Abstract
Purpose
To evaluate factors associated with progression-free and disease-specific survival in patients with paratesticular rhabdomyosarcoma, we performed a cohort study. Also, since many patients present to our institution after initial therapy, we analyzed the effects of salvage therapy for scrotal violation.
Patients and Methods
We retrospectively reviewed the records of all consecutive patients with histologically confirmed paratesticular rhabdomyosarcoma treated at our institution between 1978 and 2015. Fifty-one patients were initially identified, but two were excluded due to incomplete data for analysis. Variables evaluated for correlation with survival were TNM staging, COG-STS pretreatment staging, margins at initial resection, presence of scrotal violation, hemiscrotectomy and/or scrotal radiation. The log-rank test was used to compare survival distributions.
Results
For the analytic cohort of 49 patients, the median age and follow-up were 15.7 years (95% CI: 14.2-17.5, range: 0.8-25.1 years) and 6.9 years (95% CI: 4.4-9.0, range 0.2-37.5 years), respectively. The 5-year overall disease-specific survival was 78.7% (95% CI: 67.7-91.4%) and the progression-free survival was 66.9% (95% CI: 54.8-81.6%). Median time to recurrence was 0.9 years (95% CI: 0.7-0.9, range 0.1-6.2 years). Scrotal violation occurred in 41% (n=20) and tripled the risk of recurrence for patients not appropriately treated with either hemiscrotectomy or scrotal radiation therapy (RR=3.0, 95% CI: 1.16-7.73).
Conclusions
The strongest predictors of disease-specific survival were nodal status and distant metastasis at diagnosis. Scrotal violation remains a problem in paratesticular rhabdomyosarcoma and is a predictor of disease progression unless adequately treated. The risk of progression could be reduced with appropriate initial resection.
Keywords: Paratesticular rhabdomyosarcoma, Rhabdomyosarcoma, Scrotal radiation, Hemiscrotectomy, Scrotal violation
Introduction
In children, about 7% of all cases of genitourinary rhabdomyosarcoma are paratesticular in origin.[1, 2] These tumors arise from the mesenchymal tissue of the epididymis, spermatic cord, testis, and testicular tunica and account for 12% of pediatric scrotal tumors.[3, 4] The disease exhibits a bimodal age distribution with peaks in infancy at 3-4 months and in adolescence at about 16 years of age.[5] Prognosis for paratesticular rhabdomyosarcoma (PT-RMS) is favorable; approximately 60% to 80% of tumors are detected early while disease is localized, the majority of cases are embryonal subtype, and total resection can often be achieved.[6-8] As a result, the 5-year overall survival associated with multidisciplinary therapy, including surgical resection, chemotherapy, and radiation, exceeds 80%.
The surgical approach for PT-RMS is a radical inguinal orchiectomy with high dissection and ligation of the spermatic cord. Trans-scrotal excision or biopsy of the mass is inappropriate, as either would introduce risks of scrotal contamination with microscopic residual disease and/or residual disease in the cord from inadequate exposure. Remarkably, scrotal violations are reported to occur in up to 25% of PTRMS cases.[9] Salvage therapy after scrotal violation involves primary re-excision via an inguinal approach for residual mass or cord and wide local excision of the scrotal scar tissue, frequently resulting in a hemiscrotectomy. Alternatively, salvage therapy can include primary re-excision coupled with transposition of the contralateral testicle in anticipation of scrotal radiation therapy. The requirement of hemiscrotectomy versus irradiation of the scrotum after a scrotal violation remains unclear.[3, 10] We sought to analyze the effects of salvage therapy after scrotal violation and evaluate factors associated with progression-free and disease specific survival.
Patients and Methods
Patients
After obtaining Institutional Review Board approval for our retrospective study, we searched our surgical database and identified all consecutive patients 30 years of age or younger with a pathologically confirmed diagnosis of PT-RMS treated at our institution between January 1978 and September 2015. Two patients were excluded for incomplete data. Pathologic cellular classification of embryonal rhabdomyosarcoma was made in 48 patients and pleomorphic rhabdomyosarcoma in 1 patient. All patients were treated according to study protocols that entailed tumor resection, multi-agent chemotherapy, and/or radiation therapy.
Surgery
Surgical guidelines stipulate a radical inguinal orchiectomy for PT-RMS. Patients who underwent a transscrotal approach with incomplete resection and positive margins underwent primary re-excision via an inguinal approach. Hemiscrotectomy is prescribed for scrotal violation, direct invasion of the scrotum by the mass, palpable residual disease, and positive margins. In general, surgical salvage therapy at our institution included primary re-excision with hemiscrotectomy or primary re-excision and scrotal radiation following transposition of the contralateral testicle. After completion of electron beam radiation, the remaining testicle was placed back in the scrotum and a prosthesis was inserted. Scrotal counter-incision for delivery of extremely large masses incapable of being extirpated via inguinal approach after high ligation of the cord was not classified as a scrotal violation. The use of ipsilateral retroperitoneal lymph node dissection (iRPLND) was implemented according to guidelines in place at the time of preoperative evaluation.
Staging
The TNM staging system was used to classify the clinical status of the tumor, whereby T1 signifies tumor confined to the organ or tissue of origin (a: ≤5 cm; b: >5 cm); T2 signifies tumor involving contiguous organs and structures (a: ≤5 cm; b: >5 cm); N0 indicates that there is no clinical involvement of regional lymph nodes; N1 signifies clinical involvement of regional lymph nodes; M0 indicates that there is no metastasis; and M1 signifies presences of metastasis. The Soft Tissue Sarcoma Committee of the Children's Oncology Group (COG-STS) Pretreatment Staging System was used to classify tumors utilizing the TNM staging system and the favorable location of the tumor.
Assessment of Resection Status
Resections for tumors were classified as follows: microscopically complete tumor resection was designated R0, gross total resection with microscopic residual disease R1, and gross residual disease R2.
Assessment of Relapse
Assessment of local or metastatic relapse was performed for all patients. Local relapse was defined at the primary tumor site. Locoregional relapse was defined as tumor relapse in the inguinal or paraaortic lymph nodes. Metastatic relapse was defined as relapse in distant organs.
Statistical Analysis
The analyses are based upon data as of October 2015. Demographic data are reported as median values (95% CI, range). The 5-year progression-free and disease-specific survival rates were estimated using Kaplan-Meier analysis. For overall survival, the time from primary diagnosis to last follow-up or death was used. Calculation of progression-free survival was the time from diagnosis to the time of first relapse. The log-rank test was used to compare survival distributions. The Wilcoxon signed rank test was used to compare median values between groups. A P-value less than or equal to 0.05 was considered significant. All statistical analyses were performed using R software (version 3.2.4, R Project for Statistical Computing, Vienna, Austria; www.r-project.org), using the survival statistics and “asbio” packages.
Results
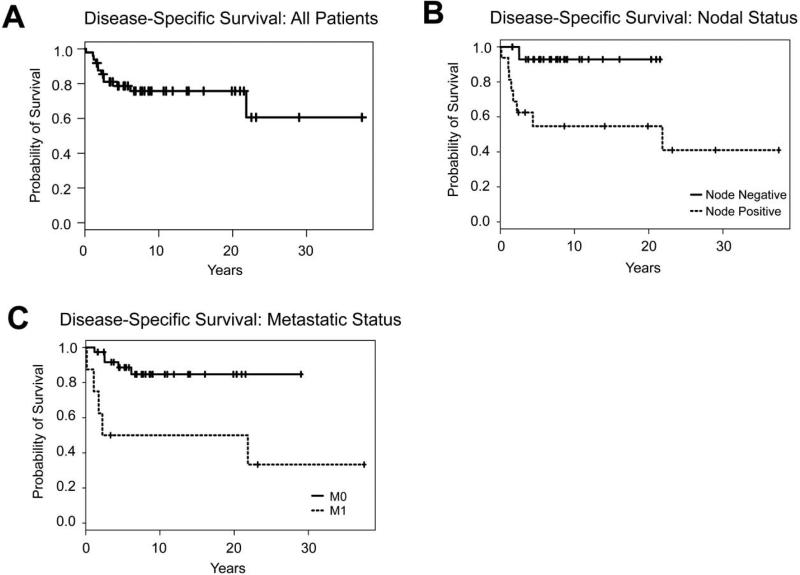
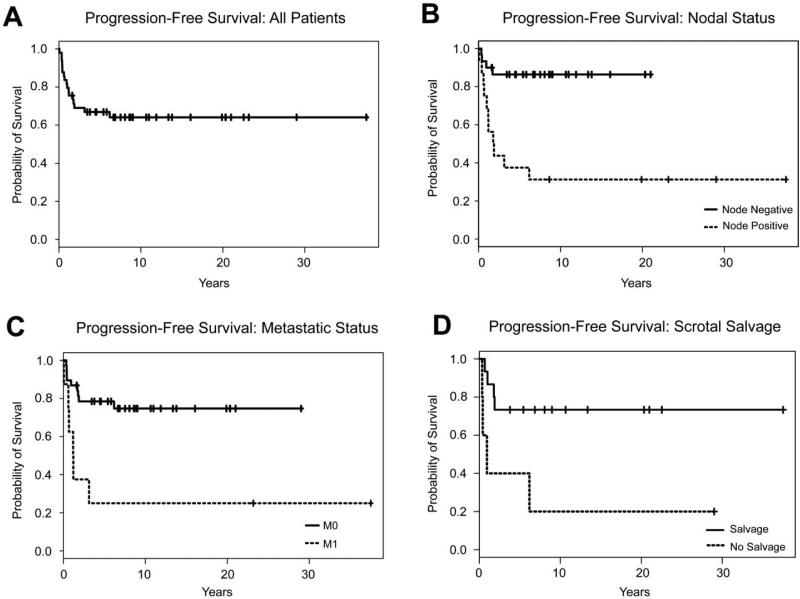
The 5-year disease-specific survival for all patients was 78.7% (95% CI: 67.7-91.4 percent) and the progression-free survival was 66.9% (95% CI: 54.8-81.6 percent) (Figures 1A, 2A). Median time to recurrence was 0.9 years (95% CI: 0.7-0.9, range 0.1-6.2 years). Median age for all patients was 15.7 years (95% CI: 14.2-17.5, range 0.8-25.1 years) and median follow-up was 6.9 years (95% CI: 4.4-9.0, range 0.2-37.5 years). Median tumor size was 4.6 cm (widest diameter) (95% CI: 3.7-6.5, range 1.2-20.0 cm). A total of 8 patients had COG-STS Pretreatment Stage IV disease. All patients received chemotherapy, 28 patients received radiation therapy either as salvage therapy or for treatment of metastatic disease, and 23 patients received iRPLND as part of their staging or for removal of disease recurrence in the retroperitoneum.
Figure 1.
Disease-specific survival was calculated using Kaplan-Meier analysis. Survival curves are shown (A) for the full cohort (N=49), (B) stratified by nodal status, and (C) stratified by the presence of metastasis.
Figure 2.
Progression-free survival was calculated using Kaplan-Meier analysis. Survival curves are shown (A) for the full cohort (N=49), (B) stratified by nodal status, (C) stratified by the presence of metastasis, and (D) stratified by presence of salvage therapy.
Variable correlation with disease-specific survival and progression-free survival are presented in Table 1. Nodal status and distant metastases at diagnosis were correlated with disease-specific and progression-free survival (Figures 1B, 1C, 2B and 2C). Appropriate scrotal treatment after scrotal violation, defined as primary re-excision with hemiscrotectomy or primary re-excision with transposition of the contralateral testicle, with subsequent scrotal radiation therapy, was correlated with progression-free survival (P=0.02, Figure 2D), but did not correlate with disease-specific survival. Tumor size and positive margin status were not associated with shorter survival or recurrence in our series.
Table 1.
Univariate analysis to assess correlation of disease and surgical variables with survival (P-value ≤0.05 statistically significant)
| Variable | Disease-Specific Survival | Progression-Free Survival |
|---|---|---|
| T-stage | 0.46 | 0.08 |
| N-stage | 0.001 | 0.001 |
| M-stage | 0.005 | 0.003 |
| COG-STS: Pretreatment Staging System | 0.005 | 0.003 |
| Scrotal Violation | 0.86 | 0.65 |
| Appropriate scrotal treatment after scrotal violation | 0.54 | 0.02 |
COG-STS = Children's Oncology Group, Soft Tissue Sarcoma Committee.
Twenty scrotal violations occurred in our study cohort; 95% of patients with scrotal violations had undergone initial resection at outside facilities and a variety of surgical approaches were used (Table 2). Fifteen patients received salvage therapy after scrotal violation, which included primary reexcision and hemiscrotectomy or primary reexcision, transposition of the contralateral testicle, and subsequent scrotal radiation therapy. Median time to salvage therapy was 19 days (95% CI: 8-39, range 0 to 472 days). In total, 4 of 15 (26.6%) patients treated with salvage therapy after scrotal violation had disease recurrence versus 4 of 5 (80%) patients not treated with salvage therapy (RR=3.0, 95% CI: 1.16-7.73). Locations of disease recurrence in patients with scrotal violation are listed in Table 3. Patient characteristics stratified by the presence of scrotal violation are summarized in Table 4. There was no significant difference in disease-specific survival for patients that received inappropriate therapy resulting in a scrotal violation (P=0.86).
Table 2.
Classification of initial surgeries resulting in scrotal violation. (n=20)
| Type of Surgery | Number of Patients (n) |
|---|---|
| Trans-scrotal tumor biopsy | 4 |
| Trans-scrotal tumorectomy | 10 |
| Inguinal tumorectomy without orchiectomy | 2 |
| Inguinal tumor biopsy without orchiectomy | 2 |
| Radical inguinal orchiectomy with trans-scrotal incision and contralateral orchiopexy | 1 |
| Radical inguinal orchiectomy with tumor rupture | 1 |
Table 3.
Location of recurrence in patients with scrotal violation based on presence or absence of salvage therapy.
| Location of disease recurrence | Salvage Therapy (n) | No Salvage Therapy (n) |
|---|---|---|
| Local | 1 | 1 |
| Regional | 2 | 1 |
| Metastatic* | 1 (lung) | 2 (lung, spine) |
Location(s) of metastatic disease provided.
Table 4.
Patient demographic and disease data based on the presence of appropriate initial surgery.
| Scrotal Violation (n=20) | Radical Inguinal Orchiectomy (n=29) | P-Value | |
|---|---|---|---|
| Median age (y) | 17.1 [7.1-17.8] | 15.4 [10.9-19.4] | 0.85 |
| <10 y (n) | 6 | 8 | |
| >10 y (n) | 14 | 21 | |
| Median follow-up (y) | 7.9 [4.4-20.3] | 5.8 [3.4-9.0] | 0.24 |
| Tumor size (n) | |||
| <5 cm in diameter | 11 | 12 | 0.28 |
| ≥5 cm in diameter | 6 | 15 | |
| Unknown | 3 | 2 | |
| Initial lymph node involvement (n) | |||
| Yes | 7 | 9 | 0.58 |
| No | 11 | 19 | |
| Unknown | 2 | 1 | |
| COG-STS Pretreatment Stage (n) | |||
| Stage I | 16 | 22 | 0.43 |
| Stage IV | 2 | 6 | |
| Unknown | 2 | 1 | |
| 5-year disease-specific survival (%) | 79.1 [62.7-99.7] | 78.2 [64.2-95.3] | 0.86 |
| 5-year progression-free survival (%) | 59.1 [40.7-85.7] | 68.3 [53.1-87.8] | 0.66 |
| Recurrence (n) | |||
| Local | 3 | 3 | 0.68 |
| Regional | 2 | 1 | |
| Distant | 3 | 5 | |
| Specialization of initial surgeon (n) | |||
| Pediatric Surgeon | 3 | 3 | 0.52 |
| Pediatric Urologist | 13 | 23 | |
| Unknown | 4 | 3 |
COG-STS = Children's Oncology Group, Soft Tissue Sarcoma Committee.
Overall, 17 patients developed recurrence (5 local, 4 regional, 8 metastatic). The number of recurrence events among patients with scrotal violation was not significantly different from the number among those who received appropriate initial therapy (P=0.68). There were 12 deaths in our patient cohort, all attributed to progression of disease. One death at 20 years of follow-up was attributable to colon adenocarcinoma in an irradiated field.
Discussion
The decision-making process for workup of and surgical approach to a scrotal mass must include the consideration of possible malignancy. Clinical exam, history of progression, radiologic and tumor markers can help narrow the differential diagnosis, which includes testicular torsion, hydrocele, inguinal hernia, mumps orchitis, and epididymo-orchitis. The surgical approach prescribed for PT-RMS is a radical inguinal orchiectomy with high dissection and ligation of the cord structures. Trans-scrotal approaches, inguinal tumorectomy without orchiectomy, or inguinal tumor biopsy are not indicated as they risk scrotal contamination.
The requirement of primary re-excision with hemiscrotectomy after scrotal violation remains unclear, as studies are generally underpowered.[3, 10, 11] In our series, a large portion of patients had a scrotal violation (41%), higher than the 25% reported in literature.[9] There was no statistical difference in age, tumor size, lymph node or metastasis status, length of follow-up, or recurrence in patients with scrotal violation compared to those who received appropriate initial surgery. Our data suggest no difference in disease-specific survival or progression-free survival with the implementation of salvage therapy after scrotal violation. In 5 patients, we identified no salvage therapy after scrotal violation. In this group, the risk of recurrence tripled, highlighting the importance of identifying and addressing cases of scrotal violation.
The overall disease-specific survival in our series reflects a progression of care standards over 37 years. Our survival data, when evaluated using time periods that mirrored the periods of the Intergroup Rhabdomyosarcoma Studies (IRS), demonstrate a survival of approximately 60% between the time periods of IRS-II to IRS-IV, 67% during the time period of IRS-V, and 79% in the time period since the completion of IRS-V. Though our overall disease-specific survival of 78.7% is lower than reports in current series, it does incorporate a high number of patients who received salvage therapy at our institution after initial therapy at outside facilities. Our data represent the largest single-institution review of PT-RMS and specific factors associated with survival.
In conclusion, children with PT-RMS require total resection via a radical inguinal orchiectomy. Scrotal violation occurs quite frequently in patients with PT-RMS, and appropriate salvage therapy appears to ameliorate the effect on disease-specific survival and progression-free survival. Our study does not have the power to definitively answer these questions, and continued effort from cooperative groups are required to address these topics. However, the frequency of scrotal violations in our series highlights the importance of educating providers on appropriate workup of scrotal masses.
William J. Hammond,
Q: Mark Davenport, London
It is an axiom of surgery never to approach a potential testicular tumour through the scrotum. When you looked back through the notes of the people referred to you what was the overriding theme - was done by a nurse assistant?
A: William J. Hammond,
The overriding theme was a presumption of a more common event either a testicular torsion, a hernia, things of this nature. With this being roughly equivalent to 8 cases per year in the entire USA, it is something that is not really on the top of a differential diagnosis amongst the providers.
Q: Mark Powis, Leeds
You say scrotal violation should be addressed by further salvage surgery. I think scrotal violation should be addressed by education of the people who are doing this in the first place. Scrotal violation is a protocol violation and these people shouldn't be doing surgery. Is there a plan to educate these people?
A: William J. Hammond,
We would like to say so! Carrying forward this study if we were able to put it out there in the literature and highlight the need to revamp education and work-up of how to deal with these scrotal masses. It would certainly help.
Funding Acknowledgement
Research at Memorial Sloan Kettering Cancer Center is supported in part by a Cancer Center Support Grant from the U.S. National Institutes of Health/National Cancer Institute (#P30 CA008748).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Level of Evidence: Level IV, retrospective study with no comparison group
REFERENCES
- 1.Crist W, Gehan EA, et al. The Third Intergroup Rhabdomyosarcoma Study. J Clin Oncol. 1995;13:610–630. doi: 10.1200/JCO.1995.13.3.610. [DOI] [PubMed] [Google Scholar]
- 2.Maurer HM, Beltangady M, et al. The Intergroup Rhabdomyosarcoma Study-I: A final report. Cancer. 1988;61:209–220. doi: 10.1002/1097-0142(19880115)61:2<209::aid-cncr2820610202>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 3.Dall'Igna P, Bisogno G, et al. Primary transcrotal excision for paratesticular rhabdomyosarcoma: is hemiscrotectomy really mandatory? Cancer. 2003;97:1981–1984. doi: 10.1002/cncr.11284. [DOI] [PubMed] [Google Scholar]
- 4.Andrassy RJ. Rhabdomyosarcoma. Semin Pediatr Surg. 1997;6:17–23. [PubMed] [Google Scholar]
- 5.Ahmed HU, Arya M, et al. Testicular and paratesticular tumours in the prepubertal population. Lancet Oncol. 2010;11:476–483. doi: 10.1016/S1470-2045(10)70012-7. [DOI] [PubMed] [Google Scholar]
- 6.Dangle PP, Correa A, et al. Current management of paratesticular rhabdomyosarcoma. Urol Oncol. 2016;34:84–92. doi: 10.1016/j.urolonc.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 7.Ferrari A, Bisogno G, Casanova M, et al. Paratesticular rhabdomyosarcoma: report from the Italian and German Cooperative Group. J Clin Oncol. 2002;20:449–455. doi: 10.1200/JCO.2002.20.2.449. [DOI] [PubMed] [Google Scholar]
- 8.Stewart RJ, Martelli H, Oberlin O, et al. Treatment of children with nonmetastatic paratesticular rhabdomyosarcoma: results of the Malignant Mesenchymal Tumors studies (MMT 84 and MMT 89) of the International Society of Pediatric Oncology. J Clin Oncol. 2003;21:793–798. doi: 10.1200/JCO.2003.06.040. [DOI] [PubMed] [Google Scholar]
- 9.Cecchetto G, De Corti F, Rogers T, et al. Surgical compliance with guidelines for paratesticular rhabdomyosarcoma (RMS). Data from the European Study on nonmetastatic RMS. J Pediatr Surg. 2012;47:2161–2162. doi: 10.1016/j.jpedsurg.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 10.Rogers DA, Rao BN, Meyer WH, et al. Indications for hemiscrotectomy in the management of genitourinary tumors in children. J Pediatr Surg. 1995;30:1437–1439. doi: 10.1016/0022-3468(95)90400-x. [DOI] [PubMed] [Google Scholar]
- 11.Seitz G, Dantonello TM, Kosztyla D, et al. Impact of hemiscrotectomy on outcome of patients with embryonal paratesticular rhabdomyosarcoma: results from the Cooperative Soft Tissue Sarcoma Group Studies CWS-86, 91, 96 and 2002P. J Urol. 2014;192:902–907. doi: 10.1016/j.juro.2014.03.005. [DOI] [PubMed] [Google Scholar]