Abstract
Neuroactive steroids modulate alcohol’s impact on brain function and behavior. Ethanol exposure alters neuroactive steroid levels in rats, humans, and some mouse strains. We conducted an exploratory analysis of the neuroactive steroids (3α,5α)-3-hydroxypregnan-20-one (3α,5α-THP), (3α,5α)-3,21-dihydroxypregnan-20-one (3α,5α-THDOC), and pregnenolone across 126–158 individuals and 19 fully inbred strains belonging to the BXD family, which were subjected to air exposure, or chronic intermittent ethanol (CIE) exposure. Neuroactive steroids were measured by gas chromatography-mass spectrometry in serum following five cycles of CIE or air exposure (CTL). Pregnenolone levels in CTLs range from 272 to 578 pg/mL (strain variation of 2.1-fold with p = 0.049 for strain main effect), with heritability of 0.20 ± 0.006 (SEM), whereas in CIE cases values range from 304 to 919 pg/mL (3.0-fold variation, p = 0.007), with heritability of 0.23 ± 0.005. 3α,5α-THP levels in CTLs range from 375 to 1055 pg/mL (2.8-fold variation, p = 0.0007), with heritability of 0.28 ± 0.01; in CIE cases they range from 460 to 1022 pg/mL (2.2-fold variation, p = 0.004), with heritability of 0.23 ± 0.005. 3α,5α-THDOC levels in CTLs range from 94 to 448 pg/mL (4.8-fold variation, p = 0.002), with heritability of 0.30 ± 0.01, whereas levels in CIE cases do not differ significantly. However, global averages across all BXD strains do not differ between CTL and CIE for any of the steroids. 3α,5α-THDOC levels were lower in females than males in both groups (CTL −53%, CIE −55%, p < 0.001). Suggestive quantitative trait loci are identified for pregnenolone and 3α,5α-THP levels. Genetic variation in 3α,5α-THP was not correlated with two-bottle choice ethanol consumption in CTL or CIE-exposed animals. However, individual variation in 3α,5α-THP correlated negatively with ethanol consumption in both groups. Moreover, strain variation in neuroactive steroid levels correlated with numerous behavioral phenotypes of anxiety sensitivity accessed in GeneNetwork, consistent with evidence that neuroactive steroids modulate anxiety-like behavior.
Keywords: neuroactive steroids; 3α,5α-THP (allopregnanolone); ethanol dependence; BXD recombinant inbred strains
Introduction
The 3α,5α-reduced metabolites of progesterone and deoxycorticosterone (DOC), (3α,5α)-3-hydroxypregnan-20-one (3α,5α-THP or allopregnanolone), and (3α,5α)- 3,21-dihydroxypregnan-20-one (3α,5α-THDOC or allotetrahydrodeoxycorticosterone) are endogenous neuroactive steroids that rapidly alter neuronal excitability via membrane receptors. Their systemic administration induces anxiolytic, antidepressant, anticonvulsant, sedative, anesthetic, and analgesic effects, mostly through action at γ-aminobutyric acid type A (GABAA) receptors (Porcu et al., 2016). Acute ethanol administration (>1.3 g/kg in rats) increases brain and plasma levels of these steroids (Serra et al., 2003; VanDoren et al., 2000), with effects that are specific to different brain regions (Cook, Dumitru, O'Buckley, & Morrow, 2014). These effects also appear to be species-specific; in fact, acute ethanol fails to alter 3α,5α-THP and 3α,5α-THDOC concentrations in the cerebral cortex, hippocampus, and plasma of C57BL/6J and DBA/2J strains of mice, or in cynomolgus monkeys plasma (Porcu et al., 2010, 2014; Porcu & Morrow, 2014). However, numerous lines of evidence suggest that ethanol's behavioral and subjective effects involve neuroactive steroids, and that ethanol-induced changes in neuroactive steroids may contribute to ethanol sensitivity and consumption (Beattie et al., 2016; Morrow & Porcu, 2009; Morrow, Porcu, Boyd, & Grant, 2006; Porcu & Morrow, 2014).
Individual differences in vulnerability to alcoholism have a strong genetic component (Schuckit, 2009). Studies in rodents indicate a shared genetic sensitivity to ethanol, anxiety, and stress/hypothalamic-pituitary-adrenal (HPA) axis response (Boehm, Reed, McKinnon, & Phillips, 2002; Crabbe, Phillips, Buck, Cunningham, & Belknap, 1999). We have previously demonstrated strong heritable differences in basal levels of the neuroactive steroid DOC (Porcu et al., 2011) across the C57BL/6 (B6) × DBA/2 (D2) (BXD) recombinant inbred mouse strains – a cohort of genetically diverse strains to study networks of phenotypes and their modulation by gene variants (Gora-Maslak et al., 1991; Wang et al., 2016; Williams et al., 2016; Williams, Gu, Qi, & Lu, 2001). DOC is a progesterone metabolite and precursor of the GABAergic neuroactive steroid 3α,5α-THDOC and of the glucocorticoid corticosterone. Its levels are elevated in rat brain and mouse plasma following acute ethanol administration (Khisti, Boyd, Kumar, & Morrow, 2005; Porcu et al., 2010), and are regulated by hypothalamic and pituitary activation of the HPA axis in both cynomolgus monkeys and humans. This regulation is altered following ethanol dependence (Porcu, Grant, Green, Rogers, & Morrow, 2006; Porcu, O'Buckley, Leslie Morrow, & Adinoff, 2008). Using GeneNetwork (www.genenetwork.org), a public repository of genetic and phenotypic data as well as a tool for multivariate analysis of complex traits (Chesler et al., 2005; Wang et al., 2016; Wang, Williams, & Manly, 2003), we previously mapped quantitative trait loci (QTLs) on chromosomes 4 and 14 that appear to modulate basal DOC levels in cerebral cortex and plasma, respectively (Porcu et al., 2011). Moreover, variation in basal DOC levels is positively correlated with increased ethanol-induced sedation, ethanol-induced ataxia, and ethanol-induced corticosterone levels–phenotypes previously characterized across these strains by several groups (data also in GeneNetwork). The finding that lines of mice with higher basal DOC levels have higher ethanol sensitivity is consistent with the hypothesis that neuroactive steroids may contribute to ethanol sensitivity and that elevated GABAergic neuroactive steroids, in response to ethanol administration, may protect against the risk for alcohol dependence (Morrow et al., 2006; Morrow & Porcu, 2009; Porcu & Morrow, 2014). Blunted elevations of neuroactive steroids following ethanol exposure would be predicted to reduce sensitivity to the anxiolytic, sedative, anticonvulsant, cognitive-impairing, and discriminative stimulus properties of ethanol (Morrow et al., 2006). Reduced sensitivity to ethanol is associated with greater risk for the development of alcoholism in individuals with genetic vulnerability to alcoholism (Schuckit, 1994; Wilhelmsen et al., 2003).
The present study is intended as an exploratory analysis of variation in serum levels of 3α,5α-THP and 3α,5α-THDOC, as well as of the neuroactive steroid precursor pregnenolone, across ethanol-dependent BXD strains that underwent chronic intermittent ethanol (CIE) or air (controls, CTL) vapor exposure plus voluntary alcohol consumption between CIE or air exposure cycles. We hypothesized that CIE exposure would alter neuroactive steroid levels in mice, but in an idiosyncratic way depending on genetic background. We evaluated the strength of the genetic effects in a well-controlled laboratory environment simply by computing heritabilities of neuroactive steroid levels under both conditions in genetically well-matched cohorts. We also tested whether variation in neuroactive steroid levels correlated to ethanol consumption. Finally, given that the study design includes many male-female pairs that are isogenic except for the obvious segregation of sex chromosomes, we were able to address sex differences with reasonable power and estimate correlations among traits across genetically diverse individuals. Indeed, we further analyzed correlations between neuroactive steroid levels and parameters of ethanol intake in the same mice, as well as phenotypic data previously determined in the BXD panel by multiple laboratories and available in GeneNetwork.
Materials and methods
Animals
Adult (12–16 weeks old upon arrival) male and female DBA/2 and BXD mice were acquired from the vivarium at the University of Tennessee Health Science Center (Memphis, TN, USA). Adult (10 weeks old upon arrival) male and female C57BL/6J mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). After arrival at the animal facility, mice were allowed to acclimate for one week. They were housed individually under 12-h light, 12-h dark cycle (light on from 0200 to 1400 h) and at a centrally controlled temperature (~22 °C) and humidity. Animals had free access to water and standard laboratory food (Harland Teklad, Madison, WI, USA) at all times. All procedures were approved by the Medical University of South Carolina Institutional Animal Care and Use Committee and adhered to the National Institutes of Health Guide for the Care and Use of Laboratory Animals (8th edition, National Research Council, 2011).
Chronic intermittent ethanol (CIE) exposure procedure
The general study design involved typically 2–4 mice per experimental cell defined by genotype, sex, and group (CIE, CTL). Body weights were recorded weekly during ethanol-drinking weeks or daily during CIE or CTL air exposure (detailed below). Mice were tested for baseline ethanol intake using a two-bottle (15% v/v ethanol vs. water) limited access (2 h/day, starting 30 min before lights off) drinking model for 6 weeks (Baseline). Ethanol bottles were prepared fresh every day and presented in 15-mL tubes (± 0.1 mL). Then, mice from each genotype received four cycles of chronic intermittent ethanol vapor exposure (CIE group) or air exposure (control group, CTL) (16 h/day × 4 days, followed by 72 h withdrawal), alternated by 5-day ethanol self-administration using the two-bottle choice procedure (Becker & Lopez, 2004; Lopez & Becker, 2005; Lopez, Griffin, Melendez, & Becker, 2012). Ethanol concentration in the inhalation chambers was uniformly set for all genotypes and monitored daily to ensure that the inhalation conditions produced stable blood ethanol concentrations (BEC) around 175 mg/dL in C57BL/6J mice. BEC was assessed once each week by sampling blood from the retro-orbital sinus immediately upon removal from the chamber. Before each 16-h ethanol exposure, intoxication was initiated in CIE mice by intraperitoneal (i.p.) administration of ethanol (1.6 g/kg) combined with the alcohol dehydrogenase inhibitor pyrazole (1 mmol/kg) in a volume of 0.02 mL/g body weight. The co-administration of pyrazole is critical to maintain a high and stable level of intoxication during each cycle of ethanol vapor exposure (Griffin, Lopez, & Becker, 2009; Griffin, Lopez, Yanke, Middaugh, & Becker, 2009). CTL mice were similarly handled, and administered the same pyrazole dose in a saline solution, prior to being placed in air inhalation chambers. Thus, all mice received the same number and timing of pyrazole injections prior to final removal from the inhalation chambers. Blood samples for neuroactive steroid assays were collected 72 h after a fifth and final CIE or CTL air exposure cycle. The data for neuroactive steroid levels and for ethanol intake reported in this study was obtained from three cohorts run in different time frames (2010–2014). Several strains were included in all cohorts to monitor for batch effects. Only those strains for which at least two replicates per treatment and per sex were obtained were included in the study. The number of mice per strain/treatment/sex ranged between 2 and 16.
Neuroactive steroid assay
3α,5α-THP, 3α,5α-THDOC, and pregnenolone levels were measured in serum samples by gas chromatography-mass spectrometry (GC-MS) as previously described (Porcu et al., 2009, 2010). Briefly, samples (100 µL) were spiked with 400 pg/mL of each deuterated internal standard and applied to C18 solid phase extraction columns (Strata C18-E, 500 mg, Phenomenex, Torrance, CA, USA) that had been preconditioned with 4 mL methanol and 4 mL distilled water. The column containing the sample was washed with 4 mL distilled water in order to remove high polar impurities. Columns were dried under vacuum for 30 min and neuroactive steroids were then eluted with 2 mL methanol. The extracts were evaporated in a speed vacuum concentrator (Thermo Fisher Scientific, Inc., Waltham, MA, USA). The dry residue was resuspended in 2 mL of ethyl acetate/methanol (80/20, v/v), and the sample was filtered through a NH2 column (Strata NH2, Phenomenex, Torrance, CA, USA) preconditioned with 4 mL of ethyl acetate and 4 mL of ethyl acetate/methanol (80/20, v/v). The neuroactive steroids passed unretained through the sorbent, and the eluate was collected. The NH2 column was further rinsed with 2 mL of the solvent mixture and the combined eluates were evaporated in the speed vacuum concentrator. Dried samples after purification were derivatized in 450 µL of ethyl acetate and 50 µL of heptafluorobutyric acid anhydride (Thermo Scientific, Waltham, MA, USA), followed by vortex mixing. Samples were allowed to react for 2 h at room temperature and were subsequently dried under a gentle stream of nitrogen. Derivatized samples were resuspended in 10 µL of heptane, and 2 µL of each sample was injected in duplicate into the GC-MS. Analysis was carried out on an Agilent 7890 gas chromatograph coupled to a 5975 mass selective detector (Agilent Technologies, Inc., Santa Clara, CA, USA) operated in negative chemical ionization mode, as previously described (Porcu et al., 2009, 2010). Neuroactive steroids were analyzed by single ion monitoring. The data acquisition was broken into retention windows corresponding to the elution of the different neuroactive steroid groups. Neuroactive steroids were quantified by interpolation of linear regression standard curves. Calibration curves were made in 300 µL distilled water spiked with 5 µL human charcoal-stripped serum (Gemini Bio- Products, Woodland, CA, USA), with 400 pg/mL of each deuterated internal standard and with the appropriate known concentration of neuroactive steroids (2, 10, 20, 50, 100, 200, 500, and 1000 pg/mL). A blank standard (5 µLhuman charcoal-stripped serum/300 µL distilled water) was also included. Calibration curves underwent the same extraction procedure as the samples. Steroid standards (>99% purity) for 3α,5α-THP and 3α,5α-THDOC were synthesized by the late Dr. R. H.Purdy (previously of Scripps Research Institute, San Diego, CA, USA). Pregnenolone standard (>99% purity) was purchased from Steraloids Inc. (Newport, RI, USA). (d4-17,21,21,21)-pregnenolone (98% purity), (d4-17,21,21,21)-3α,5α-THP, and (d3-17,21,21)-3α,5α-THDOC (>95% purity) were purchased from Cambridge Isotope Laboratories, Inc., Andover, MA, USA. Organic solvents were pesticide grade from Thermo Fisher Scientific, Inc. (Waltham, MA, USA).
Statistical and bioinformatics analysis
Only those strains for which at least two replicates per treatment/per sex were obtained were included in the analysis. The number of mice per strain/per treatment/per sex ranged between 2 and 16. Analysis was performed in male and female cases grouped together, as well as in male cases only and female cases only. Variation in neuroactive steroid levels was analyzed by one-way ANOVA, using a commercially available statistical program (GraphPad Prism 5.0, GraphPad Software, San Diego, CA, USA). Average neuroactive steroid levels in all CTL and CIE cases were compared by paired t test or by two-way ANOVA with treatment and sex as independent variables. Effect size estimates were computed by Cohen’s d for t test and η2 for ANOVA. Heritability (h2) was estimated as the ratio of the variance between strains divided by the sum of the within-strain and between-strain variances (Brigman, Mathur, Lu, Williams, & Holmes, 2009). The variance and standard error of the estimate of h2 was computed using a jackknife procedure (Williams, Strom, Rice, & Goldowitz, 1996). h2 estimates from the jackknife procedure in all CTL and all CIE cases were compared by paired t test. Genetic data were analyzed using the statistical software available in GeneNetwork, which allows for the analysis of networks of genes, transcripts, and classic phenotype data sets (Rosen, Chesler, Manly, & Williams, 2007). Datasets for neuroactive steroid levels were subjected to simple interval mapping analysis using Haley-Knott regression equations. Interval mapping was performed using the Haldane function, a 1-cM window, and marker maps for each chromosome that are very dense relative to recombination frequency in this cross. The thresholds for statistically significant (p value ~0.05) and suggestive (p value ~0.63) (Lander & Kruglyak, 1995) genome-wide linkage were determined based on permutation tests (Doerge & Churchill, 1996). Five thousand permutations were run. Spearman rank trait correlations were computed using analytical tools integrated into GeneNetwork and using data sets of numerous BXD behavioral and physiological phenotypes, since each statistic was more appropriate for some phenotypes.p values for correlation tests were not corrected for multiple tests, and nominally significant results should therefore be considered well-defined hypotheses rather than strong or independently valid results. The reason that this is the case is that GeneNetwork currently contains approximately 5000 phenotypes for BXD strains. To achieve a tolerably low FDR (p < 0.2), a correction factor of at least 100 is recommended (in other words, p < 0.0005 is likely to be significant at an FDR <0.2; see Wang et al., 2016).
Results
Neuroactive steroid levels in BXD strains
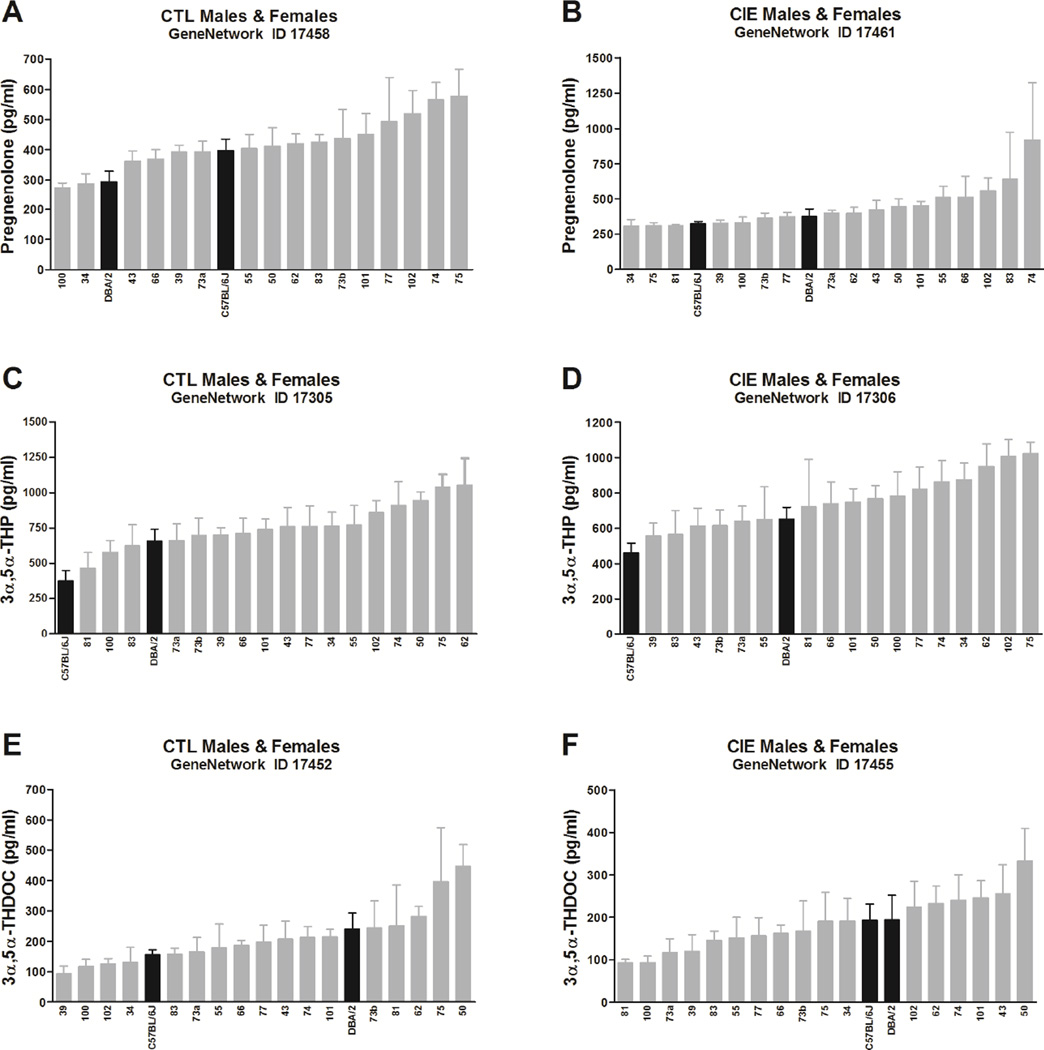
Serum pregnenolone levels in all CTL cases (414 ± 20 pg/mL, n = 18; data for BXD 81 were lost) range from 272 to 578 pg/mL (95% confidence interval 372–457), resulting in 2.1-fold variation [F(17,132) = 1.72, p = 0.049, η2 = 0.20] of this trait (Fig. 1A, Table 1), whereas levels in all CIE cases (434 ± 34 pg/mL, n = 19) range from 304 to 919 pg/mL (95% confidence interval 362–507), resulting in 3.0-fold variation [F(18,145) = 2.15, p = 0.007, η2 = 0.23] of this trait (Fig. 1B, Table 1). h2 is estimated to be 0.20 ± 0.006 in all CTL cases, and 0.23 ± 0.005 in all CIE cases (+15%, t(17) = 5.8, p < 0.0001, Cohen’s d = 1.89). Average pregnenolone levels do not differ between CTL and CIE cases [t(17) = 0.8, p = 0.41, Cohen’s d = 0.28]; however, they have a distinct pattern of variation in CTL vs. CIE cases as indicated by a lack of correlation among values of the two experimental groups (Spearman r = 0.33, p = 0.18, n = 18).
Fig. 1. Variation in basal pregnenolone (A–B), 3α,5α-THP (C–D), and 3α,5α-THDOC (E–F) levels across BXD strains.
Mice from each genotype received four cycles of chronic intermittent ethanol (CIE) vapor exposure (CIE group, B–D–F) or air exposure (CTL group, A– C–E) (16 h/day × 4 days, followed by 72-h withdrawal), alternated with 5-day drinking test cycles using a two-bottle (15% v/v ethanol vs. water) limited access (2 h/day) drinking model. Blood samples for neuroactive steroid assays were collected 72 h after a fifth CIE or air exposure cycle. Neuroactive steroid levels, assayed in all male and female cases, are expressed as pg/mL and are means ± SEM of values from 2–16 mice/strain/treatment. The x-axis reports the BXD strain number; C57BL/6J and DBA/2 are also indicated (black bars). Strains are plotted in order from the lowest to the highest levels for each of the neuroactive steroids. One-way ANOVA was used to estimate significant variation.
Table 1.
Variation and heritability for neuroactive steroid levels across BXD strains.
| ID | N | pg/mL | 95% CI | Fold- variation |
F | p | η2 | h2 | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SE | Range | Mean ± SE | Range | ||||||||
| Pregnenolone | |||||||||||
| CTL M&F | 17458 | 18 | 414 ± 20 | 272–578 | 372–457 | 2.1 | (17,132)=1.72 | 0.049 | 0.20 | 0.20 ± 0.006 | 0.17–0.25 |
| CIE M&F | 17461 | 19 | 434 ± 34 | 304–919 | 362–507 | 3.0 | (18,145)=2.15 | 0.007 | 0.23 | 0.23 ± 0.005 | 0.18–0.27 |
| CTL M | 17459 | 17 | 424 ± 31 | 288–737 | 358–490 | 2.6 | (16,69)=1.70 | 0.076 | 0.34 | 0.34 ± 0.004 | 0.31–0.38 |
| CTL F | 17460 | 17 | 399 ± 24 | 261–648 | 348–451 | 2.5 | (16,60)=1.75 | 0.071 | 0.39 | 0.39 ± 0.019 | 0.31–0.49 |
| CIE M | 17462 | 19 | 451 ± 45 | 279–1113 | 358–545 | 4.0 | (18,78)=2.02 | 0.023 | 0.38 | 0.37 ± 0.017 | 0.26–0.43 |
| CIE F | 17463 | 16 | 394 ± 25 | 253–577 | 340–448 | 2.3 | (15,64)=0.90 | 0.572 | 0.22 | 0.22 ± 0.009 | 0.20–0.31 |
| 3α,5α-THP | |||||||||||
| CTL M&F | 17305 | 19 | 741 ± 40 | 375–1055 | 657–824 | 2.8 | (18,143)=2.68 | 0.0007 | 0.28 | 0.28 ± 0.011 | 0.18–0.30 |
| CIE M&F | 17306 | 19 | 739 ± 36 | 460–1022 | 664–815 | 2.2 | (18,157)=2.27 | 0.004 | 0.23 | 0.23 ± 0.005 | 0.18–0.25 |
| CTL M | 17448 | 18 | 683 ± 47 | 254–992 | 585–782 | 3.9 | (17,78)=2.51 | 0.005 | 0.41 | 0.41 ± 0.030 | 0.24–0.44 |
| CTL F | 17449 | 17 | 818 ± 38 | 604–1360 | 737–900 | 2.3 | (16,62)=0.91 | 0.559 | 0.24 | 0.24 ± 0.004 | 0.20–0.27 |
| CIE M | 17450 | 19 | 805 ± 62 | 385–1291 | 676–935 | 3.4 | (18,87)=3.60 | <0.0001 | 0.48 | 0.48 ± 0.011 | 0.42–0.52 |
| CIE F | 17451 | 16 | 696 ± 33 | 406–929 | 625–766 | 2.3 | (15,67)=1.03 | 0.438 | 0.23 | 0.23 ± 0.008 | 0.16–0.26 |
| 3α,5α-THDOC | |||||||||||
| CTL M&F | 17452 | 19 | 211 ± 21 | 94–448 | 168–254 | 4.8 | (18,125)=2.57 | 0.001 | 0.30 | 0.30 ± 0.010 | 0.21–0.33 |
| CIE M&F | 17455 | 19 | 184 ± 14 | 93–333 | 155–214 | 3.6 | (18,144)=1.39 | 0.146 | 0.17 | 0.17 ± 0.003 | 0.13–0.19 |
| CTL M | 17453 | 18 | 247 ± 25 | 118–498 | 187–292 | 4.2 | (17,74)=2.45 | 0.006 | 0.42 | 0.42 ± 0.017 | 0.31–0.47 |
| CTL F | 17454 | 13 | 116 ± 19a | 30–243 | 75–157 | 8.2 | (12,41)=2.80 | 0.012 | 0.54 | 0.54 ± 0.024 | 0.49–0.68 |
| CIE M | 17456 | 19 | 241 ± 23 | 93–512 | 193–290 | 5.5 | (18,85)=2.48 | 0.004 | 0.40 | 0.40 ± 0.017 | 0.30–0.45 |
| CIE F | 17457 | 15 | 109 ± 18a | 41–292 | 70–148 | 7.1 | (14,55)=2.64 | 0.008 | 0.47 | 0.48 ± 0.011 | 0.45–0.57 |
Male (M) and female (F) mice from each genotype received four cycles of chronic intermittent ethanol (CIE) vapor exposure (CIE group) or air exposure (CTL group) (16 h/day × 4 days, followed by 72-h withdrawal), alternated with 5-day drinking test cycles using a two-bottle (15% v/v ethanol vs. water) limited access (2 h/day) drinking model. Blood samples for neuroactive steroid assays were collected 72 h after a fifth CIE or air exposure cycle. Neuroactive steroid levels are expressed as pg/mL. Significant genetic variation was estimated by one-way ANOVA; putative differences in average neuroactive steroid levels were estimated by two-way ANOVA, with 2 treatment and sex as independent variables. Heritability (h2) was estimated as the ratio of the variance between strains divided by the sum of the within-strain and between-strain variances. The variance and standard error of the estimate of h2 was computed using a jackknife procedure (Williams et al., 1996).
p < 0.001 vs. the respective male cases (two-way ANOVA, followed by Bonferroni post hoc test). ID refers to the GeneNetwork BXD phenotype ID for each trait.
Serum 3α,5α-THP levels in all CTL cases (741 ± 40 pg/mL, n = 19) range from 375 to 1055 pg/mL (95% confidence interval 657–824), resulting in 2.8-fold variation [F(18,143) = 2.68, p = 0.0007, η2 = 0.28] of this trait (Fig. 1C, Table 1), whereas levels in all CIE cases (739 ± 36 pg/mL, n = 19) range from 460 to 1022 pg/mL (95% confidence interval 664–815), resulting in 2.2-fold variation [F(18,157) = 2.27, p = 0.004, η2 = 0.23] of this trait (Fig. 1D, Table 1). h2 is estimated to be 0.28 ± 0.01 in all CTL cases, and 0.23 ± 0.005 in all CIE cases (−18%, t(18) = 14.6, p < 0.0001, Cohen’s d = 4.75). Average 3α,5α-THP levels do not differ between CTL and CIE cases [t(18) = 0.06, p = 0.96, Cohen’s d = 0.02]; moreover, 3α,5α-THP levels in CTL vs. CIE cases are positively correlated (Spearman r = 0.69, p = 0.01, n = 19), suggesting a similar pattern of variation, which is independent of CIE exposure.
Serum 3α,5α-THDOC levels in all CTL cases (211 ± 21 pg/mL, n = 19) range from 94 to 448 pg/mL (95% confidence interval 168–254), resulting in 4.8-fold variation [F(18,125) = 2.57, p = 0.001, η2 = 0.30] of this trait (Fig. 1E, Table 1). In contrast, 3α,5α-THDOC levels in all CIE cases (184 ± 14 pg/mL, n = 19) did not show a significant variation (Fig. 1F,Table 1). h2 is estimated to be 0.30 ± 0.010 in all CTL cases, and 0.17 ± 0.003 in all CIE cases (−45%, t(18) = 36.3, p < 0.0001, Cohen’s d = 11.78). Average 3α,5α-THDOC levels do not differ between CTL and CIE cases [t(18) = 1.52, p = 0.15, Cohen’s d = 0.49]; however, they appear to have a distinct pattern of variation as indicated by a lack of correlation among values of the two experimental groups (Spearman r = 0.41, p = 0.08, n = 19).
Significant genetic variation for neuroactive steroid levels was also observed in CTL or CIE male cases or female cases only. Thus, we analyzed putative sex differences in neuroactive steroid levels in both CTL and CIE cases. Significant genetic variation was found for pregnenolone levels in CIE male cases (451 ± 45 pg/mL, n = 19), with values ranging from 279 to 1113 pg/mL (95% confidence interval 358– 545), resulting in 4.0-fold variation [F(18,78) = 2.02, p = 0.023, η2 = 0.38] of this trait, and h2 of 0.37 ± 0.017 (Table 1). No significant variation was observed in CTL male or female cases, as well as CIE female cases (Table 1). However, two-way ANOVA for pregnenolone levels overall found no effect of CIE treatment [F(1,65) = 0.10, p = 0.75, η2 = 0.00], no effect of sex [F(1,65) = 1.49, p = 0.23, η2 = 0.02], and no interaction [F(1,65) = 0.24, p = 0.62, η2 = 0.00].
Significant genetic variation was found for 3α,5α-THP levels in male cases; in CTL male cases (683 ± 47 pg/mL, n = 18), values range from 254 to 992 pg/mL (95% confidence interval 585–782), resulting in 3.9-fold variation [F(17,78) = 2.51, p = 0.005, η2 = 0.41] of this trait, and h2 of 0.41 ± 0.030 (Table 1); in CIE male cases (805 ± 62 pg/mL, n = 19), values range from 385 to 1291 pg/mL (95% confidence interval 676–935), resulting in 3.4-fold variation [F(18,87) = 3.60, p < 0.0001, η2 = 0.48] of this trait, and h2 of 0.48 ± 0.011 (Table 1). By contrast, no significant variation was observed in CTL or CIE female cases (Table 1). Two-way ANOVA for 3α,5α-THP levels found no effect of CIE treatment [F(1,66) = 0.00007, p = 0.99, η2 = 0.00] and no effect of sex [F(1,66) = 0.07, p = 0.79, η2 = 0.00], but a significant interaction was found [F(1,65) = 6.55, p = 0.013, η2 = 0.09]. However, post hoc analysis did not reveal any significant differences between the groups.
Significant genetic variation was found for 3α,5α-THDOC levels in both CTL and CIE male cases. 3α,5α-THDOC levels in CTLs (247 ± 25 pg/mL, n = 18) range from 118 to 498 pg/mL (95% confidence interval 187–292), resulting in 4.2-fold variation [F(17,74) = 2.45, p = 0.006, η2 = 0.42] of this trait, and h2 of 0.42 ± 0.017 (Table 1); 3α,5α-THDOC levels in CIE cases (241 ± 23 pg/mL, n = 19) range from 93 to 512 pg/mL (95% confidence interval 193–290), resulting in 5.5-fold variation [F(18,85) = 2.48, p = 0.004, η2 = 0.40] of this trait, and h2 of 0.40 ± 0.017 (Table 1). A similar result was found for 3α,5α-THDOC levels in female cases. 3α,5α-THDOC levels in CTL female cases (116 ± 19 pg/mL, n = 13) range from 30 to 243 pg/mL (95% confidence interval 75–157), resulting in 8.2-fold variation [F(12,41) = 2.80, p = 0.012, η2 = 0.54] of this trait, and h2 of 0.54 ± 0.024 (Table 1); 3α,5α-THDOC levels in CIE female cases (109 ± 18 pg/mL, n = 15) range from 41 to 292 pg/mL (95% confidence interval 70–148), resulting in 7.1-fold variation [F(14,55) = 2.64, p = 0.008, η2 = 0.47] of this trait, and h2 of 0.48 ± 0.011 (Table 1). Two-way ANOVA for 3α,5α-THDOC levels found no effect of CIE treatment [F(1,61) = 0.01, p = 0.91, η2 = 0.00], a significant effect of sex [F(1,61) = 32.32, p < 0.0001, η2 = 0.35], and no interaction [F(1,61) = 0.04, p = 0.84, η2 = 0.00]. Bonferroni post hoc analysis revealed lower 3α,5α-THDOC levels in CTL females vs. CTL males (−53%, p < 0.001), as well as in CIE females vs. CIE males (−55%, p < 0.001).
Mapping QTLs for neuroactive steroid levels in BXD strains
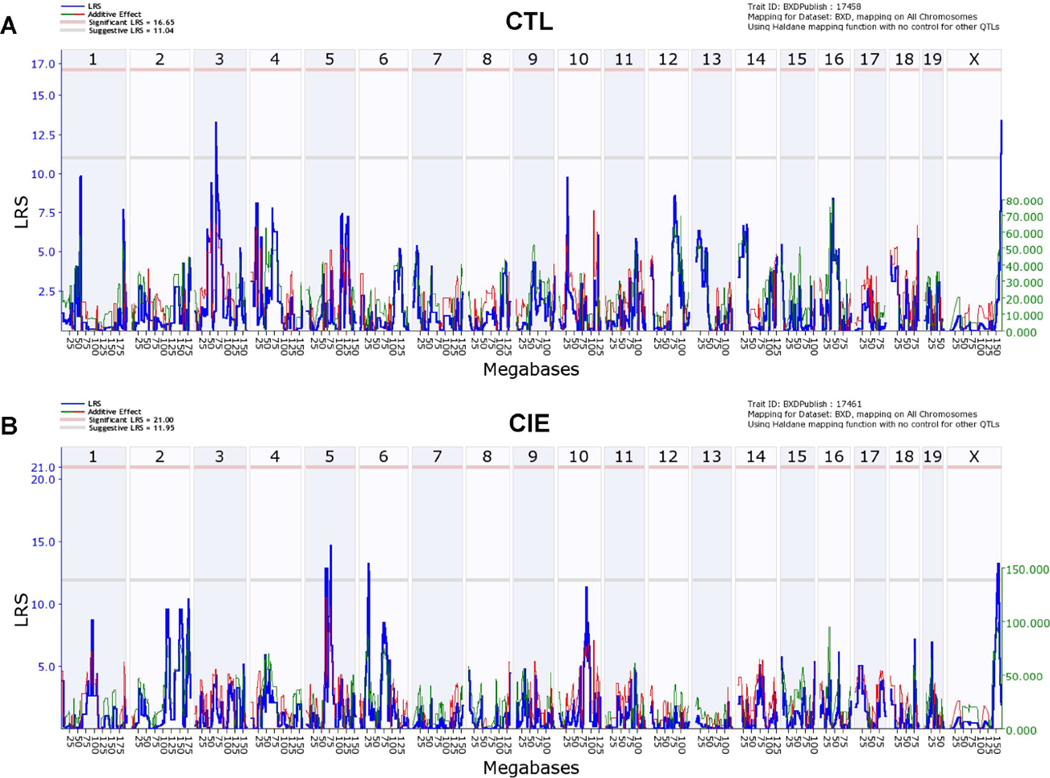
Variation in neuroactive steroid levels across the BXD strains was mapped using tools in GeneNetwork. For pregnenolone levels in all CTL cases (GeneNetwork BXD phenotype ID 17458), suggestive QTLs mapped on chromosome 3 and chromosome X, both with a likelihood ratio statistic (LRS) of 14 (Fig. 2A), while for pregnenolone levels in all CIE cases (GeneNetwork BXD phenotype ID 17461), suggestive QTLs mapped on chromosome 5 (LRS of 15), chromosome 6 (LRS of 13), and chromosome X (LRS of 13) (Fig. 2B). No QTLs were found for 3α,5α-THP levels in all CTL cases (GeneNetwork BXD phenotype ID 17305; Supplementary Fig. 1A), while for 3α,5α-THP levels in all CIE cases (GeneNetwork BXD phenotype ID 17306), a suggestive QTL mapped on chromosome 3 (LRS of 12, Supplementary Fig. 1B). Moreover, no QTLs were found for 3α,5α-THDOC levels in either CTL (GeneNetwork BXD phenotype ID 17452) or CIE cases (GeneNetwork BXD phenotype ID 17455; Supplementary Fig. 2). Because 3α,5α-THDOC levels differed by sex overall, we also mapped 3α,5α-THDOC levels separately in male and female cases. Suggestive QTLs were found for 3α,5α-THDOC levels in CIE males only (chromosomes 6 and 17, LRS of 12 and 14, respectively, Supplementary Fig. 3).
Fig. 2. Genome-wide interval mapping plots for basal pregnenolone levels across BXD strains.
Mice from each genotype received four cycles of chronic intermittent ethanol (CIE) vapor exposure (CIE group) or air exposure (CTL group) (16 h/day × 4 days, followed by 72-h withdrawal), alternated with 5-day drinking test cycles using a two-bottle (15% v/v ethanol vs. water) limited access (2 h/day) drinking model. Blood samples for pregnenolone assay were collected 72 h after a fifth CIE or air exposure cycle. (A) Likelihood ratio statistic (LRS) scores for pregnenolone levels in all CTL cases (GeneNetwork BXD phenotype ID 17458) across the entire genome show suggestive QTLs on chromosomes 3 and X (LRS of 14 for both). (B) LRS scores for pregnenolone levels in all CIE cases (GeneNetwork BXD phenotype ID 17461) across the entire genome show suggestive QTLs on chromosomes 5 (LRS of 15), 6 (LRS of 13), and X (LRS of 13). The y-axis and the thick blue lines provide the LRS of the association between the trait and the genotypes of markers. The two horizontal lines are the suggestive (gray) and significance (red) thresholds computed using 5000 permutations. A positive additive coefficient (green line) indicates that D alleles increase trait values. A negative additive coefficient (red line) indicates that B alleles increase trait values.
Correlations between neuroactive steroid levels and ethanol intake across the BXD strains
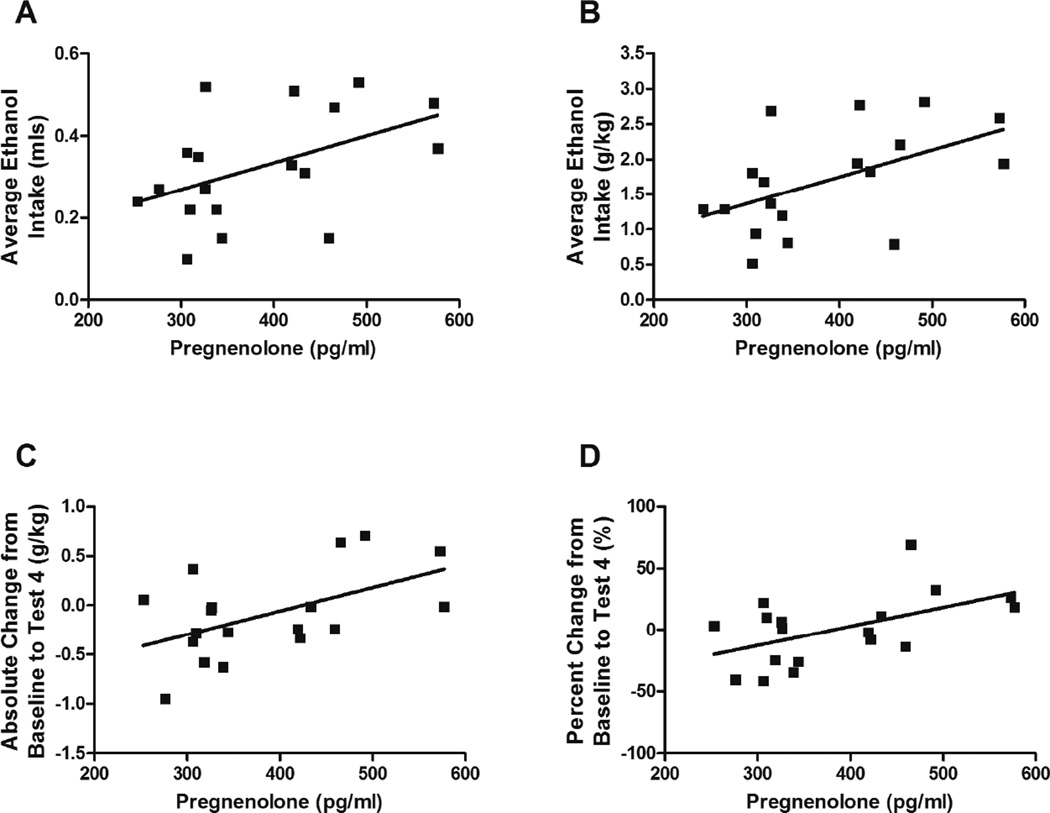
Correlation analyses were performed between neuroactive steroid levels and ethanol intake measures obtained in the same mice during the last two-bottle choice procedure following four cycles of CIE or air exposure (Test 4). A detailed analysis of ethanol intake is reported by Lopez et al., this issue. Average 3α,5α-THP and 3α,5α-THDOC levels were not correlated with average ethanol intake across the BXD strains, either CTL or CIE-exposed males and females, males only or females only. However, pregnenolone levels in CIE female cases demonstrated a positive correlation with average ethanol intake (milliliters, Spearman r = 0.48, p = 0.045, n = 18, Fig. 3A; g/kg, Spearman r = 0.53, p = 0.02, n = 18, Fig. 3B), with absolute change in ethanol intake from baseline to Test 4 (Spearman r = 0.48, p = 0.04, n = 18, Fig. 3C), and with percent change in ethanol intake from baseline to Test 4 (Spearman r = 0.52, p = 0.03, n = 18, Fig. 3D). Pregnenolone levels in the other CTL or CIE cases did not correlate with any ethanol intake measures (data not shown).
Fig. 3. Correlations between average pregnenolone levels and ethanol intake across CIE-exposed female BXD strains.
Mice from each genotype received four cycles of chronic intermittent ethanol (CIE) vapor exposure (CIE group) or air exposure (CTL group) (16 h/day × 4days, followed by 72-h withdrawal), alternated with 5-day drinking test cycles using a two-bottle (15% v/v ethanol vs. water) limited access (2 h/day) drinking model. Blood samples for pregnenolone assay were collected 72 h after a fifth CIE or air exposure cycle. Pregnenolone levels (x-axis; GeneNetwork BXD phenotype ID 17463) are expressed as pg/mL and are the average for each strain. Only data from CIE females are included in the analysis. (A) Average ethanol intake (milliliters) during Test 4 of the two-bottle choice test, Spearman r = 0.48, p = 0.045, n = 18. (B) Average ethanol intake (g/kg) during Test 4 of the two-bottle choice test, Spearman r = 0.53, p = 0.02, n = 18. (C) Absolute change in ethanol intake from baseline to Test 4, Spearman r = 0.48, p = 0.04, n = 18. (D) Percent change in ethanol intake from baseline to Test 4, Spearman r = 0.52, p = 0.03, n = 18. No significant correlations were observed between average pregnenolone levels and parameters of ethanol consumption in the other CTL or CIE cases examined.
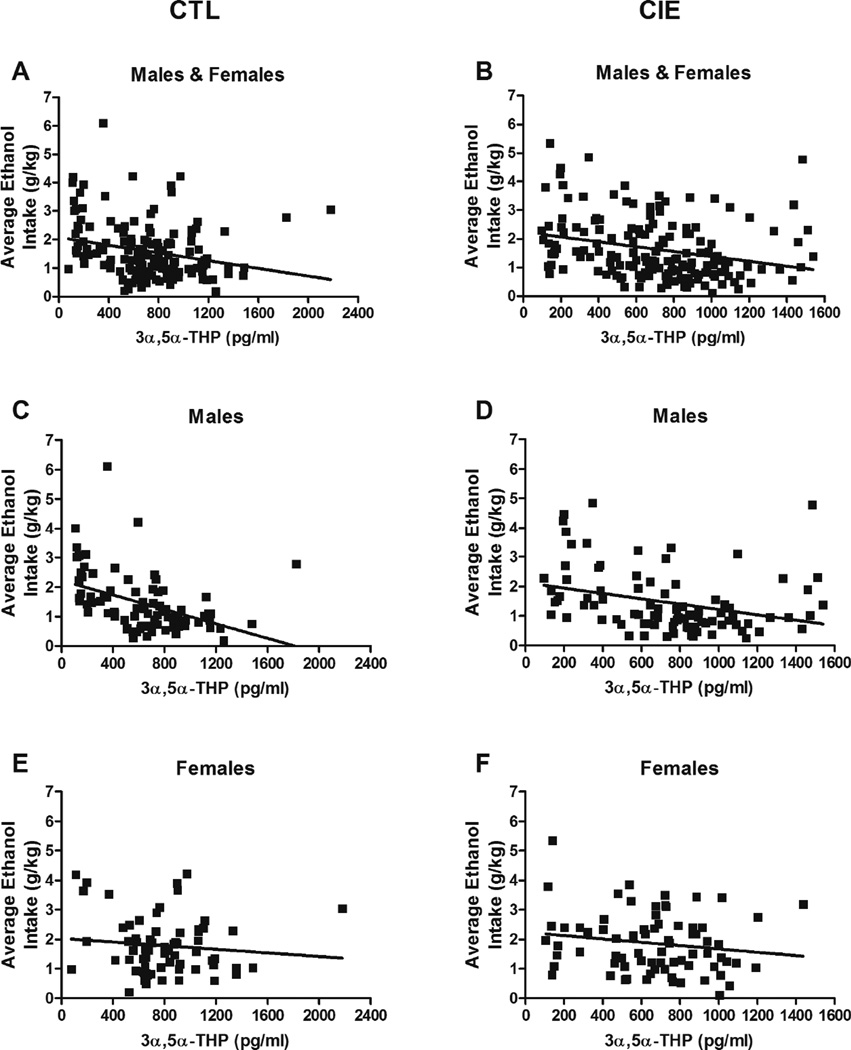
In contrast, there were consistent correlations between 3α,5α-THP levels and ethanol consumption across individual mice. 3α,5α-THP levels were negatively correlated with ethanol intake in all CTL cases (Spearman r = −0.26, p = 0.001, n = 152, Fig. 4A), in all CIE cases (Spearman r = −0.32, p < 0.0001, n = 167, Fig. 4B), in CTL males only (Spearman r = –0.51, p < 0.0001, n = 85, Fig. 4C), and in CIE males only (Spearman r = −0.38, p = 0.0001, n = 93, Fig. 4D). No correlations were found between 3α,5α-THP levels and ethanol consumption in CTL females only (Spearman r = −0.06, p = 0.61, n = 67, Fig. 4E), or CIE females only (Spearman r = −0.15, p = 0.19, n = 74, Fig. 4F). Moreover, CIE-induced changes in ethanol intake from baseline to Test 4 were negatively correlated with 3α,5α-THP levels in individual CTL male and female mice (Spearman r = −0.17, p = 0.03, n = 152; graph not shown).
Fig. 4. Correlations between 3α,5α-THP levels and ethanol intake in individual BXD strains.
Mice from each genotype received four cycles of chronic intermittent ethanol (CIE) vapor exposure (CIE group) or air exposure (CTL group) (16 h/day × 4 days, followed by 72-h withdrawal), alternated with 5-day drinking test cycles using a two-bottle (15% v/v ethanol vs. water) limited access (2 h/day) drinking model. Blood samples for 3α,5α-THP assay were collected 72 h after a fifth CIE or air exposure cycle. 3α,5α-THP levels are expressed as pg/mL and are reported for each individual mouse. Average ethanol intake (g/kg) refers to ethanol intake during Test 4 of the two-bottle choice test and is reported for each mouse. (A) Spearman r = –0.26, p = 0.001, n = 152. (B) Spearman r = –0.32, p < 0.0001, n = 167. (C) Spearman r = – 0.51, p < 0.0001, n = 85. (D) Spearman r = –0.38, p = 0.0001, n = 93. (E) Spearman r = –0.06, p = 0.61, n = 67. (F) Spearman r = –0.15, p = 0.19, n = 74.
Pregnenolone and 3α,5α-THDOC levels failed to correlate with ethanol intake across individual animals in any of the groups examined. However, a positive correlation was observed between 3α,5α-THDOC levels in all CIE cases and absolute change in ethanol intake from baseline to Test 4 (Spearman r = 0.22, p = 0.005, n = 154; graph not shown), as well as percent change in intake from baseline to Test 4 (Spearman r = 0.18, p = 0.03, n = 154; graph not shown). Likewise, a positive correlation was observed between 3α,5α-THDOC levels in male CIE cases and absolute change in ethanol intake from baseline to Test 4 (Spearman r = 0.23, p = 0.03, n = 91; graph not shown).
Trait correlations between neuroactive steroid levels and behavioral or neurochemical phenotypes across the BXD strains
One advantage of employing the BXD recombinant inbred set is the ability to test for covariation with other phenotypes that have been studied in this population. We examined correlations between neuroactive steroid levels in our study with several behavioral or neurochemical phenotypes previously characterized across the BXD strains by other independent laboratories and whose data are available in GeneNetwork (Tables 2–4). Because multiple correlations were analyzed simultaneously, we considered how to balance the risk of false discovery with the risk of false negatives and decided that Bonferroni corrections were too stringent. The gene network database contains many phenotypes that are essentially the same, such as measurements of ethanol intake or anxiety-like behavior at different time points, confounding correction procedures further. To address this dilemma, we consider correlations with p values <0.005 as most relevant, although even these will have a significant risk of false discovery. In addition, we focused on trait correlations with ethanol-related and anxiety-like behavior phenotypes because there is a rich literature showing that systemically administered neuroactive steroids have effects on these behaviors (Besheer, Lindsay, O'Buckley, Hodge, & Morrow, 2010; Bitran, Hilvers, & Kellogg, 1991; O'Dell et al., 2005; Reddy & Kulkarni, 1997; Wieland, Lan, Mirasedeghi, & Gee, 1991).
Table 2.
Trait correlations between serum pregnenolone levels and behavioral or neurochemical phenotypes across the BXD strains.
| GN ID | Phenotypes | Spearman | |||
|---|---|---|---|---|---|
| 17458 | Pregnenolone in CTL males and females | Reference | r | p | n |
| 17459 | Pregnenolone in CTL males | Present results | 0.91 | 0.0000 | 17 |
| 17460 | Pregnenolone in CTL females | Present results | 0.60 | 0.0094 | 17 |
| 17305 | 3α,5α-THP in CTL males and females | Present results | 0.50 | 0.0323 | 18 |
| 17448 | 3α,5α-THP in CTL males | Present results | 0.59 | 0.0106 | 17 |
| 12579 | Ethanol response, consumption of 15% ethanol (v/v) using two-bottle choice system (ethanol vs. water), 2-hour access, 1st week average, young adult male or female (Dec 2009 Cohort 1) [log g/kg/2h] |
Lopez M. F. et al., Unpublished |
0.87 | 0.0012 | 9 |
| 12979 | Alanine transaminase (ALT, ALAT, SGPT) level in serum following alcohol (6 gm ethanol/kg via gastric gavage) at 24 hours after gavage in young adult males and females (strain average associated with GenEx EtOH liver gene expression) [U/L] |
Rooney R. J. et al., Unpublished |
−0.60 | 0.0119 | 16 |
| 11012 | Anxiety, time in open arm of elevated plus maze in young adult males and females [sec] | (Brigman et al., 2009) | 0.89 | 0.0152 | 6 |
| 11971 | Anxiety, untreated baseline, percent entries into open arms of an elevated plus maze for males and females [%] | (Philip et al., 2010) | −0.75 | 0.0054 | 11 |
| 11713 | Anxiety, untreated baseline, percentage of entries into closed arms of a plus maze for females [%] | (Philip et al., 2010) | 0.72 | 0.0106 | 11 |
| 11714 | Anxiety, untreated baseline, percent entries into open arms of an elevated plus maze for females [%] | (Philip et al., 2010) | −0.72 | 0.0106 | 11 |
| 11723 | Anxiety, time in closed arms of an elevated plus maze for females [sec] | (Philip et al., 2010) | 0.71 | 0.0123 | 11 |
| 11715 | Anxiety, untreated baseline, percent time in closed arms of a plus maze for females [%] | (Philip et al., 2010) | 0.71 | 0.0123 | 11 |
| 11968 | Anxiety, untreated baseline, entries into closed arms of a plus maze for males and females [n] | (Philip et al., 2010) | 0.61 | 0.0454 | 11 |
| 12344 | Anxiety, baseline untreated control (BASE group), activity in closed quadrants using an elevated zero maze in 60 to 120-day-old females only during 10 min [beam breaks/sec] |
Cook M. et al., Unpublished |
−0.60 | 0.0099 | 17 |
| 12343 | Anxiety, baseline untreated control (BASE group), activity in closed quadrants using an elevated zero maze in 60 to 120-day-old females only during last 5 min [beam breaks/sec] |
Cook M. et al., Unpublished |
−0.61 | 0.0084 | 17 |
| 11013 | Anxiety, number of closed arm entries using elevated plus maze [n] | (Brigman et al., 2009) | 0.94 | 0.0023 | 6 |
| 11850 | Naloxone-induced morphine withdrawal, naloxone (30 mg/kg ip) after morphine (50 mg/kg dose ip), number of jumps for males and females [n/15 min test] |
(Philip et al., 2010) | −0.71 | 0.0080 | 12 |
| 11336 | Naloxone-induced morphine withdrawal, naloxone (30 mg/kg ip) after morphine (50 mg/kg dose ip), number of jumps for males [n/15 min test] |
(Philip et al., 2010) | 0.71 | 0.0116 | 11 |
| 11870 | Naloxone-induced morphine withdrawal, naloxone (30 mg/kg ip) after morphine (50 mg/kg dose ip), locomotion from 0–15 min after naloxone injection for males and females [n beam breaks] |
(Philip et al., 2010) | −0.59 | 0.0433 | 12 |
| 11356 | Naloxone-induced morphine withdrawal, naloxone (30 mg/kg ip) after morphine (50 mg/kg dose ip), locomotion from 0–15 min after naloxone injection for males [n beam breaks] |
(Philip et al., 2010) | −0.73 | 0.0090 | 11 |
| 11357 | Naloxone-induced morphine withdrawal, naloxone (30 mg/kg ip) after morphine (50 mg/kg dose ip), horizontal activity (distance traveled) from 0–15 min after naloxone injection for males [cm] |
(Philip et al., 2010) | −0.67 | 0.0210 | 11 |
| 13551 | Hippocampus residual weight, repeat measurement prior to dissection, statistically adjusted for variation in sex, age, body weight, and epoch (Hager et al., 2012) [residual mg] |
(Hager et al., 2012) | 0.68 | 0.0061 | 14 |
| 17461 | Pregnenolone in CIE males and females | ||||
| 17462 | Pregnenolone in CIE males | Present results | 0.88 | 0.0000 | 19 |
| 17463 | Pregnenolone in CIE females | Present results | 0.86 | 0.0000 | 16 |
| 12811 | Control for ethanol response, dopamine levels (DA) in hindbrain tissue 72 h after the 5th air control cycle of vapor chamber treatment, 16 to 18 week-old males or females (Dec 2009 Cohort 1) [ng/mg] |
Jones S. R. et al., Unpublished |
−0.49 | 0.0366 | 18 |
| 12978 | Alcohol response (6 mg/kg in saline, gastric gavage), blood alcohol concentration (BAC) 24 hr after gavage of young adult males and females (strain average associated with GenEx EtOH liver gene expression) [mg/dl] |
Rooney R. J. et al., Unpublished |
0.53 | 0.0348 | 16 |
| 12407 | Anxiety, saline treated [0.18 ml/kg i.p.] (NOS group), time in open quadrants using an elevated zero maze in 60 to 120-day-old males only during last 5 min [percentage of time] |
Cook M. et al., Unpublished |
0.48 | 0.0353 | 19 |
| 11969 | Anxiety, untreated baseline, entries into open arms of a plus maze for males and females [n] | (Philip et al., 2010) | −0.70 | 0.0142 | 11 |
| 11651 | Fear conditioning response, contextual activity for females [units] | (Philip et al., 2010) | 0.60 | 0.0370 | 12 |
| 11936 | Acoustic startle response, maximum response at 120 db for males and females (65 dB background noise, Hamilton-Kinder SM100 startle chamber, 10 msec interval, 120 dB startle stimulus, prepulse PPI control data by M Cook, UMemphis) [force] |
(Philip et al., 2010) | 0.68 | 0.0132 | 12 |
| 11937 | Acoustic startle response, maximum response at 70 db for males and females (65 dB background noise, Hamilton-Kinder SM100 startle chamber, 10 msec interval, 120 dB startle stimulus, prepulse PPI control data by M Cook, UMemphis) [force] |
(Philip et al., 2010) | 0.60 | 0.0370 | 12 |
| 11720 | Saline control response (10 ml/kg ip), motor coordination, rotarod performance following injection for females [sec] |
(Philip et al., 2010) | −0.65 | 0.0299 | 11 |
| 11977 | Saline control response (10 ml/kg ip), motor coordination, rotarod performance following injection for males and females [sec] |
(Philip et al., 2010) | −0.61 | 0.0454 | 11 |
| 11324 | Morphine response (50 mg/kg ip), locomotion (open field) from 105–120 min after injection in an activity chamber for males |
(Philip et al., 2010) | −0.68 | 0.0185 | 11 |
| 11313 | Morphine response (50 mg/kg ip), locomotion from 105–120 min after injection in an activity chamber for males [n beam breaks] |
(Philip et al., 2010) | −0.64 | 0.0334 | 11 |
| 11325 | Morphine response (50 mg/kg ip), locomotion (open field) from 120–135 min after injection in an activity chamber for males [cm] |
(Philip et al., 2010) | −0.62 | 0.0411 | 11 |
| 11268 | Adrenal zona glomerulosa width for males [um] | (Di Curzio & Goldowitz, 2011) | −0.81 | 0.0002 | 14 |
Variation in pregnenolone levels across the BXD population was linked to behavioral or neurochemical phenotypes previously characterized across these strains by other laboratories and whose data are available in GeneNetwork. GN ID refers to the GeneNetwork BXD phenotype ID for each trait.
Table 4.
Trait correlations between serum 3α,5α-THDOC levels and behavioral or neurochemical phenotypes across the BXD strains.
| GN ID | Phenotypes | Spearman | |||
|---|---|---|---|---|---|
| 17452 | 3α,5α-THDOC in CTL males and females | Reference | r | p | n |
| 17453 | 3α,5α-THDOC in CTL males | Present results | 0.93 | 0.0000 | 18 |
| 12961 | Ethanol response, ethanol intake using a 2-bottle choice test, 2h access after cycle 4 of air exposure only (control for CIE) in vapor chambers (average of 5 days ethanol intake [g/kg/2h]) |
Lopez M. F. et al., Unpublished |
0.75 | 0.0001 | 19 |
| 12963 | Ethanol response, ethanol intake using a 2-bottle choice test, 2 h access after the second cycle of air exposure (control for CIE) in vapor chamber (average of 5 days ethanol intake, young adult males or females [g/kg/2h] |
Jones S. R. et al., Unpublished |
0.56 | 0.0112 | 19 |
| 12810 | Control for ethanol response, 3,4-dihydroxyphenylacetic acid levels (DOPAC) in hindbrain tissue 72 h after the 5th air control cycle of vapor chamber treatment, 16 to 18 week-old males or females (Dec 2009 Cohort 1) [ng/mg] |
Jones S. R. et al., Unpublished |
0.60 | 0.0079 | 18 |
| 12811 | Control for ethanol response, dopamine levels (DA) in hindbrain tissue 72 h after the 5th air control cycle of vapor chamber treatment, 16 to 18 week-old males or females (Dec 2009 Cohort 1) [ng/mg] |
Jones S. R. et al., Unpublished |
0.52 | 0.0265 | 18 |
| 12813 | Control for ethanol response, homovanillic acid (HVA, a dopamine metabolite) level in hindbrain tissue 72 h after the 5th air control cycle of vapor chamber treatment, 16 to 18 week-old males or females (Dec 2009 Cohort 1) [ng/mg] |
Jones S. R. et al., Unpublished |
0.56 | 0.0137 | 18 |
| 12812 | Control for ethanol response, 5-hydroxyindoleacetic acid levels (5-HIAA, a serotonin metabolite) in hindbrain tissue 72h after the 5th air control cycle of vapor chamber treatment, 16 to 18 weeks males or females (Dec 2009 Cohort 1) [ng/mg] |
Jones S. R. et al., Unpublished |
0.56 | 0.0137 | 18 |
| 13576 | Ethanol (20% v/v) consumption using drinking in the dark (DID) method (4 hr access on day 4 of DID) in control females, within-group change (phase 2 minus phase 1) |
Jones B. C. et al., Unpublished |
−0.90 | 0.0008 | 8 |
| 12436 | Anxiety, restraint stress (15 min) and ethanol (1.8 g/kg ip) (RSE group), time in open quadrants using an elevated zero maze in 60 to 120-day-old males only during first 5 min [% time] |
Cook M. et al., Unpublished |
0.56 | 0.0133 | 18 |
| 12448 | Anxiety, restraint stress (15 min) and ethanol (1.8 g/kg ip) (RSE group), time in open quadrants using an elevated zero maze in 60 to 120-day-old males and females during 10 min [% time] |
Cook M. et al., Unpublished |
0.50 | 0.0294 | 19 |
| 12433 | Anxiety, restraint stress (15 min) and ethanol (1.8 g/kg ip) (RSE group), activity in closed quadrants using an elevated zero maze in 60 to 120-day-old females only during last 5 min [beam breaks/sec] |
Cook M. et al., Unpublished |
−0.56 | 0.0151 | 18 |
| 12446 | Anxiety, restraint stress [15 min] and ethanol (1.8 g/kg ip) (RSE group), time in open quadrants using an elevated zero maze in 60 to 120-day-old males and females during first 5 min [% time] |
Cook M. et al., Unpublished |
0.54 | 0.0147 | 19 |
| 12337 | Anxiety, baseline untreated control (BASE group), time in open quadrants using an elevated zero maze in 60 to 120-day-old females only during last 5 min [percentage of time] |
Cook M. et al., Unpublished |
0.51 | 0.0290 | 18 |
| 12357 | Anxiety, baseline untreated control (BASE group), time in open quadrants using an elevated zero maze in 60 to 120-day-old males and females during last 5 min [percentage of time] |
Cook M. et al., Unpublished |
0.59 | 0.0066 | 19 |
| 12338 | Anxiety, baseline untreated control (BASE group), time in open quadrants using an elevated zero maze in 60 to 120-day-old females only during 10 min [percentage of time] |
Cook M. et al., Unpublished |
0.60 | 0.0076 | 18 |
| 12358 | Anxiety, baseline untreated control (BASE group), time in open quadrants using an elevated zero maze in 60 to 120-day-old males and females during 10 min [percentage of time] |
Cook M. et al., Unpublished |
0.48 | 0.0361 | 19 |
| 12347 | Anxiety, baseline untreated control (BASE group), time in open quadrants using an elevated zero maze in 60 to 120-day-old males only during last 5 min [percentage of time] |
Cook M. et al., Unpublished |
0.51 | 0.0274 | 18 |
| 12336 | Anxiety, baseline untreated control (BASE group), time in open quadrants using an elevated zero maze in 60 to 120-day-old females only during first 5 min [percentage of time] |
Cook M. et al., Unpublished |
0.54 | 0.0182 | 18 |
| 12339 | Anxiety, baseline untreated control (BASE group), activity in closed quadrants using an elevated zero maze in 60 to 120-day-old females only during first 5 min [n beam breaks] |
Cook M. et al., Unpublished |
−0.57 | 0.0128 | 18 |
| 11437 | Anxiety, percentage of time in open quadrants of a zero maze for males [%] | (Philip et al., 2010) | 0.90 | 0.0000 | 12 |
| 11439 | Anxiety, time in open quadrants of a zero maze for males [sec] | (Philip et al., 2010) | 0.87 | 0.0000 | 12 |
| 11438 | Anxiety, time in closed quadrants of a zero maze for males [sec] | (Philip et al., 2010) | −0.87 | 0.0000 | 12 |
| 11526 | Novel open field behavior, vertical activity (rears) in the periphery from 0–15 min for males [n beam breaks] | (Philip et al., 2010) | 0.68 | 0.0132 | 12 |
| 11527 | Novel open field behavior, vertical activity (rears) in the periphery from 15–30 min for males [n beam breaks] | (Philip et al., 2010) | 0.72 | 0.0064 | 12 |
| 11533 | Novel open field behavior, vertical activity (rears) in the periphery from 0–60 min for males [n beam breaks] | (Philip et al., 2010) | 0.66 | 0.0163 | 12 |
| 12041 | Novel open field behavior, vertical activity (rears) in the periphery from 15–30 min for males and females [n beam breaks] |
(Philip et al., 2010) | 0.65 | 0.0199 | 12 |
| 11813 | Adult neurogenesis: BrdU-labeled cells in the rostral migratory stream 1 hr after BrdU injection for adult females [density] |
(Philip et al., 2010) | 0.86 | 0.0103 | 7 |
| 17455 | 3α,5α-THDOC in CIE males and females | ||||
| 17456 | 3α,5α-THDOC in CIE males | Present results | 0.78 | 0.0000 | 19 |
| 17454 | 3α,5α-THDOC in CTL females | Present results | 0.66 | 0.0123 | 13 |
| 17305 | 3α,5α-THP in CTL males and females | Present results | 0.57 | 0.0102 | 19 |
| 17449 | 3α,5α-THP in CTL females | Present results | 0.50 | 0.0375 | 17 |
| 17457 | 3α,5α-THDOC in CIE females | Present results | 0.65 | 0.0077 | 15 |
| 11708 | Ethanol response (2.25 g/kg ip), locomotor activity, difference in distance traveled (saline minus ethanol) from 0– 5 min for females [cm] |
(Philip et al., 2010) | −0.65 | 0.0299 | 11 |
| 13576 | Ethanol (20% v/v) consumption using drinking in the dark (DID) method (4 hr access on day 4 of DID) in control females, within-group change (phase 2 minus phase 1) |
Jones B. C. et al., Unpublished |
−0.90 | 0.0008 | 8 |
| 12368 | Anxiety, ethanol treated [1.8 g/kg i.p] (NOE group), time in open quadrants using an elevated zero maze in 60 to 120-day-old females during whole 10 min test [% time] |
Cook M. et al., Unpublished |
0.66 | 0.0023 | 18 |
| 12366 | Anxiety, ethanol treated [1.8 g/kg i.p] (NOE group), time in open quadrants using an elevated zero maze in 60 to 120-day-old females during first 5 min [% time] |
Cook M. et al., Unpublished |
0.62 | 0.0050 | 18 |
| 12438 | Anxiety, restraint stress (15 min) and ethanol (1.8 g/kg ip) (RSE group), time in open quadrants using an elevated zero maze in 60 to 120-day-old males only during 10 min [% time] |
Cook M. et al., Unpublished |
0.52 | 0.0259 | 18 |
| 12436 | Anxiety, restraint stress (15 min) and ethanol (1.8 g/kg ip) (RSE group), time in open quadrants using an elevated zero maze in 60 to 120-day-old males only during first 5 min [% time] |
Cook M. et al., Unpublished |
0.50 | 0.0349 | 18 |
| 10966 | Anxiety (E5_TLA) following restraint stress (15 min) and ethanol injection (1.8 g/kg ip), locomotor activity in light-dark box by 8–12 week-old males during a 5 min session [cm] |
Putman & Miles, Unpublished |
0.82 | 0.0202 | 7 |
| 12357 | Anxiety, baseline untreated control (BASE group), time in open quadrants using an elevated zero maze in 60 to 120-day-old males and females during last 5 min [percentage of time] |
Cook M. et al., Unpublished |
0.63 | 0.0030 | 19 |
| 12347 | Anxiety, baseline untreated control (BASE group), time in open quadrants using an elevated zero maze in 60 to 120-day-old males only during last 5 min [percentage of time] |
Cook M. et al., Unpublished |
0.58 | 0.0108 | 18 |
| 12417 | Anxiety, saline treated [0.18 ml/kg i.p.] (NOS group), time in open quadrants using an elevated zero maze in 60 to 120-day-old males and females during last 5 min [percentage of time] |
Cook M. et al., Unpublished |
0.58 | 0.0082 | 19 |
| 12337 | Anxiety, baseline untreated control (BASE group), time in open quadrants using an elevated zero maze in 60 to 120-day-old females only during last 5 min [percentage of time] |
Cook M. et al., Unpublished |
0.50 | 0.0314 | 18 |
| 12348 | Anxiety, baseline untreated control (BASE group), time in open quadrants using an elevated zero maze in 60 to 120-day-old males only during 10 min [percentage of time] |
Cook M. et al., Unpublished |
0.48 | 0.0439 | 18 |
| 12338 | Anxiety, baseline untreated control (BASE group), time in open quadrants using an elevated zero maze in 60 to 120-day-old females only during 10 min [percentage of time] |
Cook M. et al., Unpublished |
0.50 | 0.0341 | 18 |
| 12407 | Anxiety, saline treated [0.18 ml/kg i.p.] (NOS group), time in open quadrants using an elevated zero maze in 60 to 120-day-old males only during last 5 min [percentage of time] |
Cook M. et al., Unpublished |
0.53 | 0.0193 | 19 |
| 12397 | Anxiety, saline treated (0.18 ml/kg ip, NOS group), time in open quadrants only during last 5 min of test using an elevated zero maze for 60- to 120-day-old females [%] |
Cook M. et al., Unpublished |
0.49 | 0.0368 | 18 |
| 11437 | Anxiety, percentage of time in open quadrants of a zero maze for males [%] | (Philip et al., 2010) | 0.77 | 0.0022 | 12 |
| 11438 | Anxiety, time in closed quadrants of a zero maze for males [sec] | (Philip et al., 2010) | −0.74 | 0.0042 | 12 |
| 11439 | Anxiety, time in open quadrants of a zero maze for males [sec] | (Philip et al., 2010) | 0.74 | 0.0042 | 12 |
| 11952 | Anxiety, time in closed quadrants of a zero maze for males and females [sec] | (Philip et al., 2010) | −0.66 | 0.0181 | 12 |
| 11953 | Anxiety, time in open quadrants of a zero maze for males and females [sec] | (Philip et al., 2010) | 0.66 | 0.0181 | 12 |
| 11951 | Anxiety, percentage time in open quadrants of a zero maze for males and females [%] | (Philip et al., 2010) | 0.64 | 0.0219 | 12 |
| 11353 | Novel open field behavior, locomotion in the center from 0–60 min for males [n beam breaks] | (Philip et al., 2010) | 0.60 | 0.0499 | 11 |
| 11920 | Open field behavior, time in corners for males and females [min] | (Philip et al., 2010) | −0.59 | 0.0433 | 12 |
| 11853 | Naloxone-induced morphine withdrawal, naloxone (30 mg/kg ip) after morphine (50 mg/kg dose ip), change in locomotion for males and females (last 15 min of morphine test (165–180 min) minus first 15 min after naloxone) [cm difference] |
(Philip et al., 2010) | −0.74 | 0.0042 | 12 |
| 11328 | Morphine response (50 mg/kg ip), locomotion (open field) from 165–180 min after injection in an activity chamber for males [cm] |
(Philip et al., 2010) | −0.87 | 0.0001 | 11 |
| 11842 | Morphine response (50 mg/kg ip), locomotion (open field) from 165–180 min after injection in an activity chamber for males and females [cm] |
(Philip et al., 2010) | −0.73 | 0.0049 | 12 |
| 11831 | Morphine response (50 mg/kg ip), locomotion from 165–180 min after injection in an activity chamber for males and females [n beam breaks] |
(Philip et al., 2010) | −0.72 | 0.0064 | 12 |
| 11317 | Morphine response (50 mg/kg ip), locomotion from 165–180 min after injection in an activity chamber for males [n beam breaks] |
(Philip et al., 2010) | −0.82 | 0.0011 | 11 |
| 11339 | Naloxone-induced morphine withdrawal, naloxone (30 mg/kg ip) after morphine (50 mg/kg dose ip), change in locomotion for males (last 15 min of morphine test (165–180 min) minus first 15 min after naloxone) [cm difference] |
(Philip et al., 2010) | −0.78 | 0.0030 | 11 |
| 11596 | Naloxone-induced morphine withdrawal, naloxone (30 mg/kg ip) after morphine (50 mg/kg dose ip), change in locomotion for females (last 15 min of morphine test (165–180 min) minus first 15 min after naloxone) [cm difference] |
(Philip et al., 2010) | −0.73 | 0.0056 | 12 |
| 11583 | Morphine response (50 mg/kg ip), locomotion (open field) from 0–15 min after injection in an activity chamber for females [cm] |
(Philip et al., 2010) | −0.76 | 0.0031 | 12 |
| 11327 | Morphine response (50 mg/kg ip), locomotion (open field) from 150–165 min after injection in an activity chamber for males [cm] |
(Philip et al., 2010) | −0.69 | 0.0162 | 11 |
| 11841 | Morphine response (50 mg/kg ip), locomotion (open field) from 150–165 min after injections in an activity chamber for males and females [cm] |
(Philip et al., 2010) | −0.69 | 0.0118 | 12 |
| 11830 | Morphine response (50 mg/kg ip), locomotion from 150–165 min after injection in an activity chamber for males and females [n beam breaks] |
(Philip et al., 2010) | −0.69 | 0.0118 | 12 |
| 11316 | Morphine response (50 mg/kg ip), locomotion from 150–165 min after injection in an activity chamber for males [n beam breaks] |
(Philip et al., 2010) | −0.65 | 0.0267 | 11 |
| 11585 | Morphine response (50 mg/kg ip), locomotion (open field) from 165–180 min after injection in an activity chamber for females [cm] |
(Philip et al., 2010) | −0.76 | 0.0031 | 12 |
| 11572 | Morphine response (50 mg/kg ip), locomotion from 0–15 min after injection in an activity chamber for females [n beam breaks] |
(Philip et al., 2010) | −0.64 | 0.0219 | 12 |
| 11627 | Morphine response (50 mg/kg ip), vertical activity (rears) from 15–30 min after injection in an activity chamber for females [n beam breaks] |
(Philip et al., 2010) | −0.67 | 0.0147 | 12 |
| 11574 | Morphine response (50 mg/kg ip), locomotion from 165–180 min after injection in an activity chamber for females [n beam breaks] |
(Philip et al., 2010) | −0.70 | 0.0094 | 12 |
| 12890 | Activity of 13-week old females, total counts of fine movements and ambulatory activity [counts/unit time] | (Andreux et al., 2012) | 1.00 | 0.0000 | 6 |
Variation in 3α,5α-THDOC levels across the BXD population was linked to behavioral or neurochemical phenotypes previously characterized across these strains by other laboratories and whose data are available in GeneNetwork. GN ID refers to the GeneNetwork BXD phenotype ID for each trait.
In agreement with the lack of correlation between average steroid levels and ethanol consumption in BXD mouse strains in the present study, variation in 3α,5α-THP, 3α,5α-THDOC, and pregnenolone levels did not correlate with ethanol consumption or other ethanol-related behaviors previously collected by other groups (see extensive data curated in GeneNetwork). However, a few exceptions were noted for measures of ethanol consumption using the drinking in the dark paradigm. For example, 3α,5α-THP levels in CTLs co-vary with change in ethanol consumption (Jones B. C. et al., unpublished, GeneNetwork BXD phenotype ID 13576, Spearman r = –0.86, p = 0.004, n = 8; Table 3). 3α,5α-THP levels in CIE cases were positively correlated with ethanol consumption (Jones, B. C. et al., unpublished, GeneNetwork BXD phenotype ID 13565, Spearman r = 0.71, p = 0.045, n = 8; Table 3). Finally, 3α,5α-THP levels in CTL female cases were negatively correlated with ethanol consumption (GeneNetwork BXD phenotype ID 13576, Spearman r = −0.95, p = 0.00003, n = 8; data not shown). Correlations such as these, based on small sample size, should probably only be used to motivate possible validation studies.
Table 3.
Trait correlations between serum 3α,5α-THP levels and behavioral or neurochemical phenotypes across the BXD strains.
| GN ID | Phenotypes | Spearman | |||
|---|---|---|---|---|---|
| 17305 | 3α,5α-THP in CTL males and females | Reference | r | p | n |
| 17448 | 3α,5α-THP in CTL males | Present results | 0.90 | 0.0000 | 18 |
| 17449 | 3α,5α-THP in CTL females | Present results | 0.66 | 0.0030 | 17 |
| 17306 | 3α,5α-THP in CIE males and females | Present results | 0.69 | 0.0007 | 19 |
| 17450 | 3α,5α-THP in CIE males | Present results | 0.74 | 0.0001 | 19 |
| 17456 | 3α,5α-THDOC in CIE males | Present results | 0.77 | 0.0001 | 19 |
| 17455 | 3α,5α-THDOC in CIE males and females | Present results | 0.57 | 0.0100 | 19 |
| 17458 | Pregnenolone in CTL males and females | Present results | 0.50 | 0.0320 | 18 |
| 13565 | Ethanol (20% v/v) consumption using drinking in the dark (DID) method (4 hr access on day 4 of DID) in females, baseline in normally housed group, control for chronic mild stress group (Phase 1) [g/kg] |
Jones B. C. et al., Unpublished |
0.75 | 0.0299 | 8 |
| 13576 | Ethanol (20% v/v) consumption using drinking in the dark (DID) method (4 hr access on day 4 of DID) in control females, within-group change (phase 2 minus phase 1) |
Jones B. C. et al., Unpublished |
−0.86 | 0.0041 | 8 |
| 12979 | Alanine transaminase level in serum following alcohol (6 g/kg via gastric gavage) at 24 hours after gavage in young adult males and females |
Rooney R. J. et al., Unpublished |
−0.75 | 0.0004 | 16 |
| 12985 | Alanine transaminase level in serum following alcohol (6 g/kg via gastric gavage) at 24 hours after gavage in young adult males |
Rooney R. J. et al., Unpublished |
−0.59 | 0.0143 | 16 |
| 12438 | Anxiety, restraint stress (15 min) and ethanol (1.8 g/kg ip) (RSE group), time in open quadrants using an elevated zero maze in 60 to 120-day-old males only during 10 min [% time] |
Cook M. et al., Unpublished |
0.69 | 0.0011 | 18 |
| 12436 | Anxiety, restraint stress (15 min) and ethanol (1.8 g/kg ip) (RSE group), time in open quadrants using an elevated zero maze in 60 to 120-day-old males only during first 5 min [% time] |
Cook M. et al., Unpublished |
0.62 | 0.0046 | 18 |
| 12366 | Anxiety, ethanol treated [1.8 g/kg i.p] (NOE group), time in open quadrants using an elevated zero maze in 60 to 120-day-old females during first 5 min [% time] |
Cook M. et al., Unpublished |
0.49 | 0.0359 | 18 |
| 12437 | Anxiety, restraint stress (15 min) and ethanol (1.8 g/kg ip) (RSE group), time in open quadrants using an elevated zero maze in 60 to 120-day-old males only during last 5 min [% time] |
Cook M. et al., Unpublished |
0.54 | 0.0199 | 18 |
| 11454 | Anxiety, untreated baseline, entries into closed arms of a plus maze for males [n] | (Philip et al., 2010) | 0.72 | 0.0106 | 11 |
| 12337 | Anxiety, baseline untreated control (BASE group), time in open quadrants using an elevated zero maze in 60 to 120-day-old females only during last 5 min [percentage of time] |
Cook M. et al., Unpublished |
0.70 | 0.0008 | 18 |
| 12339 | Anxiety, baseline untreated control (BASE group), activity in closed quadrants using an elevated zero maze in 60 to 120-day-old females only during first 5 min [n beam breaks] |
Cook M. et al., Unpublished |
−0.51 | 0.0298 | 18 |
| 12357 | Anxiety, baseline untreated control (BASE group), time in open quadrants using an elevated zero maze in 60 to 120-day-old males and females during last 5 min [percentage of time] |
Cook M. et al., Unpublished |
0.68 | 0.0008 | 19 |
| 12338 | Anxiety, baseline untreated control (BASE group), time in open quadrants using an elevated zero maze in 60 to 120-day-old females only during 10 min [percentage of time] |
Cook M. et al., Unpublished |
0.61 | 0.0063 | 18 |
| 12345 | Anxiety, baseline untreated control (BASE group), latency to enter an open quadrant using an elevated zero maze in 60 to 120-day-old males only [sec] |
Cook M. et al., Unpublished |
0.69 | 0.0009 | 18 |
| 12336 | Anxiety, baseline untreated control (BASE group), time in open quadrants using an elevated zero maze in 60 to 120-day-old females only during first 5 min [percentage of time] |
Cook M. et al., Unpublished |
0.58 | 0.0108 | 18 |
| 12358 | Anxiety, baseline untreated control (BASE group), time in open quadrants using an elevated zero maze in 60 to 120-day-old males and females during 10 min [percentage of time] |
Cook M. et al., Unpublished |
0.53 | 0.0193 | 19 |
| 12359 | Anxiety, baseline untreated control (BASE group), activity in closed quadrants using an elevated zero maze in 60 to 120-day-old males and females during first 5 min [n beam breaks] |
Cook M. et al., Unpublished |
−0.48 | 0.0353 | 19 |
| 12417 | Anxiety, saline treated [0.18 ml/kg i.p.] (NOS group), time in open quadrants using an elevated zero maze in 60 to 120-day-old males and females during last 5 min [percentage of time] |
Cook M. et al., Unpublished |
0.71 | 0.0004 | 19 |
| 12347 | Anxiety, baseline untreated control (BASE group), time in open quadrants using an elevated zero maze in 60 to 120-day-old males only during last 5 min [percentage of time] |
Cook M. et al., Unpublished |
0.56 | 0.0142 | 19 |
| 12407 | Anxiety, saline treated [0.18 ml/kg i.p.] (NOS group), time in open quadrants using an elevated zero maze in 60 to 120-day-old males only during last 5 min [percentage of time] |
Cook M. et al., Unpublished |
0.55 | 0.0132 | 19 |
| 12418 | Anxiety, saline treated [0.18 ml/kg i.p.] (NOS group), time in open quadrants using an elevated zero maze in 60 to 120-day-old males and females during 10 min [percentage of time] |
Cook M. et al., Unpublished |
0.63 | 0.0028 | 19 |
| 11404 | Open field behavior, percentage of distance in the center for males [%] | (Philip et al., 2010) | −0.67 | 0.0147 | 12 |
| 11419 | Open field behavior, percentage of distance in the perimeter for males [%] | (Philip et al., 2010) | 0.67 | 0.0147 | 12 |
| 11918 | Open field behavior, percentage of distance in the center for males and females [%] | (Philip et al., 2010) | −0.66 | 0.0163 | 12 |
| 11933 | Open field behavior, percentage of distance in the perimeter for males and females [%] | (Philip et al., 2010) | 0.66 | 0.0163 | 12 |
| 11661 | Open field behavior, percentage of distance in the center for females [%] | (Philip et al., 2010) | −0.59 | 0.0433 | 12 |
| 11676 | Open field behavior, percentage distance in the perimeter for females [%] | (Philip et al., 2010) | 0.59 | 0.0433 | 12 |
| 11937 | Acoustic startle response, maximum response at 70 db for males and females (65 dB background noise, Hamilton-Kinder SM100 startle chamber, 10 msec interval, 120 dB startle stimulus, prepulse PPI control data by M Cook, UMemphis) [force] |
(Philip et al., 2010) | −0.72 | 0.0064 | 12 |
| 11425 | Acoustic startle response, maximum response at 85 db for males (65 dB background noise, Hamilton-Kinder SM100 startle chamber, 10 msec interval, 120 dB startle stimulus, prepulse PPI control data by M Cook, UMemphis) [force] |
(Philip et al., 2010) | −0.83 | 0.0003 | 12 |
| 11939 | Acoustic startle response, maximum response at 85 db for males and females (65 dB background noise, Hamilton-Kinder SM100 startle chamber, 10 msec interval, 120 dB startle stimulus, prepulse PPI control data by M Cook, UMemphis) [force] |
(Philip et al., 2010) | −0.83 | 0.0004 | 12 |
| 11423 | Acoustic startle response, maximum response at 70 db for males (65 dB background noise, Hamilton-Kinder SM100 startle chamber, 10 msec interval, 120 dB startle stimulus, prepulse PPI control data by M Cook, UMemphis) [force] |
(Philip et al., 2010) | −0.73 | 0.0056 | 12 |
| 11424 | Acoustic startle response, maximum response at 80 db for males (65 dB background noise, Hamilton-Kinder SM100 startle chamber, 10 msec interval, 120 dB startle stimulus, prepulse PPI control data by M Cook, UMemphis) [force] |
(Philip et al., 2010) | −0.74 | 0.0042 | 12 |
| 11938 | Acoustic startle response, maximum response at 80 db for males and females (65 dB background noise, Hamilton-Kinder SM100 startle chamber, 10 msec interval, 120 dB startle stimulus, prepulse PPI control data by M Cook, UMemphis) [force] |
(Philip et al., 2010) | −0.80 | 0.0011 | 12 |
| 11011 | Fear conditioning, freezing response to context after 48 hours [%] | (Brigman et al., 2009) | −0.89 | 0.0041 | 7 |
| 11823 | Pain response, mechanical nociception, tail clip latency for males and females [sec] | (Philip et al., 2010) | 0.62 | 0.0288 | 12 |
| 11816 | Pain sensitivity, vocalization threshold to mild foot shock for males and females [mA] | (Philip et al., 2010) | 0.63 | 0.0263 | 12 |
| 11269 | Adrenal zona glomerulosa width for females [µm] | (Di Curzio & Goldowitz, 2011) | 0.66 | 0.0083 | 14 |
| 17306 | 3α,5α-THP in CIE males and females | ||||
| 17450 | 3α,5α-THP in CIE males | Present results | 0.84 | 0.0000 | 19 |
| 17448 | 3α,5α-THP in CTL males | Present results | 0.75 | 0.0002 | 18 |
| 17305 | 3α,5α-THP in CTL males and females | Present results | 0.69 | 0.0007 | 19 |
| 17451 | 3α,5α-THP in CIE females | Present results | 0.63 | 0.0080 | 16 |
| 13565 | Ethanol (20% v/v) consumption using drinking in the dark (DID) method (4 hr access on day 4 of DID) in females, baseline in normally housed group, control for chronic mild stress group (Phase 1) [g/kg] |
Jones B. C. et al., Unpublished |
0.71 | 0.0452 | 8 |
| 12369 | Anxiety, ethanol treated [1.8 g/kg i.p] (NOE group), activity in closed quadrants using an elevated zero maze in 60 to 120-day-old females only during first 5 min [n beam breaks] |
Cook M. et al., Unpublished |
−0.70 | 0.0007 | 18 |
| 12371 | Anxiety, ethanol treated [1.8 g/kg i.p] (NOE group), activity in closed quadrants using an elevated zero maze in 60 to 120-day-old females only during 10 min [n beam breaks] |
Cook M. et al., Unpublished |
−0.74 | 0.0002 | 18 |
| 12374 | Anxiety, ethanol treated (1.8 g/kg ip) (NOE group), activity in closed quadrants using an elevated zero maze in 60 to 120-day-old females only during 10 min [beam breaks/sec] |
Cook M. et al., Unpublished |
0.58 | 0.0101 | 18 |
| 12370 | Anxiety, ethanol treated [1.8 g/kg i.p] (NOE group), activity in closed quadrants using an elevated zero maze in 60 to 120-day-old females only during last 5 min [n beam breaks] |
Cook M. et al., Unpublished |
−0.72 | 0.0004 | 18 |
| 12373 | Anxiety, ethanol treated [1.8 g/kg i.p] (NOE group), activity in closed quadrants using an elevated zero maze in 60 to 120-day-old females only during last 5 min [beam breaks/sec] |
Cook M. et al., Unpublished |
0.58 | 0.0104 | 18 |
| 12389 | Anxiety, ethanol treated [1.8 g/kg i.p] (NOE group), activity in closed quadrants using an elevated zero maze in 60 to 120-day-old males and females during first 5 min [n beam breaks] |
Cook M. et al., Unpublished |
−0.47 | 0.0421 | 19 |
| 12396 | Anxiety, saline treated [0.18 ml/kg i.p.] (NOS group), time in open quadrants using an elevated zero maze in 60 to 120-day-old females only during first 5 min [percentage of time] |
Cook M. et al., Unpublished |
0.66 | 0.0019 | 18 |
| 12337 | Anxiety, baseline untreated control (BASE group), time in open quadrants using an elevated zero maze in 60 to 120-day-old females only during last 5 min [percentage of time] |
Cook M. et al., Unpublished |
0.56 | 0.0151 | 18 |
| 12418 | Anxiety, saline treated [0.18 ml/kg i.p.] (NOS group), time in open quadrants using an elevated zero maze in 60 to 120-day-old males and females during 10 min [percentage of time] |
Cook M. et al., Unpublished |
0.56 | 0.0114 | 19 |
| 12398 | Anxiety, saline treated [0.18 ml/kg i.p.] (NOS group), time in open quadrants using an elevated zero maze in 60 to 120-day-old females only during 10 min [percentage of time] |
Cook M. et al., Unpublished |
0.60 | 0.0070 | 18 |
| 12359 | Anxiety, baseline untreated control (BASE group), activity in closed quadrants using an elevated zero maze in 60 to 120-day-old males and females during first 5 min [n beam breaks] |
Cook M. et al., Unpublished |
−0.57 | 0.0096 | 19 |
| 12417 | Anxiety, saline treated [0.18 ml/kg i.p.] (NOS group), time in open quadrants using an elevated zero maze in 60 to 120-day-old males and females during last 5 min [percentage of time] |
Cook M. et al., Unpublished |
0.63 | 0.0033 | 19 |
| 12345 | Anxiety, baseline untreated control (BASE group), latency to enter an open quadrant using an elevated zero maze in 60 to 120-day-old males only [sec] |
Cook M. et al., Unpublished |
0.59 | 0.0094 | 18 |
| 12397 | Anxiety, saline treated (0.18 ml/kg ip, NOS group), time in open quadrants only during last 5 min of test using an elevated zero maze for 60- to 120-day-old females [%] |
Cook M. et al., Unpublished |
0.53 | 0.0231 | 18 |
| 12339 | Anxiety, baseline untreated control (BASE group), activity in closed quadrants using an elevated zero maze in 60 to 120-day-old females only during first 5 min [n beam breaks] |
Cook M. et al., Unpublished |
−0.50 | 0.0332 | 18 |
| 12361 | Anxiety, baseline untreated control (BASE group), activity in closed quadrants using an elevated zero maze in 60 to 120-day-old males and females during 10 min [n beam breaks] |
Cook M. et al., Unpublished |
−0.52 | 0.0219 | 19 |
| 11455 | Anxiety, untreated baseline, entries into open arms of a plus maze for males [n] | (Philip et al., 2010) | 0.63 | 0.0371 | 11 |
| 11969 | Anxiety, untreated baseline, entries into open arms of a plus maze for males and females [n] | (Philip et al., 2010) | 0.72 | 0.0106 | 11 |
| 11936 | Acoustic startle response, maximum response at 120 db for males and females (65 dB background noise, Hamilton-Kinder SM100 startle chamber, 10 msec interval, 120 dB startle stimulus, prepulse PPI control data by M Cook, UMemphis) [force] |
(Philip et al., 2010) | −0.68 | 0.0132 | 12 |
| 11937 | Acoustic startle response, maximum response at 70 db for males and females (65 dB background noise, Hamilton-Kinder SM100 startle chamber, 10 msec interval, 120 dB startle stimulus, prepulse PPI control data by M Cook, UMemphis) [force] |
(Philip et al., 2010) | −0.67 | 0.0147 | 12 |
| 11679 | Acoustic startle response, maximum response at 120 db for females (65 dB background noise, Hamilton-Kinder SM100 startle chamber, 10 msec interval, 120 dB startle stimulus, prepulse PPI control data by M Cook, UMemphis) [force] |
(Philip et al., 2010) | −0.59 | 0.0433 | 12 |
| 11422 | Acoustic startle response, maximum response at 120 db for males (65 dB background noise, Hamilton-Kinder SM100 startle chamber, 10 msec interval, 120 dB startle stimulus, prepulse PPI control data by M Cook, UMemphis) [force] |
(Philip et al., 2010) | −0.64 | 0.0219 | 12 |
| 11423 | Acoustic startle response, maximum response at 70 db for males (65 dB background noise, Hamilton-Kinder SM100 startle chamber, 10 msec interval, 120 dB startle stimulus, prepulse PPI control data by M Cook, UMemphis) [force] |
(Philip et al., 2010) | −0.61 | 0.0341 | 12 |
| 11938 | Acoustic startle response, maximum response at 80 db for males and females (65 dB background noise, Hamilton-Kinder SM100 startle chamber, 10 msec interval, 120 dB startle stimulus, prepulse PPI control data by M Cook, UMemphis) [force] |
(Philip et al., 2010) | −0.64 | 0.0241 | 12 |
| 11424 | Acoustic startle response, maximum response at 80 db for males (65 dB background noise, Hamilton-Kinder SM100 startle chamber, 10 msec interval, 120 dB startle stimulus, prepulse PPI control data by M Cook, UMemphis) [force] |
(Philip et al., 2010) | −0.62 | 0.0313 | 12 |
| 11295 | Adrenal zona reticularis width for males [µm] | (Di Curzio & Goldowitz, 2011) | 0.59 | 0.0257 | 14 |
Variation in 3α,5α-THP levels across the BXD population was linked to behavioral or neurochemical phenotypes previously characterized across these strains by other laboratories and whose data are available in GeneNetwork. GN ID refers to the GeneNetwork BXD phenotype ID for each trait.
We also found that variation in pregnenolone levels in CTL cases was positively correlated with ethanol consumption using a two-bottle choice test (Lopez et al., unpublished, GeneNetwork BXD phenotype ID 12579; Spearman r = 0.87, p = 0.001, n = 9; Table 2). Likewise, 3α,5α-THDOC levels in the CTL group were positively correlated with ethanol intake in a two-bottle choice procedure (Lopez et al., unpublished, GeneNetwork BXD phenotype ID 12961; Spearman r = 0.75, p = 0.0001, n = 19; Jones, S. R. et al., unpublished, GeneNetwork BXD phenotype ID 12963; Spearman r = 0.56, p = 0.01, n = 19; Table 4). 3α,5α-THDOC levels in CTL males were also positively correlated with ethanol intake in a two-bottle choice procedure (Lopez et al., unpublished, GeneNetwork BXD phenotype ID 12961; Spearman r = 0.82, p = 0.000008, n = 18; Jones, S. R. et al., unpublished, GeneNetwork BXD phenotype ID 12963; Spearman r = 0.63, p = 0.004, n = 14; data not shown). However, ethanol consumption reported in GeneNetwork BXD phenotype IDs 12579, 12961, and 12963 is limited to strains from cohort 1 of this same study and does not include the other cohorts that were examined as part of this study (see also Lopez et al. this issue for more details).
Variation in neuroactive steroid levels was also positively correlated with phenotypes of anxiety-like behavior; in general, strains with higher neuroactive steroids levels are less anxious, regardless of CTL or CIE treatment (Tables 2–4), in agreement with the anxiolytic-like properties of GABAergic neuroactive steroids (Porcu et al., 2016). Neuroactive steroid levels are altered in schizophrenia, and administration of pregnenolone ameliorated symptoms in patients (Marx et al., 2009). In agreement, pregnenolone levels in CIE strains are positively correlated with pre-pulse inhibition responses (Table 2); by contrast, 3α,5α-THP levels in both CTL and CIE strains are negatively correlated with pre-pulse inhibition responses (Table 3). 3α,5α-THP exerts analgesic effects (Kavaliers & Wiebe, 1987), and in agreement, 3α,5α-THP levels in CTL strains are positively correlated with pain response, i.e., strains with higher levels are less sensitive to pain (Table 3). Finally, pregnenolone levels in both CTL and CIE strains, and 3α,5α-THDOC levels in CIE strains are negatively correlated with behavioral sensitivity to morphine (Tables 2 & 4), i.e., higher levels of steroids decrease sensitivity to morphine and to naloxone-induced morphine withdrawal, suggesting that neuroactive steroids may contribute to behavioral effects of this drug of abuse, in line with previous evidence of increased levels of neuroactive steroids following morphine administration and its withdrawal (Concas et al., 2006).
Finally, correlation analysis with phenotypes on GeneNetwork revealed that pregnenolone, 3α,5α-THP, and 3α,5α-THDOC levels in CTL or CIE cases, reported in the present study, did not always correlate with each other. For instance, in CTL cases, pregnenolone levels were positively correlated with 3α,5α-THP levels (Spearman r = 0.50, p = 0.0032, n = 18; Tables 2 & 3); however, 3α,5α-THDOC levels did not correlate with either pregnenolone or 3α,5α-THP levels (data not shown). In CIE cases, no correlations were found among either steroid. Other significant correlations were found when taking into account male or female cases only. For example, pregnenolone levels in all CTL cases were positively correlated with its levels in CTL male cases as well as in CTL female cases; moreover, they were also correlated with 3α,5α-THP levels in CTL male cases. Pregnenolone levels in all CIE cases were positively correlated with pregnenolone levels in CIE male cases and CIE female cases (see Table 2 for details). 3α,5α-THP levels in all CTL cases were positively correlated with those in CTL male cases, CTL female cases, all CIE cases, and CIE male cases. Moreover, 3α,5α-THP levels in all CTL cases were positively correlated with 3α,5α-THDOC levels in all CIE cases, and CIE male cases. 3α,5α-THP levels in all CIE cases were positively correlated with those in CIE male cases, as well as with those in all CTL cases, and CTL male cases (see Table 3 for details). 3α,5α-THDOC levels in all CTL cases were positively correlated with those in CTL male cases. Likewise, 3α,5α-THDOC levels in all CIE cases were positively correlated with those in CIE male cases and CIE female cases, as well as with those in CTL female cases. 3α,5α-THDOC levels in all CIE cases were also positively correlated with 3α,5α-THP levels in all CTL cases, and CTL female cases (see Table 4 for details).
Discussion
This study reports an exploratory analysis of the regulation of ethanol-induced levels of the neuroactive steroids 3α,5α-THP, 3α,5α-THDOC, and pregnenolone. This study also examined variation of these steroids with voluntary ethanol consumption in the test mice as well as behavioral phenotypes previously determined across the BXD strains by several independent labs. The design of this CIE study is unusual in that we have studied several strains and both sexes, but without deep replication within strain. In this respect, our work is more like an analysis of a human family or a cohort of nonhuman primates. The majority of studies using the CIE protocol in mice involves analysis of 6 to 12 cases across a single genotype – almost always the reference C57BL/6 strain or a knockout line. In contrast, we have studied a substantially larger number of cases (~140 per group) and 19 distinct genotypes. This design was intended as an initial survey that would provide a more robust and, we hope, ultimately replicable gauge of the range of variation in response to CIE treatment. We can compute heritabilities with some confidence (as in a twin study), but we cannot make strong claims about differences between specific strains. The latter would require deeper replication. Evidence for heritability of the steroid levels was in the range of 0.20–0.30; heritability estimates would have been more accurate with a larger sample size (animals and strains). However, heritability estimates are usually “confounded” by one or more factors, the most important one being environmental factors. Thus, our estimates should still be unbiased.
No strong QTLs were found for any of the steroids measured. Because levels of these steroids are controlled by the expression of multiple genes involved in biosynthesis and degradation, it is possible that QTLs for individual steroids are unlikely to be detectable or informative. Alternatively, we lacked sufficient genetic variation in the 19 BXD strains tested to detect QTLs. The use of substantially more BXD strains or a more diverse genetic reference panel may uncover genes controlling levels of neuroactive steroids. Further, there was no evidence that genetic variation in levels of these steroids was related to ethanol consumption or changes in drinking after CIE vs. air exposure, possibly due to the same limitations just mentioned. Nonetheless, individual variation in 3α,5α-THP levels was consistently found to be inversely related to ethanol consumption in all males and females, as well as male cases only.
Serum 3α,5α-THP levels in both CTL and CIE-exposed individual mice were negatively correlated with respective ethanol intake, such that lower 3α,5α-THP levels were associated with higher ethanol intake. This result is in agreement with a recent study showing that 3α,5α-THP immunoreactivity in the lateral and basolateral amygdala of cynomolgus monkeys is also negatively correlated with average daily ethanol intake, such that 3α,5α-THP immunoreactivity was associated with higher daily ethanol intake (Beattie et al., 2016). Overall, these findings support the hypothesis that elevated 3α,5α-THP levels in response to ethanol may protect against excessive drinking and the risk for alcohol dependence (Morrow et al., 2006; Morrow & Porcu, 2009; Porcu & Morrow, 2014). However, this finding appears to be specific to 3α,5α-THP, as similar correlations were not observed for 3α,5α-THDOC and pregnenolone levels.
The evidence that different suggestive QTLs were identified for pregnenolone and 3α,5α-THP levels and that no QTLs were identified for 3α,5α-THDOC levels points to a different genetic regulation of the synthesis of these neuroactive steroids following ethanol exposure, in agreement with the hypothesis that ethanol may differentially target selected neurosteroidogenic enzymes (Morrow et al., 2006; Porcu & Morrow, 2014). Suggestive QTLs for pregnenolone levels are observed on chromosomes 3 and X in all CTL cases and on chromosomes 5, 6, and X in all CIE cases, suggesting that CIE exposure changes genetic regulation of this neuroactive steroid. Likewise, a suggestive QTL for 3α,5α-THP levels is observed on chromosome 3 in CIE cases, whereas no QTLs were observed in CTL cases, once again, pointing to a different genetic regulation of neuroactive steroid synthesis following CIE exposure. Overall, these results suggest that genetic variation in all traits is polygenic and that at least two and probably four or more loci contribute to the heritable variation. However, further studies are needed for a sufficient genetic analysis and to identify more specific loci of genetic control. Indeed, the results of this study are limited by the small sample size, which does not allow resolution of QTLs with sufficient power or precision to seriously entertain candidate gene analysis (Belknap, 1998; Wang et al., 2014). It is likely that a sample size of 40 or more strains would begin to resolve single locus effects, and since there are now >100 BXD strains it should certainly be possible to define one or more candidate genes that underlie the heritable variation detected in this study. Furthermore, other mouse mapping populations, with greater recombination than the BXD panel, may uncover genes controlling neuroactive steroid levels. Moreover, the combination of genomics and bioinformatics approaches will also help to more accurately map single QTLs and any associated candidate genes (Putman et al., 2016).
Blood samples for neuroactive steroid assays were obtained during withdrawal (72 h after the last CIE exposure). We cannot rule out the possibility that genetic regulation of neuroactive steroids would have been different immediately after ethanol exposure vs. withdrawal. For instance, changes in 3α,5α-THP immunoreactivity following CIE exposure in C57BL/6J mice differ with respect to time of ethanol withdrawal: a decrease in 3α,5α-THP immunoreactivity was reported in the central nucleus of the amygdala at 8-h withdrawal only, while a decrease in medial prefrontal cortex, nucleus accumbens core, and dorsolateral striatum, as well as an increase in CA3 hippocampal area, were present only at 72-h withdrawal (Maldonado-Devincci et al., 2014).
CIE exposure is a form of chronic stress (Becker, 2012). Neuroactive steroid levels are extremely sensitive to stress (Porcu & Morrow, 2014), and exposure to chronic stress per se may have influenced the genetic regulation of their levels.
Using 47 BXD strains, we previously reported variation in basal DOC levels, a progesterone metabolite and immediate precursor of 3α,5α-THDOC. QTLs on chromosomes 4 and 14 were identified for basal DOC levels in cerebral cortex and plasma, respectively (Porcu et al., 2011). In the present study, we only identified suggestive QTLs for pregnenolone and for 3α,5α-THP serum levels, but none for 3α,5α-THDOC. The total number of strains examined in the present study (19) is smaller, compared to the previous one (47) (Porcu et al., 2011), a fact that may have limited the power for QTL detection using recombinant inbred strains (Belknap, 1998; Wang et al., 2014). Furthermore, in the previous study we looked at baseline neuroactive steroid levels in naïve mice (Porcu et al., 2011), as opposed to mice with an extensive history of ethanol exposure following five CIE cycles, which may have likely influenced genetic regulation of neuroactive steroid levels.
Variation in basal DOC levels across the BXD strains was linked to several behavioral phenotypes previously characterized in these strains, including increased ethanol-induced sedation, ethanol-induced ataxia, and ethanol-induced corticosterone levels (Porcu et al., 2011). In the present study, variation in 3α,5α-THP, 3α,5α-THDOC, and pregnenolone levels in CTL and/or CIE cases was not linked to behavioral phenotypes of ethanol sensitivity and consumption, previously determined in these strains by several groups, in agreement with the lack of correlation between average steroid levels and average ethanol consumption in the same strains of the present study. The few exceptions we found of correlations between variation in 3α,5α-THP levels and ethanol consumption using the drinking in the dark paradigm (Table 3), are limited by having only 8 female strains in common. Likewise, trait correlations between variation in pregnenolone or 3α,5α-THDOC levels and ethanol intake in the two-bottle choice paradigm are limited to strains from cohort 1 of the Lopez et al. study (see Lopez et al., this issue). Thus, differences in the experimental procedures used to assess ethanol consumption and in the BXD strains examined may account for this discrepancy. Overall, genetic variation in serum neuroactive steroid levels does not seem to correlate with behavioral phenotypes of ethanol sensitivity across the BXD strains (Porcu et al., 2010, 2014). However, acute ethanol-induced changes in cerebral cortical 3α,5α-THP appear to be related to the genetic background of the strain (Porcu & Morrow, 2014), suggesting that genetic regulation of ethanol-induced neuroactive steroid synthesis may play some role in ethanol’s behavioral effects.
Variation in 3α,5α-THP, 3α,5α-THDOC, and pregnenolone levels was also linked to other behavioral phenotypes, including anxiety, pre-pulse inhibition, pain sensitivity, and adrenal size, in agreement with the evidence that neuroactive steroids exert anxiolytic and analgesic effects, and their levels are altered in several psychiatric disorders involving stress and anxiety (Porcu et al., 2016). Finally, variation in pregnenolone and 3α,5α-THDOC levels was linked to behavioral sensitivity to morphine. This result is consistent with evidence that other drugs of abuse share with ethanol the ability to increase brain levels of neuroactive steroids, and that neuroactive steroids may contribute to sensitivity to drugs of abuse (Porcu et al., 2016). Indeed, morphine administration increases brain and plasma levels of neuroactive steroids in male Sprague-Dawley rats, as well as male C57BL/6J and DBA/2J mice (Concas et al., 2006; Porcu et al., 2014).
Males and females show different susceptibility to the effects of drugs of abuse. With respect to ethanol, C57BL/6J and DBA/2J mice show sex differences in ethanol’s discriminative stimulus effects (Shannon, Porcu, Purdy, & Grant, 2005) and in ethanol consumption, with female C57BL/6J mice consuming more ethanol than males (Finn, Beckley, Kaufman, & Ford, 2010; Melón, Wray, Moore, & Boehm, 2013). In the present study, we observed that 3α,5α-THDOC levels were lower in females than males in both CTL and CIE cases, suggesting that CIE exposure did not influence levels of this steroid. However, a significant variation in 3α,5α-THP levels was observed in both CTL and CIE cases, when grouping males and females together and also in males, but not females, only. Moreover, 3α,5α-THP levels were significantly correlated with average ethanol intake in both CTL and CIE male and female cases together, as well as in males only, but not in females only (Fig. 4). These observations suggest that variation in the levels of these steroids, and correlations between 3α,5α-THP levels and ethanol intake, were mainly driven by males, especially given that p values for the female-only group are quite high (approximately 0.5). 3α,5α-THP levels in female mice vary in relation to the estrus cycle phase (Corpéchot et al., 1997), and estrus cycle was not monitored in our experiments. We cannot rule out the possibility that the different estrus cycle phases may have influenced these results. Future studies are needed to explore putative sex differences in the ethanol-induced genetic regulation of neuroactive steroids.
In conclusion, we have reported an exploratory analysis of variation in serum levels of the neuroactive steroids 3α,5α-THP, 3α,5α-THDOC, and pregnenolone across the BXD population subjected to CIE exposure. Genetic variation in the levels of these steroids is linked to behavioral phenotypes of anxiety sensitivity, suggesting that neuroactive steroids may contribute to such sensitivity. Moreover, individual variation in 3α,5α-THP levels is inversely related to ethanol consumption under both control and CIE conditions. Future studies including a large number of strains are required to examine the hypotheses identified in this exploratory analysis and may identify the networks of genes involved in regulation of ethanol-induced neurosteroidogenesis.
Supplementary Material
Mice from each genotype received four cycles of chronic intermittent ethanol (CIE) vapor exposure (CIE group) or air exposure (CTL group) (16 h/day × 4 days, followed by 72-h withdrawal), alternated with 5-day drinking test cycles using a two-bottle (15% v/v ethanol vs. water) limited access (2 h/day) drinking model. Blood samples for 3α,5α-THP assay were collected 72 h after a fifth CIE or air exposure cycle. (A) Likelihood ratio statistic (LRS) scores for 3α,5α-THP levels in all CTL cases (GeneNetwork BXD phenotype ID 17305) across the entire genome. (B) LRS scores for 3α,5α-THP levels in all CIE cases (GeneNetwork BXD phenotype ID 17306) across the entire genome show a suggestive QTL on chromosome 3 (LRS of 12). The y-axis and the thick blue lines provide the LRS of the association between the trait and the genotypes of markers. The two horizontal lines are the suggestive (gray) and significance (red) thresholds computed using 5000 permutations. A positive additive coefficient (green line) indicates that D alleles increase trait values. A negative additive coefficient (red line) indicates that B alleles increase trait values.
Mice from each genotype received four cycles of chronic intermittent ethanol (CIE) vapor exposure (CIE group) or air exposure (CTL group) (16 h/day × 4 days, followed by 72-h withdrawal), alternated with 5-day drinking test cycles using a two-bottle (15% v/v ethanol vs. water) limited access (2 h/day) drinking model. Blood samples for 3α,5α-THDOC assay were collected 72 h after a fifth CIE or air exposure cycle. (A) Likelihood ratio statistic (LRS) scores for 3α,5α-THDOC levels in all CTL cases (GeneNetwork BXD phenotype ID 17452) across the entire genome. (B) LRS scores for 3α,5α-THDOC levels in all CIE cases (GeneNetwork BXD phenotype ID 17455) across the entire genome. The y-axis and the thick blue lines provide the LRS of the association between the trait and the genotypes of markers. The two horizontal lines are the suggestive (gray) and significance (red) thresholds computed using 5000 permutations. A positive additive coefficient (green line) indicates that D alleles increase trait values. A negative additive coefficient (red line) indicates that B alleles increase trait values.
Mice from each genotype received four cycles of chronic intermittent ethanol (CIE) vapor exposure (CIE group) or air exposure (CTL group) (16 h/day × 4 days, followed by 72-h withdrawal), alternated with 5-day drinking test cycles using a two-bottle (15% v/v ethanol vs. water) limited access (2 h/day) drinking model. Blood samples for 3α,5α-THDOC assay were collected 72 h after a fifth CIE or air exposure cycle. Likelihood ratio statistic (LRS) scores across the entire genome for 3α,5α-THDOC levels in (A) CTL male cases (GeneNetwork BXD phenotype ID 17453), (B) CIE male cases (GeneNetwork BXD phenotype ID 17456), (C) CTL female cases (GeneNetwork BXD phenotype ID 17454), and (D) CIE female cases (GeneNetwork BXD phenotype ID 17457). The y-axis and the thick blue lines provide the LRS of the association between the trait and the genotypes of markers. The two horizontal lines are the suggestive (gray) and significance (red) thresholds computed using 5000 permutations. A positive additive coefficient (green line) indicates that D alleles increase trait values. A negative additive coefficient (red line) indicates that B alleles increase trait values.
Highlights.
CIE induces marked variation in neuroactive steroid levels across the BXD cohort of mice.
3α,5α-THP levels correlate negatively with ethanol intake in CTL and CIE conditions.
Variation in neuroactive steroids is linked to phenotypes of anxiety sensitivity.
Acknowledgments
This study was supported by the National Institutes of Health (NIH) grants UO1 AA020935 (ALM), U01 AA020929 (MFL), U01 AA014095 (HCB), and P50 AA010761 (HCB). GeneNetwork and the production and genotyping of BXD strains were supported by INIA grants U01 AA016662, U01 AA013499, U24 AA013513, U01 AA014425, NIH Human Brain Project grant P20-DA 21131, and the UTHSC Center for Integrative and Translational Genomics (RWW).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andreux PA, Williams EG, Koutnikova H, Houtkooper RH, Champy MF, Henry H, et al. Systems genetics of metabolism: the use of the BXD murine reference panel for multiscalar integration of traits. Cell. 2012;150:1287–1299. doi: 10.1016/j.cell.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beattie MC, Maldonado-Devincci AM, Porcu P, O’Buckley TK, Daunais JB, Grant KA, et al. Voluntary ethanol consumption reduces GABAergic neuroactive steroid (3α,5α)3-hydroxypregnan-20-one (3α,5α-THP) in the amygdala of the cynomolgus monkey. Addiction Biology. 2016 doi: 10.1111/adb.12326. Epub Dec 2, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker HC. Effects of alcohol dependence and withdrawal on stress responsiveness and alcohol consumption. Alcohol Research: Current Reviews. 2012;34:448–458. [PMC free article] [PubMed] [Google Scholar]
- Becker HC, Lopez MF. Increased ethanol drinking after repeated chronic ethanol exposure and withdrawal experience in C57BL/6 mice. Alcoholism: Clinical and Experimental Research. 2004;28:1829–1838. doi: 10.1097/01.alc.0000149977.95306.3a. [DOI] [PubMed] [Google Scholar]
- Belknap JK. Effect of within-strain sample size on QTL detection and mapping using recombinant inbred mouse strains. Behavior Genetics. 1998;28:29–38. doi: 10.1023/a:1021404714631. [DOI] [PubMed] [Google Scholar]
- Besheer J, Lindsay TG, O'Buckley TK, Hodge CW, Morrow AL. Pregnenolone and ganaxolone reduce operant ethanol self-administration in alcohol-preferring p rats. Alcoholism: Clinical and Experimental Research. 2010;34:2044–2052. doi: 10.1111/j.1530-0277.2010.01300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitran D, Hilvers RJ, Kellogg CK. Anxiolytic effects of 3alpha-hydroxy-5 alpha[beta]-pregnan-20-one: endogenous metabolites of progesterone that are active at the GABAA receptor. Brain Research. 1991;561:157–161. doi: 10.1016/0006-8993(91)90761-j. [DOI] [PubMed] [Google Scholar]
- Boehm SL, 2nd, Reed CL, McKinnon CS, Phillips TJ. Shared genes influence sensitivity to the effects of ethanol on locomotor and anxiety-like behaviors, and the stress axis. Psychopharmacology (Berl) 2002;161:54–63. doi: 10.1007/s00213-002-1000-y. [DOI] [PubMed] [Google Scholar]
- Brigman JL, Mathur P, Lu L, Williams RW, Holmes A. Genetic relationship between anxiety-related and fear-related behaviors in BXD recombinant inbred mice. Behavioural Pharmacology. 2009;20:204–209. doi: 10.1097/FBP.0b013e32830c368c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesler EJ, Lu L, Shou S, Qu Y, Gu J, Wang J, et al. Complex trait analysis of gene expression uncovers polygenic and pleiotropic networks that modulate nervous system function. Nature Genetics. 2005;37:233–242. doi: 10.1038/ng1518. [DOI] [PubMed] [Google Scholar]
- Concas A, Sogliano C, Porcu P, Marra C, Brundu A, Biggio G. Neurosteroids in nicotine and morphine dependence. Psychopharmacology (Berl) 2006;186:281–292. doi: 10.1007/s00213-005-0111-7. [DOI] [PubMed] [Google Scholar]
- Cook JB, Dumitru AM, O'Buckley TK, Morrow AL. Ethanol administration produces divergent changes in GABAergic neuroactive steroid immunohistochemistry in the rat brain. Alcoholism: Clinical and Experimental Research. 2014;38:90–99. doi: 10.1111/acer.12223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corpéchot C, Collins BE, Carey MP, Tsouros A, Robel P, Fry JP. Brain neurosteroids during the mouse oestrous cycle. Brain Research. 1997;766:276–280. doi: 10.1016/s0006-8993(97)00749-x. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Phillips TJ, Buck KJ, Cunningham CL, Belknap JK. Identifying genes for alcohol and drug sensitivity: recent progress and future directions. Trends in Neurosciences. 1999;22:173–179. doi: 10.1016/s0166-2236(99)01393-4. [DOI] [PubMed] [Google Scholar]
- Di Curzio DL, Goldowitz D. The genetic basis of adrenal gland weight and structure in BXD recombinant inbred mice. Mammalian Genome. 2011;22:209–234. doi: 10.1007/s00335-011-9315-9. [DOI] [PubMed] [Google Scholar]
- Doerge RW, Churchill GA. Permutation tests for multiple loci affecting a quantitative character. Genetics. 1996;142:285–294. doi: 10.1093/genetics/142.1.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn DA, Beckley EH, Kaufman KR, Ford MM. Manipulation of GABAergic steroids: Sex differences in the effects on alcohol drinking- and withdrawal-related behaviors. Hormones and Behavior. 2010;57:12–22. doi: 10.1016/j.yhbeh.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gora-Maslak G, McClearn GE, Crabbe JC, Phillips TJ, Belknap JK, Plomin R. Use of recombinant inbred strains to identify quantitative trait loci in psychopharmacology. Psychopharmacology (Berl) 1991;104:413–424. doi: 10.1007/BF02245643. [DOI] [PubMed] [Google Scholar]
- Griffin WC, 3rd, Lopez MF, Becker HC. Intensity and duration of chronic ethanol exposure is critical for subsequent escalation of voluntary ethanol drinking in mice. Alcoholism: Clinical and Experimental Research. 2009;33:1893–1900. doi: 10.1111/j.1530-0277.2009.01027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin WC, 3rd, Lopez MF, Yanke AB, Middaugh LD, Becker HC. Repeated cycles of chronic intermittent ethanol exposure in mice increases voluntary ethanol drinking and ethanol concentrations in the nucleus accumbens. Psychopharmacology (Berl) 2009;201:569–580. doi: 10.1007/s00213-008-1324-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hager R, Lu L, Rosen GD, Williams RW. Genetic architecture supports mosaic brain evolution and independent brain-body size regulation. Nature Communications. 2012;3:1079. doi: 10.1038/ncomms2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavaliers M, Wiebe JP. Analgesic effects of the progesterone metabolite, 3 alpha-hydroxy-5 alpha-pregnan-20-one, and possible modes of action in mice. Brain Research. 1987;415:393–398. doi: 10.1016/0006-8993(87)90228-9. [DOI] [PubMed] [Google Scholar]
- Khisti RT, Boyd KN, Kumar S, Morrow AL. Systemic ethanol administration elevates deoxycorticosterone levels and chronic ethanol exposure attenuates this response. Brain Research. 2005;1049:104–111. doi: 10.1016/j.brainres.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Lander E, Kruglyak L. Genetic dissection of complex traits: guidelines for interpreting and reporting linkage results. Nature Genetics. 1995;11:241–247. doi: 10.1038/ng1195-241. [DOI] [PubMed] [Google Scholar]
- Lopez MF, Becker HC. Effect of pattern and number of chronic ethanol exposures on subsequent voluntary ethanol intake in C57BL/6J mice. Psychopharmacology (Berl) 2005;181:688–696. doi: 10.1007/s00213-005-0026-3. [DOI] [PubMed] [Google Scholar]
- Lopez MF, Griffin WF, 3rd, Melendez RI, Becker HC. Repeated cycles of chronic intermittent ethanol exposure leads to the development of tolerance to aversive effects of ethanol in C57BL/6J mice. Alcoholism: Clinical and Experimental Research. 2012;36:1180–1187. doi: 10.1111/j.1530-0277.2011.01717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado-Devincci AM, Cook JB, O'Buckley TK, Morrow DH, McKinley RE, Lopez MF, et al. Chronic intermittent ethanol exposure and withdrawal alters (3alpha,5alpha)-3-hydroxy-pregnan-20-one immunostaining in cortical and limbic brain regions of C57BL/6J mice. Alcoholism: Clinical and Experimental Research. 2014;38:2561–2571. doi: 10.1111/acer.12530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx CE, Keefe RS, Buchanan RW, Hamer RM, Kilts JD, Bradford DW, et al. Proof-of-concept trial with the neurosteroid pregnenolone targeting cognitive and negative symptoms in schizophrenia. Neuropsychopharmacology. 2009;34:1885–1903. doi: 10.1038/npp.2009.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melón LC, Wray KN, Moore EM, Boehm SL., 2nd Sex and age differences in heavy binge drinking and its effects on alcohol responsivity following abstinence. Pharmacology, Biochemistry, and Behavior. 2013;104:177–187. doi: 10.1016/j.pbb.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow AL, Porcu P. Neuroactive steroid biomarkers of alcohol sensitivity and alcoholism risk. In: Ritsner MS, editor. The Handbook of Neuropsychiatric Biomarkers, Endophenotypes, and Genes. Vol. 3. Dordrecht: Springer Science + Business Media B. V; 2009. pp. 47–57. [Google Scholar]
- Morrow AL, Porcu P, Boyd KN, Grant KA. Hypothalamic-pituitary-adrenal axis modulation of GABAergic neuroactive steroids influences ethanol sensitivity and drinking behavior. Dialogues in Clinical Neuroscience. 2006;8:463–477. doi: 10.31887/DCNS.2006.8.4/amorrow. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Dell LE, Purdy RH, Covey DF, Richardson HN, Roberto M, Koob GF. Epipregnanolone and a novel synthetic neuroactive steroid reduce alcohol self-administration in rats. Pharmacology, Biochemistry, and Behavior. 2005;81:543–550. doi: 10.1016/j.pbb.2005.03.020. [DOI] [PubMed] [Google Scholar]
- Philip VM, Duvvuru S, Gomero B, Ansah TA, Blaha CD, Cook MN, et al. High-throughput behavioral phenotyping in the expanded panel of BXD recombinant inbred strains. Genes, Brain, and Behavior. 2010;9:129–159. doi: 10.1111/j.1601-183X.2009.00540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porcu P, Barron AM, Frye CA, Walf AA, Yang SY, He XY, et al. Neurosteroidogenesis Today: Novel Targets for Neuroactive Steroid Synthesis and Action and Their Relevance for Translational Research. Journal of Neuroendocrinology. 2016;28:12351. doi: 10.1111/jne.12351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porcu P, Grant KA, Green HL, Rogers LS, Morrow AL. Hypothalamic-pituitary-adrenal axis and ethanol modulation of deoxycorticosterone levels in cynomolgus monkeys. Psychopharmacology (Berl) 2006;186:293–301. doi: 10.1007/s00213-005-0132-2. [DOI] [PubMed] [Google Scholar]
- Porcu P, Locci A, Santoru F, Berretti R, Morrow AL, Concas A. Failure of acute ethanol administration to alter cerebrocortical and hippocampal allopregnanolone levels in C57BL/6J and DBA/2J mice. Alcoholism: Clinical and Experimental Research. 2014;38:948–958. doi: 10.1111/acer.12329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porcu P, Morrow AL. Divergent neuroactive steroid responses to stress and ethanol in rat and mouse strains: relevance for human studies. Psychopharmacology (Berl) 2014;231:3257–3272. doi: 10.1007/s00213-014-3564-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porcu P, O'Buckley TK, Alward SE, Marx CE, Shampine LJ, Girdler SS, et al. Simultaneous quantification of GABAergic 3alpha,5alpha/3alpha,5beta neuroactive steroids in human and rat serum. Steroids. 2009;74:463–473. doi: 10.1016/j.steroids.2008.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porcu P, O'Buckley TK, Alward SE, Song SC, Grant KA, de Wit H, et al. Differential effects of ethanol on serum GABAergic 3alpha,5alpha/3alpha,5beta neuroactive steroids in mice, rats, cynomolgus monkeys, and humans. Alcoholism: Clinical and Experimental Research. 2010;34:432–442. doi: 10.1111/j.1530-0277.2009.01123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porcu P, O'Buckley TK, Morrow AL, Adinoff B. Differential hypothalamic-pituitary-adrenal activation of the neuroactive steroids pregnenolone sulfate and deoxycorticosterone in healthy controls and alcohol-dependent subjects. Psychoneuroendocrinology. 2008;33:214–226. doi: 10.1016/j.psyneuen.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porcu P, O'Buckley TK, Song SC, Harenza JL, Lu L, Wang X, et al. Genetic analysis of the neurosteroid deoxycorticosterone and its relation to alcohol phenotypes: identification of QTLs and downstream gene regulation. PLoS One. 2011;6:e18405. doi: 10.1371/journal.pone.0018405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putman AH, Wolen AR, Harenza JL, Yordanova RK, Webb BT, Chesler EJ, et al. Identification of quantitative trait loci and candidate genes for an anxiolytic-like response to ethanol in BXD recombinant inbred strains. Genes, Brain, and Behavior. 2016;15:367–381. doi: 10.1111/gbb.12289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS, Kulkarni SK. Differential anxiolytic effects of neurosteroids in the mirrored chamber behavior test in mice. Brain Research. 1997;752:61–71. doi: 10.1016/s0006-8993(96)01447-3. [DOI] [PubMed] [Google Scholar]
- Rosen GD, Chesler EJ, Manly KF, Williams RW. An informatics approach to systems neurogenetics. Methods in Molecular Biology. 2007;401:287–303. doi: 10.1007/978-1-59745-520-6_16. [DOI] [PubMed] [Google Scholar]
- Schuckit MA. Low level of response to alcohol as a predictor of future alcoholism. The American Journal of Psychiatry. 1994;151:184–189. doi: 10.1176/ajp.151.2.184. [DOI] [PubMed] [Google Scholar]
- Schuckit MA. An overview of genetic influences in alcoholism. Journal of Substance Abuse Treatment. 2009;36:S5–S14. [PubMed] [Google Scholar]
- Serra M, Pisu MG, Floris I, Cara V, Purdy RH, Biggio G. Social isolation-induced increase in the sensitivity of rats to the steroidogenic effect of ethanol. Journal of Neurochemistry. 2003;85:257–263. doi: 10.1046/j.1471-4159.2003.01680.x. [DOI] [PubMed] [Google Scholar]
- Shannon EE, Porcu P, Purdy RH, Grant KA. Characterization of the discriminative stimulus effects of the neuroactive steroid pregnanolone in DBA/2J and C57BL/6J inbred mice. The Journal of Pharmacology and Experimental Therapeutics. 2005;314:675–685. doi: 10.1124/jpet.104.082644. [DOI] [PubMed] [Google Scholar]
- VanDoren MJ, Matthews DB, Janis GC, Grobin AC, Devaud LL, Morrow AL. Neuroactive steroid 3alpha-hydroxy-5alpha-pregnan-20-one modulates electrophysiological and behavioral actions of ethanol. The Journal of Neuroscience. 2000;20:1982–1989. doi: 10.1523/JNEUROSCI.20-05-01982.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Williams RW, Manly KF. WebQTL: web-based complex trait analysis. Neuroinformatics. 2003;1:299–308. doi: 10.1385/NI:1:4:299. [DOI] [PubMed] [Google Scholar]
- Wang L, Jiao Y, Cao Y, Liu G, Wang Y, Gu W. Limitation of number of strains and persistence of false positive loci in QTL mapping using recombinant inbred strains. PLoS One. 2014;9:e102307. doi: 10.1371/journal.pone.0102307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Pandey AK, Mulligan MK, Williams EG, Mozhui K, Li Z, et al. Joint mouse-human phenome-wide association to test gene function and disease risk. Nature Communications. 2016;7:10464. doi: 10.1038/ncomms10464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieland S, Lan NC, Mirasedeghi S, Gee KW. Anxiolytic activity of the progesterone metabolite 5alpha-pregnan-3alpha-o1-20-one. Brain Research. 1991;565:263–268. doi: 10.1016/0006-8993(91)91658-n. [DOI] [PubMed] [Google Scholar]
- Wilhelmsen KC, Schuckit M, Smith TL, Lee JV, Segall SK, Feiler HS, et al. The search for genes related to a low-level response to alcohol determined by alcohol challenges. Alcoholism: Clinical and Experimental Research. 2003;27:1041–1047. doi: 10.1097/01.ALC.0000075551.02714.63. [DOI] [PubMed] [Google Scholar]
- Williams EG, Wu Y, Jha P, Dubuis S, Blattmann P, Argmann CA, et al. Systems proteomics of liver mitochondria function. Science. 2016;352:aad0189. doi: 10.1126/science.aad0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RW, Gu J, Qi S, Lu L. The genetic structure of recombinant inbred mice: high-resolution consensus maps for complex trait analysis. Genome Biology. 2001;2 doi: 10.1186/gb-2001-2-11-research0046. RESEARCH0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RW, Strom RC, Rice DS, Goldowitz D. Genetic and environmental control of variation in retinal ganglion cell number in mice. The Journal of Neuroscience. 1996;16:7193–7205. doi: 10.1523/JNEUROSCI.16-22-07193.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Mice from each genotype received four cycles of chronic intermittent ethanol (CIE) vapor exposure (CIE group) or air exposure (CTL group) (16 h/day × 4 days, followed by 72-h withdrawal), alternated with 5-day drinking test cycles using a two-bottle (15% v/v ethanol vs. water) limited access (2 h/day) drinking model. Blood samples for 3α,5α-THP assay were collected 72 h after a fifth CIE or air exposure cycle. (A) Likelihood ratio statistic (LRS) scores for 3α,5α-THP levels in all CTL cases (GeneNetwork BXD phenotype ID 17305) across the entire genome. (B) LRS scores for 3α,5α-THP levels in all CIE cases (GeneNetwork BXD phenotype ID 17306) across the entire genome show a suggestive QTL on chromosome 3 (LRS of 12). The y-axis and the thick blue lines provide the LRS of the association between the trait and the genotypes of markers. The two horizontal lines are the suggestive (gray) and significance (red) thresholds computed using 5000 permutations. A positive additive coefficient (green line) indicates that D alleles increase trait values. A negative additive coefficient (red line) indicates that B alleles increase trait values.
Mice from each genotype received four cycles of chronic intermittent ethanol (CIE) vapor exposure (CIE group) or air exposure (CTL group) (16 h/day × 4 days, followed by 72-h withdrawal), alternated with 5-day drinking test cycles using a two-bottle (15% v/v ethanol vs. water) limited access (2 h/day) drinking model. Blood samples for 3α,5α-THDOC assay were collected 72 h after a fifth CIE or air exposure cycle. (A) Likelihood ratio statistic (LRS) scores for 3α,5α-THDOC levels in all CTL cases (GeneNetwork BXD phenotype ID 17452) across the entire genome. (B) LRS scores for 3α,5α-THDOC levels in all CIE cases (GeneNetwork BXD phenotype ID 17455) across the entire genome. The y-axis and the thick blue lines provide the LRS of the association between the trait and the genotypes of markers. The two horizontal lines are the suggestive (gray) and significance (red) thresholds computed using 5000 permutations. A positive additive coefficient (green line) indicates that D alleles increase trait values. A negative additive coefficient (red line) indicates that B alleles increase trait values.
Mice from each genotype received four cycles of chronic intermittent ethanol (CIE) vapor exposure (CIE group) or air exposure (CTL group) (16 h/day × 4 days, followed by 72-h withdrawal), alternated with 5-day drinking test cycles using a two-bottle (15% v/v ethanol vs. water) limited access (2 h/day) drinking model. Blood samples for 3α,5α-THDOC assay were collected 72 h after a fifth CIE or air exposure cycle. Likelihood ratio statistic (LRS) scores across the entire genome for 3α,5α-THDOC levels in (A) CTL male cases (GeneNetwork BXD phenotype ID 17453), (B) CIE male cases (GeneNetwork BXD phenotype ID 17456), (C) CTL female cases (GeneNetwork BXD phenotype ID 17454), and (D) CIE female cases (GeneNetwork BXD phenotype ID 17457). The y-axis and the thick blue lines provide the LRS of the association between the trait and the genotypes of markers. The two horizontal lines are the suggestive (gray) and significance (red) thresholds computed using 5000 permutations. A positive additive coefficient (green line) indicates that D alleles increase trait values. A negative additive coefficient (red line) indicates that B alleles increase trait values.