The present study is the first to demonstrate that caveolin-1 can regulate DISC1 expression in neuronal models. Furthermore, the findings are consistent across three separate neuronal models that include rodent neurons (in vitro and in vivo) and human differentiated neurons derived from induced pluripotent stem cells. These findings justify further investigation regarding the modulatory role by caveolin on synaptic function and as a potential therapeutic target for the treatment of schizophrenia.
Keywords: caveolin-1, disrupted-in-schizophrenia-1, schizophrenia, synaptic plasticity, synaptic proteins, stereotactic injection
Abstract
Schizophrenia is a debilitating psychiatric disorder manifested in early adulthood. Disrupted-in-schizophrenia-1 (DISC1) is a susceptible gene for schizophrenia (Hodgkinson et al. 2004; Millar et al. 2000; St Clair et al. 1990) implicated in neuronal development, brain maturation, and neuroplasticity (Brandon and Sawa 2011; Chubb et al. 2008). Therefore, DISC1 is a promising candidate gene for schizophrenia, but the molecular mechanisms underlying its role in the pathogenesis of the disease are still poorly understood. Interestingly, caveolin-1 (Cav-1), a cholesterol binding and scaffolding protein, regulates neuronal signal transduction and promotes neuroplasticity. In this study we examined the role of Cav-1 in mediating DISC1 expression in neurons in vitro and the hippocampus in vivo. Overexpressing Cav-1 specifically in neurons using a neuron-specific synapsin promoter (SynCav1) increased expression of DISC1 and proteins involved in synaptic plasticity (PSD95, synaptobrevin, synaptophysin, neurexin, and syntaxin 1). Similarly, SynCav1-transfected differentiated human neurons derived from induced pluripotent stem cells (hiPSCs) exhibited increased expression of DISC1 and markers of synaptic plasticity. Conversely, hippocampi from Cav-1 knockout (KO) exhibited decreased expression of DISC1 and proteins involved in synaptic plasticity. Finally, SynCav1 delivery to the hippocampus of Cav-1 KO mice and Cav-1 KO neurons in culture restored expression of DISC1 and markers of synaptic plasticity. Furthermore, we found that Cav-1 coimmunoprecipitated with DISC1 in brain tissue. These findings suggest an important role by which neuron-targeted Cav-1 regulates DISC1 neurobiology with implications for synaptic plasticity. Therefore, SynCav1 might be a potential therapeutic target for restoring neuronal function in schizophrenia.
NEW & NOTEWORTHY The present study is the first to demonstrate that caveolin-1 can regulate DISC1 expression in neuronal models. Furthermore, the findings are consistent across three separate neuronal models that include rodent neurons (in vitro and in vivo) and human differentiated neurons derived from induced pluripotent stem cells. These findings justify further investigation regarding the modulatory role by caveolin on synaptic function and as a potential therapeutic target for the treatment of schizophrenia.
schizophrenia is typically manifested in late adolescence or early adulthood with an estimated prevalence of ∼1% (Saha et al. 2005). Schizophrenia is partly a genetic disorder, although it likely involves multiple recessive genes and environmental factors such as physical or psychological abuse and birth complications (Jaaro-Peled et al. 2009; Schmitt et al. 2014; Vilain et al. 2013). Although pharmacological treatments such as antipsychotics are available for schizophrenia, these classes of drugs show poor efficacy for most patients (Lieberman et al. 2005), especially in reversing cognitive abnormalities (Brown and McGrath 2011; Buchanan et al. 2007).
DISC1 is a schizophrenia-associated gene originally identified in a Scottish family (Millar et al. 2000; Muir et al. 2008; St Clair et al. 1990), and later studies have shown an increasing amount of evidence that supports the possibility that DISC1 may be a candidate gene for schizophrenia (Callicott et al. 2005; Ekelund et al. 2001; Hamshere et al. 2005; Harrison and Weinberger 2005; Hennah et al. 2003; Hodgkinson et al. 2004; Qu et al. 2007; Song et al. 2008). DISC1 protein is highly expressed in the developing brain (Schurov et al. 2004) and in the dentate gyrus of the adult hippocampus (Austin et al. 2004); it is a multifunctional protein involved in neuritogenesis and neuronal signaling (Brandon et al. 2009; Ishizuka et al. 2006; Narayan et al. 2013). DISC1 is located in multiple intracellular locations [i.e., the nucleus (Sawamura et al. 2005), mitochondria (Millar et al. 2005), and axons and synapses (Kirkpatrick et al. 2006; Miyoshi et al. 2003)]. Loss of DISC1 function causes deficits in neural development, neuronal proliferation, axonal growth, and cytoskeleton modulation, which are consistent with abnormal neural development in schizophrenia (Harrison 1997; Lewis and Levitt 2002; Lewis and Moghaddam 2006).
Proper neuronal growth (i.e., dendritic arborization, axonal guidance, and formation of synaptic contacts) and neurotransmission are dependent on a polarized membrane platform that organizes key membrane receptors, which in turn transduce extracellular cues. A necessary organizer of neuronal signaling components is the scaffolding protein caveolin-1 (Cav-1) (Head et al. 2008, 2011). Cav-1 is widely expressed in the central and peripheral nervous systems (Boulware et al. 2007; Heiman et al. 2008). Within neurons, Cav-1 regulates membrane/lipid raft (MLR) formation and neurotransmitter and neurotrophin signaling (Head et al. 2008), promotes dendritic growth and arborization (Head et al. 2011), and, when overexpressed in hippocampal neurons in vivo, augments functional neuroplasticity and improves learning and memory (Mandyam et al. 2015). To date no relevant functional role of Cav-1 in the pathogenesis of schizophrenia has been described, although CAV1 gene disruption was recently identified in some patients suffering from schizophrenia (Walsh et al. 2008). Because Cav-1 organizes and regulates neurotransmitter and neurotrophic receptor signaling pathways (Bilderback et al. 1999; Gaudreault et al. 2005; Suzuki et al. 2004) and G protein-coupled neurotransmitter receptors (Allen et al. 2007; Bhatnagar et al. 2004; Francesconi et al. 2009) necessary for proper dendritic growth and arborization (Head et al. 2011; Mandyam et al. 2015), disruption of CAV1 would likely impair neuronal signaling, leading to a schizophrenia-like phenotype. Interestingly, recent findings support the involvement of caveolin in schizophrenia; for instance, central nervous system pathologies in Cav-1 knockout (Cav-1 KO) mice are similar to those exhibited with schizophrenia (Head et al. 2008; Trushina et al. 2006), because Cav-1 KO mice demonstrate increased sensitivity to the psychotomimetic effects of N-methyl-d-aspartate receptor (NMDAR) antagonist phencyclidine (PCP) (Allen et al. 2011), a phenomenon also observed in schizophrenic patients (Lahti et al. 1995; Luby et al. 1959). Additionally, Cav-1 interacts with the serotonin receptor 5-HT2A (Bhatnagar et al. 2004), a target for atypical antipsychotic drugs (Meltzer et al. 1989). Interestingly, Cav-1 KO mice showed attenuated biochemical and behavioral actions of atypical antipsychotic drugs (Allen et al. 2011). These findings provide evidence suggesting a link between Cav-1 and schizophrenia.
In the present study, our goal was to examine if Cav-1 modulates expression of DISC1 in various neuronal systems. Interestingly, treatment of primary neurons with SynCav1 lentivirus significantly enhanced the expression of DISC1. Furthermore, human differentiated neuronal progenitor cells (NPCs) derived from induced pluripotent stem cells (hiPSCs) transfected with SynCav1 showed higher DISC1 protein expression. In addition, hippocampal homogenates from Cav-1 KO vs. wild-type (WT) mice showed a significant reduction in DISC1, and synaptic proteins such as PSD95, synaptophysin, synaptobrevin, and syntaxin 1 were also significantly reduced; however, AAV9-SynCav1 delivery to the hippocampus in vivo restored expression of these synaptic proteins.
MATERIALS AND METHODS
Animals.
All animals [C57BL/6 mice, Cav-1 KO mice (Cavtm1Mls), and rats (Jackson Laboratories, Bar Harbor, ME)] were treated in compliance with the Guide for the Care and Use of Laboratory Animals (Washington, DC: National Academy of Sciences, 2011). All animal use protocols were approved by the VA San Diego Healthcare System Institutional Animal Care and Use Committee (San Diego, CA) before any procedures were performed. Adult male mice (2–3 mo old) were housed under normal conditions with ad libitum access to food and water. The use of human cells was approved under the University of California San Diego Human Research Protection Institutional Review Board 150071.
Stereotactic injection.
Mice were anesthetized and prepared for surgery with a protocol modified from a previously described study (Mandyam et al. 2015). Hippocampus-targeted injections were controlled using Injectomate software (Neurostar, Berlin, Germany). Injections were made using a 33-gauge, 10-μl Hamilton gas-tight syringe (Hamilton, Reno, NV). At each coordinate, the needle was lowered at a rate of 0.32 mm/s. After 60 s, 0.5 μl of adeno-associated virus serotype 9 (AAV9) containing synapsin-red fluorescent protein (RFP; SynRFP) or synapsin-caveolin-1 (SynCav1) was injected over 60 s [0.5 μl/min injection rate at a viral titer of 109 genome copies/μl] at three locations (rostral to caudal) in each hippocampal hemisphere with an indwelling time of 1 min. Sagittal brain sections were stained to confirm location and spread of RFP (data not shown). Sections were also stained for hematoxylin and eosin, and histopathologic analysis did not reveal any gross morphology or cell death in the hippocampal sections (data not shown).
Primary neuron isolation and culture.
Neonatal rat neurons were isolated from hippocampi using a papain dissociation kit (Worthington Biochemical, Lakewood, NJ) as previously described (Head et al. 2008, 2011). Neurons were cultured in neurobasal A medium supplemented with B27 (2%), 250 mM GLUTMax1, and penicillin-streptomycin (1%). Cells were cultured on poly-d-lysine/laminin (2 μg/cm2)-coated plates at 37°C in 5% CO2 for 4 days before transfection with lentiviral vectors containing the synapsin promoter upstream of the CAV1 gene (SynCav1). Synapsin-green fluorescent protein (SynGFP) was used as control vector. Titer for both vectors was 109 infectious units per milliliter.
The human neurons were differentiated from the Craig Venter 4a (CV4a) neuronal progenitor cells (NPCs) derived from hiPSCs as previously described (Woodruff et al. 2013). Cells were first grown on poly-l-ornithine/laminin-coated plates. Cells were maintained in NPC base media (DMEM/F12, N2, B27, and penicillin-streptomycin) supplemented with bFGF (20 ng/ml). Cells were then differentiated for 3–4 wk in differentiating media (NPC base media supplemented with bone-derived neurotropic factor, glial cell-derived neurotropic factor, and dibutyryl cAMP). Neurons were then infected with SynCav1 or SynGFP as control.
Sample preparation and immunoblot analysis.
Mice were anesthetized with pentobarbital sodium (60 mg/kg intraperitoneally), followed by decapitation. The brain was removed and placed in ice-cold lysis buffer (150 mM Na2CO3; pH 11), minced, and homogenized with a Tissuemiser (Fisher Scientific, Waltham, MA). Homogenates were further centrifuged at 600 g to clear debris. Cell lysates were prepared in buffer (50 mM Tris·HCl, 150 mM NaCl, 1% Triton X-100; pH 7.4) supplemented with protease and phosphatase inhibitors cocktail (Cell Signaling, Beverly, MA). After 30-min incubation on ice, the cells were homogenized by a 23-gauge needle, and the lysates were cleared of debris and unbroken cells by centrifugation (800 g, 5 min at 4°C). Protein concentrations were determined using the Bio-Rad protein assay (Bio-Rad Laboratories, Hercules, CA). Equal amounts of cell lysates (10 μg) were loaded to determine expression of Cav-1, PSD95, neurexin (BD Biosciences, Franklin Lakes, NJ), syntaxin 1, synaptobrevin, synaptophysin (Abcam, Cambridge, MA), and DISC1 (Thermo Fisher Scientific, Waltham, MA). All protein expression was normalized to GAPDH (Cell Signaling Technology, Danvers, MA). Horseradish peroxidase-conjugated secondary antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Immunoblots were subsequently detected by Lumigen ECL Ultra (Lumigen, Southfield, MI). The densitometry of the different bands was further quantified using ImageJ software (National Institutes of Health, Bethesda, MD) with normalization to GAPDH.
Immunoprecipitation.
Immunoprecipitation (IP) of Cav-1 and DISC1 was performed using protein A agarose (Roche, Life Science) according to the manufacturer's protocol. In brief, brain samples were homogenized in lysis buffer. The supernatants were collected by centrifugation at 10,000 g for 10 min at 4°C. Lysates were then incubated with antibodies for either Cav-1 or DISC1 at 4°C for 3 h, followed by overnight incubation in agarose A. The immunoprecipitates were analyzed for the presence of Cav-1 and DISC1 by immunoblot assay.
Statistical analysis.
Results are means ± SE and were analyzed using GraphPad Prism 6 (GraphPad Software, San Diego, CA). We used t-tests and one-way ANOVA to compare certain paired parameters. Values of P < 0.05 were considered significant.
RESULTS
Cav-1 coimmunoprecipitates with DISC1 in brain tissue and human neurons.
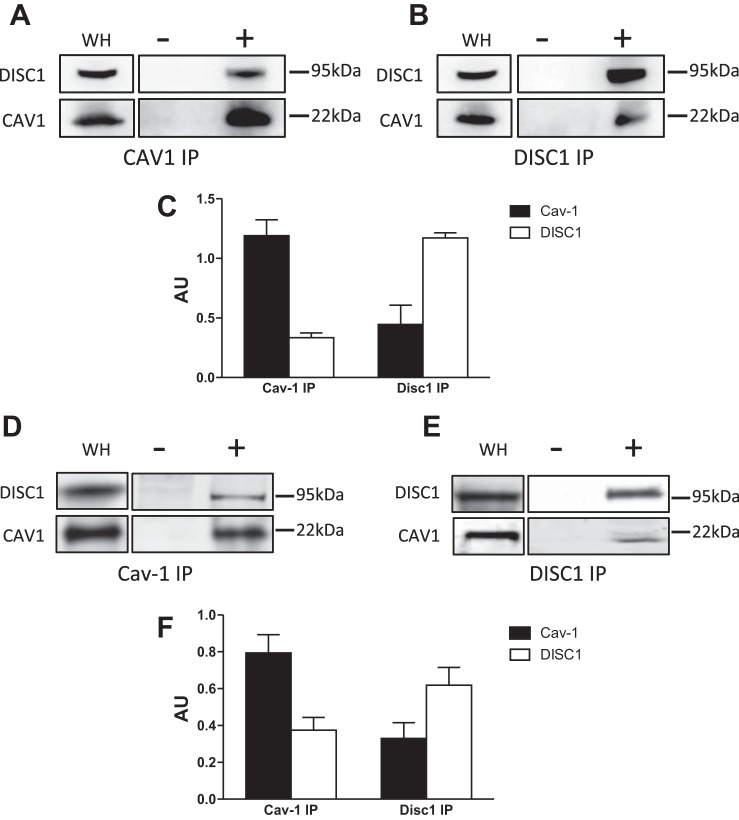
At present, there are no reports that Cav-1 and DISC1 localize to the same protein complex in neuronal tissue; thus we sought to determine if Cav-1 localizes with DISC1 in brain homogenates. Our data are the first to demonstrate that Cav-1 coimmunoprecipitated with DISC1 (Fig. 1, A and B). The DISC1 antibody specifically only detects a band at ∼95 kDa. Quantification of IP bands was normalized to the total input (Fig. 1C). To strengthen our data, we also studied the interaction between Cav-1 and DISC1 in differentiated human neuron (i.e., differentiated NPC) derived from hiPSCs. Similar to what we observed in brain homogenates, the data show that Cav-1 and DISC1 coimmunoprecipitated in human differentiated neurons (Fig. 1, D and E). Quantification of IP bands was normalized to the total input (Fig. 1F).
Fig. 1.
Caveolin-1 (Cav-1) is an interaction partner of disrupted-in-schizophrenia-1 (DISC1). Brain homogenates were immunoprecipitated with a Cav-1 or DISC1 antibodies. Immunoprecipitates were then probed for the presence of Cav-1 and DISC1 by Western blotting (representative blots from n = 3 experiments). Negative controls are incubated without antibodies (A and B). Quantification of immunoblots was normalized to the total input (C). In parallel, induced pluripotent stem cell (iPSC) homogenates were also immunoprecipitated with Cav-1 or DISC1 antibodies. IP were then probed for the presence of Cav-1 and DISC1 by Western blotting (representative blots from n = 3 experiments). Negative controls are incubated without antibodies (D and E). Quantification of immunoblots was normalized to the total input (F). AU, arbitrary units; WH, whole homogenate.
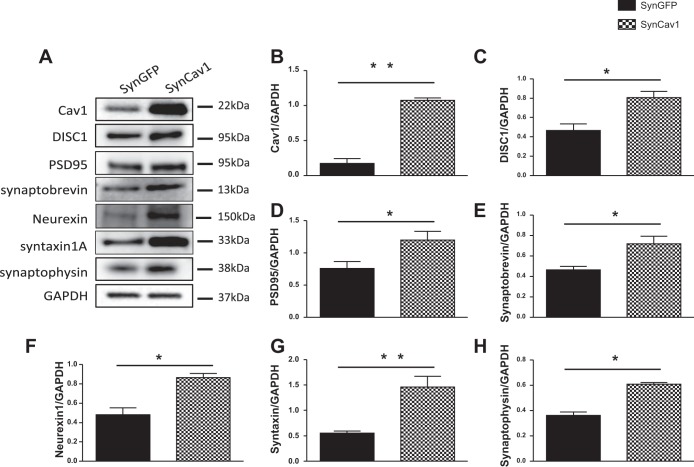
Neuron-targeted overexpression of Cav-1 (SynCav1) enhances expression of DISC1 and synaptic proteins in primary neurons.
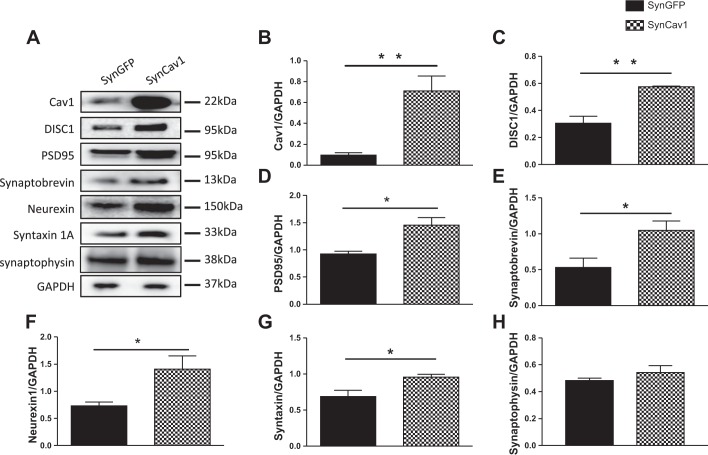
To explore the implication of Cav-1 and DISC1 on the synaptic integrity, we overexpressed Cav-1 specifically in neurons by using a synapsin promoter as previously described (Head et al. 2011). Primary neurons isolated from hippocampi of postnatal day 1–3 rats were transfected with lentiviral SynCav1, followed by immunoblot analysis. The data show that SynCav1 increased protein expression of Cav-1, DISC1, and membrane proteins necessary for synaptic plasticity and maintenance (PSD95, synaptobrevin, neurexin, and syntaxin 1). However, there was no significant change in synaptophysin expression level. (Fig. 2).
Fig. 2.
Neuron-targeted expression of caveolin-1 (Cav-1) enhances expression of disrupted-in-schizophrenia-1 (DISC1) and synaptic proteins in wild-type (WT) primary neurons. Primary rodent neurons were isolated from rats' neonatal hippocampi. Neurons were grown in culture for 4 days and infected with a lentivirus containing SynCav1 or synapsin-green fluorescent protein (SynGFP) as control (2 × 109 viral particles) for 72 h. Homogenates were immunoblotted for Cav-1, DISC1, PSD95, synaptobrevin, neurexin 1, syntaxin 1A, synaptophysin, synaptophysin, and GAPDH (A). Quantification of Western blots showed overexpression of Cav-1 protein (B). SynCav1 also significantly enhanced the protein expression of DISC1 (C) and other synaptic proteins: PSD95 (D), synaptobrevin (E), neurexin 1 (F), and syntaxin 1A (G). No significant difference was detected in synaptophysin (H). Representative blots are from n = 4 experiments. *P < 0.05; **P < 0.01, SynCav1 vs. SynGFP.
Hippocampi from Cav-1 KO mice exhibit decreased expression of DISC1 and synaptic proteins.
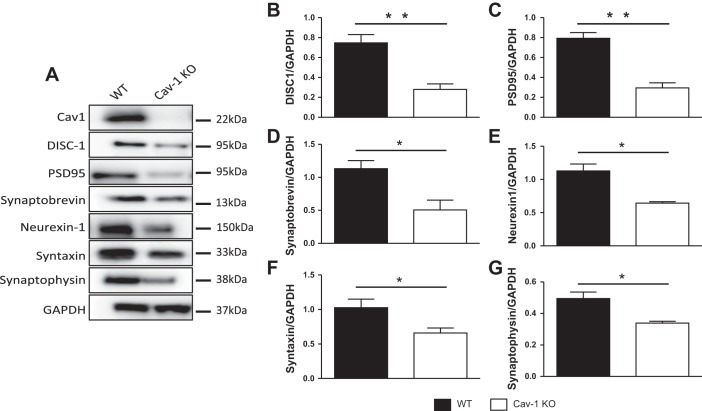
Because data in Fig. 1 showed that SynCav1 increased expression of DISC1 and synaptic proteins, we tested whether the opposite occurred in brain tissue from Cav-1 KO mice. Indeed, immunoblot data showed that loss of Cav-1 was associated with decreased protein expression of DISC1 (Fig. 3, A and B) and the synaptic proteins PSD95, synaptobrevin, neurexin 1, syntaxin, and synaptophysin (Fig. 3, A and C–G). Interestingly, loss of Cav-1 seemed to affect both post- and presynaptic proteins, suggesting decreased synaptic strength and plasticity.
Fig. 3.
Hippocampal homogenates show a caveolin-dependent reduction in disrupted-in-schizophrenia-1 (DISC1) and synaptic proteins. Hippocampi were isolated from the brains of wild-type (WT) and caveolin-1 knockout (Cav-1 KO) mice (2–3 mo). Homogenates were immunoblotted for Cav-1, DISC1, PSD95, synaptobrevin, neurexin 1, syntaxin 1A, synaptophysin, and GAPDH (A). Quantification of Western blots showed a significant decrease in the protein expression level of DISC1 (B) and other synaptic proteins: PSD95 (C), synaptobrevin (D), neurexin 1 (E), syntaxin 1A (F), and synaptophysin (G). Representative blots are from n = 4 experiments. *P < 0.05; **P < 0.01, WT vs. Cav-1 KO.
Re-expressing Cav-1 in Cav-1 KO mice increases DISC1 and synaptic proteins.
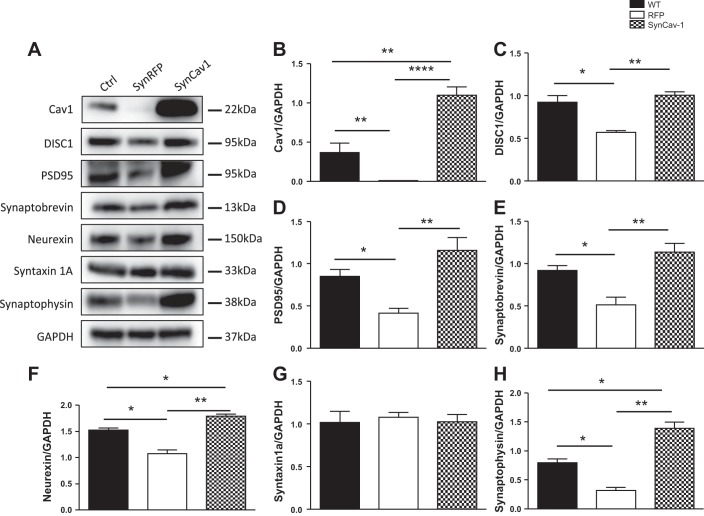
To elucidate whether reintroducing Cav-1 could reverse the effect seen with loss of Cav-1, Cav-1 KO mice underwent stereotactic injections of AAV9-SynCav1 as previously described (Mandyam et al. 2015). Successful overexpression of Cav-1 in the hippocampus was confirmed by immunoblot analysis (Fig. 4, A and B). Interestingly, re-expression of Cav-1 in Cav-1 KO hippocampi significantly increased the levels of DISC1 expression (Fig. 4, A and C) as well as expression of pre- and postsynaptic proteins such as PSD95, synaptobrevin, neurexin 1, and synaptophysin (Fig. 4, A, D–F, and H). However, there was no significant difference in protein expression levels of syntaxin (Fig. 4G). All protein expression levels were compared with control without injection.
Fig. 4.
Neuron-targeted expression of caveolin-1 (Cav-1) enhances expression of disrupted-in-schizophrenia-1 (DISC1) and synaptic proteins in caveolin-1 knockout (Cav-1 KO) mice hippocampi. Cav-1 KO mice (2 mo) were subjected to stereotactic injection of adeno-associated virus serotype 9 (AAV9) containing SynCav1 or synapsin-red fluorescent protein (SynRFP; as control). Mice were killed 1 mo later and hippocampi were collected and homogenized. Homogenates were immunoblotted for Cav-1, DISC1, PSD95, synaptobrevin, neurexin 1, syntaxin 1A, syntaxin, synaptophysin, and GAPDH (A). Western blot quantification showed that SynCav1 injection restored the Cav-1 expression (B) and significantly increased the expression levels of DISC1 (C) and other synaptic proteins: PSD95 (D), synaptobrevin (E), neurexin 1 (F) and synaptobrevin (H). No significant difference was detected in syntaxin 1A (G). Representative blots are from n = 4 experiments. *P < 0.05; **P < 0.01; ****P < 0.0001, SynCav1 vs. SynRFP.
SynCav1 enhances expression of DISC1 and synaptic proteins in differentiated human neurons derived from hiPSCs.
To investigate whether the effect from Cav-1 on DISC1 and synaptic protein could be recapitulated in a human neuronal cell model, primary human fibroblasts were reprogrammed into hiPSCs and subsequently differentiated into neurons as previously described (Woodruff et al. 2013). Interestingly, our data indicate that SynCav1-transfected human neurons (Fig. 5, A and B) exhibited increased protein expression of DISC1 (Fig. 5, A and C), as well as synaptic proteins such as PSD95, synaptophysin, neurexin, syntaxin 1A, and synaptobrevin (Fig. 5, A and D–H), results akin to what we observed in primary rodent neurons and rodent brains following SynCav1 transfection.
Fig. 5.
Neuron-targeted expression of caveolin-1 (Cav-1) enhances expression of disrupted-in-schizophrenia-1 (DISC1) and synaptic proteins in human differentiated primary neurons. The human neurons were differentiated from the Craig Venter 4a neuronal stem cells, which are derived from human induced pluripotent stem cells. Neurons were differentiated in differentiating media for 3–4 wk. After differentiation, neurons were infected by a lentivirus containing the Cav-1 driven by a synapsin promoter (HIV-synCAV1, or SynCav1) for 72 h. SynGFP served as control vector (109 viral particle from both vectors). Homogenates were immunoblotted for Cav-1, DISC1, PSD95, synaptobrevin, neurexin 1, syntaxin 1A, synaptophysin, and GAPDH (A). Quantification of Western blots showed overexpression of Cav-1 protein (B). SynCav1 also significantly enhanced the protein expression of DISC1 (C) and other synaptic proteins: PSD95 (D), synaptobrevin (E), neurexin 1 (F), syntaxin 1A(G), and synaptophysin (H). Representative blots are from n = 4 experiments. *P < 0.05; **P < 0.01, SynCav1 vs. SynGFP.
DISCUSSION
Our study has demonstrated the importance of Cav-1 in regulating DISC1 and synaptic proteins. SynCav1 enhanced DISC1 expression and synaptic proteins in primary rodent neurons as well as in rodent brains. Furthermore, Cav-1 KO mice showed a reduction in the expression levels of DISC1 and synaptic proteins. This reduced expression of synaptic proteins was reversed after AAV9-SynCav1 delivery to the hippocampus of adult mice. The most important part of our study came when we were able to reproduce the data obtained in rodent brains and primary rodent neurons with the use of differentiated human neurons derived from hiPSCs.
We have previously shown that Cav-1 is essential for maintaining and stabilizing proper synaptic signaling (Head et al. 2011) and improving learning and memory in aged rodents (Mandyam et al. 2015). Several studies from our group have shown that neuron-targeted overexpression of Cav-1 promotes neuronal survival and dendritic arborization in vitro (Head et al. 2011). Accordingly, our data showed that SynCav1 enhances expression of synaptic proteins (PSD95, neurexin, synaptobrevin, syntaxin 1, synaptophysin), important for maintaining synaptic plasticity (Calabrese et al. 2006; Guirland and Zheng 2007; Hotulainen and Hoogenraad 2010). On the other hand, loss of Cav-1 accelerates neurodegeneration (Head et al. 2010). Interestingly, our data show that loss of Cav-1 is associated with a reduction in synaptic protein expression. In fact, alteration in synaptic plasticity is responsible for many neurological and neuropsychiatric diseases (Arguello and Gogos 2012; Brennan et al. 2013; Lawrie et al. 2002; Nestler et al. 2002; Selkoe et al. 2012; Uhlhaas 2013). Taken together, our data indicate a positive role of Cav-1 in regulation of synaptic proteins and plasticity.
Risk genes and genetic mutations identified in patients with schizophrenia are involved in synaptic function (Drew et al. 2011; Frankle et al. 2003; Gogos and Gerber 2006; Lisman et al. 2008; Xu et al. 2012). Similar studies on putative mouse models of schizophrenia have strongly suggested synaptic dysfunction (Frankle et al. 2003; Fromer et al. 2014; Hall et al. 2015; Kirov et al. 2012; Xu et al. 2012). A significant amount of studies support the possibility that DISC1 may be one of the candidate genes for schizophrenia (Callicott et al. 2005; Ekelund et al. 2001; Hamshere et al. 2005; Harrison and Weinberger 2005; Hennah et al. 2003; Hodgkinson et al. 2004; Qu et al. 2007; Song et al. 2008). The importance of DISC1 in synaptic function comes from its interaction with many proteins enriched in the synapses that regulate synaptic maturation, function, and plasticity (Brandon 2007; Camargo et al. 2007). Furthermore, DISC1 mouse models display synaptic pathologies (Lee et al. 2011) and show cognitive deficits reflecting those found in schizophrenia, such as impaired working memory (Koike et al. 2006; Kvajo et al. 2008). Additionally, it has been shown that Cav-1 KO mice present with behavioral deficits similar to those seen in many schizophrenia-like symptoms such as altered motor function and altered emotion, as well as memory deficits (Colao et al. 1998; Gioiosa et al. 2008; Head et al. 2008; Trushina et al. 2006). Because there has been much interest in understanding the neurobiology of schizophrenia over the past decade (Harrison and Weinberger 2005; Owen et al. 2005; Sawa and Snyder 2002), in the present study we investigated a potential interaction between DISC1 and Cav-1 on synapse biology. Interestingly, our data indicate that DISC1 is downregulated in Cav-1 KO models and that it localizes to the same protein complex with Cav-1. Additionally, SynCav1 led to upregulation in DISC1 protein expression.
A limitation of the present study is a lack of understanding of the cellular mechanism through which Cav-1 regulates DISC1 expression. Previous work from our group has shown that Cav-1 colocalizes and coimmunoprecipitates with NMDARs and that loss of Cav-1 disrupts NMDAR-mediated signaling, NMDAR-mediated cAMP production, and NMDAR-mediated neuroprotection against oxygen-glucose deprivation (Head et al. 2008, 2011). Interestingly, others have shown in certain mouse models involving reduced NMDAR expression that these mice also have decreased DISC1 levels and exhibit schizophrenia-like mental disorders such as increased motor activity and deficits in social and sexual interactions (Mohn et al. 1999). The modulation of DISC1 by Cav-1 could also involve cAMP signaling. Previous studies have shown that DISC1 regulates cAMP production through its interaction with certain phosphodiesterases (e.g., PDE4) in postsynaptic densities (Bradshaw et al. 2011; Gamo et al. 2013; Soda et al. 2013; Wang et al. 2008). Based on our past and current findings that SynCav1 increases NMDAR and DISC1 expression and augments NMDAR-mediated cAMP production (Head et al. 2011), it is conceivable that SynCav1 could potentially reverse the schizophrenia-like behavioral phenotype in a DISC1/cAMP-dependent signaling pathway. More experiments are needed to confirm this hypothesis.
Pharmacological agents intended to treat schizophrenia are limited to antipsychotic drugs, which exert their effects through blockade of the type 2 dopaminergic receptor (Carlsson and Lindqvist 1963). However, these agents show little efficacy, suggesting a need to discover novel molecular targets and approaches to develop and improve delivery of more effective therapies. Interestingly, Cav-1 KO mice are resistant to atypical antipsychotic drugs (Allen et al. 2011). In this context, Cav-1 is a scaffold for D2-dopamine (Somkuwar et al. 2016) and 5-HT2A receptors, which represent canonical targets for typical and atypical antipsychotic drugs (Bhatnagar et al. 2004; Genedani et al. 2005; Meltzer et al. 1989; Roth et al. 2004). Cav-1 KO mice also exhibit increased sensitivity to psychomimetic effects of phencyclidine (PCP), a phenomenon observed in patients with schizophrenia (Lahti et al. 1995; Luby et al. 1959). For instance, PCP significantly disrupted prepulse inhibition (PPI; the magnitude of response as a function of repeated stimuli) in Cav-1 KO mice and increased locomotor activity (Allen et al. 2011). These findings suggest the possibility that restoring Cav-1 could in one way reduce nonresponsiveness to antipsychotics through modulation of certain molecular targets such as DISC1 or synaptic proteins. Therefore, we believe that maintaining the neuronal function by specifically targeting membrane microdomains (i.e., MLR) and associated synaptic proteins may be potential treatment for this psychiatric disorder.
In summary, the present findings are the first to demonstrate that genetic manipulation of the scaffolding protein Cav-1 specifically in neurons both in vitro and in vivo directly regulates expression of DISC1 and maintains synaptic protein expression essential for neuronal function, synapse formation, and plasticity. Neuronal Cav-1 maybe a control point for neurotransmission and neuromodulation, which are otherwise impaired in those afflicted with schizophrenia. Further understanding of how Cav-1 modulates DISC1 to maintain and organize neuronal growth, signaling, and proper function is of utmost importance to better understand and identify potential molecular targets for treating schizophrenia.
GRANTS
This work was supported by Department of Veterans Affairs Grants BX001225 (to B. P. Head), BX000783 (to D. M. Roth), and BX001963 (to H. H. Patel), and National Institutes of Health Grants NS073653 (to B. P. Head), HL091071 (to H. H. Patel), HL107200 (to H. H. Patel and D. M. Roth), HL066941 (to H. H. Patel and D. M. Roth), HL115933 (to H. H. Patel and D. M. Roth), MH094151 and MH019934 (to D. V. Jeste), and MH19934-20 (to A. Kassan).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.K., J.E., Z.Z., A.A.-Q., Q.M.N., Y.L., K.K., and E.P. performed experiments; A.K. analyzed data; A.K. interpreted results of experiments; A.K. prepared figures; A.K. drafted manuscript; A.K., D.V.J., D.M.R., P.M.P., H.H.P., and B.P.H. edited and revised manuscript; A.K. and B.P.H. approved final version of manuscript.
REFERENCES
- Allen JA, Halverson-Tamboli RA, Rasenick MM. Lipid raft microdomains and neurotransmitter signalling. Nat Rev Neurosci 8: 128–140, 2007. [DOI] [PubMed] [Google Scholar]
- Allen JA, Yadav PN, Setola V, Farrell M, Roth BL. Schizophrenia risk gene CAV1 is both pro-psychotic and required for atypical antipsychotic drug actions in vivo. Transl Psychiatry 1: e33, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arguello PA, Gogos JA. Genetic and cognitive windows into circuit mechanisms of psychiatric disease. Trends Neurosci 35: 3–13, 2012. [DOI] [PubMed] [Google Scholar]
- Austin CP, Ky B, Ma L, Morris JA, Shughrue PJ. Expression of Disrupted-In-Schizophrenia-1, a schizophrenia-associated gene, is prominent in the mouse hippocampus throughout brain development. Neuroscience 124: 3–10, 2004. [DOI] [PubMed] [Google Scholar]
- Bhatnagar A, Sheffler DJ, Kroeze WK, Compton-Toth B, Roth BL. Caveolin-1 interacts with 5-HT2A serotonin receptors and profoundly modulates the signaling of selected Gαq-coupled protein receptors. J Biol Chem 279: 34614–34623, 2004. [DOI] [PubMed] [Google Scholar]
- Bilderback TR, Gazula VR, Lisanti MP, Dobrowsky RT. Caveolin interacts with Trk A and p75(NTR) and regulates neurotrophin signaling pathways. J Biol Chem 274: 257–263, 1999. [DOI] [PubMed] [Google Scholar]
- Boulware MI, Kordasiewicz H, Mermelstein PG. Caveolin proteins are essential for distinct effects of membrane estrogen receptors in neurons. J Neurosci 27: 9941–9950, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw NJ, Soares DC, Carlyle BC, Ogawa F, Davidson-Smith H, Christie S, Mackie S, Thomson PA, Porteous DJ, Millar JK. PKA phosphorylation of NDE1 is DISC1/PDE4 dependent and modulates its interaction with LIS1 and NDEL1. J Neurosci 31: 9043–9054, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon NJ. Dissecting DISC1 function through protein-protein interactions. Biochem Soc Trans 35: 1283–1286, 2007. [DOI] [PubMed] [Google Scholar]
- Brandon NJ, Millar JK, Korth C, Sive H, Singh KK, Sawa A. Understanding the role of DISC1 in psychiatric disease and during normal development. J Neurosci 29: 12768–12775, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon NJ, Sawa A. Linking neurodevelopmental and synaptic theories of mental illness through DISC1. Nat Rev Neurosci 12: 707–722, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan AM, Harris AW, Williams LM. Functional dysconnectivity in schizophrenia and its relationship to neural synchrony. Exp Rev Neurother 13: 755–765, 2013. [DOI] [PubMed] [Google Scholar]
- Brown AS, McGrath JJ. The prevention of schizophrenia. Schizophr Bull 37: 257–261, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan RW, Javitt DC, Marder SR, Schooler NR, Gold JM, McMahon RP, Heresco-Levy U, Carpenter WT. The Cognitive and Negative Symptoms in Schizophrenia Trial (CONSIST): the efficacy of glutamatergic agents for negative symptoms and cognitive impairments. Am J Psychiatry 164: 1593–1602, 2007. [DOI] [PubMed] [Google Scholar]
- Calabrese B, Wilson MS, Halpain S. Development and regulation of dendritic spine synapses. Physiology (Bethesda) 21: 38–47, 2006. [DOI] [PubMed] [Google Scholar]
- Callicott JH, Straub RE, Pezawas L, Egan MF, Mattay VS, Hariri AR, Verchinski BA, Meyer-Lindenberg A, Balkissoon R, Kolachana B, Goldberg TE, Weinberger DR. Variation in DISC1 affects hippocampal structure and function and increases risk for schizophrenia. Proc Natl Acad Sci USA 102: 8627–8632, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camargo LM, Collura V, Rain JC, Mizuguchi K, Hermjakob H, Kerrien S, Bonnert TP, Whiting PJ, Brandon NJ. Disrupted in Schizophrenia 1 Interactome: evidence for the close connectivity of risk genes and a potential synaptic basis for schizophrenia. Mol Psychiatry 12: 74–86, 2007. [DOI] [PubMed] [Google Scholar]
- Carlsson A, Lindqvist M. Effect of chlorpromazine or haloperidol on formation of 3methoxytyramine and normetanephrine in mouse brain. Acta Pharmacol Toxicol (Copenh) 20: 140–144, 1963. [DOI] [PubMed] [Google Scholar]
- Chubb JE, Bradshaw NJ, Soares DC, Porteous DJ, Millar JK. The DISC locus in psychiatric illness. Mol Psychiatry 13: 36–64, 2008. [DOI] [PubMed] [Google Scholar]
- Colao A, Marzullo P, Ferone D, Spiezia S, Cerbone G, Marino V, Di Sarno A, Merola B, Lombardi G. Prostatic hyperplasia: an unknown feature of acromegaly. J Clin Endocrinol Metab 83: 775–779, 1998. [DOI] [PubMed] [Google Scholar]
- Drew LJ, Crabtree GW, Markx S, Stark KL, Chaverneff F, Xu B, Mukai J, Fenelon K, Hsu PK, Gogos JA, Karayiorgou M. The 22q11.2 microdeletion: fifteen years of insights into the genetic and neural complexity of psychiatric disorders. Int J Dev Neurosci 29: 259–281, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekelund J, Hovatta I, Parker A, Paunio T, Varilo T, Martin R, Suhonen J, Ellonen P, Chan G, Sinsheimer JS, Sobel E, Juvonen H, Arajarvi R, Partonen T, Suvisaari J, Lonnqvist J, Meyer J, Peltonen L. Chromosome 1 loci in Finnish schizophrenia families. Hum Mol Genet 10: 1611–1617, 2001. [DOI] [PubMed] [Google Scholar]
- Francesconi A, Kumari R, Zukin RS. Regulation of group I metabotropic glutamate receptor trafficking and signaling by the caveolar/lipid raft pathway. J Neurosci 29: 3590–3602, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankle WG, Lerma J, Laruelle M. The synaptic hypothesis of schizophrenia. Neuron 39: 205–216, 2003. [DOI] [PubMed] [Google Scholar]
- Fromer M, Pocklington AJ, Kavanagh DH, Williams HJ, Dwyer S, Gormley P, Georgieva L, Rees E, Palta P, Ruderfer DM, Carrera N, Humphreys I, Johnson JS, Roussos P, Barker DD, Banks E, Milanova V, Grant SG, Hannon E, Rose SA, Chambert K, Mahajan M, Scolnick EM, Moran JL, Kirov G, Palotie A, McCarroll SA, Holmans P, Sklar P, Owen MJ, Purcell SM, O'Donovan MC. De novo mutations in schizophrenia implicate synaptic networks. Nature 506: 179–184, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamo NJ, Duque A, Paspalas CD, Kata A, Fine R, Boven L, Bryan C, Lo T, Anighoro K, Bermudez L, Peng K, Annor A, Raja A, Mansson E, Taylor SR, Patel K, Simen AA, Arnsten AF. Role of disrupted in schizophrenia 1 (DISC1) in stress-induced prefrontal cognitive dysfunction. Transl Psychiatry 3: e328, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudreault SB, Blain JF, Gratton JP, Poirier J. A role for caveolin-1 in post-injury reactive neuronal plasticity. J Neurochem 92: 831–839, 2005. [DOI] [PubMed] [Google Scholar]
- Genedani S, Guidolin D, Leo G, Filaferro M, Torvinen M, Woods AS, Fuxe K, Ferre S, Agnati LF. Computer-assisted image analysis of caveolin-1 involvement in the internalization process of adenosine A2A-dopamine D2 receptor heterodimers. J Mol Neurosci 26: 177–184, 2005. [DOI] [PubMed] [Google Scholar]
- Gioiosa L, Raggi C, Ricceri L, Jasmin JF, Frank PG, Capozza F, Lisanti MP, Alleva E, Sargiacomo M, Laviola G. Altered emotionality, spatial memory and cholinergic function in caveolin-1 knock-out mice. Behav Brain Res 188: 255–262, 2008. [DOI] [PubMed] [Google Scholar]
- Gogos JA, Gerber DJ. Schizophrenia susceptibility genes: emergence of positional candidates and future directions. Trends Pharmacol Sci 27: 226–233, 2006. [DOI] [PubMed] [Google Scholar]
- Guirland C, Zheng JQ. Membrane lipid rafts and their role in axon guidance. Adv Exp Med Biol 621: 144–155, 2007. [DOI] [PubMed] [Google Scholar]
- Hall J, Trent S, Thomas KL, O'Donovan MC, Owen MJ. Genetic risk for schizophrenia: convergence on synaptic pathways involved in plasticity. Biol Psychiatry 77: 52–58, 2015. [DOI] [PubMed] [Google Scholar]
- Hamshere ML, Bennett P, Williams N, Segurado R, Cardno A, Norton N, Lambert D, Williams H, Kirov G, Corvin A, Holmans P, Jones L, Jones I, Gill M, O'Donovan MC, Owen MJ, Craddock N. Genomewide linkage scan in schizoaffective disorder: significant evidence for linkage at 1q42 close to DISC1, and suggestive evidence at 22q11 and 19p13. Arch Gen Psychiatry 62: 1081–1088, 2005. [DOI] [PubMed] [Google Scholar]
- Harrison PJ. Schizophrenia: a disorder of neurodevelopment? Curr Opin Neurobiol 7: 285–289, 1997. [DOI] [PubMed] [Google Scholar]
- Harrison PJ, Weinberger DR. Schizophrenia genes, gene expression, and neuropathology: on the matter of their convergence. Mol Psychiatry 10: 40–68; image 5, 2005. [DOI] [PubMed] [Google Scholar]
- Head BP, Hu Y, Finley JC, Saldana MD, Bonds JA, Miyanohara A, Niesman IR, Ali SS, Murray F, Insel PA, Roth DM, Patel HH, Patel PM. Neuron-targeted caveolin-1 protein enhances signaling and promotes arborization of primary neurons. J Biol Chem 286: 33310–33321, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head BP, Patel HH, Tsutsumi YM, Hu Y, Mejia T, Mora RC, Insel PA, Roth DM, Drummond JC, Patel PM. Caveolin-1 expression is essential for N-methyl-d-aspartate receptor-mediated Src and extracellular signal-regulated kinase 1/2 activation and protection of primary neurons from ischemic cell death. FASEB J 22: 828–840, 2008. [DOI] [PubMed] [Google Scholar]
- Head BP, Peart JN, Panneerselvam M, Yokoyama T, Pearn ML, Niesman IR, Bonds JA, Schilling JM, Miyanohara A, Headrick J, Ali SS, Roth DM, Patel PM, Patel HH. Loss of caveolin-1 accelerates neurodegeneration and aging. PLoS One 5: e15697, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiman M, Schaefer A, Gong S, Peterson JD, Day M, Ramsey KE, Suarez-Farinas M, Schwarz C, Stephan DA, Surmeier DJ, Greengard P, Heintz N. A translational profiling approach for the molecular characterization of CNS cell types. Cell 135: 738–748, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennah W, Varilo T, Kestila M, Paunio T, Arajarvi R, Haukka J, Parker A, Martin R, Levitzky S, Partonen T, Meyer J, Lonnqvist J, Peltonen L, Ekelund J. Haplotype transmission analysis provides evidence of association for DISC1 to schizophrenia and suggests sex-dependent effects. Hum Mol Genet 12: 3151–3159, 2003. [DOI] [PubMed] [Google Scholar]
- Hodgkinson CA, Goldman D, Jaeger J, Persaud S, Kane JM, Lipsky RH, Malhotra AK. Disrupted in schizophrenia 1 (DISC1): association with schizophrenia, schizoaffective disorder, and bipolar disorder. Am J Hum Genet 75: 862–872, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotulainen P, Hoogenraad CC. Actin in dendritic spines: connecting dynamics to function. J Cell Biol 189: 619–629, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizuka K, Paek M, Kamiya A, Sawa A. A review of Disrupted-In-Schizophrenia-1 (DISC1): neurodevelopment, cognition, and mental conditions. Biol Psychiatry 59: 1189–1197, 2006. [DOI] [PubMed] [Google Scholar]
- Jaaro-Peled H, Hayashi-Takagi A, Seshadri S, Kamiya A, Brandon NJ, Sawa A. Neurodevelopmental mechanisms of schizophrenia: understanding disturbed postnatal brain maturation through neuregulin-1-ErbB4 and DISC1. Trends Neurosci 32: 485–495, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick B, Xu L, Cascella N, Ozeki Y, Sawa A, Roberts RC. DISC1 immunoreactivity at the light and ultrastructural level in the human neocortex. J Comp Neurol 497: 436–450, 2006. [DOI] [PubMed] [Google Scholar]
- Kirov G, Pocklington AJ, Holmans P, Ivanov D, Ikeda M, Ruderfer D, Moran J, Chambert K, Toncheva D, Georgieva L, Grozeva D, Fjodorova M, Wollerton R, Rees E, Nikolov I, van de Lagemaat LN, Bayes A, Fernandez E, Olason PI, Bottcher Y, Komiyama NH, Collins MO, Choudhary J, Stefansson K, Stefansson H, Grant SG, Purcell S, Sklar P, O'Donovan MC, Owen MJ. De novo CNV analysis implicates specific abnormalities of postsynaptic signalling complexes in the pathogenesis of schizophrenia. Mol Psychiatry 17: 142–153, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike H, Arguello PA, Kvajo M, Karayiorgou M, Gogos JA. Disc1 is mutated in the 129S6/SvEv strain and modulates working memory in mice. Proc Natl Acad Sci USA 103: 3693–3697, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvajo M, McKellar H, Arguello PA, Drew LJ, Moore H, MacDermott AB, Karayiorgou M, Gogos JA. A mutation in mouse Disc1 that models a schizophrenia risk allele leads to specific alterations in neuronal architecture and cognition. Proc Natl Acad Sci USA 105: 7076–7081, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahti AC, Koffel B, LaPorte D, Tamminga CA. Subanesthetic doses of ketamine stimulate psychosis in schizophrenia. Neuropsychopharmacology 13: 9–19, 1995. [DOI] [PubMed] [Google Scholar]
- Lawrie SM, Buechel C, Whalley HC, Frith CD, Friston KJ, Johnstone EC. Reduced frontotemporal functional connectivity in schizophrenia associated with auditory hallucinations. Biol Psychiatry 51: 1008–1011, 2002. [DOI] [PubMed] [Google Scholar]
- Lee FH, Fadel MP, Preston-Maher K, Cordes SP, Clapcote SJ, Price DJ, Roder JC, Wong AH. Disc1 point mutations in mice affect development of the cerebral cortex. J Neurosci 31: 3197–3206, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Levitt P. Schizophrenia as a disorder of neurodevelopment. Annu Rev Neurosci 25: 409–432, 2002. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Moghaddam B. Cognitive dysfunction in schizophrenia: convergence of gamma-aminobutyric acid and glutamate alterations. Arch Neurol 63: 1372–1376, 2006. [DOI] [PubMed] [Google Scholar]
- Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, Keefe RS, Davis SM, Davis CE, Lebowitz BD, Severe J, Hsiao JK. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med 353: 1209–1223, 2005. [DOI] [PubMed] [Google Scholar]
- Lisman JE, Coyle JT, Green RW, Javitt DC, Benes FM, Heckers S, Grace AA. Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia. Trends Neurosci 31: 234–242, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby ED, Cohen BD, Rosenbaum G, Gottlieb JS, Kelley R. Study of a new schizophrenomimetic drug; sernyl. AMA Arch Neurol Psychiatry 81: 363–369, 1959. [DOI] [PubMed] [Google Scholar]
- Mandyam CD, Schilling JM, Cui W, Egawa J, Niesman IR, Kellerhals SE, Staples MC, Busija AR, Risbrough VB, Posadas E, Grogman GC, Chang JW, Roth DM, Patel PM, Patel HH, Head BP. Neuron-targeted caveolin-1 improves molecular signaling, plasticity, and behavior dependent on the hippocampus in adult and aged mice. Biol Psychiatry (October 7, 2015). doi: 10.1016/j.biopsych.2015.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer HY, Matsubara S, Lee JC. Classification of typical and atypical antipsychotic drugs on the basis of dopamine D-1, D-2 and serotonin2 pKi values. J Pharmacol Exp Ther 251: 238–246, 1989. [PubMed] [Google Scholar]
- Millar JK, James R, Christie S, Porteous DJ. Disrupted in schizophrenia 1 (DISC1): subcellular targeting and induction of ring mitochondria. Mol Cell Neurosci 30: 477–484, 2005. [DOI] [PubMed] [Google Scholar]
- Millar JK, Wilson-Annan JC, Anderson S, Christie S, Taylor MS, Semple CA, Devon RS, St Clair DM, Muir WJ, Blackwood DH, Porteous DJ. Disruption of two novel genes by a translocation co-segregating with schizophrenia. Hum Mol Genet 9: 1415–1423, 2000. [DOI] [PubMed] [Google Scholar]
- Miyoshi K, Honda A, Baba K, Taniguchi M, Oono K, Fujita T, Kuroda S, Katayama T, Tohyama M. Disrupted-In-Schizophrenia 1, a candidate gene for schizophrenia, participates in neurite outgrowth. Mol Psychiatry 8: 685–694, 2003. [DOI] [PubMed] [Google Scholar]
- Mohn AR, Gainetdinov RR, Caron MG, Koller BH. Mice with reduced NMDA receptor expression display behaviors related to schizophrenia. Cell 98: 427–436, 1999. [DOI] [PubMed] [Google Scholar]
- Muir WJ, Pickard BS, Blackwood DH. Disrupted-in-Schizophrenia-1. Curr Psychiatry Rep 10: 140–147, 2008. [DOI] [PubMed] [Google Scholar]
- Narayan S, Nakajima K, Sawa A. DISC1: a key lead in studying cortical development and associated brain disorders. Neuroscientist 19: 451–464, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of depression. Neuron 34: 13–25, 2002. [DOI] [PubMed] [Google Scholar]
- Owen MJ, Craddock N, O'Donovan MC. Schizophrenia: genes at last? Trends Genet 21: 518–525, 2005. [DOI] [PubMed] [Google Scholar]
- Qu M, Tang F, Yue W, Ruan Y, Lu T, Liu Z, Zhang H, Han Y, Zhang D, Wang F, Zhang D. Positive association of the Disrupted-in-Schizophrenia-1 gene (DISC1) with schizophrenia in the Chinese Han population. Am J Med Genet B Neuropsychiatr Genet 144B: 266–270, 2007. [DOI] [PubMed] [Google Scholar]
- Roth BL, Sheffler DJ, Kroeze WK. Magic shotguns versus magic bullets: selectively non-selective drugs for mood disorders and schizophrenia. Nat Rev Drug Discov 3: 353–359, 2004. [DOI] [PubMed] [Google Scholar]
- Saha S, Chant D, Welham J, McGrath J. A systematic review of the prevalence of schizophrenia. PLoS Med 2: e141, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawa A, Snyder SH. Schizophrenia: diverse approaches to a complex disease. Science 296: 692–695, 2002. [DOI] [PubMed] [Google Scholar]
- Sawamura N, Sawamura-Yamamoto T, Ozeki Y, Ross CA, Sawa A. A form of DISC1 enriched in nucleus: altered subcellular distribution in orbitofrontal cortex in psychosis and substance/alcohol abuse. Proc Natl Acad Sci USA 102: 1187–1192, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt A, Malchow B, Hasan A, Falkai P. The impact of environmental factors in severe psychiatric disorders. Front Neurosci 8: 19, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schurov IL, Handford EJ, Brandon NJ, Whiting PJ. Expression of disrupted in schizophrenia 1 (DISC1) protein in the adult and developing mouse brain indicates its role in neurodevelopment. Mol Psychiatry 9: 1100–1110, 2004. [DOI] [PubMed] [Google Scholar]
- Selkoe D, Mandelkow E, Holtzman D. Deciphering Alzheimer disease. Cold Spring Harb Perspect Med 2: a011460, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soda T, Frank C, Ishizuka K, Baccarella A, Park YU, Flood Z, Park SK, Sawa A, Tsai LH. DISC1-ATF4 transcriptional repression complex: dual regulation of the cAMP-PDE4 cascade by DISC1. Mol Psychiatry 18: 898–908, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somkuwar SS, Fannon MJ, Head BP, Mandyam CD. Methamphetamine reduces expression of caveolin-1 in the dorsal striatum: Implication for dysregulation of neuronal function. Neuroscience 328: 147–156, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W, Li W, Feng J, Heston LL, Scaringe WA, Sommer SS. Identification of high risk DISC1 structural variants with a 2% attributable risk for schizophrenia. Biochem Biophys Res Commun 367: 700–706, 2008. [DOI] [PubMed] [Google Scholar]
- St Clair D, Blackwood D, Muir W, Carothers A, Walker M, Spowart G, Gosden C, Evans HJ. Association within a family of a balanced autosomal translocation with major mental illness. Lancet 336: 13–16, 1990. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Numakawa T, Shimazu K, Koshimizu H, Hara T, Hatanaka H, Mei L, Lu B, Kojima M. BDNF-induced recruitment of TrkB receptor into neuronal lipid rafts: roles in synaptic modulation. J Cell Biol 167: 1205–1215, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trushina E, Du Charme J, Parisi J, McMurray CT. Neurological abnormalities in caveolin-1 knock out mice. Behav Brain Res 172: 24–32, 2006. [DOI] [PubMed] [Google Scholar]
- Uhlhaas PJ. Dysconnectivity, large-scale networks and neuronal dynamics in schizophrenia. Curr Opin Neurobiol 23: 283–290, 2013. [DOI] [PubMed] [Google Scholar]
- Vilain J, Galliot AM, Durand-Roger J, Leboyer M, Llorca PM, Schurhoff F, Szoke A. [Environmental risk factors for schizophrenia: a review]. Encephale 39: 19–28, 2013. [DOI] [PubMed] [Google Scholar]
- Walsh T, McClellan JM, McCarthy SE, Addington AM, Pierce SB, Cooper GM, Nord AS, Kusenda M, Malhotra D, Bhandari A, Stray SM, Rippey CF, Roccanova P, Makarov V, Lakshmi B, Findling RL, Sikich L, Stromberg T, Merriman B, Gogtay N, Butler P, Eckstrand K, Noory L, Gochman P, Long R, Chen Z, Davis S, Baker C, Eichler EE, Meltzer PS, Nelson SF, Singleton AB, Lee MK, Rapoport JL, King MC, Sebat J. Rare structural variants disrupt multiple genes in neurodevelopmental pathways in schizophrenia. Science 320: 539–543, 2008. [DOI] [PubMed] [Google Scholar]
- Wang Q, Jaaro-Peled H, Sawa A, Brandon NJ. How has DISC1 enabled drug discovery? Mol Cell Neurosci 37: 187–195, 2008. [DOI] [PubMed] [Google Scholar]
- Woodruff G, Young JE, Martinez FJ, Buen F, Gore A, Kinaga J, Li Z, Yuan SH, Zhang K, Goldstein LS. The presenilin-1 DeltaE9 mutation results in reduced gamma-secretase activity, but not total loss of PS1 function, in isogenic human stem cells. Cell Rep 5: 974–985, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B, Ionita-Laza I, Roos JL, Boone B, Woodrick S, Sun Y, Levy S, Gogos JA, Karayiorgou M. De novo gene mutations highlight patterns of genetic and neural complexity in schizophrenia. Nat Genet 44: 1365–1369, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]