Abstract
Chronic joint pain is a widespread problem that frequently occurs with aging and trauma. Pain occurs most often in synovial joints, the body's load bearing joints. The mechanical and molecular mechanisms contributing to synovial joint pain are reviewed using two examples, the cervical spinal facet joints and the temporomandibular joint (TMJ). Although much work has focused on the macroscale mechanics of joints in health and disease, the combined influence of tissue mechanics, molecular processes, and nociception in joint pain has only recently become a focus. Trauma and repeated loading can induce structural and biochemical changes in joints, altering their microenvironment and modifying the biomechanics of their constitutive tissues, which themselves are innervated. Peripheral pain sensors can become activated in response to changes in the joint microenvironment and relay pain signals to the spinal cord and brain where pain is processed and perceived. In some cases, pain circuitry is permanently changed, which may be a potential mechanism for sustained joint pain. However, it is most likely that alterations in both the joint microenvironment and the central nervous system (CNS) contribute to chronic pain. As such, the challenge of treating joint pain and degeneration is temporally and spatially complicated. This review summarizes anatomy, physiology, and pathophysiology of these joints and the sensory pain relays. Pain pathways are postulated to be sensitized by many factors, including degeneration and biochemical priming, with effects on thresholds for mechanical injury and/or dysfunction. Initiators of joint pain are discussed in the context of clinical challenges including the diagnosis and treatment of pain.
Introduction
Chronic joint pain is a costly and widespread problem, affecting 110 million adults in the U.S. at some point over the course of their lifetime and having an estimated total cost of $60 million annually [1,2]. However, due to the complex relationships between joint mechanics, tissue degeneration, and nociceptive signaling of pain, effective treatments for joint pain are lacking [3–6]. The challenge of defining biomechanical, physiological, and pathological mechanisms of pain is further complicated by many confounding factors that are implicated in the origin and maintenance of joint pain. This complexity also impedes the effective diagnosis of the etiology of joint injury and osteoarthritis (OA) before permanent changes in joint structure and pain signaling occur [7–9].
Synovial joints permit complex motion and often sustain loads several times greater than the weight of the human body; yet, in some cases, repeated and mechanical loading above the normal physiological conditions contribute to the initiation of acute tissue damage and degeneration [10]. Further, the local mechanical environment of joints can be substantially altered after injury or degeneration, which, in some cases, produces dysfunctional nociceptive signaling [11–14]. Neck pain is among the most prevalent chronic joint pathologies, with 30–50% of the adult population reporting pain over a 12 month period [6]. Jaw pain is also a widespread musculoskeletal condition, affecting 5–12% of the population [1]. Neck pain is most often induced by trauma and can involve damage, like loading and stretching of the facet capsular ligament, to the bilateral facet joints that provide articulations between spinal levels [15,16]. In addition, neck pain can also result from nontraumatic, cumulative exposures, such as sustained neck flexion postures [17]. Innervated by mechanoreceptors, disruption of the collagen fibers that make up the facet capsular ligament can initiate nociceptive signaling and lead to both acute and chronic pain [14,18–20]. By comparison, jaw pain is less often caused by trauma and is typically initiated by repeated atypical loading of the temporomandibular joint (TMJ) that leads to inflammatory cascades within the synovium and that can sensitize pain fibers in the joint [8,9]. Accordingly, mechanical injury can disrupt the normal microenvironment of joint tissues in several distinct ways, including via acute traumatic loading and/or stretching of the capsular ligaments of the joint or by repeated, nontraumatic loading to the tissues in the joint. Both traumatic and repeated mechanical disruption can induce structural changes to joint tissues and inflammatory cascades, which separately activate pain fibers via a modified biomechanical and biochemical environment [13,21–23].
Pain is an unpleasant sensory and emotional experience that occurs when there is potential for tissue damage to occur [24] or in response to an inciting injury as from OA [25]. In both cases, pathophysiological tissue loading triggers both peripheral and central nociceptive signaling cascades that initiate and sustain pain [25,26]. By distinction, the term “nociception” specifically refers to the physiological responses and signal transmission that encode pain [24]. Although peripheral nociceptive neurons are critical to the encoding and transmission of pain signals, the experience of pain includes both sensory and emotional components, as modulated by specific brain regions (and reviewed comprehensively elsewhere [27]). Human chronic pain can also occur in the absence of nociceptive input or tissue damage [28]; however, this review focuses on cases in which nociceptive pain is present. Since the combination of sensory, emotional, and cognitive aspects of pain contributes to the complexity of chronic pain, differentiating the relative contributions of biomechanical factors on different cells involved in the pathomechanisms of pain and injury is needed to understand and treat this complex disease.
Nociceptive neurons are pseudo-unipolar cells, with their cell body in the dorsal root ganglion (DRG) and axonal projections that innervate peripheral tissues and others that extend to the central nervous system (CNS) to transmit information to the brainstem, thalamus, and cortex of the brain. Accordingly, nociceptive signals from a joint are communicated via peripheral nerves through the DRG and are subsequently modulated by phosphorylation of intracellular kinases, synaptic receptors, and ion channels in the spinal cord and brainstem [29–31]. The thalamus receives signals from the spinal cord and brainstem, and projects information to cortical and limbic structures for further processing and signal interpretation [27]. Repeated stimulation of afferent fibers, such as those that can occur by repeated tissue loading or abnormal joint kinematics, can lead to neuron sensitization, which is an increased responsiveness to otherwise normal or typically subthreshold inputs [24,32]. In the periphery, sensitization manifests as altered nociceptive responses in the neurons in and around joints, with their activation at decreased thresholds and their increased responsiveness to stimulation [26,32]. Central sensitization in the spinal cord and brain produces sustained neuronal hyperexcitability, amplification of signals from peripheral sources, increased spontaneous activity, and reduced thresholds for activation, as well as plastic rewiring of the neuronal circuitry in and between the spinal cord and brain [24,26,33–35]. Collectively, these changes in neuronal signaling can lead to persistent pain even in the absence of any on-going tissue damage or input from peripheral nociceptive fibers [26,29].
Using the spinal facet joints and TMJ as models of pain from biomechanical mechanisms, this review attempts to summarize the current understanding of pathobiomechanical mechanisms that initiate and sustain joint pain in the peripheral and central nervous systems. It also integrates outcomes from experiments performed across cellular, tissue, and system scales, as well as proposes possible future directions for mechanistic investigation and diagnostic imaging. The review begins by highlighting relevant joint anatomy and pain mechanisms associated with dynamic loading of the spinal facet joint and repeated compressive loading of the TMJ. These two joints are highlighted for their similarities and also for the unique differences in which pathomechanisms are initiated. Tissue mechanics and neurobiological aspects of joint pain are integrated in discussions of the macro- and micromechanics of both joint tissues, degeneration, and priming of afferent nociceptors. Finally, current and emerging imaging techniques to assess early functional modifications in both joint tissue and pain circuitry of the CNS are presented.
Mechanical Injury of the Facet Capsular Ligament
The facet joints are bilateral joints located in the posterolateral region of the spine and that are responsible for providing mechanical load transmission and motion coupling throughout the full spine (Fig. 1) [22,36]. The cervical facet joint is composed of hard and soft tissues that together not only couple rotation during bending in the neck, but also transmit axial load [22,37]. The bony articular pillars that extend laterally from vertebral laminae are covered by articular cartilage and provide the opposing surfaces with frictionless surfaces to enable the smooth articulation of the joint [38,39]. The membranous synovium lines between those articular surfaces and folds into the joint space to form meniscoids that protect the articular cartilage during joint movement [40]. The facet capsular ligament encloses the articulating bones of the facets of adjacent vertebrae creating a joint space [41–43]. That ligament is composed of crimped collagen fibers which permit substantial stretch and has a large degree of laxity to permit extensive ranges of spinal bending and rotation without mechanical resistance or failure [41].
Fig. 1.
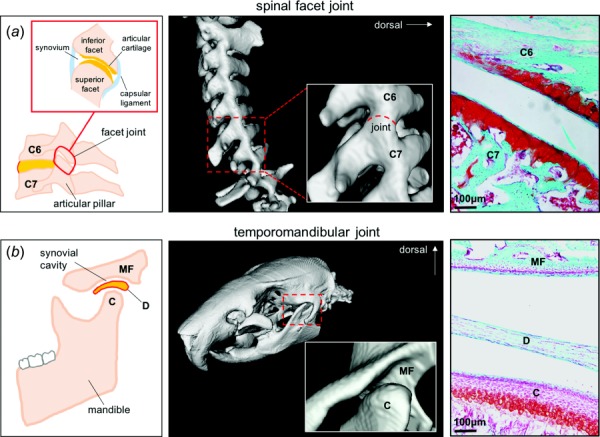
Human anatomy and rat computed tomography (CT) three-dimensional reconstructions and Safranin-O staining of tissue sections of the (a) facet joint and (b) TMJ, with important anatomy highlighted: sixth cervical vertebrae (C6), seventh cervical vertebrae (C7), mandibular fossa (MF), articular disk (D), and mandibular condyle (C)
The facet capsular ligament is innervated by mechanoreceptors that encode movement and stretch, and also by nociceptors that encode nociceptive stimuli [44–46]. Disruption of the facet capsule and other innervated tissues in the facet joint can elicit pain [44,45,47–51]. Trauma to the facet joint, as can happen by direct stretch of the facet capsule or compressive loading of the joint, can result from traumatic spine loading [52–57] and imposes excessive deformation and abnormal kinematics in the facet joint. Such mechanical injury to the facet joint can occur as the spine undergoes altered kinematics which induce excessive joint motions and/or combined loading to its tissues, as can occur when the head is axially rotated and the spine is pretorqued while the neck undergoes flexion-extension loading [57]. Dynamic spinal loading can pinch the synovial meniscoids and produce microstructural damage in the facet capsular ligament, both of which have the potential to alter the biochemical and mechanical microenvironment of the facet joint and to initiate osteoarthritic degeneration [11,19,55]. After such trauma or injury to the facet joint, patients often present with complex pain symptoms that include both physical and psychological components, with localized and widespread evoked pain, spontaneous pain, and/or psychological disturbances [58–60]. Existing psychological distress may also contribute to the maintenance of pain [60].
Although several forms of spinal loading can directly or indirectly activate the afferent fibers that innervate the facet joint structures, excessive stretch of the facet capsular ligament, in particular, induces persistent pain and modifications in neuronal signaling [18,61,62]. Peak facet capsular ligament strains of 35.4% have been observed during traumatic cervical spine loading in vitro [54], implicating mechanical damage of this ligament in pain production. Facet capsule stretch imposed by in vivo distraction of the joint in the rat produces strains below the failure limit of that ligament but that exceeds the normal physiologic range of stretch and induces chronic pain [61,63,64]. The magnitude of capsular stretch has been identified as a key modulator of the onset and maintenance of pain, with the supraphysiologic strains known to induce pain defined as those above 8–10% [63,65]. Furthermore, the severity of the sustained behavioral hypersensitivity (i.e., pain) is significantly correlated with the applied facet capsule strain [63,65]. However, complete capsule rupture, which likely also fully transects the pain fibers embedded in the facet joint, does not initiate chronic pain [61], suggesting a critical role of intact peripheral afferents in either transmitting nociceptive information to the central nervous system or in their dysfunction modulating the extensive pain system. Interestingly, such a notion highlights the complexity of this pathophysiology in that failure of this ligament is not “worse” in terms of initiating pain cascades, despite such tissue failure possibly leading to long-term alterations in the mechanical properties of that joint due to tissue scarring and/or degenerative cascades [22].
Facet joint pain in rodent models mirrors the temporal and spatial features of evoked pain in humans, emerging within hours after injury and lasting for several weeks to years in patients [66–68]. However, it should be noted that issues of scaling between the anatomic size and shape, magnitudes of loading, and relative age between animals and humans make it challenging to draw direct comparisons between species. Nevertheless, the similarities in the biomechanical metrics between human cadavers and rodents, as well as consistency between pharmacologic treatments on physiologic responses in rodents and humans, support the strengths of this work. In rodent models, excessive strains applied to the facet capsule lead to pain onset in between 6 h and 1 day which persists for up to 28 days [34,35,69,70]. The widespread hypersensitivity that patients experience following cervical spine trauma [68,71] is also detectable at the shoulders and forepaws in the rat after similar facet joint injury [61,63,72]. Although the capsular ligament is a critical mediator of pain and disability in cervical spinal trauma, pathologies in other synovial joints, such as the TMJ, may be driven by different modes of mechanical loading, including repeated compression of articular cartilage and subchondral bone [9].
Osteoarthritic Pathology of the Temporomandibular Joint
Similar to the spinal facet joints, the TMJs are synovial joints that undergo repeated physiologic loading and, in some cases, are subjected to pathological overloading. The TMJs connect the mandible to the temporal bone of the skull, permitting normal mouth opening and closing [8] (Fig. 1). The TMJ is separated into two compartments by a fibrocartilaginous articular disk surrounded by synovial fluid (Fig. 1) [73,74], which allows the mandibular condyle to articulate smoothly with the glenoid fossa of the temporal bone [8]. Since normal jaw opening imposes rotation and anterior translation of the condyle in the glenoid fossa and posterior translation of the articular disk, many different anatomic tissues experience complex local mechanical environments [8]. Injury or degeneration of any of the bone, cartilage, or disk in the TMJ can affect jaw movement, joint morphology, and, in some patients, lead to pain [8,9].
Clinically, TMJ disorder presents with many different disease pathologies, including pain in the TMJ, limited or asymmetric jaw motion, and/or TMJ sounds that are associated with motion [75,76]. Although TMJ disorders often have complex etiologies, the most common pathology affecting the joint is OA [77]. OA is often associated with parafunctional habits, such as jaw clenching [75], that increase mechanical loads on the mandible [78]. Functional overloading of the mandible can cause dysfunctional remodeling, abrasion of articular cartilage, and low-grade inflammation in the joint [77,79]. These pathological changes activate the nociceptive neurons of the trigeminal nerve that innervate bone, synovial tissue, ligaments, muscles, and capsular tissues in the TMJ [80]. For the vast majority of patients, TMJ disorder is self-resolving and requires little to no treatment. However, approximately 15% of patients develop a nonresolving, chronic form of TMJ pain that is recalcitrant to therapy, with associated physical, behavioral, and psychological symptoms [1,8]. Similar to chronic neck pain from spine trauma, human and animal studies suggest that persistent TMJ pain is due to augmented central pain processing mechanisms [60,81–85].
Abnormal TMJ loading and the associated orofacial pain have been simulated by repeated mouth-opening in the rodent [13,86]. TMJ loading reorganizes cartilage and is associated with upregulation of biochemicals involved in OA, such as vascular endothelial growth factor (VEGF) and hypoxia-inducible factor-1α (HIF-1α) [13,86,87]. Application of different loading magnitudes to the jaw, for 1 h per day for 7 days, induces distinct pain responses in the rat, suggesting a correlation between the loading magnitude and pain (Fig. 2(a)). Evaluating mechanical hyperalgesia, an increased response to stimulation, demonstrates that a 2 N load to the jaw produces acute TMJ hyperalgesia that resolves to baseline (BL) levels by day 14, while a 3.5 N load induces chronic TMJ hyperalgesia, with sustained pain for up to 28 days [13,86]. However, mechanical hyperalgesia does not measure spontaneous pain, which is an important clinical outcome and is influenced by both sensory and affective components [88]. In rodent models, spontaneous pain can be measured by evaluating facial expression using the rat grimace scale (RGS) [88,89]. Preliminary studies demonstrate a substantial increase in RGS scores at day 1 after 3.5 N loading, significantly greater pain at day 5 than sham controls with no loading, and a return to sham levels by day 13 (Fig. 2(b)). Elevated RGS scores and mechanical hyperalgesia indicate that both sensory and affective components of pain may be implicated in mechanical TMJ pain. Despite the association between certain magnitudes of TMJ loading and chronic pain, the mechanisms by which abnormal mechanics disrupts pain signaling remain unclear. Recent evidence from the same model suggests that hypoxia and inflammation increase in the TMJ after joint loading, with upregulation of HIF-1α, VEGF, matrix metalloproteinases (MMPs) [13]. These outcomes are associated with degenerative pathologies in humans [90] and in other animal models [91–93], suggesting that TMJ nociceptors may be sensitized by degenerative molecules and enzymes as a result of TMJ loading.
Fig. 2.
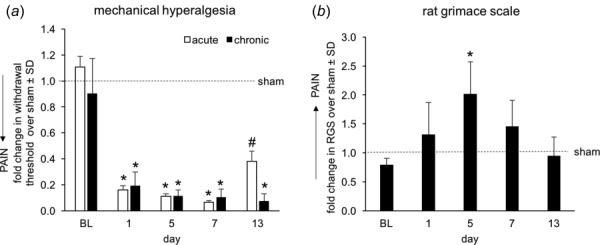
Behavioral measurements of pain in the area of the TMJ using techniques to (a) measure mechanical hyperalgesia and (b) quantify the rat grimace scale scoring for spontaneous pain levels. Both behavioral responses are shown relative to sham control responses receiving only exposure to anesthesia. For both assessments, a one-way ANOVA compared groups (n = 4/group), with the chronic exposure being different from the sham response (*p < 0.001; #p = 0.015). Baseline (BL) measurements are taken before any exposures and also serve as a control measurement.
Pain and Mechanical Dysfunction in Degenerated Joints
Degenerative joint pathologies, such as OA, are initiated by a complex combination of applied mechanical loads and biological cascades within the joint's tissues [13,92,94,95]. Both trauma and age contribute to joint degeneration [96]’ yet, the mechanisms by which pain is initiated in degenerative joints are still unclear [97–100]. The facet joints are susceptible to degeneration from supra-physiological mechanical loading and/or injury [101,102]. Further, animal models of facet joint degeneration induced by intra-articular delivery of collagenase or complete Freud's adjuvant (CFA) induce inflammation, loss of cartilage, osteophyte formation, and subchondral bone changes in the joint [94,103], and suggest that such changes may be involved in joint degeneration. In vivo studies also support that similar degenerative mechanisms may occur in the TMJ; loss of articular cartilage and remodeling of subchondral bone are reported in response to mechanical loading and intra-articular injection of CFA [13,81,86,87,104]. In particular, in degenerative TMJs, the intra-articular cartilage exhibits increased expression of VEGF, HIF-1α, and tumor necrosis factor (TNF) in the hypertrophic and mature cartilage layers [13,81,87]. Expression of MMP-13, a protein involved in collagen matrix restructuring, also increases in the TMJ after its loading [13,105], demonstrating a connection between mechanical loads and enzymatic microdamage in the TMJ articular cartilage. Sustained expression of these and other degenerative and inflammatory factors leads to cartilage thinning and changes in the underlying bone that can compromise the mechanical integrity of the overall joint and its tissues, increasing their susceptibility to mechanical injury [106].
Tissue degeneration is driven by enzymes that degrade the individual tissues and alter the mechanical and biochemical properties of the overall joint [22,107]. Enzymes like MMPs and a disintegrin and metalloprotease with Thrombospondin motifs (ADAMTSs) degrade the collagen, aggrecan, and proteoglycans that compose the extracellular matrix [108,109]. Loss of these structural proteins specifically compromises the articular cartilage and capsular ligament [108–110]. Selective digestion of constitutive components of the extracellular matrix, such as collagen, elastin, or proteoglycans, can change ligament structure by inducing crimp [111], decreasing stiffness [112], and increasing cartilage deformation in response to loading [113]. In vitro collagenase digestion that is sufficient to reduce collagen immunolabeling by 47% in a collagen gel system also decreases the stiffness (p = 0.024) and failure force (p = 0.007) of the gels under uniaxial tension to failure (Fig. 3(a)). Furthermore, that loss of collagen also results in less microstructural fiber reorganization during gel stretch, with fewer collagen fibers reoriented along the direction of loading compared to undigested control gels (p = 0.001) (Fig. 3(b)). These changes in the macroscale mechanics of collagenase-digested gels are consistent with altered mechanics of enzyme-digested native joint tissues [94,114]; both demonstrate that enzymatic reduction of constitutive matrix components compromises biomechanical behavior. Together, these studies suggest that degenerated joint tissues may have lower thresholds for mechanical injury. At the very least, it is likely that degenerated joint tissues respond differently to loading than healthy counterparts. A clinical study reported ligament tearing and fibrillation without any direct traumatic injury in patients with early signs of cartilage degeneration [115].
Fig. 3.
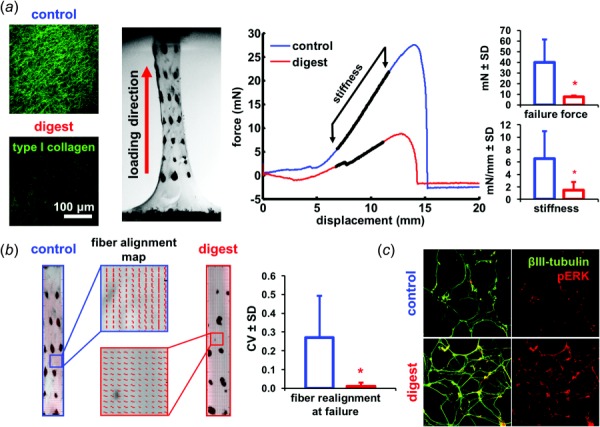
Collagenase digestion of an in vitro collagen gel system shows that collagenase digestion that is sufficient to (a) reduce collagen fiber content also decreases the failure force (*p = 0.007) and stiffness (*p = 0.024) of the gel when loaded in tension, (b) The microstructural collagen fiber reorganization at failure in the gel under tension is also altered, with the normal reorganization of collagen fibers that is evident at failure being absent and a lower circular variance (CV) (*p = 0.001). A higher CV value indicates fiber realignment toward the loading direction, and (c) The same collagenase treatment also increases the expression of phosphorylated ERK (pERK) after loading in mixed neuronal cultures.
Since the local biomechanical environment of neurons mediates their nociceptive signaling [14,116,117], and degeneration-associated tissue degradation alters the biomechanical environment of innervated joint tissues [22,111,115], degeneration may also lower thresholds for painful injury. This notion is supported by studies demonstrating that strains that induce microstructural damage correspond to those strains that induce pain in vivo and activate neurons embedded in collagen networks in vitro [11,14]. Collectively, in vitro studies suggest a relationship between the initiation of nociceptive signaling and the onset of microstructural damage, at least in capsular ligaments. Although abnormal kinematics initiate pain signaling [63,118] and degeneration alters tissue mechanics [22], excessive stretch or mechanical injury is not required for degeneration-induced pain. For example, there is not a direct correlation between the amount of structural damage (i.e., osteophytes, bone cysts, and meniscal changes) and pain levels in patients with knee OA [119]. This disconnection between pain and apparent tissue damage suggests that other molecular pathways, like inflammatory cytokines and chemokines, may be altered in the degenerative state and may more directly play a role in pain generation; indeed, inflammatory factors have been detected in the synovial fluid of OA patients [90,120].
Several other biochemical molecules involved in the degenerative cascades may act as neuronal “sensitizers” to initiate nociception. Collagenase induces features of OA degeneration in facet and knee joints [94,121]. Yet, its effects on neuronal sensitization and pain development are still undefined. Despite this, applying collagenase to mixed cell cultures of dissociated dorsal root ganglia increases neuronal expression of phosphorylated extracellular signal-regulated kinase (pERK), a signaling molecule and indicator of neuronal activation (Fig. 3(c)). Activation of ERK signaling in neurons can produce sustained hyperexcitability and altered signaling [50,122]. The increase in neuronal pERK after collagenase exposure suggests that it may have properties of direct sensitization; however, more work is needed to identify the degenerative molecules that most sensitize neurons.
Afferent Priming in Mechanically Induced Joint Pain
Although degeneration has been linked to changes in the joint biomechanics, inflammatory status, and pain, the microenvironment of a joint or the spinal cord can be altered by biochemical mediators that lead to increased nociceptive signaling. Transient insults to primary afferent nociceptors can produce sustained changes in neural transmission that can increase the constellation of nociceptive responses that are induced by later subthreshold insults (which are normally insufficient to evoke pain) [123–125]. For example, exposure to a single stimulant such as carrageenan or TNF produces only transient pain in the rat, while secondary stimulation with an inflammatory mediator (i.e., prostaglandin PGE2) induces persistent pain [126,127]. In addition to inducing pain, priming of afferent nociceptor populations can induce plastic changes in the CNS [125] that further increase susceptibility to later develop chronic pain.
Pain development is, in part, driven by the upregulation of nociceptive molecules, such as nerve growth factor (NGF) [23,128,129]. NGF is upregulated early after the induction of painfully inflamed and arthritic joints, with similar timing to inflammatory cytokines, and is responsible for the development, proliferation, and survival of neurons [130,131]. When delivered to a joint at sufficient concentrations, it can induce mechanical and thermal hypersensitivity, as well as spinal neuronal hyperexcitability, particularly in peptidergic afferents [23,129]. Accordingly, intra-articular NGF levels may be important in the onset of pain after facet joint trauma due to the high proportion of peptidergic afferents [132] and expression of the NGF receptor, trkA, in the facet joint [133]. Increased NGF accompanied by inflammation has been shown to contribute to the pain and spinal glial activation and neuronal hyperexcitability that develop after trauma to the facet joint [20,23,134]. Moreover, delivery of anti-NGF treatment to joints early after their injury blocks the development of pain [23,135], suggesting that NGF has a role in the initiation of pain. However, delaying anti-NGF administration until after pain has developed is ineffective in attenuating either pain or spinal neuronal hyperexcitability [23]. NGF appears to be an intra-articular initiator of joint injury-induced pain, but not involved in pain maintenance. Based on these findings, it is also possible that elevated levels of NGF may sensitize nociceptors, making them more susceptible to activation at lower thresholds upon future mechanical loading.
Intra-articular injection of NGF at concentrations below that which induces pain when given alone [23,128] was used to investigate if NGF increases the risk for pain from a normally non-painful physiological facet ligament stretch. NGF was injected into the bilateral C6/C7 facet joints at a dose of 1 μg in 5 μL of sterile phosphate buffered saline (PBS); 2 days after that injection, rats received either a nonpainful physiologic facet joint distraction (1 μg + D2NP; n = 6) or a sham surgery procedure (1 μg + D2sham; n = 6) [63,136,137]. Control groups were also included with a vehicle group receiving only PBS followed by a nonpainful distraction on day 2 (vehicle + D2NP; n = 6) and an NGF injection-only group (1 μg NGF; n = 6) with no joint loading. Pain was induced in the group having prior intra-articular NGF exposure, with significantly lower withdrawal thresholds than before distraction (p < 0.0012) for each day after loading (Fig. 4). Withdrawal thresholds for that group are also significantly lower (p < 0.0278) than any of the other groups on all days after physiological loading (Fig. 4). This pilot work suggests that, in some cases, large modulation in the biochemical milieu of the joint may not be required for pain to be initiated, especially if there are altered joint kinematics. Of note, if priming mechanisms, like elevated levels of NGF, indeed sensitize joint nociceptors to physiologic joint loads, then this may explain why patients who undergo moderate loading of the neck develop persistent pain symptoms [60]. Current treatments for neck pain do not target many of the potential priming mechanisms that may lead to persistent pain [60]. Developing new interventions possibly targeting molecules that can sensitize joint afferents may offer novel treatments for people who develop pain after sustaining multiple minor neck injuries. However, since it is unlikely that there is only one mediator of nociceptor sensitization, particularly across different joint pathologies, additional in vivo and in vitro studies with varied mechanical inputs may be required to define the role of other molecules in nociceptor sensitization.
Fig. 4.
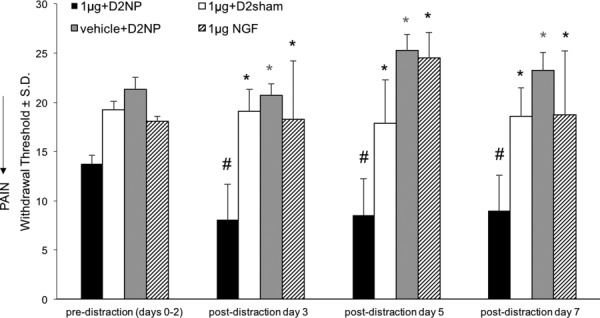
Intra-articular NGF injection prior to physiological facet joint loading induces pain, with decreased withdrawal thresholds from baseline (#p < 0.001). The groups receiving only 1 μg of NGF or a nonpainful distraction (vehicle + D2NP) does not develop pain and their withdrawal thresholds are greater than the group receiving NGF prior to physiological loading. (1 μg + D2NP) (*p < 0.05).
Integrating Macro- and Microscale Mechanics For Nociceptive Activity
Complex mechanical loading exceeding the physiologic range of a given joint can lead to persistent pain that arises from many structures in that joint [13,22,64,138]. In the neck, the facet capsular ligament is particularly vulnerable to excessive spinal motions because of the potential for ligament stretch to activate the nociceptors innervating that ligament [15,21]. The facet capsule is composed of complex multiscale fibrous structures, along with nonfibrillar materials and cells that also regulate its mechanical behavior [22,42,48]. Traumatic spinal loading, including shear, compression, and bending, can cause the facet ligament to undergo subfailure loading that produces injury, or even its failure [19,57,139–141]. Excessive stretch of the facet capsules is a common injury mechanism that produces highly heterogeneous deformations in that ligament at different spinal levels and a nonuniform strain field across individual ligaments [22,54,142]. Simulations of neck trauma using in vitro cadaveric systems indicate that horizontal acceleration can produce peak strains (∼30%) in the C6/C7 facet capsule [54], corresponding to those strains that are sufficient to produce localized altered collagen fiber kinematics and sustained pain [63,142,143]. Although cadaveric testing accurately simulates and defines the macroscopic mechanical responses and microstructural changes of the human facet joints to spinal loading, it can neither fully capture microscopic mechanics nor assess any physiological cellular responses. Nevertheless, the biomechanical and structural metrics defined by cadaveric testing, such as ligament strain, disk compression, and motion segment kinematics [22,53,57], can be used in computational, cell culture, and in vivo animal model systems to further refine our understanding of the relationship between mechanics and physiology [14,15].
The relationship between mechanics and nociception can be directly measured by neurophysiologic responses that correlate with the applied stress and strain in isolated joint capsules [144]. In fact, the same magnitude of capsule strain that induces pain has been shown to separately activate the afferents that innervate the facet capsular ligament during and after capsule stretch in vivo [45,51,63,64]. Physiologic strains of 10–15% only activate mechanoreceptors, whereas strains greater than 25% trigger the activation of mechanoreceptors, produce sustained after-discharge, and induce morphological changes in axons, including local swelling and vacuolations [51,145]. Although in vivo and ex vivo experiments provide evidence for a link between loading and mechanoreceptor activation, additional studies are required to elucidate this complex relationship.
Recent development of an in vitro neuron-collagen culture system has demonstrated that neuronal expression of pERK is correlated with the applied regional strain in the collagen matrix that surrounds neurons [14]. As the applied macroscopic strain increases, neuronal release of pERK increases substantially at strain levels that simultaneously induce collagen fiber reorganization [14]. Since strains associated with pain in vivo produce fiber realignment and elevate pERK in vitro, local peripheral biomechanics appear to align with tissue-level deformations and modulate neuronal responses that drive the development of pain. However, unlike in vivo experiments, in vitro systems can decouple the effects of inflammation, mechanics, and matrix structure on neuronal responses and enable simultaneous measurement of local strains, microscopic fiber kinematics, and cellular behaviors in response to macroscopic tissue deformation. Based on the relationships between multiscale mechanics and neuronal signaling in the facet capsule after its loading [14,51,63], it is possible to develop new in vitro models of injury. Such models enable modification of relevant mechanical and structural parameters to both understand pathomechanisms and potentially identify novel therapeutic targets that may be involved in the mechanotransduction pathways. Furthermore, the identification of critical pathomechanisms could be helpful also in developing predictive diagnostics for joint pathologies. Currently, clinical imaging collects structural information about joints, but provides little information about active biochemical processes. Therefore, outcomes from in vitro culture systems that relate joint mechanics and biochemical mediators could be an important source of information for developing clinical imaging techniques for pain.
Predictive Diagnostics in Joint Pathologies
Clinical imaging of joint pathology primarily focuses on joint anatomy to diagnose and predict patient outcomes [8,146], and does not capture active cellular processes that occur in response to joint loading and/or degeneration [8,9]. For example, patients presenting with TMJ pain are frequently screened with panoramic radiographs, and more recently, cone beam computed tomography (CBCT), an imaging technique that provides three-dimensional views of dental structures, to assess structural damage in the TMJ [8]. Anatomical signs of late-stage TMJ OA, such as erosions and osteophytes, can be identified by CBCT with high diagnostic accuracy [7,147]. Similar hallmarks of facet joint OA, including erosion of subchondral bone, cysts, joint space narrowing, and osteophytes [148], are detectable in the facet joint by CT and magnetic resonance imaging (MRI) [46,146,149,150].
Architectural changes to subchondral bone are also present in animal models of OA and are detectable by CT. Notably, alterations to bone and cartilage can be seen at early time points (2–5 weeks) in these models due to the high resolution of microCT imaging (resolution < 0.1 mm) and intensity of the model compared to clinical pathology. In the rat, 1 week of TMJ loading produces structural modifications to the TMJ condyle at day 14, which is 7 days after cessation of joint loading. Three-dimensional reconstruction of CT scans, obtained at day 14, demonstrate mild flattening of the condyle in the 3.5 N load model that produces chronic pain (Fig. 5(a)). Image subtraction between normalized scans at day 14 and baseline shows significant changes in CT image intensity in the chronic pain model compared to joints that were not loaded (Fig. 5(b)). However, there is no difference between the chronic and acute (2 N loading) pain models, suggesting that morphological changes alone may not drive persistent pain from TMJ loading. This is in accordance with clinical data that shows minimal flattening of the TMJ condyle in 35% of asymptomatic people [151].
Fig. 5.
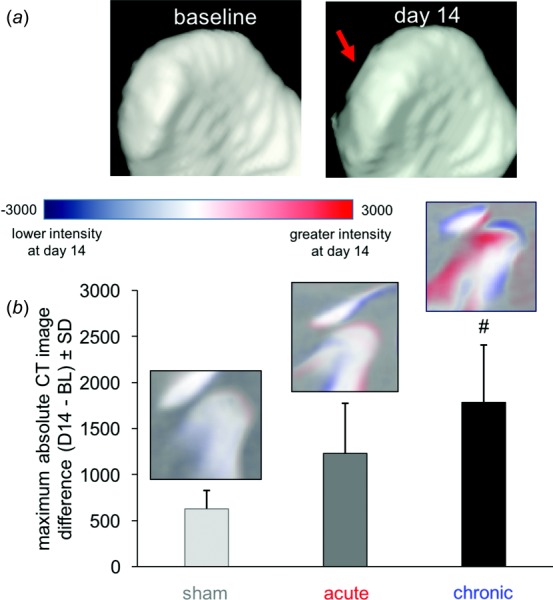
In vivo CT imaging of the TMJ showing changes in the overall joint architecture, with (a) more flattening of the TMJ condyle at day 14 with chronic pain than for sham controls and (b) quantification of changes in bone density. Changes in the CT image intensity at day 14 by image subtraction from baseline (BL) show the greatest change from controls in TMJ condyle structure with chronic pain (#p = 0.013).
The disconnection between anatomical disruption and joint pain is also reported in MRI studies of joint pathology in humans, including signs of disk displacement in both asymptomatic patients as well as patients with painful TMJs [152,153]. However, signs of disk displacements without reduction, in which the disk remains displaced with jaw opening, are more strongly associated with progressive TMJ dysfunction and pain compared to reducing disk displacements, in which the disk returns to a normal position during mouth opening [154]. Although some morphological changes, such as osteophytes and bone degeneration [79], are clinically relevant, many cases of disk displacement may be normal anatomic variants [153]. Functional imaging techniques, such as positron emission tomography (PET) and specific MRI sequences, may be able to better differentiate healthy from pathological joints and aid in earlier diagnoses [155,156]. For example, PET imaging with specific tracers, such as 18F-FDG, a glucose analog, may enable measurement of synovitis. In the rat, FDG uptake is increased in an inflammatory arthritic knee joint compared to a contralateral control joint [157]. Patients with knee OA also have increased 18F-FDG uptake in the affected joint compared to controls [156]; however, the association between tracer uptake and clinical presentations remains unclear. The uptake of the PET tracer sodium fluoride, 18F-NaF, a marker of bone perfusion and metabolism, is increased in the affected joint of patients with TMJ OA, albeit with substantial variability between patients [155]. Similar outcomes are also identified using 99Tcm hydroxymethylene diphosphonate single-photon emission computed tomography (SPECT) imaging of metabolic activity in bones of the TMJ [158]. Notably, metabolic activity of the bone does not correlate with pain scores [158], suggesting that bone activity is not strongly associated with joint nociception.
MRI, a nonradiative technique, is of particular clinical interest for imaging the joint synovium because it captures soft tissue morphology and water content. As such, novel MRI sequences for detecting particular pathologies are an active area of research [159]. Markers of active joint pathology, including synovitis, joint effusion, and interspinous ligament edema, are detectable in facet injury using fat-saturation and suppression MRI techniques [160,161]. These techniques may also be relevant to TMJ disorders, since a recent investigation using contrast-enhanced fat-suppression MRI reveals a correlation between contrast enhancement in the posterior disk attachments and severity of TMJ pain [162]. Ultrasound may also be capable of detecting active joint pathology, including synovial thickening and joint effusion, since ultrasound-detected markers of hand OA are significantly correlated with disease progression at 2 and 5 year follow-up exams [163,164]. However, the predictive value of these markers has not been verified in other joints. Despite the promise of imaging techniques that monitor the biochemical status of the joint, means for clinically measuring nociceptive dysfunction related to joint pathology are largely unstudied.
Functional brain imaging, including PET, fMRI, and diffusion tensor imaging (DTI), permit investigating functional and structural modifications of the CNS. Instead of solely relying on structures in the periphery, functional brain imaging can measure changes in neuronal activity and connectivity associated with pain [165–167] and, perhaps, predict the likelihood of transition to chronic pain. Clinical investigation using fMRI techniques shows increased brain activity in prefrontal-limbic regions in patients with knee OA that is not apparent in healthy controls [168]. In TMJ disorder, white matter alterations occur in the medullary dorsal horn of patients with pain for longer than 1 year [83], and differential brain activity is detectable in patients with painful TMJ disorder compared to controls [169]. Collectively, changes in brain activity and white matter connectivity suggest that persistent OA pain may alter both the structure and the function of the brain. However, since most of these changes have been studied in clinical populations, their time course and mechanisms are unknown.
Preliminary studies using a rat model of TMJ pain [13,170] suggest that modifications in functional brain connectivity occur within the first week corresponding to when joint pain develops. 18F-FDG PET imaging of resting-state brains was collected at baseline before any stimulation and at day 7 (after 1 week of TMJ loading). Brain networks were constructed from PET scans to assess the topological relationships between brain regions. In order to characterize the presence and organization of functional domains, community detection techniques were used to identify modules [171–173] within the brain activity networks. At day 7, when pain is present [13], there is evidence of functional reorganization of brain regions in these modules (Fig. 6); there is also a transition from all regions of the thalamus clustering in module 1 (at baseline) to splitting these regions into modules 1 and 3 (at day 7) (Fig. 6). There is similar evidence for thalamic reorganization in TMJ pain in humans [82] and it is also implicated in neuropathic pain [166]. These findings also align with research in nonhuman primates that shows somatotopic reorganization of the ventral posterior thalamus in response to peripheral nerve injury [174]. Despite evidence for early thalamic changes in brain networks due to joint loading, additional work looking at brain network signatures in acute and chronic TMJ pain could help further elucidate possible supraspinal mechanisms that contribute to the maintenance of joint pain.
Fig. 6.
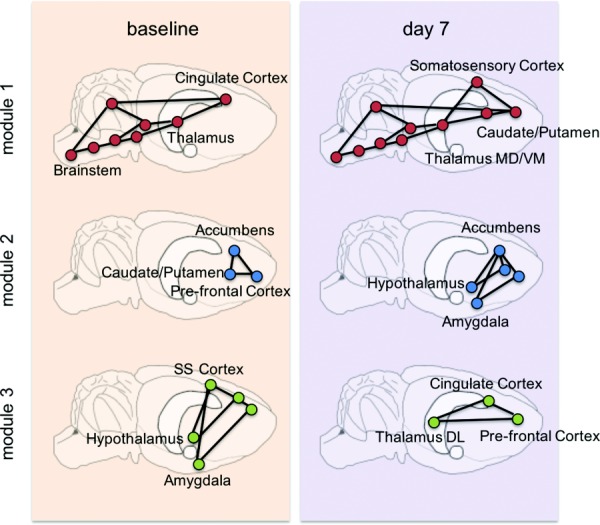
The brain can be represented as a network: a collection of brain regions (nodes; circles) and connections between them (edges; lines). Applying this methodology to TMJ loading-induced pain, modifications in functional modules between baseline and after TMJ loading (day 7) are evident, as highlighted by changes in module-affiliation of brain regions.
Summary
Facet joint and TMJ pain syndromes are complex disorders with mechanical, molecular, and nociceptive contributors. Despite some evidence of associations between these components, there is still a limited understanding of how mechanical forces that act on these joints and their tissues initiate and sustain pain. The development of in vitro systems that can specifically modulate isolated factors can be helpful in uncovering mechanisms relevant to the generation of joint pain. Such systems are particularly useful for evaluating combined effects of loading and molecular mediators on neuronal activation, such as degenerative enzymes and other potentially sensitizing molecules. However, such models must be combined with both in vivo and in silico approaches to develop the most comprehensive understanding of pain. When translating findings into in vivo findings in animals and/or humans, different aspects of the pain experience can be measured. Combined measurement of evoked and spontaneous pain responses captures both the sensory, and potentially, the affective components of pain. Further, changes in CNS connectivity that are believed to maintain pain can be measured using functional imaging approaches. Functional brain imaging of in vivo models after joint loading and with treatment may clarify mechanisms of pain centralization. Overall, diagnostics are predicted to focus on imaging of active biological processes in painful joints, targeting critical molecular processes that mediate chronic pain.
Acknowledgment
The authors acknowledge Dr. Timothy Holsgrove for help in performing the facet joint staining included in Fig. 1. This work was funded by grants from the National Institutes of Health (U01EB016638) and the Catherine Sharpe Foundation, as well as a fellowship (MMS) from the Penn Center for Musculoskeletal Disorders (NIH P30-AR06919). The authors' professional affiliation does not bias this presentation.
Contributor Information
Megan M. Sperry, Department of Bioengineering, , University of Pennsylvania, , 240 Skirkanich Hall, , 210 S. 33rd Street, , Philadelphia, PA 19104-6321 , e-mail: sperrym@seas.upenn.edu
Meagan E. Ita, Department of Bioengineering, , University of Pennsylvania, , 240 Skirkanich Hall, , 210 S. 33rd Street, , Philadelphia, PA 19104-6321 , e-mail: meita@seas.upenn.edu
Sonia Kartha, Department of Bioengineering, , University of Pennsylvania, , 240 Skirkanich Hall, , 210 S. 33rd Street, , Philadelphia, PA 19104-6321 , e-mail: skartha@seas.upenn.edu.
Sijia Zhang, Department of Bioengineering, , University of Pennsylvania, , 240 Skirkanich Hall, , 210 S. 33rd Street, , Philadelphia, PA 19104-6321 , e-mail: sijiaz@seas.upenn.edu.
Ya-Hsin Yu, Department of Endodontics, , School of Dental Medicine, , University of Pennsylvania, , 240 Skirkanich Hall, , 210 S. 33rd Street, , Philadelphia, PA 19104-6321 , e-mail: yayu@upenn.edu.
Beth Winkelstein, Departments of Bioengineering , and Neurosurgery, , University of Pennsylvania, , 240 Skirkanich Hall, , 210 S. 33rd Street, , Philadelphia, PA 19104-6321 , e-mail: winkelst@seas.upenn.edu.
References
- [1].NIDCR, 2014, “ Facial Pain,” National Institute of Dental and Craniofacial Research, Bethesda, MD, accessed Aug. 3, 2016, http://www.nidcr.nih.gov/datastatistics/finddatabytopic/facialpain/
- [2].Institute of Medicine of the National Academies, 2011, “ Relieving Pain in America,” National Academies Press, Washington DC.
- [3]. Gaskin, D. J. , and Richard, P. , 2012, “ The Economic Costs of Pain in the United States,” J. Pain, 13(8), pp. 715–724. 10.1016/j.jpain.2012.03.009 [DOI] [PubMed] [Google Scholar]
- [4]. Turk, D. C. , 2002, “ Psychological Factors in Chronic Pain: Evolution and Revolution,” J. Consult. Clin. Psychol., 70(3), pp. 678–690. 10.1037/0022-006X.70.3.678 [DOI] [PubMed] [Google Scholar]
- [5]. Breivik, H. , Collett, B. , Ventafridda, V. , and Cohen, R. , 2006, “ Survey of Chronic Pain in Europe: Prevalence, Impact on Daily Life, and Treatment,” Eur. J. Pain, 10(4), pp. 287–333. 10.1016/j.ejpain.2005.06.009 [DOI] [PubMed] [Google Scholar]
- [6]. Hogg-Johnson, S. , van der Velde, G. , Carroll, L. J. , Holm, L. W. , Cassidy, J. D. , Guzman, J. , Frcp, C. , Co, P. , Haldeman, S. , Ammendolia, C. , Carragee, E. , Hurwitz, E. , Nordin, M. , Peloso, P. , and Frcp, C. , 2010, “ The Burden and Determinants of Neck Pain in the General Population Results of the Bone and Joint Decade 2000–2010 Task Force on Neck Pain and Its Associated Disorders,” Spine, 33(4), pp. 39–51. 10.1007/s00586-008-0624-y [DOI] [PubMed] [Google Scholar]
- [7]. Hussain, A. M. , Packota, G. , Major, P. W. , and Flores-Mir, C. , 2008, “ Role of Different Imaging Modalities in Assessment of Temporomandibular Joint Erosions and Osteophytes: A Systematic Review,” Dentomaxillofacial Radiol., 37(2), pp. 63–71. 10.1259/dmfr/16932758 [DOI] [PubMed] [Google Scholar]
- [8]. Scrivani, S. J. , Keith, D. A. , and Kaban, L. B. , 2008, “ Temporomandibular Disorders,” N. Engl. J. Med., 359(25), pp. 2693–2705. 10.1056/NEJMra0802472 [DOI] [PubMed] [Google Scholar]
- [9]. Tanaka, E. , Detamore, M. S. , and Mercuri, L. G. , 2008, “ Degenerative Disorders of the Temporomandibular Joint: Etiology, Diagnosis, and Treatment,” J. Dent. Res., 87(4), pp. 296–307. 10.1177/154405910808700406 [DOI] [PubMed] [Google Scholar]
- [10]. Mow, V. C. , Ateshian, G. A. , and Spilker, R. L. , 1993, “ Biomechanics of Diarthrodial Joints: A Review of Twenty Years of Progress,” ASME J. Biomech. Eng., 115(4B), pp. 460–467. 10.1115/1.2895525 [DOI] [PubMed] [Google Scholar]
- [11]. Quinn, K. P. , and Winkelstein, B. A. , 2007, “ Cervical Facet Capsular Ligament Yield Defines the Threshold for Injury and Persistent Joint-Mediated Neck Pain,” J. Biomech., 40(10), pp. 2299–2306. 10.1016/j.jbiomech.2006.10.015 [DOI] [PubMed] [Google Scholar]
- [12]. Ko, F. C. , Dragomir, C. , Plumb, D. A. , Goldring, S. R. , Wright, T. M. , Goldring, M. B. , and van der Meulen, M. C. H. , 2013, “ In Vivo Cyclic Compression Causes Cartilage Degeneration and Subchondral Bone Changes in Mouse Tibiae,” Arthritis Rheumatol., 65(6), pp. 1569–1578. 10.1002/art.37906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13]. Kartha, S. , Zhou, T. , Granquist, E. J. , and Winkelstein, B. A. , 2016, “ Development of a Rat Model of Mechanically Induced Tunable Pain and Associated Temporomandibular Joint Responses,” J. Oral Maxillofac. Surg., 74(1), pp. 54.e1–54.e10. 10.1016/j.joms.2015.09.005 [DOI] [PubMed] [Google Scholar]
- [14]. Zhang, S. , Cao, X. , Stablow, A. M. , Shenoy, V. B. , and Winkelstein, B. A. , 2016, “ Tissue Strain Reorganizes Collagen With a Switchlike Response That Regulates Neuronal Extracellular Signal-Regulated Kinase Phosphorylation In Vitro: Implications for Ligamentous Injury and Mechanotransduction,” ASME J. Biomech. Eng., 138(2), p. 21013. 10.1115/1.4031975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15]. Winkelstein, B. , 2012, “ How can Animal Models Inform on the Transition to Chronic Symptoms in Whiplash?” Spine, 36(25S), pp. 1–16. 10.1097/BRS.0b013e3182387f96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16]. Cohen, S. P. , Huang, J. H. Y. , and Brummett, C. , 2013, “ Facet Joint Pain–Advances in Patient Selection and Treatment,” Nat. Rev. Rheumatol., 9(2), pp. 101–116. 10.1038/nrrheum.2012.198 [DOI] [PubMed] [Google Scholar]
- [17]. Côté, P. , van der Velde, G. , David Cassidy, J. , Carroll, L. J. , Hogg-Johnson, S. , Holm, L. W. , Carragee, E. J. , Haldeman, S. , Nordin, M. , Hurwitz, E. L. , Guzman, J. , and Peloso, P. M. , 2008, “ The Burden and Determinants of Neck Pain in Workers,” Eur. Spine J., 17(4), pp. 60–74. 10.1007/s00586-008-0626-9 [DOI] [PubMed] [Google Scholar]
- [18]. Lee, K. E. , Davis, M. B. , Mejilla, R. M. , and Winkelstein, B. A. , 2004, “ In Vivo Cervical Facet Capsule Distraction: Mechanical Implications for Whiplash & Neck Pain,” Stapp Car Crash J., 48, pp. 373–395.http://spinepain.seas.upenn.edu/Lee2004.pdf [DOI] [PubMed] [Google Scholar]
- [19]. Siegmund, G. P. , Winkelstein, B. A. , Ivancic, P. C. , Svensson, M. Y. , and Vasavada, A. , 2009, “ The Anatomy and Biomechanics of Acute and Chronic Whiplash Injury,” Traffic Inj. Prev., 10(2), pp. 101–112. 10.1080/15389580802593269 [DOI] [PubMed] [Google Scholar]
- [20]. Dong, L. , Smith, J. R. , and Winkelstein, B. A. , 2013, “ Ketorolac Reduces Spinal Astrocytic Activation and PAR1 Expression Associated With Attenuation of Pain After Facet Joint Injury,” J. Neurotrauma, 30(10), pp. 818–825. 10.1089/neu.2012.2600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21]. Cavanaugh, J. M. , 2006, “ Pain Generation in Lumbar and Cervical Facet Joints,” J. Bone Jt. Surg., 88(Suppl 2), pp. 63–67. 10.2106/JBJS.E.01411 [DOI] [PubMed] [Google Scholar]
- [22]. Jaumard, N. V. , Welch, W. C. , and Winkelstein, B. A. , 2011, “ Spinal Facet Joint Biomechanics and Mechanotransduction in Normal, Injury and Degenerative Conditions,” ASME J. Biomech. Eng., 133(7), p. 71010. 10.1115/1.4004493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23]. Kras, J. V. , Kartha, S. , and Winkelstein, B. A. , 2015, “ Intra-Articular Nerve Growth Factor Regulates Development, But Not Maintenance, of Injury-Induced Facet Joint Pain & Spinal Neuronal Hypersensitivity,” Osteoarthritis Cartilage, 23(11), pp. 1999–2008. 10.1016/j.joca.2015.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24]. Loeser, J. D. , and Treede, R. D. , 2008, “ The Kyoto Protocol of IASP Basic Pain Terminology,” Pain, 137(3), pp. 473–477. 10.1016/j.pain.2008.04.025 [DOI] [PubMed] [Google Scholar]
- [25]. Malfait, A.-M. , and Schnitzer, T. J. , 2013, “ Towards a Mechanism-Based Approach to Pain Management in Osteoarthritis,” Nat. Rev. Rheumatol., 9(11), pp. 654–664. 10.1038/nrrheum.2013.138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26]. Woolf, C. J. , 2011, “ Central Sensitization: Implications for the Diagnosis and Treatment of Pain,” Pain, 152(Suppl 3), pp. S2–S15. 10.1016/j.pain.2010.09.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27]. Bushnell, M. C. , Marta, Č. , and Low, L. A. , 2013, “ Cognitive and Emotional Control of Pain and Its Disruption in Chronic Pain,” Nat. Rev. Neurosci., 14(7), pp. 502–511. 10.1038/nrn3516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28]. Loeser, J. D. , and Melzack, R. , 1999, “ Pain: An Overview,” Lancet, 353(9164), pp. 1607–1609. 10.1016/S0140-6736(99)01311-2 [DOI] [PubMed] [Google Scholar]
- [29]. Woolf, C. J. , and Salter, M. W. , 2000, “ Neuronal Plasticity: Increasing the Gain in Pain,” Science, 288(5472), pp. 1765–1769. 10.1126/science.288.5472.1765 [DOI] [PubMed] [Google Scholar]
- [30]. Hu, H.-J. , and Gereau, R. W. , 2003, “ ERK Integrates PKA and PKC Signaling in Superficial Dorsal Horn Neurons—II: Modulation of Neuronal Excitability,” J. Neurophysiol., 90(3), pp. 1680–1688. 10.1152/jn.00341.2003 [DOI] [PubMed] [Google Scholar]
- [31]. Impey, S. , Obrietan, K. , and Storm, D. R. , 1999, “ Making New Connections: Role of ERK/MAP Kinase Signaling in Neuronal Plasticity,” Neuron, 23(1), pp. 11–14. 10.1016/S0896-6273(00)80747-3 [DOI] [PubMed] [Google Scholar]
- [32]. Schaible, H. G. , and Grubb, B. D. , 1993, “ Afferent and Spinal Mechanisms of Joint Pain,” Pain, 55(1), pp. 5–54. 10.1016/0304-3959(93)90183-P [DOI] [PubMed] [Google Scholar]
- [33]. Syré, P. P. , Weisshaar, C. L. , and Winkelstein, B. A. , 2014, “ Sustained Neuronal Hyperexcitability is Evident in the Thalamus After a Transient Cervical Radicular Injury,” Spine, 39(15), pp. E870–E877. 10.1097/BRS.0210030210030392 [DOI] [PubMed] [Google Scholar]
- [34]. Crosby, N. D. , Gilliland, T. M. , and Winkelstein, B. A. , 2014, “ Early Afferent Activity From the Facet Joint After Painful Trauma to Its Capsule Potentiates Neuronal Excitability and Glutamate Signaling in the Spinal Cord,” Pain, 155(9), pp. 1878–1887. 10.1016/j.pain.2014.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35]. Ita, M. , Crosby, N. , Bulka, B. , and Winkelstein, B. , 2016, “ Painful Cervical Facet Joint Injury is Accompanied by Changes in the Number of Excitatory and Inhibitory Synapses in the Superficial Dorsal Horn That Deferentially Relate to Local Tissue Injury Severity,” Spine, 39(3), pp. 207–212. 10.1097/BRS.0210030210031934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36]. Winkelstein, B. , 2006, “ Pain & Injury: Mechanisms of Central Sensitization, CNS Nociception, Injury Biomechanics, and Implications for Muscuoloskeletal Disorders,” Occupational Ergonomics Handbook, CRC Press, Boca Raton, FL, pp. 16.1–16.16. [Google Scholar]
- [37]. Yoganandan, N. , Knowles, S. A. , Maiman, D. J. , and Pintar, F. A. , 2003, “ Anatomic Study of the Morphology of Human Cervical Facet Joint,” Spine, 28(20), pp. 2317–2323. 10.1097/01.BRS.0000085356.89103.A5 [DOI] [PubMed] [Google Scholar]
- [38]. Louis, R. , 1985, “ Spinal Stability as Defined by the Three-Column Spine Concept,” Anat. Clin., 7(1), pp. 33–42. 10.1007/BF01654627 [DOI] [PubMed] [Google Scholar]
- [39]. Pal, G. P. , and Sherk, H. H. , 1988, “ The Vertical Stability of the Cervical Spine,” Spine, 13(5), pp. 447–449. 10.1097/00007632-198805000-00001 [DOI] [PubMed] [Google Scholar]
- [40]. Mercer, S. , and Bogduk, N. , 1993, “ Intra-Articular Inclusions of the Cervical Synovial Joints,” Br. J. Rheumatol., 32(8), pp. 705–710. 10.1093/rheumatology/32.8.705 [DOI] [PubMed] [Google Scholar]
- [41]. Yahia, L. , and Garzon, S. , 1993, “ Structure on the Capsular Ligaments of the Facet Joints,” Ann. Anat., 175(2), pp. 185–188. 10.1016/S0940-9602(11)80179-2 [DOI] [PubMed] [Google Scholar]
- [42]. Yamashita, T. , Minaki, Y. , Ozaktay, A. C. , Cavanaugh, J. M. , and King, A. I. , 1996, “ A Morphological Study of the Fibrous Capsule of the Human Lumbar Facet Joint,” Spine, 21(5), pp. 538–543. 10.1097/00007632-199603010-00002 [DOI] [PubMed] [Google Scholar]
- [43]. Sato, S. , Oguma, H. , Murakami, G. , and Noriyasu, S. , 2002, “ Morphometrical Study of the Joint Surface and Capsule of the Lumbar Zygapophysial Joint With Special Reference to Their Laterality,” Okajimas Folia Anat. Jpn., 79(1), pp. 43–53. 10.2535/ofaj.79.43 [DOI] [PubMed] [Google Scholar]
- [44]. McLain, R. , 1994, “ Mechanoreceptor Endings in Human Cervical Facet Joints,” Spine, 19(5), pp. 495–501. 10.1097/00007632-199403000-00001 [DOI] [PubMed] [Google Scholar]
- [45]. Chen, C. , Lu, Y. , Kallakuri, S. , Patwardhan, A. , and Cavanaugh, J. M. , 2006, “ Distribution of A-δ and C-Fiber Receptors in the Cervical Facet Joint Capsule,” J. Bone Jt. Surg., 88(8), pp. 1807–1816. 10.2106/JBJS.E.00880 [DOI] [PubMed] [Google Scholar]
- [46]. Kalichman, L. , and Hunter, D. J. , 2008, “ The Genetics of Intervertebral Disc Degeneration. Associated Genes,” Jt., Bone, Spine, 75(4), pp. 388–396. 10.1016/j.jbspin.2007.11.002 [DOI] [PubMed] [Google Scholar]
- [47]. Inami, S. , Shiga, T. , Tsujino, A. , Yabuki, T. , Okado, N. , and Ochiai, N. , 2001, “ Immunohistochemical Demonstration of Nerve Fibers in the Synovial Fold of the Human Cervical Facet Joint,” J. Orthop. Res., 19(4), pp. 593–596. 10.1016/S0736-0266(00)00048-6 [DOI] [PubMed] [Google Scholar]
- [48]. Kallakuri, S. , Li, Y. , Chen, C. , and Cavanaugh, J. M. , 2012, “ Innervation of Cervical Ventral Facet Joint Capsule: Histological Evidence,” World J. Orthop., 3(2), pp. 10–14. 10.5312/wjo.v3.i2.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49]. Kallakuri, S. , Singh, A. , Chen, C. , and Cavanaugh, J. M. , 2004, “ Demonstration of Substance P, Calcitonin Gene-Related Peptide, and Protein Gene Product 9.5 Containing Nerve Fibers in Human Cervical Facet Joint Capsules,” Spine, 29(11), pp. 1182–1186. 10.1097/00007632-200406010-00005 [DOI] [PubMed] [Google Scholar]
- [50]. Kras, J. V. , Weisshaar, C. L. , Quindlen, J. , and Winkelstein, B. A. , 2013, “ Brain-Derived Neurotrophic Factor is Upregulated in the Cervical Dorsal Root Ganglia and Spinal Cord and Contributes to the Maintenance of Pain From Facet Joint Injury in the Rat,” J. Neurosci. Res., 91(10), pp. 1312–1321. 10.1002/jnr.23254 [DOI] [PubMed] [Google Scholar]
- [51]. Lu, Y. , Chen, C. , Kallakuri, S. , Patwardhan, A. , and Cavanaugh, J. M. , 2005, “ Neurophysiological and Biomechanical Characterization of Goat Cervical Facet Joint Capsules,” J. Orthop. Res., 23(4), pp. 779–787. 10.1016/j.orthres.2005.01.002 [DOI] [PubMed] [Google Scholar]
- [52]. Anderst, W. J. , Donaldson, W. F. , Lee, J. Y. , and Kang, J. D. , 2014, “ In Vivo Cervical Facet Joint Capsule Deformation During Flexion-Extension,” Spine, 39(8), pp. E514–E520. 10.1097/BRS.0210030210030235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53]. Holsgrove, T. P. , Jaumard, N. V. , Zhu, N. , Stiansen, N. S. , Welch, W. C. , and Winkelstein, B. A. , 2016, “ Upper Cervical Spine Loading Simulating a Dynamic Low-Speed Collision Significantly Increases the Risk of Pain Compared to Quasistatic Loading With Equivalent Neck Kinematics,” ASME J. Biomech. Eng., 138(12), p. 121006. 10.1115/1.4034707 [DOI] [PubMed] [Google Scholar]
- [54]. Panjabi, M. M. , Cholewicki, J. , Nibu, K. , Grauer, J. , and Vahldiek, M. , 1998, “ Capsular Ligament Stretches During In Vitro Whiplash Simulations,” J. Spinal Disord., 11(3), pp. 227–232.http://journals.lww.com/jspinaldisorders/Abstract/1998/06000/Capsular_Ligament_Stretches_During_In_Vitro.9.aspx [PubMed] [Google Scholar]
- [55]. Pearson, A. M. , Ivancic, P. C. , Ito, S. , and Panjabi, M. M. , 2004, “ Facet Joint Kinematics and Injury Mechanisms During Simulated Whiplash,” Spine, 29(4), pp. 390–397. 10.1097/01.BRS.0000090836.50508.F7 [DOI] [PubMed] [Google Scholar]
- [56]. Sundararajan, S. , Prasad, P. , Demetropoulos, C. K. , Tashman, S. , Begeman, P. C. , Yang, K. H. , and King, A. I. , 2004, “ Effect of Head-Neck Position on Cervical Facet Stretch of Post Mortem Human Subjects During Low Speed Rear End Impacts,” Stapp Car Crash J., 48, pp. 331–372.https://trid.trb.org/view.aspx?id=755318 [DOI] [PubMed] [Google Scholar]
- [57]. Winkelstein, B. A. , Nightingale, R. W. , Richardson, W. J. , and Myers, B. S. , 2000, “ The Cervical Facet Capsule and Its Role in Whiplash Injury: A Biomechanical Investigation,” Spine, 25(10), pp. 1238–1246. 10.1097/00007632-200005150-00007 [DOI] [PubMed] [Google Scholar]
- [58]. Elliott, J. M. , Noteboom, J. T. , Flynn, T. W. , and Sterling, M. , 2009, “ Characterization of Acute and Chronic Whiplash-Associated Disorders,” J. Orthop. Sports Phys. Ther., 39(5), pp. 312–323. 10.2519/jospt.2009.2826 [DOI] [PubMed] [Google Scholar]
- [59]. Sterling, M. , Hodkinson, E. , Pettiford, C. , Souvlis, T. , and Curatolo, M. , 2008, “ Psychologic Factors are Related to Some Sensory Pain Thresholds But Not Nociceptive Flexion Reflex Threshold in Chronic Whiplash,” Clin. J. Pain, 24(2), pp. 124–130. 10.1097/AJP.0b013e31815ca293 [DOI] [PubMed] [Google Scholar]
- [60]. Sterling, M. , and Kenardy, J. , 2008, “ Physical and Psychological Aspects of Whiplash: Important Considerations for Primary Care Assessment,” Man. Ther., 13(2), pp. 93–102. 10.1016/j.math.2007.11.003 [DOI] [PubMed] [Google Scholar]
- [61]. Lee, K. E. , Davis, M. B. , and Winkelstein, B. A. , 2008, “ Capsular Ligament Involvement in the Development of Mechanical Hyperalgesia After Facet Joint Loading: Behavioral and Inflammatory Outcomes in a Rodent Model of Pain,” J. Neurotrauma, 25(11), pp. 1383–1393. 10.1089/neu.2008.0700 [DOI] [PubMed] [Google Scholar]
- [62]. Quinn, K. P. , Dong, L. , Golder, F. J. , and Winkelstein, B. A. , 2010, “ Neuronal Hyperexcitability in the Dorsal Horn After Painful Facet Joint Injury,” Pain, 151(2), pp. 414–421. 10.1016/j.pain.2010.07.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63]. Dong, L. , Quindlen, J. C. , Lipschutz, D. E. , and Winkelstein, B. A. , 2012, “ Whiplash-Like Facet Joint Loading Initiates Glutamatergic Responses in the DRG and Spinal Cord Associated With Behavioral Hypersensitivity,” Brain Res., 1461, pp. 51–63. 10.1016/j.brainres.2012.04.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64]. Lee, K. E. , Thinnes, J. H. , Gokhin, D. S. , and Winkelstein, B. A. , 2004, “ A Novel Rodent Neck Pain Model of Facet-Mediated Behavioral Hypersensitivity: Implications for Persistent Pain and Whiplash Injury,” J. Neurosci. Methods, 137(2), pp. 151–159. 10.1016/j.jneumeth.2004.02.021 [DOI] [PubMed] [Google Scholar]
- [65]. Dong, L. , and Winkelstein, B. A. , 2010, “ Simulated Whiplash Modulates Expression of the Glutamatergic System in the Spinal Cord Dynamic Cervical Facet Loading,” J. Neurotrauma, 27(1), pp. 163–174. 10.1089/neu.2009.0999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66]. Manchikanti, L. , Boswell, M. V. , Singh, V. , Pampati, V. , Damron, K. S. , and Beyer, C. D. , 2004, “ Prevalence of Facet Joint Pain in Chronic Spinal Pain of Cervical, Thoracic, and Lumbar Regions,” BMC Musculoskeletal Disord., 5(1), p. 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67]. Norris, S. , and Watt, I. , 1983, “ The Prognosis of Neck Injuries Resulting From Rear-End Vehicle Collisions,” J. Bone Jt. Surg., 65(5), pp. 608–611.http://www.bjj.boneandjoint.org.uk/content/65-B/5/608.short [DOI] [PubMed] [Google Scholar]
- [68]. Sterling, M. , Jull, G. , Vicenzino, B. , and Kenardy, J. , 2003, “ Sensory Hypersensitivity Occurs Soon After Whiplash Injury and is Associated With Poor Recovery,” Pain, 104(3), pp. 509–517. 10.1016/S0304-3959(03)00078-2 [DOI] [PubMed] [Google Scholar]
- [69]. Crosby, N. D. , Weisshaar, C. L. , and Winkelstein, B. A. , 2013, “ Spinal Neuronal Plasticity is Evident Within 1 Day After a Painful Cervical Facet Joint Injury,” Neurosci. Lett., 542, pp. 102–106. 10.1016/j.neulet.2013.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70]. Rothman, S. M. , Huang, Z. , Lee, K. E. , Weisshaar, C. L. , and Winkelstein, B. A. , 2009, “ Cytokine mRNA Expression in Painful Radiculopathy,” J. Pain, 10(1), pp. 90–99. 10.1016/j.jpain.2008.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71]. Scott, D. , Jull, G. , and Sterling, M. , 2005, “ Widespread Sensory Hypersensitivity is a Feature of Chronic Whiplash-Associated Disorder But Not Chronic Idiopathic Neck Pain,” Clin. J. Pain, 21(2), pp. 175–181. 10.1097/00002508-200503000-00009 [DOI] [PubMed] [Google Scholar]
- [72]. Takahashi, Y. , and Nakajima, Y. , 1996, “ Dermatomes in the Rat Limbs as Determined by Antidromic Stimulation of Sensory C-Fibers in Spinal Nerves,” Pain, 67(1), pp. 197–202. 10.1016/0304-3959(96)03116-8 [DOI] [PubMed] [Google Scholar]
- [73]. Rees, L. A. , 1954, “ The Structure and Function of the Mandibular Joint,” Br. Dent. J., 96(6), pp. 125–133. [Google Scholar]
- [74]. Bade, H. , 1999, “ The Function of the Disco-Muscular Apparatus in the Human Temporomandibular Joint,” Ann. Anat., 181(1), pp. 65–67. 10.1016/S0940-9602(99)80092-2 [DOI] [PubMed] [Google Scholar]
- [75]. Okeson, J. , 2004, Bell's Orofacial Pains: The Clinical Management of Orofacial Pain, Quintessence, Chicago, IL. [Google Scholar]
- [76]. Dworkin, S. , and Burgess, J. , 1987, “ Orofacial Pain of Psychogenic Origin: Current Concepts and Classification,” J. Am. Dent. Assoc., 115(4), pp. 565–571. 10.1016/S0002-8177(87)54009-0 [DOI] [PubMed] [Google Scholar]
- [77]. Mejersjö, C. , and Hollender, L. , 1984, “ Radiography of the Temporomandibular Joint in Female Patients With TMJ Pain or Dysfunction. A Seven Year Follow-Up,” Acta Radiol.: Diagn., 25(3), pp. 169–176. 10.1177/028418518402500303 [DOI] [PubMed] [Google Scholar]
- [78]. Gallo, L. M. , Chiaravalloti, G. , Iwasaki, L. R. , Nickel, J. C. , and Palla, S. , 2006, “ Mechanical Work During Stress-Field Translation in the Human TMJ,” J. Dent. Res., 85(11), pp. 1006–1010. 10.1177/154405910608501106 [DOI] [PubMed] [Google Scholar]
- [79]. Zarb, G. A. , and Carlsson, G. E. , 1999, “ Temporomandibular Disorders: Osteoarthritis,” J. Orofacial Pain, 13(4), pp. 295–306. [PubMed] [Google Scholar]
- [80]. Sessle, B. J. , 1999, “ The Neural Basis of Temporomandibular Joint and Masticatory Muscle Pain,” J. Orofacial Pain, 13(4), pp. 238–245. [PubMed] [Google Scholar]
- [81]. Suzuki, I. , Harada, T. , Asano, M. , Tsuboi, Y. , Kondo, M. , Gionhaku, N. , Kitagawa, J. , Kusama, T. , and Iwata, K. , 2007, “ Phosphorylation of ERK in Trigeminal Spinal Nucleus Neurons Following Passive Jaw Movement in Rats With Chronic Temporomandibular Joint Inflammation,” J. Orofacial Pain, 21(3), pp. 225–231.https://www.ncbi.nlm.nih.gov/pubmed/17717961 [PubMed] [Google Scholar]
- [82]. Younger, J. W. , Shen, Y. F. , Goddard, G. , and Mackey, S. C. , 2010, “ Chronic Myofascial Temporomandibular Pain is Associated With Neural Abnormalities in the Trigeminal and Limbic Systems,” Pain, 149(2), pp. 222–228. 10.1016/j.pain.2010.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83]. Wilcox, S. L. , Gustin, S. M. , Macey, P. M. , Peck, C. C. , Murray, G. M. , and Henderson, L. A. , 2015, “ Anatomical Changes Within the Medullary Dorsal Horn in Chronic Temporomandibular Disorder Pain,” Neuroimage, 117, pp. 258–266. 10.1016/j.neuroimage.2015.05.014 [DOI] [PubMed] [Google Scholar]
- [84]. Harper, D. E. , Schrepf, A. , and Clauw, D. J. , 2016, “ Pain Mechanisms and Centralized Pain in Temporomandibular Disorders,” J. Dent. Res., 95(10), pp. 1102–1108. 10.1177/0022034516657070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85]. Hawkins, J. L. , and Durham, P. L. , 2016, “ Prolonged Jaw Opening Promotes Nociception and Enhanced Cytokine Expression,” J. Oral Facial Pain Headache, 30(1), pp. 34–41. 10.11607/ofph.1557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86]. Nicoll, S. B. , Hee, C. K. , Davis, M. B. , and Winkelstein, B. A. , 2010, “ A Rat Model of Temporomandibular Joint Pain With Histopathologic Modifications,” J. Orofacial Pain, 24(3), pp. 298–304.http://spinepain.seas.upenn.edu/Nicoll_2010.pdf [PubMed] [Google Scholar]
- [87]. Tanaka, E. , Aoyama, J. , Miyauchi, M. , Takata, T. , Hanaoka, K. , Iwabe, T. , and Tanne, K. , 2005, “ Vascular Endothelial Growth Factor Plays an Important Autocrine/Paracrine Role in the Progression of Osteoarthritis,” Histochem. Cell Biol., 123(3), pp. 275–281. 10.1007/s00418-005-0773-6 [DOI] [PubMed] [Google Scholar]
- [88]. De Rantere, D. , Schuster, C. J. , Reimer, J. N. , and Pang, D. S. J. , 2016, “ The Relationship Between the Rat Grimace Scale and Mechanical Hypersensitivity Testing in Three Experimental Pain Models,” Eur. J. Pain, 20(3), pp. 417–426. 10.1002/ejp.742 [DOI] [PubMed] [Google Scholar]
- [89]. Sotocinal, S. G. , Sorge, R. E. , Zaloum, A. , Tuttle, A. H. , Martin, L. J. , Wieskopf, J. S. , Mapplebeck, J. C. , Wei, P. , Zhan, S. , Zhang, S. , McDougall, J. J. , King, O. D. , and Mogil, J. S. , 2011, “ The Rat Grimace Scale: A Partially Automated Method for Quantifying Pain in the Laboratory Rat Via Facial Expressions,” Mol. Pain, 7(1), p. 55. 10.1186/1744-8069-7-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90]. Benito, M. , Veale, D. , FitzGerald, O. , van den Berg, W. B. , and Bresnihan, B. , 2005, “ Synovial Tissue Inflammation in Early and Late Osteoarthritis,” Ann. Rheum. Dis., 64(9), pp. 1263–1267. 10.1136/ard.2004.025270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91]. Bechtold, T. E. , Saunders, C. , Decker, R. S. , Um, H. , Bin, Cottingham, N. , Salhab, I. , Kurio, N. , Billings, P. C. , Pacifici, M. , Nah, H. D. , and Koyama, E. , 2016, “ Osteophyte Formation and Matrix Mineralization in a TMJ Osteoarthritis Mouse Model are Associated With Ectopic Hedgehog Signaling,” Matrix Biol., 52–54, pp. 339–354. 10.1016/j.matbio.2016.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92]. Wang, X. D. , Kou, X. X. , Mao, J. J. , Gan, Y. H. , and Zhou, Y. H. , 2012, “ Sustained Inflammation Induces Degeneration of the Temporomandibular Joint,” J. Dent. Res., 91(5), pp. 499–505. 10.1177/0022034512441946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93]. Shen, P. , Jiao, Z. , Zheng, J. S. , Xu, W. F. , Zhang, S. Y. , Qin, A. , and Yang, C. , 2015, “ Injecting Vascular Endothelial Growth Factor Into the Temporomandibular Joint Induces Osteoarthritis in Mice,” Sci. Rep., 5, p. 16244. 10.1038/srep16244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94]. Yeh, T.-T. , Wen, Z.-H. , Lee, H.-S. , Lee, C.-H. , Yang, Z. , Jean, Y.-H. , Wu, S.-S. , Nimni, M. E. , and Han, B. , 2008, “ Intra-Articular Injection of Collagenase Induced Experimental Osteoarthritis of the Lumbar Facet Joint in Rats,” Eur. Spine J., 17(5), pp. 734–742. 10.1007/s00586-008-0594-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95]. Gong, K. , Shao, W. , Chen, H. , Wang, Z. , and Luo, Z. J. , 2011, “ Rat Model of Lumbar Facet Joint Osteoarthritis Associated With Facet-Mediated Mechanical Hyperalgesia Induced by Intra-Articular Injection of Monosodium Iodoacetate,” J. Formosan Med. Assoc., 110(3), pp. 145–152. 10.1016/S0929-6646(11)60024-7 [DOI] [PubMed] [Google Scholar]
- [96]. Loeser, R. F. , Goldring, S. R. , Scanzello, C. R. , and Goldring, M. B. , 2012, “ Osteoarthritis: A Disease of the Joint as an Organ,” Arthritis Rheumatol., 64(6), pp. 1697–1707. 10.1002/art.34453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97]. Gellhorn, A. C. , Katz, J. N. , and Suri, P. , 2012, “ Osteoarthritis of the Spine: The Facet Joints,” Nat. Rev. Rheumatol., 9(4), pp. 216–224. 10.1038/nrrheum.2012.199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98]. Neogi, T. , 2014, “ The Epidemiology and Impact of Pain in Osteoarthritis,” Osteoarthritis Cartilage, 21(9), pp. 1145–1153. 10.1016/j.joca.2013.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99]. Perrot, S. , 2015, “ Best Practice & Research Clinical Rheumatology Osteoarthritis Pain,” Best Pract. Res., Clin. Rheumatol., 29(1), pp. 90–97. 10.1016/j.berh.2015.04.017 [DOI] [PubMed] [Google Scholar]
- [100]. Malfait, A. M. , Little, C. B. , and McDougall, J. J. , 2013, “ A Commentary on Modelling Osteoarthritis Pain in Small Animals,” Osteoarthritis Cartilage, 21(9), pp. 1316–1326. 10.1016/j.joca.2013.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101]. Boszczyk, B. , Boszczyk, A. , Korge, A. , Grillhost, A. , Boos, W. , Putz, R. , Milz, S. , and Benjamin, M. , 2003, “ Immunohistochemical Analysis of the Extracellular Matrix in the Posterior Capsule of the Zygapophysial Joints in Patients With Degenerative L4–5 Motion Segment Instability,” J. Neurosurg., 99(1), pp. 27–33.http://thejns.org/doi/abs/10.3171/spi.2003.99.1.0027 [DOI] [PubMed] [Google Scholar]
- [102]. Kim, J. , Ahmadinia, K. , Li, X. , Hamilton, J. L. , Andrews, S. , Haralampus, A. , Xiao, G. , Sohn, H. , You, J. , Seo, Y. , and Gary, S. , 2015, “ Development of an Experimental Animal Model For Lower Back Pain By Percutaneous Injury-Induced Lumbar Facet Joint Osteoarthritis,” J. Cell. Physiol., 230(11), pp. 2837–2847. 10.1002/jcp.25015 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [103]. Shuang, F. , Zhu, J. , Song, K. , Hou, S. , Liu, Y. , Zhang, C. , and Tang, J. , 2014, “ Establishment of a Rat Model of Adjuvant-Induced Osteoarthritis of the Lumbar Facet Joint,” Cell Biochem. Biophys., 70(3), pp. 1545–1551. 10.1007/s12013-014-0091-5 [DOI] [PubMed] [Google Scholar]
- [104]. Kuroki, Y. , Honda, K. , Kijima, N. , Wada, T. , Arai, Y. , Matsumoto, N. , Iwata, K. , and Shirakawa, T. , 2011, “ In Vivo Morphometric Analysis of Inflammatory Condylar Changes in Rat Temporomandibular Joint,” Oral Dis., 17(5), pp. 499–507. 10.1111/j.1601-0825.2010.01782.x [DOI] [PubMed] [Google Scholar]
- [105]. Ikeda, Y. , Yonemitsu, I. , Takei, M. , Shibata, S. , and Ono, T. , 2014, “ Mechanical Loading Leads to Osteoarthritis-Like Changes in the Hypofunctional Temporomandibular Joint in Rats,” Arch. Oral Biol., 59(12), pp. 1368–1376. 10.1016/j.archoralbio.2014.08.010 [DOI] [PubMed] [Google Scholar]
- [106]. Loeser, R. F. , 2009, “ Aging and Osteoarthritis: the Role of Chondrocyte Senescence and Aging Changes in the Cartilage Matrix,” Osteoarthritis Cartilage, 17(8), pp. 971–979. 10.1016/j.joca.2009.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107]. Iatridis, J. C. , Setton, L. A. , Foster, R. J. , Rawlins, B. A. , Weidenbaum, M. , and Mow, V. C. , 1998, “ Degeneration Affects the Anisotropic and Nonlinear Behaviors of Human Anulus Fibrosus in Compression,” J. Biomech., 31(6), pp. 535–544. 10.1016/S0021-9290(98)00046-3 [DOI] [PubMed] [Google Scholar]
- [108]. Mort, J. S. , and Billington, C. J. , 2001, “ Articular Cartilage and Changes in Arthritis: Matrix Degradation,” Arthritis Res. & Therapy, 3(6), pp. 337–341. 10.1186/ar325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109]. Song, R. H. , Tortorella, M. D. , Malfait, A. M. , Alston, J. T. , Yang, Z. , Arner, E. C. , and Griggs, D. W. , 2007, “ Aggrecan Degradation in Human Articular Cartilage Explants is Mediated by Both ADAMTS-4 and ADAMTS-5,” Arthritis Rheumatol., 56(2), pp. 575–585. 10.1002/art.22334 [DOI] [PubMed] [Google Scholar]
- [110]. Pearle, A. D. , Warren, R. F. , and Rodeo, S. A. , 2005, “ Basic Science of Articular Cartilage and Osteoarthritis,” Clin. Sports Med., 24(1), pp. 1–12. 10.1016/j.csm.2004.08.007 [DOI] [PubMed] [Google Scholar]
- [111]. Grant, T. , Chen, C. , Czernuszka, J. , and Thompson, M. , 2015, “ The Mechanical, Structural, and Compositional Changes of Tendon Exposed to Elastase,” Ann. Biomed. Eng., 43(10), pp. 2477–2486. 10.1007/s10439-015-1308-5 [DOI] [PubMed] [Google Scholar]
- [112]. Barbir, A. , Michalek, A. J. , Abbott, R. D. , and Iatridis, J. C. , 2010, “ Effects of Enzymatic Digestion on Compressive Properties of Rat Intervertebral Discs,” J. Biomech., 43(6), pp. 1067–1073. 10.1016/j.jbiomech.2009.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113]. Grenier, S. , Bhargava, M. M. , and Torzilli, P. A. , 2014, “ An In Vitro Model for the Pathological Degradation of Articular Cartilage in Osteoarthritis,” J. Biomech., 47(3), pp. 645–652. 10.1016/j.jbiomech.2013.11.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114]. Adaes, S. , Ferreira-Gomes, J. , Mendonca, M. , Almeida, L. , Castro-Lopes, J. , and Neto, F. , 2015, “ Injury of Primary Afferent Neurons May Contribute to Osteoarthritis Induced Pain: An Experimental Study Using the Collagenase Model in Rats,” Osteoarthritis Cartilage, 23(6), pp. 914–924. 10.1016/j.joca.2015.02.010 [DOI] [PubMed] [Google Scholar]
- [115]. Hill, C. L. , Seo, G. S. , Gale, D. , Totterman, S. , Gale, M. E. , and Felson, D. T. , 2005, “ Cruciate Ligament Integrity in Osteoarthritis of the Knee,” Arthritis Rheumatol., 52(3), pp. 794–799. 10.1002/art.20943 [DOI] [PubMed] [Google Scholar]
- [116]. Usoskin, D. , Zilberter, M. , Linnarsson, S. , Hjerling-Leffler, J. , Uhlén, P. , Harkany, T. , and Ernfors, P. , 2010, “ En Masse in vitro Functional Profiling of the Axonal Mechanosensitivity of Sensory Neurons,” Proc. Natl. Acad. Sci. U. S. A., 107(37), pp. 16336–16341. 10.1073/pnas.0914705107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117]. Hao, J. , and Delmas, P. , 2010, “ Multiple Desensitization Mechanisms of Mechanotransducer Channels Shape Firing of Mechanosensory Neurons,” J. Neurosci., 30(40), pp. 13384–13395. 10.1523/JNEUROSCI.2926-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118]. Dong, L. , Odeleye, A. O. , Jordan-Sciutto, K. L. , and Winkelstein, B. A. , 2008, “ Painful Facet Joint Injury Induces Neuronal Stress Activation in the DRG: Implications for Cellular Mechanisms of Pain,” Neurosci. Lett., 443(2), pp. 90–94. 10.1016/j.neulet.2008.07.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119]. Torres, L. , Dunlop, D. D. , Peterfy, C. , Guermazi, A. , Prasad, P. , Hayes, K. W. , Song, J. , Cahue, S. , Chang, A. , Marshall, M. , and Sharma, L. , 2006, “ The Relationship Between Specific Tissue Lesions and Pain Severity in Persons With Knee Osteoarthritis,” Osteoarthritis Cartilage, 14(10), pp. 1033–1040. 10.1016/j.joca.2006.03.015 [DOI] [PubMed] [Google Scholar]
- [120]. Miller, R. E. , Miller, R. J. , and Malfait, A. M. , 2014, “ Osteoarthritis Joint Pain: The Cytokine Connection,” Cytokine, 70(2), pp. 185–193. 10.1016/j.cyto.2014.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121]. van der Kraan, P. M. , Vitters, E. L. , van de Putte, L. B. , and van den Berg, W. B. , 1989, “ Development of Osteoarthritic Lesions in Mice by ‘Metabolic’ and ‘Mechanical’ Alterations in the Knee Joints,” Am. J. Pathol., 135(6), pp. 1001–1014.http://europepmc.org/backend/ptpmcrender.fcgi?accid=PMC1880494&blobtype=pdf [PMC free article] [PubMed] [Google Scholar]
- [122]. Cheng, J.-K. , and Ji, R.-R. , 2008, “ Intracellular Signaling in Primary Sensory Neurons and Persistent Pain,” Neurochem. Res., 33(10), pp. 1970–1978. 10.1007/s11064-008-9711-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123]. Joseph, E. K. , and Levine, J. D. , 2010, “ Hyperalgesic Priming is Restricted to Isolectin B4-Positive Nociceptors,” Neuroscience, 169(1), pp. 431–435. 10.1016/j.neuroscience.2010.04.082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124]. Sheon, R. P. , 1997, “ Repetitive Strain Injury. 2. Diagnostic and Treatment Tips on Six Common Problems. The Goff Group,” Postgrad. Med., 102(4), pp. 72–78, 81, 85. 10.3810/pgm.1997.10.338 [DOI] [PubMed] [Google Scholar]
- [125]. Reichling, D. B. , and Levine, J. D. , 2009, “ Critical Role of Nociceptor Plasticity in Chronic Pain,” Trends Neurosci., 32(12), pp. 611–618. 10.1016/j.tins.2009.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126]. Aley, K. O. , Messing, R. O. , Mochly-Rosen, D. , and Levine, J. D. , 2000, “ Chronic Hypersensitivity for Inflammatory Nociceptor Sensitization Mediated by the Epsilon Isozyme of Protein Kinase C,” J. Neurosci., 20(12), pp. 4680–4685.http://www.jneurosci.org/content/20/12/4680.long [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127]. Parada, C. A. , Yeh, J. J. , Joseph, E. K. , and Levine, J. D. , 2003, “ Tumor Necrosis Factor Receptor Type-1 in Sensory Neurons Contributes to Induction of Chronic Enhancement of Inflammatory Hyperalgesia in Rat,” Eur. J. Neurosci., 17(9), pp. 1847–1852. 10.1046/j.1460-9568.2003.02626.x [DOI] [PubMed] [Google Scholar]
- [128]. Kras, J. V. , Weisshaar, C. L. , Pall, P. S. , and Winkelstein, B. A. , 2015, “ Pain From Intra-Articular NGF or Joint Injury in the Rat Requires Contributions From Peptidergic Joint Afferents,” Neurosci. Lett., 604, pp. 193–198. 10.1016/j.neulet.2015.07.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129]. Hoheisel, U. , Unger, T. , and Mense, S. , 2007, “ Sensitization of Rat Dorsal Horn Neurons by NGF-Induced Subthreshold Potentials and Low-Frequency Activation. A Study Employing Intracellular Recordings in vivo,” Brain Res., 1169, pp. 34–43. 10.1016/j.brainres.2007.06.054 [DOI] [PubMed] [Google Scholar]
- [130]. Barthel, C. , Yeremenko, N. , Jacobs, R. , Schmidt, R. E. , Bernateck, M. , Zeidler, H. , Tak, P. , Baeten, D. , and Rihl, M. , 2009, “ Nerve Growth Factor and Receptor Expression in Rheumatoid Arthritis and Spondyloarthritis,” Arthritis Res. Ther., 11(3), pp. 1–9. 10.1186/ar2716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131]. Orita, S. , Ishikawa, T. , Miyagi, M. , Ochiai, N. , Inoue, G. , and Eguchi, Y. , 2011, “ Pain-Related Sensory Innervation in Monoiodoacetate-Induced Osteoarthritis in Rat Knees That Gradually Develops Neuronal Injury in Addition to Inflammatory Pain,” BMC Musculoskeletal Disord., 12(134), pp. 1–12. 10.1186/1471-2474-12-134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132]. Kras, J. V. , Tanaka, K. , Gilliland, T. M. , and Winkelstein, B. A. , 2013, “ An Anatomical and Immunohistochemical Characterization of Afferents Innervating the C6-C7 Facet Joint After Painful Joint Loading in the Rat,” Spine, 38(6), pp. E325–E331. 10.1097/BRS.0b013e318285b5bb [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133]. Merighi, A. , Carmignoto, G. , Gobbo, S. , Lossi, L. , Salio, C. , Vergnano, A. M. , and Zonta, M. , 2004, “ Neurotrophins in Spinal Cord Nociceptive Pathways,” Prog. Brain Res., 146, pp. 291–321. 10.1016/S0079-6123(03)46019-6 [DOI] [PubMed] [Google Scholar]
- [134]. Mcmahon, S. B. , 2016, “ NGF as a Mediator of Inflammatory Pain,” R. Soc. Philos. Trans. Biol. Sci., 351(1338), pp. 431–440. 10.1098/rstb.1996.0039 [DOI] [PubMed] [Google Scholar]
- [135]. Mcnamee, K. E. , Burleigh, A. , Gompels, L. L. , Feldmann, M. , Allen, S. J. , Williams, R. O. , Dawbarn, D. , Vincent, T. L. , and Inglis, J. J. , 2010, “ Treatment of Murine Osteoarthritis With TrkAd5 Reveals a Pivotal Role for Nerve Growth Factor in Non-Inflammatory Joint Pain,” Pain, 149(2), pp. 386–392. 10.1016/j.pain.2010.03.002 [DOI] [PubMed] [Google Scholar]
- [136]. Crosby, N. D. , Zaucke, F. , Kras, J. V. , Dong, L. , Luo, Z. D. , and Winkelstein, B. A. , 2015, “ Thrombospondin-4 and Excitatory Synaptogenesis Promote Spinal Sensitization After Painful Mechanical Joint Injury,” Exp. Neurol., 264, pp. 111–120. 10.1016/j.expneurol.2014.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137]. Lee, K. E. , and Winkelstein, B. A. , 2009, “ Joint Distraction Magnitude Is Associated With Different Behavioral Outcomes and Substance P Levels for Cervical Facet Joint Loading in the Rat,” J. Pain, 10(4), pp. 436–445. 10.1016/j.jpain.2008.11.009 [DOI] [PubMed] [Google Scholar]
- [138]. Vincent, K. R. , Conrad, B. P. , Fregly, B. J. , and Vincent, H. K. , 2012, “ The Pathophysiology of Osteoarthritis: A Mechanical Perspective on the Knee Joint,” PM&R, 4(5 Suppl.), pp. S3–S9. 10.1016/j.pmrj.2012.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139]. Bogduk, N. , 2011, “ On Cervical Zygapophysial Joint Pain After Whiplash,” Spine, 36(25S), pp. 194–199. 10.1097/BRS.0b013e3182387f1d [DOI] [PubMed] [Google Scholar]
- [140]. Siegmund, G. P. , Myers, B. S. , Davis, M. B. , Bohnet, H. F. , and Winkelstein, B. A. , 2001, “ Mechanical Evidence of Cervical Facet Capsule Injury During Whiplash,” Spine, 26(19), pp. 2095–2101. 10.1097/00007632-200110010-00010 [DOI] [PubMed] [Google Scholar]
- [141]. Quinn, K. P. , Lee, K. E. , Ahaghotu, C. C. , and Winkelstein, B. A. , 2007, “ Structural Changes in the Cervical Facet Capsular Ligament: Potential Contributions to Pain Following Subfailure Loading,” Stapp Car Crash J., 51, pp. 169–187.http://www.seas.upenn.edu/~winkelst/Quinn_2007.pdf [DOI] [PubMed] [Google Scholar]
- [142]. Quinn, K. P. , and Winkelstein, B. A. , 2008, “ Altered Collagen Fiber Kinematics Define the Onset of Localized Ligament Damage During Loading,” J. Appl. Physiol., 105(6), pp. 1881–1888. 10.1152/japplphysiol.90792.2008 [DOI] [PubMed] [Google Scholar]
- [143]. Quinn, K. P. , and Winkelstein, B. A. , 2009, “ Vector Correlation Technique for Pixel-Wise Detection of Collagen Fiber Realignment During Injurious Tensile Loading,” J. Biomed. Opt., 14(5), pp. 54010-1–54010-10. 10.1117/1.3227037 [DOI] [PubMed] [Google Scholar]
- [144]. Khalsa, P. , Hoffman, A. , and Grigg, P. , 1996, “ Mechanical States Encoded by Stretch-Sensitive Neurons in Feline Joint Capsule,” J. Neurophysiol., 76(1), pp. 175–187.http://jn.physiology.org/content/76/1/175 [DOI] [PubMed] [Google Scholar]
- [145]. Kallakuri, S. , Singh, A. , Lu, Y. , Chen, C. , Patwardhan, A. , and Cavanaugh, J. M. , 2008, “ Tensile Stretching of Cervical Facet Joint Capsule and Related Axonal Changes,” Eur. Spine J., 17(4), pp. 556–563. 10.1007/s00586-007-0562-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146]. Weishaupt, D. , Zanetti, M. , Boos, N. , and Hodler, J. , 1999, “ MR Imaging and CT in Osteoarthritis of the Lumbar Facet Joints,” Skeletal Radiol., 28(4), pp. 215–219. 10.1007/s002560050503 [DOI] [PubMed] [Google Scholar]
- [147]. Tsiklakis, K. , Syriopoulos, K. , and Stamatakis, H. C. , 2004, “ Radiographic Examination of the Temporomandibular Joint Using Cone Beam Computed Tomography,” Dentomaxillofacial Radiol., 33(3), pp. 196–201. 10.1259/dmfr/27403192 [DOI] [PubMed] [Google Scholar]
- [148]. Pathria, M. , Sartoris, D. J. , and Resnick, D. , 1987, “ Osteoarthritis of the Facet Joints: Accuracy of Oblique Radiographic Assessment,” Radiology, 164(1), pp. 227–230. 10.1148/radiology.164.1.3588910 [DOI] [PubMed] [Google Scholar]
- [149]. Duan, C. Y. , Espinoza Orías, A. A. , Shott, S. , An, H. S. , Andersson, G. B. J. , Hu, J. Z. , Lu, H. B. , and Inoue, N. , 2011, “ In Vivo Measurement of the Subchondral Bone Thickness of Lumbar Facet Joint Using Magnetic Resonance Imaging,” Osteoarthritis Cartilage, 19(1), pp. 96–102. 10.1016/j.joca.2010.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150]. Jaumard, N. V. , Udupa, J. K. , Welch, W. C. , and Winkelstein, B. A. , 2014, “ Kinematic Magnetic Resonance Imaging to Define the Cervical Facet Joint Space for the Spine in Neutral and Torsion,” Spine, 39(8), pp. 664–672. 10.1097/BRS.0210030210030206 [DOI] [PubMed] [Google Scholar]
- [151]. Brooks, S. L. , Wesresson, P.-L. , Eriksson, L. , Hansson, L. G. , and Barsotti, J. B. , 1992, “ Prevalence of Osseous Changes in the Temporomandibular Joint of Asymptomatic Persons Without Internal Derangement,” Oral Surg., Oral Med., Oral Pathol., 73(1), pp. 118–122. 10.1016/0030-4220(92)90168-P [DOI] [PubMed] [Google Scholar]
- [152]. Rohlin, M. , Westesson, P. L. , and Eriksson, L. , 1985, “ The Correlation of Temporomandibular Joint Sounds With Joint Morphology in Fifty-Five Autopsy Specimens,” J. Oral Maxillofac. Surg., 43(3), pp. 194–200. 10.1016/0278-2391(85)90159-4 [DOI] [PubMed] [Google Scholar]
- [153]. Kircos, L. T. , Ortendahl, D. A. , Mark, A. S. , and Arakawa, M. , 1987, “ Magnetic Resonance Imaging of the TMJ Disc in Asymptomatic Volunteers,” J. Oral Maxillofac. Surg., 45(10), pp. 852–854. 10.1016/0278-2391(87)90235-7 [DOI] [PubMed] [Google Scholar]
- [154]. Katzberg, R. W. , Tallents, R. H. , and Drake, M. , 1996, “ Anatomic Disorders of the Temporomandibular Joint Disc in Asymptomatic Subjects,” J. Oral Maxillofac. Surg., 54(2), pp. 147–153. 10.1016/S0278-2391(96)90435-8 [DOI] [PubMed] [Google Scholar]
- [155]. Lee, J. W. , Lee, S. M. , Kim, S. J. , Choi, J. W. , and Baek, K. W. , 2013, “ Clinical Utility of Fluoride-18 Positron Emission Tomography/CT in Temporomandibular Disorder With Osteoarthritis: Comparisons With 99mTc-MDP Bone Scan,” Dentomaxillofacial Radiol., 42(2), pp. 1–8. 10.1259/dmfr/29292350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156]. Nakamura, H. , Masuko, K. , Yudoh, K. , Kato, T. , Nishioka, K. , Sugihara, T. , and Beppu, M. , 2007, “ Positron Emission Tomography With 18F-FDG in Osteoarthritic Knee,” Osteoarthritis Cartilage, 15(6), pp. 673–681. 10.1016/j.joca.2006.12.010 [DOI] [PubMed] [Google Scholar]
- [157]. Paquet, J. , Maskali, F. , Poussier, S. , Scala, J. , and Pinzano, A. , 2010, “ Evaluation of a Rat Knee Mono-Arthritis Using MicroPET,” Biomed. Mater. Eng., 20(3–4), pp. 195–202. 10.3233/BME-2010-0632 [DOI] [PubMed] [Google Scholar]
- [158]. Kim, J. H. , Kim, Y. K. , Kim, S. G. , Yun, P. Y. , Kim, J. D. , and Min, J. H. , 2012, “ Effectiveness of Bone Scans in the Diagnosis of Osteoarthritis of the Temporomandibular Joint,” Dentomaxillofacial Radiol., 41(3), pp. 224–229. 10.1259/dmfr/83814366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159]. Guermazi, A. , Roemer, F. W. , Haugen, I. K. , Crema, M. D. , and Hayashi, D. , 2013, “ MRI-Based Semiquantitative Scoring of Joint Pathology in Osteoarthritis,” Nat. Rev. Rheumatol., 9(4), pp. 236–251. 10.1038/nrrheum.2012.223 [DOI] [PubMed] [Google Scholar]
- [160]. Czervionke, L. F. , and Fenton, D. S. , 2008, “ Fat-Saturated MR Imaging in the Detection of Inflammatory Facet Arthropathy (Facet Synovitis) in the Lumbar Spine,” Pain Med., 9(4), pp. 400–406. 10.1111/j.1526-4637.2007.00313.x [DOI] [PubMed] [Google Scholar]
- [161]. Lakadamyali, H. , Tarhan, N. , Ergun, T. , Cakir, B. , and Agildere, A. , 2008, “ STIR Sequence for Depiction of Degenerative Changes in Posterior Stabilizing Elements in Patients With Lower Back Pain,” Musculoskeletal Imaging, 191(4), pp. 973–979.http://www.ajronline.org/doi/abs/10.2214/AJR.07.2829 [DOI] [PubMed] [Google Scholar]
- [162]. Suenaga, S. , Hamamoto, S. , Kawano, K. , Higashida, Y. , and Noikura, T. , 1996, “ Dynamic MR Imaging of the Temporomandibular Joint in Patients With Arthrosis,” Am. J. Roentgenol., 166(6), pp. 1475–1481. 10.2214/ajr.166.6.8633468 [DOI] [PubMed] [Google Scholar]
- [163]. Kortekaas, M. C. , Kwok, W. , Reijnierse, M. , and Kloppenburg, M. , 2015, “ Inflammatory Ultrasound Features Show Independent Associations With Progression of Structural Damage After Over 2 Years of Follow-Up in Patients With Hand Osteoarthritis,” Ann. Rheum. Dis., 74(9), pp. 1720–1724. 10.1136/annrheumdis-2013-205003 [DOI] [PubMed] [Google Scholar]
- [164]. Mathiessen, A. , Slatkowsky-Christensen, B. , Kvien, T. K. , Hammer, H. B. , and Haugen, I. K. , 2016, “ Ultrasound-Detected Inflammation Predicts Radiographic Progression in Hand Osteoarthritis After 5 Years,” Ann. Rheum. Dis., 75(5), pp. 825–830. 10.1136/annrheumdis-2015-207241 [DOI] [PubMed] [Google Scholar]
- [165]. Baliki, M. N. , Mansour, A. R. , Baria, A. T. , and Apkarian, A. V. , 2014, “ Functional Reorganization of the Default Mode Network Across Chronic Pain Conditions,” Plos One, 9(9), p. 106133. 10.1371/journal.pone.0106133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166]. Baliki, M. N. , Chang, P. C. , Baria, A. T. , Centeno, M. V. , and Apkarian, A. V. , 2014, “ Resting-Sate Functional Reorganization of the Rat Limbic System Following Neuropathic Injury,” Sci. Rep., 4, p. 6186. 10.1038/srep06186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167]. Kim, C.-E. , Kim, Y. K. , Chung, G. , Jeong, J. M. , Lee, D. S. , Kim, J. , and Kim, S. J. , 2014, “ Large-Scale Plastic Changes of the Brain Network in an Animal Model of Neuropathic Pain,” Neuroimage, 98, pp. 203–215. 10.1016/j.neuroimage.2014.04.063 [DOI] [PubMed] [Google Scholar]
- [168]. Parks, E. L. , Geha, P. Y. , Baliki, M. N. , Katz, J. , Schnitzer, T. J. , and Apkarian, A. V. , 2011, “ Brain Activity for Chronic Knee Osteoarthritis: Dissociating Evoked Pain From Spontaneous Pain,” Eur. J. Pain, 15(8), pp. 843.e1–14. 10.1016/j.ejpain.2010.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169]. Youssef, A. M. , Gustin, S. M. , Nash, P. G. , Reeves, J. M. , Petersen, E. T. , Peck, C. C. , Murray, G. M. , and Henderson, L. A. , 2014, “ Differential Brain Activity in Subjects With Painful Trigeminal Neuropathy and Painful Temporomandibular Disorder,” Pain, 155(3), pp. 467–475. 10.1016/j.pain.2013.11.008 [DOI] [PubMed] [Google Scholar]
- [170]. Sperry, M. M. , Kartha, S. , Granquist, E. J. , and Winkelstein, B. A. , 2016, “ Meso-Scale Reorganization of Metabolic Brain Networks is Associated With Persistent TMJ Pain,” SB3C Conference, Paper No. 59. [Google Scholar]
- [171]. Reichardt, J. , and Bornholdt, S. , 2006, “ Statistical Mechanics of Community Detection,” Phys. Rev. E, 74(1), pp. 1–16. 10.1103/PhysRevE.74.016110 [DOI] [PubMed] [Google Scholar]
- [172]. Blondel, V. D. , Guillaume, J.-L. , Lambiotte, R. , and Lefebvre, E. , 2008, “ Fast Unfolding of Communities in Large Networks,” J. of Statistical Mechanics: Theory & Experiment, 2008(10), p. P10008. 10.1088/1742-5468/2008/10/P10008 [DOI] [Google Scholar]
- [173]. Rubinov, M. , and Sporns, O. , 2010, “ Complex Network Measures of Brain Connectivity: Uses and Interpretations,” Neuroimage, 52(3), pp. 1059–1069. 10.1016/j.neuroimage.2009.10.003 [DOI] [PubMed] [Google Scholar]
- [174]. Churchill, J. D. , Arnold, L. L. , and Garraghty, P. E. , 2001, “ Somatotopic Reorganization in the Brainstem and Thalamus Following Peripheral Nerve Injury in Adult Primates,” Brain Res., 910(1), pp. 142–152. 10.1016/S0006-8993(01)02703-2 [DOI] [PubMed] [Google Scholar]


