Abstract
Background
Environmental enrichment (EE) has a beneficial effect on some neuropsychiatric disorders. In this study, we aimed to investigate whether environmental enrichment could improve the spatial learning and memory in rats with vascular dementia (VaD) and the mechanism underpinning it.
Material/Methods
Bilateral common carotid occlusion (2-vessel occlusion [2VO]) was used to develop the animal model of vascular dementia. Adult male Sprague-Dawley (SD) rats were used in the experiment and were randomly divided into 4 groups: sham group, 2VO group, sham+EE group, and 2VO+EE group (n=19/group). The 2VO group and 2VO+EE group underwent bilateral common carotid occlusion. Two different housing conditions were used in this experiment: standard environment (SE) and enriched environment (EE). Rats in the sham group and 2VO group were put into SE cages for 4 weeks, while rats in the sham+EE group and 2VO+EE group were put in EE cages for 4 weeks. The Morris water maze and Y-maze were used to assess spatial learning and memory. Apoptosis was detected by TUNEL. The damage of neurons in the hippocampus was assessed by Nissl staining. The level of wnt pathway proteins were detected by Western blot.
Results
Compared with the 2VO group, the rats in the 2VO+EE group had better behavioral performance, fewer apoptotic neurons, and more surviving neurons. Western blot analysis showed that the levels of wnt pathway proteins were higher in 2VO+EE rats than in the 2VO group.
Conclusions
Environmental enrichment can improve the spatial learning and memory in rats with vascular dementia, and the mechanism may be related to activation of the wnt/β-catenin signal pathway.
MeSH Keywords: Cognition; Dementia, Vascular; Environmental Exposure; Wnt Signaling Pathway
Background
Vascular dementia (VaD) is defined as the loss of cognitive function resulting from cerebral vascular diseases and cardiovascular pathologic changes [1], and its main manifestation is impairment in learning and memory [2]. It is the second most common cause of dementia in the elderly, after Alzheimer disease (AD), and its notable symptom is cognitive loss [1]. To date, it is still very difficult to treat the impairment of VaD, except by use of drugs approved for AD that might have some benefit in patients diagnosed with VaD [3]. Therefore, the study of treatment for VaD is important.
Environmental enrichment (EE), which was first put forward by Hebb in 1947, is a rich and stimulating environment. Since Hebb’s description, many studies on EE have been performed. Early studies have indicated that EE could induce neural and synaptic changes, like increased dendritic branching, neural density, and neural transmission [4–6]. Recently, Valero et al. found that EE has a beneficial effect on memory deficits in an AD mouse model [7]. Verret et al. also found that exposure to EE before amyloidosis onset improved cognitive functions during AD pathology [8]. EE has also been proved to ameliorate functional and molecular deficits in many other central nervous system disorders, including depression, stroke, traumatic brain injury, and schizophrenia [9], but it is still remains unknow whether EE has beneficial effects on VaD and what the mechanisms are.
The wnt/β-catenin signal pathway has emerged as a crucial pathway in neuronal self-renewal, proliferation, differentiation, maturation, and functional integration [10]. It has been proved to facilitate long-term potentiation [11] and deregulation of the wnt/β-catenin pathway has been implicated in the pathology of AD [12]. It also has been shown to be involved in behavioral modulation and several psychiatric disorders [10,13,14]. Additionally, upregulating of wnt/β-catenin is also implicated in the enhancing of cognitive functions of adult rats [15,16], but whether activation of wnt/β-catenin is a mechanism of EE is still unknown.
In this study, we aimed to investigate whether EE can improve spatial learning and memory in rats with VaD and if there is any activation of the wnt/β-catenin signal pathway.
Material and Methods
Animals and timeline of experiments
Adult Sprague-Dawley (SD) male rats (8 weeks old, 240–260 g) were obtained from the Experimental Animal Center of Chongqing Medical University. All animal procedures were approved by the Laboratory Animal Management Committee of Chongqing Medical University and were in accordance with the guidelines of the China Animal Protection Law. During animal procedures, we made efforts to minimize animal suffering. A total of 84 rats were used (dead or blinded, n=8). Rats were randomly divided into 4 groups: sham group, 2VO group, sham+EE group, and 2VO+EE group (n=19/group). The study timeline is shown in Figure 1.
Figure 1.
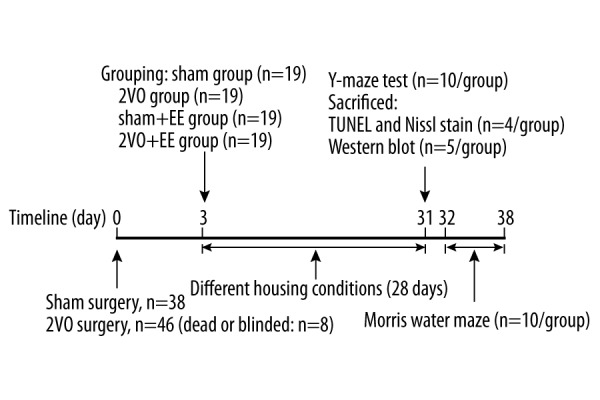
Timeline for experiments. 2VO group and 2VO+EE underwent 2VO surgery. Rats in sham group and 2VO group were put into SE cage for 28 days (4 weeks). Rats in sham+EE group and 2VO+EE group were put into EE cage for 28 days (4 weeks). 2VO – bilateral common carotid occlusion; EE – environmental enrichment.
Surgery
The rats in the 2VO and 2VO+EE groups underwent bilateral common carotid occlusion (2VO) surgery, which was used to an develop animal model for vascular dementia [17]. Chloral hydrate was used for anesthesia. Anesthetized rats were fixed supine on a heated pad, then the right common carotid artery was exposed. We separated the right common carotid artery gently from the right vagus nerve, then ligated it. The left common carotid artery was ligated in the same way. The rats in the sham and sham+EE group underwent the same surgical procedure without carotid occlusion. The survival rate was 82.61% (38 out of 46) among 2VO rats and 100% among the sham rats.
Housing conditions
Two different housing conditions were used in this experiment: standard environment (SE) and environmental enrichment (EE). SE was a standard cage (cage size: 52×36×20 cm) containing only food and water. EE consisted of a larger cage (cage size: 70×45×38 cm) containing many different objects besides food and water, including running wheels, a tunnel, a small compartment, a balance beam, stairs, and many other colorful objects (e.g., colorful plastic plate, red ball, wooden disks of varied colors and sizes, plastic cups, and hanging cubes). Rats were put into the EE cage for 12 h a day (from 9: 00 pm to 9: 00 am), 7 days a week, for 4 weeks. The objects in the EE cage were changed every day.
Morris water maze
An open circular pool (160 cm in diameter, 60 cm in height) with a camera hanging over it was filled to a depth of 25 cm with warm water (temperature was maintained at 22±1°C). Nontoxic black paint was added to make the water opaque. Four points on the perimeter of the pool were used to determine 4 quadrants of the pool. A black escape platform was placed in the middle of one of the quadrants (submerged 1 cm below the water level). The animals were faced toward the wall of the pool and placed gently into the water from 4 points for 5 days. If unable to find the platform within 60 s, the rats would be guided to the platform by the researcher and were left on the platform for 30 s. All rats were subjected to 4 trials per day for 5 consecutive days (n=10/group). On day 6, the platform was removed, and animals underwent the probe test for 60 s. The MWM performance was recorded by a video camera [18].
Y-maze test
The Y-maze is a horizontal maze consisting of 3 arms at an angle of 120° with respect to each arm. Each arm is 40 cm long, 30 cm high, and 15 cm wide. Rats were first placed within one arm and were allowed to explore the maze freely for an 8-min period. The sequence and the total number of arms entered were recorded by Behavioral Video Tracking System for each rat (n=10/group). An arm entry was counted when all 4 paws were in the arm [19]. After each trial, the Y-maze was thoroughly cleaned and wiped with alcohol to eliminate residual odors and residues. The spontaneous alternation behavior was defined as consecutive entries into all 3 arms (ABC, BCA, or CAB but not ABA, or BCB). The percentage (%) of spontaneous alternation behavior was calculated according to the following equation: The percent of alternation = (number of alternations)/(total number of arm entries − 2) ×100 (%) [20,21]. In addition, total number of arm entries was regarded as an index of motor activity [19].
Terminal Deoxynucleotidyl Transferase (TdT)-Mediated dUTP Nick-End Labeling (TUNEL)
Cell apoptosis in rat brain was detected by TUNEL according to the manufacturer’s protocol (TUNEL Detection Kit, Roche, USA). Briefly, the paraffin sections were heated and dewaxed, rehydrated, and washed 3 times in phosphate-buffered saline (PBS). Proteinase K was used to digest tissues at 37°C for 15 min. The sections were then incubated with 50 μL of TUNEL reaction mixture at 37°C for 1 h. Sections were incubated with Converter-POD solution at 37°C for 30 min. Positive cells (brown staining) and total cells in the hippocampal CA1 region were counted under a light microscope by 2 independent individuals who were blind to the experimental condition [22]. At least 5 representative CA1 fields were randomly counted per section and 3 sections were counted per brain (n=4/group). Apoptotic index (positive apoptotic cells/total cells ×100%) was used to measure apoptotic severity [23].
Nissl staining
Rats were perfused with 0.9% saline followed by 4% paraformaldehyde after being anesthetized. The brains were removed and postfixed overnight in 4% paraformaldehyde. Then the brains were embedded in paraffin, and cut into paraffin sections, which was performed with Nissl staining according to standard protocols to observe the histopathological changes of neurons. Neurons with a round cell body, Nissl substance in the cytoplasm, and visible nucleoli were considered normal neurons, while neurons with loss of Nissl substance, cavitation around the nucleus, and indiscernible nuclei were considered damaged neurons [24]. The population of normal neurons in a CA1 subfield were counted at 400× magnification by 2 independent investigators who were blind to the experimental condition in this study. The mean number of neurons was obtained by counting 3 sections per brain and 5 representative fields were randomly selected to count per section (n=4/group) [25].
Western blot
The rat hippocampus was homogenized in RIPA lysis buffer (P0013B, Beyotime, Jiangsu, China) containing PMSF. Nuclear extracts were used for the measurement of β-catenin levels (n=5/group). Protein concentrations were detected using a bicinchoninic acid protein assay kit (Beyotime, Shanghai, China). The proteins were separated by 10% gel electrophoresis and transferred to polyvinylidene fluoride (PVDF) membranes (250 mA for 90 min). The membranes were blocked with 5% milk at room temperature for 1 h and then incubated with rabbit anti-wnt3a antibody (1: 100, #bs-1700R, Bioss Inc., China), rabbit anti-GSK-3β antibody (1: 1000, #YT2082, Immunoway, USA), rabbit anti-p-GSK-3β (Ser9) antibody (1: 1000, #YP0124, Immunoway, USA), rabbit anti-β-catenin antibody (1: 2000, #NBP1-32239, Novus, USA) overnight at 4°C. After that, the membranes were exposed to secondary antibodies at 37°C for 1 h. The visualization was performed using the ECL (Beyotime Institute of Biotechnology, China) PLUS system and protein levels were measured and analyzed using Image J software.
Statistical analysis
All the data are expressed as means ± standard error of the mean (SEM). Time latency and swimming speed were analyzed by two-way repeated measures analysis of variance (ANOVA) followed by a post hoc least significant differences (LSD) multiple-comparison test. The data of probe test (time spent in target quadrant), Y-maze test (the percent of alternation), TUNEL (percent of TUNEL-positive cell), Nissl staining (number of survival cells), and Western blot (quantifications of band densities) were analyzed using oneway ANOVA followed by the Students-Newman-Keuls (SNK) multiple comparisons test. A value of P<0.05 was considered statistically significant. All statistical analyses were performed using SPSS, version 22.0 (Chicago, IL, USA).
Results
Effects of EE on learning and memory
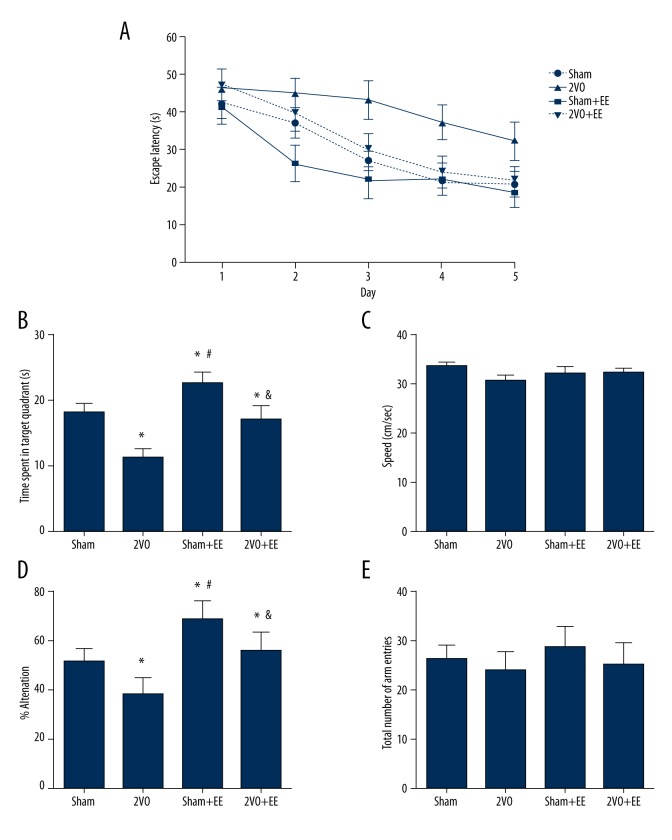
Escape latency in the 2VO group was significantly longer than in the sham group [F(3,36)=11.42; P<0.05]. Escape latency in the 2VO+EE group was significantly shorter than in the 2VO group (P<0.05, Figure 2A). These results mean that the impairment of learning ability caused by 2VO could be reduced by EE.
Figure 2.
Effects of EE on behavioral test. (A) Escape latency of 1–5 days in the sham, 2VO, sham+EE, and 2VO+EE groups. * p<0.05 versus sham rats, # p<0.05 versus 2VO rats. (B) Time spent in target quadrant in the sham, 2VO, sham+EE, and 2VO+EE groups. * p<0.05 versus sham rats, # p<0.05 versus 2VO rats, & p<0.05 versus sham+EE rats. (C) Swimming speed in Morris water maze in each group. (D) The percent of alternation in the sham, 2VO, sham+EE, and 2VO+EE group. * p<0.05 versus sham rats, # p<0.05 versus 2VO rats, & p<0.05 versus sham+EE rats. (E) Total number of arm entries of each group. Data are expressed as mean ±SEM, n=10/group. 2VO – bilateral common carotid occlusion; EE – environmental enrichment.
In the probe test, rats in the 2VO group showed significantly less time spent in the target quadrant compared to sham rats (P<0.05). Rats in the 2VO+EE group showed more time spent in the target quadrant compared to 2VO rats (P<0.05). Compared to sham rats, rats in the sham+EE group showed better performance in the probe test. (P<0.05, Figure 2B). All these results indicate that 2VO surgery impaired the memory of rats and EE could improve the memory, not only in 2VO rats, but also in sham rats.
Swimming speed was also recorded and we found that there was no significant difference in swimming speed among groups [F(3,36)=0.372, Figure 2C].
In the Y-maze test, the percent of alternation in the 2VO group significantly decreased compared to the sham group (P<0.05, Figure 2D). EE significantly increased the percent of alternation decreased by 2VO (P<0.05, Figure 2D). Compared to sham rats, rats in the sham+EE group showed higher percent of alternation (P<0.05, Figure 2D). There was no significant difference in total number of arm entries among groups (P>0.05 Figure 2E).
Effects of EE on apoptosis and survival of neurons
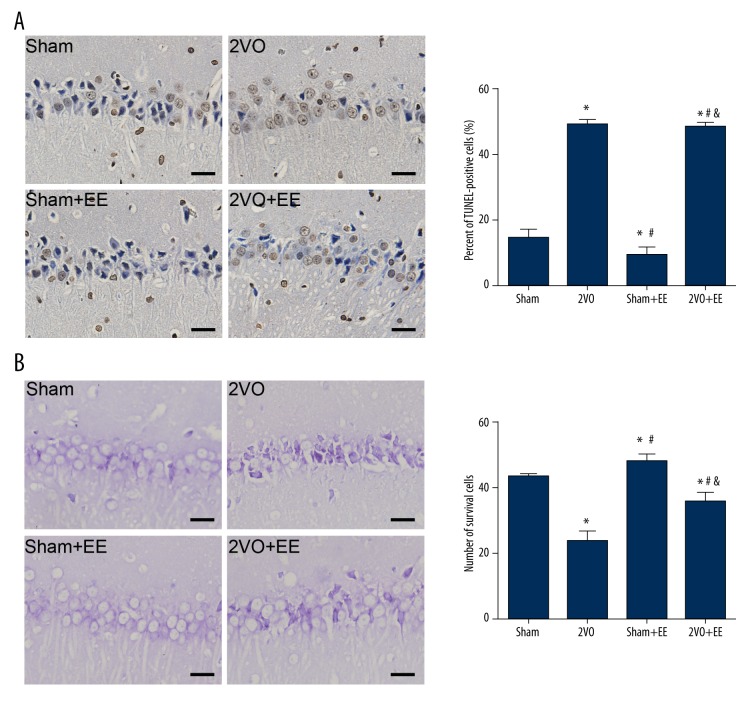
To detect apoptosis of neurons, the percent of TUNEL-positive cells in the hippocampus CA1 region was measured. The TUNEL-positive cells had brown staining in the nucleus. As shown in Figure 3A, in SE condition, carotid occlusion increased the percentage of TUNEL-positive cells compared to sham animals (sham vs. 2VO, n=4, P<0.05). EE rescued this apoptosis and had a lower percentage of TUNEL-positive cells (2VO vs. 2VO+EE P<0.05). Rats in the sham+EE group had a lower percentage of TUNEL-positive cell compared to sham animals (P<0.05).
Figure 3.
TUNEL and Nissl staining to detect the apoptotic and surviving cells. (A) Examples of TUNEL-staining sections of the hippocampus (CA1) in rats of each group and percent of TUNEL-positive cells in the sham, 2VO, sham+EE, and 2VO+EE group. * p<0.05 versus sham rats, # p<0.05 versus 2VO rats, & p<0.05 versus sham+EE rats. All images taken at 400× magnification. Scale bars: 12.5 μm. (B) Nissl staining of hippocampus (CA1). Mean (SEM) number of surviving cells in the sham, 2VO, sham+EE, and 2VO+EE group. * p<0.05 versus sham rats, # p<0.05 versus 2VO rats, & p <0.05 versus sham+EE rats. All images taken at 400× magnification. Scale bars: 12.5 μm. Data are expressed as mean ±SEM, n=4/group. 2VO – bilateral common carotid occlusion; EE – environmental enrichment.
As shown in Figure 3B, Nissl staining showed a mass of neurons with shrunken cytoplasm and karyolysis in hippocampal CA1 of 2VO rats. We measured the number of surviving neurons in hippocampal CA1 to assess the loss of neurons in each group and found that 2VO rats had fewer surviving neurons in CA1 compared to sham rats (P<0.05). However, 2VO+EE rats had more surviving neurons than in 2VO rats (P<0.05). Sham+EE rats also had more surviving neurons compared to sham animals (P<0.05).
Effects of EE on the wnt/β-catenin signal pathway
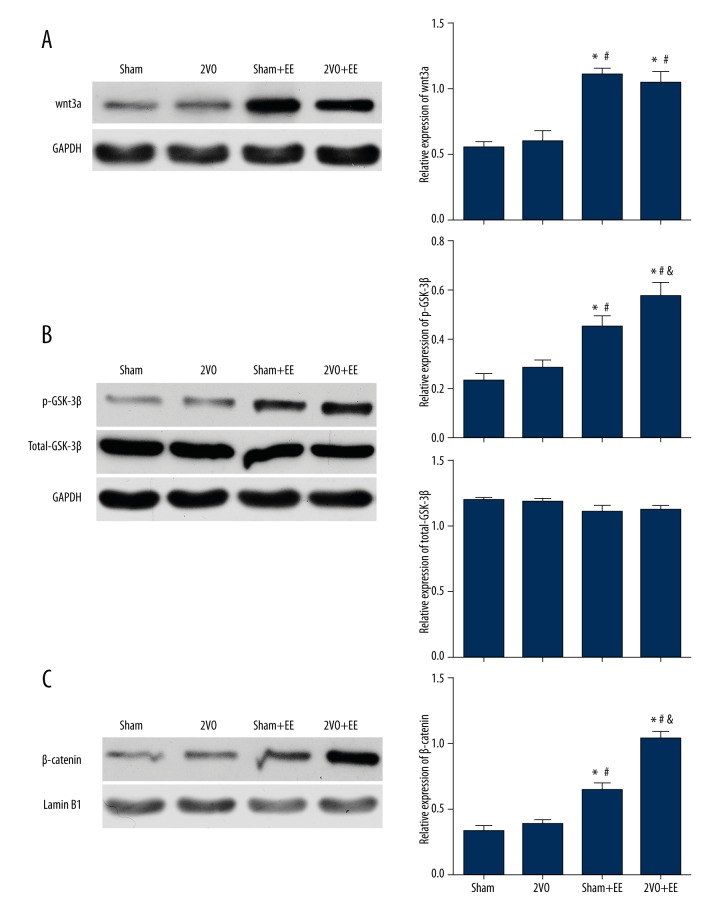
Western blot analysis was used to investigated the level of expression of wnt3a,p-GSK-3β(ser9) and β-catenin. As shown in Figure 4, we found that after 4 weeks, the sham+EE group and 2VO+EE group showed significant increases in the expression of wnt3a compared to sham and 2VO rats (P<0.05), and the expression of wnt3a is higher in 2VO+EE rats than in 2VO rats (P<0.05). There were no significant differences between 2VO and sham rats (P>0.05). We also found that after 4 weeks, rats in the 2VO group had no significant increase in expression of p-GSK-3β and β-catenin compared to sham rats (P>0.05). However, the rats in the sham+EE and 2VO+EE group both showed significant increases in expression of p-GSK-3β and β-catenin compared to the rats in SE condition (including sham and 2VO groups) (p<0.05). The expression of total-GSK-3β did not have any significant change among groups.
Figure 4.
Effects of EE on the expression of wnt3a,GSK-3β and β-catenin. (A) Representative protein bands of wnt3a from Western blot and quantifications of band densities (means ±SEM) are expressed as densitometric ratios of wnt3a to GAPDH. (B) Representative protein bands of p-GSK-3β and total-GSK-3β from Western blot, quantifications of band densities (means ±SEM) are expressed as densitometric ratios of p-GSK-3β to total-GSK-3β, and quantifications of band densities (means ±SEM) are expressed as densitometric ratios of total-GSK-3β to GAPDH. (C) Representative protein bands of β-catenin from Western blot and quantifications of band densities (Means±SEM) are expressed as densitometric ratios of β-catenin to Lamin B1. * p<0.05 versus sham rats, # p<0.05 versus 2VO rats, & p<0.05 versus sham+EE rats. Data are expressed as mean ±SEM, n=5/group. 2VO – bilateral common carotid occlusion; EE – environmental enrichment.
Discussion
Bilateral common carotid occlusion (2-VO), which causes global cerebral hypoperfusion rather than serious stroke, is a popular method to develop a rat model of vascular dementia [26]. In our study, testing with the Morris water maze and Y-maze showed that 2VO rats had worse learning and memory ability compared to sham rats. TUNEL and Nissl staining showed more apoptosis and fewer neurons in 2VO rats than in sham rats. These results are consistent with previous research [27–30] and show that the rat model of VaD used in this study is reliable.
EE consists of 3 major parts: sensory stimulation, cognitive activity, and physical exercise. Compared to rats housed in standard cage, rats housed in EE cage had more motor, sensory, and cognitive stimulation [31]. Since learning and spatial memory are 2 major facets of cognitive function [32], the Morris water maze was used to evaluate the effect of EE on cognitive function [33]. The Morris water maze test showed that the escape latency in the EE group was significantly shorter than in the SE group, which indicated learning ability of EE rats was better than that of SE rats. The results from time spent in the target quadrant showed the spatial memory of EE rats was better than that of the SE group. Swimming speed of each group was also recorded and we found there was no significant difference among groups, which showed that swimming ability of rats in each group did not affect our results. The same conclusion could be drawn from the Y-maze experiment. The Y-maze test is based on the rat’s innate disposition to alternates (a natural tendency to explore novel environment), which requires use of spatial working memory [34,35], so the Y-maze test is always used to access the spatial working memory in rats [36]. In this study, we found that 2VO surgery significantly impaired spatial working memory in rats and EE improved spatial working memory in 2VO and sham rats. There were no significant differences in total number of arm entries among groups that could eliminate the interference of motor activity.
However, EE consists of 3 major parts and each part may have different effects and mechanisms. We wondered which part is the most important in improving the spatial learning and memory and whether each part of EE has different mechanisms. Recently, Choi et al. found that exercise could delay cognitive decline by enhancing neurogenesis and increasing BDNF expression in the context of VaD [37]. Rogers et al. found exercise (Ex) and EE (without exercise) had different effects in a mouse model of anxiety with cognitive impairment, and corrected long-term spatial memory deficits are related to Ex, but not the 2 other parts of EE [38]. Sozda et al. demonstrated that exposing TBI rats to any of the 3 components individually may be more advantageous than no enrichment, but only exposure to typical EE yields optimal benefits. These findings may help clinical rehabilitation and maximize benefits, but the mechanisms of each part of EE still need to be clearly defined [39].
There are 3 major subfields of the hippocampus: dentate gyrus, CA1, and CA3. Previous studies have found that the CA1 region is an important area that correlates with spatial memory impairment and is vulnerable to the effects of 2VO [40–42]. Therefore, in the present study, the CA1 region was chosen as a major region to identify the apoptosis and neuronal survival conditions in this test. We found 2VO rats had more apoptotic neurons and less surviving ones compared to sham rats in the CA1 region, which was in accordance with previous studies [40,41,43]. 2VO+EE rats had fewer apoptotic neurons and more surviving ones compared to 2VO rats in the CA1 region, which indicates that EE can attenuate the apoptosis and increase the number of neurons in rats with VaD. We also observed the CA3 and DG region of each rats and found that there were no significant differences in apoptosis and the number of neurons among the 4 groups (P=0.378, data not shown). These results demonstrate that the CA1 region is more vulnerable to ischemia compared to CA3 and DG regions, and the effect of EE mainly depends on increasing the number of CA1 neurons.
The wnt/β-catenin signal pathway is one of the key signaling pathways involved in the regulation of cell proliferation, differentiation, and survival [44–47]. Song et al. found that hippocampal astrocytes have an active regulatory role in the mature central nervous system [48]. Lanosa et al. found that EE can increase the number of astrocytes [49]. Lie et al. reported that wnt3a, which is released by astrocytes, is expressed in the hippocampus. These studies indicate that wnt3a may play an important role in the mechanism of EE [50]. However, it has never been determined whether the stimulation of EE is accompanied by activation of the wnt/β-catenin signal pathway. In this study, Western blot analysis showed that the level of wnt3a,p-GSK-3β and β-catenin is significantly increased in sham+EE and 2VO+EE rats. Compared to sham rats, the level of wnt3a,p-GSK-3β and β-catenin in 2VO rats did not significantly increase after 4 weeks in the standard environment. These results showed that wnt/β-catenin signal pathway was activated in EE rats, which indicates that activating the wnt/β-catenin signal pathway may be an internal mechanism of the effect of EE. In addition, EE is a relative term. Compared to EE rats, SE rats, which did not have a chance to do exercise and did not have much environmental stimulation, were relatively sedentary [51]. After 4 weeks of being sedentary, the wnt/β-catenin signal pathway in 2VO rats did not to significantly increase compared to sham rats, but if the level of wnt3a,p-GSK-3β and β-catenin changed over a shorter time (e.g., 1 day, 3 days, or 1 week, not 4 weeks) still need more study.
Conclusions
The results of our study support the hypothesis that environmental enrichment has a beneficial effect on vascular dementia, and its molecular mechanisms may be related to the activation of the wnt/β-catenin signal pathway.
Acknowledgements
We thank members of our laboratory for technical support.
Footnotes
Source of support: This work was supported by the Natural Science Foundation Project (No. 81201506) and the Medical Science Research Project of Chongqing Municipal Health Bureau (No. 20142154)
References
- 1.Román GC. Vascular dementia revisited: Diagnosis, pathogenesis, treatment, and prevention. Med Clin North Am. 2002;86:477–99. doi: 10.1016/s0025-7125(02)00008-1. [DOI] [PubMed] [Google Scholar]
- 2.Jain S, Sharma B. Effect of ruthenium red, a ryanodine receptor antagonist in experimental diabetes induced vascular endothelial dysfunction and associated dementia in rats. Physiol Behav. 2016;164:140–50. doi: 10.1016/j.physbeh.2016.05.052. [DOI] [PubMed] [Google Scholar]
- 3.Marcelo A, Bix G. The potential role of perlecan domain V as novel therapy in vascular dementia. Metab Brain Dis. 2015;30:1–5. doi: 10.1007/s11011-014-9576-6. [DOI] [PubMed] [Google Scholar]
- 4.Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–95. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- 5.Rampon C, Jiang CH, Dong H, Tang YP, et al. Effects of environmental enrichment on gene expression in the brain. Proc Natl Acad Sci USA. 2000;97:12880–84. doi: 10.1073/pnas.97.23.12880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greenough WT, Volkmar FR. Pattern of dendritic branching in occipital cortex of rats reared in complex environments. Exp Neurol. 1973;40:491–504. doi: 10.1016/0014-4886(73)90090-3. [DOI] [PubMed] [Google Scholar]
- 7.Valero J, España J, Parra-Damas A, et al. Short-term environmental enrichment rescues adult neurogenesis and memory deficits in APP(Sw,Ind) transgenic mice. PLoS One. 2011;6:e16832. doi: 10.1371/journal.pone.0016832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verret L, Krezymon A, Halley H, et al. Transient enriched housing before amyloidosis onset sustains cognitive improvement in Tg2576 mice. Neurobiol Aging. 2013;34:211–25. doi: 10.1016/j.neurobiolaging.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 9.Hannan AJ. Environmental enrichment and brain repair: Harnessing the therapeutic effects of cognitive stimulation and physical activity to enhance experience-dependent plasticity. Neuropathol Appl Neurobiol. 2014;40:13–25. doi: 10.1111/nan.12102. [DOI] [PubMed] [Google Scholar]
- 10.Hussaini SM, Choi CI, Cho CH, et al. Wnt signaling in neuropsychiatric disorders: Ties with adult hippocampal neurogenesis and behavior. Neurosci Biobehav Rev. 2014;47:369–83. doi: 10.1016/j.neubiorev.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen J, Park CS, Tang SJ. Activity-dependent synaptic Wnt release regulates hippocampal long term potentiation. J Biol Chem. 2006;281:11910–16. doi: 10.1074/jbc.M511920200. [DOI] [PubMed] [Google Scholar]
- 12.Boonen RA, van Tijn P, Zivkovic D. Wnt signaling in Alzheimer’s disease: Up or down, that is the question. Ageing Res Rev. 2009;8:71–82. doi: 10.1016/j.arr.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 13.Jang MH, Kitabatake Y, Kang E, et al. Secreted frizzled-related protein 3 (sFRP3) regulates antidepressant responses in mice and humans. Mol Psychiatry. 2013;18:957–58. doi: 10.1038/mp.2012.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y, Sun Y, Wang F, et al. Downregulating the canonical Wnt/β-catenin signaling pathway attenuates the susceptibility to autism-like phenotypes by decreasing oxidative stress. Neurochem Res. 2012;37:1409–19. doi: 10.1007/s11064-012-0724-2. [DOI] [PubMed] [Google Scholar]
- 15.Vargas JY, Fuenzalida M, Inestrosa NC. In vivo activation of Wnt signaling pathway enhances cognitive function of adult mice and reverses cognitive deficits in an Alzheimer’s disease model. J Neurosci. 2014;34:2191–202. doi: 10.1523/JNEUROSCI.0862-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seib DR, Corsini NS, Ellwanger K, et al. Loss of Dickkopf-1 restores neurogenesis in old age and counteracts cognitive decline. Cell Stem Cell. 2013;12:204–14. doi: 10.1016/j.stem.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 17.Mengya X, Qingna S, Yiyi W, et al. Hydroxysafflor yellow A increases BDNF and NMDARs in the hippocampus in A vascular dementia rat model. Brain Res. 2016;1642:419–25. doi: 10.1016/j.brainres.2016.04.030. [DOI] [PubMed] [Google Scholar]
- 18.Barry JM, Tian C, Spinella A, et al. Spatial cognition following early-life seizures in rats: Performance deficits are dependent on task demands. Epilepsy Behav. 2016;60:1–6. doi: 10.1016/j.yebeh.2016.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ishola IO, Adamson FM, Adeyemi OO. Ameliorative effect of kolaviron, a biflavonoid complex from Garcinia kola seeds against scopolamine-induced memory impairment in rats: Role of antioxidant defense system. Metab Brain Dis. 2016 doi: 10.1007/s11011-016-9902-2. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 20.Xu H, You Z, Wu Z, et al. WY14643 attenuates the scopolamine-induced memory impairments in mice. Neurochem Res. 2016;41:2868–79. doi: 10.1007/s11064-016-2002-1. [DOI] [PubMed] [Google Scholar]
- 21.Sasaki-Hamada S, Hoshi M, Niwa Y, et al. Neoechinulin A induced memory improvements and antidepressant-like effects in mice. Prog Neuropsychopharmacol Biol Psychiatry. 2016;71:155–61. doi: 10.1016/j.pnpbp.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 22.Lim FT, Ogawa S, Parhar IS. Association between apoptotic neural tissue and cell proliferation in the adult teleost brain. Brain Res. 2016;1650:60–72. doi: 10.1016/j.brainres.2016.08.033. [DOI] [PubMed] [Google Scholar]
- 23.Hui H, Rao W, Zhang L, et al. Inhibition of Na(+)-K(+)-2Cl(−) Cotransporter-1 attenuates traumatic brain injury-induced neuronal apoptosis via regulation of Erk signaling. Neurochem Int. 2016;94:23–31. doi: 10.1016/j.neuint.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 24.You W, Zuo G, Shen H, Tian X, et al. Potential dual role of nuclear factor-kappa B in experimental subarachnoid hemorrhage-induced early brain injury in rabbits. Inflamm Res. 2016;65:975–84. doi: 10.1007/s00011-016-0980-8. [DOI] [PubMed] [Google Scholar]
- 25.Dong F, Yao R, Yu H, Liu Y. Neuroprotection of Ro25-6981 against ischemia/reperfusion-induced brain injury via inhibition of autophagy. Cell Mol Neurobiol. 2016 doi: 10.1007/s10571-016-0409-5. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiwa NS, Garrard P, Hainsworth AH. Experimental models of vascular dementia and vascular cognitive impairment: A systematic review. J Neurochem. 2010;115:814–28. doi: 10.1111/j.1471-4159.2010.06958.x. [DOI] [PubMed] [Google Scholar]
- 27.Shibata M, Yamasaki N, Miyakawa T, et al. Selective impairment of working memory in a mouse model of chronic cerebral hypoperfusion. Stroke. 2007;38:2826–32. doi: 10.1161/STROKEAHA.107.490151. [DOI] [PubMed] [Google Scholar]
- 28.Brown WR, Thore CR. Review: Cerebral microvascular pathology in ageing and neurodegeneration. Neuropathol Appl Neurobiol. 2011;37:56–74. doi: 10.1111/j.1365-2990.2010.01139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu X, Li Z, Yang Z, Zhang T. Decrease of synaptic plasticity associated with alteration of information flow in a rat model of vascular dementia. Neuroscience. 2012;206:136–43. doi: 10.1016/j.neuroscience.2011.12.050. [DOI] [PubMed] [Google Scholar]
- 30.Vicente E, Degerone D, Bohn L, et al. Astroglial and cognitive effects of chronic cerebral hypoperfusion in the rat. Brain Res. 2009;1251:204–12. doi: 10.1016/j.brainres.2008.11.032. [DOI] [PubMed] [Google Scholar]
- 31.van Praag H, Kempermann G, Gage FH. Neural consequences of environmental enrichment, Nature reviews. Neuroscience. 2000;1:191–98. doi: 10.1038/35044558. [DOI] [PubMed] [Google Scholar]
- 32.Leggio MG, Mandolesi L, Federico F, et al. Environmental enrichment promotes improved spatial abilities and enhanced dendritic growth in the rat. Behav Brain Res. 2005;163:78–90. doi: 10.1016/j.bbr.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 33.Brandeis R, Brandys Y, Yehuda S. The use of the Morris Water Maze in the study of memory and learning. Int J Neurosci. 1989;48:29–69. doi: 10.3109/00207458909002151. [DOI] [PubMed] [Google Scholar]
- 34.Wei Z, Chen XC, Song Y, et al. Amyloid β protein aggravates neuronal senescence and cognitive deficits in 5XFAD mouse model of Alzheimer’s disease. Chin Med J (Engl) 2016;129:1835–44. doi: 10.4103/0366-6999.186646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Satoh Y, Endo S, Ikeda T, et al. Extracellular signal-regulated kinase 2 (ERK2) knockdown mice show deficits in long-term memory; ERK2 has a specific function in learning and memory. J Neurosci. 2007;27:10765–76. doi: 10.1523/JNEUROSCI.0117-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu X, Zheng C, Li N, et al. The decrease of NMDAR subunit expression and NMDAR EPSC in hippocampus by neonatal exposure to desflurane in mice. Behav Brain Res. 2017;317:82–87. doi: 10.1016/j.bbr.2016.09.035. [DOI] [PubMed] [Google Scholar]
- 37.Choi DH, Lee KH, Lee J. Effect of exercise-induced neurogenesis on cognitive function deficit in a rat model of vascular dementia. Mol Med Rep. 2016;13:2981–90. doi: 10.3892/mmr.2016.4891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rogers J, Vo U, Buret LS, et al. Dissociating the therapeutic effects of environmental enrichment and exercise in a mouse model of anxiety with cognitive impairment. Transl Psychiatry. 2016;6:e794. doi: 10.1038/tp.2016.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sozda CN, Hoffman AN, Olsen AS, et al. Empirical comparison of typical and atypical environmental enrichment paradigms on functional and histological outcome after experimental traumatic brain injury. J Neurotrauma. 2010;27:1047–57. doi: 10.1089/neu.2010.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee CH, Park JH, Ahn JH, Won MH. Effects of melatonin on cognitive impairment and hippocampal neuronal damage in a rat model of chronic cerebral hypoperfusion. Exp Ther Med. 2016;11:2240–46. doi: 10.3892/etm.2016.3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhu B, Wang ZG, Ding J, et al. Chronic lipopolysaccharide exposure induces cognitive dysfunction without affecting BDNF expression in the rat hippocampus. Exp Ther Med. 2014;7:750–54. doi: 10.3892/etm.2014.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.De Jong GI, Farkas E, Stienstra CM, et al. Cerebral hypoperfusion yields capillary damage in the hippocampal CA1 area that correlates with spatial memory impairment. Neuroscience. 1999;91:203–10. doi: 10.1016/s0306-4522(98)00659-9. [DOI] [PubMed] [Google Scholar]
- 43.Xi Y, Wang M, Zhang W, et al. Neuronal damage, central cholinergic dysfunction and oxidative damage correlate with cognitive deficits in rats with chronic cerebral hypoperfusion. Neurobiol Learn Mem. 2014;109:7–19. doi: 10.1016/j.nlm.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 44.Grigson ER, Ozerova M, Pisklakova A, et al. Canonical Wnt pathway inhibitor ICG-001 induces cytotoxicity of multiple myeloma cells in Wnt-independent manner. PLoS One. 2015;10:e0117693. doi: 10.1371/journal.pone.0117693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wexler WM, Paucer A, Kornblum HI, et al. Endogenous Wnt signaling maintains neural progenitor cell potency. Stem Cells. 2009;27:1130–41. doi: 10.1002/stem.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sinha S, Wang Z, Ruchalski KL, et al. Lithium activates the Wnt and phosphatidylinositol 3-kinase Akt signaling pathways to promote cell survival in the absence of soluble survival factors. Am J Physiol Renal Physiol. 2005;288:F703–13. doi: 10.1152/ajprenal.00189.2004. [DOI] [PubMed] [Google Scholar]
- 47.You Z, Saims S, Chen S, et al. Wnt signaling promotes oncogenic transformation by inhibiting c-Myc-induced apoptosis. J Cell Biol. 2002;157:429–40. doi: 10.1083/jcb.200201110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Song H, Stevens CF, Gage FH. Astroglia induce neurogenesis from adult neural stem cells. Nature. 2002;417:39–44. doi: 10.1038/417039a. [DOI] [PubMed] [Google Scholar]
- 49.Lanosa XA, Santacroce I, Colombo JA. Exposure to environmental enrichment prior to a cerebral cortex stab wound attenuates the postlesional astroglia response in rats. Neuron Glia Biol. 2011;7:163–75. doi: 10.1017/S1740925X12000099. [DOI] [PubMed] [Google Scholar]
- 50.Lie DC, Colamarino SA, Song HJ, et al. Wnt signalling regulates adult hippocampal neurogenesis. Nature. 2005;437:1370–75. doi: 10.1038/nature04108. [DOI] [PubMed] [Google Scholar]
- 51.Bayod S, Mennella I, Menella I, et al. Wnt pathway regulation by long-term moderate exercise in rat hippocampus. Brain Res. 2014;1543:38–48. doi: 10.1016/j.brainres.2013.10.048. [DOI] [PubMed] [Google Scholar]