Abstract
The translation of many tissue engineering/regenerative medicine (TE/RM) therapies that demonstrate promise in vitro are delayed or abandoned due to reduced and inconsistent efficacy when implemented in more complex and clinically relevant preclinical in vivo models. Determining mechanistic reasons for impaired treatment efficacy is challenging after a regenerative therapy is implanted due to technical limitations in longitudinally measuring the progression of key environmental cues in vivo. The ability to acquire real-time measurements of environmental parameters of interest including strain, pressure, pH, temperature, oxygen tension, and specific biomarkers within the regenerative niche in situ would significantly enhance the information available to tissue engineers to monitor and evaluate mechanisms of functional healing or lack thereof. Continued advancements in material and fabrication technologies utilized by microelectromechanical systems (MEMSs) and the unique physical characteristics of passive magnetoelastic sensor platforms have created an opportunity to implant small, flexible, low-power sensors into preclinical in vivo models, and quantitatively measure environmental cues throughout healing. In this perspective article, we discuss the need for longitudinal measurements in TE/RM research, technical progress in MEMS and magnetoelastic approaches to implantable sensors, the potential application of implantable sensors to benefit preclinical TE/RM research, and the future directions of collaborative efforts at the intersection of these two important fields.
Keywords: tissue engineering, regenerative medicine, MEMS, sensors, in vivo monitoring
1. Introduction
Tissue engineering and regenerative medicine (TE/RM) comprises a wide range of therapeutic approaches, each with the intent to mimic or augment endogenous biological mechanisms to replace or repair injured tissues and organs [1].The notion of harnessing innate developmental or regenerative biology to engineer clinical therapies dates back to 1938, and in recent decades has developed into an established commercial industry [2,3]. Current TE/RM developments can largely be segmented into two approaches which are increasingly implemented in combination: (1) stimulation of intrinsic repair and (2) replacement of the injured tissue [4]. However, with the exception of tissue engineered skin products, the translation of TE/RM approaches into viable therapies for many clinical injuries has remained elusive. In particular, the regeneration of large, vascularized, and multi-tissue injuries including those as a result of chronic degeneration or trauma represents an urgent clinical need for more effective TE/RM treatment options.
The translation of many TE/RM therapies that demonstrate promise in vitro are delayed or abandoned due to reduced and inconsistent efficacy when implemented in more complex and clinically relevant preclinical in vivo models. Throughout essentially every developmental or healing process, the organization, proliferation, and differentiation of cells follow a highly coordinated spatiotemporal profile that is optimized to produce or restore functional tissue. Likewise, once a regenerative therapy is delivered in vivo, there are dynamic, bidirectional mechanical and biochemical interactions between the implant and the local injury environment which can either promote or dysregulate the healing process. Determining mechanistic reasons for impaired treatment efficacy is challenging after a regenerative therapy is implanted due to technical limitations in longitudinally measuring the progression of key environmental cues in vivo. The ability to acquire real-time measurements of environmental parameters of interest including strain, pressure, force, pH, temperature, and oxygen tension within the regenerative niche in situ would significantly enhance the information available to tissue engineers to monitor and evaluate mechanisms of functional healing or lack thereof. Moreover, correlation of such longitudinal parameters with quantitative measures of functional regeneration would provide a rational approach for the design of improved regenerative strategies.
Continued advancements in material and fabrication technologies utilized by microelectromechanical systems (MEMS) and the unique physical characteristics of passive magnetoelastic sensor platforms have created an opportunity to implant small, flexible, low-power sensors into preclinical in vivo models and quantitatively measure environmental cues throughout healing. In particular, the capabilities of implantable sensors offer three exciting applications to TE/RM research: (1) longitudinal, minimally invasive measurements of environmental parameters throughout endogenous tissue repair to define desirable biomechanical and biochemical design criteria for new regenerative therapies; (2) in vivo monitoring of novel TE/RM therapies to evaluate their ability to mimic desired spatiotemporal healing profiles, and to elucidate potential reasons for successful versus unsuccessful implementations; (3) environmentally tuned TE/RM constructs with integrated sensors that can trigger specific actions (e.g., drug or growth factor depot release) at a certain environmental threshold in a closed-loop fashion.
2. The Need for Longitudinal Measurements
The dynamic nature of healing has important implications on mechanical and chemical environments that regenerative medicine therapies seek to manipulate. The structure and composition of cells and extracellular matrix (ECM) comprising healing tissue is spatiotemporally heterogeneous. Likewise, the multiscale mechanical properties and internal stress–strain distribution evolve rapidly, particularly in regenerating load-bearing tissues. In addition, the level of vascular infiltration delivering oxygen and nutrients and removing waste is highly transient. As a result, important environmental cues known to regulate cell migration, differentiation, proliferation, apoptosis, factor secretion, and ECM deposition are continuously changing variables. To more explicitly articulate this perspective, we briefly cite large bone defect regeneration throughout the text as a relevant example which is of particular interest to our laboratories.
Bone regeneration is a highly coordinated physiological response that, in most circumstances, can functionally restore skeletal fractures without scar formation. Organized healing requires the rapid formation, morphogenesis, and remodeling of multiple tissue phenotypes over the course of several months. The regenerative process is commonly segmented into four major phases (with associated timelines) of distinct tissue formation: (1) acute inflammation and hematoma (hours and days), (2) soft callus (days and weeks), (3) woven bone (weeks and months), and (4) lamellar bone remodeling (months and years). In particular, these phases are characterized by tissue structures with distinct mechanical properties, metabolic states, and levels of vascular perfusion (Fig. 1). These three factors are critical indicators of the progression of healing, and importantly, they can be at least partially described by measurable read-outs from common sensor modalities: strain, pH, and oxygen tension, respectively.
Fig. 1.
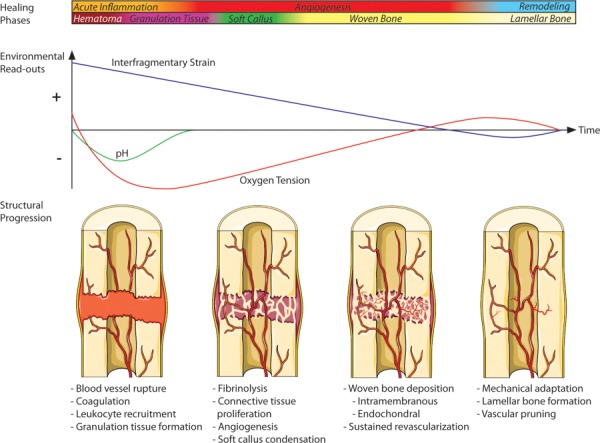
Schematic outlining the temporal profile of bone regeneration, illustrating phases of healing, structural progression in the defect, and qualitative estimates of environmental parameter profiles. Nondestructive, quantitative measurements of these environmental cues would significantly enhance fundamental understanding of the temporal progression of the bone healing environment as well as many other diseases of interest, providing a better foundation to develop and evaluate effective regenerative therapies. Created using images from Servier Medical Art, CC-BY 3.0.
During the initial injury, disruption of highly vascularized bone tissue floods the defect gap with blood which rapidly coagulates to form a hematoma. Once the initial influx of blood is consumed, glycolysis is favored over oxidative phosphorylation and the hematoma is characterized by low oxygen tension, high lactate concentration, and acidic pH [5–8]. Leukocyte recruitment and granulation tissue formation in the initial phase of healing transitions toward fibrinolysis, angiogenesis, and connective tissue migration and condensation during the soft callus phase [9]. During this time, robust angiogenesis takes place to reperfuse the defect with an adequate blood supply, a critical precursor to bone formation. The healing mechanism is dependent on the rate of revascularization and the local reestablishment of physiological oxygen tension and pH. If vascularization occurs relatively quickly, within approximately the first 2–4 weeks (dependent on the defect size and anatomical location), mineralization of the soft callus occurs primarily through direct intramembranous bone formation. If oxygen tension is restored more slowly, due to the size of the defect or external mechanical factors, healing occurs by the way of a hypertrophic cartilage intermediary which is mineralized through endochondral bone formation [10–12]. Throughout mineralization, the apparent stiffness of the fracture site steadily increases while interfragmentary motion accordingly decreases until the fracture is fully bridged [13–15]. The initial woven bone tissue is immature and characterized by an unorganized collagen–hydroxyapatite matrix and an abundant, disorganized vessel network. During the remodeling phase, both vascular and skeletal structures are gradually pruned until tissue function is completely restored [16,17].
While endogenous bone healing is typically robust, depending on the anatomical site and injury mechanism, it is estimated that up to 10% of fractures do not heal spontaneously and result in nonunion [18]. Comorbidities and circumstances that increase the likelihood of nonunion are extensive and include smoking, diabetes, infection, nutritional deficiency, old age, poly-trauma, mechanical instability, and critically sized segmental bone loss [19]. Risk factors all contribute to dysregulate the temporal progression of the regenerative program, although the ability to detect when and where the dysregulation initiates is a key technical limitation to clinical practice and preclinical research. In cases of chronic fracture nonunion or acute critical bone loss, surgeons are left with strikingly few therapeutic options to stimulate healing, and clinicians primarily must rely on autologous or allogenic bone graft sources, which possess a number of inherent limitations [20,21]. To develop improved therapies, tissue engineers would benefit from nondestructive measurement platforms that can longitudinally quantify mechanical and chemical parameters within the healing environment to aid in the identification of critical thresholds that are detrimental to healing and to isolate time-points where dysregulation occurs. Common quantitative, nondestructive preclinical assays are intended to evaluate overall healing (e.g., computed tomography measurements of bone volume and architecture), which are highly dependent on the animal and injury model being investigated. They do not quantify the actual mechanical and biochemical cues regulating injury progression. Since local measurements of environmental signals are typically not measured directly, a meaningfully quantitative description of an ideal versus a challenged healing environment is difficult to generalize across multiple studies.
While longitudinal evaluation platforms improve the ability to evaluate physiological processes in their entirety, they also offer additional benefits by maximizing data acquired from a single study subject which consequently could reduce the number of animals required for an adequately powered study. Numerous innovative nondestructive or minimally invasive experimental techniques have been developed to measure important parameters such as tissue mechanical properties, oxygen tension, and pH in vivo. Such techniques are often image-based and include ultrasound strain imaging, shear wave elastography, and magnetic resonance imaging (MRI) [22–26]. Nonimage based techniques to measure mechanical strains or properties of structural tissues such as healing bones utilize external fixation hardware which are subjected to estimated physiological loads by a mechanical testing instrument [27]. While still nondestructive, the aforementioned methods are typically limited to snapshot measurements acquired relatively infrequently due to practical limits on the number of occasions animals can be safely anesthetized to perform the measurement. Additionally, imaging methods often rely on expensive equipment and typically must be conducted one specimen at a time. Thus, such techniques are inherently low-throughput and costly for studies utilizing large sample numbers.
Finite element (FE) models and numerical computational fluid dynamics (CFD) simulations based on in vivo imaging or simplified representations of healing tissue structures offer unique insights into the spatial distribution of stress, strain, and oxygen tension within the tissue at the healing site. Additionally, computational models allow for parametric analyses to study the sensitivity of the tissue structure and the relative importance of distinct variables in a tissue-engineered construct (i.e., scaffold modulus, porosity, and initial growth factor concentration). However, the results of such computational models are affected drastically by the initial boundary conditions imposed during the definition of the model. Boundary conditions such as external load magnitudes and oxygen concentration profiles are often based on simplified assumptions lacking experimental validation, which can compromise the accuracy of conclusions obtained by simulations [28,29].
Other approaches to overcome limitations in longitudinal in vivo measurement techniques while maintaining some aspects of in vivo complexity include top–down ex vivo models such as explant cultures, as well as bottom–up organ-on-a-chip in vitro systems [30,31]. These experimental platforms are especially valuable to precisely study cellular and molecular mechanisms of healing while approximating physiological conditions. However, they cannot fully recapitulate in vivo complexities which ultimately determine the clinical efficacy of TE/RM therapies. Such factors include important interactions between integrated tissues and organs, transport challenges imposed by large injuries that must be re-infiltrated with a functional vasculature, and systemic inflammatory responses, which are especially relevant to traumatic injuries and diseases that result in a chronically dysregulated immune system. To engineer such complex tissues, the desirable spatiotemporal biomechanical and biochemical characteristics of the regenerative niche in vivo must be elucidated. While small animal models are advantageous compared to the aforementioned in vitro approaches for regenerative medicine, the pertinence of preclinical animal studies is limited to the degree that the selected organism (rodent, ruminant, mammal, etc.), strain (breeding and genetic background), and injury or disease model recapitulates the human scenario, since these characteristics all factor in to inform the functional design and evaluation criteria for newly developed therapies. Thus, consideration must be given to important model traits such as size, metabolic rate, sex effects, rate of relevant tissue growth and development throughout lifetime, and comorbidities of interest (e.g., obesity and diabetes). Given these important limitations, there remains a distinct need for in vivo technical platforms that permit detailed longitudinal analyses of environmental cues known to regulate the healing response and characterize its progression in robust preclinical models.
Thanks to continued technological advances, novel implantable sensor techniques are a promising approach to facilitate in vivo measurements for numerous injuries and diseases of interest. Besides those mentioned earlier, a number of sensor modalities could provide valuable insight into healing processes including pressure for intraocular and intracranial pathologies, pH and temperature for infections, glucose for diabetes, and even specific relevant biomarkers for inflammation, matrix production, or vascularization. Implanted as well as externally mounted probes and sensors have been implemented in the past, but have typically been limited to large animals due to the size constraints imposed by cheaper and higher-throughput preclinical rodent and mouse models [13,15,32–34]. However, miniaturization coupled with novel passive and active sensor designs have now progressed to the point that sensing platforms can be readily fabricated at feasible size scales for small animal model applications. Once deployed, microfabricated sensors can wirelessly transmit quantitative measurements in real-time, eliminating the need to anesthetize the animal or disrupt normal activity. Thus, the frequency and duration of data acquisition is primarily limited by the power consumption of the sensor and wireless telemetry relative to the capacity of the power source (implanted battery, if active circuity is utilized). The data acquired from implanted sensors quantify the temporal profile of the parameter of interest, but there are limitations to the scale and resolution that spatially heterogeneous environmental factors can be measured due to the size of the probe since a locally positioned sensor or even a sensor array can only provide a discrete number of spatial measurement(s). To better describe spatial variations throughout a tissue, sensor measurements could also be applied as a validated time-varying boundary condition at their respective position(s) for image-based computational models of healing tissues, better capturing the dynamics of the in vivo response. In Secs. 3–5, we discuss technical progress in MEMS and non-MEMS approaches to implantable sensors, the potential application of implantable sensors to benefit TE/RM research, and the future directions of efforts at the intersection of these two important fields.
3. Technical Advancements in Implantable MEMS
MEMS technology grew from complementary metal–oxide–semiconductor (CMOS) transistor fabrication technologies that were developed for the integrated circuit (IC) industry. Traditionally, MEMS encompasses a suite of fabrication technologies that can produce devices possessing characteristic lengths at the micron scale; even if the overall device is larger, key enabling features are often at this scale, much as an IC chip can be large compared to the size of an individual transistor. MEMS typically can have structural and/or transduction functionality. Further, since the roots of MEMS are in the IC industry, MEMS can be colocated with circuits and power sources to create complex electromechanical systems at small scales.
The correspondence of the size scales of MEMS and the size scales of many biological systems make MEMS technology an attractive option for biological implants that can transduce physical parameters to quantitative external read-outs thereby guiding scientific investigation as well as clinical treatment. As this new application area develops, the use of materials outside the traditional IC industry in MEMS fabrication has become more widespread [35–37]. The emerging next generation of implantable MEMS systems encompasses self-powering capability, increased intelligence, and biodegradable devices for biomedicine [38–41].
As discussed above, in the early days of implantable MEMS, material selection and fabrication approaches were substantially derived from the CMOS industry, resulting in uniformity and reproducibility in manufacturing processes [40]. Biomedical devices fabricated from traditional microelectronics materials, such as silicon and ceramics, have been extensively used with devices ranging from bench top testing to animal deployment to approved clinical use [39–42]. The prevalent medical device markets utilizing MEMS technologies are cardiovascular monitoring and pacing [43–45], and neuromodulation electrodes [46–48]. A critical difference between consumer electronics and implantable devices is the environment in which the device must operate; the device must interface with the external physiological environment while maintaining stability and functionality (i.e., without its performance being altered or degraded). Hermetically sealed micromachined silicon and silicon/ceramic packages [40,49] as well as polymer encapsulation [36,40] have been used to achieve these goals. Interconnection techniques such as flip chip bonding, wire-bonding, and conductive epoxy, which have been employed in the semiconductor packaging and assembly industries, are also used for the manufacturing of implantable devices. Communication with the implantable device is typically achieved by inductive coupling. The design strategies, fabrication techniques, and materials implemented in implantable MEMS are the same as those commonly used in traditional CMOS microfabrication technologies [37,40,50].
The traditional CMOS-inspired implantable MEMS, however, feature many limitations for medical applications. Since implantable devices have to interface with the local physiological environment in order to function, and since the body is a dynamic system responding to this foreign device, achieving the requisite sensitivity, specificity, accuracy, and stability is often countered by host inflammatory or fibrous encapsulation response to chronic implants [37,51]. Extensive research has focused on improving implant biocompatibility, through physical and chemical modifications to tissue-device interfaces with the goal of prolonging device lifetime [36–40,48,52–54]. The mechanisms governing device biocompatibility depend not only on many details, such as material processing, device geometry, surface treatment, and material impurities, but also on the physiological location and duration of use. In recent years, technological advances in material science have driven the efforts toward overcoming these challenges. Materials aimed at reducing tissue damage inflicted by the device at the implant site, better emulating the material properties of the host tissue, and improving biocompatibility have been investigated. These include flexible and polymeric materials, hybrid composites, and biological materials, such as proteins, cells, and tissues, that may be considered for implantable applications in regenerative medicine and tissue engineering [53].
Flexible implantable MEMS devices are very attractive for medical applications, as their less rigid nature may reduce local damage and thereby improve the host foreign body response. Flexible devices that can be bent, stretched, or twisted to adapt to the local tissue geometry can minimize irritation and improve conformal contact with the physiological environment [55]. The availability of low-cost manufacturing and rapid prototyping methods with plastic materials has also contributed to the development of flexible devices. The use of flexible polymeric materials, such as parylene and polyimide, for clinical applications continues to increase steadily due to their biocompatibility and ease of processing with traditional microfabrication technologies [36]. In 2001, a polyimide-based multichannel intracortical electrode array was manufactured with standard, planar, photolithographic, and CMOS-compatible techniques. Polyimide served as the mechanically flexible substrate that was manipulated into unique three-dimensional designs. The array was electrically interfaced with an integrated polyimide cable to provide efficient contact points for a high density of channels [56]. Another example is a wireless, passive, radio frequency pressure sensor for long-range continuous intraocular pressure monitoring for glaucoma patients. The sensor featured parylene-C (poly-chloro-p-xylene) as the encapsulant and sensing membrane. The flexible coil substrate can be folded into a smaller form factor to enable minimally invasive implantation (e.g., catheter-based deployment) and, subsequently, can naturally unfold to its original state without damage. Long-term and short-term device testing in a six-month in vivo model and acute ex vivo model, respectively, verified the feasibility and efficacy of the sensor, including robust fixation and long-term biocompatibility in the intraocular environment [57].
Biodegradable devices are sensors and actuators that break down after a targeted functional lifetime into nontoxic components that may either be resorbed or expelled by the body. This distinguishing feature may overcome the complications associated with permanent implants for applications that are transient in nature, such as bone healing. Further, the resorbable nature eliminates the need for secondary surgery to extract the implant. A typical example is the passive wireless pressure sensor demonstrated by Luo et al. [58,59]. The pressure sensor comprises flexible plates bearing inductor windings to form a resonant electrical circuit with the capacitor and to magnetically couple with an external loop. Zinc/iron bilayers were used as the sensor conductor material, and biodegradable polymers poly-L-lactide (PLLA) and polycaprolactone (PCL) were used as dielectric and structural materials. The fabricated sensor demonstrated a linear frequency response with external applied pressure. The functional lifetime of the sensors was approximately 4 days, and can be tailored by the choice of polymer encapsulation and area ratio of the bilayer galvanic couple.
Previously reported biodegradable MEMS sensors (i.e., as described above) are mostly passive, with no need for an internal power source or circuitry, and by necessity limited in functionality [60]. It is therefore useful to consider the incorporation of active elements to achieve a full electrical system. The power consumption of an active device, however, demands the exploration of biodegradable batteries as viable energy sources [61–63]. Tsang et al. [64] presented a magnesium/iron battery featuring PCL as the packaging and functional material. Compared with medical-grade nondegradable lithium-ion batteries at similar size scales, the PCL-coated Mg/Fe batteries showed superior performance of up to six times higher in energy density and 1–2 orders of magnitude reduction in volume. More recently, a biodegradable battery featuring a solid electrolyte of sodium chloride and PCL was demonstrated by the same group [63]. This approach harnesses the body fluid that diffuses into the cell as an element of the electrolyte, and the large excess of ionic material suspended in the PCL holds intracellular conditions constant to achieve a constant discharge profile in the presence of varying external aqueous conditions.
In order to achieve a full electrical system, active devices and packaging for device-level integration must be addressed. Toward these ends, Zhang et al. [65] presented the development of conductive polymer-based biodegradable electrical interconnects comprising Fe microparticles and PCL as the conductor and insulating matrix, respectively. The electrical resistivity and the mechanical and electrochemical properties of the interconnects were investigated during physiological degradation. Tensile and adhesion tests were also performed to confirm the interconnect viability. This work demonstrates fully biodegradable MEMS components which are critical to ultimately achieve a physiologically integrated MEMS system possessing multiple sensing modalities. Further material advances have supported dually biodegradable and flexible electronics. A variety of structures in the form of meshes, webs, and high-aspect-ratio nanopillars have been developed to form an active, functional layer to facilitate reliable and conformal interfacing. A representative example can be found in the work by Kim et al. [66], which presented an ultrathin electronic system featuring bioresorbable silk fibroin as the supporting substrate. Specialized mesh designs and ultrathin forms for the electronics ensured minimal stresses on the tissue and highly conformal coverage, even for complex curvilinear surfaces, as confirmed by experimental and theoretical studies. Future iterations of biodegradable sensors must also consider the specific physiological compartment they will occupy and the tissues they will have direct contact with, as highly vascularized tissues will result in much faster device breakdown compared to less vascular tissues like cartilage, significantly altering the functional lifespan of the sensor.
The work in biomimetic devices encompassing biological materials remains relatively unexplored, and presents many new and exciting possibilities. The direct use of biological materials, such as proteins, cells, and tissues, to attain native mechanical and chemical properties might reduce tissue inflammation and local damage; these technologies would open a broad spectrum of opportunities in regenerative medicine, such as minimally obstructive deep brain implants, artificial organs, hybrid sensing devices, and tools to promote tissue integration. An example presented by Shen et al. [67] is the extracellular matrix-based implantable neural electrodes. Microfabrication strategies were developed for the micropatterning and processing of collagen and implemented to develop extracellular matrix-based intracortical electrodes. The design rendered the implants sufficiently rigid for penetration into the target brain region. The device subsequently softened from hydration after insertion so that the mechanical properties of the electrode better matched that of brain tissue than traditional silicon-based intracortical recording devices and, thereby, reduced inflammation and device-induced mechanical strain in the tissue.
4. Magnetoelastic Materials as Passive Implantable Sensors
As discussed in Sec. 3, passive (battery and circuitry-free) sensors [68–71] are well suited for monitoring in vivo conditions in certain medical implants [72–74] since they do not require an internal power source and are generally more robust and reliable due to their simple design [72,74]. A particularly intriguing example of passive sensors not necessarily fabricated utilizing MEMS techniques are those based on magnetoelastic materials [75], which are a class of magnetic materials that can efficiently convert magnetic to mechanical energies and vice versa. A common type of magnetoelastic sensor, typically made of a strip or wire of magnetoelastic material, undergoes mechanical resonance when energized with an AC magnetic field at its resonant frequency (kHz–MHz). Since the sensor's resonant frequency changes with applied stress, this type of sensor, known as a magnetoelastic resonance sensor [68], is commonly used to measure small mass loading or pressure which may be ideal in preclinical small animal and tissue engineering applications [72]. Due to the dampening of the sensor's resonance at high mechanical loading, a different sensor design based on magnetic induction is commonly employed when the expected applied mass loading is approaching the mass of the sensor. For this design, the sensor is usually adhered or deposited to the substrate, and then exposed to a low frequency magnetic AC field (tens to hundreds of Hz) to become magnetized. The magnetized sensor in turn generates a secondary magnetic field that is sensitive to applied mechanical loads. This type of sensor has been applied to monitor force loading at bone fixation plates [73] or medical sutures [74].
Essentially, magnetoelastic sensors are strain or pressure sensors that can wirelessly gather physical information in real-time, making them ideal for use in musculoskeletal TE/RM where the mechanical environment is a critical regulator of healing outcomes. These sensors are best suited for peri- or postoperative monitoring of orthopedic repairs where implants are already utilized. Due to the simple design of these sensors (typically simple strips or wires [68]), they can be incorporated into the existing orthopedic implants without significantly affecting the implants' functionality. Furthermore, magnetoelastic sensors are not limited to monitoring mechanical deformation. For example, magnetoelastic resonance sensors have been used to investigate and monitor cell adhesion on an implant [76], allowing the study of postoperative orthopedic regeneration behavior such as integration between bone/tissue and the implant. In addition, by incorporating chemically responsive surface materials which alter the resonant frequency of the magnetoelastic construct, a biochemical sensor can be developed to monitor concentrations of certain bioactive molecules (e.g., glucose [68]) at surgical sites.
Magnetoelastic materials can also be deployed as implantable actuators [77,78]. By incorporating a high-strain magnetoelastic material such as Terfenol-D to orthopedic implants, it is possible to produce a mechanically active, externally controlled fixation device for bone fracture repair. Furthermore, it is also found that small mechanical perturbations, such as those generated by the magnetoelastic resonance sensor [77], can affect cell behavior. Thus, the incorporation of a magnetoelastic vibration layer near the outer surface of an orthopedic implant can mechanically stimulate the healing environment by means of a remotely activated external magnetic field.
A challenge for magnetoelastic materials is the lack of understanding of their biocompatibility when integrated into an implantable device. Investigations on passivating the material with coatings such as parylene-C have been promising [79], but a naturally biocompatible magnetoelastic material is desirable. Recent work has shown that the iron-gallium magnetoelastic alloy is noncytotoxic, although its long-term biocompatibility has yet to be validated [75].
Geometry and material components of magnetoelastic sensors and actuators can be tailored to quantify and produce both large and small mechanical perturbations. The passive, electronic-free nature and simple design of magnetoelastic materials offer distinct advantages over complex technologies to monitor certain regenerative environments, with particular promise for orthopedic applications. With continuing development, it is expected that this class of sensor/actuator technology will be instrumental in facilitating real-time monitoring and precision control of the mechanical environment for a variety of TE/RM applications.
5. Implantable Sensors Applications in TE/RM
A large body of research has been demonstrated on various biomedical applications of sensors, but most efforts have focused on clinical monitoring rather than TE/RM or preclinical applications [80,81]. Implantable sensors differ significantly in their designs and fabrication techniques, but the endpoint sensing modalities include biopotential [82], electrical impedance [83], pressure [84,85], flow [86,87], strain [88], oxygen [89], pH [83], and glucose [90,91].
Clinical diagnostics have greatly benefited from implantable sensors, as it enables in situ monitoring of physiological metrics to track the progression of or recovery from a disease. Sensing mechanisms for implantable MEMS sensors include mechanical [84], optical [85], magnetic [92], and electrochemical detection methods [89], as well as combinations thereof, which underscore the appeal of MEMS technology; implantable MEMS can transduce a physiological input into an electrical output, oftentimes requiring only a small sample or stimulus. In spite of the formidable challenges of avoiding adverse tissue response to implants, the goal of in vivo sensing has largely been achieved for a subset of clinical applications. Pressure sensors are a wonderful example of such, as they have been extensively demonstrated for arterial [93–95], intraocular [96], and intracranial [97,98] pressure monitoring. Pressure sensors have further been presented to indirectly detect aneurysms [99] and restenosis [100], as well as to identify optimal settings for pacemakers [45]. Flow and glucose sensors have been investigated for diagnosing cardiovascular diseases [87] and continuous glucose monitoring [101], respectively. Due to continued advancements in clinical sensing, we see an exciting opportunity to leverage and adapt implantable sensors to enhance the preclinical development and evaluation of novel TE/RM therapies for a number of relevant diseases.
Oftentimes, multiple conditions or cues, such as physical, chemical, and biological, are relevant in evaluating how tissue engineered constructs perform in vivo. MEMS offers notable advantages over alternative types of implantable systems for tissue engineering and regenerative medicine due to the spectrum of supported sensing modalities, compactness of size, and amenability to integration [102–104]. For example, the role the local environment in bone healing could be more deeply understood by longitudinal monitoring of strain, oxygen tension, and pH within the defect. This could be enabled by the development of a multimodal sensor system designed for a preclinical animal model. Strain sensors have previously been deployed to evaluate mechanical strains on internal and external fixation instrumentation in humans and sheep, which show promise for clinical monitoring [13–15,105]; however, implementations have primarily been limited to long bones of the leg where there is a substantial hardware footprint for sensor integration. The role of local oxygen tension is of particular interest to fracture healing. In 1972, Brighton and Krebs measured oxygen tension in a rabbit fibular fracture using platinum microelectrodes which were not implanted but inserted at each time point [6]. They noted marked differences and temporal trends of the oxygen tension in the hematoma, woven bone, and intact diaphyseal bone. Epari et al. revisited this approach with new technology in 2008, utilizing a percutaneously mounted, commercial multimodal catheter probing the fracture gap to simultaneously measure pressure, oxygen tension, and temperature in a sheep tibial defect over a 10 day period [106]. While larger canine and ovine models are preferable to assess human-scale orthopedic and spine implants due to improved biomechanical similarity, small animal models possess important relative cost and throughput advantages, which makes them better suited for investigating newer and less-established therapeutic strategies for a wide range of diseases. Miniaturization of sensors and telemetry sufficient for small animal models, which are the primary test-bed for novel tissue engineering therapies, has not been demonstrated. Continued efforts toward preclinical sensors for bone healing and a host of other relevant injury and disease models would substantially inform a more quantitative understanding of the healing environment encountered in vivo, and also help to elucidate mechanistic reasons when different healing outcomes are observed between preclinical models. A rendering of one approach to implement an implantable oxygen tension or strain sensor platform in a rodent femoral bone defect model is illustrated and described in Fig. 2.
Fig. 2.
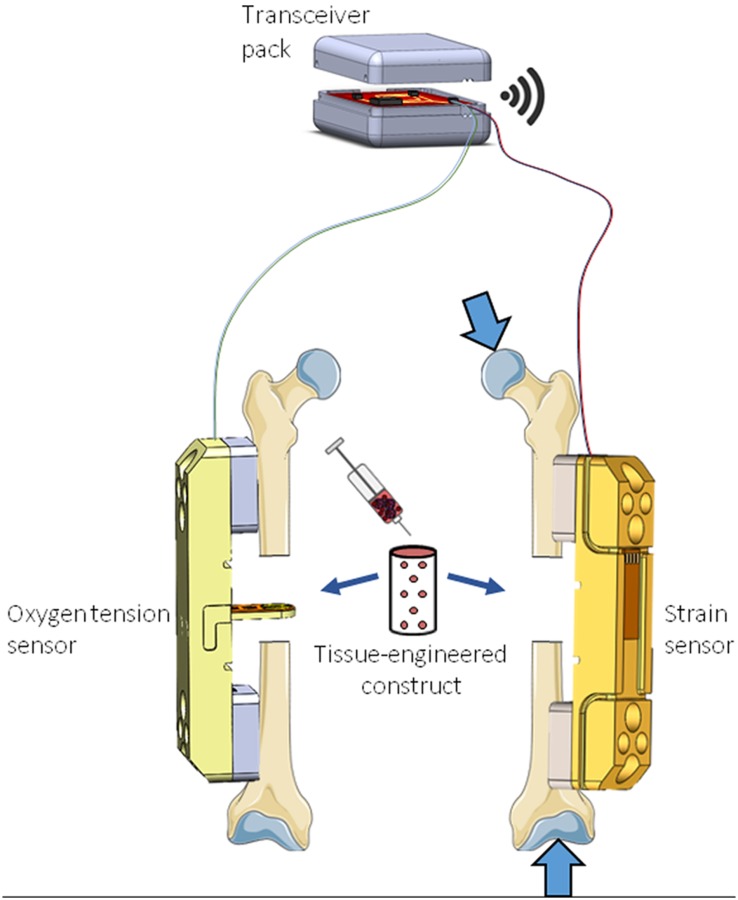
Rendering of one approach which could be implemented to implant sensors in a rodent femoral defect model to measure oxygen tension and/or strain during bone regeneration. Animal injury models utilizing structural implants are particularly advantageous for implantable devices because they provide a stable foundation to anchor the sensor. Depending on the size constraints of the anatomical space under investigation, transceiver and circuitry components could be packaged within a single device or subcutaneous wires could be routed to a remote transceiver pack mounted either intraperitoneally or subcutaneously. Created using images from Servier Medical Art, CC-BY 3.0.
Reactive oxygen species and inflammation (nitric oxide and pH) can also be monitored by electrochemical or optical sensors [107]. All sensors mentioned above can be integrated into one intelligent system for data collection and transmission. The use of multiple detection mechanisms, such as electrochemical and optical, can minimize crosstalk between different types of sensors or validation and calibration for sensors of the same modality. This illustrates that certain attributes of MEMS, in this case their ability to detect and transduce various mechanical and chemical cues mediating bone healing into electrical signals, can be leveraged and applied toward a broad spectrum of tissue engineering and regenerative medicine applications. The microscale nature of MEMS devices can be designed to meet the physical constraints of preclinical animal models, which are oftentimes rodents or other small animals, to enable in situ, real-time sensing of physiological cues continuously within an animal and, thus, overcome the limitations of ex vivo endpoint measurements. Alternatively, the trend toward more biomimetic and physiologically inspired devices supports their integration with TE/RM constructs to enable local evaluation of the therapeutic efficacy of TE/RM constructs in preclinical and clinical settings.
It is critical that sensors disturb the natural healing environment as little as possible to ensure that valid measurements are acquired. To ensure that novel implantable devices do not prompt fibrous encapsulation or actively irritate the tissue to a degree that alters the course of healing, the host response should be rigorously validated by histological evaluations against “sensor-free” controls for increased fibrous tissue growth and for localization of pro-inflammatory immune cells within the tissue of interest at the conclusion of the study. Additionally, sensors should be characterized under controlled ex vivo conditions before and after implantation to ensure their sensitivity or functionality does not drift over time due to interactions with the surrounding tissue. Migration of the sensor within the tissue is another factor that could potentially compromise the validity of the resultant data. Thus, TE/RM applications where a structural implant is used (e.g., orthopedic fixation hardware, vascular stent, and tissue scaffold) are advantageous since they can act as a foundation to anchor the sensor in the healing environment (as in Fig. 2). Including specific attachment features in the device design such as loops to accommodate sutures may be required. Additionally, longitudinal radiography could serve as a valuable tool to track implant migration throughout a study. To mitigate the risk of implant-induced infection, preclinical sensors must also be able to endure sterilization processes. While autoclaving is preferred and compatible with some conventional sensor materials and designs, novel sensors housing delicate chemical species or dissolvable materials may have to rely on more delicate sterilization approaches such as ethylene oxide or gamma irradiation and test their efficacy. In such cases, consultation of GMP or ISO standards may serve as a helpful guide.
In the design of an implantable system that facilitates tissue regeneration, a pivotal factor is the interplay between the targeted tissue and the implant. The integration of implantable sensors with TE/RM technologies can support this endeavor by providing a closed loop system for customizing regenerative therapies. A common example is the incorporation of a sensor into a drug delivery system so that the timing and rate of drug delivery can be tuned by changes in certain local physiological conditions. The integration of sensing components into responsive polymeric systems for controlled drug release has been the subject of extensive research [108]. Reports in the literature include systems triggered by the application of ultrasound [109], changes in pH [110,111], temperature [112], analyte concentrations [113,114], and electric [115] or magnetic [116] fields. The delivered molecules are diverse, including low molecular weight drugs, nucleic acids, peptides, and proteins, for the accelerated regeneration of tissues [52,54].
As the fields of TE/RM and implantable sensors continue to grow, emerging technologies should consider the union of these two areas for smart, multifunctional regenerative therapies and preclinical tools for better understanding and modulating the complex biological world.
6. Conclusions and Future Directions
While significant strides have been made in the field of implantable sensors, key challenges and opportunities remain in developing implantable devices for continuous, in vivo monitoring. Whereas the early incarnations of implantable sensors were technology-driven devices, enabled by traditional CMOS-based materials and microfabrication technologies, recent developments in implantable MEMS highlight a trend toward more application-driven device design. Figure 3 illustrates this progression in implantable sensors, where direct offshoots of traditional CMOS technology included silicon microelectrode arrays for neuromodulation and ceramic-based pressure sensors for cardiac monitoring that are commercially available. In fact, most sensing modalities have corresponding commercial or near-commercial devices featuring traditional CMOS materials and processing. The implementation of sensors in TE/RM applications will significantly benefit from the advances in implantable sensors research. These can be grossly categorized into a bottom–up versus a top–down change in the sensor design. Specifically, recent developments in flexible, biodegradable, and biomimetic sensors were mainly designed from a bottom–up, materials-level approach. Whether the goal was to minimize the deleterious response to the device, to overcome the negative effects of permanent implants or to better emulate the physiological tissue, the underlying motivation in these cases was to develop more physiologically compatible devices. The critical advancements to achieve this goal were primarily the introduction of new materials, as well as the development of corresponding fabrication technologies for the micropatterning and integration of these materials with standard MEMS processing. This application-driven approach has been a stark departure from early iterations of implantable sensors, which were largely technology driven. Further support of this application-driven endeavor is the emphasis on multisensing or multifunctional devices to detect multiple cues and/or dually sense and actuate, thereby providing both monitoring and treatment. These efforts toward smart therapies and preclinical tools can be classified as top–down, systems integration approaches.
Fig. 3.
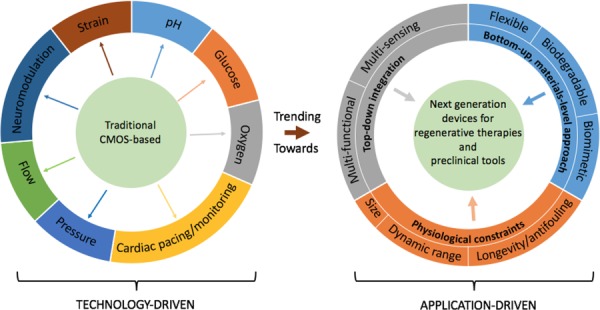
Advancements in implantable sensors. Early iterations of implantable sensors featured materials and design approaches that were direct outgrowth from traditional CMOS processing, as denoted by the outward-oriented arrows. However, research developments at the materials-, device- and systems-level have paved the road toward more application-driven, physiologically motivated designs. The integration of these approaches, along with addressing physiological constraints and representative testing, will be necessary for the development of next-generation, implantable sensors for smart regenerative therapies and preclinical tools.
Lastly, the road to next-generation regenerative therapies and preclinical tools must tailor device design toward physiological constraints. This final thrust would ensure that the device addresses the real, physiological challenges and constraints of the design space. These include physical size and footprint, dynamic range, biostability, and pertinent testing. While most implantable strain sensors were designed to satisfy the physical constraints of a human body, sensors used for the preclinical models face more stringent size constraints because preclinical animal models are often conducted in rodents or small animals. Second, the dynamic range of a sensing parameter may either be very large or unknown. To the sensor engineer, a working range is necessary for device design. However, this information may be unknown and, hence, motivate the need for the device. For example, the mechanical stiffness of bone may change across orders of magnitude throughout the time course of bone regeneration and remodeling. For a segmental bone defect model, where the fracture gap in a rodent femur is 5–8 mm, the transition from an empty gap to a mineralized bone would span a large range in mechanical stiffness. Consequently, a strain sensor monitoring the deformation across a fixation plate must vary strains across orders of magnitude due to the extent of load sharing between the bone defect and the plate. Together with the desired strain resolution when operating at the lower spectrum of the dynamic range, these constraints point toward the challenges of design for preclinical in vivo models. While preclinical applications of implantable sensors have been the primary focus of this article, it is critical to acknowledge that investigators may ultimately seek to translate their devices to the clinic. For such cases, consultation of United States Food and Drug Administration regulatory pathways should be considered from the outset of device development. Further, whether for clinical or preclinical applications, the next-generation sensors for TE/RM must directly address biostability and functionality by moving beyond benchtop to in vivo testing as the endpoint for device characterization. A majority of the established literature in implantable sensors does not pursue in vivo device characterization even though the variability associated with a full animal system and host inflammatory response are just as important as the device specifications for designing an implantable system.
The development of smart, regenerative therapies and better investigatory tools for understanding tissue regeneration presents an exciting future for implantable sensors. The recognition of a need for more physiologically motivated technology, whether pursued from a bottom–up materials or top–down systems approach, has been a major step toward these goals. However, to realize these technologies for preclinical and clinical uses, more integrative and collaborative efforts must be established between biology and engineering in order to design not only for a specific sensing modality, but also to design specifically for an in vivo system.
Acknowledgment
This work was supported by a research partnership between Children's Healthcare of Atlanta and the Georgia Institute of Technology and grants from the National Institutes of Health (NIH R21 AR066322; NIH R01 AR069297) and the National Science Foundation (NSF CMMI—1400065). BSK was supported by the Cell and Tissue Engineering NIH Biotechnology Training Grant (T32-GM008433).
Contributor Information
Brett S. Klosterhoff, George W. Woodruff School , of Mechanical Engineering, , Georgia Institute of Technology, , Atlanta, GA 30332; Parker H. Petit Institute for , Bioengineering and Bioscience, , Georgia Institute of Technology, , Atlanta, GA 30332
Melissa Tsang, School of Electrical and Computer Engineering, , Georgia Institute of Technology, , Atlanta, GA 30332.
Didi She, Department of Electrical and , Systems Engineering, , University of Pennsylvania, , Philadelphia, PA 19104.
Keat Ghee Ong, Department of Biomedical Engineering, , Michigan Technological University, , Houghton, MI 49931.
Mark G. Allen, School of Electrical and Computer Engineering, , Georgia Institute of Technology, , Atlanta, GA 30332; Department of Electrical and , Systems Engineering, , University of Pennsylvania, , Philadelphia, PA 19104
Nick J. Willett, Parker H. Petit Institute for , Bioengineering and Bioscience, , Georgia Institute of Technology, , Atlanta, GA 30332; Department of Orthopaedics, , Emory University, , Atlanta, GA 30303; Atlanta Veteran's Affairs Medical Center, , Decatur, GA 30033; Wallace H. Coulter Department , of Biomedical Engineering, , Georgia Institute of Technology , and Emory University, , Atlanta, GA 30332
Robert E. Guldberg, George W. Woodruff School , of Mechanical Engineering, , Georgia Institute of Technology, , Atlanta, GA 30332; Parker H. Petit Institute for , Bioengineering and Bioscience, , Georgia Institute of Technology, , Atlanta, GA 30332
References
- [1]. Nerem, R. M. , 2006, “ Tissue Engineering: The Hope, the Hype, and the Future,” Tissue Eng., 12(5), pp. 1143–1150. 10.1089/ten.2006.12.1143 [DOI] [PubMed] [Google Scholar]
- [2]. Carrel, A. , and Lindbergh, C. , 1938, “ The Culture of Organs,” Can. Med. Assoc. J., 39(4), p. 416. [Google Scholar]
- [3]. Lysaght, M. J. , Jaklenec, A. , and Deweerd, E. , 2008, “ Great Expectations: Private Sector Activity in Tissue Engineering, Regenerative Medicine, and Stem Cell Therapeutics,” Tissue Eng. Part A, 14(2), pp. 305–315. 10.1089/tea.2007.0267 [DOI] [PubMed] [Google Scholar]
- [4]. Guldberg, R. E. , 2009, “ Spatiotemporal Delivery Strategies for Promoting Musculoskeletal Tissue Regeneration,” J. Bone Miner. Res., 24(9), pp. 1507–1511. 10.1359/jbmr.090801 [DOI] [PubMed] [Google Scholar]
- [5]. Wray, J. B. , 1970, “ The Biochemical Characteristics of the Fracture Hematoma in Man,” Surg., Gynecol. Obstet., 130(5), pp. 847–852. [PubMed] [Google Scholar]
- [6]. Brighton, C. T. , and Krebs, A. G. , 1972, “ Oxygen Tension of Healing Fractures in the Rabbit,” J. Bone Jt. Surg. Am., 54(2), pp. 323–332. 10.2106/00004623-197254020-00010 [DOI] [PubMed] [Google Scholar]
- [7]. Brighton, C. T. , and Krebs, A. G. , 1972, “ Oxygen Tension of Nonunion of Fractured Femurs in the Rabbit,” Surg. Gynecol. Obstet., 135(3), pp. 379–385. [PubMed] [Google Scholar]
- [8]. Kolar, P. , Gaber, T. , Perka, C. , Duda, G. N. , and Buttgereit, F. , 2011, “ Human Early Fracture Hematoma is Characterized by Inflammation and Hypoxia,” Clin. Orthop. Relat. Res., 469(11), pp. 3118–3126. 10.1007/s11999-011-1865-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9]. Yuasa, M. , Mignemi, N. A. , Nyman, J. S. , Duvall, C. L. , Schwartz, H. S. , Okawa, A. , Yoshii, T. , Bhattacharjee, G. , Zhao, C. , Bible, J. E. , Obremskey, W. T. , Flick, M. J. , Degen, J. L. , Barnett, J. V. , Cates, J. M. M. , and Schoenecker, J. G. , 2015, “ Fibrinolysis is Essential for Fracture Repair and Prevention of Heterotopic Ossification,” J. Clin. Invest., 125(8), pp. 3117–3131. 10.1172/JCI80313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10]. Boerckel, J. D. , Uhrig, B. A. , Willett, N. J. , Huebsch, N. , and Guldberg, R. E. , 2011, “ Mechanical Regulation of Vascular Growth and Tissue Regeneration In Vivo,” Proc. Natl. Acad. Sci., 108(37), pp. E674–E680. 10.1073/pnas.1107019108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11]. Boerckel, J. D. , Kolambkar, Y. M. , Stevens, H. Y. , Lin, A. S. P. , Dupont, K. M. , and Guldberg, R. E. , 2012, “ Effects of In Vivo Mechanical Loading on Large Bone Defect Regeneration,” J. Orthop. Res., 30(7), pp. 1067–1075. 10.1002/jor.22042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12]. Lienau, J. , Schmidt-Bleek, K. , Peters, A. , Haschke, F. , Duda, G. N. , Perka, C. , Bail, H. J. , Schütze, N. , Jakob, F. , and Schell, H. , 2009, “ Differential Regulation of Blood Vessel Formation Between Standard and Delayed Bone Healing,” J. Orthop. Res., 27(9), pp. 1133–1140. 10.1002/jor.20870 [DOI] [PubMed] [Google Scholar]
- [13]. Claes, L. E. , and Cunningham, J. L. , 2009, “ Monitoring the Mechanical Properties of Healing Bone,” Clin. Orthop. Relat. Res., 467(8), pp. 1964–1971. 10.1007/s11999-009-0752-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14]. Seide, K. , Aljudaibi, M. , Weinrich, N. , Kowald, B. , Jürgens, C. , Müller, J. , and Faschingbauer, M. , 2012, “ Telemetric Assessment of Bone Healing With an Instrumented Internal Fixator: A Preliminary Study,” J. Bone Jt. Surg. Br., 94(3), pp. 398–404. 10.1302/0301-620X.94B3.27550 [DOI] [PubMed] [Google Scholar]
- [15]. McGilvray, K. C. , Unal, E. , Troyer, K. L. , Santoni, B. G. , Palmer, R. H. , Easley, J. T. , Demir, H. V. , and Puttlitz, C. M. , 2015, “ Implantable Microelectromechanical Sensors for Diagnostic Monitoring and Post-Surgical Prediction of Bone Fracture Healing,” J. Orthop. Res., 33(10), pp. 1439–1446. 10.1002/jor.22918 [DOI] [PubMed] [Google Scholar]
- [16]. Claes, L. , Recknagel, S. , and Ignatius, A. , 2012, “ Fracture Healing Under Healthy and Inflammatory Conditions,” Nat. Rev. Rheumatol., 8(3), pp. 133–143. 10.1038/nrrheum.2012.1 [DOI] [PubMed] [Google Scholar]
- [17]. Korn, C. , and Augustin, H. G. , 2015, “ Mechanisms of Vessel Pruning and Regression,” Dev. Cell, 34(1), pp. 5–17. 10.1016/j.devcel.2015.06.004 [DOI] [PubMed] [Google Scholar]
- [18]. Tzioupis, C. , and Giannoudis, P. V. , 2007, “ Prevalence of Long-Bone Non-Unions,” Injury, 38(Suppl. 2), pp. S3–S9. 10.1016/S0020-1383(07)80003-9 [DOI] [PubMed] [Google Scholar]
- [19]. Hak, D. J. , Fitzpatrick, D. , Bishop, J. A. , Marsh, J. L. , Tilp, S. , Schnettler, R. , Simpson, H. , and Alt, V. , 2014, “ Delayed Union and Nonunions: Epidemiology, Clinical Issues, and Financial Aspects,” Injury, 45(Suppl. 2), pp. S3–S7. 10.1016/j.injury.2014.04.002 [DOI] [PubMed] [Google Scholar]
- [20]. Tang, D. , Tare, R. S. , Yang, L.-Y. , Williams, D. F. , Ou, K.-L. , and Oreffo, R. O. C. , 2016, “ Biofabrication of Bone Tissue: Approaches, Challenges and Translation for Bone Regeneration,” Biomaterials, 83, pp. 363–382. 10.1016/j.biomaterials.2016.01.024 [DOI] [PubMed] [Google Scholar]
- [21]. Amini, A. R. , Laurencin, C. T. , and Nukavarapu, S. P. , 2012, “ Bone Tissue Engineering: Recent Advances and Challenges,” Crit. Rev. Biomed. Eng., 40(5), pp. 363–408. 10.1615/CritRevBiomedEng.v40.i5.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22]. Sebag, F. , Vaillant-Lombard, J. , Berbis, J. , Griset, V. , Henry, J. F. , Petit, P. , and Oliver, C. , 2010, “ Shear Wave Elastography: A New Ultrasound Imaging Mode for the Differential Diagnosis of Benign and Malignant Thyroid Nodules,” J. Clin. Endocrinol. Metab., 95(12), pp. 5281–5288. 10.1210/jc.2010-0766 [DOI] [PubMed] [Google Scholar]
- [23]. Weidemann, F. , Eyskens, B. , Jamal, F. , Mertens, L. , Kowalski, M. , D'Hooge, J. , Bijnens, B. , Gewillig, M. , Rademakers, F. , Hatle, L. , and Sutherland, G. R. , 2002, “ Quantification of Regional Left and Right Ventricular Radial and Longitudinal Function in Healthy Children Using Ultrasound-Based Strain Rate and Strain Imaging,” J. Am. Soc. Echocardiography, 15(1), pp. 20–28. 10.1067/mje.2002.116532 [DOI] [PubMed] [Google Scholar]
- [24]. Mason, R. P. , Antich, P. P. , Babcock, E. E. , Constantinescu, A. , Peschke, P. , and Hahn, E. W. , 1994, “ Non-Invasive Determination of Tumor Oxygen Tension and Local Variation With Growth,” Int. J. Radiat. Oncol., 29(1), pp. 95–103. 10.1016/0360-3016(94)90231-3 [DOI] [PubMed] [Google Scholar]
- [25]. Zhang, X. , Lin, Y. , and Gillies, R. J. , 2010, “ Tumor pH and Its Measurement,” J. Nucl. Med., 51(8), pp. 1167–1170. 10.2967/jnumed.109.068981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26]. Gallagher, F. A. , Kettunen, M. I , Day , S. E., Hu , D.-E., Ardenkjaer-Larsen , J. H., Zandt , in't R., Jensen, P. R. , Karlsson, M. , Golman, K. , Lerche, M. H. , and Brindle, K. M. , 2008, “ Magnetic Resonance Imaging of pH In Vivo Using Hyperpolarized 13C-Labelled Bicarbonate,” Nature, 453(7197), pp. 940–943. 10.1038/nature07017 [DOI] [PubMed] [Google Scholar]
- [27]. Wulsten, D. , Glatt, V. , Ellinghaus, A. , Schmidt-Bleek, K. , Petersen, A. , Schell, H. , Lienau, J. , Sebald, W. , Plöger, F. , Seemann, P. , and Duda, G. N. , 2011, “ Time Kinetics of Bone Defect Healing in Response to BMP-2 and GDF-5 Characterised by in vivo Biomechanics,” Eur. Cell. Mater., 21, pp. 177–192. 10.22203/eCM.v021a14 [DOI] [PubMed] [Google Scholar]
- [28]. Anderson, A. E. , Ellis, B. J. , and Weiss, J. A. , 2007, “ Verification, Validation and Sensitivity Studies in Computational Biomechanics,” Comput. Methods Biomech. Biomed. Eng., 10(3), pp. 171–184. 10.1080/10255840601160484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29]. Henninger, H. B. , Reese, S. P. , Anderson, A. E. , and Weiss, J. A. , 2010, “ Validation of Computational Models in Biomechanics,” Proc. Inst. Mech. Eng., Part H, 224(7), pp. 801–812. 10.1243/09544119JEIM649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30]. Brown, G. N. , Sattler, R. L. , and Guo, X. E. , 2016, “ Experimental Studies of Bone Mechanoadaptation: Bridging In Vitro and In Vivo Studies With Multiscale Systems,” Interface Focus, 6(1), p. 20150071. 10.1098/rsfs.2015.0071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31]. Bhatia, S. N. , and Ingber, D. E. , 2014, “ Microfluidic Organs-On-Chips,” Nat. Biotechnol., 32(8), pp. 760–772. 10.1038/nbt.2989 [DOI] [PubMed] [Google Scholar]
- [32]. Claes, L. E. , Claes, L. E. , Heigele, C. A. , Heigele, C. A. , Neidlinger-Wilke, C. , Neidlinger-Wilke, C. , Kaspar, D. , Kaspar, D. , Seidl, W. , Seidl, W. , Margevicius, K. J. , Margevicius, K. J. , Augat, P. , and Augat, P. , 1998, “ Effects of Mechanical Factors on the Fracture Healing Process,” Clin. Orthop. Relat. Res., Oct(355Suppl.), pp. S132–S147. 10.1097/00003086-199810001-00015 [DOI] [PubMed] [Google Scholar]
- [33]. Epari, D. R. , Lienau, J. , Schell, H. , Witt, F. , and Duda, G. N. , 2008, “ Pressure, Oxygen Tension and Temperature in the Periosteal Callus During Bone Healing—An In Vivo Study in Sheep,” Bone, 43(4), pp. 734–739. 10.1016/j.bone.2008.06.007 [DOI] [PubMed] [Google Scholar]
- [34]. Szivek, J. A. , Ruth, J. T. , Heden, G. J. , Martinez, M. A. , Diggins, N. H. , and Wenger, K. H. , 2016, “ Determination of Joint Loads Using New Sensate Scaffolds for Regenerating Large Cartilage Defects in the Knee,” J. Biomed. Mater. Res., Part B, epub. 10.1002/jbm.b.33677 [DOI] [PubMed] [Google Scholar]
- [35]. Rebello, K. J. , 2004, “ Applications of MEMS in Surgery,” Proc. IEEE, 92(1), pp. 43–55. 10.1109/JPROC.2003.820536 [DOI] [Google Scholar]
- [36]. Pang, C. , Lee, C. , and Suh, K. Y. , 2013, “ Recent Advances in Flexible Sensors for Wearable and Implantable Devices,” J. Appl. Polym. Sci., 130(3), pp. 1429–1441. 10.1002/app.39461 [DOI] [Google Scholar]
- [37]. Bashir, R. , 2004, “ BioMEMS: State-of-the-Art in Detection, Opportunities and Prospects,” Adv. Drug Delivery Rev., 56(11), pp. 1565–1586. 10.1016/j.addr.2004.03.002 [DOI] [PubMed] [Google Scholar]
- [38]. Du, H. , and Bogue, R. , 2007, “ MEMS Sensors: Past, Present and Future,” Sens. Rev., 27(1), pp. 7–13.http://www.dsif.fee.unicamp.br/~fabiano/IE012/Material%20complementar/silicon%20sensors%20past%20present%20and%20future.pdf [Google Scholar]
- [39]. Grayson, A. C. R. , Shawgo, R. S. , Johnson, A. M. , Flynn, N. T. , Li, Y. , Cima, M. J. , and Langer, R. , 2004, “ A BioMEMS Review: MEMS Technology for Physiologically Integrated Devices,” Proc. IEEE, 92(1), pp. 6–21. 10.1109/JPROC.2003.820534 [DOI] [Google Scholar]
- [40]. Receveur, R. A. M. , Lindemans, F. W. , and De Rooij, N. F. , 2007, “ Microsystem Technologies for Implantable Applications,” J. Micromech. Microeng., 17(5), pp. R50–R80. 10.1088/0960-1317/17/5/R02 [DOI] [Google Scholar]
- [41]. Wise, K. D. , 2007, “ Integrated Sensors, MEMS, and Microsystems: Reflections on a Fantastic Voyage,” Sens. Actuators, A, 136(1), pp. 39–50. 10.1016/j.sna.2007.02.013 [DOI] [Google Scholar]
- [42]. Allen, M. G. , 2014, “ Microfabricated Implantable Wireless Microsystems: Permanent and Biodegradable Implementations,” IEEE International Conference Micro Electro Mechanical Systems, San Francisco, CA, Jan. 26–30, pp. 1–4. 10.1109/MEMSYS.2014.6765558 [DOI] [Google Scholar]
- [43]. Langenfeld, H. , Krein, A. , Kirstein, M. , and Binner, L. , 1998, “ Peak Endocardial Acceleration-Based Clinical Testing of the ‘BEST’ DDDR Pacemaker. European PEA Clinical Investigation Group,” Pacing Clin. Electrophysiol., 21(11 Pt 2), pp. 2187–2191. 10.1111/j.1540-8159.1998.tb01150.x [DOI] [PubMed] [Google Scholar]
- [44]. Dimarco, J. P. , and Mower, M. , 2003, “ Implantable Cardioverter–Defibrillators,” New Engl. J. Med., 349, pp. 1836–1847. 10.1056/NEJMra035432 [DOI] [PubMed] [Google Scholar]
- [45]. Magalski, A. , Adamson, P. , Gadler, F. , Böehm, M. , Steinhaus, D. , Reynolds, D. , Vlach, K. , Linde, C. , Cremers, B. , Sparks, B. , and Bennett, T. , 2002, “ Continuous Ambulatory Right Heart Pressure Measurements With an Implantable Hemodynamic Monitor: A Multicenter, 12-Month Follow-Up Study of Patients With Chronic Heart Failure,” J. Card. Failure, 8(2), pp. 63–70. 10.1054/jcaf.2002.32373 [DOI] [PubMed] [Google Scholar]
- [46]. Kipke, D. R. , Vetter, R. J. , Williams, J. C. , and Hetke, J. F. , 2003, “ Silicon-Substrate Intracortical Microelectrode Arrays for Long-Term Recording of Neuronal Spike Activity in Cerebral Cortex,” IEEE Trans. Neural Syst. Rehabil. Eng., 11(2), pp. 151–155. 10.1109/TNSRE.2003.814443 [DOI] [PubMed] [Google Scholar]
- [47]. Schmidt, E. M. , Bak, M. J. , Hambrecht, F. T. , Kufta, C. V. , O'Rourke, D. K. , and Vallabhanath, P. , 1996, “ Feasibility of a Visual Prosthesis for the Blind Based on Intracortical Microstimulation of the Visual Cortex,” Brain, 119(5), pp. 507–522. 10.1093/brain/119.2.507 [DOI] [PubMed] [Google Scholar]
- [48]. Zeng, F. G. , Rebscher, S. , Harrison, W. , Sun, X. , and Feng, H. , 2008, “ Cochlear Implants: System Design, Integration, and Evaluation,” IEEE Rev. Biomed. Eng., 1, pp. 115–142. 10.1109/RBME.2008.2008250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49]. Ziaie, B. , Von Arx, J. A. , Dokmeci, M. R. , and Najafi, K. , 1996, “ A Hermetic Glass-Silicon Micropackage With High-Density On-Chip Feedthroughs for Sensors and Actuators,” J. Microelectromech. Syst., 5(3), pp. 166–179. 10.1109/84.536623 [DOI] [Google Scholar]
- [50]. Najafi, K. , 2007, “ Packaging of Implantable Microsystems,” Sixth IEEE Sensors Conference, Atlanta, Oct. 28–30, pp. 58–63. 10.1109/ICSENS.2007.4388335 [DOI] [Google Scholar]
- [51]. Gilleo, Ken, ET-Trends., L. L. C. , and Warwick, R. I. , 2005, “ MEMS in Medicine,” Circuits Assembly, 16(8), pp. 1–10.http://www.allflexinc.com/PDF/Medical%20Electronics-MEMS.pdf [Google Scholar]
- [52]. Steichen, S. D. , Caldorera-Moore, M. , and Peppas, N. A. , 2013, “ A Review of Current Nanoparticle and Targeting Moieties for the Delivery of Cancer Therapeutics,” Off. J. Eur. Fed. Pharm. Sci., 48(3), pp. 416–427. 10.1016/j.ejps.2012.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53]. Jivani, R. R. , Lakhtaria, G. J. , Patadiya, D. D. , Patel, L. D. , Jivani, N. P. , and Jhala, B. P. , 2014, “ Biomedical Microelectromechanical Systems (BioMEMS): Revolution in Drug Delivery and Analytical Techniques,” Saudi Pharm. J., 24(1), pp. 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [54]. Tng, D. J. H. , Hu, R. , Song, P. , Roy, I. , and Yong, K. T. , 2012, “ Approaches and Challenges of Engineering Implantable Microelectromechanical Systems (MEMS) Drug Delivery Systems for In Vitro and In Vivo Applications,” Micromachines, 3(4), pp. 615–631. 10.3390/mi3040615 [DOI] [Google Scholar]
- [55]. Viventi, J. , Kim, D.-H. , Vigeland, L. , Frechette, E. S. , Blanco, J. A. , Kim, Y.-S. , Avrin, A. E. , Tiruvadi, V . R. , Hwang, S.-W. , Vanleer, A. C. , Wulsin, D. F. , Davis, K. , Gelber, C. E. , Palmer, L. , Van der Spiegel, J. , Wu, J. , Xiao, J. , Huang, Y. , Contreras, D. , Rogers, J. A. , and Litt, B. , 2011, “ Flexible, Foldable, Actively Multiplexed, High-Density Electrode Array for Mapping Brain Activity In Vivo,” Nat. Neurosci., 14(12), pp. 1599–1605. 10.1038/nn.2973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56]. Rousche, P. J. , Pellinen, D. S. , Pivin, D. P. , Williams, J. C. , Vetter, R. J. , and Kipke, D. R. , 2001, “ Flexible Polyimide-Based Intracortical Electrode Arrays With Bioactive Capability,” IEEE Trans. Biomed. Eng., 48(3), pp. 361–370. 10.1109/10.914800 [DOI] [PubMed] [Google Scholar]
- [57]. Chen, P. J. , Saati, S. , Varma, R. , Humayun, M. S. , and Tai, Y. C. , 2010, “ Wireless Intraocular Pressure Sensing Using Microfabricated Minimally Invasive Flexible-Coiled LC Sensor Implant,” J. Microelectromech. Syst., 19(4), pp. 721–734. 10.1109/JMEMS.2010.2049825 [DOI] [Google Scholar]
- [58]. Luo, M. , Song, C. J. , Herrault, F. , and Allen, M. G. , 2014, “ A Microfabricated RF Wireless Pressure Sensor Made Completely of Biodegradable Materials,” Journal of Microelectromechanical Systems, 23(1), pp. 4–13. 10.1109/JMEMS.2013.2290111 [DOI] [Google Scholar]
- [59]. Luo, M. , Martinez, A. W. , Song, C. , Herrault, F. , and Allen, M. G. , 2014, “ A Microfabricated Wireless RF Pressure Sensor Made Completely of Biodegradable Materials,” J. Microelectromech. Syst., 23(1), pp. 4–13. 10.1109/JMEMS.2013.2290111 [DOI] [Google Scholar]
- [60]. Boutry, C. M. , Chandrahalim, H. , Streit, P. , Schinhammer, M. , Hänzi, A. C. , and Hierold, C. , 2013, “ Characterization of Miniaturized RLC Resonators Made of Biodegradable Materials for Wireless Implant Applications,” Sens. Actuators, A, 189, pp. 344–355. 10.1016/j.sna.2012.08.039 [DOI] [Google Scholar]
- [61]. Heller, A. , 2006, “ Potentially Implantable Miniature Batteries,” Anal. Bioanal. Chem., 385(3), pp. 469–473. 10.1007/s00216-006-0326-4 [DOI] [PubMed] [Google Scholar]
- [62]. Yin, L. , Huang, X. , Xu, H. , Zhang, Y. , Lam, J. , Cheng, J. , and Rogers, J. A. , 2014, “ Materials, Designs, and Operational Characteristics for Fully Biodegradable Primary Batteries,” Adv. Mater., 26(23), pp. 3879–3884. 10.1002/adma.201306304 [DOI] [PubMed] [Google Scholar]
- [63]. She, D. , Tsang, M. , Kim, J. K. , and Allen, M. G. , 2015, “ Immobilized Electrolyte Biodegradable Batteries for Implantable MEMS,” 18th International Conference on Solid-State Sensors, Actuators and Microsystems (TRANSDUCERS), Anchorage, Alaska, June 21–25, pp. 494–497. 10.1109/TRANSDUCERS.2015.7180968 [DOI] [Google Scholar]
- [64]. Tsang, M. , Armutlulu, A. , Martinez, A. W. , Allen, S. A. B. , and Allen, M. G. , 2015, “ Biodegradable Magnesium/Iron Batteries With Polycaprolactone Encapsulation: A Microfabricated Power Source for Transient Implantable Devices,” Microsyst. Nanoeng., 1, p. 15024. 10.1038/micronano.2015.24 [DOI] [Google Scholar]
- [65]. Zhang, T. , Tsang, M. , and Allen, M. G. , 2016, “ Biodegradable Electrical Interconnects for Transient Implantable Systems,” Solid-State Sensor, Actuator, Microsystems Work, Philadelphia, PA, Oct. 24–27.
- [66]. Kim, D.-H. , Viventi, J. , Amsden, J. J. , Xiao, J. , Vigeland, L. , Kim, Y.-S. , Blanco, J. A. , Panilaitis, B. , Frechette, E. S. , Contreras, D. , Kaplan, D. L. , Omenetto, F. G. , Huang, Y. , Hwang, K.-C. , Zakin, M. R. , Litt, B. , and Rogers, J. A. , 2010, “ Dissolvable Films of Silk Fibroin for Ultrathin Conformal Bio-Integrated Electronics,” Nat. Mater., 9(6), pp. 511–517. 10.1038/nmat2745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67]. Shen, W. , Karumbaiah, L. , Liu, X. , Saxena, T. , Chen, S. , Patkar, R. , Bellamkonda, R. V. , and Allen, M. G. , 2015, “ Extracellular Matrix-Based Intracortical Microelectrodes: Toward a Microfabricated Neural Interface Based on Natural Materials,” Microsyst. Nanoeng., 1, p. 15010. 10.1038/micronano.2015.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68]. Grimes, C. A. , Roy, S. C. , Rani, S. , and Cai, Q. , 2011, “ Theory, Instrumentation and Applications of Magnetoelastic Resonance Sensors: A Review,” Sensors (Basel), 11(3), pp. 2809–2844. 10.3390/s110302809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69]. Pereles, B. D. , Dienhart, T. , Sansom, T. , Johnston, K. , and Ong, K. G. , 2012, “ A Wireless, Passive Load Cell Based on Magnetoelastic Resonance,” Smart Mater. Struct., 21(7), p. 075018. 10.1088/0964-1726/21/7/075018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70]. Pereles, B. D. , DeRouin, A. J. , and Ong, K. G. , 2015, “ Partially Loaded Magnetoelastic Sensors With Customizable Sensitivities for Large Force Measurements,” IEEE Sens. J., 15(1), pp. 591–597. 10.1109/JSEN.2014.2348500 [DOI] [Google Scholar]
- [71]. Nakamura, T. , Inoue, Y. , Kim, D. , Matsuhisa, N. , Yokota, T. , Sekitani, T. , Someya, T. , and Sekino, M. , 2014, “ Basic Characteristics of Implantable Flexible Pressure Sensor for Wireless Readout Using MRI,” 36th Annual International Conference of the Engineering in Medicine and Biology Society, IEEE, pp. 2338–2341. 10.1109/EMBC.2014.6944089 [DOI] [PubMed] [Google Scholar]
- [72]. Green, S. R. , Kwon, R. S. , Elta, G. H. , and Gianchandani, Y. B. , 2013, “ in vivo and In Situ Evaluation of a Wireless Magnetoelastic Sensor Array for Plastic Biliary Stent Monitoring,” Biomed. Microdevices, 15(3), pp. 509–517. 10.1007/s10544-013-9750-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73]. Oess, N. P. , Weisse, B. , and Nelson, B. J. , 2009, “ Magnetoelastic Strain Sensor for Optimized Assessment of Bone Fracture Fixation,” IEEE Sens. J., 9(8), pp. 961–968. 10.1109/JSEN.2009.2025575 [DOI] [Google Scholar]
- [74]. DeRouin, A. , Pacella, N. , Zhao, C. , An, K.-N. , and Ong, K. , 2015, “ A Wireless Sensor for Real-Time Monitoring of Tensile Force on Sutured Wound Sites,” IEEE Trans. Biomed. Eng., 63(8), pp. 1665–1671. 10.1109/TBME.2015.2470248 [DOI] [PubMed] [Google Scholar]
- [75]. Holmes, H. R. , DeRouin, A. , Wright, S. , Riedemann, T. M. , Lograsso, T. A. , Rajachar, R. M. , and Ong, K. G. , 2014, “ Biodegradation and Biocompatibility of Mechanically Active Magnetoelastic Materials,” Smart Mater. Struct., 23(9), p. 095036. 10.1088/0964-1726/23/9/095036 [DOI] [Google Scholar]
- [76]. Vlaisavljevich, E. , Holmes, H. R. , Tan, E. L. , Qian, Z. , Trierweiler, S. , Ong, K. G. , and Rajachar, R. M. , 2013, “ Magnetoelastic Vibrational Biomaterials for Real-Time Monitoring and Modulation of the Host Response,” J. Mater. Sci. Mater. Med., 24(4), pp. 1093–1104. 10.1007/s10856-013-4854-0 [DOI] [PubMed] [Google Scholar]
- [77]. Vlaisavljevich, E. , Janka, L. P. , Ong, K. G. , and Rajachar, R. M. , 2011, “ Magnetoelastic Materials as Novel Bioactive Coatings for the Control of Cell Adhesion,” IEEE Trans. Biomed. Eng., 58(3), pp. 698–704. 10.1109/TBME.2010.2093131 [DOI] [PubMed] [Google Scholar]
- [78]. Pepakayala, V. , Stein, J. , and Gianchandani, Y. , 2015, “ Resonant Magnetoelastic Microstructures for Wireless Actuation of Liquid Flow on 3D Surfaces and Use in Glaucoma Drainage Implants,” Microsyst. Nanoeng., 1, p. 15032. 10.1038/micronano.2015.32 [DOI] [Google Scholar]
- [79]. Trierweiler, S. , Holmes, H. , Pereles, B. , Rajachar, R. , and Ong, K. G. , 2013, “ Remotely Activated, Vibrational Magnetoelastic Array System for Controlling Cell Adhesion,” J. Biomed. Sci. Eng., 06(4), pp. 478–482. 10.4236/jbise.2013.64060 [DOI] [Google Scholar]
- [80]. Chew, D. J. , Zhu, L. , Delivopoulos, E. , Minev, I . R. , Musick, K. M. , Mosse, C. A. , Craggs, M. , Donaldson, N. , Lacour, S. P. , McMahon, S. B. , and Fawcett, J. W. , 2013, “ A Microchannel Neuroprosthesis for Bladder Control After Spinal Cord Injury in Rat,” Sci. Transl. Med., 5(210), pp. 210–155. 10.1126/scitranslmed.3007186 [DOI] [PubMed] [Google Scholar]
- [81]. Chow, E. Y. , Chlebowski, A. L. , Chakraborty, S. , Chappell, W. J. , and Irazoqui, P. P. , 2010, “ Fully Wireless Implantable Cardiovascular Pressure Monitor Integrated With a Medical Stent,” IEEE Trans. Biomed. Eng., 57(6), pp. 1487–1496. 10.1109/TBME.2010.2041058 [DOI] [PubMed] [Google Scholar]
- [82]. Griss, P. , Enoksson, P. , Tolvanen-Laakso, H. K. , Meriläinen, P. , Ollmar, S. , and Stemme, G. , 2001, “ Micromachined Electrodes for Biopotential Measurements,” J. Microelectromech. Syst., 10(1), pp. 10–16. 10.1109/84.911086 [DOI] [Google Scholar]
- [83]. Cao, H. , Landge, V. , Tata, U. , Seo, Y. S. , Rao, S. , Tang, S. J. , Tibbals, H. F. , Spechler, S. , and Chiao, J. C. , 2012, “ An Implantable, Batteryless, and Wireless Capsule With Integrated Impedance and pH Sensors for Gastroesophageal Reflux Monitoring,” IEEE Trans. Biomed. Eng., 59(12 Part 2), pp. 3131–3139. 10.1109/TBME.2012.2214773 [DOI] [PubMed] [Google Scholar]
- [84]. Troughton, R. W. , Ritzema, J. , Eigler, N. L. , Melton, I . C. , Krum, H. , Adamson, P. B. , Kar, S. , Shah, P. K. , Whiting, J. S. , Heywood, J. T. , Rosero, S. , Singh, J. P. , Saxon, L. , Matthews, R. , Crozier, I . G. , and Abraham, W. T. , 2011, “ Direct Left Atrial Pressure Monitoring in Severe Heart Failure: Long-Term Sensor Performance,” J. Cardiovasc. Transl. Res., 4(1), pp. 3–13. 10.1007/s12265-010-9229-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85]. Totsu, K. , Haga, Y. , and Esashi, M. , 2003, “ Vacuum Sealed Ultra Miniature Fiber-Optic Pressure Sensor Using White Light Interferometry,” 12th International Conference Solid-State Sensors, Actuators Microsystems, (TRANSDUCERS), Boston, June 8–12, pp. 931–934. 10.1109/SENSOR.2003.1215628 [DOI] [Google Scholar]
- [86]. Lal, A. , 2001, “ Integrated Pressure and Flow Sensor in Silicon-Based Ultrasonic Surgical Actuator,” IEEE Ultrasonics Symposium. An International Symposium, Oct. 7–10, pp. 1373–1376. 10.1109/ULTSYM.2001.991976 [DOI] [Google Scholar]
- [87]. Hong, M. K. , Wong, S. C. , Mintz, G. S. , Popma, J. J. , Kent, K. M. , Pichard, A. D. , Satler, L. F. , Leon, M. B. , and Tobis, J. M. , 1995, “ Can Coronary Flow Parameters After Stent Placement Predict Restenosis?,” Catheterization Cardiovasc. Diagn., 36(3), pp. 278–282. 10.1002/ccd.1810360321 [DOI] [PubMed] [Google Scholar]
- [88]. Umbrecht, F. , Wendlandt, M. , Juncker, D. , Hierold, C. , and Neuenschwander, J. , 2005, “ A Wireless Implantable Passive Strain Sensor System,” IEEE Sensors, pp. 20–23. [Google Scholar]
- [89]. Mahutte, C. K. , 1998, “ On-Line Arterial Blood Gas Analysis With Optodes: Current Status,” Clin. Biochem., 31(3), pp. 119–130. 10.1016/S0009-9120(98)00009-5 [DOI] [PubMed] [Google Scholar]
- [90]. Kim, Y. T. , Kim, Y.-Y. , and Jun, C.-H. , 1999, “ Needle-Shaped Glucose Sensor With Multi-Cell Electrode Fabricated by Surface Micromachining,” Proc. SPIE 680, pp. 924–930. [Google Scholar]
- [91]. Mastrototaro, J. J. , Cooper, K. W. , Soundararajan, G. , Sanders, J. B. , and Shah, R. V. , 2006, “ Clinical Experience With an Integrated Continuous Glucose Sensor/Insulin Pump Platform: A Feasibility Study,” Adv. Ther., 23(5), pp. 725–732. 10.1007/BF02850312 [DOI] [PubMed] [Google Scholar]
- [92]. Ling, Y. , Pong, T. , Vassiliou, C. C. , Huang, P. L. , and Cima, M. J. , 2011, “ Implantable Magnetic Relaxation Sensors Measure Cumulative Exposure to Cardiac Biomarkers,” Nat. Biotechnol., 29(3), pp. 273–277. 10.1038/nbt.1780 [DOI] [PubMed] [Google Scholar]
- [93]. DeHennis, A. D. , and Wise, K. D. , 2006, “ A Fully Integrated Multisite Pressure Sensor for Wireless Arterial Flow Characterization,” J. Microelectromech. Syst., 15(3), pp. 678–685. 10.1109/JMEMS.2006.876668 [DOI] [Google Scholar]
- [94]. Ritzema-Carter, J. L. T. , Smyth, D. , Troughton, R. W. , Crozier, I . G. , Melton, I . C. , Richards, A. M. , Eigler, N. , Whiting, J. , Kar, S. , Krum, H. , and Abraham, W. T. , 2006, “ Dynamic Myocardial Ischemia Caused by Circumflex Artery Stenosis Detected by a New Implantable Left Atrial Pressure Monitoring Device,” Circulation, 113(15), pp. 705–707. 10.1161/CIRCULATIONAHA.105.572040 [DOI] [PubMed] [Google Scholar]
- [95]. Schnakenberg, U. , Kruger, C. , Pfeffer, J. G. , Mokwa, W. , Vom Bogel, G. , Gunther, R. , and Schmitz-Rode, T. , 2004, “ Intravascular Pressure Monitoring System,” Sens. Actuators, A, 110(1–3), pp. 61–67. 10.1016/j.sna.2003.04.001 [DOI] [Google Scholar]
- [96]. Twa, M. D. , Roberts, C. J. , Karol, H. J. , Mahmoud, A. M. , Weber, P. A. , and Small, R. H. , 2010, “ Evaluation of a Contact Lens-Embedded Sensor for Intraocular Pressure Measurement,” J. Glaucoma, 19(6), pp. 382–390. 10.1097/IJG.0b013e3181c4ac3d [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97]. Miyake, H. , Ohta, T. , Kajimoto, Y. , and Matsukawa, M. , 1997, “ A New Ventriculoperitoneal Shunt With a Telemetric Intracranial Pressure Sensor: Clinical Experience in 94 Patients With Hydrocephalus,” Neurosurgery, 40(5), pp. 931–935. 10.1097/00006123-199705000-00009 [DOI] [PubMed] [Google Scholar]
- [98]. Signorini, D. F. , Shad, A. , Piper, I. R. , and Statham, P. F. , 1998, “ A Clinical Evaluation of the Codman MicroSensor for Intracranial Pressure Monitoring,” Br. J. Neurosurg., 12(3), pp. 223–227. 10.1080/02688699845032 [DOI] [PubMed] [Google Scholar]
- [99]. Milner, R. , 2006, “ Remote Pressure Sensing for Thoracic Endografts,” Endovascular Today, pp. 1–3.http://evtoday.com/pdfs/EVT0206_F1_Milner.pdf [Google Scholar]
- [100]. Takahata, K. , DeHennis, A. , Wise, K. D. , and Gianchandani, Y. B. , 2004, “ A Wireless Microsensor for Monitoring Flow and Pressure in a Blood Vessel Utilizing a Dual-Inductor Antenna Stent and Two Pressure Sensors,” 17th IEEE International Conference on Micro Electro Mechanical Systems, Maastricht, Germany, Jan. 25–29, pp. 216–219. 10.1109/MEMS.2004.1290561 [DOI] [Google Scholar]
- [101].Renard, 2004, “ Implantable Glucose Sensors for Diabetes Monitoring,” Minimally Invasive Ther. Allied Technol., 13(2), pp. 78–86. 10.1080/13645700410026993 [DOI] [PubMed] [Google Scholar]
- [102]. Receveur, R. A. M. , Marxer, C. R. , Woering, R. , Larik, V. C. M. H. , and de Rooij, N. F. , 2005, “ Laterally Moving Bistable MEMS DC Switch for Biomedical Applications,” J. Microelectromech. Syst., 14(5), pp. 1089–1098. 10.1109/JMEMS.2005.851843 [DOI] [Google Scholar]
- [103]. Schwarz, M. , Ewe, L. , Hauschild, R. , Hosticka, B. J. , Huppertz, J. , Kolnsberg, S. , Mokwa, W. , and Trieu, H. K. , 2000, “ Single Chip CMOS Imagers and Flexible Microelectronic Stimulators for a Retina Implant System,” Sens. Actuators, A, 83(1), pp. 40–46. 10.1016/S0924-4247(00)00290-9 [DOI] [Google Scholar]
- [104]. Siwapornsathain, E. , Lal, A. , and Binard, J. , 2002, “ A Telemetry and Sensor Platform for Ambulatory Urodynamics,” 2nd Annual International IEEE-EMBS Special Topic Conference Microtechnologies in Medicine Biology, pp. 283–287. [Google Scholar]
- [105]. D'Lima, D. D. , Fregly, B. J. , and Colwell, C. W. , 2013, “ Implantable Sensor Technology: Measuring Bone and Joint Biomechanics of Daily Life In Vivo,” Arthritis Res. Ther., 15(1), p. 203. 10.1186/ar4138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106]. Epari, D. R. , Lienau, J. , Schell, H. , Witt, F. , and Duda, G. N. , 2008, “ Pressure, Oxygen Tension and Temperature in the Periosteal Callus During Bone Healing-An In Vivo Study in Sheep,” Bone, 43(4), pp. 734–739. 10.1016/j.bone.2008.06.007 [DOI] [PubMed] [Google Scholar]
- [107]. Frost, M. C. , and Meyerhoff, M. E. , 2002, “ Implantable Chemical Sensors for Real-Time Clinical Monitoring: Progress and Challenges,” Curr. Opin. Chem. Biol., 6(5), pp. 633–641. 10.1016/S1367-5931(02)00371-X [DOI] [PubMed] [Google Scholar]
- [108]. Langer, R. , 1998, “ Drug Delivery and Targeting,” Nature, 392(6679), pp. 5–10. [PubMed] [Google Scholar]
- [109]. Azagury, A. , Khoury, L. , Enden, G. , and Kost, J. , 2014, “ Ultrasound Mediated Transdermal Drug Delivery,” Adv. Drug Delivery Rev., 72, pp. 127–143. 10.1016/j.addr.2014.01.007 [DOI] [PubMed] [Google Scholar]
- [110]. Gao, W. , Chan, J. , and Farokhzad, O. C. , 2010, “ pH-Responsive Nanoparticles for Drug Delivery,” Mol. Pharm., 7(6), pp. 1913–1920. 10.1021/mp100253e [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111]. Liu, J. , Huang, Y. , Kumar, A. , Tan, A. , Jin, S. , Mozhi, A. , and Liang, X. J. , 2014, “ PH-Sensitive Nano-Systems for Drug Delivery in Cancer Therapy,” Biotechnol. Adv., 32(4), pp. 693–710. 10.1016/j.biotechadv.2013.11.009 [DOI] [PubMed] [Google Scholar]
- [112]. Bikram, M. , Gobin, A. M. , Whitmire, R. E. , and West, J. L. , 2007, “ Temperature-Sensitive Hydrogels With SiO2-Au Nanoshells for Controlled Drug Delivery,” J. Controlled Release, 123(3), pp. 219–227. 10.1016/j.jconrel.2007.08.013 [DOI] [PubMed] [Google Scholar]
- [113]. Koo, A. N. , Lee, H. J. , Kim, S. E. , Chang, J. H. , Park, C. , Kim, C. , Park, J. H. , and Lee, S. C. , 2008, “ Disulfide-Cross-Linked PEG-Poly(Amino Acid)s Copolymer Micelles for Glutathione-Mediated Intracellular Drug Delivery,” Chem. Commun. (Cambridge)., 2008(48), pp. 6570–6572. 10.1039/b815918a [DOI] [PubMed] [Google Scholar]
- [114]. Banerjee, J. , Hanson, A. J. , Gadam, B. , Elegbede, A. I. , Tobwala, S. , Ganguly, B. , Wagh, A. V. , Muhonen, W. W. , Law, B. , Shabb, J. B. , Srivastava, D. K. , and Mallik, S. , 2009, “ Release of Liposomal Contents by Cell-Secreted Matrix Metalloproteinase-9,” Bioconjugate Chem., 20(7), pp. 1332–1339. 10.1021/bc9000646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115]. Ge, J. , Neofytou, E. , Cahill, T. J. , Beygui, R. E. , and Zare, R. N. , 2012, “ Drug Release From Electric-Field-Responsive Nanoparticles,” ACS Nano, 6(1), pp. 227–233. 10.1021/nn203430m [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116]. Cai, K. , Luo, Z. , Hu, Y. , Chen, X. , Liao, Y. , Yang, L. , and Deng, L. , 2009, “ Magnetically Triggered Reversible Controlled Drug Delivery From Microfabricated Polymeric Multireservoir Devices,” Adv. Mater., 21(40), pp. 4045–4049. 10.1002/adma.200900593 [DOI] [Google Scholar]


