Abstract
A-kinase anchoring proteins (AKAPs) are thought to be passive members of protein complexes that coordinate the association of cAMP-dependent protein kinase A (PKA) with cellular substrates to facilitate targeted PKA protein phosphorylation. IKs, the slow heart postassium current, is carried by the IKs potassium channel, a substrate for PKA phosphorylation in response to sympathetic nerve stimulation, is a macromolecular complex that includes the KCNQ1 α subunit, the KCNE1 regulatory subunit, and the AKAP Yotiao. Disruption of this regulation by mutation in the long QT syndrome is associated with elevated risk of sudden death. Here, we have studied the effects of the AKAP Yotiao on the function of the IKs channel that had been mutated to simulate channel phosphorylation, and we report direct AKAP-mediated alteration of channel function distinct from its role in the coordination of channel phosphorylation by PKA. These data reveal previously undescribed actions of Yotiao that occur subsequent to channel phosphorylation and provide evidence that this adaptor protein also may serve as an effector in regulating this important ion channel.
Keywords: adaptor, KCNQ1, KCNE1
The ubiquitous second messenger cAMP transduces extracellular receptor-activated signals into a plethora of intracellular events, many of which are mediated through protein kinase A (PKA)-dependent protein phosphorylation. Intracellular specificity and cAMP-dependent diversity now are recognized to be mediated largely by A-kinase anchoring proteins (AKAPs), which, together with specific substrates, create macromolecular signaling complexes to control the PKA phosphorylation state of targeted proteins (1-4). Although they are essential in creating targeted signaling environments, it is not so clear that the only role of AKAPs is to act as scaffolds in molecular complexes. Is it possible that, like other regulatory protein subunits, AKAPs also may participate in direct control of protein function?
Ion channels, exchangers, and G protein-coupled receptors form a subset of proteins that are PKA-phosphorylated and for which a number of AKAPs have been reported to contribute to targeting of PKA phosphorylation (5-15). IKs is a slow heart potassium current carried by the IKs potassium channel, critically important in the regulation of the cardiac action potential, particularly in the face of sympathetic nervous system stimulation (16), which belongs to this subset of proteins. The IKs PKA macromolecular complex consists of an α subunit (KCNQ1), a regulatory subunit (KCNE1), and the AKAP Yotiao, which binds to a leucine zipper (LZ) motif in the KCNQ1 C terminus and in turn binds PKA and protein phosphatase 1 (17). Disruption of the complex by mutation renders the channel functionally insensitive to cAMP-dependent regulation (17) and elevates the risk of exercise-induced sudden death in mutation carriers (18, 19). PKA phosphorylation of Ser-27 in the KCNQ1 N terminus accounts for most of the functional modulation of IKs by the sympathetic nervous system, and mutation of KCNQ1 residue Ser-27 to Asp (S27D) reconstitutes most of these effects of PKA on expressed channel function (20, 21). Here, we took advantage of the relative simplicity of the IKs complex and mutation of Ser-27 in KCNQ1 to test the hypothesis that Yotiao may play a role in the regulation of channel function in addition to coordinating the assembly of the IKs signaling complex.
Methods
Molecular Biology. The N terminus of KCNQ1 was amplified and subcloned into the GST vector pGEX (Amersham Pharmacia) at BamHI and XhoI sites. A restriction site encoding for XmaI was introduced at base 59 while maintaining the correct amino acid sequence. Mutations at residue Ser-27 were performed by PCR using the forward primer GGCCCGGGGCCCGGCGGGGCXXXGCGGGCCTGGCCAAGAAGTGCCCCTTCTCGC (where XXX is the introduced codon sequence taking the place of the serine at position 27) and a reverse primer encoding for the naturally encoding XhoI site at nucleotide 340. The efficiency of the PCR was enhanced by the inclusion of 10% DMSO. The resultant PCR product was subcloned back into the pGEX vector. The reformed N terminus was ligated back into the full-length KCNQ1 channel in pcDNA 3.1 (Invitrogen). All cDNA clones for transfection were subcloned into the mammalian expression vector pcDNA 3.1. All sequence manipulations were confirmed by using the chain termination method in the DNA Sequencing Facility at Columbia University.
Cell Culture and Transfection. Chinese hamster ovary (CHO) cells (American Type Culture Collection) were cultured in Ham's F12 medium. Cells were transiently transfected with cDNAs for KCNQ1, KCNE1, CD8, and Yotiao (0.4, 0.4, 0.4, and 2 μg, respectively) by using Lipofectamine with Lipofectamine-Plus reagents (Invitrogen) as we reported in ref. 20. Transfected cells were plated on small Petri dishes and cultured in an incubator in the presence of 5% CO2. Dynabeads M-450 anti-CD8 beads (1 μg/ml, Dynal, Oslo) were used to visually identify transfected cells. Electrophysiological measurements were carried out 48 h after transfection.
Electrophysiology. Currents were recorded by using the whole-cell patch-clamp technique (17, 22, 23). Cells plated in culture dishes were placed on the stage of an inverted microscope (IMT-2, Olympus), and the CHO medium was replaced by Tyrode's solution (132 mM NaCl/4.8 mM KCl/1.2 mM MgCl2/2 mM CaCl2/5 mM glucose/10 mM Hepes, pH 7.4) including Dynabeads M-450 before measurement of currents. All measurements were performed at room temperature. In whole-cell patch-clamp experiments, the tip resistance of the microelectrodes (borosilicate glass capillaries) was 2-4 MΩ when filled with the internal solution composed of 110 mM K-aspartate, 5 mM ATP-K2, 11 mM EGTA, 10 mM Hepes, 1 mM CaCl2, and 1 mM MgCl2 (pH 7.3). Series resistance was 2-8 MΩ.
Currents were recorded by using the whole-cell patch-clamp technique with Axopatch 200-A amplifiers (Axon Instruments, Union City, CA), sampled online, and stored on a computer hard disk. Recording of currents was started 1 min after rupture of the membrane. After rupture, internal solutions without cAMP (control) or with 0.2 mM cAMP plus 0.2 μM okadaic acid (OA) (Calbiochem) were dialyzed for 12 min after first measurements were made. The protein kinase inhibitor peptide (PKI) (Sigma) was dissolved in the pipette solution (20 μM). The PKC inhibitor chelerythrine (20 μM; Sigma) was added to the external solution 30 min before recordings and maintained during the recording period. This chelerythrine concentration and application procedure previously has been shown to inhibit PKC-dependent modulation of Na+ channels (24).
KCNQ1/KCNE1 channel tail-current amplitude was monitored by analysis of peak deactivating tail current recorded at -40 mV after 2-s activating pulses to 60 mV. The holding potential was -65 mV, and pulse frequency was 0.067 Hz. We show and analyze current traces for each combination of subunits and constructs 12 min after recording was started.
Deactivation time constants (τ) were obtained by fits to data recorded 12 min after recording was started. Data were fit with functions containing single exponential time components by using origin (Version 7.0, Microcal, Northhampton, MA) or pclamp (Version 8.02, Axon Instruments, Burlingame, CA) software. pclamp was used both to generate voltage-clamp protocols and acquire data. Statistical significance was assessed with Student's t test for simple comparisons and with ANOVA and Bonferroni's test for multiple comparisons; differences of P < 0.05 were considered to be significant. Data for statistical analysis between with and without Yotiao or drugs or between with and without LZm were from paired experiments from CHO cells prepared on the same day.
Results
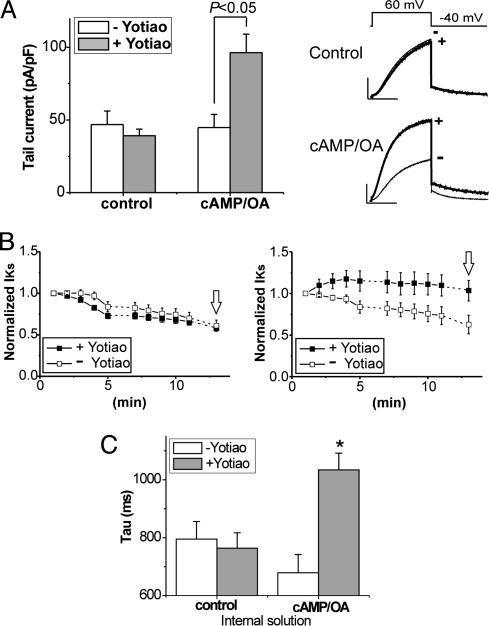
Fig. 1, which summarizes the influence of Yotiao on the response of IKs channels to cAMP, shows that coexpression of Yotiao does not affect IKs channel activity under control recording conditions but significantly affects channel function when cells are dialyzed with cAMP and the nonspecific phosphatase inhibitor OA. Fig. 1 A illustrates mean current traces (Right) and mean ± SEM current summarized as bars (Left). Fig. 1B, in which currents were measured at various times after initializing recording, normalized to initial current, and plotted vs. dialysis time, illustrates the influence of Yotiao on the change in current amplitude (rundown) that occurs during cell dialysis. The data indicate that coexpression of Yotiao affects neither the time course of channel rundown nor IKs magnitude during dialysis when recording with a cAMP-free pipette (Fig. 1B Left) but slows the time course of channel rundown and consequently increases current amplitude (Fig. 1 A) when cells are dialyzed with cAMP/OA (Fig. 1B Right). Cell dialysis with cAMP/OA also slows IKs channel deactivation (current tail) if and only if Yotiao is present (Fig. 1C). These effects are consistent with a regulatory response (channel phosphorylation) that develops as cAMP/OA diffuses into the cell in the presence, but not in the absence, of Yotiao.
Fig. 1.
Yotiao is required for functional response of IKs channels to cAMP. (A) Yotiao coexpression increases KCNQ1/KCNE1 channel currents with (cAMP/OA) but not without (control) cAMP (0.2 mM) and OA (0.2 μM) dialysis. Shown are records and analysis obtained 12 min after membrane rupture. (Left) The bar graphs plot mean tail-current amplitude minus (open bars) or plus (filled bars) Yotiao coexpression (control: -Yotiao, n = 10, and +Yotiao, n = 20; cAMP/OA: -Yotiao, n = 8, and +Yotiao, n = 12). (Right) Mean current traces elicited by test pulses (+60 mV, -40 mV return) are superimposed without (-) or with (+) Yotiao coexpression for control (Upper) and with cAMP/OA (Lower). (Scale: 100 pA/pF, 1 s.) (B) Mean ± SEM tail-current amplitude (normalized to amplitude recorded 1 min after membrane rupture) is plotted vs. time after membrane rupture. Cells cotransfected with KCNQ1/KCNE1 (open squares) and KCNQ1/KCNE1/Yotiao (filled squares) were dialyzed either by internal solution without cAMP/OA (control, Left) or with cAMP/OA (Right). Data in A were obtained at times indicated by open arrows. Number of experiments for each condition are as in A. (C) Time constants of IKS deactivation are slowed by Yotiao expression (+Yotiao) in the presence (cAMP/OA) but not in the absence (control) of cAMP. *, P < 0.05, Student's t test vs. -Yotiao. Data for statistical analysis were from experiments with CHO cells prepared on the same day. In all figures, channel activity was measured by whole-cell patch-clamp procedures in CHO cells transiently transfected (see Methods) with channel constructs as indicated in legends.
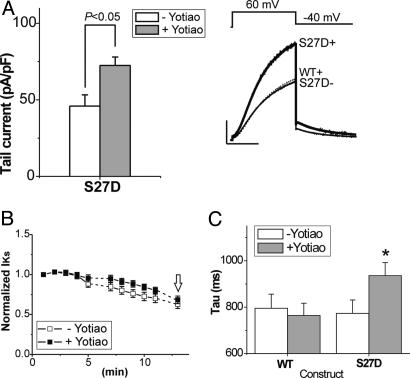
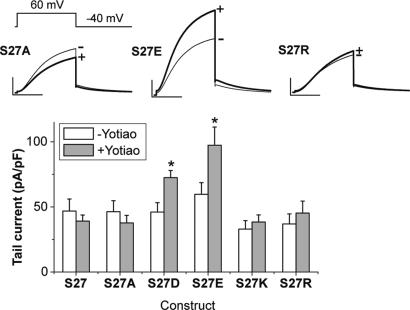
If recruitment of PKA and protein phosphatase 1 to the microenvironment of the channel to phosphorylate/dephosphorylate the KCNQ1 subunit is not the only role of Yotiao, other roles of the adaptor protein might be revealed under conditions in which channel phosphorylation is simulated by mutation. Fig. 2 summarizes the first set of experiments to test for this possibility. The figure shows the effects of Yotiao on currents recorded in cells expressing KCNE1 and the S27D mutant KCNQ1 (S27D), which simulates PKA phosphorylation of KCNQ1 Ser-27. Repeating experimental protocols illustrated in Fig. 1 reveals a significant increase in current (Fig. 2 A) and a slowing of channel deactivation (Fig. 2C) in cells expressing Yotiao compared with Yotiao-free cells, despite the fact that the pipette recording solution omitted cAMP/OA. Additionally, there is no effect of Yotiao on the plot of normalized current vs. dialysis time or the change in current during dialysis (Fig. 2B). These data are consistent with a Yotiao-dependent effect on current amplitude and kinetics that does not develop with dialysis time but, instead, is apparent immediately upon cell rupture at the beginning of whole-cell recording. These effects of Yotiao do not occur if Ser-27 is not mutated to Asp (see dashed trace in Fig. 2 A and bar graphs in Fig. 1 A and C), further suggesting an effect of Yotiao on IKs channel function that may occur subsequent to, and in addition to, phosphorylation-altered charge at KCNQ1 residue 27. In support of this interpretation are the data summarized in Fig. 3, which show that coexpression of Yotiao with mutated KCNQ1 constructs significantly increases current density only if Ser-27 is replaced by another negatively charged amino acid (Glu).
Fig. 2.
Phosphorylation-independent regulation of IKs channels by Yotiao. The S27D KCNQ1 mutation was used to mimic PKA phosphorylation of IKs channels in the absence of cAMP. (A) S27D KCNQ1/KCNE1 channel currents are enhanced by Yotiao coexpression. Shown are a bar graph summary of mean tail-current amplitude (Left) and mean currents (Right) recorded in cells cotransfected with S27D KCNQ1/KCNE1 (S27D-, open bar, n = 22) and S27D KCNQ1/KCNE1/Yotiao (S27D+, filled bar, n = 20). The cAMP/OA-free control solution was used for dialysis. Mean WT KCNQ1/KCNE1/Yotiao current in control (dotted line, n = 20) is superimposed with the S27D IKs traces for comparison. (Scale bars: 100 pA/pF, 1 s.) (B) Yotiao coexpression does not influence rundown of IKs channels in which α subunits harbor the S27D mutation. Data were collected and analyzed as in Fig. 1. The open arrow, which indicates time of measurement of currents in A, reveals no effect of Yotiao expression on current rundown as indicated by normalized current, but a significant effect on current amplitude in A. (C) Yotiao coexpression slows deactivation kinetics of IKs channels with S27D-mutated α subunits but not WT α subunits. Mean ± SEM deactivation time constants (see Methods) are plotted for each construct +Yotiao expression (filled bars) and -Yotiao expression (open bars). The numbers of experiments were as follows: WT, -Yotiao, n = 10, and +Yotiao, n = 20; S27D -Yotiao, n = 22, and +Yotiao, n = 20. *, P < 0.05 for +Yotiao vs. -Yotiao, Student's t test.
Fig. 3.
Yotiao-mediated changes in KCNQ1/KCNE1 function require a negative charge at KCNQ1 residue 27. Ser-27 in the KCNQ1 N terminus was mutated to Ala (S27A), Asp (S27D), Glu (S27E), Lys (S27K), and Arg (S27R) and coexpressed with KCNE1 ± Yotiao. Currents were measured as in other figures and illustrated as average traces for select mutations in Upper. Cells were transfected with KCNE1, the indicated KCNQ1 construct, with (+) or without (-) Yotiao. The numbers of experiments for each construct were as follows: WT, -, n = 10, and +, n = 20; S27A, -, n = 8, and +, n = 6; S27D, -, n = 22, and +, n = 20; S27E, - and +, n = 9; S27K, - and +, n = 7; S27R, - and +, n = 7. The bar graphs compare tail currents recorded at -40 mV after 2-s pulses to +60 mV for each combination (open bars, -Yotiao; filled bars, +Yotiao). *, P < 0.05 vs. -Yotiao.
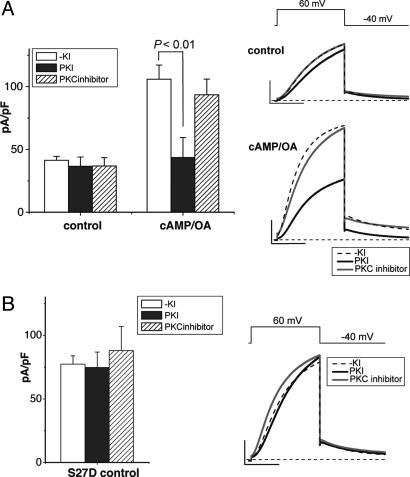
Despite the fact that we have not stimulated either PKA or PKC pathways in the experiments described above, we wanted to rule out the possibility that the effects of Yotiao on the IKs channel were not due to Yotiao-dependent phosphorylation at another site(s) that is required to interact with phosphorylated Ser-27 to alter channel function. In this case, basal phosphorylation of a putative site would be consistent with our data. To test for this possibility, we carried out experiments in which we inhibited PKA pathways by dialyzing cells with a peptide PKA inhibitor (PKI) and inhibited PKC pathways by external application of the PKC inhibitor chelerythrine and tested for changes in currents for cells expressing the S27D construct plus KCNE1 Yotiao (Fig. 4). Control experiments carried out for cells expressing wild-type (WT) KCNQ1/KCNE1 plus Yotiao revealed no effect of either inhibitor on basal currents but selective inhibition of the cAMP-dependent increase in IKs current by PKI. These data indicate that dialysis with PKI can be used to inhibit PKA pathways in these experiments (Fig. 4A). We then tested these reagents on cells expressing S27D KCNQ1/KCNE1 plus Yotiao, and, as indicated in Fig. 4B, there was no effect on these currents by either PKI or chelerythrine, supporting the view that Yotiao-mediated effects of S27D do not require interactions with other PKA- or PKC-phosphorylated residues.
Fig. 4.
Basal PKA or PKC phosphorylation of additional residues is not required for Yotiao-dependent modulation of S27D channels. Effects of a peptide PKA inhibitor (PKI) and a PKC inhibitor (chelerythrine) were tested as indicated in Methods. (Left) Currents and summary data, obtained as in Fig. 1, are shown ± kinase inhibitors (without kinase inhibitors: -KI, open column; +PKI, filled column; +PKC inhibitor, hatched column). (Right) Representative averaged current traces (-KI, dashed line; +PKI, black line; +PKC inhibitor, gray line). (Scale bars: 100 pA/PF, 1 s.) (A) Experiments carried out in CHO cells transfected with WT KCNQ1/KCNE1/Yotiao. P < 0.01, ANOVA and Bonferroni's test. The numbers of experiments were as follows: without cAMP/OA (control), -KI, n = 20; PKI and PKC inhibitor, n = 6; and with cAMP/OA, -KI, n = 16; PKI and PKC inhibitor, n = 6. (B) Experiments in CHO cells expressing S27D KCNQ1/KCNE1/Yotiao. The number of experiments were as follows: -KI, n = 23; PKI, n = 6; PKC inhibitor, n = 7. There is no significant difference among the three groups of data.
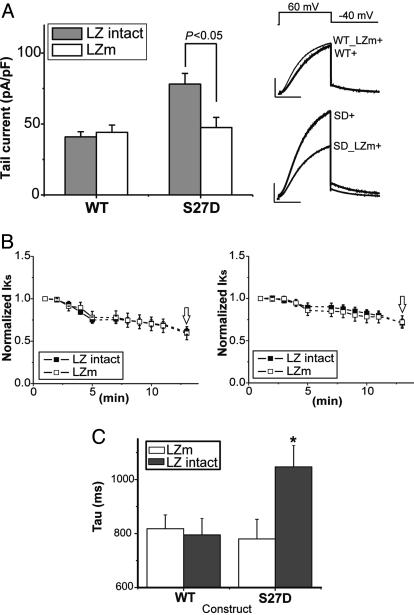
Is assembly of Yotiao and KCNQ1 necessary to modulate channel gating? To address this question, we studied channels in which we used a double-alanine mutation of the LZ motif in KCNQ1 (LZm; Leu-602 → Ala, Ile-609 → Ala), which previously has been shown to disrupt the Yotiao/KCNQ1 protein-protein interaction and ablate the response of expressed channels to PKA phosphorylation (17). We also engineered this mutation into the S27D KCNQ1 construct to allow comparison of the effects of mutating the LZ motif on channels with an aspartate at position 27 (S27D_LZm) vs. the naturally occurring (WT) serine at position 27 (S27_LZm). Several points are apparent in Fig. 5, which summarizes data from these experiments. First, mutation of the C-terminal LZ significantly reduces currents when Ser-27 is replaced by Asp (Fig. 5A, S27D) but does not affect the amplitude of currents when Ser-27 is not mutated (Fig. 5A, WT). Second, there is no significant difference between the amplitude of current measured in cells expressing WT KCNQ1 channels (with or without the C-terminal LZ intact) and S27D KCNQ1 channels in which the C-terminal domain LZ is disrupted. Third, there is no effect of Yotiao on WT channel kinetics, but deactivation time course of S27D channels is significantly slower than WT channels if and only if Yotiao is coexpressed and the KCNQ1 C-terminal domain LZ is intact (Fig. 5C). Finally, disruption of the LZ motif does not affect the time course of channel rundown of WT or S27D KCNQ1 channels (Fig. 5B). These data suggest that Yotiao modulates the gating of KCNQ1/KCNE1 channels in a manner that depends not only on the presence of a negative charge at residue 27 (S27D) but also depends on the binding of Yotiao to the KCNQ1 by means of an intact C-terminal LZ motif.
Fig. 5.
Yotiao-KCNQ1 intermolecular interactions are necessary for phosphorylation-independent IKs regulation. The KCNQ1 C-terminal LZ was mutated (see text) with Ser-27 intact (WT_LZm) and with Ser-27 mutated to Asp (SD_LZm) to test the importance of Yotiao/KCNQ1 interactions in channel regulation. (A) Mean ± SEM bar graphs of tail-current amplitude (Left) and mean current traces (Right) are shown for experiments in which the effects of mutation of the LZ in modulating channels with α subunits with Ser-27 intact (WT) or with the S27D mutation (S27D). Filled bars indicate expression of α subunits with the LZ motif intact, and open bars represent results with the LZm (_LZm) described in the text. The numbers of experiments were as follows: WT, n = 22; WT_LZm, n = 9; SD+, n = 12; S27D_LZm, n = 20. (Scale bars: 100 pA/pF, 1 s). (B) LZm-mediated Yotiao/KCNQ1 disruption does not affect channel rundown in WT (S27, Left) and S27D (Right). Data were collected and analyzed as in Fig. 1; open arrows indicate points where data were recorded in A. (C) Disruption of Yotiao/KCNQ1 interaction by LZm slows deactivation kinetics of IKs channels with S27D-mutated α subunits but not WT α subunits. Mean ± SEM deactivation time constants (see Methods) are plotted for each construct for α subunits with intact (filled) and mutated (open) C-terminal LZ motifs. The numbers of experiments were as follows: WT, n = 22; WT_LZm, n = 9; S27D, n = 12; S27D_LZm, n = 20. *, P < 0.05, S27D vs. S27D_LZm, Student's t test. In all experiments, KCNE1 and Yotiao (indicated by +) were coexpressed with the KCNQ1 constructs noted.
Discussion
It is now well established that macromolecular signaling complexes, coordinated by the binding of adaptor proteins to target proteins, are essential in creating microsignaling environments of many proteins, including ion channels (5, 8, 10, 11, 17, 25). Disruption of signaling complexes by genetic defects can lead to sudden cardiac death (18, 19), kidney disease (26), and cystic fibrosis (12), and genetic variation in AKAPs may raise the risk of susceptibility in complex diseases (27).
The principal role of this protein family is thought to be the establishment of compartmentalized signaling environments by the recruitment of PKA and phosphatases to target proteins. These microenvironments enable rapid responses to cellular signaling and regulation of distinct targeted proteins (28). In at least one previous case, it has been reported that recruitment of these enzymes is also required for autophosphorylation of the AKAP as well as its associated target protein (29). Here, however, we have shown that coordination of targeted signaling environments is not the only role of adaptor proteins. We found in the case of the KCNQ1/KCNE1/Yotiao macromolecular complex that Yotiao is also necessary to translate the phosphorylated KCNQ1 subunit into altered channel activity. Thus, Yotiao functions not only to anchor and recruit PKA but also to modulate the ion channel in a manner distinct from PKA or PKC phosphorylation. Key to these experiments was our previous finding that the S27D mutation of KCNQ1 could reconstitute most of the responses of the KCNQ1/KCNE1/Yotiao complex to cAMP (20). It is very clear from our experiments that coexpression of Yotiao with KCNQ1 and KCNE1 has no effect on basal channel activity, that is, activity recorded in the absence of exogenous cAMP/OA. Under these conditions, it also does not matter whether Yotiao is able to interact with the C-terminal domain of KCNQ1 because we find no difference in channel activity when we replace WT KCNQ1 with KCNQ1 harboring a mutation in the C-terminal domain LZ motif that uncouples KCNQ1 and Yotiao (17). These effects of Yotiao were seen only when Ser-27 was replaced by a negatively charged residue and only if the KCNQ1 LZ motif was intact. This action is not affected by application of a PKA inhibitor (PKI) or PKC inhibitor, ruling out contributions from endogenous PKA or PKC phosphorylation of other putative KCNQ1 PKA substrates (Fig. 4). Our results raise the possibility that Yotiao, when bound to the KCNQ1 C-terminal domain, mediates interactions that are markedly affected by the addition of negative charge to the KCNQ1 N terminus that accompanies PKA phosphorylation of Ser-27. This result implies dual roles of Yotiao: (i) a scaffolding protein to recruit PKA and protein phosphatase 1 to the channel to control its phosphorylation state; and (ii) a participant in translating the phosphorylation-induced change in charge at residue 27 of KCNQ1 into altered channel activity. The second role requires PKA phosphorylation of Ser-27 as a prerequisite and depends on physical interactions between Yotiao and the channel. Although we have ruled out a role of PKA and PKC in this second effect by the use of specific inhibitors, it is possible that Yotiao partners with other, as yet unidentified, protein kinases to induce the PKA-independent actions. Our results thus demonstrate a previously undescribed role of an AKAP in alteration of channel function in a manner other than mere coordination of molecules involved in phosphorylation or dephosphorylation. Our data suggest that adaptor proteins may have more importance in the regulation of channel function subsequent to channel phosphorylation than previously thought.
Acknowledgments
We thank Drs. Steven A. Siegelbaum, Lei Chen, and Colleen E. Clancy for reading and providing helpful suggestions for the manuscript. This work was supported by National Institutes of Health Grants 1R01-HL 44365 and 1P01-HL-30557 (both to R.S.K.), and J.K. was supported by the Japan Society for the Promotion of Science.
Author contributions: J.K. and R.S.K. designed research; J.K. performed research; H.K.M. and J.R. contributed new reagents/analytic tools; J.K. and R.S.K. analyzed data; and J.R. and R.S.K. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: AKAP, A-kinase anchoring protein; CHO, Chinese hamster ovary; LZ, leucine zipper; OA, okadaic acid; PKA, protein kinase A.
References
- 1.Michel, J. J. & Scott, J. D. (2002) Annu. Rev. Pharmacol. Toxicol. 42, 235-257. [DOI] [PubMed] [Google Scholar]
- 2.Bauman, A. L. & Scott, J. D. (2002) Nat. Cell Biol. 4, E203-E206. [DOI] [PubMed] [Google Scholar]
- 3.Angelo, R. & Rubin, C. S. (1998) J. Biol. Chem. 273, 14633-14643. [DOI] [PubMed] [Google Scholar]
- 4.Reinitz, C. A., Bianco, R. A. & Shabb, J. B. (1997) Arch. Biochem. Biophys. 348, 391-402. [DOI] [PubMed] [Google Scholar]
- 5.Marx, S. O., Reiken, S., Hisamatsu, Y., Jayaraman, T., Burkhoff, D., Rosemblit, N. & Marks, A. R. (2000) Cell 101, 365-376. [DOI] [PubMed] [Google Scholar]
- 6.Marx, S. O., Reiken, S., Hisamatsu, Y., Gaburjakova, M., Gaburjakova, J., Yang, Y. M., Rosemblit, N. & Marks, A. R. (2001) J. Cell Biol. 153, 699-708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Malbon, C. C., Tao, J. & Wang, H. Y. (2004) Biochem. J. 379, 1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Westphal, R. S., Tavalin, S. J., Lin, J. W., Alto, N. M., Fraser, I. D., Langeberg, L. K., Sheng, M. & Scott, J. D. (1999) Science 285, 93-96. [DOI] [PubMed] [Google Scholar]
- 9.Colledge, M., Dean, R. A., Scott, G. K., Langeberg, L. K., Huganir, R. L. & Scott, J. D. (2000) Neuron 27, 107-119. [DOI] [PubMed] [Google Scholar]
- 10.Hoshi, N., Zhang, J. S., Omaki, M., Takeuchi, T., Yokoyama, S., Wanaverbecq, N., Langeberg, L. K., Yoneda, Y., Scott, J. D., Brown, D. A. & Higashida, H. (2003) Nat. Neurosci. 6, 564-571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schulze, D. H., Muqhal, M., Lederer, W. J. & Ruknudin, A. M. (2003) J. Biol. Chem. 278, 28849-28855. [DOI] [PubMed] [Google Scholar]
- 12.Sun, F., Hug, M. J., Bradbury, N. A. & Frizzell, R. A. (2000) J. Biol. Chem. 275, 14360-14366. [DOI] [PubMed] [Google Scholar]
- 13.Tibbs, V. C., Gray, P. C., Catterall, W. A. & Murphy, B. J. (1998) J. Biol. Chem. 273, 25783-25788. [DOI] [PubMed] [Google Scholar]
- 14.Cantrell, A. R., Tibbs, V. C., Yu, F. H., Murphy, B. J., Sharp, E. M., Qu, Y., Catterall, W. A. & Scheuer, T. (2002) Mol. Cell. Neurosci. 21, 63-80. [DOI] [PubMed] [Google Scholar]
- 15.Gray, P. C., Scott, J. D. & Catterall, W. A. (1998) Curr. Opin. Neurobiol. 8, 330-334. [DOI] [PubMed] [Google Scholar]
- 16.Kass, R. S. & Wiegers, S. E. (1982) J. Physiol. 322, 541-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marx, S. O., Kurokawa, J., Reiken, S., Motoike, H., D'Armiento, J., Marks, A. R. & Kass, R. S. (2002) Science 295, 496-499. [DOI] [PubMed] [Google Scholar]
- 18.Paavonen, K. J., Swan, H., Piippo, K., Hokkanen, L., Laitinen, P., Viitasalo, M., Toivonen, L. & Kontula, K. (2001) Heart 86, 39-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Piippo, K., Swan, H., Pasternack, M., Chapman, H., Paavonen, K., Viitasalo, M., Toivonen, L. & Kontula, K. (2001) J. Am. Coll. Cardiol. 37, 562-568. [DOI] [PubMed] [Google Scholar]
- 20.Kurokawa, J., Chen, L. & Kass, R. S. (2003) Proc. Natl. Acad. Sci. USA 100, 2122-2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang, T., Kanki, H. & Roden, D. M. (2003) Circulation 108, 132-134. [DOI] [PubMed] [Google Scholar]
- 22.Wang, W., Xia, J. & Kass, R. S. (1998) J. Biol. Chem. 273, 34069-34074. [DOI] [PubMed] [Google Scholar]
- 23.Hamill, O. P., Marty, A., Neher, E., Sakmann, B. & Sigworth, F. J. (1981) Pflügers Arch. 391, 85-100. [DOI] [PubMed] [Google Scholar]
- 24.Tateyama, M., Kurokawa, J., Terrenoire, C., Rivolta, I. & Kass, R. S. (2003) Circulation 107, 3216-3222. [DOI] [PubMed] [Google Scholar]
- 25.Hulme, J. T., Ahn, M., Hauschka, S. D., Scheuer, T. & Catterall, W. A. (2002) J. Biol. Chem. 277, 4079-4087. [DOI] [PubMed] [Google Scholar]
- 26.Orellana, S. A., Quinones, A. M. & Mandapat, M. L. (2003) Pediatr. Res. 54, 406-412. [DOI] [PubMed] [Google Scholar]
- 27.Kammerer, S., Burns-Hamuro, L. L., Ma, Y., Hamon, S. C., Canaves, J. M., Shi, M. M., Nelson, M. R., Sing, C. F., Cantor, C. R., Taylor, S. S. & Braun, A. (2003) Proc. Natl. Acad. Sci. USA 100, 4066-4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kass, R. S., Kurokawa, J., Marx, S. O. & Marks, A. R. (2003) Trends Cardiovasc. Med. 13, 52-56. [DOI] [PubMed] [Google Scholar]
- 29.Zakhary, D. R., Fink, M. A., Ruehr, M. L. & Bond, M. (2000) J. Biol. Chem. 275, 41389-41395. [DOI] [PubMed] [Google Scholar]