Jackson A. Is exposure to e-cigarette communication associated with perceived harms of e-cigarette secondhand vapour? Results from a national survey of U.S. adults. BMJ Open. 2015;5(3):e007134. doi: 10.1136/bmjopen-2014-007134. http://dx.doi.org/10.1136/bmjopen-2014-007134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Trumbo CW, Kim SJ. The effect of electronic cigarette advertising on intended use among college students. Addict Behav. 2015;46:77–81. doi: 10.1016/j.addbeh.2015.03.005. http://dx.doi.org/10.1016/j.addbeh.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 404.Amato MS, Boyle RG, Levy D. How to define e-cigarette prevalence? Finding clues in the use frequency distribution. Tob Control. 2016;25(e1):e24–e29. doi: 10.1136/tobaccocontrol-2015-052236. http://dx.doi.org/10.1136/tobaccocontrol-2015-052236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Anand V, McGinty KL, O'Brien K, Guenthner G, Hahn E, Martin CA. E-cigarette Use and Beliefs Among Urban Public High School Students in North Carolina. J Adolesc Health. 2015;57(1):46–51. doi: 10.1016/j.jadohealth.2015.03.018. http://dx.doi.org/10.1016/j.jadohealth.2015.03.018. [DOI] [PubMed] [Google Scholar]
- 406.Babineau K, Taylor K, Clancy L. Electronic Cigarette Use among Irish Youth: A Cross Sectional Study of Prevalence and Associated Factors. PLoS One. 2015;10(5):e0126419. doi: 10.1371/journal.pone.0126419. http://dx.doi.org/10.1371/journal.pone.0126419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Baeza-Loya S, Viswanath H, Carter A, et al. Perceptions about e-cigarette safety may lead to e-smoking during pregnancy. Bull Menninger Clin. 2014;78(3):243–252. doi: 10.1521/bumc.2014.78.3.243. http://dx.doi.org/10.1521/bumc.2014.78.3.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Barrington-Trimis JL, Berhane K, Unger JB, et al. Psychosocial Factors Associated With Adolescent Electronic Cigarette and Cigarette Use. Pediatrics. 2015;136(2):308–317. doi: 10.1542/peds.2015-0639. http://dx.doi.org/10.1542/peds.2015-0639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Berg CJ, Barr DB, Stratton E, Escoffery C, Kegler M. Attitudes toward E-Cigarettes, Reasons for Initiating E-Cigarette Use, and Changes in Smoking Behavior after Initiation: A Pilot Longitudinal Study of Regular Cigarette Smokers. Open J Prev Med. 2014;4(10):789–800. doi: 10.4236/ojpm.2014.410089. http://dx.doi.org/10.4236/ojpm.2014.410089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Cataldo JK, Petersen AB, Hunter M, Wang J, Sheon N. E-cigarette marketing and older smokers: road to renormalization. Am J Health Behav. 2015;39(3):361–371. doi: 10.5993/AJHB.39.3.9. http://dx.doi.org/10.5993/AJHB.39.3.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Chaffee BW, Gansky SA, Halpern-Felsher B, Couch ET, Essex G, Walsh MM. Conditional risk assessment of adolescents' electronic cigarette perceptions. Am J Health Behav. 2015;39(3):421–432. doi: 10.5993/AJHB.39.3.14. http://dx.doi.org/10.5993/AJHB.39.3.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Cheney MK, Gowin M, Wann TF. Vapor Store Owner Beliefs and Messages to Customers. Nicotine Tob Res. 2016;18(5):694–699. doi: 10.1093/ntr/ntv129. http://dx.doi.org/10.1093/ntr/ntv129. [DOI] [PubMed] [Google Scholar]
- 413.Chu KH, Valente TW. How different countries addressed the sudden growth of e-cigarettes in an online tobacco control community. BMJ Open. 2015;5(5):e007654. doi: 10.1136/bmjopen-2015-007654. http://dx.doi.org/10.1136/bmjopen-2015-007654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Eastwood B, Dockrell MJ, Arnott D, et al. Electronic cigarette use in young people in Great Britain 2013–2014. Public Health. 2015;129(9):1150–1156. doi: 10.1016/j.puhe.2015.07.009. http://dx.doi.org/10.1016/j.puhe.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 415.Guillet S, Sicard S, Meynard JB, Mayet A. Electronic cigarette: use and perceptions among French military nurses in 2013. Swiss Med Wkly. 2015;145:w14137. doi: 10.4414/smw.2015.14137. http://dx.doi.org/10.4414/smw.2015.14137. [DOI] [PubMed] [Google Scholar]
- 416.Harrell PT, Simmons VN, Pineiro B, et al. E-cigarettes and expectancies: why do some users keep smoking? Addiction. 2015;110(11):1833–1843. doi: 10.1111/add.13043. http://dx.doi.org/10.1111/add.13043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Kiviniemi MT, Kozlowski LT. Deficiencies in public understanding about tobacco harm reduction: results from a United States national survey. Harm Reduct J. 2015;12:21. doi: 10.1186/s12954-015-0055-0. http://dx.doi.org/10.1186/s12954-015-0055-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Li J, Newcombe R, Walton D. The prevalence, correlates and reasons for using electronic cigarettes among New Zealand adults. Addict Behav. 2015;45:245–251. doi: 10.1016/j.addbeh.2015.02.006. http://dx.doi.org/10.1016/j.addbeh.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 419.Li J, Newcombe R, Walton D. Contextual Information Around the First Use of Electronic Cigarettes Among New Zealand Smokers and Recent Quitters. Nicotine Tob Res. 2016;18(5):737–738. doi: 10.1093/ntr/ntv143. http://dx.doi.org/10.1093/ntr/ntv143. [DOI] [PubMed] [Google Scholar]
- 420.Palipudi KM, Mbulo L, Morton J, et al. Awareness and Current Use of Electronic Cigarettes in Indonesia, Malaysia, Qatar, and Greece: Findings From 2011–2013 Global Adult Tobacco Surveys. Nicotine Tob Res. 2016;18(4):501–507. doi: 10.1093/ntr/ntv081. http://dx.doi.org/10.1093/ntr/ntv081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Peters EN, Harrell PT, Hendricks PS, O'Grady KE, Pickworth WB, Vocci FJ. Electronic cigarettes in adults in outpatient substance use treatment: Awareness, perceptions, use, and reasons for use. Am J Addict. 2015;24(3):233–239. doi: 10.1111/ajad.12206. http://dx.doi.org/10.1111/ajad.12206. [DOI] [PubMed] [Google Scholar]
- 422.Shiplo S, Czoli CD, Hammond D. E-cigarette use in Canada: prevalence and patterns of use in a regulated market. BMJ Open. 2015;5(8):e007971. doi: 10.1136/bmjopen-2015-007971. http://dx.doi.org/10.1136/bmjopen-2015-007971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Stein MD, Caviness CM, Grimone K, Audet D, Borges A, Anderson BJ. E-cigarette knowledge, attitudes, and use in opioid dependent smokers. J Subst Abuse Treat. 2015;52:73–77. doi: 10.1016/j.jsat.2014.11.002. http://dx.doi.org/10.1016/j.jsat.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Link AR, Cawkwell PB, Shelley DR, Sherman SE. An Exploration of Online Behaviors and Social Media Use Among Hookah and Electronic-Cigarette Users. Addict Behav Rep. 2015;2:37–40. doi: 10.1016/j.abrep.2015.05.006. http://dx.doi.org/10.1016/j.abrep.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Little MA, Derefinko KJ, Bursac Z, et al. Prevalence and Correlates of Tobacco and Nicotine Containing Product Use in a Sample of United States Air Force Trainees. Nicotine Tob Res. 2016;18(4):416–423. doi: 10.1093/ntr/ntv090. http://dx.doi.org/10.1093/ntr/ntv090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Mark KS, Farquhar B, Chisolm MS, Coleman-Cowger VH, Terplan M. Knowledge, Attitudes, and Practice of Electronic Cigarette Use Among Pregnant Women. J Addict Med. 2015;9(4):266–272. doi: 10.1097/ADM.0000000000000128. http://dx.doi.org/10.1097/ADM.0000000000000128. [DOI] [PubMed] [Google Scholar]
- 427.Nelson VA, Goniewicz ML, Beard E, et al. Comparison of the characteristics of long-term users of electronic cigarettes versus nicotine replacement therapy: A cross-sectional survey of English ex-smokers and current smokers. Drug Alcohol Depend. 2015;153:300–305. doi: 10.1016/j.drugalcdep.2015.05.005. http://dx.doi.org/10.1016/j.drugalcdep.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Oncken CA, Litt MD, McLaughlin LD, Burki NA. Nicotine concentrations with electronic cigarette use: effects of sex and flavor. Nicotine Tob Res. 2015;17(4):473–478. doi: 10.1093/ntr/ntu232. http://dx.doi.org/10.1093/ntr/ntu232. [DOI] [PubMed] [Google Scholar]
- 429.Perkins KA, Karelitz JL, Michael VC. Reinforcement enhancing effects of acute nicotine via electronic cigarettes. Drug Alcohol Depend. 2015;153:104–108. doi: 10.1016/j.drugalcdep.2015.05.041. http://dx.doi.org/10.1016/j.drugalcdep.2015.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Roditis ML, Halpern-Felsher B. Adolescents' Perceptions of Risks and Benefits of Conventional Cigarettes, E-cigarettes, and Marijuana: A Qualitative Analysis. J Adolesc Health. 2015;57(2):179–185. doi: 10.1016/j.jadohealth.2015.04.002. http://dx.doi.org/10.1016/j.jadohealth.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Steinberg MB, Zimmermann MH, Delnevo CD, et al. E-Cigarette Versus Nicotine Inhaler: Comparing the Perceptions and Experiences of Inhaled Nicotine Devices. J Gen Intern Med. 2014;29(11):1444–1450. doi: 10.1007/s11606-014-2889-7. http://dx.doi.org/10.1007/s11606-014-2889-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Suris JC, Berchtold A, Akre C. Reasons to use e-cigarettes and associations with other substances among adolescents in Switzerland. Drug Alcohol Depend. 2015;153:140–144. doi: 10.1016/j.drugalcdep.2015.05.034. http://dx.doi.org/10.1016/j.drugalcdep.2015.05.034. [DOI] [PubMed] [Google Scholar]
- 433.Sussman S, Garcia R, Cruz TB, Baezconde-Garbanati L, Pentz MA, Unger JB. Consumers' perceptions of vape shops in Southern California: an analysis of online Yelp reviews. Tob Induc Dis. 2014;12(1):22. doi: 10.1186/s12971-014-0022-7. http://dx.doi.org/10.1186/s12971-014-0022-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Sutfin EL, Reboussin BA, Debinski B, Wagoner KG, Spangler J, Wolfson M. The Impact of Trying Electronic Cigarettes on Cigarette Smoking by College Students: A Prospective Analysis. Am J Public Health. 2015;105(8):e83–e89. doi: 10.2105/AJPH.2015.302707. http://dx.doi.org/10.2105/AJPH.2015.302707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Beard E, Brose LS, Brown J, West R, McEwen A. How are the English Stop Smoking Services responding to growth in use of electronic cigarettes? Patient Educ Couns. 2014;94(2):276–281. doi: 10.1016/j.pec.2013.10.022. http://dx.doi.org/10.1016/j.pec.2013.10.022. [DOI] [PubMed] [Google Scholar]
- 436.Bowker K, Campbell KA, Coleman T, Lewis S, Naughton F, Cooper S. Understanding Pregnant Smokers' Adherence to Nicotine Replacement Therapy During a Quit Attempt: A Qualitative Study. Nicotine Tob Res. 2016;18(5):906–912. doi: 10.1093/ntr/ntv205. http://dx.doi.org/10.1093/ntr/ntv205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Chen AT, Zhu SH. What Online Communities Can Tell Us About Electronic Cigarettes and Hookah Use: A Study Using Text Mining and Visualization Techniques. J Med Internet Res. 2015;17(9):e220. doi: 10.2196/jmir.4517. http://dx.doi.org/10.2196/jmir.4517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Eadie D, Stead M, MacKintosh AM, et al. E-cigarette marketing in UK stores: an observational audit and retailers' views. BMJ Open. 2015;5(9):e008547. doi: 10.1136/bmjopen-2015-008547. http://dx.doi.org/10.1136/bmjopen-2015-008547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Garbutt JM, Miller W, Dodd S, Bobenhouse N, Sterkel R, Strunk RC. Parental Use of Electronic Cigarettes. Acad Pediatr. 2015;15(6):599–604. doi: 10.1016/j.acap.2015.06.013. http://dx.doi.org/10.1016/j.acap.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Huang J, Kim Y, Vera L, Emery SL. Electronic Cigarettes Among Priority Populations: Role of Smoking Cessation and Tobacco Control Policies. Am J Prev Med. 2016;50(2):199–209. doi: 10.1016/j.amepre.2015.06.032. http://dx.doi.org/10.1016/j.amepre.2015.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Jiang N, Chen J, Wang MP, et al. Electronic cigarette awareness and use among adults in Hong Kong. Addict Behav. 2015;52:34–38. doi: 10.1016/j.addbeh.2015.08.008. http://dx.doi.org/10.1016/j.addbeh.2015.08.008. [DOI] [PubMed] [Google Scholar]
- 442.Li J, Newcombe R, Newcombe R, Walton D. Susceptibility to e-cigarette use among never-users: findings from a survey of New Zealand adult smokers and ex-smokers. N Z Med J. 2015;128(1421):65–68. [PubMed] [Google Scholar]
- 443.Pepper JK, Gilkey MB, Brewer NT. Physicians' Counseling of Adolescents Regarding E-Cigarette Use. J Adolesc Health. 2015;57(6):580–586. doi: 10.1016/j.jadohealth.2015.06.017. http://dx.doi.org/10.1016/j.jadohealth.2015.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Pineiro B, Correa JB, Simmons VN, et al. Gender differences in use and expectancies of e-cigarettes: Online survey results. Addict Behav. 2015;52:91–97. doi: 10.1016/j.addbeh.2015.09.006. http://dx.doi.org/10.1016/j.addbeh.2015.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Pokhrel P, Herzog TA, Muranaka N, Regmi S, Fagan P. Contexts of cigarette and e-cigarette use among dual users: a qualitative study. BMC Public Health. 2015;15:859. doi: 10.1186/s12889-015-2198-z. http://dx.doi.org/10.1186/s12889-015-2198-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.Rass O, Pacek LR, Johnson PS, Johnson MW. Characterizing Use Patterns and Perceptions of Relative Harm in Dual Users of Electronic and Tobacco Cigarettes. Exp Clin Psychopharmacol. 2015;23(6):494–503. doi: 10.1037/pha0000050. http://dx.doi.org/10.1037/pha0000050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Ruther T, Wissen F, Linhardt A, Aichert DS, Pogarell O, de Vries H. Electronic Cigarettes-Attitudes and Use in Germany. Nicotine Tob Res. 2016;18(5):660–669. doi: 10.1093/ntr/ntv188. http://dx.doi.org/10.1093/ntr/ntv188. [DOI] [PubMed] [Google Scholar]
- 448.Twyman L, Bonevski B, Paul C, Bryant J, Gartner C, Guillaumier A. Electronic Cigarettes: Awareness, Recent Use, and Attitudes Within a Sample of Socioeconomically Disadvantaged Australian Smokers. Nicotine Tob Res. 2016;18(5):670–677. doi: 10.1093/ntr/ntv183. http://dx.doi.org/10.1093/ntr/ntv183. [DOI] [PubMed] [Google Scholar]
- 449.Wackowski OA, Delnevo CD. Young Adults' Risk Perceptions of Various Tobacco Products Relative to Cigarettes: Results From the National Young Adult Health Survey. Health Educ Behav. 2016;43(3):328–336. doi: 10.1177/1090198115599988. http://dx.doi.org/10.1177/1090198115599988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Zhou S, Van Devanter N, Fenstermaker M, Cawkwell P, Sherman S, Weitzman M. A Study of the Use, Knowledge, and Beliefs About Cigarettes and Alternative Tobacco Products Among Students at One U.S. Medical School. Acad Med. 2015;90(12):1713–1719. doi: 10.1097/ACM.0000000000000873. http://dx.doi.org/10.1097/ACM.0000000000000873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Ayers JW, Ribisl KM, Brownstein JS. Tracking the rise in popularity of electronic nicotine delivery systems (electronic cigarettes) using search query surveillance. Am J Prev Med. 2011;40(4):448–453. doi: 10.1016/j.amepre.2010.12.007. http://dx.doi.org/10.1016/j.amepre.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 452.Martinez-Sanchez JM, Ballbe M, Fu M, et al. Attitudes towards Electronic Cigarettes Regulation in Indoor Workplaces and Selected Public and Private Places: A Population-Based Cross-Sectional Study. PLoS One. 2014;9(12):e114256. doi: 10.1371/journal.pone.0114256. http://dx.doi.org/10.1371/journal.pone.0114256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453.Abo-Elkheir OI, Sobh E. Knowledge about electronic cigarettes and its perception: a community survey, Egypt. Respir Res. 2016;17(1):58. doi: 10.1186/s12931-016-0365-0. http://dx.doi.org/10.1186/s12931-016-0365-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.Allem JP, Unger JB, Garcia R, Baezconde-Garbanati L, Sussman S. Tobacco Attitudes and Behaviors of Vape Shop Retailers in Los Angeles. Am J Health Behav. 2015;39(6):794–798. doi: 10.5993/AJHB.39.6.7. http://dx.doi.org/10.5993/AJHB.39.6.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Andler R, Guignard R, Wilquin JL, Beck F, Richard JB, Nguyen-Thanh V. Electronic cigarette use in France in 2014. Int J Public Health. 2016;61(2):159–165. doi: 10.1007/s00038-015-0773-9. http://dx.doi.org/10.1007/s00038-015-0773-9. [DOI] [PubMed] [Google Scholar]
- 456.Baggett TP, Campbell EG, Chang Y, Rigotti NA. Other tobacco product and electronic cigarette use among homeless cigarette smokers. Addict Behav. 2016;60:124–130. doi: 10.1016/j.addbeh.2016.04.006. http://dx.doi.org/10.1016/j.addbeh.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457.Basch CH, Kecojevic A, Menafro A. Provision of information regarding electronic cigarettes from shop employees in New York City. Public Health. 2016;136:175–177. doi: 10.1016/j.puhe.2016.03.034. http://dx.doi.org/10.1016/j.puhe.2016.03.034. [DOI] [PubMed] [Google Scholar]
- 458.Bascombe TM, Scott KN, Ballard D, Smith SA, Thompson W, Berg CJ. Primary healthcare provider knowledge, beliefs and clinic-based practices regarding alternative tobacco products and marijuana: a qualitative study. Health Educ Res. 2016;31(3):375–383. doi: 10.1093/her/cyv103. http://dx.doi.org/10.1093/her/cyv103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459.Berg CJ. Preferred flavors and reasons for e-cigarette use and discontinued use among never, current, and former smokers. Int J Public Health. 2016;61(2):225–236. doi: 10.1007/s00038-015-0764-x. http://dx.doi.org/10.1007/s00038-015-0764-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.Best C, van der Sluijs W, Haseen F, et al. Does exposure to cigarette brands increase the likelihood of adolescent e-cigarette use? A cross-sectional study. BMJ Open. 2016;6(2):e008734. doi: 10.1136/bmjopen-2015-008734. http://dx.doi.org/10.1136/bmjopen-2015-008734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461.Biener L, Song E, Sutfin EL, Spangler J, Wolfson M. Electronic Cigarette Trial and Use among Young Adults: Reasons for Trial and Cessation of Vaping. Int J Environ Res Public Health. 2015;12(12):16019–16026. doi: 10.3390/ijerph121215039. http://dx.doi.org/10.3390/ijerph121215039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462.Brose LS, Brown J, Hitchman SC, McNeill A. Perceived relative harm of electronic cigarettes over time and impact on subsequent use. A survey with 1-year and 2-year follow-ups. Drug Alcohol Depend. 2015;157:106–111. doi: 10.1016/j.drugalcdep.2015.10.014. http://dx.doi.org/10.1016/j.drugalcdep.2015.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463.Busch AM, Leavens EL, Wagener TL, Buckley ML, Tooley EM. Prevalence, Reasons for Use, and Risk Perception of Electronic Cigarettes Among Post-Acute Coronary Syndrome Smokers. J Cardiopulm Rehabil Prev. 2016;36(5):352–357. doi: 10.1097/HCR.0000000000000179. http://dx.doi.org/10.1097/HCR.0000000000000179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.Case K, Crook B, Lazard A, Mackert M. Formative Research to Identify Perceptions of E-cigarettes in College Students: Implications for Future Health Communication Campaigns. J Am Coll Health. 2016;64(5):380–389. doi: 10.1080/07448481.2016.1158180. http://dx.doi.org/10.1080/07448481.2016.1158180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465.Chen C, Zhuang YL, Zhu SH. E-Cigarette Design Preference and Smoking Cessation: A U.S. Population Study. Am J Prev Med. 2016;51(3):356–363. doi: 10.1016/j.amepre.2016.02.002. http://dx.doi.org/10.1016/j.amepre.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 466.Cheney MK, Gowin M, Wann TF. Electronic Cigarette Use in Straight-to-Work Young Adults. Am J Health Behav. 2016;40(2):268–279. doi: 10.5993/AJHB.40.2.12. http://dx.doi.org/10.5993/AJHB.40.2.12. [DOI] [PubMed] [Google Scholar]
- 467.Coleman BN, Johnson SE, Tessman GK, et al. "It's not smoke. It's not tar. It's not 4000 chemicals. Case closed": Exploring attitudes, beliefs, and perceived social norms of e-cigarette use among adult users. Drug Alcohol Depend. 2016;159:80–85. doi: 10.1016/j.drugalcdep.2015.11.028. http://dx.doi.org/10.1016/j.drugalcdep.2015.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468.Cooper M, Case KR, Loukas A, Creamer MR, Perry CL. E-cigarette Dual Users, Exclusive Users and Perceptions of Tobacco Products. Am J Health Behav. 2016;40(1):108–116. doi: 10.5993/AJHB.40.1.12. http://dx.doi.org/10.5993/AJHB.40.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 469.Cummins S, Leischow S, Bailey L, et al. Knowledge and beliefs about electronic cigarettes among quitline cessation staff. Addict Behav. 2016;60:78–83. doi: 10.1016/j.addbeh.2016.03.031. http://dx.doi.org/10.1016/j.addbeh.2016.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 470.Czoli CD, Goniewicz M, Islam T, Kotnowski K, Hammond D. Consumer preferences for electronic cigarettes: results from a discrete choice experiment. Tob Control. 2016;25(e1):e30–e36. doi: 10.1136/tobaccocontrol-2015-052422. http://dx.doi.org/10.1136/tobaccocontrol-2015-052422. [DOI] [PubMed] [Google Scholar]
- 471.de Andrade M, Angus K, Hastings G. Teenage perceptions of electronic cigarettes in Scottish tobacco-education school interventions: co-production and innovative engagement through a pop-up radio project. Perspect Public Health. 2016;136(5):288–293. doi: 10.1177/1757913915612109. http://dx.doi.org/10.1177/1757913915612109. [DOI] [PubMed] [Google Scholar]
- 472.Dunlop S, Lyons C, Dessaix A, Currow D. How are tobacco smokers using e-cigarettes? Patterns of use, reasons for use and places of purchase in New South Wales. Med J Aust. 2016;204(9):355. doi: 10.5694/mja15.01156. http://dx.doi.org/10.5694/mja15.01156. [DOI] [PubMed] [Google Scholar]
- 473.El-Shahawy O, Brown R, Elston Lafata J. Primary Care Physicians' Beliefs and Practices Regarding E-Cigarette Use by Patients Who Smoke: A Qualitative Assessment. Int J Environ Res Public Health. 2016;13(5):445. doi: 10.3390/ijerph13050445. http://dx.doi.org/10.3390/ijerph13050445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 474.Fallin A, Miller A, Assef S, Ashford K. Perceptions of Electronic Cigarettes Among Medicaid-Eligible Pregnant and Postpartum Women. J Obstet Gynecol Neonatal Nurs. 2016;45(3):320–325. doi: 10.1016/j.jogn.2016.02.009. http://dx.doi.org/10.1016/j.jogn.2016.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 475.Ford A, MacKintosh AM, Bauld L, Moodie C, Hastings G. Adolescents' responses to the promotion and flavouring of e-cigarettes. Int J Public Health. 2016;61(2):215–224. doi: 10.1007/s00038-015-0769-5. http://dx.doi.org/10.1007/s00038-015-0769-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 476.Goniewicz ML, Leigh NJ, Gawron M, et al. Dual use of electronic and tobacco cigarettes among adolescents: a cross-sectional study in Poland. Int J Public Health. 2016;61(2):189–197. doi: 10.1007/s00038-015-0756-x. http://dx.doi.org/10.1007/s00038-015-0756-x. [DOI] [PubMed] [Google Scholar]
- 477.Hammal F, Finegan BA. Exploring Attitudes of Children 12–17 Years of Age Toward Electronic Cigarettes. J Community Health. 2016;41(5):962–968. doi: 10.1007/s10900-016-0178-6. http://dx.doi.org/10.1007/s10900-016-0178-6. [DOI] [PubMed] [Google Scholar]
- 478.Hefner K, Rosenheck R, Merrel J, Coffman M, Valentine G, Sofuoglu M. E-cigarette Use in Veterans Seeking Mental Health and/or Substance Use Services. J Dual Diagn. 2016;12(2):109–117. doi: 10.1080/15504263.2016.1172895. http://dx.doi.org/10.1080/15504263.2016.1172895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 479.Hiratsuka VY, Avey JP, Trinidad SB, Beans JA, Robinson RF. Views on electronic cigarette use in tobacco screening and cessation in an Alaska Native healthcare setting. Int J Circumpolar Health. 2015;74:27794. doi: 10.3402/ijch.v74.27794. http://dx.doi.org/10.3402/ijch.v74.27794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 480.Hiscock R, Bauld L, Arnott D, Dockrell M, Ross L, McEwen A. Views from the Coalface: What Do English Stop Smoking Service Personnel Think about E-Cigarettes? Int J Environ Res Public Health. 2015;12(12):16157–16167. doi: 10.3390/ijerph121215048. http://dx.doi.org/10.3390/ijerph121215048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 481.Kahr MK, Padgett S, Shope CD, et al. A qualitative assessment of the perceived risks of electronic cigarette and hookah use in pregnancy. BMC Public Health. 2015;15:1273. doi: 10.1186/s12889-015-2586-4. http://dx.doi.org/10.1186/s12889-015-2586-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 482.Lazuras L, Muzi M, Grano C, Lucidi F. E-cigarettes as smoking cessation aids: a survey among practitioners in Italy. Int J Public Health. 2016;61(2):243–248. doi: 10.1007/s00038-015-0772-x. http://dx.doi.org/10.1007/s00038-015-0772-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 483.Martinez-Sanchez JM, Fu M, Martin-Sanchez JC, Ballbe M, Salto E, Fernandez E. Perception of electronic cigarettes in the general population: does their usefulness outweigh their risks? BMJ Open. 2015;5(11):e009218. doi: 10.1136/bmjopen-2015-009218. http://dx.doi.org/10.1136/bmjopen-2015-009218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 484.Moysidou A, Farsalinos KE, Voudris V, Merakou K, Kourea K, Barbouni A. Knowledge and Perceptions about Nicotine, Nicotine Replacement Therapies and Electronic Cigarettes among Healthcare Professionals in Greece. Int J Environ Res Public Health. 2016;13(5):514. doi: 10.3390/ijerph13050514. http://dx.doi.org/10.3390/ijerph13050514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 485.Nayak P, Kemp CB, Redmon P. A Qualitative Study of Vape Shop Operators' Perceptions of Risks and Benefits of E-Cigarette Use and Attitude Toward Their Potential Regulation by the U.S. Food and Drug Administration, Florida, Georgia, South Carolina, or North Carolina, 2015. Prev Chronic Dis. 2016;13:E68. doi: 10.5888/pcd13.160071. http://dx.doi.org/10.5888/pcd13.160071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 486.Nolan M, Leischow S, Croghan I, et al. Feasibility of Electronic Nicotine Delivery Systems in Surgical Patients. Nicotine Tob Res. 2016;18(8):1757–1762. doi: 10.1093/ntr/ntw003. http://dx.doi.org/10.1093/ntr/ntw003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 487.Noland M, Ickes MJ, Rayens MK, Butler K, Wiggins AT, Hahn EJ. Social influences on use of cigarettes, e-cigarettes, and hookah by college students. J Am Coll Health. 2016;64(4):319–328. doi: 10.1080/07448481.2016.1138478. http://dx.doi.org/10.1080/07448481.2016.1138478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 488.Pechacek TF, Nayak P, Gregory KR, Weaver SR, Eriksen MP. The Potential That Electronic Nicotine Delivery Systems Can be a Disruptive Technology: Results From a National Survey. Nicotine Tob Res. 2016;18(10):1989–1997. doi: 10.1093/ntr/ntw102. http://dx.doi.org/10.1093/ntr/ntw102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 489.Penzes M, Foley KL, Balazs P, Urban R. Intention to Experiment With E-Cigarettes in a Cross-Sectional Survey of Undergraduate University Students in Hungary. Subst Use Misuse. 2016;51(9):1083–1092. doi: 10.3109/10826084.2016.1160116. http://dx.doi.org/10.3109/10826084.2016.1160116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 490.Roditis M, Delucchi K, Cash D, Halpern-Felsher B. Adolescents' Perceptions of Health Risks, Social Risks, and Benefits Differ Across Tobacco Products. J Adolesc Health. 2016;58(5):558–566. doi: 10.1016/j.jadohealth.2016.01.012. http://dx.doi.org/10.1016/j.jadohealth.2016.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 491.Saddleson ML, Kozlowski LT, Giovino GA, et al. Enjoyment and other reasons for electronic cigarette use: Results from college students in New York. Addict Behav. 2016;54:33–39. doi: 10.1016/j.addbeh.2015.11.012. http://dx.doi.org/10.1016/j.addbeh.2015.11.012. [DOI] [PubMed] [Google Scholar]
- 492.Sherratt FC, Newson L, Field JK. Electronic cigarettes: a survey of perceived patient use and attitudes among members of the British thoracic oncology group. Respir Res. 2016;17(1):55. doi: 10.1186/s12931-016-0367-y. http://dx.doi.org/10.1186/s12931-016-0367-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 493.Sherratt FC, Newson L, Marcus MW, Field JK, Robinson J. Perceptions towards electronic cigarettes for smoking cessation among Stop Smoking Service users. Br J Health Psychol. 2016;21(2):421–433. doi: 10.1111/bjhp.12177. http://dx.doi.org/10.1111/bjhp.12177. [DOI] [PubMed] [Google Scholar]
- 494.Tabuchi T, Kiyohara K, Hoshino T, Bekki K, Inaba Y, Kunugita N. Awareness and use of electronic cigarettes and heat-not-burn tobacco products in Japan. Addiction. 2016;111(4):706–713. doi: 10.1111/add.13231. http://dx.doi.org/10.1111/add.13231. [DOI] [PubMed] [Google Scholar]
- 495.Tan AS, Lee CJ, Bigman CA. Comparison of beliefs about e-cigarettes' harms and benefits among never users and ever users of e-cigarettes. Drug Alcohol Depend. 2016;158:67–75. doi: 10.1016/j.drugalcdep.2015.11.003. http://dx.doi.org/10.1016/j.drugalcdep.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 496.Tavolacci MP, Vasiliu A, Romo L, Kotbagi G, Kern L, Ladner J. Patterns of electronic cigarette use in current and ever users among college students in France: a cross-sectional study. BMJ Open. 2016;6(5):e011344. doi: 10.1136/bmjopen-2016-011344. http://dx.doi.org/10.1136/bmjopen-2016-011344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 497.Thrasher JF, Abad-Vivero EN, Barrientos-Gutierrez I, et al. Prevalence and Correlates of E-Cigarette Perceptions and Trial Among Early Adolescents in Mexico. J Adolesc Health. 2016;58(3):358–365. doi: 10.1016/j.jadohealth.2015.11.008. http://dx.doi.org/10.1016/j.jadohealth.2015.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 498.VanDevanter N, Zhou S, Katigbak C, Naegle M, Sherman S, Weitzman M. Knowledge, Beliefs, Behaviors, and Social Norms Related to Use of Alternative Tobacco Products Among Undergraduate and Graduate Nursing Students in an Urban U.S. University Setting. J Nurs Scholarsh. 2016;48(2):147–153. doi: 10.1111/jnu.12192. http://dx.doi.org/10.1111/jnu.12192. [DOI] [PubMed] [Google Scholar]
- 499.Wagoner KG, Cornacchione J, Wiseman KD, Teal R, Moracco KE, Sutfin E. E-cigarettes, hookah pens and vapes: Adolescent and young adult perceptions of Electronic Nicotine Delivery Systems. Nicotine Tob Res. 2016;18(10):2006–2012. doi: 10.1093/ntr/ntw095. http://dx.doi.org/10.1093/ntr/ntw095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 500.Wang MP, Li WH, Jiang N, et al. E-Cigarette Awareness, Perceptions and Use among Community-Recruited Smokers in Hong Kong. PLoS One. 2015;10(10):e0141683. doi: 10.1371/journal.pone.0141683. http://dx.doi.org/10.1371/journal.pone.0141683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 501.Wiseman KD, Cornacchione J, Wagoner KG, et al. Adolescents' and Young Adults' Knowledge and Beliefs About Constituents in Novel Tobacco Products. Nicotine Tob Res. 2016;18(7):1581–1587. doi: 10.1093/ntr/ntw009. http://dx.doi.org/10.1093/ntr/ntw009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 502.Wong LP, Mohamad Shakir SM, Alias H, Aghamohammadi N, Hoe VC. Reasons for Using Electronic Cigarettes and Intentions to Quit Among Electronic Cigarette Users in Malaysia. J Community Health. doi: 10.1007/s10900-016-0196-4. In press. Online May 4, 2016 http://dx.doi.org/10.1007/s10900-016-0196-4. [DOI] [PubMed] [Google Scholar]
- 503.Yong HH, Borland R, Balmford J, et al. Prevalence and Correlates of the Belief That Electronic Cigarettes are a Lot Less Harmful Than Conventional Cigarettes Under the Different Regulatory Environments of Australia and the United Kingdom. Nicotine Tob Res. doi: 10.1093/ntr/ntw137. In press. Online May 17, 2016 http://dx.doi.org/10.1093/ntr/ntw137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 504.Zarobkiewicz MK, Wawryk-Gawda E, Wozniakowski MM, Slawinski MA, Jodlowska-Jedrych B. Tobacco smokers and electronic cigarettes users among Polish universities students. Rocz Panstw Zakl Hig. 2016;67(1):75–80. [PubMed] [Google Scholar]
- 505.Margolis KA, Nguyen AB, Slavit WI, King BA. E-cigarette curiosity among U.S. middle and high school students: Findings from the 2014 national youth tobacco survey. Prev Med. 2016;89:1–6. doi: 10.1016/j.ypmed.2016.05.001. http://dx.doi.org/10.1016/j.ypmed.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 506.Agaku IT, Ayo-Yusuf OA. The Effect of Exposure to Pro-Tobacco Advertising on Experimentation With Emerging Tobacco Products Among U.S. Adolescents. Health Educ Behav. 2013;41(3):275–280. doi: 10.1177/1090198113511817. http://dx.doi.org/10.1177/1090198113511817. [DOI] [PubMed] [Google Scholar]
- 507.Agaku IT, King BA, Husten CG, et al. Tobacco product use among adults--United States, 2012–2013. MMWR Morb Mortal Wkly Rep. 2014;63(25):542–547. [PMC free article] [PubMed] [Google Scholar]
- 508.Allem JP, Forster M, Neiberger A, Unger JB. Characteristics of emerging adulthood and e-cigarette use: Findings from a pilot study. Addict Behav. 2015;50:40–44. doi: 10.1016/j.addbeh.2015.06.023. http://dx.doi.org/10.1016/j.addbeh.2015.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 509.Arrazola RA, Neff LJ, Kennedy SM, et al. Tobacco use among middle and high school students--United States, 2013. MMWR Morb Mortal Wkly Rep. 2014;63(45):1021–1026. [PMC free article] [PubMed] [Google Scholar]
- 510.Arrazola RA, Singh T, Corey CG, et al. Tobacco use among middle and high school students - United States, 2011–2014. MMWR Morb Mortal Wkly Rep. 2015;64(14):381–385. [PMC free article] [PubMed] [Google Scholar]
- 511.Barnett TE, Soule EK, Forrest JR, Porter L, Tomar SL. Adolescent Electronic Cigarette Use: Associations With Conventional Cigarette and Hookah Smoking. Am J Prev Med. 2015;49(2):199–206. doi: 10.1016/j.amepre.2015.02.013. http://dx.doi.org/10.1016/j.amepre.2015.02.013. [DOI] [PubMed] [Google Scholar]
- 512.Bartoli F, Carretta D, Crocamo C, et al. Prevalence and Correlates of Binge Drinking among Young Adults Using Alcohol: A Cross-Sectional Survey. Biomed Res Int. 2014;2014:930795. doi: 10.1155/2014/930795. http://dx.doi.org/10.1155/2014/930795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 513.Behar RZ, Hua M, Talbot P. Puffing topography and nicotine intake of electronic cigarette users. PLoS One. 2015;10(2):e0117222. doi: 10.1371/journal.pone.0117222. http://dx.doi.org/10.1371/journal.pone.0117222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 514.Biener L, Hargraves JL. A longitudinal study of electronic cigarette use among a population-based sample of adult smokers: association with smoking cessation and motivation to quit. Nicotine Tob Res. 2015;17(2):127–133. doi: 10.1093/ntr/ntu200. http://dx.doi.org/10.1093/ntr/ntu200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 515.Boyle RG, Amato MS, Rode P, Kinney AM, St Claire AW, Taylor K. Tobacco Use among Minnesota Adults, 2014. Am J Health Behav. 2015;39(5):674–679. doi: 10.5993/AJHB.39.5.9. http://dx.doi.org/10.5993/AJHB.39.5.9. [DOI] [PubMed] [Google Scholar]
- 516.Brose LS, Hitchman SC, Brown J, West R, McNeill A. Is the use of electronic cigarettes while smoking associated with smoking cessation attempts, cessation and reduced cigarette consumption? A survey with a 1-year follow-up. Addiction. 2015;110(7):1160–1168. doi: 10.1111/add.12917. http://dx.doi.org/10.1111/add.12917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 517.Bunnell RE, Agaku IT, Arrazola RA, et al. Intentions to smoke cigarettes among never-smoking U.S. middle and high school electronic cigarette users: National Youth Tobacco Survey, 2011–2013. Nicotine Tob Res. 2015;17(2):228–235. doi: 10.1093/ntr/ntu166. http://dx.doi.org/10.1093/ntr/ntu166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 518.Butler KM, Ickes MJ, Rayens MK, Wiggins AT, Hahn EJ. Polytobacco Use Among College Students. Nicotine Tob Res. 2016;18(2):163–169. doi: 10.1093/ntr/ntv056. http://dx.doi.org/10.1093/ntr/ntv056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 519.Camenga DR, Delmerico J, Kong G, et al. Trends in use of electronic nicotine delivery systems by adolescents. Addict Behav. 2014;39(1):338–340. doi: 10.1016/j.addbeh.2013.09.014. http://dx.doi.org/10.1016/j.addbeh.2013.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 520.Camenga DR, Kong G, Cavallo DA, et al. Alternate Tobacco Product and Drug Use Among Adolescents Who Use Electronic Cigarettes, Cigarettes Only, and Never Smokers. J Adolesc Health. 2014;55(4):588–591. doi: 10.1016/j.jadohealth.2014.06.016. http://dx.doi.org/10.1016/j.jadohealth.2014.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 521.CDC. Tobacco product use among middle and high school students--United States, 2011 and 2012. MMWR Morb Mortal Wkly Rep. 2013;62(45):893–897. [PMC free article] [PubMed] [Google Scholar]
- 522.Choi K, Forster JL. Authors' response. Am J Prev Med. 2014;46(6):e58–e59. doi: 10.1016/j.amepre.2014.02.013. http://dx.doi.org/10.1016/j.amepre.2014.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 523.Cohn A, Villanti A, Richardson A, et al. The association between alcohol, marijuana use, and new and emerging tobacco products in a young adult population. Addict Behav. 2015;48:79–88. doi: 10.1016/j.addbeh.2015.02.005. http://dx.doi.org/10.1016/j.addbeh.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 524.Coleman BN, Apelberg BJ, Ambrose BK, et al. Association between electronic cigarette use and openness to cigarette smoking among U.S. young adults. Nicotine Tob Res. 2015;17(2):212–218. doi: 10.1093/ntr/ntu211. http://dx.doi.org/10.1093/ntr/ntu211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 525.Cooper M, Case KR, Loukas A. E-cigarette use among Texas youth: Results from the 2014 Texas Youth Tobacco Survey. Addict Behav. 2015;50:173–177. doi: 10.1016/j.addbeh.2015.06.034. http://dx.doi.org/10.1016/j.addbeh.2015.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 526.Corey CG, Wang B, Johnson SE, et al. Notes from the field: electronic cigarette use among middle and high school students - United States, 2011–2012. MMWR Morb Mortal Wkly Rep. 2013;62(35):729–730. [PMC free article] [PubMed] [Google Scholar]
- 527.Cummins SE, Zhu SH, Tedeschi GJ, Gamst AC, Myers MG. Use of e-cigarettes by individuals with mental health conditions. Tob Control. 2014;23(Suppl 3):iii48–iii53. doi: 10.1136/tobaccocontrol-2013-051511. http://dx.doi.org/10.1136/tobaccocontrol-2013-051511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 528.Czoli CD, Hammond D, Reid JL, Cole AG, Leatherdale ST. Use of Conventional and Alternative Tobacco and Nicotine Products Among a Sample of Canadian Youth. J Adolesc Health. 2015;57(1):123–125. doi: 10.1016/j.jadohealth.2015.03.006. http://dx.doi.org/10.1016/j.jadohealth.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 529.Dawkins L, Munafo M, Christoforou G, Olumegbon N, Soar K. The effects of e-cigarette visual appearance on craving and withdrawal symptoms in abstinent smokers. Psychol Addict Behav. 2016;30(1):101–105. doi: 10.1037/adb0000112. http://dx.doi.org/10.1037/adb0000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 530.Delnevo CD, Villanti AC, Wackowski OA, Gundersen DA, Giovenco DP. The influence of menthol, e-cigarettes and other tobacco products on young adults' self-reported changes in past year smoking. Tob Control. 2015;25(5):571–574. doi: 10.1136/tobaccocontrol-2015-052325. http://dx.doi.org/10.1136/tobaccocontrol-2015-052325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 531.Douptcheva N, Gmel G, Studer J, Deline S, Etter JF. Use of electronic cigarettes among young Swiss men. J Epidemiol Community Health. 2013;67(12):1075–1076. doi: 10.1136/jech-2013-203152. http://dx.doi.org/10.1136/jech-2013-203152. [DOI] [PubMed] [Google Scholar]
- 532.Dutra LM, Glantz SA. Electronic cigarettes and conventional cigarette use among U.S. adolescents: a cross-sectional study. JAMA Pediatr. 2014;168(7):610–617. doi: 10.1001/jamapediatrics.2013.5488. http://dx.doi.org/10.1001/jamapediatrics.2013.5488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 533.Emory K, Kim Y, Buchting F, Vera L, Huang J, Emery SL. Intragroup Variance in Lesbian, Gay, and Bisexual Tobacco Use Behaviors: Evidence That Subgroups Matter, Notably Bisexual Women. Nicotine Tob Res. 2016;18(6):1494–1501. doi: 10.1093/ntr/ntv208. http://dx.doi.org/10.1093/ntr/ntv208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 534.Erickson DJ, Lenk KM, Forster JL. Latent Classes of Young Adults Based on Use of Multiple Types of Tobacco and Nicotine Products. Nicotine Tob Res. 2014;16(8):1056–1062. doi: 10.1093/ntr/ntu024. http://dx.doi.org/10.1093/ntr/ntu024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 535.Etter JF. Electronic cigarettes and cannabis: an exploratory study. Eur Addict Res. 2015;21(3):124–130. doi: 10.1159/000369791. http://dx.doi.org/10.1159/000369791. [DOI] [PubMed] [Google Scholar]
- 536.Etter JF, Bullen C. A longitudinal study of electronic cigarette users. Addict Behav. 2014;39(2):491–494. doi: 10.1016/j.addbeh.2013.10.028. http://dx.doi.org/10.1016/j.addbeh.2013.10.028. [DOI] [PubMed] [Google Scholar]
- 537.Farsalinos KE, Romagna G, Tsiapras D, Kyrzopoulos S, Spyrou A, Voudris V. Impact of flavour variability on electronic cigarette use experience: an internet survey. Int J Environ Res Public Health. 2013;10(12):7272–7282. doi: 10.3390/ijerph10127272. http://dx.doi.org/10.3390/ijerph10127272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 538.Farsalinos KE, Romagna G, Tsiapras D, Kyrzopoulos S, Voudris V. Evaluation of electronic cigarette use (vaping) topography and estimation of liquid consumption: implications for research protocol standards definition and for public health authorities' regulation. Int J Environ Res Public Health. 2013;10(6):2500–2514. doi: 10.3390/ijerph10062500. http://dx.doi.org/10.3390/ijerph10062500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 539.Fotiou A, Kanavou E, Stavrou M, Richardson C, Kokkevi A. Prevalence and correlates of electronic cigarette use among adolescents in Greece: A preliminary cross-sectional analysis of nationwide survey data. Addict Behav. 2015;51:88–92. doi: 10.1016/j.addbeh.2015.07.021. http://dx.doi.org/10.1016/j.addbeh.2015.07.021. [DOI] [PubMed] [Google Scholar]
- 540.Foulds J, Veldheer S, Yingst J, et al. Development of a questionnaire for assessing dependence on electronic cigarettes among a large sample of ex-smoking E-cigarette users. Nicotine Tob Res. 2015;17(2):186–192. doi: 10.1093/ntr/ntu204. http://dx.doi.org/10.1093/ntr/ntu204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 541.Giovenco DP, Lewis MJ, Delnevo CD. Factors Associated with E-cigarette Use: A National Population Survey of Current and Former Smokers. Am J Prev Med. 2014;47(4):476–480. doi: 10.1016/j.amepre.2014.04.009. http://dx.doi.org/10.1016/j.amepre.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 542.Goniewicz ML, Gawron M, Nadolska J, Balwicki L, Sobczak A. Rise in electronic cigarette use among adolescents in Poland. J Adolesc Health. 2014;55(5):713–715. doi: 10.1016/j.jadohealth.2014.07.015. http://dx.doi.org/10.1016/j.jadohealth.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 543.Goniewicz ML, Zielinska-Danch W. Electronic cigarette use among teenagers and young adults in Poland. Pediatrics. 2012;130(4):e879–e885. doi: 10.1542/peds.2011-3448. http://dx.doi.org/10.1542/peds.2011-3448. [DOI] [PubMed] [Google Scholar]
- 544.Hampson SE, Andrews JA, Severson HH, Barckley M. Prospective Predictors of Novel Tobacco and Nicotine Product Use in Emerging Adulthood. J Adolesc Health. 2015;57(2):186–191. doi: 10.1016/j.jadohealth.2015.04.015. http://dx.doi.org/10.1016/j.jadohealth.2015.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 545.Hanewinkel R, Isensee B. Risk factors for e-cigarette, conventional cigarette, and dual use in German adolescents: a cohort study. Prev Med. 2015;74:59–62. doi: 10.1016/j.ypmed.2015.03.006. http://dx.doi.org/10.1016/j.ypmed.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 546.Hershberger AR, Karyadi KA, VanderVeen JD, Cyders MA. Combined expectancies of alcohol and e-cigarette use relate to higher alcohol use. Addict Behav. 2015;52:13–21. doi: 10.1016/j.addbeh.2015.08.005. http://dx.doi.org/10.1016/j.addbeh.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 547.Heydari G, Masjedi M, Ahmady AE, et al. Assessment of Different Quit Smoking Methods Selected by Patients in Tobacco Cessation Centers in Iran. Int J Prev Med. 2015;6(1):81. doi: 10.4103/2008-7802.164118. http://dx.doi.org/10.4103/2008-7802.164118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 548.Hitchman SC, Brose LS, Brown J, Robson D, McNeill A. Associations Between E-Cigarette Type, Frequency of Use, and Quitting Smoking: Findings From a Longitudinal Online Panel Survey in Great Britain. Nicotine Tob Res. 2015;17(10):1187–1194. doi: 10.1093/ntr/ntv078. http://dx.doi.org/10.1093/ntr/ntv078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 549.Hughes K, Bellis MA, Hardcastle KA, et al. Associations between e-cigarette access and smoking and drinking behaviours in teenagers. BMC Public Health. 2015;15:244. doi: 10.1186/s12889-015-1618-4. http://dx.doi.org/10.1186/s12889-015-1618-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 550.Jawad M, Power G. Waterpipe tobacco and electronic cigarette use in a southeast London adult sample: a cross-sectional analysis. J Public Health (Oxf) 2015;38(2):e114–e121. doi: 10.1093/pubmed/fdv106. http://dx.doi.org/10.1093/pubmed/fdv106. [DOI] [PubMed] [Google Scholar]
- 551.Kalkhoran S, Grana RA, Neilands TB, Ling PM. Dual Use of Smokeless Tobacco or E-cigarettes with Cigarettes and Cessation. Am J Health Behav. 2015;39(2):277–284. doi: 10.5993/AJHB.39.2.14. http://dx.doi.org/10.5993/AJHB.39.2.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 552.Kasza KA, Bansal-Travers M, O'Connor RJ, et al. Cigarette smokers' use of unconventional tobacco products and associations with quitting activity: findings from the ITC-4 U.S. cohort. Nicotine Tob Res. 2014;16(6):672–681. doi: 10.1093/ntr/ntt212. http://dx.doi.org/10.1093/ntr/ntt212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 553.King AC, Smith LJ, McNamara PJ, Matthews AK, Fridberg DJ. Passive exposure to electronic cigarette (e-cigarette) use increases desire for combustible and e-cigarettes in young adult smokers. Tob Control. 2015;24(5):501–504. doi: 10.1136/tobaccocontrol-2014-051563. http://dx.doi.org/10.1136/tobaccocontrol-2014-051563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 554.Kristjansson AL, Mann MJ, Sigfusdottir ID. Licit and Illicit Substance Use by Adolescent E-Cigarette Users Compared with Conventional Cigarette Smokers, Dual Users, and Nonusers. J Adolesc Health. 2015;57(5):562–564. doi: 10.1016/j.jadohealth.2015.07.014. http://dx.doi.org/10.1016/j.jadohealth.2015.07.014. [DOI] [PubMed] [Google Scholar]
- 555.Lee S, Grana RA, Glantz SA. Electronic cigarette use among Korean adolescents: a cross-sectional study of market penetration, dual use, and relationship to quit attempts and former smoking. J Adolesc Health. 2014;54(6):684–690. doi: 10.1016/j.jadohealth.2013.11.003. http://dx.doi.org/10.1016/j.jadohealth.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 556.Lee YH, Gawron M, Goniewicz ML. Changes in puffing behavior among smokers who switched from tobacco to electronic cigarettes. Addict Behav. 2015;48:1–4. doi: 10.1016/j.addbeh.2015.04.003. http://dx.doi.org/10.1016/j.addbeh.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 557.Lee YO, Hebert CJ, Nonnemaker JM, Kim AE. Multiple tobacco product use among adults in the United States: Cigarettes, cigars, electronic cigarettes, hookah, smokeless tobacco, and snus. Prev Med. 2014;62:14–19. doi: 10.1016/j.ypmed.2014.01.014. http://dx.doi.org/10.1016/j.ypmed.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 558.Lee YO, Hebert CJ, Nonnemaker JM, Kim AE. Youth Tobacco Product Use in the United States. Pediatrics. 2015;135(3) doi: 10.1542/peds.2014-3202. http://dx.doi.org/10.1542/peds.2014-3202. [DOI] [PubMed] [Google Scholar]
- 559.Lessard J, Henrie J, Livingston JA, Leonard KE, Colder CR, Eiden RD. Correlates of ever having used electronic cigarettes among older adolescent children of alcoholic fathers. Nicotine Tob Res. 2014;16(12):1656–1660. doi: 10.1093/ntr/ntu148. http://dx.doi.org/10.1093/ntr/ntu148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 560.Leventhal AM, Strong DR, Kirkpatrick MG, et al. Association of Electronic Cigarette Use With Initiation of Combustible Tobacco Product Smoking in Early Adolescence. JAMA. 2015;314(7):700–707. doi: 10.1001/jama.2015.8950. http://dx.doi.org/10.1001/jama.2015.8950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 561.Lippert AM. Do Adolescent Smokers Use E-Cigarettes to Help Them Quit? The Sociodemographic Correlates and Cessation Motivations of U.S. Adolescent E-Cigarette Use. Am J Health Promot. 2015;29(6):374–379. doi: 10.4278/ajhp.131120-QUAN-595. http://dx.doi.org/10.4278/ajhp.131120-QUAN-595. [DOI] [PubMed] [Google Scholar]
- 562.Little MA, Derefinko KJ, Colvin L, et al. The Prevalence of E-cigarette Use in a Sample of U.S. Air Force Recruits. Am J Prev Med. 2015;49(3):402–408. doi: 10.1016/j.amepre.2015.02.019. http://dx.doi.org/10.1016/j.amepre.2015.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 563.Littlefield AK, Gottlieb JC, Cohen LM, Trotter DR. Electronic Cigarette Use Among College Students: Links to Gender, Race/Ethnicity, Smoking, and Heavy Drinking. J Am Coll Health. 2015;63(8):523–529. doi: 10.1080/07448481.2015.1043130. http://dx.doi.org/10.1080/07448481.2015.1043130. [DOI] [PubMed] [Google Scholar]
- 564.Lopez AA, Hiler MM, Soule EK, et al. Effects of Electronic Cigarette Liquid Nicotine Concentration on Plasma Nicotine and Puff Topography in Tobacco Cigarette Smokers: A Preliminary Report. Nicotine Tob Res. 2016;18(5):720–723. doi: 10.1093/ntr/ntv182. http://dx.doi.org/10.1093/ntr/ntv182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 565.Lotrean LM. Use of electronic cigarettes among Romanian university students: a cross-sectional study. BMC Public Health. 2015;15:358. doi: 10.1186/s12889-015-1713-6. http://dx.doi.org/10.1186/s12889-015-1713-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 566.Loukas A, Batanova M, Fernandez A, Agarwal D. Changes in use of cigarettes and non-cigarette alternative products among college students. Addict Behav. 2015;49:46–51. doi: 10.1016/j.addbeh.2015.05.005. http://dx.doi.org/10.1016/j.addbeh.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 567.McMillen R, Maduka J, Winickoff J. Use of emerging tobacco products in the United States. J Environ Public Health. 2012;2012:989474. doi: 10.1155/2012/989474. http://dx.doi.org/10.1155/2012/989474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 568.McMillen RC, Gottlieb MA, Shaefer RM, Winickoff JP, Klein JD. Trends in Electronic Cigarette Use Among U.S. Adults: Use is Increasing in Both Smokers and Nonsmokers. Nicotine Tob Res. 2015;17(10):1195–1202. doi: 10.1093/ntr/ntu213. http://dx.doi.org/10.1093/ntr/ntu213. [DOI] [PubMed] [Google Scholar]
- 569.Meier EM, Tackett AP, Miller MB, Grant DM, Wagener TL. Which nicotine products are gateways to regular use? First-tried tobacco and current use in college students. Am J Prev Med. 2015;48(1 Suppl 1):S86–S93. doi: 10.1016/j.amepre.2014.09.018. http://dx.doi.org/10.1016/j.amepre.2014.09.018. [DOI] [PubMed] [Google Scholar]
- 570.Miech RA, O'Malley PM, Johnston LD, Patrick ME. E-Cigarettes and the Drug Use Patterns of Adolescents. Nicotine Tob Res. 2016;18(5):654–659. doi: 10.1093/ntr/ntv217. http://dx.doi.org/10.1093/ntr/ntv217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 571.Moore G, Hewitt G, Evans J, et al. Electronic-cigarette use among young people in Wales: evidence from two cross-sectional surveys. BMJ Open. 2015;5(4):e007072. doi: 10.1136/bmjopen-2014-007072. http://dx.doi.org/10.1136/bmjopen-2014-007072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 572.Pentz MA, Shin H, Riggs N, Unger JB, Collison KL, Chou CP. Parent, peer, and executive function relationships to early adolescent e-cigarette use: a substance use pathway? Addict Behav. 2015;42:73–78. doi: 10.1016/j.addbeh.2014.10.040. http://dx.doi.org/10.1016/j.addbeh.2014.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 573.Pokhrel P, Little MA, Fagan P, Muranaka N, Herzog TA. Electronic cigarette use outcome expectancies among college students. Addict Behav. 2014;39(6):1062–1065. doi: 10.1016/j.addbeh.2014.02.014. http://dx.doi.org/10.1016/j.addbeh.2014.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 574.Polosa R, Caponnetto P, Cibella F, Le-Houezec J. Quit and smoking reduction rates in vape shop consumers: a prospective 12-month survey. Int J Environ Res Public Health. 2015;12(4):3428–3438. doi: 10.3390/ijerph120403428. http://dx.doi.org/10.3390/ijerph120403428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 575.Popova L, Ling PM. Alternative tobacco product use and smoking cessation: a national study. Am J Public Health. 2013;103(5):923–930. doi: 10.2105/AJPH.2012.301070. http://dx.doi.org/10.2105/AJPH.2012.301070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 576.Porter L, Duke J, Hennon M, et al. Electronic Cigarette and Traditional Cigarette Use among Middle and High School Students in Florida, 2011–2014. PLoS One. 2015;10(5):e0124385. doi: 10.1371/journal.pone.0124385. http://dx.doi.org/10.1371/journal.pone.0124385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 577.Prochaska JJ, Grana RA. E-Cigarette Use among Smokers with Serious Mental Illness. PLoS One. 2014;9(11):e113013. doi: 10.1371/journal.pone.0113013. http://dx.doi.org/10.1371/journal.pone.0113013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 578.Pulvers K, Hayes RB, Scheuermann TS, et al. Tobacco Use, Quitting Behavior, and Health Characteristics Among Current Electronic Cigarette Users in a National Tri-Ethnic Adult Stable Smoker Sample. Nicotine Tob Res. 2015;17(9):1085–1095. doi: 10.1093/ntr/ntu241. http://dx.doi.org/10.1093/ntr/ntu241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 579.Ramo DE, Young-Wolff KC, Prochaska JJ. Prevalence and correlates of electronic-cigarette use in young adults: findings from three studies over five years. Addict Behav. 2015;41:142–147. doi: 10.1016/j.addbeh.2014.10.019. http://dx.doi.org/10.1016/j.addbeh.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 580.Ramoa CP, Hiler MM, Spindle TR, et al. Electronic cigarette nicotine delivery can exceed that of combustible cigarettes: a preliminary report. Tob Control. 2016;25(e1):e6–e9. doi: 10.1136/tobaccocontrol-2015-052447. http://dx.doi.org/10.1136/tobaccocontrol-2015-052447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 581.Reid JL, Rynard VL, Czoli CD, Hammond D. Who is using e-cigarettes in Canada? Nationally representative data on the prevalence of e-cigarette use among Canadians. Prev Med. 2015;81:180–183. doi: 10.1016/j.ypmed.2015.08.019. http://dx.doi.org/10.1016/j.ypmed.2015.08.019. [DOI] [PubMed] [Google Scholar]
- 582.Rigotti NA, Harrington KF, Richter K, et al. Increasing Prevalence of Electronic Cigarette Use Among Smokers Hospitalized in 5 U.S. Cities, 2010–2013. Nicotine Tob Res. 2015;17(2):236–244. doi: 10.1093/ntr/ntu138. http://dx.doi.org/10.1093/ntr/ntu138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 583.Robinson RJ, Hensel EC, Morabito PN, Roundtree KA. Electronic Cigarette Topography in the Natural Environment. PLoS One. 2015;10(6):e0129296. doi: 10.1371/journal.pone.0129296. http://dx.doi.org/10.1371/journal.pone.0129296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 584.Saddleson ML, Kozlowski LT, Giovino GA, et al. Risky behaviors, e-cigarette use and susceptibility of use among college students. Drug Alcohol Depend. 2015;149:25–30. doi: 10.1016/j.drugalcdep.2015.01.001. http://dx.doi.org/10.1016/j.drugalcdep.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 585.Soneji S, Sargent J, Tanski S. Multiple tobacco product use among U.S. adolescents and young adults. Tob Control. 2016;25(2):174–180. doi: 10.1136/tobaccocontrol-2014-051638. http://dx.doi.org/10.1136/tobaccocontrol-2014-051638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 586.Stillman FA, Soong A, Zheng LY, Navas-Acien A. E-cigarette use in air transit: self-reported data from U.S. flight attendants. Tob Control. 2015;24(4):417–418. doi: 10.1136/tobaccocontrol-2013-051514. http://dx.doi.org/10.1136/tobaccocontrol-2013-051514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 587.Strong DR, Myers M, Linke S, et al. Gender differences influence overweight smokers' experimentation with electronic nicotine delivery systems. Addict Behav. 2015;49:20–25. doi: 10.1016/j.addbeh.2015.05.003. http://dx.doi.org/10.1016/j.addbeh.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 588.Tackett AP, Lechner WV, Meier E, et al. Biochemically verified smoking cessation and vaping beliefs among vape store customers. Addiction. 2015;110(5):868–874. doi: 10.1111/add.12878. http://dx.doi.org/10.1111/add.12878. [DOI] [PubMed] [Google Scholar]
- 589.Tami-Maury I, Lin MT, Lapham HL, et al. A pilot study to assess tobacco use among sexual minorities in Houston, Texas. Am J Addict. 2015;24(5):391–395. doi: 10.1111/ajad.12244. http://dx.doi.org/10.1111/ajad.12244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 590.Vardavas CI, Filippidis FT, Agaku IT. Determinants and prevalence of e-cigarette use throughout the European Union: a secondary analysis of 26 566 youth and adults from 27 Countries. Tob Control. 2015;24(5):442–448. doi: 10.1136/tobaccocontrol-2013-051394. http://dx.doi.org/10.1136/tobaccocontrol-2013-051394. [DOI] [PubMed] [Google Scholar]
- 591.Wang MP, Ho SY, Leung LT, Lam TH. Electronic cigarette use and its association with smoking in Hong Kong Chinese adolescents. Addict Behav. 2015;50:124–127. doi: 10.1016/j.addbeh.2015.06.037. http://dx.doi.org/10.1016/j.addbeh.2015.06.037. [DOI] [PubMed] [Google Scholar]
- 592.White J, Li J, Newcombe R, Walton D. Tripling use of electronic cigarettes among New Zealand adolescents between 2012 and 2014. J Adolesc Health. 2015;56(5):522–528. doi: 10.1016/j.jadohealth.2015.01.022. http://dx.doi.org/10.1016/j.jadohealth.2015.01.022. [DOI] [PubMed] [Google Scholar]
- 593.Wills TA, Knight R, Williams RJ, Pagano I, Sargent JD. Risk factors for exclusive e-cigarette use and dual e-cigarette use and tobacco use in adolescents. Pediatrics. 2015;135(1):e43–e51. doi: 10.1542/peds.2014-0760. http://dx.doi.org/10.1542/peds.2014-0760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 594.Wills TA, Sargent JD, Knight R, Pagano I, Gibbons FX. E-cigarette use and willingness to smoke: a sample of adolescent non-smokers. Tob Control. 2016;25(e1):e52–e59. doi: 10.1136/tobaccocontrol-2015-052349. http://dx.doi.org/10.1136/tobaccocontrol-2015-052349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 595.Yingst JM, Veldheer S, Hrabovsky S, Nichols TT, Wilson SJ, Foulds J. Factors Associated With Electronic Cigarette Users' Device Preferences and Transition From First Generation to Advanced Generation Devices. Nicotine Tob Res. 2015;17(10):1242–1246. doi: 10.1093/ntr/ntv052. http://dx.doi.org/10.1093/ntr/ntv052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 596.Alcala HE, Albert SL, Ortega AN. E-cigarette use and disparities by race, citizenship status and language among adolescents. Addict Behav. 2016;57:30–34. doi: 10.1016/j.addbeh.2016.01.014. http://dx.doi.org/10.1016/j.addbeh.2016.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 597.Ali M, Gray TR, Martinez DJ, Curry LE, Horn KA. Risk Profiles of Youth Single, Dual, and Poly Tobacco Users. Nicotine Tob Res. 2016;18(7):1614–1621. doi: 10.1093/ntr/ntw028. http://dx.doi.org/10.1093/ntr/ntw028. [DOI] [PubMed] [Google Scholar]
- 598.Barrington-Trimis JL, Berhane K, Unger JB, et al. The E-cigarette Social Environment, E-cigarette Use, and Susceptibility to Cigarette Smoking. J Adolesc Health. 2016;59(1):75–80. doi: 10.1016/j.jadohealth.2016.03.019. http://dx.doi.org/10.1016/j.jadohealth.2016.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 599.Berg CJ, Haardorfer R, Lewis M, et al. DECOY: Documenting Experiences with Cigarettes and Other Tobacco in Young Adults. Am J Health Behav. 2016;40(3):310–321. doi: 10.5993/AJHB.40.3.3. http://dx.doi.org/10.5993/AJHB.40.3.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 600.Bostean G, Trinidad DR, McCarthy WJ. E-Cigarette Use Among Never-Smoking California Students. Am J Public Health. 2015;105(12):2423–2425. doi: 10.2105/AJPH.2015.302899. http://dx.doi.org/10.2105/AJPH.2015.302899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 601.Caraballo RS, Jamal A, Nguyen KH, Kuiper NM, Arrazola RA. Electronic Nicotine Delivery System Use Among U.S. Adults, 2014. Am J Prev Med. 2016;50(2):226–229. doi: 10.1016/j.amepre.2015.09.013. http://dx.doi.org/10.1016/j.amepre.2015.09.013. [DOI] [PubMed] [Google Scholar]
- 602.Cardenas VM, Evans VL, Balamurugan A, Faramawi MF, Delongchamp RR, Wheeler JG. Use of electronic nicotine delivery systems and recent initiation of smoking among U.S. youth. Int J Public Health. 2016;61(2):237–241. doi: 10.1007/s00038-015-0783-7. http://dx.doi.org/10.1007/s00038-015-0783-7. [DOI] [PubMed] [Google Scholar]
- 603.Cherng ST, Tam J, Christine PJ, Meza R. Modeling the Effects of E-Cigarettes on Smoking Behavior: Implications for Future Adult Smoking Prevalence. Epidemiology. 2016;27(6):819–826. doi: 10.1097/EDE.0000000000000497. http://dx.doi.org/10.1097/EDE.0000000000000497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 604.Cho JH, Paik SY. Association between Electronic Cigarette Use and Asthma among High School Students in South Korea. PLoS One. 2016;11(3):e0151022. doi: 10.1371/journal.pone.0151022. http://dx.doi.org/10.1371/journal.pone.0151022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 605.Choi K, Bernat D. E-Cigarette Use Among Florida Youth With and Without Asthma. Am J Prev Med. 2016;51(4):446–453. doi: 10.1016/j.amepre.2016.03.010. http://dx.doi.org/10.1016/j.amepre.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 606.Cooper M, Harrell MB, Perry CL. A Qualitative Approach to Understanding Real-World Electronic Cigarette Use: Implications for Measurement and Regulation. Prev Chronic Dis. 2016;13:E07. doi: 10.5888/pcd13.150502. http://dx.doi.org/10.5888/pcd13.150502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 607.Corey CG, Ambrose BK, Apelberg BJ, King BA. Flavored Tobacco Product Use Among Middle and High School Students--United States, 2014. MMWR Morb Mortal Wkly Rep. 2015;64(38):1066–1070. doi: 10.15585/mmwr.mm6438a2. http://dx.doi.org/10.15585/mmwr.mm6438a2. [DOI] [PubMed] [Google Scholar]
- 608.Corsi DJ, Lippert AM. An examination of the shift in school-level clustering of U.S. adolescent electronic cigarette use and its multilevel correlates, 2011–2013. Health Place. 2016;38:30–38. doi: 10.1016/j.healthplace.2015.12.007. http://dx.doi.org/10.1016/j.healthplace.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 609.Dautzenberg B, Berlin I, Tanguy ML, Rieu N, Birkui P. Factors associated with experimentation of electronic cigarettes among Parisian teenagers in 2013. Tob Induc Dis. 2015;13:40. doi: 10.1186/s12971-015-0065-4. http://dx.doi.org/10.1186/s12971-015-0065-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 610.Delnevo CD, Giovenco DP, Steinberg MB, et al. Patterns of electronic cigarette use among adults in the United States. Nicotine Tob Res. 2016;18(5):715–719. doi: 10.1093/ntr/ntv237. http://dx.doi.org/10.1093/ntr/ntv237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 611.Doran N, Brikmanis K. Expectancies for and use of e-cigarettes and hookah among young adult non-daily smokers. Addict Behav. 2016;60:154–159. doi: 10.1016/j.addbeh.2016.04.008. http://dx.doi.org/10.1016/j.addbeh.2016.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 612.Etter JF. Characteristics of users and usage of different types of electronic cigarettes: findings from an online survey. Addiction. 2016;111(4):724–733. doi: 10.1111/add.13240. http://dx.doi.org/10.1111/add.13240. [DOI] [PubMed] [Google Scholar]
- 613.Filippidis FT, Laverty AA, Gerovasili V, Vardavas CI. Two-year trends and predictors of e-cigarette use in 27 European Union member states. Tob Control. doi: 10.1136/tobaccocontrol-2015-052771. In press. Online May 24, 2016. http://dx.doi.org/10.1136/tobaccocontrol-2015-052771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 614.Gilreath TD, Leventhal A, Barrington-Trimis JL, et al. Patterns of Alternative Tobacco Product Use: Emergence of Hookah and E-cigarettes as Preferred Products Amongst Youth. J Adolesc Health. 2016;58(2):181–185. doi: 10.1016/j.jadohealth.2015.10.001. http://dx.doi.org/10.1016/j.jadohealth.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 615.Gmel G, Baggio S, Mohler-Kuo M, Daeppen JB, Studer J. E-cigarette use in young Swiss men: is vaping an effective way of reducing or quitting smoking? Swiss Med Wkly. 2016;146:w14271. doi: 10.4414/smw.2016.14271. http://dx.doi.org/10.4414/smw.2016.14271. [DOI] [PubMed] [Google Scholar]
- 616.Harrold TC, Maag AK, Thackway S, Mitchell J, Taylor LK. Prevalence of e-cigarette users in New South Wales. Med J Aust. 2015;203(8):326. doi: 10.5694/mja15.00652. http://dx.doi.org/10.5694/mja15.00652. [DOI] [PubMed] [Google Scholar]
- 617.Huh J, Leventhal AM. Intraindividual covariation between e-cigarette and combustible cigarette use in Korean American emerging adults. Psychol Addict Behav. 2016;30(2):246–251. doi: 10.1037/adb0000141. http://dx.doi.org/10.1037/adb0000141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 618.Huh J, Leventhal AM. Progression of Poly-tobacco Product Use Patterns in Adolescents. Am J Prev Med. 2016;51(4):513–517. doi: 10.1016/j.amepre.2016.04.004. http://dx.doi.org/10.1016/j.amepre.2016.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 619.Johnson SE, Holder-Hayes E, Tessman GK, King BA, Alexander T, Zhao X. Tobacco Product Use Among Sexual Minority Adults: Findings From the 2012–2013 National Adult Tobacco Survey. Am J Prev Med. 2016;50(4):e91–e100. doi: 10.1016/j.amepre.2015.07.041. http://dx.doi.org/10.1016/j.amepre.2015.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 620.Kalkhoran S, Glantz SA. Modeling the Health Effects of Expanding e-Cigarette Sales in the United States and United Kingdom: A Monte Carlo Analysis. JAMA Intern Med. 2015;175(10):1671–1680. doi: 10.1001/jamainternmed.2015.4209. http://dx.doi.org/10.1001/jamainternmed.2015.4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 621.Kalkhoran S, Padilla JL, Neilands TB, Ling PM. Multiple tobacco product use among young adult bar patrons in New Mexico. Prev Med. 2016;83:16–21. doi: 10.1016/j.ypmed.2015.11.024. http://dx.doi.org/10.1016/j.ypmed.2015.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 622.Kilibarda B, Mravcik V, Martens MS. E-cigarette use among Serbian adults: prevalence and user characteristics. Int J Public Health. 2016;61(2):167–175. doi: 10.1007/s00038-016-0787-y. http://dx.doi.org/10.1007/s00038-016-0787-y. [DOI] [PubMed] [Google Scholar]
- 623.Kingsbury JH, Parks MJ, Amato MS, Boyle RG. Deniers and Admitters: Examining Smoker Identities in a Changing Tobacco Landscape. Nicotine Tob Res. 2016;18(11):2130–2137. doi: 10.1093/ntr/ntw110. http://dx.doi.org/10.1093/ntr/ntw110. [DOI] [PubMed] [Google Scholar]
- 624.La Torre G, Mipatrini D. Country-level correlates of e-cigarette use in the European Union. Int J Public Health. 2016;61(2):269–275. doi: 10.1007/s00038-016-0792-1. http://dx.doi.org/10.1007/s00038-016-0792-1. [DOI] [PubMed] [Google Scholar]
- 625.Lee JA, Kim SH, Cho HJ. Electronic cigarette use among Korean adults. Int J Public Health. 2016;61(2):151–157. doi: 10.1007/s00038-015-0763-y. http://dx.doi.org/10.1007/s00038-015-0763-y. [DOI] [PubMed] [Google Scholar]
- 626.Leventhal AM, Strong DR, Sussman S, et al. Psychiatric comorbidity in adolescent electronic and conventional cigarette use. J Psychiatr Res. 2016;73:71–78. doi: 10.1016/j.jpsychires.2015.11.008. http://dx.doi.org/10.1016/j.jpsychires.2015.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 627.Lidon-Moyano C, Martinez-Sanchez JM, Fu M, Ballbe M, Martin-Sanchez JC, Fernandez E. Prevalence and user profile of electronic cigarettes in Spain (2014) Gaceta sanitaria / S.E.S.P.A.S. 2016 doi: 10.1016/j.gaceta.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 628.Lippert AM. Temporal Changes in the Correlates of U.S. Adolescent Electronic Cigarette Use and Utilization in Tobacco Cessation, 2011 to 2013. Health Educ Behav. doi: 10.1177/1090198116650150. In press. Online May 25, 2016. http://dx.doi.org/10.1177/1090198116650150. [DOI] [PubMed] [Google Scholar]
- 629.Mantey DS, Cooper MR, Clendennen SL, Pasch KE, Perry CL. E-Cigarette Marketing Exposure Is Associated With E-Cigarette Use Among U.S. Youth. J Adolesc Health. 2016;58(6):686–690. doi: 10.1016/j.jadohealth.2016.03.003. http://dx.doi.org/10.1016/j.jadohealth.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 630.Mays D, Arrazola RA, Tworek C, Rolle IV, Neff LJ, Portnoy DB. Openness to Using Non-cigarette Tobacco Products Among U.S. Young Adults. Am J Prev Med. 2016;50(4):528–534. doi: 10.1016/j.amepre.2015.08.015. http://dx.doi.org/10.1016/j.amepre.2015.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 631.Morean ME, Kong G, Camenga DR, Cavallo DA, Simon P, Krishnan-Sarin S. Latent class analysis of current e-cigarette and other substance use in high school students. Drug Alcohol Depend. 2016;161:292–297. doi: 10.1016/j.drugalcdep.2016.02.018. http://dx.doi.org/10.1016/j.drugalcdep.2016.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 632.Nadasan V, Foley KL, Penzes M, et al. Use of electronic cigarettes and alternative tobacco products among Romanian adolescents. Int J Public Health. 2016;61(2):199–207. doi: 10.1007/s00038-015-0774-8. http://dx.doi.org/10.1007/s00038-015-0774-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 633.Neff LJ, Arrazola RA, Caraballo RS, et al. Frequency of Tobacco Use Among Middle and High School Students - United States, 2014. MMWR Morb Mortal Wkly Rep. 2015;64(38):1061–1065. doi: 10.15585/mmwr.mm6438a1. http://dx.doi.org/10.15585/mmwr.mm6438a1. [DOI] [PubMed] [Google Scholar]
- 634.Ooms GI, Bosdriesz JR, Portrait FR, Kunst AE. Sociodemographic Differences in the Use of Electronic Nicotine Delivery Systems in the European Union. Nicotine Tob Res. 2016;18(5):724–729. doi: 10.1093/ntr/ntv215. http://dx.doi.org/10.1093/ntr/ntv215. [DOI] [PubMed] [Google Scholar]
- 635.Park JY, Seo DC, Lin HC. E-Cigarette Use and Intention to Initiate or Quit Smoking Among U.S. Youths. Am J Public Health. 2016;106(4):672–678. doi: 10.2105/AJPH.2015.302994. http://dx.doi.org/10.2105/AJPH.2015.302994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 636.Primack BA, Soneji S, Stoolmiller M, Fine MJ, Sargent JD. Progression to Traditional Cigarette Smoking After Electronic Cigarette Use Among U.S. Adolescents and Young Adults. JAMA Pediatr. 2015;169(11):1018–1023. doi: 10.1001/jamapediatrics.2015.1742. http://dx.doi.org/10.1001/jamapediatrics.2015.1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 637.Rennie LJ, Bazillier-Bruneau C, Rouesse J. Harm Reduction or Harm Introduction? Prevalence and Correlates of E-Cigarette Use Among French Adolescents. J Adolesc Health. 2016;58(4):440–445. doi: 10.1016/j.jadohealth.2015.12.013. http://dx.doi.org/10.1016/j.jadohealth.2015.12.013. [DOI] [PubMed] [Google Scholar]
- 638.Salloum RG, Getz KR, Tan AS, et al. Use of Electronic Cigarettes Among Cancer Survivors in the U.S. Am J Prev Med. doi: 10.1016/j.amepre.2016.04.015. In press. Online May 27, 2016. http://dx.doi.org/10.1016/j.amepre.2016.04.015. [DOI] [PubMed] [Google Scholar]
- 639.Schoenborn CA, Gindi RM. Electronic Cigarette Use Among Adults: United States, 2014. U.S. DHHS, CDC, National Center for Health Statistics; 2015. Oct, [PubMed] [Google Scholar]
- 640.Singh T, Arrazola RA, Corey CG, et al. Tobacco Use Among Middle and High School Students - United States, 2011–2015. MMWR Morb Mortal Wkly Rep. 2016;65(14):361–367. doi: 10.15585/mmwr.mm6514a1. http://dx.doi.org/10.15585/mmwr.mm6514a1. [DOI] [PubMed] [Google Scholar]
- 641.Sutherland R, Sindicich N, Entwistle G, et al. Tobacco and e-cigarette use amongst illicit drug users in Australia. Drug Alcohol Depend. 2016;159:35–41. doi: 10.1016/j.drugalcdep.2015.10.035. http://dx.doi.org/10.1016/j.drugalcdep.2015.10.035. [DOI] [PubMed] [Google Scholar]
- 642.Unger JB, Soto DW, Leventhal A. E-cigarette use and subsequent cigarette and marijuana use among Hispanic young adults. Drug Alcohol Depend. 2016;163:261–264. doi: 10.1016/j.drugalcdep.2016.04.027. http://dx.doi.org/10.1016/j.drugalcdep.2016.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 643.Warner KE. Frequency of E-Cigarette Use and Cigarette Smoking by American Students in 2014. Am J Prev Med. 2016;51(2):179–184. doi: 10.1016/j.amepre.2015.12.004. http://dx.doi.org/10.1016/j.amepre.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 644.Weaver SR, Majeed BA, Pechacek TF, Nyman AL, Gregory KR, Eriksen MP. Use of electronic nicotine delivery systems and other tobacco products among USA adults, 2014: results from a national survey. Int J Public Health. 2016;61(2):177–188. doi: 10.1007/s00038-015-0761-0. http://dx.doi.org/10.1007/s00038-015-0761-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 645.Wills TA, Knight R, Sargent JD, Gibbons FX, Pagano I, Williams RJ. Longitudinal study of e-cigarette use and onset of cigarette smoking among high school students in Hawaii. Tob Control. doi: 10.1136/tobaccocontrol-2015-052705. In press. Online January 25, 2016. http://dx.doi.org/10.1136/tobaccocontrol-2015-052705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 646.Zhang X, Pu J. E-cigarette use among U.S. adolescents: secondhand smoke at home matters. Int J Public Health. 2016;61(2):209–213. doi: 10.1007/s00038-015-0784-6. http://dx.doi.org/10.1007/s00038-015-0784-6. [DOI] [PubMed] [Google Scholar]
- 647.Action on Smoking and Health. Use of electronic cigarettes among children in Great Britain. 2015 Aug [Google Scholar]
- 648.Johnston LD, O'Malley PM, Miech RA, Bachman JG, Schulenberg JE. Monitoring the Future National Survey Results on Drug Use 1975–2015: 2015 Overview Key Findings on Adolescent Drug Use. Ann Arbor, MI: University of Michigan; 2016. [Google Scholar]
- 649.Richardson A, Williams V, Rath J, Villanti AC, Vallone D. The Next Generation of Users: Prevalence and Longitudinal Patterns of Tobacco Use Among U.S. Young Adults. Am J Public Health. 2014;104(8):1429–1436. doi: 10.2105/AJPH.2013.301802. http://dx.doi.org/10.2105/AJPH.2013.301802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 650.Borland R. Understanding Hard to Maintain Behaviour Change: A dual-process approach. Oxford: Wiley-Blackwell, Addiction Press; 2014. [Google Scholar]
- 651.Benowitz NL. Clinical pharmacology of nicotine: implications for understanding, preventing, and treating tobacco addiction. Clin Pharmacol Ther. 2008;83(4):531–541. doi: 10.1038/clpt.2008.3. http://dx.doi.org/10.1038/clpt.2008.3. [DOI] [PubMed] [Google Scholar]
- 652.Benowitz NL. Nicotine addiction. N Engl J Med. 2010;362(24):2295–2303. doi: 10.1056/NEJMra0809890. http://dx.doi.org/10.1056/NEJMra0809890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 653.Adriaens K, Van Gucht D, Declerck P, Baeyens F. Effectiveness of the electronic cigarette: An eight-week Flemish study with six-month follow-up on smoking reduction, craving and experienced benefits and complaints. Int J Environ Res Public Health. 2014;11(11):11220–11248. doi: 10.3390/ijerph111111220. http://dx.doi.org/10.3390/ijerph111111220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 654.Etter JF. A longitudinal study of cotinine in long-term daily users of e-cigarettes. Drug Alcohol Depend. 2016;160:218–221. doi: 10.1016/j.drugalcdep.2016.01.003. http://dx.doi.org/10.1016/j.drugalcdep.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 655.Etter JF. Throat hit in users of the electronic cigarette: An exploratory study. Psychol Addict Behav. 2016;30(1):93–100. doi: 10.1037/adb0000137. http://dx.doi.org/10.1037/adb0000137. [DOI] [PubMed] [Google Scholar]
- 656.Walele T, Sharma G, Savioz R, Martin C, Williams J. A randomised, crossover study on an electronic vapour product, a nicotine inhalator and a conventional cigarette. Part A: Pharmacokinetics. Regul Toxicol Pharmacol. 2016;74:187–192. doi: 10.1016/j.yrtph.2015.12.003. http://dx.doi.org/10.1016/j.yrtph.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 657.Velez de Mendizabal N, Jones DR, Jahn A, Bies RR, Brown JW. Nicotine and cotinine exposure from electronic cigarettes: a population approach. Clin Pharmacokinet. 2015;54(6):615–626. doi: 10.1007/s40262-014-0221-7. http://dx.doi.org/10.1007/s40262-014-0221-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 658.Grace RC, Kivell BM, Laugesen M. Gender differences in satisfaction ratings for nicotine electronic cigarettes by first-time users. Addict Behav. 2015;50:140–143. doi: 10.1016/j.addbeh.2015.06.027. http://dx.doi.org/10.1016/j.addbeh.2015.06.027. [DOI] [PubMed] [Google Scholar]
- 659.Lechner WV, Meier E, Wiener JL, et al. The comparative efficacy of first- versus second-generation electronic cigarettes in reducing symptoms of nicotine withdrawal. Addiction. 2015;110(5):862–867. doi: 10.1111/add.12870. http://dx.doi.org/10.1111/add.12870. [DOI] [PubMed] [Google Scholar]
- 660.Rosbrook K, Green BG. Sensory Effects of Menthol and Nicotine in an E-Cigarette. Nicotine Tob Res. 2016;18(7):1588–1595. doi: 10.1093/ntr/ntw019. http://dx.doi.org/10.1093/ntr/ntw019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 661.Penn State Electronic Cigarette Dependence Index. https://smokingcessationleadership.ucsf.edu/sites/smokingcessationleadership.ucsf.edu/files/Documents/Webinars/FINAL_webinar_40_042314.pdf. [Google Scholar]
- 662.Etter JF, Eissenberg T. Dependence levels in users of electronic cigarettes, nicotine gums and tobacco cigarettes. Drug Alcohol Depend. 2015;147:68–75. doi: 10.1016/j.drugalcdep.2014.12.007. http://dx.doi.org/10.1016/j.drugalcdep.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 663.Bullen C, Howe C, Laugesen M, et al. Electronic cigarettes for smoking cessation: a randomised controlled trial. Lancet. 2013;382(9905):1629–1637. doi: 10.1016/S0140-6736(13)61842-5. http://dx.doi.org/10.1016/S0140-6736(13)61842-5. [DOI] [PubMed] [Google Scholar]
- 664.O'Brien B, Knight-West O, Walker N, Parag V, Bullen C. E-cigarettes versus NRT for smoking reduction or cessation in people with mental illness: secondary analysis of data from the ASCEND trial. Tob Induc Dis. 2015;13(1):5. doi: 10.1186/s12971-015-0030-2. http://dx.doi.org/10.1186/s12971-015-0030-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 665.Tseng TY, Ostroff JS, Campo A, et al. A Randomized Trial Comparing the Effect of Nicotine Versus Placebo Electronic Cigarettes on Smoking Reduction Among Young Adult Smokers. Nicotine Tob Res. 2016;18(10):1937–1943. doi: 10.1093/ntr/ntw017. http://dx.doi.org/10.1093/ntr/ntw017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 666.Treating Tobacco Use and Dependence: 2008 Update. Rockville, MD: U.S. DHHS; 2008. Tobacco Use and Dependence Guideline Panel. [Google Scholar]
- 667.Grana RA, Popova L, Ling PM. A Longitudinal Analysis of Electronic Cigarette Use and Smoking Cessation. JAMA Intern Med. 2014;174(5):812–813. doi: 10.1001/jamainternmed.2014.187. http://dx.doi.org/10.1001/jamainternmed.2014.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 668.Pearson JL, Stanton CA, Cha S, Niaura RS, Luta G, Graham AL. E-Cigarettes and Smoking Cessation: Insights and Cautions From a Secondary Analysis of Data From a Study of Online Treatment-Seeking Smokers. Nicotine Tob Res. 2015;17(10):1219–1227. doi: 10.1093/ntr/ntu269. http://dx.doi.org/10.1093/ntr/ntu269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 669.Al-Delaimy WK, Myers MG, Leas EC, Strong DR, Hofstetter CR. E-cigarette use in the past and quitting behavior in the future: a population-based study. Am J Public Health. 2015;105(6):1213–1219. doi: 10.2105/AJPH.2014.302482. http://dx.doi.org/10.2105/AJPH.2014.302482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 670.Vickerman KA, Schauer GL, Malarcher AM, Zhang L, Mowery P, Nash CM. Reasons for Electronic Nicotine Delivery System use and smoking abstinence at 6 months: a descriptive study of callers to employer and health plan-sponsored quitlines. Tob Control. doi: 10.1136/tobaccocontrol-2015-052734. In press. Online April 12, 2016. http://dx.doi.org/10.1136/tobaccocontrol-2015-052734. [DOI] [PubMed] [Google Scholar]
- 671.Polosa R, Caponnetto P, Morjaria JB, Papale G, Campagna D, Russo C. Effect of an electronic nicotine delivery device (e-Cigarette) on smoking reduction and cessation: a prospective 6-month pilot study. BMC Public Health. 2011;11:786. doi: 10.1186/1471-2458-11-786. http://dx.doi.org/10.1186/1471-2458-11-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 672.Polosa R, Morjaria JB, Caponnetto P, et al. Effectiveness and tolerability of electronic cigarette in real-life: a 24-month prospective observational study. Intern Emerg Med. 2014;9(5):537–546. doi: 10.1007/s11739-013-0977-z. http://dx.doi.org/10.1007/s11739-013-0977-z. [DOI] [PubMed] [Google Scholar]
- 673.Pacifici R, Pichini S, Graziano S, Pellegrini M, Massaro G, Beatrice F. Successful Nicotine Intake in Medical Assisted Use of E-Cigarettes: A Pilot Study. Int J Environ Res Public Health. 2015;12(7):7638–7646. doi: 10.3390/ijerph120707638. http://dx.doi.org/10.3390/ijerph120707638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 674.Stein MD, Caviness C, Grimone K, Audet D, Anderson BJ, Bailey GL. An Open Trial of Electronic Cigarettes for Smoking Cessation Among Methadone-Maintained Smokers. Nicotine Tob Res. 2016;18(5):1157–1162. doi: 10.1093/ntr/ntv267. http://dx.doi.org/10.1093/ntr/ntv267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 675.James SA, Meier EM, Wagener TL, Smith KM, Neas BR, Beebe LA. E-Cigarettes for Immediate Smoking Substitution in Women Diagnosed with Cervical Dysplasia and Associated Disorders. Int J Environ Res Public Health. 2016;13(3):288. doi: 10.3390/ijerph13030288. http://dx.doi.org/10.3390/ijerph13030288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 676.Siegel MB, Tanwar KL, Wood KS. Electronic cigarettes as a smoking-cessation: tool results from an online survey. Am J Prev Med. 2011;40(4):472–475. doi: 10.1016/j.amepre.2010.12.006. http://dx.doi.org/10.1016/j.amepre.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 677.Brown J, Beard E, Kotz D, Michie S, West R. Real-world effectiveness of e-cigarettes when used to aid smoking cessation: a cross-sectional population study. Addiction. 2014;109(9):1531–1540. doi: 10.1111/add.12623. http://dx.doi.org/10.1111/add.12623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 678.Lechner WV, Tackett AP, Grant DM, Tahirkheli NN, Driskill LM, Wagener TL. Effects of duration of electronic cigarette use. Nicotine Tob Res. 2015;17(2):180–185. doi: 10.1093/ntr/ntu061. http://dx.doi.org/10.1093/ntr/ntu061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 679.Christensen T, Welsh E, Faseru B. Profile of e-cigarette use and its relationship with cigarette quit attempts and abstinence in Kansas adults. Prev Med. 2014;69C:90–94. doi: 10.1016/j.ypmed.2014.09.005. http://dx.doi.org/10.1016/j.ypmed.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 680.Borderud SP, Li Y, Burkhalter JE, Sheffer CE, Ostroff JS. Electronic cigarette use among patients with cancer: Characteristics of electronic cigarette users and their smoking cessation outcomes. Cancer. 2014;120(22):3527–3535. doi: 10.1002/cncr.28811. http://dx.doi.org/10.1002/cncr.28811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 681.McQueen N, Partington EJ, Harrington KF, Rosenthal EL, Carroll WR, Schmalbach CE. Smoking Cessation and Electronic Cigarette Use among Head and Neck Cancer Patients. Otolaryngol Head Neck Surg. 2016;154(1):73–79. doi: 10.1177/0194599815613279. http://dx.doi.org/10.1177/0194599815613279. [DOI] [PubMed] [Google Scholar]
- 682.Truth Initiative. Where We Stand: FDA Can Balance Potential Benefits and Harms of ENDS (comments submitted to FDA in July 2015) 2015 http://truthinitiative.org/sites/default/files/2015.06.30%20E-Cig%20FDA%20Workshop%20Docket%20FINAL.pdf. [Google Scholar]
- 683.Shiffman S, Brockwell SE, Pillitteri JL, Gitchell JG. Use of smoking-cessation treatments in the United States. Am J Prev Med. 2008;34(2):102–111. doi: 10.1016/j.amepre.2007.09.033. http://dx.doi.org/10.1016/j.amepre.2007.09.033. [DOI] [PubMed] [Google Scholar]
- 684.Alberg AJ, Patnaik JL, May JW, et al. Nicotine replacement therapy use among a cohort of smokers. J Addict Dis. 2005;24(1):101–113. doi: 10.1300/J069v24n01_09. http://dx.doi.org/10.1300/J069v24n01_09. [DOI] [PubMed] [Google Scholar]
- 685.Pierce JP, Gilpin EA. Impact of over-the-counter sales on effectiveness of pharmaceutical aids for smoking cessation. JAMA. 2002;288(10):1260–1264. doi: 10.1001/jama.288.10.1260. http://dx.doi.org/10.1001/jama.288.10.1260. [DOI] [PubMed] [Google Scholar]
- 686.Silagy C, Lancaster T, Stead L, Mant D, Fowler G. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev. 2004;(3):CD000146. doi: 10.1002/14651858.CD000146.pub2. http://dx.doi.org/10.1002/14651858.cd000146.pub2. [DOI] [PubMed] [Google Scholar]
- 687.Campaign for Tobacco-Free Kids. 7 ways e-cigarette companies are copying Big Tobacco's playbook (or 7 reasons FDA should quickly regulate e-cigarettes) [Accessed November 8];2013 www.tobaccofreekids.org/tobacco_unfiltered/post/2013_10_02_ecigarettes. [Google Scholar]
- 688.Cobb CO, Vansickel AR, Blank MD, Jentink K, Travers MJ, Eissenberg T. Indoor air quality in Virginia waterpipe cafes. Tob Control. 2013;22(5):338–343. doi: 10.1136/tobaccocontrol-2011-050350. http://dx.doi.org/10.1136/tobaccocontrol-2011-050350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 689.Duke JC, Lee YO, Kim AE, et al. Exposure to electronic cigarette television advertisements among youth and young adults. Pediatrics. 2014;134(1):e29–e36. doi: 10.1542/peds.2014-0269. http://dx.doi.org/10.1542/peds.2014-0269. [DOI] [PubMed] [Google Scholar]
- 690.Hua M, Yip H, Talbot P. Mining data on usage of electronic nicotine delivery systems (ENDS) from YouTube videos. Tob Control. 2013;22(2):103–106. doi: 10.1136/tobaccocontrol-2011-050226. http://dx.doi.org/10.1136/tobaccocontrol-2011-050226. [DOI] [PubMed] [Google Scholar]
- 691.Huang J, Kornfield R, Szczypka G, Emery SL. A cross-sectional examination of marketing of electronic cigarettes on Twitter. Tob Control. 2014;23(Suppl 3):iii26–iii30. doi: 10.1136/tobaccocontrol-2014-051551. http://dx.doi.org/10.1136/tobaccocontrol-2014-051551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 692.Kamerow D. Big Tobacco lights up e-cigarettes. BMJ (Clinical Research Ed) 2013;346:f3418. doi: 10.1136/bmj.f3418. http://dx.doi.org/10.1136/bmj.f3418. [DOI] [PubMed] [Google Scholar]
- 693.Kim AE, Lee YO, Shafer P, Nonnemaker J, Makarenko O. Adult smokers' receptivity to a television advert for electronic nicotine delivery systems. Tob Control. 2015;24(2):132–135. doi: 10.1136/tobaccocontrol-2013-051130. http://dx.doi.org/10.1136/tobaccocontrol-2013-051130. [DOI] [PubMed] [Google Scholar]
- 694.Kress M. Big three moving quickly in growing e-cigarette presence. Convenience Store News. 2013 [Google Scholar]
- 695.Kuschner WG, Reddy S, Mehrotra N, Paintal HS. Electronic cigarettes and thirdhand tobacco smoke: two emerging health care challenges for the primary care provider. Int J Gen Med. 2011;4:115–120. doi: 10.2147/IJGM.S16908. http://dx.doi.org/10.2147/IJGM.S16908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 696.Lee YO, Kim AE. 'Vape shops' and 'E-Cigarette lounges' open across the USA to promote ENDS. Tob Control. 2015;24(4):410–412. doi: 10.1136/tobaccocontrol-2013-051437. http://dx.doi.org/10.1136/tobaccocontrol-2013-051437. [DOI] [PubMed] [Google Scholar]
- 697.Paek HJ, Kim S, Hove T, Huh JY. Reduced harm or another gateway to smoking? source, message, and information characteristics of E-cigarette videos on YouTube. J Health Commun. 2014;19(5):545–560. doi: 10.1080/10810730.2013.821560. http://dx.doi.org/10.1080/10810730.2013.821560. [DOI] [PubMed] [Google Scholar]
- 698.Pepper JK, Emery SL, Ribisl KM, Southwell BG, Brewer NT. Effects of advertisements on smokers' interest in trying e-cigarettes: the roles of product comparison and visual cues. Tob Control. 2014;23(Suppl 3):iii31–iii36. doi: 10.1136/tobaccocontrol-2014-051718. http://dx.doi.org/10.1136/tobaccocontrol-2014-051718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 699.Richardson A, Ganz O, Stalgaitis C, Abrams D, Vallone D. Noncombustible tobacco product advertising: how companies are selling the new face of tobacco. Nicotine Tob Res. 2014;16(5):606–614. doi: 10.1093/ntr/ntt200. http://dx.doi.org/10.1093/ntr/ntt200. [DOI] [PubMed] [Google Scholar]
- 700.Salloum RG, Osman A, Maziak W, Thrasher JF. How popular is waterpipe tobacco smoking? Findings from internet search queries. Tob Control. 2015;24(5):509–513. doi: 10.1136/tobaccocontrol-2014-051675. http://dx.doi.org/10.1136/tobaccocontrol-2014-051675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 701.Zhu SH, Sun JY, Bonnevie E, et al. Four hundred and sixty brands of e-cigarettes and counting: implications for product regulation. Tob Control. 2014;23(Suppl 3):iii3–iii9. doi: 10.1136/tobaccocontrol-2014-051670. http://dx.doi.org/10.1136/tobaccocontrol-2014-051670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 702.Ganz O, Cantrell J, Moon-Howard J, Aidala A, Kirchner TR, Vallone D. Electronic cigarette advertising at the point-of-sale: a gap in tobacco control research. Tob Control. 2015;24(e1):e110–e112. doi: 10.1136/tobaccocontrol-2013-051337. http://dx.doi.org/10.1136/tobaccocontrol-2013-051337. [DOI] [PubMed] [Google Scholar]
- 703.Hahn EJ, Begley K, Gokun Y, Johnson AO, Mundy ME, Rayens MK. Electronic Cigarette Retail Outlets and Proximity to Schools. Am J Health Promot. 2015;29(6):380–383. doi: 10.4278/ajhp.130627-ARB-335. http://dx.doi.org/10.4278/ajhp.130627-ARB-335. [DOI] [PubMed] [Google Scholar]
- 704.Hsu R, Myers AE, Ribisl KM, Marteau TM. An observational study of retail availability and in-store marketing of e-cigarettes in London: potential to undermine recent tobacco control gains? BMJ Open. 2013;3(12):e004085. doi: 10.1136/bmjopen-2013-004085. http://dx.doi.org/10.1136/bmjopen-2013-004085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 705.Wagoner KG, Song EY, Egan KL, et al. E-cigarette Availability and Promotion Among Retail Outlets Near College Campuses in Two Southeastern States. Nicotine Tob Res. 2014;16(8):1150–1155. doi: 10.1093/ntr/ntu081. http://dx.doi.org/10.1093/ntr/ntu081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 706.Cranwell J, Murray R, Lewis S, Leonardi-Bee J, Dockrell M, Britton J. Adolescents' exposure to tobacco and alcohol content in YouTube music videos. Addiction. 2015;110(4):703–711. doi: 10.1111/add.12835. http://dx.doi.org/10.1111/add.12835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 707.Luo C, Zheng X, Zeng DD, Leischow S. Portrayal of electronic cigarettes on YouTube. BMC Public Health. 2014;14:1028. doi: 10.1186/1471-2458-14-1028. http://dx.doi.org/10.1186/1471-2458-14-1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 708.Rooke C, Amos A. News media representations of electronic cigarettes: an analysis of newspaper coverage in the UK and Scotland. Tob Control. 2014;23(6):507–512. doi: 10.1136/tobaccocontrol-2013-051043. http://dx.doi.org/10.1136/tobaccocontrol-2013-051043. [DOI] [PubMed] [Google Scholar]
- 709.Yao T, Jiang N, Grana R, Ling PM, Glantz SA. A content analysis of electronic cigarette manufacturer websites in China. Tob Control. 25(2):188–194. doi: 10.1136/tobaccocontrol-2014-051840. http://dx.doi.org/10.1136/tobaccocontrol-2014-051840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 710.Tsai FJ, Sainbayar B. Portrayal of tobacco in Mongolian language YouTube videos: policy gaps. Tob Control. 25(4):480–482. doi: 10.1136/tobaccocontrol-2014-052032. http://dx.doi.org/10.1136/tobaccocontrol-2014-052032. [DOI] [PubMed] [Google Scholar]
- 711.Cobb NK, Brookover J, Cobb CO. Forensic analysis of online marketing for electronic nicotine delivery systems. Tob Control. 2015;24(2):128–131. doi: 10.1136/tobaccocontrol-2013-051185. http://dx.doi.org/10.1136/tobaccocontrol-2013-051185. [DOI] [PubMed] [Google Scholar]
- 712.Farrelly MC, Duke JC, Crankshaw EC, et al. A Randomized Trial of the Effect of E-cigarette TV Advertisements on Intentions to Use E-cigarettes. Am J Prev Med. 2015;49(5):686–693. doi: 10.1016/j.amepre.2015.05.010. http://dx.doi.org/10.1016/j.amepre.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 713.Jo CL, Kornfield R, Kim Y, Emery S, Ribisl KM. Price-related promotions for tobacco products on Twitter. Tob Control. 2016;25(4):476–479. doi: 10.1136/tobaccocontrol-2015-052260. http://dx.doi.org/10.1136/tobaccocontrol-2015-052260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 714.Sanders-Jackson A, Schleicher NC, Fortmann SP, Henriksen L. Effect of warning statements in e-cigarette advertisements: an experiment with young adults in the U.S. Addiction. 2015;110(12):2015–2024. doi: 10.1111/add.12838. http://dx.doi.org/10.1111/add.12838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 715.Mackey TK, Miner A, Cuomo RE. Exploring the e-cigarette e-commerce marketplace: Identifying Internet e-cigarette marketing characteristics and regulatory gaps. Drug Alcohol Depend. 2015;156:97–103. doi: 10.1016/j.drugalcdep.2015.08.032. http://dx.doi.org/10.1016/j.drugalcdep.2015.08.032. [DOI] [PubMed] [Google Scholar]
- 716.Roberts ME, Berman ML, Slater MD, Hinton A, Ferketich AK. Point-of-sale tobacco marketing in rural and urban Ohio: Could the new landscape of Tobacco products widen inequalities? Prev Med. 2015;81:232–235. doi: 10.1016/j.ypmed.2015.08.024. http://dx.doi.org/10.1016/j.ypmed.2015.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 717.Tan AS, Lee CJ, Bigman CA. Public support for selected e-cigarette regulations and associations with overall information exposure and contradictory information exposure about e-cigarettes: Findings from a national survey of U.S. adults. Prev Med. 2015;81:268–274. doi: 10.1016/j.ypmed.2015.09.009. http://dx.doi.org/10.1016/j.ypmed.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 718.Morris DS, Fiala SC. Online electronic cigarette retailers can do more to prevent accidental poisonings. Tob Control. 2015;24(4):415–416. doi: 10.1136/tobaccocontrol-2014-051779. http://dx.doi.org/10.1136/tobaccocontrol-2014-051779. [DOI] [PubMed] [Google Scholar]
- 719.Pepper JK, Emery SL, Ribisl KM, Brewer NT. How U.S. Adults Find Out About Electronic Cigarettes: Implications for Public Health Messages. Nicotine Tob Res. 2014;16(8):1140–1144. doi: 10.1093/ntr/ntu060. http://dx.doi.org/10.1093/ntr/ntu060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 720.Villanti AC, Rath JM, Williams VF, et al. Impact of Exposure to Electronic Cigarette Advertising on Susceptibility and Trial of Electronic Cigarettes and Cigarettes in U.S. Young Adults: A Randomized Controlled Trial. Nicotine Tob Res. 2016;18(5):1331–1339. doi: 10.1093/ntr/ntv235. http://dx.doi.org/10.1093/ntr/ntv235. [DOI] [PubMed] [Google Scholar]
- 721.Allem JP, Escobedo P, Chu KH, Soto DW, Cruz TB, Unger JB. Campaigns and counter campaigns: reactions on Twitter to e-cigarette education. Tob Control. doi: 10.1136/tobaccocontrol-2015-052757. In press. Online March 8, 2016. http://dx.doi.org/10.1136/tobaccocontrol-2015-052757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 722.Basch CH, Mongiovi J, Hillyer GC, MacDonald Z, Basch CE. YouTube videos related to e-cigarette safety and related health risks: implications for preventing and emerging epidemic. Public Health. 2016;132:57–59. doi: 10.1016/j.puhe.2015.12.003. http://dx.doi.org/10.1016/j.puhe.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 723.Best C, Haseen F, van der Sluijs W, et al. Relationship between e-cigarette point of sale recall and e-cigarette use in secondary school children: a cross-sectional study. BMC Public Health. 2016;16(1):310. doi: 10.1186/s12889-016-2968-2. http://dx.doi.org/10.1186/s12889-016-2968-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 724.Cantrell J, Emelle B, Ganz O, Hair EC, Vallone D. Rapid increase in e-cigarette advertising spending as Altria's MarkTen enters the marketplace. Tob Control. 2016;25(e1):e16–e18. doi: 10.1136/tobaccocontrol-2015-052532. http://dx.doi.org/10.1136/tobaccocontrol-2015-052532. [DOI] [PubMed] [Google Scholar]
- 725.Chu KH, Unger JB, Allem JP, et al. Diffusion of Messages from an Electronic Cigarette Brand to Potential Users through Twitter. PLoS One. 2015;10(12):e0145387. doi: 10.1371/journal.pone.0145387. http://dx.doi.org/10.1371/journal.pone.0145387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 726.Cole-Lewis H, Pugatch J, Sanders A, et al. Social Listening: A Content Analysis of E-Cigarette Discussions on Twitter. J Med Internet Res. 2015;17(10):e243. doi: 10.2196/jmir.4969. http://dx.doi.org/10.2196/jmir.4969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 727.Dai H, Hao J. Mining social media data for opinion polarities about electronic cigarettes. Tob Control. doi: 10.1136/tobaccocontrol-2015-052818. In press. Online March 15, 2016. http://dx.doi.org/10.1136/tobaccocontrol-2015-052818. [DOI] [PubMed] [Google Scholar]
- 728.Duke JC, Allen JA, Eggers ME, Nonnemaker J, Farrelly MC. Exploring Differences in Youth Perceptions of the Effectiveness of Electronic Cigarette Television Advertisements. Nicotine Tob Res. 2016;18(5):1382–1386. doi: 10.1093/ntr/ntv264. http://dx.doi.org/10.1093/ntr/ntv264. [DOI] [PubMed] [Google Scholar]
- 729.Hammond D, White CM, Czoli CD, Martin CL, Magennis P, Shiplo S. Retail availability and marketing of electronic cigarettes in Canada. Can J Public Health. 2015;106(6):e408–e412. doi: 10.17269/CJPH.106.5105. http://dx.doi.org/10.17269/cjph.106.5105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 730.Hosler AS, Done DH, Michaels IH, Guarasi DC, Kammer JR. Longitudinal Trends in Tobacco Availability, Tobacco Advertising, and Ownership Changes of Food Stores, Albany, New York, 2003–2015. Prev Chronic Dis. 2016;13:E62. doi: 10.5888/pcd13.160002. http://dx.doi.org/10.5888/pcd13.160002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 731.Huang J, Kornfield R, Emery SL. 100 Million Views of Electronic Cigarette YouTube Videos and Counting: Quantification, Content Evaluation, and Engagement Levels of Videos. J Med Internet Res. 2016;18(3):e67. doi: 10.2196/jmir.4265. http://dx.doi.org/10.2196/jmir.4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 732.Jiang N, Ho SY, Lam TH. Electronic cigarette marketing tactics in mainland China. Tob Control. doi: 10.1136/tobaccocontrol-2015-052824. In press. Online April 12, 2016. http://dx.doi.org/10.1136/tobaccocontrol-2015-052824. [DOI] [PubMed] [Google Scholar]
- 733.Kim AE, Hopper T, Simpson S, et al. Using Twitter Data to Gain Insights into E-cigarette Marketing and Locations of Use: An Infoveillance Study. J Med Internet Res. 2015;17(11):e251. doi: 10.2196/jmir.4466. http://dx.doi.org/10.2196/jmir.4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 734.Mays D, Smith C, Johnson AC, Tercyak KP, Niaura RS. An experimental study of the effects of electronic cigarette warnings on young adult nonsmokers' perceptions and behavioral intentions. Tob Induc Dis. 2016;14:17. doi: 10.1186/s12971-016-0083-x. http://dx.doi.org/10.1186/s12971-016-0083-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 735.Nagelhout GE, Heijndijk SM, Cummings KM, et al. E-cigarette advertisements, and associations with the use of e-cigarettes and disapproval or quitting of smoking: Findings from the International Tobacco Control (ITC) Netherlands Survey. Int J Drug Policy. 2016;29:73–79. doi: 10.1016/j.drugpo.2015.12.015. http://dx.doi.org/10.1016/j.drugpo.2015.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 736.Pokhrel P, Fagan P, Herzog TA, et al. E-cigarette advertising exposure and implicit attitudes among young adult non-smokers. Drug Alcohol Depend. 2016;163:134–140. doi: 10.1016/j.drugalcdep.2016.04.008. http://dx.doi.org/10.1016/j.drugalcdep.2016.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 737.Popova L, Linde BD, Bursac Z, et al. Testing antismoking messages for Air Force trainees. Tob Control. doi: 10.1136/tobaccocontrol-2015-052477. In press. Online October 19, 2015. http://dx.doi.org/10.1136/tobaccocontrol-2015-052477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 738.Ramamurthi D, Fadadu RP, Jackler RK. Electronic cigarette marketers manipulate antitobacco advertisements to promote vaping. Tob Control. doi: 10.1136/tobaccocontrol-2015-052661. In press. Online November 6, 2015. http://dx.doi.org/10.1136/tobaccocontrol-2015-052661. [DOI] [PubMed] [Google Scholar]
- 739.Romito LM, Hurwich RA, Eckert GJ. A Snapshot of the Depiction of Electronic Cigarettes in YouTube Videos. Am J Health Behav. 2015;39(6):823–831. doi: 10.5993/AJHB.39.6.10. http://dx.doi.org/10.5993/AJHB.39.6.10. [DOI] [PubMed] [Google Scholar]
- 740.Ayers JW, Althouse BM, Allem JP, Leas EC, Dredze M, Williams RS. Revisiting the Rise of Electronic Nicotine Delivery Systems Using Search Query Surveillance. Am J Prev Med. 2016;50(6):e173–e181. doi: 10.1016/j.amepre.2015.12.008. http://dx.doi.org/10.1016/j.amepre.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 741.Singh T, Agaku IT, Arrazola RA, et al. Exposure to Advertisements and Electronic Cigarette Use Among U.S. Middle and High School Students. Pediatrics. 2016;137(5) doi: 10.1542/peds.2015-4155. http://dx.doi.org/10.1542/peds.2015-4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 742.Singh T, Marynak K, Arrazola RA, Cox S, Rolle IV, King BA. Vital Signs: Exposure to Electronic Cigarette Advertising Among Middle School and High School Students - United States, 2014. MMWR Morb Mortal Wkly Rep. 2016;64(52):1403–1408. doi: 10.15585/mmwr.mm6452a3. http://dx.doi.org/10.15585/mmwr.mm6452a3. [DOI] [PubMed] [Google Scholar]
- 743.van der Tempel J, Noormohamed A, Schwartz R, Norman C, Malas M, Zawertailo L. Vape, quit, tweet? Electronic cigarettes and smoking cessation on Twitter. Int J Public Health. 2016;61(2):249–256. doi: 10.1007/s00038-016-0791-2. http://dx.doi.org/10.1007/s00038-016-0791-2. [DOI] [PubMed] [Google Scholar]
- 744.Vasiljevic M, Petrescu DC, Marteau TM. Impact of advertisements promoting candy-like flavoured e-cigarettes on appeal of tobacco smoking among children: an experimental study. Tob Control. doi: 10.1136/tobaccocontrol-2015-052593. In press. Online January 17, 2016. http://dx.doi.org/10.1136/tobaccocontrol-2015-052593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 745.Wang L, Zhan Y, Li Q, Zeng DD, Leischow SJ, Okamoto J. An Examination of Electronic Cigarette Content on Social Media: Analysis of E-Cigarette Flavor Content on Reddit. Int J Environ Res Public Health. 2015;12(11):14916–14935. doi: 10.3390/ijerph121114916. http://dx.doi.org/10.3390/ijerph121114916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 746.Williams RS. VapeCons: E-cigarette user conventions. J Public Health Policy. 2015;36(4):440–451. doi: 10.1057/jphp.2015.31. http://dx.doi.org/10.1057/jphp.2015.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 747.Pesko MF, Kenkel DS, Wang H, Hughes JM. The effect of potential electronic nicotine delivery system regulations on nicotine product selection. Addiction. 2016;111(4):734–744. doi: 10.1111/add.13257. http://dx.doi.org/10.1111/add.13257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 748.Kim AE, Arnold KY, Makarenko O. E-cigarette Advertising Expenditures in the U.S., 2011–2012. Am J Prev Med. 2014;46(4):409–412. doi: 10.1016/j.amepre.2013.11.003. http://dx.doi.org/10.1016/j.amepre.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 749.Kornfield R, Huang J, Vera L, Emery SL. Rapidly increasing promotional expenditures for e-cigarettes. Tob Control. 2015;24(2):110–111. doi: 10.1136/tobaccocontrol-2014-051580. http://dx.doi.org/10.1136/tobaccocontrol-2014-051580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 750.Richardson A, Ganz O, Vallone D. Tobacco on the web: surveillance and characterisation of online tobacco and e-cigarette advertising. Tob Control. 2015;24(4):341–347. doi: 10.1136/tobaccocontrol-2013-051246. http://dx.doi.org/10.1136/tobaccocontrol-2013-051246. [DOI] [PubMed] [Google Scholar]
- 751.eCigs b. blu cartridges. [Accessed July 2, 2013];2013 www.blucigs.com/store/cartridges. [Google Scholar]
- 752.Food and Drug Administration. Transcript for FDA's media briefing on electronic cigarettes Moderator: Judy Leon July 22, 2009. 1:30 pm CT. 2009 [Google Scholar]
- 753.Grana RA, Ling PM. "Smoking revolution": a content analysis of electronic cigarette retail websites. Am J Prev Med. 2014;46(4):395–403. doi: 10.1016/j.amepre.2013.12.010. http://dx.doi.org/10.1016/j.amepre.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 754.NJOY. NJOY. [Accessed July 3];2013 www.njoy.com/ [Google Scholar]
- 755.USA GSI. Green Smoke. 2013 www.greensmoke.com/ [Google Scholar]
- 756.Bagcchi S. E-cigarette market expands online. Lancet Oncol. 2014;15(8):e313. doi: 10.1016/s1470-2045(14)70297-9. http://dx.doi.org/10.1016/S1470-2045(14)70297-9. [DOI] [PubMed] [Google Scholar]
- 757.Huang J, Tauras J, Chaloupka FJ. The impact of price and tobacco control policies on the demand for electronic nicotine delivery systems. Tob Control. 2014;23(Suppl 3):iii41–iii47. doi: 10.1136/tobaccocontrol-2013-051515. http://dx.doi.org/10.1136/tobaccocontrol-2013-051515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 758.Rose SW, Barker DC, D'Angelo H, et al. The availability of electronic cigarettes in U.S. retail outlets, 2012: results of two national studies. Tob Control. 2014;23(Suppl 3):iii10–iii16. doi: 10.1136/tobaccocontrol-2013-051461. http://dx.doi.org/10.1136/tobaccocontrol-2013-051461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 759.Seidenberg AB, Hong W, Liu J, Noel JK, Rees VW. Availability and range of tobacco products for sale in Massachusetts pharmacies. Tob Control. 2013;22(6):372–375. doi: 10.1136/tobaccocontrol-2012-050591. http://dx.doi.org/10.1136/tobaccocontrol-2012-050591. [DOI] [PubMed] [Google Scholar]
- 760.Giovenco DP, Hammond D, Corey CG, Ambrose BK, Delnevo CD. E-Cigarette Market Trends in Traditional U.S. Retail Channels, 2012–2013. Nicotine Tob Res. 2015;17(10):1279–1283. doi: 10.1093/ntr/ntu282. http://dx.doi.org/10.1093/ntr/ntu282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 761.Grace RC, Kivell BM, Laugesen M. Estimating cross-price elasticity of e-cigarettes using a simulated demand procedure. Nicotine Tob Res. 2015;17(5):592–598. doi: 10.1093/ntr/ntu268. http://dx.doi.org/10.1093/ntr/ntu268. [DOI] [PubMed] [Google Scholar]
- 762.Hahn EJ, Begley K, Gokun Y, Johnson AO, Mundy ME, Rayens MK. Electronic Cigarette Retail Outlets and Proximity to Schools. Am J Health Promot. 2015;29(6):380–383. doi: 10.4278/ajhp.130627-ARB-335. http://dx.doi.org/10.4278/ajhp.130627-ARB-335. [DOI] [PubMed] [Google Scholar]
- 763.Williams RS, Derrick J, Ribisl KM. Electronic cigarette sales to minors via the internet. JAMA Pediatr. 2015;169(3):e1563. doi: 10.1001/jamapediatrics.2015.63. http://dx.doi.org/10.1001/jamapediatrics.2015.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 764.Barnoya J, Jin L, Hudmon KS, Schootman M. Nicotine replacement therapy, tobacco products, and electronic cigarettes in pharmacies in St. Louis, Missouri. J Am Pharm Assoc (2003) 2015;55(4):405–412. doi: 10.1331/JAPhA.2015.14230. http://dx.doi.org/10.1331/JAPhA.2015.14230. [DOI] [PubMed] [Google Scholar]
- 765.Loomis BR, Rogers T, King BA, et al. National and State-Specific Sales and Prices for Electronic Cigarettes-U.S., 2012–2013. Am J Prev Med. 2016;50(1):18–29. doi: 10.1016/j.amepre.2015.05.003. http://dx.doi.org/10.1016/j.amepre.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 766.Laugesen M, Grace RC. Excise, electronic cigarettes and nicotine reduction to reduce smoking prevalence in New Zealand by 2025. N Z Med J. 2015;128(1420):72–74. [PubMed] [Google Scholar]
- 767.Cuomo RE, Miner A, Mackey TK. Pricing and sales tax collection policies for e-cigarette starter kits and disposable products sold online. Drug Alcohol Rev. 2016;35:110–114. doi: 10.1111/dar.12353. http://dx.doi.org/10.1111/dar.12353. [DOI] [PubMed] [Google Scholar]
- 768.Liber AC, Drope JM, Stoklosa M. Combustible cigarettes cost less to use than e-cigarettes: global evidence and tax policy implications. Tob Control. doi: 10.1136/tobaccocontrol-2015-052874. In press. Online March 28, 2016. http://dx.doi.org/10.1136/tobaccocontrol-2015-052874. [DOI] [PubMed] [Google Scholar]
- 769.Nikitin D, Timberlake DS, Williams RS. Is the E-Liquid Industry Regulating Itself? A Look at E-Liquid Internet Vendors in the United States. Nicotine Tob Res. 2016;18(10):1967–1972. doi: 10.1093/ntr/ntw091. http://dx.doi.org/10.1093/ntr/ntw091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 770.Quisenberry AJ, Koffarnus MN, Hatz LE, Epstein LH, Bickel WK. The Experimental Tobacco Marketplace I: Substitutability as a Function of the Price of Conventional Cigarettes. Nicotine Tob Res. 2016;18(7):1642–1648. doi: 10.1093/ntr/ntv230. http://dx.doi.org/10.1093/ntr/ntv230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 771.Seidenberg AB, Jo CL, Ribisl KM. Differences in the design and sale of e-cigarettes by cigarette manufacturers and non-cigarette manufacturers in the USA. Tob Control. 2016;25(e1):e3–e5. doi: 10.1136/tobaccocontrol-2015-052375. http://dx.doi.org/10.1136/tobaccocontrol-2015-052375. [DOI] [PubMed] [Google Scholar]
- 772.Soneji S, Gerling M, Yang J, Sargent J. Online Electronic Cigarette Marketing-Violation of Self-regulated Standards by Tobacco Companies. JAMA Pediatr. 2016;170(5):511–512. doi: 10.1001/jamapediatrics.2015.4501. http://dx.doi.org/10.1001/jamapediatrics.2015.4501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 773.Stoklosa M, Drope J, Chaloupka FJ. Prices and E-Cigarette Demand: Evidence From the European Union. Nicotine Tob Res. 2016;18(10):1973–1980. doi: 10.1093/ntr/ntw109. http://dx.doi.org/10.1093/ntr/ntw109. [DOI] [PubMed] [Google Scholar]
- 774.Sussman S, Allem JP, Garcia J, et al. Who walks into vape shops in Southern California?: a naturalistic observation of customers. Tob Induc Dis. 2016;14:18. doi: 10.1186/s12971-016-0082-y. http://dx.doi.org/10.1186/s12971-016-0082-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 775.Stafford N. Hanover bans e-cigarette use in civic offices amid calls for better safety data. BMJ. 2012;344:e3. doi: 10.1136/bmj.e3. http://dx.doi.org/10.1136/bmj.e3. [DOI] [PubMed] [Google Scholar]
- 776.Paradise J. Electronic cigarettes: smoke-free laws, sale restrictions, and the public health. Am J Public Health. 2014;104(6):e17–e18. doi: 10.2105/AJPH.2014.301890. http://dx.doi.org/10.2105/AJPH.2014.301890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 777.Gourdet CK, Chriqui JF, Chaloupka FJ. A baseline understanding of state laws governing e-cigarettes. Tob Control. 2014;23(Suppl 3):iii37–iii40. doi: 10.1136/tobaccocontrol-2013-051459. http://dx.doi.org/10.1136/tobaccocontrol-2013-051459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 778.Lempert LK, Grana R, Glantz SA. The importance of product definitions in U.S. e-cigarette laws and regulations. Tob Control. 2016;25(e1):e44–e51. doi: 10.1136/tobaccocontrol-2014-051913. http://dx.doi.org/10.1136/tobaccocontrol-2014-051913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 779.Marynak K, Holmes CB, King BA, Promoff G, Bunnell R, McAfee T. State laws prohibiting sales to minors and indoor use of electronic nicotine delivery systems--United States, November 2014. MMWR Morb Mortal Wkly Rep. 2014;63(49):1145–1150. [PMC free article] [PubMed] [Google Scholar]
- 780.Dobbs PD, Hammig B, Sudduth A. 2015 Legislative Update of E-Cigarette Youth Access and Exposure Laws. Prev Med. 2016;88:90–94. doi: 10.1016/j.ypmed.2016.03.010. http://dx.doi.org/10.1016/j.ypmed.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 781.Cahn Z. France acts on electronic cigarettes. J Public Health Policy. 2013;34(4):560–564. doi: 10.1057/jphp.2013.32. http://dx.doi.org/10.1057/jphp.2013.32. [DOI] [PubMed] [Google Scholar]
- 782.Capasso L, Gualano MR, Flacco ME, Siliquini R, Manzoli L. E-cigarette regulations in Italy: fluctuating and confusing. Lancet. 2014;383(9932):1883. doi: 10.1016/S0140-6736(14)60908-9. http://dx.doi.org/10.1016/S0140-6736(14)60908-9. [DOI] [PubMed] [Google Scholar]
- 783.Hall W, Gartner C. Should Australia reconsider its ban on the sale of electronic nicotine delivery systems? Lancet Respir Med. 2014;2(8):602–604. doi: 10.1016/S2213-2600(14)70155-9. http://dx.doi.org/10.1016/S2213-2600(14)70155-9. [DOI] [PubMed] [Google Scholar]
- 784.Buonocore F, Marques Gomes AC, Nabhani-Gebara S, Barton SJ, Calabrese G. Labelling of electronic cigarettes: regulations and current practice. Tob Control. doi: 10.1136/tobaccocontrol-2015-052683. In press. Online January 20, 2016. http://dx.doi.org/10.1136/tobaccocontrol-2015-052683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 785.Miller A. E-cigarette ban proposed in Toronto. CMAJ. 2014;186(13):E481–E482. doi: 10.1503/cmaj.109-4873. http://dx.doi.org/10.1503/cmaj.109-4873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 786.Boyers LN, Karimkhani C, Riggs J, Dellavalle RP. U.S. campus and university debit card policies regarding tobacco and electronic cigarettes. Tob Control. 2015;24(6):623–624. doi: 10.1136/tobaccocontrol-2014-051957. http://dx.doi.org/10.1136/tobaccocontrol-2014-051957. [DOI] [PubMed] [Google Scholar]
- 787.Tan AS, Bigman CA, Sanders-Jackson A. Sociodemographic correlates of self-reported exposure to e-cigarette communications and its association with public support for smoke-free and vape-free policies: results from a national survey of U.S. adults. Tob Control. 2015;24(6):574–581. doi: 10.1136/tobaccocontrol-2014-051685. http://dx.doi.org/10.1136/tobaccocontrol-2014-051685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 788.Blaser J, Cornuz J. Experts' consensus on use of electronic cigarettes: a Delphi survey from Switzerland. BMJ Open. 2015;5(4):e007197. doi: 10.1136/bmjopen-2014-007197. http://dx.doi.org/10.1136/bmjopen-2014-007197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 789.Harris JK, Moreland-Russell S, Choucair B, Mansour R, Staub M, Simmons K. Tweeting for and against public health policy: response to the Chicago Department of Public Health's electronic cigarette Twitter campaign. J Med Internet Res. 2014;16(10):e238. doi: 10.2196/jmir.3622. http://dx.doi.org/10.2196/jmir.3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 790.Farrimond H. E-cigarette regulation and policy: UK vapers' perspectives. Addiction. 2016;111(6):1077–1083. doi: 10.1111/add.13322. http://dx.doi.org/10.1111/add.13322. [DOI] [PubMed] [Google Scholar]
- 791.Mello S, Bigman CA, Sanders-Jackson A, Tan AS. Perceived Harm of Secondhand Electronic Cigarette Vapors and Policy Support to Restrict Public Vaping: Results From a National Survey of U.S. Adults. Nicotine Tob Res. 2016;18(5):686–693. doi: 10.1093/ntr/ntv232. http://dx.doi.org/10.1093/ntr/ntv232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 792.Patterson C, Hilton S, Weishaar H. Who thinks what about e-cigarette regulation? A content analysis of UK newspapers. Addiction. 2016;111(7):1267–1274. doi: 10.1111/add.13320. http://dx.doi.org/10.1111/add.13320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 793.Unger JB, Barker D, Baezconde-Garbanati L, Soto DW, Sussman S. Support for electronic cigarette regulations among California voters. Tob Control. doi: 10.1136/tobaccocontrol-2016-052918. In press. Online May 20, 2016. http://dx.doi.org/10.1136/tobaccocontrol-2016-052918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 794.Weishaar H, Trevisan F, Hilton S. "Maybe they should regulate them quite strictly until they know the true dangers": A focus group study exploring UK adolescents' views on e-cigarette regulation. Addiction. 2016;111(9):1637–1645. doi: 10.1111/add.13377. http://dx.doi.org/10.1111/add.13377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 795.Schmitt CL, Lee YO, Curry LE, Farrelly MC, Rogers T. Research support for effective state and community tobacco control programme response to electronic nicotine delivery systems. Tob Control. 2014;23(Suppl 3):iii54–iii57. doi: 10.1136/tobaccocontrol-2013-051460. http://dx.doi.org/10.1136/tobaccocontrol-2013-051460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 796.Meernik C, Baker HM, Paci K, Fischer-Brown I, Dunlap D, Goldstein AO. Electronic Cigarettes on Hospital Campuses. Int J Environ Res Public Health. 2016;13(1):87. doi: 10.3390/ijerph13010087. http://dx.doi.org/10.3390/ijerph13010087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 797.Klein EG, Kennedy RD, Berman M. Tobacco Control Policies in Outdoor Areas of High Volume American Transit Systems. J Community Health. 2014;39(4):660–667. doi: 10.1007/s10900-014-9873-3. http://dx.doi.org/10.1007/s10900-014-9873-3. [DOI] [PubMed] [Google Scholar]
- 798.Huang J, Zheng R, Emery S. Assessing the impact of the national smoking ban in indoor public places in china: evidence from quit smoking related online searches. PLoS One. 2013;8(6):e65577. doi: 10.1371/journal.pone.0065577. http://dx.doi.org/10.1371/journal.pone.0065577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 799.Jo CL, Ayers JW, Althouse BM, Emery S, Huang J, Ribisl KM. U.S. consumer interest in non-cigarette tobacco products spikes around the 2009 federal tobacco tax increase. Tob Control. 2015;24(4):395–399. doi: 10.1136/tobaccocontrol-2013-051261. http://dx.doi.org/10.1136/tobaccocontrol-2013-051261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 800.Goniewicz ML, Kosmider L, Delijewski M, Knysak J, Ochota P, Sobczak A. The impact of the 2010 Polish smoke-free legislation on the popularity and sales of electronic cigarettes. Eur J Public Health. 2014;24(3):471–473. doi: 10.1093/eurpub/ckt214. http://dx.doi.org/10.1093/eurpub/ckt214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 801.Lidon-Moyano C, Martin-Sanchez JC, Saliba P, Graffelman J, Martinez-Sanchez JM. Correlation between tobacco control policies, consumption of rolled tobacco and e-cigarettes, and intention to quit conventional tobacco, in Europe. Tob Control. doi: 10.1136/tobaccocontrol-2015-052482. In press. Online February 17, 2016. http://dx.doi.org/10.1136/tobaccocontrol-2015-052482. [DOI] [PubMed] [Google Scholar]
- 802.Wackowski OA, Delnevo CD, Pearson JL. Switching to E-Cigarettes in the Event of a Menthol Cigarette Ban. Nicotine Tob Res. 2015;17(10):1286–1287. doi: 10.1093/ntr/ntv021. http://dx.doi.org/10.1093/ntr/ntv021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 803.Friedman AS. How does electronic cigarette access affect adolescent smoking? J Health Econ. 2015;44:300–308. doi: 10.1016/j.jhealeco.2015.10.003. http://dx.doi.org/10.1016/j.jhealeco.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 804.Pesko MF, Hughes JM, Faisal FS. The influence of electronic cigarette age purchasing restrictions on adolescent tobacco and marijuana use. Prev Med. 2016;87:207–212. doi: 10.1016/j.ypmed.2016.02.001. http://dx.doi.org/10.1016/j.ypmed.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 805.Nonnemaker J, Kim AE, Lee YO, MacMonegle A. Quantifying how smokers value attributes of electronic cigarettes. Tob Control. 2016;25(e1):e37–e43. doi: 10.1136/tobaccocontrol-2015-052511. http://dx.doi.org/10.1136/tobaccocontrol-2015-052511. [DOI] [PubMed] [Google Scholar]
- 806.Tuchman AE. Advertising and Demand for Addictive Goods: The Effects of E-Cigarette Advertising. Stanford University; 2016. [Google Scholar]
- 807.Monitoring the Future - The University of Michigan. Table 28: Lifetime use of cigarettes by use of e-cigarettes in last 30 days: Grade 12, 2014. Ann Arbor, MI: 2015. [Google Scholar]
- 808.Monitoring the Future - The University of Michigan. Table 29: Use of cigarettes by e-cigarettes in last 30 days: Grade 12, 2014. Ann Arbor, MI: 2015. [Google Scholar]
- 809.Monitoring the Future - The University of Michigan. Table 1: Trends in prevalence of use of cigarettes in grades 8, 10, and 12. Ann Arbor, MI: 2014. [Google Scholar]
- 810.Action on Smoking and Health. Use of electronic cigarettes (vapourisers) among adults in Great Britain. 2015 May [Google Scholar]
- 811.Johnston LD, O'Malley PM, Miech RA, Bachman JG, Schulenberg JE. Monitoring the Future national survey results on drug use: 1975–2014: Overview, key findings on adolescent drug use. Ann Arbor, MI: 2015. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.