Abstract
Background
Outcomes after surgical pulmonary valve replacement (PVR) in patients with congenital cardiac disease are limited by long-term valve deterioration, which may be hastened by turbulent flow. The use of the Trifecta valve (St. Jude Medical, Little Canada, MN) at our institution (Duke University Medical Center, Durham, NC) appears to result in low postimplantation transvalvular gradients. This study was performed to compare the early transvalvular gradient associated with the Trifecta valve with that associated with two other valves commonly used for PVR.
Methods
We performed a single institution review of patients undergoing PVR with the Perimount valve (Edwards Lifesciences, Irvine, CA), the Biocor valve (St. Jude Medical), or the Trifecta valve between November 1993 and January 2014. Multivariable linear regression modeling was used to determine the adjusted association between valve type and transvalvular gradient as determined by early postoperative echocardiography.
Results
A total of 186 patients met study criteria; 54 (29%) received a Biocor valve, 87 (47%) received a Perimount valve, and 45 (24%) received a Trifecta valve. There were no baseline differences among the groups, but the peak transvalvular gradient was significantly decreased among patients with the Trifecta valve. After adjustment for age, valve size, patients’ weight, and time to the assessment, as compared with the Trifecta valve, the Biocor valve was associated with a 57% higher peak valve gradient (p < 0.01), whereas the Perimount valve was associated with a 26% higher peak valve gradient (p = 0.04).
Conclusions
PVR for congenital heart disease with the Trifecta bioprosthetic valve is associated with a reduced early transvalvular gradient. This finding may be associated with reduced valve deterioration over time.
Right ventricular outflow tract reconstruction is a part of many operations for congenital heart disease. Unfortunately, pulmonary stenosis, pulmonary insufficiency, or both are a common long-term complication of this procedure [1]. Because continually increasing numbers of patients survive through childhood after surgical repair of congenital heart operations, growing numbers of patients will present with this complication again in the future [2]. Changes in recommendations have led to an increase in the number of patients for whom pulmonary valve replacement (PVR) is indicated because PVR has been shown to improve both right and left ventricular function, as well as lead to a reduction in symptoms [3, 4]. Despite excellent outcomes after PVR in this population (including 1 and 5-year mortality rates of 1% and 2%, respectively), long-term outcomes are unfortunately limited by pulmonary valve deterioration, which can reach 25% by 5 years [5].
It has been hypothesized that turbulent flow may increase the rate of pulmonary valve deterioration. This is supported by findings that younger patients, and patients with oversized valves, tend to have higher rates of pulmonary valve deterioration [5–7]. At our institution (Duke University Medical Center, Durham, NC), we have used several bioprosthetic valves for PVR in patients with congenital heart disease. Recently, we have used the Perimount valve (Edwards Lifesciences, Irvine, CA) and the Biocor valve (St. Jude Medical, Little Canada, MN). Beginning in 2011, the Trifecta aortic pericardial valve (St. Jude Medical) became our preferred bioprosthesis because of our perception that this valve was associated with a reduced transvalvular gradient in the immediate postoperative period. We speculated that using a Trifecta valve for PVR could lead to delayed long-term valve deterioration, a finding also seen among adult patients receiving this valve for aortic valve replacement [8]. In addition, we also began using this valve because of its larger effective orifice area, which may facilitate easier “valve-in-valve” percutaneous valve replacement if needed at a later date. We performed the present study to formally evaluate the transvalvular gradient associated with the Trifecta valve, as well as compare it with two other devices with which we have substantial experience. We hypothesized that the Trifecta valve would have significantly reduced postoperative transvalvular gradients as compared with the Perimount and Biocor valves.
Patients and Methods
Patient Population
The study was performed as a retrospective chart review. Patients who received the Perimount valve, the Biocor valve (including patients who received both the Biocor porcine bioprosthesis and its later iteration, the Epic porcine bioprosthesis), or the Trifecta valve at a single tertiary care institution from November 1993 to January 2014 for PVR in congenital heart disease were included. Patients were excluded if they died within 30 days of the operative procedure because these patients were unable to receive a postoperative echocardiogram to assess valve performance. The institutional review board at Duke University Medical Center approved this study before data extraction.
Operative Procedure
The details of our methods of PVR in patients with congenital heart disease have previously been described [6]. In brief, PVR is performed using cardiopulmonary bypass (at normothermia or with mild hypothermia). The pulmonary annulus is visualized through a longitudinal arteriotomy or incision in the conduit, sometimes extending slightly into the right ventricle. The largest valve prosthesis that will reasonably fit within the outflow tract (with the caveat of not excessively oversizing in younger patients) is selected. A patch arterioplasty is used as needed to allow placement of the appropriately sized prosthesis (at least 25 mm in all adults) without deformation of the valve stent posts. If there is a concern that external compression of the valve may lead to deformity, the valve is implanted more distally, or less commonly proximally, to reduce this risk. If the valve is implanted more proximally in the right ventricular outflow tract, the possibility of right coronary artery compression is considered. Running polypropylene suture without pledgets is used to secure the valve in place. The pulmonary artery is closed with selective use of a patch arterioplasty. Patients are instructed to take aspirin for 3 months after the procedure to reduce the risk of thrombotic or embolic events. After this, anticoagulation and antiplatelet therapy are given at the discretion of the cardiologist.
Variables
Patients’ characteristics including age, sex, race, diagnosis, and weight at the time of intervention were extracted, as well as operative characteristics including valve type, valve size, and operative time. The indexed valve size was also determined by dividing the valve diameter by the weight of each patient. We acknowledge that the traditional indexed valve size is determined by body surface area and not by weight; however, because of the retrospective nature of this study and the variables recorded in the medical records, we were unable to determine body surface area for some of the subjects in this study and therefore substituted weight as a surrogate. The transvalvular gradient according to the first postoperative echocardiogram was recorded, along with the time from the PVR to the imaging study. The first postoperative echocardiogram was chosen to avoid confounding the data by the variable follow-up times for each valve.
Statistical Analysis
Baseline characteristics were compared among groups using the χ2 test or Fisher’s exact test as appropriate for categorical variables and the Kruskal-Wallis test for continuous variables. Multivariable linear regression was then performed to determine the adjusted association between valve type and transvalvular gradient. Variables included in this model were determined a priori based on clinical significance and included age, valve size, weight at the time of operation, and time from operation to echocardiographic assessment. Model assumptions were tested and transformations were used as needed to ensure that all assumptions were met. Two sensitivity analyses were also performed in which heart rate and hemoglobin near the time of echocardiography were included in the model in place of the time from the procedure to the echocardiogram, to ensure that the variation in time to this imaging was not responsible for the variation in peak valve gradient as a result of variations in cardiopulmonary physiology related to the proximity to the operation. Finally, to determine whether the effective valve orifice was responsible for the differences in peak transvalvular gradients, a final model was created in which valve size was replaced with the internal valve area indexed to the patient’s weight. A p value of 0.05 was used to define statistical significance. Complete case analysis was used for all adjusted models. All statistical analyses were performed with R software (version 3.2.1, R Foundation for Statistical Computing, Vienna, Austria).
Results
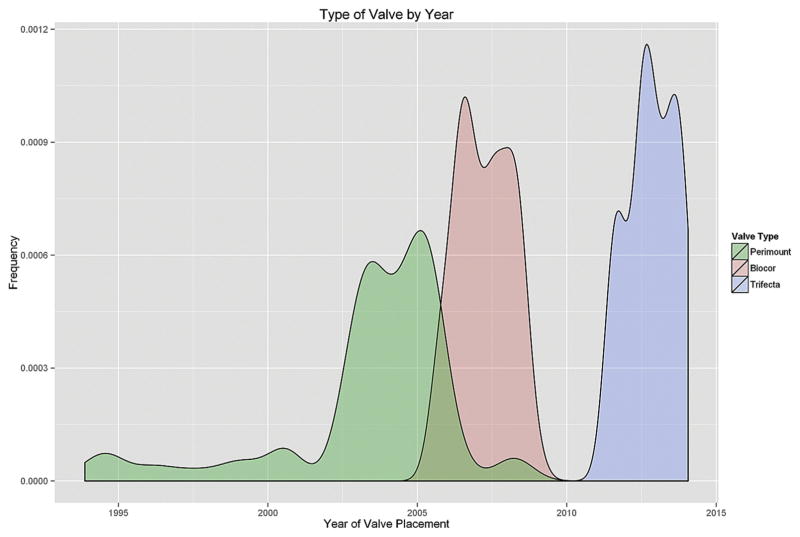
A total of 186 patients met study criteria, of whom 54 (29%) received a Biocor valve, 87 (47%) received a Perimount valve, and 45 (24%) received a Trifecta valve (Fig 1). The median age at PVR was 19 years (interquartile range [IQR], 11, 39). The median valve size was 27 mm (IQR, 24, 27). The majority of patients had a baseline diagnosis of tetralogy of Fallot (114; 61%).
Fig 1.
Use of the Biocor (St. Jude Medical, Little Canada, MN), Perimount (Edwards Lifesciences, Irvine, CA), and Trifecta (St. Jude Medical) valves at our institution (Duke University Medical Center, Durham, NC) over time.
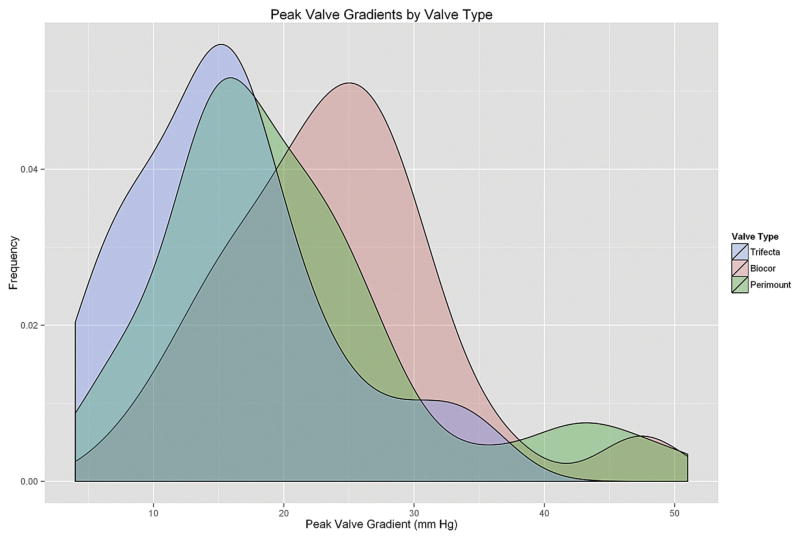
There were no significant differences in age, sex, weight at the time of operation, indexed valve size, diagnosis, or valve size among groups (Table 1). There was a significant difference in operative time, with a median time of 336 minutes (IQR, 273, 372) for the Trifecta valve as compared with only 240 minutes (IQR, 184, 300) and 236 minutes (IQR, 185, 270) for the Biocor and Perimount valves, respectively (p < 0.01). There was also a significant difference in the peak valve gradient seen on postoperative echocardiography (Fig 2). The median peak valve gradient of the Trifecta valve was 16 mm Hg (IQR, 10, 20) as compared with 24 mm Hg (IQR,18, 28) and 18 mm Hg (IQR, 14, 24) for the Biocor and Perimount valves, respectively (p < 0.01).
Table 1.
Patient-Related and Operative Characteristics by Valve Typea
| Variable | Overall | Trifecta (St. Jude) | Biocor (St. Jude) | Perimount (Edwards Lifesciences) | p Value |
|---|---|---|---|---|---|
| Number | 186 | 45 (24%) | 54 (29%) | 87 (47%) | ... |
| Age (years) | 18.7 (11, 39) | 26 (12, 46) | 20 (14, 36) | 17 (10, 37) | 0.22 |
| Female sex | 71 (38%) | 17 (38%) | 17 (32%) | 35 (40%) | 0.58 |
| Weight at operation (kg) | 60 (40, 78) | 73 (40, 85) | 61 (50, 78) | 58 (33, 75) | 0.19 |
| Diagnosis | 0.48 | ||||
| Tetralogy of Fallot | 115 (61%) | 33 (73%) | 32 (59%) | 49 (56%) | |
| Truncus arteriosus | 6 (3.2%) | 1 (2.2%) | 1 (1.9%) | 4 (4.6%) | |
| Pulmonary stenosis | 16 (8.5%) | 4 (8.9%) | 3 (5.6%) | 9 (10%) | |
| Pulmonary atresia with VSD | 11 (5.9%) | 0 (0.0%) | 3 (5.6%) | 8 (9.2%) | |
| Pulmonary atresia without VSD | 8 (4.3%) | 1 (2.2%) | 3 (5.6%) | 4 (4.6%) | |
| Other | 32 (17%) | 6 (13%) | 12 (22%) | 13 (15%) | |
| Valve size (mm) | 27 (23, 27) | 27 (25, 27) | 27 (25, 27) | 25 (23, 27) | 0.87 |
| Indexed valve size (mm/Kg) | 0.43 (0.34, 0.60) | 0.37 (0.32, 0.54) | 0.43 (0.34, 0.52) | 0.45 (0.35, 0.64) | 0.21 |
| Cardiopulmonary bypass time (min) | 102 (73, 135) | 106 (76, 129) | 107 (76, 136) | 96 (69, 133) | 0.48 |
| Operative time (min) | 251 (202, 331) | 336 (273, 372) | 242 (185, 306) | 236 (185, 270) | <0.01 |
| Peak valve gradient (mm Hg) | 20 (15, 26) | 16 (10, 20) | 24 (18, 28) | 19 (14, 25) | <0.01 |
| Time to echocardiography (days) | 34.5 (4, 238) | 36 (4, 163) | 6 (3, 45) | 54 (4, 526) | <0.01 |
Continuous variables are presented as median (interquartile range), whereas categorical variables are presented as frequency (percentage).
VSD = ventricular septal defect.
Fig 2.
Peak valve gradients (mm Hg) by type of valve.
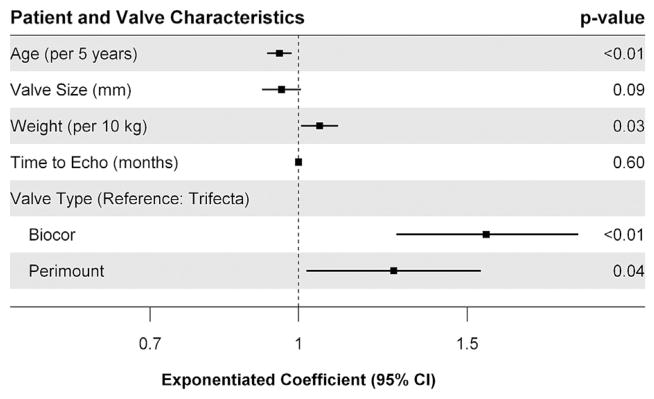
On adjustment with multivariable linear regression, the initial model did not meet assumptions, thus necessitating a logarithmic transformation of the outcome. Because of the use of complete case analysis, 62 patients were excluded from the multivariable analysis. After transformation, increasing age was found to be significantly associated with a reduction in peak valvular gradient (exponentiated coefficient/5 years, 0.95; 95% confidence interval [CI], 0.93, 0.98) (Fig 3). Increasing weight was found to be significantly associated with an increase in peak valvular gradient (exponentiated coefficient/10 kg, 1.05; 95% CI, 1.01, 1.10). There was no significant association between valve size or time to echocardiographic assessment and peak valvular gradient. When comparing the Biocor valve with the Trifecta valve, the Biocor valve was associated with a 57% higher peak valve gradient (exponentiated coefficient, 1.57; 95% CI, 1.27, 1.96). When comparing the Perimount valve with the Trifecta valve, the Perimount valve was associated with a 26% higher peak valve gradient (exponentiated coefficient, 1.26; 95% CI, 1.02, 1.55).
Fig 3.
Patient-related and valve characteristics associated with peak transvalvular gradient postoperatively after adjustment. (CI = confidence interval; Echo = echocardiography.)
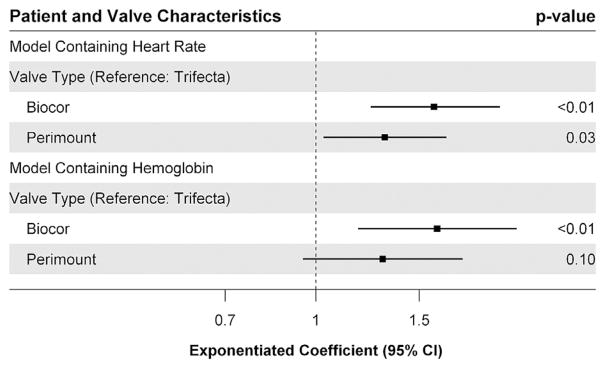
Out of concern that patients receiving echocardiograms at varying postoperative time points have different physiologic features secondary to variations in heart rate and hemoglobin immediately after the operation, both of these variables were substituted in the model in place of the time from the operation to the echocardiogram. An additional 22 patients were missing data on heart rate at the time of their echocardiogram, thus leaving 102 patients for analysis. Heart rate was not found to be associated with significant variation in peak valve gradient (exponentiated coefficient, 1.00; 95% CI, 0.99, 1.01). Furthermore in this model, the Biocor valve (exponentiated coefficient, 1.59; 95% CI, 1.24, 2.06) and the Perimount valve (exponentiated coefficient, 1.31; 95% CI, 1.03, 1.67) remained associated with significantly increased peak valve gradients as compared with the Trifecta valve (Fig 4). Hemoglobin values around the time of echocardiogram were available for 61 patients. Hemoglobin around the time of the echocardiogram was also not found to be associated with a significant difference in peak valve gradient (exponentiated coefficient, 0.96; 95% CI, 0.89, 1.03). However, once again, the Biocor valve was associated with significantly increased peak valve gradient (exponentiated coefficient, 1.61; 95% CI, 0.95, 1.78). In this model, the Perimount valve was associated with a nonsignificantly increased peak valve gradient (exponentiated coefficient, 1.30; 95% CI, 0.95, 1.78).
Fig 4.
Results of the sensitivity analyses after the inclusion of heart rate and hemoglobin in the model in place of time to echocardiographic assessment. (CI = confidence interval.)
Finally, to determine whether the effective valve orifice was responsible for the variation seen in peak transvalvular gradient among the different valve types, the valve size was replaced by the internal valve area indexed to the patient’s weight in the initial model. Once again, the Biocor valve was associated with a 50% higher peak valve gradient (exponentiated coefficient, 1.50; 95% CI, 1.21, 1.87) when compared with the Trifecta valve. When comparing the Perimount valve with the Trifecta valve, the Perimount valve was associated with a nonsignificant 23% higher peak valve gradient (exponentiated coefficient, 1.23; 95% CI, 0.99, 1.52).
Comment
In this study, we demonstrated that when used for PVR in patients with congenital heart disease, the Trifecta aortic pericardial valve is associated with significantly reduced transvalvular gradients. Furthermore, this difference remains even after adjustment for patient-related and operative characteristics including both valve size and patient’s weight at time of operation. Even after the substitution of heart rate around the time of the echocardiogram in the model in place of time to the imaging procedure, the findings were still consistent. Only with the substitution of hemoglobin in the model and when incorporating the effective valve orifice into the model did the association become nonsignificant for the Perimount valve. This finding was as likely the result of an issue of statistical power as it was the reflection of an actual change in the association. Given the shorter time to follow-up with the Trifecta valve along with the amount of time necessary to observe valve deterioration (studies have demonstrated a median time to valve deterioration of roughly 6 years), we do not yet have the ability to determine whether this will be directly related to reduced rates of valve deterioration [5]. Further study will be necessary to determine whether this relationship exists.
The basis of our findings may be secondary to the valve design. The Biocor valve is a porcine stented bioprosthesis first introduced in 1982, whereas the Perimount valve consists of bovine pericardial leaflets mounted on a cobalt-chromium stent [9, 10]. It is possible that this is the reason that the Biocor valve had significantly higher transvalvular gradients as compared with both the Perimount and Trifecta valves. Another difference in valve design is related to coverage of the valve posts. Both the Biocor and Perimount valves have cloth-covered posts. In contrast, the Trifecta valve incorporates several new design elements, including a supraannular sewing cuff, as well as a pericardium-covered post [11]. Finally, the pericardial leaflets of the Trifecta valve are attached to the exterior of the valve stent, thereby increasing the effective orifice area of the valve as compared with other valves of similar size [12].
Other studies have also demonstrated that the Trifecta valve may be associated with reduced transvalvular gradients, although these studies did not investigate the use of this valve for PVR. These other studies were conducted in different patient populations, less at risk for requiring reoperation for valve deterioration during their lifetime than patients with congenital heart disease, and they examined the use of the valve in the aortic position for which the valve prosthesis was designed. Dell’Aquila and colleagues [13] compared mean transvalvular gradients of the Trifecta valve with the Mosaic aortic bioprosthesis (Medtronic, Minneapolis, MN) and the Perimount valve among adult patients undergoing aortic valve replacement and found substantially reduced gradients associated with the Trifecta valve. Ruggieri and colleagues [14] also published their 3-year results using the Trifecta valve for aortic valve replacement in adult patients and found very low peak transvalvular gradients, even out to 3 years. Nonetheless, all these studies focused on adult patients and valve implantation in the aortic position.
Another finding of our study is our demonstration that younger age is associated with increased transvalvular gradients, even after adjustment. As discussed earlier, other studies have demonstrated that younger age is associated with increased prosthesis deterioration [5, 15]. Our demonstration that age is also associated with increased peak transvalvular gradients supports the notion of a relationship between immediate transvalvular gradient and long-term valve deterioration. Although it is not clear why age is a risk factor for valve deterioration, different theories exist. It may be secondary to the increased growth rates of younger children; however, this would not necessarily explain our findings [5]. Other data support that younger children may have accelerated calcification of the valve, which leads to higher transvalvular gradients and, again, earlier failure [16, 17].
Our study has important limitations, First, because they derive from a single-center analysis, our findings may not be generalizable to other centers. Second, as discussed previously, because of the more recent release of the Trifecta valve as compared with other valves, we have limited long-term data regarding pulmonary valve deterioration, as well as limited data on earlier valve-related events such as infective endocarditis and thromboembolic events. Therefore, we could not determine how differences in transvalvular gradients or the valves themselves were related to the need for reoperation or other important long-term outcomes. Third, there is substantial variation in the time to echocardiography by valve type. This may have led to variations in echocardiographic performance resulting from differences in hemodynamic parameters such as hemoglobin concentration or heart rate. We attempted to adjust for this issue by including the time to echocardiogram in the model. In addition, we performed sensitivity analyses among patients who had heart rate and hemoglobin data available and included these variables in the model. Despite these adjustments, a significant difference in transvalvular gradient was still apparent by valve type. Finally, unlike in studies performed in adult patients, there is substantial heterogeneity among patients with congenital heart disease beyond what could be accounted for by statistical methodology. This heterogeneity makes it difficult to be certain that valve type alone was responsible for our findings.
In conclusion, we have demonstrated that the Trifecta prosthetic valve is safe and effective for PVR, and that the immediate transvalvular gradient associated with the use of this valve in patients with congenital heart disease appears to be lower than for previously used stented bioprostheses. This finding is likely related to substantial changes in valve design. It is possible that this finding may extrapolate to significantly delayed long-term valve deterioration, which is of much greater concern in the typically young adult population receiving PVR than in the older counterparts undergoing bioprosthetic aortic valve replacement. Longer-term assessment will be necessary to determine whether this potential advantage of the Trifecta valve for PVR is realized.
Acknowledgments
Dr Gulack is supported by the Cardiothoracic Surgery Trials Network, 5U01HL088953-05, funded by the National Institutes of Health.
Footnotes
Presented at the Sixty-second Annual Meeting of the Southern Thoracic Surgical Association, Orlando, FL, Nov 4-7, 2015.
References
- 1.Therrien J, Marx GR, Gatzoulis MA. Late problems in tetralogy of Fallot: recognition, management, and prevention. Cardiol Clin. 2002;20:395–404. doi: 10.1016/s0733-8651(02)00010-3. [DOI] [PubMed] [Google Scholar]
- 2.Boneva RS, Botto LD, Moore CA, Yang Q, Correa A, Erickson JD. Mortality associated with congenital heart defects in the United States trends and racial disparities, 1979–1997. Circulation. 2001;103:2376–81. doi: 10.1161/01.cir.103.19.2376. [DOI] [PubMed] [Google Scholar]
- 3.Ferraz Cavalcanti PE, Sa MP, Santos CA, et al. Pulmonary valve replacement after operative repair of tetralogy of Fallot: meta-analysis and meta-regression of 3,118 patients from 48 studies. J Am Coll Cardiol. 2013;62:2227–43. doi: 10.1016/j.jacc.2013.04.107. [DOI] [PubMed] [Google Scholar]
- 4.Warnes CA, Williams RG, Bashore TM, et al. ACC/AHA 2008 guidelines for the management of adults with congenital heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Develop Guidelines on the Management of Adults With Congenital Heart Disease). Developed in collaboration with the American Society of Echocardiography, Heart Rhythm Society, International Society for Adult Congenital Heart Disease, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2008;52:e143–263. doi: 10.1016/j.jacc.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 5.Chen PC, Sager MS, Zurakowski D, et al. Younger age and valve oversizing are predictors of structural valve deterioration after pulmonary valve replacement in patients with tetralogy of Fallot. J Thorac Cardiovasc Surg. 2012;143:352–60. doi: 10.1016/j.jtcvs.2011.10.079. [DOI] [PubMed] [Google Scholar]
- 6.Chen XJ, Smith PB, Jaggers J, Lodge AJ. Bioprosthetic pulmonary valve replacement: contemporary analysis of a large, single-center series of 170 cases. J Thorac Cardiovasc Surg. 2013;146:1461–6. doi: 10.1016/j.jtcvs.2012.09.081. [DOI] [PubMed] [Google Scholar]
- 7.Caldarone CA, McCrindle BW, Van Arsdell GS, et al. Independent factors associated with longevity of prosthetic pulmonary valves and valved conduits. J Thorac Cardiovasc Surg. 2000;120:1022–30. doi: 10.1067/mtc.2000.110684. [DOI] [PubMed] [Google Scholar]
- 8.Modi A, Budra M, Miskolczi S, et al. Hemodynamic performance of Trifecta: single-center experience of 400 patients. Asian Cardiovasc Thorac Ann. 2015;23:140–5. doi: 10.1177/0218492314533684. [DOI] [PubMed] [Google Scholar]
- 9.Eichinger WB, Hettich IM, Ruzicka DJ, et al. Twenty-year experience with the St. Jude medical Biocor bioprosthesis in the aortic position. Ann Thorac Surg. 2008;86:1204–10. doi: 10.1016/j.athoracsur.2008.05.058. [DOI] [PubMed] [Google Scholar]
- 10.Bourguignon T, Bouquiaux-Stablo AL, Candolfi P, et al. Very long-term outcomes of the Carpentier-Edwards Perimount valve in aortic position. Ann Thorac Surg. 2015;99:831–7. doi: 10.1016/j.athoracsur.2014.09.030. [DOI] [PubMed] [Google Scholar]
- 11.Bavaria JE, Desai ND, Cheung A, et al. The St Jude Medical Trifecta aortic pericardial valve: results from a global, multicenter, prospective clinical study. J Thorac Cardiovasc Surg. 2014;147:590–7. doi: 10.1016/j.jtcvs.2012.12.087. [DOI] [PubMed] [Google Scholar]
- 12.Permanyer E, Estigarribia AJ, Ysasi A, Herrero E, Semper O, Llorens R. St Jude Medical Trifecta aortic valve perioperative performance in 200 patients. Interact Cardiovasc Thorac Surg. 2013;17:669–72. doi: 10.1093/icvts/ivt270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dell’Aquila AM, Schlarb D, Schneider SR, et al. Clinical and echocardiographic outcomes after implantation of the Trifecta aortic bioprosthesis: an initial single-centre experience. Interact Cardovasc Thorac Surg. 2013;16:112–5. doi: 10.1093/icvts/ivs460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ruggieri VG, Anselmi A, Chabanne C, et al. Three-year haemodynamic performance of the St Jude Trifecta bioprosthesis. Eur J Cardiothorac Surg. 2016;49:972–7. doi: 10.1093/ejcts/ezv211. [DOI] [PubMed] [Google Scholar]
- 15.Zubairi R, Malik S, Jaquiss RD, Imamura M, Gossett J, Morrow WR. Risk factors for prosthesis failure in pulmonary valve replacement. Ann Thorac Surg. 2011;91:561–5. doi: 10.1016/j.athoracsur.2010.07.111. [DOI] [PubMed] [Google Scholar]
- 16.Geha AS, Laks H, Stansel H, Jr, et al. Late failure of porcine valve heterografts in children. J Thorac Cardiovasc Surg. 1979;78:351–64. [PubMed] [Google Scholar]
- 17.Ferrans VJ, Boyce SW, Billingham ME, Jones M, Ishihara T, Roberts WC. Calcific deposits in porcine bioprostheses: structure and pathogenesis. Am J Cardiol. 1980;46:721–34. doi: 10.1016/0002-9149(80)90421-x. [DOI] [PubMed] [Google Scholar]