Abstract
Many positive signalling pathways of osteoclastogenesis have been characterized, but negative signalling pathways are less well studied. Here we show by microarray and RNAi that guanine nucleotide-binding protein subunit α13 (Gα13) is a negative regulator of osteoclastogenesis. Osteoclast-lineage-specific Gna13 conditional knockout mice have a severe osteoporosis phenotype. Gna13-deficiency triggers a drastic increase in both osteoclast number and activity (hyper-activation), mechanistically through decreased RhoA activity and enhanced Akt/GSK3β/NFATc1 signalling. Consistently, Akt inhibition or RhoA activation rescues hyper-activation of Gna13-deficient osteoclasts, and RhoA inhibition mimics the osteoclast hyperactivation resulting from Gna13-deficiency. Notably, Gα13 gain-of-function inhibits Akt activation and osteoclastogenesis, and protects mice from pathological bone loss in disease models. Collectively, we reveal that Gα13 is a master endogenous negative switch for osteoclastogenesis through regulation of the RhoA/Akt/GSK3β/NFATc1 signalling pathway, and that manipulating Gα13 activity might be a therapeutic strategy for bone diseases.
Osteoclasts are bone resorptive cells and an attractive target to treat diseases characterized by excessive bone loss, but little is known about osteoclast inhibition. Here the authors identify Gα13 as an intracellular inhibitor of osteoclastogenesis that can prevent bone loss in mice via Akt activation and inhibition of RhoA signalling.
Osteoclasts are the principal, if not exclusive, bone-resorbing cells1,2,3. A balance between bone formation by osteoblasts and bone resorption by osteoclasts is critical to maintain normal bone density and mineral homeostasis1,2,3. Osteoclastogenesis is initiated by signals transmitted by the receptor activator of nuclear factor-κB (RANK) ligand (RANKL) and macrophage colony-stimulating factor (M-CSF)1,2,3,4. RANKL and M-CSF, binding to their receptors, RANK and cFms, respectively, on the surface of osteoclast precursors, activates many key transcription factors including nuclear factor of activated T-cells, c1 (NFATc1) and CCAAT/enhancer-binding protein alpha (C/EBPα)1,2,3,4. Our previous work on cathepsin K5,6, Atp6i (refs 7, 8), C/EBPα9 and RGS10 (ref. 10) has contributed to the discovery of positive regulatory machinery controlling osteoclast differentiation and function. Notably, excessive osteoclast activity is responsible for various bone diseases including osteoporosis, rheumatoid arthritis and Paget's disease1,2,3. Thus, we anticipate that hyperactivation of osteoclasts should be antagonized by intrinsic negative regulators, which have received limited attention, to maintain normal bone homeostasis. A decrease in the expression of negative osteoclast regulators might trigger the bone loss associated with many pathological bone disorders.
Guanine nucleotide-binding protein subunit α13 (Gα13; encoded by Gna13) belongs to the G12 subfamily of the G protein superfamily. Gα13 regulates cell cytoskeleton organization by regulating the RhoGEF-RhoGTPase signalling pathway11,12. Here we utilize a genome-wide screening strategy and characterize Gα13 as an intrinsic negative regulator of osteoclast formation and activity. Absence of Gα13 favours osteoclast formation and enlarges osteoclast size, leading to an osteopenia phenotype in mice. On the other hand, constitutively active Gα13 blocks the formation of multinucleated osteoclasts and their bone resorptive activity. We reveal that Akt-mediated signalling, regulated by RhoA, is critical for the function of Gα13 in osteoclasts.
Results
Gna13 silencing promotes osteoclastogenesis
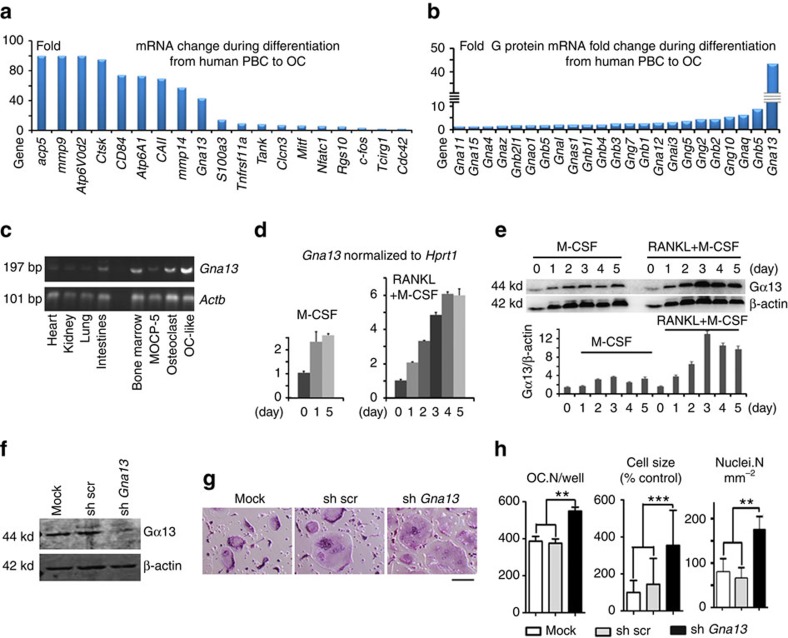
Given the limited attention attributed to the investigation of negative regulators of osteoclastogenesis, we performed a genome-wide screening to identify factors that negatively regulate osteoclast formation (Fig. 1). Towards this end, we compared gene expression in human blood monocyte-derived osteoclasts with that in their precursors, and found that Gna13 was not only drastically induced by RANKL, but also its expression pattern was comparable to various osteoclast genes (Fig. 1a), and much higher than the genes encoding several other G proteins (Fig. 1b). Moreover, gene expression analysis confirmed that Gna13 was highly expressed in osteoclasts and osteoclast-like cells derived from MOCP-5, an osteoclast precursor cell line that was generated in our lab13, as compared with several tissues, such as heart, kidney, lung and intestine (Fig. 1c). Furthermore, using murine bone marrow monocytes (BMMs), which are widely used as primary osteoclast precursors, we demonstrated that whereas Gα13 expression was only mildly induced by M-CSF (∼2-fold at both mRNA and protein level), its expression was strongly induced by combined stimulation with M-CSF and RANKL (∼6-fold at mRNA level and 8-fold at protein level) (Fig. 1d,e). These results indicated that Gα13 might have important roles in osteoclasts. Hence, we then silenced Gα13 expression by lentiviral-mediated expression of short hairpin RNA against Gna13 in BMMs. The knockdown efficiency (∼90%) was confirmed by immunobloting as compared with control cells (Fig. 1f). Interestingly, our data showed that Gα13 silencing strongly increased osteoclast formation and also drastically enhanced osteoclast size (Fig. 1g,h). These results suggested that Gα13 might be a negative regulator of bone resorption.
Figure 1. Gα13 which is induced by RANKL and M-CSF inhibits osteoclastogenesis.
(a,b) Microarray profile of gene expression during osteoclast differentiation. OC, osteoclasts; PBM, peripheral blood monocytes. (c) Semi-quantitative reverse transcription–PCR to detect Gna13 mRNA level in murine tissues and cells. (d) Quantitative reverse transcription–PCR to analyse time-course expression of Gna13, normalized to Hprt1, in murine bone marrow monocytes (BMMs) under the treatment of M-CSF, or M-CSF and RANKL; N≥4. (e) Western blot to analyse time-course expression of Gα13 in BMMs under the treatment of M-CSF or M-CSF and RANKL, and its quantification in lower panel; N≥4. (f) Western blot to analyse Gα13 expression in non-infected BMMs (mock) and BMMs infected with lentivirus expressing scrambled short hairpin RNA (shRNA) (sh-src) or shRNA targeting Gna13 (sh-Gna13). (g) TRAP staining to detect osteoclast formation of mock, sh-src and sh-Gna13 BMMs. (h) Quantification of cell number (OC.N/well and nuclei.N mm−2; N≥10) and relative cell size (N≥100) in g. OC.N/well, osteoclast (TRAP positive multinucleated cell) number per well. Nuclei.N mm−2, osteoclast nuclei number per mm2. Results in d,e,h are expressed as mean±s.d.; **P≤0.01; ***P≤0.001 (Student's t-test). Scale bars, 200 μm.
Depletion of Gna13 causes osteopenia in mice
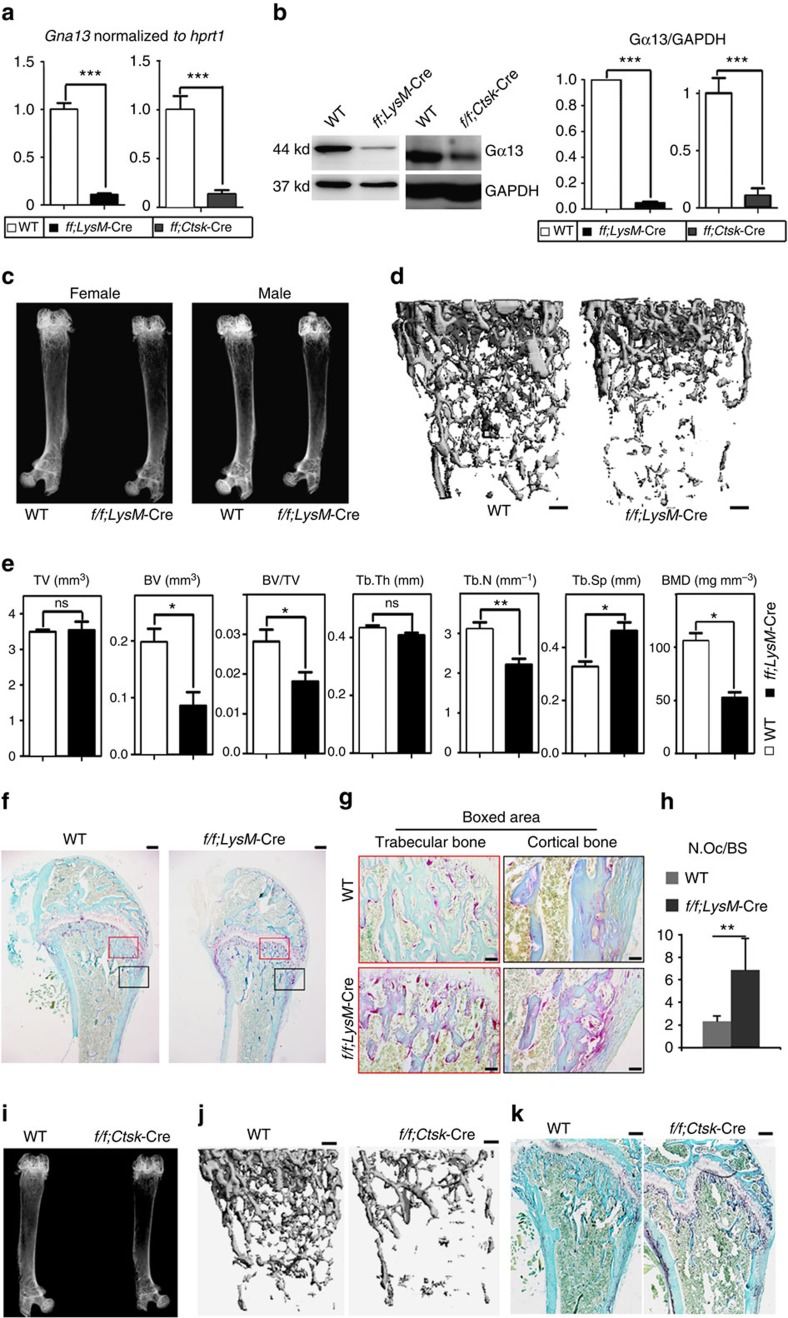
To further investigate the role of Gα13 in osteoclast formation and activity, we generated knock-out mice through specific deletion of Gna13 in the osteoclast lineage (Fig. 2). Mice bearing loxP sites encompassing the Gna13 exon2 (Gna13f/f mice)14 were crossed with those expressing Cre recombinase driven by the lysozyme M promoter (LysM-Cre mice) or the Cathepsin K promoter (Ctsk-Cre mice). The offspring were intercrossed to get Gna13f/fLysM-Cre mice or Gna13f/fCtsk-Cre mice and wild-type (WT) mice. Mouse genotypes were confirmed by PCR (Supplementary Fig. 1a–c). The deletion of Gα13 in Gna13f/fLysM-Cre BMMs and Gna13f/fCtsk-Cre pre-osteoclasts was confirmed by quantitative PCR and immunoblotting (Fig. 2a,b). While LysM-Cre deletes Gna13 in the osteoclast precursors (Fig. 2a,b), Ctsk-Cre works at a late stage of osteoclast differentiation (Supplementary Fig. 1d).
Figure 2. Osteoclast-specific Gna13-CKO mice displayed osteoporosis.
(a) Quantitative reverse transcription–PCR confirmed deletion of Gna13 mRNA in day-1 Gna13f/fLysM-Cre (f/f;LysM-Cre) and day-4 Gna13f/fCtsk-Cre (f/f;Ctsk-Cre) pre-osteoclasts; N=6. (b) Western blot confirmed deletion of Gα13 protein in Gna13f/fLysM-Cre BMMs and day-5 Gna13f/fCtsk-Cre pre-osteoclasts. Quantification is co-presented in the right panels; N=6. (c) Radiographs of two-month-old wild type (WT) and Gna13f/fLysM-Cre male and female mice femurs. (d) μ-CT analysis of two-month-old WT and Gna13f/fLysM-Cre female mice femurs. (e) Quantification from d; N=6. BMD, bone mineral density; BV, bone volume; Tb.Th, trabecular bone thickness; Tb.N, trabecular bone number; Tb.Sp, trabecular bone space; TV, tissue volume. (f) TRAP staining of 2-month-old WT and Gna13f/fLysM-Cre female mice femurs. (g) High magnification images of f. (h) Quantification of N.Oc/BS (osteoclast number / bone surface) in f; N=6. (i) Radiographs of two-month-old WT and Gna13f/fCtsk-Cre female mice femurs. (j) μ-CT analysis of two-month-old WT and Gna13f/fCtsk-Cre female mice femurs. (k) TRAP staining of 2-month-old WT and Gna13f/fCtsk-Cre female mice femurs. WT, wild-type. Results were expressed as mean±s.d.; *P≤0.05; **P≤0.01; ***P≤0.001; ns, not significant (Student's t-test). Scale bars in d,f,j,k, 200 μm; Scale bar in g, 20 μm.
Two-month-old WT and Gna13f/fLysM-Cre or Gna13f/fCtsk-Cre mice have no obvious gross morphological changes. As assessed by X-ray analysis, skeletal mass was decreased in distal femurs of both male and female Gna13f/fLysM-Cre mice as compared with those of WT littermates (Fig. 2c). Quantitative microtomography (μ-CT) analysis showed that Gna13f/fLysM-Cre mice exhibited ∼30% reduction in bone volume/tissue volume (BV/TV) and trabecular number (Tb.N) as well as ∼40% increase in trabecular space (Tb.Sp), ∼50% decreased in bone mineral density (BMD) as compared with WT littermates (Fig. 2d,e). Tartrate-resistant acid phosphatase (TRAP) activity was markedly increased in the primary spongiosa of 2-month old Gna13f/fLysM-Cre mouse femur as compared with that of WT littermates (Fig. 2f–h). Histomorphometric parameters of distal femurs showed that, in the trabecular bone area, Gna13f/fLysM-Cre mice had ∼3-fold increased in osteoclast numbers per bone surface (N.Oc/BS) as compared with WT littermates (Fig. 2g). Interestingly, osteoclast number was even more drastically increased in the cortical bone area in the mutant mice (Fig. 2f,g). Similarly, X-ray and μ-CT analyses showed that skeletal mass was decreased in distal femurs of 2-month-old Gna13f/fCtsk-Cre mice (Fig. 2i,j, Supplementary Fig. 2c). TRAP staining showed ∼6-fold increase in osteoclast numbers in femoral sections of Gna13f/fCtsk-Cre mice as compared with that of WT controls (Fig. 2k, Supplementary Fig. 1d–f). Histomorphometry based on Trichrome staining showed that trabecular bone number was reduced without much change of osteoblast number and osteoblast surface per bone surface in the Gna13f/fCtsk-Cre mice, confirming that the reduced bone density was not caused by decreased bone formation (Supplementary Fig. 2a,b). In fact, as measured by calcein labelling (Supplementary Fig. 2d), mineral apposition rate was increased by about 35% in Gna13f/fCtsk-Cre mice. Consistently, serum alkaline phosphatase (ALP) was also increased by about 35% in Gna13f/fCtsk-Cre mice (Supplementary Fig. 2e). The accelerated bone formation and remodelling might be caused by robust osteoclast formation. These results show that osteoclast-lineage-specific Gna13 deletion promotes osteoclastic bone resorption leading to lower bone density in vivo.
Gna13-depletion hyper-activates osteoclast
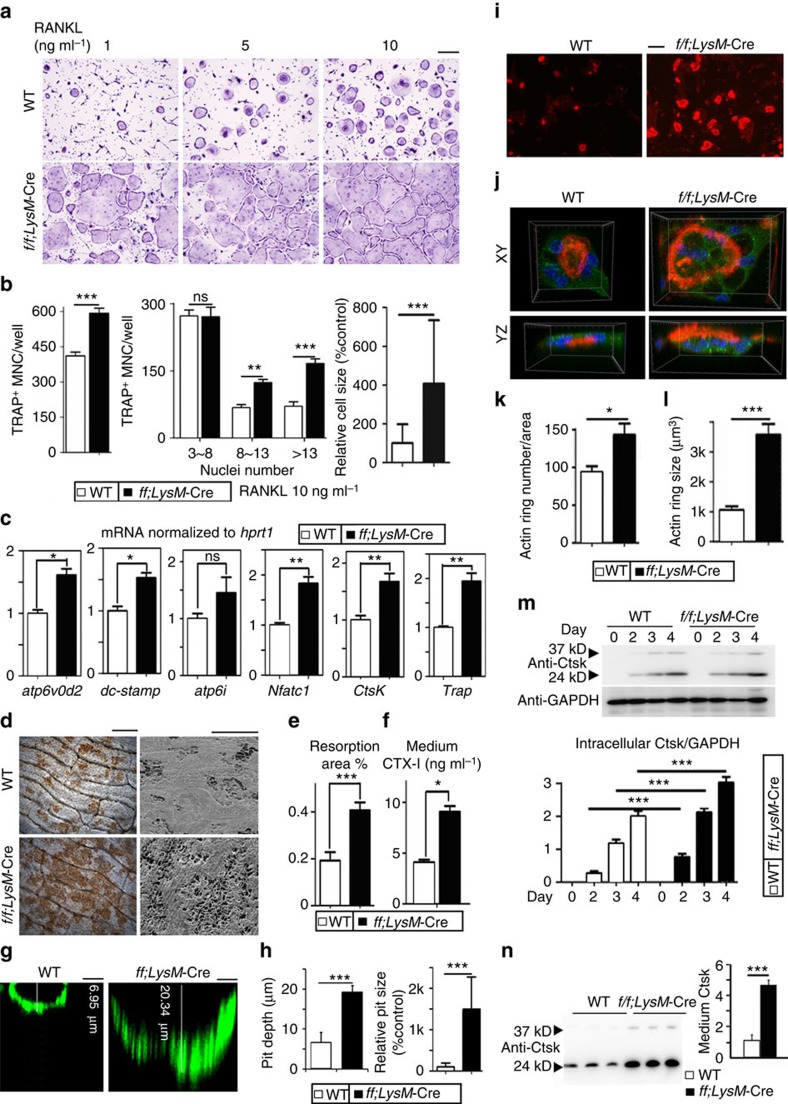
Given our data that Gna13 deficiency in vivo led to an increase in osteoclast development (Fig. 2), we examined the effects of using lower doses of RANKL in osteoclastogenesis under Gna13 deficiency. Unlike WT BMMs, Gna13-deficient cells formed osteoclasts when attended by permissive RANKL dose (one tenth of the optimum 10 ng ml−1 RANKL) (Fig. 3a) and formed 25% more osteoclasts at 10 ng ml−1 RANKL (Fig. 3a,b). Further, BMMs deficient in Gna13 generated osteoclast earlier (∼day 3) than WT BMMs (∼day 5) (Supplementary Fig. 3a,b). Gna13 deficiency not only increased the sensitivity of BMMs to RANKL, but also increased the size of osteoclasts (Fig. 3a,b). More osteoclasts with 3–8 nuclei or >13 nuclei were observed in the Gna13-deficient group (increased by 50% and 100%, respectively) (Fig. 3b). Consistently, gene expression analysis revealed that the expression of the osteoclast genes encoding NFATc1, Ctsk, Acp5, Atp6v0d2 and dc-stamp was higher in Gna13f/fLysM-Cre, but not in WT, BMMs cultured with M-CSF and RANKL for 3 days (Fig. 3c). Importantly, among the aforementioned genes, dc-stamp and Atp6v0d2 are known to have roles in osteoclast fusion15,16, indicating that Gα13 negatively regulates osteoclast formation by also promoting the fusion of mononucleated osteoclasts into multinucleated osteoclasts.
Figure 3. Loss of Gα13 promotes osteoclast formation and function.
(a) TRAP staining to detect osteoclast formation from WT and Gna13f/fLysM-Cre (f/f;LysM-Cre) BMMs treated by M-CSF and different doses of RANKL for 5 days. (b) Quantification of cell number and relative cell size in a, RANKL=10 ng ml−1 group; N=6. TRAP+ MNC, TRAP positive multinucleated cell. (d) Quantitative reverse transcription–PCR analysis of atp6v0d2, dc-stamp, atp6i, nfatc1, ctsk and trap expression in WT and Gna13f/fLysM-Cre osteoclasts; N=6. (e) WGA (wheat germ agglutinin) staining and SEM (scanning electron microscope) analysis of bone slides to detect bone resorption pits. (f) Quantification of bone resorption area in e; N=6. (g) Confocal microscopy of FITC-WGA stained bone resorption pits. (h) Quantification of bone resorption pit depth and relative pit size in g. Culture medium CTX-I (type I collagen carboxy-terminal peptide) concentration measured by ELISA. (i) Fluorescence microscopy of F-actin ring stain in mature osteoclasts. (j) Confocal microscopy of F-actin ring (red)/Ctsk (green)/nuclei (blue) staining in mature osteoclasts. (k) Quantification of F-actin ring number in i; N=6. (l) Quantification of F-actin ring volume in j; N=30. (m) Western blot to detect intracellular Ctsk protein level, and its quantification (lower panel); N=3. (n) Western blot to detect Ctsk concentration in the culture medium, and its quantification (right panel); N=3. Results are expressed as mean±s.d.; *P≤0.05; **P≤0.01; ***P≤0.001 (Student's t-test or ANOVA analysis). Scale bars in a,i,d, 200 μm; Scale bar in g, 5 μm.
To further analyse the role of Gα13 in osteoclastic bone resorption, we performed in vitro bone resorption assays and analysed bone resorption pits by wheat germ agglutinin (WGA) staining and scanning electron microscope (Fig. 3d). Data showed that the total resorption area by Gna13f/fLysM-Cre osteoclasts was significantly higher than that of WT osteoclasts (Fig. 3e). Collagen I C-terminal telopeptide (CTX-I), released during bone resorption17, were drastically increased in Gna13f/fLysM-Cre cell culture medium (Fig. 3f). In addition, WGA-fluorescein isothiocyanate (FITC) staining showed that pits resorbed by Gna13f/fLysM-Cre osteoclasts are also larger (∼14-fold) and deeper (∼3-fold) than those by WT cells (Fig. 3g,h, Supplementary Fig. 4). Collectively, the results showed that Gα13 negatively regulated osteoclastic bone resorption.
Next, we co-stained the osteoclasts on bone slides with Rhodamine-conjugated-Phalloidin (red), to analyse F-actin ring formation, a critical structure of mature osteoclasts18, anti-Ctsk antibody (green) and Hoechst (blue) (Fig.3i,j). Data showed that Gna13-deficient osteoclasts exhibited 1.5-fold increased F-actin ring formation (Fig. 3i,k), and the average size of F-actin rings was also about threefold larger in these cells than control cells (Fig. 3j,l).
Furthermore, immunostaining showed a more polarized location of Ctsk in Gna13f/fLysM-Cre cells (Fig. 3j), indicating a change in Ctsk transportation and secretion. We thus analysed intracellular and medium Ctsk by western blot (Fig. 3m,n). Data showed a slight increase (∼1.5-fold) in intracellular Ctsk level in Gna13f/fLysM-Cre osteoclasts (Fig. 3m). However, secreted Ctsk concentration in the culture medium by Gna13f/fLysM-Cre osteoclasts was drastically elevated (∼4-fold) (Fig. 3n). These results demonstrate that Gna13-deficient osteoclasts had enlarged actin ring with increased Ctsk secretion, both of which would contribute to enhanced bone resorption besides enhanced osteoclast formation.
We performed TUNEL staining on day-6 osteoclasts (Supplementary Fig. 3c,e) and cytokine/serum-starved day-5 osteoclasts (Supplementary Fig. 3d,f), and similar apoptosis nuclei ratio are observed in the WT and mutant cells. Similar survival rate was observed in the WT and Gna13f/fLysM-Cre cells when the osteoclasts were extensively cultured for 7 days (Supplementary Fig. 3h,i). Data indicated that Gna13-deficiency might not influence osteoclast apoptosis. Further, acridine Orange staining showed that acidification is similar between WT and Gna13f/fLysM-Cre osteoclasts (Supplementary Fig. 3g).
Gα13-RhoA brakes Akt-GSK3β-NFATc1 signalling
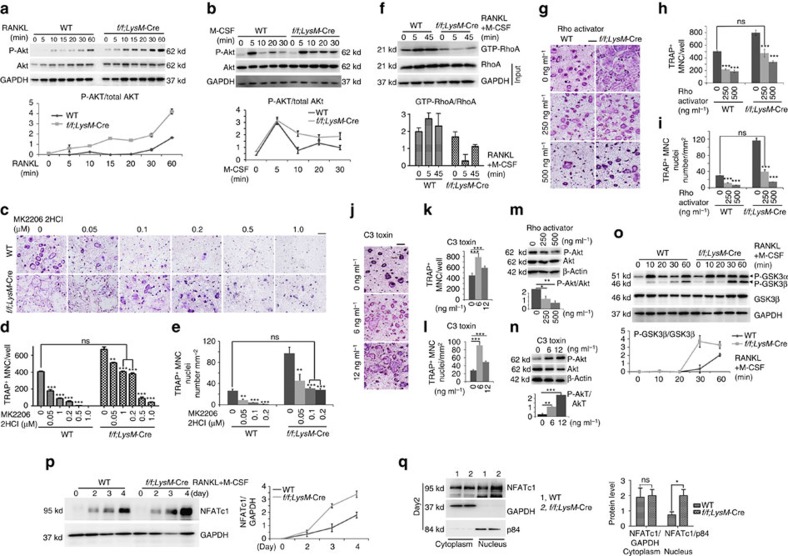
RANKL and M-CSF orchestrate multiple signalling pathways to promote osteoclast formation1,2,3. Expression of RANKL receptor (RANK) and M-CSF receptor (cFms) were similar in WT and Gna13-deficient cells (Supplementary Fig. 3j,k). To analyse signal transduction in response to RANKL or M-CSF stimulation (including Akt, p38, JNK and Erk activation), BMMs were starved for 5 h and then stimulated with RANKL or M-CSF for 5–60 min (Fig. 4a,b). Akt phosphorylation was enhanced in Gna13f/fLysM-Cre cells by either RANKL or M-CSF as compared with WT BMMs (Fig. 4a,b). Nonetheless, activation of the p38, JNK and Erk signalling pathways were similar between WT and Gna13f/fLysM-Cre cells (Supplementary Fig. 5).
Figure 4. Knockout of Gna13 enhances Akt-GSK3β-Nfatc1 signalling through RhoA.
(a) Western blot analysis to detect Akt phosphorylation induced by RANKL in WT and Gna13f/fLysM-Cre (f/f;LysM-Cre) monocytes/macrophages. N=3. (b) Western blot analysis to detect Akt phosphorylation induced by M-CSF in WT and Gna13f/fLysM-Cre monocytes/macrophages. Lower panel in a–b, Quantification of phospho-Akt level versus total Akt level; N=3. (c) TRAP staining to detect osteoclastogenesis of WT and Gna13-deficient osteoclasts treated by MK2206 2HCl at different doses during differentiation. (d) Quantification of TRAP+ MNC number per well in c; N=6. (e) Quantification of TRAP+ nuclei number per mm2 in c; N=6. (f) RhoA activity in WT and Gna13f/fLysM-Cre pre-osteoclasts upon RANKL and M-CSF stimulation, and its quantification; N=3. (g) TRAP staining of WT and Gna13f/fLysM-Cre osteoclasts treated by Rho activator II at different doses during differentiation. (h) Quantification of TRAP+ MNC number per well in g; N=6. (i) Quantification of TRAP+ nuclei number per mm2 in g; N=6. (j) TRAP staining of WT and Gna13f/fLysM-Cre osteoclasts treated by different doses of RhoA inhibitor (C3 toxin) (0, 6, 12 ng ml−1) during differentiation. (k) Quantification of TRAP+ MNC number per well in j; N=6. (l) Quantification of TRAP+ nuclei number per mm2 in j; N=6. (m) Akt phosphorylation in pre-osteoclasts treated different doses of Rho activator II. (n) Akt phosphorylation in pre-osteoclasts treated different doses of C3 toxin (0, 1, 3, 6 ng ml−1). Lower panel in m–n, Quantification of phospho-Akt level versus total Akt level; N=3. (o) GSK3β phosphorylation induced by RANKL and M-CSF. Lower panel, Quantification of phospho-GSK3β level versus total GSK3β level; N=3. (p) Western blot analysis of NFATc1 expression in WT and Gna13f/fLysM-Cre osteoclast precursors. (q) NFATc1, GAPDH (cytoplasm loading control) and p84 (nuclei loading control) in Nuclei and cytoplasm lysates of WT and Gna13f/fLysM-Cre pre-osteoclasts. Right panels in p–q, Quantification of NFATc1 level versus loading control; N=3. Results were expressed as mean±s.d.; *P≤0.05; **P≤0.01; ***P≤0.001 (Student's t-test or ANOVA analysis). Scale bars, 200 μm.
Given the drastic increase in Akt activation, we then applied the Akt inhibitor MK2206 2HCl to attenuate Akt activity, which inhibited osteoclastogenesis in a dose-dependent manner (Fig. 4c–e). Notably, Gna13-deficient cells were more resistant to the Akt inhibitory effect on osteoclastogenesis. In the presence of 0.1∼0.2 μM MK2206 2HCl (Fig. 4d,e), Gna13-deficient cells formed osteoclasts at normal level comparatively to WT cells. The results indicate that Gna13 deficiency promotes osteoclastogenesis by increasing Akt activity.
Studies have demonstrated that Gα13 activates RhoA through RhoGEF (p115RhoGEF, LARG et al.)11,12, and RhoA can inhibit Akt activity19. Consistently, RhoA activity was dramatically downregulated in Gna13-deficient cells (Fig. 4f). In all, 250 ng ml−1 Rho activator II corrected the hyper-activation of Gna13-deficient osteoclasts (Fig. 4g–i). In addition, Rho activator II could attenuate Akt activation in osteoclasts (Fig. 4m). On the other hand, in the presence of 6–12 ng ml−1 Rho inhibitor, cell-permeable C3 toxin, osteoclast differentiation was enhanced (Fig. 4j–l) and Akt activation was also increased (Fig. 4n). Thus, Gα13 regulated Akt activity through RhoA so as to promote osteoclastogenesis.
It has been established that the PI3K/Akt-GSK3β-NFATc1 signalling cascade is critical for osteoclast differentiation20,21. Specifically, Akt can inhibit GSK3β, which can promote NFATc1 translocation from the nucleus into the cytoplasm and thereby abrogate osteoclast differentiation. Thus, we characterized GSK3β phosphorylation level and NFATc1 nuclei translocation in WT and Gna13f/fLysM-Cre cells. We found that GSK3α/β phosphorylation was hyper-activated in Gna13f/fLysM-Cre monocyte/macrophage in response to RANKL and M-CSF (Fig. 4o). NFATc1 expression increased during osteoclastogenesis in WT and Gna13f/fLysM-Cre cells, and more quickly in Gna13f/fLysM-Cre cells (Fig. 4p). Consistently, while similar amounts of NFATc1 were observed in the cytoplasm, much more NFATc1 was observed in the Gna13f/fLysM-Cre nucleus than WT (Fig. 4q). The results demonstrated that Gα13 mediates Akt-GSK3β-NFATc1 signalling to promote osteoclast gene expression.
Gα13CA inhibits osteoclastogenesis
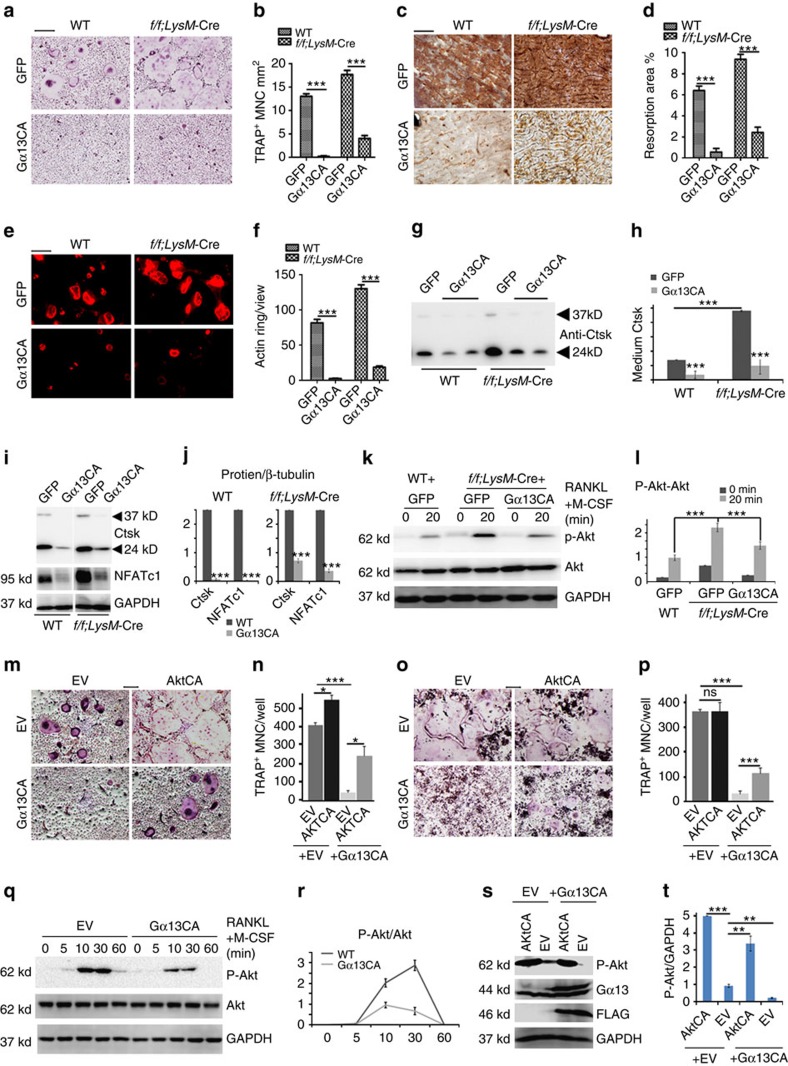
To confirm the inhibitory effect of Gα13 on osteoclastogenesis, we overexpressed a constitutively active form of Gα13 (Gα13CA) in BMMs and RAW264.7 cells (Fig. 5). Moreover, we overexpressed Gα13CA in WT and Gna13f/fLysM-Cre pre-osteoclasts using lentivirus22. A successful infection (>90% infection) was assessed by green fluorescent protein expression in control groups (Supplementary Fig. 6a,c), and Gα13CA overexpression was confirmed by western blot (Supplementary Fig. 6b,d,e). Importantly, Gα13CA overexpression drastically blocked osteoclast fusion, inhibited bone resorption and impaired F-actin ring formation in WT and Gna13f/f;LysM-Cre cells (Fig. 5a–f). Additionally, the secretion of Ctsk was decreased after Gα13CA overexpression in WT and Gna13-deficient osteoclasts (Fig. 5g,h). Ctsk and NFATc1 protein levels were also decreased after Gα13CA overexpression (Fig. 5i,j). Furthermore, Gα13CA overexpression attenuated Akt activation in Gna13f/fLysM-Cre cells, but did not affect the activation of p38 and ERK (Fig. 5k,l; Supplementary Fig. 7a). Gα13CA overexpression in WT BMMs also drastically inhibited osteoclastogenesis (Fig. 5m left panels; 5n). Interestingly, overexpression of a constitutively active form of Akt (AktCA) could partially rescue the inhibitory effects of Gα13CA (Fig. 5m right panels; 5n). These results demonstrate that Gα13CA inhibited osteoclastogenesis by inhibiting Akt activation in BMMs.
Figure 5. Gα13 gain-of-function inhibits osteoclast formation and function in vitro.
(a) TRAP staining to detect osteoclastogenesis of WT and Gna13f/fLysM-Cre (f/f;LysM-Cre) cells transfected with lentivirus overexpressing green fluorescent protein (GFP) and Gα13CA. (b) Quantification of TRAP+ MNC number per mm2 in a; N=6. (c) WGA staining to detect bone resorption of WT and Gna13f/fLysM-Cre cells overexpressing GFP and Gα13CA. (d) Quantification of bone resorption area per total area in c; N=6. (e) Rhodamine-conjugated-Phalloidin staining to detect F-actin ring formation of WT and Gna13f/fLysM-Cre cells transfected with lentivirus overexpressing GFP and Gα13CA. (f) Quantification of F-actin ring number per view in e; N=6. (g,i,k) Western blot to detect medium Ctsk (g), osteoclast-specific gene expression (i) Akt phosphorylation induced by RANKL and M-CSF (k) in WT and Gna13f/fLysM-Cre osteoclasts (overexpressing GFP or Gα13CA). (h,j,l) Quantification of protein level in g–l; N=3. (m,o) TRAP staining to detect osteoclastogenesis of BMMs (m) and RAW264.7 cells (o), which were transfected with control retrovirus and retrovirus overexpressing 3xFLAG-Gα13CA, AktCA or 3xFLAG-Gα13CA+AktCA. (n,p) Quantification of TRAP+ MNC per well in n,m and in p,o; N=6. (q,r) Western blot analysis of RANKL induced Akt phosphorylation in RAW264.7 cells transfected with control retrovirus and retrovirus overexpressing Gα13CA, and its quantification; N=3. (s,t) Western blot confirmation of AktCA and Gα13CA overexpression in RAW264.7 cells, and its quantification; N=3. EV, empty vesicle control. Results were expressed as mean±s.d.; *P≤0.05; **P≤0.01; ***P≤0.001 (Student's t-test or ANOVA analysis). Scale bars, 200 μm.
Furthermore, we infected RAW264.7 cells with the same retrovirus we used in BMMs in Fig. 5m,o. Similarly, Gα13CA overexpression inhibited osteoclast-like cell formation (Fig. 5o left panels; 5p), which could be rescued by AktCA overexpression (Fig. 5o right panels; 5p). Akt activation was greatly decreased in RAW264.7 cells overexpressing Gα13CA (Fig. 5q,r), while the activation of p38 and Erk remains unchanged (Supplementary Fig. 7b). The overexpression of Gα13CA and AktCA was confirmed by western blot (Fig. 5s,t). The results indicated that Gα13CA also inhibited osteoclast-like cells formation from the RAW264.7 cells by inhibiting Akt phosphorylation.
Gα13CA protects mice from pathological bone loss
To investigate Gα13 clinical implication potential, we overexpressed Gα13CA locally in mice using adeno-associated virus (AAV) gene expression to investigate its effects in protecting against bone degradation. AAV vectors have advantages compared with many other viral and non-viral based gene delivery platforms in both function and safety for correcting genetic-based diseases23. To test the use of AAV-Gα13CA, we utilized three disease models of bone loss: the human tumour-necrosis factorα (hTNFα)-transgene expressing mouse model of autoimmune arthritis24 (named TNFα-RA) (Fig. 6), ovariectomized (OVX) animal model of osteoporosis, (Fig. 7a–d) and calvaria-adjacent lipopolysaccharide (LPS)-injection mouse model of osteolysis (Fig. 7e,f)25. Successful infection of AAV in vivo was monitored by yellow fluorescent protein (YFP) expression (Supplementary Figs 8 and 9a). Successful overexpression of G13CA in vivo was confirmed by immunofluorescence staining (Supplementary Fig. 9b).
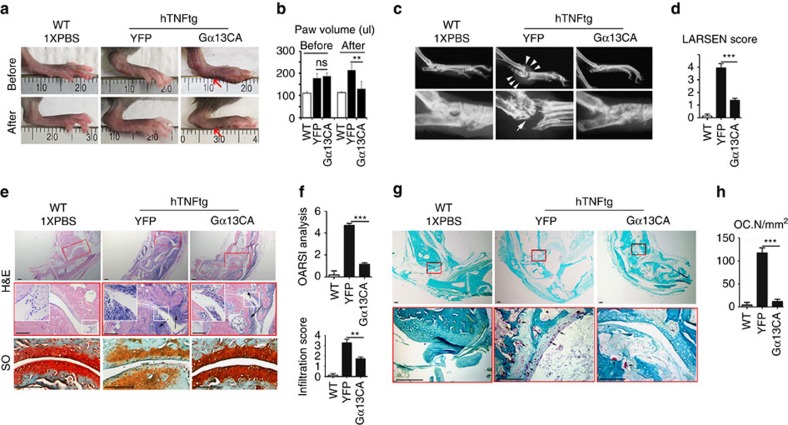
Figure 6. Gα13 protects Rhematoid arthritis mice from inflammatory bone loss.
(a–h) Histology analysis of 18-week-old male WT and hTNFtg Rheumatoid arthritis mouse ankles. hTNFtg mouse ankles were injected with AAV-Gα13CA or AAV-YFP. (a) Photographic images before and after AAV treatment. The red arrows showed paw swelling is relieved after AAV-Gα13CA treatment. (b) Quantification of hind paw volume in a; N=8. (c,d) Radiographic images; White arrows mark bone destruction. (d) Quantification of bone destruction (Larsen grade) in a; N=8. (e) H&E staining (black arrows mark monocyte infiltration) and Safranin O (SO) staining (black arrows mark articular cartilage damage). (f) Quantification of cartilage damage (OARSI grade) and inflammation (infiltration score) in e; N=8. (g) TRAP staining; black arrows mark TRAP positive cells. (h) Quantification of osteoclast number (Oc.N) in g; N=8. Results were expressed as mean±s.d.; *P≤0.05; **P≤0.01; ***P≤0.001 (Student's t-test). scale bars in e,g 200 μm.
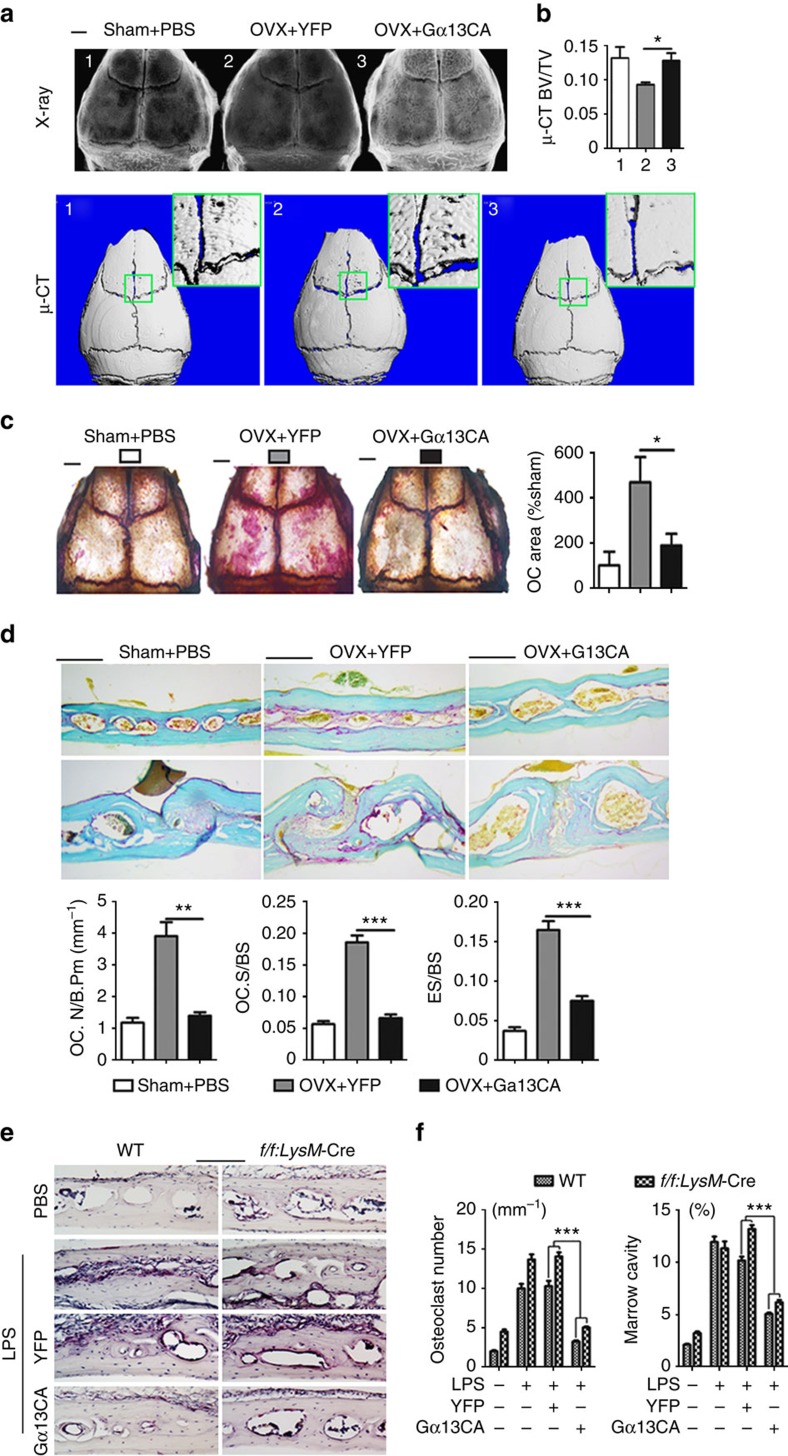
Figure 7. Gα13 protects mice from OVX- and LPS-induced bone loss.
(a–d) Histology analysis of calvarial bones from 13-week-old female sham-operated mice (control), and ovariectomized (OVX) mice injected with AAV-YFP or AAV-Gα13CA. (a) Radiographic (higher panels) and μ-CT analysis (lower panels). High-magnification images are co-presented in the right corners. (b) Quantification of bone volume per tissue volume (BV/TV); N=3. (c) Whole calvaria TRAP staining; Quantification of relative TRAP positive area is co-presented on the right panel; N=4. (d) TRAP staining of calvaria frozen sections. Quantification of osteoclast and bone resorption metrics are presented in lower panel; OC.N/B.Pm, osteoclast number per bone perimeter; OC.N/BS, osteoclast number per bone surface; ES/BS, eroded surface per bone surface; N=4. (e) TRAP staining using frozen sections of calvarial bones from 8-week-old male WT and Gna13f/fLysM-Cre (f/f;LysM-Cre) mice treated with PBS, LPS, LPS+AAV-YFP or LPS+AAV-Gα13CA. (f) Quantification of TRAP-positive cells per bone surface and marrow cavity area in e; N=6. Results were expressed as mean±s.d.; *P≤0.05; **P≤0.01; ***P≤0.001 (Student's t-test or ANOVA analysis). scale bars in a,c 200 μm; scale bars in d,e, 1 mm.
TNFα-RA mice were accompanied by excessive osteoclast formation and bone destruction (Fig. 6). Local administration of AAV-Gα13CA to ankles greatly reduced bone loss, as assessed by X-ray in the TNFα-RA mice as compared with those injected with AAV-YFP (Fig. 6c,d). Not surprisingly, osteoclast number was dramatically decreased in TNFα-RA mice with local injection of AAV-Gα13CA to ankles (Fig. 6g,h). Unexpectedly, local administration of AAV-Gα13CA also relieved ankle swelling (Fig. 6a,b), cartilage destruction (Fig. 6e lower panels, Fig. 6f) and inflammation cell infiltration (Fig. 6e upper and middle panels, Fig. 6f) in TNFα-RA mice. Hence, reduced bone loss and decreased osteoclast number might be a combined effect of Gα13CA and inhibited inflammation.
OVX-induced bone loss and osteoclast number increased due to estrogen depletion (Fig. 7a–d, sham+PBS group compared with OVX+YFP group). OVX mice were subjected to calvaria-adjacent subcutaneous injection of AAV-YFP or AAV-Gα13CA. As assessed by X-ray and u-CT, application of AAV-Gα13CA reduced OVX-induced bone loss (Fig. 7a,b). As assessed by TRAP staining of whole calvarial and calvarial sections, AAV-Gα13CA dramatically reduced OVX-induced osteoclast number increase (Fig. 7c,d) as compared with YFP group.
In the LPS-injection model, both WT and Gna13f/fLysM-Cre mice were subjected to calvaria-adjacent subcutaneous injection of LPS or PBS control (Fig. 7e,f). Compared with local injection of PBS, LPS induced a dramatic increase in osteoclast number and bone resorption (Fig. 7e,f). The absence of Gα13 further promoted the induction of osteoclastogenesis and bone loss in both PBS and LPS treated mice (Fig. 7e,f). Local administration of AAV-Gα13CA to calvaria had a marked therapeutic effect on osteoclast formation and bone destruction by LPS, compared with local injection of AAV-YFP control (Fig. 7e,f). These results confirm that it is possible to overexpress Gα13CA in vivo to target pathologic bone loss.
Full images of western blots are presented in Supplementary Fig. 10.
Discussion
We proposed a model that Gα13-RhoA antagonizes osteoclast formation and activity by attenuating the Akt-GSK3β-NFATc1 signalling axis (Fig. 8). Briefly, as a downstream of RANK and c-Fms, Akt phosphorylates and inactivates GSK3β, which has a role in NFATc1 nuclei exportation, so as to promote osteoclast differentiation; Meanwhile, Gα13 activates RhoA which in turn inhibits Akt phosphorylation and activity, so as to antagonize the over-activation of osteoclast (Fig. 8). The critical role of PI3K-Akt activity in osteoclast differentiation and activation is well-documented20,21,26,27,28,29. Although several endogenous factors that oppose osteoclast differentiation were described, most are downregulated under osteoclastogenic condition. In contrast, Gα13 is induced by the combined stimulation of RANKL and M-CSF, and in turn inhibit osteoclast differentiation so as to avoid over-activated osteoclast and excessive bone loss. Hence, our data reveal that Gα13 is an intrinsic control mechanism during osteoclastogenesis. In addition, we comprehensively study and report a signalling cascade (Gα13-RhoA-Akt-GSK3β-NFATc1) that negatively regulates osteoclastogenesis and osteoclast function. Most importantly, our results from both loss-of-function and gain-of-function strategies proved that Gα13 is a key negative regulator and main switch in osteoclastogenesis.
Figure 8. Working model of Gα13 as a negative regulator in osteoclast.
CNF toxin, Rho activator; C3 toxin, Rho inhibitor.
It was reported that RhoA activity is essential for podosome formation and osteoclastic bone resorption30. On the other hand, it was also reported that increased RhoA activity destabilizes the sealing zone in osteoclasts, and inhibition of RhoA stabilizes the sealing zone correlating with acetylation and stabilization of the microtubule network in these cells31,32,33,34. Our study showed that inhibition of RhoA activity through Gα13 knockout, RhoA inhibitor and RNA interference favours osteoclastogenesis, while RhoA activator inhibits osteoclastogenesis. Hence, these studies suggest that RhoA is the downstream effector of Gα13 to negatively regulate osteoclastogenesis. Our finding is underscored by genetic evidences that have suggested an important role for the RhoGEF-RhoGTPase (for example, Arhgef3-RhoA) pathway in osteoporosis35,36,37,38. Genome-wide linkage studies have identified chromosome region 3p14-p21, in which RHOA gene was located, as a quantitative trait locus for BMD35,36. One RHOA single-nucleotide polymorphism (SNP) rs17595772, and one ARHGEF3 SNP were reported to be significantly associated with decreased BMD) in postmenopausal women37,38. However, the mechanisms underlying the correlation between RhoGEF-RhoGTPase pathway and BMD remain to be explored.
Current anti-resorptive agents (for example, bisphosphonates and denosumab) are effective but far from ideal. The major problem of bisphosphonates is their permanent deleterious affect on normal bone remodelling. Agents preventing bone resorption by inhibiting late differentiation of osteoclasts without affecting normal bone remodelling are more desired, which are unlikely to interfere with the coupling of osteoblastic bone formation to osteoclastic bone resorption, critical for maintaining normal bone homeostasis39. Here we showed that overexpression of Gα13CA markely inhibits osteoclast formation and bone resorption both in vitro and in vivo. Importantly, although multi-nuclear cell formation was blocked, Gα13CA over-expressing monocytes were TRAP+ stained (Fig. 5a), indicating that Gα13CA regulated osteoclast differentiation at a late stage and could be a good target for bone loss without affecting normal bone remodelling. Interestingly, Gα13 might have dual functions in inflammatory disease models (TNFα-RA and LPS-injection models), by concurrently inhibiting osteoclast differentiation and inflammation so as to achieve the ultimate outcome of bone loss protection. Hence, activating Gα13, an endogenous osteoclast inhibitor, may serve as a novel therapeutic approach targeting bone loss in various bone pathological states.
Methods
Mice
Gna13flox/flox (Gna13f/f) mice were provided by Dr S. Offermanns (Max-Planck-Institute for Heart and Lung Research, Bad Nauheim, Germany)14. LysM-Cre transgenic mice were purchased from the Jackson laboratory. Ctsk-Cre knock-in mice were provided by Dr S. Kato (University of Tokyo, Tokyo, Japan)40. To obtain mice with Gna13-deficient macrophages, Gna13f/+LysM-Cre males were bred with Gna13f/+LysM-Cre females. To obtain mice with Gna13-deficient osteoclasts, Gna13f/+Ctsk-Cre males were bred with Gna13f/f females. In all experiments, Gna13f/+LysM-Cre or Gna13f/+Ctsk-Cre mice were compared with their WT littermates (Gna13f/+, Gna13f/f, Gna13+/+LysM-Cre or Ctsk-Cre mice). All mice were on a pure C57BL/6 background. All animal studies were approved by the Institutional Animal Care and Use Committee (IACUC) at University of Alabama at Birmingham (UAB), Birmingham, USA.
GeneChip analysis
Human peripheral blood mononuclear cells were cultured for 7 days with human RANKL and human M-CSF. GeneChip data were analysed using Affymetrics scanner and accompanying gene expression software.
Radiographic analysis.
For X-ray analysis, excised 2-month-old mouse femurs were scanned using a high-resolution soft X-ray system (Faxitron Model MX-20) at 30 kV. For Micro-Computed Tomography (μ-CT) analysis, excised 2-month-old mouse femurs were scanned using the Scanco CT40 desktop cone-beam μ-CT scanner (Scanco Medical AG, Brüttisellen, Switzerland). The scan of the trabecular bone was performed from the growth plate, and consisted of 310 slices (12 μm per slice) and 200 slices were analysed using the CT Evaluation Program (v5.0A, Scanco Medical). The scan and analysis of the cortical bone was performed at the midshaft of the femur and consisted of 25 slices (12 μm per slice).
Histology
Histology was performed as previously described40,41. Briefly, the femurs of 2-month-old mice were fixed with 70% ethanol followed by plastic embedding and Golden trichrome staining, or with 10% neutral buffered formalin followed by decalcification in 10% EDTA for 2 weeks, paraffin embedding and TRAP staining using Phosphatase, Leukocyte Acid (TRAP) kit (Sigma-Aldrich, 387A-1KT). Osteoclastic and osteoblastic perimeters were measured and analysed using Osteomeasure in a blinded fashion.
In-vivo calcein labelling
Calcein labelling was previously described41. Briefly, 2-month-old mice were intraperitoneally injected with 20 mg kg−1 of calcein in a 2% sodium bicarbonate solution, 8 days and 2 days before killing of mice. Calvarias were fixed in 4% PFA, soaked in 30% glucose in PB, embedded in OCT and frozen sectioned. Mineral apposition rate is the distance between the midpoints of the two labels divided by the time between the midpoints of the interval.
Serum ALP assay
Two-month old mouse serum was collected after 6-hour fasting, and the serum ALP activity was detected and quantified using Alkaline Phosphatase Assay Kit (Colorimetric) (ab83369) according to the manufacturer's instructions.
In vitro osteoclast differentiation assay
Mature osteoclasts were generated as described10. Briefly, isolated BMMs from C57BL/6 mice were cultured in MEM α (pH 6.9) containing 10% FBS, 10 ng ml−1 recombinant RANKL and 10 ng ml−1 recombinant M-CSF for 5 days. Recombinant murine M-CSF and RANKL were obtained from R&D systems, Inc. Cell culture medium was obtained from Gibco, Life Technologies Corporation. Mature osteoclasts were characterized by staining for TRAP activity using a commercial kit (Sigma-Aldrich, 387A-1KT) and TRAP+ MNCs were enumerated per well in a 24-well plate.
In vitro bone resorption assay
For bone resorption assay, osteoclasts were seeded on bovine bone slides. Concentration of bovine cross-linked C-telopeptide of type I collagen (CTX-1) in the medium was measured using CrossLaps for Culture ELISA (CTX-I) kit (Immunodiagnostic Systems Limited) following the manufacturer's instructions. Bone resorption pits were sonicated in PBS, stained with 2 ug ml−1 WGA-lectin (Sigma-Aldrich, L-3892) for two hours and then using DAB peroxidase (horseradish peroxidase) Substrate Kit (Vector laboratories, SK-4100) (ref. 42). To analyse bone resorption pits depth and volume, bone slides were sonicated in PBS, stained with 2 ug ml−1 FITC-WGA (Sigma-Aldrich, L-4895) staining for 2 h and visualized by confocal microscopy43.
Cellular immunofluorescent staining
For F-actin ring staining, osteoclasts on bovine bone slides were incubated with 2 U ml−1 Texas Red-X Phalloidin (Life Technologies, T7471). For anti-Ctsk immunofluorescence stain, osteoclasts on bovine bone slides were fixed by 4% formaldehyde, permeabilized with 0.2% Triton X-100, blocked with 5% goat serum and 100 μg ml−1 unconjugated AffiniPure Fab Fragment Goat Anti-Mouse IgG (H+L) (Jackson ImmunoResearch Labs), incubated with mouse anti-mouse Ctsk primary antibody (1:100, Santa Cruz sc-48353) and then with FITC goat anti-mouse IgG (H+L) secondary antibody. Nuclei were visualized with 1 μg ml−1 DAPI (4',6-diamidino-2-phenylindole; Sigma-Aldrich, D9542). Three-dimensional images were taken by confocal microscopy and constructed by Imaris software44.
Inhibitors and activators treatment.
MK-2206 2HCl was purchased from Selleckchem Inc. (Cat# S1078). Rho Inhibitor I (cell-permeable exoenzyme C3 transferase, or cell-permeable C3 toxin) was purchased from Cytoskeleton Inc. (Cat# CT04). Rho activator II was purchased from Cytoskeleton Inc. (Cat# CN03). Stock solutions were prepared according to the manufacturer's instructions. During differentiation from BMMs to mature osteoclasts, the cells were treated continuously by those inhibitors or activators at indicated doses.
Quantitative reverse transcription–PCR (qPCR) assay
Total RNA was isolated from cultured cells with TRIzol reagent (Life Technologies, 15596018). Mouse complementary DNA (cDNA) was reverse-transcribed from 0.5 μg total RNA with SuperScript VILO Master Mix (Life Technologies, 11755050). Quantitative reverse transcription–PCR performed on StepOne Real-Time PCR System (Life Technologies, Applied Biosystems) using the TaqMan Gene Expression assays (Life Technologies, Applied Biosystems) or SYBR Green reagents (Life Technologies, 4385610). Taqman probe numbers and Primer sequences are as followings: Ctsk (Mm00484039_m1); Acp5 (Mm00475698_m1), Atp6i (Mm00469394_m1), Nfatc1 (Mm00479445_m1), Hprt1 (Mm00446968_m1), Hprt1 (5′-GGTGGAGATGATCTCTCAACTTTAA-3′, 5′-AGGAAAGCAAAGTCTGCATTGTT-3′), Atp6v0d2 (5′-GAAGCTGTCAACATTGCAGA-3′, 5′-TCACCGTGATCCTTGCAGAAT-3′), DC-stamp (5′-GGGGACTTATGTGTTTCCACG-3′, 5′-ACAAAGCAACAGACTCCCAAAT-3′), Gna13 (5′-TGCTGGTAGATGCCCGAGA-3′, 5′-GATCGTAGGCATTCTGTATACCA-3′), β-actin (5′-TGACGTTGACATCCGTAAAGAC-3′, 5′-TGCTAGGAGCCAGAGCAGTAA-3′).
Western blotting and phosphorylation assay
Cell lysates preparation and SDS–polyacrylamide gel electrophoresis /western blotting analysis were carried out according to a standard protocol10,45. For the phosphorylation assay, BMMs and pre-osteoclasts (derived from BMMs with 2-day treatment of RANKL and M-CSF) were starved for 5 h and stimulated with RANKL or M-CSF for different times. Proteins were harvested in cell lysis buffer supplemented with proteinase inhibitor cocktail (Sigma-Aldrich Co. LLC, 1:100) and phosphatase inhibitor cocktail 2 (P-5726, Sigma-Aldrich Co. LLC, 1:100). To extract nuclear and cytoplasmic proteins, NE-PER Nuclear and Cytoplasmic Extraction Reagent Kit (Thermo Fisher Scientific Inc) was used. Western blot was performed using following antibodies:, anti-p84 mouse IgG (GeneTex GTX70220, 1:1,000), anti-IκBα rabbit IgG (Cell Signaling Technology 4812, 1:1,000), anti-phospho-IκBα (Ser32) rabbit IgG (Cell Signaling Technology 2859, 1:1,000), anti-p38 rabbit IgG (Cell Signaling Technology 9212, 1:1,000), anti-phospho-p38 (Thr180/Tyr182) rabbit IgG (Cell Signaling Technology 4631, 1:1,000), anti-Erk rabbit IgG (Cell Signaling Technology 4370, 1:1,000), anti-phospho-Erk (Thr202/Tyr204) rabbit IgG (Cell Signaling Technology 4695, 1:1,000), anti-Akt rabbit IgG (Cell Signaling Technology 4685, 1:1,000), anti-phospho-Akt (Thr308) rabbit IgG (Cell Signaling Technology 2965, 1:1,000), anti-JNK rabbit IgG (Cell Signaling Technology 9251, 1:1,000), anti-phospho-JNK (Thr183/Tyr185) rabbit IgG (Cell Signaling Technology 9258, 1:1,000), anti-c-Jun rabbit IgG (Cell Signaling Technology 9165), anti-phospho-c-Jun (Ser73) rabbit IgG (Cell Signaling Technology 9164, 1:1,000), anti- GSK3β rabbit IgG (Cell Signaling Technology 9315, 1:1,000), anti-phospho-GSK3α/β (Ser21/9) rabbit IgG (Cell Signaling Technology 9331, 1:1,000), anti-Gα13 rabbit IgG(Santa Cruz sc-410, 1:500), anti-Ctsk mouse IgG (Santa Cruz sc-48353, 1:500), anti-NFATc1 mouse IgG (Santa Cruz sc-7294, 1:500), anti-GAPDH mouse IgG (Santa Cruz sc-166574, 1:500), anti-β-Actin mouse monoclonal IgG (Santa Cruz sc-81178, 1:500), Goat anti-rabbit IgG-HRP (Santa Cruz sc-200416, 1:2,500), Rabbit anti-mouse IgG-HRP (Santa Cruz sc358917, 1:2,500). Full images of western blots are presented in Supplementary Fig. 10.
RhoA activation assay
Pre-osteoclasts (derived from BMMs with 2-day treatment of RANKL and M-CSF) were starved for 4 h and stimulated by RANKL and M-CSF for different times. Cell lysates was harvested and RhoA-GTP activity was analysed using commercial kits (Rho Activation Assay Biochem Kit, Cytoskeleton, Inc.) according to the manufacturer's instructions.
Constructs and virus production and infection
TRC lentiviral mouse Gna13 targeted short hairpin RNA (Clone ID: TRCN0000098146, target sequence 5′-CGCGATCAACACAGAGAACAT-3′), TRC Lentiviral pLKO.1 Empty Vector and mouse Gna13 full-length cDNA were purchased from Open Biosystems, Thermo Fisher Scientific Inc. Lentiviral pLB vector and packaging plasmids (pCMV-VSV-G and pCMV-Dr8.92) were purchased from Addgene. pHR-EF-IRES-Bla (referred to as pHR in the following text) was a gift from Dr Ling Tian in Dr W. Timothy Garvey's lab and used for lentivirus-mediated overexpression22. Gα13 constitutively active form (Gα13 Q226L, referred to as Gα13CA in the text) full-length cDNA was purchased from Missouri cDNA Resource Center. Akt constitutively active form cDNA was purchased from addgene. Gα13CA was ligated into pHR-EF-IRES-Bla at the BamHI and XhoI sites and substituted the green fluorescent protein fragments, to construct pHR.Gα13CA vector. Retrovirus vector pMXs-ires-puro was a gift from Dr Xu Feng. A 3xFLAG sequence was inserted into pMXs-ires-puro to construct pMXs-3xFLAG-ires-puro vector. Gα13CA was ligated into pMXs-3xFLAG-ires-puro at the EcoRI and NotI sites to construct pMXs-3xFLAG-G13CA-ires-puro vector (referred to as pMXs-Gα13CA). AktCA was ligated into pMXs-ires-puro vector to construct pMXs-AktCA vector. For lentivirus production, lentivirus vectors (with 10% pLB vector or 10% pHR vector) and packaging plasmids were co-transfected into HEK-293T cells using a calcium phosphate co-precipitation method. The lentiviral supernatant was harvested 48–72 h post-transfection. For retrovirus production, pMXs vectors was transfected into 293GPG cells (gift from Dr Xu Feng) using a calcium phosphate co-precipitation method and retrovirus supernatant were harvested between 48–96 h (ref. 46). Gα13CA was ligated into pAAV-CMV vector from Invitrogen at EcoRI and SalI sites to produce pAAV-CMV-G13CA vector (referred to as pAAV-G13CA). Lentivirus and retrovirus titers were determined by transfecting HEK293T cells with serial dilutions of virus supernatant. AAV was produced and tittered according to the manufacturer's instructions (Life Technology, Inc). BMMs or RAW264.7 cells were transduced with virus supernatant in the presence of 8 μg ml−1 polybrene (Sigma) for 24 h before induced with RANKL.
OVX-induced bone destruction and AAV-G13CA treatment
OVX or sham was performed on two-month-old female mice. One week later and two weeks later those mice were administered with a local calvarial injection of 30 ul AAV (titer=109-10 ml−1) expressing YFP or G13CA. Mice were harvested 5 weeks after OVX operation and fixed in 4% PFA. Calvaria bone were analysed by X-ray, u-CT and whole-mount TRAP staining. Calvarial were also decalcified for 3 days, immersed in 30% sucrose overnight and then submitted to frozen section and TRAP staining and immunofluorescent staining.
Immunofluorescent staining
Frozen sections were fixed in 4% PFA for half an hour and heat-induced antigen retrieval was performed (abcam, ab973). Sections were incubated with mouse IgG blocking solution and universal blocking solution, and then overnight with primary antibodies: anti-YFP rabbit IgG (Santa Cruz sc-410, 1:100) or anti-Gα13 rabbit IgG (Santa Cruz sc-410, 1:100) and anti-Ctsk mouse IgG (Santa Cruz sc-48353, 1:200). Ctsk was then labelled by biotin-anti-mouse IgG (Vector laboratories, BMK-2202) and streptavidin-FITC (sigma). YFP and Gα13 were labelled by TR-goat-anti-rabbit IgG.
LPS-induced bone destruction and AAV-G13CA treatment
Seven-week-old female mice were administered with a local calvarial injection of AAV expressing YFP or G13CA. Six days later those mice were administered with a local calvarial injection of LPS (sigma) at 25 mg kg−1 body weight or PBS and analysed after 5 days following a previously described model25. For analysis, calvarial with skin tissue were fixed in 4% PFA for 6 h at 4 °C, decalcified for 3 days, immersed in 30% sucrose overnight and then submitted to frozen section and TRAP staining.
TNFα-induced rheumatoid arthritis and AAV-Gα13CA treatment
13-week old WT and human-TNFα-transgenic (hTNFtg) mice was injected with AAV (titer=109-10 ml−1) expressing YFP or G13CA over ankles. The injection was performed four times every week successively. Photographic and radiographic analysis were performed before and 35 days after the first injection. Samples were harvested 35 days after the first injection, and Safranin O (SO) stain, H&E stain and TRAP stain were performed. Hind paw volume was quantified by water displacement method47. Bone destruction in X-ray was quantified by Larsen method in RA lesion area48. Cartilage destruction was assessed using safranin O staining and was measured as OARSI grade in ankle joint area49.
Statistics
Data represent mean±s.d. Analysis was performed using GraphPad Prism, version 5. Statistical significance was assessed using a two-tailed Student's t-test or ANOVA analysis, as indicated in the figure legend, considering a P value≤0.05 as significant.
Data availability
The authors declare that the data supporting the findings of this study are available within the article and its Supplementary Information files.
Additional information
How to cite this article: Wu, M. et al. Gα13 negatively controls osteoclastogenesis through inhibition of the Akt-GSK3β-NFATc1 signalling pathway. Nat. Commun. 8, 13700 doi: 10.1038/ncomms13700 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Supplementary Figures 1-10.
Acknowledgments
We would like to thank Dr S. Offermanns from Max-Planck-Institute for kindly giving us the Gna13f/f mice. We would like to thank Dr Garvey from UAB for kindly giving us the pHR-EF-IRES-Bla vector, and Dr Xu Feng from UAB for kindly giving us the pMXs-3xFLAG-ires-puro retroviral vector and 293GPG retroviral packaging cell line. We also would like to thank Dr Joel Jules, Matthew McConnell and Dr Jay M. McDonald for their extensive reading, discussion and excellent assistance with this manuscript. We appreciate the assistance of the Center for Metabolic Bone Disease at the University of Alabama at Birmingham (UAB) (P30 AR046031). We are also grateful for the assistance of the UAB Small Animal Phenotyping Core, the UAB Metabolism Core, the UAB Vector Production Facility and the UAB Neuroscience Molecular Detection Core Laboratory (P30 NS0474666). This work was supported by NIH grants R01-AR-044741 (Y-.P.L.) and R01-DE-023813 (Y-.P.L.).
Footnotes
Author contributions Y-.P.L., M.W. and W.C. designed the experiments. M.W., W.C., Y.L., G.Z. and L.H. conducted the studies. M.W., W.C., Y.L., G.Z., L.H. and Y-.P.L. collected the data and analysed the data. M.W., W.C. and Y-.P.L. drafted the manuscript. All authors approved the final version of the manuscript for submission.
References
- Takayanagi H. New immune connections in osteoclast formation. Ann. N. Y. Acad. Sci. 1192, 117–123 (2010). [DOI] [PubMed] [Google Scholar]
- Boyle W. J., Simonet W. S. & Lacey D. L. Osteoclast differentiation and activation. Nature 423, 337–342 (2003). [DOI] [PubMed] [Google Scholar]
- Teitelbaum S. L. & Ross F. P. Genetic regulation of osteoclast development and function. Nat. Rev. 4, 638–649 (2003). [DOI] [PubMed] [Google Scholar]
- Chen W. et al. C/EBPalpha regulates osteoclast lineage commitment. Proc. Natl Acad. Sci. USA 110, 7294–7299 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W. et al. Novel pycnodysostosis mouse model uncovers cathepsin K function as a potential regulator of osteoclast apoptosis and senescence. Hum. Mol. Genet. 16, 410–423 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y. P. et al. Cloning and complete coding sequence of a novel human cathepsin expressed in giant cells of osteoclastomas. J. Bone Miner. Res. 10, 1197–1202 (1995). [DOI] [PubMed] [Google Scholar]
- Li Y. P., Chen W. & Stashenko P. Molecular cloning and characterization of a putative novel human osteoclast-specific 116-kDa vacuolar proton pump subunit. Biochem. Biophys. Res. Commun. 218, 813–821 (1996). [DOI] [PubMed] [Google Scholar]
- Li Y. P., Chen W., Liang Y., Li E. & Stashenko P. Atp6i-deficient mice exhibit severe osteopetrosis due to loss of osteoclast-mediated extracellular acidification. Nat. Genet. 23, 447–451 (1999). [DOI] [PubMed] [Google Scholar]
- Chen W. et al. C/EBPα regulates osteoclast lineage commitment. Proc. Natl Acad. Sci. 110, 7294–7299 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S. & Li Y. P. RGS10-null mutation impairs osteoclast differentiation resulting from the loss of [Ca2+]i oscillation regulation. Genes Dev. 21, 1803–1816 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siehler S. Regulation of RhoGEF proteins by G12/13-coupled receptors. Br. J. Pharmacol. 158, 41–49 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worzfeld T., Wettschureck N. & Offermanns S. G(12)/G(13)-mediated signalling in mammalian physiology and disease. Trends Pharmacol. Sci. 29, 582–589 (2008). [DOI] [PubMed] [Google Scholar]
- Chen W. & Li Y. P. Generation of mouse osteoclastogenic cell lines immortalized with SV40 large T antigen. J. Bone Miner. Res. 13, 1112–1123 (1998). [DOI] [PubMed] [Google Scholar]
- Moers A. et al. G13 is an essential mediator of platelet activation in hemostasis and thrombosis. Nat. Med. 9, 1418–1422 (2003). [DOI] [PubMed] [Google Scholar]
- Yagi M. et al. DC-STAMP is essential for cell-cell fusion in osteoclasts and foreign body giant cells. J. Exp. Med. 202, 345–351 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. H. et al. v-ATPase V0 subunit d2-deficient mice exhibit impaired osteoclast fusion and increased bone formation. Nat. Med. 12, 1403–1409 (2006). [DOI] [PubMed] [Google Scholar]
- Zhuo Y., Gauthier J. Y., Black W. C., Percival M. D. & Duong L. T. Inhibition of bone resorption by the cathepsin K inhibitor odanacatib is fully reversible. Bone 67, 269–280 (2014). [DOI] [PubMed] [Google Scholar]
- Kanehisa J. et al. A band of F-actin containing podosomes is involved in bone resorption by osteoclasts. Bone 11, 287–293 (1990). [DOI] [PubMed] [Google Scholar]
- Muppidi J. R. et al. Loss of signalling via G[agr]13 in germinal centre B-cell-derived lymphoma. Nature 516, 254–258 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon J. B. et al. Akt induces osteoclast differentiation through regulating the GSK3beta/NFATc1 signaling cascade. J. Immunol. 188, 163–169 (2012). [DOI] [PubMed] [Google Scholar]
- Jang H. D., Noh J. Y., Shin J. H., Lin J. J. & Lee S. Y. PTEN regulation by the Akt/GSK-3beta axis during RANKL signaling. Bone 55, 126–131 (2013). [DOI] [PubMed] [Google Scholar]
- Zhang W. et al. TRIB3 mediates glucose-induced insulin resistance via a mechanism that requires the hexosamine biosynthetic pathway. Diabetes 62, 4192–4200 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daya S. & Berns K. I. Gene therapy using adeno-associated virus vectors. Clin. Microbiol. Rev. 21, 583–593 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keffer J. et al. Transgenic mice expressing human tumour necrosis factor: a predictive genetic model of arthritis. EMBO J. 10, 4025–4031 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang C. Y., Kyritsis G., Graves D. T. & Amar S. Interleukin-1 and tumor necrosis factor activities partially account for calvarial bone resorption induced by local injection of lipopolysaccharide. Infect. Immun. 67, 4231–4236 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilkington M. F., Sims S. M. & Dixon S. J. Wortmannin inhibits spreading and chemotaxis of rat osteoclasts in vitro. J. Bone Miner. Res. 13, 688–694 (1998). [DOI] [PubMed] [Google Scholar]
- Nakamura I. et al. Phosphatidylinositol-3 kinase is involved in ruffled border formation in osteoclasts. J. Cell. Physiol. 172, 230–239 (1997). [DOI] [PubMed] [Google Scholar]
- Shinohara M. et al. Class IA phosphatidylinositol 3-kinase regulates osteoclastic bone resorption through protein kinase B-mediated vesicle transport. J. Bone Miner. Res. 27, 2464–2475 (2012). [DOI] [PubMed] [Google Scholar]
- Takeshita S. et al. SHIP-deficient mice are severely osteoporotic due to increased numbers of hyper-resorptive osteoclasts. Nat. Med. 8, 943–949 (2002). [DOI] [PubMed] [Google Scholar]
- Chellaiah M. A. et al. Rho-A is critical for osteoclast podosome organization, motility, and bone resorption. J. Biol. Chem. 275, 11993–12002 (2000). [DOI] [PubMed] [Google Scholar]
- Destaing O. et al. A novel Rho-mDia2-HDAC6 pathway controls podosome patterning through microtubule acetylation in osteoclasts. J. Cell Sci. 118, 2901–2911 (2005). [DOI] [PubMed] [Google Scholar]
- Ory S., Brazier H., Pawlak G. & Blangy A. Rho GTPases in osteoclasts: orchestrators of podosome arrangement. Eur. J. Cell Biol. 87, 469–477 (2008). [DOI] [PubMed] [Google Scholar]
- Ory S., Munari-Silem Y., Fort P. & Jurdic P. Rho and Rac exert antagonistic functions on spreading of macrophage-derived multinucleated cells and are not required for actin fiber formation. J. Cell Sci. 113, (Pt 7): 1177–1188 (2000). [DOI] [PubMed] [Google Scholar]
- Granot-Attas S., Luxenburg C., Finkelshtein E. & Elson A. Protein tyrosine phosphatase epsilon regulates integrin-mediated podosome stability in osteoclasts by activating Src. Mol. Biol. Cell 20, 4324–4334 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioannidis J. P. et al. Meta-analysis of genome-wide scans provides evidence for sex- and site-specific regulation of bone mass. J. Bone Miner. Res. 22, 173–183 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson S. G. et al. Comparison of genome screens for two independent cohorts provides replication of suggestive linkage of bone mineral density to 3p21 and 1p36. Am. J. Hum. Genet. 72, 144–155 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullin B. H. et al. Further genetic evidence suggesting a role for the RhoGTPase-RhoGEF pathway in osteoporosis. Bone 45, 387–391 (2009). [DOI] [PubMed] [Google Scholar]
- Mullin B. H. et al. Identification of a role for the ARHGEF3 gene in postmenopausal osteoporosis. Am. J. Hum. Genet. 82, 1262–1269 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin T. J. & Sims N. A. Osteoclast-derived activity in the coupling of bone formation to resorption. Trends Mol. Med. 11, 76–81 (2005). [DOI] [PubMed] [Google Scholar]
- Nakamura T. et al. Estrogen prevents bone loss via estrogen receptor alpha and induction of Fas ligand in osteoclasts. Cell 130, 811–823 (2007). [DOI] [PubMed] [Google Scholar]
- Su X. et al. The ADP receptor P2RY12 regulates osteoclast function and pathologic bone remodeling. J. Clin. Invest. 122, 3579–3592 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H. et al. Synaptotagmin VII regulates bone remodeling by modulating osteoclast and osteoblast secretion. Dev. Cell 14, 914–925 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSelm C. J. et al. Autophagy proteins regulate the secretory component of osteoclastic bone resorption. Dev. Cell 21, 966–974 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D. Q. et al. V-ATPase subunit ATP6AP1 (Ac45) regulates osteoclast differentiation, extracellular acidification, lysosomal trafficking, and protease exocytosis in osteoclast-mediated bone resorption. J. Bone Miner. Res. 27, 1695–1707 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng S. et al. Atp6v1c1 is an essential component of the osteoclast proton pump and in F-actin ring formation in osteoclasts. Biochem. J. 417, 195–203 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ory D. S., Neugeboren B. A. & Mulligan R. C. A stable human-derived packaging cell line for production of high titer retrovirus/vesicular stomatitis virus G pseudotypes. Proc. Natl Acad. Sci. USA 93, 11400–11406 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asagiri M. et al. Cathepsin K-dependent toll-like receptor 9 signaling revealed in experimental arthritis. Science 319, 624–627 (2008). [DOI] [PubMed] [Google Scholar]
- Boini S. & Guillemin F. Radiographic scoring methods as outcome measures in rheumatoid arthritis: properties and advantages. Ann. Rheum. Dis. 60, 817–827 (2001). [PMC free article] [PubMed] [Google Scholar]
- Glasson S. S., Chambers M. G., Van Den Berg W. B. & Little C. B. The OARSI histopathology initiative—recommendations for histological assessments of osteoarthritis in the mouse. Osteoarthr. Cartil. 18, S17–S23 (2010). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures 1-10.
Data Availability Statement
The authors declare that the data supporting the findings of this study are available within the article and its Supplementary Information files.