Abstract
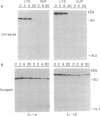
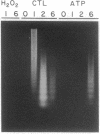
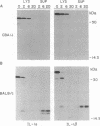
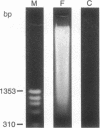
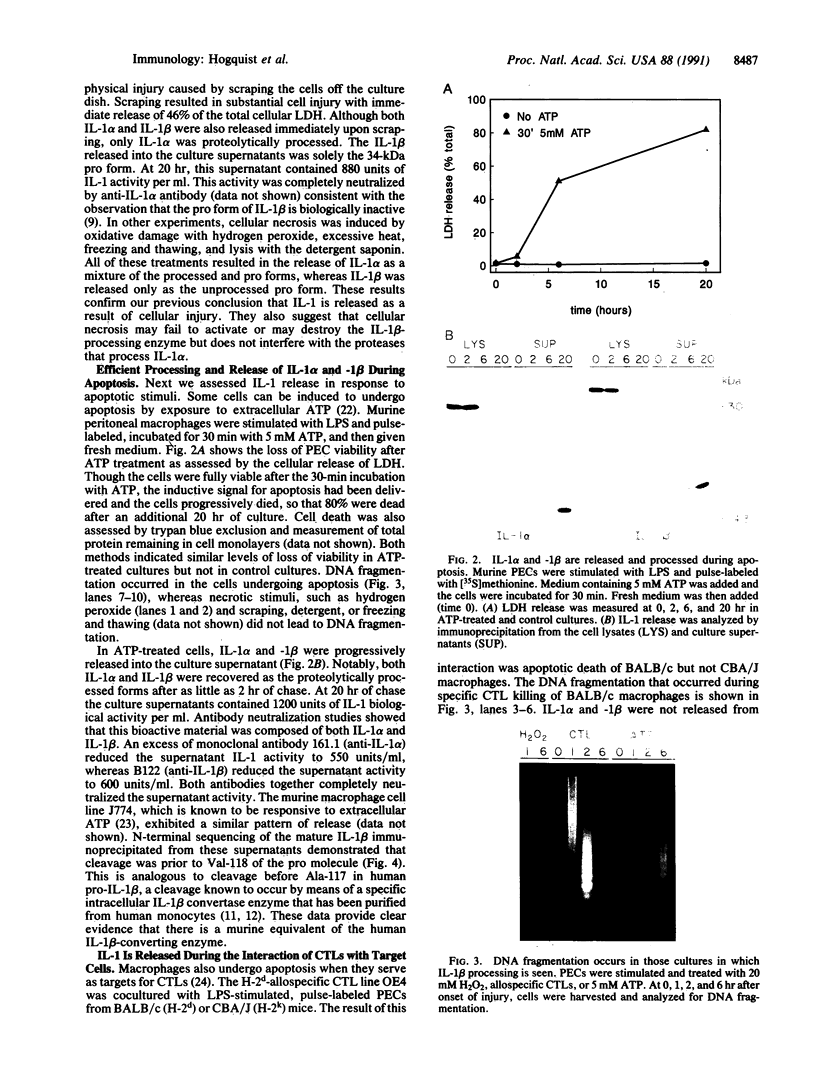
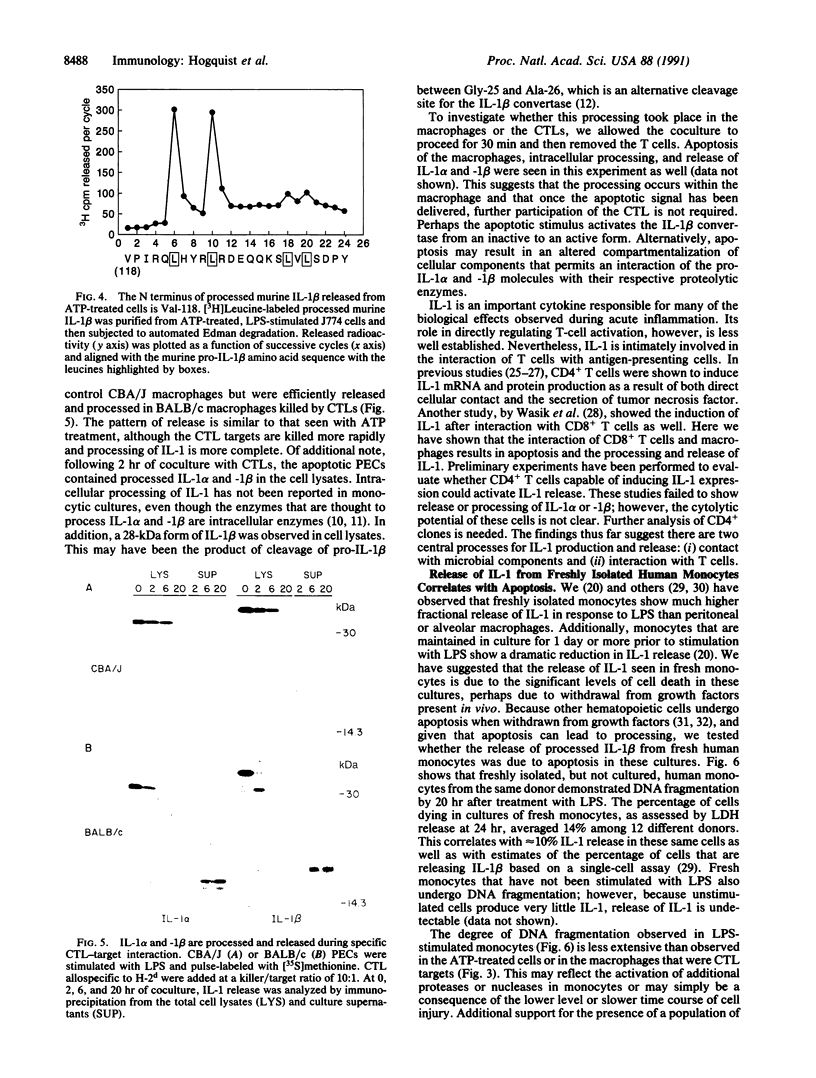
Interleukin (IL-) 1 alpha and 1 beta are synthesized as 31- to 34-kDa pro molecules. They are released from monocytes and macrophages as proteolytically processed 17-kDa mature molecules that bind with high affinity to specific receptors on target cells. IL-1 is not released via the classic secretory pathway. The pro molecules are synthesized as cytosolic proteins without signal peptides. Although the proteases that convert the pro molecules to the mature forms are cytosolic enzymes, processed IL-1 is not detected associated with the cell but is found only in culture supernatants. We demonstrate here that release of IL-1 is efficiently induced by cell injury. When the injury causes cellular necrosis, IL-1 alpha is released as a mixture of unprocessed and processed molecules but IL-1 beta is released exclusively as the biologically inactive pro form. In contrast, when cells undergo apoptosis, maturation of both IL-1 alpha and IL-1 beta is efficient. When apoptosis is rapid, as in macrophages that are targets for allospecific cytotoxic T lymphocytes, processing is observed to occur intracellularly. These findings suggest that cell injury is an important physiologic stimulus for release of IL-1. The nature of the injury profoundly affects the forms of IL-1 that are released.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beuscher H. U., Günther C., Röllinghoff M. IL-1 beta is secreted by activated murine macrophages as biologically inactive precursor. J Immunol. 1990 Mar 15;144(6):2179–2183. [PubMed] [Google Scholar]
- Black R. A., Kronheim S. R., Sleath P. R. Activation of interleukin-1 beta by a co-induced protease. FEBS Lett. 1989 Apr 24;247(2):386–390. doi: 10.1016/0014-5793(89)81376-6. [DOI] [PubMed] [Google Scholar]
- Bugler B., Amalric F., Prats H. Alternative initiation of translation determines cytoplasmic or nuclear localization of basic fibroblast growth factor. Mol Cell Biol. 1991 Jan;11(1):573–577. doi: 10.1128/mcb.11.1.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhlbrigge R. C., Hogquist K. A., Unanue E. R., Chaplin D. D. Molecular biology and genetics of interleukin-1. Year Immunol. 1989;5:21–37. [PubMed] [Google Scholar]
- Fuhlbrigge R. C., Sheehan K. C., Schreiber R. D., Chaplin D. D., Unanue E. R. Monoclonal antibodies to murine IL-1 alpha. Production, characterization, and inhibition of membrane-associated IL-1 activity. J Immunol. 1988 Oct 15;141(8):2643–2650. [PubMed] [Google Scholar]
- Gery I., Davies P., Derr J., Krett N., Barranger J. A. Relationship between production and release of lymphocyte-activating factor (interleukin 1) by murine macrophages. 1. Effects of various agents. Cell Immunol. 1981 Nov 1;64(2):293–303. doi: 10.1016/0008-8749(81)90481-0. [DOI] [PubMed] [Google Scholar]
- Hazuda D. J., Strickler J., Kueppers F., Simon P. L., Young P. R. Processing of precursor interleukin 1 beta and inflammatory disease. J Biol Chem. 1990 Apr 15;265(11):6318–6322. [PubMed] [Google Scholar]
- Hazuda D. J., Strickler J., Simon P., Young P. R. Structure-function mapping of interleukin 1 precursors. Cleavage leads to a conformational change in the mature protein. J Biol Chem. 1991 Apr 15;266(11):7081–7086. [PubMed] [Google Scholar]
- Hogquist K. A., Nett M. A., Sheehan K. C., Pendleton K. D., Schreiber R. D., Chaplin D. D. Generation of monoclonal antibodies to murine IL-1 beta and demonstration of IL-1 in vivo. J Immunol. 1991 Mar 1;146(5):1534–1540. [PubMed] [Google Scholar]
- Kobayashi Y., Yamamoto K., Saido T., Kawasaki H., Oppenheim J. J., Matsushima K. Identification of calcium-activated neutral protease as a processing enzyme of human interleukin 1 alpha. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5548–5552. doi: 10.1073/pnas.87.14.5548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostura M. J., Tocci M. J., Limjuco G., Chin J., Cameron P., Hillman A. G., Chartrain N. A., Schmidt J. A. Identification of a monocyte specific pre-interleukin 1 beta convertase activity. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5227–5231. doi: 10.1073/pnas.86.14.5227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurt-Jones E. A., Beller D. I., Mizel S. B., Unanue E. R. Identification of a membrane-associated interleukin 1 in macrophages. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1204–1208. doi: 10.1073/pnas.82.4.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Landis R. C., Friedman M. L., Fisher R. I., Ellis T. M. Induction of human monocyte IL-1 mRNA and secretion during anti-CD3 mitogenesis requires two distinct T cell-derived signals. J Immunol. 1991 Jan 1;146(1):128–135. [PubMed] [Google Scholar]
- Lewis C. E., McCarthy S. P., Lorenzen J., McGee J. O. Heterogeneity among human mononuclear phagocytes in their secretion of lysozyme, interleukin 1 and type-beta transforming growth factor: a quantitative analysis at the single-cell level. Eur J Immunol. 1989 Nov;19(11):2037–2043. doi: 10.1002/eji.1830191111. [DOI] [PubMed] [Google Scholar]
- Lomedico P. T., Kilian P. L., Gubler U., Stern A. S., Chizzonite R. Molecular biology of interleukin-1. Cold Spring Harb Symp Quant Biol. 1986;51(Pt 1):631–639. doi: 10.1101/sqb.1986.051.01.075. [DOI] [PubMed] [Google Scholar]
- MacDonald H. R., Sekaly R. P., Kanagawa O., Thiernesse N., Taswell C., Cerottini J. C., Weiss A., Glasebrook A. L., Engers H. D., Kelso A. Cytolytic T lymphocyte clones. Immunobiology. 1982 Mar;161(1-2):84–106. doi: 10.1016/S0171-2985(82)80020-X. [DOI] [PubMed] [Google Scholar]
- Maier J. A., Voulalas P., Roeder D., Maciag T. Extension of the life-span of human endothelial cells by an interleukin-1 alpha antisense oligomer. Science. 1990 Sep 28;249(4976):1570–1574. doi: 10.1126/science.2218499. [DOI] [PubMed] [Google Scholar]
- Matsushima K., Taguchi M., Kovacs E. J., Young H. A., Oppenheim J. J. Intracellular localization of human monocyte associated interleukin 1 (IL 1) activity and release of biologically active IL 1 from monocytes by trypsin and plasmin. J Immunol. 1986 Apr 15;136(8):2883–2891. [PubMed] [Google Scholar]
- Mosley B., Urdal D. L., Prickett K. S., Larsen A., Cosman D., Conlon P. J., Gillis S., Dower S. K. The interleukin-1 receptor binds the human interleukin-1 alpha precursor but not the interleukin-1 beta precursor. J Biol Chem. 1987 Mar 5;262(7):2941–2944. [PubMed] [Google Scholar]
- Rodriguez-Tarduchy G., Collins M., López-Rivas A. Regulation of apoptosis in interleukin-3-dependent hemopoietic cells by interleukin-3 and calcium ionophores. EMBO J. 1990 Sep;9(9):2997–3002. doi: 10.1002/j.1460-2075.1990.tb07492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubartelli A., Cozzolino F., Talio M., Sitia R. A novel secretory pathway for interleukin-1 beta, a protein lacking a signal sequence. EMBO J. 1990 May;9(5):1503–1510. doi: 10.1002/j.1460-2075.1990.tb08268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J. H. Internal disintegration model of cytotoxic lymphocyte-induced target damage. Immunol Rev. 1983;72:97–118. doi: 10.1111/j.1600-065x.1983.tb01074.x. [DOI] [PubMed] [Google Scholar]
- Searle J., Kerr J. F., Bishop C. J. Necrosis and apoptosis: distinct modes of cell death with fundamentally different significance. Pathol Annu. 1982;17(Pt 2):229–259. [PubMed] [Google Scholar]
- Steinberg T. H., Newman A. S., Swanson J. A., Silverstein S. C. ATP4- permeabilizes the plasma membrane of mouse macrophages to fluorescent dyes. J Biol Chem. 1987 Jun 25;262(18):8884–8888. [PubMed] [Google Scholar]
- Suttles J., Giri J. G., Mizel S. B. IL-1 secretion by macrophages. Enhancement of IL-1 secretion and processing by calcium ionophores. J Immunol. 1990 Jan 1;144(1):175–182. [PubMed] [Google Scholar]
- Wasik M. A., Donnelly R. P., Beller D. I. Lymphokine-independent induction of macrophage membrane IL-1 by autoreactive T cells recognizing either class I or class II MHC determinants. J Immunol. 1988 Nov 15;141(10):3456–3462. [PubMed] [Google Scholar]
- Weaver C. T., Duncan L. M., Unanue E. R. T cell induction of macrophage IL-1 during antigen presentation. Characterization of a lymphokine mediator and comparison of TH1 and TH2 subsets. J Immunol. 1989 May 15;142(10):3469–3476. [PubMed] [Google Scholar]
- Weaver C. T., Unanue E. R. T cell induction of membrane IL 1 on macrophages. J Immunol. 1986 Dec 15;137(12):3868–3873. [PubMed] [Google Scholar]
- Wewers M. D., Herzyk D. J. Alveolar macrophages differ from blood monocytes in human IL-1 beta release. Quantitation by enzyme-linked immunoassay. J Immunol. 1989 Sep 1;143(5):1635–1641. [PubMed] [Google Scholar]
- Williams G. T., Smith C. A., Spooncer E., Dexter T. M., Taylor D. R. Haemopoietic colony stimulating factors promote cell survival by suppressing apoptosis. Nature. 1990 Jan 4;343(6253):76–79. doi: 10.1038/343076a0. [DOI] [PubMed] [Google Scholar]
- Young P. R., Hazuda D. J., Simon P. L. Human interleukin 1 beta is not secreted from hamster fibroblasts when expressed constitutively from a transfected cDNA. J Cell Biol. 1988 Aug;107(2):447–456. doi: 10.1083/jcb.107.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanovello P., Bronte V., Rosato A., Pizzo P., Di Virgilio F. Responses of mouse lymphocytes to extracellular ATP. II. Extracellular ATP causes cell type-dependent lysis and DNA fragmentation. J Immunol. 1990 Sep 1;145(5):1545–1550. [PubMed] [Google Scholar]
- Zhu X., Komiya H., Chirino A., Faham S., Fox G. M., Arakawa T., Hsu B. T., Rees D. C. Three-dimensional structures of acidic and basic fibroblast growth factors. Science. 1991 Jan 4;251(4989):90–93. doi: 10.1126/science.1702556. [DOI] [PubMed] [Google Scholar]