Abstract
Background
Combined oral contraceptives (COCs) have not been shown to have major effects on lipid and carbohydrate metabolism in normal-weight women. However, we have limited information about the effects on women at high risk for cardiovascular disease and diabetes due to being overweight and obese.
Objectives
To evaluate the effects of second and third generation contraceptive pills on lipid and carbohydrate metabolism in overweight and obese women.
Patients and Methods
This triple-blind controlled trial was performed on 137 healthy women aged 18 - 40 years with a body mass index of 25-34.9 (kg/m2) who were referred to health centers in Tabriz, Iran from 2014 to 2015. The women were randomly divided into groups who were to take 30 mcg ethinyl estradiol/150 mcg levonorgestrel (EE/LGN) (n = 69) or 30 mcg ethinyl estradiol/150 mcg desogestrel (EE/DSG) (n = 68) with an allocation ratio of 1: 1 for three cycles. As primary outcomes, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), and fasting plasma glucose (FPG) were assessed; total cholesterol (TC), triglycerides (TG), and 2-hour plasma glucose in the 75-g oral glucose tolerance test (2-hour 75-g OGTT) were assessed as secondary outcomes.
Results
The differences in lipid and carbohydrate parameters were not significant between the two groups, except for HDL-C (Adjusted MD (CI95%) = 7.00 (2.98 to 11.02)). HDL-C decreased with EE/LGN (P = 0.016) and increased with EE/DSG (P = 0.004). LDL-C and TC increased in both groups, whereas TG increased only with EE/DSG (P < 0.05). Compared with the baseline, FPG levels did not differ significantly in both groups, but EE/DSG increased 2-hour 75-g OGTT (P = 0.010).
Conclusions
We observed no significant differences between the two groups in lipid and carbohydrate metabolism, except for HDL-C. Considering the importance of overweight and obese women’s health, studies with longer follow-up periods are recommended in this respect.
Keywords: Levonorgestrel, Desogestrel, Overweight, Obesity, Lipid metabolism, Carbohydrate Metabolism
1. Background
Since the introduction of the first combined oral contraceptives (COCs) in the 1960s (1), extensive research has been conducted on the non-contraceptive effects of these pills, including their effects on lipid and carbohydrate metabolism (2-4). The effects of COCs on lipid and carbohydrate metabolism depend on the dose of estrogen and progestin and the androgenicity of progestin (5, 6). Old COCs contained high amount of hormones (high-dose pills) and caused adverse changes in lipid and carbohydrate metabolism (7), while low-dose COCs have little effect in changing lipid (5) and carbohydrate (8) metabolism. Estrogen positively changes the lipid metabolism, including increasing the high-density lipoprotein cholesterol (HDL-C) levels and decreasing the low-density lipoprotein cholesterol levels (LDL-C) (9). Depending on their type and androgenic levels, progestins counteract these changes (6). Second generation progestins, including levonorgestrel (LNG), have androgenic properties, decreasing HDL-C and increasing LDL-C, while third generation progestins, including desogestrel (DSG), have fewer androgenic properties, increasing HDL-C and decreasing LDL-C (6, 7). Second generation progestins in combination with 30 mcg or more of ethinyl estradiol (EE) cause subclinical abnormalities in carbohydrate metabolism by reducing peripheral insulin receptors (10). Third generation progestins, however, have negligible effects on carbohydrate metabolism (10, 11). Lipid metabolism disorders and glucose intolerance are risk factors for cardiovascular disease (CVD) and diabetes (12).
According to the world health organization, the prevalence of overweightness and obesity is increasing worldwide (13). Due to increased dyslipidemia (14) and glucose intolerance (15, 16), overweightness and obesity are risk factors for CVD and diabetes. As reported in the last published review in the Cochrane database, most studies have not included overweight and obese women, so information about these women who use COCs and are more prone to diabetes and CVD is insufficient (8).
2. Objectives
The aim of this study was to compare the effects of commonly used second (30 mcg EE/150 mcg LGN) and third generation (30 mcg EE/150 mcg DSG) COCs on lipid and carbohydrate metabolism in overweight and obese women.
3. Patients and Methods
This was a triple-blind clinical trial conducted on 137 women referred to governmental healthcare centers in Tabriz, Iran during June 2014 to July 2015. After obtaining approval from the ethics committee of Tabriz University of Medical Sciences (9317) and registration in the Iranian registry of clinical trials (IRCT201402266709N15), and by complying with the ethical guidelines of the Helsinki declaration, sampling started from the center with the highest number of client referrals and continued on to the three other centers until the desired sample size was obtained.
The inclusion criteria consisted of non-smoking married women between 18 and 40 years of age, having regular and spontaneous menstrual cycles varying from 21 to 35 days, a body mass index (BMI) between 25 - 34.9 (kg/m2), fasting plasma glucose (FPG) < 126 (mg/dL), 2-hour plasma glucose in the 75-g oral glucose tolerance test (2-hour 75-g OGTT) < 200 (mg/dL), LDL-C < 160 (mg/dL) and triglycerides (TG) < 250 (mg/dL), and total cholesterol (TC) < 250 (mg/dL). Exclusion criteria included the existence of absolute and relative contraindications for COCs (17), use of hormonal contraception methods in the past three months, taking lipid-lowering drugs, and having thyroid and renal diseases.
Before recruitment, the inclusion and exclusion criteria checklist and a demographic-anthropometric-obstetric questionnaire were completed after explanation of the objectives and study method. The validity of the forms was confirmed by ten faculty members, and the test-retest method was used to confirm their reliability (r = 0.96).
After obtaining informed written consent, participants were randomly allocated into two groups receiving either second generation contraceptive pills containing 30 mcg EE/150 mcg LGN or third generation contraceptive pills containing 30 mcg EE/150 mcg DSG (Ovocept LD® and Marolin®, respectively, Aburaihan pharmaceutical company, Tehran, Iran).
Allocation sequence was determined by a computer-generated randomization scheme with block sizes of four and six, and an allocation ratio of 1: 1. Participants were stratified by BMI class. Each participant was given an opaque sealed envelope of the same size and shape as the others containing three packages of 21 LD or Marolin pills. Envelopes were numbered consecutively from one to 46 for women with class I obesity and from 47 to 137 for overweight women. Pills were of the same color, size, and appearance and were produced by Aburaihan pharmaceutical company. Generation of the allocation sequence and preparation of the envelopes was done by an individual not involved in the study.
Pills were consumed from the first day of menstruation to day 21 for three cycles with seven-day pill-free intervals between cycles. Participants were instructed to take one tablet daily at the same time, ideally at bed time. Participants, the researcher, and the analyzer were unaware of the assignments of participants to each of the groups.
3.1. Assessment of Blood Pressure and Anthropometric Indices
Blood pressure (BP) and BMI were measured before and three months after intervention. After 10 minutes of rest, BP values were measured on the right arm with patients in the sitting position using a Mercury sphygmomanometer (Microlife, Switzerland) with an accuracy of 5 mmHg; BP was measured twice with at least a 30-second interval, and the average value was reported. Weights and heights were measured using a scale-stadiometer (Seca, Germany) with accuracies of 0.1 kg and 0.1 cm, respectively. During the measurements, participants had light clothing and no shoes on. BMI was calculated as weight in kilograms divided by the square of their height in meters.
3.2. Blood Samples and Biochemical Evaluation
Before and three months after intervention, venous blood samples were obtained from the brachial area in two stages and in two different test tubes: 1.5 mL blood after 12 hours of fasting for HDL-C, LDL-C, TC, TG, and FPG tests, and 2.2 hours after taking 75 g dry glucose mixed with 300 mL of water in 2 mL blood for OGTT. At each stage, blood samples were kept at room temperature for 10 - 15 minutes to clot. Blood serums were then isolated at room temperature through centrifuging at 3500 rpm for 10 minutes (Behdad Universal Centrifuges, Tehran, Iran) and transferred to microtubes (1.5 mL). Each day, serum samples were transferred in ice to the laboratory of the pharmaceutical research center of Tabriz University of Medical Sciences for testing.
Levels of HDL-C, TC, TG, FPG, and 2-hour 75-g OGTT were measured using Pars-Azmun kits (Pars Azmun Co, Tehran, Iran) and using the enzymatic method with an autoanalyzer (Alcyon 300, USA Abbott model). Serum LDL-C levels were calculated using the William Friedewald formula (18). A Tru Cal HDL/LDL calibrator was used for HDL-C Pars-Azmone kits, and a Tru Cal U (Multi Calibrator) calibrator for TC, TG, and FPG Pars-Azmone kits.
3.3. Outcomes
The primary outcome variables included mean levels of HDL-C, LDL-C, and FPG, and the secondary outcome variables included mean levels of TC, TG, and 2-hour 75-g OGTT in the LNG or DSG groups of overweight and obese women three months after intervention.
3.4. Sample Size and Analysis
Because of the lack of Persian studies in various databases in Iran about the serum levels of FPG, HDL-C, and LDL-C in overweight and obese women who take second or third generation oral contraceptives, the sample size was determined using results from a pilot study on 20 overweight and 10 obese women. Serum levels of FPG, HDL-C, and LDL-C were measured before the intervention.
Sample size was calculated using G-Power (G-Power Version 3.1.2, Germany). With two-sided α-0.05, β-0.5, m1 = 76.73 (mean FPG serum level before intervention), m2 = 65.22 (mean FPG serum level after intervention assuming a 15% decrease), and sd1 = sd2 = 7.27, the sample size required was 11 for each group, and by considering m1 = 45.12 (mean HDL-C serum level before intervention), m2 = 51.75 (mean HDL-C serum level after intervention assuming a 15% increase) and sd1 = sd2 = 9.47, the sample size was 55 women for each group. With two-sided α-0.05, β-0.20, m1 = 98.12 (mean LDL-C serum level before intervention), m2 = 83.40 (mean LDL-C serum level after intervention assuming 15% decrease), and sd1 = sd2 = 27.3, the sample size was 54 women for each group. Finally, to account for 25% probability of study drop out, the sample size was calculated to be a total of 137 women.
Statistical analyses were performed using SPSS 13 (SPSS Inc., Chicago, IL, USA). P < 0.05 was considered significant. Data are presented as mean (standard deviation, SD) or median (interquartile range) and frequency (percent) for quantitative and qualitative variables, respectively. The Kolmogorov-Smirnov test, skewness, and kurtosis were used to assess the normality of the data’s distribution. A chi-square test, a trend chi-square test, an exact chi-square test, and Fisher’s exact test were used to compare the qualitative variables between the two groups. A T-test and a paired t-test were used to compare quantitative variables between and within groups, respectively. In the case of abnormal data, the Mann-Whitney test and the Wilcoxon signed-rank test were used to compare quantitative variables between and within the groups, respectively. Analysis of covariance (ANCOVA) was used to compare the clinical and paraclinical characteristics between the two groups at three months after intervention, with control for baseline values and adjusting for BMI.
4. Results
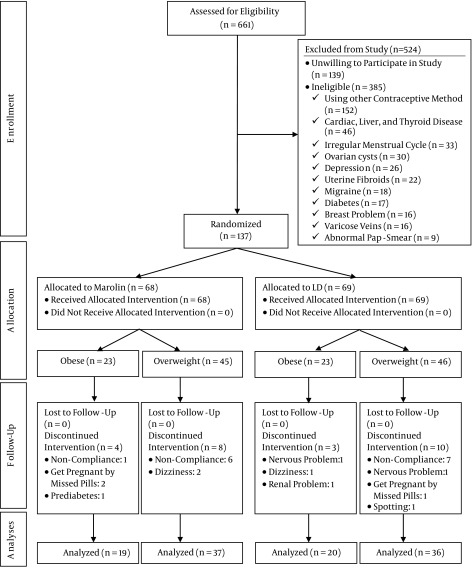
Of the 661 women who were assessed for eligibility, 385 women did not meet the eligibility criteria, and 139 declined to participate in the study. Finally, 137 women participated, out of whom 69 and 68 women were randomized into the LNG and DSG groups, respectively. Thirteen participants in the LNG group and 12 in the DSG group discontinued intervention. Finally, 56 participants were evaluated in each group (Figure 1). Both groups were similar in terms of socio-demographic characteristics (Table 1).
Figure 1. Flowchart of Participants.
Table 1. Demographic Characteristics of the Participants Receiving Second Generation (LD) or Third Generation (Marolin) COCs at Baselinea.
| Characteristics | LD® (n = 69) | Marolin® (n = 68) | Statistical Indicators |
|---|---|---|---|
| Age, y, mean ± SD | 28.85 ± 5.01 | 29.97 ± 6.21 | T- test = -1.15, df = 128.49, P = 0.250 |
| Age of menarche, y, mean ± SD | 13 ± 1.65 | 12.76 ± 2.03 | T-test = 0.743, df = 135, P = 0.459 |
| Weight, kg, mean ± SD | 71.18 ± 7.51 | 70.36 ± 6.52 | T-test = 2.079, df = 135, P = 0.493 |
| Education level | x2 for trend = 0.01, df = 1, P = 0.904 | ||
| Elementary | 19 (27.5) | 18 (26.5) | |
| Guidance | 17 (24.6) | 14 (20.6) | |
| High school | 29 (42) | 35 (51.5) | |
| University | 4 (5.8) | 1 (1.5) | |
| Job | Exact x2 test = 0.645, df = 1, P = 0.759 | ||
| Housewife | 61 (88.4) | 59 (86.8) | |
| Work at home | 4 (8.7) | 2 (8.7) | |
| Work outside the home | 1 (2.2) | 1 (4.3) | |
| Level of income | x2 for trend = 1.39, df = 1, P = 0.237 | ||
| Insufficient | 17 (24.6) | 13 (19.1) | |
| Relatively Sufficient | 50 (72.5) | 50 (73.5) | |
| Sufficient | 2 (2.9) | 5 (1.5) | |
| Previous pregnancy | Fisher’s exact test = 0.32, df = 1, P = 1 | ||
| Yes | 67 (97.1) | 67 (98.5) | |
| No | 2 (2.9) | 1 (1.5) | |
| Children | Fisher’s exact test = 1, df = 1, P = 0.619 | ||
| Yes | 66 (95.7) | 67 (98.5) | |
| No | 3 (4.3) | 1 (1.5) | |
| Previous contraceptive method | Exact x2 test = 2.11, df = 3, P = 0.573 | ||
| Condom | 21 (30.4) | 21 (30.9) | |
| Withdrawal | 35 (50.7) | 38 (55.9) | |
| No method | 6 (8.7) | 2 (2.9) | |
| IUD | 7 (10.1) | 7 (10.3) |
aValues are expressed as No. (%) unless otherwise indicatd.
4.1. Body Mass Index and Blood Pressure
At baseline, no significant difference was observed in BMI and systolic and diastolic BP between the two groups (Table 2). Compared with the baseline, no statistically significant difference was observed in BMI and diastolic BP in both groups, and in systolic BP in the LNG group. However, systolic BP significantly decreased in the DSG group (P = 0.012). The Mann-Whitney test showed no significant difference in BMI and systolic and diastolic BP between the two groups after intervention (Table 3).
Table 2. Clinical and Paraclinical Characteristics of the Participants Receiving Second Generation (LD) or Third Generation (Marolin) COCs Before Intervention.
| Characteristics | LD® Mean (SD)a | Marolin® Mean (SD)a | Statistical Indicators |
|---|---|---|---|
| BMI, kg/m 2 | 28.5 (2.9) | 28.7 (2.78) | Tb = -0.4, df = 135, P = 0.638 |
| Systolic BP, mmHg | 100 (95 to 105)c | 100 (100 to 105)c | Zd = -1.31, P = 0.190 |
| Dystolic BP, mmHg | 60 (60 to 70)c | 65 (60 to70)c | Zd = -85, P = 0.393 |
| HDL-C, mg/dL | 47.9 (10.71) | 45.78 (9.83) | Tb= 1.2, df = 135, P=0.230 |
| LDL-C, mg/dL | 93.69 (28.25) | 99.93 (29.06) | Tb = -1.27, df = 135, P = 0.205 |
| TC, mg/dL | 158.55 (28.99) | 164.88 (31.32) | Tb = -1.22, df = 135, P = 0.222 |
| TG, mg/dL | 84.33 (37.17) | 95.5 (39.29) | Tb = -1.70, df = 135, P = 0.090 |
| FPG, mg/dL | 78.37 (9.87) | 80.38 (10.51) | Tb = -1.15, df = 135, P = 0.252 |
| 2-hour 75-g OGTT, mg/dL | 91.62 (16.15) | 85.69 (19.59) | Tb = 1.93, df = 135, P = 0.055 |
aMean (Standard Deviation)
bT-test
cMedian (P25 to P75)
dMann-Whitney U test
Table 3. Clinical and Paraclinical Characteristics of the Participants Receiving Second Generation (LD) or Third Generation (Marolin) COCs After Intervention.
| Characteristics | LD® Mean (SD)a | Marolin® Mean (SD)a | Adjusted MD (95%CI)b | Statistical Indicators |
|---|---|---|---|---|
| BMI, kg/m2 | ||||
| Before intervention | 28.61 (2.83) | 28.69 (2.79) | ||
| After intervention | 28.69 (3.03) | 28.55 (3.07) | -0.22 (-0.5 to 0.06) | Fc = 2.33, df = 1, P = 0.129 |
| Pd | 0.394 | 0.205 | ||
| Systolic BP, mmHg | ||||
| Before intervention | 95 (100 to 105)e | 100 (100 to 105)e | ||
| After intervention | 100 (100 to 105)e | 100 (100 to 103.75)e | - | Zf = -0.10, P = 0.913 |
| Pg | 0.624 | 0.012 | ||
| Dystolic BP, mmHg | ||||
| Before intervention | 60 (60 to 70)e | 65 (60 to 70)e | ||
| After intervention | 65 (60 to 70)e | 65 (60 to 70)e | - | Zf = -0.14, P = 0.882 |
| Pg | 0.145 | 0.176 | ||
| HDL-C, mg/dL | ||||
| Before intervention | 48.62 (10.83) | 47.08 (9.94) | ||
| After intervention | 45.31 (12.18) | 51.71 (12.2) | 7 (2.98 to 11.02) | Fc = 11.94, df = 1, P = 0.001 |
| Pd | 0.016 | 0.004 | ||
| LDL-C, mg/dL | ||||
| Before intervention | 94.17 (28.08) | 97.81 (30.39) | ||
| After intervention | 107 (33.80) | 105.3 (28.33) | -6.83 (-16.5 to 2.92) | Fc = 1.92, df = 1, P = 0.168 |
| Pd | 0.001 | 0.033 | ||
| TC, mg/dL | ||||
| Before intervention | 160.44 (28.38) | 163.44 (32.96) | ||
| After intervention | 171.41 (36.25) | 178.23 (32.12) | 2.09 (-8.7 to 12.86) | Fc = 0.14, df = 1, P = 0.701 |
| Pd | 0.005 | 0.001 | ||
| TG, mg/dL | ||||
| Before intervention | 88.21 (40.06) | 92.73 (10.30) | ||
| After intervention | 93.57 (37.17) | 106.08 (42.41) | 9.9 (-1.06 to 20.86) | Fc = 3.2, df = 1, P = 0.076 |
| Pd | 0.125 | 0.005 | ||
| FPG, mg/dL | ||||
| Before intervention | 78.71 (10) | 79.69 (9.35) | -0.51 (-3.32 to 2.29) | Fc = 0.13, df = 1, P = 0.716 |
| After intervention | 78.82 (8.71) | 78.16 (7.03) | ||
| Pd | 0.931 | 0.235 | ||
| 2-hour 75-g OGTT, mg/dL | ||||
| Before intervention | 92.41 (16.12) | 83.37 (16.8) | ||
| After intervention | 93 (16.52) | 88.85 (16.82) | 1.11 (-4.54 to 6.76) | Fc = 0.15, df = 1, P = 0.698 |
| Pd | 0.784 | 0.010 |
aMean (Standard Deviation).
bMean Difference (Confidence Interval).
cANCOVA.
dPaired T-test.
eMedian (P25 to P75).
fMann-Whitney U test.
gWilcoxon signed-rank test.
4.2. Lipid Metabolism
Before intervention, no statistically significant difference was found between the two groups in HDL-C, LDL-C, TC, and TG levels (Table 2). ANCOVA adjusted for baseline values and BMI showed that HDL-C in the DSG group was significantly higher than in the LNG group (adjusted MD (CI95%) = 7.00 (2.98 to 11.02). However, no difference was observed in mean LDL-C (adjusted MD (CI95%) = -6.83 (-16.5 to 2.92)), TC (Adjusted MD (CI95%) = 2.09 (-8.7 to 12.86)), or TG (Adjusted MD (CI95%) = 9.9 (-1.06 to 20.86)) (Table 3).
Serum HDL-C levels were significantly reduced in the LNG group (P = 0.016) and significantly increased in the DSG group (P = 0.004). The mean LDL-C was significantly increased in both groups, while the increase was greater in the LNG group (P = 0.001) than in the DSG group (P = 0.033). Both the LNG and DSG groups had significantly increased TC levels (P = 0.005 and P = 0.001, respectively). However, serum TG levels significantly increased only in the DSG group (P = 0.005) (Table 3).
4.3. Carbohydrate Metabolism
At baseline, no statistically significant differences were found between the two groups in FPG and 2-hour 75-g OGTT (Table 2). ANCOVA adjusted for baseline values and BMI showed no significant difference in FPG (Adjusted MD (CI95%) = -0.51 (-3.32 to 2.29)) and 2-hour 75-g OGTT (Adjusted MD (CI95%) = 1.11 (-4.54 to 6.67)) between the two groups three months after intervention. In the DSG group, 2-hour 75-g OGTT was significantly increased compared with the baseline values (P = 0.01) (Table 3).
5. Discussion
In the present study, the effects of second generation COCs containing 30 mcg EE/150 mcg LNG and third generation COCs containing 30 mcg EE/150 mcg DSG on lipid and carbohydrate metabolism in overweight and obese women were examined over the course of a three-month period. Both types of COCs were found to have similar effects on lipid and carbohydrate parameters, except for HDL-C levels, in overweight and obese women.
Besides the contraceptive effects of COCs, their hormone components can cause changes in lipid metabolism (9). Due to their low androgenic effects and the favorable changes induced by estrogen, third generation progestins increase HDL-C and TG and reduce LDL-C. On the other hand, second generation progestins with androgenic activity and dominant progestin can lead to adverse effects and make the lipid profile unfavorable (6, 9). Impaired lipid and lipoprotein metabolism are associated with CVD (19).
In the present study, although no significant difference was observed in the lipid metabolism except for HDL-C in the intergroup comparison, the intragroup comparison showed significant differences in most lipid parameters compared with the baseline. In line with previous studies (20-22), HDL-C levels decreased by about 7% in the LNG group and increased by about 10% in the DSG group, which can be due to the androgenic effects of the second generation COCs compared with the low androgenic effects of the third generation COCs. In two other studies, similar rises in HDL-C levels had also been observed in the DSG groups (10, 23). Levels of HDL-C have been shown to be inversely associated with CVD (19, 24), so that a low HDL-C level is associated with an increased risk of CVD, particularly coronary heart disease (CHD) (19). In this study, increased LDL-C levels were observed in both groups. The increase was more pronounced in the LNG group than in the DSG group (14% vs. 7.7%). Our findings were inconsistent with the results of the study conducted by Van Rooijen et al. (22), which reported unchanged levels of LDL-C after receiving 30 mcg EE/150 mcg DSG or 30mcg EE/150 mcg LNG for two cycles in both groups, and with the study conducted by Foulun et al. (25), where LDL-C levels in women with BMI < 24 (kg/m2) were increased with the use of triphasic pills of 30, 40, 30 mcg EE/50, 75, 125 mcg LNG, and remained unchanged in the 20 mcg EE/150 mcg DSG group. Also, in additional studies, the serum levels of LDL-C were unchanged in women with BMI < 25 (kg/m2) after six months of taking DSG-containing pills (10, 23). The differences in these findings can be attributed to the doses of EE and progestin, the duration of the intervention, and the diets, lifestyles, and BMIs of the participants in the studies.
In line with some previous studies (20, 21, 25), the TC and TG levels were increased in both intervention groups; however, the increase in TG in the LNG group was not significant. The increase in TC and TG in the DSG group (9% and 14.3%, respectively) was greater than that of the LNG group (6% and 6.8%, respectively). Although elevated TG levels are associated with an increased risk of atherosclerosis and CHD (26), increased TG levels parallel to increases in HDL-C levels and without increases in LDL-C levels will not increase the risk of atherosclerosis (23). In previous studies, this lipid pattern was observed for DSG-containing COCs (20, 22, 23), but not for LNG-containing ones (20, 22). In this study, such a pattern was not observed in the lipid parameters in any of the two groups, so that in the DSG group, LDL-C was also increased in addition to TG and HDL-C. According to the National Cholesterol Education Program, elevated LDL-C levels are the major cause of CHD (19). The reason for the differences in the LDL-C parameters in the DSG group compared with previous studies (10, 20, 22,23, 27) might have resulted from the participation of overweight and obese women in this study, who are at higher risk for dyslipidemia, including decreased HDL-C and increased LDL-C and TG (14).
Glucose intolerance is a risk factor for diabetes type II (28) and CVD (29). Although the impact of COCs on carbohydrate metabolism is associated with progestin’s androgenicity (11), after three months, no significant difference was observed in FPG and 2-hour 75-g OGTT levels between the two groups. In comparison with the baseline value, 2-h 75-g OGTT increased in the DSG group. Despite the 6.5%-increase in 2-hour 75-g OGTT glucose levels in the DSG group, the FPG and 2-hour 75-g OGTT levels remained in the normal range for all women, and the observed changes were not indicative of impaired fasting glucose or impaired glucose tolerance. In general, our findings are consistent with those of previous studies (10, 21, 25).
Although our study enrolled overweight and obese women, it is consistent with the findings from the Cochrane database review that aimed at evaluating the effects of steroid contraceptives on carbohydrate metabolism in women without diabetes mellitus; in this review study, no statistically significant differences were found in carbohydrate metabolism among normal-weight women who consumed contraceptives (8). In a recently published study, no statistically significant difference was observed in the fasting glucose levels between obese and normal-weight women during three months of intervention (30). Similarly, in a study by Cheang et al., there were no significant differences in FPG between lean and obese women after six months (31). These findings may indicate that the changes in carbohydrate metabolism in overweight and obese women are similar to those of normal-weight women who take COCs.
The triple-blind design of the study and adjustment for baseline values and BMI in the analyses strengthens the validity of the results. Given that obese and overweight women were enrolled in this study, it is hoped that the findings will be useful, given the increasing trend of overweightness and obesity worldwide.
With respect to the limitations, diet could have affected the results of our study in both groups, which can be considered a potential confounding factor. Although the random assignment of individuals to the groups may have prevented such a limitation, it is suggested that the effects of diet in future studies be controlled. It is also important to point out that, due to financial and time constraints, the effects of COCs on lipid and carbohydrate metabolism were studied for only three months. Given that the effects of COCs may be observed over longer periods of time, further studies on overweight and obese women with longer follow-up periods are recommended.
5.1. Conclusions
In comparison, second and third generation contraceptives caused similar changes in lipid and carbohydrate metabolism in overweight and obese women, except for HDL-C levels. Intragroup evaluations showed the adverse effects of both COCs on lipid metabolism in overweight and obese women. In fact, the patterns of changes caused by both contraceptives on the lipid parameters may increase the risk of CVD. Changes in carbohydrate parameters were negligible in both groups and not clinically significant. However, due to the high prevalence of overweightness and obesity and considering the importance of overweight and obese women’s health (especially given that they are at higher risk for CVD and diabetes), the results are unreliable since COCs can be consumed for a long time. Given the importance of lipid and carbohydrate metabolism and its significant effects on women’s health, consulting and training for overweight and obese women who want to use this method is essential before the COCs are administered.
Acknowledgments
The researchers would hereby like to thank the authorities and officials of the school of nursing and midwifery and the deputy research council of Tabriz University of Medical Sciences for their financial aid, and also all those who helped us with this study. The authors express their thanks to the women who participated in this study and the midwives who were referred as the study patients.
Footnotes
Authors’ Contribution:Study concept and design, Mahnaz Shahnazi, Azizeh Farshbaf-Khalili, and Samira Pourzeinali-Beilankouh; analysis and interpretation of data, Mahnaz Shahnazi, Azizeh Farshbaf-Khalili; Samira Pourzeinali-Beilankouh, and Farza Sadrimehr; drafting of the manuscript, Samira Pourzeinali-Beilankouh; critical revision of the manuscript for important intellectual content, Mahnaz Shahnazi and Azizeh Farshbaf-Khalili; statistical analysis, Mahnaz Shahnazi, Azizeh Farshbaf-Khalili, Samira Pourzeinali-Beilankouh, and Farnaz Sadrimehr.
Declaration of Interest:The authors report no declarations of interest.
Funding/Support:This study was supported by the research deputy of Tabriz University of Medical Sciences. This study was based on a Master of Science thesis approved by the research deputy of Tabriz University of Medical Science and Health Services (Grant No. 5/4/1129).
References
- 1.Junod SW. [cited Agu 7];FDA's approval of the first oral contraceptive, enovid. 2009 Available from: http://www.fda.gov/AboutFDA/WhatWeDo/History/ProductRegulation/SelectionsFromFDLIUpdateSeriesonFDAHistory/ucm092009.htm.
- 2.Hasan Sidd Y, Naz F, Jyoti S, Afzal M. Biochemical Effects of Oral Contraceptives among Users: A Review. Int J Pharmacol. 2012;8(5):314–20. doi: 10.3923/ijp.2012.314.320. [DOI] [Google Scholar]
- 3.Prasad RN, Liew D, Ratnam SS. Comparative metabolic effects of three types of combined oral contraceptive pills in Chinese women. Contraception. 1989;39(1):21–35. doi: 10.1016/0010-7824(89)90013-9. [DOI] [PubMed] [Google Scholar]
- 4.Gaspard UJ, Lefebvre PJ. Clinical aspects of the relationship between oral contraceptives, abnormalities in carbohydrate metabolism, and the development of cardiovascular disease. Am J Obstet Gynecol. 1990;163(1 Pt 2):334–43. doi: 10.1016/0002-9378(90)90578-u. [DOI] [PubMed] [Google Scholar]
- 5.Berek JS, Novak E. Berek and Novak's Gynecology. 15th ed. Philadelphia: Lippincott Williams and Wilkins; 2012. [Google Scholar]
- 6.Sitruk-Ware R, Nath A. Metabolic effects of contraceptive steroids. Rev Endocr Metab Disord. 2011;12(2):63–75. doi: 10.1007/s11154-011-9182-4. [DOI] [PubMed] [Google Scholar]
- 7.Speroff L, Fritz MA. Clinical gynecologic endocrinology and infertility. 8th ed. Philadelphia: Lippincott Williams and Wilkins; 2012. [Google Scholar]
- 8.Lopez LM, Grimes DA, Schulz KF. Steroidal contraceptives: effect on carbohydrate metabolism in women without diabetes mellitus. Cochrane Database Syst Rev. 2014;(4):CD006133. doi: 10.1002/14651858.CD006133.pub5. [DOI] [PubMed] [Google Scholar]
- 9.Sitruk-Ware R, Nath A. Characteristics and metabolic effects of estrogen and progestins contained in oral contraceptive pills. Best Pract Res Clin Endocrinol Metab. 2013;27(1):13–24. doi: 10.1016/j.beem.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 10.Cagnacci A, Ferrari S, Tirelli A, Zanin R, Volpe A. Insulin sensitivity and lipid metabolism with oral contraceptives containing chlormadinone acetate or desogestrel: a randomized trial. Contraception. 2009;79(2):111–6. doi: 10.1016/j.contraception.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 11.Guerra JA, Lopez-Mu-oz F, Alamo C. Progestins in combined contraceptives. J Exp Clin Med. 2013;5(2):51–5. [Google Scholar]
- 12.Rader DJ. Effect of insulin resistance, dyslipidemia, and intra-abdominal adiposity on the development of cardiovascular disease and diabetes mellitus. Am J Med. 2007;120(3 Suppl 1):S12–8. doi: 10.1016/j.amjmed.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 13.World Health Organization.. [cited Sep 1];Obesity and overweight . 2015 Available from: http://www.who.int/mediacentre/factsheets/fs311/
- 14.Klop B, Elte JW, Cabezas MC. Dyslipidemia in obesity: mechanisms and potential targets. Nutrients. 2013;5(4):1218–40. doi: 10.3390/nu5041218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bacha F, Lee S, Gungor N, Arslanian SA. From pre-diabetes to type 2 diabetes in obese youth: pathophysiological characteristics along the spectrum of glucose dysregulation. Diabetes Care. 2010;33(10):2225–31. doi: 10.2337/dc10-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neeland IJ, Turer AT, Ayers CR, Powell-Wiley TM, Vega GL, Farzaneh-Far R, et al. Dysfunctional adiposity and the risk of prediabetes and type 2 diabetes in obese adults. JAMA. 2012;308(11):1150–9. doi: 10.1001/2012.jama.11132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Health Ministry of Iran.. Instruction of contraception methods in isalamic republic of iran [in persian] 2008 Tehran.
- 18.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin clem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 19.National Cholesterol Education Program Expert Panel on Detection E, Treatment of High Blood Cholesterol in A. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143–421. [PubMed] [Google Scholar]
- 20.Kemmeren JM, Algra A, Grobbee DE. Effect of second and third generation oral contraceptives on lipid metabolism in the absence or presence of the factor V Leiden mutation. J Intern Med. 2001;250(5):441–8. doi: 10.1046/j.1365-2796.2001.00906.x. [DOI] [PubMed] [Google Scholar]
- 21.Knopp RH, Broyles FE, Cheung M, Moore K, Marcovina S, Chandler WL. Comparison of the lipoprotein, carbohydrate, and hemostatic effects of phasic oral contraceptives containing desogestrel or levonorgestrel. Contraception. 2001;63(1):1–11. doi: 10.1016/s0010-7824(00)00196-7. [DOI] [PubMed] [Google Scholar]
- 22.van Rooijen M, von Schoultz B, Silveira A, Hamsten A, Bremme K. Different effects of oral contraceptives containing levonorgestrel or desogestrel on plasma lipoproteins and coagulation factor VII. Am J Obstet Gynecol. 2002;186(1):44–8. doi: 10.1067/mob.2002.119179. [DOI] [PubMed] [Google Scholar]
- 23.Klipping C, Marr J. Effects of two combined oral contraceptives containing ethinyl estradiol 20 microg combined with either drospirenone or desogestrel on lipids, hemostatic parameters and carbohydrate metabolism. Contraception. 2005;71(6):409–16. doi: 10.1016/j.contraception.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 24.deGoma EM, Leeper NJ, Heidenreich PA. Clinical significance of high-density lipoprotein cholesterol in patients with low low-density lipoprotein cholesterol. J Am Coll Cardiol. 2008;51(1):49–55. doi: 10.1016/j.jacc.2007.07.086. [DOI] [PubMed] [Google Scholar]
- 25.Foulon T, Payen N, Laporte F, Bijaoui S, Dupont G, Roland F, et al. Effects of two low-dose oral contraceptives containing ethinylestradiol and either desogestrel or levonorgestrel on serum lipids and lipoproteins with particular regard to LDL size. Contraception. 2001;64(1):11–6. doi: 10.1016/s0010-7824(01)00224-4. [DOI] [PubMed] [Google Scholar]
- 26.Talayero BG, Sacks FM. The role of triglycerides in atherosclerosis. Curr Cardiol Rep. 2011;13(6):544–52. doi: 10.1007/s11886-011-0220-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Winkler UH, Sudik R. The effects of two monophasic oral contraceptives containing 30 mcg of ethinyl estradiol and either 2 mg of chlormadinone acetate or 0.15 mg of desogestrel on lipid, hormone and metabolic parameters. Contraception. 2009;79(1):15–23. doi: 10.1016/j.contraception.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 28.Tabak AG, Herder C, Rathmann W, Brunner EJ, Kivimaki M. Prediabetes: a high-risk state for diabetes development. Lancet. 2012;379(9833):2279–90. doi: 10.1016/S0140-6736(12)60283-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ford ES, Zhao G, Li C. Pre-diabetes and the risk for cardiovascular disease: a systematic review of the evidence. J Am Coll Cardiol. 2010;55(13):1310–7. doi: 10.1016/j.jacc.2009.10.060. [DOI] [PubMed] [Google Scholar]
- 30.Beasley A, Estes C, Guerrero J, Westhoff C. The effect of obesity and low-dose oral contraceptives on carbohydrate and lipid metabolism. Contraception. 2012;85(5):446–52. doi: 10.1016/j.contraception.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 31.Cheang KI, Essah PA, Sharma S, Wickham E3, Nestler JE. Divergent effects of a combined hormonal oral contraceptive on insulin sensitivity in lean versus obese women. Fertil Steril. 2011;96(2):353–359 e1. doi: 10.1016/j.fertnstert.2011.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]