Abstract
Aims
Given the high prevalence of psychotropic medication use in people with dementia and the potential for different prescribing practices in men and women, our study aimed to investigate sex differences in psychotropic medication use in older adults with Alzheimer's disease (AD) living in the US and Finland.
Methods
We used data collected between 2005 and 2011 as part of the National Alzheimer's Coordinating Center (NACC) in the US, and Medication use and Alzheimer's disease (MEDALZ) cohorts in Finland. We evaluated psychotropic medication use (antidepressant, antipsychotic, anxiolytic, sedative, or hypnotic) in participants aged 65 years or older. We employed multivariable logistic regression adjusted for demographics, co-morbidities, and other medications to estimate the magnitude of the association (adjusted odds ratio [aOR] with 95% confidence intervals [CIs]) according to sex.
Results
We included 1099 NACC participants (502 [45.68%] men, 597 [54.32%] women), and 67,049 participants from the MEDALZ cohort (22,961 [34.24%] men, 44,088 [65.75%] women). Women were more likely than men to use psychotropic medications: US, 46.2% vs. 33.1%, p < 0.001; Finland, 45.3% vs. 36.1%, p < 0.001; aOR was 2.06 (95% CI 1.58–2.70) in the US cohort and 1.38 (95% CI 1.33–1.43) in the Finnish cohort. Similarly, of the different psychotropic medications, women were more likely to use antidepressants (aOR-US: 2.16 [1.44–3.25], Finland: 1.52 [1.45–1.58]) and anxiolytics (aOR-US: 2.16 [1.83–3.96], Finland: 1.17 [1.13-1.23]) than men.
Conclusionl
Older women with AD are more likely to use psychotropic medications than older men, regardless of study population and country. Approaches to mitigate psychotropic medication use need to consider different prescribing habits observed in older women vs. men with AD.
1 Introduction
Current evidence suggests a high prevalence of psychotropic medication use among people with dementia. In a study of older people with dementia in the US, 57% used one or more psychotropic medications consistently over a 1-year period, regardless of whether they were living in the community or nursing homes (NH) [1]. Other studies have reported a similar prevalence, with 51% of community-dwelling older people with Alzheimer's disease (AD) in Finland being exposed to anticholinergic and sedative medications (mostly psychotropic medications) [2]. Alarmingly, psychotropic polypharmacy is highly prevalent in older people with dementia, with a recent study reporting an increase from 42% in 2004 to 50% in 2013 in patients being dispensed two or more psychotropic medications [3]. The observed high prevalence of psychotropic medications is of concern given the potential for significant harm and the limited evidence on efficacy in older people with dementia [4, 5]. Data from meta-analyses suggest that antipsychotic use in older people with dementia is associated with greater mortality [6], and evidence from well-designed observational studies suggests an association with a range of dose-dependent adverse events, including impaired physical function, frailty, increased risk of hospitalization, and mortality [2, 7, 8].
Factors contributing to psychotropic medication use and subsequent poor clinical outcomes in older people with dementia are complex. In particular, there are limited data on the role of biological sex in determining psychotropic medication utilization patterns in older people with cognitive impairment. However, sex is an important factor to consider in clinical practice as it can influence healthcare, the choice of pharmacological treatments, and patient outcomes [9, 10]. Disability and morbidity are more prevalent in older women than men; therefore, this may explain why older women are more likely to use more medications overall [11]. For instance, one study found that potentially inappropriate medication use was more common in women (24.6%) than in men (19.3%) [12]. Moreover, older women were at 50% higher odds of receiving three or more psychotropic medications than men (adjusted odds ratio 1.50; 95% confidence interval [CI] 1.47–1.53). Current evidence suggests that women with dementia are more likely to use certain psychotropic medications such as antipsychotics than men [13]. There are also differences in behavioral and psychological symptoms of dementia with men being more physically aggressive, apathetic, and regressive, while women tend to display depression, anxiety, and agitation more frequently [14, 15]. This may result in sex differences in medication utilization patterns, with men being more likely to use antipsychotics or a high dose [16]. Older men with dementia are more likely to experience adverse events when given antipsychotics than older women [15, 17].
To date, no study has explicitly compared the role of sex on psychotropic prescribing practices in older people with AD living in different countries. Our focus on comparing sex differences in psychotropic medication prescribing between countries was driven by the need to document patterns across populations with different healthcare systems, and potentially identifying country-specific factors for psychotropic medication use that could help guide targeted intervention to optimize medication use in this population [18]. Therefore, the aim of this study was to evaluate sex differences in psychotropic medication prescribing (referred to as ‘use’ or ‘utilization’ hereafter) in older adults with AD living in the US and Finland. We also aimed to examine sociodemographic and clinical characteristics that contribute to these differences.
2 Methods
2.1 Study Design and Population
Our investigation was conducted using data from two cohorts: the National Alzheimer's Coordinating Center (NACC) in the US and the Medication use and Alzheimer's disease (MEDALZ) in Finland. Cohort data use was approved by local ethics committees at each institution and owing to the de-identified nature of the data included in our current study, informed consent was waived. Specifically, for the US data, the Alzheimer's Disease Research Center Clinical/Research Core protocol was approved by the Institutional Review Board of the University of Kentucky. Ethics committee approval was not required for the MEDALZ cohort according to Finnish legislation as only register-based data were used and persons were not contacted.
2.1.1 US Cohort
The NACC was established in 1999 through the funding of Alzheimer's Disease Centers (ADC) by the National Institute on Aging. Since inception of the Uniform Data Set in 2005, 34 past and present ADC throughout the US collected self-reported information and conducted standardized cognitive and behavioral assessments in participants with the full range of cognitive functioning, from normal to dementia. Participants are recruited through clinician or self-referral (patients or family members), or through active community recruitment strategies following ADC-specific recruitment protocols. Detailed descriptions of the cohort as well as the various instruments and assessments used for data collection are described elsewhere [19–22]. Briefly, NACC UDS data includes information on sociodemographic characteristics, family history, medical history, and medication use. In addition, participants undergo neuropsychological evaluations using validated instruments.
For this investigation, we used UDS enrollment and yearly follow-up visits data collected between 2005 and 2011 to reflect the similar time window available from the Finnish cohort. We identified 1169 participants newly diagnosed with AD during the cohort follow-up (i.e., free of dementia at enrollment), of which 1099 were aged ≥65 years. A participant was considered to have AD if they met the criteria for dementia and had probable AD as the primary clinical diagnosis based on the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association (NINCDS/ADRDA) criteria [23]. Subjects without dementia diagnosis or those with non-AD dementia were excluded from this analysis.
2.1.2 Finnish Cohort
The MEDALZ cohort is a population-based register of all community-dwelling individuals diagnosed with AD between 2005 and 2011 in Finland. The MEDALZ cohort collected information from several nationwide registers including the Prescription Register (1995–2012), Special Reimbursement Register (1972–2012), Hospital Discharge Register (1972–2012), and Statistics Finland (socioeconomic data since 1970 and causes of death 2005–12). The Prescription Register includes information on reimbursed purchases of medications classified according to the Anatomical Therapeutic Chemical classification system [24]. The register includes data on community-dwelling individuals (i.e., medications used in hospital or NH are not recorded). Older adults with AD have been identified from the Special Reimbursement Register [16, 25–28].
From this Finnish cohort, of the 70,718 adults diagnosed with AD between 2005 and 2011, our study included 67,049 individuals aged ≥65 years who were alive at the time when medication utilization was assessed (i.e., 6 months after the diagnosis). Similarly to the US cohort, the AD diagnosis was based on the NINCDS/ADRDA and Diagnostic and Statistical Manual of Mental Disorders, 4th edition criteria [23, 29]. In Finland, current guidelines for care recommend that all persons with clinically verified AD should be prescribed anti-dementia drugs unless there are contraindications for use [30].
2.2 Study Variables
The index date was defined as the first visit when the patient was identified with AD during the cohort follow-up (US cohort) or 6 months after the date of diagnosis (Finnish cohort). Medication exposure in the US cohort was measured from self-reported data using the ‘brown bag’ medication review approach (i.e., the participant or a family member were asked to bring all the medications to the research assessment) on prescription and over-the-counter medications for the 2-week window preceding the index date [21]. Medication exposure in the MEDALZ data was defined as medications used during a 2-week period before the index date from register-based data. The use periods were modeled with the PRE2DUP method as previously described [31–33]. Exposure to psychotropic medication was defined as the use of an antidepressant, antipsychotic, or an anxiolytic, sedative, or hypnotic. We also assessed the use of medications approved for AD treatment and the total number of medications used at the index date for each person in the two cohorts.
Sociodemographic characteristics included race, education, living situation, and type of residence for the US cohort and socioeconomic position for the Finnish cohort. Race was categorized as White, Black, or other. Education was categorized as high-school degree or less, college education, or graduate education. Living situation was defined as living alone, living with spouse or partner, or other living arrangements. Residence type was described as single family residence, retirement community, assisted living (assisted living, boarding home, adult family home, and skilled nursing facility, or NH), or other. Occupational socioeconomic position was defined for those included in the Finnish cohort as the highest recorded position since 1972 and was obtained from the censuses maintained by Statistics Finland. A six-category variable was constructed with the following categories “managerial/professional”, “office worker”, “farming/forestry”, “sales/industry/cleaning”, “unknown”, and “did not respond” [34, 35].
Cognitive evaluation information was available only from the US data and included the clinical dementia rating (CDR) [36], and the Mini-Mental State Examination (MMSE) [37]. In our study, we included the standard global CDR score categorized as no impairment (CDR = 0), questionable impairment (CDR = 0.5), mild impairment (CDR = 1), and moderate tosevere impairment (CDR = 2 or 3). MMSE score was categorized as normal (MMSE = 27–30), mild impairment (MMSE = 21–26), moderate impairment (MMSE = 11–20),or severe impairment (MMSE = 0–10).
Behavioral symptoms available only from the US data included information collected as part of the Neuropsychiatric Inventory Questionnaire [38, 39]; specifically, for our study, the analyses included indicators for the presence of symptoms of delusions, hallucinations, agitation or aggression, depression or dysphoria, and anxiety.
Co-morbidities were measured at the index date for both cohorts. For the US cohort, indicators were created based on the health history at the index date visit. For the Finnish cohort, the Special Reimbursement Register data were used for defining co-morbidities. Some co-morbidities were available for both cohorts (cardiovascular diseases, diabetes mellitus, stroke, history of seizures [US cohort] or epilepsy [Finnish cohort]), but some of them were only available for the US cohort (obesity, depression in the previous 2 years and Geriatric Depression Scale [40], psychiatric diagnosis, urinary incontinence) or for the Finnish cohort (history of hospital-treated depression, rheumatoid arthritis, asthma/chronic obstructive pulmonary disease, history of hip fracture). Cardiovascular diseases indicators included chronic heart failure, arterial hypertension, coronary artery disease, and chronic arrhythmia. History of stroke and hip fracture were collected from the Hospital Discharge Register for the Finnish cohort. Genetic information (apolipoprotein E allele) was available for US cohort participants.
2.3 Statistical Analyses
Baseline characteristics were compared using descriptive statistics (mean, standard deviation [SD], and range for continuous variables, and proportions for categorical variables). We used the t test (or the Mann–Whitney test if normality assumptions were not met) to determine statistical association with continuous variables, and the chi-square statistic to document the statistical association with categorical variables. Prevalence of psychotropic medication use by sex, overall, and for each of the different medication classes included in this category was evaluated for both cohorts. We also investigated whether age (<8 0 and ≥80 years), in addition to sex, influenced our results.
We used unconditional logistic regression to estimate the association between sex and psychotropic medication use. Two different multivariable models were developed for each cohort to address confounding and to determine the adjusted estimates for odds ratio (aOR) and the 95% CI. The first model included baseline characteristics available from both countries (Table 1). The second model added country-specific information to those included in the first model (Table 1 plus country-specific information from Table 2). All analyses were conducted at the level of statistical significance of 0.05 using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA) [41].
Table 1. Baseline characteristics.
| Baseline characteristics | US cohort | Finnish cohorta | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Male (N = 502) | Female (N = 597) | p value | Male (N = 22,961) | Female (N = 44,088) | p value | |
| Age at diagnosis (years) | 0.175 | <0.001 | ||||
| Mean (SD) | 79.8 (6.64) | 80.4 (8.02) | 79.5 (6.0) | 81.2 (6.1) | ||
| Minimum, maximum | 65, 98 | 65, 104 | 65, 101 | 65, 103 | ||
| Co-morbidities, N (%) | ||||||
| Cardiovascular disease | 382 (76.1) | 411 (68.8) | 0.008 | 12,481 (54.4; 53.7–55.0) | 22,601 (51.3; 50.1–51.7) | <0.001 |
| Stroke | 34 (6.8) | 38 (6.4) | 0.786 | 3050 (13.3; 12.9–13.7) | 3958 (9.0; 8.7–9.3) | <0.001 |
| Seizures (US cohort)/epilepsy (Finnish cohort) | 11 (2.2) | 13 (2.2) | 0.988 | 603 (2.6; 2.4–2.8) | 809 (1.8; 1.7–2.0) | <0.001 |
| Diabetes mellitus | 74 (14.7) | 62 (10.4) | 0.029 | 3764 (16.4; 15.9–16.9) | 5536 (12.6; 12.3–12.9) | <0.001 |
| Number of medications, N (%) | 0.128 | <0.001 | ||||
| Mean (SD) | 7 (3.59) | 6.6 (3.79) | 5.5 (3.3) | 5.8 (3.4) | ||
| Minimum, maximum | 0, 20 | 0, 21 | 0, 23 | 0, 23 | ||
| Number of medication categories, N (%) | 0.008 | <0.001 | ||||
| 0 | 9 (1.8) | 18 (3.1) | 835 (3.6; 3.4–3.9) | 1354 (3.1; 2.9–3.2) | ||
| 1–4 | 110 (22.4) | 175 (29.8) | 8809 (38.4; 37.7–39.0) | 15,759 (35.7; 35.3–36.2) | ||
| 5–9 | 269 (54.7) | 267 (45.5) | 10,493 (45.7; 45.1–46.3) | 20,872 (47.3; 46.9–47.8) | ||
| 10 and more | 104 (21.1) | 127 (21.6) | 2824 (12.3; 11.9–12.7) | 6103 (13.8; 13.5–14.2) | ||
| Cognitive enhancers, N (%) | ||||||
| Any approved drugs for AD treatment | 324 (64.5) | 312 (52.3) | <0.001 | 15,973 (69.6; 69.0–70.2) | 29,425 (66.7; 66.3–67.2) | <0.001 |
| Memantine | 151 (30.1) | 131 (21.9) | 0.007 | 3280 (14.3; 13.8–14.7) | 5777 (13.1; 12.8–13.4) | <0.001 |
| Cholinesterase inhibitors | 297 (59.2) | 288 (48.2) | <0.001 | 13,341 (58.1; 57.5–58.7) | 24,850 (56.4; 55.9–56.8) | <0.001 |
AD Alzheimer's disease, CI confidence interval, SD standard deviation
For the Finnish cohort, table includes N (%) with 95% CI for %
Table 2. Cohort-specific characteristics at the index date.
| Baseline characteristicsa | Male, N (%) | Female, N (%)b | p value | |
|---|---|---|---|---|
| US cohort | ||||
| Race | 0.032 | |||
| White | 446 (88.8) | 491 (82.2) | ||
| Black | 34 (6.8) | 65 (10.9) | ||
| Other | 22 (4.4) | 41 (6.9) | ||
| Education | <0.001 | |||
| High school or less | 111 (22.21) | 203 (34.0) | ||
| College | 189 (37.95) | 244 (40.87) | ||
| Graduate | 202 (40.24) | 150 (25.13) | ||
| Living situation | <0.001 | |||
| Lives alone | 49 (9.8) | 214 (35.8) | ||
| Lives with spouse or partner | 408 (81.3) | 256 (42.9) | ||
| Other living arrangements | 45 (9.0) | 127 (21.3) | ||
| Type of residence | 0.017 | |||
| Single family residence | 439 (87.5) | 485 (81.2) | ||
| Retirement community | 38 (7.6) | 55 (9.2) | ||
| Assisted livinga | 19 (3.8) | 47 (7.9) | ||
| Other | 6 (1.2) | 10 (1.7) | ||
| Level of independence | 0.651 | |||
| Able to live independently | 184 (36.7) | 199 (33.3) | ||
| Requires some assistance with complex activities | 247 (49.2) | 297 (49.7) | ||
| Requires some assistance with basic activities | 60 (12.0) | 88 (14.7) | ||
| Completely dependent | 11 (2.2) | 13 (2.2) | ||
| BMI | <0.001 | |||
| Mean (SD) | 25.8 (3.63) | 24.6 (4.72) | ||
| Q1, median, Q3 | 23, 25, 28 | 21, 24, 27 | ||
| BMI categories | <0.001 | |||
| Obese | 64 (14.4) | 68 (13.6) | ||
| Global clinical dementia rating | 0.776 | |||
| No impairment | 0 (0) | 0 (0) | ||
| Questionable impairment | 272 (54.2) | 327 (54.8) | ||
| Mild impairment | 207 (41.2) | 237 (39.7) | ||
| Moderate/severe impairment | 23 (4.6) | 33 (5.5) | ||
| MMSE category | 0.232 | |||
| Normal | 112 (23.0) | 119 (21.3) | ||
| Mild | 277 (57.0) | 348 (62.4) | ||
| Moderate | 81 (16.7) | 72 (12.9) | ||
| Severe | 2 (0.4) | 3 (0.5) | ||
| Unknown | 14 (2.9) | 16 (2.9) | ||
| Behavioral symptoms | ||||
| Delusions | 36 (7.4) | 50 (8.6) | 0.481 | |
| Hallucinations | 11 (2.3) | 21 (3.6) | 0.199 | |
| Agitation or aggression | 137 (28.3) | 158 (27.2) | 0.693 | |
| Depression or dysphoria | 157 (32.4) | 231 (39.8) | 0.013 | |
| Anxiety | 157 (32.4) | 178 (30.7) | 0.541 | |
| Geriatric Depression Scale score categories | 0.616 | |||
| 0–4 | 405 (84.2) | 455 (81.1) | ||
| 5–9 | 59 (12.3) | 84 (15.0) | ||
| 10–15 | 9 (1.9) | 12 (2.1) | ||
| Unknown | 8 (1.7) | 10 (1.8) | ||
| Co-morbidities | ||||
| Depression within the past 2 years | 178 (35.5) | 279 (46.7) | <0.001 | |
| Psychiatric disorders | 29 (5.8) | 53 (8.9) | 0.051 | |
| Transient ischemic attack | 33 (6.6) | 44 (7.4) | 0.606 | |
| Incontinence: urinary | 84 (16.7) | 150 (25.1) | <0.001 | |
| Apolipoprotein e4 alleles | 0.119 | |||
| No e4 allele | 196 (39.0) | 214 (35.8) | ||
| Any copy of e4 allele | 225 (44.8) | 258 (43.2) | ||
| Finnish cohort | ||||
| Occupational socioeconomic position | <0.001 | |||
| Office worker | 714 (3.1; 2.9–3.3) | 4954 (11.2; 11.0–11.5) | ||
| Farming/forestry | 5195 (22.6; 22.1–23.2) | 7748 (17.6; 17.2 –17.9) | ||
| Sales/industry/cleaning | 10,528 (45.9; 45.2–46.5) | 18,228 (41.3; 40.9–41.8) | ||
| Unknown | 245 (1.1; 0.9–1.2) | 5318 (12.1; 11.8–12.4) | ||
| Did not respond | 159 (0.7; 0.6–0.8) | 353 (0.8; 0.7–0.9) | ||
| Co-morbidities | ||||
| Asthma/COPD | 2077 (9.1; 8.7–9.4) | 3908 (8.9; 8.6–9.1) | 0.434 | |
| Rheumatoid arthritis | 750 (3.3; 3.0–3.5) | 2319 (5.3; 5.1–5.5) | <0.001 | |
| History of hip fracture | 764 (3.3; 3.1–3.6) | 3351 (7.6; 7.4–7.9) | <0.001 | |
| Hospital-treated depression | 519 (2.3; 2.1–2.5) | 1700 (3.9; 3.7–4.1) | ||
BMI body mass index, CI confidence interval, COPD chronic obstructive pulmonary disorder, MMSE Mini-Mental State Examination, SD standard deviation
Columns display N (%), unless otherwise specified
For the Finnish cohort, table includes N (%) with 95% CI for %
3 Results
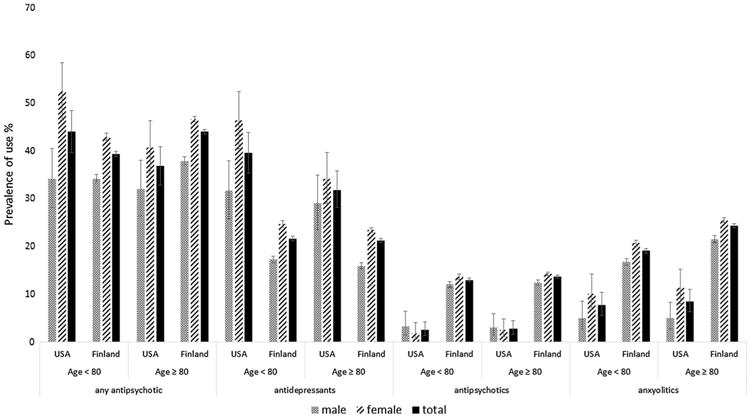
The US cohort included 502 (45.68%) men and 597 (54.32%) women with AD, whereas the Finnish cohort included 22,961 (34.24%) men and 44,088 (65.75%) women with AD. Table 1 shows the distribution of baseline characteristics by sex for the information available from both cohorts. Additional country-specific baseline characteristics are included in Table 2. Mean (SD) age for participants at the index date was 80.2 (7.43) for the US cohort and 80.6 (6.1) for the Finnish cohort. Men had a statistically significantly higher prevalence of cardiovascular disease and diabetes (both countries), as well as stroke and epilepsy (Finland); there were no sex differences for stroke and seizure history for US cohort participants. When comparing the number of medications used, in the US, the prevalence of using five or more medications was higher in men than in women (Table 1). In addition, men in the US cohort were more likely to report the use of cognitive enhancers than women (Table 1). When comparing psychotropic medication use by sex, women were more likely to use psychotropic medications than men in both the US (46.2% [95% CI 42.2–50.3] vs. 33.1% [95% CI 28.9–37.4], p < 0.001) and Finland (45.3% [95% CI 44.8–45.8] vs. 36.1% [95% CI 35.5–36.7], p < 0.001) (Table 1; Fig. 1). In relation to medication classes, the sex difference was observed in both countries for antidepressants and anxiolytics, while sex was associated with antipsychotic use in Finland only. When evaluating sex differences by age, the pattern of use when comparing men and women remained the same for the two age groups investigated (Fig. 2).
Fig. 1. Exposure to psychotropic medications according to sex in the US and Finnish cohorts.
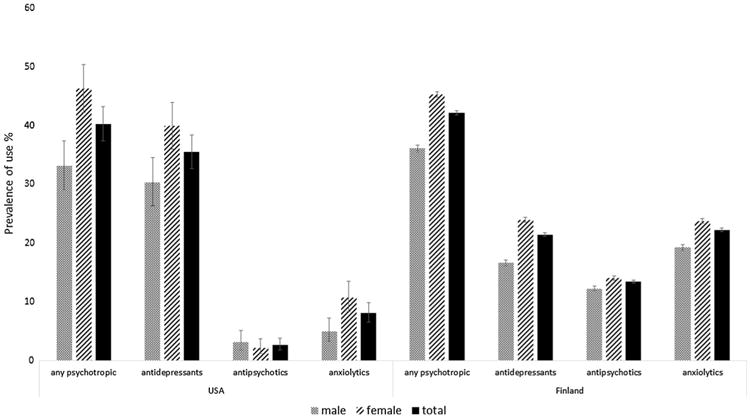
Fig. 2. Exposure to psychotropic medications according to sex and age in the US and Finnish cohorts.
Of the behavioral symptoms evaluated from the NACC cohort, depression/ dysphoria was most commonly reported by women (Table 2). No sex differences were observed between men and women concerning other behavioral symptoms.
The results of the logistic regression analysis are presented in Table 3. Specifically, when comparing women with men in the US cohort, the unadjusted odds ratio was 1.74 (95% CI 1.36–2.23), while the aOR was 2.08 (95% CI 1.59–2.71) in the limited data model, and 2.29 (95% CI 1.56–3.37) in the extended data model. The estimates from the Finnish data were odds ratio = 1.47 (95% CI 1.42–1.52) in the unadjusted analysis and odds ratio = 1.39 (95% CI 1.34–1.44) and odds ratio = 1.38 (95% CI 1.33–1.43) for the limited data model and the extended data model, respectively. Similarly, in both countries, women were more likely to use antidepressants or anxiolytics as compared with men, in both unadjusted and adjusted analyses. However, when looking at antipsychotic use, there was not a statistically significant difference between women and men in the US cohort (Table 3). However, women were more likely than men to report using antipsychotics in the Finnish cohort (Table 3).
Table 3. Psychotropic medication use in women as compared with men.
| Country | Unadjusted OR (95% CI) | Adjusted OR (95% CI) Limited dataa | Adjusted OR (95% CI) Extended datab | |
|---|---|---|---|---|
| Any psychotropic medication use | US | 1.74 (1.36–2.23) | 2.08 (1.59–2.71) | 2.29 (1.56–3.37) |
| Finland | 1.47 (1.42–1.52) | 1.39 (1.34–1.44) | 1.34 (1.29–1.39) | |
| Antidepressant use | US | 1.51 (1.18–1.93) | 1.76 (1.34–2.3) | 2.16 (1.44–3.25) |
| Finland | 1.58 (1.52–1.65) | 1.52 (1.46–1.59) | 1.48 (1.41–1.55) | |
| Antipsychotic use | US | 0.85 (0.48–1.52) | 0.79 (0.37–1.71) | 0.61 (0.2–1.82) |
| Finland | 1.17 (1.12–1.23) | 1.09 (1.04–1.15) | 1.05 (1.01–1.11) | |
| Anxiolytic use | US | 1.99 (1.31–3.05) | 2.43 (1.49–3.98) | 2.16 (1.83–3.96) |
| Finland | 1.31 (1.26–1.36) | 1.18 (1.13–1.23) | 1.14 (1.09–1.19) |
4 Discussion
To our knowledge, this is the first study to explicitly investigate psychotropic medication use according to sex in older adults with AD. We found that psychotropic medication use was more common among older women than older men with AD in both the US (46 vs. 33%) and Finland (45 vs. 36%). After adjusting for covariates, older women with AD were two times more likely to receive psychotropic medications in the US cohort (aOR = 2.06; 95% CI 1.58–2.70) and 1.4 times more likely (aOR = 1.38; 95% CI 1.33–1.43) in the Finnish cohort, compared with older men. Despite differences in medication data collection approaches across study cohorts (i.e., self-reported in the US vs. prescription registry data in Finland), it is important to note that sex differences were apparent in both cohorts. Self-reported data are subject to bias and may have resulted in underreporting. However, considering that the medication inventory in the US cohort was conducted using the brown-bag approach, the potential misclassification of medication use by sex is likely non-differential and thus probably underestimating the real sex difference. Although it was not the primary focus of our article, it is worth noting that, in the US cohort, polypharmacy (five or more medications) was more common among men than women, and men were more likely to use cognitive enhancers than women. The reason for sex differences in cognitive enhancers use should be further investigated in future studies.
The findings of our study are similar to recent studies comparing the use of cognitive enhancers and psychotropic medications in women and men [1, 13, 42]. In a study evaluating the prevalence of psychotropic medications use across different settings by Medicare beneficiaries in the US, sex was an important factor associated with use [1]. Similarly, in a European study, women with dementia were more likely to use antipsychotics than men [13]. A study using the National Health and Nutrition Examination Survey in the US found that for all race categories, older women were more likely to report psychotropic medication use than men [42]. In a recent study of community-dwelling older adults living in Canada, older women were at 16% higher odds of receiving potentially inappropriate medications, measured using 2015 Beers Criteria than older men [43]. These differences were mostly the result of older women using psychotropic medications including antidepressants and benzodiazepines. Older women are more likely like to have more co-morbidities than men; therefore, this may in part explain differences in psychotropic medication use. Moreover, the importance of considering personality traits as a potential factor influencing psychotropic medication use has been also highlighted [44]. Future studies investigating the role of sex in psychotropic prescribing patterns should account for personality traits.
In our study, in the US cohort, psychotropic medication use consisted mainly of antidepressant use, whereas antipsychotics and anxiolytics were less commonly used among both men and women. In Finland, anxiolytics were the most frequently used psychotropic medication among men, while antidepressants and anxiolytics were the most commonly used classes among women (Fig. 1). The prevalence of behavioral symptoms might explain the observed difference between men and women. Previous studies have reported that women experience behavioral symptoms, especially depressive symptoms, more frequently than men [14, 45, 46]. We compared the prevalence of behavioral symptoms in the US cohort and only depression and dysphoria were more frequently reported by women. We also adjusted our regression model for behavioral symptoms in the US cohort and the association between female sex and psychotropic medication use persisted. Antipsychotic use was significantly more frequent in the Finnish cohort than in the US cohort. This discrepancy might be related to AD disease severity or may reflect national treatment practices and restricted use of antipsychotics in the US. Previous research evaluating antipsychotic use in US veterans with dementia living in the community showed that 14–15% of veterans had outpatient prescriptions for an antipsychotic [47]. In our US cohort, 95% of the participants had questionable or mild impairment (CDR global = 0.5 or 1), and a small proportion had behavioral symptoms, which can explain the low prevalence of antipsychotics. Furthermore, when looking at use by level of impairment, prevalence increased from 1.17% for a CDR global score of 0.5–3.6% for CDR of 1, and to 34.62% for a CDR of 2 or 3. In Finland, clinical care guidelines recommend antipsychotics and anxiolytics only for short-term use and if non-pharmacological options are not effective [30]. However for the Finnish cohort, we did not have information on disease severity at the time we measured antipsychotic use and we could not evaluate if the same pattern would be identified in the US cohort. Both cohorts included persons newly diagnosed with AD, and the mean age was similar.
Importantly, there is a need for more research to elucidate the role of sex and its impact on clinical outcomes among older people with and without dementia. Recent findings on the association between antipsychotics and mood stabilizers and impairment on activities of daily living in older women suggest sex differences in response to psychotropic medication among older people with dementia living in NH [48]. In addition, it would be important to investigate whether the observed sex differences in the use of psychotropic medications are consistent over time.
4.1 Strengths and Limitations
4.1.1 US Cohort
An important strength of the NACC data comes from the use of validated instruments and standardized testing to collect patient-reported information and to conduct in-depth cognitive evaluations in all study participants. One of the limitations in using these data stems from the collection of data at enrollment and yearly after. Our approach of defining the index date as the date a participant was first identified with AD during follow-up in the cohort does not necessarily coincide with the time she/he was first diagnosed with AD. In addition, given that medication use asked about current medications taken by the participant (i.e., within 14 days of the visit), we could not ascertain psychotropic medication exposure (and other medications) accurately. Participants may have been misclassified as non-users in the situation in which they started and stopped treatment between two consecutive study visits. Last, considering the recruitment strategies for the NACC, participants enrolled in this cohort were not necessarily a representative random sample for the entire US population of patients with or without cognitive impairment. Participants in the NACC cohort, and therefore in our study, were generally more educated, had higher incomes, and were more likely to receive care in academic hospitals and clinics. Thus, careful consideration is needed when attempting to generalize our findings to all older adults with AD living in the US.
4.1.2 Finnish Cohort
The MEDALZ cohort included all community-dwelling people with clinically verified AD diagnosis in Finland. The strengths of these nationwide data result from the inclusion of all socioeconomic classes and the fact that medication use is assessed from registers, thus limiting recall bias or under-reporting. Limitations of the MEDALZ data relate to limitations of registers used in data collection. Register-based data do not include clinical information on behavioral symptoms and other indications for psychotropic medication use. Furthermore, we could not assess MMSE or the severity of AD, although all persons had a similar time period since AD diagnosis. However, possible delays or sex differences in the diagnostic process could not be studied. While dispensing data are considered a more accurate estimate of medication exposure than other sources of prescribing data, they may not reflect the actual medication use.
5 Conclusion
We found that older women with AD are more likely to report psychotropic medication use than older men in both countries. These findings may suggest that approaches to mitigate psychotropic medication use among older people with dementia should consider different prescribing habits observed in women vs. men. Further research is needed to elucidate underlying risk factors for the differences in psychotropic medication use in women and men with AD.
Key Points.
Older women with dementia were more likely to use psychotropic medications than older men in the US (46 vs. 33%) and Finland (45 vs. 36%).
The difference between women and men with regard to psychotropic medication use persisted even after accounting for behavioral symptoms and other important confounders.
Our results suggest that sex needs to be taken into consideration when prescribing to older people with dementia.
Acknowledgments
Funding: This work was supported by Grant No. K12 DA035150 (Building Interdisciplinary Research Careers in Women's Health) from the National Institutes of Health, Office of Women's Health Research and the National Institute on Drug Abuse (DM) and National Health and Medical Research Early Career Fellowship (DG). The NACC database is funded by the National Institute on Aging (NIA)/National Institutes of Health (NIH) Grant U01 AG016976. NACC data are contributed to by the NIA-funded ADCs: P30 AG019610 (PI Eric Reiman, MD), P30 AG013846 (PI Neil Kowall, MD), P50 AG008702 (PI Scott Small, MD), P50 AG025688 (PI Allan Levey, MD, PhD), P50 AG047266 (PI Todd Golde, MD, PhD), P30 AG010133 (PI Andrew Saykin, PsyD), P50 AG005146 (PI Marilyn Albert, PhD), P50 AG005134 (PI Bradley Hyman, MD, PhD), P50 AG016574 (PI Ronald Petersen, MD, PhD), P50 AG005138 (PI Mary Sano, PhD), P30 AG008051 (PI Steven Ferris, PhD), P30 AG013854 (PI M. Marsel Mesulam, MD), P30 AG008017 (PI Jeffrey Kaye, MD), P30 AG010161 (PI David Bennett, MD), P50 AG047366 (PI Victor Henderson, MD, MS), P30 AG010129 (PI Charles DeCarli, MD), P50 AG016573 (PI Frank LaFerla, PhD), P50 AG016570 (PI Marie-Francoise Chesselet, MD, PhD), P50 AG005131 (PI Douglas Galasko, MD), P50 AG023501 (PI Bruce Miller, MD), P30 AG035982 (PI Russell Swerdlow, MD), P30 AG028383 (PI Linda Van Eldik, PhD), P30 AG010124 (PI John Trojanowski, MD, PhD), P50 AG005133 (PI Oscar Lopez, MD), P50 AG005142 (PI Helena Chui, MD), P30 AG012300 (PI Roger Rosenberg, MD), P50 AG005136 (PI Thomas Montine, MD, PhD), P50 AG033514 (PI Sanjay Asthana, MD, FRCP), P50 AG005681 (PI John Morris, MD), and P50 AG047270 (PI Stephen Strittmatter, MD, PhD).
HT participated in research projects funded by Janssen with grants paid to an employer institution outside of this work. AT participated in research projects funded by Janssen with grants paid to Karolinska Institutet outside of this work. JT has served as a consultant to Lundbeck, Organon, Janssen-Cilag, Eli Lilly, AstraZeneca, F. Hoffman-La Roche, and Bristol-Myers Squibb, and has received fees for giving expert opinions to Bristol-Myers Squibb and GlaxoSmithKline, lecture fees from Janssen-Cilag, Bristol-Myers Squibb, Eli Lilly, Pfizer, Lundbeck, GlaxoSmithKline, AstraZeneca, and Novartis; and a grant from Stanley Foundation. JT is a member of the advisory boards for AstraZeneca, Janssen-Cilag, and Otsuka. SH received lecture fees from MSD and Professio for providing lectures concerning medications in old age.
Footnotes
Author contributions: DM: study concept and design, acquisition of data, data analysis and interpretation, and preparation and editing of manuscript. HT: study concept and design, acquisition of data, data analysis and interpretation, and preparation and editing of manuscript. AMT: acquisition of data and interpretation, critical revising of the manuscript for important intellectual content, and final approval of the version to be published. AT: acquisition of data and interpretation, critical revising of the manuscript for important intellectual content, and final approval of the version to be published. JT: acquisition of data and interpretation, critical revising of the manuscript for important intellectual content, and final approval of the version to be published. SH: study concept and design, acquisition of data, data analysis and interpretation, and preparation and editing of manuscript. QW: data analysis, critical revising of the manuscript for important intellectual content, and final approval of the version to be published. GJ: study concept and design, interpretation of study findings, critical revising of the manuscript for important intellectual content, and final approval of the version to be published. DG: study concept and design, interpretation of findings, and preparation and editing of manuscript.
Conflict of interest: DM, AMT, QW, GJ and DG declare no conflicts of interest.
Compliance with Ethical Standards: Ethical approval: Cohort data use was approved by local ethics committees at each institution and owing to the de-identified nature of the data included in our current study, informed consent was waived. Specifically, for the US data, the Alzheimer's Disease Research Center Clinical/Research Core protocol was approved by the Institutional Review Board of the University of Kentucky. Ethics committee approval was not required for the MEDALZ cohort according to Finnish legislation as only register-based data were used and persons were not contacted.
Sponsor's role: None.
References
- 1.Rattinger GB, et al. Pharmacotherapeutic management of dementia across settings of care. J Am Geriatr Soc. 2013;61(5):723–33. doi: 10.1111/jgs.12210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gnjidic D, et al. Impact of high risk drug use on hospitalization and mortality in older people with and without Alzheimer's disease: a national population cohort study. PLoS One. 2014;9(1):e83224. doi: 10.1371/journal.pone.0083224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vasudev A, et al. Trends in psychotropic dispensing among older adults with dementia living in long-term care facilities: 2004–2013. Am J Geriatr Psychiatry. 2015;23(12):1259–69. doi: 10.1016/j.jagp.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 4.Ballard C, Waite J. The effectiveness of atypical antipsychotics for the treatment of aggression and psychosis in Alzheimer's disease. Cochrane Database Syst Rev. 2006;(1):CD003476. doi: 10.1002/14651858.CD003476.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banerjee S, et al. Sertraline or mirtazapine for depression in dementia (HTA-SADD): a randomised, multicentre, double-blind, placebo-controlled trial. Lancet. 2011;378(9789):403–11. doi: 10.1016/S0140-6736(11)60830-1. [DOI] [PubMed] [Google Scholar]
- 6.Schneider LS, Dagerman KS, Insel P. Risk of death with atypical antipsychotic drug treatment for dementia: meta-analysis of randomized placebo-controlled trials. JAMA. 2005;294(15):1934–43. doi: 10.1001/jama.294.15.1934. [DOI] [PubMed] [Google Scholar]
- 7.Gnjidic D, et al. Drug Burden Index associated with function in community dwelling older people living in Finland: a cross-sectional study. Ann Med. 2012;44:458–67. doi: 10.3109/07853890.2011.573499. [DOI] [PubMed] [Google Scholar]
- 8.Gnjidic D, et al. High risk prescribing and incidence of frailty among older community-dwelling men. Clin Pharmacol Ther. 2012;91:521–8. doi: 10.1038/clpt.2011.258. [DOI] [PubMed] [Google Scholar]
- 9.Legato MJ, Johnson PA, Manson JE. Consideration of sex differences in medicine to improve health care and patient outcomes. JAMA. 2016;316(18):1865–6. doi: 10.1001/jama.2016.13995. [DOI] [PubMed] [Google Scholar]
- 10.Clayton JA, Tannenbaum C. Reporting sex, gender, or both in clinical research? JAMA. 2016;316(18):1863–4. doi: 10.1001/jama.2016.16405. [DOI] [PubMed] [Google Scholar]
- 11.Bierman AS, et al. Sex differences in inappropriate prescribing among elderly veterans. Am J Geriatr Pharmacother. 2007;5(2):147–61. doi: 10.1016/j.amjopharm.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 12.Johnell K, Weitoft GR, Fastbom J. Sex differences in inappropriate drug use: a register-based study of over 600,000 older people. Ann Pharmacother. 2009;43(7):1233–8. doi: 10.1345/aph.1M147. [DOI] [PubMed] [Google Scholar]
- 13.Foebel AD, et al. Quality of care in European home care programs using the second generation interRAI Home Care Quality Indicators (HCQIs) BMC Geriatr. 2015;15(1):148. doi: 10.1186/s12877-015-0146-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lovheim H, et al. Sex differences in the prevalence of behavioral and psychological symptoms of dementia. Int Psychogeriatr. 2009;21(3):469–75. doi: 10.1017/S1041610209008497. [DOI] [PubMed] [Google Scholar]
- 15.Zuidema SU, et al. Predictors of neuropsychiatric symptoms in nursing home patients: influence of gender and dementia severity. Int J Geriatr Psychiatry. 2009;24(10):1079–86. doi: 10.1002/gps.2225. [DOI] [PubMed] [Google Scholar]
- 16.Taipale H, et al. Antipsychotic doses among community-dwelling persons with Alzheimer disease in Finland. J Clin Psychopharmacol. 2014;34(4):435–40. doi: 10.1097/JCP.0000000000000133. [DOI] [PubMed] [Google Scholar]
- 17.Rochon PA, et al. Older men with dementia are at greater risk than women of serious events after initiating antipsychotic therapy. J Am Geriatr Soc. 2013;61(1):55–61. doi: 10.1111/jgs.12061. [DOI] [PubMed] [Google Scholar]
- 18.Lucas C, Byles J, Martin JH. Medicines optimisation in older people: taking age and sex into account. Maturitas. 2016;93:114–20. doi: 10.1016/j.maturitas.2016.06.021. [DOI] [PubMed] [Google Scholar]
- 19.Beekly DL, et al. The National Alzheimer's Coordinating Center (NACC) database: the uniform data set. Alzheimer Dis Assoc Disord. 2007;21(3):249–58. doi: 10.1097/WAD.0b013e318142774e. [DOI] [PubMed] [Google Scholar]
- 20.Beekly DL, et al. The National Alzheimer's Coordinating Center (NACC) database: an Alzheimer disease database. Alzheimer Dis Assoc Disord. 2004;18(4):270–7. [PubMed] [Google Scholar]
- 21.Morris JC, et al. The Uniform Data Set (UDS): clinical and cognitive variables and descriptive data from Alzheimer Disease Centers. Alzheimer Dis Assoc Disord. 2006;20(4):210–6. doi: 10.1097/01.wad.0000213865.09806.92. [DOI] [PubMed] [Google Scholar]
- 22.Weintraub S, et al. The Alzheimer's Disease Centers' Uniform Data Set (UDS): the neuropsychologic test battery. Alzheimer Dis Assoc Disord. 2009;23(2):91–101. doi: 10.1097/WAD.0b013e318191c7dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McKhann G, et al. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34(7):939–44. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 24.WHO Collobarating Centre for Drug Statistics Methodology. Anatomical Therapeutic Chemical classification system: structure and principles. [Accessed 15 Nov 2016];2015 http://www.whocc.no/atc/structure_and_principles/
- 25.Taipale H, et al. High prevalence of psychotropic drug use among persons with and without Alzheimer's disease in Finnish nationwide cohort. Eur Neuropsychopharmacol. 2014;24(11):1729–37. doi: 10.1016/j.euroneuro.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 26.Taipale H, et al. Antipsychotic polypharmacy among a nation-wide sample of community-dwelling persons with Alzheimer's disease. J Alzheimers Dis. 2014;41(4):1223–8. doi: 10.3233/JAD-140282. [DOI] [PubMed] [Google Scholar]
- 27.Taipale H, et al. Antidementia drug use among community-dwelling individuals with Alzheimer's disease in Finland: a nationwide register-based study. Int Clin Psychopharmacol. 2014;29(4):216–23. doi: 10.1097/YIC.0000000000000032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tolppanen AM, et al. Use of existing data sources in clinical epidemiology: Finnish health care registers in Alzheimer's disease research. The Medication use among persons with Alzheimer's Disease (MEDALZ-2005) study. Clin Epidemiol. 2013;5:277–85. doi: 10.2147/CLEP.S46622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 30.Duodecim FMS. Current care: memory disorders [in Finnish with English summary] [Accessed 15 Nov 2016];2010 http:\\www.kaypahoito.fi.
- 31.Taipale H, et al. Long-term use of benzodiazepines and related drugs among community-dwelling individuals with and without Alzheimer's disease. Int Clin Psychopharmacol. 2015;30(4):202–8. doi: 10.1097/YIC.0000000000000080. [DOI] [PubMed] [Google Scholar]
- 32.Taipale H, et al. Hospital care and drug costs from five years before until two years after the diagnosis of Alzheimer's disease in a Finnish nationwide cohort. Scand J Public Health. 2016;44(2):150–8. doi: 10.1177/1403494815614705. [DOI] [PubMed] [Google Scholar]
- 33.Tanskanen A, et al. From prescription drug purchases to drug use periods: a second generation method (PRE2DUP) BMC Med Inform Decis Mak. 2015;15:21. doi: 10.1186/s12911-015-0140-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Galobardes B, et al. Indicators of socioeconomic position (part 1) J Epidemiol Commun Health. 2006;60(1):7–12. doi: 10.1136/jech.2004.023531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Galobardes B, et al. Indicators of socioeconomic position (part 2) J Epidemiol Commun Health. 2006;60(2):95–101. doi: 10.1136/jech.2004.028092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43(11):2412–4. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 37.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician”. J Psychiatr Res. 1975;12(3):189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 38.Cummings JL, et al. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44(12):2308–14. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- 39.Kaufer DI, et al. Validation of the NPI-Q, a brief clinical form of the Neuropsychiatric Inventory. J Neuropsychiatry Clin Neurosci. 2000;12(2):233–9. doi: 10.1176/jnp.12.2.233. [DOI] [PubMed] [Google Scholar]
- 40.Sheikh JI, Yesavage JA. Geriatric Depression Scale (GDS): recent evidence and development of a shorter version. Clin Gerontol. 1986;5(1/2):65–173. [Google Scholar]
- 41.SAS Institute Inc. SAS version 9.4. Cary: SAS Institute Inc.; 2013. [Google Scholar]
- 42.Paulose-Ram R, et al. Trends in psychotropic medication use among U.S. adults. Pharmacoepidemiol Drug Saf. 2007;16(5):560–70. doi: 10.1002/pds.1367. [DOI] [PubMed] [Google Scholar]
- 43.Morgan SG, et al. Sex differences in the risk of receiving potentially inappropriate prescriptions among older adults. Age Ageing. 2016;45(4):535–42. doi: 10.1093/ageing/afw074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Petrovic M, et al. Personality traits and socio-epidemiological status of hospitalised elderly benzodiazepine users. Int J Geriatr Psychiatry. 2002;17(8):733–8. doi: 10.1002/gps.677. [DOI] [PubMed] [Google Scholar]
- 45.Kitamura T, et al. Gender differences in clinical manifestations and outcomes among hospitalized patients with behavioral and psychological symptoms of dementia. J Clin Psychiatry. 2012;73(12):1548–54. doi: 10.4088/JCP.11m07614. [DOI] [PubMed] [Google Scholar]
- 46.Steinberg M, et al. Risk factors for neuropsychiatric symptoms in dementia: the Cache County Study. Int J Geriatr Psychiatry. 2006;21(9):824–30. doi: 10.1002/gps.1567. [DOI] [PubMed] [Google Scholar]
- 47.Kales HC, et al. Trends in antipsychotic use in dementia 1999-2007. Arch Gen Psychiatry. 2011;68(2):190–7. doi: 10.1001/archgenpsychiatry.2010.200. [DOI] [PubMed] [Google Scholar]
- 48.Dutcher SK, et al. Effect of medications on physical function and cognition in nursing home residents with dementia. J Am Geriatr Soc. 2014;62(6):1046–55. doi: 10.1111/jgs.12838. [DOI] [PMC free article] [PubMed] [Google Scholar]


