Abstract
Objective
Cardiac guidelines recommend that the decision to perform coronary angiography (CA) in patients with Non-ST-Elevation Acute Coronary Syndrome (NST-ACS) is based on multiple factors. It is, however, unknown how cardiologists weigh these factors in their decision-making. The aim was to investigate the importance of different clinical characteristics, including information derived from risk scores, in the decision-making of Dutch cardiologists regarding performing CA in patients with suspected NST-ACS.
Design
A web-based survey containing clinical vignettes.
Setting and participants
Registered Dutch cardiologists were approached to complete the survey, in which they were asked to indicate whether they would perform CA for 8 vignettes describing 7 clinical factors: age, renal function, known coronary artery disease, persistent chest pain, presence of risk factors, ECG findings and troponin levels. Cardiologists were divided into two groups: group 1 received vignettes ‘without’ a risk score present, while group 2 completed vignettes ‘with’ a risk score present.
Results
129 (of 946) cardiologists responded. In both groups, elevated troponin levels and typical ischaemic changes (p<0.001) made cardiologists decide more often to perform CA. Severe renal dysfunction (p<0.001) made cardiologists more hesitant to decide on CA. Age and risk score could not be assessed independently, as these factors were strongly associated. Inspecting the factors together showed, for example, that cardiologists were more hesitant to perform CA in elderly patients with high-risk scores than in younger patients with intermediate risk scores.
Conclusions
When deciding to perform in-hospital CA (≤72 hours after patient admission) in patients with suspected NST-ACS, cardiologists tend to rely mostly on troponin levels, ECG changes and renal function. Future research should focus on why CA is less often recommended in patients with severe renal dysfunction, and in elderly patients with high-risk scores. In addition, the impact of age and risk score on decision-making should be further investigated.
Strengths and limitations of this study.
This study provides insight into how cardiologists weigh clinical information in deciding on performing coronary angiography (CA) or not in patients with suspected Non-ST-Elevation Acute Coronary Syndrome.
The decision had to be made on the basis of seven attributes, while in clinical practice cardiologists may take into account other aspects in their decision-making as well. However, clinical vignette studies have been shown to be the most practical, cost-effective and at the same time thorough and valid approach to measure the process of decision-making.
The response rate on the web-based survey, containing clinical vignettes, was low. Nevertheless, despite the wider CIs of ORs, several significant associations were found. Therefore, this study provides further insight into decision processes of cardiologists offering a valuable contribution to the modest number of studies conducted in the field of decision-making in cardiology so far.
Background
The management of patients with Non-ST-Elevation Acute Coronary Syndrome (NST-ACS), including Non-ST-Elevation Myocardial Infarction (NSTEMI) and Unstable Angina (UA), is challenging. Physicians deal with the difficult task of identifying patients at high risk for adverse cardiac events who would benefit most from invasive therapies, such as percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG), while preventing unnecessary invasive procedures in low risk patients in whom conservative therapies are appropriate.1 Recent guidelines from the European Society of Cardiology (ESC) for the management of patients with NST-ACS recommend that cardiologists base their decision regarding coronary angiography (CA) and subsequent treatments on multiple factors, including a patient’s cardiac history, risk factors for coronary artery disease (CAD), results from physical examination, laboratory results and ECG findings.1 2 Furthermore, it is recommended that physicians use objective risk scoring instruments, such as Global Registry of Acute Coronary Events (GRACE) or Thrombolysis in Myocardial Infarction (TIMI), in guiding risk stratification and management.1–5 In patients at intermediate or high risk for cardiac adverse events, CA within 72 or 24 hours, respectively, after hospital admission is indicated, except in case of severe contra-indications such as active bleeding or the presence of major comorbidities.1 2 Timing of CA and, if indicated, subsequent revascularisation should thus be based on the patient's risk status. Previous studies, however, demonstrated that patients at high risk for cardiac adverse events were often less likely to undergo CA than low-risk patients.6–11 A possible explanation for such a treatment risk paradox may be the cardiologists’ reluctance to perform invasive procedures in patients with high-risk features, such as high age and acute heart failure, because of a perceived increased risk of procedure-related adverse events (ie, contrast-induced kidney injury, bleeding, stroke or even death).1 2 12–14 Further, a recent study in 13 Dutch hospitals showed that compliance to cardiac risk scores in clinical practice is relatively low and that risk score use varies largely between hospitals.15 However, data were collected retrospectively, and it is therefore unknown whether the information derived by using a cardiac risk score actually influenced the cardiologists’ treatment decisions in this recent study. The exact importance of various clinical characteristics and risk score outcomes on the decision to perform prompt invasive management remains unclear. Therefore, the aim of this study was to investigate the relative importance of different clinical characteristics, including information derived from risk scores, in the decision-making of Dutch cardiologists regarding performing CA in patients with suspected NST-ACS.
Methods
This study used a binary choice experiment to study the relative importance of different clinical characteristics, including information derived from risk scores, in the decision-making of Dutch cardiologists.
Survey
A web-based survey containing the binary choice experiments was sent to all 946 cardiologists who were registered in the Dutch directory of physicians in the year 2014. The survey started with an informed consent procedure, explaining the purpose of the study and the option to decline participation. To describe respondents’ characteristics, each cardiologist was subsequently asked to register his/her age, gender and working experience in years. In addition, they were asked whether they are employed in a hospital with a teaching status (yes/no), with revascularisation options (no, PCI or PCI/CABG) and whether they used a cardiac risk score at the coronary care unit. Responding cardiologists who were retired or no longer active in practice were excluded from analysis. For a detailed description of the study, we refer to the previously published study protocol.16
Factors: selection and choice of levels
The binary choice experiments consisted of vignettes of clinical cases. Based on literature review and expert opinion, seven essential factors representing clinical characteristics were identified on which cardiologists were likely to base their decision to perform CA, that is,: age, renal function, known CAD, persistent chest pain, presence of risk factors for CAD (ie, diabetes mellitus, hypertension, hypercholesterolaemia, smoking and a positive family history), electrocardiogram findings and high sensitive troponin levels. Respondents were instructed to interpret the factor troponin levels (positive/negative) according to their own hospital standards. The factors have different levels, which are depicted in table 1.
Table 1.
Final selection of factors and their levels
| Clinical setting: patient with suspected NST-ACS is admitted for observation in the hospital. Decision: ‘would you perform coronary angiography within 72 hours in this patient?’ | |||
|---|---|---|---|
| Factors | Factor levels |
||
| Age | <70 years 65 years in clinical vignette |
70–80 years 75 years in clinical vignette |
>80 years 85 years in clinical vignette |
| Renal function | No renal dysfunction | Mild to moderate renal dysfunction | Severe renal dysfunction |
| Known coronary artery disease | No | Yes | |
| Persistent chest pain | No | Yes | |
| Risk factors* | No risk factors | One risk factor | >One risk factor |
| ECG | Normal | Atypical changes | Typical ischaemic changes |
| Troponin† | Normal at repeated measures | Significant rise and/or ‘rise and fall’ | |
*Diabetes mellitus, hypertension, hypercholesterolaemia, smoking and a positive family history.
†According to cardiologists’ own hospital standards.
NST-ACS,
In addition to the aforementioned factors, the patient's cardiac risk of adverse events was estimated for every clinical vignette by using the GRACE 2.0 risk score leading to the following risk categories: low, intermediate and high.17 This was accomplished by entering the values present in the vignette, and entering similar values of ‘severity’ for the remaining parameters (ie, diuretic use, heart rate, systolic blood pressure, Killip class and cardiac arrest at admission) in every vignette.
The sample of cardiologists was divided into two groups before the start of the survey.16 One group completed the vignettes ‘without’ a cardiac risk score being present (group 1), while the other group completed the vignettes ‘with’ a cardiac risk score present (group 2). Cardiologists in the latter group were instructed that the reported risk categories were generated by the risk score that they apply in their own practice, as it was not specified that it was the result of the GRACE 2.0 risk score.
Experimental design
The vignettes were systematically varied on the aforementioned clinical factors (factorial design): age, renal function, known CAD, persistent chest pain, presence of risk factors, ECG findings and troponin levels. When combining all factors and factor levels, 23×34=648 unique clinical vignettes were created (full factorial design). From these vignettes, a G-optimal design of 64 vignettes was selected that allowed for estimation of all main effects, employing the computer algorithm implemented by Wheeler.18 The 64 scenarios were randomly allocated to eight blocks containing eight vignettes each. Cardiologists were randomly assigned to a block of eight vignettes. For each of the eight vignettes included in the survey, cardiologists were asked to decide whether they would perform CA within 72 hours after patient admission or would not perform CA (yes or no).
Data analysis
The strength of associations between independent variables (ie, factors) and decisions of cardiologists in the survey (yes/no CA) were estimated using a generalised linear mixed model (GLMM) for binary response data, and expressed as ORs (ORs). Random effects for cardiologists were added to this model to account for the clustering of data within cardiologists. Separate GLMM models were created for group 1 (vignettes without a risk score) and group 2 (vignettes with a risk score). The various factors, the variable risk score (group 2 only) and the block factor, were simultaneously entered as fixed effects to the model. Since the number of blocks is relatively small, blocks were not introduced as random effects in the model as the associated component of variance cannot be estimated with acceptable accuracy. For that reason, block effects were introduced as fixed effects in the analysis. As a check for partial confounding/near multicollinearity, in table 4, ORs from multivariable analyses (and their SEs and CIs) were compared with ORs from univariable analyses (at all times including fixed block effects and random cardiologists effects in the model). Significance tests were based on the likelihood ratio test. In addition, for independent factors with three factor levels, pairwise comparisons, that is, level 1 vs 2, level 1 vs 3, and level 2 vs 3, were made using the Wald test. Effect sizes were expressed in terms of ORs and their associated 95% CI. p Values equal to or below 0.05 were considered significant. The impact of the presence of the risk score on a cardiologist’s decision was studied by comparing ORs and p values of the analyses of group 1 with group 2. The analyses with the GLMM were conducted in R for windows (V.3.1.3) (R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, 2013. http://www.R-project.org/ (accessed Jan 2014)).
Table 4.
Univariable and multivariable associations between a positive decision to order a CA and factors using Generalised Linear Mixed Models (GLMM)
| Factor | GROUP 1 (n=57 cardiologists) |
GROUP 2 (n=54 cardiologists) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariable analysis |
Multivariable analysis |
Univariable analysis |
Multivariable analysis |
|||||||||
| Raw % CA=yes‡ | OR | 95% CI LL-UL | OR | 95% CI LL–UL | p Value* | Raw % CA=yes‡ | OR | 95% CI LL–UL | OR | 95% CI LL–UL | p Value* | |
| Troponin levels | ||||||||||||
| Elevated | 80.6 | 13.7 | 8.25 to 22.69 | 66.90 | 26.29 to 170.25 | ≤0.001 | 84.9 | 11.57 | 7.14 to 18.76 | 55.80 | 22.50 to 138.36 | ≤0.001 |
| Normal | 26.9 | 1.0 | 1.0 | 33.9 | 1.0 | 1.0 | ||||||
| ECG changes† | ≤0.001 | ≤0.001 | ||||||||||
| Aspecific | 43.0 | 1.04 | 0.66 to 1.65 | 1.13 | 0.54 to 2.39 | 0.74 | 49.3 | 1.13 | 0.71 to 1.82 | 2.00 | 0.88 to 4.56 | 0.10 |
| Typical ischaemic | 74.7 | 4.17 | 2.51 to 6.92 | 15.39 | 6.37 to 37.17 | ≤0.001 | 77.7 | 4.05 | 2.41 to 6.79 | 16.40 | 4.80 to 56.05 | ≤0.001 |
| Normal | 42.8 | 1.0 | 1.0 | 46.4 | 1.0 | 1.0 | ||||||
| Age† | ≤0.001 | 0.50 | ||||||||||
| 70–80 years | 65.0 | 1.81 | 1.13 to 2.89 | 1.21 | 0.58 to 2.49 | 0.61 | 72.8 | 2.58 | 1.56 to 4.26 | 1.08 | 0.28 to 4.18 | 0.91 |
| >80 years | 42.0 | 0.72 | 0.45 to 1.14 | 0.14 | 0.05 to 0.34 | ≤0.001 | 49.7 | 0.97 | 0.60 to 1.55 | 0.58 | 0.10 to 3.48 | 0.55 |
| <70 years | 51.7 | 1.0 | 1.0 | 51.4 | 1.0 | 1.0 | ||||||
| Presence of risk factors† | 0.43 | 0.45 | ||||||||||
| 1 risk factor | 51.0 | 1.01 | 0.64 to 1.61 | 0.82 | 0.35 to 1.91 | 0.65 | 59.3 | 1.07 | 0.66 to 1.74 | 0.69 | 0.30 to 1.59 | 0.38 |
| >1 risk factor | 58.2 | 1.40 | 0.88 to 2.22 | 1.32 | 0.61 to 2.85 | 0.48 | 59.0 | 1.08 | 0.68 to 1.72 | 1.09 | 0.50 to 2.39 | 0.83 |
| No risk factors | 49.7 | 1.0 | 1.0 | 56.0 | 1.0 | 1.0 | ||||||
| Renal dysfunction† | ≤0.001 | ≤0.001 | ||||||||||
| Mild to moderate | 54.1 | 0.62 | 0.38 to 0.99 | 0.52 | 0.23 to 1.18 | 0.12 | 64.3 | 1.36 | 0.84 to 2.21 | 2.17 | 0.84 to 5.60 | 0.11 |
| Severe | 39.4 | 0.33 | 0.21 to 0.54 | 0.09 | 0.04 to 0.21 | ≤0.001 | 51.4 | 0.76 | 0.47 to 1.21 | 0.25 | 0.10 to 0.63 | ≤0.001 |
| No | 66.0 | 1.0 | 1.0 | 58.7 | 1.0 | 1.0 | ||||||
| Previous CAD | ||||||||||||
| Yes | 61.4 | 2.08 | 1.42 to 3.04 | 2.80 | 1.45 to 5.42 | ≤0.01 | 57.3 | 0.93 | 0.63 to 1.37 | 0.94 | 0.50 to 1.78 | 0.75 |
| No | 44.1 | 1.0 | 1.0 | 59.0 | 1.0 | 1.0 | ||||||
| Persistent chest pain | ||||||||||||
| Yes | 63.2 | 2.29 | 1.57 to 3.36 | 4.90 | 2.36 to 10.16 | 0.000 | 68.4 | 2.36 | 1.56 to 3.51 | 3.79 | 1.92 to 7.51 | 0.000 |
| No | 43.6 | 1.0 | 1.0 | 48.2 | 1.0 | 1.0 | ||||||
| Risk score outcome† | NA | 0.02 | ||||||||||
| Intermediate | NA | NA | NA | NA | NA | 63.7 | 4.41 | 2.60 to 7.49 | 4.40 | 0.84 to 22.93 | 0.08 | |
| High | NA. | NA | NA | NA | NA | 71.8 | 6.32 | 3.21 to 12.42 | 1.27 | 0.09 to 18.20 | 0.86 | |
| Low | NA | NA | NA | NA | NA | 32.7 | 1.0 | 1.0 | ||||
Associations comparing level 2 vs 3 for the different factors were.
▪ ECG changes: typical ischaemic versus aspecific (GROUP 1: p 0.000, OR 13.57, CI 95% 5.65 to 32.60)/(GROUP 2: p 0.000, OR 8.20, CI 95% 2.87 to 23.46).
▪ Age: >80 years versus 70–80 years (GROUP 1: p 0.000, OR 0.11, CI 95% 0.05 to 0.27)/(GROUP 2: p 0.25, OR 0.53, CI 95% 0.18 to 1.55).
▪ Presence of risk factors: >1 risk factor versus 1 risk factor (GROUP 1: p 0.21, OR 1.61, CI 95% 0.77 to 3.36)/(GROUP 2: p 0.22, OR 1.56, CI 95% 0.76 to 3.32).
▪ Renal dysfunction: severe versus mild to moderate (GROUP 1: p 0.000, OR 0.17, CI 95% 0.07 to 0.39)/(GROUP 2: p 0.000, OR 0.12, CI 95% 0.05 to 0.26).
▪ Risk score outcome: high versus intermediate (GROUP 2: p 0.14, OR 0.29, CI 95% 0.06 to 1.50).
*Significance tests for independent factors were based on the loglikelihood ratio test (in bold).
†Significance tests for independent factors with three levels of pairwise comparisons, that is, level 1 vs 2, level 1 vs 3, and level 2 vs 3, were based on the Wald test (in italic).
‡ Raw percentages of patients receiving CA for each level of a factor are presented. Eg, in group 1, for factor Troponin, 222 vignettes are in the category ‘elevated’ and 179 of these received CA, leading to 179/222×100=80.6%.
CAD, coronary artery disease; LL, lower limit; LRT, loglikelihood ratio test; NA, not applicable; UL, upper limit.
The multivariable GLMMs of the two groups were used to determine the relative importance of each factor in deciding on CA. Relative importance refers to the contribution of a specific factor to the total deviance (−2×log likelihood) of the multivariable model. It was calculated by taking the difference between the deviances of the multivariable model with all factors present and a model with one of the factors of interest removed. The resulting differences were converted to percentages for each factor by dividing the difference by the sum of contributions of all independent factors, multiplying by 100.19 Interpretation of relative importance measures is similar to the percentage of variance accounted for in ordinary regression.
In the study protocol, we considered the degree of perceived certainty of decisions as a possible covariate in the GLMM.16 Effectively, this implies that results are ‘corrected’ for uncertainty. However, since uncertainty is an integral part of the decision process, analyses that ‘corrected’ for uncertainty led to results that could not be properly interpreted and the variable was not included in the analyses.
Results
Study population
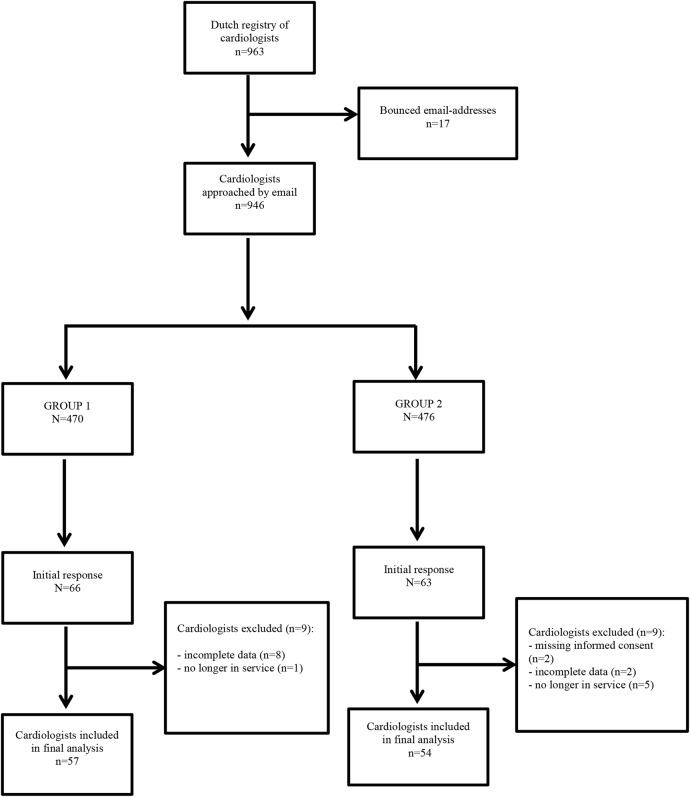
A total of 946 Dutch cardiologists, 470 in group 1 and 476 in group 2, were approached by email to complete the survey. A total of seven reminders were sent between June and October 2014. Eventually, 14% (66/470) and 13% (63/476) of the cardiologists responded. In each group, the answers of nine participants were not eligible for analysis, due to missing informed consent, incomplete data or because cardiologists were not active in practice anymore (figure 1). The final sample consisted of 57 cardiologists in group 1 and 54 cardiologists in group 2. The majority of cardiologists who completed the survey were male, had more than 10 years of clinical experience, and were employed in a hospital with both PCI and CABG options. There were no significant differences in characteristics of the cardiologists between group 1 and group 2 (table 2). Detailed information regarding responses of cardiologists on the clinical vignettes is provided as supplementary material (see online supplementary appendix A).
Figure 1.
Flow chart of respondent selection and survey response.
Table 2.
Demographics of participating cardiologists
| GROUP 1* (n=57) | GROUP 2† (n=54) | p Value‡ | |
|---|---|---|---|
| Gender | 0.803 | ||
| Male | 48 (84.2%) | 44 (81.5%) | |
| Age≈ | 50.0 (42.0–59.0) | 49.5 (41.0–55.0) | 0.125 |
| <50 years | 26 (45.6%) | 27 (50.0%) | |
| ≥50 years | 31 (54.4%) | 27 (50.0%) | |
| Working years≈ | 12.0 (7.0–24.0) | 11.0 (5.0–21.0) | 0.172 |
| <5 | 7 (12.3%) | 11 (20.4%) | |
| 5–10 | 18 (31.6%) | 16 (29.6%) | |
| >10 | 32 (56.1%) | 27 (50.0%) | |
| Revascularisation options | 0.805 | ||
| No | 18 (31.6%) | 15 (27.8%) | |
| Yes, PCI | 13 (22.8%) | 11 (20.4%) | |
| Yes, PCI and CABG | 26 (45.6%) | 28 (51.9%) | |
| Teaching hospital | 0.424 | ||
| Yes | 35 (61.4%) | 38 (70.4%) | |
| Use of risk score at CCU§ | 0.177 | ||
| Yes | 41 (71.9%) | 45 (83.3%) |
≈Median and accompanied 25th and 75th centile. All other data are presented in n (%).
*Group 1 refers to the group of responding cardiologists receiving the set of vignettes without a risk score present.
†Group 2 refers to the group of responding cardiologists receiving the set of vignettes with a risk score present.
‡Goodness of fit test for continuous variables with the Mann-Whitney U test, and for categorical variables with Pearson's χ2 test or Fisher's Exact Test.
§GRACE, TIMI, FRISC and HEART risk score.
CABG, coronary artery bypass grafting; CCU, coronary care unit; FRISC, fast revascularisation in instability in coronary disease; GRACE, Global Registry of Acute Coronary Events; PCI, percutaneous coronary intervention; TIMI, Thrombolysis in Myocardial Infarction.
Relative importance of clinical factors
Group 1: vignettes without risk score present
For group 1, the following factors affected cardiologists’ decisions to perform CA within 72 hours the strongest (in decreasing order): troponin levels (48.9%), ECG changes (17.9%), renal function (11.8%), age (9.5%), persistent chest pain (6.4%), previous CAD (2.9%) and presence of risk factors (0.5%; table 3). When changing from one level of a factor to another, the probability for deciding to perform CA may be relatively strongly affected, that is, for troponin levels or modestly affected, that is, for presence of risk factors. This is what is reflected in the percentage for relative importance of a factor and in the estimated ORs.
Table 3.
Importance of each factor in deciding whether or not to perform coronary angiography
| Group 1 | Group 2 | ||||||
|---|---|---|---|---|---|---|---|
| Order of importance | Factor | Explained deviance%* | p Value† | Order of importance | Factor | Explained deviance%* | p Value† |
| 1. | Troponin | 48.9% | ≤0.001 | 1. | Troponin | 49.6% | ≤0.001 |
| 2. | ECG | 17.9% | ≤0.001 | 2. | Renal function | 14.9% | ≤0.001 |
| 3. | Renal function | 11.8% | ≤0.001 | 3. | Risk score outcome | 14.3%∞ | 0.02 |
| 4. | Age | 9.5% | ≤0.001 | 4. | ECG | 9.8% | ≤0.001 |
| 5. | Persistent chest pain | 6.4% | ≤0.001 | 5. | Persistent chest pain | 6.7% | ≤0.001 |
| 6. | Previous CAD | 2.9% | ≤0.001 | 6. | Risk factors | 0.7% | 0.45 |
| 7. | Risk factors | 0.5% | 0.43 | 7. | Age | 0.6%∞ | 0.50 |
| 8. | Risk score outcome | NA | NA | 8. | Previous CAD | 0.0% | 0.75 |
| (Blocks)‡ | (2.1%) | – | (Blocks)‡ | (3.4%) | – | ||
| Total | 100% | Total | 100% |
*Loglikelihood from a single attribute divided by the sum of partial loglikelihoods of all factors.
†p Value based on the loglikelihood test. ∞Factor risk score outcome is partially confounded by factor age, and therefore its impact cannot be interpreted.
‡Explained deviance due to the allocation of cardiologists to blocks in the experimental design.
CAD, coronary artery disease; NA, not applicable.
bmjopen-2016-011213supp.pdf (292.4KB, pdf)
Of the two factors affecting cardiologists’ decisions the strongest, patients with a significant rise and/or ‘rise and fall’ of troponin levels, or with typical ischaemic changes on the ECG, were more likely to receive CA compared to patients with normal troponin levels or with no changes or atypical changes on the ECG. Severe renal dysfunction compared to no renal dysfunction or mild to moderate renal dysfunction, and older age (>80 years) compared to younger patients (<70 and 70–80 years) made cardiologists decide less often to perform CA. Presence of persistent symptoms of chest pain or a history of CAD hardly seemed to affect cardiologists’ decisions. The presence of risk factors was not significantly (p=0.43) associated with the decision whether or not to perform CA. The strengths of the multivariable associations are presented in terms of ORs and associated 95% CIs. Also in parentheses, the ORs and CIs of the univariable analyses are presented for comparison (table 4).
Group 2: vignettes with risk score present
For group 2, the following factors impacted cardiologists’ decisions to perform CA within 72 hours the strongest (in decreasing order): troponin levels (49.6%), renal function (14.9%), risk score (14.3%), ECG changes (9.8%), persistent chest pain (6.7%), presence of risk factors (0.7%), age (0.6%) and previous CAD (0.00%; table 3).
Cardiologists decided more often to perform CA in patients with a significant rise and/or ‘rise and fall’ of troponin levels than in patients with normal troponin levels. In patients with severe renal dysfunction, cardiologists were less likely to perform CA compared to patients with no or mild to moderate renal dysfunction. For patients with typical ischaemic changes on the ECG, cardiologists decided more often to perform CA than for patients with no changes or for patients with aspecific ECG changes. Cardiologists were also more likely to perform CA for patients with persistent symptoms of chest pain than for patients without such symptoms. Presence of risk factors, age and previous CAD was not significantly associated with the decision to perform CA, with p values ranging between 0.45 and 0.75. The strengths of the multivariable associations are presented in terms of ORs and associated 95% CIs. Also in parentheses, the ORs and CIs of the univariable analyses are presented for comparison (table 4).
Information derived from a cardiac risk score was in the top three factors that influenced cardiologists’ decisions the most. Although the likelihood ratio test suggested a significant effect of the availability of a risk score on the decision to perform CA (p=0.02), subsequent pairwise comparisons between the three levels of risk score with the Wald test did not provide conclusive evidence about the nature of this effect. Associated p values of the Wald test were all above 0.05. Further analyses revealed that there was a strong association (ie, partial confounding) between the provision of a risk score and a patient's age as presented in the vignette. Conclusions about the contributions of age and risk score by inspecting these factors separately could therefore not be made. The combined factor for age and risk score, however, was significantly associated with the decision to perform CA (p=0.003). This despite problems with convergence of the multivariable model, possibly related to fairly extreme probabilities connected to age lower than 70 years and low-risk score, and age higher than 80 years and high-risk score. In elderly patients (>80 years) with high-risk scores, cardiologists were more hesitant in their decision to perform CA than in younger patients with intermediate risk scores; OR of 0.15 (95% CI 0.05 to 0.46) for 70–80 years versus age older than 80 and OR of 0.13 (95% CI 0.04 to 0.83) for the comparison of patients younger than 70 and older than 80 years. Further, in younger patients (<70 years) with low risk scores, cardiologists were more likely to decide on performing CA than in patients aged between 70 and 80 years with intermediate risk scores (OR=4.58, 95% CI 1.88 to 11.14).
Block effects
Although block effects are significant (p≤0.05), the percentage explained deviance for blocks was relatively small: 2.1% in group 1 and 3.4% in group 2. For group 2, the analysis without blocks in the model yielded similar results, except for factor risk score: the percentage explained deviance for risk score dropped from 14.3% to 3.8%. Again, we have to concede that conclusions with respect to the impact of risk score alone on performing CA cannot be drawn with sufficient confidence.
Discussion
When deciding to perform in-hospital CA (within 72 hours after patient admission) in patients with suspected NST-ACS, cardiologists tend to rely mostly on the following three sources of clinical information: troponin levels, ECG changes and renal function. In our binary choice experiment, cardiologists decided more often to perform CA in vignettes representing patients with elevated troponin levels and in patients with typical ischaemic changes on the ECG. In contrast, in vignettes representing patients with severe renal dysfunction, cardiologists seemed to be more hesitant to perform CA. Persistent symptoms of chest pain, previous CAD and the presence of risk factors had limited impact on the decision whether or not to perform CA. Since effects of risk score were strongly associated (ie, partial confounding) with age, no firm conclusions could be drawn about the separate contributions of risk score and age on cardiologists’ decisions.
With CA, there is a small risk for complications. It is therefore recommended by the guidelines that physicians take several criteria into account when assessing a patient and subsequently deciding on a conservative or invasive approach.1 2 20 21 In the current study, troponin and ECG changes were considered most important in decision-making, which is in line with the guideline recommendations where both factors are defined as primary features of high risk for adverse cardiac events, and thus with a clear indication for invasive management.1 2 The guidelines consider patients with (severe) renal dysfunction as high risk for adverse cardiac events as well, and therefore recommend invasive treatment. However, the results in our study suggest that cardiologists were less likely to opt for CA in patients with severe renal dysfunction compared to patients with mild to moderate or no renal dysfunction. This treatment risk paradox, in which patients at low risk for adverse cardiac events are more likely to receive invasive treatment than high-risk patients, has been reported before in patients with NST-ACS with renal dysfunction.22–24 Although several studies demonstrated that invasive treatment in patients with severe renal dysfunction was associated with a reduction in rehospitalisation together with a significant reduction or trends of reduced risk for death and reinfarction,12 25–27 cardiologists seem to be hesitant to perform CA. A possible explanation may be that cardiologists are hesitant to perform CA, as severe renal dysfunction is associated with an increased risk of complications.1 21 Another explanation could relate to the available scientific evidence regarding the benefits of early invasive therapy in patients with NST-ACS with renal dysfunction. For instance, in an editorial on this topic, the author points out that there is conflicting evidence regarding the benefits of early invasive management in this patient group, and that the majority of studies have observational study designs (instead of experimental designs) which can encompass an increased risk of confounding and/or have relatively small study samples.28
Just as in patients with severe renal dysfunction, a treatment risk paradox was present in elderly patients at high risk for adverse cardiac events based on a cardiac risk score outcome. Cardiologists seemed to be more hesitant to opt for CA in patients over 80 years with a high-risk score than in patients at intermediate risk and of a younger age. As mentioned before, perceived increased risk for complications of treatment and less benefit for the older patient and patients with renal dysfunction probably plays a role here. Future research should focus on why in these specific patient groups the guidelines are not adhered to.
It has been suggested before that cardiologists may not take all predictors of adverse cardiac events into account when deciding on CA.9 29 30 This was also the case in our study, where information regarding a patient's cardiac history and presence of risk factors hardly influenced cardiologists’ decision-making. Cardiac risk scores incorporate all important clinical factors, and therefore could be, when actively used in practice, a solution to the aforementioned treatment-risk paradox. In the past decade, several prospective studies demonstrated that risk scores were superior to clinical assessment by the physician alone.30–32 This emphasises the importance of multifactorial risk assessment as recommended by the guidelines. Further prospective research regarding the impact of these scores on decision-making and patient outcomes is necessary, given that in this study we were not able to determine the exact impact of risk score on decision-making.
Study limitations
Several limitations should be taken into account.
First, although cardiologists were repeatedly contacted, the response rate was low. It was described in the study protocol that a response rate of 40%, resulting in 385 cardiologists, would be sufficient to estimate main effects. This sample rate is, however, not reached. Nevertheless, despite the wider CIs of ORs, several significant associations were found. Therefore, this study provides further insight into decision processes of cardiologists offering a valuable contribution to the modest number of studies conducted in the field of decision-making in cardiology so far.
Second, possibly only cardiologists with an affinity for scientific research participated (ie, selection bias). The study sample consisted mainly of cardiologists who were male, 50 years or older and with more than 10 years of experience in clinical practice. However, this pattern was the same for both groups of cardiologists, and thus comparable in demographics. Unfortunately, statistics regarding the average age and years in practice of all cardiologists in the Netherlands were not available, making an assessment of the generalisability of the study results difficult.
Third, despite our study design, it remained difficult to determine individual contributions of age and risk score as these factors were strongly associated (ie, hampered by confounding).
Fourth, the decisions made on the basis of vignettes can be different from decisions made in a real-life situation in clinical practice where the patient can actually be observed at the coronary care unit. In addition, in daily practice other factors—not included in this vignette study—might influence cardiologists’ decisions. However, results were generally consistent with findings from earlier studies. Further, clinical vignette studies have been shown to be the most practical, cost-effective and at the same time thorough and valid approach to measure the process of decision-making.33 34
Finally, the time frame in which cardiologists were asked to decide on CA was set on ‘performing CA within 72 hours after patient admission (in-hospital)’. Given the recommendations in the latest guidelines,2 in which it is not so much a question ‘if’ CA should be performed but rather ‘when’, it can be debated that timing of CA is also of interest to investigate. For instance, by adding more variation in response categories, for example, immediately, within 24 hours or within 72 hours. However, the aim was not to measure whether the ‘correct’ decision was made, but to gain insight into which factors influence decisions the most. Furthermore, the latest guidelines were published after data collection was finished and it can be argued that the 2011 guidelines are still up to date, as implementation of guidelines in practice takes a considerable amount of time.
Conclusions
When deciding to perform in-hospital CA (within 72 hours after patient admission) in patients with suspected NST-ACS, cardiologists tend to rely mostly on the following three sources of clinical information: troponin levels, ECG changes and renal function. The importance of age and risk score in separation was difficult to assess, due to strong association between these factors. However, in elderly patients at high risk of adverse cardiac events according to a risk score, cardiologists seemed to be more hesitant to perform CA than in younger patients with intermediate risk scores. This hesitance to perform CA was also seen in patients with severe renal dysfunction. Future research should focus on decision-making regarding CA in these patient groups, and on the impact of age and risk scores on decision-making.
Footnotes
Contributors: JE and JMP carried out data collection. JE performed statistical analysis and drafted the manuscript. JE and JMP prepared the manuscript for publication. IvdW provided substantial support in statistical analysis. IvdW, JBR, MCdB, JJHB, MJC, WJT, RU and CW made substantial contributions to interpretation of the data and revised the manuscript critically for important intellectual content. All authors made substantial contributions to the conception and design of the study. All authors read and approved the final manuscript.
Funding: The study was funded by the Dutch Ministry of Public Health, Welfare and Sports. The study sponsor had no role in the study design, collection, analysis and interpretation of the data, or in the writing of the manuscript and decision to submit the manuscript for publication.
Competing interests: None declared.
Ethics approval: The study protocol was reviewed and approved by the medical ethical committee of the VU University Medical Center, Amsterdam (protocol number: 2014008). A waiver of active informed consent was granted, as the study concerned completely anonymised data.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.Hamm CW, Bassand JP, Agewall S et al. . ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST segment elevation: the Task Force for the management of acute coronary syndromes (ACS) in patients presenting without persistent ST segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2011;32:2999–3054. 10.1093/eurheartj/ehr236 [DOI] [PubMed] [Google Scholar]
- 2.Roffi M, Patrono C, Collet JP et al. , Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST segment Elevation of the European Society of Cardiology. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST segment Elevation of the European Society of Cardiology (ESC). Eur Heart J 2016;37:267–315. 10.1093/eurheartj/ehv320 [DOI] [PubMed] [Google Scholar]
- 3.Granger CB, Goldberg RJ, Dabbous O et al. , Global Registry of Acute Coronary Events Investigators. Predictors of hospital mortality in the global registry of acute coronary events. Arch Intern Med 2003;163:2345–53. 10.1001/archinte.163.19.2345 [DOI] [PubMed] [Google Scholar]
- 4.Fox KA, Dabbous OH, Goldberg RJ et al. . Prediction of risk of death and myocardial infarction in the six months after presentation with acute coronary syndrome: prospective multinational observational study (GRACE). BMJ 2006;333:1091 10.1136/bmj.38985.646481.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Antman EM, Cohen M, Bernink PJ et al. . The TIMI risk score for unstable angina/non-ST elevation MI: a method for prognostication and therapeutic decision-making. JAMA 2000;284:835–42. [DOI] [PubMed] [Google Scholar]
- 6.Yan AT, Yan RT, Tan M et al. . In-hospital revascularization and one-year outcome of acute coronary syndrome patients stratified by the GRACE risk score. Am J Cardiol 2005;96:913–16. 10.1016/j.amjcard.2005.05.046 [DOI] [PubMed] [Google Scholar]
- 7.Yan AT, Yan RT, Tan M et al. . Management patterns in relation to risk stratification among patients with non-ST elevation acute coronary syndromes. Arch Intern Med 2007;167:1009–16. 10.1001/archinte.167.10.1009 [DOI] [PubMed] [Google Scholar]
- 8.Roe MT, Peterson ED, Newby LK et al. . The influence of risk status on guideline adherence for patients with non-ST segment elevation acute coronary syndromes. Am Heart J 2006;151:1205–13. 10.1016/j.ahj.2005.08.006 [DOI] [PubMed] [Google Scholar]
- 9.Fox KA, Anderson FA Jr, Dabbous OH et al. , GRACE investigators. Intervention in acute coronary syndromes: do patients undergo intervention on the basis of their risk characteristics? The Global Registry of Acute Coronary Events (GRACE). Heart 2007;93:177–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heras M, Bueno H, Bardají A et al. , DESCARTES Investigators. Magnitude and consequences of undertreatment of high-risk patients with non-ST segment elevation acute coronary syndromes: insights from the DESCARTES Registry. Heart 2006;92:1571–6. 10.1136/hrt.2005.079673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Motivala AA, Cannon CP, Srinivas VS et al. . Changes in myocardial infarction guideline adherence as a function of patient risk: an end to paradoxical care? J Am Coll Cardiol 2011;58:1760–5. 10.1016/j.jacc.2011.06.050 [DOI] [PubMed] [Google Scholar]
- 12.Szummer K, Lundman P, Jacobson SH et al. . Relation between renal function, presentation, use of therapies and in-hospital complications in acute coronary syndrome: data from the SWEDEHEART register. J Intern Med 2010;268:40–9. 10.1111/j.1365-2796.2009.02204.x [DOI] [PubMed] [Google Scholar]
- 13.Ezekowitz J, McAlister FA, Humphries KH et al. . The association among renal insufficiency pharmacotherapy, and outcomes in 6,427 patients with heart failure and coronary artery disease. J Am Coll Cardiol 2004;44:1587–92. 10.1016/j.jacc.2004.06.072 [DOI] [PubMed] [Google Scholar]
- 14.Farshid A, Brieger D, Hyun K et al. . Characteristics and clinical course of STEMI patients who received no reperfusion in the Australia and New Zealand SNAPSHOT ACS registry. Heart Lung Circ 2016;25:132–9. 10.1016/j.hlc.2015.08.004 [DOI] [PubMed] [Google Scholar]
- 15.Engel J, van der Wulp I, de Bruijne MC et al. . A cross-sectional multicenter study of cardiac risk score use in the management of unstable angina and non-ST-elevation myocardial infarction. BMJ Open 2015;5:e008523 10.1136/bmjopen-2015-008523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Engel J, van der Wulp I, Poldervaart JM et al. . Clinical decision-making of cardiologists regarding admission and treatment of patients with suspected unstable angina or non-ST-elevation myocardial infarction: protocol of a clinical vignette study. BMJ Open 2015;5:e006441 10.1136/bmjopen-2014-006441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fox KAA, FitzGerald G, Puymirat E et al. . Should patients with acute coronary disease be stratified for management according to their risk? Derivation, external validation and outcomes using the updated GRACE risk score. BMJ Open 2014;4:e004425 10.1136/bmjopen-2013-004425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wheeler RE. Comments on algorithmic design 2004–2009. http://cran.r-project.org/web/packages/AlgDesign/vignettes/AlgDesign.pdf (accessed Jan 2014).
- 19.Crouch GI, Louviere JJ. The determinants of convention site selection: a logistic choice model from experimental data. J Travel Res 2004;43:118–30. 10.1177/0047287504268233 [DOI] [Google Scholar]
- 20.Kolh P, Windecker S, Alfonso F et al. , Task Force on Myocardial Revascularization of the European Society of Cardiology and the European Association for Cardio-Thoracic Surgery; European Association of Percutaneous Cardiovascular Interventions. 2014 ESC/EACTS Guidelines on myocardial revascularization: the task force on myocardial revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur J Cardiothorac Surg 2014;46:517–92. 10.1093/ejcts/ezu366 [DOI] [PubMed] [Google Scholar]
- 21.Tavakol M, Ashraf S, Brener SJ. Risk and complications of coronary angiography: a comprehensive review. Glob J Health Sci 2012;4:65–93. 10.5539/gjhs.v4n1p65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Charytan D, Mauri L, Agarwal A et al. . The use of invasive cardiac procedures after acute myocardial infarction in long-term dialysis patients. Am Heart J 2006;152:558–64. 10.1016/j.ahj.2006.02.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chertow GM, Normand SL, McNeil BJ. “Renalism”: inappropriately low rates of coronary angiography in elderly individuals with renal insufficiency. J Am Soc Nephrol 2004;15:2462–8. 10.1097/01.ASN.0000135969.33773.0B [DOI] [PubMed] [Google Scholar]
- 24.Szummer K, Lundman P, Jacobson SH et al. , SWEDEHEART. Influence of renal function on the effects of early revacularisation in non-ST-elevation myocardial infarction: data from the Swedish Web-System for Enhancement and Development of Evidence-Based Care in Heart Disease Evaluated According to Recommended Therapies (SWEDEHEART). Circulation 2009;120:851–8. 10.1161/CIRCULATIONAHA.108.838169 [DOI] [PubMed] [Google Scholar]
- 25.Charytan DM, Wallentin L, Lagerqvist B et al. . Early angiography in patients with chronic kidney disease: a collaborative systematic review. Clin J Am Soc Nephrol 2009;4:1032–43. 10.2215/CJN.05551008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keeley EC, Kadakia R, Soman S et al. . Analysis of long-term survival after revascularization in patients with chronic kidney disease presenting with acute coronary syndromes. Am J Cardiol 2003;92:509–14. 10.1016/S0002-9149(03)00716-1 [DOI] [PubMed] [Google Scholar]
- 27.Huang HD, Alam M, Hamzeh I et al. . Patients with severe chronic kidney disease benefit from early revascularization after acute coronary syndrome. Int J Cardiol 2013;168:3741–6. 10.1016/j.ijcard.2013.06.013 [DOI] [PubMed] [Google Scholar]
- 28.Cannon CP. Should we manage patients with non-ST segment elevation myocardial infarction with renal failure with an invasive strategy? Circulation 2009;120:828–30. 10.1161/CIRCULATIONAHA.109.888602 [DOI] [PubMed] [Google Scholar]
- 29.Yan AT, Yan RT, Tan M et al. . Risk scores for risk stratification in acute coronary syndromes: useful but simpler is not necessarily better. Eur Heart J 2007;28:1072–8. 10.1093/eurheartj/ehm004 [DOI] [PubMed] [Google Scholar]
- 30.Ramsay G, Podogrodzka M, McClure C et al. . Risk prediction in patients presenting with suspected cardiac pain: the GRACE and TIMI risk scores versus clinical evaluation. QJM 2007;100:11–18. 10.1093/qjmed/hcl133 [DOI] [PubMed] [Google Scholar]
- 31.Chew DP, Juergens C, French J et al. , Predict study Investigators. An examination of clinical intuition in risk assessment among acute coronary syndromes patients: observations from a prospective multi-center international observational registry. Int J Cardiol 2014;171:209–16. 10.1016/j.ijcard.2013.12.010 [DOI] [PubMed] [Google Scholar]
- 32.Lee CH, Tan M, Yan AT et al. , Canadian Acute Coronary Syndromes (ACS) Registry II Investigators. Use of cardiac catheterization for non-ST segment elevation acute coronary syndromes according to initial risk: reasons why physicians choose not to refer their patients. Arch Intern Med 2008;168:291–6. 10.1001/archinternmed.2007.78 [DOI] [PubMed] [Google Scholar]
- 33.Peabody JW, Luck J, Glassman P et al. . Comparison of vignettes, standardized patients, and chart abstraction: a prospective validation study of 3 methods for measuring quality. JAMA 2000;283:1715–22. 10.1001/jama.283.13.1715 [DOI] [PubMed] [Google Scholar]
- 34.Peabody JW, Luck J, Glassman P et al. . Measuring the quality of physician practice by using clinical vignettes: a prospective validation study. Ann Intern Med 2004;141:771–80. 10.7326/0003-4819-141-10-200411160-00008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2016-011213supp.pdf (292.4KB, pdf)