Abstract
The role of serum cystatin C (Scys) for the detection of acute kidney injury (AKI) has not been fully discussed. This meta-analysis was aimed to investigate the overall diagnostic accuracy of Scys for AKI in adults, and further identify factors affecting its performance. Studies before Sept. 2016 were retrieved from PubMed, Embase, Web of Science and the Cochrane Library. A total of 30 prospective cohort studies (involving 4247 adults from 15 countries, 982 patients occurring AKI) were included. The revised Quality Assessment for Studies of Diagnostic Accuracy (QUADAS-2) tools demonstrated no significant bias had influenced the methodological quality of the included studies. Scys showed a high predictive power for all-cause AKI, that the area under the receiver operating characteristic curve was 0.89. The detailed assessment parameters, such as sensitivity, specificity, positive likelihood ratio, negative likelihood ratio and diagnostic odds ratio for Scys were 0.82, 0.82, 4.6, 0.22 and 21, respectively. Although Scys could be slightly influenced by the following factors: settings, AKI diagnostic criteria, ethnicity, determination method, age and gender, these factors above did not reach statistically significance. In conclusion, Scys could be a vital promising marker to screen out AKI.
Acute kidney injury (AKI) has been recognized as an independent risk factor for prolonged hospital stay, new-onset chronic kidney disease (CKD) and increased mortality rate1,2. Seriously, the prevalence of AKI is increasing in recent years3,4: nearly 3–20% for general inpatients, 30–60% for intensive care unit (ICU) patients.
To earlier and more accurate screen out the severe disease, diagnosis criteria for AKI have been updated three times: the RIFLE (Risk, Injury, Failure, Loss, End-Stage Kidney Disease) criteria in 2004 year, AKIN (Acute Kidney Injury Network) criteria in 2007 year, and the newly 2012 KDIGO (Kidney Disease Improving Global Outcomes) criteria5,6,7. It should be mentioned that all of the three criteria use the same kidney function assessment index: serum creatinine (Scr) and urine output.
However, both Scr and urine output are known as insensitive and nonspecific parameters for renal function evaluation. Thus, a great variety of bio-markers has been identified and then applied in the clinical settings in recent years8,9,10. Among the potential markers, serum cystatin C (Scys) performs a consistent accuracy in various conditions. For both the healthy individuals and CKD patients, Scys has ever been proposed as a superior marker to Scr to evaluate glomerular filtration rate (GFR)11,12.
Recently, based on its physiological metabolism characteristics, that the life cycle of Scys is merely half of that of Scr (1.5–2 hours vs. 4 hours). Namely, once renal function is fluctuating, Scys changes much earlier than Scr13,14. Zhang, et al.15 performed a meta-analysis to compare serum and/or urine cystatin C for diagnosis of AKI, and then they found Scys appeared to be a better biomarker in the prediction of AKI.
Since then, concerns focused on Scys for AKI prediction have been accelerating. However, with accumulating evidence, conflicting results have raised. Wan et al.16 reported that the predict value (the area under the receiver operating characteristic curve, AUROC) of Scys was 0.974, with high sensitivity and specificity, which were similar in Liu’s17, Yim’s18 and other studies. In contrast, another studies indicated a negative results. GaygIsIz et al.19 and Martensson et al.20 found that the predict values (AUROC) of Scys was 0.67, with low sensitivity and specificity. Based on these controversial results, we conducted the present meta-analysis to investigate the overall diagnostic accuracy of Scys for AKI, and further identify which factors affecting its performance.
Results
Literature search
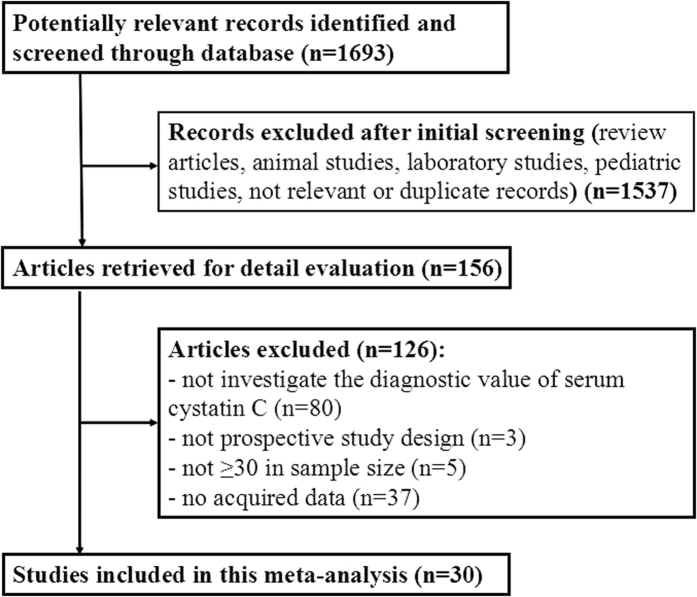
Our research initially identified 1693 citations, among which, 1537 were excluded as they were review articles, animal studies, laboratory reports, pediatric studies, not relevant and duplicate records. Thirty studies16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45 finally met the inclusion criteria via full-text evaluation from 156 potentially eligible citations. Seven studies21,22,23,24,25,26,27 were selected from the previous meta-analysis (Zhang, et al. 2011)15 and an additional 23 studies16,17,18,19,20,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45 were complemented in the present meta-analysis. A flow chart of the identification and selection process is shown in Fig. 1.
Figure 1. Flow chart of study selection.
Subjects characteristics and quality assessment
The main characteristics of the included studies were summarized in Tables 1 and 2. A total of 4,247 patients (mean age 61.6 years, male 70.9%) from 15 countries were enrolled in the 30 studies. The overall AKI incidence was 23.1% (982/4247, varied from 6.0% to 54.3%). The top three settings prone to AKI were 32.3% after cardiac surgery, 28.5% in ICU/cardiac care unit (CCU) and 13.8% in contrast-induced nephropathy (CIN). The elderly and non-elderly patients suffered from an almost similar AKI prevalence (23.1% vs. 22.6%, P > 0.05).
Table 1. Basic characteristics of the selected AKI studies for Scys.
| Study | Country | Clinical setting | No. of patients | AKI incidence (%) | Mean age (y) | Males (%) | Definition of AKI | Scys assay method |
|---|---|---|---|---|---|---|---|---|
| Herget-Rosenthal S.21 | Germany | ICU | 85 | 51.8 | 66.6 | 63.9 | RIFLE-R | Immunonephelometric assay |
| Ling Q.22 | China | LTx | 30 | 43.3 | 47 | 90 | GFR < 80 mL/min/1.73 m2 | NR |
| Kato K.28 | Japan | CIN | 87 | 20.7 | 67 | 71.3 | Scr↑ > 25% or > 0.5 mg/dL within 48 h | PENIA |
| Liang X. L.23 | China | CS | 132 | 22.0 | NR | NR | RIFLE ≥ R | PETIA |
| Haase-Fielitz. A.29 | Australia | CS | 100 | 23.0 | 69.5 | 61 | RIFLE ≥ R | Immunonephelometric assay |
| Haase M.24 | Australia | CS | 100 | 46.0 | 71 | 61 | AKIN ≥ 1 | Immunonephelometric assay |
| Nejat M.25 | New Zealand | ICU | 318 | 6.0 | 60 | 61.1 | RIFLE ≥ R | PENIA |
| Briguori C.26 | Italy | CIN | 410 | 8.3 | 70 | 83.9 | Scr↑ ≥ 0.3 mg/dL from baseline | Dade Behring N Latex Scys assay |
| Stoto K.27 | Portugal | ED | 616 | 21.1 | 59.1 | 62.7 | RIFLE ≥ R & AKIN ≥ 1 | PENIA |
| Torregrosa L.30 | Spain | CIN | 89 | 13.5 | 62.6 | 75.3 | RIFLE ≥ R | Immunonephelometric assay |
| Spain | CS | 46 | 30.4 | 68.8 | 73.9 | RIFLE ≥ R | Immunonephelometric assay | |
| Chen T. H.31 | Taiwan | CCU | 150 | 28.7 | 66 | 75.3 | AKIN ≥ 1 | ELISA |
| Liu X. L.32 | China | CIN | 311 | 12.5 | 58.9 | 63.7 | KDIGO | ELISA |
| Hsiao P. G.33 | Taiwan | AMI | 96 | 17.7 | 63 | 90.6 | AKIN ≥ 1 | ELISA |
| Kokkoris S.34 | Greece | ICU | 100 | 36.0 | 49* & 63* | 57 | RIFLE ≥ R | Immunonephelometric assay |
| Aydoğdu M.35 | Turkey | ICU | 151 | 41.2 | 68.1 | 64.9 | RIFLE ≥ R | PENIA |
| Alharazy S. M.36 | Malaysia | CIN | 100 | 11.0 | 60.4 | 79 | Scr↑ ≥ 25% from baseline in 48 hours | PENIA |
| Wan Z. H.16 | China | ACLF | 56 | 14.3 | 44 | 71.4 | AKIN ≥ 1 | PENIA |
| Padhy M.37 | India | CIN | 60 | 50.0 | 55.9 | 73.3 | KDIGO | ELISA |
| Ghonemy T. A.38 | Egypt | CS | 50 | 34.0 | 44.4 | 64 | RIFLE ≥ R | ELISA |
| Yang H. T.39 | Korea | MBI | 90 | 34.4 | 49.3 | 85.6 | RIFLE ≥ R | Immunoturbidimetric assay |
| Prowle J. R.40 | Australia | CPB | 93 | 26.9 | 70* | 69 | RIFLE ≥ R | Immunonephelometric assay |
| Arun O.41 | Turkey | CS | 30 | 53.3 | 71.9 | 73.3 | KDIGO | Immunonephelometric assay |
| Tung Y. C.42 | Taiwan | ED | 189 | 19.6 | 62.3 | 86.6 | AKIN ≥ 1 | ELISA |
| Yim H.18 | Korea | BICU | 97 | 41.2 | 47 | 80.4 | AKIN ≥ 1 | NR |
| Chen J. Z.43 | China | PNE | 89 | 31.5 | 48.9 | 66.3 | AKIN ≥ 1 | ELISA |
| Liu Y. J.17 | China | CS | 35 | 54.3 | 52.2 | 34.3 | AKIN ≥ 1 | PETIA |
| Peng L.44 | China | CIN | 196 | 14.8 | 70.4 | 68.4 | KDIGO | PETIA |
| Gong M. M.45 | China | ICU | 176 | 40.3 | 55.1 | 61.9 | KDIGO | ELISA |
| GaygIsIz U.19 | Turkey | ICU | 72 | 26.4 | 64.6 | 72.2 | RIFLE ≥ R | PENIA |
| Martensson J.20 | Australia | ICU | 93 | 22.6 | 50* & 66* | 71 | KDIGO | PETIA |
Abbreviations: ACLE, acute-on-chronic liver failure; AMI, acute myocardial infarction; AKI, acute kidney injury; BICU, burn intensive care unit; CCU, coronary care unit; CIN, contrast-induced nephropathy; CPB, cardiopulmonary bypass; CS, cardiac surgery; ED, emergency department; ELISA, enzyme-linked immunosorbent assay; GFR, glomerular filtration rate; KDIGO, Kidney Disease: Improving Global Outcomes; LTx, liver transplantation; MBI, major burn injury; NR, not reported; PENIA, particle-enhanced nephelometric immunoassay; PETIA, particle-enhanced turbidimetric immunoassay; PNE, Partial nephrectomy; RIFLE, risk-injury-failure-loss-end stage renal disease; Scr, serum creatinine; Scys, serum cystatin C.
*Median age (year).
Table 2. The accuracy of Scys at various blood sampling point-in-time and cut-off value.
| Study | Blood sampling point-in-time | Scys cutoff value | Test results | Sensitivity (%) | Specificity (%) | AUROC (95% CI) | |||
|---|---|---|---|---|---|---|---|---|---|
| TP | FP | FN | TN | ||||||
| Herget-Rosenthal S.21 | On day after kidney injury | ↑ ≥ 50% from baseline | 43 | 3 | 1 | 38 | 98 | 93 | 0.99(0.98, 1.00) |
| 24 h before kidney injury | ↑ ≥ 50% from baseline | 36 | 2 | 8 | 39 | 82 | 95 | 0.97(0.94, 0.99) | |
| 24 h before kidney injury | ↑ ≥ 50% from baseline | 24 | 2 | 20 | 39 | 55 | 95 | 0.82(0.71, 0.92) | |
| Ling Q.22 | Post-Tx d 1,4, &7 | 1.57 mg/L | 11 | 3 | 2 | 14 | 84.6 | 84.5 | 0.94(0.86, 0.98) |
| Kato K.28 | Before,1,2,3 days after catheterization | 1.2 mg/L | 17 | 10 | 1 | 59 | 94.7 | 84.8 | 0.93 |
| Liang X. L.23 | Postoperative d1 | ↑ ≥ 50% from baseline | 27 | 5 | 2 | 98 | 92 | 95 | 0.99(0.98, 1.01) |
| Haase-Fielitz A.29 | On ICU admission | 1.1 mg/L | 18 | 11 | 5 | 66 | 77 | 86 | 0.83(0.68, 0.98) |
| 24 h after CPB | 1.2 mg/L | 21 | 28 | 2 | 49 | 91 | 64 | 0.84(0.75, 0.93) | |
| Haase M.24 | 6 h after CPB | 1.1 mg/L | 34 | 18 | 12 | 36 | 74 | 67 | 0.76(0.61, 0.91) |
| Nejat M.25 | On ICU admission | 0.8 mg/L | 18 | 123 | 1 | 176 | 95 | 59 | 0.80(0.71, 0.88) |
| Briguori C.26 | 24 h after CM exposure | ↑ ≥ 10% from baseline | 34 | 53 | 0 | 323 | 100 | 85.9 | 0.92 |
| Soto K27 | On ED admission | 0.98 mg/L | 106 | 113 | 24 | 373 | 81.4 | 76.7 | 0.87(0.83, 0.90) |
| 6 h after ED admission | 0.98 mg/L | 106 | 112 | 24 | 374 | 81.6 | 77.0 | 0.87(0.83, 0.91) | |
| 12 h after ED admission | 0.98 mg/L | 106 | 104 | 24 | 382 | 81.6 | 78.5 | 0.88(0.84, 0.91) | |
| 24 h after ED admission | 0.98 mg/L | 103 | 109 | 27 | 377 | 79.5 | 77.5 | 0.86(0.82, 0.90) | |
| 48 h after ED admission | 0.98 mg/L | 105 | 97 | 25 | 389 | 81 | 80.1 | 0.87(0.83, 0.91) | |
| Torregrosa L.30 | 12 h after CAG | 0.8 mg/L | 11 | 18 | 1 | 59 | 89 | 76 | 0.87(0.68, 1.06) |
| 12 h after CS | 0.8 mg/L | 9 | 12 | 5 | 20 | 64 | 64 | 0.68(0.46, 0.88) | |
| Chen T. H.31 | On CCU admission | 1.8 mg/L | 33 | 10 | 10 | 97 | 77 | 91 | 0.90(0.83, 0.96) |
| Liu X. L.32 | On hospital admission | 475 ng/mL | 22 | 54 | 17 | 218 | 57.1 | 80.1 | 0.63(0.53, 0.73) |
| 24 h after CM administration | 503 ng/mL | 22 | 108 | 17 | 164 | 57.1 | 60.2 | 0.63(0.54, 0.72) | |
| Hsiao P. G.33 | On 24 h of AMI after PCI | 1364 mg/L | 12 | 11 | 5 | 68 | 69.2 | 85.9 | 0.864 |
| Kokkoris S.34 | On ICU admission | 1.04 mg/L | 22 | 12 | 14 | 52 | 61.1 | 81.2 | 0.75(0.65, 0.83) |
| Aydoğdu M.35 | Within first 24 h of ICU admission | 1.5 mg/L | 46 | 28 | 17 | 60 | 73 | 68 | 0.82 |
| Alharazy S. M.36 | At 24 h after CM exposure | 0.19 mg/L | 7 | 10 | 4 | 79 | 63.64 | 88.76 | 0.80(0.70, 0.87) |
| Wan Z. H.16 | On Center admission | 1.21 mg/L | 8 | 6 | 0 | 42 | 100 | 87.5 | 0.97(0.85, 1.00) |
| Padhy M.37 | 0 h of angioplasty procedure | 0.504 mg/L | 20 | 11 | 10 | 19 | 66 | 63 | 0.70 |
| 4 h of angioplasty procedure | 0.517 mg/L | 20 | 10 | 10 | 20 | 66.7 | 66.6 | 0.72 | |
| 24 h of angioplasty procedure | 0.994 mg/L | 30 | 1 | 0 | 29 | 100 | 96.7 | 1.00 | |
| 48 h of angioplasty procedure | 0.961 mg/L | 28 | 1 | 2 | 29 | 93.3 | 96.7 | 0.99 | |
| Ghonemy T. A.38 | 3 h after CS | 2.65 ng/dL | 9 | 9 | 8 | 24 | 54.7 | 72.7 | NR |
| 6 h after CS | 2.65 ng/dL | 13 | 8 | 4 | 25 | 75.2 | 75.8 | NR | |
| Yang H. T.39 | 12 h from admission | 0.70 mg/L | 22 | 21 | 9 | 38 | 70.4 | 65.2 | 0.75 |
| 24 h from admission | 0.75 mg/L | 16 | 23 | 15 | 36 | 50.0 | 61.8 | 0.73 | |
| Prowle J. R.40 | 4.5 h after CPB | 1.24 mg/L | 19 | 25 | 6 | 43 | 76.0 | 63.2 | 0.69(0.56, 0.82) |
| 24 h after CPB | 1.57 mg/L | 16 | 15 | 9 | 53 | 64.0 | 77.9 | 0.72(0.59, 0.85) | |
| Arun O.41 | At 1 h after CS | 0.76 mg/dL | 12 | 5 | 4 | 9 | 75 | 65 | 0.74 |
| At 12 h after CS | 0.98 mg/dL | 12 | 3 | 4 | 11 | 75 | 80 | 0.77 | |
| At 24 h after CS | 0.83 mg/dL | 15 | 3 | 1 | 11 | 93 | 79 | 0.79 | |
| At 48 h after CS | 0.73 mg/dL | 13 | 4 | 3 | 10 | 81 | 72 | 0.72 | |
| Tung Y. C.42 | On ED admission | 1.6 mg/L | 29 | 47 | 8 | 105 | 79 | 69 | 0.73(0.63, 0.81) |
| Yim H.18 | At 7 d after Burn | 0.75 mg/L | 31 | 14 | 9 | 43 | 76.3 | 75.4 | 0.81(0.71, 0.91) |
| At 14 d after Burn | 0.85 mg/L | 36 | 10 | 4 | 47 | 89.5 | 82.5 | 0.91(0.84, 0.97) | |
| Chen J. Z.43 | At 24 h after PNE | 0.98 mg/L | 20 | 16 | 8 | 45 | 71.4 | 73.8 | 0.79(0.70, 0.89) |
| At 48 h after PNE | 1.005 mg/L | 19 | 21 | 9 | 40 | 67.9 | 65.6 | 0.76(0.66, 0.86) | |
| Liu Y. J.17 | 0 h after CS | 0.965 mg/L | 14 | 4 | 5 | 12 | 73.7 | 75.0 | 0.74(0.57, 0.91) |
| 4 h after CS | 1.150 mg/L | 15 | 4 | 4 | 12 | 78.9 | 75.0 | 0.86(0.74, 0.98) | |
| 24 h after CS | 1.275 mg/L | 18 | 2 | 1 | 14 | 94.7 | 87.5 | 0.97(0.97, 1.02) | |
| 48 h after CS | 1.405 mg/L | 18 | 2 | 1 | 14 | 94.7 | 87.5 | 0.97(0.93, 1.02) | |
| 72 h after CS | 1.380 mg/L | 18 | 3 | 1 | 13 | 94.7 | 81.2 | 0.94(0.87, 1.02) | |
| Peng L.44 | 24 h after PCI | ↑ ≥ 15% from baseline | 10 | 10 | 19 | 157 | 34.48 | 94.01 | 0.66(0.55, 0.77) |
| 48 h after PCI | ↑ ≥ 15% from baseline | 12 | 12 | 17 | 155 | 41.38 | 92.86 | 0.78(0.70, 0.87) | |
| Gong M. M.45 | On ICU admission | 1.54 mg/L | 54 | 4 | 17 | 101 | 76.1 | 96.2 | 0.90(0.86, 0.95) |
| GaygIsIz U19 | within the first 24–48 h of ICU admission | 0.94 mg/L | 12 | 18 | 7 | 35 | 63 | 66 | 0.67 (0.53, 0.81) |
| Martensson J.20 | within 48 h of ICU admission | 1.1 mg/l | 12 | 17 | 9 | 55 | 55 | 76 | 0.67 (0.54-0.81) |
Abbreviations: AKI, acute kidney injury; AUROC, the area under the receiver operating characteristic curve; CAG, coronary angiography; CM, contrast medium; CPB, cardiopulmonary bypass; CS, cardiac surgery; DOR, diagnostic odds ratio; Scys, serum cystatin C; TP, true positive; FP, false positive; FN, false negative; TN, true negative; Tx, transplant; ICU, intensive care unit; ED, emergency department; PCI, percutaneous coronary intervention; PNE, Partial nephrectomy; NR, not reported.
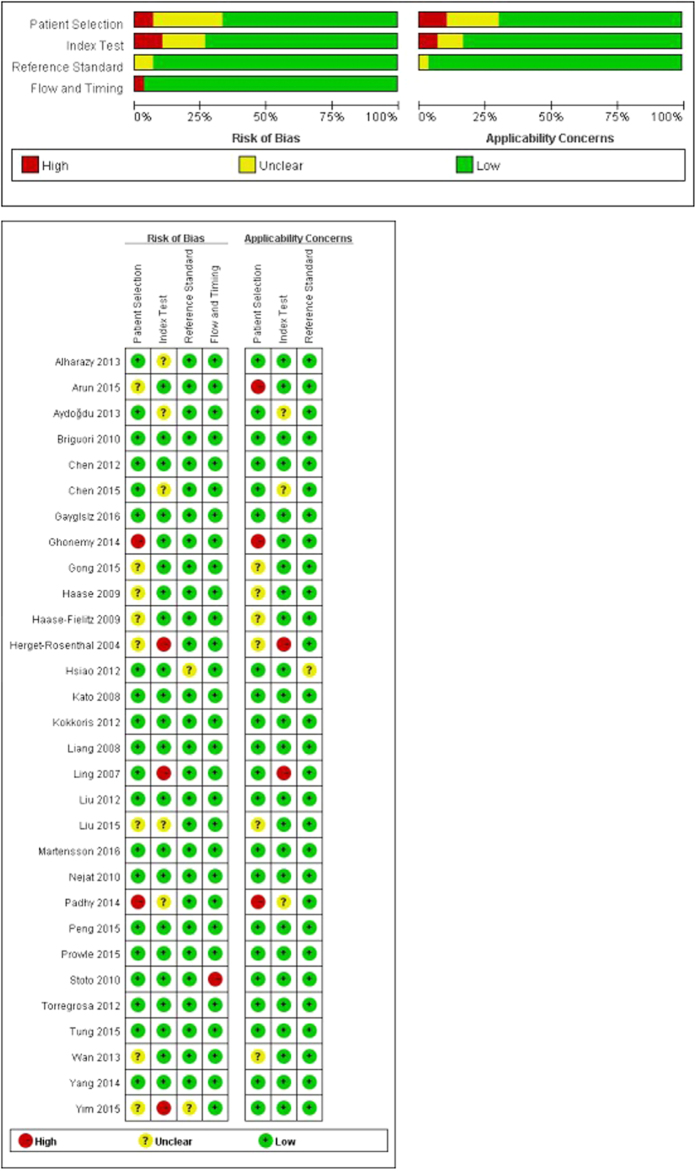
The second version of the Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) plot demonstrated no significant bias had influenced the methodological quality of the included studies (Fig. 2).
Figure 2. Assessment of the methodological quality of the selected studies by the Quality Assessment of Diagnostic Accuracy Studies tool, version 2 (QUADAS-2).
Predictive value of Scys for AKI
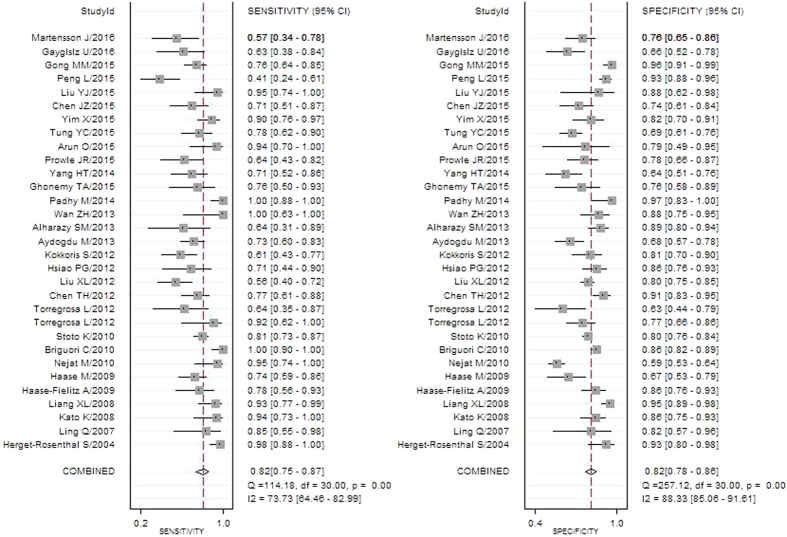
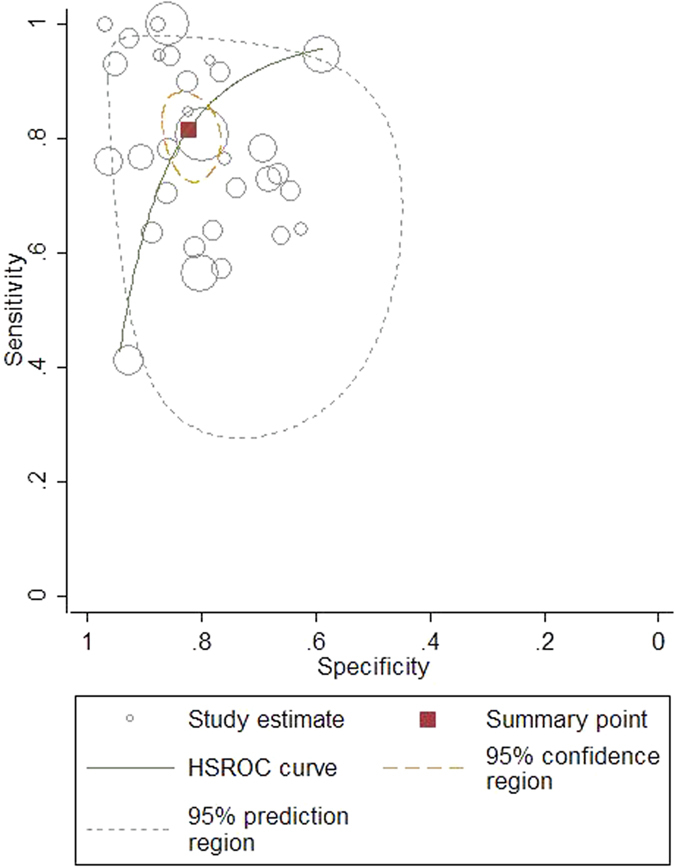
The pooled diagnostic accuracy of Scys was listed in Table 3 and Fig. 3. The overall diagnostic sensitivity and specificity was 0.82 (95% CI: 0.75 to 0.87) and 0.82 (95% CI: 0.78 to 0.86), respectively. The pooled positive and negative likelihood ratios were 4.6 (95% CI: 3.6–5.9) and 0.22 (95% CI: 0.16–0.31), respectively and the DOR was 21 (95% CI: 12–35). The overall area under the receiver operating characteristic curve (AUROC) reached 0.89. All the results above revealed a good diagnostic accuracy of Scys to screen out AKI (Fig. 4).
Table 3. Pooled diagnostic accuracy of Scys in various AKI subgroup studies.
| Settings | AKI Pts/Total Pts; No. of studies | Sensitivity (95% CI) | Specificity (95% CI) | DOR (95% CI) | AUROC (95% CI) | I2 | Likelihood Ratio (95% CI) |
RDOR | P Value | |
|---|---|---|---|---|---|---|---|---|---|---|
| Positive | Negative | |||||||||
| Across all settings | 982/4247; 30 | 0.82 (0.75, 0.87) | 0.82 (0.78, 0.86) | 21 (12, 35) | 0.89 (0.86, 0.91) | 96 | 4.6 (3.6, 5.9) | 0.22 (0.16, 0.31) | ||
| Subgroups | ||||||||||
| 1.AKI setting: | ||||||||||
| CS-AKI | 189/586; 8 | 0.81 (0.71, 0.89) | 0.82 (0.72, 0.89) | 20 (7, 57) | 0.89 (0.86, 0.91) | 0 | 4.5 (2.7, 7.8) | 0.23 (0.13, 0.39) | 1.49 (0.65, 3.40) | 0.327 |
| ICU/CCU-AKI | 340/1194; 9 | 0.77 (0.66, 0.85) | 0.82 (0.72, 0.89) | 16 (6, 38) | 0.86 (0.83, 0.89) | 89 | 4.3 (2.5, 7.4) | 0.28 (0.18, 0.44) | ||
| CIN | 173/1253; 7 | 0.90 (0.61, 0.98) | 0.87 (0.82, 0.90) | 61(10, 388) | 0.90 (0.88, 0.93) | 95 | 6.7 (4.7, 9.7) | 0.11 (0.02, 0.57) | ||
| CIN | 173/1253; 7 | 0.90 (0.61, 0.98) | 0.87 (0.82, 0.90) | 61(10, 388) | 0.90 (0.88, 0.93) | 95 | 6.7 (4.7, 9.7) | 0.11 (0.02, 0.57) | 2.24 (0.65, 7.79) | 0.194 |
| Exp-CIN | 809/2994; 23 | 0.79 (0.74, 0.84) | 0.81 (0.75, 0.85) | 16(10, 26) | 0.87 (0.84, 0.89) | 85 | 4.1 (3.1, 5.4) | 0.26 (0.20, 0.34) | ||
| 2.AKI diagnostic criteria: | ||||||||||
| KDIGO | 206/866; 6 | 0.78 (0.49, 0.93) | 0.90 (0.81, 0.95) | 31 (6, 174) | 0.92 (0.90, 0.94) | 77 | 7.7 (3.4, 17.7) | 0.25 (0.09, 0.70) | 0.60 (0.29, 1.22) | 0.147 |
| AKIN ≥ 1 | 238/812; 8 | 0.81 (0.74, 0.86) | 0.81 (0.74, 0.87) | 18 (9, 35) | 0.86 (0.83, 0.89) | 0 | 4.3 (3.0, 6.4) | 0.24 (0.17, 0.34) | ||
| RIFLE ≥ R | 284/1241; 11 | 0.75 (0.67, 0.82) | 0.76 (0.67, 0.82) | 10 (5, 18) | 0.82 (0.72, 0.85) | 64 | 2.1 (2.2, 4.4) | 0.32 (0.23, 0.46) | ||
| 3.region: | ||||||||||
| Asia | 578/2197; 20 | 0.81 (0.73, 0.87) | 0.85 (0.80, 0.89) | 24 (12, 45) | 0.90 (0.87, 0.92) | 93 | 5.3 (3.8, 7.5) | 0.23 (0.15, 0.33) | 1.69 (0.60, 4.78) | 0.307 |
| Non-Asia | 404/2050; 10 | 0.83 (0.70, 0.91) | 0.78 (0.71, 0.83) | 17 (7, 43) | 0.85 (0.82, 0.88) | 82 | 3.7 (2.7, 5.2) | 0.22 (0.11, 0.42) | ||
| 4.Scys assay: | ||||||||||
| PETIA | 129/546; 5 | 0.76 (0.51, 0.91) | 0.87 (0.74, 0.94) | 21 (4, 103) | 0.90 (0.87, 0.92) | 88 | 5.8 (2.5, 13.5) | 0.27 (0.11, 0.67) | 1.21 (0.56, 2.60) | 0.614 |
| PENIA | 484/2043; 14 | 0.82 (0.31, 0.81) | 0.78 (0.72, 0.83) | 16 (8, 31) | 0.86 (0.83, 0.89) | 62 | 3.8 (2.8, 5.0) | 0.24 (0.15, 0.37) | ||
| ELISA | 282/1121; 8 | 0.77 (0.66, 0.85) | 0.86 (0.77, 0.92) | 21 (8, 55) | 0.88 (0.85, 0.90) | 72 | 5.6 (3.0, 10.3) | 0.27 (0.17, 0.42) | ||
| 5.participant mean age: | ||||||||||
| ≤60 years | 445/1928; 12 | 0.85 (0.75, 0.91) | 0.82 (0.74, 0.88) | 26 (11, 61) | 0.90 (0.87, 0.93) | 89 | 4.8 (3.1, 7.4) | 0.19 (0.11, 0.33) | 1.24 (0.43, 1.56) | 0.684 |
| >60 years | 451/1994; 15 | 0.80 (0.70, 0.88) | 0.82 (0.76, 0.88) | 18 (9, 36) | 0.88 (0.84, 0.90) | 93 | 4.4 (3.2, 5.9) | 0.24 (0.15, 0.38) | ||
| 6.male rate: | ||||||||||
| ≤70% | 604/2512; 14 | 0.78 (0.67, 0.86) | 0.83 (0.78, 0.88) | 18 (9, 36) | 0.88 (0.85, 0.91) | 94 | 4.7 (3.4, 6.5) | 0.26 (0.17, 0.41) | 0.96 (0.37, 2.49) | 0.925 |
| >70% | 349/1603; 15 | 0.83 (0.75, 0.90) | 0.80 (0.73, 0.85) | 20 (10, 41) | 0.88 (0.85, 0.91) | 77 | 4.1 (3.0, 5.7) | 0.21 (0.13, 0.33) | ||
| 7.sample size: | ||||||||||
| ≤100 | 488/1598; 20 | 0.82 (0.74, 0.88) | 0.81 (0.77, 0.85) | 20 (10, 39) | 0.88 (0.85, 0.91) | 42 | 4.4 (3.3, 5.8) | 0.22 (0.14, 0.33) | 0.59 (0.21, 1.65) | 0.304 |
| >100 | 494/2649; 10 | 0.81 (0.67, 0.89) | 0.85 (0.76, 0.91) | 23 (10, 54) | 0.90 (0.87, 0.92) | 96 | 5.3 (3.3, 8.6) | 0.23 (0.13, 0.40) | ||
Abbreviations: AKI, acute kidney injury; AUROC, the area under the receiver operating characteristic curve; CCU, coronary care unit; CIN, contrast-induced nephropathy; CS, cardiac surgery; DOR, diagnostic odd ratio; ELISA, enzyme-linked immunosorbent assay; KDIGO, Kidney Disease: Improving Global Outcomes; PENIA, particle-enhanced nephelometric immunoassay; PETIA, particle-enhanced turbidimetric immunoassay; Pts, patients; RIFLE, risk-injury-failure-loss-end stage renal disease; Scr, serum creatinine; Scys, serum cystatin C.
Figure 3. Forest plot of the pooled sensitivity and specificity of serum cystatin C to predict all-cause acute kidney injury.
Figure 4. Hierarchical summary receiver operating characteristic (HSROC) plot of serum cystatin C to predict acute kidney injury across all settings.
Threshold analysis and meta-regression analysis
The Spearman correlation coefficient between the pooled sensitivity and 1-specitity was −0.277 (P = 0.131), indicating no threshold effect. Meta-regression analysis showed the following factors irrelevant with accuracy of Scys for AKI: settings, diagnostic criteria, region, Scys determination method, participant mean age, gender, sample size (Table 3).
Influence factors affecting Scys of AKI
Various Scys blood sampling point-in-time, cut-off value, and determination method resulted in various Scys predictive value for AKI by subgroup analysis (Tables 1,2,3,4). Foremost, Twenty-four hours after AKI occurrence to adopt the blood seems to be an optimal time, with sensitivity of 0.82, specificity of 0.83, DOR of 23, and AUROC of 0.89 (Table 4). Besides, 50% elevated from baseline could be an ideal cut-off value to predict AKI, with AUROC 0.99 (Table 2). Last but not the least, PETIA performed better than other two determination methods, with sensitivity 0.76, specificity 0.87 and AUROC 0.90 (Table 3). In addition, several factors other than Scys assay itself were also analyzed in this study, such as AKI diagnostic criteria, region, gender, age and sample size and et al. Based on the newly KDIGO criteria, Scys performed the best accuracy, with sensitivity 0.78, specificity 0.90 and AUROC 0.92 (Table 3). However, these factors mentioned above were not the origin of possible sources of heterogeneity by meta-regression analysis.
Table 4. Diagnostic accuracy of Scys in predicting AKI at different time points.
| Time | Study number | Sensitivity (95% CI) | Specificity (95% CI) | DOR (95% CI) | I2 | AUROC (95% CI) |
|---|---|---|---|---|---|---|
| All settings 0 h | 12 | 0.79 (0.70, 0.86) | 0.82 (0.74, 0.88) | 17 (9, 35) | 92 | 0.88 (0.84, 0.90) |
| 1–12 h | 9 | 0.75 (0.70, 0.80) | 0.72 (0.68, 0.76) | 8 (5,12) | 0 | 0.80 (0.76, 0.83) |
| 24 h | 16 | 0.82 (0.69, 0.90) | 0.83 (0.76, 0.89) | 23 (9, 57) | 95 | 0.89 (0.86, 0.92) |
| 48 h | 7 | 0.76 (0.60, 0.88) | 0.87 (0.76, 0.93) | 21 (5, 58) | 94 | 0.89 (0.86, 0.92) |
| 1–6 h after cardiac surgery | 5 | 0.73 (0.65, 0.80) | 0.68 (0.62, 0.74) | 6 (4, 9) | 100 | 0.77 (0.73, 0.80) |
| 12–24 h after cardiac surgery | 6 | 0.85 (0.72, 0.92) | 0.80 (0.68, 0,89) | 23 (7, 77) | 7 | 0.90 (0.87, 0.92) |
Abbreviations: AKI, acute kidney injury; AUROC, the area under the receiver operating characteristic curve; DOR, diagnostic odds ratio; Scys, serum cystatin C.
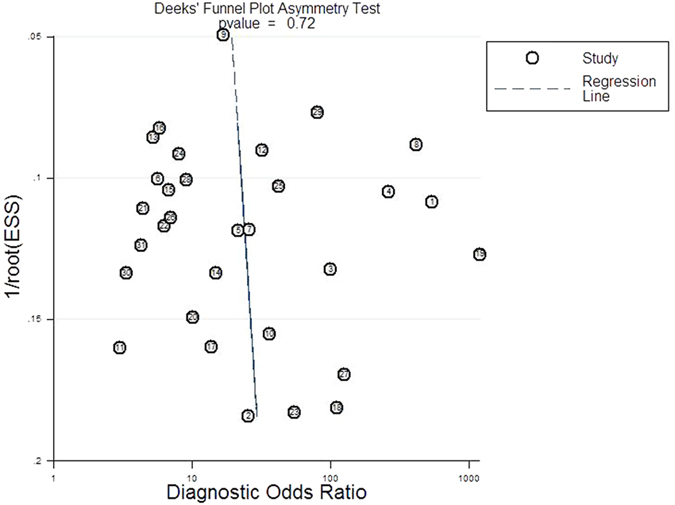
Publication bias
No publication bias and high symmetry of the included studies were proved by Deeks’ funnel plot asymmetry test (P = 0.72; Fig. 5).
Figure 5. Deeks’ funnel plot to analyze the likelihood of publication bias.
Discussion
The overall AKI incidence in this study was 23.1% (982/4247), similar to the prevalence reported in Siew’ study4, indicating the disease is still not in control and prevented. The mean age in this meta-analysis achieved 61 years old, demonstrating more attention should be taken on the susceptible population: the elderly. Various setting, various AKI incidence. The top three settings prone to AKI were cardiovascular surgery, ICU/CCU and radiology intervention department.
Facing to the severe reality, early diagnosis is crucial to prevent and relieve the prognosis of AKI. Scys has been known to be an ideal marker to assess renal function in CKD patients46,47. Whether it is a satisfactory marker to predict AKI is still in debate. Thus, to comprehensively and objectively evaluate the value of Scys predicting AKI, this meta-analysis set a rigorous inclusion and exclusion criteria at the very start. One of the essential selected condition should be prospective cohort studies. After literature searching, 30 studies finally were included. The pooled sensitivity, specificity and AUROC of Scys was 0.82, 0.82 and 0.89, respectively. These diagnostic efficiency demonstrated that Scys would be an excellent bio-marker for the all-cause AKI prediction.
Further subgroups analysis indicated several influence factors should be noted. Different Scys blood sampling point-in-time, cut-off value, and determination method, different Scys predictive value for AKI. If an AKI event would occur, it could be suggested that Scys should be determined by PETIA method at 24-hours after the possible AKI event, referring the diagnostic criteria-50% elevated from baseline. Compared with the previous studies results, this advice is rational and acceptable. The blood sampling point-in-time was another focus. Among the various time point, 24-hours point after AKI might be a preferable selection.
Otherwise, factors potentially influencing Scys were also assessed in this study. Three main criteria to diagnose AKI were presented in Table 3. The newly KDIGO criteria performed an increased diagnostic accuracy in Thomas’ study48. The subgroup analysis in this study also confirmed its superiority, that the newly KDIGO criteria showed higher specificity and AUROC than the RIFLE criteria and AKIN criteria.
As reported, the most common cause of AKI is acute tubular necrosis (ATN), which could be caused by prolonged hypotension, sepsis, surgery, nephrotoxic medications, and contrast media in hospitalized patients49,50. Among the three main causes of AKI in this meta-analysis, Scys performed the best accuracy in CIN-AKI. The probable reason might be that kidney injury and hemodynamic disorder induced by CIN-AKI is less serious and complicate than that by CS-AKI and ICU-AKI. CIN could be the most simple, but also the most important AKI model to ascertain the value of Scys. To our knowledge, CIN is the third leading cause of AKI in hospitalized patients51,52. There is a variety of novel bio-markers have been proposed to diagnose CIN. Among them, Neutrophil gelatinaseassociated lipocalin (NGAL), interleukin-18 (IL-18) and Scys are the most known promising bio-markers53,54. However, the former two biomarker determination method have not yet been established in clinical laboratories. Thus, according to the results of this study, Scys could be the optimal marker predicting various AKI.
It should be mentioned in the end, the same as the previous CKD studies proved55, Scys was not significantly influenced by gender and age in this AKI-related study, as well. Moreover, although settings, AKI diagnostic criteria, race and assay method might play a little bit of influence on the accuracy of Scys, it did not reach statistical significance. Thus, these results above showed that Scys could be a nice marker, not only for CKD diagnosis, but for AKI prediction.
For all meta-analyses, heterogeneity is a potential problem when interpreting the results. The I2 statistic was 96% in our meta-analysis, indicating significant heterogeneity across the included studies. One major source of heterogeneity is the threshold effect in which different cut-offs are used in the included studies. The Spearman correlation analysis in our study indicated no threshold effect related heterogeneity exit. Furthermore, meta-regression analysis results revealed that factors potentially affecting Scys did not participate in the heterogeneity (p > 0.05; Table 3). Thus, we considered that the heterogeneity may be related to additional factors, such as specified ethnicity (except from the two race in this study), kidney function, and etc. However, these factors is difficult to unify and analyze.
In summary, this meta-analysis demonstrated that Scys shows a good diagnostic performance for predicting all-cause AKI. Several factors could affect the predictive value of Scys for AKI, but not reach significant differences. More randomized controlled trials in multicenter are in need to further investigate the accuracy of Scys.
Methods
Data sources and search strategy
In accordance with the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines56, we searched PubMed, EMBASE, Web of Science and the Cochrane Library from the inception to September 2016.
The following terms were used: “AKI, acute kidney injury, acute renal failure, acute renal insufficiency, acute renal dysfunction and cystatin C”. References of the selected studies were further screened manually to identify whether additional eligible articles were available or not.
Study selection
The inclusion criteria of this study were composed of the following characteristics: (1) prospective cohort study, (2) adults, (3) sample size ≥ 30, (4) original data of sensitivity and specificity, (5) AKI diagnostic criteria. If any disagreement existed, two investigators would check and discuss about the full text.
Authors were contacted when there were incomplete or missing data. Ethics approval and patients consent were not in need for this study.
Data extraction and quality assessment
Two investigators (Z.Z.Y. and X.H.P.) independently extracted information from each article using a standardized collection form. Collected parameters included the first author, publication year, clinical setting, region, age, gender, AKI diagnostic criteria, Scys determination method, Scys cut-off value, sensitivity and specificity. Differences were resolved by consensus or the third researcher (W.H.Z.).
We investigated the methodological quality of the present study using the second version of the Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2)57. QUADAS-2 assesses the risk of bias and applicability in four domains: Patient selection (consecutive or random sample enrolled, case–control design and inappropriate exclusions avoided); Index test (blinded interpretation of the Rules); Reference standard (correctly excluded a fracture and blinded interpretation); and Flow and timing (appropriate interval between application of the Rules and reference standard, all patients received the reference standard and were included in the analysis).
Statistical analysis
A bivariate meta-analytic approach was used to pool sensitivity, specificity, DOR, PLR, and NLR. Subsequently, the respective hierarchical summary receiver operating characteristic (HSROC) curves was constructed to plot sensitivity versus specificity, and then calculate the area under the curve. The highest Youden index (sensitivity + 1-specificity) of every included studies was chosen to end pooled in various Scys measurement times58. We used the I2 statistic to evaluate the heterogeneity59, and the I2 > 75% is supposed of significant heterogeneity, the threshold analysis and meta-regression analysis were further used to identify possible sources of heterogeneity. Publication bias was estimated by Deeks’ funnel plot asymmetry test60. All the data processing and analysis were performed using the midas and metandi commands of Stata/SE version 12.0 (Stata Corp LP, College Station, TX) and Meta-Disc 1.4 for Windows (XI Cochrane Colloquium, Barcelona, Spain). QUADAS-2 quality assessment was descriptively analyzed using Review Manager 5.3 (The Cochrane Collaboration, Copenhagen, Denmark). P < 0.05 was considered of statistical significance.
Additional Information
How to cite this article: Yong, Z. et al. Predictive value of serum cystatin C for acute kidney injury in adults: a meta-analysis of prospective cohort trials. Sci. Rep. 7, 41012; doi: 10.1038/srep41012 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Acknowledgments
The present study was supported by the grants from the Major State Basic Research Development Program of China (2013CB530803), the National Natural Science Foundation of China (H0511-81370843), Chinese Society of Nephrology (15020020590), the Innovation of Science and Technology Achievement Transformation Fund of Jiangsu Province (BL2012066), and the Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (JX10231801).
Footnotes
Author Contributions Conception and design: Z.Z.Y., X.H.P. and W.H.Z. Development of methodology: Z.Z.Y., X.H.P., B.Z. Acquisition of data: Z.Z.Y., X.H.P., B.Z. Analysis and interpretation of data: Z.Z.Y., X.H.P. and H.C.Y. Writing, review and/or revision of the manuscript: Z.Z.Y., X.H.P. and W.H.Z.
References
- Chawla L. & Kimmel P. Acute kidney injury and chronic kidney disease: an integrated clinical syndrome. Kidney Int 82, 516–524 (2012). [DOI] [PubMed] [Google Scholar]
- Gallagher M. et al. Long-term survival and dialysis dependency following acute kidney injury in intensive care: extended follow-up of a randomized controlled trial. Plos Medicine 11, e1001601 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lameire N. et al. Acute kidney injury: an increasing global concern. Lancet 382, 170–179 (2013). [DOI] [PubMed] [Google Scholar]
- Siew E. & Davenport A. The growth of acute kidney injury: a rising tide or just closer attention to detail? Kidney Int 87, 46–61 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellomo R. et al. Acute Dialysis Quality Initiative workgroup. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 8, R204–212 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta R. et al. Acute Kidney Injury Network. Acute kidney injury network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 11, R31 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- KDIGO. Clinical practice guideline for acute kidney injury section 2: AKI definition. Kidney Int Suppl 2, 19–36 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srisawat N. et al. Thai Lepto-AKI study group. Neutrophil gelatinase associated lipocalin(NGAL) in leptospirosis acute kidney injury: a multicenter study in thailand. PLoS One 10, e0143367 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisula S. et al. FINNAKI Study Group. Predictive value of urine interleukin-18 in the evolution and outcome of acute kidney injury in critically ill adult patients. Br J Anaesth 114, 460–468 (2015). [DOI] [PubMed] [Google Scholar]
- Parikh C. et al. TRIBE-AKI Consortium. Performance of kidney injury molecule-1 and liver fatty acid-binding protein and combined biomarkers of AKI after cardiac surgery. Clin J Am Soc Nephrol 8, 1079–1088 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filler G. et al. Cystatin C as a marker of GFR: history, indication and future research. Clin Biochem 38, 1–8 (2005). [DOI] [PubMed] [Google Scholar]
- Shlipak M. et al. Cystatin C and the risk of death and cardiovascular events among elderly persons. N Engl J Med. 352, 2049-2060 (2005). [DOI] [PubMed] [Google Scholar]
- Odutayo A. & Cherney D. Cystatin C and acute changes in glomerular filtration rate. Clin Nephrol 78, 64–75 (2012). [DOI] [PubMed] [Google Scholar]
- Sjöström P., Tidman M. & Jones I. The shorter T1/2 of cystatin C explains the earlier change of its serum level compared to serum creatinine. Clin Nephrol 62, 241–242 (2004). [DOI] [PubMed] [Google Scholar]
- Zhang Z., Lu B., Sheng X. & Jin N. Cystatin C in prediction of acute kidney injury: A systemic review and meta-analysis. Am J Kidney Dis 58, 356e65 (2011). [DOI] [PubMed] [Google Scholar]
- Wan Z. et al. Cystatin C is a biomarker for predicting acute kidney injury in patients with acute-on-chronic liver failure. World J Gastroenterol 19, 9432–9438 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y. et al. Klotho: a novel and early biomarker of acute kidney injury after cardiac valve replacement surgery in adults. Int J Clin Exp Med 8, 7351–7358 (2015). [PMC free article] [PubMed] [Google Scholar]
- Yim H. et al. Serum cystatin C and microalbuminuria in burn patients with acute kidney injury. Eur J Clin Invest 45, 594–600 (2015). [DOI] [PubMed] [Google Scholar]
- Gaygısız Ü. et al. Can admission serum cystatin C level be an early marker subclinical acute kidney injury in critical care patients? Scand J Clin Lab Invest 76, 143–150 (2016). [DOI] [PubMed] [Google Scholar]
- Mårtensson J. et al. Plasma endostatin may improve acute kidney injury risk prediction in critically ill patients. Ann Intensive Care 6, 6 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herget-Rosenthal S. et al. Early detection of acute renal failure by serum cystatin C. Kidney Int 66, 115–1122 (2004). [DOI] [PubMed] [Google Scholar]
- Ling Q. et al. Alternative definition of acute kidney injury following liver transplantation: based on serum creatinine and cystatin C levels. Transplant Proc 39, 3257–3260 (2007). [DOI] [PubMed] [Google Scholar]
- Liang X. et al. Prospective study of cystatin C for diagnosis of acute kidney injury after cardiac surgery. Nan Fang Yi Ke Da Xue Xue Bao. 28, 2154–2156 (2008). [PubMed] [Google Scholar]
- Haase M. et al. Novel Biomarkers Early Predict the Severity of Acute Kidney Injury After Cardiac Surgery in Adults. Ann Thorac Surg. 88, 124–130 (2009). [DOI] [PubMed] [Google Scholar]
- Nejat M., Pickering J., Walker R. & Endre Z. Rapid detection of acute kidney injury by plasma cystatin C in the intensive care unit. Nephrol Dial Transplant. 25, 3283–3289 (2010). [DOI] [PubMed] [Google Scholar]
- Briguori C. et al. Cystatin C and Contrast-Induced Acute Kidney Injury. Circulation. 121, 2117–2122 (2010). [DOI] [PubMed] [Google Scholar]
- Soto K. et al. Cystatin C as a marker of acute kidney injury in the emergency department. Clin J Am Soc Nephrol 5, 1745–1754 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K. et al. Valuable markers for contrast-induced nephropathy in patients undergoing cardiac catheterization. Circ J 72, 1499–1505 (2008). [DOI] [PubMed] [Google Scholar]
- Haase-Fielitz A. et al. Novel and conventional serum biomarkers predicting acute kidney injury in adult cardiac surgery-A prospective cohort study. Crit Care Med. 37, 553–560 (2009). [DOI] [PubMed] [Google Scholar]
- Torregrosa I. et al. Early biomarkers of acute kidney failure after heart angiography or heart surgery in patients with acute coronary syndrome or acute heart failure. Nefrologia 32, 44–52 (2012). [DOI] [PubMed] [Google Scholar]
- Chen T. et al. Acute kidney injury biomarkers for patients in a coronary care unit: A prospective cohort study. PLoS One 7, e32328 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X. et al. Plasma neutrophil-gelatinase-associated lipocalin and cystatin C could early diagnose contrast-induced acute kidney injury in patients with renal insufficiency undergoing an elective percutaneous coronary intervention. Chin Med J (Engl) 125, 1051–1056 (2012). [PubMed] [Google Scholar]
- Hsiao P. et al. Early prediction of acute kidney injury in patients with acute myocardial injury. J Crit Care 27, 525.e1–7 (2012). [DOI] [PubMed] [Google Scholar]
- Kokkoris S. et al. Combination of renal biomarkers predicts acute kidney injury in critically ill adults. Ren Fail 34, 1100–1108 (2012). [DOI] [PubMed] [Google Scholar]
- Aydoğdu M. et al. The use of plasma and urine neutrophil gelatinase-associated lipocalin (NGAL) and Cystatin C in early diagnosis of septic acute kidney injury in critically ill patients. Dis Markers 34, 237–246 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alharazy S. et al. Serum neutrophil gelatinase-associated lipocalin and cystatin C are early biomarkers of contrast-induced nephropathy after coronary angiography in patients with chronic kidney disease. Angiology 65, 436–442 (2014). [DOI] [PubMed] [Google Scholar]
- Padhy M. et al. Serum neutrophil gelatinase associated lipocalin (NGAL) and cystatin C as early predictors of contrast-induced acute kidney injury in patients undergoing percutaneous coronary intervention. Clin Chim Acta 435, 48–52 (2014). [DOI] [PubMed] [Google Scholar]
- Ghonemy T. & Amro G. Plasma neutrophil gelatinase-associated lipocalin (NGAL) and plasma cystatin C (CysC) as biomarker of acute kidney injury after cardiac surgery. Saudi J Kidney Dis Transpl 25, 582–588 (2014). [DOI] [PubMed] [Google Scholar]
- Yang H. et al. Assessment of biochemical markers in the early post-burn period for predicting acute kidney injury and mortality in patients with major burn injury: comparison of serum creatinine, serum cystatin-C, plasma and urine neutrophil gelatinase-associated lipocalin. Crit Care 18, R151 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prowle J. et al. Combination of biomarkers for diagnosis of acute kidney injury after cardiopulmonary bypass. Ren Fail 37, 408–416 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aruna O. et al. Renal effects of coronary artery bypass graft surgery in diabetic and nondiabetic patients: A study with urinary neutrophil gelatinase-associated lipocalin and serum cystatin C. Kidney Blood Press Res 40, 141–152 (2015). [DOI] [PubMed] [Google Scholar]
- Tung Y., Chang C., Chen Y. & Chu P. Combined biomarker analysis for risk of acute kidney injury in patients with ST segment elevation myocardial infarction. PLoS One 10, e0125282 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Lin J. & Lin C. Serum and urinary biomarkers for predicting acute kidney injury after partial nephrectomy. Clin Invest Med 38, E82–9 (2015). [DOI] [PubMed] [Google Scholar]
- Peng L. et al. Diagnostic value of cystatin C in contrast-induced acute kidney injury after percutaneous coronary intervention. Zhonghua Nei Ke Za Zhi 54, 188–192 (2015). [PubMed] [Google Scholar]
- Gong M., Yang Y. & Zhang S. Value of acute renal injury associated biomarkers for patients in intensive care unit. Zhong Nan Da Xue Xue Bao Yi Xue Ban 40, 1083–1088 (2015). [DOI] [PubMed] [Google Scholar]
- Dharnidharka V., Kwon C. & Stevens G. Serum cystatin C is superior to serum creatinine as a marker of kidney function: a meta-analysis. Am J Kidney Dis 40, 221–226 (2002). [DOI] [PubMed] [Google Scholar]
- Roos J. et al. Diagnostic accuracy of cystatin C compared to serum creatinine for the estimation of renal dysfunction in adults and children: a meta-analysis. Clin Biochem 40, 383–391 (2007). [DOI] [PubMed] [Google Scholar]
- Thomas M. et al. The definition of acute kidney injury and its use in practice. Kidney Int 87, 62–73 (2015). [DOI] [PubMed] [Google Scholar]
- Nash K., Hafeez A. & Hou S. Hospital-acquired renal insufficiency. Am J Kidney Dis 39, 930–936 (2002). [DOI] [PubMed] [Google Scholar]
- Eftekhari P. Evaluation of acute kidney injury in the hospital setting. Prim Care 41, 779–802 (2014). [DOI] [PubMed] [Google Scholar]
- Wichmann J. et al. Contrast-induced nephropathy. Circulation 132, 1931–1936 (2015). [DOI] [PubMed] [Google Scholar]
- Chalikias G., Drosos I. & Tziakas D. Contrast-induced acute kidney injury: an update. Cardiovasc Drugs Ther 30, 215–228 (2016). [DOI] [PubMed] [Google Scholar]
- Connolly M. et al. Novel biomarkers of acute kidney injury after contrast coronary angiography. Cardiol Rev 23, 240–246 (2015). [DOI] [PubMed] [Google Scholar]
- Briguori C., Quintavalle C., Donnarumma E. & Condorelli G. Novel biomarkers for contrast-induced acute kidney injury. Biomed Res Int 2014, 568738 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei L. et al. Diagnostic accuracy of serum cystatin C in chronic kidney disease: a meta-analysis. Clin Nephrol 84, 86–94 (2015). [DOI] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J. & Altman D. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 151, 264–269 (2009). [DOI] [PubMed] [Google Scholar]
- Whiting P. et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 155, 529–536 (2011). [DOI] [PubMed] [Google Scholar]
- Schisterman E., Perkins N., Liu A. & Bondell H. Optimal cut-point and its corresponding Youden index to discriminate individuals using pooled blood samples. Epidemiology 16, 73–81 (2005). [DOI] [PubMed] [Google Scholar]
- Higgins J. & Thompson S. Quantifying heterogeneity in a meta-analysis. Stat Med 21, 1539–1558 (2002). [DOI] [PubMed] [Google Scholar]
- Peters J., Sutton A., Jones D., Abrams K. & Rushton L. Comparison of two methods to detect publication bias in meta-analysis. JAMA 295, 676–680 (2006). [DOI] [PubMed] [Google Scholar]