Abstract
Cardiopulmonary resuscitation (CPR) duration until return of spontaneous circulation (ROSC) influences survival and neurologic outcomes after delivery room (DR) CPR. High quality chest compressions (CC) improve cerebral and myocardial perfusion. Improved myocardial perfusion increases the likelihood of a faster ROSC. Thus, optimizing CC quality may improve outcomes both by preserving cerebral blood flow during CPR and by reducing the recovery time. CC quality is determined by rate, CC to ventilation (C:V) ratio, and applied force, which are influenced by the CC provider. Thus, provider performance should be taken into account. Neonatal resuscitation guidelines recommend a 3:1 C:V ratio. CCs should be delivered at a rate of 90/min synchronized with ventilations at a rate of 30/min to achieve a total of 120 events/min. Despite a lack of scientific evidence supporting this, the investigation of alternative CC interventions in human neonates is ethically challenging. Also, the infrequent occurrence of extensive CPR measures in the DR make randomized controlled trials difficult to perform. Thus, many biomechanical aspects of CC have been investigated in animal and manikin models. Despite mathematical and physiological rationales that higher rates and uninterrupted CC improve CPR hemodynamics, studies indicate that provider fatigue is more pronounced when CC are performed continuously compared to when a pause is inserted after every third CC as currently recommended. A higher rate (e.g., 120/min) is also more fatiguing, which affects CC quality. In post-transitional piglets with asphyxia-induced cardiac arrest, there was no benefit of performing continuous CC at a rate of 90/min. Not only rate but duty cycle, i.e., the duration of CC/total cycle time, is a known determinant of CC effectiveness. However, duty cycle cannot be controlled with manual CC. Mechanical/automated CC in neonatal CPR has not been explored, and feedback systems are under-investigated in this population. Evidence indicates that providers perform CC at rates both higher and lower than recommended. Video recording of DR CRP has been increasingly applied and observational studies of what is actually done in relation to outcomes could be useful. Different CC rates and ratios should also be investigated under controlled experimental conditions in animals during perinatal transition.
Keywords: newborn, cardiopulmonary resuscitation, chest compression, manikins, piglet
Introduction
Observational data indicate that prolonged cardiopulmonary resuscitation (CPR) in the delivery room (DR) is associated with poor survival and neurologic outcomes (1). High quality chest compressions (CC) improve cerebral and myocardial perfusion. Improved cerebral perfusion ensures brain cell survival during CPR, whereas enhanced myocardial perfusion increases the likelihood of a fast return of spontaneous circulation (ROSC). Thus, optimizing CC quality may improve outcomes both by preserving cerebral blood flow during cardiac arrest and by reducing recovery time. The effectiveness of CC is influenced by (i) CC rate, (ii) CC to ventilation (C:V) ratio, and (iii) applied force, which are all influenced by the CC provider (Figure 1). Thus, besides CPR mechanics and mathematical modeling, educational, emotional, and physical aspects of provider performance need to be considered when addressing the optimal CC algorithm. Ultimately, the physiological effects in the infant determine the most optimal CC intervention.
Figure 1.
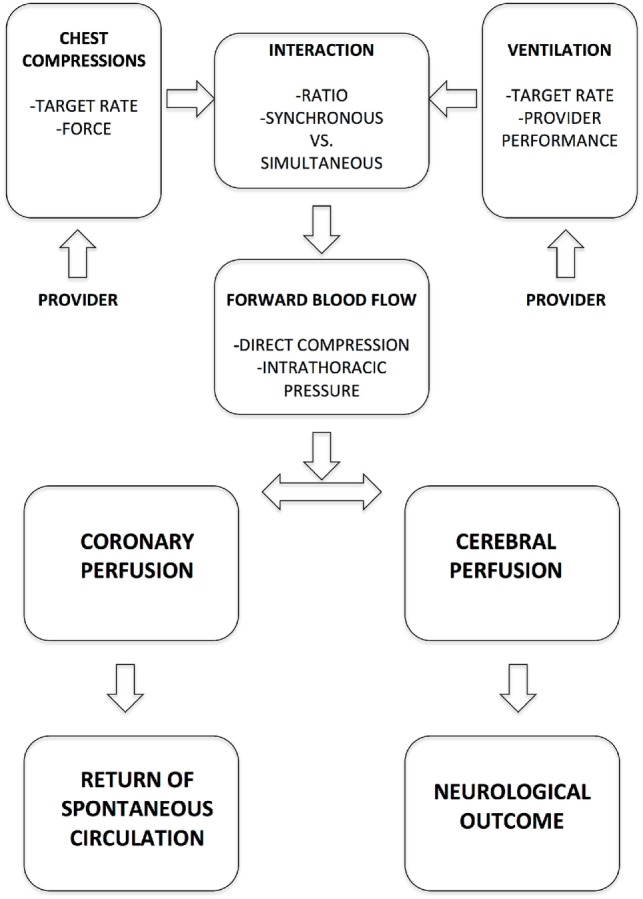
Key determinants of chest compression effectiveness in delivery room cardiopulmonary resuscitation.
In animals with ventricular fibrillation (VF) cardiac arrest, adequately oxygenated blood can be circulated with CC for up to 4–6 min even without assisted ventilation (2). In addition, pediatric and adult animal data demonstrate better coronary perfusion pressures (CPP) with less CC interruption (3–6). Thus, more uninterrupted CC in a series is generally regarded to be beneficial. However, because cardiovascular collapse in the DR is almost invariably due to hypoxia, neonatal resuscitation guidelines recommend a 3:1 C:V ratio (7). CCs should be delivered at a rate of 90/min synchronized with ventilations at a rate of 30/min to achieve a total of 120 events/min (7). There is a lack of scientific evidence to support this standard of care in neonatal CPR, and the unique physiology of perinatal transition as well as cerebral and cardiovascular immaturity call for special considerations in DR CPR. However, investigations of alternative CC interventions in the critically ill human neonate are ethically challenging. Also, the infrequent occurrence of extensive CPR in the DR makes randomized controlled trials difficult to perform. Thus, many biomechanical aspects of CC have been studied in animal and manikin models. In such models, operator performance can also be assessed in a systematic fashion. Because of physical similarities between piglets and human infants including broader chest compared to other non-primate species, and similar chest size and stiffness (8), the porcine model of neonatal CPR has been extensively studied. The aim of this review is to provide an overview about the current knowledge of optimal CC rate and C:V ratio during neonatal CPR.
A summary of studies investigating different CC rates and C:V ratios in neonatal CPR is presented in Table 1.
Table 1.
Summary of studies exploring different CC rates and C:V ratios in neonatal resuscitation.
| Reference | Design | Subjects | Interventions | Outcomes | Conclusion |
|---|---|---|---|---|---|
| Solevåg et al. (13) | Randomized controlled animal trial | Piglets 1–3 days of age weight 1.7–2.4 kg (n = 32) | Asphyxiated piglets were randomized to 3:1 C:V CPR or CCaV CPR (CC rate 90/min) | Time to ROSC was similar for 3:1 C:V CPR and CCaV CPR; p = 0.84 | Overall recovery may be similar, but CCaV might impair myocardial perfusion compared to 3:1 C:V CPR |
| Post mortem analysis of left ventricle lactate was increased in the CCaV group | |||||
| Solevåg et al. (11) | Randomized controlled animal trial | Piglets 12–36 h old (n = 32) | Asphyxiated piglets were randomized to receive a C:V ratio of 3:1 or 9:3 | Time to ROSC (median and interquartile range) was 150 (115–180) s vs. 148 (116–195) s for 3:1 and 9:3, respectively (p = 0.74). There were no differences in diastolic blood pressure (DBP) during compression cycles or in markers of hypoxia and inflammation | The C:V ratio 9:3 was not better than 3:1 |
| Solevåg et al. (12) | Randomized controlled animal trial | Piglets 12–36 h old (n = 22) | Asphyxiated piglets were randomized to receive a C:V ratio 3:1 or 15:2 | Mean (SD) increase in DBP (mmHg) during compression cycles was significantly higher with 15:2 than 3:1 C:V [7.1 (2.8) vs. 4.8 (2.6)]. Median (interquartile range) time to ROSC for the 3:1 group was 150 (140–180) s, and 195 (145–358) s for the 15:2 group | The C:V ratio 15:2 is not better than 3:1 in neonatal resuscitation |
| Dannevig et al. (9) | Randomized controlled animal trial | Piglets 12–36 h old (n = 94) | Asphyxiated piglets were resuscitated with a C:V ratio of 3:1, 9:3, or 15:2 | Interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), and S100 in CSF, and gene expression of matrix metalloproteinases (MMPs), intercellular adhesion molecule-1 (ICAM-1), caspase 3, IL-6 and TNF-α in hippocampus and frontal cortex tissue were similar across C:V groups | Higher C:V ratios did not change the brain inflammatory response compared with the 3:1 C:V ratio |
| Dannevig et al. (17) | Randomized controlled animal trial | Piglets 12–36 h old (n = 72) | Asphyxiated piglets were resuscitated with a C:V ratio of 3:1, 9:3, or 15:2 | IL-8 and TNF-α in BAL fluid and MMP2, MMP9, ICAM-1, and TNFα in lung tissue were similar across C:V groups | Higher C:V ratios did not change the lung inflammatory response compared with the 3:1 C:V ratio |
| Boldingh et al. (30) | Randomized crossover manikin trial | Doctors, nurses, and midwifes (n = 34) | 5 min CPR, with either a 3:1 C:V ratio or CCaV (CC rate 120/min). All participants performed all interventions in a randomized order | The CC proportion with adequate depth was 90.5% for the 3:1 C:V ratio and 60.1% in CCaV | CCaV is more exhausting than a 3:1 C:V ratio |
| CCaV resulted in a greater increase in rescuer heart rate and mean arterial blood pressure, and perceived fatigue, compared to 3:1 C:V CPR | |||||
| Li et al. (28) | Randomized crossover manikin trial | Neonatologists, neonatal-perinatal fellows, neonatal nurse practitioners, and registered nurses (n = 30) | 10 min CPR with a 3:1 C:V ratio, CCaV-90 (CCaV at a 90/min rate), and CC at a rate of 120/min (CCaV-120) (CCaV at a 120/min rate). All participants performed all interventions in a randomized order | Peak CC pressure decreased significantly after 156, 96, and 72 s in the 3:1, CCaV-90, and CCaV-120 groups, respectively | 3:1 C:V CPR was the least fatiguing and the most preferred method |
| Boldingh et al. (29) | Randomized crossover manikin trial | Doctors, nurses, midwives, and last-year medical students (n = 84) | 2 min of CPR with C:V ratios 3:1, 9:3, or 15:2—and CCaV (CC rate 120/min). All participants performed all interventions in a randomized order | 3:1 C:V and 9:3 C:V were comparable in terms of CC and ventilation dynamics | The results of the study support the currently recommended 3:1 C:V ratio |
| The 15:2 C:V ratio resulted in less ventilation vs. the 3:1 C:V ratio | |||||
| The mean CC depth with CCaV vs. the 3:1 method was 32.7 vs. 34.6 mm (p < 0.001) | |||||
| There was a significant decrease in CC depth from baseline after 60 s (p = 0.025) with CCaV | |||||
| The two-person CRP coordination was rated easiest with the 3:1 C:V ratio | |||||
| Solevåg et al. (31) | Randomized crossover manikin trial | Two medical students | Ten times 2 min 3:1 C:V CPR, 9:3 C:V, and 15:2 C:V—and CCaV (CC rate 120/min) were performed in a randomized order | Minute ventilation in mL/kg was significantly lower at the C:V ratios 9:3 [140 (134–144)] and 15:2 [77 (74–83)] vs. 3:1 [191 (183–199)] | Higher C:V ratios than 3:1 compromised ventilation |
CC, chest compression; CPR, cardiopulmonary resuscitation; CCaV, continuous chest compression and asynchronous ventilation; ROSC, return of spontaneous circulation; CSF, cerebrospinal fluid; BAL, bronchoalveolar lavage.
Piglets
Hemodynamic Effects of CC
Biomarkers of Cerebral Perfusion
As an indirect measure of cerebral “well-being” during CPR, Dannevig et al. (9) measured inflammation markers [interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), and S100] in cerebrospinal fluid (CSF), and gene expression of matrix metalloproteinases (MMPs), intercellular adhesion molecule-1 (ICAM-1), caspase 3, IL-6, and TNF-α in hippocampus and frontal cortex tissue. They found that the CSF cytokine concentrations and tissue quantitative real-time PCR in the three C:V ratio groups 3:1, 9:3, and 15:2 were not different (9). This might indicate that in asphyxia, higher C:V ratios do not improve cerebral perfusion in asphyxiated infants and contrasts to the improved neurological outcomes when CC are not being interrupted in VF cardiac arrest (10).
Myocardial Perfusion
Diastolic blood pressure (DBP) is a proxy for myocardial perfusion during CPR. Solevåg et al. reported similar DBP between 3:1 and 9:3 C:V ratio CPR in a piglet model of asphyxia-induced cardiac arrest (11). Interestingly, using a 15:2 C:V ratio resulted in a higher mean (SD) DBP of 7.1 (2.8) vs. 4.8 (2.6) mmHg during standard 3:1 C:V CPR (p = 0.004) (12). Unfortunately, time to ROSC was similar between 15:2 and 3:1 C:V CPR. In a similar study (13), CCaV had similar DBP compared to 3:1 C:V CPR. Post mortem analysis of left ventricle lactate was increased in the CCaV group, which might indicate either anaerobic metabolism or physical trauma, or a combination of both (13).
Physical Trauma/Inflammation
There is a lack of knowledge about lung injury resulting from neonatal CPR. Clinical studies reported an increase in inflammatory markers in blood early after thoracic trauma (14–16). Dannevig et al. (17) analyzed IL-8 and TNF-α in bronchoalveolar lavage (BAL) fluid and MMP2, MMP9, ICAM-1, and TNFα in lung tissue of asphyxiated piglets after CPR. They observed no differences in IL-8 or gene expression in lung tissue between piglets resuscitated with a C:V ratio of 3:1 or 9:3. However, median (IQR) TNF-α was 1.26 (0.92–1.55) pg/ml in the 9:3 group vs. 0.88 (0.67–1.28) pg/ml in the 3:1 group (p = 0.047). Furthermore, inflammatory markers in BAL and lung tissue were increased in piglets where CC were initiated after a 30-s ventilation period compared to 60 and 90 s suggesting that a higher total number of CC during CPR might negatively influence outcome through inflammatory pathways (17).
Epinephrine
Through its α-adrenergic effects, epinephrine mediates peripheral vasoconstriction while forward flow is generated with CC (18), thus elevating the aortic pressure and CPP in pigs (19). Epinephrine has been thought to be essential for ROSC in severe perinatal compromise (20). However, recent experimental data in lambs (21) and piglets (11, 12, 22, 23) are conflicting. Pytte et al. (24) speculated that the hemodynamic effects of epinephrine depend on CC quality, which might explain the conflicting results in different animal models. In asphyxiated piglets, Linner et al. (23) and McNamara (25) found that ROSC could be achieved with CC alone without epinephrine, whereas Solevåg et al. (11, 12) reported that piglets almost invariably required epinephrine for ROSC. Similar findings were made in lambs by Sobotka et al. (21). One important difference between the study protocols was that Linner et al. (23) targeted CC to a mean arterial blood pressure (MAP) of 35–40 mmHg, whereas Solevåg et al. (11, 12) targeted the CC to a MAP of 20 mmHg. McNamara (25) did not specify a pressure target but aimed to compress the chest by one-third of the anteroposterior diameter. Lambs have different chest geometry than piglets, and the two models are not easily comparable in terms of CC quality (26).
Manikins
CC Rate
Neonatal CPR guidelines recommend 120 events/min, which comprise 90 CC and 30 inflations (7). CC duty cycle is the CC duration divided by total cycle time. Increasing the CC rate increases the effective duty cycle by decreasing the total cycle time provided that the CC duration remains constant (8). In fact, mathematical models suggest that the optimal CC rate in newborn infants should exceed 120/min (27). Manikin studies have investigated the feasibility of performing neonatal CC at different rates. Li et al. (28) showed that even though it was possible for neonatal staff to perform continuous CC at rates of 90 and 120/min, a significant decay in CC pressure occurred after 96 and 72 s, respectively. In contrast, when performing standard 3:1 C:V CPR, significant decay occurred only after 156 s. This means that good quality CC might be maintained for more than twice as long with 3:1 C:V CPR compared with uninterrupted CC at a rate of 120/min (CCaV-120). In addition, the 3-min CC depth decline was 50% if CC was performed at a rate of 120/min vs. 30% at a rate of 90/min (28). Similarly, Boldingh et al. (29) found that the CC depth was lower in CCaV-120 compared with the 3:1 C:V ratio method, with a significant decline in CC depth from baseline after 60 s in CCaV-120 vs. no significant decline after 120 s in 3:1 C:V CPR. Boldingh et al. (29) did not investigate continuous CC at a rate of 90/min, but quite unanimously, studies indicate that rescuer fatigue is more pronounced when CC are performed continuously compared to when a pause is inserted after every third CC as currently recommended (28, 29). This is further supported by the preference of resuscitators of the 3:1 C:V ratio over CCaV (28–30), and the two-person coordination is easier with the 3:1 C:V ratio (29). On the other hand, CCaV has been shown to improve minute ventilation during manikin CPR (29, 31).
Educational, Emotional, and Physical Aspects of Operator Performance
Educational
The International Liaison Committee on Resuscitation stated, in their 2015 treatment recommendations (7), that despite very low quality evidence in favor of the 3:1 C:V ratio, they chose to retain the recommendation for educational reasons, as the value of consistency of the algorithm was thought to be significant. There is a lack of data demonstrating educational advantages of alternative C:V ratios.
Emotional
Rescuers’ perceptions of self-efficacy may influence skills acquisition and retention. “Perceived control,” determined by knowledge, competencies, skills, ability, and experience, is a determinant of behavior (32). Education about CC dynamics and physiology, as well as practical CC training can therefore both through knowledge and skills acquisition, and also through enhanced perceived control, positively influence CC performance. Perceived control in the DR may also alleviate the stress experienced during CPR. Some investigators have tried to incorporate the element of stress in simulated CC scenarios (29), but practicing CC in an artificial simulated environment can never replicate the stress of real-life CPR.
Physical
Boldingh et al. (30) assessed heart rate (HR), MAP, and respiratory rate of rescuers as measures of physical fatigue during CPR with either 3:1 C:V CPR or CCaV-120. In addition, they investigated whether body mass index (BMI) and weekly physical activity influenced CC performance. CCaV-120 resulted in a greater increase in rescuer HR and MAP compared to a 3:1 C:V ratio. In agreement with this, continuous CC were perceived as being more fatiguing. Weekly physical activity and BMI did not seem to influence CC performance.
Discussion
In asphyxiated newborn piglets, harm caused by the trauma of CC may counterbalance the beneficial effects of more CC on hemodynamics seen in pediatric and adult animals. The reasons for this are at least twofold: (1) Dean et al. (33) speculated that “standard” CPR is already quite effective in animals with small and compliant chests. Thus, increasing CC rates may not have additive effects in infant CPR, (2) as the cause of profound bradycardia or cardiac arrest in the DR is almost invariably asphyxia, the inflammatory cascade is already highly activated at the time CPR with CC is commenced. The additional trauma of performing CC may add to the injury of vital organs and result in a poor outcome.
The asphyxial etiology of arrest in the DR is of course complicating the picture in a multitude of other ways. Piglet studies suggest that in severe asphyxia, the systemic vascular resistance (SVR) initially increases and then decreases (34). Almost concurrently with the drop in SVR, the MAP declines (34). Aaltonen et al. (34) speculated that this drop in MAP could not be explained by a reduced left ventricular output. This is supported by results from Li et al. (35) that cardiac output in asphyxiated newborn piglets after CPR was not different from control piglets. Thus, a more likely reason for hypotension in the asphyxic neonate is the profound acidosis and lack of substrate (ATP). ATP is required for maintaining vascular tone, and sustained asphyxia will eventually result in maximal vasodilation (36). Thus, blood flow generated with CC preferentially goes through a dilated aorta and into the peripheral circulation rather than into the smaller, higher-resistance coronary arteries (36). This is a plausible explanation for why attempts at increasing coronary perfusion by increasing the C:V ratio or even CC rate has not been successful in models of perinatal asphyxia. CCaV has not proven to be of benefit in asphyxiated piglets, unless they are combined with a sustained inflation (35, 37). Manikin studies support that continuous CC and asynchronous standard positive pressure ventilation might not provide benefit. In fact, this method may result in suboptimal CC quality (28, 29). Rescuers typically prefer standard 3:1 C:V CPR (28, 29).
Knowledge Gaps
Effects of CC in the Absence of a Functioning Cerebral Autoregulation
As cerebral oxygen delivery depends on cerebral blood flow and arterial oxygen content, cerebral oxygen delivery is maintained during hypoxia by an increase in cerebral blood flow (38). However, even brief hypoxia (20 min) results in impairment of cerebral blood flow autoregulation, especially during hypotension episodes in animal models (39, 40). Rosenberg (41) demonstrated in newborn lambs that after asphyxia, the lambs exhibited a markedly impaired vasodilation in response to hypoxia, and no cerebral vasodilation with systemic hypotension (41), quite different from adult models where cerebral autoregulation is intact after asphyxia (42). These differences between newborns and adults could be the result of maturation and should be taken into account when attempts are made to establish the best CC strategy in asphyxiated infants. Since asphyxia causes an impairment of cerebral autoregulation in the newborn infant, the risk of brain damage resulting from fluctuations in blood pressure caused by CC and inotropes should not outweigh the benefits of improved coronary and systemic perfusion. The focus of CC research has been on systemic and coronary hemodynamics with the primary endpoint often being ROSC. Even though neurological adverse effects of epinephrine in preterm infants have been described (43, 44), less attention has been given to the effects of CC alone on cerebral circulation in asphyxiated term infants.
Preterm Infants
Preterm infants requiring CPR with CC have a high mortality and many of the infants that survive develop neurodevelopmental impairment (45). A fragile germinal matrix and poor cerebral autoregulation in preterm infants predispose for intraventricular hemorrhage and may be among the reasons why preterm infants potentially need different CPR strategies compared to term or near-term infants.
Oxygen Fractions
One of the proposed mechanisms behind the impaired cerebral autoregulation after asphyxia is vascular endothelial injury secondary to oxygen-free radical production during reperfusion (46). Hyperoxia during CPR in perinatal asphyxia is detrimental in a multitude of other ways. This has been extensively studied in animals and humans needing positive pressure ventilation. Far less is known about the balance between damage caused by hypoxia and hyperoxia when CC are needed (47).
Duty Cycle and Automated CC
Duty cycle, i.e., the duration of CC/total cycle time, is a known determinant of CC effectiveness in pediatric and adult models. In animals, the optimal CC rate and duty cycle differ between pediatric and adult models. This may also be the case in human children and adults and should thus be studied further. However, duty cycle cannot be controlled with manual CC. Mechanic CC in neonatal CPR has not been thoroughly explored.
CC Depth and Force
Most studies of CC efficacy in neonatal CPR have used CC depth as measure of CC quality. However, the CC depth that optimizes cerebral and myocardial perfusion remains unknown. Even though some studies have assessed leaning during CC administration, little attention has been given to over-compression of the chest, i.e., the slogan from adult CPR “push hard and fast” (48) has not been properly challenged in neonatal CPR. The depth of CC can be translated into compressive force, which is related to intrathoracic pressure (49). Feedback systems for both depth and force during neonatal CPR are insufficiently explored and deserve attention (50).
Future Research Objectives
In real-life resuscitation, it is not uncommon that CC and assisted ventilations are being performed in a more or less asynchronous fashion. Evidence indicates that compliance with the algorithm is poor (51–53). Video recording of DR CRP has been increasingly applied and observational studies of what is actually done in relation to outcomes could potentially be useful.
The extensively used manikin models cannot replicate the complex mechanisms of antegrade blood flow during CPR. Manikins are one-dimensional with respect to the fact that blood flow during CC is a product of direct compression forces and the complex changes in intrathoracic pressure that occur. To reliably determine the effects of different CC interventions on regional and systemic hemodynamics, the use of transitioning animal models that more accurately replicate the newborn circulation with patent fetal shunts is required.
In conclusion, the very unique physiology of perinatal transition and asphyxia make DR resuscitation different from resuscitation at any time later in life. Most of what we know about CC dynamics from pediatric and adult basic and clinical research has to be challenged in appropriate neonatal models. Thus far, no CC study has been performed in a transitioning animal model, and is urgently needed.
Author Contributions
AS and GS: substantial contributions to all of the following: (1) the conception and design of the manuscript, (2) drafting the manuscript or revising it critically for important intellectual content, and (3) final approval of the version to be submitted.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
We would like to thank the public for donation to our funding agencies: GS is a recipient of the Heart and Stroke Foundation/University of Alberta Professorship of Neonatal Resuscitation and a Heart and Stroke Foundation Canada and a Heart and Stroke Foundation Alberta New Investigator Award. The authors have no financial relationships relevant to this article to disclose. No current funding source for this study. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Abbreviations
CPR, cardiopulmonary resuscitation; DR, delivery room; CC, chest compressions; ROSC, return of spontaneous circulation; C:V ratio, chest compression to ventilation ratio; VF, ventricular fibrillation; CPP, coronary perfusion pressure; CSF, cerebrospinal fluid; CCaV, continuous CC with asynchronous ventilation; DBP, diastolic blood pressure; MAP, mean arterial blood pressure.
References
- 1.Harrington DJ, Redman CW, Moulden M, Greenwood CE. The long-term outcome in surviving infants with Apgar zero at 10 minutes: a systematic review of the literature and hospital-based cohort. Am J Obstet Gynecol (2007) 196:463.e1–5. 10.1016/j.ajog.2006.10.877 [DOI] [PubMed] [Google Scholar]
- 2.Chandra NC, Gruben KG, Tsitlik JE, Brower R, Guerci AD, Halperin HH, et al. Observations of ventilation during resuscitation in a canine model. Circulation (1994) 90:3070–5. 10.1161/01.CIR.90.6.3070 [DOI] [PubMed] [Google Scholar]
- 3.Ewy GA, Zuercher M, Hilwig RW, Sanders AB, Berg RA, Otto CW, et al. Improved neurological outcome with continuous chest compressions compared with 30:2 compressions-to-ventilations cardiopulmonary resuscitation in a realistic swine model of out-of-hospital cardiac arrest. Circulation (2007) 116:2525–30. 10.1161/CIRCULATIONAHA.107.711820 [DOI] [PubMed] [Google Scholar]
- 4.Kern KB, Hilwig R, Berg RA, Ewy GA. Efficacy of chest compression-only BLS CPR in the presence of an occluded airway. Resuscitation (1998) 39:179–88. 10.1016/S0300-9572(98)00141-5 [DOI] [PubMed] [Google Scholar]
- 5.Kern KB, Hilwig RW, Berg RA, Sanders AB, Ewy GA. Importance of continuous chest compressions during cardiopulmonary resuscitation: improved outcome during a simulated single lay-rescuer scenario. Circulation (2002) 105:645–9. 10.1161/hc0502.102963 [DOI] [PubMed] [Google Scholar]
- 6.Berg RA, Sanders AB, Kern KB, Hilwig RW, Heidenreich JW, Porter ME, et al. Adverse hemodynamic effects of interrupting chest compressions for rescue breathing during cardiopulmonary resuscitation for ventricular fibrillation cardiac arrest. Circulation (2001) 104:2465–70. 10.1161/hc4501.098926 [DOI] [PubMed] [Google Scholar]
- 7.Perlman JM, Wyllie J, Kattwinkel J, Wyckoff MH, Aziz K, Guinsburg R, et al. Part 7: neonatal resuscitation: 2015 international consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations. Circulation (2015) 132:S204–41. 10.1161/CIR.0000000000000276 [DOI] [PubMed] [Google Scholar]
- 8.Dean JM, Koehler RC, Schleien CL, Atchison D, Gervais H, Berkowitz I, et al. Improved blood flow during prolonged cardiopulmonary resuscitation with 30% duty cycle in infant pigs. Circulation (1991) 84:896–904. 10.1161/01.CIR.84.2.896 [DOI] [PubMed] [Google Scholar]
- 9.Dannevig I, Solevag AL, Sonerud T, Saugstad OD, Nakstad B. Brain inflammation induced by severe asphyxia in newborn pigs and the impact of alternative resuscitation strategies on the newborn central nervous system. Pediatr Res (2013) 73:163–70. 10.1038/pr.2012.167 [DOI] [PubMed] [Google Scholar]
- 10.Xanthos T, Karatzas T, Stroumpoulis K, Lelovas P, Simitsis P, Vlachos I, et al. Continuous chest compressions improve survival and neurologic outcome in a swine model of prolonged ventricular fibrillation. Am J Emerg Med (2012) 30:1389–94. 10.1016/j.ajem.2011.10.008 [DOI] [PubMed] [Google Scholar]
- 11.Solevåg AL, Dannevig I, Wyckoff M, Saugstad OD, Nakstad B. Extended series of cardiac compressions during CPR in a swine model of perinatal asphyxia. Resuscitation (2010) 81:1571–6. 10.1016/j.resuscitation.2010.06.007 [DOI] [PubMed] [Google Scholar]
- 12.Solevåg AL, Dannevig I, Wyckoff M, Saugstad OD, Nakstad B. Return of spontaneous circulation with a compression: ventilation ratio of 15:2 versus 3:1 in newborn pigs with cardiac arrest due to asphyxia. Arch Dis Child Fetal Neonatal Ed (2011) 96:F417–21. 10.1136/adc.2010.200386 [DOI] [PubMed] [Google Scholar]
- 13.Solevåg AL, Schmölzer GM, O’Reilly M, Lu M, Lee TF, Hornberger LK, et al. Myocardial perfusion and oxidative stress after 21% vs. 100% oxygen ventilation and uninterrupted chest compressions in severely asphyxiated piglets. Resuscitation (2016) 106:7–13. 10.1016/j.resuscitation.2016.06.014 [DOI] [PubMed] [Google Scholar]
- 14.Gebhard F, Pfetsch H, Steinbach G, Strecker W, Kinzl L, Bruckner UB. Is interleukin 6 an early marker of injury severity following major trauma in humans? Arch Surg (2000) 135:291–5. 10.1001/archsurg.135.3.291 [DOI] [PubMed] [Google Scholar]
- 15.Strecker W, Gebhard F, Perl M, Rager J, Buttenschon K, Kinzl L, et al. Biochemical characterization of individual injury pattern and injury severity. Injury (2003) 34:879–87. 10.1016/S0020-1383(03)00022-6 [DOI] [PubMed] [Google Scholar]
- 16.Yamada T, Hisanaga M, Nakajima Y, Kanehiro H, Watanabe A, Ohyama T, et al. Serum interleukin-6, interleukin-8, hepatocyte growth factor, and nitric oxide changes during thoracic surgery. World J Surg (1998) 22:783–90. 10.1007/s002689900470 [DOI] [PubMed] [Google Scholar]
- 17.Dannevig I, Solevag AL, Saugstad OD, Nakstad B. Lung injury in asphyxiated newborn pigs resuscitated from cardiac arrest – the impact of supplementary oxygen, longer ventilation intervals and chest compressions at different compression-to-ventilation ratios. Open Respir Med J (2012) 6:89–96. 10.2174/1874306401206010089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown CG, Werman HA, Davis EA, Katz S, Hamlin RL. The effect of high-dose phenylephrine versus epinephrine on regional cerebral blood flow during CPR. Ann Emerg Med (1987) 16:743–8. 10.1016/S0196-0644(87)80566-8 [DOI] [PubMed] [Google Scholar]
- 19.Burnett AM, Segal N, Salzman JG, McKnite MS, Frascone RJ. Potential negative effects of epinephrine on carotid blood flow and ETCO2 during active compression-decompression CPR utilizing an impedance threshold device. Resuscitation (2012) 83:1021–4. 10.1016/j.resuscitation.2012.03.018 [DOI] [PubMed] [Google Scholar]
- 20.Wyckoff MH, Perlman JM. Use of high-dose epinephrine and sodium bicarbonate during neonatal resuscitation: is there proven benefit? Clin Perinatol (2006) 33:141–51, viii–ix. 10.1016/j.clp.2005.11.016 [DOI] [PubMed] [Google Scholar]
- 21.Sobotka KS, Polglase GR, Schmolzer GM, Davis PG, Klingenberg C, Hooper SB. Effects of chest compressions on cardiovascular and cerebral haemodynamics in asphyxiated near-term lambs. Pediatr Res (2015) 78:395–400. 10.1038/pr.2015.117 [DOI] [PubMed] [Google Scholar]
- 22.McNamara PJ, Engelberts D, Finelli M, Adeli K, Kavanagh BP. Vasopressin improves survival compared with epinephrine in a neonatal piglet model of asphyxial cardiac arrest. Pediatr Res (2014) 75:738–48. 10.1038/pr.2014.38 [DOI] [PubMed] [Google Scholar]
- 23.Linner R, Werner O, Perez-de-Sa V, Cunha-Goncalves D. Early adrenaline administration does not improve circulatory recovery during resuscitation from severe asphyxia in newborn piglets. Resuscitation (2012) 83:1298–303. 10.1016/j.resuscitation.2012.02.030 [DOI] [PubMed] [Google Scholar]
- 24.Pytte M, Kramer-Johansen J, Eilevstjonn J, Eriksen M, Stromme TA, Godang K, et al. Haemodynamic effects of adrenaline (epinephrine) depend on chest compression quality during cardiopulmonary resuscitation in pigs. Resuscitation (2006) 71:369–78. 10.1016/j.resuscitation.2006.05.003 [DOI] [PubMed] [Google Scholar]
- 25.McNamara PJ. Cardiopulmonary resuscitation for neonates: evidence for compressions? Resuscitation (2008) 78:241–2. 10.1016/j.resuscitation.2008.03.009 [DOI] [PubMed] [Google Scholar]
- 26.Solevåg AL, Cheung PY, Lie H, O’Reilly M, Aziz K, Nakstad B, et al. Chest compressions in newborn animal models: a review. Resuscitation (2015) 96:151–5. 10.1016/j.resuscitation.2015.08.001 [DOI] [PubMed] [Google Scholar]
- 27.Babbs CF, Meyer A, Nadkarni V. Neonatal CPR: room at the top – a mathematical study of optimal chest compression frequency versus body size. Resuscitation (2009) 80:1280–4. 10.1016/j.resuscitation.2009.07.014 [DOI] [PubMed] [Google Scholar]
- 28.Li ES, Cheung PY, O’Reilly M, Aziz K, Schmolzer GM. Rescuer fatigue during simulated neonatal cardiopulmonary resuscitation. Perinatol (2014) 35:142–5. 10.1038/jp.2014.165 [DOI] [PubMed] [Google Scholar]
- 29.Boldingh AM, Solevåg AL, Aasen E, Nakstad B. Resuscitators who compared four simulated infant cardiopulmonary resuscitation methods favoured the three to one compression to ventilation ratio. Acta Paediatr (2016) 105(8):910–6. 10.1111/apa.13339 [DOI] [PubMed] [Google Scholar]
- 30.Boldingh AM, Jensen TH, Bjorbekk AT, Solevag AL, Nakstad B. Rescuers’ physical fatigue with different chest compression to ventilation methods during simulated infant cardiopulmonary resuscitation. J Matern Fetal Neonatal Med (2016) 29:3202–7. 10.3109/14767058.2015.1119115 [DOI] [PubMed] [Google Scholar]
- 31.Solevåg AL, Madland JM, Gjaerum E, Nakstad B. Minute ventilation at different compression to ventilation ratios, different ventilation rates, and continuous chest compressions with asynchronous ventilation in a newborn manikin. Scand J Trauma Resusc Emerg Med (2012) 20:73. 10.1186/1757-7241-20-73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ajzen I. The theory of planed behaviour. Organiz Behav Human Decision Processes (1991) 50:179–211. [Google Scholar]
- 33.Dean JM, Koehler RC, Schleien CL, Berkowitz I, Michael JR, Atchison D, et al. Age-related effects of compression rate and duration in cardiopulmonary resuscitation. J ApplPhysiol (1990) 68:554–60. [DOI] [PubMed] [Google Scholar]
- 34.Aaltonen M, Soukka H, Halkola L, Jalonen J, Holopainen IE, Kero P, et al. Asphyxia aggravates systemic hypotension but not pulmonary hypertension in piglets with meconium aspiration. Pediatr Res (2003) 53:473–8. 10.1203/01.PDR.0000049514.02607.03 [DOI] [PubMed] [Google Scholar]
- 35.Li ES, Cheung PY, Lee TF, Lu M, O’Reilly M, Schmölzer GM. Return of spontaneous circulation is not affected by different chest compression rates superimposed with sustained inflations during cardiopulmonary resuscitation in newborn piglets. PLoS One (2016) 11:e0157249. 10.1371/journal.pone.0157249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kapadia VS, Wyckoff MH. Drugs during delivery room resuscitation – what, when and why? Semin Fetal Neonatal Med (2013) 18:357–61. 10.1016/j.siny.2013.08.001 [DOI] [PubMed] [Google Scholar]
- 37.Schmölzer GM, O’Reilly M, Labossiere J, Lee TF, Cowan S, Qin S, et al. Cardiopulmonary resuscitation with chest compressions during sustained inflations: a new technique of neonatal resuscitation that improves recovery and survival in a neonatal porcine model. Circulation (2013) 128:2495–503. 10.1161/CIRCULATIONAHA.113.002289 [DOI] [PubMed] [Google Scholar]
- 38.Jones MD, Jr, Traystman RJ. Cerebral oxygenation of the fetus, newborn, and adult. Semin Perinatol (1984) 8:205–16. [PubMed] [Google Scholar]
- 39.Tweed A, Cote J, Lou H, Gregory G, Wade J. Impairment of cerebral blood flow autoregulation in the newborn lamb by hypoxia. Pediatr Res (1986) 20:516–9. 10.1203/00006450-198606000-00007 [DOI] [PubMed] [Google Scholar]
- 40.Short BL, Walker LK, Traystman RJ. Impaired cerebral autoregulation in the newborn lamb during recovery from severe, prolonged hypoxia, combined with carotid artery and jugular vein ligation. Crit Care Med (1994) 22:1262–8. 10.1097/00003246-199408000-00010 [DOI] [PubMed] [Google Scholar]
- 41.Rosenberg AA. Regulation of cerebral blood flow after asphyxia in neonatal lambs. Stroke (1988) 19:239–44. 10.1161/01.STR.19.2.239 [DOI] [PubMed] [Google Scholar]
- 42.Nemoto EM, Snyder JV, Carroll RG, Morita H. Global ischemia in dogs: cerebrovascular CO2 reactivity and autoregulation. Stroke (1975) 6:425–31. 10.1161/01.STR.6.4.425 [DOI] [PubMed] [Google Scholar]
- 43.Frontanes A, Garcia-Fragoso L, Garcia I, Rivera J, Valcarcel M. Outcome of very-low-birth-weight infants who received epinephrine in the delivery room. Resuscitation (2011) 82:427–30. 10.1016/j.resuscitation.2010.11.020 [DOI] [PubMed] [Google Scholar]
- 44.Pinto M, Solevåg AL, O’Reilly M, Aziz K, Cheung PY, Schmölzer GM. Evidence on adrenaline use in resuscitation and its relevance to newborn infants: a non-systematic review. Neonatology (2016) 111:37–44. 10.1159/000447960 [DOI] [PubMed] [Google Scholar]
- 45.Wyckoff MH, Salhab WA, Heyne RJ, Kendrick DE, Stoll BJ, Laptook AR, et al. Outcome of extremely low birth weight infants who received delivery room cardiopulmonary resuscitation. J Pediatr (2012) 160:239–244.e2. 10.1016/j.jpeds.2011.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kontos HA, George E. Brown memorial lecture. Oxygen radicals in cerebral vascular injury. Circ Res (1985) 57:508–16. 10.1161/01.RES.57.4.508 [DOI] [PubMed] [Google Scholar]
- 47.Solevåg AL, Dannevig I, Nakstad B, Saugstad OD. Resuscitation of severely asphyctic newborn pigs with cardiac arrest by using 21% or 100% oxygen. Neonatology (2010) 98:64–72. 10.1159/000275560 [DOI] [PubMed] [Google Scholar]
- 48.Scholefield BR, Clinton RO. Push hard and fast, until I tell you not to. Resuscitation (2013) 84:1007–8. 10.1016/j.resuscitation.2013.05.011 [DOI] [PubMed] [Google Scholar]
- 49.Cheung PY, Schmölzer GM. Learning not to lean when you push … some hard-pressed issues of cardiac compressions during cardiopulmonary resuscitation of neonates. Resuscitation (2013) 84:1637–8. 10.1016/j.resuscitation.2013.09.014 [DOI] [PubMed] [Google Scholar]
- 50.Solevåg AL, Cheung PY, Li E, Aziz K, O’Reilly M, Fu B, et al. Quantifying force application to a newborn manikin during simulated cardiopulmonary resuscitation. J Matern Fetal Neonatal Med (2016) 29:1770–2. 10.3109/14767058.2015.1061498 [DOI] [PubMed] [Google Scholar]
- 51.Li ES, Cheung PY, Pichler G, Aziz K, Schmölzer GM. Respiratory function and near infrared spectroscopy recording during cardiopulmonary resuscitation in an extremely preterm newborn. Neonatology (2014) 105:200–4. 10.1159/000357609 [DOI] [PubMed] [Google Scholar]
- 52.Whyte SD, Sinha AK, Wyllie JP. Neonatal resuscitation – a practical assessment. Resuscitation (1999) 40:21–5. 10.1016/S0300-9572(98)00143-9 [DOI] [PubMed] [Google Scholar]
- 53.Foglia E, Patel J, Niles D, Aasland PH, Nadkarni V, Ades A. Provider adherence to neonatal resuscitation program recommendations for coordinated neonatal chest compressions and ventilations. Analg Resusc (2013) (Suppl 1). 10.4172/2324-903X.S1-010 [DOI] [PMC free article] [PubMed] [Google Scholar]


