Abstract
Rates of alcohol use disorders (AUDs) differ between men and women, and there is also marked variation between sexes in the effects of acute and chronic alcohol. In parallel to observations in humans, prior studies in rodents have described male/female differences across a range of ethanol-related behaviors, including ethanol drinking. Nonetheless, there remain gaps in our knowledge of the role of sex in moderating the effects of ethanol, particularly in models of chronic ethanol exposure. The goal of the current study was to assess various behavioral sequelae of exposing female C57BL/6J mice to chronic intermittent ethanol (CIE) via ethanol vapors. Following four weeks of CIE exposure, adult male and female mice were compared for ethanol drinking in a two-bottle paradigm, for sensitivity to acute ethanol intoxication (via loss of righting reflex [LORR]) and for anxiety-like behaviors in the novelty-suppressed feeding and marble burying assays. Next, adult and adolescent females were tested on two different two-bottle drinking preparations (fixed or escalating ethanol concentration) after CIE. Results showed that males and females exhibited significantly blunted ethanol-induced LORR following CIE, whereas only males showed increased anxiety-like behavior after CIE. Increased ethanol drinking after CIE was also specific to males, but high baseline drinking in females may have occluded detection of a CIE-induced effect. The failure to observe elevated drinking in females in response to CIE was also seen in females exposed to CIE during adolescence, regardless of whether a fixed or escalating ethanol-concentration two-bottle procedure was employed. Collectively, these data add to the literature on sex differences in ethanol-related behaviors and provide a foundation for future studies examining how the neural consequences of CIE might differ between males and females.
Keywords: alcohol, gender, sex, addiction, mouse, drinking
Introduction
Rates of diagnosed alcohol use disorders (AUDs) are lower in women than men (Goldstein, Smith, Dawson, & Grant, 2015). Women are also more sensitive to alcohol intoxication and withdrawal and show evidence of more neurotoxicity because of alcohol abuse (reviewed in Hommer, 2003). Despite these differences, however, the etiological and mechanistic factors underling sex differences in risk for AUDs still remain poorly understood. Moreover, the impact of biological factors (e.g., ovarian hormone alterations during the estrous cycle) on the risk for developing AUDs is also unclear. Indeed, there are a number of studies describing how factors, including alcohol metabolism and pharmacokinetics, can differ at different phases of the estrous cycle (Baraona et al., 2001; Morin & Forger, 1982; Mumenthaler, Taylor, O’Hara, & Yesavage, 1999; Roberts, Smith, Weiss, Rivier, & Koob, 1998), although there is no consensus on how the estrous cycle might contribute to sex-related differences in AUDs (reviewed in Devaud, Risinger, & Selvage, 2006 and Lynch, Roth, & Carroll, 2002).
Rodents provide an important model species for studying the neural and genetic basis of AUDs, and sex differences in ethanol drinking and other behavioral responses to ethanol have been well documented both in rats and mice (reviewed in Becker & Koob, 2016; Lynch et al., 2002). For example, previous studies have shown that, as compared to males, female rodents exhibit enhanced place-preference for ethanol (Torres, Walker, Beas, & O’Dell, 2014) and reduced signs of withdrawal, including attenuated anxiety-like behavior and plasma corticosterone levels (e.g., Alele & Devaud, 2007; Devaud & Chadda, 2001; Janis, Devaud, Mitsuyama, & Morrow, 1998; Lopez, Grahame, & Becker, 2011; Overstreet, Knapp, & Breese, 2004; Reilly, Koirala, & Devaud, 2009; Strong, Kaufman, Crabbe, & Finn, 2009; Tanchuck-Nipper et al., 2015; Varlinskaya & Spear, 2004; Veatch, Wright, & Randall, 2007) (but see Morales, McGinnis, & McCool, 2015). Furthermore, many (e.g., Aufrère, Le Bourhis, & Beaugé, 1997; Lancaster, Brown, Coker, Elliott, & Wren, 1996; Lancaster & Spiegel, 1992; Li & Lumeng, 1984; McKinzie et al., 1998; Moore & Lynch, 2015; Vengeliene, Vollmayr, Henn, & Spanagel, 2005; Vetter, Doremus-Fitzwater, & Spear, 2007), although not all (e.g., Schramm-Sapyta et al., 2014; Varlinskaya & Spear, 2002), prior studies have shown that females drink more ethanol than males.
The chronic intermittent ethanol (CIE) procedure is a tractable method for modeling the behavioral and neural sequelae of a history of a chronic alcohol exposure, and may be of value for furthering current understanding of sex differences in risk for AUDs. In this procedure, subjects are repeatedly maintained at high blood ethanol concentrations (BECs) for extended periods (e.g., sixteen hours) interspersed by forced withdrawals (Becker, 2013; Goldstein & Pal, 1971; O’Dell, Roberts, Smith, & Koob, 2004). Previous work has described an array of neural and behavioral effects of CIE in male mice of several strains (though predominantly C57BL/6J) and various lines of male or sex-balanced groups of gene mutant mice (Becker, 2013). The reported behavioral effects of CIE in mice include elevated ethanol drinking (Becker & Lopez, 2004; Carrara-Nascimento, Lopez, Becker, Olive, & Camarini, 2013; Dhaher, Finn, Snelling, & Hitzemann, 2007; Finn et al., 2007; Griffin, Lopez, & Becker, 2009; Griffin, Lopez, Yanke, Middaugh, & Becker, 2009; Holmes et al., 2012; Lopez & Becker, 2005; McCool & Chappell, 2015), increased seizure susceptibility and anxiety-like behavior (Becker, 1994; Becker, Diaz-Granados, & Hale, 1997; Becker, Diaz-Granados, & Weathersby, 1997; Becker & Hale, 1993; Morales et al., 2015), tolerance to acute ethanol intoxication (Daut et al., 2015), and alterations in appetitive and aversive learning (DePoy et al., 2013, 2015; Holmes et al., 2012; Radke et al., 2015). In terms of sex differences, one study reported that male but not female HAP-2 mice showed increased ethanol drinking and withdrawal signs following CIE (Lopez et al., 2011). Beyond these observations, however, little is currently known about sex differences in the behavioral consequences of CIE.
The goal of the current study was to determine multiple behavioral changes resulting from CIE in female mice. We first compared adult and adolescent, male and female sentinel mice in order to evaluate the potential effects of sex (e.g., effects of the estrous cycle) and age on CIE-induced BECs. Next, we compared adult male and female mice for ethanol drinking, sensitivity to acute ethanol intoxication, and anxiety-like behavior following CIE. We then examined CIE effects on ethanol drinking in two different drinking paradigms (fixed and escalating concentration), in adult and adolescent females in view of adolescence being a period of heightened risk for AUDs and the increasing abuse of alcohol among young women (Khan et al., 2013).
Materials and methods
Subjects
Subjects were female and male C57BL/6J mice obtained at either 21 ± 4 days (adolescent group) or 49 ± 4 days (adult group) of age from The Jackson Laboratory (Bar Harbor, ME, USA). Mice were housed, by sex, 2/cage in a temperature- (72 ± 5 °F) and humidity- (45 ± 15%) controlled vivarium under a 12-hour light/dark cycle (lights on at 0600 h), and acclimated to the vivarium for at least 1 week prior to experimentation. The numbers of mice used in each experiment are given in the figure legends. Experimental procedures were approved by both the National Institute on Alcohol Abuse and Alcoholism (NIAAA) Animal Care and Use Committee and the University of North Carolina (UNC) at Chapel Hill Institutional Animal Care and Use Committee. Experiments were performed at NIAAA facilities, with the exception of anxiety-related behavioral experiments, which were performed at UNC facilities. All procedures followed the US National Institutes of Health guidelines outlined in Using Animals in Intramural Research.
Chronic intermittent ethanol (CIE) exposure
Chronic ethanol exposure was achieved via vapor inhalation, as previously described (Becker & Lopez, 2004; DePoy et al., 2015). Mice were placed in standard mouse cages in Plexiglas® vapor chambers (60 × 36 × 60 cm, PlasLabs, Lansing, MI, USA) and exposed to ethanol volatized by passing air through a vaporization stone submerged in ethanol (95%) and mixed with fresh air to deliver 19–22 mg ethanol/L of air at a rate of ~10 L per minute. Ethanol delivery parameters were designed to produce blood ethanol concentrations (BECs) of 175 ± 25 mg/dL (unless otherwise specified) – confirmed weekly via blood samples taken from age/sex-matched ‘sentinel’ mice. Blood samples were collected into heparinized glass capillary tubes using a lancet to prick the submandibular vein (Golde, Gollobin, & Rodriguez, 2005). Blood samples were centrifuged at 15,000 rpm for 30 min at 4 °C prior to the measurement of BECs using the Analox AM1 alcohol analyzer (Analox Instruments USA, Lunenburg, MA, USA). Vapor exposure parameters did not require alteration to attain comparable BECs across different sex and age groups. This was determined by exposing two separate cohorts (cohort 1 = adult female mice while monitoring the estrous cycle, target BECs = 175 ± 25 mg/dL; cohort 2 = female and male mice, both adolescent and adult, target BECs = 195 ± 25 mg/dL) of mice to CIE and measuring their respective BECs daily, throughout exposure (see Tables 1 and 2).
Table 1. BECs as a function of estrous cycle.
BECs from adult female mice did not significantly change as a function of the cycle of the estrous cycle at which they were measured. Samples were measured on each day of CIE-exposure and are presented as averages over the four-week CIE-exposure period.
| Metestrus | Diestrus | Proestrus | Estrous | Mean | |
|---|---|---|---|---|---|
| Mean BECs (mg/dL) | 185.46 | 141.35 | 169.13 | 200.45 | 174.10 |
| SEM | 19.51 | 24.49 | 9.455 | 8.67 | 9.80 |
| Sample size (n) | 5 | 4 | 4 | 4 | 17 |
Table 2. Effects of age and sex on CIE-induced BECs.
BECs from mice did not differ significantly throughout the four-week exposure period regardless of age or sex.
| Adolescent Female | Adult Female | Adolescent Male | Adult Male | Mean | |
|---|---|---|---|---|---|
| Mean BECs (mg/dL) | 190.08 | 191.48 | 199.82 | 199.22 | 195.15 |
| SEM | 19.51 | 24.49 | 9.45 | 8.67 | 12.65 |
| Sample size (n) | 5 | 5 | 5 | 5 | 20 |
In order to induce intoxication and stabilize BECs, the ethanol group received intraperitoneal (i.p.) injections of 71.6 mg/kg of the alcohol dehydrogenase inhibitor pyrazole (Sigma, St. Louis, MO, USA) combined with 1.5 g/kg 20% (v/v) ethanol, in a volume of 10 mL/kg body weight, prior to placement in the chambers. Air controls received an injection of 68.1 mg/kg pyrazole and were placed in dedicated chambers (located adjacent to the ethanol chambers) in which air was exchanged at a rate of ~10 L/minute. Each CIE and air exposure lasted 16 hours per day (in at 1700 h, 1 hour before start of the 12-hour circadian dark phase, out at 0900 h), followed by an 8-hour withdrawal. There were four consecutive days of exposure (Monday-Friday) followed by a longer, 80-hour, withdrawal (Friday-Monday). This was repeated for a total of four cycles.
Estrous cycle monitoring
The estrous cycle was monitored during each day of CIE exposure at 0900 hours in a dedicated cohort of adult female mice that did not undergo any other experimentation. BECs were measured from this cohort using the same method as described above. BECs were averaged over the four-week exposure period and are presented according to the corresponding estrous stage at the time of blood collection (see Table 1 for results). Vaginal gavage with 0.9% phosphate-buffered saline was used to dislodge vaginal cells for collection. Vaginal cytology was examined using an Olympus BX41 microscope with a 4X objective (Olympus, Center Valley, PA, USA). The stage of estrous was determined by identification of three distinct vaginal cells: 1) nucleated epithelial cells, 2) anucleated cornified cells, and 3) leukocytes (Caligioni, 2009).
Effects of CIE on sensitivity to acute ethanol intoxication
Sensitivity to the acute intoxicating effects of ethanol was assayed using the loss of righting reflex (LORR) test, as previously described (Chesler et al., 2012). One cohort of adult females and males was exposed to four weeks of CIE – with exposure parameters set to achieve BECs of 150 ± 25 mg/dL and tested for LORR the day after the final exposure. One-day post-CIE was chosen based on prior evidence that reduced sensitivity to LORR (i.e., tolerance) is evident at this time point in adult male C57BL/6J mice (Daut et al., 2015). A separate cohort of both adolescent and adult, females and males was exposed to four weeks of CIE – with exposure parameters set to achieve lower BECs of 100 ± 25 mg/dL (in order to avoid a ‘floor effect’ and increase the ability of detecting reduced sensitivity in the adolescent and/or female groups) and again tested for LORR the day after the final exposure.
For the LORR test, mice were injected i.p. with 3.5 g/kg 20% (v/v) ethanol and placed into the supine position in a ‘V’-shaped chamber. LORR duration was measured as the time from injection to the time when the mouse was able to self-right onto all four paws twice within 30 seconds. Immediately after recovery, the mouse was sacrificed via cervical dislocation followed by rapid decapitation for trunk blood collection. Blood was centrifuged in a microcentrifuge at 4 °C for 30 minutes at a speed of 13,000 rpm and analyzed for BECs using the Analox AM1 alcohol analyzer.
Effects of CIE on anxiety-like behavior
Adult male and female mice were given four weeks of CIE exposure and tested in the novelty-suppressed feeding (NSF) test three days later, and then after another three-day interval, the marble burying test. Exposure parameters were set to achieve relatively high BECs of 175 ± 25 mg/dL, given prior data showing anxiety-like behavior is insensitive to the effects of four weeks of CIE when tested in the light-dark exploration test three days after CIE in male C57BL/6J mice (Holmes et al., 2012).
The NSF test was conducted as previously described (Kiselycznyk, Svenningsson, Delpire, & Holmes, 2011). Two days before testing (i.e., one day post-CIE), mice were given, in the home-cage, three pieces of the colored, highly palatable food to be used in the NSF test. Sixteen hours before testing, mice were food deprived. The NSF test was conducted in a polycarbonate cage (28 × 17 cm × 14 cm) placed in a sound-attenuated behavioral cabinet under 20-lux lighting. Cage bedding was placed on the floor and changed between subjects. A circular piece of filter paper (7-cm diameter) was placed in the center of the cage, and three pieces of the colored food were placed on the paper. Mice were placed in a corner of the cage and latency to feed was recorded. Mice that took more than 10 minutes to feed (n = 2) were excluded from the data analysis. Immediately after starting to feed (but before any significant consumption), mice were removed from the testing cage and returned to their home cage, where they were offered another three pieces of the colored food to determine consumption in a non-anxiety-provoking situation.
The marble burying test was conducted as previously described (Deacon, 2006; Zhao et al., 2006). The test was conducted in a polycarbonate cage (28 × 17 cm × 14 cm) lined with 5-cm deep bedding (changed between subjects) and placed in a sound-attenuated behavioral cabinet under 20-lux lighting. Twelve marbles were placed atop the bedding arranged in a 4 × 3 array. Mice were introduced to a corner of the cage, and removed after 30 minutes. The number of marbles buried by bedding to at least 2/3 of their depth was counted.
Effects of CIE on ethanol drinking
Ethanol drinking was assessed using a 24-hour access two-bottle choice procedure, as previously described (Boyce-Rustay, Janos, & Holmes, 2008). Ethanol-naive mice were individually housed in ‘Space Saver’ cages (Model 1145T with Model 1145T482SUDB Polysulfone cage top, Tecniplast, Buguggiate, Italy) and offered two bottles: one containing 15% (v/v) ethanol in water (unless otherwise specified) and the other containing tap water. Every two days, mice were weighed and ethanol and water consumption measured, correcting for evaporation and spillage measured from empty ‘dummy’ cages adjacent to the test cages. The left/right position of the bottles was switched to control for side bias. Food was available ad libitum. Three separate experiments were conducted in separate cohorts of mice to assess the effects of CIE on drinking under various conditions. For each experiment, daily ethanol consumption was calculated in g per kg of body weight. Relative preference for the ethanol-containing solution over water was calculated as a percentage of total fluid consumption.
We first compared adult male and female mice that were given two weeks of two-bottle drinking prior to four weeks of CIE exposure – with exposure parameters set to achieve BECs of 100 ± 25 mg/dL. Drinking of a 15% ethanol solution was then assessed for two weeks, beginning 72 hours after the final CIE exposure. Next, we compared adolescent and adult female mice that were given four weeks of CIE exposure and then assessed for two weeks of 15% ethanol solution two-bottle drinking, beginning 72 hours after the final CIE exposure. Because there was no pre-drinking experience in this experiment, the exposure parameters were set to achieve lower BECs of 100 ± 25 mg/dL to avoid the potential of a high CIE concentration producing an aversion to drinking in mice with no prior history of drinking (Lopez, Griffin, Melendez, & Becker, 2012). A second cohort of adult and adolescent females was tested in the same manner, with the exception that the concentration of ethanol was increased by 3% (3, 6, 9, 12, 15%) every four days and then finally by 5% (20%) for two days. As we did not find evidence of aversion in the prior experiment, the CIE-exposure parameters were set to achieve higher BECs of 175 ± 25 mg/dL.
Statistical analysis
The effects of age and sex were analyzed using Student’s t test or two-factor analysis of variances (ANOVA), followed by Sidak’s multiple comparison post hoc analysis. The effects of age or sex and ethanol concentration or time were analyzed using two-factor ANOVA, with repeated measures for ethanol concentration and time. The statistical threshold was set at p < .05.
Results
Effects of sex and age on CIE-induced BECs
In adult female mice, CIE-induced BECs did not significantly differ as a function of stage of estrous cycle during the four-week exposure period (Table 1). Moreover, there were no significant differences between CIE-induced BECs when comparing female and male mice, regardless of age during exposure (Table 2).
Effects of CIE on sensitivity to acute ethanol intoxication
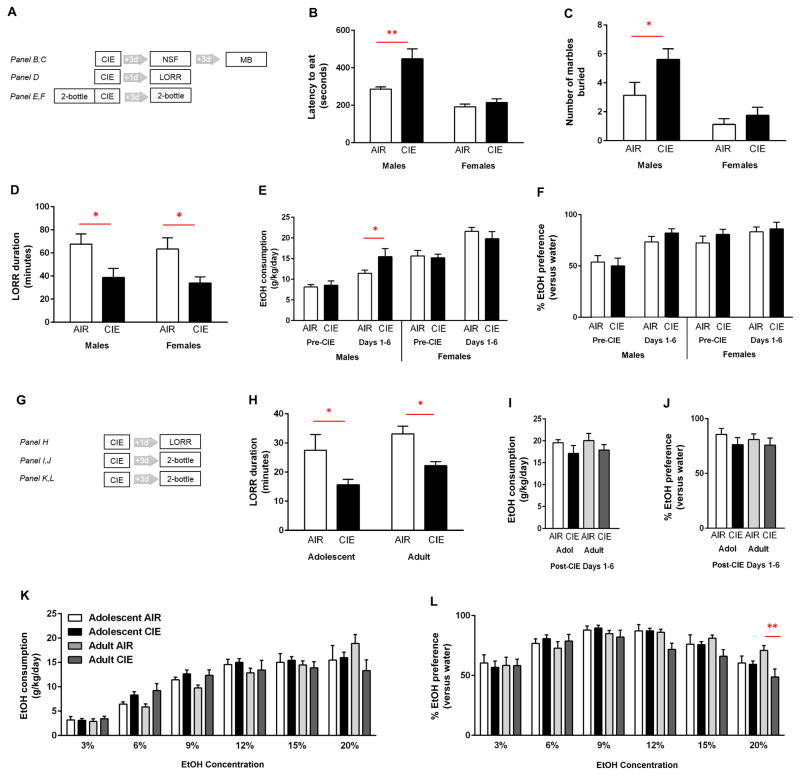
In adult female and male mice, two-factor ANOVA revealed a main effect of CIE exposure [F(1,48) = 12.34, p = 0.0010], but not of sex, on the duration to regain LORR. Sidak’s multiple comparison post hoc analysis revealed a shorter LORR duration in male [t(1,48) = 2.359, p = 0.0449] and female [t(1,48) = 2.624, p = 0.0233] CIE-exposed mice, as compared to air controls (Fig. 1D).
Fig. 1. Effects of CIE in female mice.
(A) Experimental schematic for data shown in panels B–F. (B) CIE-exposed males, but not females, had a longer latency to feed in the novelty-suppressed feeding test (C) and buried more marbles (n = 5–8 per group). (D) LORR duration was shorter in CIE-exposed adult male and female mice, relative to air controls (n = 11–15 per group). Ethanol (15%) drinking (E), but not preference (F), was higher in females than males before and after CIE, and higher in CIE-exposed males than air controls (n = 8–12 per group). (G) Experimental schematic for data shown in panels H–L. (H) LORR duration was shorter in CIE-exposed adult and adolescent female mice, as compared to air controls (n = 10 per group). Ethanol (15%) drinking (I), and preference (J), were no different between adult and adolescent females, regardless of CIE exposure (n = 7–8 per group). Drinking of (K) and preference for (L) increasing ethanol concentrations did not differ between adult and adolescent females. Ethanol drinking preference was lower (20% ethanol concentration) in adult CIE-exposed females than similarly aged air controls (n = 7–8 per group). Data are means ± SEM. *p < .05, **p < .01 versus air controls
In adolescent and adult female mice, two-factor ANOVA revealed a main effect of CIE exposure [F(1,36) = 12.37, p = 0.0012], but not of age [F(1,36) = 3.541, p = 0.0680], on the duration of LORR. Sidak’s multiple comparison post hoc analysis revealed a shorter LORR duration in adolescent [t(1,36) = 2.596, p = 0.0270] and adult [t(1,36) = 2.378, p = 0.0452] female mice, as compared to air controls (Fig. 1H).
Effects of CIE on anxiety-like behavior
In adult female and male mice, two-factor ANOVA revealed main effects of sex [F(1,25) = 23.93, p < 0.0001] and CIE exposure [F(1,25) = 7.505, p = 0.0112] on the latency to feed in a novel cage in the NSF test. There was a trend for an interaction between the two factors [F(1,25) = 4.210, p = 0.0508]. Sidak’s multiple comparison post hoc analysis revealed that CIE-exposed male mice, but not female mice, exhibited a greater latency to feed than air-exposed controls [t(1,25) = 3.187, p = 0.0077] (Fig. 1B). Neither sex showed CIE-induced changes in the amount of food consumed in the home cage (data not shown).
In the same adult female and male mice, two-factor ANOVA revealed main effects of sex [F(1,27) = 20.32, p = 0.0001] and CIE exposure [F(1,25) = 5.649, p = 0.0248] on the number of marbles buried (Fig. 1C). Sidak’s multiple comparison post hoc analysis revealed that CIE-exposed male mice, but not female mice, buried more marbles than air-exposed controls [t(1,27) = 2.640, p = 0.0270].
Effects of CIE on ethanol drinking
In adult male mice, two-factor ANOVA revealed main effects of pre- versus post-exposure [F(1,44) = 20.46, p < 0.0001] and of CIE exposure [F(1,44) = 4.59, p = 0.0376], as well as a trend toward an interaction of these two main effects [F(1,44) = 3.155, p = 0.0826], on ethanol consumption. Two-factor ANOVA revealed a main effect of pre- versus post-exposure [F(1,44) = 18.61, p < 0.0001] on preference for ethanol over water. Sidak’s multiple comparison post hoc analysis revealed that CIE-exposed male mice consumed more ethanol following exposure than air-exposed controls [t(1,44) = 2.344, p = 0.0467] (Fig. 1E), while ethanol preference did not change (Fig. 1F).
In adult female mice, two-factor ANOVA revealed a main effect of pre- versus post-exposure [F(1,28) = 17.73, p = 0.0002] but not of CIE exposure on ethanol consumption. CIE exposure did not significantly alter ethanol consumption in female mice (Fig. 1E). There were no significant effects on ethanol preference (Fig. 1F).
In adolescent and adult female mice, two-factor ANOVA revealed main effects of concentration [F(5,167) = 60.25, p < 0.0001] but not of age, on ethanol consumption. Consumption did not differ between the age groups (Fig. 1K). Two-factor ANOVA revealed main effects of concentration [F(5,167) = 23.41, p < 0.0001] and CIE exposure [F(3,167) = 3.731, p = 0.0125] on ethanol preference. Sidak’s multiple comparison post hoc analysis revealed that CIE-exposed adult female mice preferred ethanol (20% concentration) less than air-exposed controls [t(1,167) = 4.610, p = 0.0073] (Fig. 1L).
Discussion
The results of the current study provide novel insights into sex differences in the behavioral effects of chronic exposure to ethanol.
A heightened level of anxiety during withdrawal is thought to be a major factor underlying relapse and can be observed in rodents following chronic ethanol exposure (Koob, 2003). However, consistent with prior studies in rats using other chronic ethanol models (Overstreet et al., 2004; Reilly et al., 2009), we found that female C57BL/6J mice were resistant to the anxiety-like effects of CIE. Specifically, while CIE-exposed males exhibited increased anxiety-like behavior in two separate assays, NSF and marble burying, which persisted for up to six days after exposure, females did not. To our knowledge, these are the first data on the use of the NSF and marble burying tests to assess anxiety-related changes resulting from chronic ethanol, and they encourage use of these tests in future studies given that other assays, such as the light-dark exploration assay, appear to be less sensitive to CIE effects in male C57BL/6J mice (Holmes et al., 2012). An obvious avenue for future studies will be to delineate the mechanisms underlying this apparent protection of females from the anxiety-inducing effects of CIE, for example, with regard to emerging evidence implicating sex steroids and hormones (Becker & Koob, 2016; Strong et al., 2009). Of note in this context, we did not observe variation in CIE-induced BECs across different stages of the estrous cycle, as some (Baraona et al., 2001) but not other (Robinson, Brunner, & Gonzales, 2002) prior studies have seen in rats, or any evidence of an ethanol-related disruption in cyclicity that others have also reported in rats (Emanuele, LaPaglia, Steiner, Kirsteins, & Emanuele, 2001; Krueger, Bo, & Rudeen, 1983; Sanchis, Esquifino, & Guerri, 1985).
The current data show that sex-divergent effects of CIE seen for anxiety-like behavior did not extend to other behavioral measures currently examined. LORR duration, taken as an index of sensitivity to the acute intoxicating effects of an ethanol challenge, did not differ between males and females, either in CIE-exposed mice or in air-exposed controls. The absence of an overall sex difference on this measure contrasts with the recent finding of shorter LORR duration in females to a similar ethanol challenge dose as used here (Tanchuck-Nipper et al., 2015). Given that genetic background strongly influences mouse LORR (Boyce-Rustay et al., 2008; Crabbe, 2012; Crabbe, Metten, Cameron, & Wahlsten, 2005) and the mice tested by Tanchuck-Nipper and colleagues were on a C57BL/6J × 129/SvJ background (as opposed to the pure C57BL/6J background used here), a plausible explanation for this discrepancy is that sex differences in the LORR are background-dependent. This would be another interesting question to explore in follow-up work, perhaps similar to what other studies have shown reporting the influence of genetic background on response to ethanol in mice (Fish, DiBerto, Krouse, Robinson, & Malanga, 2014). Aside from this question, we found that LORR durations were significantly shortened by CIE in both males and females – consistent with the development of tolerance to this measure of intoxication (as previously reported in rats and male C57BL/6J mice [Daut et al., 2015; Walls, Macklin, & Devaud, 2012]). These data argue that the resistance to the anxiogenic-like effects of CIE in females is not simply due to a general insensitivity to the chronic ethanol exposure. This conclusion should be qualified, however, by the fact that LORR was tested sooner post-CIE than anxiety-like behavior, and it therefore remains possible that the LORR response recovers more quickly in females, as it does in chronically exposed rats (Walls et al., 2012).
Nonetheless, these LORR data also bear upon our finding that females failed to show an increase in ethanol drinking after CIE, even under exposure conditions that produced clear increases in males (as had been reported in earlier studies [Becker & Lopez, 2004; Carrara-Nascimento et al., 2013; Dhaher et al., 2007; Finn et al., 2007; Griffin, Lopez, & Becker, 2009; Griffin, Lopez, Yanke, et al., 2009; Holmes et al., 2012; Lopez & Becker, 2005; McCool & Chappell, 2015]). This finding resembles an earlier report that females from high- and low-ethanol-preferring selected lines did not increase drinking after CIE, though males from these lines (unlike C57BL/6J males) also failed to do so (Lopez et al., 2011). One interpretation of these data is that female mice are protected from chronic ethanol effects on drinking, as they are from its effects on anxiety-like behavior and withdrawal hyperexcitability (Alele & Devaud, 2007; Devaud & Chadda, 2001; Janis et al., 1998; Lopez et al., 2011; Overstreet et al., 2004; Reilly et al., 2009; Strong et al., 2009; Tanchuck-Nipper et al., 2015; Varlinskaya & Spear, 2004; Veatch et al., 2007). Indeed, it is possible that these various behavioral effects are related, such that elevated withdrawal severity and anxiety-like behavior is a driver of elevated drinking in males that is absent in females.
One caveat to this explanation, however, is that, consistent with numerous previous studies in rats (e.g., Aufrère et al., 1997; Lancaster et al., 1996; Lancaster & Spiegel, 1992; Li & Lumeng, 1984; McKinzie et al., 1998; Moore & Lynch, 2015; Vengeliene et al., 2005; Vetter et al., 2007) (although see Schramm-Sapyta et al., 2014; Varlinskaya & Spear, 2002), female C57BL/6J mice drank significantly more ethanol than males before any CIE exposure. As such, high basal levels of drinking in the females could have artificially prevented our ability to detect further increases due to CIE (i.e., caused a ‘ceiling effect’). The authors of previous studies have posited a similar explanation for the difficultly in detecting increases in drinking in female rats after CIE (Morales et al., 2015) and other environmental insults, such as stress (Butler, Carter, & Weiner, 2014; Rosenwasser, McCulley, & Fecteau, 2014). There are, however, examples of elevated drinking, despite high basal levels, in females rats after prolonged (two or more weeks) forced ethanol abstinence (McKinzie et al., 1998; Vengeliene et al., 2005), indicating that there are at least some experimental conditions in which drinking can be driven higher in females.
In an effort to reduce basal levels of drinking in females, and thereby increase the likelihood of detecting a CIE-related increase, we tested a group of female mice that were not offered ethanol to drink prior to CIE – working under the assumption that exposing naïve animals to CIE would induce a partial aversion to ethanol and lessen post-CIE drinking. Contrary to our prediction, females with no prior drinking showed levels of post-CIE drinking of a 15% percent ethanol concentration that were, if anything, greater than those that had been given previous drinking experience. Similarly, progressively increasing the concentration of ethanol offered also failed to reveal a CIE-induced elevation in females that had not been given an opportunity to drink before CIE exposure began. In fact, under these test parameters, CIE-exposed females drank less than air-exposed controls when they reached the highest (20%) concentration. This finding may warrant replication and further examination as it hints at the possibility that CIE may actually decrease drinking in females under certain conditions, e.g., when offered high drinking concentrations or at long periods since CIE. This would not be without precedent; one recent study showed that CIE-exposed female rats drank less across weeks of intermittent two-bottle access (whereas males escalated drinking) (Morales et al., 2015).
Another finding from the current study was that a cohort of adult female mice did not exhibit significantly different BECs throughout estrous during CIE exposure. While one study in rats reported that BECs were altered throughout the estrous cycle following exposure to ethanol (Baraona et al., 2001), another report, also in rats, reported the opposite (Robinson et al., 2002). Furthermore, while others have reported that chronic ethanol causes disruptions in cyclicity of estrous in rats (Emanuele et al., 2001; Krueger et al., 1983; Sanchis et al., 1985), we did not observe any such disruption in mice (data not shown) – though this remains to be replicated in a larger sample.
Adolescence is a period of vulnerability to AUDs, and young women in particular are abusing alcohol at historically high levels (Khan et al., 2013). Adolescent rodents also display contrasting behavioral and neural responses to ethanol when compared to adults (e.g., Crews, Braun, Hoplight, Switzer, & Knapp, 2000; Hefner & Holmes, 2007; Lancaster et al., 1996; Melón, Wray, Moore, & Boehm, 2013; Varlinskaya, Truxell, & Spear, 2015), some of which vary as a function of sex (reviewed in Spear, 2000). The current experiments did not, however, demonstrate differential effects of CIE in females as a function of whether exposure was during adolescence or adulthood. This finding differs from the increased drinking reported in rats with adolescent ethanol exposure (Acevedo, Molina, Nizhnikov, Spear, & Pautassi, 2010; Pascual, Boix, Felipo, & Guerri, 2009), but resembles the absence of drinking differences reported in male C57BL/6J mice given CIE exposure during adolescence (Carrara-Nascimento et al., 2013). Thus, the potential effects of CIE exposure during adolescence on this behavioral measure may differ between rats and mice, and conditions under which adolescent exposure might produce long-lasting effects on drinking remain to be determined.
In summary, the main aim of the current study was to assess effects of chronic ethanol exposure, using the CIE vapor method, in female C57BL/6J mice. Results confirmed and extended previous studies in rats and mice by showing that females were protected against the anxiogenic-like effects of withdrawal from chronic ethanol exposure. By contrast, sensitivity to the acute intoxicating effects of ethanol, as measured by LORR, was no different between the sexes. Unlike males, CIE failed to produce elevated (two-bottle) drinking in female mice. This profile in females was seen against a background of high basal drinking and across a variety of experimental conditions: i.e., with and without pre-CIE drinking experience and with either fixed or escalating post-CIE ethanol concentrations. Lastly, we found that when exposed to CIE during adolescence, females again showed no change in ethanol drinking as adults. Taken together, the current findings replicate and extend the literature on the CIE model and the effect of sex on various behavioral sequelae of chronic ethanol exposure. This work may prove useful to future studies aimed at investigating how males and females differ in their risk for AUDs.
Highlights.
Behavioral effects of CIE were assessed in male and female C57BL/6J mice.
CIE reduced sensitivity to ethanol-induced LORR in males and females.
CIE increased anxiety-like behavior in males but not females.
CIE did not increase ethanol drinking above baseline in adult or adolescent females.
These data show sex differences in behavioral effects of CIE.
Acknowledgments
Research was supported by the National Institute on Alcohol Abuse and Alcoholism Intramural Research Program. We would like to thank Erica Bush and Erica Sagalyn for providing excellent technical assistance during the CIE vapor exposure procedure.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acevedo MB, Molina JC, Nizhnikov ME, Spear NE, Pautassi RM. High ethanol dose during early adolescence induces locomotor activation and increases subsequent ethanol intake during late adolescence. Developmental Psychobiology. 2010;52:424–440. doi: 10.1002/dev.20444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alele PE, Devaud LL. Sex differences in steroid modulation of ethanol withdrawal in male and female rats. The Journal of Pharmacology and Experimental Therapeutics. 2007;320:427–436. doi: 10.1124/jpet.106.107896. [DOI] [PubMed] [Google Scholar]
- Aufrère G, Le Bourhis B, Beaugé F. Ethanol intake after chronic intoxication by inhalation of ethanol vapour in rats: behavioural dependence. Alcohol. 1997;14:247–253. doi: 10.1016/s0741-8329(96)00175-9. [DOI] [PubMed] [Google Scholar]
- Baraona E, Abittan CS, Dohmen K, Moretti M, Pozzato G, Chayes ZW, et al. Gender differences in pharmacokinetics of alcohol. Alcoholism: Clinical and Experimental Research. 2001;25:502–507. [PubMed] [Google Scholar]
- Becker HC. Positive relationship between the number of prior ethanol withdrawal episodes and the severity of subsequent withdrawal seizures. Psychopharmacology. 1994;116:26–32. doi: 10.1007/BF02244867. [DOI] [PubMed] [Google Scholar]
- Becker HC. Animal models of excessive alcohol consumption in rodents. Current Topics in Behavioral Neurosciences. 2013;13:355–377. doi: 10.1007/7854_2012_203. [DOI] [PubMed] [Google Scholar]
- Becker HC, Diaz-Granados JL, Hale RL. Exacerbation of ethanol withdrawal seizures in mice with a history of multiple withdrawal experience. Pharmacology, Biochemistry, and Behavior. 1997;57:179–183. doi: 10.1016/s0091-3057(96)00303-6. [DOI] [PubMed] [Google Scholar]
- Becker HC, Diaz-Granados JL, Weathersby RT. Repeated ethanol withdrawal experience increases the severity and duration of subsequent withdrawal seizures in mice. Alcohol. 1997;14:319–326. doi: 10.1016/s0741-8329(97)87949-9. [DOI] [PubMed] [Google Scholar]
- Becker HC, Hale RL. Repeated episodes of ethanol withdrawal potentiate the severity of subsequent withdrawal seizures: an animal model of alcohol withdrawal “kindling”. Alcoholism: Clinical and Experimental Research. 1993;17:94–98. doi: 10.1111/j.1530-0277.1993.tb00731.x. [DOI] [PubMed] [Google Scholar]
- Becker HC, Lopez MF. Increased ethanol drinking after repeated chronic ethanol exposure and withdrawal experience in C57BL/6 mice. Alcoholism: Clinical and Experimental Research. 2004;28:1829–1838. doi: 10.1097/01.alc.0000149977.95306.3a. [DOI] [PubMed] [Google Scholar]
- Becker JB, Koob GF. Sex Differences in Animal Models: Focus on Addiction. Pharmacological Reviews. 2016;68:242–263. doi: 10.1124/pr.115.011163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce-Rustay JM, Janos AL, Holmes A. Effects of chronic swim stress on EtOH-related behaviors in C57BL/6J, DBA/2J and BALB/cByJ mice. Behavioural Brain Research. 2008;186:133–137. doi: 10.1016/j.bbr.2007.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler TR, Carter E, Weiner JL. Adolescent social isolation does not lead to persistent increases in anxiety-like behavior or ethanol intake in female long-evans rats. Alcoholism: Clinical and Experimental Research. 2014;38:2199–2207. doi: 10.1111/acer.12476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caligioni CS. Assessing Reproductive Status/Stages in Mice. Current Protocols in Neuroscience. 2009;48:4I:A.4I.1–A.4I.8. doi: 10.1002/0471142301.nsa04is48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrara-Nascimento PF, Lopez MF, Becker HC, Olive MF, Camarini R. Similar ethanol drinking in adolescent and adult C57BL/6J mice after chronic ethanol exposure and withdrawal. Alcoholism: Clinical and Experimental Research. 2013;37:961–968. doi: 10.1111/acer.12056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesler EJ, Plitt A, Fisher D, Hurd B, Lederle L, Bubier JA, et al. Quantitative trait loci for sensitivity to ethanol intoxication in a C57BL/6J×129S1/SvImJ inbred mouse cross. Mammalian Genome. 2012;23:305–321. doi: 10.1007/s00335-012-9394-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC. Translational behaviour-genetic studies of alcohol: are we there yet? Genes, Brain, and Behavior. 2012;11:375–386. doi: 10.1111/j.1601-183X.2012.00798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC, Metten P, Cameron AJ, Wahlsten D. An analysis of the genetics of alcohol intoxication in inbred mice. Neuroscience and Biobehavioral Reviews. 2005;28:785–802. doi: 10.1016/j.neubiorev.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Crews FT, Braun CJ, Hoplight B, Switzer RC, 3rd, Knapp DJ. Binge ethanol consumption causes differential brain damage in young adolescent rats compared with adult rats. Alcoholism: Clinical and Experimental Research. 2000;24:1712–1723. [PubMed] [Google Scholar]
- Daut RA, Busch EF, Ihne J, Fisher D, Mishina M, Grant SG, et al. Tolerance to ethanol intoxication after chronic ethanol: role of GluN2A and PSD-95. Addiction Biology. 2015;20:259–262. doi: 10.1111/adb.12110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deacon RM. Digging and marble burying in mice: simple methods for in vivo identification of biological impacts. Nature Protocols. 2006;1:122–124. doi: 10.1038/nprot.2006.20. [DOI] [PubMed] [Google Scholar]
- DePoy L, Daut R, Brigman JL, MacPherson K, Crowley N, Gunduz-Cinar O, et al. Chronic alcohol produces neuroadaptations to prime dorsal striatal learning. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:14783–14788. doi: 10.1073/pnas.1308198110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePoy L, Daut R, Wright T, Camp M, Crowley N, Noronha B, et al. Chronic alcohol alters rewarded behaviors and striatal plasticity. Addiction Biology. 2015;20:345–348. doi: 10.1111/adb.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaud LL, Chadda R. Sex differences in rats in the development of and recovery from ethanol dependence assessed by changes in seizure susceptibility. Alcoholism: Clinical and Experimental Research. 2001;25:1689–1696. [PubMed] [Google Scholar]
- Devaud LL, Risinger FO, Selvage D. Impact of the hormonal milieu on the neurobiology of alcohol dependence and withdrawal. The Journal of General Psychology. 2006;133:337–356. doi: 10.3200/GENP.133.4.337-356. [DOI] [PubMed] [Google Scholar]
- Dhaher R, Finn D, Snelling C, Hitzemann R. Lesions of the extended amygdala in C57BL/6J mice do not block the intermittent ethanol vapor-induced increase in ethanol consumption. Alcoholism: Clinical and Experimental Research. 2007;32:197–208. doi: 10.1111/j.1530-0277.2007.00566.x. [DOI] [PubMed] [Google Scholar]
- Emanuele NV, LaPaglia N, Steiner J, Kirsteins L, Emanuele MA. Effect of chronic ethanol exposure on female rat reproductive cyclicity and hormone secretion. Alcoholism: Clinical and Experimental Research. 2001;25:1025–1029. [PubMed] [Google Scholar]
- Finn DA, Snelling C, Fretwell AM, Tanchuck MA, Underwood L, Cole M, et al. Increased drinking during withdrawal from intermittent ethanol exposure is blocked by the CRF receptor antagonist D-Phe-CRF(12–41) Alcoholism: Clinical and Experimental Research. 2007;31:939–949. doi: 10.1111/j.1530-0277.2007.00379.x. [DOI] [PubMed] [Google Scholar]
- Fish EW, DiBerto JF, Krouse MC, Robinson JE, Melanga CJ. Different contributions of dopamine D1 and D2 receptor activity to alcohol potentiation of brain stimulation reward in C57BL/6J and DBA/2J mice. Journal Pharmacology and Experimental Therapeutics. 2014;350:322–329. doi: 10.1124/jpet.114.216135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golde WT, Gollobin P, Rodriguez LL. A rapid, simple, and humane method for submandibular bleeding of mice using a lancet. Lab Animal. 2005;34:39–43. doi: 10.1038/laban1005-39. [DOI] [PubMed] [Google Scholar]
- Goldstein DB, Pal N. Alcohol dependence produced in mice by inhalation of ethanol: grading the withdrawal reaction. Science. 1971;172:288–290. doi: 10.1126/science.172.3980.288. [DOI] [PubMed] [Google Scholar]
- Goldstein RB, Smith SM, Dawson DA, Grant BF. Sociodemographic and psychiatric diagnostic predictors of 3-year incidence of DSM-IV substance use disorders among men and women in the National Epidemiologic Survey on Alcohol and Related Conditions. Psychology of Addictive Behaviors. 2015;29:924–932. doi: 10.1037/adb0000080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin WC, 3rd, Lopez MF, Becker HC. Intensity and duration of chronic ethanol exposure is critical for subsequent escalation of voluntary ethanol drinking in mice. Alcoholism: Clinical and Experimental Research. 2009;33:1893–1900. doi: 10.1111/j.1530-0277.2009.01027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin WC, 3rd, Lopez MF, Yanke AB, Middaugh LD, Becker HC. Repeated cycles of chronic intermittent ethanol exposure in mice increases voluntary ethanol drinking and ethanol concentrations in the nucleus accumbens. Psychopharmacology. 2009;201:569–580. doi: 10.1007/s00213-008-1324-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefner K, Holmes A. An investigation of the behavioral actions of ethanol across adolescence in mice. Psychopharmacology. 2007;191:311–322. doi: 10.1007/s00213-006-0646-2. [DOI] [PubMed] [Google Scholar]
- Holmes A, Fitzgerald PJ, MacPherson KP, DeBrouse L, Colacicco G, Flynn SM, et al. Chronic alcohol remodels prefrontal neurons and disrupts NMDAR-mediated fear extinction encoding. Nature Neuroscience. 2012;15:1359–1361. doi: 10.1038/nn.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hommer DW. Male and female sensitivity to alcohol-induced brain damage. Alcohol Research & Health. 2003;27:181–185. [PMC free article] [PubMed] [Google Scholar]
- Janis GC, Devaud LL, Mitsuyama H, Morrow AL. Effects of chronic ethanol consumption and withdrawal on the neuroactive steroid 3alpha-hydroxy-5alpha-pregnan-20-one in male and female rats. Alcoholism: Clinical and Experimental Research. 1998;22:2055–2061. [PubMed] [Google Scholar]
- Khan S, Okuda M, Hasin DS, Secades-Villa R, Keyes K, Lin KH, et al. Gender differences in lifetime alcohol dependence: results from the national epidemiologic survey on alcohol and related conditions. Alcoholism: Clinical and Experimental Research. 2013;37:1696–1705. doi: 10.1111/acer.12158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiselycznyk C, Svenningsson P, Delpire E, Holmes A. Genetic, pharmacological and lesion analyses reveal a selective role for corticohippocampal GLUN2B in a novel repeated swim stress paradigm. Neuroscience. 2011;193:259–268. doi: 10.1016/j.neuroscience.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Alcoholism: allostasis and beyond. Alcoholism: Clinical and Experimental Research. 2003;27:232–243. doi: 10.1097/01.ALC.0000057122.36127.C2. [DOI] [PubMed] [Google Scholar]
- Krueger WA, Bo WJ, Rudeen PK. Estrous cyclicity in rats fed an ethanol diet for four months. Pharmacology, Biochemistry, and Behavior. 1983;19:583–585. doi: 10.1016/0091-3057(83)90331-3. [DOI] [PubMed] [Google Scholar]
- Lancaster FE, Brown TD, Coker KL, Elliott JA, Wren SB. Sex differences in alcohol preference and drinking patterns emerge during the early postpubertal period. Alcoholism: Clinical and Experimental Research. 1996;20:1043–1049. doi: 10.1111/j.1530-0277.1996.tb01945.x. [DOI] [PubMed] [Google Scholar]
- Lancaster FE, Spiegel KS. Sex differences in pattern of drinking. Alcohol. 1992;9:415–420. doi: 10.1016/0741-8329(92)90041-8. [DOI] [PubMed] [Google Scholar]
- Li TK, Lumeng L. Alcohol preference and voluntary alcohol intakes of inbred rat strains and the National Institutes of Health heterogeneous stock of rats. Alcoholism: Clinical and Experimental Research. 1984;8:485–486. doi: 10.1111/j.1530-0277.1984.tb05708.x. [DOI] [PubMed] [Google Scholar]
- Lopez MF, Becker HC. Effect of pattern and number of chronic ethanol exposures on subsequent voluntary ethanol intake in C57BL/6J mice. Psychopharmacology. 2005;181:688–696. doi: 10.1007/s00213-005-0026-3. [DOI] [PubMed] [Google Scholar]
- Lopez MF, Grahame NJ, Becker HC. Development of ethanol withdrawal-related sensitization and relapse drinking in mice selected for high- or low-ethanol preference. Alcoholism: Clinical and Experimental Research. 2011;35:953–962. doi: 10.1111/j.1530-0277.2010.01426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez MF, Griffin WC, 3rd, Melendez RI, Becker HC. Repeated cycles of chronic intermittent ethanol exposure leads to the development of tolerance to aversive effects of ethanol in C57BL/6J mice. Alcoholism: Clinical and Experimental Research. 2012;36:1180–1187. doi: 10.1111/j.1530-0277.2011.01717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ, Roth ME, Carroll ME. Biological basis of sex differences in drug abuse: preclinical and clinical studies. Psychopharmacology. 2002;164:121–137. doi: 10.1007/s00213-002-1183-2. [DOI] [PubMed] [Google Scholar]
- McCool BA, Chappell AM. Chronic intermittent ethanol inhalation increases ethanol self-administration in both C57BL/6J and DBA/2J mice. Alcohol. 2015;49:111–120. doi: 10.1016/j.alcohol.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinzie DL, Nowak KL, Murphy JM, Li TK, Lumeng L, McBride WJ. Development of alcohol drinking behavior in rat lines selectively bred for divergent alcohol preference. Alcoholism: Clinical and Experimental Research. 1998;22:1584–1590. [PubMed] [Google Scholar]
- Melón LC, Wray KN, Moore EM, Boehm SL., 2nd Sex and age differences in heavy binge drinking and its effects on alcohol responsivity following abstinence. Pharmacology, Biochemistry, and Behavior. 2013;104:177–187. doi: 10.1016/j.pbb.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore CF, Lynch WJ. Alcohol preferring (P) rats as a model for examining sex differences in alcohol use disorder and its treatment. Pharmacology, Biochemistry, and Behavior. 2015;132:1–9. doi: 10.1016/j.pbb.2015.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales M, McGinnis MM, McCool BA. Chronic ethanol exposure increases voluntary home cage intake in adult male, but not female, Long-Evans rats. Pharmacology, Biochemistry, and Behavior. 2015;139:67–76. doi: 10.1016/j.pbb.2015.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin LP, Forger NG. Endocrine control of ethanol intake by rats or hamsters: relative contributions of the ovaries, adrenals and steroids. Pharmacology, Biochemistry, and Behavior. 1982;17:529–537. doi: 10.1016/0091-3057(82)90315-x. [DOI] [PubMed] [Google Scholar]
- Mumenthaler MS, Taylor JL, O’Hara R, Yesavage JA. Gender differences in moderate drinking effects. Alcohol Research & Health. 1999;23:55–64. [PMC free article] [PubMed] [Google Scholar]
- O’Dell LE, Roberts AJ, Smith RT, Koob GF. Enhanced alcohol self-administration after intermittent versus continuous alcohol vapor exposure. Alcoholism: Clinical and Experimental Research. 2004;28:1676–1682. doi: 10.1097/01.alc.0000145781.11923.4e. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Breese GR. Similar anxiety-like responses in male and female rats exposed to repeated withdrawals from ethanol. Pharmacology, Biochemistry, and Behavior. 2004;78:459–464. doi: 10.1016/j.pbb.2004.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual M, Boix J, Felipo V, Guerri C. Repeated alcohol administration during adolescence causes changes in the mesolimbic dopaminergic and glutamatergic systems and promotes alcohol intake in the adult rat. Journal of Neurochemistry. 2009;108:920–931. doi: 10.1111/j.1471-4159.2008.05835.x. [DOI] [PubMed] [Google Scholar]
- Radke AK, Jury NJ, Kocharian A, Marcinkiewcz CA, Lowery-Gionta EG, Pleil KE, et al. Chronic EtOH effects on putative measures of compulsive behavior in mice. Addiction Biology. 2015 doi: 10.1111/adb.12342. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly W, Koirala B, Devaud LL. Sex differences in acoustic startle responses and seizure thresholds between ethanol-withdrawn male and female rats. Alcohol and Alcoholism. 2009;44:561–566. doi: 10.1093/alcalc/agp049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AJ, Smith AD, Weiss F, Rivier C, Koob GF. Estrous cycle effects on operant responding for ethanol in female rats. Alcoholism: Clinical and Experimental Research. 1998;22:1564–1569. [PubMed] [Google Scholar]
- Robinson DL, Brunner LJ, Gonzales RA. Effect of gender and estrous cycle on the pharmacokinetics of ethanol in the rat brain. Alcoholism: Clinical and Experimental Research. 2002;26:165–172. [PubMed] [Google Scholar]
- Rosenwasser AM, McCulley WD, 3rd, Fecteau M. Circadian activity rhythms and voluntary ethanol intake in male and female ethanol-preferring rats: effects of long-term ethanol access. Alcohol. 2014;48:647–655. doi: 10.1016/j.alcohol.2014.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchis R, Esquifino A, Guerri C. Chronic ethanol intake modifies cyclicity and alters prolactin and LH levels. Pharmacology, Biochemistry, and Behavior. 1985;23:221–224. doi: 10.1016/0091-3057(85)90560-x. [DOI] [PubMed] [Google Scholar]
- Schramm-Sapyta NL, Francis R, MacDonald A, Keistler C, O’Neill L, Kuhn CM. Effect of sex on ethanol consumption and conditioned taste aversion in adolescent and adult rats. Psychopharmacology. 2014;231:1831–1839. doi: 10.1007/s00213-013-3319-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neuroscience and Biobehavioral Reviews. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Strong MN, Kaufman KR, Crabbe JC, Finn DA. Sex differences in acute ethanol withdrawal severity after adrenalectomy and gonadectomy in Withdrawal Seizure-Prone and Withdrawal Seizure-Resistant mice. Alcohol. 2009;43:367–377. doi: 10.1016/j.alcohol.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanchuck-Nipper MA, Ford MM, Hertzberg A, Beadles-Bohling A, Cozzoli DK, Finn DA. Sex Differences in Ethanol’s Anxiolytic Effect and Chronic Ethanol Withdrawal Severity in Mice with a Null Mutation of the 5α-Reductase Type 1 Gene. Behavior Genetics. 2015;45:354–367. doi: 10.1007/s10519-014-9691-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres OV, Walker EM, Beas BS, O’Dell LE. Female rats display enhanced rewarding effects of ethanol that are hormone dependent. Alcoholism: Clinical and Experimental Research. 2014;38:108–115. doi: 10.1111/acer.12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Acute effects of ethanol on social behavior of adolescent and adult rats: role of familiarity of the test situation. Alcoholism: Clinical and Experimental Research. 2002;26:1502–1511. doi: 10.1097/01.ALC.0000034033.95701.E3. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Acute ethanol withdrawal (hangover) and social behavior in adolescent and adult male and female Sprague-Dawley rats. Alcoholism: Clinical and Experimental Research. 2004;28:40–50. doi: 10.1097/01.ALC.0000108655.51087.DF. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Truxell EM, Spear LP. Ethanol intake under social circumstances or alone in sprague-dawley rats: impact of age, sex, social activity, and social anxiety-like behavior. Alcoholism: Clinical and Experimental Research. 2015;39:117–125. doi: 10.1111/acer.12604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veatch LM, Wright TM, Randall CL. Only male mice show sensitization of handling-induced convulsions across repeated ethanol withdrawal cycles. Alcoholism: Clinical and Experimental Research. 2007;31:477–485. doi: 10.1111/j.1530-0277.2006.00328.x. [DOI] [PubMed] [Google Scholar]
- Vengeliene V, Vollmayr B, Henn FA, Spanagel R. Voluntary alcohol intake in two rat lines selectively bred for learned helpless and non-helpless behavior. Psychopharmacology. 2005;178:125–132. doi: 10.1007/s00213-004-2013-5. [DOI] [PubMed] [Google Scholar]
- Vetter CS, Doremus-Fitzwater TL, Spear LP. Time course of elevated ethanol intake in adolescent relative to adult rats under continuous, voluntary-access conditions. Alcoholism: Clinical and Experimental Research. 2007;31:1159–1168. doi: 10.1111/j.1530-0277.2007.00417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walls SA, Macklin ZL, Devaud LL. Ethanol-induced loss-of-righting response during ethanol withdrawal in male and female rats: associations with alterations in Arc labeling. Alcoholism: Clinical and Experimental Research. 2012;36:234–241. doi: 10.1111/j.1530-0277.2011.01613.x. [DOI] [PubMed] [Google Scholar]
- Zhao S, Edwards J, Carroll J, Wiedholz L, Millstein RA, Jaing C, et al. Insertion mutation at the C-terminus of the serotonin transporter disrupts brain serotonin function and emotion-related behaviors in mice. Neuroscience. 2006;140:321–334. doi: 10.1016/j.neuroscience.2006.01.049. [DOI] [PubMed] [Google Scholar]